“Don’t await the perfection of Plato’s Republic, but be content with the smallest step forward, and regard that result as no mean achievement.” (Marcus Aurelius, Meditations)
Artificial Intelligence and AKI: Vast Opportunities for an Unresolved Problem
AKI is common in hospitalized adults and children, with increased morbidity, mortality, hospital length of stay, and healthcare costs (1,2). Although clinicians have increasingly recognized the scale and effect of AKI, significant challenges remain in reducing AKI incidence and improving outcomes. AKI is a heterogeneous cluster of pathophysiologic processes that result in the rise of serum creatinine and/or drop in urine output. The diagnosis of AKI is often delayed by the reliance upon these often late and inaccurate biomarkers of kidney function (3). The various pathophysiologies within the syndrome of AKI can stymie both the clinician and the implementation of artificial intelligence (AI) to improve recognition and outcomes. AI uses the science of informatics to augment human performance in the assessment and management of disease. AI has multiple foreseeable uses in the setting of AKI to improve patient care (Figure 1). Future AI-based AKI endeavors should (1) focus on the elimination of care and knowledge gaps identified by both patients and clinicians, (2) design and validate fair and equitable models for short- and long-term AKI risk stratification, and (3) improve patient-centered outcomes among AKI survivors. Beyond direct patient care, AI has key roles in moving AKI research forward and connecting the practicing clinician with researchers and industry partners.
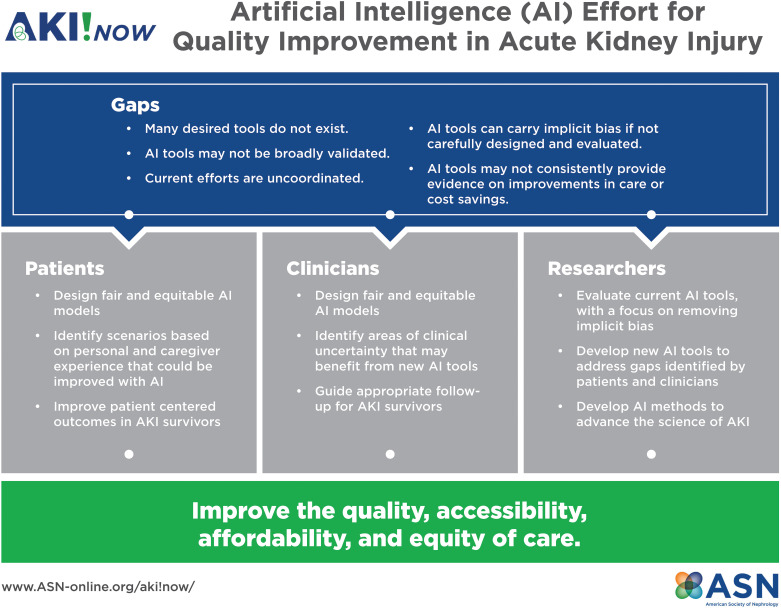
Figure 1.
The figure demonstrates the goals of the AKI!Now Artificial Intelligence Workgroup, which seeks to identify gaps in AKI care that can be answered through artificial intelligence. The group will focus on issues/questions that will improve patient-centered outcomes and improve clinical care. We will engage patients, clinicians, and researchers in the hope of improving the quality, accessibility, and equity of care. AI, artificial intelligence.
