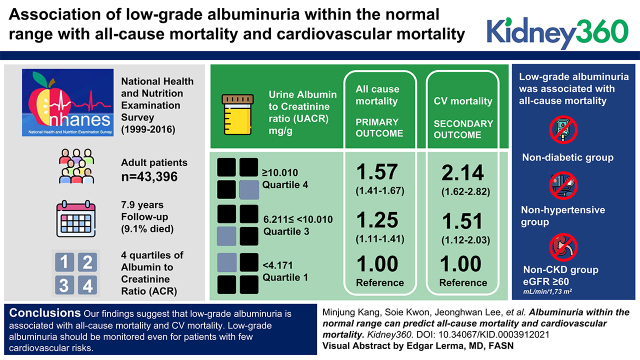
Key Points
Despite interest in low-grade albuminuria and poor clinical outcomes, evidence from a large-scale population is lacking.
In this large cohort study, low-grade albuminuria was associated with all-cause and cardiovascular mortality.
In the general population, low-grade albuminuria should be carefully monitored.
Keywords: clinical nephrology, albuminuria, cardiovascular diseases, cardiovascular system, mortality, normoalbuminuria, reference values
Visual Abstract
Abstract
Background
Despite interest in low-grade albuminuria and poor clinical outcomes, evidence from a large-scale population is lacking. Therefore, we identified the association of low-grade albuminuria within the normal range with all-cause and cardiovascular (CV) mortality.
Methods
After excluding individuals with urine albumin-creatinine ratio (ACR) ≥30 mg/g (n=6094), this cohort study analyzed 43,396 adults who participated in the National Health and Nutrition Examination Survey (1999–2016). Participants were divided into four quartiles of ACR. The primary outcome was all-cause mortality, and the secondary outcome was CV mortality. Multivariable Cox proportional hazards models were used.
Results
During a median 7.9 years of follow-up, 3516 (9%) participants died. Compared with the reference group (Q1, ACR <4.171 mg/g), low-grade albuminuria groups were associated with all-cause mortality (Q3, ACR ≥6.211 to <10.010 mg/g, hazard ratio [HR], 1.25 [95% CI, 1.11 to 1.41]; Q4, ACR ≥10.010 mg/g, HR, 1.57 [95% CI, 1.41 to 1.76]) in a multivariable hazards model. A similar pattern was also seen in the association of low-grade albuminuria with CV mortality. Subgroup analyses showed that low-grade albuminuria was also associated with all-cause mortality in the nondiabetic group, nonhypertensive group, and non-CKD group (eGFR ≥60 ml/min per 1.73 m2).
Conclusions
Our findings suggest that low-grade albuminuria is associated with all-cause and CV mortality. Low-grade albuminuria should be monitored, even for patients with low CV risk.
Introduction
Albuminuria is a known predictor of all-cause and cardiovascular (CV) mortality (1,2). Thus, the importance of albuminuria has been emphasized and monitoring of albuminuria is recommended in patients who are high risk, such as those with diabetes mellitus (DM) or CKD (3,4). In patients with diabetes, albuminuria is a characteristic finding of diabetic kidney disease (DKD) (5,6). Previously, albuminuria was thought to be a sequential process of DKD that followed glomerular hyperfiltration (7); recently, however, albuminuria was also considered to be an activated state of DKD, that is, an already deteriorated state (8). Albuminuria is a marker of kidney damage (9). Therefore, if albuminuria (urine albumin excretion rate ≥30 mg/24 h; random urine albumin-creatinine ratio [ACR] ≥30 mg/g [3 mg/mmol]) persists for >3 months, it is defined as CKD. In addition, albuminuria was reported to be associated with obesity (10) and dementia in elderly individuals (11).
Albuminuria could be caused by a variety of factors. Endothelial dysfunction could cause albuminuria by increasing glomerular pressure and permeability of the glomerular basement membrane (12). Moreover, albuminuria could be caused by dietary habits. Eating more protein and fewer lipids of vegetable origin could cause albuminuria (13). Genetic variants related to albuminuria were also discovered through a genome-wide association study (14). For example, rs116907128 and rs1801239 are genetic variants associated with ACR in the general adult population, and rs13427836 and rs649529 are genetic variants associated with ACR in the DM population.
In the current guidelines, the lowest level of albuminuria considered to be of clinical importance is microalbuminuria with an ACR of ≥30 mg/g (3 mg/mmol) (9). Most guidelines recommend screening for ACR in patients who are at risk, such as those with DM, hypertension, obesity, or CKD; those who are smokers; and elderly individuals (9,15–17). However, some previous studies have garnered interest for albuminuria and poor clinical outcomes in individuals with normoalbuminuria (ACR <30 mg/g). Several previous studies showed that low-grade albuminuria was associated with all-cause mortality (18), left ventricular hypertrophy (19), incident heart failure events (20), and DKD (21). An observational study from the Atherosclerosis Risk in Communities (ARIC) study demonstrated that high-normal albuminuria was associated with heart failure in patients with low CV risk (20). However, these studies were limited to patients who were high risk, such as those with DKD (21) and hypertension (19), and those who were elderly (18,20). Additionally, the previous studies did not analyze >40,000 subjects as we have done in this study.
We analyzed the general United States adult population without albuminuria (ACR <30 mg/g), not restricted to specific age groups. We sought to show that albuminuria within the normal range (i.e., low-grade albuminuria) is associated with all-cause and CV mortality using a dataset from the National Health and Nutrition Examination Survey (NHANES).
