Key Points
Kidney alterations in AKI are challenging to study directly due to the need for kidney biopsy or postmortem analysis to obtain cells.
Urine scRNAseq can be used to noninvasively characterize cellular diversity and identify altered pathways in the setting of COVID-19 AKI.
This study provides preliminary evidence that SARS-CoV-2 is capable of directly infecting urothelial cells.
Keywords: acute kidney injury and ICU nephrology, acute kidney injury, basic science, COVID-19, SARS-CoV-2, single-cell RNA sequencing, urine
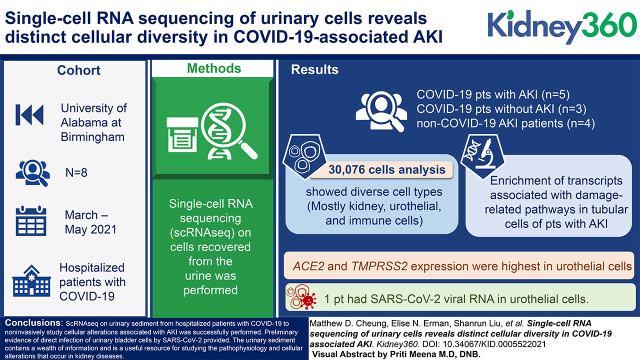
Visual Abstract
Abstract
Background
AKI is a common sequela of infection with SARS-CoV-2 and contributes to the severity and mortality from COVID-19. Here, we tested the hypothesis that kidney alterations induced by COVID-19–associated AKI could be detected in cells collected from urine.
Methods
We performed single-cell RNA sequencing (scRNAseq) on cells recovered from the urine of eight hospitalized patients with COVID-19 with (n=5) or without AKI (n=3) as well as four patients with non–COVID-19 AKI (n=4) to assess differences in cellular composition and gene expression during AKI.
Results
Analysis of 30,076 cells revealed a diverse array of cell types, most of which were kidney, urothelial, and immune cells. Pathway analysis of tubular cells from patients with AKI showed enrichment of transcripts associated with damage-related pathways compared with those without AKI. ACE2 and TMPRSS2 expression was highest in urothelial cells among cell types recovered. Notably, in one patient, we detected SARS-CoV-2 viral RNA in urothelial cells. These same cells were enriched for transcripts associated with antiviral and anti-inflammatory pathways.
Conclusions
We successfully performed scRNAseq on urinary sediment from hospitalized patients with COVID-19 to noninvasively study cellular alterations associated with AKI and established a dataset that includes both injured and uninjured kidney cells. Additionally, we provide preliminary evidence of direct infection of urinary bladder cells by SARS-CoV-2. The urinary sediment contains a wealth of information and is a useful resource for studying the pathophysiology and cellular alterations that occur in kidney diseases.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection triggers pathology across multiple systems, including the kidney, and AKI is associated with significant morbidity and mortality in coronavirus disease 2019 (COVID-19) (1–5). Multiple studies have demonstrated high rates of AKI among hospitalized patients with COVID-19 (6), with some reporting up to 50% of infected individuals developing AKI (7–9). The primary SARS-CoV-2 receptor ACE2 is expressed on epithelial cells throughout the urinary system, including proximal tubule cells and urothelial cells (10–13), although it is unclear if AKI in patients with COVID-19 is due to direct viral infection of the proximal tubules or is a result of the systemic response to SARS-CoV-2 (14–22). Similarly, it is unclear if SARS-CoV-2 can cause viral cystitis via direct infection of urothelial cells, although this possibility has been proposed (23–26). Despite the high prevalence of COVID-19–associated AKI, the underlying cellular alterations that occur in the setting of AKI remain poorly understood.
COVID-19–associated AKI has remained largely understudied as access to kidney tissue requires biopsy or postmortem analysis (27). Recent studies have detected a diverse array of kidney, bladder, and immune cells in the urine (28–30). Thus, the urine may offer valuable insight to noninvasively study kidney changes during COVID-19–associated AKI. Here, we performed single-cell RNA sequencing (scRNAseq) to characterize the cellular diversity in the urine of hospitalized patients with COVID-19 with and without AKI. We tested the hypothesis that kidney alterations in COVID-19–associated AKI could be detected in cells collected from urine. We also collected samples from patients without COVID-19 and with AKI (non–COVID-19 AKI). We found several inflammatory immune cell populations and differentially activated pathways in COVID-19–associated AKI as well as preliminary evidence for direct infection of urothelial cells by SARS-CoV-2.
Materials and Methods
Participants and Variables
Adults aged 18 years old and older were screened during admission or transfer to the University of Alabama at Birmingham (UAB) hospital between March and May 2021. Cases of AKI were identified using the Kidney Disease Improving Global Outcomes definition as a rise in serum creatinine (sCr) >0.3 mg/dl within 48 hours or >1.5× baseline creatinine. Controls with no change in creatinine were selected on the basis of age and sex matching where possible and processed with each respective AKI sample. Baseline sCr was determined using the most recent sCr value 7–365 days prior to hospitalization. Additional clinical data regarding demographics, medical history, clinical characteristics, and laboratory values were extracted from patient charts through the UAB Center for Clinical and Translational Sciences i2b2 team. Eight hospitalized patients with COVID-19, five with AKI and three without AKI, were included. This study and the specimen collections were approved by the UAB Institutional Review Board. The UAB Acute Nephrology Consult Team also collected samples from patients with non–COVID-19 AKI (n=4) to compare cellular changes with COVID-19–associated AKI. These were collected under a different institutional review board protocol that allows for collection of remnant urine samples and thus, are anonymous.