An Ounce of Prevention
The feasibility of employing AI in predicting AKI has been shown in numerous care settings. Tomašev et al. (4) demonstrated the power of partnership between researchers and industry (Google’s subsidiary DeepMind) to leverage large datasets to create a continuous prediction model of AKI in US veterans. One major limitation of this study was the lack of diversity in the study/validation patient population. Koyner et al. (5) used electronic health record (EHR) data to develop a prediction tool that can predict stage 2 AKI a median of 41 hours before a rise in serum creatinine and the need for dialysis within 2 days. The tool performed well across numerous adult care settings and was also valuable in predicting AKI in adults with pre-existing kidney disease, making its use potentially applicable in multiple clinical scenarios (5). Simonov et al. (6) implemented an AKI prediction tool across several institutions that used the same EHR in diverse settings. The model performed well at predicting AKI within 24 hours and the need for dialysis and mortality (6). Other groups have shown the ability to reduce the time to AKI diagnosis in specific clinical settings, including in adults with severe burn (7) and acute pancreatitis (8), postoperative patients (9–11), and patients with cardiac disorders (12,13). Although such prediction tools have the potential to enable care optimization and to mitigate ongoing renal insults, investigators first need to demonstrate that implementation of such tools is reproducible and improves meaningful patient-centered outcomes, and these findings require external validation for broader application. The dissimilarity in EHR systems makes this process challenging. Additional challenges to a wider implementation strategy include substantial up-front costs across systems, without demonstrable healthcare savings for the institution and barriers to key stakeholder buy-in and utilization. As models are increasingly validated in multiple systems, some of these hurdles will be easier to clear. Further, systematic, actionable interventions supported by these risk-prediction tools must also be rigorously tested before widespread implementation.
An Inconvenient Truth: Not All AKI Is Preventable
Although prevention is enticing, many patients arrive to the hospital with evolving AKI. However, AI can be leveraged to provide alerts within the EHR to notify providers as soon as criteria are met and prompt the use of bundled care measures to mitigate further kidney injury and promote recovery. The feasibility of using AI to reduce the rate, duration, and intensity of AKI in progress and to reduce morbidity, mortality, and hospital costs has been shown by Al-Jaghbeer et al. (14). In this study, using automated clinical decision support (CDS), patients with AKI had a slight but significant reduction in mortality, hospital length of stay, and dialysis requirements after implementation of the CDS tool. However, not all studies using CDS/AI have demonstrated improved outcomes. Recently, a 6030 adult patient, double-blind, multicenter, parallel, randomized control trial demonstrated that an EHR-based “pop-up” alert for AKI with an associated order set did not improve a composite end point of AKI progression, receipt of dialysis, or death within 14 days (15). Future studies should attempt to use AI and CDS to identify patients at high risk for AKI in a timely fashion, but also pair this timely recognition with a series of well-validated AKI interventions. Careful patient and outcome selection, and balancing measures, including alert fatigue, must be incorporated into the design of AI tools meant to improve care. Best practices to use CDS in AKI care have been recently reviewed elsewhere (16).
In children, the Assessment of Worldwide Acute Kidney Injury, Renal Angina, and Epidemiology study demonstrated the limitations of recognizing AKI without including urine output criteria (17). Jin et al. (18) showed an improvement in AKI recognition in patients who underwent close monitoring and documentation of urine output in the EHR. These findings highlight the potential for novel device integration within the EHR to improve urine output monitoring and allowing the inclusion of urine output criteria within AKI alert tools, something that has been missing in other AKI predictive tools (4,5).
An even greater opportunity to influence care lies in AI’s potential to prompt appropriate postdischarge follow-up for patients at high risk of rehospitalizations, recurrent AKI, cardiorenal complications, or other comorbidities after hospital discharge. Growing evidence suggests that patients who suffer an episode of AKI warrant not only kidney (19) but also cardiac (20) monitoring. After adjusting for confounders, patients with AKI have been shown to have an increased risk of major adverse cardiac events, such as stroke and heart failure, in the years after discharge (20–23). Semler et al. (24) demonstrated the feasibility of AI to predict major adverse kidney events by 30 days. Supervised machine learning and data science could be deployed for risk stratification of post-AKI complications, such as fractures, heart failure, or progression to CKD, and could, therefore, guide evidence-based long-term follow-up without overburdening patients or the healthcare system. Machine learning is a type of AI in which the systems can adapt, learn, and improve over time without following specific instructions. A strength of supervised machine learning is that data science can be leveraged to predict long-term outcomes in a heterogeneous cluster of diseases, such as AKI, rather than in a single disease process. This can be leveraged further for research purposes to evaluate subphenotypes within AKI, their respective long-term sequelae, and their candidacy for personalized interventions (25,26). Widespread implementation of AI tools is not without risk, however. The Epic Sepsis Model has been incorporated for use in hundreds of US hospitals to identify patients with sepsis. Peer review adjudication of the system has demonstrated the overall suboptimal performance of the tool, thus highlighting the need for rigorous design, testing, and iterative process improvement of independent and external validation before implementation (27). Even after implementation, evolving clinical data are likely to warrant an iterative approach to AI tools to keep apace with best practice.
