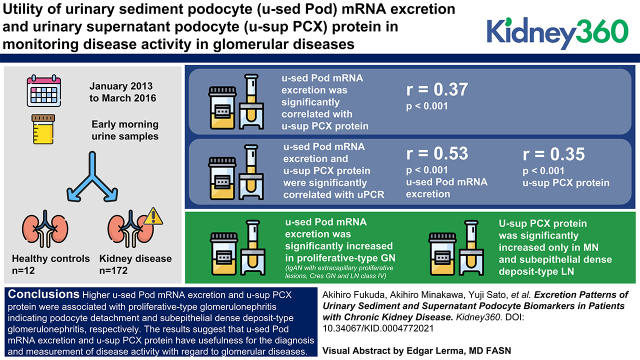
Key Points
Higher urinary sediment podocyte mRNA excretion is associated with proliferative-type GN indicating podocyte detachment.
Higher urinary supernatant podocyte protein is associated with subepithelial dense deposit–type GN.
These podocyte biomarkers have usefulness for the diagnosis and measurement of disease activity in glomerular diseases.
Keywords: chronic kidney disease, podocyte, proteinuria, urinary sediment podocyte mRNA, urinary supernatant podocyte protein
Visual Abstract
Abstract
Background
Podocyte depletion causes glomerulosclerosis, and persistent podocyte loss drives progression to ESKD. Urinary sediment podocin (u-sed Pod) mRNA excretion and urinary supernatant podocalyxin (u-sup PCX) protein have been used to monitor disease activity in glomerular diseases. However, the differences in these markers among pathologies have not been investigated. We examined the roles of these markers in kidney diseases.
Methods
From January 2013 to March 2016, early morning urine samples were collected from 12 healthy controls and 172 patients with kidney disease (n=15 patients with minor glomerular abnormality with mild proteinuria and/or microscopic hematuria, n=15 with minimal change nephrotic syndrome [MCNS], n=15 with membranous nephropathy [MN], n=60 with IgA nephropathy [IgAN], n=19 with crescentic GN [Cres GN], n=10 with lupus nephritis [LN], and n=38 with other kidney diseases). We examined u-sed Pod mRNA excretion, u-sup PCX protein, and the urinary protein-creatinine ratio (u-PCR).
Results
u-sed Pod mRNA excretion was significantly correlated with u-sup PCX protein (r=0.37, P<0.001). Both u-sed Pod mRNA excretion and u-sup PCX protein were significantly correlated with u-PCR (r=0.53, P<0.001 and r=0.35, P<0.001, respectively). Interestingly, u-sed Pod mRNA excretion was significantly increased in proliferative-type GN—including IgAN with extracapillary proliferative lesions, Cres GN, and LN class IV—and significantly correlated with the rate of crescent formation, whereas u-sup PCX protein was significantly increased only in those with MN and subepithelial dense deposit–type LN compared with controls.
Conclusions
Higher u-sed Pod mRNA excretion and u-sup PCX protein were associated with proliferative-type GN, indicating podocyte detachment and subepithelial dense deposit–type GN, respectively. The results suggest that u-sed Pod mRNA excretion and u-sup PCX protein have usefulness for the diagnosis and measurement of disease activity with regard to glomerular diseases.
Introduction
Proteinuria and/or albuminuria have served as diagnostic and monitoring tools for kidney diseases in the clinic for many years. Proteinuria increases early in glomerular injury and can potentially serve as an early and predictive marker of future progression. However, proteinuria is caused by many mechanisms, including various forms of glomerular injury, tubulointerstitial injury, and physiologic processes that enhance glomerular filtration of protein (1). Proteinuria is, therefore, not a specific biomarker for any single kidney disease. However, it is a viable tool for monitoring kidney injury and the response to treatment.
Glomerular diseases, including diabetic kidney disease and hypertension, comprise >80% of the causes of ESKD. Compelling data now support the concept that podocyte injury and depletion cause glomerulosclerosis, and that persistent podocyte loss drives most forms of progression of glomerular diseases (2–18). Podocytes reside on the urinary space side of the glomerular basement membrane, therefore, as they detach or die, their products can be identified in urine. Thus, in the last quarter century, we and other investigators reported that podocyte products in urine (such as urinary sediment podocin [u-sed Pod] mRNA excretion and urinary supernatant podocalyxin protein) could be potential biomarkers of glomerular disease activity and progression (19–32). However, no study has investigated the differences in these urinary podocyte biomarkers. We examined the importance of these markers in various kidney diseases.
Materials and Methods
Ethical Considerations
This study was conducted according to the principles of the Declaration of Helsinki and was approved by the institutional review board of the University of Miyazaki Hospital (number 2014-055). Informed consent was obtained from all subjects.
Collection and Histologic Evaluation of Samples from Patients with Kidney Disease
From January 2013 to March 2016, urine samples were collected in the morning from 184 consecutive patients with kidney disease: 15 patients with minor glomerular abnormality with mild proteinuria and/or microscopic hematuria, 15 with minimal change nephrotic syndrome (MCNS), 15 with membranous nephropathy (MN), 60 with IgAN, 19 with crescentic GN (Cres GN), 10 with lupus nephritis (LN), 38 with other kidney diseases (n=7 with mesangial proliferative GN [IgA negative], n=5 with IgA vasculitis, n=4 with FSGS, n=3 with secondary IgA nephropathy, n=3 with membranoproliferative GN, n=3 with interstitial nephritis, n=2 with nephrosclerosis, n=2 with AL amyloidosis, n=2 with postinfectious GN, n=1 with endocapillary proliferative GN, n=1 with light chain deposition disease, n=1 with C3 glomerulopathy, n=1 with obesity-related glomerulopathy, n=1 with diabetic nephropathy, n=1 with bone marrow transplantation nephropathy, n=1 with familial nephropathy associated with hyperuricemia), and 12 healthy volunteers. Patients with minor glomerular abnormality were those with mild proteinuria and microscopic hematuria (mostly qualitative 1+, quantitative mean 0.3 g/gCr) found during physical examination, and those with minor glomerular changes observed on a renal biopsy specimen. The healthy volunteers had never been diagnosed with any underlying disease in past medical checkups and had not been diagnosed with any urinary abnormalities or renal dysfunction in the last year. On the basis of this information, we determined that the healthy volunteers did not have kidney disease or hypertension, and only urine samples were collected. The eGFR was estimated by the isotope dilution mass spectrometry–traceable Modification of Diet in Renal Disease method adjusted for the Japanese population (194×serum creatinine−1.094×age−0.287×0.739 [if female]) (33). The clinical parameters of the patients with kidney disease and healthy controls are shown in Table 1. The urinary protein-creatinine ratio (u-PCR), u-sed Pod (podocin) mRNA–factored urinary creatinine concentration (u-sed Pod mRNA), and urinary supernatant podocalyxin protein–factored urinary creatinine concentration (u-sup PCX protein) were measured. The Oxford classification (34) system was used to evaluate the histologic findings of patients with IgAN. The minimal number of glomerular profiles evaluated per section was eight, according to the Oxford classification system. The rate of crescent formation in patients with Cres GN was counted as the percentage of glomeruli with cellular and fibrocellular crescents. MN was classified into stages I–IV according to the Churg stage classification (35) on the basis of electron microscopic findings. Patients with LN were evaluated for the presence of subepithelial and subendothelial deposits on the basis of electron microscopic findings. Histology slides were evaluated by two investigators blinded to sample identity.
