Key Points
Remdesivir was not approved in patients with eGFR <30 ml/min per 1.73 m2, and safety data are extremely limited.
Compared with matched controls who did not receive remdesivir, there was no increased risk of cardiac, kidney, liver, or neurologic adverse events.
Hyperglycemia was more common in remdesivir-treated patients; this may be explained by concomitant dexamethasone use.
Keywords: chronic kidney disease, acute kidney injury, antiviral, COVID-19, dialysis, propensity score, remdesivir, SARS-CoV-2
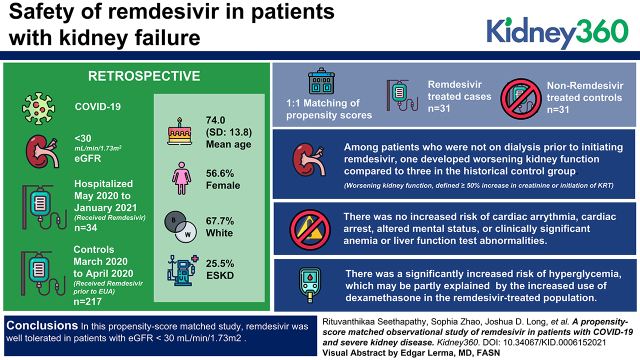
Visual Abstract
Abstract
Background
Remdesivir is not currently approved for patients with eGFR <30 ml/min per 1.73 m2. We aimed to determine the safety of remdesivir in patients with kidney failure.
Methods
This study was a retrospective cohort study of patients with COVID-19 hospitalized between May 2020 and January 2021 with eGFR <30 ml/min per 1.73 m2 who received remdesivir and historical controls with COVID-19 hospitalized between March 1, 2020 and April 30, 2020 prior to the emergency use authorization of remdesivir within a large health care system. Patients were 1:1 matched by propensity scores accounting for factors associated with treatment assignment. Adverse events and hospital outcomes were recorded by manual chart review.
Results
The overall cohort included 34 hospitalized patients who initiated remdesivir within 72 hours of hospital admission with eGFR<30 ml/min per 1.73 m2 and 217 COVID-19 controls with eGFR <30 ml/min per 1.73 m2. The propensity score–matched cohort included 31 remdesivir-treated patients and 31 nonremdesivir-treated controls. The mean age was 74.0 (SD=13.8) years, 57% were women, and 68% were white participants. A total of 26% had ESKD. Among patients who were not on dialysis prior to initiating remdesivir, one developed worsening kidney function (defined as ≥50% increase in creatinine or initiation of KRT) compared with three in the historical control group. There was no increased risk of cardiac arrythmia, cardiac arrest, altered mental status, or clinically significant anemia or liver function test abnormalities. There was a significantly increased risk of hyperglycemia, which may be partly explained by the increased use of dexamethasone in the remdesivir-treated population.
Conclusions
In this propensity score–matched study, remdesivir was well tolerated in patients with eGFR <30 ml/min per 1.73 m2.
Introduction
There have been over 200 million cases of coronavirus disease 2019 (COVID-19) worldwide as of August 10, 2021 (1). Severe COVID-19 can cause multiorgan injury (2,3), and AKI has emerged as a common complication of COVID-19. In the first wave of the pandemic, multiple studies demonstrated that the rate of AKI among hospitalized patients was between 17% and 37%; however, with improvements in care, the rate of AKI has decreased (4–11). Nevertheless, patients with low eGFR due to either AKI or preexisting CKD and patients with ESKD have a significantly increased risk of death from COVID-19 (12,13).
Remdesivir is the only antiviral treatment that has been approved for the treatment of COVID-19 in patients with severe COVID-19. However, because of concerns about the drug’s potential toxicity in patients with kidney disease related to both metabolism and elimination of remdesivir and the potential accumulation of its sulfobutylether-β-cyclodextrin (SBECD) excipient, the Food and Drug Administration approval states that remdesivir is not recommended in patients with eGFR <30 ml/min per 1.73 m2. Remdesivir is a prodrug that, after it is metabolized to remdesivir triphosphate, acts as an analog of ATP, competing for incorporation by RNA-dependent RNA polymerase and interfering with viral RNA replication. Rare but serious side effects of remdesivir include transaminitis and hypersensitivity/infusion reactions (14).
Given the limited duration of treatment (5 days) and relatively low concentration of SBECD excipient, it has been suggested that the benefits of remdesivir may outweigh risks in select patients with eGFR <30 ml/min per 1.73 m2 who present early in their disease course when antiviral therapy is most likely to be effective (15). Thus, within our health care network, off-label remdesivir was offered to patients with eGFR <30 ml/min per 1.73 m2 when infectious diseases experts and nephrologists agreed that these potential benefits outweighed risks. There is a small number of case series of remdesivir use in patients with eGFR <30 ml/min per 1.73 m2; however, no prior series included matched comparators. To address this knowledge gap, we sought to compare the risk of key safety events and hospital outcomes in patients treated with remdesivir with propensity score–matched patients who did not receive remdesivir during the early phase of the first COVID-19 surge in Boston, Massachusetts when remdesivir was not yet available (March 5, 2020 to April 30, 2020).
