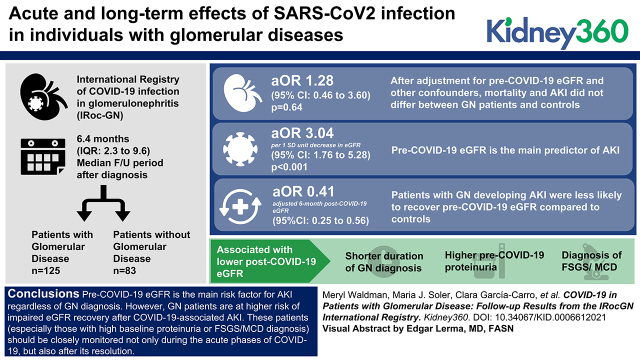
Key Points
Mortality and incidence of AKI do not differ between coronavirus disease 2019 (COVID-19) patients with or without glomerular diseases.
The main predictor of AKI is pre-COVID-19 eGFR, independent of the presence of GN.
Incomplete kidney function recovery after COVID-19-associated AKI is more common in GN patients than in controls.
Keywords: glomerular and tubulointerstitial diseases, COVID-19, follow-up studies, glomerular disease, kidney glomerulus, registries
Visual Abstract
Abstract
Background
The acute and long-term effects of severe acute respiratory syndrome coronavirus 2 infection in individuals with GN are still unclear. To address this relevant issue, we created the International Registry of COVID-19 infection in GN.
Methods
We collected serial information on kidney-related and -unrelated outcomes from 125 GN patients (63 hospitalized and 62 outpatients) and 83 non-GN hospitalized patients with coronavirus disease 2019 (COVID-19) and a median follow-up period of 6.4 (interquartile range 2.3–9.6) months after diagnosis. We used logistic regression for the analyses of clinical outcomes and linear mixed models for the longitudinal analyses of eGFR. All multiple regression models were adjusted for age, sex, ethnicity, and renin-angiotensin-aldosterone system inhibitor use.
Results
After adjustment for pre-COVID-19 eGFR and other confounders, mortality and AKI did not differ between GN patients and controls (adjusted odds ratio for AKI=1.28; 95% confidence interval [CI], 0.46 to 3.60; P=0.64). The main predictor of AKI was pre-COVID-19 eGFR (adjusted odds ratio per 1 SD unit decrease in eGFR=3.04; 95% CI, 1.76 to 5.28; P<0.001). GN patients developing AKI were less likely to recover pre-COVID-19 eGFR compared with controls (adjusted 6-month post-COVID-19 eGFR=0.41; 95% CI, 0.25 to 0.56; times pre-COVID-19 eGFR). Shorter duration of GN diagnosis, higher pre-COVID-19 proteinuria, and diagnosis of focal segmental glomerulosclerosis or minimal change disease were associated with a lower post-COVID-19 eGFR.
Conclusions
Pre-COVID-19 eGFR is the main risk factor for AKI regardless of GN diagnosis. However, GN patients are at higher risk of impaired eGFR recovery after COVID-19-associated AKI. These patients (especially those with high baseline proteinuria or a diagnosis of focal segmental glomerulosclerosis or minimal change disease) should be closely monitored not only during the acute phases of COVID-19 but also after its resolution.
Introduction
The International Registry of COVID-19 infection in GN (IRoc-GN) was created shortly after the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was declared a pandemic in March 2020 by the World Health Organization. The purpose of the registry was to examine the short- and long-term effect of coronavirus disease 2019 (COVID-19) in patients with underlying GN and identify risk factors for unfavorable outcomes.
Our initial report comparing 40 GN patients and 80 controls with SARS-CoV2 infection showed that the GN cohort had higher overall rates of mortality (15% versus 5%, respectively) and AKI (39% versus 14%, respectively) (1). Immunosuppressive therapy at presentation was not associated with greater mortality or AKI in the GN cohort, but more pronounced hypoalbuminemia at presentation and shorter duration of glomerular disease were associated with greater risk of AKI and need for kidney replacement therapy (KRT) in GN patients.
Enrollment of new patients in the IRoc-GN registry and collection of longitudinal data continued throughout the pandemic. Herein, we present the results of our follow-up study that was aimed to extend the findings of our initial report, describe the spectrum of COVID-19 in a larger GN cohort, and explore the interactions of underlying GN, immunosuppressive medications, and other determinants on susceptibility and outcomes of COVID-19 (AKI, need for KRT, or death). In addition, we aimed to characterize kidney recovery after COVID-19-associated AKI in GN patients and the longer-term consequences of COVID-19 on kidney prognosis.
Methods
Study Design and Participants
Details of this registry have been previously described (1). We used data collected from April 20, 2020, to April 20, 2021, via a secure public survey (https://redcapsurvey.niddk.nih.gov/surveys/?s=FPM87NK7T4) on REDCap (Research Electronic Data Capture) (2).
Briefly, we included patients with biopsy-proven GN diagnosed with COVID-19 managed as inpatients or outpatients from centers participating in the IRoc-GN registry. For each GN patient entered in the registry, reporters were asked to enter at least one age- and sex-matched control patient who was positive for SARS-CoV-2 but without GN and with an eGFR of >60 ml/min per 1.73 m2. Controls were hospitalized within ±2 weeks of the GN patients to account for changes in treatment strategies over time that may influence outcomes. Patients on maintenance hemodialysis before infection and kidney transplant recipients were excluded.
Data Collection
The initial survey collected data on pre-COVID-19 renal parameters, glomerular disease diagnosis, immunosuppressive medications, COVID-19-related symptoms, COVID-19-directed therapies, management of immunosuppression during infection, outcomes, the maximum level of care, nonrenal complications, AKI, need for KRT, disposition (i.e., recovery or death), and laboratory parameters. Links to follow-up surveys were automatically generated and sent by REDCap at predefined intervals (8 weeks) to collect longitudinal data on patient status and kidney outcomes after infection. With this combination of surveys, renal parameters were collected at various time points: (1) pre COVID-19 (latest available before infection onset), (2) at COVID-19 presentation, (3) peak, (4) post COVID-19 as defined by the absence of or marked improvement in original COVID-19-related symptoms or signs, and (5) during extended follow-up after recovery from acute infection. All data were checked for quality by three physicians (M.W., P.C., and U.M.).
Statistical Analyses
We compared baseline differences between groups in continuous variables using the Kruskal–Wallis test for three-group equality testing and the Mann–Whitney test for pairwise testing. We compared baseline differences between groups in categorical variables using Fisher’s exact test. We used Wilcoxon matched-pairs signed-rank test to compare crude paired continuous measurements over follow-up.
We used logistic regression to examine the association between glomerular disease status (indicator variable for patients with history of glomerular disease) and the clinical outcomes of AKI, KRT, and death (primary end points). This analysis was limited to hospitalized patients (i.e., glomerular disease patients versus controls). We calculated crude odds ratios (OR), OR adjusted for pre-COVID-19 eGFR, and OR additionally adjusted for demographic characteristics (age, continuous variable; men, indicator variable for men; ethnicity, indicator variable for non-White ethnicity), and renin-angiotensin-aldosterone system inhibitor (RAASi) use (indicator variable for their use). We deemed that RAASi use is a potential confounder because it had different distribution between patients with glomerular disease and controls (and within glomerular disease patients, between patients with different disease severity), and may also be associated with increased risk of AKI and KRT. Models that additionally adjusted for diabetes, obesity, and hypertension provided similar results. However, to avoid having to report several regression models that included more variables than the data could support, we did not report them in the final results.
The analysis of recovery of kidney function was based on the multiple regression models examining the effect of each variable on post-COVID-19 eGFR analyzed as longitudinal (repeated measures) continuous variable. Because the longitudinal eGFR measurements were unbalanced between patients, and because there was inconsistency among subjects in timing of the eGFR assessment, we examined longitudinal eGFR using random effects regression models estimated via restricted maximum likelihood. These models estimated eGFR as a linear change over time, which provided a good fit to the data points (Supplemental Figures 1 and 2).
We performed all of the analyses using Stata v17 (StataCorp, College Station, TX). The Stata code for all of the analyses is freely available at https://github.com/UMaggiore/iROC-GN.
A two-sided P value of <0.05 was regarded as statistically significant. Unless otherwise stated, we reported nominal P values without adjustment for multiple testing.
Further details on statistical analyses are included in the Supplemental Methods.
Results
Study Population
The study population included 125 patients with a history of GN diagnosed with COVID-19 (63 requiring hospitalization and 62 managed as outpatients) and 83 patients without GN who developed COVID-19 requiring hospitalization (Ctrl hospitalized). Baseline kidney function (pre-COVID-19 eGFR) was assessed at a median of 3.7 months (interquartile range 1.8–6.2 months) before infection. After COVID-19, the median follow-up of kidney function was 6.4 months (interquartile range 2.3–9.6).
The characteristics of the study population are reported in Table 1. Compared with Ctrl hospitalized patients, GN hospitalized patients had lower eGFR, serum albumin, and hemoglobin pre-COVID-19 and at time of admission (Table 1 and Supplemental Table 1). GN hospitalized patients also had lower eGFR, lower serum albumin, and higher proteinuria levels pre-COVID-19 and at COVID-19 diagnosis than GN outpatients (Table 1).
Table 1.