Table 1.
Clinical profiles of the patients with kidney disease and healthy controls
| Parameter | Control, n=12 |
Minor, n=15 |
Minimal Change Nephrotic Syndrome, n=15 |
Membranous Nephropathy, n=15 |
IgA Nephropathy, n=60 |
Crescentic Glomerulonephritis, n=19 |
Lupus Nephritis, n=10 |
Others, n=38 |
|---|---|---|---|---|---|---|---|---|
| Age, yr | 34±5 | 45±18 | 54±18 | 65±16 | 41±15 | 65±15 | 40±16 | 54±15 |
| Sex, M/F | 1/11 | 9/6 | 7/8 | 6/9 | 24/36 | 10/9 | 1/9 | 18/20 |
| SBP, mm HG | N/A | 115±15 | 119±24 | 124±18 | 115±14 | 134±21 | 119±16 | 118±19 |
| DBP, mm HG | N/A | 71±12 | 72±14 | 71±12 | 71±11 | 74±11 | 76±11 | 70±14 |
| Serum Alb, g/dl | N/A | 4.0±0.6 | 1.5±0.3 | 2.3±0.7 | 3.9±0.5 | 2.9±0.7 | 2.7±0.6 | 3.2±0.9 |
| Serum Cre, mg/dl | N/A | 0.77±0.10 | 1.44±1.16 | 1.00±0.88 | 1.05±1.11 | 2.35±1.82 | 0.81±0.27 | 1.41±1.23 |
| eGFR, ml/min per 1.73 m2 | N/A | 81.3±18.9 | 51.7±27.8 | 67.2±25.7 | 70.9±25.5 | 33.8±26.4 | 74.2±24.5 | 52.6±25.1 |
| U-Pro/Cre, g/gCre | 0.04±0.03 | 0.30±0.46 | 6.69±5.38 | 3.42±2.83 | 0.71±1.14 | 1.76±2.25 | 2.23±1.18 | 2.62±3.22 |
| Drug use, % | ||||||||
| ARB or ACE-I | 0 | 26 | 7 | 40 | 22 | 26 | 40 | 34 |
| CCB | 0 | 13 | 27 | 60 | 18 | 58 | 30 | 47 |
| Immunosuppressives | 0 | 7 | 33 | 13 | 2 | 21 | 70 | 8 |
Values are given as means±SD or percentages. Minor, minor glomerular abnormality with mild proteinuria and/or microscopic hematuria; M, male; F, female; SBP, systolic BP; N/A, not applicable; DBP, diastolic BP; Alb, albumin; Cre, creatinine; U-pro/Cre, urinary protein to creatinine ratio; ARB, angiotensin receptor blocker; ACE-I, angiotensin-converting enzyme inhibitor; CCB, calcium channel blockers.
Extraction of RNA from Human Urinary Sediment
Urine samples were collected in the morning and centrifuged at 4°C for 15 minutes at 3200×g in a tabletop centrifuge. The supernatant was removed, the pellet suspended in 1.5 ml of diethyl pyrocarbonate–treated PBS, and centrifuged at 12,000×g for 5 minutes at 4°C. The washed pellet was resuspended in RLT/β-mercaptoethanol buffer (RNeasy Kit; Qiagen, Germantown, MD) and frozen at −80°C until RNA extraction (16,23,28).
RNA Preparation and Quantitative RT-PCR
Total urinary sediment was purified using an RNeasy Mini Kit (catalog number 74106; Qiagen). cDNA was transcribed from sample total RNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitation of the podocin mRNA abundance was performed with a LightCycler 96 system (Roche Molecular Systems, Mannheim, Germany) using FastStart Essential DNA Probe Master Mix (Roche Molecular Systems) in a final volume of 10 μl per reaction. The TaqMan probe (Applied Biosystems) used was for human NPHS2 (podocin; cat. no. Hs00922492_m1). Data were from 2 μg samples of cDNA measured in duplicate. cDNA standard curves were constructed using serially diluted standards as described previously (16,23,28). The concentration of u-sed Pod mRNA was standardized by the creatinine concentration and was expressed as molar per grams of creatinine.
Quantitation of u-sup PCX Protein Concentration
u-sup PCX was measured by a sandwich ELISA, as described previously (31,36). To construct the sandwich-type ELISA, the protein-G–bound fraction from ascitic fluid was used as the capture antibody for ELISA plates and was labeled with horseradish peroxidase. The urine samples were mixed and incubated with an equal volume of sample buffer (0.4 M N-tris[hydroxymethyl]methyl-2-aminoethanesulfonic acid–sodium hydroxide buffer containing 0.04 M EDTA and 0.4% Triton X-100, pH 7.0). ELISA was performed using 100 μl of treated urine samples. The u-sup PCX concentration was standardized to the creatinine concentration and expressed as nanograms per micromole of creatinine.
Statistical Analysis
Statistical analysis was performed using Prism software, version 6.0 (GraphPad Software, La Jolla, CA). The clinical parameters of the patients with kidney disease and urinary measurements are given as means±SD. Differences between the two groups were evaluated using the Mann–Whitney U test, and those among more than two groups were evaluated by the Kruskal–Wallis test. When the result of the Kruskal–Wallis test was significant, the Dunn test was performed for post hoc analysis. Correlations between parameters were assessed by single regression analysis (Spearman rank correlation). A P value <0.05 was considered indicative of statistical significance.
Results
Correlation between u-sed Pod mRNA Excretion, u-sup PCX Protein, and Proteinuria
Table 1 shows the clinical parameters of the healthy controls (n=12) and patients with kidney disease (minor glomerular abnormality with mild proteinuria and/or microscopic hematuria, n=15; MCNS, n=15; MN, n=15; IgAN, n=60; Cres GN, n=19; LN, n=10; and others [details are provided in the Materials and Methods], n=38) in this cross-sectional study. A low serum albumin level and nephrotic range of proteinuria were observed in those with MCNS and MN, and eGFR was decreased in those with Cres GN. The antihypertensive and immunosuppressive medications used at the time of urine collection are shown in Table 1.
u-sed Pod mRNA excretion was significantly correlated with u-sup PCX protein (r=0.37, P<0.001). u-sed Pod mRNA excretion and u-sup PCX protein were significantly correlated with u-PCR (r=0.53, P<0.001 and r=0.35, P<0.001, respectively; Figure 1). These data suggest that urinary podocyte biomarkers are correlated with proteinuria in kidney diseases.
Figure 1.
