CKD Management
CKD management relies exclusively on the referent nephrologist in collaboration with primary care physicians and other specialists (e.g., diabetologist, cardiologist). Patient care is on the basis of outpatient clinics in an ambulatory mode, with periodical consultations, clinical assessments, laboratory tests, or imaging monitoring with medicine prescription. From CKD stages 3–5, including RRT, all costs related to patient monitoring and treatment are covered by the universal social security insurance system, under the regime of long-lasting or chronic disease (Affection Longue Durée, list of 30 pathologies covered) (1).
Patients with CKD may benefit from dialysis care when necessary. It is at the discretion of the nephrologist to decide when and how to start RRT. For all patients, the nephrologist must follow the recommendations and implement best clinical practices developed by professional experts of the Haute Autorité de Santé (HAS) (2). The referent practitioner must present and explain all therapeutic modalities (e.g., RRT on the basis of facility, preemptive transplant), with their benefits and risks. The treatment modality choice must be shared with the patient and/or relatives, and noted in the medical file. When RRT is indicated, the patient is then referred to the care-provider partner of the network habilitated to provide the adequate therapeutic option. More information on CKD management in France can be found in a recent review of Nephrology in France (3), as part of a Worldwide Nephrology Project (4).
RRT: Legal Framework
Dialysis facilities are part of the national health service network serving the whole population under authority of the Ministry of Solidarity and Health, represented regionally by Regional Health Agencies (ARS). Renal care providers operate under three legal entities, namely public hospitals including academic hospitals, private for-profit (e.g., privately owned clinics or part of a chain) and private not-for-profit (e.g., associations). Total dialysis stations are provided through 891 facilities shared by 433 private not-for-profit entities (49%), 216 private for-profit entities (24%), 102 public hospitals (11%), 58 academic public hospitals (7%), and 82 mixed public-private entities (9%). Creation and distribution of dialysis units through various legal entities is established regionally every 5 years by ARS, according to the population’s needs defined by Réseau Epidémiologie et Information en Néphrologie registry. Legal entities then contract with ARS to provide the care service for the number of patients defined. Dialysis units belong to the health care facilities network, which is subject to regular certification (5 years) by the HAS. Nephrologists serving in these dialysis facilities must be certified, registered to French National Medical Council, and periodically accredited by a national organization (SIAM, HAS) that checks the acquisition of continuous medical education credits.
Dialysis-related costs are fully covered by the National Health Insurance Fund (Caisse Nationale Assurance Maladie) as part of the French Social Security System, no matter who the care provider is (public, private for-profit, or private not-for-profit), according to a yearly tariff established on the basis of facility, not modality. This is discussed later in the Funding of RRT section.
