Introduction
Kidney disease affects >10% of the population in the United States and leads to a high risk of mortality and morbidity. The kidney has immense potential for regeneration in the appropriate environment. Providing an appropriate environment has been a very active area of research in nephrology for the development of biomarker and disease modeling. Cell-based screens rely on the function of these kidney cells in culture systems. This often leads to the failure of screening hits translating into mouse models for testing its efficacy in disease. Identification of nephrotoxic therapeutic agents before the clinical stage would allow modifications to maintain efficacy with lower kidney toxicity (1). Early identification will reduce nearly 20% of the drugs that fail in phase 3 clinical trials, improving the drug development pipeline (2). Thus, better biomarkers and disease modeling systems are needed for proficient drug discovery.
Cell Culture Systems and Cell Function
The apical surface of kidney proximal tubular epithelial cells (PTECs) is exposed to constant glomerular flow, which promotes cytoskeletal rearrangements, alters the expression of apical and basolateral transporters, and modulates sodium transport in vivo (3–5). At the same time, the basolateral surface of kidney PTECs is exposed to the interstitial space, promoting the movement of amino acids, glucose, active transport of ions, and drugs across the epithelium. In cell culture systems, both the above-mentioned components are lacking. The Transwell culture system mimics crossepithelial transport (6–8), but lacks fluid flow and the apical function of in vivo–like kidney functions. Kidney-on-a-chip is a fluid-flowing device consisting of a structural component, often made of polydimethyl siloxane, and a microfluidic component either attached to a pump or syringe with constant flow pressure (9–11). Several investigators, including those in our group, have examined the microfluidic kidney-on-a-chip platform, which provides the fluid flow component to PTECs and glomerular cells, and shows the functional and structural improvements of these two cell types (12–19). Kidney-on-a-chip promotes actin cytoskeleton rearrangement and tight junction protein expression, and improves cellular transport functions compared with static cultures (15,17,20,21).
Biomarkers in Cell Culture Systems
Biomarkers play a critical role in the assessment of cellular function, particularly in the culture system. Many biomarkers that show sensitivity in in vivo settings fail to show sensitivity in vitro. This limits assessment of the cell function. Two main cell types that are well studied in the kidney are renal PTECs and podocytes that are damaged in response to various stimuli, including many investigational drugs. PTECs play an important role in active clearance, intracellular concentration, reabsorption, and interstitial accumulation of drugs, thus making them more susceptible to injury (22,23).
Another prominent segment of the kidney that is prone to frequent injuries is the glomerulus, which filters electrolytes and fluids from the blood, retaining plasma proteins (24,25). The two specialized cell types in the glomerulus that show injury are the glomerular endothelial cells and podocytes.
We found kidney-on-a-chip using PTECs show a significant increase in biomarkers such as kidney injury molecule-1 (KIM-1) and heme oxygenase-1, compared with static culture after injury (20). In this issue, Vormann et al. modeled ischemia reperfusion injury using a vessel-on-a-chip platform to assess the functionality of PTECs in a combination of human endothelial cells. The authors found increased sensitivity of cisplatin, tobramycin, and cyclosporin A to PTECs. Moreover, adenosine treatment rescued PTECs from ischemia reperfusion injury. Thus, kidney-on-a-chip shows promise for the development of predictive biomarkers for the preclinical screening of nephrotoxic compounds.
Disease Modeling
Disease modeling is a critical parameter for screening compounds to identify a drug for preclinical studies.
Modeling Tubulointerstitial Disease
Kidney-on-a-chip shows promise for disease modeling, owing to its closer resemblance to in vivo conditions. Investigators, including us, have shown that kidney-on-a-chip shows a better response to injury markers in response to nephrotoxic compounds (20,26,27). We found that expression of KIM-1 after nephrotoxic compound treatment shows a significant increase compared with static culture (20,26). Many compounds are biotransformed by the liver, producing nephrotoxic products (28,29). Chang et al. modeled human hepatocytes and PTECs to investigate the biotransformation of aristolochic acid–on-a-chip, and found that biotransformation of aristolochic acid by hepatocytes increases the toxicity toward kidney cells, as shown by increased cell injury markers, including KIM-1 expression (12).