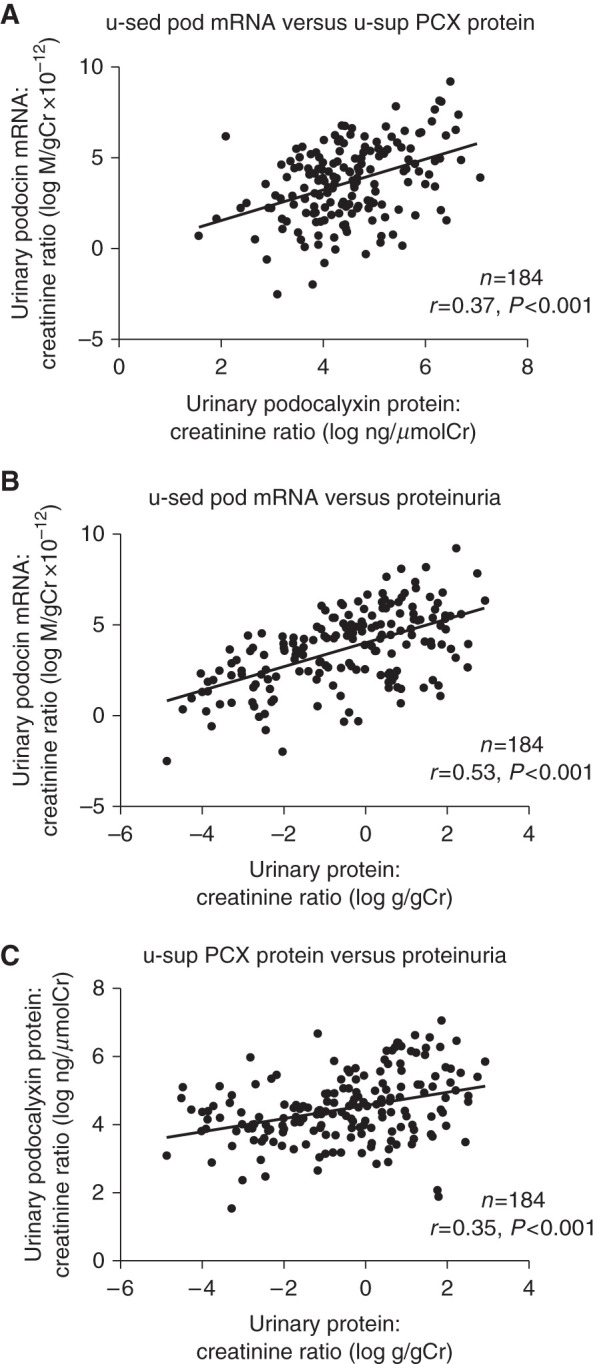
Urinary podocyte markers was significantly correlated with proteinuria. (A) Relationship between urinary sediment podocyte (podocin) (u-sed Pod) mRNA excretion and urinary supernatant podocyte (podocalyxin) (u-sup PCX) protein. (B) Relationship between u-sed Pod mRNA excretion and proteinuria. (C) Relationship between u-sup PCX protein and proteinuria. u-sed Pod mRNA excretion was significantly correlated with u-sup PCX protein (r=0.37, P<0.001). u-sed Pod mRNA excretion and u-sup PCX protein were significantly correlated with u-PCR (r=0.53, P<0.001 and r=0.35, P<0.001, respectively). Cr, creatinine.
u-sed Pod mRNA Excretion and u-sup PCX Protein in Glomerular Diseases
We selected representative glomerular diseases (134 patients; 15 with minor glomerular abnormality with mild proteinuria and/or microscopic hematuria, 15 with MCNS, 15 with MN, 60 with IgAN, 19 with Cres GN, and 10 with LN) to assess the difference between u-sed Pod mRNA excretion and u-sup PCX protein. Diseases with fewer than eight cases were excluded due to the difficulty of statistical analysis. Proteinuria was significantly increased in all of the glomerular diseases. u-sed Pod mRNA excretion was also significantly increased in all of the glomerular diseases compared with the controls, and was further increased in IgAN (29-fold), Cres GN (76-fold), and LN (190-fold); however, u-sup PCX protein was significantly increased only in MN (5.1-fold) and LN (5.5-fold) compared with the controls (Figure 2). These data indicate that u-sed Pod mRNA excretion and u-sup PCX protein could provide different information.
Figure 2.
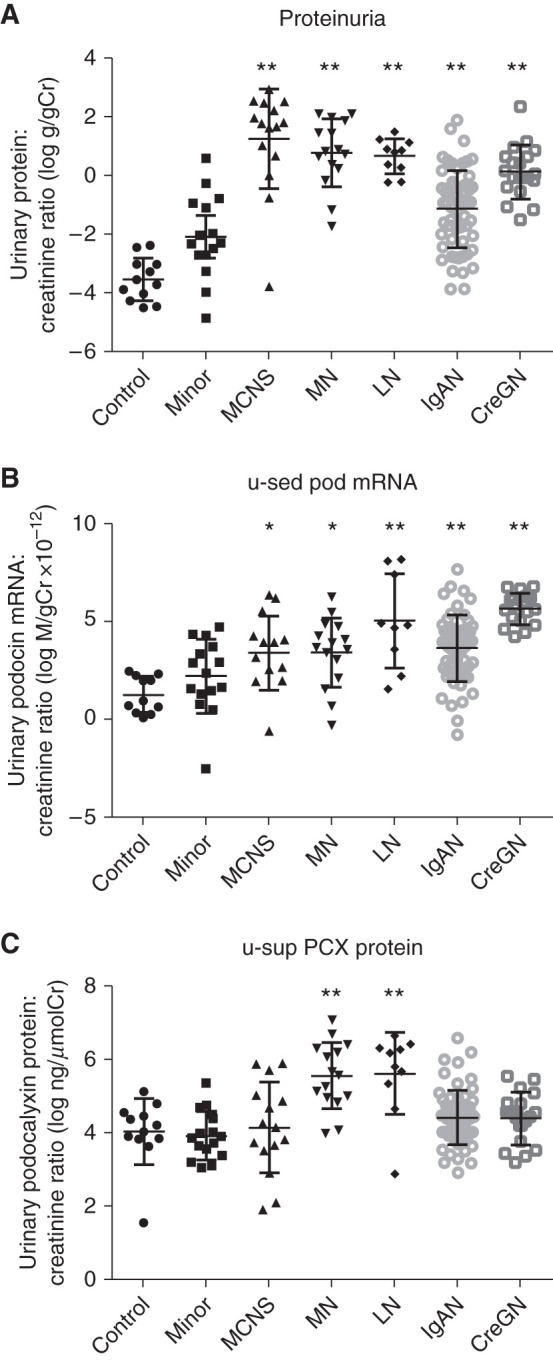
U-sed pod mRNA and u-sup PCX was different excretion pattern in glomerular diseases. (A) Proteinuria in glomerular diseases. (B) u-sed Pod mRNA excretion in glomerular diseases. (C) u-sup PCX protein in glomerular diseases. Proteinuria was significantly increased in all of the glomerular diseases. u-sed Pod mRNA excretion was also significantly increased in all of the glomerular diseases compared with controls, and further increased in IgA nephropathy (IgAN), crescentic GN (CreGN), and lupus nephritis (LN); however, u-sup PCX protein was significantly increased only in those with membranous nephropathy (MN) and LN compared with the controls. *P<0.05, **P<0.01 versus controls, assessed by Kruskal–Wallis test followed by Dunn test. Cr, creatinine; MCNS, minimal change nephrotic syndrome; minor, minor glomerular abnormality with mild proteinuria and/or microscopic hematuria.
