Key Points
Respiratory disease was associated with nonrecovery and renal recovery was associated with survival in AKI-2/3 patients with COVID-19.
Machine Learning algorithms can predict AKI and recovery from COVID-19-associated AKI-2/3 and identify key predictors.
At 12-month follow-up in moderate/severe AKI survivors, no difference in CKD between COVID-positive and -negative patients was observed.
Keywords: AKI and ICU nephrology, AKI, CKD, COVID-19, Machine Learning, mortality, recovery
Visual Abstract
Abstract
Background
Severe AKI is strongly associated with poor outcomes in coronavirus disease 2019 (COVID-19), but data on renal recovery are lacking.
Methods
We retrospectively analyzed these associations in 3299 hospitalized patients (1338 with COVID-19 and 1961 with acute respiratory illness but who tested negative for COVID-19). Uni- and multivariable analyses were used to study mortality and recovery after Kidney Disease Improving Global Outcomes Stages 2 and 3 AKI (AKI-2/3), and Machine Learning was used to predict AKI and recovery using admission data. Long-term renal function and other outcomes were studied in a subgroup of AKI-2/3 survivors.
Results
Among the 172 COVID-19-negative patients with AKI-2/3, 74% had partial and 44% complete renal recovery, whereas 12% died. Among 255 COVID-19 positive patients with AKI-2/3, lower recovery and higher mortality were noted (51% partial renal recovery, 25% complete renal recovery, 24% died). On multivariable analysis, intensive care unit admission and acute respiratory distress syndrome were associated with nonrecovery, and recovery was significantly associated with survival in COVID-19-positive patients. With Machine Learning, we were able to predict recovery from COVID-19-associated AKI-2/3 with an average precision of 0.62, and the strongest predictors of recovery were initial arterial partial pressure of oxygen and carbon dioxide, serum creatinine, potassium, lymphocyte count, and creatine phosphokinase. At 12-month follow-up, among 52 survivors with AKI-2/3, 26% COVID-19-positive and 24% COVID-19-negative patients had incident or progressive CKD.
Conclusions
Recovery from COVID-19-associated moderate/severe AKI can be predicted using admission data and is associated with severity of respiratory disease and in-hospital death. The risk of CKD might be similar between COVID-19-positive and -negative patients.
Introduction
Patients hospitalized with coronavirus disease 2019 (COVID-19) often develop sepsis, leading to a cytokine storm and subsequent organ dysfunction (1). AKI is a hallmark of COVID-19 and a major risk factor associated with poor outcomes in hospitalized patients (2–5). To our knowledge, no study has compared the factors and outcomes associated with short- and long-term renal recovery after AKI in hospitalized patients with and without COVID-19.
Most outcome studies of recovery after AKI in the pre-COVID era show favorable outcomes with renal recovery (6), including lower risk of progression to kidney failure (7) or death (8). Other data suggest that AKI, even in the presence of renal recovery, is associated with long-term mortality (7,9). Among patients with COVID-19, factors associated with recovery after AKI include remission of proteinuria (10), baseline CKD (11), right heart failure (12), and oxygenation status (12). We previously reported the lower mortality in those who had recovered from COVID-19-associated AKI compared with those had not recovered (13). However, this study was limited by a small sample size, lack of a COVID-negative group, and short follow-up (13).
Early prediction of AKI and recovery in COVID-19 can allow better preventive management, hospital resource allocation, and patient prognostication in this pandemic where acute surges in hospitalizations are seen. Machine Learning (ML) approaches of AKI prediction using electronic health records (EHR) data have been reported in multiple settings (14–17), with greater accuracy for predicting more severe AKI and closer time points to AKI onset (18–21). To the best of our knowledge, the use of ML models to predict recovery from COVID-19 AKI has not been previously reported. AKI is a risk factor for the development of CKD (22), including in those with complete recovery (23); however, there are very few studies that have reported the incidence of post-AKI CKD in the setting of COVID-19 (24,25).
The severity of AKI has been associated with worse outcomes (26,27), including in patients with COVID-19 (26). In this study, we investigated the factors and outcomes associated with renal recovery in patients with Kidney Disease Improving Global Outcomes (KDIGO) Stages 2 and 3 AKI (AKI-2/3) hospitalized in the setting of COVID-19. We compared patients who tested negative for COVID-19 using approximately 100 variables around the time of hospitalization. We also investigated if ML can be used to predict moderate/severe AKI and subsequent recovery in patients with and without COVID-19 using 57 data features at the time of hospitalization. Finally, in a subgroup of survivors with AKI-2/3, we investigated the presence of new-onset or progressive CKD using serum creatinine (SCr) and eGFR data more than 3 months after hospital discharge.
Materials and Methods
Study Design and Participants
We conducted a retrospective cohort study on patients hospitalized at Stony Brook University Medical Center from March 7, 2020, to July 31, 2020. COVID-19 was diagnosed by at least one positive result for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on PCR testing of nasopharyngeal samples. Patients who were hospitalized with suggestive symptoms of COVID-19 but subsequently reported a negative PCR were placed in the control (COVID-19 negative) group. All patients were followed until a final disposition of discharge alive from the hospital or in-hospital death. A subgroup of survivors with AKI-2/3 were followed for 12 months post discharge. We excluded patients who were ≤18 years of age, pregnant, or who had ESKD, including chronic dialysis or kidney transplant. The study was approved by the Stony Brook University Institutional Review Board.
Data Collection and Definition of Variables
Information pertaining to data collection and the definition of variables is detailed in the Supplemental Materials and Methods.
AKI, Recovery, and Post-AKI CKD definitions
AKI was defined as a rise in SCr of ≥1.5 times the baseline to maximum in-hospital SCr based on the KDIGO definition (28). More than 80% of patients did not have a prehospitalization SCr available. Therefore, we used the lowest SCr in the hospital as the “baseline” as has been previously used in COVID-19 studies (29). Further discussion on the rationale of using this baseline SCr criteria is provided in the Supplemental Materials and Methods. RRT was defined as the need for either hemodialysis or continuous kidney replacement therapy, or both. AKI classes for in-hospital AKI were assigned for each patient based on the KDIGO criteria (28). Class 1 was assigned for an acute increase in SCr by 50%, Class 2 for an increase by 100%, and Class 3 for an increase of 200% or if the patient required RRT during hospitalization. In this study, we focused on AKI-2/3, and the rationale is further detailed in the Supplemental Materials and Methods.
Recovery from AKI was defined in two ways. For the main analysis, we used the definition of Recovery-1 based on the Acute Dialysis Quality Initiative criteria (30) (final SCr at discharge returned to ≤50% above baseline SCr). For sensitivity analysis, we used the Recovery-2 definition based on a stricter criterion defined by Bucaloiu et al. (31) (final SCr at discharge returned to ≤10% above baseline SCr). For both analyses, if a patient died before recovery, then he/she was treated as not recovered. In addition, patients with RRT requiring AKI had to get off RRT at least 3 days before discharge, and for those without RRT requiring AKI, the final SCr should be <50% of the maximum SCr. These two criteria were applied to both recovery definitions. The rationale of using these recovery definitions is further detailed in the Supplemental Materials and Methods.
A subgroup analysis was done on AKI-2/3 survivors who were followed for 12 months post discharge. For each patient, the most recent post-discharge outpatient SCr and eGFR values were used and were collected >90 days post discharge after index hospitalization. Post-AKI CKD was diagnosed if the patient’s latest outpatient SCr value remained >10% above baseline SCr plus a final eGFR <60 ml/min per 1.73 m2. CKD was further divided into “incident CKD” if the patient had no history of CKD before hospitalization and “progressive CKD” if the patient had a history of CKD before hospitalization based on EHR documentation.
Statistical Analyses
All statistical analyses were performed using R v3.6.0 (The R Foundation for Statistical Computing, Vienna, Austria). Univariate logistic regression was used to select potential explanatory variables for the outcomes. For multivariable analysis, variables with too many missing values (>5%) were removed. Variables that were significant based on univariate analysis were placed in the order of clinical importance (demographics, comorbid conditions, severity of illness, medications, and other measures), and then backward stepwise logistic regression was used to select the best model based on Akaike information criterion (32). Variable categories of clinical importance (demographics, comorbid conditions, and severity of illness) were forced to be kept in the final model. A cutoff of P<0.05 was considered statistically significant.
ML Analysis
We considered the task of predicting if a patient will develop AKI and recovery from AKI during their hospital stay using data at the time of hospitalization (first 48 hours of the Emergency Department visit) in the cohort. Our ML models were based on XGBoost, a state-of-the-art algorithm that can handle both numerical and categorical attributes and has been previously used in ML-based predictive modeling studies in COVID-19 (33). We calculated the precision–recall curve and calculated the average precision (AP), which are standard metrics to measure the performance of predictors. SHAP (SHapley Additive exPlanations), an established method to explain individual predictions (34), including kidney disease (35), was used to identify predictors of AKI and recovery. Further details of the ML methodology are described in the Supplemental Materials and Methods.
Results
Comparison of Patients with and without COVID-19
Out of 1338 patients with COVID-19, 43% were women, 42% non-White, and 18% Hispanic, whereas among the 1961 COVID-19-negative patients, 45% were women, 17% non-White, and 4% Hispanic. Among patients with COVID-19, 553 (41%) were diagnosed with AKI, 255 (19%) had moderate/severe AKI (AKI-2/3), and 118 (9%) had severe AKI (AKI-3). Among the COVID-19-negative patients, 474 (24%) had AKI, 172 (9%) had AKI-2/3, and only 67 (3%) had AKI-3 (Supplemental Figure 1). In the AKI-3 group, among patients with COVID-19, 35 (3% of total cohort and 6% of all AKI patients) required RRT, whereas among COVID-19-negative patients, only nine patients (0.46% and 2%, respectively) required RRT. Comparison of the characteristics of patients with and without COVID-19 is detailed in Supplemental Table 1.
