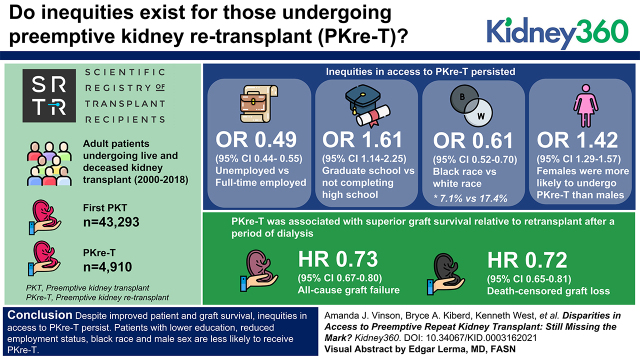
Key Points
Preemptive kidney transplant (PKT) is associated with improved survival versus transplant after a period of dialysis.
There are inequities in access to repeat PKT.
Those with lower education or socioeconomic status, those of Black or Hispanic race, and men are less likely to receive repeat PKT.
Keywords: transplantation, deceased donor, disparity, kidney, living donors, predictors, preemptive, repeat transplant, transplantation
Visual Abstract
Abstract
Background
The need for repeat transplant due to failing kidney allografts is increasing over time. The benefit of preemptive kidney retransplant (PKre-T) is controversial. Marginalized populations are less likely to undergo their first transplant preemptively; however, whether inequities exist for those undergoing PKre-T is unknown.
Methods
We performed a cohort study of adult patients undergoing live and deceased kidney transplant in the United States from 2000 to 2018 identified using the Scientific Registry of Transplant Recipients, and we identified patients with first preemptive kidney transplant (PKT) and PKre-T. In the primary analysis, a multivariable logistic regression was used to identify independent predictors of PKre-T. In secondary analyses, multivariable Cox models were used to determine the association of PKre-T with death-censored and all-cause graft loss.
Results
In total, 4910 (15.5%) patients underwent PKre-T, and 43,293 (19.1%) underwent first PKT. Inequities in access to PKre-T persisted (OR, 0.49; 95% CI, 0.44 to 0.55 for unemployed versus full time; OR, 1.61; 95% CI, 1.14 to 2.25 for graduate school versus not completing high school; OR, 0.61; 95% CI, 0.52 to 0.70 for Black versus White race); 7.1% of all transplanted Black patients received PKre-T versus 17.4% of White patients. Women were more likely to undergo PKre-T than men (OR, 1.42; 95% CI, 1.29 to 1.57). PKre-T was associated with superior graft survival relative to retransplant after a period of dialysis (HR, 0.73; 95% CI, 0.67 to 0.80 for all-cause graft failure; HR, 0.72; 95% CI, 0.65 to 0.81 for death-censored graft loss).
Conclusions
Despite improved patient and graft survival, inequities in access to PKre-T persist. Patients with lower education, patients with reduced employment status, patients of Black race, and men are less likely to receive PKre-T.
Introduction
Preemptive kidney transplant (PKT) has been shown to result in superior patient and graft survival, less rejection, better quality of life, and lower societal costs compared with undergoing transplant after dialysis initiation (1–3). As such, a focus on PKT as a first-line strategy for management of end-stage kidney disease (ESKD) is promoted by most renal consensus guidelines, including those of Kidney Disease Improving Global Outcomes (4), the Canadian Society of Transplantation (5), and the British Transplant Society (BTS) (6).
In spite of significant improvements in graft survival, increasing proportions of patients are undergoing second or third kidney transplants, with previous graft failure now the fourth leading indication for transplant (7). Access to second kidney transplant is more restricted than first kidney transplant due in part to increased recipient sensitization to HLA proteins and higher comorbidity burden in candidates for retransplantation versus those who are transplant naïve. Interestingly, whether preemptive kidney retransplant (PKre-T) is associated with improved patient and/or graft outcomes is controversial (8,9). Nonetheless, most studies have shown that PKre-T is also associated with improved outcomes compared with transplantation after a return to dialysis (8,10). Thus, despite the mixed signals, the BTS guidelines for management of a failing kidney transplant recommend PKre-T for patients with a first failed graft that lasted longer than 1 year (11).
Consistently, marginalized populations, including those with lower education, with lower socioeconomic status, and of non-White race, have been disadvantaged in terms of referral for transplant and if referred, less frequently undergo PKT (1). This has been proposed to reflect, in part, inequities in access to specialized CKD care (1). It has been shown that early access to specialized CKD care and patient education is associated with a significantly higher rate of first PKT (12–15), with patients three times as likely to be preemptively transplanted if kidney transplant is discussed >12 months prior to dialysis start (15).
Many kidney transplant recipients are followed in specialized clinics by health care providers with expertise in CKD and transplant care, and all repeat transplant recipients will by definition have had prior exposure to kidney transplant. Therefore, it is reasonable to surmise that these patients may receive superior CKD care and more prompt referral for repeat transplantation compared with the general incident ESKD population (16). Thus, access to PKre-T may be more equitable than has been shown for first PKT.
Therefore, in this study, we examine if racial and socioeconomic disparities in access to PKre-T persist and how these compare with the disparities noted for access to first PKT in patients with incident ESKD.
Materials and Methods
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the United States submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of OPTN and SRTR contractors.
The study population included patients receiving a first or repeat (at least one prior failed transplant) solitary kidney transplant in the United States between January 1, 2000 and December 31, 2017. We excluded patients younger than 18 years of age at the time of either transplant and those receiving multiple organs.
Outcome
The outcome of interest was PKre-T, defined as repeat transplantation (including second and third transplants) without a period of maintenance dialysis after the preceding kidney transplant had failed. As a comparator, we examined predictors of first PKT.
Covariates
Recipient factors were collected from SRTR, including age, race, sex, body mass index, education achieved, employment status, ESKD cause, medical comorbidities (coronary artery disease [defined as angina or documented coronary disease], hypertension, peripheral vascular disease, or diabetes), donor type (live versus deceased), panel reactive antibody (PRA) status, and ABO blood group. Dialysis vintage (time from initiation of maintenance dialysis to transplant; vintage =0 if PKT) was determined, and patients were identified as undergoing either PKT or non-PKT (a first transplant after a period of dialysis) or either PKre-T or non–PKre-T (a repeat transplant after a period of dialysis following the preceding graft's failure).
