Key Points
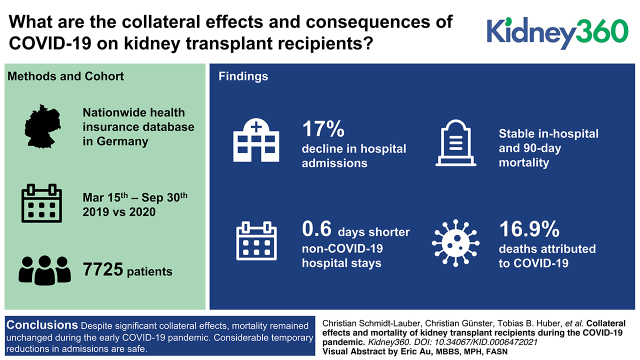
Despite significant collateral effects on kidney transplant recipients during the early COVID-19 pandemic, mortality remained unchanged.
Considerable temporary reductions in admissions are safe, whereas reducing immunosuppression results in increased allograft rejection risk.
Keywords: transplantation, acute allograft rejection, clinical epidemiology, COVID-19, epidemiology and outcomes, immunosuppression, kidney transplantation, mortality, transplant recipients, transplantation
Visual Abstract
Abstract
Background
Collateral effects and consequences of the coronavirus disease 19 (COVID-19) pandemic on kidney transplant recipients remain widely unknown.
Methods
This retrospective cohort study examined changes in admission rates, incidences of diseases leading to hospitalization, in-patient procedures, and maintenance medication in long-term kidney transplant recipients with functioning graft during the early COVID-19 pandemic in Germany. Data were derived from a nationwide health insurance database. Analysis was performed from March 15 to September 30 and compared the years 2019 and 2020. Effects on mortality and adverse allograft events were compared with COVID-19-attributed effects.
Results
A total of 7725 patients were included in the final analysis. Admissions declined in 2020 by 17%, with the main dip during a 3-month lockdown (–31%) but without a subsequent rebound. Incidences for hospitalization did not increase for any investigated disease entities, whereas decreasing trends were noted for non-COVID-19 pulmonary and urogenital infections (incidence rate ratio 0.8, 95% CI, 0.62 to 1.03, and 0.82, 95% CI, 0.65 to 1.04, respectively). Non-COVID-19 hospital stays were 0.6 days shorter (P=0.03) and not complicated by increased dialysis, ventilation, or intensive care treatment rates. In-hospital and 90-day mortality remained stable. Incidences of severe COVID-19 requiring hospitalization was 0.09 per 1000 patient-days, and in-hospital mortality was 9%. A third (31%) of patients with calcineurin-inhibitor medication and without being hospitalized for COVID-19 reduced doses by at least 25%, which was associated with an increased allograft rejection risk (adjusted hazard ratio 1.29, 95% CI, 1.02 to 1.63). COVID-19 caused 17% of all deaths but had no significant association with allograft rejections. All-cause mortality remained stable (incidence rate ratio 1.15, 95% CI, 0.91 to 1.46), also when restricting analysis to patients with no or outpatient-treated COVID-19 (0.97, 95% CI, 0.76 to 1.25).
Conclusion
Despite significant collateral effects, mortality remained unchanged during the early COVID-19 pandemic. Considerable temporary reductions in admissions are safe, whereas reducing immunosuppression results in increased allograft rejection risk.
Introduction
Since emerging at the beginning of 2020, coronavirus disease 19 (COVID-19) has led to an ongoing pandemic that has dramatically affected medical care around the globe (1). Due to the contagious nature of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), millions of infections with many deaths have occurred. This has led to adaptations of the lives of both individuals and society to reduce viral transmission and to prevent severe disease courses (2,3). Both COVID-19 and these adaptations have dramatically influenced medical care. Studies on the general population have shown that hospital admissions for non-COVID-19 disease entities and the redemption of maintenance medication decreased considerably during the pandemic (4–7). Further, hospital admissions showed longer symptom-to-door time and reduced procedure rates (8). Data indicate an excess mortality rate at the population level during the pandemic, which is attributed not only to COVID-19 but also to non-COVID-19-related deaths, arguing for fatal collateral effects (9).
Kidney transplant recipients seem to be especially vulnerable, with an increased COVID-19-related mortality over the general population (10). Therefore, many transplant centers have developed protocols to protect kidney transplant recipients or candidates by pausing living transplantation programs, reducing medical contacts with infection risk, or postponing elective procedures (11,12). Additionally, ideas were raised to reduce immunosuppression in patients with COVID-19 risk or contact (13). Given the high burden of comorbidity in kidney transplant recipients, these ideas and adaptions might also cause harm. The drastic reduction of admissions for cardiovascular diseases as observed in the general population raises particular concern, considering the high cardiovascular risk of kidney transplant recipients (14,15). However, the collateral effects of the COVID-19 pandemic on kidney transplant recipients remain unknown. A comparison of collateral and COVID-19-related consequences is crucial to balance transmission protection and to maintain specific medical care. This applies both for future pandemics and for potential future COVID-19 waves. This study investigates the hypothesis of relevant collateral changes in the medical care of long-term kidney transplant recipients during the COVID-19 pandemic. The effect of these changes is compared with SARS-CoV-2-related effects.
Materials and Methods
Study Design and Data Collection
This retrospective cohort study is composed of anonymized nationwide administrative claim data of the German Local Health Care Funds (Allgemeine Ortskrankenkassen [AOK]). AOK is the largest sickness fund within Germany, providing insurance for 32% of the national population (16). Membership is open to the entire population without conditions of working status, income, age, or comorbidities (17). Although individuals insured with AOK constitute a significant proportion of the overall German population, they probably have a slightly lower general health status (18). As per the law, data on inpatient treatments, including dates, diagnoses, and procedures, must be disclosed to sickness funds. Outpatient treatments and prescriptions must be reported to apply for reimbursement. Beyond claims, core data consist of age, sex, insurance status, and day of death. Diagnoses are coded according to the 10th revision of the International Classification of Diseases (ICD-10) German Modification, and procedures are coded according to the International Classification of Procedures in Medicine, the “Operationen- und Prozedurenschlüssel” (OPS). Reimbursable outpatient treatments are defined by the “Einheitlicher Bewertungsmassstab” (EBM), and outpatient prescription by the World Health Organization ATC/DDD Index 2019/2020 (German modification). The local ethics committee of the Ärztekammer Hamburg approved this study (WF-022/21).