u-sed Pod mRNA Excretion, u-sup PCX Protein, and Relationship to Histopathologic Findings in Glomerular Diseases
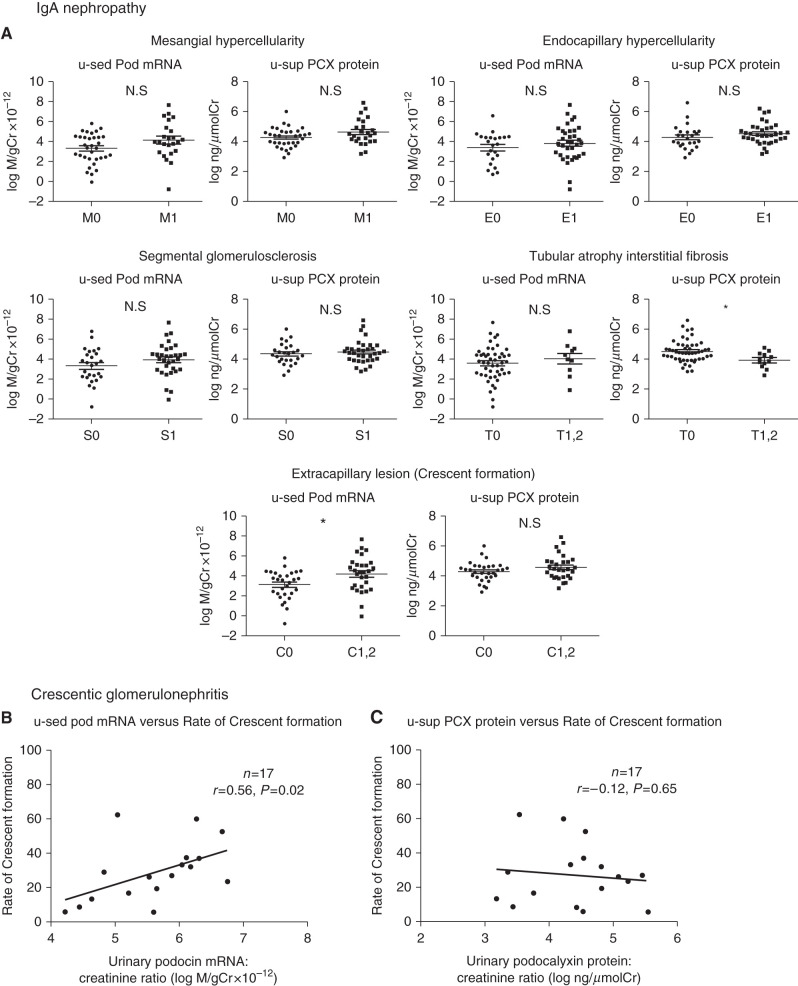
First, we evaluated the proliferative glomerular diseases: IgAN and Cres GN. u-sed Pod mRNA excretion was significantly increased only with extracapillary proliferative lesions in the Oxford classification, whereas u-sup PCX protein did not differ significantly in any lesions in IgAN (Figure 3A). Furthermore, u-sed Pod mRNA excretion, but not u-sup PCX protein, was correlated significantly with the rate of crescent formation (n=17, r=0.56, P=0.02, and n=17, r=−0.12, P=0.65, respectively; two patients were excluded because a renal biopsy was not performed) in those with Cres GN (Figure 3, B and C). These results suggest that u-sed Pod mRNA excretion is strongly associated with extracapillary proliferative lesions, as we have reported previously (23,27).
Figure 3.
U-sed pod mRNA, but not u-sup P CX was significantly correlated with the rate of crescent formation in IgAN and Cres GN. (A) u-sed Pod mRNA excretion and u-sup PCX protein in the Oxford IgAN histologic classification (M0, n=35; M1, n=25; E0, n=25; E1, n=35; S0, n=26; S1, n=34; T0, n=50; T1 and 2, n=10; C0, n=31; C1 and 2, n=29). u-sed Pod mRNA excretion, but not u-sup PCX protein, was significantly increased in the presence of extracapillary proliferative lesions. (B) Relationship between u-sed Pod mRNA excretion and rate of crescent formation in crescentic GN (Cres GN). (C) Relationship between u-sup PCX protein and rate of crescent formation in Cres GN. u-sed Pod mRNA excretion, but not sup-PCX protein, was significantly correlated with the rate of crescent formation (u-sed Pod mRNA excretion: n=17, r=0.56, P=0.02; u-sup PCX protein: n=17, r=−0.12, P=0.65). *P<0.05 by Mann–Whitney U test. Cr, creatinine.
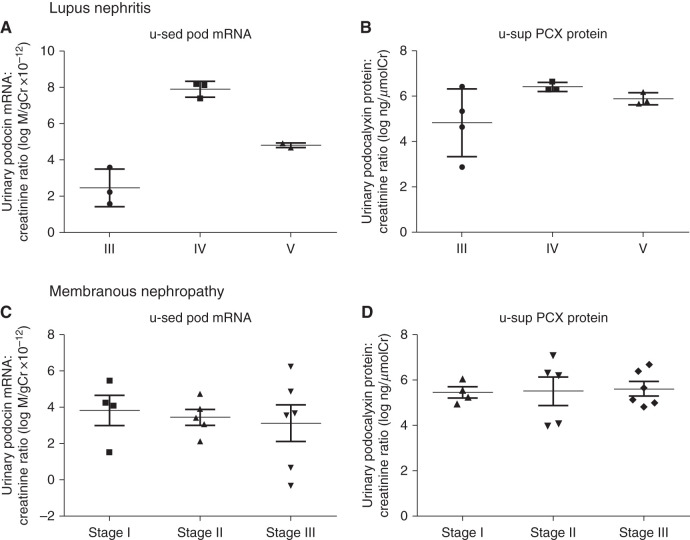
Next, we evaluated LN, which has proliferative and nonproliferative forms. u-sed Pod mRNA excretion and u-sup PCX protein were significantly increased in patients with LN compared with the controls (190- and 5.5-fold, respectively). We examined differences in urinary podocyte markers among pathologic classifications of LN. Table 2 shows the clinical parameters, urinary biomarkers, and areas of dense deposition in ten patients with LN. The ratio of males to females was 1:9. The histologic classification was class III in four patients, IV in three patients, and V in three patients. The mean eGFR was >60 ml/min per 1.73 m2; there was nephrotic-range proteinuria in class IV. u-sed Pod mRNA excretion was increased in class IV, whereas u-sup PCX protein was increased in the presence of subepithelial dense deposition but not subendothelial dense deposition (Figure 4, A and B, Table 2). Although the sample size was small, these data suggest that higher u-sed Pod mRNA excretion and u-sup PCX protein might be associated with proliferative-type GN (class III and IV: 260-fold versus control) and subepithelial dense deposit GN (6.9-fold versus control), respectively. We found that u-sup PCX protein was increased in subepithelial dense deposit–type LN, we next validated this finding in MN. Table 3 shows the clinical parameters, urinary biomarkers, and histologic stages of 15 patients with MN. The ratio of males to females was 6:9. The histologic stage was class I in four patients, II in five patients, III in six patients, and IV in no patients. The mean eGFR was 67.2 ml/min per 1.73 m2 and mean proteinuria was 3.4 g/gCr. Both u-sed Pod mRNA excretion and u-sup PCX protein were increased compared with the controls. There was no difference in the two podocyte markers according to classification stage (Figure 4, C and D, Table 3); however, u-sup PCX protein in MN was increased 5.1-fold compared with the controls and to those with proliferative glomerular disease (1.6-fold for IgAN and 1.4-fold for Cres GN). Furthermore, when patients with IgAN, Cres GN, MN, and LN were divided into mesangial proliferative type, extracapillary proliferative type, and subepithelial dense deposit type (LN class IV was excluded because it corresponded to both the proliferative and nonproliferative types), u-sed Pod mRNA excretion was increased in the extracapillary proliferative type, and u-sup PCX protein was increased in the subepithelial dense deposit type (Figure 5). These results are consistent with those in LN, in which higher u-sed Pod mRNA excretion and u-sup PCX protein were associated with proliferative-type GN and subepithelial dense deposit–type GN, respectively.