Analyses
Descriptive statistics were used to report baseline characteristics stratified by first or repeat PKT.
Primary Analysis
The adjusted odds ratio (OR) for PKre-T (versus receiving a repeat transplant nonpreemptively [non–PKre-T]) was determined using multivariable logistic regression examining the covariates listed above. For a comparison, the adjusted odds of first PKT were also examined in those without a prior transplant, relative to non-PKT.
Sensitivity Analyses
Using multivariable logistic regression in those undergoing repeat transplant, we determined the adjusted odds of (1) prior PKT and (2) first graft survival time with PKre-T.
We also determined if the type of health care provider follow-up (transplant center physician, nontransplant center specialty physician, primary care physician, or other) at the time of first graft failure was associated with the proportion of patients receiving a subsequent PKre-T, with significant differences explored using chi-squared tests.
Given the possibility that those patients with and without a live kidney donor may represent systemically different populations, we repeated our primary analysis separately in those undergoing live and deceased donor repeat transplants.
In patients undergoing PKT and PKre-T, we determined the eGFR using the Modification of Diet in Renal Disease (MDRD) equation (adjusting for sex and race) on the basis of the most recent recipient creatinine prior to transplant. MDRD has been previously validated and is accurate in kidney transplant recipients (17). Significant differences were determined using the Wilcoxon rank sum test (for race; eGFR between (1) Black and White race, (2) other and White race, and (3) Hispanic and White race were compared separately).
Secondary Analyses
In a secondary analysis, we examined the hazard ratio (HR) for death-censored graft failure and all-cause graft failure associated with PKre-T (versus non–PKre-T), adjusting for the above listed covariates and HLA mismatch. For comparison, we repeated the above analysis in those undergoing first kidney transplant. Data for all-cause graft failure were displayed graphically using Kaplan–Meier survival curves for first and repeat PKTs versus non-PKT and non–PKre-T, respectively. Proportionality of hazards was confirmed visually, and significant differences were determined using the log-rank test.
The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. All statistical analyses were performed using Stata version 14.2 (Stata Corp., College Station, TX). Institutional ethics approval to conduct this study was provided by the Nova Scotia Health Authority Research Ethics Board.
Results
Over the study period, a total of 31,610 patients underwent repeat kidney transplant (at least one prior failed graft), of whom 4910 (15.5%) were transplanted preemptively (PKre-T) without a period of maintenance dialysis. This is compared with 226,693 patients who underwent first kidney transplant over the same period, 43,293 (19.1%) of whom were transplanted preemptively (PKT). Baseline characteristics for those undergoing first and repeat PKTs are shown in Table 1; 61.1% and 53.1% of first and repeat PKTs, respectively, were from live kidney donors.
Table 1.
Baseline characteristics for first and repeat preemptive kidney transplant recipients
| Patient Characteristics | First Transplant | Repeat Transplant | ||
|---|---|---|---|---|
| Preemptive, N=43,293 (19.1%) | All, N=226,693 | Preemptive, N=4910 (15.5%) | All, N=31,610 | |
| Employment | ||||
| Full time | 14,728 (30.8) | 47,900 | 1464 (22.2) | 6596 |
| Part time/full time | 3753 (28.7) | 13,066 | 376 (17.7) | 2215 |
| Part time | 1935 (18.5) | 10,484 | 242 (13.5) | 1798 |
| Unemployed | 11,848 (10.5) | 112,736 | 1700 (9.9) | 17,148 |
| Retired | 4866 (16.9) | 28,741 | 288 (17.7) | 1820 |
| Education | ||||
| None/grade school | 863 (6.5) | 13,227 | 80 (8.1) | 983 |
| High school | 11,856 (13.6) | 87,290 | 1261 (11.0) | 11,432 |
| College/technical school | 8966 (17.8) | 50,408 | 1022 (13.3) | 7662 |
| Bachelor degree | 8529 (24.4) | 34,919 | 963 (18.0) | 5337 |
| Graduate school | 4789 (31.3) | 15,292 | 443 (22.8) | 1941 |
| Men | 22,637 (16.3) | 138,628 | 2299 (12.5) | 18,448 |
| Women | 16,663 (18.9) | 88,065 | 2054 (15.6) | 13,162 |
| Race | ||||
| White | 33,036 (21.8) | 118,241 | 3399 (17.4) | 19,536 |
| Black | 4341 (7.4) | 58,631 | 486 (7.1) | 6821 |
| Hispanic | 3163 (9.5) | 33,405 | 298 (8.2) | 3654 |
| Other | 1921 (11.7) | 16,413 | 170 (10.6) | 1599 |
| Comorbidities | ||||
| Coronary artery disease | 1925 (11.6) | 16,630 | 182 (10.5) | 1741 |
| Hypertension | 27,781 (15.9) | 174,415 | 3,060 (13.2) | 23,145 |
| Peripheral vascular disease | 1318 (11.2) | 11,822 | 169 (12.8) | 1321 |
| Diabetes | 9348 (12.6) | 74,155 | 995 (15.6) | 6374 |
| Body mass index, kg/m2 | ||||
| <18.5 | 909 (21.3) | 4271 | 174 (16.5) | 1052 |
| 18.5 to <25 | 11,603 (21.9) | 53,017 | 1801 (16.6) | 10,855 |
| 25 to <30 | 13,007 (22.1) | 58,826 | 1228 (16.0) | 7823 |
| 30 to <35 | 8053 (20.5) | 39,300 | 629 (16.4) | 3846 |
| ≥35 | 3370 (16.7) | 20,182 | 238 (13.3) | 1783 |
| PRA | ||||
| <20 | 27,788 (17.3) | 160,597 | 2521 (20.9) | 12,078 |
| 20 to <80 | 2783 (15.9) | 17,499 | 761 (9.9) | 7707 |
| ≥80 | 685 (12.8) | 5356 | 283 (5.4) | 5265 |
| Deceased donor | 12,853 (9.0) | 142,266 | 1747 (8.1) | 21,598 |
| Live donor | 26,447 (31.3) | 84,427 | 2606 (26.0) | 10,012 |
| Age, yr | ||||
| <40 | 7833 (16.4) | 47,637 | 1384 (12.5) | 11,088 |
| 40 to <60 | 19,385 (17.7) | 109,642 | 2339 (14.5) | 16,149 |
| ≥60 | 12,082 (17.4) | 69,414 | 630 (14.4) | 4373 |
| ABO | ||||
| O | 16,073 (15.8) | 101,652 | 1743 (12.3) | 14,149 |
| A | 16,427 (19.5) | 84,216 | 1892 (15.2) | 12,486 |
| B | 4718 (15.6) | 30,253 | 456 (13.1) | 3475 |
| AB | 2082 (19.7) | 10,572 | 262 (17.5) | 1500 |
Missing: employment first KTR (n=13,766), second KTR (n=2123); highest education first KTR (n=25,557), second KTR (n=4255); race first KTR (n=3), recipient CAD first KTR (n=42,163), second KTR (n=5990); recipient hypertension first KTR (n=27,851), second KTR (n=4388); recipient PVD first KTR (n=8478), second KTR (n=1372); recipient diabetes first KTR (n=2129), second KTR (n=606); body mass index first KTR (n=2354), second KTR (n=283); PRA first KTR (n=43,241), second KTR (n=6560). PRA, panel reactive antibody. KTR, kidney transplant recipient; CAD, coronary artery disease; PVD, peripheral vascular disease.