Study Population
The study population comprised adult long-term kidney transplant recipients with functioning graft. This population was chosen to investigate patients in a stable post-transplant situation without natural fluctuations in medical care. Thus, patients aged ≥18 years on January 1, 2017, who received kidney transplantation after 1998 and at least 1 year before the study period were included (OPS 5–555 and ICD Z94.0 after January 1, 2017). Patients with graft loss and re-dialysis dependence defined as dialysis treatment within 6 months before the study period were excluded (OPS 8–853, 8–854, 8–855, 8–857, EBM 40823–40828 or surcharge 13602, 13610, 13611). To ensure adequate comparison and a stable post-transplant situation, these criteria were dynamically applied before the investigated periods in 2019 and 2020, respectively. Patients not continuously insured with the AOK since January 1, 2017, were excluded to assure data integrity.
Study Period and COVID-19 Incidences
The study period started on March 15, 2019 and terminated on September 30, 2020. The first SARS-CoV-2 infection in Germany was reported on February 27, 2020, followed by rapidly increasing COVID-19 incidences (19). In mid-March, the German Federal and State Governments released guidelines restricting social contacts, which led to a nationwide lockdown (20,21). Hospitals were urged to keep capacity for the care of COVID-19 patients, postponing elective procedures (21). Due to decreasing incidence, social and medical restrictions were gradually relaxed during June, followed by a period of low incidences until late September (19). Thus, we investigated the period from March 15, 2020, onward in comparison to the preceding year and further divided this period into two phases: the lockdown phase from March 15 to June 15 and the post-lockdown phase from June 16 to September 30.
Outcome Measures
For baseline description, we report age, sex, and comorbidities assessed by a combination of outpatient diagnoses and prescriptions in the preceding calendar year (22). Hospital stays were only counted if the admission and discharge dates were within the study period. As patients might have had several hospitals stays due to transfer between hospitals, we counted patients with adjacent completed hospital stays as one incidence. Incidences of selected diseases leading to hospitalization were assessed using ICD-10 principal diagnoses codes (Supplemental Table 1). For in-hospital treatments, we show length of hospital stay, procedures according to OPS codes (Supplemental Table 1), ventilation days, in-hospital mortality, and mortality within 90 days after admission. Changes in maintenance medication as assessed by ATC codes of redeemed prescriptions (Supplemental Table 1) were investigated by prescription rates and dose changes. Prescription was analyzed by comparing rates of patients with a corresponding redemption, and dose alterations were calculated by changes in defined daily doses (DDD) among patients with at least one prescription of the investigated drug. Further, we present all-cause mortality rates over the study period.
Statistical Analyses
Counts and percentages are reported for categorical variables and means and SD, and medians and interquartile ranges (IQR) are reported for continuous variables. Hospital admissions were analyzed by total admissions per week. Further, we report rates for hospital admissions, redeemed outpatient medications and all-cause mortality. Incidences of selected incident diseases entities leading to hospitalization and hospitalized COVID-19 are presented as incidence rate ratios (IRR). For the analysis of trends between 2019 and 2020, rate ratios with 95% confidence intervals (95% CI) of hospital admissions, redeemed outpatient medications, and all-cause mortality and IRR with 95% CI of the investigated disease entities leading to hospitalization were compared using Poisson regression models. Robust sandwich-variance estimator according to Huber and White was applied to account for potential overdispersion.
Characteristics of hospital stays between the investigated periods and characteristics of patients with or without COVID-19 were compared using a univariate chi-squared test for categorical variables and corrected using Fisher’s exact test for numbers less than five. The t test was used for normally distributed continuous variables, and the Mann–Whitney U test was used for non-normally distributed continuous variables. Cox regression models were used to calculate unadjusted and adjusted hazard ratios for allograft rejections of patients with a 25% reduction in steroids, calcineurin inhibitors (CNI), or both. Analyses were performed using Stata v16.0 (StataCorp, College Station, TX).
Results
Baseline Characteristics and Admission Rates
We identified 9739 patients who received a kidney transplantation at least 12 months before the study period. We excluded 1941 patients due to dialysis within the preceding 6 months, and 73 due to incomplete data (Supplemental Figure 1). A total of 7725 patients were investigated. The study population consisted of 38% women and had a mean age of 56.1 years (Table 1). Most frequent comorbidities were hypertension (85%), coronary artery disease (24%), diabetes (20%), and obesity (19%). Between the investigated periods of 2019 and 2020, 883 patients died or lost graft function, and 661 patients were additionally included because they received transplantation at least 12 months before.
Table 1.
Baseline characteristics of the study population
| Characteristics | Study Population (N=7725) |
|---|---|
| Sex, n (%) | |
| Men | 4805 (62) |
| Women | 2920 (38) |
| Age, yr | |
| Mean (SD) | 56.1 (13.5) |
| Median (IQR) | 57.1 (48–66) |
| Comorbidities, n (%) | |
| Hypertension | 6561 (85) |
| Coronary artery disease | 1846 (24) |
| Diabetes | 1520 (20) |
| Obesity | 1448 (19) |
| Congestive heart failure | 1283 (17) |
| Chronic liver disease | 1156 (15) |
| Chronic obstructive pulmonary disease | 648 (8) |
| Asthma | 478 (6) |
| Cancer | 245 (3) |
| Dementia | 112 (1) |
IQR, interquartile range.
From March 15 to September 30, 2020, there were 3063 admissions within 1,455,137 patient-days compared with 3800 admissions within 1,494,353 patient-days in the corresponding period in 2019 (rate ratio 0.83, 95% CI, 0.77 to 0.89; Figure 1). Investigation of total weekly admissions showed that hospitalizations decreased during the first 12 weeks of observation and equalized over the remaining period. A general trend toward declining admissions in the summer months was also noted. The distribution corresponded well to the defined subphases and translated into a rate ratio for admissions of 0.69 (95% CI, 0.63 to 0.75) in the lockdown and 0.97 (95% CI, 0.90 to 1.05) in the post-lockdown phase compared with 2019 (Figure 1).
Figure 1.
Total weekly admissions and admission rates in 2019 and 2020. The lockdown phase is from March 15 to June 15 (weeks 1–14), and the post-lockdown phase is from June 16 to September 30 (weeks 15–27). CI, confidence interval.