Materials and Methods
Patient Population and Data Acquisition
We conducted an observational, retrospective cohort study of hospitalized adults with COVID-19 within the Mass General Brigham (MGB) health care system located in the Boston region. Inclusion criteria for remdesivir-treated patients were (1) receipt of remdesivir within 72 hours of hospital admission and (2) receipt of KRT or eGFR <30 ml/min per 1.73 m2 on the basis of their creatinine level just prior to the first dose of remdesivir administration. eGFR was calculated via the Chronic Kidney Disease Epidemiology Collaboration equation (16). Patients were excluded if they had begun remdesivir prior to transfer to our health care system from an outside hospital because we could not evaluate baseline laboratory studies in these patients. Potential historical comparators were identified using the MGB Research Patients Data Registry (RPDR) to identify consecutive patients diagnosed with COVID-19 by RT-PCR between March 1, 2020 and April 30, 2020 who were hospitalized for >24 hours and had eGFR <30 ml/ min per 1.73 m2 within the first 72 hours of admission. We developed inclusion/exclusion criteria a priori to identify candidates who would have qualified for remdesivir had they been admitted after the emergency use authorization (EUA) when remdesivir became available. Inclusion criteria for historical comparators included (1) admission for COVID-19 with oxygen saturation ≤94% on room air or requiring supplemental oxygen within 72 hours of admission and (2) eGFR <30 ml/min per 1.73 m2 or receiving KRT within the first 72 hours of admission. Exclusion criteria included (1) evidence of decompensated liver disease within 72 hours of admission, (2) enrollment in a placebo-controlled trial of remdesivir, (3) initiation of hospice care prior to receiving COVID-19–directed therapies, or (4) transfer from an outside facility.
Patient characteristics, including demographics, laboratory tests, diagnosis, and medications, were obtained from MGB RPDR, which is the central data repository of MGB used for research and quality improvement purposes (17,18). Race/ethnicity was defined by incorporating race data and primary language spoken using an algorithm shown in Supplemental Table 1. Pretreatment creatinine was defined by the serum creatinine value just prior to remdesivir initiation. “Prehospital” baseline creatinine was determined by chart review according to the following algorithm. When available, the closest outpatient creatinine value between 14 and 365 days prior to admission was used; if not available, then the minimum serum creatinine during the hospital stay was used. CKD was defined as a “prehospital” baseline creatinine that corresponded to an eGFR <60 ml/min per 1.73 m2. Comorbidities were defined by the presence of at least two diagnosis International Classification of Diseases (ICD) 9/10 codes using the “diagnoses domain” (Supplemental Table 2). Baseline medications were defined by at least one instance of a prescribed medication within 1 year of baseline obtained from the “medications domain” (Supplemental Table 3). Laboratory studies were defined by the worst value within 72 hours of admission. The sequential organ failure assessment (SOFA) score was calculated according to the equation by Vincent et al. (19) using the most severe values obtained prior to remdesivir initiation or within 72 hours from admission for patients and controls, respectively.
Manual chart review was performed by two physicians (R.S. and M.E.S.) who reviewed each physician and nursing note to determine adverse events for all case and control patients. Remdesivir-treated patients were chart reviewed for their entire remdesivir course plus 48 hours afterward (typically corresponding to 7 days), and control patients were chart reviewed for the first 7 days of hospitalization. Clinical adverse events of interest were cardiac arrythmia, cardiac arrest, seizure, and altered mental status. Laboratory adverse events of interest were transaminitis (more than five times the upper limit of normal for aspartase aminotransferase [AST] and alanine aminotransferase [ALT]), worsening kidney function, anemia (hemoglobin <8 g/dl), and hyperglycemia (blood glucose >200 mg/dl). Worsening kidney function was defined as a rise in serum creatinine ≥50% for all patients who were not on KRT prior to baseline. For remdesivir-treated patients, the pretreatment creatinine was the value just prior to the first administered dose of remdesivir; for control patients, the admission creatinine was used. All adverse events of interest were defined a priori.
Our institutional review board approved the protocol and waived the need for informed consent. Research was conducted in accordance with the Helsinki Declaration.
Statistical Analyses
Baseline characteristics were described using means and SDs or medians and interquartile ranges for continuous variables and frequencies and percentages for categorical variables.
To minimize bias from nonrandomized treatment assignment, remdesivir-treated patients were matched to nonremdesivir-treated controls using a propensity score (PS). PSs were first determined on the basis of a multivariate logistic regression model that estimated the probability of receiving remdesivir. The covariates included in the logistic regression model were age; sex; race/ethnicity; SOFA score; invasive mechanical ventilation within 72 hours after admission; pretreatment/admission creatinine level; and history of hypertension, diabetes mellitus, ESKD, and prior solid organ transplantation. For patients with ESKD, the admission creatinine level was imputed as 10 mg/dl because the creatinine values for these patients are modified by dialysis. We then matched the remdesivir-treated patients and nonremdesivir-treated controls using 1:1 nearest neighbor greedy matching without replacement and a caliper of 0.1 SD of the PS. Standardized differences were calculated for the patients and controls before and after matching (20,21).
Following PS matching, patient outcomes were examined using the McNemar test for binary outcomes (proportion experiencing clinical adverse events, 28-day all-cause mortality) and paired t test or Wilcoxon signed rank test for continuous outcomes (lowest hemoglobin and highest AST, ALT, and blood glucose), as appropriate. Statistical analyses were performed using SAS Version 16. All P values were two sided, and P=0.05 was considered statistically significant.
Results
Cohorts and Study Time Line
There were 40 individuals with eGFR <30 ml/min per 1.73 m2 who received remdesivir at MGB between May 10, 2020 and Jan 31, 2021. Four patients were excluded due to beginning remdesivir >72 hours after admission, and two were excluded because they began remdesivir at an outside hospital prior to transfer to our health care system. There were 34 remdesivir-treated patients with eGFR <30 ml/min per 1.73 m2 who met inclusion criteria (Figure 1).
Figure 1.
Patient flow and exclusions. COVID-19, coronavirus disease 2019; EUA, emergency use authorization; O2, oxygen.