Specimen Collection and Processing
All steps were performed on ice. Urine was collected either as a voided specimen or from a urinary catheter. Samples were immediately transferred to a biosafety level 2+ laboratory for processing. Urine samples were transferred to a 50-ml conical tube and centrifuged at 1000×g for 10 minutes at 4°C. Cell pellets were washed with ice-cold PBS, filtered through a 40-µm filter, and centrifuged again. Live cells were purified using the MACS Debris Removal Kit (Miltenyi Biotec) followed by the EasySep Annexin V Dead Cell Removal Kit (StemCell). Briefly, cells were resuspended in ice-cold PBS and mixed with debris removal solution. Cold PBS was overlaid on the mixture and centrifuged at 300×g for 10 minutes at 4°C. The top two phases were aspirated, and then, remaining cells were washed with PBS. Cells were resuspended and mixed with Dead Cell Removal Cocktail, Biotin Selection Cocktail, and then, RapidSpheres before separation in an EasySep magnet. Cells were washed and resuspended in 52 µl of PBS (no calcium or magnesium) +0.04% BSA (Fisher Scientific) for scRNAseq processing.
scRNAseq
Purified cells were transferred to the UAB Flow Cytometry and Single Cell Core and immediately processed using the Chromium 3′ Single-Cell RNA Sequencing Kit (10× Genomics) according to the manufacturer’s instructions. The cell suspension was counted, combined with the 10× Chromium reagent mixture, and loaded into a microfluidic single-cell partitioning device in which lysis and reverse transcription occur in microdroplets. The resulting cDNA was amplified by PCR and subsequently processed to yield bar-coded sequencing libraries. Paired-end sequencing was carried out on the Illumina NovaSeq6000 or NextSeq500 sequencing platform (Illumina). Reads were processed using the 10× Genomics Cell Ranger Single-Cell Software Suite version 6.0 on the UAB Cheaha High Performance Computing Cluster. BCL files were converted to FASTQ files using the CellRanger mkfastq function. CellRanger mkfastq was used to align FASTQ files to a custom genome consisting of the hg38 human genome (GRCh38.p13) with the SARS-CoV-2 genome (NC_045512.2) inserted as an exon (31). The genes table, barcodes table, and transcriptional expression matrices were created for the analysis indicated below.
Data Analyses
Analyses were carried out using packages created for the R statistical analysis environment (version 4.06). Data were primarily analyzed in Seurat version 3.2.3 (32,33) and its associated dependencies. Data from each individual patient were imported using the Read10× function and then structured into a Seurat object using CreateSeuratObject. For quality control, cells with unique feature counts over 2500 or under 200 and cells with mitochondrial proportions >15% were filtered out. Data were normalized using LogNormalize and scaled to prepare for linear dimensional reduction. Objects from individual patients were labeled with unique group identifications and then merged into a single object using the Seurat merge function. Patient samples were integrated with the RunHarmony function using the Harmony R package (34). Principal component analysis was performed, and then, cells were clustered on the basis of differential gene expression as determined by the Seurat FindAllMarkers function set to a resolution of one. Dimensional reduction was performed using uniform manifold approximation and projection. Cell types were identified by comparing the differentially expressed transcripts for each cluster with known transcripts associated with specific cell types (29,35,36). The Escape R package was used to run Gene Set Enrichment Analysis (GSEA) (37). WebGestaltR was used for gene ontology analysis to identify pathways using the Biologic Process and Kyoto Encyclopedia of Genes and Genomes databases (38).
Results
We performed scRNAseq on urine sediment from eight hospitalized patients with COVID-19, five with AKI and three without AKI. Four additional urine samples from hospitalized patients without COVID-19 and with AKI were also collected to compare potential differences in AKI in the context of COVID-19. The average sCr in patients with COVID-19–associated AKI was 2.4 mg/dl as compared with 0.83 mg/dl in control patients at the time of collection. Notably, two of the patients with COVID-19–associated AKI had prior diagnoses of CKD, which has been shown to worsen outcomes from COVID-19 (39,40). Comorbidities and other variables differed slightly between the AKI and no AKI control populations (Table 1).
Table 1.
Baseline characteristics and demographics of study participants
| Variable | Coronavirus Disease 2019 AKI | Coronavirus Disease 2019 No AKI Control | ||||||
|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
| Age, yr | 32 | 62 | 46 | 49 | 81 | 60 | 62 | 38 |
| Sex | M | M | F | M | M | M | F | M |
| Race | AA | AA | AA | AA | White | White | AA | White |
| BMI, kg/m2 | 32.1 | 40.5 | 26.6 | 28.4 | 35.2 | 29.1 | 34.2 | 58.0 |
| sCr baseline, mg/dl | 1.8 | 1.3 | 0.8 | 1.2 | 0.7 | 0.8 | 0.7 | 0.8 |
| sCr at collection, mg/dl | 3.5 | 2.1 | 1.5 | 2.6 | 2.3 | 0.8 | 0.8 | 0.9 |
| Comorbidities | ||||||||
| Diabetes | X | X | X | X | X | |||
| HTN | X | X | X | X | ||||
| Hyperlipidemia | X | X | X | X | X | |||
| CAD | X | X | X | |||||
| COPD/asthma | X | |||||||
| Baseline GFR <60 | X | X | X | |||||
| Foley catheter | X | |||||||
AKI was defined using the Kidney Disease Improving Global Outcomes criteria of rise in sCr of >0.3 mg/dl or 1.5× baseline. Clinical data from patients with noncoronavirus disease 2019 AKI (patients 9–12) were not obtained as samples were collected anonymously. M, male; F, female; AA, Black; BMI, body mass index, sCR, serum creatinine; X, patients with comorbidity; HTN, hypertension; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease.