The technical operating conditions of dialysis units have been revised by law and what are known as “2002 September decrees” (5,6). They apply to all renal care providers. These decrees specify the technical platforms and quotas of minimum caregivers to ensure safety and care quality for patients: (1) the in-center hemodialysis (CHD) facility mainly supports patients whose health conditions (e.g., comorbidity, disabilities) require the permanent presence of doctor. It also specifies the number of patients per nephrologist and the number of nurses and/or caregivers per series of patients treated. The center must be located geographically within a health care facility, with full hospital beds, and emergency and continuous care services. The HD center has, on its own or by agreement, the services of a laboratory and imaging unit. The number of patients treated per day and per dialysis station is limited to three. The certified nurse to patient ratio is 1:4. (2) Satellite in-center HD units, namely medicalized dialysis units, do not require a continuous medical presence during the session. However, without being physically present, the medical team must be able to intervene during the session, while maintaining security. The nephrologist visit is carried out one to three times a week. Up to three patients can be treated per machine, per day. The certified nurse to patient ratio is 1:4 with a care helper. (3) Self-care HD (SCHD) units offer unassisted or nurse-assisted HD. (3a) Unassisted SCHD is intended for patients who are autonomous, and can provide their own treatment themselves. The permanent presence of a certified nurse is necessary to help patients when needed. The certified nurse to patient ratio is 1:6, with care helpers. A personal and not sharable dialysis machine is assigned to each patient to provide more flexibility and a wide range of treatment time schedules. (3b) Assisted SCHD is offered to patients who are semiautonomous and require the assistance of a certified nurse to perform certain actions (for example, arteriovenous fistula puncture). The maximum number of patients treated in this case is two per shift, per day. The unit has a permanent nurse. The certified nurse to patient ratio is 1:6 optionally with a care helper. The visit of the nephrologist, during the session, is provided at least once a quarter in simple SCHD, and at least once a month in assisted SCHD. In addition, an external consultation must be carried out at least once a quarter. (4) Home therapy relies on HD and home peritoneal dialysis (PD). Home HD is offered to patients who are autonomous and capable of performing all of the necessary actions for their treatment, in the presence of a person who can assist them. PD is provided under two options (unassisted or assisted) for patients able to treat themselves at home. For unassisted PD, patients receive a monthly allowance for costs related to treatment (such as electricity, water, media) and loss of financial resources. In assisted PD, the patient does not receive this allowance, but private nurse care fees for patients are paid for by the social security system. For home-based therapy, the care provider is responsible for training, monitoring, logistical support, and repatriation to the clinic if there are