Comparison of Patients with and without AKI-2/3
Patients with AKI-2/3 (compared with those without) had greater severity of illness and death (Table 1). On multivariable analysis, in both patient groups (COVID-19 positive and negative), those with intensive care unit (ICU) admission, greater length of hospital stay, and sepsis had significantly higher odds of having AKI-2/3, whereas those receiving anticoagulation had lower odds (Figure 1, A and B). Older age, mechanical ventilation (MV) and higher initial blood urea nitrogen were specifically associated with COVID-19-associated AKI-2/3 (Figure 1A). Sensitivity analysis restricted to patients with AKI-3 showed mostly similar associations (Supplemental Figure 2, A and B, Supplemental Table 2).
Table 1.
Univariate analysis of patients with and without AKI-2/3 in presence and absence of COVID-19
| Variables | COVID-19 Negative (N=1961) | COVID-19 Positive (N=1338) | ||||
|---|---|---|---|---|---|---|
| No AKI-2/3 | AKI-2/3 | No AKI-2/3 | AKI-2/3 | |||
| (N=1789) | (N=172) | P Value | (N=1083) | (N=255) | P Value | |
| Demographics | ||||||
| Sex, n (%) | ||||||
| Men | 996 (55.67) | 92 (53.49) | 601 (55.49) | 161 (63.14) | ||
| Women | 793 (44.33) | 80 (46.51) | 0.5819 | 482 (44.51) | 94 (36.86) | 0.0269a |
| Race, n (%) | ||||||
| White | 1476 (82.5) | 152 (88.37) | 632 (58.36) | 148 (58.04) | ||
| Non-White | 313 (17.5) | 20 (11.63) | 0.0522 | 451 (41.64) | 107 (41.96) | 0.9264 |
| Ethnicity, n (%) | ||||||
| Non-Hispanic | 1708 (95.47) | 169 (98.26) | 875 (80.79) | 219 (85.88) | ||
| Hispanic | 81 (4.53) | 3 (1.74) | 0.0976 | 208 (19.21) | 36 (14.12) | 0.0594 |
| Age, yr, mean (SD) | 63.25 (19.42) | 66.92 (17.25) | 0.0173a | 60.87 (18.26) | 65.76 (15.48) | 1.00E-04a |
| Comorbid conditions, n (%) | ||||||
| DM | 440 (24.59) | 57 (33.14) | 0.0144a | 292 (26.96) | 112 (43.92) | 0a |
| HF | 343 (19.17) | 49 (28.49) | 0.0038a | 150 (13.85) | 51 (20) | 0.014a |
| CKD | 256 (14.31) | 38 (22.09) | 0.0068a | 124 (11.45) | 61 (23.92) | 0a |
| COPD | 259 (14.48) | 34 (19.77) | 0.0644 | 112 (10.34) | 38 (14.9) | 0.0389a |
| HTN | 858 (47.96) | 79 (45.93) | 0.6109 | 448 (41.37) | 123 (48.24) | 0.0464a |
| CAD | 483 (27) | 47 (27.33) | 0.9265 | 191 (17.64) | 57 (22.35) | 0.0819 |
| Cancer | 294 (16.43) | 32 (18.6) | 0.4655 | 74 (6.83) | 24 (9.41) | 0.1568 |
| Asthma | 109 (6.09) | 11 (6.4) | 0.8744 | 81 (7.48) | 16 (6.27) | 0.505 |
| BMI, mean (SD), kg/m2 | 29.41 (9.82) | 29.58 (16.1) | 0.8382 | 29.68 (9.63) | 29.57 (7.34) | 0.8784 |
| Severity of illness | ||||||
| Length of hospital stay, mean (SD), d | 6.33 (5.16) | 13.52 (10.17) | 0a | 8.98 (6.61) | 21.23 (15.48) | 0a |
| ICU admission, n (%) | 275 (15.37) | 72 (41.86) | 0a | 129 (11.91) | 163 (63.92) | 0a |
| Length of ICU stay, mean (SD), d | 7.77 (14.43) | 15.74 (21.66) | 9.00E-04a | 10.77 (15.91) | 17.6 (15.68) | 7.00E-04a |
| MV, n (%) | 146 (8.16) | 24 (13.95) | 0.0109a | 87 (8.03) | 72 (28.24) | 0a |
| MV days, mean (SD) | 4.03 (3.58) | 13.32 (12.5) | 0a | 8.58 (5.62) | 17.94 (12.54) | 0a |
| ARD, n (%) | 296 (16.55) | 61 (35.47) | 0a | 723 (66.76) | 221 (86.67) | 0a |
| ARDS, n (%) | 2 (0.11) | 4 (2.33) | 4.00E-04a | 99 (9.14) | 103 (40.39) | 0a |
| Vasopressor, n (%) | 232 (12.97) | 37 (21.51) | 0.0021a | 23 (2.12) | 18 (7.06) | 1.00E-04a |
| Sepsis, n (%) | 163 (9.11) | 59 (34.3) | 0a | 257 (23.73) | 161 (63.14) | 0a |
| Medications, n (%) | ||||||
| ACEI | 285 (15.93) | 42 (24.42) | 0.0047a | 153 (14.13) | 75 (29.41) | 0a |
| ARB | 321 (17.94) | 19 (11.05) | 0.0241a | 177 (16.34) | 52 (20.39) | 0.1233 |
| AC | 1488 (83.17) | 110 (63.95) | 0a | 1018 (94) | 171 (67.06) | 0a |
| NSAIDs | 644 (36) | 65 (37.79) | 0.6402 | 260 (24.01) | 91 (35.69) | 2.00E-04a |
| Remdesivir | 0 (0) | 0 (0) | NA | 12 (1.11) | 8 (3.14) | 0.0215a |
| Hydroxychloroquine | 56 (3.13) | 9 (5.23) | 0.1458 | 714 (65.93) | 203 (79.61) | 0a |
| Tocilizumab | 0 (0) | 0 (0) | NA | 84 (7.76) | 58 (22.75) | 0a |
| Azithromycin | 227 (12.69) | 29 (16.86) | 0.1224 | 502 (46.35) | 150 (58.82) | 4.00E-04a |
| Vitals, mean (SD) | ||||||
| SBP, mm Hg | 132.77 (28.24) | 135.05 (33.22) | 0.3215 | 132.88 (27.65) | 136.85 (30.56) | 0.0439a |
| Oral temperature, °C | 37.12 (0.73) | 36.99 (0.59) | 0.0324a | 37.14 (0.75) | 37.11 (0.77) | 0.518 |
| Respiratory measures, mean (SD) | ||||||
| FIO2 | 57.91 (24.58) | 64.65 (27.52) | 0.0924 | 57.55 (26.5) | 67.76 (26.45) | 8.00E-04a |
| Renal labs, mean (SD) | ||||||
| BUN, mg/dl | 22.79 (20.02) | 27.15 (20.48) | 0.0074a | 20.94 (17.85) | 24.21 (17.07) | 0.0101a |
| K, mmol/L | 4.19 (0.62) | 4.27 (0.73) | 0.1052 | 4.15 (0.58) | 4.25 (0.65) | 0.0181a |
| HCO3, mmol/L | 23.74 (4.15) | 23.38 (4.46) | 0.2822 | 24.03 (3.86) | 23.28 (4.83) | 0.0078a |
| iCa, mg/dl | 4.54 (0.57) | 4.4 (0.55) | 0.1994 | 4.58 (0.47) | 4.41 (0.48) | 0.0259a |
| Phos, mg/dl | 3.39 (1.12) | 3.63 (1.46) | 0.0132a | 3.28 (1.06) | 3.56 (1.45) | 0.0014a |
| Inflammatory labs, mean (SD) | ||||||
| Ferritin, ng/ml | 775.34 (1194.39) | 1211.87 (2730.12) | 0.0089a | 783.02 (1183.33) | 1067.04 (1650.39) | 0.0148a |
| Serum albumin, g/dl | 3.85 (0.59) | 3.79 (0.57) | 0.1963 | 3.83 (0.59) | 3.74 (0.65) | 0.035a |
| CRP, mg/dl | 8.56 (9.23) | 6.84 (7.71) | 0.0664 | 8.55 (9.08) | 10.16 (10.41) | 0.0406a |
| Other labs, mean (SD) | ||||||
| Hb, g/dl | 12.96 (2.34) | 12.39 (2.9) | 0.0029a | 13.04 (2.28) | 12.72 (2.67) | 0.0557a |
| Lactate, mmol/L | 2 (1.95) | 2.39 (2.58) | 0.0523 | 1.9 (1.69) | 2.59 (3.3) | 3.00E-04a |
| INR | 1.31 (0.8) | 1.26 (0.5) | 0.3936 | 1.27 (0.62) | 1.4 (1) | 0.0204a |
| LDH, IU/L | 328.4 (254.43) | 468.22 (450.42) | 0.0346a | 285.12 (138.86) | 283.61 (116.62) | 0.9487 |
| Death, n (%) | 99 (5.53) | 20 (11.63) | 0.0018a | 87 (8.03) | 61 (23.92) | 0a |
Categorical variables presented as a count with associated percentage; continuous variables presented as value with SD. P values <0.0001 are labeled as 0. All variables were included until the “Severity of illness” section; for the rest, only significant variables were kept for simplicity. AKI-2/3, AKI Stages 2 and 3; COVID-19, coronavirus disease 2019; DM, diabetes mellitus; HF, heart failure; COPD, chronic obstructive pulmonary disease; HTN, hypertension; CAD, coronary artery disease; BMI, body mass index; ICU, intensive care unit; MV, mechanical ventilation; ARD, acute respiratory disease; ARDS, acute respiratory distress syndrome; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; AC, anticoagulation; NSAIDs, nonsteroidal anti-inflammatory drugs; SBP, systolic blood pressure; FIO2, fraction of inspired oxygen; K, potassium; HCO3, bicarbonate; iCa, ionized calcium; Phos, phosphorus; CRP, C-reactive protein; Hb, hemoglobin; INR, International Normalized Ratio; LDH, lactate dehydrogenase.