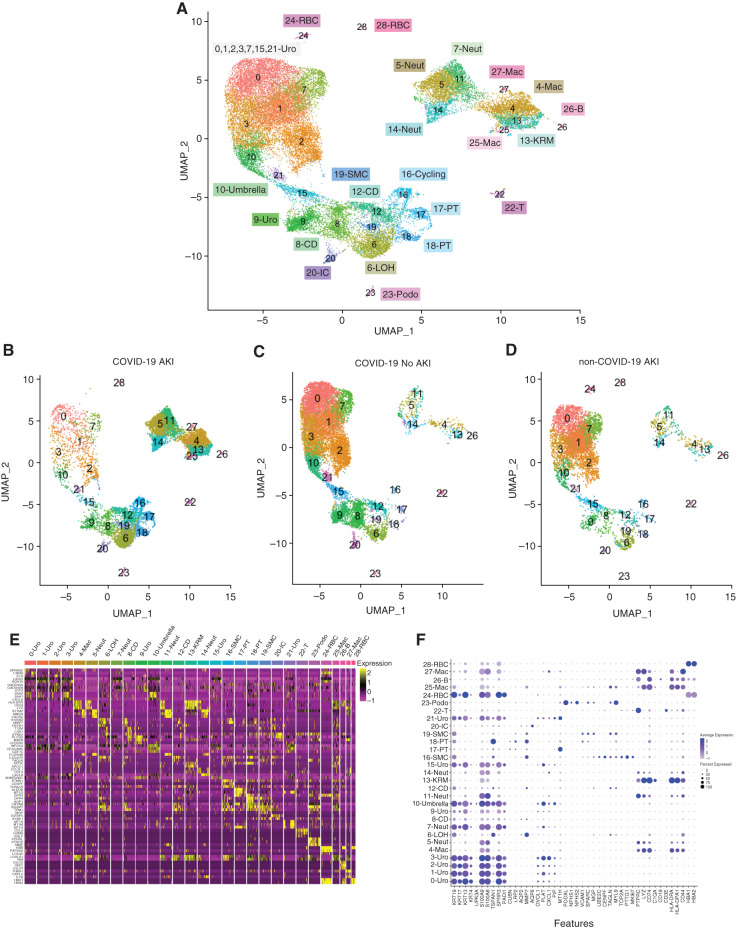
Consistent with prior reports (29), cell numbers were highly heterogeneous between patients (Supplemental Figure 1, A and B). In total, 65,234 cells were sequenced, and 30,076 cells were retained through quality control. Integrated uniform manifold approximation and projections of all 12 patient samples revealed a diverse array of kidney, urothelial, and immune cells (Figure 1, A, E, and F). We were able to detect most kidney cells, including podocytes and VCAM1+ kidney cells, demonstrating the ability to noninvasively study the kidney through analysis of urine. There is clear heterogeneity in the urothelial cell population, which falls outside the scope of this study but warrants further investigation. The cell types captured varied between AKI and non-AKI groups (Figure 1, B–D) as well as between each patient (Supplemental Figure 1C). As expected, larger numbers of immune cells were detected in the COVID-19–associated AKI samples.
Figure 1.
Single-cell RNA sequencing of urine from patients with coronavirus disease 2019 (COVID-19) with and without AKI and patients with non–COVID-19 AKI. (A) Integrated uniform manifold approximation and projection (UMAP) plot of 30,076 cells sequenced from patient urine sediment. (B–D) UMAP plot subsetted by COVID-19 and AKI status showing the difference in collected cell populations. Cell-type labels are the same. (E) Heat map of differentially expressed genes of integrated clusters. (F) Dot plot of known marker genes for identification of cells. B, B lymphocyte; CD, collecting duct; IC, intercalated cell; KRM, kidney resident macrophage; LOH, loop of Henle; Mac, macrophage; Neut, neutrophil; Podo, podocyte; PT, proximal tubule; RBC, red blood cell; SMC, smooth muscle cell; T, T lymphocyte; Uro, urothelial.
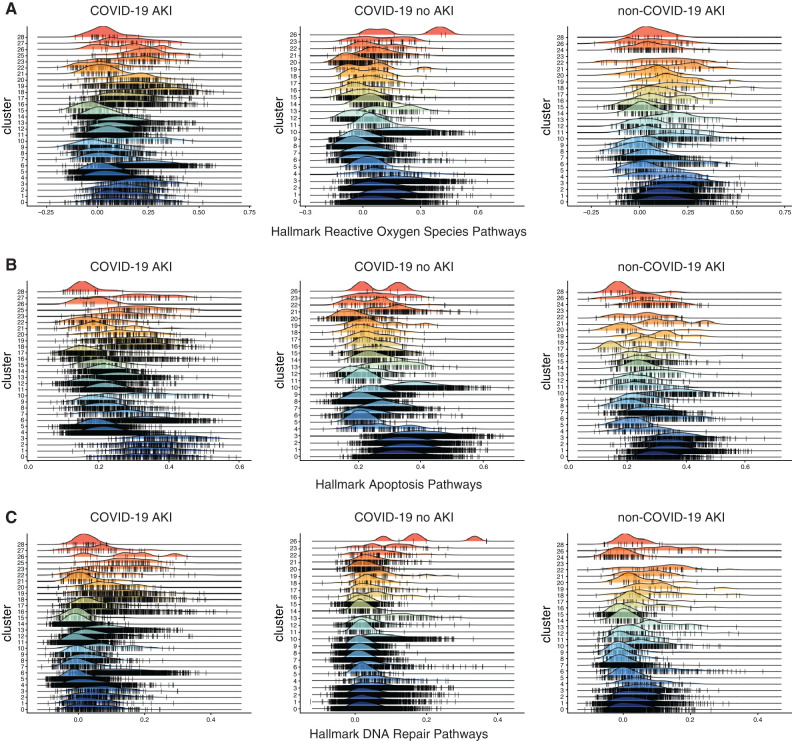
GSEA revealed upregulation of apoptotic genes, IFN response elements, and various other signaling pathways in both COVID-19–associated and non–COVID-19 AKI and non-AKI control samples (Figure 2C, Supplemental Figure 2). Because of the high energy requirements and mitochondrial content, the proximal tubule is one of the structures most sensitive to insult during AKI (41), and its severity of injury is related to overall outcomes (42). Pathway analysis of proximal tubule cells from patients with AKI revealed enrichment of pathways related to apoptosis, DNA repair, and cellular responses to reactive oxygen species and stress in contrast to patients without AKI (Figure 2, A and B). Similar pathway upregulation was detected in other kidney tubular cells. Pathways related to damage were upregulated in the setting of AKI, but enrichment of reactive oxygen species, DNA damage, and apoptotic pathways in the proximal tubules, loop of Henle, and collecting duct was more apparent in COVID-19–associated AKI in comparison with the non–COVID-19 AKI samples. We suspect that there are many overlapping pathways in AKI due to the combination of injury mechanisms, including ischemia, inflammation, or drug toxicity. The additional enrichment of damage-related pathways in COVID-19–associated AKI may be due to the other inflammatory processes occurring in the COVID-19 disease process. This may explain the metabolic differences seen in urine of patients with COVID-19–associated AKI (43).