Historical Comparators
There were 1854 individuals who tested positive for COVID-19 and were hospitalized within MGB between March 5, 2020 and April 30, 2020. After applying the exclusions shown in Figure 1, we included 217 potential historical comparators who were hospitalized with COVID-19 and an eGFR <30 ml/min per 1.73 m2 or receiving KRT (either chronically or acutely) within the first 72 hours of admission.
Table 1 provides descriptive statistics for the remdesivir-treated patients and the historical comparator patients before and after matching. Prior to matching, there were substantial differences between the cohorts; historical comparators had higher SOFA scores, lower admission creatinine levels, and higher requirement for mechanical ventilation within 72 hours, reflecting the high acuity of COVID-19 in patients presenting in the first wave in Boston, Massachusetts (March and April 2020). A sufficiently close match was found for 31 (of 34) remdesivir-treated patients. After matching, some of the patient characteristics achieved good balance between the patients and their comparators with a standardized difference <0.1, whereas other characteristics had suboptimal balance, with major imbalance observed in sex and the prevalence of ESKD at baseline (Table 1) (21). The clinical events and laboratory findings in the three unmatched remdesivir-treated patients are shown in Supplemental Table 4.
Table 1.
Patient characteristics pre- and postmatch
| Recipient Variables (Matched) | Patients on Remdesivir Prematch, N=34 | Controls Prematch, N=217 | Patients on Remdesivir Postmatch, N=31 | Controls Postmatch, N=31 | Standard Differencea |
|---|---|---|---|---|---|
| Age, yr, mean (SD) | 70.2 (16.5) | 74.6 (13.2) | 71.4 (16.6) | 72.0 (15.3) | −0.0382 |
| Women | 22 (64.7%) | 120 (55.3%) | 21 (67.7%) | 17 (54.8%) | 0.2673 |
| White race | 17 (50.0%) | 153 (70.5%) | 16 (51.6%) | 14 (45.2%) | 0.1294 |
| SOFA score, mean (SD) | 6.5 (2.4) | 7.8 (3.7) | 6.6 (2.5) | 6.3 (2.9) | 0.1311 |
| Admission creatinine, mg/dl, mean (SD)b | 3.1 (2.4) | 2.9 (1.7) | 3.2 (2.5) | 3.2 (2.7) | −0.0030 |
| Hypertension | 32 (94.1%) | 192 (88.5%) | 29 (93.5%) | 30 (96.8%) | −0.1508 |
| Diabetes mellitus | 29 (85.3%) | 138 (63.6%) | 26 (83.9%) | 26 (83.9%) | 0 |
| ESKD | 14 (41.2%) | 50 (23%) | 13 (41.9%) | 10 (32.3%) | 0.2013 |
| SOT | 4 (11.8%) | 5 (2.3%) | 2 (6.5%) | 2 (6.5%) | 0 |
| Mechanical ventilation within first 72 h | 7 (20.6%) | 68 (31.3%) | 7 (22.6%) | 7 (22.6%) | 0 |
Each remdesivir-treated patient was matched 1:1 with a potential comparator who was admitted in March or April 2020 prior to the emergency use authorization of remdesivir. The results presented here are the means (SDs) or counts (percentages). SOFA, sequential organ failure assessment; SOT, solid organ transplant.
A standardized (mean) difference is a measure of distance between two group means in terms of a variables and is a conventional metric used to evaluate the quality of a match. By convention, a standardized mean difference of <0.1 is considered a well-balanced covariate.
Admission creatinine for patients not on dialysis only. For patients on KRT prior to baseline, a baseline creatinine of 10 mg/dl was imputed for the purpose of matching.
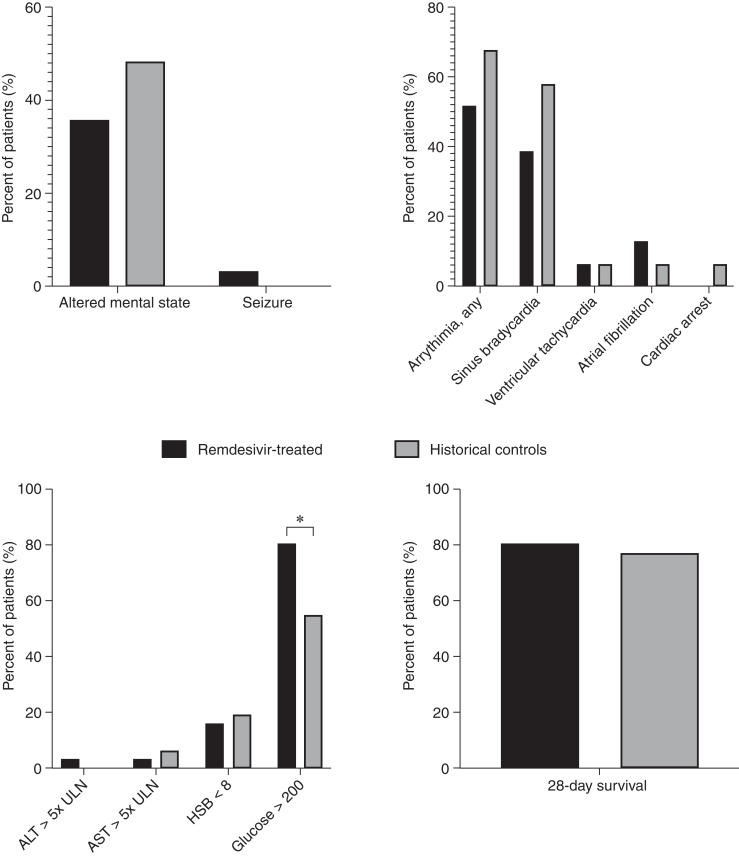
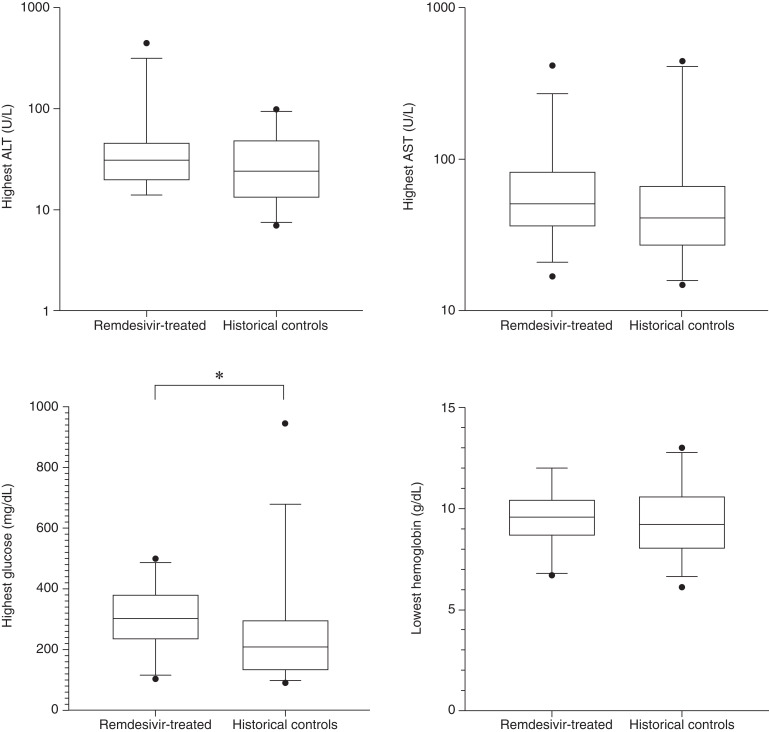
Figure 2 displays the incidence of clinical adverse events of interest in the 31 remdesivir-treated patients and 31 matched historical comparators. There were no significant differences in adverse events with the exception of an increased incidence of hyperglycemia (glucose >200 mg/dl) in patients treated with remdesivir. Hyperglycemia occurred in 81% of patients treated with remdesivir compared with 55% of controls (P=0.03). However, a total of 25 of 31 (81%) remdesivir-treated patients also received dexamethasone concurrently compared with three (10%) controls. Among the six remdesivir-treated patients who did not receive dexamethasone, three (50%) also had hyperglycemia. There were no documented infusion reactions. Figure 3 compares the most extreme values of the laboratory adverse events of interest during the study period (lowest hemoglobin and highest AST, ALT, and glucose). Among the laboratory findings, there were no significant differences between lowest hemoglobin or peak ALT; only peak glucose was significantly different.