Primary Analysis
Education and Income Status
Patient characteristics associated with receiving a PKre-T (and PKT) are shown in Table 2. For PKre-T, employment status was significantly associated with receipt of a PKre-T (OR, 0.49; 95% confidence interval [95% CI], 0.44 to 0.55 for unemployed versus full time). Likewise, a higher formal education was associated with greater odds of undergoing PKre-T (OR, 1.60; 95% CI, 1.14 to 2.25 for graduate school versus those not completing high school). The same trend existed for first PKT (OR, 0.37; 95% CI, 0.36 to 0.39 for unemployed and OR, 2.65; 95% CI, 2.38 to 2.95 for graduate school).
Table 2.
Adjusted odds ratio of receiving first and repeat preemptive kidney transplants
| Patient Characteristics | Preemptive First Transplant, N=124,238 | Preemptive Second Transplant, N=16,186 | ||
|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | P Value | Odds Ratio (95% Confidence Interval) | P Value | |
| Employment | ||||
| Full time | Reference | — | Reference | — |
| Part time/full time | 0.82 (0.74 to 0.91) | <0.001 | 1.03 (0.78 to 1.38) | 0.82 |
| Part time | 0.55 (0.51 to 0.58) | <0.001 | 0.64 (0.53 to 0.77) | <0.001 |
| Unemployed | 0.37 (0.36 to 0.39) | <0.001 | 0.49 (0.44 to 0.55) | <0.001 |
| Retired | 0.53 (0.50 to 0.56) | <0.001 | 0.74 (0.60 to 0.92) | 0.006 |
| Education | ||||
| None/grade school | Reference | — | Reference | — |
| High school | 1.49 (1.35 to 1.64) | <0.001 | 1.01 (0.74 to 1.38) | 0.93 |
| College/technical school | 1.73 (1.56 to 1.91) | <0.001 | 1.06 (0.77 to 1.46) | 0.71 |
| Bachelor degree | 2.08 (1.87 to 2.30) | <0.001 | 1.33 (0.97 to 1.83) | 0.08 |
| Graduate school | 2.65 (2.38 to 2.95) | <0.001 | 1.60 (1.14 to 2.25) | 0.007 |
| Women | 1.45 (1.40 to 1.50) | <0.001 | 1.42 (1.29 to 1.57) | <0.001 |
| Race | ||||
| White | Reference | — | Reference | — |
| Black | 0.37 (0.36 to 0.39) | <0.001 | 0.61 (0.52 to 0.70) | <0.001 |
| Hispanic | 0.50 (0.47 to 0.52) | <0.001 | 0.63 (0.52 to 0.75) | <0.001 |
| Other | 0.48 (0.44 to 0.51) | <0.001 | 0.74 (0.58 to 0.95) | 0.02 |
| Comorbidities | ||||
| Coronary artery disease | 0.69 (0.64 to 0.73) | <0.001 | 0.60 (0.49 to 0.74) | <0.001 |
| Hypertension | 0.89 (0.86 to 0.94) | <0.001 | 1.14 (0.99 to 1.31) | 0.07 |
| Peripheral vascular disease | 0.78 (0.71 to 0.84) | <0.001 | 1.11 (0.88 to 1.40) | 0.38 |
| Diabetes | 0.76 (0.73 to 0.79) | <0.001 | 1.17 (1.04 to 1.32) | 0.008 |
| Body mass index, kg/m2 | ||||
| <18.5 | Reference | — | Reference | — |
| 18.5 to <25 | 1.16 (1.04 to 1.29) | 0.009 | 1.25 (0.97 to 1.60) | 0.08 |
| 25 to <30 | 1.24 (1.12 to 1.39) | <0.001 | 1.25 (0.97 to 1.61) | 0.09 |
| 30 to <35 | 1.21 (1.08 to 1.35) | 0.001 | 1.45 (1.11 to 1.90) | 0.006 |
| ≥35 | 1.03 (0.91 to 1.16) | 0.63 | 1.11 (0.81 to 1.52) | 0.51 |
| PRA | ||||
| <20 | Reference | — | Reference | — |
| 20–80 | 1.04 (0.98 to 1.10) | 0.21 | 0.48 (0.43 to 0.54) | <0.001 |
| ≥80 | 0.98 (0.88 to 1.10) | 0.75 | 0.29 (0.25 to 0.34) | <0.001 |
| Live donor | 3.83 (3.70 to 3.96) | <0.001 | 2.65 (2.40 to 2.93) | <0.001 |
| Age, yr | ||||
| <40 | Reference | — | Reference | — |
| 40–60 | 1.33 (1.28 to 1.39) | <0.001 | 1.25 (1.12 to 1.39) | <0.001 |
| ≥60 | 1.66 (1.57 to 1.76) | <0.001 | 1.41 (1.18 to 1.68) | <0.001 |
| ABO | ||||
| O | Reference | — | Reference | — |
| A | 1.19 (1.15 to 1.23) | <0.001 | 1.18 (1.06 to 1.30) | 0.002 |
| B | 1.06 (1.00 to 1.11) | 0.04 | 1.05 (0.89 to 1.24) | 0.58 |
| AB | 1.53 (1.41 to 1.65) | <0.001 | 1.68 (1.36 to 2.07) | <0.001 |
—, no data; PRA, panel reactive antibody.