Characteristics of Hospitalizations
Characteristics of hospital stays were investigated for all non-COVID-19 admissions to analyze collateral effects (Table 2). Length of hospital stay was slightly shorter during the pandemic than it was in the preceding year (–0.6 days, P=0.03). There was no difference in dialysis (5.3% in 2019 versus 6.1% in 2020), ventilation (1.3% versus 1.3% for noninvasive and 1.8% versus 1.7% for invasive ventilation), or intensive care treatments (1.1% versus 0.9%). This equally applied for the lockdown and post-lockdown phases. In-hospital (2.2% versus 2.7%) and 90-day mortality (5.0% versus 5.6%) also remained unchanged during the pandemic.
Table 2.
Characteristics of hospital stays in 2019 and 2020 restricted non-COVID-19 admissions
| Characteristics | Total | Lockdown | Post-Lockdown | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2019 (N=3800) | 2020 Non-COVID-19 (N=2922) | P Value | 2019 (N=1875) | 2020 Non-COVID-19 (N=1174) | P Value | 2019 (N=1925) | 2020 Non-COVID-19 (N=1748) | P Value | |
| Length of hospital stay, d | |||||||||
| Mean (SD) | 9.7 (12.4) | 9.1 (10.8) | 0.03 | 10.5 (13.9) | 10.1 (12.3) | 0.39 | 8.9 (10.6) | 8.4 (9.6) | 0.14 |
| Median (IQR) | 6 (3–11) | 6 (3–10) | 7 (3–11) | 6 (3–11) | 6 (3–10) | 6 (3–10) | |||
| Procedures, n (%) | |||||||||
| Intensive care unit | 42 (1) | 25 (0.9) | 0.31 | 18 (1) | 10 (0.9) | 0.76 | 24 (2) | 15 (0.9) | 0.25 |
| Dialysis | 201 (5) | 179 (6) | 0.14 | 100 (5) | 78 (7) | 0.13 | 101 (5) | 101 (6) | 0.48 |
| Invasive ventilation | 69 (2) | 51 (2) | 0.83 | 40 (2) | 27 (2) | 0.76 | 29 (2) | 24 (1) | 0.74 |
| Noninvasive ventilation | 51 (1) | 37 (1) | 0.79 | 34 (2) | 16 (1) | 0.34 | 17 (1) | 21 (1) | 0.34 |
| Tracheostomy | 12 (0.3) | 7 (0.2) | 0.56 | 9 (0.5) | 3 (0.3) | 0.39 | 3 (0.2) | 4 (0.2) | 0.72 |
| Extracorporal membrane oxygenation | 2 (0.1) | 1 (0.0) | 1.00 | 1 (0.1) | 1 (0.1) | 1.00 | 1 (0.1) | 0 (0.0) | 1.00 |
| Ventilation, d | |||||||||
| Mean (SD) | 1.5 (1.1) | 1.4 (0.8) | 0.43 | 1.6 (1.3) | 1.3 (0.5) | 0.21 | 1.4 (0.8) | 1.5 (0.9) | 0.68 |
| Median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–1) | 1 (1–2) | 1 (1–2) | |||
| Deaths, n (%) | |||||||||
| In-hospital mortality | 84 (2) | 79 (3) | 0.19 | 44 (2) | 36 (3) | 0.23 | 40 (2) | 43 (3) | 0.44 |
| 90-day mortality | 183 (5) | 164 (6) | 0.14 | 85 (5) | 73 (6) | 0.04 | 98 (5) | 91 (5) | 0.88 |
Total refers to the period from March 15 to September 30, whereas the lockdown phase is from March 15 to June 15 and the post-lockdown phase is from June 16 to September 30. IQR, interquartile range; COVID-19, coronavirus disease 2019.
Hospitalizations with COVID-19 had a mean length of 15.8 days. In-hospital rates of invasive ventilation and dialysis were each 11% (Supplemental Figure 2). In-hospital and 90-day mortality for admitted COVID-19 patients was 9% and 12%, respectively.
Incidences of Disease Entities Leading to Hospitalization
Hospitalizations for non-COVID-19 infectious diseases, such as pulmonary (IRR 0.8, 95% CI, 0.62 to 1.03) and urogenital infections (IRR 0.82, 95% CI, 0.65 to 1.04), showed a decreasing trend during the pandemic (Table 3). For cardiovascular diseases and allograft rejections, this trend was restricted to the lockdown phase, whereas overall incidence rates remained virtually constant (IRR 0.91, 95% CI, 0.75 to 1.12 for cardiovascular diseases: IRR 0.95, 95% CI, 0.82 to 1.10 for allograft rejections). None of the diseases showed an increase after the lockdown period. The IRR of hospitalized COVID-19 was 0.09 per 1000 patient-days over the total period (129 patients) and 0.12 in the lockdown phase (85 patients). In 16 patients, COVID-19 and allograft rejections occurred within the same hospital stay, but there was no patient where a hospitalized COVID-19 disease preceded an admission for an allograft rejection.
Table 3.
Incidence rates and incidence rate ratios of disease entities leading to hospitalization in 2019 and 2020
| Principal Diagnoses | Total | Lockdown | Post-Lockdown | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence Rate 2019 | Incidence Rate 2020 | Incidence Rate Ratio (95% Confidence Interval) | P Value | Incidence Rate 2019 | Incidence Rate 2020 | Incidence Rate Ratio (95% Confidence Interval) | P Value | Incidence Rate 2019 | Incidence Rate 2020 | Incidence Rate Ratio (95% Confidence Interval) | P Value | |
| Allograft rejections | 0.25 | 0.24 | 0.95 (0.82 to 1.10) | 0.51 | 0.29 | 0.24 | 0.84 (0.69 to 1.04) | 0.11 | 0.28 | 0.28 | 1.00 (0.83 to 1.21) | 0.97 |
| Cardiovascular diseases | 0.14 | 0.12 | 0.91 (0.75 to 1.12) | 0.38 | 0.17 | 0.15 | 0.89 (0.68 to 1.16) | 0.39 | 0.13 | 0.12 | 0.97 (0.73 to 1.28) | 0.83 |
| Urogenital infections | 0.11 | 0.09 | 0.82 (0.65 to 1.04) | 0.10 | 0.11 | 0.08 | 0.78 (0.55 to 1.10) | 0.16 | 0.11 | 0.10 | 0.92 (0.68 to 1.24) | 0.57 |
| Pulmonary infections | 0.09 | 0.07 | 0.80 (0.62 to 1.03) | 0.08 | 0.12 | 0.10 | 0.83 (0.60 to 1.14) | 0.24 | 0.07 | 0.05 | 0.74 (0.49 to 1.12) | 0.15 |
| Sepsis | 0.07 | 0.05 | a | 0.07 | 0.05 | a | 0.07 | 0.06 | a | |||
Incidence rates are given per 1000 patient-days. Total refers to the period from March 15 to September 30, whereas the lockdown phase is from March 15 to June 15 and the post-lockdown phase is from June 16 to September 30.