P values <0.05 were considered significant.
Figure 1.
Forest plots of patients with versus without AKI Stages 2 and 3 (AKI-2/3). (A) Forest plot showing the multivariable analysis of coronavirus disease 2019 (COVID-19)-positive patients with versus without AKI-2/3. (B) Forest plot showing the multivariable analysis of COVID-19-negative patients with versus without AKI-2/3.
Comparison of Patients with and without Recovery after AKI-2/3
Out of a total of 255 patients with COVID-19 who had AKI-2/3, 129 (51%) had at least partial renal recovery (Recovery-1), whereas 126 (49%) did not. Among the 172 patients with AKI-2/3 who tested negative for COVID-19, 128 (74%) had renal recovery, whereas 44 (26%) did not (Supplemental Figure 1, Table 2). In our sensitivity analysis (Recovery-2), out of a total of 255 patients with AKI-2/3 in the setting of COVID-19, 63 (25%) had complete renal recovery, whereas 192 (75%) did not (Supplemental Table 3). Among the 172 patients with AKI-2/3 who tested negative for COVID-19, 76 (44%) had renal recovery, whereas 96 (56%) did not (Supplemental Table 3).
Table 2.
Univariate analysis of patients with and without Recovery-1 from AKI-2/3 in presence and absence of COVID-19
| Variables | COVID-19 Negative (N=172) | P Value | COVID-19 Positive (N=255) | P Value | ||
|---|---|---|---|---|---|---|
| No Recovery | Recovery-1 | No Recovery | Recovery-1 | |||
| (N1=44) | (N2=128) | (N1=126) | (N2=129) | |||
| Demographics | ||||||
| Sex, n (%) | ||||||
| Men | 23 (52.27) | 69 (53.91) | 84 (66.67) | 77 (59.69) | ||
| Women | 21 (47.73) | 59 (46.09) | 0.8514 | 42 (33.33) | 52 (40.31) | 0.2488 |
| Race, n (%) | ||||||
| White | 39 (88.64) | 113 (88.28) | 63 (50) | 85 (65.89) | ||
| Non-White | 5 (11.36) | 15 (11.72) | 0.9495 | 63 (50) | 44 (34.11) | 0.0105a |
| Ethnicity, n (%) | ||||||
| Non-Hispanic | 44 (100) | 125 (97.66) | 102 (80.95) | 117 (90.7) | ||
| Hispanic | 0 (0) | 3 (2.34) | 0.9862 | 24 (19.05) | 12 (9.3) | 0.0283a |
| Age, yr, mean (SD) | 70.89 (15.56) | 65.55 (17.65) | 0.0794 | 64.94 (15.18) | 66.55 (15.79) | 0.4073 |
| Comorbid conditions, n (%) | ||||||
| DM | 12 (27.27) | 45 (35.16) | 0.3393 | 48 (38.1) | 64 (49.61) | 0.0646 |
| HF | 18 (40.91) | 31 (24.22) | 0.0365a | 24 (19.05) | 27 (20.93) | 0.7072 |
| CKD | 11 (25) | 27 (21.09) | 0.5905 | 31 (24.6) | 30 (23.26) | 0.8009 |
| COPD | 13 (29.55) | 21 (16.41) | 0.0625 | 15 (11.9) | 23 (17.83) | 0.1867 |
| HTN | 16 (36.36) | 63 (49.22) | 0.142 | 62 (49.21) | 61 (47.29) | 0.7591 |
| CAD | 15 (34.09) | 32 (25) | 0.245 | 31 (24.6) | 26 (20.16) | 0.3946 |
| Cancer | 10 (22.73) | 22 (17.19) | 0.4168 | 9 (7.14) | 15 (11.63) | 0.2242 |
| Asthma | 2 (4.55) | 9 (7.03) | 0.5641 | 9 (7.14) | 7 (5.43) | 0.5731 |
| BMI, mean (SD), kg/m2 | 28.31 (5.58) | 30.03 (18.44) | 0.5689 | 28.99 (6.39) | 30.1 (8.09) | 0.2564 |
| Severity of illness | ||||||
| Length of hospital stay, mean (SD), d | 12.82 (8.79) | 13.77 (10.62) | 0.5937 | 22.6 (16.2) | 19.89 (14.69) | 0.1636 |
| ICU admission, n (%) | 25 (56.82) | 47 (36.72) | 0.0212a | 102 (80.95) | 61 (47.29) | 0a |
| Length of ICU stay, mean (SD), d | 11.12 (13.65) | 18.19 (24.69) | 0.2078 | 18.54 (16.81) | 16.03 (13.59) | 0.3253 |
| MV, n (%) | 9 (20.45) | 15 (11.72) | 0.1541 | 44 (34.92) | 28 (21.71) | 0.02a |
| MV days, mean (SD) | 11 (11.11) | 14.52 (13.21) | 0.395 | 17.54 (13.08) | 18.79 (11.4) | 0.5739 |
| ARD, n (%) | 22 (50) | 39 (30.47) | 0.021a | 119 (94.44) | 102 (79.07) | 7.00E-04a |
| ARDS, n (%) | 2 (4.55) | 2 (1.56) | 0.2794 | 67 (53.17) | 36 (27.91) | 0a |
| Vasopressor, n (%) | 8 (18.18) | 29 (22.66) | 0.534 | 8 (6.35) | 10 (7.75) | 0.6624 |
| Sepsis, n (%) | 18 (40.91) | 41 (32.03) | 0.2859 | 87 (69.05) | 74 (57.36) | 0.054 |
| Medications, n (%) | ||||||
| Hydroxychloroquine | 3 (6.82) | 6 (4.69) | 0.5862 | 108 (85.71) | 95 (73.64) | 0.0182a |
| Tocilizumab | 0 (0) | 0 (0) | NA | 41 (32.54) | 17 (13.18) | 3.00E-04a |
| Azithromycin | 7 (15.91) | 22 (17.19) | 0.8451 | 89 (70.63) | 61 (47.29) | 2.00E-04a |
| Respiratory measures, mean (SD) | ||||||
| Pulse Ox | 94.77 (6.95) | 95.88 (4.16) | 0.2185 | 94.04 (6.03) | 95.9 (4.19) | 0.0066a |
| Renal labs, mean (SD) | ||||||
| iCa, mg/dl | 4 (0.68) | 4.56 (0.41) | 0.0407a | 4.32 (0.56) | 4.53 (0.33) | 0.0542 |
| Serum Osm, mOsm/kg | 302.25 (19) | 294 (26.72) | 0.5521 | 306.59 (29.39) | 285.48 (16.22) | 0.0105a |
| Urine Na, mEq/L | 47.69 (34.68) | 50.74 (38.89) | 0.7981 | 42.78 (33) | 58.41 (37.83) | 0.0321a |
| Inflammatory labs, mean (SD) | ||||||
| Ferritin, ng/ml | 1374.13 (4431.31) | 1155.52 (1851.46) | 0.7311 | 1433.56 (2075.43) | 691.79 (923.63) | 0.01a |
| Other labs, mean (SD) | ||||||
| Lactate, mmol/L | 3.28 (4.03) | 2 (1.48) | 0.0327a | 2.64 (3.51) | 2.54 (3.08) | 0.8394 |
Categorical variables presented as a count with associated percentage; continuous variables presented as value with SD. P values <0.0001 are labeled as 0. All variables were included until and including the “Severity of illness” section; for the rest, only significant variables were kept for simplicity. AKI-2/3, AKI Stages 2 and 3; COVID-19, coronavirus disease 2019; DM, diabetes mellitus; HF, heart failure; COPD, chronic obstructive lung disease; HTN, hypertension; CAD, coronary artery disease; BMI, body mass index; ICU, intensive care unit; MV, mechanical ventilation; ARD, acute respiratory disease; ARDS, acute respiratory distress syndrome; Ox, oxygenation; iCa, ionized calcium; Osm, osmolality; Na, sodium.
P values <0.05 were considered significant.
Among COVID-19-positive patients, we compared those with Recovery-1 after AKI-2/3 with those without Recovery-1 (Table 2). On multivariable analysis (Figure 2A), only ICU admission remained significantly associated with lower odds of Recovery-1 (odds ratio [OR]=0.35; 95% confidence interval [CI], 0.18 to 0.67]). Among COVID-19-negative patients (Figure 2B), no association was noted.
Figure 2.
Forest plots of patients with versus without Recovery-1. (A) Forest plot showing the multivariable analysis of COVID-19-positive patients with versus without Recovery-1 after AKI-2/3. (B) Forest plot showing the multivariable analysis of COVID-19 negative patients with versus without Recovery-1 after AKI-2/3. Recovery-1 was defined as the final serum creatinine (SCr) at discharge returning to ≤50% above baseline SCr.
The associations with Recovery-2 were similar to associations with Recovery-1 on univariate analysis (Supplemental Table 3). On multivariable analysis (Supplemental Figure 3A), among COVID-19-positive patients, acute respiratory distress syndrome (ARDS) was significantly associated with lower odds of recovery (OR=0.28; 95% CI, 0.12 to 0.70). Diabetes mellitus was associated with borderline higher odds of recovery (OR=2.05; 95% CI, 1.04 to 4.05). Among COVID-19-negative patients (Supplemental Figure 3B), ICU admission was association with lower odds of recovery (OR=0.52; 95% CI, 0.28 to 0.97).