Figure 2.
Enrichment of damage-related pathways in cells from patients with AKI. Gene Set Enrichment Analysis of each cluster of cells from the integrated UMAP for pathways, including (A) hallmark reactive oxygen species pathways, (B) hallmark apoptosis pathways, and (C) hallmark DNA repair pathways in patients with COVID-19 AKI (left panels), COVID-19 no AKI controls (center panels), and patients with non–COVID-19 AKI (right panels). Cluster identifications are as follows: 1, urothelial; 2, urothelial; 3, urothelial; 4, macrophage; 5, MHC class 2–positive neutrophil; 6, loop of Henle; 7, neutrophil; 8, collecting duct; 9, urothelial; 10, umbrella; 11, neutrophil; 12, collecting duct; 13, kidney resident macrophage; 14, neutrophil; 15, urothelial; 16, cycling; 17, proximal tubule; 18, proximal tubule; 19, smooth muscle cell; 20, intercalated cell; 21, urothelial; 22, T cell; 23, podocyte; 24, red blood cell; 25, macrophage; 26, B cell; 27, macrophage; 28, red blood cell.
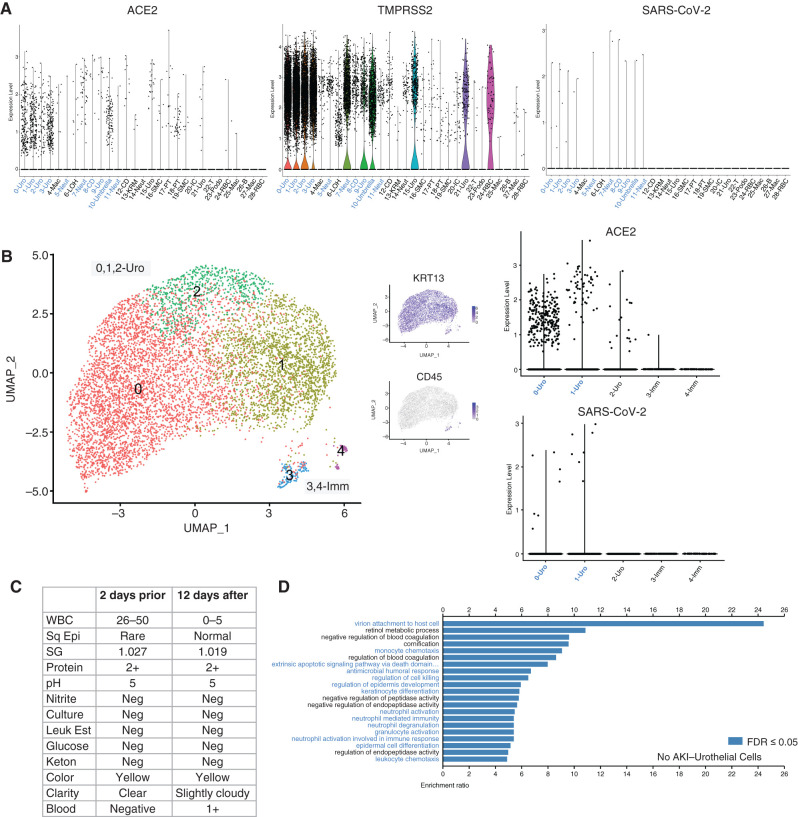
The viral entry receptor, ACE2, is abundantly present in a variety of tissues, including the kidneys. ACE2 and TMPRSS2 transcripts were detected on various epithelial cells from the kidney and were particularly abundant in urothelial cells (Figure 3A). Because SARS-CoV-2 is an RNA virus with a poly-A tail (44), we created a customized genome for alignment where the SARS-CoV-2 genome was added as an exon to the hg38 human genome. Interestingly, although we did not detect any SARS-CoV-2 viral transcripts in the kidney cells in patients with AKI, we did detect trace transcripts in the urothelial cells of patient 7 (who had COVID-19 without AKI), suggesting direct infection of these bladder cells (Figure 3A). Individual analysis of patient 7 revealed few kidney-derived cells. Most of the cells isolated were immune and urothelial cells. Viral RNA was found exclusively in urothelial cells with high ACE2 expression (Figure 3B, Supplemental Figure 3). Urinalysis of this patient showed leukocyturia without evidence of a bacterial infection (negative leukocyte esterase and nitrite) (Figure 3C). Pathway analysis of the urothelial cells revealed enrichment of pathways involved in type 1 IFN signaling, an acute inflammatory response, and leukocyte responses (Figure 3D, Supplemental Figure 2B), which is consistent with a viral infection of these cells.