Baseline characteristics of the study population
| Study Group | P Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control Hospitalized (N=83) | Glomerular Neuropathy Hospitalized (N=63) | Glomerular Neuropathy Outpatients (N=62) | ||||||||
| Age, yr | 80 | 60.0 | 14.4 | 60 | 62.4 | 15.9 | 60 | 45.4 | 12.9 | <0.001b,c |
| Women | 29 | 35% | 28 | 44% | 36 | 58% | 0.02b | |||
| Race | ||||||||||
| White | 59 | 71% | 38 | 60% | 28 | 45% | 0.05b | |||
| Black | 5 | 6% | 3 | 5% | 4 | 7% | ||||
| Asian | 0 | 0% | 3 | 5% | 3 | 5% | ||||
| Other | 18 | 22% | 19 | 30% | 27 | 44% | ||||
| Unknown | 1 | 1% | 0 | 0% | 0 | 0% | ||||
| Ethnicity | ||||||||||
| Hispanic/Latino | 20 | 24% | 19 | 31% | 26 | 42% | 0.03b | |||
| BMI, kg/m2 | 68 | 30.2 | 7.4 | 50 | 27.6 | 4.9 | 53 | 27.2 | 5.6 | 0.06 |
| Comorbidities | ||||||||||
| Hypertension | 45 | 54% | 43 | 68% | 29 | 47% | 0.05c | |||
| Diabetes | 23 | 28% | 14 | 22% | 5 | 8% | 0.009b,c | |||
| Cardiovascular disease | 16 | 19% | 12 | 19% | 4 | 7% | 0.06 | |||
| Asthma | 6 | 7% | 3 | 5% | 1 | 2% | 0.34 | |||
| COPD | 4 | 5% | 7 | 11% | 2 | 3% | 0.23 | |||
| Liver disease | 4 | 5% | 3 | 5% | 1 | 2% | 0.66 | |||
| Cancerd | 4 | 5% | 7 | 11% | 0 | 0% | 0.02c | |||
| HIV/AIDS | 0 | 0% | 0 | 0% | 1 | 2% | 0.3 | |||
| Rheumatoid arthritis | 0 | 0% | 3 | 5% | 0 | 0% | 0.05 | |||
| Smoking habit | 5 | 6% | 1 | 2% | 1 | 2% | 0.3 | |||
| Use of RAASi | 3 | 4% | 17 | 27% | 22 | 36% | <0.001a,b,c | |||
| Clinical presentation | ||||||||||
| Fever | 66 | 80% | 46 | 73% | 31 | 50% | 0.001b,c | |||
| Dyspnea | 53 | 64% | 34 | 54% | 8 | 13% | <0.001b,c | |||
| Cough | 27 | 33% | 32 | 51% | 28 | 45% | 0.07 | |||
| GI symptoms | 26 | 31% | 13 | 21% | 11 | 18% | 0.14 | |||
| Anosmia | 15 | 18% | 4 | 6% | 12 | 19% | 0.06 | |||
| Fatigue | 8 | 10% | 19 | 30% | 24 | 39% | <0.001a,b | |||
| Myalgia | 8 | 10% | 14 | 22% | 20 | 32% | 0.003b | |||
| Anorexia | 4 | 5% | 12 | 19% | 7 | 11% | 0.02a | |||
| Chills | 4 | 5% | 9 | 14% | 5 | 8% | 0.12 | |||
| Sore throat | 3 | 4% | 2 | 3% | 8 | 13% | 0.05 | |||
| Nasal congestion | 2 | 2% | 3 | 5% | 8 | 13% | 0.04b | |||
| Neurologic symptoms | 0 | 0.0% | 1 | 2% | 0 | 0% | 0.6 | |||
| Pre-COVID-19 kidney parameters | ||||||||||
| sCr, mg/dl | 74 | 1.1 | 0.9 | 62 | 1.8 | 1.7 | 62 | 1.5 | 1.6 | <0.001a,c |
| eGFR, ml/min per 1.73 m2 | 71 | 80.0 | 25.8 | 59 | 52.9 | 28.7 | 60 | 73.0 | 31.0 | <0.001a,c |
| Serum albumin, g/dl | 42 | 3.9 | 0.6 | 55 | 3.7 | 0.6 | 59 | 4.1 | 0.7 | 0.002a,c |
| Proteinuria, g/d | 13 | 0.1 | 0.3 | 52 | 2.6 | 3.9 | 56 | 1.4 | 2.3 | <0.001a,b,c |
| Kidney parameters at presentation/ admission | ||||||||||
| sCr, mg/dl | 80 | 1.5 | 2.0 | 56 | 2.7 | 2.8 | 30 | 1.5 | 1.3 | <0.001a,c |
| eGFR, ml/min per 1.73 m2 | 77 | 76.2 | 32.0 | 53 | 46.1 | 32.4 | 30 | 72.0 | 35.8 | <0.001a,c |
| Laboratory parameters during COVID-19 | ||||||||||
| Serum albumin, g/dl | 64 | 3.5 | 0.8 | 47 | 3.0 | 0.9 | 24 | 4.0 | 0.6 | <0.001a,b,c |
| Proteinuria, g/d | 5 | 0.1 | 0.3 | 14 | 5.1 | 6.3 | 11 | 0.3 | 0.4 | 0.001a,c |
| White blood cells, n/μl | 43 | 8208.8 | 3581.1 | 52 | 8476.9 | 5274.4 | 38 | 6510.3 | 2341.6 | 0.12 |
| Lymphocytes, n/μl | 65 | 1126.5 | 1705.5 | 57 | 1730.9 | 2510.1 | 37 | 1533.4 | 1856.5 | 0.13 |
| Neutrophils, n/μl | 43 | 7105.1 | 6144.9 | 51 | 6496.2 | 3884.9 | 36 | 4726.6 | 2836.2 | 0.02b,c |
| Hemoglobin, g/dl | 43 | 13.1 | 2.6 | 52 | 11.5 | 2.4 | 39 | 12.8 | 1.6 | 0.002a,c |
| Platelets, n/μl | 43 | 248,720.9 | 132,425.1 | 52 | 230,230.8 | 105,626.2 | 39 | 243,820.5 | 72,710.6 | 0.57 |
| Ferritin, ng/ml | 64 | 1128.8 | 1527.2 | 38 | 927.2 | 840.4 | 23 | 260.1 | 231.6 | <0.001b,c |
| CRP, mg/l | 79 | 111.0 | 121.4 | 57 | 81.6 | 77.4 | 24 | 11.7 | 21.6 | <0.001b,c |
| D-Dimer, ng/ml | 70 | 1382.2 | 1984.5 | 41 | 1721.2 | 1787.0 | 11 | 613.7 | 731.4 | 0.010b,c |
Continuous data are reported as number of nonmissing variables, mean (SD); categorical data are reported as number of nonmissing variables and percentages. Due to the distribution of serum creatinine, which, unlike eGFR, is highly skewed on the right, and due to missing values in eGFR, mean serum creatinine and mean eGFR may erroneously appear inconsistent with each other. For continuous data, P values refer to Kruskal–Wallis test for any difference between the three groups, and Mann–Whitney for pairwise two-sample comparisons, for categorical data, to Fisher’s exact test. GN, history of glomerular disease; BMI, body mass index; sCr, serum creatinine; SLE, systemic lupus erythematosus; RAASi, renin-angiotensin-aldosterone system inhibitors; GI, gastrointestinal; COVID-19, coronavirus disease 2019; CRP, C-reactive protein.
The test for pairwise differences between the groups are indicated by superscripts as follows:.
P<0.05 Ctrl hospitalized versus GN hospitalized.
P<0.05 Ctrl hospitalized versus GN outpatients.
P<0.05 GN hospitalized versus GN outpatients.
None of the patients with cancer were on active chemotherapy treatment.
GN hospitalized patients and GN outpatients had similar distributions in the type of glomerular disease (Table 2). However, compared with GN outpatients, GN hospitalized patients were more likely to have received rituximab before COVID-19 diagnosis, to be on calcineurin inhibitors at the time of COVID-19, and to have a shorter duration of GN disease (<6 months; Table 2), whereas RAASi therapy was less common (Table 1). The percentage of patients with active GN disease at COVID-19 onset was similar between the two groups (Table 2). Tapering or withdrawal of immunosuppression during COVID-19 was uncommon and did not statistically differ between GN outpatients and GN hospitalized patients, with the exception of mycophenolate mofetil, which was more frequently reduced or discontinued in GN hospitalized patients (Table 2).
Table 2.