Comparison of Patients with and without Recovery after AKI-3
In the sensitivity analysis restricted to patients with AKI-3, out of a total of 118 patients with COVID-19 who had AKI-3, 43 (37%) had partial renal recovery, whereas 75 (63%) did not (Recovery-1; Supplemental Table 4). Among the 67 patients with AKI-3 who tested negative for COVID-19, 47 (70%) had renal recovery, whereas 20 (29%) did not (Supplemental Table 4). Among COVID-19 positive patients, 24 (20%) had complete renal recovery (Recovery-2), whereas 94 (80%) did not (Supplemental Table 5). Among the COVID-19-negative patients, 31 (46%) had renal recovery, whereas 36 (54%) did not.
We compared those with and without Recovery-1 and Recovery-2 after AKI-3 with those without (Supplemental Tables 4 and 5), and the results of multivariable analysis are presented in Supplemental Figures 4, A and B and 5, A and B.
Analysis of Death in Patients with and without Recovery after AKI-2/3
Among patients with COVID-19, 148 (11%) of the 1338 patients died, and among COVID-19-negative patients, 119 (6%) of the 1961 patients died (Supplemental Table 6). Among AKI-2/3 patients with COVID-19, 61 (24%) of the 255 patients died, whereas among COVID-19-negative patients, 20 (12%) of the 172 patients died (Table 3). In COVID-19-positive patients, both Recovery-1 and Recovery-2 were significantly associated with survival, whereas in COVID-19-negative patients, only Recovery-1 was significantly associated with survival (Table 3).
Table 3.
Univariate analysis comparing death in patients with and without AKI-2/3 in presence and absence of COVID-19
| Variables | Covid-19 Negative (N=172) | P Value | Covid-19 Positive (N=255) | P Value | ||
|---|---|---|---|---|---|---|
| No Death | Death | No Death | Death | |||
| (N1=152) | (N2=20) | (N1=194) | (N2=61) | |||
| Demographics | ||||||
| Sex, n (%) | ||||||
| Men | 79 (51.97) | 13 (65) | 114 (58.76) | 47 (77.05) | ||
| Women | 73 (48.03) | 7 (35) | 0.2764 | 80 (41.24) | 14 (22.95) | 0.0111a |
| Race, n (%) | ||||||
| White | 134 (88.16) | 18 (90) | 116 (59.79) | 32 (52.46) | ||
| Non-White | 18 (11.84) | 2 (10) | 0.8093 | 78 (40.21) | 29 (47.54) | 0.3121 |
| Ethnicity, n (%) | ||||||
| Non-Hispanic | 149 (98.03) | 20 (100) | 169 (87.11) | 50 (81.97) | ||
| Hispanic | 3 (1.97) | 0 (0) | 0.9916 | 25 (12.89) | 11 (18.03) | 0.3162 |
| Age, mean (SD) | 66.62 (16.85) | 69.15 (20.42) | 0.5379 | 67.08 (15.14) | 61.56 (15.92) | 0.0164a |
| Comorbid conditions, n (%) | ||||||
| DM | 49 (32.24) | 8 (40) | 0.4895 | 92 (47.42) | 20 (32.79) | 0.0462a |
| HF | 43 (28.29) | 6 (30) | 0.8734 | 46 (23.71) | 5 (8.2) | 0.012a |
| CKD | 30 (19.74) | 8 (40) | 0.046a | 51 (26.29) | 10 (16.39) | 0.1177 |
| COPD | 29 (19.08) | 5 (25) | 0.5335 | 29 (14.95) | 9 (14.75) | 0.9703 |
| HTN | 73 (48.03) | 6 (30) | 0.1352 | 95 (48.97) | 28 (45.9) | 0.6759 |
| CAD | 38 (25) | 9 (45) | 0.0652 | 43 (22.16) | 14 (22.95) | 0.8978 |
| Cancer | 28 (18.42) | 4 (20) | 0.8646 | 22 (11.34) | 2 (3.28) | 0.078 |
| Asthma | 11 (7.24) | 0 (0) | 0.9896 | 8 (4.12) | 8 (13.11) | 0.0165a |
| BMI, mean (SD), kg/m2 | 29.84 (16.8) | 27.17 (6.26) | 0.5366 | 29.4 (7.21) | 30.15 (7.78) | 0.5154 |
| Severity of illness | ||||||
| Length of hospital stay, mean (SD), d | 12.99 (9.63) | 17.55 (13.18) | 0.0689 | 20.22 (15.52) | 24.44 (15.03) | 0.0658 |
| ICU admission, n (%) | 57 (37.5) | 15 (75) | 0.003a | 115 (59.28) | 48 (78.69) | 0.007a |
| Length of ICU stay, mean (SD), d | 16.65 (23.93) | 12.27 (8.57) | 0.4909 | 17.1 (16.21) | 18.79 (14.43) | 0.5312 |
| MV, n (%) | 9 (5.92) | 15 (75) | 0a | 21 (10.82) | 51 (83.61) | 0a |
| MV days, mean (SD) | 13.41 (12.3) | 13.08 (13.53) | 0.9379 | 16.84 (11.89) | 20.1 (13.59) | 0.1367 |
| ARD, n (%) | 51 (33.55) | 10 (50) | 0.1538 | 163 (84.02) | 58 (95.08) | 0.0368a |
| ARDS, n (%) | 3 (1.97) | 1 (5) | 0.4155 | 68 (35.05) | 35 (57.38) | 0.0023a |
| Vasopressor, n (%) | 31 (20.39) | 6 (30) | 0.3297 | 14 (7.22) | 4 (6.56) | 0.8609 |
| Sepsis, n (%) | 50 (32.89) | 9 (45) | 0.2873 | 117 (60.31) | 44 (72.13) | 0.0971 |
| Medications, n (%) | ||||||
| ACEI | 37 (24.34) | 5 (25) | 0.9487 | 70 (36.08) | 5 (8.2) | 2.00E-04a |
| AC | 93 (61.18) | 17 (85) | 0.0483a | 119 (61.34) | 52 (85.25) | 9.00E-04a |
| NSAIDs | 53 (34.87) | 12 (60) | 0.0344a | 68 (35.05) | 23 (37.7) | 0.706 |
| Vitals, mean (SD) | ||||||
| SBP, mm Hg | 135.89 (33.63) | 128.6 (29.92) | 0.3553 | 139.39 (30.83) | 128.67 (28.39) | 0.0186a |
| DBP, mm Hg | 76.7 (16.22) | 70.55 (12.75) | 0.1061 | 77.16 (14.26) | 70.85 (12) | 0.0027a |
| MAP, mm Hg | 97.07 (18.2) | 91.85 (16.44) | 0.2229 | 96.15 (18.26) | 88.24 (13.59) | 0.0028a |
| Oral temperature, °C | 36.97 (0.58) | 37.14 (0.75) | 0.3068 | 37.02 (0.71) | 37.45 (0.92) | 8.00E-04a |
| Respiratory rate (breaths per minute) | 19.36 (4.5) | 25.2 (13.08) | 0.0018 a | 19.85 (4.81) | 25.84 (13.12) | 0a |
| Respiratory measures, mean (SD) | ||||||
| Pulse Ox | 96.24 (3.55) | 90.7 (9.92) | 7.00E-04a | 96.06 (3.82) | 91.56 (7.37) | 0 |
| Renal labs, mean (SD) | ||||||
| Na, mmol/L | 137.5 (5.1) | 134.9 (5.82) | 0.039a | 137.42 (4.75) | 134.7 (5.79) | 4.00E-04 |
| Cl, mmol/L | 99.42 (6.27) | 95.65 (6.17) | 0.016a | 99.42 (5.71) | 96.15 (6.81) | 4.00E-04 |
| HCO3, mmol/L | 23.69 (4.27) | 21 (5.25) | 0.0138a | 24.03 (4.27) | 20.9 (5.69) | 0 |
| Phos, mg/dl | 3.53 (1.28) | 4.41 (2.31) | 0.0238a | 3.43 (1.25) | 3.95 (1.88) | 0.0258 |
| Mg, mg/dl | 1.99 (0.35) | 2.19 (0.41) | 0.0282a | 1.98 (0.32) | 2.14 (0.43) | 0.0049 |
| Urine Na, mEq/L | 57.08 (39.58) | 26.33 (14.14) | 0.0314a | 60.91 (37.82) | 37.75 (29.69) | 0.0019 |
| Urine RBCs (per HPF) | 7.99 (24.24) | 40.39 (69.84) | 0.0075a | 17.37 (42.05) | 24.58 (43.65) | 0.3015 |
| Inflammatory labs, mean (SD) | ||||||
| Ferritin, ng/ml | 850.81 (1562.35) | 3544.82 (5994.73) | 0.0322a | 813.65 (1451.99) | 1611.36 (1915.27) | 0.0089a |
| Lymphocyte count, K/μl | 1.32 (0.8) | 0.87 (0.38) | 0.0424a | 1.46 (1.08) | 0.76 (0.46) | 0a |
| ESR, mm/h | 47.97 (32.74) | 72.25 (23.63) | 0.0661 | 44.21 (32.51) | 57.45 (30.15) | 0.0432a |
| CRP, mg/dl | 5.39 (5.79) | 14.8 (11.6) | 2.00E-04a | 8.09 (9.38) | 14.61 (11.18) | 3.00E-04a |
| Other labs, mean (SD) | ||||||
| Lactate, mmol/L | 2.03 (1.94) | 4.41 (4.35) | 0.0072a | 2.05 (2) | 3.76 (4.92) | 0.0077a |
| BNP, pg/ml | 1947.37 (3522.84) | 4756.67 (9475.9) | 0.0748a | 2959.07 (9177.11) | 4193.52 (7608.25) | 0.4189 |
| LDH, IU/L | 344.24 (269.16) | 819.5 (678.6) | 0.0601 | 242.88 (72.48) | 451.62 (116.71) | 0.0052a |
| AST, IU/L | 41.21 (48.23) | 129.2 (172.14) | 8.00E-04a | 44.12 (61.18) | 84.95 (124.55) | 0.0126a |
| ALT, IU/L | 32.05 (37.29) | 98 (200.78) | 0.034a | 37.07 (67.05) | 43.82 (39.15) | 0.4648 |
| Recovery-1, n (%) | 117 (76.97) | 11 (55) | 0.0396a | 110 (56.7) | 19 (31.15) | 7.00E-04a |
| Recovery-2, n (%) | 71 (46.71) | 5 (25) | 0.0741 | 56 (28.87) | 7 (11.48) | 0.0082a |
Categorical variables presented as a count with associated percentage; continuous variables presented as value with SD. P values <0.0001 are labeled 0. All variables were included until and including “Severity of illness” section; for the rest, only significant variables were kept for simplicity. AKI-2/3, AKI Stages 2 and 3; COVID-19, coronavirus disease 2019; DM, diabetes mellitus; HF, heart failure; COPD, chronic obstructive pulmonary disease; HTN, hypertension; CAD, coronary artery disease; BMI, body mass index; ICU, intensive care unit; MV, mechanical ventilation; ARD, acute respiratory disease; ARDS, acute respiratory distress syndrome; ACEI, angiotensin converting enzyme inhibitor; AC, anticoagulation; NSAIDs, nonsteroidal anti-inflammatory drugs; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; Ox, oxygenation; Na, sodium; Cl, chloride; HCO3, bicarbonate; Phos, phosphorus; Mg, magnesium; RBCs, red blood cells; HPF, high power field; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; BNP, brain natriuretic peptide; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
P values <0.05 were considered significant.