Figure 3.
Detection of direct infection of urothelial cells by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). (A) Violin plots of ACE2, TMPRSS2, and SARS-CoV-2 gene expression among sequenced cell types. Cell types with identified SARS-CoV-2 gene expression are identified in blue to identify overlap between SARS-CoV-2 receptor and gene expression. (B) Individual analysis of patient 7: UMAP plots and ACE2 and SARS-CoV-2 expression identifying the presence of SARS-CoV-2 gene expression within urothelial cells. (C) Urinalysis laboratory values from urine samples taken from patient 7 2 days prior and 12 days after urine collection for single-cell RNA sequencing. (D) Gene enrichment analysis using the WebGestaltR Biologic Process to identify upregulated pathways in the urothelial cells of patient 7. Pathways involved in viral infection and immune response are shown in blue with a false discovery rate (FDR) of ≤0.05. Sq Epi, squamous epithelial cells; Leuk Est, leukocyte esterase; lmm, immune cells; Neg, negative; SG, specific gravity; WBC, white blood cell.
Lack of detection of viral RNA in proximal tubule cells in the urine does not rule out direct infection by SARS-CoV-2. Other groups have reported evidence for and against viral tropism in the kidney (14–22). The detection of viral RNA in urothelial cells and the enrichment of transcripts associated with antiviral pathways in these cells suggest direct infection and a possible case of viral cystitis. Viral cystitis due to SARS-CoV-2 has been noted, but evidence of direct infection has been lacking. The mechanism of entry would be from the basal side of the urothelial cells if from the blood or the luminal side if from the urine. A previous study suggested that ACE2 is expressed on both the basal and luminal surfaces of the urinary bladder (23). The detection of viral cystitis using scRNAseq is consistent with the clinical characteristics from patient 7 (Figure 3C). Although the virus was only detected in a small number of cells, we suspect that the majority of virally infected cells could have been filtered out during the urine processing and data analysis quality control steps in which we removed dead and dying cells.
Discussion
A limitation of this study is the small number of patients sampled. Sample collections were restricted to new hospital admissions to control for other variables, such as the various medications used as treatments for COVID-19. Hospitalized patients with COVID-19 and without AKI were used as controls because healthy controls do not secrete many cells in urine. An additional limitation is having to process urine before sequencing; however, this greatly increased the proportion of live cells obtained. Here, cells were readily detectable in the urine of infected patients without kidney injury. This is significant because most scRNAseq studies have focused on damaged kidneys, leading to few datasets available to study uninjured kidney cells. AKI has many different causes, and only a subset may be caused by direct kidney infection by SARS-CoV-2, making it difficult to draw broad conclusions about AKI in COVID-19. Additionally, it is possible that direct viral infection of kidney cells occurs later in the infection process than was captured in this study. Subsequent studies will investigate urinary cell changes over the course of illness with sequential samples taken before, during, and after AKI.
We successfully performed scRNAseq on urinary sediment from hospitalized patients with COVID-19. This allowed for noninvasive studies of cellular changes occurring during AKI, a frequent manifestation of COVID-19. The urinary cell composition and upregulated pathways in hospitalized patients with COVID-19 drastically differ in those with AKI versus those without AKI. We also provide preliminary evidence of a potential case of viral cystitis through direct infection of urinary bladder cells. Analysis of urinary cells may provide a useful avenue to understanding the pathophysiology and cellular alterations that occur in kidney diseases.
Disclosures
A. Agarwal reports consultancy agreements with Akebia Therapeutics (served on an expert panel to review new therapeutics on the basis of the hypoxia-inducible factor pathway for AKI), Dynamed (reviewed content related to AKI for Dynamed and reviewed and updated materials prepared by the Dynamed editorial team for AKI topics), and Reata Pharmaceuticals (served as a consultant); ownership interest in Goldilocks Therapeutics, Inc.; research funding from the Genzyme/Sanofi Fabry fellowship award; and honoraria from Akebia Therapeutics, Emory, the University of Southern California, and Vanderbilt. A. Agarwal also reports scientific advisor or membership as an editorial board member for American Journal of Physiology–Renal Physiology, Kidney International, and Laboratory Investigation; as an advisory board member of Goldilocks Therapeutics, Inc. (a New York–based company investigating the delivery of drugs in the kidney using nanotechnology for acute kidney disease and CKD); as an external evaluation panel member for the Kidney Precision Medicine Program; and as an advisory board member of Alpha Young, LLC and Angion. All remaining authors have nothing to disclose.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants P30 DK079337 (to A. Agarwal), R01 DK118932 (to A. Agarwal), and R01 DK59600 (to A. Agarwal); National Institute of Allergy and Infectious Diseases grant T32 AI007051 (to M.D. Cheung); National Institute of General Medical Sciences grant T32-GM-008361 (to M.D. Cheung); American Heart Association grant 827257 (to E.N. Erman); and Anderson Innovation Award (to J.F. George).
Acknowledgments
The authors thank Ms. Wanda Hall, Ms. Rachael Shevin, and Dr. Chiao-Wang Sun for assistance in collection and processing of urine specimens; Mr. Robert Johnson and the Center for Clinical and Translational Sciences (CCTS) Informatics for Integrating Biology and the Bedside (i2b2) team for processing the clinical data; Dr. Katie Bean, Dr. Winn Seay, Dr. Clare Lyas, and the Acute Nephrology Consult Team for collection of non–COVID-19 AKI urine; Dr. Caroline Kelly for diligent proofreading; and Dr. Kelly Andringa and Ms. Mozella Kerley for administrative assistance. We acknowledge the UAB Flow Cytometry and Single Cell Core (National Institutes of Health [NIH] grant P30-AR-04831), the UAB Heflin Genomics Core (NIH grant 5P30CA013148-48), and CCTS, National Center for Advancing Translational Sciences (NIH grant UL1TR003096).