Baseline characteristics of glomerular neuropathy patients: diagnosis, treatment, and disease duration
| Admission Status | P Value | ||||
|---|---|---|---|---|---|
| Outpatients | Hospitalized | ||||
| SLE GN or vasculitis (systemic GN) | 26 | 42% | 26 | 41% | >0.99 |
| Lupus nephritis | 19 | 31% | 11 | 18% | 0.1 |
| Vasculitis | 7 | 11% | 15 | 24% | 0.1 |
| IgA nephropathy | 13 | 21% | 5 | 8% | 0.04 |
| FSGS or MCD | 11 | 18% | 9 | 14% | 0.63 |
| Membranous nephropathy | 8 | 13% | 5 | 8% | 0.4 |
| Amyloidosis/fibrillary GN | 2 | 3% | 2 | 3% | >0.99 |
| Thrombotic microangiopathy | 1 | 2% | 0 | 0% | 0.5 |
| Membranoproliferative GN | 0 | 0% | 4 | 6% | 0.12 |
| Post-infectious GN | 0 | 0% | 2 | 3% | 0.5 |
| Not specified | 1 | 2% | 10 | 16% | 0.009 |
| Immunosuppression at time of COVID-19 | 38 | 61% | 35 | 56% | 0.59 |
| Prednisone | 23 | 37% | 23 | 37% | >0.99 |
| Prednisone dose, mg/d | 8.6 (7.4) | 15.7 (22.0) | 0.21 | ||
| MMF | 15 | 24% | 11 | 18% | 0.39 |
| AZA | 1 | 2% | 4 | 6% | 0.37 |
| RTX | 1 | 2% | 11 | 18% | 0.004 |
| CNI | 2 | 3% | 15 | 24% | 0.001 |
| Therapy reduction/withdrawal during COVID-19a | |||||
| Prednisone | 0 | 0% | 1 | 4% | >0.99 |
| MMF | 3 | 20% | 8 | 73% | 0.007 |
| AZA | 0 | 0% | 3 | 75% | 0.24 |
| CNI | 1 | 50% | 1 | 7% | 0.47 |
| Duration of GN disease | |||||
| 1–6 mo | 5 | 8% | 13 | 27% | 0.004 |
| 6–12 mo | 0 | 0% | 6 | 12% | |
| 12–24 mo | 9 | 15% | 5 | 10% | |
| 2–5 yr | 17 | 28% | 6 | 12% | |
| >5 yr | 29 | 48% | 19 | 39% | |
| Active GN disease at COVID-19 onset | 5 | 8% | 8 | 18% | 0.23 |
Continuous data are reported as number of non-missing variables, mean (SD); categorical data are reported as number of nonmissing variables and percentages. Active GN disease was defined based on reporter assessment of patient’s clinical and laboratory features. P values refer to Fisher’s exact and to Cochran–Armitage test for trend (with exact P values) for GN disease. GN, history of glomerular disease; MCD, minimal change disease; SLE, systemic lupus erythematosus; MMF, mycophenolate mofetil; AZA, azathioprine; CNI, calcineurin inhibitor; RTX, rituximab.
Out of patients on immunosuppression at time of COVID-19.
At disease presentation, GN outpatients had less severe inflammatory indexes compared with GN hospitalized patients (C-reactive protein, serum ferritin, and D-dimer levels), whereas the same characteristics were similar between GN hospitalized patients and Ctrl hospitalized patients (Table 1). The rates of in-hospital complications (i.e., need for intubation or inotropes/vasopressors, superimposed bacterial infections, or thrombotic and cardiovascular complications) were similar between GN hospitalized patients and Ctrl hospitalized patients, but the duration of hospitalization was slightly longer in GN patients (mean 16.3 versus 14.4 days, respectively; P=0.03; Supplemental Table 1). As predicted on the basis of lower disease severity, fewer GN outpatients received any COVID-19 treatment compared with GN hospitalized patients and Ctrl hospitalized patients (Supplemental Table 2). Within hospitalized patients, fewer GN hospitalized patients received hydroxychloroquine and azithromycin than Ctrl hospitalized patients (Supplemental Table 2).
Clinical Outcomes in Patients with Glomerular Disease
We assessed the effect of glomerular disease on COVID-19 outcomes by comparing GN hospitalized with Ctrl hospitalized patients before and after adjusting for potential confounders (Table 3). Primary end points were incidence of AKI, initiation of KRT, and death. Incidence of AKI, KRT, and death were 46% versus 19%, 13% versus 10%, and 19% versus 10% in GN hospitalized patients versus Ctrl hospitalized patients, respectively. In crude analyses, GN hospitalized patients had increased odds of AKI (OR=3.44; 95% confidence interval [CI], 1.58 to 7.47; P=0.002) but not of KRT or death (Table 3). However, after adjusting for pre-COVID-19 eGFR and additional confounders (age, sex, non-White ethnicity, and RAASi use), the odds of AKI were similar between groups (OR=1.28; 95% CI, 0.43 to 3.60; P=0.64; Table 3). The adjusted analyses confirmed that OR for KRT and death were similar between GN hospitalized patients and Ctrl hospitalized patients (Table 3).
Table 3.
Association between presence of glomerular disease and clinical outcomes AKI, KRT, and death
| AKI | KRT | Death | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude | eGFR Adjusted | Fully Adjusted | Crude | eGFR Adjusted | Fully Adjusted | Crude | eGFR Adjusted | Fully Adjusted | |
| Preexisting GN | 3.44a | 1.62 | 1.28 | 1.43 | 0.68 | 0.60 | 1.80 | 0.90 | 0.80 |
| (1.58 to 7.47) | (0.66 to 4.00) | (0.46 to 3.60) | (0.49 to 4.22) | (0.19 to 2.36) | (0.15 to 2.38) | (0.67 to 4.83) | (0.29 to 2.79) | (0.23 to 2.81) | |
| 0.002 | 0.29 | 0.64 | 0.51 | 0.54 | 0.46 | 0.24 | 0.85 | 0.73 | |
| Prior eGFR (per 1 SD unit decrease) | 2.68a | 3.04 | 2.31b | 2.39b | 2.21a | 1.76 | |||
| (1.64 to 4.37) | (1.76 to 5.28) | (1.21 to 4.44) | (1.20 to 4.78) | (1.22 to 3.99) | (0.91 to 3.37) | ||||
| <0.001 | <0.001 | 0.01 | 0.01 | 0.009 | 0.09 | ||||
| Age (per 1 SD unit increase) | 0.84 | 0.90 | 2.33b | ||||||
| (0.48 to 1.47) | (0.46 to 1.76) | (1.10 to 4.94) | |||||||
| 0.54 | 0.75 | 0.03 | |||||||
| Sex | 1.81 | 1.04 | 0.77 | ||||||
| (0.68 to 4.79) | (0.31 to 3.50) | (0.25 to 2.35) | |||||||
| 0.23 | 0.95 | 0.65 | |||||||
| Non-White ethnicity | 3.06b | 1.50 | 4.62b | ||||||
| (1.12 to 8.34) | (0.41 to 5.57) | (1.30 to 16.44) | |||||||
| 0.03 | 0.54 | 0.02 | |||||||
| RAASi use | 1.55 | 1.08 | 0.87 | ||||||
| (0.44 to 5.51) | (0.19 to 6.21) | (0.15 to 4.99) | |||||||
| 0.5 | 0.93 | 0.88 | |||||||
Data shown are odds ratios from logistic regression models examining the association between history of glomerular disease and the clinical outcomes AKI, KRT, and death, comparing GN hospitalized patients with Ctrl hospitalized patients. For each outcome, three regression models are reported: crude model (no adjustment), eGFR adj. (adjusted for pre-COVID-19 eGFR), and fully adj. (additionally adjusted for age, sex, non-White ethnicity, and RAASi use). Odds ratios associated with eGFR are expressed per 1 SD unit (approximately 30 ml/min per 1.73 m2) decrease. Therefore, an odds ratio for AKI associated with prior eGFR (i.e., pre-COVID-19 eGFR) of 3.04 means that the odds of AKI increases by 3.04 times every 30 ml/min per 1.73 m2 decrease of pre-COVID-19 eGFR; the odds ratio for age is expressed per 1 SD (approximately 15 years) increase in age. Numbers in brackets represent 95% confidence intervals; the numbers below the brackets are the associated P values. Ctrl, control; KRT, kidney replacement therapy; RAASi, renin-angiotensin-aldosterone system inhibitors.
P<0.01.
P<0.05.
Pre-COVID-19 eGFR was the major determinant of clinical outcomes in adjusted analyses: for every 1 SD unit decrease in eGFR (approximately 30 ml/min per 1.73 m2 decrease), the adjusted OR was 3.04 (95% CI, 1.76 to 5.28; P<0.001) for AKI, 2.39 (95% CI, 1.20 to 4.78; P=0.014) for KRT, and 1.76 (95% CI, 0.91 to 3.37; P=0.09; Table 3) for death.
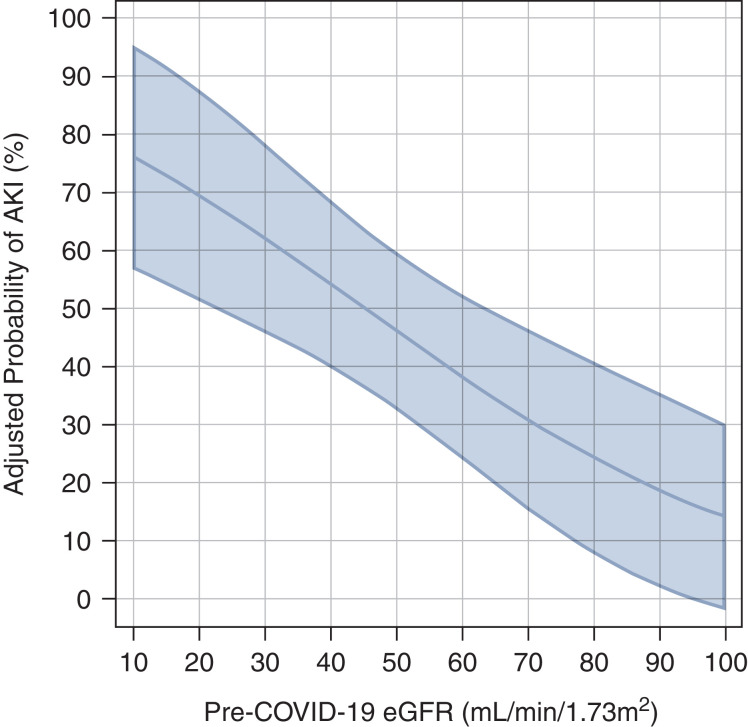
The multivariable adjusted relationship between pre-COVID-19 eGFR and the probability of AKI is reported in Figure 1: patients with pre-COVID-19 eGFR <30 ml/min per 1.73 m2 had a probability of developing AKI during their hospital stay of >50%. In the adjusted analyses, the rate of death increased with age (OR per 1 SD unit increase, which is approximately 15 years=2.33; 95% CI, 1.10 to 4.94; P=0.03) and in patients with non-White ethnicity (OR=4.62; 95% CI, 1.30 to 16.44; P=0.02; Table 3).