Figure 2.
Percentage of patients experiencing clinical events of interest. Clinical outcomes were adjudicated by two physicians who reviewed each physician and clinical nursing note for the 5-day remdesivir course +48 hours after remdesivir treatment (black bars) and the first 7 days of admission for historical comparators (gray bars). The only clinical event that was significantly increased in patients treated with remdesivir was the incidence of hyperglycemia (defined by glucose >200), which occurred in 81% of patients treated with remdesivir compared to 55% of controls. A total of 25 of 31 (81%) remdesivir-treated patients also received dexamethasone concurrently compared with three of 31 (10%) controls. Among the six remdesivir-treated patients who did not receive dexamethasone, three (50%) also had hyperglycemia >200 mg/dl. There were no significant differences in any of the other adverse events. ALT, alanine aminotransferase; AST, aspartate aminotransferase; HGB, hemoglobin; ULN, Upper limit of normal.
Figure 3.
Box plots showing laboratory results in remdesivir-treated patients and controls. Box plots for the highest ALT, AST, and glucose and lowest hemoglobin for remdesivir-treated patients show interquartile range (box), median (line), and whiskers with 5th and 95th percentiles. Outliers are shown with solid circles. Only peak glucose significantly differed between matched remdesivir-treated patients and historical controls.
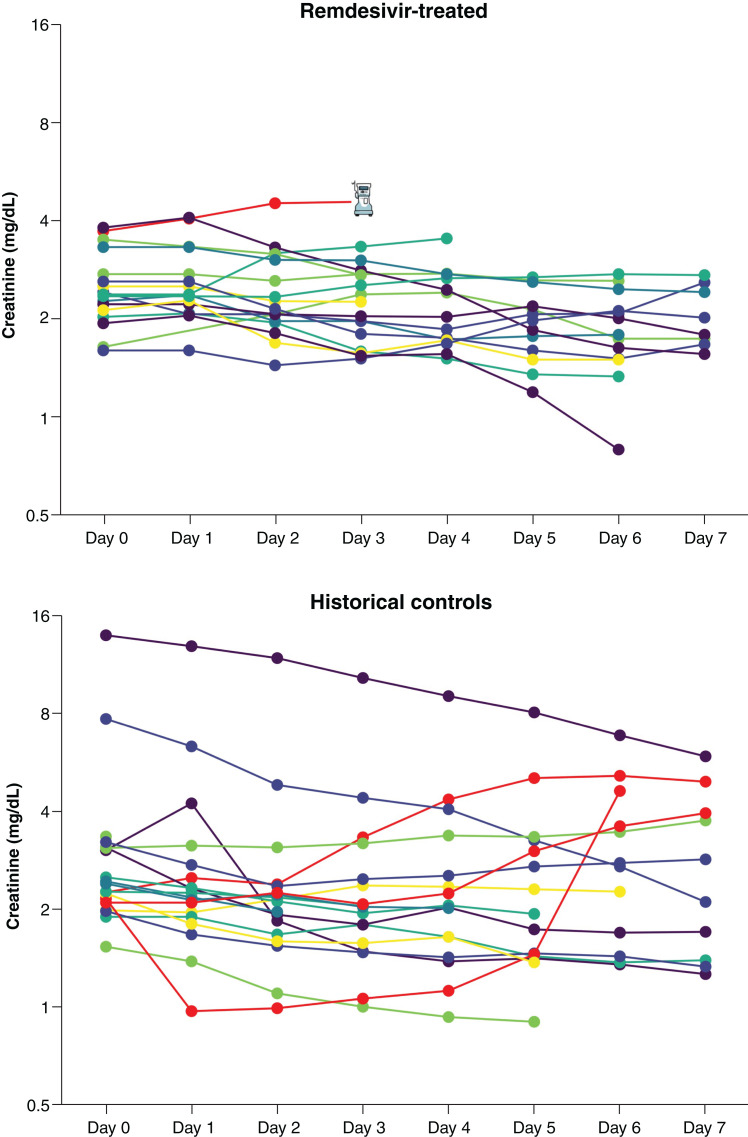
In-hospital creatinine trajectories are shown for the patients who were not on KRT at baseline in Figure 4. Among remdesivir-treated patients (Figure 4), only one patient met predefined criteria for worsening kidney function due to initiation of KRT. AKI in this patients was likely related to COVID-19 itself, shock requiring vasopressor medications, and concomitantly, receiving multiple nephrotoxins, including tacrolimus, vancomycin, and high-dose furosemide. Among the untreated historical comparators, three patients experienced a >50% increase in serum creatinine (shown in red in Figure 4). Of them, the first patient likely had AKI due to a combination of COVID-19 infection, hemorrhagic shock, lisinopril use, and poor oral intake prior to admission. The second patient had septic shock from intraabdominal infection and also was concurrently receiving lisinopril and hydrochlorothiazide. The third patient also experienced AKI attributed to septic shock.
Figure 4.
Creatinine trends among patients who were not on KRT at baseline. Serum creatinine values for each patient throughout the study period are shown on a log2 scale. Patients shown in red had ≥50% rise in serum creatinine or initiation of KRT. Initiation of KRT in one remdesivir-treated patient is demarcated by a dialysis machine icon.
Remdesivir was discontinued early in four patients (14%) due to safety concerns: twice due to elevated transaminase levels and twice due to concerns regarding low eGFR, despite neither of these two patients experiencing ≥50% increase in serum creatinine. The first had decompensated congestive heart failure in addition to COVID-19, was receiving high-dose continuous furosemide infusion, and had a rise in creatinine from 2.39 to 3.28 mg/dl prior to discontinuation of remdesivir. The second had an improving creatinine from 2.53 to 2.28 mg/dl; however, the medical team withheld further doses of remdesivir due to eGFR remaining <30 ml/min per 1.73 m2.
Overall mortality rate during the hospital stay was 19% (six of 31) in remdesivir-treated patients and 23% (seven of 31) in historical comparators (P=0.71).
Discussion