Materials and Methods
Study Participants
We analyzed adults (≥18 years) who participated in NHANES from 1999 to 2016. Among the 53,348 adult NHANES participants from 1999 to 2016, we excluded those without a random urine ACR (n=3858) and those with an ACR of ≥30 mg/g (n=6094), which constituted 12% of the whole adult cohort (Supplemental Figure 1). As a result, 43,396 adults were included in the analysis. This observational cohort study was approved by the institutional review board of Seoul National University Boramae Medical Center (approval number 07-2021-3).
Data Collection and Definitions
Information related to demographics, laboratory values, and mortality was obtained from the NHANES database (https://www.cdc.gov/nchs/nhanes/index.htm; demographic, examination, questionnaire, and laboratory dataset) in March 2019.
DM was defined as a history of DM, fasting glucose level >126 mg/dl, or random glucose level >200 mg/dl. Hypertension was defined as at least two measurements with a systolic BP >140 mm Hg or diastolic BP >90 mm Hg, a history of hypertension, or current use of antihypertensive medications. CV event was defined as the composite of congestive heart failure, coronary heart disease, angina, history of heart attack, and stroke. Serum and urine creatinine levels were measured by the Jaffe rate method with standardization to the isotope dilution mass spectrometry reference method (22). Urine albumin levels were measured by solid-phase fluorescent immunoassay. In the surveys of 1999–2000 and 2005–2006, corrected serum creatinine levels were used (23,24). The eGFR was calculated using the CKD Epidemiology Collaboration equation (25). Participants were categorized according to quartile of ACR.
The primary outcome was all-cause mortality on the basis of the data retrieved from the NHANES linked to National Death Index data. The secondary outcome was CV mortality, which was defined as death due to heart disease or cerebrovascular disease. The code of causes of death follows the International Statistical Classification of Diseases, Injuries, and Causes of Death, Tenth Revision guidelines. The codes for heart disease were I00–I09, I11, I13, and I20–I51; the codes for cerebrovascular disease were I60–I69. The participants who were lost to follow-up were censored at the time of last visit. Survival time was calculated from the date of interview to the date of death or the end of the mortality period.
Statistical Analyses
All statistical analyses were performed using SPSS software (version 25; IBM Corp., Armonk, NY) and R software (version 3.6.3; www.r-project.org/; R Foundation for Statistical Computing, Vienna, Austria). Categoric variables are presented as numbers and percentages. Continuous variables are presented as the mean±SD or as medians with interquartile ranges (IQRs). The Kolmogorov–Smirnov test was used to test for normality. To compare the baseline characteristics according to quartile of ACR, the chi-squared test was used for categoric variables (or Fisher exact test if the chi-squared test was not applicable), whereas one-way ANOVA was used for continuous variables (or the Kruskal–Wallis test if ANOVA was not applicable). For P values for trend, the linear-by-linear association was used for categoric variables, and the Jonckheere–Terpstra test was used for continuous variables. Kaplan–Meier curves were drawn according to quartile of ACR, and they were compared using the log-rank test. The hazard ratios (HRs) of all-cause and CV mortality were analyzed using Cox proportional hazards models. Model 1 was a univariable model. Model 2 was adjusted for sociodemographic information (age, sex, race, and education). Model 3 was further adjusted for body mass index (BMI), baseline eGFR, smoking, and comorbidities such as DM, hypertension, and CV event. The percentages of missing values of education, BMI, baseline eGFR, smoking, and CV event were 8%, 1%, 5%, 6%, and 8%, respectively. The multivariable models were analyzed except for covariates with missing values. A cubic spline curve was used to demonstrate the association between continuous ACR and mortality. The reference was set to ACR 3.00 mg/g, which was within the first quartile of ACR (<4.171 mg/g). Subgroup analyses were performed. We performed subgroup analyses regarding age group, sex, DM, hypertension, obesity (BMI ≥30 kg/m2), baseline eGFR, education status, and smoking status. Subgroup analyses were also performed in the group without DM, CKD (eGFR <60 ml/min per 1.73 m2), and hypertension, and in the groups with at least one of these conditions. A P value <0.05 was considered statistically significant.
Results
Baseline Characteristics
The baseline characteristics according to quartile of ACR are shown in Table 1. Overall, the median (IQR) age was 44 (29–61) years, and the number of male participants was 20,988 (48%). As the ACR increased, the patients were older and more likely to be female (P for trend <0.001). Our data consisted of 44% non-Hispanic White, 21% non-Hispanic Black, 19% Mexican American, and 8% other Hispanic individuals. Participants who were obese were more likely to have a high ACR than participants who were not obese (P for trend <0.001). Groups with higher ACR tended to have more individuals with DM, hypertension, and CV events (P for trend <0.001). A higher ACR was associated with more people with high school education or lower and fewer current smokers (P for trend <0.001). More participants with CKD (eGFR <60 ml/min per 1.73 m2) tended to have a higher ACR (P for trend <0.001).
Table 1.