Figure 1.
Probability of developing AKI as a function of eGFR before coronavirus disease 2019 (COVID-19). The probability is based on a regression model, which is adjusted for age, sex, non-White ethnicity, and use of renin-angiotensin-aldosterone system inhibitors. The model is estimated only in hospitalized patients with glomerular disease. The shadowed area represents the 95% confidence interval (CI). The adjusted odds ratio of AKI per 1 SD unit decreases in eGFR (approximately 15 ml/min per 1.73 m2) was 2.88 (95% CI, 1.38 to 6.02; P=0.005).
Determinants of Clinical Outcomes in Patients with Glomerular Disease
We assessed the effect of determinants of clinical outcomes (AKI, KRT, or death) in patients with glomerular disease by performing analyses on GN hospitalized patients and GN outpatients pooled, after adjusting for potential confounders, including hospitalization status. Similar to the results above, pre-COVID-19 eGFR was the only significant determinant of AKI, KRT, and death (OR for death associated with 1 SD unit decrease in eGFR=3.00; 95% CI, 1.15 to 7.85; P=0.03), the latter being also associated with older age (OR for death associated with 1 SD increase in age=3.97; 95% CI, 1.47 to 10.74; P=0.007). In the crude analysis, only serum albumin was associated with AKI, and the presence of SLE was associated with the need for KRT (Supplemental Table 3). However, in the adjusted analyses, serum albumin, urinary protein excretion, and immunosuppressive drugs (azathioprine, calcineurin inhibitors, mycophenolate, rituximab, or steroids) were not significantly associated with the main clinical outcomes (Supplemental Table 3). In addition, the duration of glomerular disease diagnosis and type of GN (including a comparison of systemic GN versus renal limited GN) were not significantly associated with the main clinical outcomes (Supplemental Table 3).
Effect of Presence of Glomerular Disease on Recovery of Kidney Function Post-COVID-19
We assessed the effect of presence of glomerular disease on post-COVID-19 kidney function by comparing GN hospitalized patients with Ctrl hospitalized patients after adjusting for potential confounders. Kidney function recovery was assessed as a categorical outcome (i.e., any post-COVID-19 eGFR within –10% of pre-COVID-19 eGFR [baseline]). Overall, the rate of kidney function recovery was similar in GN hospitalized patients and Ctrl hospitalized patients (OR=1.47; 95% CI, 0.57 to 3.77; P=0.43; Table 4). In adjusted models, the only borderline statistically significant determinant of kidney function recovery was pre-COVID-19 eGFR (OR of kidney function recovery per 1 SD unit eGFR decrease=0.55; 95% CI, 0.33 to 0.90; P=0.02; Table 4).
Table 4.
Association between history of glomerular disease with kidney function recovery, and determinants of kidney function recovery in patients with history of glomerular disease
| Glomerular Neuropathy Hospitalized versus Control Hospitalized | Glomerular Neuropathy Hospitalized and Glomerular Neuropathy Outpatients Pooled | ||||
|---|---|---|---|---|---|
| Crude | eGFR Adjusted | Fully Adjusted | eGFR | Full Model | |
| Preexisting GN | 1.23 | 1.60 | 1.47 | ||
| (0.62 to 2.43) | (0.66 to 3.86) | (0.57 to 3.77) | |||
| 0.55 | 0.3 | 0.43 | |||
| Prior eGFR (per 1 SD unit decrease) | 0.57b | 0.55b | 0.55a | 0.59b | |
| (0.36 to 0.90) | (0.33 to 0.90) | (0.35 to 0.86) | (0.36 to 0.95) | ||
| 0.02 | 0.02 | 0.009 | 0.03 | ||
| Age (per 1 SD unit increase) | 1.10 | 1.02 | |||
| (0.66 to 1.82) | (0.61 to 1.73) | ||||
| 0.73 | 0.93 | ||||
| Sex | 0.93 | 1.25 | |||
| (0.40 to 2.15) | (0.49 to 3.18) | ||||
| 0.86 | 0.64 | ||||
| Non-White ethnicity | 1.78 | 2.73b | |||
| (0.69 to 4.60) | (1.03 to 7.28) | ||||
| 0.24 | 0.04 | ||||
| RAASi use | 1.07 | 1.24 | |||
| (0.31 to 3.67) | (0.44 to 3.46) | ||||
| 0.92 | 0.69 | ||||
Data shown are odds ratios from logistic regression models examining the association between history of glomerular disease and recovery of kidney disease (i.e., at least one eGFR values with –10% of pre-COVID-19 eGFR [baseline]), comparing GN hospitalized patients with Ctrl hospitalized patients. For each outcome, three regression models are reported: crude model (no adjustment), eGFR adj. (adjusted for pre-COVID-19 eGFR), and fully adj. (additionally adjusted for age, sex, non-White ethnicity, and RAASi use). On the right, the same logistic model is fitted in GN hospitalized patients and GN outpatients (pooled) for examining prior eGFR (i.e., pre-COVID-19). The model is fitted before (eGFR model), and after (full model) adjusting for the other covariates. Odds ratios associated with eGFR are expressed per 1 SD unit (approximately 30 ml/min per 1.73 m2) decrease. Therefore, an odds ratio associated with a prior eGFR of 0.55 means that the odds of recovery decreases by 0.55 times every 30 ml/min per 1.73 m2 decrease of pre-COVID-19 eGFR; the odds ratio for age is expressed per 1 SD (approximately 15 years) increase in age. Numbers in brackets represent 95% confidence intervals; the numbers below the brackets are the associated P values. Crtl, control; GN, history of glomerular disease; RAASi, renin–angiotensin–aldosterone system inhibitors.
P<0.01.
P<0.05.
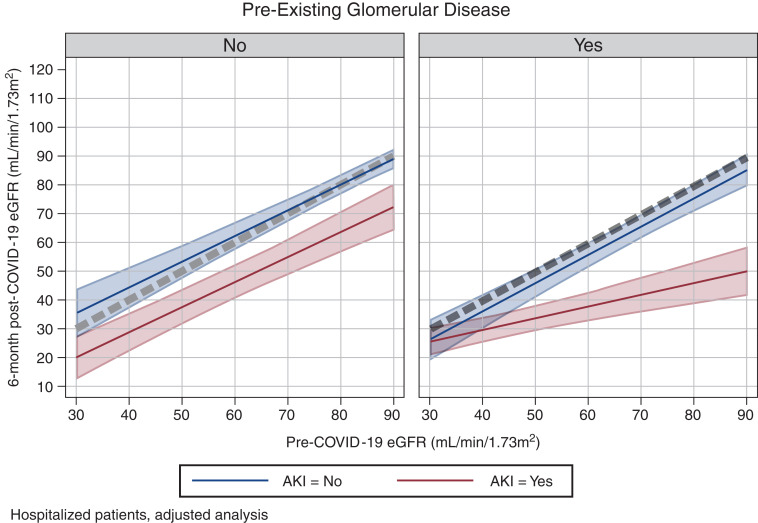
We next analyzed data using kidney function as a continuous outcome (by comparing pre- and post-COVID-19 eGFR). Overall, there was no significant difference in the correlation between pre- and post-COVID-19 eGFR between GN hospitalized patients and Ctrl hospitalized patients. However, when we stratified patients on the basis of development of AKI, the correlation between pre- and post-COVID-19 eGFR significantly differed between GN hospitalized patients and Ctrl hospitalized patients. In particular, we found that Ctrl hospitalized patients who developed AKI had a full recovery of eGFR (to pre-COVID-19 levels) by 6 months after COVID-19. In contrast, kidney recovery was only partial in GN hospitalized patients who developed AKI during hospitalization. This is demonstrated by the four-way interaction term between GN versus Ctrl, time, AKI, and pre-COVID-19 eGFR, which was statistically significant (P=0.03). To have a visual appraisal of the interaction term, we estimated the adjusted coefficient of the relationship between pre-COVID-19 eGFR and 6-month post-COVID-19 eGFR according to the history of glomerular disease and AKI, and plotted the results predicted by the multiple regression model (Supplemental Table 4 and Figure 2). For all of the groups, except GN hospitalized patients who had AKI, the coefficient between pre-COVID-19 and 6-month post-COVID-19 was close to one (Supplemental Table 4) and the line close to the line of identity (the line of identity between pre-COVID-19 and 6-month post-COVID-19 eGFR is the dotted line in Figure 2): Ctrl hospitalized patients without AKI: 0.90 (95% CI, 0.75 to 1.04); GN hospitalized patients without AKI: 0.87 (95% CI, 0.70 to 1.04); Ctrl hospitalized patients with AKI: 0.98 (95% CI, 0.82 to 1.14); and GN hospitalized patients with AKI: 0.41 (95% CI, 0.25 to 0.56). The intercept was not statistically significantly different from zero in all four groups (data not shown). The visual representation of how the four-way interaction in terms of changes in eGFR over time is shown in Supplemental Figure 3.
Figure 2.