issues. A specialized coordinating nurse must be able to provide 24-hour and 7-day operational support. In all cases, so-called “out-of-center” dialysis units and the unit care providers must have, on their own or by convention, fallback protocols with specialized care structures capable of ensuring immediate care if there are complications and/or medical emergencies. (5) Training and repatriation units are intended to teach, train, and install equipment for patients at home when they are ready to perform their own treatment, and to ensure repatriation and continuity of care in case there are technical or medical issues. For serious medical issues, the patient should be referred to a secondary-level hospital or clinic.
Epidemiology of RRT: Updated End 2019
Epidemiologic data on RRT in France including overseas territories are captured, monitored, and analyzed via the national Réseau Epidémiologie et Information en Néphrologie registry on behalf of the Biomedical Agency, established in 2002 (7,8).
Global RRT: Overview
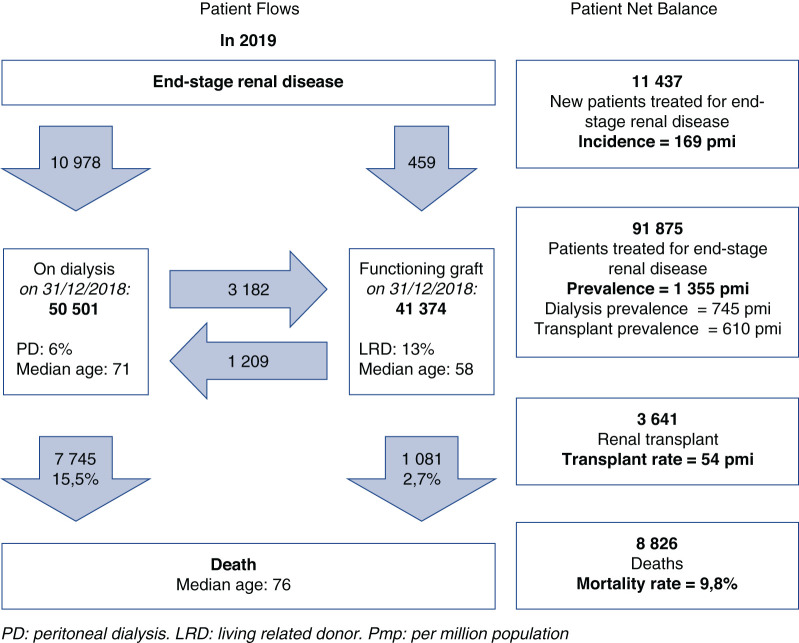
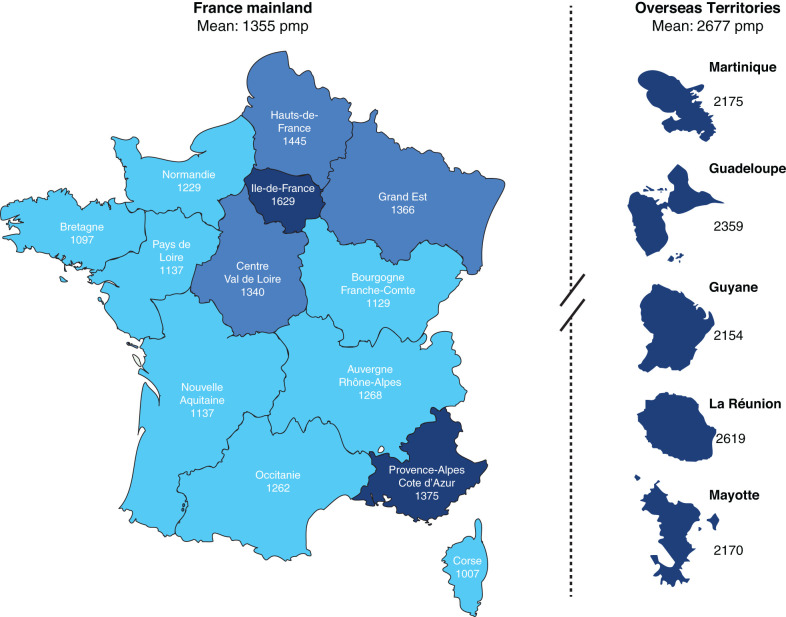
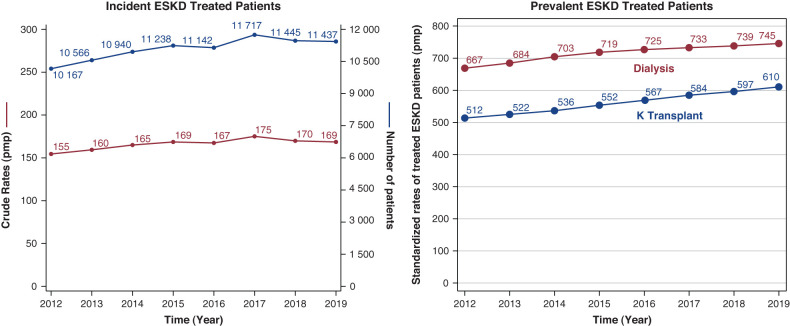
On December 31, 2019, 91,875 patients were receiving a RRT in France, 55% on dialysis and 45% living with a functional renal transplant as presented in Figure 1 (9). The overall standardized prevalence was 1355 per million population (pmp). It was 1.7-fold higher in males. Prevalence was subject to regional variations with seven regions (five overseas) above and seven below the national rate, as presented in Figure 2. The renal transplant share varied from <40% in three regions to >50% in five regions, and from 15% to 32% in overseas regions. The overall sex and age standardized prevalences were 44,700 and 610 pmp, respectively, for PD, HD, and transplantation, with marked regional variations. Trends in incidence and prevalent standardized rates of patients with ESKD treated in France through 2012–2019 are presented in Figure 3.
Figure 1.
Incoming/outgoing patients with ESKD flows in 2019. Patient flows were aligned on December 31, 2019. pmp, per million population; pmi, per million inhabitants; PD, peritoneal dialysis; LRD, living related donor.
Figure 2.
Territorial organization and standardized prevalence rate (pmp) of patients treated for ESKD heat map color. pmp, per million population.
Figure 3.
Trend in incidence and prevalent rates of patients treated for ESKD (2012–2019). pmp, per million population.
In 2019, 11,437 new patients with ESKD started RRT. The overall incidence of RRT was 169 pmp (dialysis, 162 pmp; preemptive transplantation, 7 pmp). The median age at RRT initiation was 71 years. Those patients present a higher rate of comorbidities, especially diabetes (48% of new patients) and cardiovascular diseases (57% of new patients), which increases with age. The first treatment option remains in-center HD. RRT started as an emergency in 30% of patients. The hemoglobin level at the start of RRT offers an interesting proxy indicator of timely appropriate CKD management before dialysis initiation. As indicated, 67% of patients presenting an underprovided follow-up during advanced CKD management have a hemoglobin level <10 g/dl, whereas only 48% of patients on HD and 27% patients on PD with an appropriate follow-up presented such a condition.