Baseline participant characteristics
| Variable |
Total (n=43,396) |
Urine Albumin-Creatinine Ratio, mg/g | P for Trend | |||
|---|---|---|---|---|---|---|
| <4.171 (n=10,849) | 4.171 to <6.211 (n=10,848) | 6.211 to <10.010 (n=10,931) | ≥10.010 (n=10,768) | |||
| Age, yr, median (IQR) | 44 (29–61) | 38 (26–52) | 43 (29–58) | 46 (30–62) | 52 (34–68) | <0.001 |
| Male, n (%) | 20,988 (48) | 7195 (66) | 5328 (49) | 4344 (40) | 4121 (38) | <0.001 |
| Race and ethnicity, n (%) | <0.001 | |||||
| Mexican American | 8203 (19) | 1757 (16) | 2097 (19) | 2244 (21) | 2105 (20) | |
| Other Hispanic | 3498 (8) | 785 (7) | 881 (8) | 920 (8) | 912 (9) | |
| Non-Hispanic White | 19,088 (44) | 4671 (43) | 4830 (45) | 4796 (44) | 4791 (45) | |
| Non-Hispanic Black | 9022 (21) | 2806 (26) | 2126 (20) | 2010 (18) | 2080 (19) | |
| Other race | 3585 (8) | 830 (8) | 914 (8) | 961 (9) | 880 (8) | |
| Body mass index, kg/m2, n (%) | <0.001 | |||||
| <30 | 28,427 (66) | 7317 (68) | 7259 (68) | 7098 (66) | 6753 (64) | |
| ≥30 | 14,431 (34) | 3428 (32) | 3475 (32) | 3693 (34) | 3835 (36) | |
| Diabetes mellitus, n (%) | 4351 (10) | 562 (5) | 842 (8) | 1144 (11) | 1803 (17) | <0.001 |
| Hypertension, n (%) | 15,227 (35) | 2626 (24) | 3315 (31) | 4121 (38) | 5165 (48) | <0.001 |
| Cardiovascular event, n (%) | 3474 (9) | 549 (6) | 685 (7) | 913 (9) | 1327 (13) | <0.001 |
| Education, n (%) | <0.001 | |||||
| High school or lower | 19,801 (46) | 4425 (41) | 4781 (44) | 5157 (47) | 5438 (51) | |
| College or graduate | 20,228 (47) | 5377 (50) | 5231 (48) | 4983 (46) | 4637 (43) | |
| Smoking, n (%) | <0.001 | |||||
| Current smoker | 8574 (21) | 2293 (22) | 2156 (21) | 2115 (20) | 2010 (19) | |
| Exsmoker | 9591 (23) | 2179 (21) | 2359 (23) | 2433 (23) | 2620 (25) | |
| Never smoker | 22,419 (54) | 5468 (53) | 5645 (54) | 5720 (54) | 5586 (53) | |
| Laboratory findings, median (IQR) | ||||||
| uACR, mg/g | 6.21 (4.17–10.00) | 3.26 (2.67–3.73) | 5.11 (4.63–5.63) | 7.69 (6.88–8.71) | 14.83 (11.91–19.83) | <0.001 |
| Fasting glucose, mg/dl | 97.7 (90.7–106.1) | 96.0 (90.0–103.0) | 97.0 (90.1–105.0) | 98.0 (90.7–107.0) | 99.3 (92.0–112.0) | <0.001 |
| eGFR <60 ml/min per 1.73 m2, n (%) | 2,830 (7) | 506 (5) | 530 (5) | 696 (7) | 1098 (11) | <0.001 |
IQR, interquartile range; uACR, random urine albumin-creatinine ratio; eGFR, estimated glomerular filtration rate.
Albuminuria and Mortality
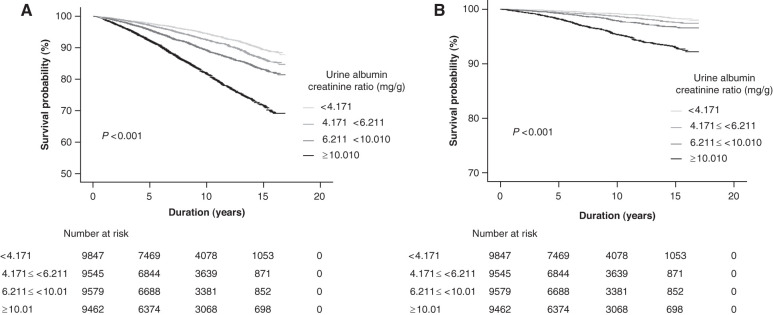
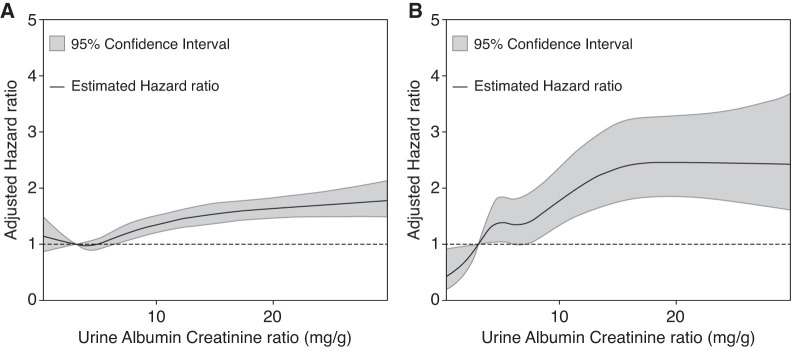
During a median (IQR) follow-up of 7.9 (4.4−12.0) years, 3516 (9%) participants died. The Kaplan–Meier curve showed that the higher the ACR was the lower the survival probability for all-cause mortality (log-rank P<0.001) (Figure 1A). The survival probability for CV mortality showed a similar pattern (Figure 1B). The incidence rates of all-cause mortality from the lowest to the highest quartile of ACR were 539 (6.18 per 1000 person-years), 645 (8.05 per 1000 person-years), 865 (11.11 per 1000 person-years), and 1467 (19.87 per 1000 person-years), respectively. The crude HRs of all-cause mortality (compared with that of the lowest quartile) were 1.31 (95% CI, 1.17 to 1.47), 1.82 (95% CI, 1.64 to 2.03), and 3.30 (95% CI, 2.99 to 3.64) for the second, third, and fourth quartiles of ACR, respectively (model 1 of all-cause mortality in Table 2). After adjustments for sociodemographic information, BMI, smoking, baseline eGFR, and comorbidities, the HRs for all-cause mortality were significant for the third and fourth quartiles of ACR (models 2 and 3 of all-cause mortality in Table 2). The spline curve clearly showed a relatively linear relationship between ACR and the risk of all-cause mortality (Figure 2A).