Visual representation of the results of the four-way interaction term from the longitudinal mixed model on eGFR between GN and Ctrl hospitalized patients, time, AKI, and pre-COVID-19 eGFR (P=0.02). The figure represents the predicted relationship between pre- and post-COVID-19 eGFR after stratification of hospitalized patients according to the absence (left panel) and presence (right panel) of a history of glomerular disease (Ctrl hospitalized and GN hospitalized, respectively, throughout the text). Patients are additionally stratified on the basis of those who did not develop (blue) or who developed (red) AKI during their hospital stay. The dotted gray line represents the line of identity between pre- and post-COVID-19 eGFR (i.e., full recovery of kidney function). Although Ctrl hospitalized patients had a full recovery of eGFR at 6 months after COVID-19, the recovery was only partial in GN hospitalized patients who developed AKI during their hospital stay. For all of the groups, except GN hospitalized patients who had AKI, the coefficient between pre-COVID-19 and 6-month post-COVID-19 was close to one and the line close to the line of identity: Ctrl hospitalized patients without AKI: 0.90 (95% CI, 0.75 to 1.04); GN hospitalized patients without AKI: 0.87 (95% CI, 0.70 to 1.04); Ctrl hospitalized patients with AKI: 0.98 (95% CI, 0.82 to 1.14); GN hospitalized patients with AKI: 0.41 (95% CI, 0.25 to 0.56; see Supplemental Table 4). The visual representation of the four-way interaction term as eGFR change over time is reported in Supplemental Figure 3.
In the multivariable regression models, determinants that were significantly associated with lower post-COVID-19 eGFR were older age (–3.5 ml/min per 1.73 m2 per 1 SD unit increase) and non-White ethnicity (–5.4 ml/min per 1.73 m2).
Determinants of Kidney Function Recovery in Patients with Glomerular Disease
Among infected GN patients, pre-COVID-19 eGFR and serum albumin decreased only in those who were hospitalized (Supplemental Table 5). The only borderline significant determinant of kidney function recovery was pre-COVID-19 eGFR (OR of kidney function recovery per 1 SD unit decrease of eGFR=0.62 (95% CI, 0.38 to 1.01; P=0.06; Table 4). In the adjusted analyses, serum albumin, urinary protein, type of immunosuppressive drugs (azathioprine, mycophenolate, calcineurin inhibitors, rituximab, or steroids), and duration and type of glomerular disease were not significantly associated with the rate of kidney function recovery (categorical variable; Supplemental Table 6). At variance, when we examined the effect of the same determinants of post-COVID-19 eGFR (continuous variable), we found that higher baseline proteinuria (–2.1 ml/min per 1.73 m2 per 1 SD unit increase) and, more importantly, preexisting FSGS or minimal change disease (MCD) diagnosis (–7.7 ml/min per 1.73 m2) were associated with significantly lower post-COVID-19 eGFR (Supplemental Table 7).
Discussion
To our knowledge, this is the first analysis of both the short- and long-term outcomes of COVID-19 infection among GN patients. On the basis of a meta-analysis from 2020, the pooled incidence of COVID-19-associated AKI among hospitalized patients was 29% (95% CI, 19.8 to 39.5) in the United States and Europe, although the reported ranges are quite broad (3). In our study, nearly half of the hospitalized GN patients experienced AKI, suggesting that the incidence of COVID-19-associated AKI in GN patients is at the higher range of estimates of AKI reported among hospitalized patients (4–8). However, our data indicate that the presence of underlying GN does not confer an independent risk for COVID-19-associated AKI. In line with the well-established association between preexisting CKD and risk of in-hospital AKI in general (9–11), and in the setting of COVID-19 (4,12–14), we showed that pre-COVID-19 eGFR is the main determinant of AKI and of need for KRT.
There was no difference in mortality between GN and control patients. Both in-hospital AKI and the presence of CKD have been consistently associated with higher COVID-19-associated mortality (5,15–20). The similar adjusted rates of AKI between the GN and control cohorts may partly explain the observed lack of difference in mortality in this study. AKI and death were almost entirely confined to hospitalized patients. Although we do not have information on COVID-19 severity scores, these data suggest that milder cases of COVID-19 in GN patients managed in the outpatient setting have a more benign outcome than hospitalized patients.
Importantly, our results indicate that whereas controls with COVID-19-associated AKI tend to show recovery of kidney function by 6 months, GN patients with AKI have slower, incomplete kidney recovery and persistently lower eGFR at longer-term follow-up. Longitudinal outcome and recovery data on patients with COVID-19-associated AKI are still limited (4,21,22). One recent cohort study reported accelerated eGFR decline and lower rates of kidney recovery after hospitalization in patients with COVID-19-associated AKI compared with AKI for other reasons, independent of comorbidities or AKI severity (21). The etiology of the observed slower and incomplete kidney recovery in GN patients compared with control patients after COVID-19-associated AKI is likely multifactorial (20). It is noteworthy that a shorter duration of GN diagnosis was associated with both a greater risk of AKI and slower kidney recovery. In addition, a higher degree of pre-COVID-19 proteinuria and a history of FSGS/MCD were associated with decreased kidney recovery and lower eGFR post-COVID-19 at follow-up. These interesting observations set the basis for future studies aimed to better define GN subgroups that are particularly vulnerable to CKD progression after COVID-19-associated AKI.
Several pathophysiological pathways are believed to be activated in the context of SARS-CoV-2 infection leading to kidney injury, and a spectrum of histologic changes in the glomerular, tubulointerstitial, and vascular compartments of the kidney have been described. The extent to which baseline patient characteristics (i.e., type, duration, or immunologic activity of underlying GN, proteinuria, and kidney gene expression profiles) contribute to the type of AKI or subsequent recovery warrants further study. The lack of kidney biopsies in the GN and control cohorts with COVID-19-associated AKI precludes comparisons. However, it is conceivable that GN patients with underlying immune dysregulation, kidney immune cell infiltration, and prothrombotic tendencies are more prone to endothelial dysfunction, coagulopathy, and complement activation or have greater sensitivity of podocytes and tubules to the effects of SARS-CoV-2, which may affect renal prognosis compared with those without GN. Superimposed on limited renal reserve, persistence of proteinuria, hematuria, and maladaptive repair mechanisms in GN patients after AKI may also promote renal fibrosis, leading to incomplete kidney recovery (23,24). Limited serial proteinuria values and relevant serological data in our study preclude meaningful conclusions regarding the contribution of changes in GN activity or GN relapses (possibly related to reduction of immunosuppression during infection or to the infection itself) in delayed kidney recovery.
Our data do not indicate an association between immunosuppressive treatment before COVID-19, level of pre-COVID-19 proteinuria, or the type of GN (systemic versus kidney limited) on the primary outcomes of mortality and AKI. However, hospitalized GN patients had higher pre-COVID-19 proteinuria and lower serum albumin than those managed as outpatients. In addition, a greater proportion of hospitalized patients were on calcineurin inhibitors at the time of COVID-19 or received rituximab within 6 months preceding infection. The published data regarding the influence of longitudinal immunosuppression on COVID-19 susceptibility and outcomes has been changing and contradictory. Although our data regarding calcineurin inhibitors and rituximab are too limited to draw firm conclusions, these observations deserve further study, particularly in light of the importance of B cell depleting therapy for GN. Some early reports suggested no effect of rituximab or a slightly increased risk of hospitalization from COVID‐19 (25–29). More recently studies have reported unfavorable prognosis particularly in those with shorter duration between last rituximab infusion and infection (30–33). These conflicting results may reflect heterogeneity in the diseases treated with rituximab, intensity of B cell depletion and IgG levels at infection onset, and number of previous treatments. Similarly, the data regarding calcineurin inhibitors has been mixed. In a large Danish cohort study, treatment with cyclosporine or tacrolimus was associated with a significantly increased risk of hospitalization (34). In contrast, a retrospective study from Spain indicated beneficial effects of cyclosporine in COVID-19 (76% reduction in mortality) (35). This preliminary evidence has boosted interest in these drugs for treatment of COVID-19, and there are several ongoing clinical trials (36). Results of such trials may influence treatment decisions of underlying GN during the ongoing pandemic (37).
Among GN patients, those of non-White ethnicity had a higher adjusted mortality risk—a finding consistent with many published reports that have highlighted race/ethnicity-related differences in COVID-19 rates and outcomes. Research to disentangle the various complex factors that contribute to ethnic variability is ongoing, although socioeconomic and sociodemographic factors appear to play important roles (19,33,38–40).
Our study has many strengths. There is global representation with a diverse population. It focuses on a subpopulation of CKD patients with unique (and potentially dynamic) baseline clinical characteristics and treatment challenges for which little data is currently available. The study is reflective of the various presentations of COVID-19 in GN patients managed as inpatients and outpatients. We also analyzed longitudinal laboratory data extending from pre-COVID-19 through recovery. This is particularly relevant because the 25th Consensus Conference of the Acute Disease Quality Initiative highlighted the natural history of kidney sequelae after COVID-19-associated AKI as major research recommendations (41,42). Importantly, inclusion of a control group of non-GN patients strengthened the validity of the findings. However, there are some caveats that should be considered. The sample size remains relatively small despite our large network. There were missing data at various time points, particularly for GN patients managed as outpatients. Serial laboratory tests (i.e., proteinuria) that would provide a better understanding of GN relapses or changes in disease activity post-COVID-19 were not consistently performed. Also, the control and GN groups were not perfectly matched, and we chose to include only hospitalized controls due to limitations in data collection in outpatients. Adjustments for confounders allowed us to minimize the bias of the unbalanced control characteristics, but it is possible that differences between the two groups persist that cannot be fully accounted for.