On December 31, 2019, 50,337 patients with prevalent ESKD, including in overseas territories, were maintained on dialysis (HD 47,324 and PD 2999). Interestingly, the prevalence of patients on dialysis in overseas territories is almost twice that of mainland France (1355 pmp versus 2677 pmp). It is interesting to note patients aged >65 years account for 55% of the patients undergoing dialysis (median age 71 years, stable since 2012). These patients present a high rate of comorbidity, especially diabetes (44% of patients, increasing since 2012) and cardiovascular comorbidities (59% of patients), which increases with the patient’s age.
Dialysis Modalities: Repartition and Specificities
The distribution of dialysis modalities and the location of patients with prevalent ESKD treated in France by the end of 2019 is presented in Table 1.
Table 1.
Overview of prevalent patients receiving RRT according to treatment modality and location
| Treatment Modality |
Mainland France |
Overseas France |
All France | |
|---|---|---|---|---|
|
n pts (%) |
46,924 (100) |
3413 (100) |
50,337 (100) |
|
| In-center HD | In-center +UDM |
36,407 (78) |
2642 (77) |
39,049 (78) |
| Self-care HD | Self-Care HD | 230 (0.5) |
5 (0.1) |
235 (0.5) |
| Assisted Self-Care HD |
6559 (14) |
640 (19) |
7199 (14) |
|
| Home treatment | Home HD | 538 (1) |
13 (0.4) |
551 (1) |
| Unassisted PD | 1466 (3) |
34 (1) |
1500 (3) |
|
| Assisted PD | 1231 (3) |
66 (2) |
1297 (3) |
|
| Unknown PD | 162 (0.3) |
9 (0.3) |
171 (0.3) |
|
| Training center | HD+PD | 360 (0.8) |
12 (0.4) |
372 (0.7) |
HD, hemodialysis; UDM, medicalized dialysis units; PD, peritoneal dialysis.
In-center HD is the main treatment modality in France, accounting for 78% of dialysis performed in the outpatient clinic, either in-center (53%) or in a satellite unit (24%).
Self-care HD represents 15% of the total performed, either unassisted (0.5%) or assisted (14%).
Home treatment represents 7% of HD (1%) and PD (6%). On December 31, 2019, the overall prevalent use of PD modality was 2999, predominantly in mainland France, with 2883 patients accounting for 96% of the total share (Table 4). The overall prevalence of PD has remained stable, with a share of about 6% of patients on dialysis. PD represents the main home therapy, relying on continuous ambulatory PD (CAPD) or automated PD (APD), with a share of 58% and 35%, respectively, with 7% mixed or unknown. These figures are in close agreement with the most recent report of the Registre de Dialyse Péritonéale de Langue Française (10). Even if the number of patients treated by PD is stable, the relative share trend declined by 2% per year between 2012 and 2019 (the annual percent change between 2012 and 2019 was −2%). This trend is also observed for patients who were treated with self-care dialysis. Home HD treatment increased, with a share of 1% of patients on dialysis, about 551 patients, mainly linked to the development of more frequent and daily HD (annual percent change between 2012 and 2019 was 9.5%). Frequent or daily HD is performed exclusively at home or temporarily within training centers as a rescue process. Reimbursement of frequent dialysis is on the basis of the number of sessions delivered per week, but the social security system is planning to move to a weekly flat rate, due to the increase in the prevalence of this modality.
Table 4.
Distribution of patients treated with peritoneal dialysis according to peritoneal dialysis modality (automated peritoneal dialysis/continuous ambulatory peritoneal dialysis) and nurse assistance
| Peritoneal Dialysis Modality | Total | Automated Peritoneal Dialysis (%) | Continuous Ambulatory Peritoneal Dialysis (%) | |||
|---|---|---|---|---|---|---|
| n | Unassisted | Assisted | Unassisted | Assisted | Unknown | |
| Mainland | 2883 | 808 (28) | 189 (7) | 658 (23) | 1042 (36) | 186 (7) |
| Overseas | 116 | 25 (22) | 24 (21) | 9 (8) | 42 (36) | 16 (14) |
| All France | 2999 | 833 (28) | 213 (7) | 667 (22) | 1084 (36) | 202 (7) |
Training centers are part of the home therapy service provided mainly by private not-for-profit clinics to permit implementation of home HD and PD therapies. On December 31, 372 patients were trained for home treatment, either on HD or PD.