Criteria for sepsis coding changed between 2019 and 2020 impeding direct comparison.
Changes in Maintenance Medication
For the evaluation of collateral effects on maintenance medication, drug analyses were restricted to patients without severe and hospitalized COVID-19. Rates of redeemed prescriptions did not decrease for any of the investigated cardiovascular or immunosuppressive drugs during the pandemic. The rate of patients with statin and belatacept therapy slightly increased in 2020 (IRR 1.05, 95% CI, 1.02 to 1.08 for statins; IRR 1.39, 95% CI, 1.07 to 1.81 for belatacept; Table 4).
Table 4.
Changes in redeemed prescription of maintenance medication for patients without hospitalized COVID-19
| Medication | 2019 | 2020 Non-COVID-19 | Rate Ratio (95% Confidence Interval) | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Patients (N=7725), n (%) | Rate per 1000 Patient-days | Defined Daily Doses, Mean (SD) | Patients (N=7383), n (%) | Rate per 1000 Patient-days | DDD, Mean (SD) | |||
| Cardiovascular drugs | ||||||||
| Statin | 3965 (51) | 2.65 | 4043 (54) | 2.78 | 1.05 (1.02 to 1.08) | 0.003 | ||
| 145.4 (106.8) | 148.7 (112.6) | 0.3 | ||||||
| Calcium antagonist | 3426 (44) | 2.29 | 3311 (44) | 2.28 | 0.99 (0.96 to 1.03) | 0.71 | ||
| 219.1 (115.7) | 215.5 (115.2) | 0.13 | ||||||
| ACE-I | 2284 (30) | 1.53 | 2108 (28) | 1.45 | 0.95 (0.90 to 1.00) | 0.29 | ||
| 328.7 (224.8) | 333.1 (231.0) | 0.48 | ||||||
| ARB | 2267 (29) | 1.52 | 2278 (30) | 1.57 | 1.03 (0.99 to 1.09) | 0.18 | ||
| 261.7 (199.8) | 258.6 (192.6) | 0.84 | ||||||
| Antiplatelet agent | 1747 (23) | 1.17 | 1689 (23) | 1.15 | 0.99 (0.93 to 1.05) | 0.66 | ||
| 148.7 (73.9) | 147.8 (70.6) | 0.87 | ||||||
| Immunosuppressive drugs | ||||||||
| Belatacept | 94 (1) | 0.06 | 127 (2) | 0.09 | 1.36 (1.04 to 1.78) | 0.02 | ||
| 174.3 (62.7) | 179.6 (60.1) | 0.88 | ||||||
| Tacrolimus | 5271 (68) | 3.53 | 5147 (69) | 3.54 | 1.00 (0.98 to 1.02) | 0.84 | ||
| 99.6 (72.2) | 94.8 (69.9) | <0.001 | ||||||
| Cyclosporine | 1569 (20) | 1.05 | 1473 (20) | 1.01 | 0.96 (0.90 to 1.02) | 0.21 | ||
| 83.8 (42.1) | 79.9 (38.9) | 0.02 | ||||||
| Mycophenolate | 5713 (74) | 3.82 | 5480 (73) | 3.77 | 0.99 (0.97 to 1.00) | 0.15 | ||
| 85.6 (44.6) | 85.8 (45.9) | 0.21 | ||||||
| Azathioprine | 269 (4) | 0.18 | 249 (3) | 0.17 | 0.95 (0.80 to 1.13) | 0.54 | ||
| 83.1 (61.0) | 80.8 (45.4) | 0.49 | ||||||
| Everolimus | 401 (5) | 0.27 | 426 (6) | 0.29 | 1.09 (0.95 to 1.24) | 0.23 | ||
| 210.7 (122.7) | 201.9 (134.0) | 0.17 | ||||||
| Sirolimus | 269 (4) | 0.18 | 245 (3) | 0.17 | 0.93 (0.79 to 1.11) | 0.43 | ||
| 74.2 (50.3) | 71.0 (54.4) | 0.15 | ||||||
| Steroids | 4797 (62) | 3.21 | 4585 (61) | 3.14 | 0.98 (0.96 to 1.01) | 0.07 | ||
| 79.4 (46.3) | 77.0 (42.6) | <0.001 | ||||||
Percentages of total numbers refer to all patients without hospitalized COVID-19. Doses are presented in relation to patients with at least one prescription of the investigated drug. Data refers to the period from March 15 to September 30. ACE-I, angiotensin-converting-enzyme inhibitors; ARB, angiotensin II receptor blockers; COVID-19, coronavirus disease 2019.
Analysis of drug dosing showed a prevailing reduction of immunosuppressive drugs, whereas doses of cardiovascular drugs remained stable (Table 4). Dose reduction was most distinctive for CNI and steroids. DDD of tacrolimus and cyclosporine were each reduced by 5% (–4.8 DDD, P=0.001 and –3.9 DDD, P=0.02, respectively) and DDD of steroids by 3% (–2.4 DDD, P<0.001). These declines were driven by a considerable proportion of patients reducing steroids (25% of patients with at least one prescription) or CNI (31%) by at least 25%. These patients had an increased allograft rejection risk compared with patients with <25% dose reduction (hazard ratio [HR] 1.31, 95% CI, 1.02 to 1.67 for reduction of steroids; HR 1.37, 95% CI, 1.09 to 1.63 for CNI; and HR 1.40, 95% CI, 1.11 to 1.75 for both; Figure 2). Even after adjustment for age, time since transplantation, allograft rejection in the preceding year, and the not-investigated immunosuppressive, reductions of CNI (HR 1.29, 95% CI, 1.02 to 1.63) and steroids and CNI (HR 1.32, 95% CI, 1.05 to 1.66) were significantly associated with an increased allograft rejection risk. For steroids after adjustment, this association only showed a nonsignificant trend (HR 1.21, 95% CI, 0.94 to 1.55).
Figure 2.
HR for allograft rejections of patients with a 25% reduction in steroids, CNI, or both. HR were calculated in comparison to patients with <25% dose reduction. Data refers to the period from March 15 to September 30. Adjustment was performed for age, time since transplantation, and rejection in the preceding 24 months. CNI, calcineurin inhibitor; HR, hazard ratio.