In this retrospective cohort study, we examined the risk of clinical and laboratory adverse events in hospitalized patients with COVID-19 who had eGFR <30 ml/min per 1.73 m2 and received remdesivir compared with historical controls who were hospitalized prior to EUA for remdesivir. This is the first report that used matched historical comparators with eGFR <30 ml/min per 1.73 m2. Our PS-matched algorithm was designed to predict both the chances of receiving remdesivir and the chances of adverse in-hospital outcomes (22). Our study also included a detailed chart review to ensure accurate matching on appropriate indicators of severity of illness bolstered by comprehensive review of all physician and nursing notes by two physicians for both patients and matched controls to ensure rigorous capture and adjudication of predefined adverse events. We found no significant difference in key clinical adverse events of interest. Lowest hemoglobin and highest AST and ALT values were similar between remdesivir-treated patients and historical comparators, whereas hyperglycemia was more common in remdesivir-treated patients. The rate of hyperglycemia was extremely high in the cohort treated with remdesivir; however, 81% of these patients also concurrently received dexamethasone compared with 10% of historical control patients. This is because all control patients were admitted in March or April 2020, prior to the routine use of dexamethasone for COVID-19 at our center. This change in the standard of care occurred in June 2020 with the release of data from the Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, after which hospitalized patients with severe COVID-19 routinely received dexamethasone (23). Our study was not powered to show treatment effectiveness, and the finding of insignificantly improved mortality in remdesivir-treated patients must be considered in light of the changing standard of care for COVID-19 over the study period and the known improvement in in-hospital mortality over time among hospitalized patients with COVID-19 (24).
Although a prior study demonstrated that remdesivir may be associated with bradycardia (25), we found no difference between the rates of bradycardia in remdesivir-treated patients and matched controls. This is reassuring given that patients with eGFR <30 ml/min per 1.73 m2 are likely to have higher exposure to the active metabolite of remdesivir and are more likely to have cardiovascular disease at baseline. Overall, the high rate of clinical events in the control group reflects the severity of COVID-19 among hospitalized patients as well as the high comorbidity burden and frailty of patients with eGFR <30 ml/min per 1.73 m2; this highlights the importance of benchmarking comparisons against appropriately matched controls.
Prior single-center and multicenter patient series of off-label remdesivir use in patients with ESKD have suggested that it is safe and well tolerated. Our finding that clinically significant transaminase elevations were rare and not increased with remdesivir treatment confirms all prior reports of remdesivir in patients with eGFR <30 ml/min per 1.73 m2 (26–29). Pettit et al. (27) found no significant increase in adverse events or increases in ALT, AST, or serum creatinine when remdesivir was given to patients with eGFR <30 ml/min per 1.73 m2 compared with patients with eGFR >30 ml/min per 1.73 m2, and they found that none of the transaminase or creatinine elevations were attributed to remdesivir use. Estiverne et al. (30) found that among 18 patients with eGFR <30 ml/min per 1.73 m2 who received remdesivir, there were two cases of transaminitis (ALT or AST more than five times the upper limit of normal), both of which were attributed to shock liver. Aiswarya et al. (29) studied 48 patients with ESKD on dialysis treated with remdesivir at a single hospital in India and noted that remdesivir was well tolerated, but one patient experienced acute coronary syndrome after the first dose of remdesivir. Relatedly, our study provides reassurance, as there were no cases of cardiac arrest and no increased risk of cardiac arrythmia in the remdesivir-treated patients compared with historical controls.