The Kids Are Alright
Significant progress has been made in pediatric care along the risk-stratification/prediction/alert/follow-up continuum. In developing the Nephrotoxic Injury Negated by Just in time Action (NINJA) alert program, Goldstein et al. (28) demonstrated a sustained reduction in the development of nephrotoxin-mediated AKI among hospitalized pediatric patients. The program has since undergone multicenter validation, has been incorporated throughout numerous pediatric centers, and has been expanded to the intensive care (including neonatal and cardiac) settings (29). Owing to its promising multicenter validation, NINJA has also been adopted for nationwide implementation by the Children’s Hospitals Solutions for Patient Safety program. One of the strengths of the NINJA program is the ability for each center to tailor its implementation to fit within its workflow and structure, enabling flexibility for success in a variety of environments, agnostic to software/hardware requirements. Perhaps a more significant strength is the collaborative nature of the program’s network, with centers routinely sharing ideas and tools to improve clinical outcomes at programs throughout the country and updating the tool as advancements in the understanding of nephrotoxin-mediated AKI become available. The NINJA program has also investigated balancing measures and demonstrated that implementation of the stewardship program did not result in an increase in mortality, sepsis-associated complications, or increased length of stay. Importantly, we remain convinced that the NINJA program will require further validation before implementation in adult populations.
Meanwhile, the renal angina index has been incorporated into the EHR of several pediatric intensive care units to alert providers of patients at high risk of developing AKI within 48 hours of admission (30). Matsuura et al. (31) have demonstrated the potential utilization of the renal angina index combined with urine biomarkers to predict AKI in hospitalized adults who are noncritically ill, and Ortiz-Soriano et al. (32) have recently demonstrated the utility of a modified renal angina index to predict stage 2 or 3 AKI in adults who are critically ill. These efforts highlight the potential collaboration between adult and pediatric providers in breaking down siloes to improve care in AKI across the life span. Menon et al. (33) have also successfully implemented an AKI alert tool to increase AKI recognition, provide decision support, and improve bundle compliance with Kidney Disease Improving Global Outcomes recommendations in pediatric patients who are hospitalized. This resource has also been implemented at other pediatric centers, with collaboration fostered through the NINJA network. Quality improvement has been emphasized in many of these efforts and has facilitated widespread implementation and buy-in throughout the pediatric community. Along these lines, Mottes (34) has developed a dashboard to incorporate machine data into quality improvement work for acute RRT in children who are critically ill. The development and implementation of quality assurance systems for continuous RRT in the adult critically ill population have also demonstrated sustainability in tracking treatment deliverables and reducing filter resource utilization (35). Incorporating dialysis machine data (blood flow rates, machine pressures, etc.) into the EHR is feasible via existing technology and can provide further data for supervised machine learning systems to improve care. Apart from dialysis therapy, AI tools have the potential to recognize patients who may benefit from dialysis initiation and/or cessation, assist with anticoagulation therapy delivery and monitoring, and prompt early recognition of dialysis access issues, which could collectively trigger associated best practice alerts to providers and effective dialysis delivery to patients.
Conclusions: The Pursuit of Perfection Is the Enemy of Progress
Even small changes in clinical practice may have incredible effects on patient outcomes and healthcare costs. In adults, AKI is estimated to cost the US healthcare system between $5.4 and $20 billion per year (36). This estimate implies that, despite the relatively small effects on mortality and length of stay reported in Al-Jahgbeer et al.’s (14) CDS tools, widespread implementation throughout the United States could save millions of dollars per year, not accounting for the cost of post-AKI care, which was not included in these studies, or the effect of AKI on quality of life. Health economists have described the possible significant improvement in cost savings in the US healthcare system with slight 1% incremental improvements (37). Implementing such strategies along the continuum of AKI-related AI resources, such as risk stratification and prediction, alerts, decision support, and long-term follow-up, could substantially improve patient outcomes and reduce the burden of AKI on the healthcare system (38). Collaborative opportunities exist throughout the clinical healthcare systems—both within and between adult and pediatric centers—and through partnerships with industry to make steady progress along these lines. The likelihood of finding a “one-size-fits-all” approach is low, and we think we should attempt one best tool for each one of these tasks: whether AI based or not, each tool must be designed for its own purpose and rigorously tested for sensitivity, specificity, and accuracy.