Mortality and COVID-19 Infections
In total, there were 132 deaths in 2019 and 142 in 2020. This translates into almost equal all-cause mortality rates for the total period (IRR 1.15, 95% CI, 0.91 to 1.46) and for the lockdown (IRR 1.11, 95% CI, 0.78 to 1.57) and post-lockdown phases (IRR 1.11, 95% CI, 0.80 to 1.53) compared with the previous year (Figure 3). When restricting analysis to patients with no or outpatient-treated mild SRAS-CoV-2 infections, mortality for all investigated periods showed a marginal decreasing trend but did not differ significantly from analysis including hospitalized COVID-19 patients or from the preceding year. Of the 142 deaths in 2020, 24 (17%) were accounted for by patients hospitalized with COVID-19. Patients with hospitalized SARS-CoV-2 infections were older and more likely to have diabetes, coronary artery disease, chronic liver disease, or chronic obstructive pulmonary disease (Supplemental Table 2). Steroid use in maintenance medication was also associated with being hospitalized with COVID-19, whereas mycophenolate therapy was less frequent among these patients (Supplemental Table 2).
Figure 3.
Rate ratios for total all-cause mortality in 2020 compared with 2019. Total refers to the period from March 15 to September 30, whereas the lockdown phase is from March 15 to June 15 and the post-lockdown phase from June 16 to September 30. Analyses were performed to all patients and patients without hospitalized COVID-19 (non-COVID-19). COVID-19, coronavirus disease 2019; RR, rate ratio.
Discussion
This study shows a 17% decline in hospital admissions without adverse consequences for long-term kidney transplant recipients having functioning grafts during the early COVID-19 pandemic in Germany. Significant changes in immunosuppressive medications were rare but were associated with an increased allograft rejection risk. All-cause mortality remained unchanged during the pandemic.
The observed decrease in admission rates corresponds to reported trends in the general population (5,6). This decrease mainly affected non-COVID-19-related infectious diseases, which might be attributed to better hygiene, mask wearing, and social distancing (23). Admissions for cardiovascular diseases and allograft rejections remained rather stable, reflecting the high risk of these diseases for kidney transplant recipients. However, the absence of a rebound for any of the investigated disease entities after the lockdown suggests no adverse consequences of the decline in admissions. This is further underlined by stable in-hospital complications, even during the lockdown phase. Given that most acute or subacute graft failures and dialysis treatments for kidney transplant recipients are treated as inpatients in Germany, our findings indicate that graft loss rates also remained stable during the pandemic. Together, a temporary decrease in admissions appears safe. This could also have implications for the general care of long-term kidney transplant recipients because a relevant number of admitted patients might be treated as outpatients.
In contrast to distinct effects on admissions, there were only subtle changes in maintenance medication. Despite debates on its influence on COVID-19 (24), there was no decline in the redemption of angiotensin-converting-enzyme inhibitors. Statins were used more frequently in 2020, which is likely due to stricter guidelines on lipid-lowering therapy (25). Although dose reductions were overall minor, the prevailing reduction of immunosuppressives compared with cardiovascular drugs argues for specific effects. As our analysis was restricted to patients who were not hospitalized with COVID-19 and to outpatients, COVID-19 treatment was generally rare in transplant recipients (26). This finding is likely caused by collateral effects. Although our study cannot identify the exact collateral reason of this observation, general caution during the pandemic, former COVID-19 contacts, and more relaxed medical care with fewer CNI-level controls might have contributed. The preference for changes in CNI dosing could be explained by the convenient possibility of adjusting target trough levels, and steroid reduction could be explained by the association of long-term steroid use with worse COVID-19 outcomes (26,27). Minor total effects on these drugs were due to a quarter of patients reducing doses by at least 25%. For CNI, this reduction resulted in an increased allograft rejection risk, even after adjusting for known risk factors. This finding is in line with the literature because adequate CNI target levels are crucial to prevent allograft rejections (28). In accordance with data showing no or only slightly increased rejection rates after late steroid withdrawal, the association of steroid reduction with allograft rejections showed a nonsignificant trend and had no additional effect on CNI reduction (29).
Despite emerging data, it remains unclear whether kidney transplant recipients have an increased risk for COVID-19 infections and adverse disease courses (30–32). Data are still sparse, especially regarding incidence rates. During the study period, Germany reported a general COVID-19 incidence of 0.35% (19) with a 17% hospitalization rate (33). Compared with our collective, this translates into a 28-fold increase in kidney transplant recipients being hospitalized with COVID-19 compared with the general population. A recent study investigated hospitalized COVID-19 courses on the general population of the same insurance database in an overlapping period, allowing direct comparison (34). In-hospital mortality in this study was 22% and thus nearly doubled compared with our collective. In contrast, dialysis rates were only 6%, translating into a two-fold higher risk in kidney transplant recipients. The low coincidence of allograft rejection and COVID-19 observed in our study argues against an immunologic reason for higher dialysis rates in kidney transplant recipients. In fact, the higher risk for severe AKI is probably caused by a predisposition to the different mechanisms of COVID-19-related kidney injury such as direct or indirect tubular damage (35). A lower threshold for admissions in kidney transplant recipients, as shown in recent reports, could explain the differences in mortality (26). Known risk factors for COVID-19 such as age, diabetes, chronic obstructive pulmonary disease, dementia, and cardiac and liver diseases also applied to our collective (36). Further, maintenance medication with steroids was associated with the risk of being hospitalized with COVID-19. This supports findings correlating long-term steroid use to worse COVID-19 outcomes (26,27). Whereas some studies identified high doses of mycophenolate as a risk factor for COVID-19 in solid organ transplant recipients, most found no correlation (26,37–39). In contrast, our study argues for a negative association of mycophenolate with COVID-19. This protective effect is supported by in vitro data showing inhibition of coronaviruses by mycophenolate (40). Ultimately, it might be an effect of lower doses being protective and higher doses posing a risk for COVID-19.
Although COVID-19 had a high mortality rate and accounted for 17% of all observed deaths in our collective, all-cause mortality remained stable. This also applied to patients without severe COVID-19 courses, indicating no collateral effects on mortality.