Two analyses of the World Health Organization pharmacovigilance database have identified that reports of AKI are more commonly reported after remdesivir use compared with other medications used to treated COVID-19 (hydroxycholoroquine, dexamethasone, and tocilizumab) (31,32); however, reporting bias exists in pharmacovigilance databases. Wongboonsin et al. (33) reported a case of worsening kidney function in a remdesivir-treated patient with biopsy-proven osmotic tubulopathy, which can occur when the kidney tubules become overwhelmed with an indigestible carbohydrate load, suggesting that there is a risk of nephrotoxicity from accumulation of the SBECD excipient; this is the only reported case to date in the literature, and this patient also had severe collapsing focal segmental glomerulopathy triggered by COVID-19, suggesting that osmotic tubulopathy is rare (33). Estiverne et al. (30) also reported one patient with worsening kidney function that was possibly attributed to remdesivir. However, it is important to note that this and all prior series have not had historical comparators with COVID-19 who did not receive remdesivir, and it is important to note that COVID-19 is associated with high rates of AKI in hospitalized patients (4,34). In this regard, our comparison of creatinine trends between remdesivir-treated patients and historical controls in this study is reassuring; the vast majority of remdesivir-treated patients had stable to improving serum creatinine (Figure 4). Biancalana et al. (35) evaluated a cohort of 80 elderly patients receiving remdesivir and found that many experienced a rise in eGFR during treatment; they speculated that remdesivir might promptly counteract severe acute respiratory syndrome coronavirus 2–mediated injury and attenuate systemic inflammation. Buxeda et al. (36) reported that remdesivir was safe in a series of 51 kidney transplant recipients and that early use (within 48 hours of hospitalization) was associated with a trend toward shortened hospital length of stay.
Our study had several limitations. This is a single health care system, and therefore, this limits generalizability; despite this, our matched cohort was racially and ethnically diverse. Our sample size was small, and we were only able to find appropriate matches for 31 of the 34 remdesivir-treated patients; even so, there were still differences between the matched groups. We chose historical controls from the first wave (prior to remdesivir’s EUA) because selecting controls who did not receive remdesivir after EUA would have introduced significant indication bias; however, the rapidly changing standard of care during the pandemic, including use of corticosteroids and changes in supportive care, makes it challenging to identify appropriate controls. A much larger study will be needed to have a robust estimate of safety. However, this study is among the largest series of off-label use in this population. Our study is also limited by the retrospective ascertainment of clinical outcomes and the fact that investigators were not blinded to treatment assignment. The fact that safety outcomes were predefined and that every physician and nursing note was reviewed by two investigators minimizes this bias.
Acute kidney disease and CKD are among the most important risk factors for adverse outcomes in patients with COVID-19. Exclusion of patients with kidney disease from clinical trials of lifesaving therapies is an important problem that the COVID-19 pandemic has highlighted (37,38). Although conclusive data on the safety of remdesivir among individuals with eGFR <30 ml/min per 1.73 m2 are lacking, our report adds to a growing body of data suggesting that a short course of remdesivir may be safe in the patients with AKI, advanced CKD, and ESKD. Definitive safety and efficacy data will follow from the Study to Evaluate the Efficacy and Safety of Remdesivir in Participants with Severely Reduced Kidney Function Who Are Hospitalized for Coronavirus Disease 2019, a randomized, controlled trial recruiting patients with severe COVID-19 who have eGFR <30 ml/min per 1.73 m2 (39).
Disclosures
M.E. Sise reports consultancy agreements with Bioporto; research funding from Abbvie, EMD-Serono, Gilead Sciences, and Merck; honoraria from the International Society of Hemodialysis for the Hemodialysis University Lecture; and scientific advisor or membership with Gilead Sciences as a scientific advisory board member and Travere Therapeutics as an scientific advisory board member. All remaining authors have nothing to disclose.
Funding
This work was funded by a Gilead Sciences (Gilead) investigator-initiated grant to Massachusetts General Hospital (to M.E. Sise).
Acknowledgments
All aspects of the study (protocol, data capture, analysis, and manuscript preparation) were performed by M.E. Sise and her coinvestigators at Massachusetts General Hospital.
Author Contributions
M.E. Sise conceptualized the study; J.D. Long, R. Seethapathy, M.E. Sise, I.A. Strohbehn, and S. Zhao were responsible for data curation; R. Seethapathy and M.E. Sise were responsible for investigation; M.E. Sise and S. Zhao were responsible for formal analysis; M.E. Sise was responsible for methodology; M.E. Sise was responsible for project administration; M.E. Sise and I.A. Strohbehn were responsible for resources; I.A. Strohbehn was responsible for software; M.E. Sise was responsible for validation; M.E. Sise was responsible for visualization; M.E. Sise was responsible for funding acquisition; M.E. Sise provided supervision; R. Seethapathy and M.E. Sise wrote the original draft; and J.D. Long, R. Seethapathy, M.E. Sise, I.A. Strohbehn, and S. Zhao reviewed and edited the manuscript.
Supplemental Material
This article contains supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0006152021/-/DCSupplemental.