In light of the growing recognition that AI-based algorithms can, unfortunately, absorb and perpetuate racial biases, care and intention needs to be present throughout the design and validation phases to test and customize these tools to serve multifaceted and diverse patient populations. Otherwise, AI tools are at risk of absorbing implicit biases and perpetuating healthcare inequity, such as when supervised machine learning risks obfuscating race with specific social determinants of health. Conversely, supervised machine learning poses a potential strength in serving diverse populations because AI interventions have the potential to be tailored to the populations they serve. Thus, rather than implementing a general risk prediction or alert tool, AI can foster individualized care. Our workgroup recommends that experts in healthcare equity and patients/caregivers be involved early in the design and implementation phases of AI initiatives to capitalize on the potential strengths of AI and avoid inequities in care.
The AKI!Now initiative of the American Society of Nephrology (ASN) envisions a collaborative future within pediatric and adult healthcare systems working together to leverage basic science, data science, and quality initiatives to improve early recognition and treatment of AKI and to reduce the disease burden of individual patients and the healthcare system as a whole (3). The AI Workgroup of the AKI!Now initiative welcomes your ideas and collaboration to improve the care we all provide as part of the medical community. Our immediate and long-term goals are laid out in Table 1. For more information or to get involved, please reach out to EPC@asn-online.org or via https://www.asn-online.org/aki!now/.
Table 1.
Short- and long-term goals for the AKI-NOW! Artificial Intelligence Workgroup
| Short- and Long-Term Goals |
|---|
| Short-term goals (next 2 years) |
| Complete one to two AKI!Now webinars, which help define the current state of AI in AKI and set the stage for future projects through: |
| Establishing and consolidating nomenclature including common data elements. |
| Identifying use cases for AI (risk assessment, drug dosing, RRT). |
| Proposing a framework for the development and validation of predictive models. |
| Establish a web-based platform for ASN members to ask questions, communicate with “AI experts,” and use resources around AI tools (risk calculators, clinical decision support tools, etc.). |
| Use patient advocates to better understand which issues around the intersection of AI and AKI are important to patients and caregivers. |
| Provide content for ASN’s Kidney Week and other nephrology meetings in 2021 and 2022. |
| Establish a clear position and plan to educate providers and patients on the role of implicit bias and algorithmic fairness in AI and ensure future efforts work to eliminate biases and promote fairness/equity. |
| Long-term goals (2–5 years) |
| Establish a platform/collaborative network to test and validate novel AKI models and other AI tools. The first step in this process must include working on benchmarking existing AKI predictive models across our institutions. |
| Partner with industry sponsors to support: |
| AKI!NOW platform, which can help distribute/implement validated tools. |
| Clinical trials to test tools. |
| Develop AI tools to establish baseline quality and cost-of-care metrics around AKI and acute dialysis patient care. |
AI, artificial intelligence; ASN, American Society of Nephrology.