Modeling Glomerular Disease
Coculture of podocin and glomerular endothelial cells–on-a-chip showed a better injury response to puromycin as shown by albumin leakage; the serum from patients with membranous nephropathy (MN) showed increased albumin leakage (30). In addition, mechanistically, podocytes showed activation of signaling similar to MN, such as mislocalized nephrin, due to the activation of complement signaling molecule, C3d (31), leading to the loss of a slit diaphragm structure (32). Further, it was found that treatment with a therapeutic agent, α-melanocortin stimulating hormone, known to ameliorate MN, reduces albumin leakage in the kidney-on-a-chip platform (30). Finally, kidney-on-a-chip shows promising results for modeling podocytes from genetic kidney disease (30). The summary of cells used to model segments of nephron is shown in Table 1.
Table 1.
Kidney-/organ-on-a-chip platforms and cell types tested
| Cells | Segment | Author/Reference |
|---|---|---|
| RPTECs | Proximal tubule | Adler et al. (2016) (20) |
| RPTECs | Proximal tubule | Jang et al. (2013) (17) |
| RPTECs | Proximal tubule | Ferrell et al. (2018) (37) |
| RPTECs/NIH3T3 | Proximal tubule/fibroblast | Oo et al. (2011) (38) |
| HK-2 | Proximal tubule | Zhou et al. (2015) (39) |
| RPTECs, HK2 | Proximal tubule | Frohlich et al. (2012) (15) |
| MDCK, GC-ASC | Proximal/distal tubule | Huang et al. (2012) (40) |
| ARPCs | Proximal tubule | Sciancalepore et al. (2014) (41) |
| MDCK, IMCD | Proximal tubule/collecting duct | Jang et al. (2010) (42) |
| IMCD | Collecting duct | Jang et al. (2011) (43) |
| Mouse glomerular endothelial cells (GEnCs) and mouse podocytes (MPC-5) | Glomerulus | Zhou et al. (2016) (19) |
| Rat primary glomerular endothelial cells | Glomerulus | Wang et al. (2017) (44) |
| iPSC derived | Glomerulus | Musah et al. (2017) (45) |
| Ci-PTEC | Proximal tubule | Vriend et al. (2018) (46) |
| Human and rat PTECs and hepatocytes | Hepatocytes/proximal tubule | Chang et al. (2017) (12) |
| Human primary kidney cells, HK-2 | Kidney/proximal tubule | Hoppensack et al. (2014) (47) |
| iPSC derived | Glomerulus/proximal tubule/vascular | Homan et al. (2019) (35) |
| Human primary podocytes/glomerular endothelial cells and immortalized podocytes | Glomerulus | Petrosyan et al. (2019) (30) |
| OK | Renal epithelium | Ferrell et al. (2013) (48) |
| HREC and MDCK | Renal epithelium | Ferrell et al. (2010) (49) |
| MDCK | Renal epithelium | Jayagopal et al. (2019) (50) |
| PTEC-TERT1 | Proximal tubule | Homan et al. (2016) (10) |
| LLC-PK1 | Proximal tubule | Essig et al. (2001) (14) |
| OK, LLC-PK1, and MDCK | Proximal tubule | Raghavan et al. (2014) (51) |
RPTECs, renal proximal tubule epithelial cells; iPSC, induced pluripotent stem cell; Ci-PTEC, conditionally immortalized proximal tubular epithelial cell line; OK, opossum kidney.
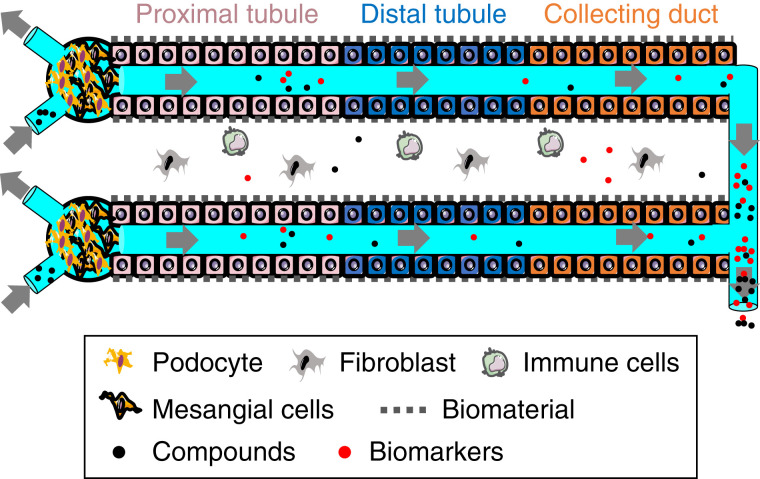
The development of kidney organoids provides additional benefits, where kidney tubular and glomerular-like structures develop in the dish (33,34). The addition of a microfluidic component to the kidney organoids showed vascularization and early maturation, indicating the addition of a flow system mimics in vivo–like conditions, compared with static organoid culture (35). A schematic of kidney-on-a-chip for compound screening and biomarker evaluation is shown in Figure 1.