Race code definitions. Download Supplemental Table 1, PDF file, 136 KB (135.9KB, pdf)
Diagnosis codes. Download Supplemental Table 2, PDF file, 136 KB (135.9KB, pdf)
Medication list. Download Supplemental Table 3, PDF file, 136 KB (135.9KB, pdf)
Characteristics and outcomes in the three unmatched remdesivir-treated patients. Download Supplemental Table 4, PDF file, 136 KB (135.9KB, pdf)
References
- 1.Johns Hopkins University of Medicine Coronavirus Resource Center : COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU), 2021. Available at: https://coronavirus.jhu.edu/map.html. Accessed August 10, 2021
- 2.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB: Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383: 590–592, 2020. 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020. 10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KDNorthwell COVID-19 Research ConsortiumNorthwell Nephrology COVID-19 Research Consortium : Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020. 10.1016/j.kint.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP; the Northwell COVID-19 Research Consortium : Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323: 2052–2059, 2020. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohamed MMB, Lukitsch I, Torres-Ortiz AE, Walker JB, Vipin Varghese V, Hernandez-Arroyo CF, Alqudsi M, LeDoux JR, Velez JCQ: Acute kidney injury associated with coronavirus disease 2019 in urban New Orleans. Kidney360 1: 614–622, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y, Bowe B, Maddukuri G, Al-Aly Z: Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: Cohort study. BMJ 371: m4677, 2020. 10.1136/bmj.m4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens JS, King KL, Robbins-Juarez SY, Khairallah P, Toma K, Alvarado Verduzco H, Daniel E, Douglas D, Moses AA, Peleg Y, Starakiewicz P, Li MT, Kim DW, Yu K, Qian L, Shah VH, O’Donnell MR, Cummings MJ, Zucker J, Natarajan K, Perotte A, Tsapepas D, Krzysztof K, Dube G, Siddall E, Shirazian S, Nickolas TL, Rao MK, Barasch JM, Valeri AM, Radhakrishnan J, Gharavi AG, Husain SA, Mohan S: High rate of renal recovery in survivors of COVID-19 associated acute renal failure requiring renal replacement therapy. PLoS One 15: e0244131, 2020. 10.1371/journal.pone.0244131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins-Juarez SY, Qian L, King KL, Stevens JS, Husain SA, Radhakrishnan J, Mohan S: Outcomes for patients with COVID-19 and acute kidney injury: A systematic review and meta-analysis. Kidney Int Rep 5: 1149–1160, 2020. 10.1016/j.ekir.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charytan DM, Parnia S, Khatri M, Petrilli CM, Jones S, Benstein J, Horwitz LI: Decreasing incidence of acute kidney injury in patients with COVID-19 critical illness in New York City. Kidney Int Rep 6: 916–927, 2021. 10.1016/j.ekir.2021.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellepiane S, Vaid A, Jaladanki SK, Coca S, Fayad ZA, Charney AW, Bottinger EP, He JC, Glicksberg BS, Chan L, Nadkarni G: Acute kidney injury in patients hospitalized with COVID-19 in New York City: Temporal trends from March 2020 to April 2021. Kidney Med 3: 877–879, 2021. 10.1016/j.xkme.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, Paranjpe I, Somani S, Richter F, Miotto R, Lala A, Kia A, Timsina P, Li L, Freeman R, Chen R, Narula J, Just AC, Horowitz C, Fayad Z, Cordon-Cardo C, Schadt E, Levin MA, Reich DL, Fuster V, Murphy B, He JC, Charney AW, Böttinger EP, Glicksberg BS, Coca SG, Nadkarni GN; Mount Sinai COVID Informatics Center (MSCIC) : AKI in hospitalized patients with COVID-19. J Am Nephrol Soc 32: 151–160, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flythe JE, Assimon MM, Tugman MJ, Chang EH, Gupta S, Shah J, Sosa MA, Renaghan AD, Melamed ML, Wilson FP, Neyra JA, Rashidi A, Boyle SM, Anand S, Christov M, Thomas LF, Edmonston D, Leaf DE; STOP-COVID Investigators : Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis 77: 190–203.e1, 2021. 10.1053/j.ajkd.2020.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilead : Veklury Prescribing Information, 2021. Available at: https://www.gilead.com/-/media/files/pdfs/medicines/COVID-19/veklury/veklury_pi.pdf. Accessed August 10, 2021
- 15.Adamsick ML, Gandhi RG, Bidell MR, Elshaboury RH, Bhattacharyya RP, Kim AY, Nigwekar S, Rhee EP, Sise ME: Remdesivir in patients with acute or chronic kidney disease and COVID-19. J Am Soc Nephrol 31: 1384–1386, 2020. 10.1681/ASN.2020050589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA: Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 55: 622–627, 2010. 10.1053/j.ajkd.2010.02.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee CM, Bhan I, Alexander EK, Brunelli SM: Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med 172: 153–159, 2012. 10.1001/archinternmed.2011.677 [DOI] [PubMed] [Google Scholar]
- 18.McMahon GM, Zeng X, Waikar SS: A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med 173: 1821–1828, 2013. 10.1001/jamainternmed.2013.9774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710, 1996. 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 20.Austin PC: An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46: 399–424, 2011. 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbaum PR, Rubin DB: Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 39: 33–38, 1985 [Google Scholar]