Disclosures
J. Cerda reports serving as chair of the ASN’s AKI!Now Initiative, as a scientific advisor for or member of the International Society of Nephrology (ISN) AKI Committee and cochair of the 0by25 Initiative, and as cochair of the ISN’s advisory committee; serving as associate director of the ASN AKI!Now Initiative and of the ISN’s 0by25 Initiative, and as a member of the ASN Online AKI Community; and having ownership interest in Capital District Renal Physicians (as shareholder). S. L. Goldstein reports having consultancy agreements with Akebia, Baxter Healthcare, Bayer, Bioporto Inc., CHF Solutions, Fresenius, Kaneka Inc., La Jolla Pharmaceuticals, MediBeacon, Medtronic, Otsuka, Reata, and Renibus; receiving research funding from Baxter Healthcare, Bioporto, and CHF Solutions; receiving honoraria from, and serving on speakers bureaus for, Baxter Healthcare and Fresenius; having ownership interest in, and serving as a scientific advisor for or member of, MediBeacon; and having patents and inventions with Vigilanz. K. B. Kashani reports having consultancy agreements with AM Pharma; serving as a scientific advisor for or member of Baxter, GE, La Jolla Inc., and MediBeacon Inc.; receiving research funding from La Jolla Inc.; having patents and inventions with MediBeacon; and receiving honoraria from Nikkiso. J. L. Koyner reports receiving honoraria from Acute Disease Quality Initiative (ADQI), ASN, and Society of Critical Care Medicine; serving as a scientific advisor for or member of the American Journal of Nephrology and Kidney360; being listed on a patent for Pi Glutathione-S-Transferase (GST) to detect severe AKI after cardiac surgery with Argutus Medical; having consultancy agreements with Astute Medical, Baxter, Novartis, and Mallinckrodt; receiving research funding from Astute Medical, Fresenius Medical, National Institutes of Health, and NxStage Medical; serving on the scientific advisory boards for the National Kidney Foundation (NKF) of Illinois and for the NKF; and serving on a speakers bureau for NxStage Medical. S. Menon reports having consultancy agreements with AKI Foundation and Nuwellis Inc. (CHF Solutions). G.N. Nadkarni reports receiving honoraria from AstraZeneca, BioVie, Lexicon, and Reata; having consultancy agreements with AstraZeneca, BioVie, Pensieve Health, Reata, Renalytix AI, Siemens, and Variant Bio; receiving research funding from Goldfinch Bio and Renalytix; having ownership interest in and serving as a scientific advisor for or member of, Pensieve Health and Renalytix; and having patents and inventions with Renalytix. J.A. Neyra reports serving as guest editor for Critical Care Nephrology in Advances in Chronic Kidney Disease, on the editorial board for Advances in Chronic Kidney Disease and Kidney360, and as a section editor for Clinical Nephrology; and having consultancy agreements with Baxter Healthcare Inc., Biomedical Insights, and Leadiant Biosciences. N.I. Pannu reports receiving honoraria from ADQI for the AKI meeting 2020 (US$1000.00 honorarium) and for the AKI CRRT meeting 2021 (provided microphone and lamp for speakers), ISN (as speaker, covered expenses 2019), and World Congress of Nephrology (as speaker, covered expenses in 2019 and 2020); having other interests in/relationships with Amgen (funded quality improvement initiative in ESKD); having consultancy agreements with GE; and serving as a scientific advisor for or membership of Kidney Foundation of Canada, Northern Alberta branch (on board of directors). K. Singh reports having other interests in/relationships with Blue Cross Blue Shield of Michigan (receive salary support through the University of Michigan for work done on the Michigan Urological Surgery Improvement Collaborative); receiving honoraria from Harvard University (for education provided in the Safety, Quality, Informatics, and Leadership program); and receiving research funding from Teva Pharmaceutical Ltd. D.E. Soranno reports having consultancy agreements with Levin & Perconti Attorneys at Law. The remaining author has nothing to disclose.
Funding
None.
Acknowledgments
We wish to thank the rest of the AKI!Now team and Ms. Melissa West, Ms. Susan Stark, Ms. Bonnie Freshly, and Mr. Javier Rivera for their support.