Author Contributions
A. Agarwal, M.D. Cheung, J.C. Edberg, N.B. Erdmann, and S. Liu conceptualized the study; A. Agarwal, M.D. Cheung, N.B. Erdmann, E.N. Erman, J.F. George, G. Ghajar-Rahimi, and K.H. Moore were responsible for data curation; M.D. Cheung, E.N. Erman, J.F. George, G. Ghajar-Rahimi, and K.H. Moore were responsible for formal analysis; A. Agarwal, M.D. Cheung, J.F. George, G. Ghajar-Rahimi, and K.H. Moore were responsible for investigation; A. Agarwal, M.D. Cheung, N.B. Erdmann, and S. Liu were responsible for methodology; A. Agarwal was responsible for project administration; A. Agarwal and J.F. George were responsible for resources; J.F. George was responsible for validation; M.D. Cheung was responsible for visualization; A. Agarwal and J.F. George were responsible for funding acquisition; A. Agarwal, J.C. Edberg, and J.F. George provided supervision; A. Agarwal, M.D. Cheung, E.N. Erman, and J.F. George wrote the original draft; and A. Agarwal, M.D. Cheung, J.C. Edberg, N.B. Erdmann, E.N. Erman, J.F. George, G. Ghajar-Rahimi, S. Liu, and K.H. Moore reviewed and edited the manuscript.
Data Sharing Statement
The scRNAseq data generated in this paper are available in the Gene Expression Omnibus under accession number GSE180595.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0005522021/-/DCSupplemental.
Heterogeneity of cells captured from each patient sample. Download Supplemental Figure 1, PDF file, 828 KB (827.4KB, pdf)
Gene Set Enrichment Analysis of hallmark pathways in both AKI and no AKI control clusters. Download Supplemental Figure 2, PDF file, 828 KB (827.4KB, pdf)
Heat map of differentially expressed genes from cells from patient 7. Download Supplemental Figure 3, PDF file, 828 KB (827.4KB, pdf)
References
- 1.Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, Swaminathan S; COVID-19 and ACE2 in Cardiovascular, Lung, and Kidney Working Group : Acute kidney injury in COVID-19: Emerging evidence of a distinct pathophysiology. J Am Soc Nephrol 31: 1380–1383, 2020. 10.1681/ASN.2020040419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xia P, Wen Y, Duan Y, Su H, Cao W, Xiao M, Ma J, Zhou Y, Chen G, Jiang W, Wu H, Hu Y, Xu S, Cai H, Liu Z, Zhou X, Du B, Wang J, Li T, Yan X, Chen L, Liang Z, Zhang S, Zhang C, Qin Y, Wang G, Li X: Clinicopathological features and outcomes of acute kidney injury in critically ill COVID-19 with prolonged disease course: A retrospective cohort. J Am Soc Nephrol 31: 2205–2221, 2020. 10.1681/ASN.2020040426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wald R, Bagshaw SM: COVID-19-associated acute kidney injury: Learning from the first wave. J Am Soc Nephrol 32: 4–6, 2021. 10.1681/ASN.2020101401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teoh JY-C, Yip TC-F, Lui GC-Y, Wong VW-S, Chow VC-Y, Ho TH-Y, Li TC-M, Tse Y-K, Chiu PK-F, Ng C-F, Hui DS-C, Chan HL-Y, Szeto C-C, Wong GL-H: Risks of AKI and major adverse clinical outcomes in patients with severe acute respiratory syndrome or coronavirus disease 2019. J Am Soc Nephrol 32: 961–971, 2021. 10.1681/ASN.2020071097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP; the Northwell COVID-19 Research Consortium : Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323: 2052–2059, 2020. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium : Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020. 10.1016/j.kint.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, Paranjpe I, Somani S, Richter F, Miotto R, Lala A, Kia A, Timsina P, Li L, Freeman R, Chen R, Narula J, Just AC, Horowitz C, Fayad Z, Cordon-Cardo C, Schadt E, Levin MA, Reich DL, Fuster V, Murphy B, He JC, Charney AW, Böttinger EP, Glicksberg BS, Coca SG, Nadkarni GN; Mount Sinai COVID Informatics Center (MSCIC) : AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 32: 151–160, 2021. 10.1681/ASN.2020050615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns TS, Abramowitz MK, Levy R, Kumar N, Mokrzycki MH, Coco M, Dominguez M, Prudhvi K, Golestaneh L: AKI in hospitalized patients with and without COVID-19: A comparison study. J Am Soc Nephrol 31: 2145–2157, 2020. 10.1681/ASN.2020040509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birkelo BC, Parr SK, Perkins AM, Greevy RA Jr., Hung AM, Shah SC, Arroyo JP, Denton J, Vincz AJ, Matheny ME, Siew ED: Comparison of COVID-19 versus influenza on the incidence, features, and recovery from acute kidney injury in hospitalized United States veterans. Kidney Int 100: 894–905, 2021. 10.1016/j.kint.2021.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW: Extrapulmonary manifestations of COVID-19. Nat Med 26: 1017–1032, 2020. 10.1038/s41591-020-0968-3 [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparks MA, South AM, Badley AD, Baker-Smith CM, Batlle D, Bozkurt B, Cattaneo R, Crowley SD, Dell’Italia LJ, Ford AL, Griendling K, Gurley SB, Kasner SE, Murray JA, Nath KA, Pfeffer MA, Rangaswami J, Taylor WR, Garovic VD: Severe acute respiratory syndrome coronavirus 2, COVID-19, and the renin-angiotensin system: Pressing needs and best research practices. Hypertension 76: 1350–1367, 2020. 