Backup hospitalization and/or alternative options are required for all patients on dialysis as part of the service engagement to ARS. Depending on the cause and/or severity of the medical issue, patients are referred to primary or secondary care units.
HD Prescription
Vascular Access
The vascular access used in 77% of patients who are prevalent is a native arteriovenous fistula, whereas a tunneled central venous catheter is used in the remaining 23%.
Treatment Schedule and Time
Three sessions per week is the standard treatment in 93% of patients, with 4% of patients undergoing ≤2 sessions per week, reflecting either the start of the RRT program, or end-of-life patient management. In total, 2% of patients are on daily dialysis (>4 sessions per week). The duration of the sessions is 4 hours in 72% of patients, between 3 and 4 hours for 19%, >4 hours for 7%, and <3 hours for 2%. Long dialysis of >6 hours is performed in 0.7% of patients. The distribution of weekly treatment times and session numbers is presented in Table 2.
Table 2.
Distribution of patients treated with hemodialysis according to weekly treatment time and session number
| n pts (%) | <12 Hours/ Week | =12 Hours/ Week | >12 Hours/ Week |
|---|---|---|---|
| Mainland France | 9157 (21) | 30,796 (70) | 4088 (9) |
| Overseas France | 648 (20) | 2473 (75) | 176 (5) |
| All France | 9805 (21) | 33,269 (70) | 4264 (9) |
| <3 sessions/ wk | =3 sessions/ wk | >3 sessions/ wk | |
| Mainland France | 1799 (4) | 40,893 (93) | 1349 (3) |
| Overseas France | 45 (1) | 3198 (97) | 54 (2) |
| All France | 1844 (4) | 44,091 (93) | 1403 (3) |
Hemodialyzers are single use, and made of high-flux synthetic membranes, with a median surface of 1.80 m2. The reuse of dialyzers and/or disposable dialysis material equipment has been prohibited in France since 1995.
Hemodiafiltration is used in 35% of patients, with differences ranging from 1% to 70%, depending on the region, but increasing steadily since 2012 (annual percent change between 2012 and 2016 +13% and between 2016 and 2019 +2%). The online production method referring to cold sterilization of fresh dialysate (two sterilizing ultrafilters in series) with CE-certified hemodiafiltration machines is almost the only substitution method currently used. We refer interested readers to recent reviews detailing technical and practical aspects (11,12). The median substitution volume is 21 (18%–28%) liters per session in postdilution mode. The distribution of various treatment modalities is presented in Table 3.
Table 3.
Distribution of patients treated with hemodialysis according to treatment modality
| Treatment Modality, n pts (%) | Mainland France |
Overseas France |
All France |
|---|---|---|---|
| 44,029 (100) |
3295 (100) |
47,324 (100) |
|
| Conventional high-flux HD | 27,970 (64) |
2192 (67) |
30,162 (64) |
| Online hemodiafiltration | 15,593 (35) |
1089 (33) |
16,682 (35) |
| Acetate-free biofiltration | 72 (0.2) |
0 (0.0) |
72 (0.2) |
| Online hemofiltration | 58 (0.1) |
1 (0.0) |
59 (0.1) |
| Frequent and slow daily HD (≥5 sessions/wk) | 336 (0.8) |
13 (0.4) |
349 (0.7) |
HD, hemodialysis.
Systemic anticoagulation is performed in almost 80% of patients with low molecular weight heparin and the rest uses unfragmented heparin or another modality as needed.
Electrolytic Dialysate
Dialysis dose delivered
The median electrolytic composition in mmol/L of the bicarbonate dialysate is as follows: Na 138, K 2.0, HCO3 35, Ca 1.5, Mg 0.5, and acetate (80%) or citrate (20%) as an acidifier. The median Kt/V for patients having three HD sessions per week varies from 1.4 to 1.5, depending on the measurement method. The percentage of patients >1.2 corresponding to the targets of adequate minimum dialysis according to the recommendations varies from 73% to 89%, depending on the method. The percentage of patients with a Kt/V >1.2 is higher in patients aged >75 years. It is logically more important in patients with an arteriovenous fistula than in patients with a catheter.