Race and Ethnicity
Inequities in access to PKre-T were observed for different races. Specifically, Black patients (OR, 0.61; 95% CI, 0.52 to 0.70) and Hispanic patients (OR, 0.63; 95% CI, 0.52 to 0.75) had lower likelihoods of PKre-T compared with White patients. Of those who underwent first and repeat transplants, only 7.4% and 7.1% of Black patients, respectively, and 9.5% and 8.2% of Hispanic patients, respectively, received their transplants preemptively compared with 27.9% and 17.4% of White patients, respectively (Supplemental Figure 1).
Other Predictive Factors
Women were associated with higher odds of PKre-T (and PKT), as was having a live donor. Those with comorbidities were less likely to receive a first PKT, but only coronary artery disease was associated with a reduced odds of PKre-T. A high PRA was associated with a lower likelihood of PKre-T (OR, 0.29; 95% CI, 0.25 to 0.34 for PRA >80% versus <20%), but there was no association between PRA and first PKT.
Sensitivity Analysis
Prior Transplant Management
A prior PKT was associated with a higher odds of subsequent PKre-T in adjusted analyses (OR, 2.01; 95% CI, 1.73 to 2.35). Overall, 33.1% of patients with a prior PKT were retransplanted preemptively compared with 14.8% of patients without a prior PKT (P<0.001). Additionally, longer survival time for the preceding kidney graft was also significantly associated with a higher likelihood of PKre-T (OR, 1.04; 95% CI, 1.03 to 1.05 for each year of graft survival). In those with data, there was a significantly higher incidence of PKre-T in those followed at a transplant center when their earlier graft failed (11.5%) compared with those followed by nontransplant center specialty physicians (7.6%) or primary care physicians (5.9%; P<0.001) (Supplemental Table 1).
Donor Type (Live versus Deceased)
In both live and deceased donor transplants, men, unemployment, and Black and Hispanic race were associated with lower odds of PKre-T (and PKT) compared with transplant after a period of maintenance dialysis (Table 3).
Table 3.
Adjusted odds ratio of receiving repeat preemptive kidney transplant separately among those undergoing live and deceased donor transplant
| Patient Characteristics | Live Donor Preemptive Second Transplant, N=5436 | Deceased Donor Preemptive Second Transplant, N=10,750 | ||
|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | P Value | Odds Ratio (95% Confidence Interval) | P Value | |
| Employment | ||||
| Full time | Reference | — | Reference | — |
| Part time/full time | 1.06 (0.72 to 1.56) | 0.78 | 1.00 (0.64 to 1.55) | >0.99 |
| Part time | 0.61 (0.48 to 0.78) | <0.001 | 0.70 (0.52 to 0.94) | 0.02 |
| Unemployed | 0.44 (0.38 to 0.51) | <0.001 | 0.57 (0.48 to 0.68) | <0.001 |
| Retired | 0.62 (0.45 to 0.86) | 0.005 | 0.87 (0.66 to 1.15) | 0.33 |
| Education | ||||
| None/grade school | Reference | — | Reference | — |
| High school | 1.19 (0.71 to 1.99) | 0.51 | 0.93 (0.63 to 1.37) | 0.71 |
| College/technical school | 1.20 (0.72 to 2.02) | 0.49 | 1.04 (0.69 to 1.55) | 0.87 |
| Bachelor degree | 1.66 (0.98 to 2.79) | 0.06 | 1.12 (0.74 to 1.68) | 0.60 |
| Graduate school | 1.75 (1.02 to 3.01) | 0.04 | 1.66 (1.06 to 2.60) | 0.03 |
| Women | 1.40 (1.22 to 1.60) | <0.001 | 1.46 (1.26 to 1.68) | <0.001 |
| Race | ||||
| White | Reference | — | Reference | — |
| Black | 0.56 (0.44 to 0.72) | <0.001 | 0.63 (0.52 to 0.75) | <0.001 |
| Hispanic | 0.71 (0.56 to 0.90) | 0.004 | 0.53 (0.39 to 0.70) | <0.001 |
| Other | 0.60 (0.40 to 0.87) | 0.007 | 0.85 (0.63 to 1.16) | 0.31 |
| Comorbidities | ||||
| Coronary artery disease | 0.67 (0.51 to 0.89) | 0.005 | 0.55 (0.40 to 0.75) | <0.001 |
| Hypertension | 1.00 (0.83 to 1.20) | 0.99 | 1.36 (1.09 to 1.70) | 0.007 |
| Peripheral vascular disease | 1.06 (0.76 to 1.49) | 0.73 | 1.14 (0.83 to 1.57) | 0.41 |
| Diabetes | 1.05 (0.90 to 1.24) | 0.53 | 1.31 (1.10 to 1.55) | 0.002 |
| Body mass index, kg/m2 | ||||
| <18.5 | Reference | — | Reference | — |
| 18.5 to <25 | 1.12 (0.83 to 1.51) | 0.47 | 1.50 (0.95 to 2.37) | 0.08 |
| 25 to <30 | 1.10 (0.80 to 1.51) | 0.55 | 1.54 (0.97 to 2.44) | 0.07 |
| 30 to <35 | 1.25 (0.87 to 1.76) | 0.20 | 1.82 (1.13 to 2.92) | 0.01 |
| ≥35 | 1.15 (0.77 to 1.72) | 0.49 | 1.17 (0.67 to 1.99) | 0.56 |
| PRA | ||||
| <20 | Reference | — | Reference | — |
| 20–80 | 0.57 (0.49 to 0.66) | <0.001 | 0.40 (0.33 to 0.47) | <0.001 |
| >80 | 0.25 (0.19 to 0.34) | <0.001 | 0.31 (0.25 to 0.38) | <0.001 |
| Age, yr | ||||
| <40 | Reference | — | Reference | — |
| 40–60 | 1.13 (0.98 to 1.31) | 0.08 | 1.45 (1.22 to 1.73) | <0.001 |
| >60 | 1.12 (0.85 to 1.46) | 0.43 | 1.71 (1.33 to 2.20) | <0.001 |
| ABO | ||||
| O | Reference | — | Reference | — |
| A | 1.10 (0.96 to 1.27) | 0.18 | 1.27 (1.09 to 1.49) | 0.003 |
| B | 0.92 (0.74 to 1.16) | 0.49 | 1.24 (0.97 to 1.58) | 0.09 |
| AB | 1.49 (1.09 to 2.04) | 0.01 | 1.89 (1.42 to 2.51) | <0.001 |
—, no data; PRA, panel reactive antibody.