10.1161/HYPERTENSIONAHA.120.15948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nath KA, Singh RD, Grande JP, Garovic VD, Croatt AJ, Ackerman AW, Barry MA, Agarwal A: Expression of ACE2 in the intact and acutely injured kidney. Kidney360 2: 1095–1106, 2021. 10.34067/KID.0001562021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farkash EA, Wilson AM, Jentzen JM: Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol 31: 1683–1687, 2020. 10.1681/ASN.2020040432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santoriello D, Khairallah P, Bomback AS, Xu K, Kudose S, Batal I, Barasch J, Radhakrishnan J, D’Agati V, Markowitz G: Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol 31: 2158–2167, 2020. 10.1681/ASN.2020050744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, Canetta P, Ratner LE, Marasa M, Gharavi AG, Stokes MB, Markowitz GS, D’Agati VD: Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol 31: 1959–1968, 2020. 10.1681/ASN.2020060802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, Khanin Y, Madireddy V, Larsen CP, Jhaveri KD, Bijol V; Northwell Nephrology COVID-19 Research Consortium : COVID-19-associated kidney injury: A case series of kidney biopsy findings. J Am Soc Nephrol 31: 1948–1958, 2020. 10.1681/ASN.2020050699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golmai P, Larsen CP, DeVita MV, Wahl SJ, Weins A, Rennke HG, Bijol V, Rosenstock JL: Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol 31: 1944–1947, 2020. 10.1681/ASN.2020050683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan S, Chen L, Yang C-R, Raghuram V, Khundmiri SJ, Knepper MA: Does SARS-CoV-2 infect the kidney? J Am Soc Nephrol 31: 2746–2748, 2020. 10.1681/ASN.2020081229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George S, Pal AC, Gagnon J, Timalsina S, Singh P, Vydyam P, Munshi M, Chiu JE, Renard I, Harden CA, Ott IM, Watkins AE, Vogels CBF, Lu P, Tokuyama M, Venkataraman A, Casanovas-Massana A, Wyllie AL, Rao V, Campbell M, Farhadian SF, Grubaugh ND, Dela Cruz CS, Ko AI, Berna Perez AZ, Akaho EH, Moledina DG, Testani J, John AR, Ledizet M, Mamoun CB; Yale IMPACT Team : Evidence for SARS-CoV-2 spike protein in the urine of COVID-19 patients. Kidney360 2: 922–934, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su H, Yang M, Wan C, Yi L-X, Tang F, Zhu H-Y, Yi F, Yang H-C, Fogo AB, Nie X, Zhang C: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020. 10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB: Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383: 590–592, 2020. 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mumm J-N, Osterman A, Ruzicka M, Stihl C, Vilsmaier T, Munker D, Khatamzas E, Giessen-Jung C, Stief C, Staehler M, Rodler S: Urinary frequency as a possibly overlooked symptom in COVID-19 patients: Does SARS-CoV-2 cause viral cystitis? Eur Urol 78: 624–628, 2020. 10.1016/j.eururo.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamb LE, Dhar N, Timar R, Wills M, Dhar S, Chancellor MB: COVID-19 inflammation results in urine cytokine elevation and causes COVID-19 associated cystitis (CAC). Med Hypotheses 145: 110375, 2020. 10.1016/j.mehy.2020.110375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin W, Fan J, Hu L-F, Zhang Y, Ooi JD, Meng T, Jin P, Ding X, Peng L-K, Song L, Tang R, Xiao Z, Ao X, Xiao X-C, Zhou Q-L, Xiao P, Zhong Y: Single-cell analysis of angiotensin-converting enzyme II expression in human kidneys and bladders reveals a potential route of 2019 novel coronavirus infection. Chin Med J (Engl) 134: 935–943, 2021. 10.1097/CM9.0000000000001439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhar N, Dhar S, Timar R, Lucas S, Lamb LE, Chancellor MB: De novo urinary symptoms associated with COVID-19: COVID-19-associated cystitis. J Clin Med Res 12: 681–682, 2020. 10.14740/jocmr4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivero J, Merino-López M, Olmedo R, Garrido-Roldan R, Moguel B, Rojas G, Chavez-Morales A, Alvarez-Maldonado P, Duarte-Molina P, Castaño-Guerra R, Ruiz-Lopez IK, Soria-Castro E, Luna C, Bonilla-Méndez A, Baranda F, Zabal C, Madero M, Valdez-Ortiz R, Soto-Abraham MV, Vazquez-Rangel A: Association between postmortem kidney biopsy findings and acute kidney injury from patients with SARS-CoV-2 (COVID-19). Clin J Am Soc Nephrol 16: 685–693, 2021. 10.2215/CJN.16281020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon R, Otto EA, Sealfon R, Nair V, Wong AK, Theesfeld CL, Chen X, Wang Y, Boppana AS, Luo J, Yang Y, Kasson PM, Schaub JA, Berthier CC, Eddy S, Lienczewski CC, Godfrey B, Dagenais SL, Sohaney R, Hartman J, Fermin D, Subramanian L, Looker HC, Harder JL, Mariani LH, Hodgin JB, Sexton JZ, Wobus CE, Naik AS, Nelson RG, Troyanskaya OG, Kretzler M: SARS-CoV-2 receptor networks in diabetic and COVID-19-associated kidney disease. Kidney Int 98: 1502–1518, 2020. 10.1016/j.kint.2020.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abedini A, Zhu YO, Chatterjee S, Halasz G, Devalaraja-Narashimha K, Shrestha R, S. Balzer M, Park J, Zhou T, Ma Z, Sullivan KM, Hu H, Sheng X, Liu H, Wei Y, Boustany-Kari CM, Patel U, Almaani S, Palmer M, Townsend R, Blady S, Hogan J, Morton L, Susztak K; TRIDENT Study Investigators : Urinary single-cell profiling captures the cellular diversity of the kidney. J Am Soc Nephrol 32: 614–627, 2021. 