Author Contributions
J. Koyner, and D.E. Soranno wrote the original draft; and all authors conceptualized the study and reviewed and edited the manuscript.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005. 10.1681/ASN.2004090740 [DOI] [PubMed] [Google Scholar]
- 2.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; AWARE Investigators : Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376: 11–20, 2017. 10.1056/NEJMoa1611391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu KD, Goldstein SL, Vijayan A, Parikh CR, Kashani K, Okusa MD, Agarwal A, Cerdá J; AKI!Now Initiative of the American Society of Nephrology : AKI!Now Initiative: Recommendations for awareness, recognition, and management of AKI. Clin J Am Soc Nephrol 15: 1838–1847, 2020. 10.2215/CJN.15611219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomašev N, Glorot X, Rae JW, Zielinski M, Askham H, Saraiva A, Mottram A, Meyer C, Ravuri S, Protsyuk I, Connell A, Hughes CO, Karthikesalingam A, Cornebise J, Montgomery H, Rees G, Laing C, Baker CR, Peterson K, Reeves R, Hassabis D, King D, Suleyman M, Back T, Nielson C, Ledsam JR, Mohamed S: A clinically applicable approach to continuous prediction of future acute kidney injury. Nature 572: 116–119, 2019. 10.1038/s41586-019-1390-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koyner JL, Carey KA, Edelson DP, Churpek MM: The development of a machine learning inpatient acute kidney injury prediction model. Crit Care Med 46: 1070–1077, 2018. 10.1097/CCM.0000000000003123 [DOI] [PubMed] [Google Scholar]
- 6.Simonov M, Ugwuowo U, Moreira E, Yamamoto Y, Biswas A, Martin M, Testani J, Wilson FP: A simple real-time model for predicting acute kidney injury in hospitalized patients in the US: A descriptive modeling study. PLoS Med 16: e1002861, 2019. 10.1371/journal.pmed.1002861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran NK, Sen S, Palmieri TL, Lima K, Falwell S, Wajda J, Rashidi HH: Artificial intelligence and machine learning for predicting acute kidney injury in severely burned patients: A proof of concept. Burns 45: 1350–1358, 2019. 10.1016/j.burns.2019.03.021 [DOI] [PubMed] [Google Scholar]
- 8.Qu C, Gao L, Yu XQ, Wei M, Fang GQ, He J, Cao LX, Ke L, Tong ZH, Li WQ: Machine learning models of acute kidney injury prediction in acute pancreatitis patients. Gastroenterol Res Pract 2020: 3431290, 2020. 10.1155/2020/3431290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thottakkara P, Ozrazgat-Baslanti T, Hupf BB, Rashidi P, Pardalos P, Momcilovic P, Bihorac A: Application of machine learning techniques to high-dimensional clinical data to forecast postoperative complications. PLoS One 11: e0155705, 2016. 10.1371/journal.pone.0155705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adhikari L, Ozrazgat-Baslanti T, Ruppert M, Madushani RWMA, Paliwal S, Hashemighouchani H, Zheng F, Tao M, Lopes JM, Li X, Rashidi P, Bihorac A: Improved predictive models for acute kidney injury with IDEA: Intraoperative data embedded analytics. PLoS One 14: e0214904, 2019. 10.1371/journal.pone.0214904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta S, Loftus TJ, Ruppert MM, Giordano C, Upchurch GR Jr, Rashidi P, Ozrazgat-Baslanti T, Bihorac A: Added value of intraoperative data for predicting postoperative complications: The MySurgeryRisk PostOp Extension. J Surg Res 254: 350–363, 2020. 10.1016/j.jss.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng PY, Chen YT, Wang CH, Chiu KM, Peng YS, Hsu SP, Chen KL, Yang CY, Lee OK: Prediction of the development of acute kidney injury following cardiac surgery by machine learning. Crit Care 24: 478, 2020. 10.1186/s13054-020-03179-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rank N, Pfahringer B, Kempfert J, Stamm C, Kühne T, Schoenrath F, Falk V, Eickhoff C, Meyer A: Deep-learning-based real-time prediction of acute kidney injury outperforms human predictive performance. NPJ Digit Med 3: 139, 2020. 