Table 2.
Clinical parameters, urinary podocyte markers, and areas of dense deposition in patients with lupus nephritis
| Patient | Sex | Age, yr | Class | eGFR, ml/min per 1.73 m2 |
Systolic BP, mm Hg |
Diastolic BP, mm Hg |
Total Protein, g/dl |
Albumin, g/dl |
Urinary Protein-Creatinine Ratio, g/gCre | log PodCR | log PCX | Subendothelial Dense Deposition | Subepithelial Dense Deposition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 37 | III | 85.8 | 110 | 54 | 5.7 | 3.0 | 1.46 | 2.22 | 4.66 | 1+ | — |
| 2 | F | 30 | III | 56.5 | 109 | 73 | 5.1 | 2.6 | 2.21 | 1.57 | 6.42 | 2+ | +/− |
| 3 | F | 35 | III | 42.7 | 108 | 76 | 7.0 | 3.8 | 1.31 | 3.58 | 2.87 | 1+ | — |
| 4 | F | 66 | III | 75.3 | 138 | 77 | 6.2 | 2.2 | 0.80 | N/A | 5.35 | 2+ | +/− |
| 5 | F | 16 | IV | 87.3 | 114 | 77 | 5.2 | 2.5 | 3.42 | 7.39 | 6.65 | 2+ | 1+ |
| 6 | F | 41 | IV | 37.4 | 119 | 74 | 7.8 | 1.7 | 4.43 | 8.18 | 6.28 | 3+ | 2+ |
| 7 | M | 27 | IV | 88.9 | 139 | 95 | 5.5 | 2.9 | 2.44 | 8.11 | 6.32 | 2+ | 1+ |
| 8 | F | 39 | V | 102.4 | 135 | 81 | 5.8 | 3.1 | 2.37 | 4.69 | 5.80 | — | 3+ |
| 9 | F | 44 | V | 108.0 | 92 | 68 | 6.2 | 2.9 | 0.79 | 4.92 | 5.68 | — | 3+ |
| 10 | F | 66 | V | 57.3 | 129 | 89 | 5.2 | 2.2 | 3.10 | 4.82 | 6.19 | — | 3+ |
log PodCR, log u-sed pod mRNA; log PCX, log u-sup PCX protein; F, female; 1+, weak; —, no data; 2+, intermediate; +/−, partially weak; N/A, not applicable; 3+, strong; M, male.
Figure 4.
U-sed pod mRNA was increased in class IV, and u-sup PCX was increased in subepithelial dense deposit-type in LN, but there was no difference in the both podocyte markers according to MN classification stage. (A) u-sed Pod mRNA excretion by histopathologic classification in patients with LN. (B) u-sup PCX protein by histopathologic classification in patients with LN. u-sed pod mRNA excretion was increased in class IV, and u-sup PCX protein was increased in subepithelial dense deposit–type LN. (C) u-sed Pod mRNA excretion by histopathologic stage in patients with MN. (D) u-sup PCX protein by histopathologic classification in patients with LN. There was no difference in the podocyte markers according to classification stage (Churg stage classification). Cr, creatinine.
Table 3.
Clinical parameters, urinary podocyte markers, and classification of histologic stage in patients with membranous nephropathy
| Patient | Sex | Age, yr | eGFR, ml/min per 1.73 m2 |
Systolic BP, mm Hg |
Diastolic BP, mm Hg |
Total Protein, g/dl |
Albumin, g/dl |
Urinary Protein Creatinine Ratio, g/gCr |
log PodCR | log PCX | Histologic Stage |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 71 | 67.6 | 142 | 75 | 4.7 | 2.0 | 0.31 | 4.88 | 6.70 | III |
| 2 | F | 78 | 46.1 | 137 | 63 | 6.5 | 3.4 | 0.18 | 3.08 | 4.08 | II |
| 3 | M | 83 | 11.8 | 152 | 82 | 5.0 | 1.7 | 7.30 | 4.75 | 3.99 | II |
| 4 | M | 68 | 41.3 | 124 | 80 | 8.6 | 1.9 | 8.11 | 5.48 | 5.26 | I |
| 5 | F | 78 | 74.5 | 118 | 72 | 6.1 | 3.0 | 1.44 | 4.26 | 5.58 | I |
| 6 | F | 35 | 95.6 | 72 | 39 | 4.7 | 1.5 | 4.24 | 1.54 | 4.97 | I |
| 7 | M | 59 | 96.4 | 126 | 87 | 6.7 | 3.1 | 2.40 | 0.69 | 5.14 | III |
| 8 | F | 71 | 35.2 | 120 | 68 | 4.4 | 1.7 | 8.12 | 3.69 | 5.67 | III |
| 9 | F | 33 | 62.0 | 117 | 75 | 5.7 | 3.5 | 0.84 | −0.31 | 4.84 | III |
| 10 | M | 76 | 73.5 | 131 | 81 | 6.0 | 2.5 | 1.27 | 3.57 | 5.01 | III |
| 11 | F | 71 | 106.2 | 121 | 70 | 4.7 | 2.3 | 2.28 | 6.25 | 6.41 | III |
| 12 | M | 63 | 65.5 | 111 | 82 | 4.6 | 1.8 | 2.05 | 2.13 | 6.30 | II |
| 13 | F | 66 | 94.0 | 128 | 68 | 5.4 | 2.4 | 4.29 | 4.09 | 6.07 | I |
| 14 | M | 42 | 70.2 | 129 | 68 | 4.4 | 1.8 | 1.91 | 3.42 | 6.19 | II |
| 15 | F | 74 | 67.9 | 130 | 55 | 4.5 | 1.9 | 6.49 | 3.92 | 7.08 | II |
Pt, patient; log PodCR, log u-sed pod mRNA; log PCX, log u-sup PCX protein; F, female; M, male.
Figure 5.