Dialysis Vintage
The length of RRT in patients on prevalent dialysis in 2019 was a median of 3.2 years. The dialysis vintage varies significantly from one region to another, according to the transplantation rate and patients’ characteristics. In total, 48% of all patients have a total treatment duration of ≤2 years. This distribution is a reflection of patients treated exclusively by dialysis for any reason (e.g., hyperimmunized, high risk) and regional transplant activity. Among patients treated for >20 years, 86% received a kidney transplant at least once.
Virus
In 2019, among new patients starting dialysis, the prevalence of chronic carriers of the Hepatitis B virus, Hepatitis C virus (HCV), and HIV viruses was 0.7%, 1%, and 0.9%, respectively, with a significant decrease over the past decade. Among the 93 new patients with the HIV virus, 29 had AIDS, and 45% of these patients were treated in Ile-de-France. No geographic isolation is requested for patients who were chronic virus bearers. However, specific disinfection and reinforced hygiene measures for HD machines and dialysis material are required, as is specific virus monitoring when patients move location. Note, for patients with chronic virus contamination, this is much more important in overseas territories, where there is a prevalence for Hepatitis B virus, HCV, and HIV of 1%, 0.5%, and 2%, respectively. It is forecasted that the prevalence of HCV, for example, decreases with access to and generalization of anti-HCV treatment.
Outcome
The overall survival rate of patients on incident HD (n=58,446) equipped with an arteriovenous access at initiation starting in 2002–2018, at 1, 3, 5, and 10 years, was 90% (89–90), 72% (72–73), 57% (57–58), and 35% (35–36), respectively. The survival probability in patients with diabetes and cardiac problems, and in the elderly, is 10%–20% lower and 5%–10% better in patients who are younger and have no comorbidities.
PD Prescription
PD is performed exclusively at home on a daily basis, either with support of a cycler as automated PD (APD) or manually as CAPD with a bag and transfer set systems. APD is the most used modality, with 59% of patients. Unassisted accounts for 51% of patients on PD, whereas assisted modality accounted for 44% and is mostly used in patients on CAPD; 5% had information missing, as shown on Table 5. PD solutions are multiple and prescription relies on various associations (dextrose 1%, 2%, and 4%) with biocompatible solutions, including bicarbonate-buffered and low glucose degradation product solutions, and icodextrin-based solution for long-dwell exchange in almost 80% of patients. Median exchange volume in CAPD is 6.2 L/day (0.5–12) and in APD is 10.1 L/day (1.0–18.0). The type of PD solutions and daily exchange volume are tailored to the peritoneum membrane permeability test and residual kidney function to fit the patient’s needs. Support may be given to PD care givers by the Francophone PD registry (Registre de Dialyse Péritonéale de Langue Française), which that captures data and provides feedback support to PD centers (13) on a voluntary basis.
Table 5.
Dialysis reimbursement tariff according to treatment modality (March 2021)
| Type of Dialysis Facility | Tariff, US$ |
|---|---|
| In-center HD (per session) | 302.69 |
| Medicalized HD (per session) | 301.54 |
| Self-care HD Facility (per session) | 295.09 |
| Home HD (per session) | 261.58 |
| Automated PD (per week) | 858.05 |
| Continuous APD (per week) | 667.83 |
| Training home HD (per session) | 462.60 |
| Training APD (per day) | 461.42 |
| Training CAPD (per day) | 430.00 |
Conversion rate US$ to €1.2. HD, hemodialysis; PD, peritoneal dialysis; APD, automated peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis.
Funding of RRT
Kidney disease and chronic renal failure are listed as 19th out of the 30 recognized chronic diseases (Affection Longue Durée, list of 30 pathologies covered). CKD is considered an “exonerating” disease totally covered by the universal national health insurance system (Caisse Nationale Assurance Maladie and regional branches Caisse Régionale Assurance Maladie [CRAM]) (2,3). As soon as a patient is diagnosed with kidney disease, their general practitioner and referent nephrologist must document the clinical case to the CRAM medical office. After this, CKD is recognized and covered by the social security system. All rights will appear on the patient’s electronic card (Vital Card) and open the rights for the patient to be treated with all costs related to kidney disease fully covered. Thanks to its personal electronic card (Vital Card), each patient may benefit from health insurance funding for CKD care. A patient with CKD has no out-of-pocket expenses for their renal care. Full coverage of renal care remains true across all kidney disease stages, including dialysis and transplantation.