Figure 1.
High urine albumin-creatinine ratio was associated with low survival probability. (A) The survival probability for all-cause mortality. (B) The survival probability for cardiovascular mortality.
Table 2.
Risk of mortality according to quartile of random albumin-creatinine ratio
| Outcome | Urine Albumin-Creatinine Ratio (mg/g) | |||||||
|---|---|---|---|---|---|---|---|---|
| Quartile 1 (<4.171) | Quartile 2 (4.171 to <6.211) | Quartile 3 (6.211 to <10.010) | Quartile 4 (≥10.010) | |||||
| Hazard Ratio (95% Confidence Interval) | P | Hazard Ratio (95% Confidence Interval) | P | Hazard Ratio (95% Confidence Interval) | P | Hazard Ratio (95% Confidence Interval) | P | |
| All-cause mortality | ||||||||
| Model 1a | 1.0 (ref) | 1.31 (1.17 to 1.47) | <0.001 | 1.82 (1.64 to 2.03) | <0.001 | 3.30 (2.99 to 3.64) | <0.001 | |
| Model 2b | 1.0 (ref) | 1.08 (0.96 to 1.21) | 0.18 | 1.27 (1.14 to 1.43) | <0.001 | 1.68 (1.51 to 1.87) | <0.001 | |
| Model 3c | 1.0 (ref) | 1.08 (0.95 to 1.22) | 0.20 | 1.25 (1.11 to 1.41) | <0.001 | 1.57 (1.41 to 1.76) | <0.001 | |
| Number of events, % | 539 (6) | 645 (7) | 865 (9) | 1467 (16) | ||||
| Cardiovascular mortality | ||||||||
| Model 1a | 1.0 (ref) | 1.52 (1.14 to 2.03) | 0.004 | 2.20 (1.68 to 2.88) | <0.001 | 4.98 (3.90 to 6.36) | <0.001 | |
| Model 2b | 1.0 (ref) | 1.18 (0.88 to 1.57) | 0.26 | 1.43 (1.09 to 1.88) | 0.01 | 2.20 (1.71 to 2.83) | <0.001 | |
| Model 3c | 1.0 (ref) | 1.28 (0.94 to 1.75) | 0.11 | 1.51 (1.12 to 2.03) | 0.006 | 2.14 (1.62 to 2.82) | <0.001 | |
| Number of events, % | 80 (0.8) | 111 (1) | 155 (2) | 330 (4) | ||||
ref, reference.
Unadjusted.
Adjusted for age, sex, race, and education.
Model 2 plus adjustment for body mass index, smoking, diabetes mellitus, hypertension, cardiovascular event, and baseline eGFR.
Figure 2.
There was a linear association of the urine albumin-creatinine ratio with mortality. (A) All-cause mortality. (B) Cardiovascular mortality.
During the follow-up period, 676 (2%) participants died of heart disease or cerebrovascular disease. The incidence rates of CV mortality from the lowest to the highest quartile of ACR were 80 (0.91 per 1000 person-years), 111 (1.38 per 1000 person-years), 155 (1.99 per 1000 person-years), and 330 (4.46 per 1000 person-years), respectively. The results for CV mortality were similar to those of all-cause mortality. The crude HRs for CV mortality (compared with that of the lowest quartile) were 1.52 (95% CI, 1.14 to 2.03), 2.20 (95% CI, 1.68 to 2.88), and 4.98 (95% CI, 3.90 to 6.36) for the second, third, and fourth quartiles of ACR, respectively (model 1 of CV mortality in Table 2). After adjustments for sociodemographic information, BMI, smoking, baseline eGFR, and comorbidities, the HRs for CV mortality were significant for the third and fourth quartiles of ACR (models 2 and 3 of CV mortality in Table 2). The spline curve clearly showed a relatively linear relationship between ACR and the risk of CV mortality (Figure 2B).
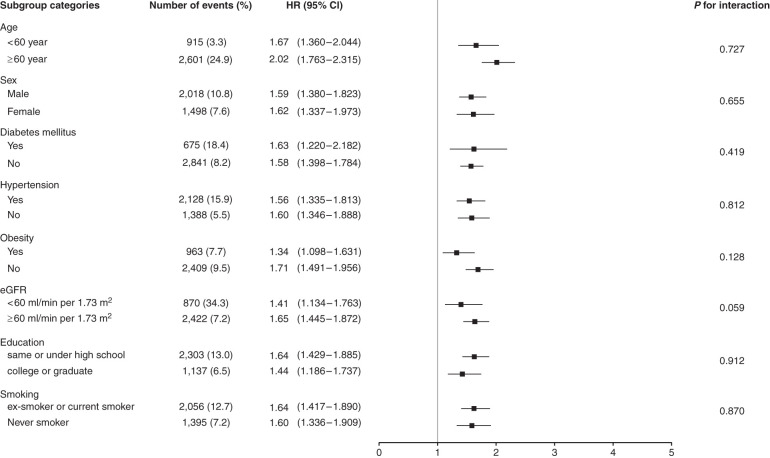
Subgroup Analyses
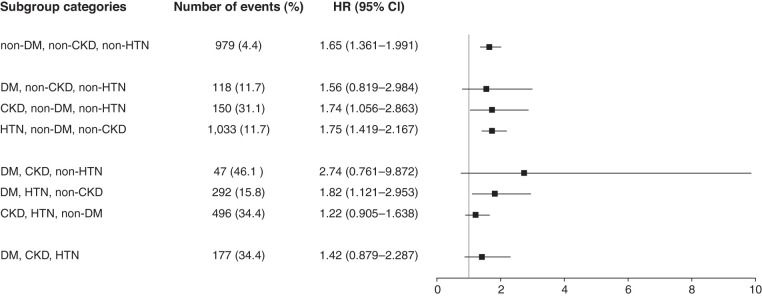
To assess modification effects of subgroups on the association between ACR and all-cause mortality, analyses were performed in subgroups stratified by age (<60 or ≥60 years old), sex (male or female), DM (with or without), hypertension (with or without), obesity (BMI ≥30 kg/m2 or <30 kg/m2), eGFR (<60 or ≥60 ml/min per 1.73 m2), education, and smoking. The risk of the fourth quartile of ACR (≥10.010 mg/g) was compared with the risk of the first quartile of ACR. P values for interactions were nonsignificant for the subgroups by age, sex, DM, hypertension, obesity, eGFR, education, and smoking, suggesting the increased risk of all-cause mortality associated with low-grade albuminuria (ACR ≥10.010 mg/g) was evident regardless of these factors (Figure 3). For sensitivity analysis, we performed a subgroup analysis on the risk of the third quartile (ACR of 6.211 to <10.010 mg/g), not the risk of the fourth quartile. Similar results of subgroup analyses were observed when the risks of the third quartile (ACR 6.211 to <10.010 mg/g) were compared with the risk of the first quartile of ACR (Supplemental Figure 2). Subgroup analyses were also performed in the group without DM, CKD (eGFR <60 ml/min per 1.73 m2), and hypertension, and in the groups with at least one of these conditions (Figure 4). In subgroup analyses, compared with the reference group (ACR <4.171 mg/g), we analyzed the association of low-grade albuminuria (ACR ≥10.010 mg/g) with all-cause mortality and found the association was significant in individuals with low CV risks.