Age and Sex at Transplant
The median age of all patients undergoing repeat transplant was younger than for first transplant, irrespective of whether or not the transplant was preemptive (Supplemental Table 2).
Fewer women than men underwent first and repeat transplants; however, women had relatively higher rates of PKre-T and PKT versus men (Supplemental Figure 1).
GFR at Transplant
The median eGFR at the time of transplant was significantly higher at the time of repeat versus first PKT (15.1 versus 13.9 ml/min per 1.73 m2; P<0.001) (Supplemental Table 3). Women underwent first and repeat PKTs at significantly lower eGFR than did men, and White patients had a significantly lower eGFR than Black and Hispanic patients at the time of first PKT (Supplemental Table 3).
Secondary Analyses: Outcomes with PKre-T and PKT
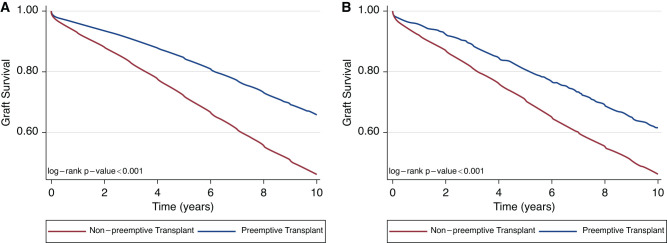
PKre-T remained associated with an adjusted graft survival benefit relative to undergoing non–PKre-T (HR, 0.73; 95% CI, 0.67 to 0.80 for all-cause graft failure; HR, 0.72; 95% CI, 0.65 to 0.81 for death-censored graft loss) (Supplemental Table 4). Likewise, first PKT was associated with better all-cause graft survival (HR, 0.66; 95% CI, 0.64 to 0.68) and death-censored graft failure (HR, 0.67; 95% CI, 0.64 to 0.70). Kaplan–Meier survival curves for all-cause graft failure are demonstrated in Figure 1A (PKre-T versus non–PKre-T) and Figure 1B (PKT versus non PKT).
Figure 1.
All-cause graft loss for repeat and first preemptive kidney transplant versus nonpreemptive repeat and first kidney transplant, respectively. (A) PKre-T versus non–PKre-T; (B) PKT versus non PKT. PKre-T, preemptive kidney retransplant; PKT, preemptive kidney transplant.
Discussion
In this study, we show that disparities in access to PKre-T persist. Despite the established benefits with first PKT, significant disparities in access to transplant exist for first-time transplant recipients, with marginalized populations having significantly lower transplant referral rates and once referred, lower PKT rates (1,18). Historically, Black patients have been shown to be waitlisted for transplant prior to dialysis start nearly 50% less frequently than White patients (15). This finding persists even after adjustments for age, sex, education, and socioeconomic status. Although the reason for these discrepancies in access is likely multifold, at least some of the disparity in PKT is felt to relate to differences in access to pretransplant CKD care.
Therefore, it is perhaps surprising that a significant disparity in access to PKre-T persists in those undergoing repeat transplant. Although race-based inequities in access to post-transplant care may endure, they would be expected to be somewhat minimized in that most patients with a kidney transplant will have at least some knowledge of the process and exposure to a specialist in the field. It is, therefore, discouraging that despite patient and health care provider knowledge about kidney transplant as an option for ESKD management, patients are still not undergoing PKre-T in an equitable manner. Inequities in access to PKre-T are pervasive, with those with lower education and lower employment status, men, those of non-White race, and younger individuals continuing to have lower rates of repeat PKT. Of those patients who eventually underwent repeat transplant, Black patients were 39% less likely and Hispanic patients were 37% less likely to be transplanted preemptively than White patients. Considering there is already a race-based disparity in who ultimately receives a transplant (19,20), this will be a clear underestimate of PKre-T among all those with a first failed graft. This effect does not appear to be related to a systemic difference in access to live donors. Importantly, Black recipients are less likely to undergo living donor transplant than their White counterparts, a disparity that has been increasing with time (21). Despite this, even when restricting the analysis to Black patients who eventually underwent live donor transplant, they were still significantly less likely than White patients to receive a PKre-T.
The association of women with a higher odds of both first and repeat PKTs is hard to reconcile. Although controversial, women have previously been associated with a higher likelihood of first adult PKT but always without explanation (1,22,23). Conversely, boys are more likely than girls to undergo PKT in pediatric recipients (24). It is known that compared with men, women with ESKD are less likely to be referred for kidney transplant, have less activation on the waiting list once referred, and are less likely to undergo transplant once activated (25–27). Women also experience higher presensitization compared with men on account of pregnancy, and it has been postulated that women may be perceived by care providers to be less eligible for transplant than men, even with similar or reduced comorbidity burden (28). Potential explanations for more first and repeat PKTs in women than men may include a faster rate of progression to ESKD in men (29) that precludes appropriate preparation for PKT. However, we found that men undergo PKT at a significantly higher eGFR than women do.