Reimbursement Policy and Tariff
During RRT, the cost of treatment includes dialysis reimbursement fees, use of disposable material, intradialytic medications (e.g., erythropoetin stimulating agent, iron), and physician fees (private for-profit), but also during the interdialytic period, medicine prescription, specialized consultations, laboratory tests, and imaging tests when needed. Renal replacement fees are on the basis of the facility (CHD, self-care HD, home HD and PD, training centers), and not on the modality itself. Reimbursement tariffs are presented in Table 5. In other words, HD or hemodiafiltration receive the same reimbursement fees. It is of interest to note transportation to the dialysis unit benefits from an additional specific reimbursement by the CRAM (4).
Conclusions
By the end of 2019, 91,875 patients were receiving RRT in France (55% on dialysis and 45% with a functional renal transplant). The overall crude prevalence was 1355 pmp. Out of this population, 50,337 patients with prevalent ESKD, including those in overseas territories, were maintained on dialysis (HD 47,338 and PD 2999). The prevalence of treated ESKD is subject to regional variations with nine regions (six overseas) above and 14 below the national rate. In 2019, 11,437 new patients with ESKD started RRT. For example, the prevalence of patients on dialysis in overseas territories is almost twice that of mainland France (1553 pmp versus 2677 pmp). The overall incidence of RRT was 169 pmp (dialysis 162 pmp; preemptive transplantation 7 pmp). Home HD treatment increased, with a share of 1% of patients on dialysis, whereas PD treatment remained stable, with a share of 6% of patients on dialysis. Assisted home PD treatment is viable option to facilitate home therapy. As indicated, the most-used RRT option in France remains in-center HD (78%), followed by self-care HD (15%), and home therapy (8%). A fact sheet summary of RRT in France closing 2019 is shown in Table 6.
Table 6.
RRT in France: Fact sheet closing 2019
| Population | 66.6 million inhabitants |
| Total number of nephrologists | 1657 (2017) |
| National Society of Nephrology Société Francophone de Néphrologie Dialyze et Transplantation |
https://www.sfndt.org/ |
| Renal Epidemiologic & Information Network - Agency Biomedical | https://www.agence-biomedecine.fr/R-E-I-N-Reseau-Epidemiologique-et-Information-en-Nephrologie |
| National Renal Foundation Foundation for Medical Research |
https://www.frm.org/fondation/fondations-abritees/fondations-de-chercheurs/fondation-du-rein |
| Registre de Dialyze Peritoneale de Langue Française et Hémodialyse à Domicile | https://www.rdplf.org/ |
| Incidence of ESKD | 162 pmp (2019) |
| Prevalence of ESKD | Mainland 1355 pmp (2019) Overseas 2677 pmp (2019) |
| Total number of patients treated for ESKD (dialysis and transplant) | 91,875 patients (2019) 50,501 (dialysis), 41,374 (transplant) |
| Total number of patients in dialysis (all modalities) |
50,337 (2019) |
| Number of patients in hemodialysis(including hemodiafiltration)a | 47,338 (2019) |
| Number of patients in peritoneal dialysis (APD and CAPD)b |
2999 (2019) |
| Number of renal transplantations per yeara | 3641 (2019) |
APD, automated peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; pmp, per million population.
Number of patients in hemodialysis.
Number of patients in peritoneal dialysis.
Kidney replacement therapy fees and related costs are fully covered by the Universal National Health Insurance Fund as part of French Social Security, through mainland and overseas territories.
Various incentives from National Health Authorities are being considered to promote conservative and protective kidney care, increase out-center RRT (home, self-care) acceptance, and promote kidney transplant rate, including living related donors, to reduce the global economic burden of kidney care.
Disclosures
B. Canaud reports having consultancy agreements with Fresenius Medical Care as Senior Chief Scientist; and reports having patents and inventions for an implantable vascular access port. The remaining author has nothing to disclose.
Funding
None.