In conclusion, in a diverse international cohort of patients with COVID-19, mortality and AKI did not differ between GN patients and controls. The main predictor of AKI was pre-COVID-19 eGFR. Incomplete kidney recovery after COVID-19-associated AKI was more common in GN patients compared with controls. Shorter duration of GN diagnosis, higher grade proteinuria, and FSGS/MCD diagnosis were associated with impaired kidney recovery during longer-term follow-up. These findings remain highly relevant despite the availability of COVID-19 vaccines in light of the rising rates of COVID-19 spread of the delta variant and other variants of concern, impaired vaccine response among immunocompromised patients (43–47), breakthrough infections among vaccinated (48,49), and waning vaccine immunity over time.
Disclosures
O. Bestard reports patents and inventions with Oxford Immunotec and is associate editor of Transplant International and Frontiers in Immunology. A. Bruchfeld reports consultancy agreements with AstraZeneca, Chemocentryx, Fresenius, and Merck; a research grant from AstraZeneca; honoraria from Bayer, Chemocentryx, Fresenius, Merck, and Vifor; and is a member of the ERA-EDTA scientific advisory board 2018–2024, chair of the ERA-EDTA Immunonephrology Working Group, and vice-chair of the Swedish Renal Fund. G. Comai reports honoraria from Alexion, Astellas, and Novartis. P. Cravedi reports honoraria as an advisor for Chinook Therapeutics and is associate editor for the Journal of Nephrology and the American Journal of Transplantation. G. Fernandez Juarez reports research funding from Instituto Salud Calos III and honoraria from Alexion and GSK. E. Fiaccadori is on the editorial board of the Journal of Nephrology and Blood Purification and is a member of the Italian Society of Nephrology and the European Society of Parenteral and Enteral Nutrition. O. Flossmann reports consultancy agreements with Vifor Pharma has other interests/relationships with the British Medical Association, European Vasculitis Society, Renal Association (UK), Royal College of Physicians London, and UK Ireland Vasculitis Society. C. García-Carro reports consultancy agreements with Astellas, AstraZeneca, Boehringer Ingelheim Lilly, Esteve, Novartis and Baxter, Novo Nordisk, and Otsuka; honoraria from Astellas, AstraZeneca, Boehringer Ingelheim Lilly, Esteve, Novartis and Baxter, Novo Nordisk, and Otsuka; and is a scientific advisor for or member of AstraZeneca, Boehringer Ingelheim Lilly, Mundipharma, and Novo Nordisk. M. Griffith reports honoraria from Retrophin’s advisory board. A. J. Hamilton is on the editorial board of the Journal of Kidney Care and is a member of the SONG-Kids Life Participation Expert Working Group. T. Leach reports honoraria from Janssen for conference attendance in 2012 and is a scientific advisor to the National Institute for Health and Care Excellence. L. Lightstone reports consultancy agreements with Achillion, Alexion, AstraZeneca, Aurinia, BMS, GSK, Kezar, Novartis, Pfizer, and Roche; honoraria from Alexion, AstraZeneca, BMS, GSK, and Pfizer; is a scientific advisor or member of EU Exec Lupus Nephritis Trials Network; is on the advisory board of Nature Reviews Nephrology; participates in a speakers’ bureau for Alexion and GSK; is a trustee of Kidney Research UK 2018–2022; is an executive member of the International Society of Nephrology 2021–2023; is deputy chair of Western Regional Board of the International Society of Nephrology; and is a clinical expert representing the Renal Association response to NICE on STA for belimumab in lupus 2020–2021. U. Maggiore reports consultancy agreements with Biotest, Chiesi, GSK, Hansa, Novartis, Sandoz, and Takeda. J. Manrique is a scientific advisor for or member of AstraZeneca, Boehringer, and Viphor Pharma. E. Morales reports consultancy agreements with Alexion, Celgene, Viphor Fresenius, and Vifor Pharma. J.A. Niño-Cruz reports research funding from Pfizer Scientific Institute and participates in a speakers’ bureau for Takeda and Roche. A. Ortiz reports consultancy agreements with Retrophin and Sanofi Genzyme; research funding from AstraZeneca, Mundipharma, and Sanofi Genzyme; honoraria from Advicciene, Alexion, Amgen, Amicus, Astellas, AstraZeneca, Bayer, Chiesi, Fresenius Medical Care, Idorsia, Kyowa Kirin, Menarini, Otsuka, Sanofi Genzyme, and Vifor Fresenius Medical Care Renal Pharma; is a member of the Spanish Society of Nephrology; is editor-in-chief of the Clinical Kidney Journal; is on the editorial boards of the Journal of Nephrology, Journal of the American Society of Nephrology, and Peritoneal Dialysis International; is a member of SOMANE and ERA Councils; is on the board of directors for IIS-Fundacion Jimenez Diaz UAM; is on the scientific advisory board of the Dutch Kidney Foundation; honoraria listed above are for speaker engagements: Advicciene, Alexion, Astellas, AstraZeneca, Amicus, Amgen, Bayer, Chiesi, Fresenius Medical Care, Idorsia, Kyowa Kirin, Menarini, Otsuka, Sanofi Genzyme, and Vifor Fresenius Medical Care Renal Pharma. S. Sinha reports consultancy agreements with Sanifit; research funding from Amgen, AstraZeneca, and Ethicon; and honoraria from AstraZeneca, Bayer, Napp Pharmaceuticals, Novartis, and Sanofi Genzyme. M.F. Slon-Roblero reports consultancy agreements with Baxter, Fresenius, and Nipro; and honoraria from Baxter, Fresenius, and Nipro. M.J. Soler reports consultancy agreements with AstraZeneca, Bayer, Boehringer, Esteve, Jansen, Mundipharma, Novo Nordisk, Travere, and ICU; research funding from Abbvie and Boehringer; honoraria from AstraZeneca, Boehringer, Esteve, FMC, Jansen, ICU Medical, Mundipharma, Novo Nordisk, Otsuka, and Travere; patents and inventions: U691ES00; is a scientific advisor for or member of BMC Nephrology and Clinical Kidney Journal; is a former member of ERA-EDTA Council; participates in a speakers’ bureau for AstraZeneca, Bayer, Boehringer, Esteve, FMC, Jansen, Mundipharma, Novo Nordisk, and Vifor; is a member of the Sociedad Española de Nefrología and Sociedad Catalana de Nefrologia; and is elected editor-in-chief of the Clinical Kidney Journal. All remaining authors have nothing to disclose.
Funding
This research was supported in part by the Intramural Research Program of the NIH, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. P. Cravedi is supported by NIH R01 Grants DK119431 and AI132949.
Acknowledgments
The authors thank Matthew Breymaier (NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development) for his assistance with creation of the registry using REDCap. Thank you to Dr. Damien Ashby, and all in the ICHNT Renal COVID Group, for use of the COVID database and care of the patients.
Author Contributions
P. Cravedi and M. Waldman conceived and oversaw the project; I. Agraz, A. Avello, O. Bestard, C. Bini, A. Bruchfeld, K.L. Budge, C. Cantarelli, C. Chrysochou, G. Comai, M. Delsante, G. Fernández Juárez, R. Fernandez-Prado, E. Fiaccadori, O. Flossman, R. García-Agudo, C. Garcia-Carro, C. Geddes, M. Griffith, A.J. Hamilton, L. Howard, G. La Manna, T. Leach, L. Lightstone, J. Manrique, S. Marinaki, A.J. Martinez-Rueda, C. Martin Vasa, L. Martinez Valenzuela, E. Morales, J.A. Niño-Cruz, A. Ortiz, C. Rabasco Ruiz, M. Sierra-Carpio, S. Sinha, M.F. Slon, M.J. Soler, J. Torras, and T. Turner-Stokes collected the data; U. Maggiore performed all of the statistical analyses; P. Cravedi, U. Maggiore, and M. Waldman analyzed the data and wrote the initial draft of the manuscript; all of the authors reviewed and approved the final version of the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0006612021/-/DCSupplemental.