On multivariable analysis, in both AKI-2/3 groups (with COVID-19 [Figure 3] or without COVID-19 [Figure 4]), those with MV had significantly higher odds of death. Among COVID-19-positive patients, higher initial blood urea nitrogen was specifically associated with death, whereas women and Recovery-1 (partial) were associated with survival (Figure 3A). However, the association of Recovery-2 (complete) with survival in patients with COVID-19 was no longer statistically significant (Figure 3B). Among COVID-19-negative patients, a higher initial respiratory rate was associated with death, and the association of Recovery-1 and Recovery-2 with survival was not statistically significant (Figure 4, A and B).
Figure 3.
Forest plots of COVID-19-positive patients analyzing the association of Recovery-1 and Recovery-2 with death. (A) Forest plot showing the multivariable analysis of death in COVID-19-positive patients analyzing the association of Recovery-1 after AKI-2/3. (B) Forest plot showing the multivariable analysis of death in COVID-19-positive patients analyzing the association of Recovery-2 after AKI-2/3. Recovery-2 was defined as the final SCr at discharge returning to ≤10% above baseline SCr.
Figure 4.
Forest plots of COVID-19-negative patients analyzing the association of Recovery-1 and Recovery-2 with death. (A) Forest plot showing the multivariable analysis of death in COVID-19-negative patients analyzing the association of Recovery-1 after AKI-2/3. (B) Forest plot showing the multivariable analysis of death in COVID-19-negative patients analyzing the association of Recovery-2 after AKI-2/3.
Analysis of Death in Patients with and without Recovery after AKI-3
Among AKI-3 patients with COVID-19, 32 (27%) of the 118 patients died, whereas among COVID-19-negative patients, eight (12%) of the 67 patients died (Supplemental Table 7). On multivariable analysis, the associations between Recovery-1 and Recover-2 and death were similar to patients with AKI-2/3 (Supplemental Figures 6 and 7).
Prediction of AKI-2/3 and Recovery Using ML Algorithms
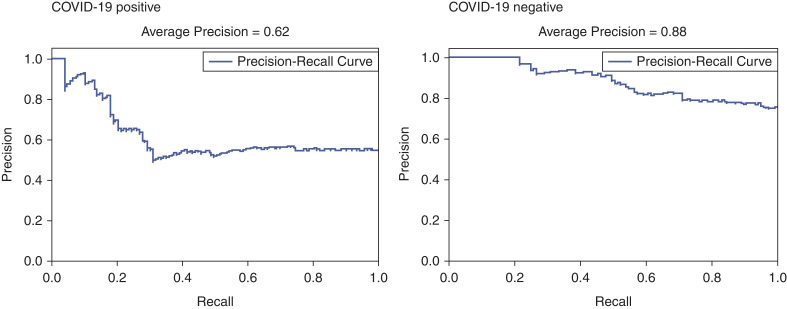
In patients with and without COVID-19, we were able to predict AKI-2/3 with an AP of 0.36 and 0.23, respectively (Supplemental Figure 8), and we were able to predict AKI-3 with an AP of 0.47 and 0.40, respectively (Supplemental Figure 9). For recovery prediction, we focused on Recovery-1 (partial) because Recovery-2 (complete) was achieved in a small sample size. In patients with COVID-19-associated AKI-2/3 and AKI-3, we were able to predict Recovery-1 with an AP of 0.62 (Figure 5) and 0.61 (Supplemental Figure 10), respectively, whereas in COVID-19-negative patients, we were able to predict Recovery-1 with an AP of 0.88 (Figure 6 and Supplemental Figure 10).
Figure 5.
Precision–recall (PR) curves for recovery after AKI-2/3 in patients with and without COVID-19.
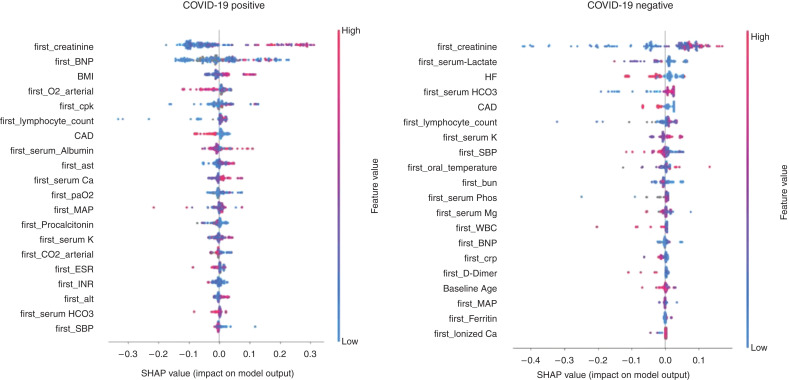
Figure 6.
SHapley Additive exPlanations (SHAP) plots for recovery after AKI-2/3 in patients with and without COVID-19.
Among patients with AKI-2/3, the key recovery predictors are shown in the SHAP plots in Figure 6, and among patients with AKI-3, they are shown in Supplemental Figure 11. The measures that predicted recovery among both AKI-2/3 and AKI-3 patients with COVID-19 were initial respiratory measures (arterial partial pressure of oxygen and carbon dioxide) and initial labs (SCr, potassium, lymphocyte count, and creatine phosphokinase).
The performance metrics of each of the tasks in the ML-based prediction models are summarized in Supplemental Table 8.
Twelve-Month Follow-Up of a Subgroup of Survivors with AKI-2/3
Among 227 AKI-2/3 survivors followed for 12 months after their index hospitalization, COVID-19 AKI survivors were more likely to be non-White, Hispanic, with less baseline CKD and greater severity of illness (MV, ARDS, vasopressor use, and length of hospital stay) during hospitalization compared with COVID-19-negative survivors (all P≤0.01). COVID-19-negative AKI survivors were more likely to be readmitted to hospital (P<0.001), although no difference was noted in rehospitalization with AKI among the two groups. Out of 161 COVID-19-positive AKI survivors, 25 (16%) died after being discharged from hospital for COVID-19 compared with only one out of 66 (2%) COVID-19-negative AKI survivors (P<0.001).
Forty-five (28%) of the COVID-19-positive and 22 (33%) of the COVID-19-negative patients had a SCr and eGFR measure >90 days after discharge (Table 4). The median number of days from discharge to follow-up SCr was 326.5 days for those with COVID-19 at presentation, and 300 days for those without COVID-19 at presentation. COVID-19-positive AKI survivors had no difference in the rate of incident or progressive CKD compared with COVID-19-negative AKI survivors (16% versus 18%, respectively; P=0.99; Table 4).
Table 4.
CKD outcomes in AKI-2/3 survivors at 12-month follow-up
| CKD Outcomes | COVID-19 Positive | COVID-19 Negative | P Value |
|---|---|---|---|
| Total number of patients followed for 12 months | 161 | 66 | |
| Number of patients with >90-day post-discharge creatinine data | 45 (28.0%) | 22 (33.3%) | |
| History of CKD | 5 (11.1%) | 2 (9.1%) | 0.99 |
| CKD | |||
| Incident/progressive CKD | 7 (15.6%) | 4 (18.2%) | 0.99 |
| No incident/progressive CKD | 38 (84.6%) | 18 (81.8%) |
Discussion
To our knowledge, this is one of the first detailed comparisons of in-hospital recovery and of long-term outcomes after moderate/severe AKI in hospitalized patients with and without COVID-19. Our key findings are as follows. (1) COVID-19-associated AKI-2/3 was associated with lower recovery and greater mortality compared with AKI-2/3 without COVID-19. (2) ICU admission was associated with non-recovery in all AKI-2/3 patients, whereas ARDS was specific to those with COVID-19. (3) Among both COVID-positive and -negative patients with AKI-2/3, renal recovery was significantly associated with survival, but this association was further observed only in patients with COVID-19 after adjusting for other key variables. (4) Sensitivity analysis using a stricter criterion of renal recovery (Recovery-2, complete) and restricting patients to severe AKI (AKI-3) were mostly consistent with our main analysis. (5) Using ML algorithms, we were able to predict AKI and recovery from COVID-19-associated AKI-2/3 and identified key predictors. (6) At 10-month follow-up in moderate/severe AKI survivors, no difference in CKD between COVID-positive and -negative patients was observed.