10.1681/ASN.2020050757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arazi A, Rao DA, Berthier CC, Davidson A, Liu Y, Hoover PJ, Chicoine A, Eisenhaure TM, Jonsson AH, Li S, Lieb DJ, Zhang F, Slowikowski K, Browne EP, Noma A, Sutherby D, Steelman S, Smilek DE, Tosta P, Apruzzese W, Massarotti E, Dall’Era M, Park M, Kamen DL, Furie RA, Payan-Schober F, Pendergraft WF 3rd, McInnis EA, Buyon JP, Petri MA, Putterman C, Kalunian KC, Woodle ES, Lederer JA, Hildeman DA, Nusbaum C, Raychaudhuri S, Kretzler M, Anolik JH, Brenner MB, Wofsy D, Hacohen N, Diamond B; Accelerating Medicines Partnership in SLE network : The immune cell landscape in kidneys of patients with lupus nephritis [published erratum appears in Nat Immunol 20: 1404, 2019]. Nat Immunol 20: 902–914, 2019. 10.1038/s41590-019-0398-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speranza E, Williamson BN, Feldmann F, Sturdevant GL, Pérez-Pérez L, Meade-White K, Smith BJ, Lovaglio J, Martens C, Munster VJ, Okumura A, Shaia C, Feldmann H, Best SM, de Wit E: Single-cell RNA sequencing reveals SARS-CoV-2 infection dynamics in lungs of African green monkeys. Sci Transl Med 13: eabe8146, 2021. 10.1126/scitranslmed.abe8146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R: Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 36: 411–420, 2018. 10.1038/nbt.4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, Hao Y, Stoeckius M, Smibert P, Satija R: Comprehensive integration of single-cell data. Cell 177: 1888–1902.e21, 2019. 10.1016/j.cell.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, Baglaenko Y, Brenner M, Loh PR, Raychaudhuri S: Fast, sensitive and accurate integration of single-cell data with Harmony. Nat Methods 16: 1289–1296, 2019. 10.1038/s41592-019-0619-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Z, Liao J, Chen Y, Zou C, Zhang H, Cheng J, Liu D, Li T, Zhang Q, Li J, Yang X, Ye Y, Huang Z, Long X, Yang R, Mo Z: Single-cell transcriptomic map of the human and mouse bladders. J Am Soc Nephrol 30: 2159–2176, 2019. 10.1681/ASN.2019040335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu H, Malone AF, Donnelly EL, Kirita Y, Uchimura K, Ramakrishnan SM, Gaut JP, Humphreys BD: Single-cell transcriptomics of a human kidney allograft biopsy specimen defines a diverse inflammatory response. J Am Soc Nephrol 29: 2069–2080, 2018. 10.1681/ASN.2018020125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borcherding N, Vishwakarma A, Voigt AP, Bellizzi A, Kaplan J, Nepple K, Salem AK, Jenkins RW, Zakharia Y, Zhang W: Mapping the immune environment in clear cell renal carcinoma by single-cell genomics. Commun Biol 4: 122, 2021. 10.1038/s42003-020-01625-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Vasaikar S, Shi Z, Greer M, Zhang B: WebGestalt 2017: A more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res 45[W1]: W130–W137, 2017. 10.1093/nar/gkx356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada T, Mikami T, Chopra N, Miyashita H, Chernyavsky S, Miyashita S: Patients with chronic kidney disease have a poorer prognosis of coronavirus disease 2019 (COVID-19): An experience in New York City. Int Urol Nephrol 52: 1405–1406, 2020. 10.1007/s11255-020-02494-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henry BM, Lippi G: Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol 52: 1193–1194, 2020. 10.1007/s11255-020-02451-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chevalier RL: The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomerulotubular junction. Am J Physiol Renal Physiol 311: F145–F161, 2016. 10.1152/ajprenal.00164.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takaori K, Nakamura J, Yamamoto S, Nakata H, Sato Y, Takase M, Nameta M, Yamamoto T, Economides AN, Kohno K, Haga H, Sharma K, Yanagita M: Severity and frequency of proximal tubule injury determines renal prognosis. J Am Soc Nephrol 27: 2393–2406, 2016. 10.1681/ASN.2015060647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raines NH, Cheung MD, Wilson LS, Edberg JC, Erdmann NB, Schmaier AA, Berryhill TF, Manickas-Hill Z, Li JZ, Yu XG, Agarwal A, Barnes S, Parikh SM: NAD+ biosynthetic impairment and urinary metabolomic alterations observed in hospitalized adults with COVID-19–related acute kidney injury [published online ahead of print September 14, 2021]. Kidney Int Rep 10.1016/j.ekir.2021.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim D, Lee J-Y, Yang J-S, Kim JW, Kim VN, Chang H: The architecture of SARS-CoV-2 transcriptome. Cell 181: 914–921.e10, 2020. 10.1016/j.cell.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Heterogeneity of cells captured from each patient sample. Download Supplemental Figure 1, PDF file, 828 KB (827.4KB, pdf)
Gene Set Enrichment Analysis of hallmark pathways in both AKI and no AKI control clusters. Download Supplemental Figure 2, PDF file, 828 KB (827.4KB, pdf)
Heat map of differentially expressed genes from cells from patient 7. Download Supplemental Figure 3, PDF file, 828 KB (827.4KB, pdf)