10.1038/s41746-020-00346-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Jaghbeer M, Dealmeida D, Bilderback A, Ambrosino R, Kellum JA: Clinical decision support for in-hospital AKI. J Am Soc Nephrol 29: 654–660, 2018. 10.1681/ASN.2017070765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson FP, Martin M, Yamamoto Y, Partridge C, Moreira E, Arora T, Biswas A, Feldman H, Garg AX, Greenberg JH, Hinchcliff M, Latham S, Li F, Lin H, Mansour SG, Moledina DG, Palevsky PM, Parikh CR, Simonov M, Testani J, Ugwuowo U: Electronic health record alerts for acute kidney injury: Multicenter, randomized clinical trial. BMJ 372: m4786, 2021. 10.1136/bmj.m4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen ED, Menon S: For whom the bell tolls: Acute kidney injury and electronic alerts for the pediatric nephrologist. Front Pediatr 9: 628096, 2021. 10.3389/fped.2021.628096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaddourah A, Basu RK, Goldstein SL, Sutherland SM; Assessment of Worldwide Acute Kidney Injury, Renal Angina and, Epidemiology (AWARE) Investigators : Oliguria and acute kidney injury in critically ill children: Implications for diagnosis and outcomes. Pediatr Crit Care Med 20: 332–339, 2019. 10.1097/PCC.0000000000001866 [DOI] [PubMed] [Google Scholar]
- 18.Jin K, Murugan R, Sileanu FE, Foldes E, Priyanka P, Clermont G, Kellum JA: Intensive monitoring of urine output is associated with increased detection of acute kidney injury and improved outcomes. Chest 152: 972–979, 2017. 10.1016/j.chest.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 19.Chawla LS: Acute kidney injury leading to chronic kidney disease and long-term outcomes of acute kidney injury: The best opportunity to mitigate acute kidney injury? Contrib Nephrol 174: 182–190, 2011. 10.1159/000329396 [DOI] [PubMed] [Google Scholar]
- 20.Go AS, Hsu CY, Yang J, Tan TC, Zheng S, Ordonez JD, Liu KD: Acute kidney injury and risk of heart failure and atherosclerotic events. Clin J Am Soc Nephrol 13: 833–841, 2018. 10.2215/CJN.12591117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bansal N, Matheny ME, Greevy RA Jr, Eden SK, Perkins AM, Parr SK, Fly J, Abdel-Kader K, Himmelfarb J, Hung AM, Speroff T, Ikizler TA, Siew ED: Acute kidney injury and risk of incident heart failure among US veterans. Am J Kidney Dis 71: 236–245, 2018. 10.1053/j.ajkd.2017.08.027 [DOI] [PubMed] [Google Scholar]
- 22.Wu VC, Huang TM, Lai CF, Shiao CC, Lin YF, Chu TS, Wu PC, Chao CT, Wang JY, Kao TW, Young GH, Tsai PR, Tsai HB, Wang CL, Wu MS, Chiang WC, Tsai IJ, Hu FC, Lin SL, Chen YM, Tsai TJ, Ko WJ, Wu KD: Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int 80: 1222–1230, 2011. 10.1038/ki.2011.259 [DOI] [PubMed] [Google Scholar]
- 23.Gammelager H, Christiansen CF, Johansen MB, Tønnesen E, Jespersen B, Sørensen HT: Three-year risk of cardiovascular disease among intensive care patients with acute kidney injury: A population-based cohort study. Crit Care 18: 492, 2014. 10.1186/s13054-014-0492-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semler MW, Rice TW, Shaw AD, Siew ED, Self WH, Kumar AB, Byrne DW, Ehrenfeld JM, Wanderer JP: Identification of major adverse kidney events within the electronic health record. J Med Syst 40: 167, 2016. 10.1007/s10916-016-0528-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatraju PK, Zelnick LR, Herting J, Katz R, Mikacenic C, Kosamo S, Morrell ED, Robinson-Cohen C, Calfee CS, Christie JD, Liu KD, Matthay MA, Hahn WO, Dmyterko V, Slivinski NSJ, Russell JA, Walley KR, Christiani DC, Liles WC, Himmelfarb J, Wurfel MM: Identification of acute kidney injury subphenotypes with differing molecular signatures and responses to vasopressin therapy. Am J Respir Crit Care Med 199: 863–872, 2019. 