Some initial studies from China at the start of the COVID-19 pandemic reported a variable AKI recovery rate (17%–46%) (10,12); however, subsequent studies report a higher recovery rate from 64% to 87% in AKI survivors (36,37) and 41% overall (38), although some of these studies were restricted to critically ill patients. The recovery rates noted in our study of COVID-19-associated AKI-2/3 were 51% (partial) and 25% (complete). Unlike previous studies, we compared recovery after AKI-2/3 in COVID-19-negative patients.
We found that ARDS was associated with non-recovery in AKI-2/3 patients with COVID-19, suggesting that severe lung disease in COVID-19 is associated with a lower chance of AKI recovery. This association was not noted in our non-COVID-19 group after adjusting for covariates but has been previously reported in critically ill patients without COVID-19 (39). We also observed that among patients with AKI-2/3, renal recovery was significantly associated with survival; however, this association was observed only in patients with COVID-19 after adjusting for covariates. This finding, if replicated in other studies, has potentially important prognostic implications. Therapies directed at enhancing renal recovery can be tested in clinical trials with the goal of improving survival in patients with COVID-19.
Using state-of-the-art ML techniques, we asked whether AKI and recovery can be predicted using data at the time of hospitalization. Whereas the AP in the models for AKI was low, their prediction performance is much higher than AKI incidence, e.g., AKI-2/3 incidence was 19% in COVID-19-positive patients versus our AP of 0.36. We were able to predict more severe AKI (AKI-3) with greater accuracy as was previously observed (18–21). For recovery prediction, we used the cohort of patients who were diagnosed with AKI. The AP was only slightly higher than the incidence of recovery noted in our cohort. This means that it is relatively more difficult to predict recovery using only admission data; however, as can be seen from the precision–recall curves (Figure 5), the precision values of our models at 20% recall are high at around 80%. This suggests that there is a small population of AKI patients where our recovery prediction models can identify recovery with a high level of accuracy. The key predictors for recovery in patients with COVID-19-associated AKI-2/3 were coronary artery disease, body mass index, blood pressure, respiratory status, and labs (SCr, brain natriuretic peptide, lymphocyte count, potassium, creatine phosphokinase, albumin, procalcitonin, calcium, International Normalized Ratio, aspartate aminotransferase, and alanine aminotransferase). These findings, if replicated in our cohorts, can be used to develop robust predictive models and clinical decision support systems (40) that can be used to guide preventive therapies and patient prognostication.
Approximately 10%–30% of COVID-19 patients are known to develop long COVID, which includes symptoms of post-acute sequalae of SARS-CoV2 (41) and organ injury, including kidney disease (Rando HM, Bennett TD, Byrd JB, Bramante C, Callahan TJ, Chute CG, Davis HE, Deer R, Gagnier J, Koraishy FM, Liu F, McMurry JA, Moffit JA, Pfaff ER, Reese JT, Relevo R, Robinson PN, Saltz JH, Solomonides A, Sule A, Topaloglu U, Haendel MA: Challenges in defining long COVID: Striking differences across literature, electronic health records, and patient-reported information. medRxiv 2021. https://doi.org/10.1101/2021.03.20.21253896) (42). Whereas AKI is an established independent risk factor for CKD (43), COVID-19 studies have mostly reported the persistence of renal dysfunction at discharge (44,45). Two studies report a 3–6 months follow-up after COVID-19-associated AKI. Hulstrom et al. found that inpatient AKI severity was associated with higher CKD stages (24), whereas Nugent et al. reported that patients with COVID-19–associated AKI had a greater rate of GFR decrease compared with non-COVID-19 patients with AKI (25). At the time of this report, we report longer 12-month follow-up data on SCr and GFR measures on AKI-2/3 survivors, where the incidence of CKD was noted not to be statistically different between the COVID-19-negative and -positive groups. Whereas ours is one of the first studies reporting long-term renal outcomes associated with COVID-19 AKI, further multicenter studies with larger sample sizes and longer follow-up are required to analyze this COVID-19 AKI/CKD relationship further.
A strength of our study was the use of two different criteria of renal recovery: partial (Recovery-1) (30). and complete (Recovery-2) (31). Various definitions of renal recovery have been used in COVID-19 studies, and multicenter studies will be required to compare the outcomes associated with different recovery definitions in COVID-19-associated AKI. Other major strengths include the inclusion of multiple covariates (around 100), including data on medication, respiratory measures, vitals, and multiple laboratory values that are often not captured in larger multicenter studies using different EHR systems leading to issues with data harmonization. This granularity of clinical data allowed us to conduct a robust multivariable analysis of the association of AKI and recovery with outcomes. We also report a control group of patients admitted during the first wave (when isolation measures were delayed due to a delay in the reporting of SARS-CoV-2 PCR tests). We believe that this was a better control group for comparative analysis than using a retrospective cohort because the hospitalization period was the same as our patients with COVID-19, and the COVID-19-negative patients initially presented with symptoms suggestive of COVID-19.
Our study has several limitations. The use of the lowest SCr in the hospital as the baseline would capture not only incident AKI patients in the hospital but also those with AKI at the time of hospitalization who subsequently recovered. However, our approach has its limitations because AKI patients at admission who did not recover were included in the “no AKI” control group. The restriction of our study cohort to AKI-2/3 removes mild rises in SCr misdiagnosed as AKI by the KDIGO criteria; however, the limitation of this approach is the inclusion of true mild AKI patients in the “no AKI” control group. In addition, our AKI diagnosis relied on baseline and maximum SCr measures since we did not have access to daily SCr measures in our dataset to apply the time variable of 48 hour or 7-day diagnostic criteria of KDIGO. Other limitations of this study include retrospective analysis in a single-center study and incomplete data for inflammatory biomarkers and for urinary data; hence, these data were not included in the multivariable analysis. We did not have urine output data available for accurate definition of AKI, which was limited to only SCr measures. Whereas our 12-month follow-up data are among the longest reported thus far, the analysis was limited to only a small subgroup of survivors with AKI-2/3 because patients were often followed up at non-SBU clinics. We were also not able to use urinalysis and renal imaging criteria to diagnose CKD-1 or -2 and relied solely on SCr and eGFR, which might lead to underdiagnosis of true post-AKI CKD. We used ICD-9/10 codes to define prevalent CKD on index admission, which is another limitation of our study due to the possibility of misclassification bias, but it was the most objective way to define CKD present on index admission due to the lack of baseline GFR prehospitalization. Due to the limited frequency of ARDS in COVID-negative patients in our cohort, we were underpowered to observe significant associations with recovery. We do not have information on the trajectory of AKI due to lack of daily SCr data. Our initial hospitalization period was from the first wave of the pandemic when there were no new reported variants of SARS-CoV-2, including the delta variant. Finally, in this study, if a patient died before recovery, then the person was treated as not recovered, meaning that the models are predicting survival and recovery at hospital discharge.
In conclusion, non-recovery after moderate/severe AKI is associated with ARDS and in-hospital death in patients with COVID-19. Moderate/severe AKI and recovery can be predicted using admission data with ML algorithms, thereby informing clinicians of patient prognosis. COVID-19-associated AKI-2/3 is associated with the risk of CKD. These findings need to be validated in large multicenter cohorts.
Disclosures
All authors have nothing to disclose.
Funding
None.
Acknowledgment
We would like to acknowledge valuable insights into analysis provided by Dr. Mary Saltz.
Footnotes
This article contains a podcast at https://www.asn-online.org/media/podcast/K360/2022_02_24_KID0005342021.mp3.
Author Contributions
S. Sun, R.R. Annadi, I. Chaudhri, R. Moffit, and K. Munir were responsible for the formal analysis; J. Hajagos and S. Sun curated the data; M. Hoai, F.M. Koraishy, R. Moffit, and J. Saltz supervised the study; F.M. Koraishy, S.K. Mallipattu, and R. Moffit conceptualized the study; R.R. Annadi, F.M. Koraishy, and S. Sun were responsible for the methodology, validation, and writing the original draft of the manuscript; F.M. Koraishy was responsible for the project administration; F.M. Koraishy. R. Moffit and S.K. Mallipattu were responsible for resources; R.R. Annadi and S. Sun were responsible for the software and for visualization of the study; and all authors reviewed, edited, and approved the final version of the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0005342021/-/DCSupplemental.