Figure 1.
Schematic for functional kidney-on-a-chip for compound screening and evaluation of biomarkers.
Future Challenges
Although kidney-on-a-chip systems show promise for biomarker and nephrotoxicity assessment, they need optimization for high throughput screening. The optimization of a new cell culture system is often challenging and requires optimization for seeding density and modulation of flow. There is an immense need for improving the imaging of the kidney-on-a-chip using high magnification microscopes to understand the biologic process. Coculture of different cells from the kidney improves cellular functions (36), but lacks circulatory and immune cells. Kidney-on-a-chip provides an opportunity to add circulatory immune cells (T cells, B cells, macrophages, and neutrophils) and nonimmune (fibrocyte) cells into the circulation. The addition of these cells will mimic in vivo–like signaling crosstalk among these cells and creates a microenvironment with secretory proteins, such as TGF-β (fibrosis), TNF-α (acute kidney injury and fibrosis), KIM-1 (proximal tubular injury), and neutrophil gelatinase–associated lipocalin (tubular injury marker) in the culture medium, and are crucial for disease modeling. Thus, efforts are being made to create a complete kidney-on-a-chip with circulatory cells and kidney cells for disease modeling. Developing a complete nephron by combining cells from the different segments of kidney such as the glomerulus, PTECs, distal tubules, and collecting ducts will allow us to investigate segment-specific nephrotoxic preclinical compounds. With the combined efforts of bioengineers developing new biomaterials, and cell biologists using them to create the in vivo–like microenvironment, we anticipate great advances in drug screening and disease modeling in the coming years.
Disclosures
A.K. Ajay reports being a founder of CompEdge and Sense Research Foundation; this conflict of interest is managed by the Mass General Brigham office of external collaboration.
Funding
This work was supported by an American Heart Association Career Development Grant (19CDA34780005) and Brigham and Women’s Hospital funds.
Acknowledgments
The content of this article reflects the personal experience and views of the author and should not be considered medical advice or recommendations. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the author.
Footnotes
See related article, “Modelling and Prevention of AKI through Ischemia and Reperfusion in a Combined Human Renal Proximal Tubule/Blood Vessel-on-a-Chip,” on pages 217–231.
Author Contributions
A.K. Ajay conceptualized he study, was responsible for the formal analysis, funding acquisition, investigation, project administration, resources, and software, provided supervision, wrote the original draft, and reviewed and edited the manuscript.
References
- 1.Chu X, Bleasby K, Evers R: Species differences in drug transporters and implications for translating preclinical findings to humans. Expert Opin Drug Metab Toxicol 9: 237–252, 2013. 10.1517/17425255.2013.741589 [DOI] [PubMed] [Google Scholar]
- 2.Parasrampuria DA, Benet LZ, Sharma A: Why drugs fail in late stages of development: Case study analyses from the last decade and recommendations. AAPS J 20: 46, 2018. 10.1208/s12248-018-0204-y [DOI] [PubMed] [Google Scholar]
- 3.Duan Y, Gotoh N, Yan Q, Du Z, Weinstein AM, Wang T, Weinbaum S: Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical junctional complexes. Proc Natl Acad Sci U S A 105: 11418–11423, 2008. 10.1073/pnas.0804954105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan Y, Weinstein AM, Weinbaum S, Wang T: Shear stress-induced changes of membrane transporter localization and expression in mouse proximal tubule cells. Proc Natl Acad Sci U S A 107: 21860–21865, 2010. 10.1073/pnas.1015751107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du Z, Duan Y, Yan Q, Weinstein AM, Weinbaum S, Wang T: Mechanosensory function of microvilli of the kidney proximal tubule. Proc Natl Acad Sci U S A 101: 13068–13073, 2004. 10.1073/pnas.0405179101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elwi AN, Damaraju VL, Kuzma ML, Mowles DA, Baldwin SA, Young JD, Sawyer MB, Cass CE: Transepithelial fluxes of adenosine and 2′-deoxyadenosine across human renal proximal tubule cells: Roles of nucleoside transporters hENT1, hENT2, and hCNT3. Am J Physiol Renal Physiol 296: F1439–F1451, 2009. 10.1152/ajprenal.90411.2008 [DOI] [PubMed] [Google Scholar]