Figure 3.
The increased risk of all-cause mortality associated with low-grade albuminuria (ACR ≥10.010 mg/g) was evident regardless of age, sex, diabetes mellitus, hypertension, obesity, eGFR, education, and smoking.
Figure 4.
The association of low-grade albuminuria with all-cause mortality was significant in individuals with low cardiovascular risks.
Sensitivity Analyses
For sensitivity analyses, we drew cubic spline curves with different reference points of ACR. The cubic spline curves demonstrated a relatively linear relationship between ACR and the risk of all-cause mortality for the reference points of 4.00 mg/g, 5.00 mg/g, and 6.00 mg/g (Supplemental Figure 3).
Discussion
Although there was interest in the association between low-grade albuminuria and poor clinical outcomes, the current guidelines consider ACR ≥30 mg/g to be clinically meaningful albuminuria (9). In this study, we found that albuminuria in the normoalbuminuria category (ACR <30 mg/g) was associated with all-cause and CV mortality. Risks of all-cause mortality were prominent in the non-DM, nonhypertensive, and non-CKD groups.
A previous study showed that, even in the normoalbuminuria category (ACR <30 mg/g), the prevalence of low eGFR (<60 ml/min per 1.73 m2) and increased urine α1-microglobulin–creatinine ratio, an indicator of renal tubular dysfunction, was high in the elderly Chinese population (26). This study suggested the possibility that renal damage may have already occurred in normoalbuminuria. However, the degree of renal injury was not analyzed by subdividing the normoalbuminuria category in that study. In another study, the condition of patients with DM and renal dysfunction (eGFR <60 ml/min per 1.73 m2) but without albuminuria was even named normoalbuminuric DKD because the prevalence of renal dysfunction could not be ignored, ranging from 21% to 63% (21,27). Another study showed that low-grade albuminuria (8.1–29.6 mg/g in males and 11.8–28.9 mg/g in females) was associated with left ventricular hypertrophy and left ventricular dysfunction in patients with hypertension (19). Collectively, these studies considered normoalbuminuria a pathologic state and suggested a need for monitoring. However, these studies analyzed <1000 subjects and, of these, few patients were in the normoalbuminuria category. In addition, it was difficult to generalize the results of these studies because they were limited to patients with diseases such as DM (21) or hypertension (19). Moreover, there were studies that showed the association between albuminuria within normoalbuminuria and poor clinical outcome in the general population. The Reasons for Geographic and Racial Differences in Stroke study showed that low-grade albuminuria (ACR 10–30 mg/g) increased the risk of all-cause mortality (28), and the ARIC study showed that low-grade albuminuria (ACR 10–30 mg/g) increased the risks of CV events and all-cause mortality in the elderly population (mean age, 63 years) (18). However, these studies used ACR <10 mg/g as a reference, and the relationship between albuminuria and mortality was not analyzed in categories with ACR <10 mg/g. The prevention of Renal and Vascular End Stage Disease study, which analyzed 40,548 subjects, showed that albuminuria was a predictor of all-cause mortality in the general population (29). However, that study analyzed >40,000 subjects with microalbuminuria, macroalbuminuria, and normoalbuminuria, and the enrollment period of subjects was shorter than that in our study. In addition, subjects were limited to the city of Groningen, The Netherlands, and various races were not analyzed. On the other hand, it was also shown that low-grade albuminuria and mortality were associated in the group with low CV risks. In a nondiabetic, normotensive, Japanese group, low-grade albuminuria (ACR ≥9.6 mg/g for men, ≥12.0 mg/g for women) was associated with CV disease and all-cause mortality (30). In addition, an ARIC study showed that intermediate normal albuminuria (ACR 5–9 mg/g) was associated with incident heart failure, defined as heart failure–related hospitalization or death in participants with low CV risks (20). These studies suggested that the association between albuminuria and CV mortality did not necessarily mediate CV diseases because this association was significant even in participants with low CV risks. These results were confirmed in this study using a US nationally representative study population.