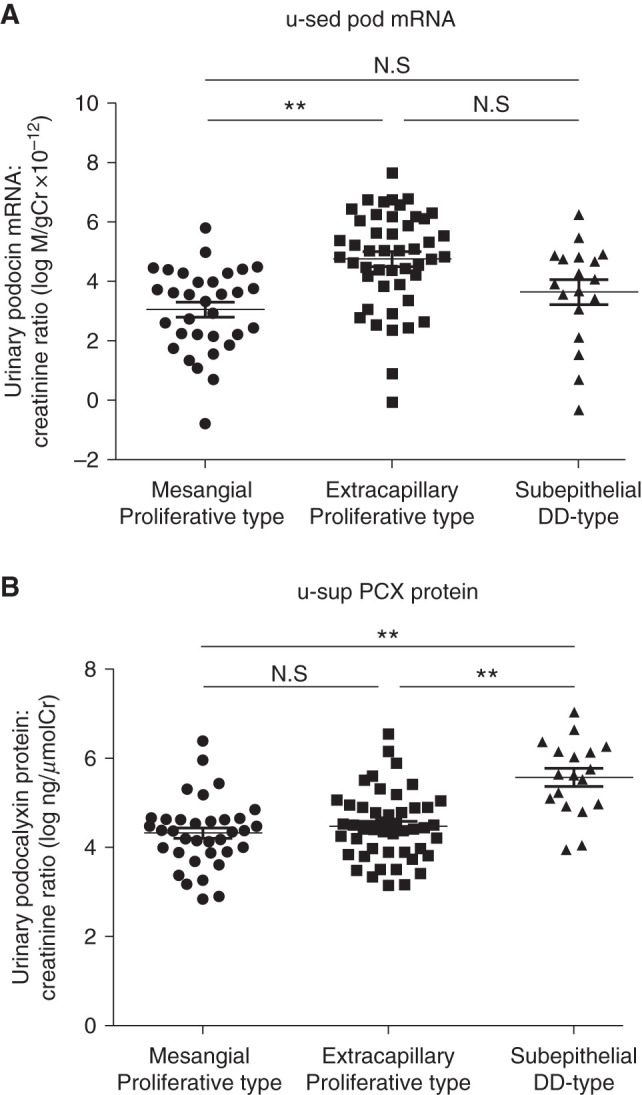
U-sed pod mRNA was increased in the extracapillary proliferative type, and u-sup PCX protein was increased in the subepithelial dense deposit type. (A) u-sed Pod mRNA excretion by histopathologic type (mesangial proliferative type, extracapillary proliferative type, and epithelial dense deposit [DD] type) in patients with IgAN, Cres GN, MN, and LN. (B) u-sup PCX protein by histopathologic type (mesangial proliferative type, extracapillary proliferative type, and epithelial dense deposit type) in patients with IgAN, Cres GN, MN, and LN. u-sed pod mRNA excretion was increased in the extracapillary proliferative type, and u-sup PCX protein was increased in the subepithelial dense deposit type. **P<0.01 versus controls, by Kruskal–Wallis test followed by the Dunn test. Cr, creatinine.
There was a significant correlation between urinary podocyte markers (u-sed Pod mRNA excretion and u-sup PCX protein) and proteinuria (Figure 1). Therefore, we evaluated the association between podocyte depletion and proteinuria in MCNS, which had the highest frequency of proteinuria. Supplemental Table 1 shows the clinical parameters, urinary biomarkers, and the presence or absence of relapse in 15 patients with MCNS. The ratio of males to females was 7:8. The mean eGFR was 51.7 ml/min per 1.73 m2 and mean proteinuria was 6.7 g/gCr. Four of the 15 patients did not have nephrotic-range proteinuria, but these patients had been on steroids or other immunosuppressive drugs at the time of urine sample collection. No patient was found to have FSGS during the observation period, and urinary podocyte markers did not differ between patients with and without relapse. u-sed Pod mRNA excretion was significantly correlated with proteinuria, whereas u-sup PCX protein was not (Supplemental Figure 1). These results suggest that patients with massive proteinuria may have some podocyte detachment even in MCNS, although this may be influenced by the fact that many patients have poor renal function (mean eGFR <60 ml/min per 1.73 m2) at the time of urine collection.
Discussion
We assessed u-sed Pod mRNA excretion and u-sup PCX protein in various kidney diseases. Both of these biomarkers have potential for the diagnosis and monitoring of glomerular diseases (19–32). We reported detection of specific biomarkers in urine pellets using mRNA technology (11–18,21–28). This approach has several advantages in that it is potentially quantitative, is sensitive and specific, and can be multiplexed to measure several mRNAs simultaneously. We also reported detection of urinary podocyte mRNA in rat models of several glomerular diseases and human diseases, including IgAN, ANCA-associated GN, and diabetic nephropathy. Furthermore, the glomerular podocyte loss rate was quantitatively related to the podocyte detachment rate measured by urinary pellet mRNAs (11–18,21–28). These reports suggested that u-sed Pod mRNA is a potential biomarker for the monitoring of glomerular diseases ranging from mild to severe podocyte detachment. Hara et al. (29) reported two structural elements in urine sediment—podocalyxin-positive cells and podocalyxin-positive subcellular granular structures—using an anti-podocalyxin antibody. These granular structures originated from podocyte microvilli or vesicle-like structures derived from podocytes, as determined by light and electron microscopy. These granular structures in urine were derived from the apical portion of podocyte cell membranes and quantified by ELISA (u-sup PCX). They concluded that the detection of microvesicles in urine by this ELISA (u-sup PCX) could be useful for diagnosing and monitoring early-stage glomerular disease (29–32).
u-sed Pod mRNA excretion was significantly increased in patients with all types of glomerular diseases, whereas u-sup PCX protein was increased only in those with MN and LN compared with the controls. Patients with IgAN with extracapillary proliferative lesions, those with Cres GN, and those with LN class IV, in which we expected severe podocyte detachment, showed significantly increased u-sed Pod mRNA excretion. In addition, u-sed Pod mRNA excretion, but not u-sup PCX protein, was correlated significantly with the rate of crescent formation in those with Cres GN. These results suggest that u-sed Pod mRNA excretion correlates with histologic podocyte depletion and may be useful as a marker of podocyte detachment. Furthermore, u-sed Pod mRNA excretion was significantly correlated with proteinuria, even in patients with MCNS, which is not thought to cause podocyte depletion. These results suggest that massive proteinuria itself may have temporary podocyte detachment, and persistence of which may lead to podocyte loss. In contrast, u-sup PCX protein was increased in those with subepithelial dense deposition–type LN and MN compared with the controls. Previous reports have also shown that u-sup PCX protein is high in patients with MN and LN class V (36).
Hara et al. (29,36) reported that the u-sup PCX protein does not originate from cell debris of the detached podocyte, but is thought to be a vesicle structure derived from apical cell membrane shedding from microvilli of the injured podocyte. In idiopathic MN, antibodies such as anti–phospholipase A2 receptor 1 and anti–thrombospondin type-1 domain–containing 7A have been reported to be involved in the pathogenesis of the disease.. It is thought that immune complexes containing these antibodies are formed in the subepithelium and activate the complement, resulting in podocyte damage and the development of MN (37,38). We suspect that immune complexes deposited in the subepithelial region cause podocyte injury via complement activation, resulting in vesicle shedding from microvilli and high levels of u-sup PCX protein in MN and LN; however, when cell death does not occur (mild podocyte loss), u-sed Pod mRNA excretion does not increase. The detailed mechanism of podocyte damage by complement activation in subepithelial dense deposit–type GN requires further investigation in the recently developed nephropathy models involving the phospholipase A2 receptor and thrombospondin type-1 domain–containing 7A (39,40). Furthermore, patients with MCNS have massive proteinuria, as do those with MN, but u-sup PCX protein was not increased in this study. These results support the above concept and indicate that u-sed Pod mRNA excretion and u-sup PCX protein could provide different information.