Download Supplemental Materials and Methods, PDF file, 2.1 MB (2.1MB, pdf)
Flow chart of the study cohort. Download Supplemental Figure 1, PDF file, 2.1 MB (2.1MB, pdf)
(A) Forest plot showing the multivariable analysis of COVID-19-positive patients with versus without AKI-3. (B) Forest plot showing the multivariable analysis of COVID-19-negative patients with versus without AKI-3. Download Supplemental Figure 2, PDF file, 2.1 MB (2.1MB, pdf)
(A) Forest plot showing the multivariable analysis of COVID-19-positive patients with versus without Recovery-2 after AKI-2/3. (B) Forest plot showing the multivariable analysis of COVID-19-negative patients with versus without Recovery-2 after AKI-2/3. Download Supplemental Figure 3, PDF file, 2.1 MB (2.1MB, pdf)
(A) Forest plot showing the multivariable analysis of COVID-19-positive patients with versus without Recovery-1 after AKI-3. (B) Forest plot showing the multivariable analysis of COVID-19-negative patients with versus without Recovery-1 after AKI-3. Download Supplemental Figure 4, PDF file, 2.1 MB (2.1MB, pdf)
(A) Forest plot showing the multivariable analysis of COVID-19-positive patients with versus without Recovery-2 after AKI-3. (B) Forest plot showing the multivariable analysis of COVID-19-negative patients with versus without Recovery-2 after AKI-3. Download Supplemental Figure 5, PDF file, 2.1 MB (2.1MB, pdf)
(A) Forest plot showing the multivariable analysis of death in COVID-19-positive patients analyzing the association of Recovery-1 after AKI-3. (B) Forest plot showing the multivariable analysis of death in COVID-19-positive patients analyzing the association of Recovery-2 after AKI-3. Download Supplemental Figure 6, PDF file, 2.1 MB (2.1MB, pdf)
(A) Forest plot showing the multivariable analysis of death in COVID-19-negative patients analyzing the association of Recovery-1 after AKI-3. (B) Forest plot showing the multivariable analysis of death in COVID-19-negative patients analyzing the association of Recovery-2 after AKI-3. Download Supplemental Figure 7, PDF file, 2.1 MB (2.1MB, pdf)
Precision–recall (PR) curves for patients with AKI-2/3 with and without COVID-19. Download Supplemental Figure 8, PDF file, 2.1 MB (2.1MB, pdf)
Precision–recall (PR) curves for patients with AKI-3 with and without COVID-19. Download Supplemental Figure 9, PDF file, 2.1 MB (2.1MB, pdf)
Precision–recall (PR) curves for patients with recovery from AKI-3 with and without COVID-19. Download Supplemental Figure 10, PDF file, 2.1 MB (2.1MB, pdf)
SHapley Additive exPlanations (SHAP) plots for patients with recovery after AKI-3 with and without COVID-19. Download Supplemental Figure 11, PDF file, 2.1 MB (2.1MB, pdf)
Univariate analysis of patients in presence and absence of COVID-19. Download Supplemental Table 1, PDF file, 2.1 MB (2.1MB, pdf)
Univariate analysis of patients with and without AKI-3 in presence and absence of COVID-19. Download Supplemental Table 2, PDF file, 2.1 MB (2.1MB, pdf)
Univariate analysis of patients with and without Recovery-2 from AKI-2/3 in presence and absence of COVID-19. Download Supplemental Table 3, PDF file, 2.1 MB (2.1MB, pdf)
Univariate analysis of patients with and without Recovery-1 from AKI-3 in presence and absence of COVID-19. Download Supplemental Table 4, PDF file, 2.1 MB (2.1MB, pdf)
Univariate analysis of patients with and without Recovery-2 from AKI-3 in presence and absence of COVID-19. Download Supplemental Table 5, PDF file, 2.1 MB (2.1MB, pdf)
Univariate analysis comparing death in all patients with and without COVID-19. Download Supplemental Table 6, PDF file, 2.1 MB (2.1MB, pdf)
Univariate analysis comparing death in patients with and without AKI-3 in presence and absence of COVID-19. Download Supplemental Table 7, PDF file, 2.1 MB (2.1MB, pdf)
Supplemental Materials and Methods Table X: list of all of the variables used for ML algorithms and the % missingness in each variable. Download Supplemental Table 8, PDF file, 2.1 MB (2.1MB, pdf)
References
- 1.Beltrán-García J, Osca-Verdegal R, Pallardó FV, Ferreres J, Rodríguez M, Mulet S, Ferrando-Sánchez C, Carbonell N, García-Giménez JL: Sepsis and coronavirus disease 2019: Common features and anti-inflammatory therapeutic approaches. Crit Care Med 48: 1841–1844, 2020. 10.1097/CCM.0000000000004625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stony Brook COVID-19 Research Consortium : Geospatial distribution and predictors of mortality in hospitalized patients with COVID-19: A cohort study. Open Forum Infect Dis 7: ofaa436, 2020. 10.1093/ofid/ofaa436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; Northwell COVID-19 Research Consortium : Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020. 10.1016/j.kint.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP; Northwell COVID-19 Research Consortium : Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323: 2052–2059, 2020. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiorentino M, Tohme FA, Wang S, Murugan R, Angus DC, Kellum JA: Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLoS One 13: e0198269, 2018. 10.1371/journal.pone.0198269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pannu N, James M, Hemmelgarn B, Klarenbach S; Alberta Kidney Disease Network : Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 8: 194–202, 2013. 10.2215/CJN.06480612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellum JA, Chawla LS, Keener C, Singbartl K, Palevsky PM, Pike FL, Yealy DM, Huang DT, Angus DC; ProCESS and ProGReSS-AKI Investigators : The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med 193: 281–287, 2016. 10.1164/rccm.201505-0995OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A: Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 119: 2444–2453, 2009. 10.1161/CIRCULATIONAHA.108.800011 [DOI] [PubMed] [Google Scholar]
- 10.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, Ma Z, Huang Y, Liu W, Yao Y, Zeng R, Xu G: Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31: 1157–1165, 2020. 10.1681/ASN.2020030276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng JH, Hirsch JS, Hazzan A, Wanchoo R, Shah HH, Malieckal DA, Ross DW, Sharma P, Sakhiya V, Fishbane S, Jhaveri KD; Northwell Nephrology COVID-19 Research Consortium : Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis 77: 204–215.e1, 2021. 10.1053/j.ajkd.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sang L, Chen S, Zheng X, Guan W, Zhang Z, Liang W, Zhong M, Jiang L, Pan C, Zhang W, Xia J, Chen N, Wu W, Wu H, Xu Y, Liu X, Liu X, He J, Li S, Zhang D, Zhong N, Li Y: The incidence, risk factors and prognosis of acute kidney injury in severe and critically ill patients with COVID-19 in mainland China: A retrospective study. BMC Pulm Med 20: 290, 2020. 10.1186/s12890-020-01305-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaudhri I, Moffitt R, Taub E, Annadi RR, Hoai M, Bolotova O, Yoo J, Dhaliwal S, Sahib H, Daccueil F, Hajagos J, Saltz M, Saltz J, Mallipattu SK, Koraishy FM: Association of proteinuria and hematuria with acute kidney injury and mortality in hospitalized patients with COVID-19. Kidney Blood Press Res 45: 1018–1032, 2020. 10.1159/000511946 [DOI] [PubMed] [Google Scholar]
- 14.Huang C, Murugiah K, Mahajan S, Li SX, Dhruva SS, Haimovich JS, Wang Y, Schulz WL, Testani JM, Wilson FP, Mena CI, Masoudi FA, Rumsfeld JS, Spertus JA, Mortazavi BJ, Krumholz HM: Enhancing the prediction of acute kidney injury risk after percutaneous coronary intervention using Machine Learning techniques: A retrospective cohort study. PLoS Med 15: e1002703, 2018. 10.1371/journal.pmed.1002703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim NE, McCarthy CP, Shrestha S, Gaggin HK, Mukai R, Magaret CA, Rhyne RF, Januzzi JL Jr: A clinical, proteomics, and artificial intelligence-driven model to predict acute kidney injury in patients undergoing coronary angiography. Clin Cardiol 42: 292–298, 2019. 10.1002/clc.23143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin WJ, Yi YH, Guan XF, Zhou LY, Wang JL, Li DY, Zuo XC: Preprocedural prediction model for contrast-induced nephropathy patients. J Am Heart Assoc 6: e004498, 2017. 10.1161/JAHA.116.004498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Ho KM, Hong Y: Machine Learning for the prediction of volume responsiveness in patients with oliguric acute kidney injury in critical care. Crit Care 23: 112, 2019. 10.1186/s13054-019-2411-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Argyropoulos A, Townley S, Upton PM, Dickinson S, Pollard AS: Identifying on admission patients likely to develop acute kidney injury in hospital. BMC Nephrol 20: 56, 2019. 10.1186/s12882-019-1237-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koyner JL, Carey KA, Edelson DP, Churpek MM: The development of a Machine Learning inpatient acute kidney injury prediction model. Crit Care Med 46: 1070–1077, 2018. 10.1097/CCM.0000000000003123 [DOI] [PubMed] [Google Scholar]
- 20.Cheng P, Waitman LR, Hu Y, Liu M: Predicting inpatient acute kidney injury over different time horizons: How early and accurate? AMIA Annu Symp Proc 2017: 565–574, 2018 [PMC free article] [PubMed] [Google Scholar]