To the best of our knowledge, this is the first study evaluating collateral effects of the COVID-19 pandemic in comparison to COVID-19-related consequences on kidney transplant recipients. The study population constitutes one of the largest transplant collectives investigated during the pandemic and comprises about 30% of the estimated total number of kidney transplant recipients with a functioning graft in Germany (41,42). Regarding most patient characteristics, our collective is highly similar to other studies on long-term kidney transplant recipients in Germany and other countries (43,44). Despite its strengths, our study has several limitations. Our study investigates patients in a long-term situation after kidney transplantation during the early COVID-19 pandemic in Germany. It remains to be investigated whether observed effects also apply to patients without stable graft function or other countries with different COVID-19 incidences. Further, information on outpatient treatments was restricted to redeemed medication. Effects of mild and outpatient-treated COVID-19 could thus not be investigated. However, this bias is probably minor because reports showed that <10% of SARS-CoV-2-positive kidney transplant recipients were treated as outpatients (26). Data on laboratory results were also not available in our database. We thus cannot clarify whether more relaxed control of laboratory data, including CNI levels, contributed to the observed changes. Stockpiling at the beginning of the pandemic also cannot be excluded. Future retrospective studies including laboratory and outpatient data will help in part to overcome these limitations. Further, prospective studies during future waves are needed to clarify the nature of changes during the pandemic.
In conclusion, despite considerable changes in admission rates, collateral effects during the early COVID-19 pandemic had no influence on mortality or graft loss rates. Thus, a temporary drastic reduction of hospital admissions appears safe. However, reducing immunosuppressive maintenance medication poses a relevant risk for allograft rejections and should be restricted to special situations. COVID-19 mortality is high but did not significantly influence all-cause mortality.
Disclosures
T.B. Huber has consultancy agreements with Astrazeneca, Boehringer-Ingelheim, DaVita, Deerfield, Fresenius Medical Care, GoldfinchBio, MantraBio, Novartis, and Retrophin, and received research funding from Amicus and Fresenius Medical Care. All remaining authors have nothing to disclose.
Funding
F. Grahammer was supported by the DFG (CRC 1192 and GR 3933/1-1). T.B. Huber was supported by the DFG (CRC1192, HU 1016/8-2, HU 1016/11-1, and HU 1016/12-1), the BMBF (STOP-FSGS-01GM1901C and NephrESA-031L0191E), and DEFEAT PANDEMIcs (01KX2021) that is part of the National Network University Medicine (NUM).
Author Contributions
F. Grahammer, C. Günster, T. Huber, C. Schmidt-Lauber, and M. Spoden conceptualized the study and reviewed and edited the manuscript; F. Grahammer and C. Schmidt-Lauber were responsible for project administration; F. Grahammer and C. Günster supervised the study; F. Grahammer, C. Günster, and T. Huber were responsible for validation; C. Schmidt-Lauber and M. Spoden were responsible for data curation, formal analysis, and investigation; and C. Schmidt-Lauber was responsible for methodology, and wrote the original draft.
Data Sharing Statement
The data used in this study cannot be made available in the manuscript, the supplemental files, or in a public repository due to German data protection laws (Bundesdatenschutzgesetz). Therefore, they are stored on a secure drive in the Wissenschaftliches Institut der AOK to facilitate replication of the results. Generally, access to data of statutory health insurance funds for research purposes is possible only under the conditions defined in German Social Law (SGB V §287). Requests for data access can be sent as a formal proposal specifying the recipient and purpose of the data transfer to the appropriate data protection agency. Access to the data used in this study can only be provided to external parties under the conditions of the cooperation contract of this research project and after written approval by the AOK. For assistance in obtaining access to the data, please contact wido@wido.bv.aok.de.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0006472021/-/DCSupplemental.
Patient disposition. Download Supplemental Figure 1, PDF file, 345 KB (344.7KB, pdf)
Characteristics of hospitalized COVID-19 patients. Download Supplemental Figure 2, PDF file, 345 KB (344.7KB, pdf)
Definition of procedures, diagnoses, and drugs. Download Supplemental Table 1, PDF file, 345 KB (344.7KB, pdf)
Characteristics of patients with and without hospitalized COVID-19. Download Supplemental Table 2, PDF file, 345 KB (344.7KB, pdf)
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team : A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733, 2020. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Driscoll M, Ribeiro Dos Santos G, Wang L, Cummings DAT, Azman AS, Paireau J, Fontanet A, Cauchemez S, Salje H: Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 590: 140–145, 2021. 10.1038/s41586-020-2918-0 [DOI] [PubMed] [Google Scholar]