Alternatively, the higher preemptive rates in women may reflect reduced access or disinclination to dialysis (which has been shown in women patients with native kidney disease) (30) and thereby, less competing risk for PKT. This would be supported by the significantly lower eGFR in women than men at the time of both first and repeat PKT. Alternatively, it may reflect bias in that only the most robust women with high health literacy and self-advocation are considered eligible and thus, referred for repeat transplant and PKre-T. Regardless of the cause, given the sex-discrepant inequitable access to kidney transplantation in women compared with men, the better PKT rates may not be a true advantage but instead, may reflect unrecognized disadvantage elsewhere.
A recent paper by Schold et al. (31) was the first to examine disparities in the fate of patients after a primary failed transplant, similarly demonstrating that patients of Black and Hispanic race, men, those of older age, obese patients, and publicly insured patients were less likely to be preemptively activated on the transplant waiting list or retransplanted. Our studies differ, however, in that we examined independent predictors or PKre-T, whereas their earlier analysis was instead driven by predictors of preemptive waitlisting (with only 3% of their study population undergoing PKre-T). Although disparate barriers to preemptive waitlisting will inevitably reduce access to PKre-T, potential for additional barriers to PKre-T among those who are eligible and ultimately, undergo repeat transplant is a separate question that has not been previously explored.
Furthermore, we demonstrated that having access to a living donor did not influence the inequities in those who underwent PKre-T, which is an important and novel finding.
PKT has been shown to result in superior outcomes compared with being transplanted after a period of maintenance dialysis (1). However, the benefit of PKre-T is controversial, with some studies demonstrating increased graft failure compared with repeat transplant after return to dialysis (9). In this study, we show a benefit in both all-cause graft loss and death-censored graft failure with PKre-T relative to undergoing repeat transplant after a period of maintenance dialysis.
A potential limitation of this study is that our population was restricted to only those who eventually underwent kidney transplant. It is known that there is inequity in access to referral for kidney transplant, with marginalized populations referred less frequently (19,20,31). Therefore, exploring PKT in a cohort that included only those who ultimately were transplanted does not account for the inequity in those marginalized patients who were never referred for transplant, which would only accentuate the disparity in access to PKT and PKre-T. Additionally, there is noted increased morbidity and mortality with return to dialysis after transplant failure compared with starting dialysis when transplant naïve (16). Thus, a period of return to dialysis after a failed transplant may render some patients transplant ineligible who may otherwise have qualified for a second PKT. The proportion of patients who died or were withdrawn from the waiting list after a first failed graft was not explored, but again, it would likely exacerbate the disparities observed in PKre-T (32). Finally, we hoped to explore if location of follow-up at the time of a failing graft was associated with an increased incidence of PKre-T versus return to dialysis. Unfortunately, there were significant missing data that cannot be assumed to be missing at random; therefore, even though there was a signal that those patients followed in a transplant center were significantly more likely to undergo PKre-T, this will require future confirmatory analyses in a more complete dataset.
This study is novel in that it is the first to examine predictors of preemptive repeat transplant. The incidence of failed prior transplant as an indication for transplant has been increasing over time, and currently, a failed graft is the fourth leading indication for transplant in the United States (7).
Therefore, understanding ways to optimize outcomes for recipients of repeat transplant is a critical area for study.
Given the graft survival benefit with PKre-T, physicians and care providers must remain vigilant to seek opportunities to list patients, when appropriate, for repeat transplant, preemptively.
Awareness of differences in access to transplant is the first step toward addressing these disparities and working toward more equitable access to PKT and PKre-T for all patients, regardless of race, sex, age, or socioeconomic status. Appropriate timely transplant referral should be the expectation for all but especially for those followed by a specialized care team with expertise in transplantation and knowledge of the benefits that PKT and PKre-T provide over return to dialysis. We as transplant experts must hold ourselves accountable to ensure this is an equal priority in the care of all patients with a failing graft.
Disclosures
B.J. Foster reports research funding as a coinvestigator from two grants sponsored by Astellas Canada. R.B. Mannon reports research funding from Astellas, CareDx, CSL Behring, Mallinckrodt, Quark Pharmaceuticals, and Transplant Genomics, Inc.; honoraria from CSL Behring, Hansa, Novartis, Sanofi, and Vitaerris; scientific advisor or membership with the steering committee of the Vitaeris VKTX01 Clazakizumab for the Treatment of Chronic Active Antibody Mediated Rejection in Kidney Transplant Recipients (IMAGINE) Trial; and other interests/relationships as the chair of Data Safety Monitoring Board for the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health, member of the American Society of Nephrology (ASN) Grants Committee, chair of the ASN Policy and Advocacy Committee, chair of Women in Transplantation, member of the Program Committee TTS 2020 and 2022, and cochair of the SRTR Review Committee. K.K. Tennankore reports consultancy agreements with AstraZeneca, Baxter, Bayer, Janssen, and Otsuka; research funding from Astellas Canada and Otsuka Canada; honoraria from Astra Zeneca, Bayer, and Otsuka; scientific advisor or membership as an associate editor of the Canadian Journal of Kidney Health and Disease; and speakers bureau for AstraZeneca, Baxter, and Bayer. A.J. Vinson reports consultancy agreements with Paladin Labs Inc. and research funding from Paladin Labs Inc. K. West reports consultancy agreements with Envarsus Canada and honoraria from Envarsus Canada. The remaining author has nothing to disclose.
Funding
None.
Acknowledgments
The data reported here have been supplied by the Hennepin Healthcare Research Institute as the contractor for SRTR.
The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by SRTR or the United States Government.
Footnotes
See related editorial, “A Second Chance at Transplant First: Preemptive Repeat Kidney Transplantation,” on pages 11–13.
Author Contributions
A.J. Vinson conceptualized the study; B.J. Foster, B.A. Kiberd, R.B. Mannon, K.K. Tennankore, and K. West were responsible for methodology; A.J. Vinson was responsible for formal analysis; A.J. Vinson wrote the original draft; and B.J. Foster, B.A. Kiberd, R.B. Mannon, K.K. Tennankore, A.J. Vinson and K. West reviewed and edited the manuscript.