- 7.Hering-Smith KS, Schiro FR, Pajor AM, Hamm LL: Calcium sensitivity of dicarboxylate transport in cultured proximal tubule cells. Am J Physiol Renal Physiol 300: F425–F432, 2011. 10.1152/ajprenal.00036.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang M, Ramsey CR, Knox FG: The paracellular permeability of opossum kidney cells, a proximal tubule cell line. Kidney Int 56: 2304–2308, 1999. 10.1046/j.1523-1755.1999.00787.x [DOI] [PubMed] [Google Scholar]
- 9.Duffy DC, McDonald JC, Schueller OJ, Whitesides GM: Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem 70: 4974–4984, 1998. 10.1021/ac980656z [DOI] [PubMed] [Google Scholar]
- 10.Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, Lewis JA: Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep 6: 34845, 2016. 10.1038/srep34845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber EJ, Chapron A, Chapron BD, Voellinger JL, Lidberg KA, Yeung CK, Wang Z, Yamaura Y, Hailey DW, Neumann T, Shen DD, Thummel KE, Muczynski KA, Himmelfarb J, Kelly EJ: Development of a microphysiological model of human kidney proximal tubule function. Kidney Int 90: 627–637, 2016. 10.1016/j.kint.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang SY, Weber EJ, Sidorenko VS, Chapron A, Yeung CK, Gao C, Mao Q, Shen D, Wang J, Rosenquist TA, Dickman KG, Neumann T, Grollman AP, Kelly EJ, Himmelfarb J, Eaton DL: Human liver-kidney model elucidates the mechanisms of aristolochic acid nephrotoxicity. JCI Insight 2: 95978, 2017. 10.1172/jci.insight.95978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE: Microengineered physiological biomimicry: Organs-on-chips. Lab Chip 12: 2156–2164, 2012. 10.1039/c2lc40089h [DOI] [PubMed] [Google Scholar]
- 14.Essig M, Terzi F, Burtin M, Friedlander G: Mechanical strains induced by tubular flow affect the phenotype of proximal tubular cells. Am J Physiol Renal Physiol 281: F751–F762, 2001. 10.1152/ajprenal.2001.281.4.F751 [DOI] [PubMed] [Google Scholar]
- 15.Frohlich EM, Zhang X, Charest JL: The use of controlled surface topography and flow-induced shear stress to influence renal epithelial cell function. Integr Biol 4: 75–83, 2012. 10.1039/C1IB00096A [DOI] [PubMed] [Google Scholar]
- 16.Kelly EJ, Wang Z, Voellinger JL, Yeung CK, Shen DD, Thummel KE, Zheng Y, Ligresti G, Eaton DL, Muczynski KA, Duffield JS, Neumann T, Tourovskaia A, Fauver M, Kramer G, Asp E, Himmelfarb J: Innovations in preclinical biology: Ex vivo engineering of a human kidney tissue microperfusion system. Stem Cell Res Ther 4[Suppl 1]: S17, 2013. 10.1186/scrt378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang KJ, Mehr AP, Hamilton GA, McPartlin LA, Chung S, Suh KY, Ingber DE: Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol 5: 1119–1129, 2013. 10.1039/c3ib40049b [DOI] [PubMed] [Google Scholar]
- 18.Wilmer MJ, Ng CP, Lanz HL, Vulto P, Suter-Dick L, Masereeuw R: Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol 34: 156–170, 2016. 10.1016/j.tibtech.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 19.Zhou M, Zhang X, Wen X, Wu T, Wang W, Yang M, Wang J, Fang M, Lin B, Lin H: Development of a functional glomerulus at the organ level on a chip to mimic hypertensive nephropathy. Sci Rep 6: 31771, 2016. 10.1038/srep31771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adler M, Ramm S, Hafner M, Muhlich JL, Gottwald EM, Weber E, Jaklic A, Ajay AK, Svoboda D, Auerbach S, Kelly EJ, Himmelfarb J, Vaidya VS: A quantitative approach to screen for nephrotoxic compounds in vitro. J Am Soc Nephrol 27: 1015–1028, 2016. 10.1681/ASN.2015010060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatia SN, Ingber DE: Microfluidic organs-on-chips. Nat Biotechnol 32: 760–772, 2014. 10.1038/nbt.2989 [DOI] [PubMed] [Google Scholar]