The risk of all-cause mortality was higher in low-grade albuminuria (ACR ≥6.211 mg/g) than in the reference group (ACR <4.171 mg/g). Similar patterns were observed between low-grade albuminuria and CV mortality. It is suggested that albuminuria should be carefully monitored for individuals with an ACR of <10 mg/g and those with an ACR of 10–30 mg/g. Albuminuria has been associated with hypertension (31), DM (8), dyslipidemia (32), obesity (33,34), CKD (9), and activation of the renin-angiotensin-aldosterone system (35), which are traditional risk factors for CV disease. Low-grade albuminuria was also associated with other risk factors for CV disease, such as frailty in the elderly (36), pulmonary arterial hypertension (37), and left ventricular hypertrophy (19). Collectively, these risk factors could contribute to all-cause and CV mortality. Moreover, non-CV mortality could also contribute to all-cause mortality. Cancer mortality is the main cause of death worldwide and in the United States (38,39). In our data, the main causes of death were malignant neoplasms and heart disease. Indeed, albuminuria was associated with cancer incidence (40). Albuminuria was also associated with inflammation, which is a risk factor for cancer, and is also considered a manifestation of paraneoplastic syndrome (40). Therefore, albuminuria could affect the incidence of malignant neoplasms and subsequently increase the risk of all-cause mortality.
In the groups with few risk factors, such as the non-DM, nonhypertensive, and non-CKD groups (eGFR ≥60 ml/min per 1.73 m2), low-grade albuminuria (ACR ≥10.010 mg/g) was associated with the risk of all-cause mortality. We also confirmed the results from previous studies that showed that low-grade albuminuria was associated with mortality in non-DM groups (20,30). Furthermore, low-grade albuminuria was reported to be associated with the risk of developing nonalcoholic fatty liver disease and liver fibrosis, especially in non-DM groups (41). Albumin leakage through the vessel wall might induce an inflammatory response (42), which induces nonalcoholic fatty liver disease and liver fibrosis (43). In our study, subgroup analyses showed that the patients in the non-CKD group had a higher risk of mortality than the patients in the CKD group. The proportions of patients with high-risk factors, such as old age, DM, hypertension, and CV disease, were higher in the CKD group compared with the non-CKD group. The very high prevalence of such risk factors obscures the effect of albuminuria, an indicator for endothelial damage, on mortality, even with statistical adjustments. Endothelial damage was also observed in the non-CKD group. It has been reported that subnormal renal function (eGFR 61–90 ml/min per 1.73 m2) could predict CV events in the diabetic group with normoalbuminuria (44). Collectively, these findings suggest that, even in individuals with few CV risks that have not yet been screened for albuminuria, it is necessary to carefully monitor for the occurrence of low-grade albuminuria.
The strengths of this study include the use of the United States nationally representative, large-scale study population. Nevertheless, this study has several limitations. By measuring baseline ACR only once, each albuminuria status could be misclassified. Because it is an observational study, there were limitations in concluding causal relationships.
In conclusion, low-grade albuminuria is associated with an increased risk of all-cause and CV mortality. In particular, risk was most pronounced in the non-DM, nonhypertensive, and non-CKD groups. Our findings suggest that low-grade albuminuria is associated with mortality and should be monitored even in groups with low CV risks.
Disclosures
All authors have nothing to disclosure.
Funding
This work was supported by a Seoul National University research grant (SRnD 800-20200496) and by the Seoul National University Hospital research fund (03-2020-2130).
Author Contributions
E. Bae, M. Kang, D. K. Kim, E.Y. Kim, Y.C. Kim, S. Kwon, J. Lee, J.P. Lee, C.S. Lim, and J.I. Shin were responsible for methodology; E. Bae, M. Kang, D.K. Kim, E.Y. Kim, S. Kwon, J. Lee, J.P. Lee, C.S. Lim, and J.Y. Park were responsible for formal analysis; J.P. Lee were responsible for funding acquisition; M. Kang, Y.C. Kim, J.P. Lee, and J.I. Shin reviewed and edited the manuscript; M. Kang wrote the original draft and were responsible for data curation and visualization; M. Kang, S. Kwon, and J.P. Lee conceptualized the study; J.P. Lee provided supervision; J.Y. Park was responsible for investigation; and all authors read and approved the final manuscript.
Data Sharing Statement
All data is included in the manuscript and/or supporting information.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0003912021/-/DCSupplemental.