Download Supplemental Methods, PDF file, 894 KB (893.5KB, pdf)
Download Supplemental Reference, PDF file, 894 KB (893.5KB, pdf)
Analysis on the goodness of fit of the linear random-coefficient mixed model on eGFR. Download Supplemental Figure 1, PDF file, 894 KB (893.5KB, pdf)
Data used for longitudinal analyses. Download Supplemental Figure 2, PDF file, 894 KB (893.5KB, pdf)
Visual representation of the results of the four-way interaction term from the longitudinal mixed model on eGFR between GN patients and Ctrl hospitalized patients, time, AKI, and pre-COVID-19 eGFR. Download Supplemental Figure 3, PDF file, 894 KB (893.5KB, pdf)
Complications during hospital stay. Download Supplemental Table 1, PDF file, 894 KB (893.5KB, pdf)
Treatments for COVID-19. Download Supplemental Table 2, PDF file, 894 KB (893.5KB, pdf)
Association of various determinants with clinical outcomes AKI, kidney replacement therapy, and death in the overall cohort of GN patients. Download Supplemental Table 3, PDF file, 894 KB (893.5KB, pdf)
Estimated relationship between pre- and post-COVID-19 eGFR at 6 months after COVID-19 admission. Download Supplemental Table 4, PDF file, 894 KB (893.5KB, pdf)
Serial kidney parameters in patients with glomerular disease based on admission status. Download Supplemental Table 5, PDF file, 894 KB (893.5KB, pdf)
Analysis on determinants of kidney function recovery (categorical variable) in GN patients. Download Supplemental Table 6, PDF file, 894 KB (893.5KB, pdf)
Analysis on the determinants of post-COVID-19 eGFR (continuous variables). Download Supplemental Table 7, PDF file, 894 KB (893.5KB, pdf)
References
- 1.Waldman M, Soler MJ, García-Carro C, Lightstone L, Turner-Stokes T, Griffith M, Torras J, Valenzuela LM, Bestard O, Geddes C, Flossmann O, Budge KL, Cantarelli C, Fiaccadori E, Delsante M, Morales E, Gutierrez E, Niño-Cruz JA, Martinez-Rueda AJ, Comai G, Bini C, La Manna G, Slon MF, Manrique J, Agraz I, Sinaii N, Cravedi P: Results from the IRoc-GN international registry of patients with COVID-19 and glomerular disease suggest close monitoring. Kidney Int 99: 227–237, 2021. 10.1016/j.kint.2020.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN; REDCap Consortium : The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 95: 103208, 2019. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu EL, Janse RJ, de Jong Y, van der Endt VHW, Milders J, van der Willik EM, de Rooij ENM, Dekkers OM, Rotmans JI, van Diepen M: Acute kidney injury and kidney replacement therapy in COVID-19: A systematic review and meta-analysis. Clin Kidney J 13: 550–563, 2020. 10.1093/ckj/sfaa160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, Paranjpe I, Somani S, Richter F, Miotto R, Lala A, Kia A, Timsina P, Li L, Freeman R, Chen R, Narula J, Just AC, Horowitz C, Fayad Z, Cordon-Cardo C, Schadt E, Levin MA, Reich DL, Fuster V, Murphy B, He JC, Charney AW, Böttinger EP, Glicksberg BS, Coca SG, Nadkarni GN; Mount Sinai COVID Informatics Center (MSCIC) : AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 32: 151–160, 2021. 10.1681/ASN.2020050615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng JH, Hirsch JS, Hazzan A, Wanchoo R, Shah HH, Malieckal DA, Ross DW, Sharma P, Sakhiya V, Fishbane S, Jhaveri KD; Northwell Nephrology COVID-19 Research Consortium : Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis 77: 204–215.e1, 2021. 10.1053/j.ajkd.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns TS, Abramowitz MK, Levy R, Kumar N, Mokrzycki MH, Coco M, Dominguez M, Prudhvi K, Golestaneh L: AKI in hospitalized patients with and without COVID-19: A comparison study. J Am Soc Nephrol 31: 2145–2157, 2020. 10.1681/ASN.2020040509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins-Juarez SY, Qian L, King KL, Stevens JS, Husain SA, Radhakrishnan J, Mohan S: Outcomes for patients with COVID-19 and acute kidney injury: A systematic review and meta-analysis. Kidney Int Rep 5: 1149–1160, 2020. 10.1016/j.ekir.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan KW, Yu KY, Lee PW, Lai KN, Tang SC: Global REnal involvement of CORonavirus Disease 2019 (RECORD): A systematic review and meta-analysis of incidence, risk factors, and clinical outcomes. Front Med (Lausanne) 8: 678200, 2021. 10.3389/fmed.2021.678200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grams ME, Sang Y, Ballew SH, Gansevoort RT, Kimm H, Kovesdy CP, Naimark D, Oien C, Smith DH, Coresh J, Sarnak MJ, Stengel B, Tonelli M; CKD Prognosis Consortium : A meta-analysis of the association of estimated GFR, albuminuria, age, race, and sex with acute kidney injury. Am J Kidney Dis 66: 591–601, 2015. 10.1053/j.ajkd.2015.02.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James MT, Grams ME, Woodward M, Elley CR, Green JA, Wheeler DC, de Jong P, Gansevoort RT, Levey AS, Warnock DG, Sarnak MJ; CKD Prognosis Consortium : A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis 66: 602–612, 2015. 10.1053/j.ajkd.2015.02.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James MT, Hemmelgarn BR, Wiebe N, Pannu N, Manns BJ, Klarenbach SW, Tonelli M; Alberta Kidney Disease Network : Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: A cohort study. Lancet 376: 2096–2103, 2010. 10.1016/S0140-6736(10)61271-8 [DOI] [PubMed] [Google Scholar]
- 12.Flythe JE, Assimon MM, Tugman MJ, Chang EH, Gupta S, Shah J, Sosa MA, Renaghan AD, Melamed ML, Wilson FP, Neyra JA, Rashidi A, Boyle SM, Anand S, Christov M, Thomas LF, Edmonston D, Leaf DE; STOP-COVID Investigators : Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis 77: 190–203.e1, 2021. 10.1053/j.ajkd.2020.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh J, Malik P, Patel N, Pothuru S, Israni A, Chakinala RC, Hussain MR, Chidharla A, Patel H, Patel SK, Rabbani R, Patel U, Chugh S, Kichloo A: Kidney disease and COVID-19 disease severity-systematic review and meta-analysis [published online ahead of print April 23, 2021]. Clin Exp Med 10.1007/s10238-021-00715-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibertoni D, Reno C, Rucci P, Fantini MP, Buscaroli A, Mosconi G, Rigotti A, Giudicissi A, Mambelli E, Righini M, Zambianchi L, Santoro A, Bravi F, Altini M: COVID-19 incidence and mortality in non-dialysis chronic kidney disease patients. PLoS One 16: e0254525, 2021. 10.1371/journal.pone.0254525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ERA-EDTA Council; ERACODA Working Group : Chronic kidney disease is a key risk factor for severe COVID-19: A call to action by the ERA-EDTA. Nephrol Dial Transplant 36: 87–94, 2021. 10.1093/ndt/gfaa314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson N, Nelveg-Kristensen K-E, Freese Ballegaard E, Feldt-Rasmussen B, Hornum M, Kamper A-L, Gislason G, Torp-Pedersen C: Increased vulnerability to COVID-19 in chronic kidney disease. J Intern Med 290: 166–178, 2021. 10.1111/joim.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, Sutherland A, Puri S, Srivastava A, Leonberg-Yoo A, Shehata AM, Flythe JE, Rashidi A, Schenck EJ, Goyal N, Hedayati SS, Dy R, Bansal A, Athavale A, Nguyen HB, Vijayan A, Charytan DM, Schulze CE, Joo MJ, Friedman AN, Zhang J, Sosa MA, Judd E, Velez JCQ, Mallappallil M, Redfern RE, Bansal AD, Neyra JA, Liu KD, Renaghan AD, Christov M, Molnar MZ, Sharma S, Kamal O, Boateng JO, Short SAP, Admon AJ, Sise ME, Wang W, Parikh CR, Leaf DE; STOP-COVID Investigators : AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol 32: 161–176, 2021. 10.1681/ASN.2020060897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B: Factors associated with COVID-19-related death using OpenSAFELY. Nature 584: 430–436, 2020. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pakhchanian H, Raiker R, Mukherjee A, Khan A, Singh S, Chatterjee A: Outcomes of COVID-19 in CKD patients: A multicenter electronic medical record cohort study. Clin J Am Soc Nephrol 16: 785–786, 2021. 10.2215/CJN.13820820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nugent J, Aklilu A, Yamamoto Y, Simonov M, Li F, Biswas A, Ghazi L, Greenberg H, Mansour G, Moledina G, Wilson FP: Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw Open 4: e211095, 2021. 10.1001/jamanetworkopen.2021.1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowe B, Xie Y, Xu E, Al-Aly Z: Kidney outcomes in long COVID. J Am Soc Nephrol 32: 2851–2862, 2021. 10.1681/ASN.2021060734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, Kellum JA, Ronco C; ADQI XIII Work Group : Progression after AKI: Understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol 27: 687–697, 2016. 10.1681/ASN.2015030309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenoglio R, Sciascia S, Baldovino S, Roccatello D: Acute kidney injury associated with glomerular diseases. Curr Opin Crit Care 25: 573–579, 2019. 10.1097/MCC.0000000000000675 [DOI] [PubMed] [Google Scholar]
- 25.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, Izadi Z, Jacobsohn L, Katz P, Lawson-Tovey S, Mateus EF, Rush S, Schmajuk G, Simard J, Strangfeld A, Trupin L, Wysham KD, Bhana S, Costello W, Grainger R, Hausmann JS, Liew JW, Sirotich E, Sufka P, Wallace ZS, Yazdany J, Machado PM, Robinson PC; COVID-19 Global Rheumatology Alliance : Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: Data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 79: 859–866, 2020. 10.1136/annrheumdis-2020-217871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angeletti A, Drovandi S, Sanguineri F, Santaniello M, Ferrando G, Forno R, Cipresso G, Caridi G, Riella LV, Cravedi P, Ghiggeri GM: COVID-19 in children with nephrotic syndrome on anti-CD20 chronic immunosuppression. Clin J Am Soc Nephrol 15: 1494–1495, 2020. 10.2215/CJN.06400420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salter A, Fox RJ, Newsome SD, Halper J, Li DKB, Kanellis P, Costello K, Bebo B, Rammohan K, Cutter GR, Cross AH: Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol 78: 699–708, 2021. 10.1001/jamaneurol.2021.0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suárez-Díaz S, Morán-Castaño C, Coto-Hernández R, Mozo-Avellaneda L, Suárez-Cuervo C, Caminal-Montero L: Mild COVID-19 in ANCA-associated vasculitis treated with rituximab. Ann Rheum Dis 80: e99, 2020. 10.1136/annrheumdis-2020-218246 [DOI] [PubMed] [Google Scholar]