Acknowledgments
The authors thank REIN and BioMedical Agency for providing access to database and analyses. AIDER-SANTE, Anne-Valérie Boulet & CLINIFUTUR, Jeanne Loyher for providing us documentary information.
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the author(s).
Author Contributions
B. Canaud and C. Couchoud conceptualized the study; C. Couchoud was responsible for the data curation; and B. Canaud wrote the original draft, and reviewed and edited the manuscript.
References
- 1.Canaud B, Choukroun G: Décret n° 2011-77 du 19 janvier 2011 portant actualisation de la liste et des critères médicaux utilisés pour la définition des affections ouvrant droit à la suppression de la participation de l'assuré. (JORF n°0017 du 21 janvier 2011). Available at: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000023456250. Accessed June 28, 2021
- 2.Diagnostic de l’insuffisance rénale chronique chez l’adulte. ANAES/Service des recommandations et références professionnelles/Septembre 2002. Available at: https://www.has-sante.fr/upload/docs/application/pdf/irc_chez_ladulte_2002-_recommandations.pdf. Accessed June 28, 2021
- 3.Canaud B, Choukroun G: Nephrology in France. In: Nephrology Worldwide, edited by Moura-Neto JA, Divino-Filho JC, Ronco C, Basel, Springer Nature Switzerland AG, 2021, pp 521–541 [Google Scholar]
- 4.Moura-Neto JA, Divino-Filho JC, Ronco C, editors: Nephrology Worldwide, Basel, Springer Nature Switzerland AG, 2021 [Google Scholar]
- 5.Décret n°2002-1198 du 23 septembre 2002 relatif aux conditions techniques de fonctionnement des établissements de santé qui exercent l'activité de traitement de l'insuffisance rénale chronique par la pratique de l'épuration extrarénale et modifiant le code de la santé publique (troisième partie: Décrets). Available at: https://www.legifrance.gouv.fr/loda/id/JORFTEXT000000780502/. Accessed June 28, 2021
- 6.Décret n°2002-1197 du 23 septembre 2002 relatif à l'activité de traitement de l'insuffisance rénale chronique par la pratique de l'épuration extrarénale et modifiant le code de la santé publique (deuxième partie: Décrets en Conseil d'Etat). Available at: https://www.legifrance.gouv.fr/loda/id/JORFTEXT000000232864/. Accessed June 28, 2021
- 7.Stengel B: Landais P et les membres du groupe de travail du projet de Réseau Epidémiologie et Information en Néphrologie (REIN). Recueil d’information sur la prise en charge de l’insuffisance rénale terminale. Nephrologie 20: 29–40, 1999 [PubMed] [Google Scholar]
- 8.Landais P, Simonet A, Guillon D, Jacquelinet C, Ben Saïd M, Mugnier C, Simonet M: SIMS REIN: Un système d’information multi-sources pour l’insuffisance rénale terminale. (SIMS REIN: A multi-source information system for end-stage renal disease) C R Biol 325: 515–528, 2002. 10.1016/S1631-0691(02)01456-7 [DOI] [PubMed] [Google Scholar]
- 9.Rapport annuel REIN 2019 V1 Preliminary Report. Available at: https://www.agence-biomedecine.fr/IMG/pdf/rapport_rein_2019_2021-10-14.pdf. Accessed June 6, 2021
- 10.Verger C, Veniez G, Padernoz M-C, Fabre E: Home dialysis in French speaking countries in 2020 (RDPLF database). Bull Dial Domic 24: 55–60, 2021 [Google Scholar]
- 11.Canaud B, Vienken J, Ash S, Ward RA; Kidney Health Initiative HDF Workgroup : Hemodiafiltration to address unmet medical needs ESKD patients. Clin J Am Soc Nephrol 13: 1435–1443, 2018. 10.2215/CJN.12631117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward RA, Vienken J, Silverstein DM, Ash S, Canaud B; Kidney Health Initiative HDF Workgroup : Regulatory considerations for hemodiafiltration in the United States. Clin J Am Soc Nephrol 13: 1444–1449, 2018. 10.2215/CJN.12641117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Registre de Dialyse Peritoneale de Langue Française et Hémodialyse à Domicile. Available at: https://www.rdplf.org/. Accessed June 28, 2021