10.1164/rccm.201807-1346OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaudhary K, Vaid A, Duffy Á, Paranjpe I, Jaladanki S, Paranjpe M, Johnson K, Gokhale A, Pattharanitima P, Chauhan K, O’Hagan R, Van Vleck T, Coca SG, Cooper R, Glicksberg B, Bottinger EP, Chan L, Nadkarni GN: Utilization of deep learning for subphenotype identification in sepsis-associated acute kidney injury. Clin J Am Soc Nephrol 15: 1557–1565, 2020. 10.2215/CJN.09330819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong A, Otles E, Donnelly JP, Krumm A, McCullough J, DeTroyer-Cooley O, Pestrue J, Phillips M, Konye J, Penoza C, Ghous M, Singh K: External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med 181: 1065–1070, 2021. 10.1001/jamainternmed.2021.2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, Kirkendall ES: A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int 90: 212–221, 2016. 10.1016/j.kint.2016.03.031 [DOI] [PubMed] [Google Scholar]
- 29.Goldstein SL, Dahale D, Kirkendall ES, Mottes T, Kaplan H, Muething S, Askenazi DJ, Henderson T, Dill L, Somers MJG, Kerr J, Gilarde J, Zaritsky J, Bica V, Brophy PD, Misurac J, Hackbarth R, Steinke J, Mooney J, Ogrin S, Chadha V, Warady B, Ogden R, Hoebing W, Symons J, Yonekawa K, Menon S, Abrams L, Sutherland S, Weng P, Zhang F, Walsh K: A prospective multi-center quality improvement initiative (NINJA) indicates a reduction in nephrotoxic acute kidney injury in hospitalized children. Kidney Int 97: 580–588, 2020. 10.1016/j.kint.2019.10.015 [DOI] [PubMed] [Google Scholar]
- 30.Basu RK, Kaddourah A, Goldstein SL; AWARE Study Investigators : Assessment of a renal angina index for prediction of severe acute kidney injury in critically ill children: A multicentre, multinational, prospective observational study. Lancet Child Adolesc Health 2: 112–120, 2018. 10.1016/S2352-4642(17)30181-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuura R, Srisawat N, Claure-Del Granado R, Doi K, Yoshida T, Nangaku M, Noiri E: Use of the renal angina index in determining acute kidney injury. Kidney Int Rep 3: 677–683, 2018. 10.1016/j.ekir.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortiz-Soriano V, Kabir S, Claure-Del Granado R, Stromberg A, Toto RD, Moe OW, Goldstein SL, Neyra JA: Assessment of a modified renal angina index for AKI prediction in critically ill adults [published online ahead of print February 19, 2021]. Nephrol Dial Transplant 10.1093/ndt/gfab049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menon S, Tarrago R, Carlin K, Wu H, Yonekawa K: Impact of integrated clinical decision support systems in the management of pediatric acute kidney injury: A pilot study. Pediatr Res 89: 1164–1170, 2020. 10.1038/s41390-020-1046-8 [DOI] [PubMed] [Google Scholar]
- 34.Mottes TA: Does your program know its AKI and CRRT epidemiology? The case for a dashboard. Front Pediatr 8: 80, 2020. 10.3389/fped.2020.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruiz EF, Ortiz-Soriano VM, Talbott M, Klein BA, Thompson Bastin ML, Mayer KP, Price EB, Dorfman R, Adams BN, Fryman L, Neyra JA; University of Kentucky CRRT Quality Assurance Group : Development, implementation and outcomes of a quality assurance system for the provision of continuous renal replacement therapy in the intensive care unit. Sci Rep 10: 20616, 2020. 10.1038/s41598-020-76785-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silver SA, Chertow GM: The economic consequences of acute kidney injury. Nephron 137: 297–301, 2017. 10.1159/000475607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper Z, Scott Morton F: 1% steps for health care reform: Implications for health care policy and for researchers. Health Serv Res 56: 346–349, 2021. 10.1111/1475-6773.13658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewkowicz D, Wohlbrandt A, Boettinger E: Economic impact of clinical decision support interventions based on electronic health records. BMC Health Serv Res 20: 871, 2020. 10.1186/s12913-020-05688-3 [DOI] [PMC free article] [PubMed] [Google Scholar]