- 22.Schetz M, Dasta J, Goldstein S, Golper T: Drug-induced acute kidney injury. Curr Opin Crit Care 11: 555–565, 2005. 10.1097/01.ccx.0000184300.68383.95 [DOI] [PubMed] [Google Scholar]
- 23.Perazella MA: Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol 4: 1275–1283, 2009. 10.2215/CJN.02050309 [DOI] [PubMed] [Google Scholar]
- 24.Scott RP, Quaggin SE: Review series: The cell biology of renal filtration. J Cell Biol 209: 199–210, 2015. 10.1083/jcb.201410017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suh JH, Miner JH: The glomerular basement membrane as a barrier to albumin. Nat Rev Nephrol 9: 470–477, 2013. 10.1038/nrneph.2013.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S, LesherPerez SC, Kim BC, Yamanishi C, Labuz JM, Leung B, Takayama S: Pharmacokinetic profile that reduces nephrotoxicity of gentamicin in a perfused kidney-on-a-chip. Biofabrication 8: 015021, 2016. 10.1088/1758-5090/8/1/015021 [DOI] [PubMed] [Google Scholar]
- 27.Vormann MK, Vriend J, Lanz HL, Gijzen L, van den Heuvel A, Hutter S, Joore J, Trietsch SJ, Stuut C, Nieskens TTG, Peters JGP, Ramp D, Caj M, Russel FGM, Jacobsen B, Roth A, Lu S, Polli JW, Naidoo AA, Vulto P, Masereeuw R, Wilmer MJ, Suter-Dick L: Implementation of a human renal proximal tubule on a chip for nephrotoxicity and drug interaction studies. J Pharm Sci 110: 1601–1614, 2021. 10.1016/j.xphs.2021.01.028 [DOI] [PubMed] [Google Scholar]
- 28.Skipper PL, Obiedzinski MW, Tannenbaum SR, Miller DW, Mitchum RK, Kadlubar FF: Identification of the major serum albumin adduct formed by 4-aminobiphenyl in vivo in rats. Cancer Res 45: 5122–5127, 1985 [PubMed] [Google Scholar]
- 29.Bendadani C, Meinl W, Monien B, Dobbernack G, Florian S, Engst W, Nolden T, Himmelbauer H, Glatt H: Determination of sulfotransferase forms involved in the metabolic activation of the genotoxicant 1-hydroxymethylpyrene using bacterially expressed enzymes and genetically modified mouse models. Chem Res Toxicol 27: 1060–1069, 2014. 10.1021/tx500129g [DOI] [PubMed] [Google Scholar]
- 30.Petrosyan A, Cravedi P, Villani V, Angeletti A, Manrique J, Renieri A, De Filippo RE, Perin L, Da Sacco S: A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. [Published correction appears in Nat Commun 10: 4791, 2019.] Nat Commun 10: 3656, 2019. 10.1038/s41467-019-11577-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrence MG, Altenburg MK, Sanford R, Willett JD, Bleasdale B, Ballou B, Wilder J, Li F, Miner JH, Berg UB, Smithies O: Permeation of macromolecules into the renal glomerular basement membrane and capture by the tubules. Proc Natl Acad Sci U S A 114: 2958–2963, 2017. 10.1073/pnas.1616457114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan H, Takeuchi E, Taylor GA, McLaughlin M, Brown D, Salant DJ: Nephrin dissociates from actin, and its expression is reduced in early experimental membranous nephropathy. J Am Soc Nephrol 13: 946–956, 2002. 10.1681/ASN.V134946 [DOI] [PubMed] [Google Scholar]
- 33.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV: Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33: 1193–1200, 2015. 10.1038/nbt.3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH: Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. [Published correction appears in Nature 536: 7615, 2016.] Nature 526: 564–568, 2015. 10.1038/nature15695 [DOI] [PubMed] [Google Scholar]
- 35.Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R: Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16: 255–262, 2019. 10.1038/s41592-019-0325-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nugraha B, Mohr MA, Ponti A, Emmert MY, Weibel F, Hoerstrup SP, Moll S, Certa U, Prunotto M, Pantazis P: Monitoring and manipulating cellular crosstalk during kidney fibrosis inside a 3D in vitro co-culture. Sci Rep 7: 14490, 2017. 10.1038/s41598-017-12683-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrell N, Cheng J, Miao S, Roy S, Fissell WH: Orbital shear stress regulates differentiation and barrier function of primary renal tubular epithelial cells. ASAIO J 64: 766–772, 2018. 10.1097/MAT.0000000000000723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oo ZY, Deng R, Hu M, Ni M, Kandasamy K, bin Ibrahim MS, Ying JY, Zink D: The performance of primary human renal cells in hollow fiber bioreactors for bioartificial kidneys. Biomaterials 32: 8806–8815, 2011. 10.1016/j.biomaterials.2011.08.030 [DOI] [PubMed] [Google Scholar]