- 21.Tomašev N, Glorot X, Rae JW, Zielinski M, Askham H, Saraiva A, Mottram A, Meyer C, Ravuri S, Protsyuk I, Connell A, Hughes CO, Karthikesalingam A, Cornebise J, Montgomery H, Rees G, Laing C, Baker CR, Peterson K, Reeves R, Hassabis D, King D, Suleyman M, Back T, Nielson C, Ledsam JR, Mohamed S: A clinically applicable approach to continuous prediction of future acute kidney injury. Nature 572: 116–119, 2019. 10.1038/s41586-019-1390-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu RK, Hsu CY: The role of acute kidney injury in chronic kidney disease. Semin Nephrol 36: 283–292, 2016. 10.1016/j.semnephrol.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones J, Holmen J, De Graauw J, Jovanovich A, Thornton S, Chonchol M: Association of complete recovery from acute kidney injury with incident CKD Stage 3 and all-cause mortality. Am J Kidney Dis 60: 402–408, 2012. 10.1053/j.ajkd.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hultström M, Lipcsey M, Wallin E, Larsson IM, Larsson A, Frithiof R: Severe acute kidney injury associated with progression of chronic kidney disease after critical COVID-19. Crit Care 25: 37, 2021. 10.1186/s13054-021-03461-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nugent J, Aklilu A, Yamamoto Y, Simonov M, Li F, Biswas A, Ghazi L, Greenberg H, Mansour G, Moledina G, Wilson FP: Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw Open 4: e211095, 2021. 10.1001/jamanetworkopen.2021.1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin S, Orieux A, Clouzeau B, Rigothier C, Combe C, Gruson D, Boyer A: The incidence of chronic kidney disease three years after non-severe acute kidney injury in critically ill patients: A single-center cohort study. J Clin Med 8: 2215, 2019. 10.3390/jcm8122215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao CT, Tsai HB, Wu CY, Lin YF, Hsu NC, Chen JS, Hung KY: The severity of initial acute kidney injury at admission of geriatric patients significantly correlates with subsequent in-hospital complications. Sci Rep 5: 13925, 2015. 10.1038/srep13925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Notice. Kidney Int Suppl 2: 1, 2012. 10.1038/kisup.2012.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns TS, Abramowitz MK, Levy R, Kumar N, Mokrzycki MH, Coco M, Dominguez M, Prudhvi K, Golestaneh L: AKI in hospitalized patients with and without COVID-19: A comparison study. J Am Soc Nephrol 31: 2145–2157, 2020. 10.1681/ASN.2020040509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA; Acute Disease Quality Initiative Workgroup 16 : Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 13: 241–257, 2017. 10.1038/nrneph.2017.2 [DOI] [PubMed] [Google Scholar]
- 31.Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE 2nd, Perkins RM: Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81: 477–485, 2012. 10.1038/ki.2011.405 [DOI] [PubMed] [Google Scholar]
- 32.Vrieze SI: Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods 17: 228–243, 2012. 10.1037/a0027127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan X, Zhang B, Fu M, Li M, Yuan X, Zhu Y, Peng J, Guo H, Lu Y: Clinical and inflammatory features based Machine Learning model for fatal risk prediction of hospitalized COVID-19 patients: Results from a retrospective cohort study. Ann Med 53: 257–266, 2021. 10.1080/07853890.2020.1868564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundberg S, Lee S-I: A unified approach to interpreting model predictions. Presented at the 31st Conference on Neural Information Processing Systems (NIPS 2017), Long Beach, CA, December 4–9, 2017
- 35.Li Y, Chen T, Chen T, Li X, Zeng C, Liu Z, Xie G: An interpretable Machine Learning survival model for predicting long-term kidney outcomes in IgA nephropathy. AMIA Annu Symp Proc 2020: 737–746, 2021 [PMC free article] [PubMed] [Google Scholar]
- 36.Hittesdorf E, Panzer O, Wang D, Stevens JS, Hastie J, Jordan DA, Yoh N, Eiseman KA, Elisman K, Wagener G: Mortality and renal outcomes of patients with severe COVID-19 treated in a provisional intensive care unit. J Crit Care 62: 172–175, 2021. 10.1016/j.jcrc.2020.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilbers TJ, Koning MV: Renal replacement therapy in critically ill patients with COVID-19: A retrospective study investigating mortality, renal recovery and filter lifetime. J Crit Care 60: 103–105, 2020. 10.1016/j.jcrc.2020.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens JS, King KL, Robbins-Juarez SY, Khairallah P, Toma K, Alvarado Verduzco H, Daniel E, Douglas D, Moses AA, Peleg Y, Starakiewicz P, Li MT, Kim DW, Yu K, Qian L, Shah VH, O’Donnell MR, Cummings MJ, Zucker J, Natarajan K, Perotte A, Tsapepas D, Krzysztof K, Dube G, Siddall E, Shirazian S, Nickolas TL, Rao MK, Barasch JM, Valeri AM, Radhakrishnan J, Gharavi AG, Husain SA, Mohan S: High rate of renal recovery in survivors of COVID-19 associated acute renal failure requiring renal replacement therapy. PLoS One 15: e0244131, 2020. 10.1371/journal.pone.0244131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darmon M, Clec’h C, Adrie C, Argaud L, Allaouchiche B, Azoulay E, Bouadma L, Garrouste-Orgeas M, Haouache H, Schwebel C, Goldgran-Toledano D, Khallel H, Dumenil AS, Jamali S, Souweine B, Zeni F, Cohen Y, Timsit JF: Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin J Am Soc Nephrol 9: 1347–1353, 2014. 10.2215/CJN.08300813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park S, Lee H: Acute kidney injury prediction models: Current concepts and future strategies. Curr Opin Nephrol Hypertens 28: 552–559, 2019. 10.1097/MNH.0000000000000536 [DOI] [PubMed] [Google Scholar]
- 41.Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, Chu HY: Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 4: e210830, 2021. 10.1001/jamanetworkopen.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.SeyedAlinaghi S, Afsahi AM, MohsseniPour M, Behnezhad F, Salehi MA, Barzegary A, Mirzapour P, Mehraeen E, Dadras O: Late complications of COVID-19; A systematic review of current evidence. Arch Acad Emerg Med 9: e14, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012. 10.1038/ki.2011.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yichun Cheng RL, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G: Kidney impairment is associated with in-hospital death of COVID-19 patients. Kidney Int 97: 829–838, 2020. 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, Paranjpe I, Somani S, Richter F, Miotto R, Lala A, Kia A, Timsina P, Li L, Freeman R, Chen R, Narula J, Just AC, Horowitz C, Fayad Z, Cordon-Cardo C, Schadt E, Levin MA, Reich DL, Fuster V, Murphy B, He JC, Charney AW, Böttinger EP, Glicksberg BS, Coca SG, Nadkarni GN; Mount Sinai COVID Informatics Center (MSCIC) : Acute kidney injury in hospitalized patients with COVID-19. J Am Soc Nephrol 32: 151–160, 2021. 10.1681/ASN.2020050615 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Download Supplemental Materials and Methods, PDF file, 2.1 MB (2.1MB, pdf)
Flow chart of the study cohort. Download Supplemental Figure 1, PDF file, 2.1 MB (2.1MB, pdf)
(A) Forest plot showing the multivariable analysis of COVID-19-positive patients with versus without AKI-3. (B) Forest plot showing the multivariable analysis of COVID-19-negative patients with versus without AKI-3. Download Supplemental Figure 2, PDF file, 2.1 MB (2.1MB, pdf)
(A) Forest plot showing the multivariable analysis of COVID-19-positive patients with versus without Recovery-2 after AKI-2/3. (B) Forest plot showing the multivariable analysis of COVID-19-negative patients with versus without Recovery-2 after AKI-2/3. Download Supplemental Figure 3, PDF file, 2.1 MB (2.1MB, pdf)
(A) Forest plot showing the multivariable analysis of COVID-19-positive patients with versus without Recovery-1 after AKI-3. (B) Forest plot showing the multivariable analysis of COVID-19-negative patients with versus without Recovery-1 after AKI-3. Download Supplemental Figure 4, PDF file, 2.1 MB (2.1MB, pdf)
(A) Forest plot showing the multivariable analysis of COVID-19-positive patients with versus without Recovery-2 after AKI-3. (B) Forest plot showing the multivariable analysis of COVID-19-negative patients with versus without Recovery-2 after AKI-3. Download Supplemental Figure 5, PDF file, 2.1 MB (2.1MB, pdf)
(A) Forest plot showing the multivariable analysis of death in COVID-19-positive patients analyzing the association of Recovery-1 after AKI-3. (B) Forest plot showing the multivariable analysis of death in COVID-19-positive patients analyzing the association of Recovery-2 after AKI-3. Download Supplemental Figure 6, PDF file, 2.1 MB (2.1MB, pdf)
(A) Forest plot showing the multivariable analysis of death in COVID-19-negative patients analyzing the association of Recovery-1 after AKI-3. (B) Forest plot showing the multivariable analysis of death in COVID-19-negative patients analyzing the association of Recovery-2 after AKI-3. Download Supplemental Figure 7, PDF file, 2.1 MB (2.1MB, pdf)
Precision–recall (PR) curves for patients with AKI-2/3 with and without COVID-19. Download Supplemental Figure 8, PDF file, 2.1 MB (2.1MB, pdf)
Precision–recall (PR) curves for patients with AKI-3 with and without COVID-19. Download Supplemental Figure 9, PDF file, 2.1 MB (2.1MB, pdf)
Precision–recall (PR) curves for patients with recovery from AKI-3 with and without COVID-19. Download Supplemental Figure 10, PDF file, 2.1 MB (2.1MB, pdf)
SHapley Additive exPlanations (SHAP) plots for patients with recovery after AKI-3 with and without COVID-19. Download Supplemental Figure 11, PDF file, 2.1 MB (2.1MB, pdf)
Univariate analysis of patients in presence and absence of COVID-19. Download Supplemental Table 1, PDF file, 2.1 MB (2.1MB, pdf)
Univariate analysis of patients with and without AKI-3 in presence and absence of COVID-19. Download Supplemental Table 2, PDF file, 2.1 MB (2.1MB, pdf)
Univariate analysis of patients with and without Recovery-2 from AKI-2/3 in presence and absence of COVID-19. Download Supplemental Table 3, PDF file, 2.1 MB (2.1MB, pdf)
Univariate analysis of patients with and without Recovery-1 from AKI-3 in presence and absence of COVID-19. Download Supplemental Table 4, PDF file, 2.1 MB (2.1MB, pdf)
Univariate analysis of patients with and without Recovery-2 from AKI-3 in presence and absence of COVID-19. Download Supplemental Table 5, PDF file, 2.1 MB (2.1MB, pdf)
Univariate analysis comparing death in all patients with and without COVID-19. Download Supplemental Table 6, PDF file, 2.1 MB (2.1MB, pdf)
Univariate analysis comparing death in patients with and without AKI-3 in presence and absence of COVID-19. Download Supplemental Table 7, PDF file, 2.1 MB (2.1MB, pdf)
Supplemental Materials and Methods Table X: list of all of the variables used for ML algorithms and the % missingness in each variable. Download Supplemental Table 8, PDF file, 2.1 MB (2.1MB, pdf)