Supplemental Material
This article contains supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0003162021/-/DCSupplemental.
The proportion of transplanted patients who underwent preemptive first and repeat kidney transplant by recipient factors. *P value <0.001 for first preemptive kidney transplant (PKT); ψP value <0.001 for repeat PKT; λP value <0.05 for repeat PKT. Download Supplemental Figure 1, PDF file, 132 KB (131KB, pdf)
The proportion of patients undergoing preemptive kidney retransplant stratified by where follow-up care was last provided at the time the preceding graft failed. Download Supplemental Table 1, PDF file, 132 KB (131KB, pdf)
Median age and sex proportion at the time of first and repeat preemptive and nonpreemptive kidney transplants. Download Supplemental Table 2, PDF file, 132 KB (131KB, pdf)
Last available eGFR prior to preemptive kidney transplant (by MDRD equation). Download Supplemental Table 3, PDF file, 132 KB (131KB, pdf)
Adjusted hazard ratio for death-censored graft failure with preemptive retransplant versus nonpreemptive retransplant and preemptive first transplant versus nonpreemptive first transplant. Download Supplemental Table 4, PDF file, 132 KB (131KB, pdf)
References
- 1.King KL, Husain SA, Jin Z, Brennan C, Mohan S: Trends in disparities in preemptive kidney transplantation in the United States. Clin J Am Soc Nephrol 14: 1500–1511, 2019. 10.2215/CJN.03140319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramowicz D, Hazzan M, Maggiore U, Peruzzi L, Cochat P, Oberbauer R, Haller MC, Van Biesen W; Descartes Working Group and the European Renal Best Practice (ERBP) Advisory Board : Does pre-emptive transplantation versus post start of dialysis transplantation with a kidney from a living donor improve outcomes after transplantation? A systematic literature review and position statement by the Descartes Working Group and ERBP. Nephrol Dial Transplant 31: 691–697, 2016. 10.1093/ndt/gfv378 [DOI] [PubMed] [Google Scholar]
- 3.Kostro JZ, Hellmann A, Kobiela J, Skóra I, Lichodziejewska-Niemierko M, Dębska-Ślizień A, Śledziński Z: Quality of life after kidney transplantation: A prospective study. Transplant Proc 48: 50–54, 2016. 10.1016/j.transproceed.2015.10.058 [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes Transplant Work Group : KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Available at https://kdigo.org/wp-content/uploads/2018/08/KDIGO-Txp-Candidate-GL-FINAL.pdf. Accessed August 10, 2021
- 5.Knoll G, Cockfield S, Blydt-Hansen T, Baran D, Kiberd B, Landsberg D, Rush D, Cole E; Kidney Transplant Working Group of the Canadian Society of Transplantation : Canadian Society of Transplantation consensus guidelines on eligibility for kidney transplantation. CMAJ 173: 1181–1184, 2005. 10.1503/cmaj.051291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews PA, Burnapp L: British Transplantation Society/Renal Association UK guidelines for living donor kidney transplantation 2018: Summary of updated guidance. Transplantation 102: e307, 2018. 10.1097/TP.0000000000002253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Castro S, Foutz J, Wainright JL, Snyder JJ, Kasiske BL, Israni AK: OPTN/SRTR 2018 annual data report: Kidney. Am J Transplant 20[Suppl S1]: 20–130, 2020. 10.1111/ajt.15672 [DOI] [PubMed] [Google Scholar]
- 8.Johnston O, Rose CL, Gill JS, Gill JS: Risks and benefits of preemptive second kidney transplantation. Transplantation 95: 705–710, 2013. 10.1097/TP.0b013e31827a938f [DOI] [PubMed] [Google Scholar]
- 9.Goldfarb-Rumyantzev AS, Hurdle JF, Baird BC, Stoddard G, Wang Z, Scandling JD, Barenbaum LL, Cheung AK: The role of pre-emptive re-transplant in graft and recipient outcome. Nephrol Dial Transplant 21: 1355–1364, 2006. 10.1093/ndt/gfk061 [DOI] [PubMed] [Google Scholar]
- 10.Girerd S, Girerd N, Duarte K, Giral M, Legendre C, Mourad G, Garrigue V, Morelon E, Buron F, Kamar N, Del Bello A, Ladrière M, Kessler M, Frimat L: Preemptive second kidney transplantation is associated with better graft survival compared with non-preemptive second transplantation: A multicenter French 2000–2014 cohort study. Transpl Int 31: 408–423, 2018. 10.1111/tri.13105 [DOI] [PubMed] [Google Scholar]
- 11.Andrews PA; Standards Committee of the British Transplantation Society : Summary of the British Transplantation Society guidelines for management of the failing kidney transplant. Transplantation 98: 1130–1133, 2014. 10.1097/TP.0000000000000426 [DOI] [PubMed] [Google Scholar]
- 12.Fishbane S, Agoritsas S, Bellucci A, Halinski C, Shah HH, Sakhiya V, Balsam L: Augmented nurse care management in CKD stages 4 to 5: A randomized trial. Am J Kidney Dis 70: 498–505, 2017. 10.1053/j.ajkd.2017.02.366 [DOI] [PubMed] [Google Scholar]
- 13.Khosla N, Gordon E, Nishi L, Ghossein C: Impact of a chronic kidney disease clinic on preemptive kidney transplantation and transplant wait times. Prog Transplant 20: 216–220, 2010. 10.1177/152692481002000304 [DOI] [PubMed] [Google Scholar]
- 14.Knight RJ, Teeter LD, Graviss EA, Patel SJ, DeVos JM, Moore LW, Gaber AO: Barriers to preemptive renal transplantation: A single center questionnaire study. Transplantation 99: 576–579, 2015. 10.1097/TP.0000000000000357 [DOI] [PubMed] [Google Scholar]
- 15.Kutner NG, Zhang R, Huang Y, Johansen KL: Impact of race on predialysis discussions and kidney transplant preemptive wait-listing. Am J Nephrol 35: 305–311, 2012. 10.1159/000336891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill JS, Abichandani R, Khan S, Kausz AT, Pereira BJ: Opportunities to improve the care of patients with kidney transplant failure. Kidney Int 61: 2193–2200, 2002. 10.1046/j.1523-1755.2002.00373.x [DOI] [PubMed] [Google Scholar]