- 39.Zhou M, Ma H, Lin H, Qin J: Induction of epithelial-to-mesenchymal transition in proximal tubular epithelial cells on microfluidic devices. Biomaterials 35: 1390–1401, 2014. 10.1016/j.biomaterials.2013.10.070 [DOI] [PubMed] [Google Scholar]
- 40.Huang HC, Chang YJ, Chen WC, Harn HI, Tang MJ, Wu CC: Enhancement of renal epithelial cell functions through microfluidic-based coculture with adipose-derived stem cells. Tissue Eng Part A 19: 2024–2034, 2013. 10.1089/ten.tea.2012.0605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sciancalepore AG, Sallustio F, Girardo S, Gioia Passione L, Camposeo A, Mele E, Di Lorenzo M, Costantino V, Schena FP, Pisignano D: A bioartificial renal tubule device embedding human renal stem/progenitor cells. [Published correction appears in PLoS One 10: e0128261, 2015.] PLoS One 9: e87496, 2014. 10.1371/journal.pone.0087496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jang KJ, Suh KY: A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 10: 36–42, 2010. 10.1039/B907515A [DOI] [PubMed] [Google Scholar]
- 43.Jang KJ, Cho HS, Kang DH, Bae WG, Kwon TH, Suh KY: Fluid-shear-stress-induced translocation of aquaporin-2 and reorganization of actin cytoskeleton in renal tubular epithelial cells. Integr Biol 3: 134–141, 2011. 10.1039/C0IB00018C [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Tao T, Su W, Yu H, Yu Y, Qin J: A disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab Chip 17: 1749–1760, 2017. 10.1039/C7LC00134G [DOI] [PubMed] [Google Scholar]
- 45.Musah S, Dimitrakakis N, Camacho DM, Church GM, Ingber DE: Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a Glomerulus Chip. Nat Protoc 13: 1662–1685, 2018. 10.1038/s41596-018-0007-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vriend J, Nieskens TTG, Vormann MK, van den Berge BT, van den Heuvel A, Russel FGM, Suter-Dick L, Lanz HL, Vulto P, Masereeuw R, Wilmer MJ: Screening of drug-transporter interactions in a 3D microfluidic renal proximal tubule on a chip. AAPS J 20: 87, 2018. 10.1208/s12248-018-0247-0 [DOI] [PubMed] [Google Scholar]
- 47.Hoppensack A, Kazanecki CC, Colter D, Gosiewska A, Schanz J, Walles H, Schenke-Layland K: A human in vitro model that mimics the renal proximal tubule. Tissue Eng Part C Methods 20: 599–609, 2014. 10.1089/ten.tec.2013.0446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrell N, Ricci KB, Groszek J, Marmerstein JT, Fissell WH: Albumin handling by renal tubular epithelial cells in a microfluidic bioreactor. Biotechnol Bioeng 109: 797–803, 2012. 10.1002/bit.24339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrell N, Desai RR, Fleischman AJ, Roy S, Humes HD, Fissell WH: A microfluidic bioreactor with integrated transepithelial electrical resistance (TEER) measurement electrodes for evaluation of renal epithelial cells. Biotechnol Bioeng 107: 707–716, 2010. 10.1002/bit.22835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jayagopal A, Brakeman PR, Soler P, Ferrell N, Fissell W, Kroetz DL, Roy S: Apical shear stress enhanced organic cation transport in human OCT2/MATE1-transfected madin-darby canine kidney cells involves ciliary sensing. J Pharmacol Exp Ther 369: 523–530, 2019. 10.1124/jpet.118.255026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raghavan V, Rbaibi Y, Pastor-Soler NM, Carattino MD, Weisz OA: Shear stress-dependent regulation of apical endocytosis in renal proximal tubule cells mediated by primary cilia. [Published correction appears in Proc Natl Acad Sci USA 113: e0128261, 2015.] Proc Natl Acad Sci U S A 111: 8506–8511, 2014. 10.1073/pnas.1402195111 [DOI] [PMC free article] [PubMed] [Google Scholar]