Flow diagram of study cohort. Download Supplemental Figure 1, PDF file, 386 KB (385.1KB, pdf)
Subgroup associations of urine albumin creatine ratio with all-cause mortality. Download Supplemental Figure 2, PDF file, 386 KB (385.1KB, pdf)
The relationship between the urine albumin-creatinine ratio and mortality. Download Supplemental Figure 3, PDF file, 386 KB (385.1KB, pdf)
References
- 1.Anyanwagu U, Donnelly R, Idris I: Albuminuria regression and all-cause mortality among insulin-treated patients with type 2 diabetes: Analysis of a large UK primary care cohort. Am J Nephrol 49: 146–155, 2019. 10.1159/000496276 [DOI] [PubMed] [Google Scholar]
- 2.Fangel MV, Nielsen PB, Kristensen JK, Larsen TB, Overvad TF, Lip GY, Jensen MB: Albuminuria and risk of cardiovascular events and mortality in a general population of patients with type 2 diabetes without cardiovascular disease: A Danish cohort study. Am J Med 133: e269–e279, 2020. 10.1016/j.amjmed.2019.10.042 [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Mann JF, Pogue J, Dinneen SF, Hallé JP, Hoogwerf B, Joyce C, Rashkow A, Young J, Zinman B, Yusuf S; The HOPE Study Investigators : Prevalence and determinants of microalbuminuria in high-risk diabetic and nondiabetic patients in the Heart Outcomes Prevention Evaluation Study. Diabetes Care 23: B35–B39, 2000 [PubMed] [Google Scholar]
- 4.Levey AS, Becker C, Inker LA: Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 313: 837–846, 2015. 10.1001/jama.2015.0602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin YC, Chang YH, Yang SY, Wu KD, Chu TS: Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc 117: 662–675, 2018. 10.1016/j.jfma.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 6.Satirapoj B, Adler SG: Comprehensive approach to diabetic nephropathy. Kidney Res Clin Pract 33: 121–131, 2014. 10.1016/j.krcp.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mogensen CE: How to protect the kidney in diabetic patients: With special reference to IDDM. Diabetes 46: S104–S111, 1997. 10.2337/diab.46.2.S104 [DOI] [PubMed] [Google Scholar]
- 8.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group : Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74 [published correction appears in Diabetes 60: 1823, 2011]. Diabetes 55: 1832–1839, 2006. 10.2337/db05-1620 [DOI] [PubMed] [Google Scholar]
- 9.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63: 713–735, 2014. 10.1053/j.ajkd.2014.01.416 [DOI] [PubMed] [Google Scholar]
- 10.Ren M, Sun K, Li F, Qi YQ, Lin DZ, Li N, Li Y, Yan L: Association between obesity measures and albuminuria: A population-based study. J Diabetes Complications 30: 451–456, 2016. 10.1016/j.jdiacomp.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 11.Takae K, Hata J, Ohara T, Yoshida D, Shibata M, Mukai N, Hirakawa Y, Kishimoto H, Tsuruya K, Kitazono T, Kiyohara Y, Ninomiya T: Albuminuria increases the risks for both alzheimer disease and vascular dementia in community-dwelling Japanese elderly: The Hisayama Study. J Am Heart Assoc 7: e006693, 2018. 10.1161/JAHA.117.006693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stehouwer CD: Endothelial dysfunction in diabetic nephropathy: State of the art and potential significance for non-diabetic renal disease. Nephrol Dial Transplant 19: 778–781, 2004. 10.1093/ndt/gfh015 [DOI] [PubMed] [Google Scholar]
- 13.Almeida JC, Zelmanovitz T, Vaz JS, Steemburgo T, Perassolo MS, Gross JL, Azevedo MJ: Sources of protein and polyunsaturated fatty acids of the diet and microalbuminuria in type 2 diabetes mellitus. J Am Coll Nutr 27: 528–537, 2008. 10.1080/07315724.2008.10719735 [DOI] [PubMed] [Google Scholar]
- 14.Pattaro C: Genome-wide association studies of albuminuria: Towards genetic stratification in diabetes? J Nephrol 31: 475–487, 2018. 10.1007/s40620-017-0437-3 [DOI] [PubMed] [Google Scholar]
- 15.Royal Australian College of General Practitioners : Prevention of vascular and metabolic disease-kidney disease. In: Guidelines for preventive activities in general practice 9th edition. East Melbourne, Australia, Royal Australian College of General Practitioners, 2016. [Google Scholar]
- 16.Caring for Australians with Renal Impairment (CARI) : The CARI guidelines. Urine protein as diagnostic test: Performance characteristics of tests used in the initial evaluation of patients at risk of renal disease. Nephrology (Carlton) 9: S8–S14, 2004. 10.1111/j.1440-1797.2004.00312.x [DOI] [PubMed] [Google Scholar]
- 17.National Collaborating Centre for Chronic Conditions (UK) : Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care, London, Royal College of Physicians, 2008 [PubMed] [Google Scholar]
- 18.Waheed S, Matsushita K, Sang Y, Hoogeveen R, Ballantyne C, Coresh J, Astor BC: Combined association of albuminuria and cystatin C-based estimated GFR with mortality, coronary heart disease, and heart failure outcomes: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 60: 207–216, 2012. 10.1053/j.ajkd.2012.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T, Zhong H, Lian G, Cai X, Gong J, Ye C, Xie L: Low-grade albuminuria is associated with left ventricular hypertrophy and diastolic dysfunction in patients with hypertension. Kidney Blood Press Res 44: 590–603, 2019. 10.1159/000500782 [DOI] [PubMed] [Google Scholar]
- 20.Blecker S, Matsushita K, Köttgen A, Loehr LR, Bertoni AG, Boulware LE, Coresh J: High-normal albuminuria and risk of heart failure in the community. Am J Kidney Dis 58: 47–55, 2011. 10.1053/j.ajkd.2011.02.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pichaiwong W, Homsuwan W, Leelahavanichkul A: The prevalence of normoalbuminuria and renal impairment in type 2 diabetes mellitus. Clin Nephrol 92: 73–80, 2019. 10.5414/CN109606 [DOI] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics, Centers for Disease Control and Prevention : National Health and Nutrition Examination Survey; NHANES 2013-2014 Laboratory Data; Albumin & Creatinine - Urine. Available at: https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=2013-2014. Accessed December 2, 2021