- 22.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T: Variable selection for propensity score models. Am J Epidemiol 163: 1149–1156, 2006. 10.1093/aje/kwj149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ; RECOVERY Collaborative Group : Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384: 693–704, 2021. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asch DA, Sheils NE, Islam MN, Chen Y, Werner RM, Buresh J, Doshi JA: Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med 181: 471–478, 2021. 10.1001/jamainternmed.2020.8193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Touafchia A, Bagheri H, Carrié D, Durrieu G, Sommet A, Chouchana L, Montastruc F: Serious bradycardia and remdesivir for coronavirus 2019 (COVID-19): A new safety concerns. Clin Microbiol Infect 27: 791.e5–791.e8, 2021. 10.1016/j.cmi.2021.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thakare S, Gandhi C, Modi T, Bose S, Deb S, Saxena N, Katyal A, Patil A, Patil S, Pajai A, Bajpai D, Jamale T: Safety of remdesivir in patients with acute kidney injury or CKD. Kidney Int Rep 6: 206–210, 2021. 10.1016/j.ekir.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettit NN, Pisano J, Nguyen CT, Lew AK, Hazra A, Sherer R, Mullane K: Remdesivir use in the setting of severe renal impairment: A theoretical concern or real risk? Clin Infect Dis 73: e3990–e3995,2021. 10.1093/cid/ciaa1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ackley TW, McManus D, Topal JE, Cicali B, Shah S: A valid warning or clinical lore: An evaluation of safety outcomes of remdesivir in patients with impaired renal function from a multicenter matched cohort [published correction appears in Antimicrob Agents Chemother 65: e0094321, 2021]. Antimicrob Agents Chemother 65: e02290-20, 2021. 10.1128/AAC.02290-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aiswarya D, Arumugam V, Dineshkumar T, Gopalakrishnan N, Lamech TM, Nithya G, Sastry BVRH, Vathsalyan P, Dhanapriya J, Sakthirajan R: Use of remdesivir in patients with COVID-19 on hemodialysis: A study of safety and tolerance. Kidney Int Rep 6: 586–593, 2021. 10.1016/j.ekir.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estiverne C, Strohbehn IA, Mithani Z, Hirsch JS, Wanchoo R, Goyal PG, Lee Dryden-Peterson S, Pearson JC, Kubiak DW, Letourneau AR, Bhattacharyya R, Jhaveri KD, Sise ME: Remdesivir in patients with estimated GFR <30 ml/min per 1.73 m2 or on renal replacement therapy. Kidney Int Rep 6: 835–838, 2021. 10.1016/j.ekir.2020.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chouchana L, Preta LH, Tisseyre M, Terrier B, Treluyer JM, Montastruc F: Kidney disorders as serious adverse drug reactions of remdesivir in coronavirus disease 2019: A retrospective case-noncase study. Kidney Int 99: 1235–1236, 2021. 10.1016/j.kint.2021.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gérard AO, Laurain A, Fresse A, Parassol N, Muzzone M, Rocher F, Esnault VLM, Drici MD: Remdesivir and acute renal failure: A potential safety signal from disproportionality analysis of the WHO safety database. Clin Pharmacol Ther 109: 1021–1024, 2021. 10.1002/cpt.2145 [DOI] [PubMed] [Google Scholar]
- 33.Wongboonsin J, Shah SI, Marty FM, Mount DB, Rennke HG, Murakami N: Osmotic tubulopathy in a patient with COVID-19 treated with remdesivir. Kidney Int Rep 6: 1987–1991, 2021. 10.1016/j.ekir.2021.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strohbehn IA, Zhao S, Seethapathy H, Lee M, Rusibamayila N, Allegretti AS, Parada XV, Sise ME: Acute kidney injury incidence, recovery, and long-term kidney outcomes among hospitalized patients with COVID-19 and influenza. Kidney Int Rep 6: 2565–2574, 2021. 10.1016/j.ekir.2021.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biancalana E, Chiriacò M, Sciarrone P, Mengozzi A, Mechelli S, Taddei S, Solini A: Remdesivir, renal function and short-term clinical outcomes in elderly COVID-19 pneumonia patients: A single-centre study. Clin Interv Aging 16: 1037–1046, 2021. 10.2147/CIA.S313028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buxeda A, Arias-Cabrales C, Pérez-Sáez MJ, Cacho J, Cabello Pelegrin S, Melilli E, Aladrén MJ, Galeano C, Lorenzo I, Mazuecos A, Saura IM, Franco A, Ruiz-Fuentes MDC, Sánchez-Cámara LA, Siverio O, Martin ML, González-García E, López V, Martin-Moreno PL, Moina I, Moral Berrio E, Moreso F, Portolés JM, Santana-Estupiñán R, Zárraga S, Canal C, Sánchez-Álvarez E, Pascual J, Crespo M: Use and safety of remdesivir in kidney transplant recipients with COVID-19. Kidney Int Rep 6: 2305–2315, 2021. 10.1016/j.ekir.2021.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Major R, Selvaskandan H, Makkeyah YM, Hull K, Kuverji A, Graham-Brown M: The exclusion of patients with CKD in prospectively registered interventional trials for COVID-19: A rapid review of international registry data. J Am Soc Nephrol 31: 2250–2252, 2020. 10.1681/ASN.2020060877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chewcharat A, Chang YT, Sise ME, Bhattacharyya RP, Murray MB, Nigwekar SU: Phase-3 randomized controlled trials on exclusion of participants with kidney disease in COVID-19. Kidney Int Rep 6: 196–199, 2021. 10.1016/j.ekir.2020.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ClinicalTrials.gov : Study to Evaluate the Efficacy and Safety of Remdesivir in Participants with Severely Reduced Kidney Function Who Are Hospitalized for Coronavirus Disease 2019 (COVID-19) (REDPINE), 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT04745351. Accessed August 10, 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Race code definitions. Download Supplemental Table 1, PDF file, 136 KB (135.9KB, pdf)
Diagnosis codes. Download Supplemental Table 2, PDF file, 136 KB (135.9KB, pdf)
Medication list. Download Supplemental Table 3, PDF file, 136 KB (135.9KB, pdf)
Characteristics and outcomes in the three unmatched remdesivir-treated patients. Download Supplemental Table 4, PDF file, 136 KB (135.9KB, pdf)