- 17.Salvador CL, Hartmann A, Åsberg A, Bergan S, Rowe AD, Mørkrid L: Estimating glomerular filtration rate in kidney transplant recipients: Comparing a novel equation with commonly used equations in this population. Transplant Direct 3: e332, 2017. 10.1097/TXD.0000000000000742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee CM, Lertdumrongluk P, Streja E, Park J, Moradi H, Lau WL, Norris KC, Nissenson AR, Amin AN, Kovesdy CP, Kalantar-Zadeh K: Impact of age, race and ethnicity on dialysis patient survival and kidney transplantation disparities. Am J Nephrol 39: 183–194, 2014. 10.1159/000358497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni S, Ladin K, Haakinson D, Greene E, Li L, Deng Y: Association of racial disparities with access to kidney transplant after the implementation of the new kidney allocation system. JAMA Surg 154: 618–625, 2019. 10.1001/jamasurg.2019.0512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, Weissman JS, David-Kasdan JA, Carlson D, Fuller J, Marsh D, Conti RM: Racial disparities in access to renal transplantation–Clinically appropriate or due to underuse or overuse? N Engl J Med 343: 1537–1544, 2000. 10.1056/NEJM200011233432106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purnell TS, Luo X, Cooper LA, Massie AB, Kucirka LM, Henderson ML, Gordon EJ, Crews DC, Boulware LE, Segev DL: Association of race and ethnicity with live donor kidney transplantation in the United States from 1995 to 2014. JAMA 319: 49–61, 2018. 10.1001/jama.2017.19152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jay CL, Dean PG, Helmick RA, Stegall MD: Reassessing preemptive kidney transplantation in the United States: Are we making progress? Transplantation 100: 1120–1127, 2016. 10.1097/TP.0000000000000944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT: Preemptive kidney transplantation: The advantage and the advantaged. J Am Soc Nephrol 13: 1358–1364, 2002. 10.1097/01.ASN.0000013295.11876.C9 [DOI] [PubMed] [Google Scholar]
- 24.Amaral S, Sayed BA, Kutner N, Patzer RE: Preemptive kidney transplantation is associated with survival benefits among pediatric patients with end-stage renal disease. Kidney Int 90: 1100–1108, 2016. 10.1016/j.kint.2016.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg PP, Furth SL, Fivush BA, Powe NR: Impact of gender on access to the renal transplant waiting list for pediatric and adult patients. J Am Soc Nephrol 11: 958–964, 2000. 10.1681/ASN.V115958 [DOI] [PubMed] [Google Scholar]
- 26.Monson RS, Kemerley P, Walczak D, Benedetti E, Oberholzer J, Danielson KK: Disparities in completion rates of the medical prerenal transplant evaluation by race or ethnicity and gender. Transplantation 99: 236–242, 2015. 10.1097/TP.0000000000000271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander GC, Sehgal AR: Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA 280: 1148–1152, 1998. 10.1001/jama.280.13.1148 [DOI] [PubMed] [Google Scholar]
- 28.Ahearn P, Johansen KL, Tan JC, McCulloch CE, Grimes BA, Ku E: Sex disparity in deceased-donor kidney transplant access by cause of kidney disease. Clin J Am Soc Nephrol 16: 241–250, 2021. 10.2215/CJN.09140620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tangri N, Grams ME, Levey AS, Coresh J, Appel LJ, Astor BC, Chodick G, Collins AJ, Djurdjev O, Elley CR, Evans M, Garg AX, Hallan SI, Inker LA, Ito S, Jee SH, Kovesdy CP, Kronenberg F, Heerspink HJ, Marks A, Nadkarni GN, Navaneethan SD, Nelson RG, Titze S, Sarnak MJ, Stengel B, Woodward M, Iseki K; CKD Prognosis Consortium : Multinational assessment of accuracy of equations for predicting risk of kidney failure: A meta-analysis. JAMA 315: 164–174, 2016. 10.1001/jama.2015.18202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hecking M, Bieber BA, Ethier J, Kautzky-Willer A, Sunder-Plassmann G, Säemann MD, Ramirez SP, Gillespie BW, Pisoni RL, Robinson BM, Port FK: Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: The Dialysis Outcomes and Practice Patterns Study (DOPPS). PLoS Med 11: e1001750, 2014. 10.1371/journal.pmed.1001750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schold JD, Augustine JJ, Huml AM, O’Toole J, Sedor JR, Poggio ED: Modest rates and wide variation in timely access to repeat kidney transplantation in the United States. Am J Transplant 20: 769–778, 2020. 10.1111/ajt.15646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfe RA, Ashby VB, Milford EL, Bloembergen WE, Agodoa LY, Held PJ, Port FK: Differences in access to cadaveric renal transplantation in the United States. Am J Kidney Dis 36: 1025–1033, 2000. 10.1053/ajkd.2000.19106 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The proportion of transplanted patients who underwent preemptive first and repeat kidney transplant by recipient factors. *P value <0.001 for first preemptive kidney transplant (PKT); ψP value <0.001 for repeat PKT; λP value <0.05 for repeat PKT. Download Supplemental Figure 1, PDF file, 132 KB (131KB, pdf)
The proportion of patients undergoing preemptive kidney retransplant stratified by where follow-up care was last provided at the time the preceding graft failed. Download Supplemental Table 1, PDF file, 132 KB (131KB, pdf)
Median age and sex proportion at the time of first and repeat preemptive and nonpreemptive kidney transplants. Download Supplemental Table 2, PDF file, 132 KB (131KB, pdf)
Last available eGFR prior to preemptive kidney transplant (by MDRD equation). Download Supplemental Table 3, PDF file, 132 KB (131KB, pdf)
Adjusted hazard ratio for death-censored graft failure with preemptive retransplant versus nonpreemptive retransplant and preemptive first transplant versus nonpreemptive first transplant. Download Supplemental Table 4, PDF file, 132 KB (131KB, pdf)