A limitation of this study is that urinary podocyte marker measurements were determined on the basis of a single urine spot and a small sample size. Serial urinary podocyte markers would be more useful and reliable. In addition, many of the patients were transferred to other hospitals and were thus lost to follow-up. Few patients required RRT during the observation period, hampering analysis of their prognosis. Therefore, a long-term prognostic study is required. Despite this limitation, we found that urinary podocyte biomarkers could be useful for monitoring the activity of glomerular diseases.
In summary, we demonstrated that u-sed Pod mRNA excretion was increased in multiple glomerular diseases and that u-sup PCX protein was increased in MN and LN. Furthermore, a high level of u-sed Pod mRNA excretion was associated with severe proliferative glomerular diseases, such as IgAN with extracapillary proliferative lesions, Cres GN, and LN class IV; and a high level of u-sup PCX protein was associated with subepithelial dense deposit–type GN. These data suggest that u-sed Pod mRNA biomarkers and u-sup PCX protein have potential as diagnostic and disease activity markers in glomerular diseases.
Disclosures
S. Fujimoto reports receiving research funding from Chugai-Pharm Co. Ltd., Kissei-Pharm Co. Ltd, Kyowa-Kirin Co. Ltd., Otsuka-Pharm Co. Ltd., Sumitomo-Dainippon-Pharma Co. Ltd., and Teijin-Pharm Co. Ltd.; and serving as a scientific advisor for, or member of, Kyowa-Kirin Co. Ltd. H. Shibata reports serving on a speakers bureau for Daiichisankyo Company and receiving honoraria from Daiichisankyo Company and Mochida Pharmaceuticals. All remaining authors have nothing to disclose.
Funding
This work was supported by JSPS KAKENHI grants JP16K19198 and JP19K16983.
Acknowledgments
The authors thank Mr. Hiroyuki Kurosawa of Denka Co., Ltd., Niigata, Japan, for ELISA.
Some data from this study were presented at Kidney Week 2017, the annual meeting of the American Society of Nephrology, November 2–5, 2017, in New Orleans, Los Angeles.
We thank Textcheck (http://www.textcheck.com) for conducting English language editing.
Author Contributions
S. Fujimoto reviewed and edited the manuscript; A. Fukuda wrote the original draft and was responsible for funding acquisition and investigation; A. Fukuda and S. Fujimoto were responsible for project administration; A. Fukuda, A. Minakawa, Y. Sato, and H. Shibata were responsible for data curation and formal analysis; M. Hara and S. Fujimoto conceptualized the study and provided supervision; and all authors approved the final version of the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0004772021/-/DCSupplemental.
Relationship between urinary podocyte markers and proteinuria in patients with MCNS. Download Supplemental Figure 1, PDF file, 76 KB (75.4KB, pdf)
Clinical parameters, urinary podocyte markers, with or without relapse, and use of immunosuppressive drugs in patients with MCNS. Download Supplemental Table 1, PDF file, 76 KB (75.4KB, pdf)
References
- 1.Barratt J, Topham P: Urine proteomics: The present and future of measuring urinary protein components in disease. CMAJ 177: 361–368, 2007. 10.1503/cmaj.061590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998. 10.1046/j.1523-1755.1998.00044.x [DOI] [PubMed] [Google Scholar]
- 3.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007. 10.1038/sj.ki.5002222 [DOI] [PubMed] [Google Scholar]
- 4.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997. 10.1172/JCI119163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer TW, Bennett PH, Nelson RG: Podocyte number predicts long-term urinary albumin excretion in Pima Indians with type II diabetes and microalbuminuria. Diabetologia 42: 1341–1344, 1999. 10.1007/s001250051447 [DOI] [PubMed] [Google Scholar]
- 6.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R: Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968, 2001. 10.1046/j.1523-1755.2001.060003957.x [DOI] [PubMed] [Google Scholar]
- 7.Kriz W: Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech 57: 189–195, 2002. 10.1002/jemt.10072 [DOI] [PubMed] [Google Scholar]
- 8.White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, De Cosmo S, Viberti G: Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes 51: 3083–3089, 2002. 10.2337/diabetes.51.10.3083 [DOI] [PubMed] [Google Scholar]
- 9.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005. 10.1681/ASN.2005010055 [DOI] [PubMed] [Google Scholar]
- 10.Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I: Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol 16: 1013–1023, 2005. 10.1681/ASN.2004080720 [DOI] [PubMed] [Google Scholar]
- 11.Sato Y, Wharram BL, Lee SK, Wickman L, Goyal M, Venkatareddy M, Chang JW, Wiggins JE, Lienczewski C, Kretzler M, Wiggins RC: Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol 20: 1041–1052, 2009. 10.1681/ASN.2007121328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuda A, Wickman LT, Venkatareddy MP, Sato Y, Chowdhury MA, Wang SQ, Shedden KA, Dysko RC, Wiggins JE, Wiggins RC: Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int 81: 40–55, 2012. 10.1038/ki.2011.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, Wickman LT, Wiggins JE, Muchayi T, Fingar D, Shedden KA, Inoki K, Wiggins RC: Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol 23: 1351–1363, 2012. 10.1681/ASN.2012030271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Hodgin JB, Afshinnia F, Wang SQ, Wickman L, Chowdhury M, Nishizono R, Kikuchi M, Huang Y, Samaniego M, Wiggins RC: The two kidney to one kidney transition and transplant glomerulopathy: A podocyte perspective. J Am Soc Nephrol 26: 1450–1465, 2015. 10.1681/ASN.2014030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O’Connor C, Yang Y, Meadowbrooke C, Chowdhury M, Kikuchi M, Wiggins JE, Wiggins RC: Glomerular aging and focal global glomerulosclerosis: A podometric perspective. J Am Soc Nephrol 26: 3162–3178, 2015. 10.1681/ASN.2014080752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuda A, Minakawa A, Sato Y, Iwakiri T, Iwatsubo S, Komatsu H, Kikuchi M, Kitamura K, Wiggins RC, Fujimoto S: Urinary podocyte and TGF-β1 mRNA as markers for disease activity and progression in anti-glomerular basement membrane nephritis. Nephrol Dial Transplant 32: 1818–1830, 2017. 10.1093/ndt/gfx047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishizono R, Kikuchi M, Wang SQ, Chowdhury M, Nair V, Hartman J, Fukuda A, Wickman L, Hodgin JB, Bitzer M, Naik A, Wiggins J, Kretzler M, Wiggins RC: FSGS as an adaptive response to growth-induced podocyte stress. J Am Soc Nephrol 28: 2931–2945, 2017. 10.1681/ASN.2017020174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minakawa A, Fukuda A, Sato Y, Kikuchi M, Kitamura K, Wiggins RC, Fujimoto S: Podocyte hypertrophic stress and detachment precedes hyperglycemia or albuminuria in a rat model of obesity and type2 diabetes-associated nephropathy. Sci Rep 9: 18485, 2019. 10.1038/s41598-019-54692-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szeto CC, Lai KB, Chow KM, Szeto CY, Yip TW, Woo KS, Li PK, Lai FM: Messenger RNA expression of glomerular podocyte markers in the urinary sediment of acquired proteinuric diseases. Clin Chim Acta 361: 182–190, 2005. 10.1016/j.cccn.2005.05.016 [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Lai FM, Lai KB, Chow KM, Li KT, Szeto CC: Messenger RNA expression of podocyte-associated molecules in the urinary sediment of patients with diabetic nephropathy. Nephron Clin Pract 106: c169–c179, 2007. 10.1159/000104428 [DOI] [PubMed] [Google Scholar]
