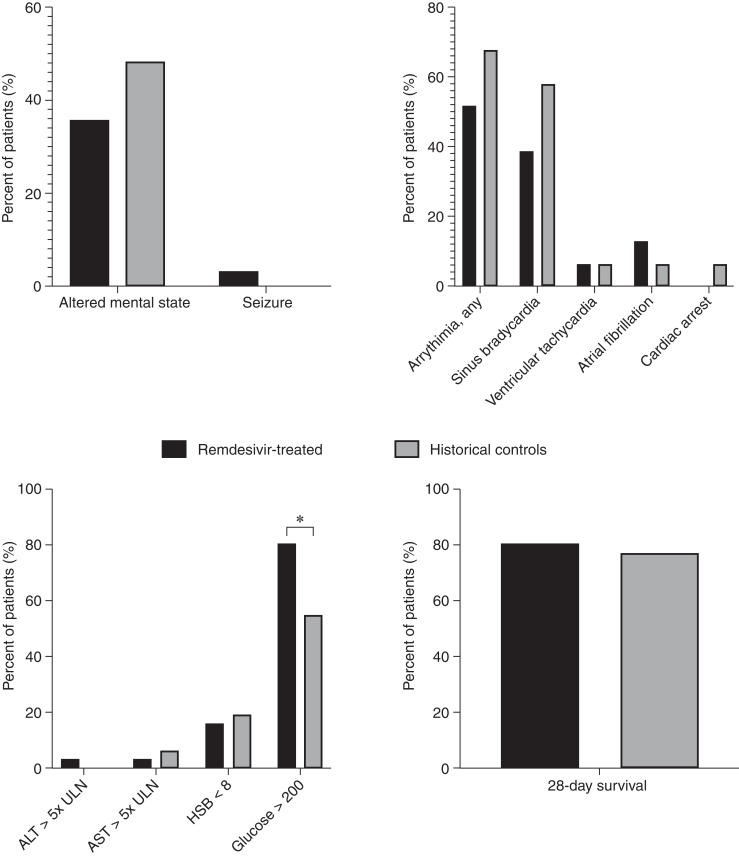
Figure 2.
Percentage of patients experiencing clinical events of interest. Clinical outcomes were adjudicated by two physicians who reviewed each physician and clinical nursing note for the 5-day remdesivir course +48 hours after remdesivir treatment (black bars) and the first 7 days of admission for historical comparators (gray bars). The only clinical event that was significantly increased in patients treated with remdesivir was the incidence of hyperglycemia (defined by glucose >200), which occurred in 81% of patients treated with remdesivir compared to 55% of controls. A total of 25 of 31 (81%) remdesivir-treated patients also received dexamethasone concurrently compared with three of 31 (10%) controls. Among the six remdesivir-treated patients who did not receive dexamethasone, three (50%) also had hyperglycemia >200 mg/dl. There were no significant differences in any of the other adverse events. ALT, alanine aminotransferase; AST, aspartate aminotransferase; HGB, hemoglobin; ULN, Upper limit of normal.