- 29.Sahraian MA, Azimi A, Navardi S, Ala S, Naser Moghadasi A: Evaluation of the rate of COVID-19 infection, hospitalization and death among Iranian patients with multiple sclerosis. Mult Scler Relat Disord 46: 102472, 2020. 10.1016/j.msard.2020.102472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loarce-Martos J, García-Fernández A, López-Gutiérrez F, García-García V, Calvo-Sanz L, Del Bosque-Granero I, Terán-Tinedo MA, Boteanu A, Bachiller-Corral J, Vázquez-Díaz M: High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: A descriptive study. Rheumatol Int 40: 2015–2021, 2020. 10.1007/s00296-020-04699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FAI2R/SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors : Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: Data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis 80: 527–538, 2020. 10.1136/annrheumdis-2020-218310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones JM, Faruqi AJ, Sullivan JK, Calabrese C, Calabrese LH: COVID-19 outcomes in patients undergoing B cell depletion therapy and those with humoral immunodeficiency states: A scoping review. Pathog Immun 6: 76–103, 2021. 10.20411/pai.v6i1.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, Mateus EF, Richez C, Santos MJ, Schmajuk G, Scirè CA, Sirotich E, Sparks JA, Sufka P, Thomas T, Trupin L, Wallace ZS, Al-Adely S, Bachiller-Corral J, Bhana S, Cacoub P, Carmona L, Costello R, Costello W, Gossec L, Grainger R, Hachulla E, Hasseli R, Hausmann JS, Hyrich KL, Izadi Z, Jacobsohn L, Katz P, Kearsley-Fleet L, Robinson PC, Yazdany J, Machado PM; COVID-19 Global Rheumatology Alliance : Factors associated with COVID-19-related death in people with rheumatic diseases: Results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 80: 930–942, 2021. 10.1136/annrheumdis-2020-219498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nørgård BM, Nielsen J, Knudsen T, Nielsen RG, Larsen MD, Jølving LR, Kjeldsen J: Hospitalization for COVID-19 in patients treated with selected immunosuppressant and immunomodulating agents, compared to the general population: A Danish cohort study. Br J Clin Pharmacol 87: 2111–2120, 2021. 10.1111/bcp.14622 [DOI] [PubMed] [Google Scholar]
- 35.Guisado-Vasco P, Valderas-Ortega S, Carralón-González MM, Roda-Santacruz A, González-Cortijo L, Sotres-Fernández G, Martí-Ballesteros EM, Luque-Pinilla JM, Almagro-Casado E, La Coma-Lanuza FJ, Barrena-Puertas R, Malo-Benages EJ, Monforte-Gómez MJ, Diez-Munar R, Merino-Lanza E, Comeche-Casanova L, Remirez-de-Esparza-Otero M, Correyero-Plaza M, Recio-Rodríguez M, Rodríguez-López M, Sánchez-Manzano MD, Andreu-Vázquez C, Thuissard-Vasallo IJ, María-Tomé JME, Carnevali-Ruiz D: Clinical characteristics and outcomes among hospitalized adults with severe COVID-19 admitted to a tertiary medical center and receiving antiviral, antimalarials, glucocorticoids, or immunomodulation with tocilizumab or cyclosporine: A retrospective observational study (COQUIMA cohort). EClinicalMedicine 28: 100591, 2020. 10.1016/j.eclinm.2020.100591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ClinicalTrials.gov : Cyclosporidine, COVID-10. Available at: https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=cyclosporine&cntry=&state=&city=&dist=. Accessed September 17, 2021.
- 37.Willicombe M, Thomas D, McAdoo S: COVID-19 and calcineurin inhibitors: Should they get left out in the storm? J Am Soc Nephrol 31: 1145–1146, 2020. 10.1681/ASN.2020030348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yehia BR, Winegar A, Fogel R, Fakih M, Ottenbacher A, Jesser C, Bufalino A, Huang R-H, Cacchione J: Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw Open 3: e2018039, 2020. 10.1001/jamanetworkopen.2020.18039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S: Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open 3: e2029058, 2020. 10.1001/jamanetworkopen.2020.29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin J, Agarwala N, Kundu P, Harvey B, Zhang Y, Wallace E, Chatterjee N: Individual and community-level risk for COVID-19 mortality in the United States. Nat Med 27: 264–269, 2021. 10.1038/s41591-020-01191-8 [DOI] [PubMed] [Google Scholar]
- 41.Nadim MK, Forni LG, Mehta RL, Connor MJ Jr, Liu KD, Ostermann M, Rimmelé T, Zarbock A, Bell S, Bihorac A, Cantaluppi V, Hoste E, Husain-Syed F, Germain MJ, Goldstein SL, Gupta S, Joannidis M, Kashani K, Koyner JL, Legrand M, Lumlertgul N, Mohan S, Pannu N, Peng Z, Perez-Fernandez XL, Pickkers P, Prowle J, Reis T, Srisawat N, Tolwani A, Vijayan A, Villa G, Yang L, Ronco C, Kellum JA: COVID-19-associated acute kidney injury: Consensus report of the 25th Acute Disease Quality Initiative (ADQI) workgroup [published correction appears in Nat Rev Nephrol 16: 765, 2020 10.1038/s41581-020-00372-5]. Nat Rev Nephrol 16: 747–764, 2020. 10.1038/s41581-020-00356-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vijayan, A, Abdel-Rahman, EM, Liu, KD, Goldstein, SL, Agarwal, A, Okusa, MD, Cerda, J; AKI!NOW Steering Committee : Recovery after critical illness and acute kidney injury. Clin J Am Soc Nephrol 16: 1601–1609, 2021. 10.2215/CJN.19601220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A: Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N Engl J Med 385: 661–662, 2021. 10.1056/NEJMc2108861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM: Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 325: 2204–2206, 2021. 10.1001/jama.2021.7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marinaki S, Adamopoulos S, Degiannis D, Roussos S, Pavlopoulou ID, Hatzakis A, Boletis IN: Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant 21: 2913–2915, 2021. 10.1111/ajt.16607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geisen UM, Berner DK, Tran F, Sümbül M, Vullriede L, Ciripoi M, Reid HM, Schaffarzyk A, Longardt AC, Franzenburg J, Hoff P, Schirmer JH, Zeuner R, Friedrichs A, Steinbach A, Knies C, Markewitz RD, Morrison PJ, Gerdes S, Schreiber S, Hoyer BF: Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 80: 1306–1311, 2021. 10.1136/annrheumdis-2021-220272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, Demissie EG, El-Qunni AA, Haile A, Huang K, Kinnett B, Liebeskind MJ, Liu Z, McMorrow LE, Paez D, Pawar N, Perantie DC, Schriefer RE, Sides SE, Thapa M, Gergely M, Abushamma S, Akuse S, Klebert M, Mitchell L, Nix D, Graf J, Taylor KE, Chahin S, Ciorba MA, Katz P, Matloubian M, O’Halloran JA, Presti RM, Wu GF, Whelan SPJ, Buchser WJ, Gensler LS, Nakamura MC, Ellebedy AH, Kim AHJ: Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: A prospective cohort study. Ann Intern Med 174: 1572–1585, 2021. 10.7326/M21-1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsapepas D, Paget K, Mohan S, Cohen DJ, Husain SA: Clinically significant COVID-19 following SARS-CoV-2 vaccination in kidney transplant recipients. Am J Kidney Dis 78: 314–317, 2021. 10.1053/j.ajkd.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ali NM, Alnazari N, Mehta SA, Boyarsky B, Avery RK, Segev DL, Montgomery RA, Stewart ZA: Development of COVID-19 infection in transplant recipients after SARS-CoV-2 vaccination. Transplantation 105: e104–e106, 2021. 10.1097/TP.0000000000003836 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Download Supplemental Methods, PDF file, 894 KB (893.5KB, pdf)
Download Supplemental Reference, PDF file, 894 KB (893.5KB, pdf)
Analysis on the goodness of fit of the linear random-coefficient mixed model on eGFR. Download Supplemental Figure 1, PDF file, 894 KB (893.5KB, pdf)
Data used for longitudinal analyses. Download Supplemental Figure 2, PDF file, 894 KB (893.5KB, pdf)
Visual representation of the results of the four-way interaction term from the longitudinal mixed model on eGFR between GN patients and Ctrl hospitalized patients, time, AKI, and pre-COVID-19 eGFR. Download Supplemental Figure 3, PDF file, 894 KB (893.5KB, pdf)
Complications during hospital stay. Download Supplemental Table 1, PDF file, 894 KB (893.5KB, pdf)
Treatments for COVID-19. Download Supplemental Table 2, PDF file, 894 KB (893.5KB, pdf)
Association of various determinants with clinical outcomes AKI, kidney replacement therapy, and death in the overall cohort of GN patients. Download Supplemental Table 3, PDF file, 894 KB (893.5KB, pdf)
Estimated relationship between pre- and post-COVID-19 eGFR at 6 months after COVID-19 admission. Download Supplemental Table 4, PDF file, 894 KB (893.5KB, pdf)
Serial kidney parameters in patients with glomerular disease based on admission status. Download Supplemental Table 5, PDF file, 894 KB (893.5KB, pdf)
Analysis on determinants of kidney function recovery (categorical variable) in GN patients. Download Supplemental Table 6, PDF file, 894 KB (893.5KB, pdf)
Analysis on the determinants of post-COVID-19 eGFR (continuous variables). Download Supplemental Table 7, PDF file, 894 KB (893.5KB, pdf)