- 3.Honein MA, Christie A, Rose DA, Brooks JT, Meaney-Delman D, Cohn A, Sauber-Schatz EK, Walker A, McDonald LC, Liburd LC, Hall JE, Fry AM, Hall AJ, Gupta N, Kuhnert WL, Yoon PW, Gundlapalli AV, Beach MJ, Walke HT; CDC COVID-19 Response Team : Summary of guidance for public health strategies to address high levels of community transmission of SARS-CoV-2 and related deaths, December 2020. MMWR Morb Mortal Wkly Rep 69: 1860–1867, 2020. 10.15585/mmwr.mm6949e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeffery MM, D’Onofrio G, Paek H, Platts-Mills TF, Soares WE 3rd, Hoppe JA, Genes N, Nath B, Melnick ER: Trends in emergency department visits and hospital admissions in health care systems in 5 states in the first months of the COVID-19 pandemic in the US. JAMA Intern Med 180: 1328–1333, 2020. 10.1001/jamainternmed.2020.3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodilsen J, Nielsen PB, Søgaard M, Dalager-Pedersen M, Speiser LOZ, Yndigegn T, Nielsen H, Larsen TB, Skjøth F: Hospital admission and mortality rates for non-covid diseases in Denmark during covid-19 pandemic: Nationwide population based cohort study. BMJ 373: n1135, 2021. 10.1136/bmj.n1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapsner LA, Kampf MO, Seuchter SA, Gruendner J, Gulden C, Mate S, Mang JM, Schüttler C, Deppenwiese N, Krause L, Zöller D, Balig J, Fuchs T, Fischer P, Haverkamp C, Holderried M, Mayer G, Stenzhorn H, Stolnicu A, Storck M, Storf H, Zohner J, Kohlbacher O, Strzelczyk A, Schüttler J, Acker T, Boeker M, Kaisers UX, Kestler HA, Prokosch H-U: Reduced rate of inpatient hospital admissions in 18 German university hospitals during the COVID-19 lockdown. Front Public Health 8: 594117, 2021. 10.3389/fpubh.2020.594117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaduganathan M, van Meijgaard J, Mehra MR, Joseph J, O’Donnell CJ, Warraich HJ: Prescription fill patterns for commonly used drugs during the COVID-19 pandemic in the United States. JAMA 323: 2524–2526, 2020. 10.1001/jama.2020.9184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiss P, Carcel C, Hockham C, Peters SAE: The impact of the COVID-19 pandemic on the care and management of patients with acute cardiovascular disease: A systematic review. Eur Heart J Qual Care Clin Outcomes 7: 18–27, 2021. 10.1093/ehjqcco/qcaa084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woolf SH, Chapman DA, Sabo RT, Zimmerman EB: Excess deaths from COVID-19 and other causes in the US, March 1, 2020, to January 2, 2021. JAMA 325: 1786–1789, 2021. 10.1001/jama.2021.5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thaunat O, Legeai C, Anglicheau D, Couzi L, Blancho G, Hazzan M, Pastural M, Savoye E, Bayer F, Morelon E, Le Meur Y, Bastien O, Caillard S; French Nationwide Registry of Solid Organ Transplant Recipients with COVID-19 : IMPact of the COVID-19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT). Kidney Int 98: 1568–1577, 2020. 10.1016/j.kint.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alasfar S, Avery RK: The impact of COVID-19 on kidney transplantation. Nat Rev Nephrol 16: 568–569, 2020. 10.1038/s41581-020-00340-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aziz F, Jorgenson MR, Garg N, Mohamed M, Djamali A, Mandelbrot D, Parajuli S: The care of kidney transplant recipients during a global pandemic: Challenges and strategies for success. Transplant Rev (Orlando) 34: 100567, 2020. 10.1016/j.trre.2020.100567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kronbichler A, Gauckler P, Windpessl M, Il Shin J, Jha V, Rovin BH, Oberbauer R: COVID-19: Implications for immunosuppression in kidney disease and transplantation. Nat Rev Nephrol 16: 365–367, 2020. 10.1038/s41581-020-0305-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Filippo O, D’Ascenzo F, Angelini F, Bocchino PP, Conrotto F, Saglietto A, Secco GG, Campo G, Gallone G, Verardi R, Gaido L, Iannaccone M, Galvani M, Ugo F, Barbero U, Infantino V, Olivotti L, Mennuni M, Gili S, Infusino F, Vercellino M, Zucchetti O, Casella G, Giammaria M, Boccuzzi G, Tolomeo P, Doronzo B, Senatore G, Grosso Marra W, Rognoni A, Trabattoni D, Franchin L, Borin A, Bruno F, Galluzzo A, Gambino A, Nicolino A, Truffa Giachet A, Sardella G, Fedele F, Monticone S, Montefusco A, Omedè P, Pennone M, Patti G, Mancone M, De Ferrari GM: Reduced rate of hospital admissions for ACS during Covid-19 outbreak in Northern Italy. N Engl J Med 383: 88–89, 2020. 10.1056/NEJMc2009166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Primessnig U, Pieske BM, Sherif M: Increased mortality and worse cardiac outcome of acute myocardial infarction during the early COVID-19 pandemic. ESC Heart Fail 8: 333–343, 2021. 10.1002/ehf2.13075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bundesgesundheitsministerium : Gesetzliche Krankenversicherung Mitglieder, mitversicherte Angehörige und Krankenstand Monatswerte Januar–April, 2020. Available at: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Statistiken/GKV/Mitglieder_Versicherte/KM1_Januar_bis_April_2020.pdf. Accessed November 7, 2021
- 17.Busse R, Blümel M, Knieps F, Bärnighausen T: Statutory health insurance in Germany: A health system shaped by 135 years of solidarity, self-governance, and competition. Lancet 390: 882–897, 2017. 10.1016/S0140-6736(17)31280-1 [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann F, Koller D: [Different regions, differently insured populations? Socio-demographic and health-related differences between insurance funds]. Gesundheitswesen 79: e1–e9, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Robert Koch Institut : Täglicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19), 2021. Available at: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Okt_2020/2020-10-01-de.pdf?__blob=publicationFile. Accessed November 7, 2021
- 20.Osterloh F: Coronavirus: Krankenhäuser verschieben planbare Eingriffe. Dtsch Arztebl 117: A-575, 2020 [Google Scholar]
- 21.Die Bundesregierung : Pressemitteilung 96: Vereinbarung zwischen der Bundesregierung und den Regierungschefinnen und Regierungschefs der Bundesländer angesichts der Corona-Epidemie in Deutschland, 2020. Available at: https://www.bundesregierung.de/breg-de/suche/vereinbarung-zwischen-der-bundesregierung-und-den-regierungschefinnen-und-regierungschefs-der-bundeslaender-angesichts-der-corona-epidemie-in-deutschland-1730934. Accessed July 10, 2021
- 22.Schröder, H, Brückner, G, Schüssel, K, Breitkreuz, J, Schlotmann, A, Günster, C: Gesundheitliche Beeinträchtigungen - Vorerkrankungen mit erhöhtem Risiko für schwere Verläufe von COVID-19, 2020. Available at: https://www.wido.de/fileadmin/Dateien/Dokumente/News/wido_dat_correct_paper_covid-19_2020.pdf. Accessed April 15, 2020.