- 21.Fukuda A, Wickman LT, Venkatareddy MP, Wang SQ, Chowdhury MA, Wiggins JE, Shedden KA, Wiggins RC: Urine podocin:nephrin mRNA ratio (PNR) as a podocyte stress biomarker. Nephrol Dial Transplant 27: 4079–4087, 2012. 10.1093/ndt/gfs313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickman L, Afshinnia F, Wang SQ, Yang Y, Wang F, Chowdhury M, Graham D, Hawkins J, Nishizono R, Tanzer M, Wiggins J, Escobar GA, Rovin B, Song P, Gipson D, Kershaw D, Wiggins RC: Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases. J Am Soc Nephrol 24: 2081–2095, 2013. 10.1681/ASN.2013020173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuda A, Sato Y, Iwakiri T, Komatsu H, Kikuchi M, Kitamura K, Wiggins RC, Fujimoto S: Urine podocyte mRNAs mark disease activity in IgA nephropathy. Nephrol Dial Transplant 30: 1140–1150, 2015. 10.1093/ndt/gfv104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding F, Wickman L, Wang SQ, Zhang Y, Wang F, Afshinnia F, Hodgin J, Ding J, Wiggins RC: Accelerated podocyte detachment and progressive podocyte loss from glomeruli with age in Alport syndrome. Kidney Int 92: 1515–1525, 2017. 10.1016/j.kint.2017.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naik AS, Afshinnia F, Aqeel J, Cibrik DM, Samaniego M, Wickman L, Wang SQ, Chowdhury M, Wiggins RC: Accelerated podocyte detachment early after kidney transplantation is related to long-term allograft loss of function. Nephrol Dial Transplant 34: 1232–1239, 2019. 10.1093/ndt/gfy350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naik AS, Le D, Aqeel J, Wang SQ, Chowdhury M, Walters LM, Cibrik DM, Samaniego M, Wiggins RC: Podocyte stress and detachment measured in urine are related to mean arterial pressure in healthy humans. Kidney Int 98: 699–707, 2020. 10.1016/j.kint.2020.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minakawa A, Fukuda A, Kikuchi M, Sato Y, Sato Y, Kitamura K, Fujimoto S: Urinary podocyte mRNA is a potent biomarker of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Clin Exp Nephrol 24: 242–252, 2020. 10.1007/s10157-019-01823-5 [DOI] [PubMed] [Google Scholar]
- 28.Fukuda A, Minakawa A, Kikuchi M, Sato Y, Nagatomo M, Nakamura S, Mizoguchi T, Fukunaga N, Shibata H, Naik AS, Wiggins RC, Fujimoto S: Urinary podocyte mRNAs precede microalbuminuria as a progression risk marker in human type 2 diabetic nephropathy. Sci Rep 10: 18209, 2020. 10.1038/s41598-020-75320-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara M, Yanagihara T, Kihara I, Higashi K, Fujimoto K, Kajita T: Apical cell membranes are shed into urine from injured podocytes: A novel phenomenon of podocyte injury. J Am Soc Nephrol 16: 408–416, 2005. 10.1681/ASN.2004070564 [DOI] [PubMed] [Google Scholar]
- 30.Hara M, Yanagihara T, Kihara I: Cumulative excretion of urinary podocytes reflects disease progression in IgA nephropathy and Schönlein-Henoch purpura nephritis. Clin J Am Soc Nephrol 2: 231–238, 2007. 10.2215/CJN.01470506 [DOI] [PubMed] [Google Scholar]
- 31.Hara M, Yamagata K, Tomino Y, Saito A, Hirayama Y, Ogasawara S, Kurosawa H, Sekine S, Yan K: Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: Establishment of a highly sensitive ELISA to detect urinary podocalyxin. Diabetologia 55: 2913–2919, 2012. 10.1007/s00125-012-2661-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hara M, Oohara K, Dai DF, Liapis H: Mitotic catastrophe causes podocyte loss in the urine of human diabetes. Am J Pathol 189: 248–257, 2019. 10.1016/j.ajpath.2018.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009. 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 34.Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, Liu ZH, Roberts IS, Yuzawa Y, Zhang H, Feehally J; IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology SocietyConference Participants : Oxford Classification of IgA nephropathy 2016: An update from the IgA Nephropathy Classification Working Group. Kidney Int 91: 1014–1021, 2017. 10.1016/j.kint.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 35.Ehrenreich T, Churg J: Pathology of membranous nephropathy. In: The pathology Annual No. 3, edited by Sommers SC, New York, Appleton-Century-Crofts, 1968, pp 145–186. [Google Scholar]
- 36.Hara M, Yanagihara T, Hirayama Y, Ogasawara S, Kurosawa H, Sekine S, Kihara I: Podocyte membrane vesicles in urine originate from tip vesiculation of podocyte microvilli. Hum Pathol 41: 1265–1275, 2010. 10.1016/j.humpath.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 37.Ponticelli C, Glassock RJ: Glomerular diseases: Membranous nephropathy--A modern view. Clin J Am Soc Nephrol 9: 609–616, 2014. 10.2215/CJN.04160413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imaizumi T, Nakatochi M, Akiyama S, Yamaguchi M, Kurosawa H, Hirayama Y, Katsuno T, Tsuboi N, Hara M, Maruyama S: Urinary podocalyxin as a biomarker to diagnose membranous nephropathy. PLoS One 11: e0163507, 2016. 10.1371/journal.pone.0163507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomas NM, Meyer-Schwesinger C, von Spiegel H, Kotb AM, Zahner G, Hoxha E, Helmchen U, Endlich N, Koch-Nolte F, Stahl RAK: A heterologous model of thrombospondin type 1 domain-containing 7A-associated membranous nephropathy. J Am Soc Nephrol 28: 3262–3277, 2017. 10.1681/ASN.2017010030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer-Schwesinger C, Tomas NM, Dehde S, Seifert L, Hermans-Borgmeyer I, Wiech T, Koch-Nolte F, Huber TB, Zahner G: A novel mouse model of phospholipase A2 receptor 1-associated membranous nephropathy mimics podocyte injury in patients. Kidney Int 97: 913–919, 2020. 10.1016/j.kint.2019.10.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relationship between urinary podocyte markers and proteinuria in patients with MCNS. Download Supplemental Figure 1, PDF file, 76 KB (75.4KB, pdf)
Clinical parameters, urinary podocyte markers, with or without relapse, and use of immunosuppressive drugs in patients with MCNS. Download Supplemental Table 1, PDF file, 76 KB (75.4KB, pdf)