- 23.Hoerger TJ, Simpson SA, Yarnoff BO, Pavkov ME, Ríos Burrows N, Saydah SH, Williams DE, Zhuo X: The future burden of CKD in the United States: A simulation model for the CDC CKD Initiative. Am J Kidney Dis 65: 403–411, 2015. 10.1053/j.ajkd.2014.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, Coresh J: Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis 50: 918–926, 2007. 10.1053/j.ajkd.2007.08.020 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Lou Y, Ma Y, Shan X: Estimating the glomerular filtration rate and tubular dysfunction in an elderly population with normoalbuminuria in China. Clin Chim Acta 495: 377–381, 2019. 10.1016/j.cca.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Wang C, Hu C, Han Y, Zhao L, Zhu X, Xiao L, Sun L: Normoalbuminuric diabetic kidney disease. Front Med 11: 310–318, 2017. 10.1007/s11684-017-0542-7 [DOI] [PubMed] [Google Scholar]
- 28.Warnock DG, Muntner P, McCullough PA, Zhang X, McClure LA, Zakai N, Cushman M, Newsome BB, Kewalramani R, Steffes MW, Howard G, McClellan WM; REGARDS Investigators : Kidney function, albuminuria, and all-cause mortality in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. Am J Kidney Dis 56: 861–871, 2010. 10.1053/j.ajkd.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE; Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group : Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777–1782, 2002. 10.1161/01.CIR.0000031732.78052.81 [DOI] [PubMed] [Google Scholar]
- 30.Tanaka F, Komi R, Makita S, Onoda T, Tanno K, Ohsawa M, Itai K, Sakata K, Omama S, Yoshida Y, Ogasawara K, Ishibashi Y, Kuribayashi T, Okayama A, Nakamura M; Iwate-Kenco Study Group : Low-grade albuminuria and incidence of cardiovascular disease and all-cause mortality in nondiabetic and normotensive individuals. J Hypertens 34: 506–512, discussion 512, 2016. 10.1097/HJH.0000000000000809 [DOI] [PubMed] [Google Scholar]
- 31.Wang TJ, Evans JC, Meigs JB, Rifai N, Fox CS, D’Agostino RB, Levy D, Vasan RS: Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation 111: 1370–1376, 2005. 10.1161/01.CIR.0000158434.69180.2D [DOI] [PubMed] [Google Scholar]
- 32.Shankar A, Klein R, Moss SE, Klein BE, Wong TY: The relationship between albuminuria and hypercholesterolemia. J Nephrol 17: 658–665, 2004 [PubMed] [Google Scholar]
- 33.Sharma K: The link between obesity and albuminuria: Adiponectin and podocyte dysfunction. Kidney Int 76: 145–148, 2009. 10.1038/ki.2009.137 [DOI] [PubMed] [Google Scholar]
- 34.Ahn SY, Kim DK, Han SS, Park JH, Shin SJ, Lee SH, Choi BS, Lim CS, Kim S, Chin HJ: Weight loss has an additive effect on the proteinuria reduction of angiotensin II receptor blockers in hypertensive patients with chronic kidney disease. Kidney Res Clin Pract 37: 49–58, 2018. 10.23876/j.krcp.2018.37.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobre D, Nimade S, de Zeeuw D: Albuminuria in heart failure: What do we really know? Curr Opin Cardiol 24: 148–154, 2009. 10.1097/HCO.0b013e328323aa9a [DOI] [PubMed] [Google Scholar]
- 36.Chang CC, Hsu CY, Chang TY, Huang PH, Liu LK, Chen LK, Chen JW, Lin SJ: Association between low-grade albuminuria and frailty among community-dwelling middle-aged and older people: A cross-sectional analysis from I-Lan Longitudinal Aging Study. Sci Rep 6: 39434, 2016. 10.1038/srep39434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nickel NP, de Jesus Perez VA, Zamanian RT, Fessel JP, Cogan JD, Hamid R, West JD, de Caestecker MP, Yang H, Austin ED: Low-grade albuminuria in pulmonary arterial hypertension. Pulm Circ 9: 2045894018824564, 2019. 10.1177/2045894018824564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.GBD 2015 Mortality and Causes of Death Collaborators : Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1459–1544, 2016. 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park S, Lee S, Kim Y, Lee Y, Kang MW, Han K, Han SS, Lee H, Lee JP, Joo KW, Lim CS, Kim YS, Kim DK: Risk of cancer in pre-dialysis chronic kidney disease: A nationwide population-based study with a matched control group. Kidney Res Clin Pract 38: 60–70, 2019. 10.23876/j.krcp.18.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jørgensen L, Heuch I, Jenssen T, Jacobsen BK: Association of albuminuria and cancer incidence. J Am Soc Nephrol 19: 992–998, 2008. 10.1681/ASN.2007060712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xin Z, Liu S, Niu J, Xu M, Wang T, Lu J, Chen Y, Wang W, Ning G, Bi Y, Xu Y, Li M, Zhao Z: The association of low-grade albuminuria with incident nonalcoholic fatty liver disease and non-invasive markers of liver fibrosis by glycemic status. Liver Int 41: 422–423, 2021. 10.1111/liv.14322 [DOI] [PubMed] [Google Scholar]
- 42.Ruggenenti P, Remuzzi G: Time to abandon microalbuminuria? Kidney Int 70: 1214–1222, 2006. 10.1038/sj.ki.5001729 [DOI] [PubMed] [Google Scholar]
- 43.Engin A: Non-alcoholic fatty liver disease. Adv Exp Med Biol 960: 443–467, 2017. 10.1007/978-3-319-48382-5_19 [DOI] [PubMed] [Google Scholar]
- 44.Hsieh YT, Kuo JF, Su SL, Chen JF, Chen HC, Hsieh MC: Subnormal estimated glomerular filtration rate strongly predict incident cardiovascular events in type 2 diabetic Chinese population with normoalbuminuria. Medicine (Baltimore) 95: e2200, 2016. 10.1097/MD.0000000000002200 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow diagram of study cohort. Download Supplemental Figure 1, PDF file, 386 KB (385.1KB, pdf)
Subgroup associations of urine albumin creatine ratio with all-cause mortality. Download Supplemental Figure 2, PDF file, 386 KB (385.1KB, pdf)
The relationship between the urine albumin-creatinine ratio and mortality. Download Supplemental Figure 3, PDF file, 386 KB (385.1KB, pdf)