- 23.Chiu N-C, Chi H, Tai Y-L, Peng C-C, Tseng C-Y, Chen C-C, Tan BF, Lin C-Y: Impact of wearing masks, hand hygiene, and social distancing on influenza, enterovirus, and all-cause pneumonia during the coronavirus pandemic: Retrospective national epidemiological surveillance study. J Med Internet Res 22: e21257, 2020. 10.2196/21257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD: Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med 382: 1653–1659, 2020. 10.1056/NEJMsr2005760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O; ESC Scientific Document Group : 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J 41: 111–188, 2020. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 26.Elias M, Pievani D, Randoux C, Louis K, Denis B, Delion A, Le Goff O, Antoine C, Greze C, Pillebout E, Abboud I, Glotz D, Daugas E, Lefaucheur C: COVID-19 infection in kidney transplant recipients: Disease incidence and clinical outcomes. J Am Soc Nephrol 31: 2413–2423, 2020. 10.1681/ASN.2020050639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, Izadi Z, Jacobsohn L, Katz P, Lawson-Tovey S, Mateus EF, Rush S, Schmajuk G, Simard J, Strangfeld A, Trupin L, Wysham KD, Bhana S, Costello W, Grainger R, Hausmann JS, Liew JW, Sirotich E, Sufka P, Wallace ZS, Yazdany J, Machado PM, Robinson PC; COVID-19 Global Rheumatology Alliance : Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: Data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 79: 859–866, 2020. 10.1136/annrheumdis-2020-217871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaynor JJ, Ciancio G, Guerra G, Sageshima J, Roth D, Goldstein MJ, Chen L, Kupin W, Mattiazzi A, Tueros L, Flores S, Hanson L, Ruiz P, Vianna R, Burke GW 3rd: Lower tacrolimus trough levels are associated with subsequently higher acute rejection risk during the first 12 months after kidney transplantation. Transpl Int 29: 216–226, 2016. 10.1111/tri.12699 [DOI] [PubMed] [Google Scholar]
- 29.Opelz G, Döhler B, Laux G; Collaborative Transplant Study : Long-term prospective study of steroid withdrawal in kidney and heart transplant recipients. Am J Transplant 5: 720–728, 2005. 10.1111/j.1600-6143.2004.00765.x [DOI] [PubMed] [Google Scholar]
- 30.Rinaldi M, Bartoletti M, Bussini L, Pancaldi L, Pascale R, Comai G, Morelli M, Ravaioli M, Cescon M, Cristini F, Viale P, Giannella M: COVID-19 in solid organ transplant recipients: No difference in survival compared to general population. Transpl Infect Dis 23: e13421, 2021. 10.1111/tid.13421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molnar MZ, Bhalla A, Azhar A, Tsujita M, Talwar M, Balaraman V, Sodhi A, Kadaria D, Eason JD, Hayek SS, Coca SG, Shaefi S, Neyra JA, Gupta S, Leaf DE, Kovesdy CP; STOP-COVID Investigators : Outcomes of critically ill solid organ transplant patients with COVID-19 in the United States. Am J Transplant 20: 3061–3071, 2020. 10.1111/ajt.16280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craig-Schapiro R, Salinas T, Lubetzky M, Abel BT, Sultan S, Lee JR, Kapur S, Aull MJ, Dadhania DM: COVID-19 outcomes in patients waitlisted for kidney transplantation and kidney transplant recipients. Am J Transplant 21: 3061–3071, 2021. 10.1111/ajt.16351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert Koch Institut : Coronavirus disease 2019 (COVID-19) daily situation report of the Robert Koch Institute, 2020. Available at: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/2020-07-15-en.pdf?__blob=publicationFile. Accessed November 7, 2021
- 34.Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, Klauber J, Janssens U, Marx G, Weber-Carstens S, Kluge S, Pfeifer M, Grabenhenrich L, Welte T, Busse R: Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: An observational study. Lancet Respir Med 8: 853–862, 2020. 10.1016/S2213-2600(20)30316-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nadim MK, Forni LG, Mehta RL, Connor MJ Jr, Liu KD, Ostermann M, Rimmelé T, Zarbock A, Bell S, Bihorac A, Cantaluppi V, Hoste E, Husain-Syed F, Germain MJ, Goldstein SL, Gupta S, Joannidis M, Kashani K, Koyner JL, Legrand M, Lumlertgul N, Mohan S, Pannu N, Peng Z, Perez-Fernandez XL, Pickkers P, Prowle J, Reis T, Srisawat N, Tolwani A, Vijayan A, Villa G, Yang L, Ronco C, Kellum JA: COVID-19-associated acute kidney injury: Consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup [published correction appears in Nat Rev Nephrol 16: 765, 2020 10.1038/s41581-020-00372-5]. Nat Rev Nephrol 16: 747–764, 2020. 10.1038/s41581-020-00356-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B: Factors associated with COVID-19-related death using OpenSAFELY. Nature 584: 430–436, 2020. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA: Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol 74: 148–155, 2021. 10.1016/j.jhep.2020.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, Catana MA, Cargill T, Dhanasekaran R, García-Juárez I, Hagström H, Kennedy JM, Marshall A, Masson S, Mercer CJ, Perumalswami PV, Ruiz I, Thaker S, Ufere NN, Barnes E, Barritt AS 4th, Moon AM: Outcomes following SARS-CoV-2 infection in liver transplant recipients: An international registry study. Lancet Gastroenterol Hepatol 5: 1008–1016, 2020. 10.1016/S2468-1253(20)30271-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bottio T, Bagozzi L, Fiocco A, Nadali M, Caraffa R, Bifulco O, Ponzoni M, Lombardi CM, Metra M, Russo CF, Frigerio M, Masciocco G, Potena L, Loforte A, Pacini D, Faggian G, Onorati F, Sponga S, Livi U, Iacovoni A, Terzi A, Senni M, Rinaldi M, Boffini M, Marro M, Jorgji V, Carrozzini M, Gerosa G: COVID-19 in heart transplant recipients: A multicenter analysis of the Northern Italian outbreak. JACC Heart Fail 9: 52–61, 2021. 10.1016/j.jchf.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao D, Zhou F, Luo L, Xu M, Wang H, Xia J, Gao Y, Cai L, Wang Z, Yin P, Wang Y, Tang L, Deng J, Mei H, Hu Y: Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: A retrospective cohort study. Lancet Haematol 7: e671–e678, 2020. 10.1016/S2352-3026(20)30217-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coemans M, Süsal C, Döhler B, Anglicheau D, Giral M, Bestard O, Legendre C, Emonds MP, Kuypers D, Molenberghs G, Verbeke G, Naesens M: Analyses of the short- and long-term graft survival after kidney transplantation in Europe between 1986 and 2015. Kidney Int 94: 964–973, 2018. 10.1016/j.kint.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 42.Deutsche Stiftung Organtransplantation : Statistiken & Berichte, 2021. Available at: https://dso.de/organspende/statistiken-berichte/organspende. Accessed November 5, 2021)
- 43.Bae S, McAdams-DeMarco MA, Massie AB, Ahn JB, Werbel WA, Brennan DC, Lentine KL, Durand CM, Segev DL: Early changes in kidney transplant immunosuppression regimens during the COVID-19 pandemic. Transplantation 105: 170–176, 2021. 10.1097/TP.0000000000003502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herrmann KH, Meier-Kriesche U, Neubauer AS: Real world data in health technology assessments in kidney transplants in Germany: Use of routinely collected data to address epidemiologic questions in kidney transplants in the AMNOG process in Germany. Ger Med Sci 16: Doc01, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient disposition. Download Supplemental Figure 1, PDF file, 345 KB (344.7KB, pdf)
Characteristics of hospitalized COVID-19 patients. Download Supplemental Figure 2, PDF file, 345 KB (344.7KB, pdf)
Definition of procedures, diagnoses, and drugs. Download Supplemental Table 1, PDF file, 345 KB (344.7KB, pdf)
Characteristics of patients with and without hospitalized COVID-19. Download Supplemental Table 2, PDF file, 345 KB (344.7KB, pdf)