Abstract
The association between cardiovascular (CV) disease and anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is well documented. The recent work by Massicotte-Azarniouch et al. confirms the risk and adds to the existing evidence by describing the highest risk in the first 3 months after diagnosis. In this review, we aim to put their findings into perspective and formulate implications for the care of AAV patients. We discuss mechanisms for increased CV disease in AAV, including the impact of traditional risk factors and disease-related risks such as renal impairment and anti-myeloperoxidase (MPO) ANCA serotype. We also provide a brief primer on the impact of inflammatory-driven endothelial dysfunction and platelet activation on accelerated atherosclerosis in AAV patients. These features alongside the impact of disease activity and systemic inflammation provide potential explanations to why the incidence of CV events is highest in the first 3 months from diagnosis. We suggest future avenues of research, provide some suggestions to address and treat CV risk based on current evidence, and highlight the importance of addressing this topic early on. Addressing modifiable risk factors, dialogue with patients, patient information and a structured approach overall will be key to improve CV outcomes in AAV.
Keywords: cardiovascular, medication, prognosis, treatment, vasculitis
INTRODUCTION
The association between cardiovascular disease (CVD) and chronic inflammatory conditions is well described [1] with a robust evidence base particularly around systemic lupus erythematosus [2]. There is growing recognition of a similar cardiovascular (CV) risk in patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated small vessel vasculitis (AAV). Multiple studies, including long-term follow-ups from large randomized control trials, have corroborated a high CV risk in AAV, with evidence of premature atherosclerosis independent of traditional risk factors and with increased rates of CV events such as myocardial infarction (MI) and stroke [3–6]. A recent meta-analysis has since placed the risk of all CV events in AAV patients as being 65% higher compared with the general population [7]. In this issue of the journal, Massicotte-Azarniouch et al. [8] undertook a large retrospective cohort study evaluating CV outcomes in patients with a new diagnosis of AAV compared with a non-AAV population. Their study benefits from a strong study design with propensity score matching and demonstrated a significantly increased CV risk amongst those with AAV. The CV risk was highest within the first year of diagnosis, with peak risk during the initial 3-month period. This well-conducted study supports existing evidence, highlighting AAV patients as an at-risk group, and is the first to stratify risk from the time of diagnosis. Here, we aim to put this study into perspective, provide a brief primer on the existing body of literature around CVD in AAV and highlight areas of uncertainty. We briefly review risk factors, both traditional and those relating to vasculitis disease activity. We also suggest further areas of research into this important aspect of the management of patients with AAV. On a background of much improved treatment options, we also provide clinicians with a practical approach to CV risk reduction in AAV.
Traditional risk factors
When considering predictors of CVD in the AAV population, pre-existing traditional risk factors, including hypertension, dyslipidaemia and a relevant family history, have all been shown to be significant and highly prevalent [7, 9–10]. Advancing age has also been demonstrated as a contributing factor in an at-risk population with a typical peak disease onset in the sixth and seventh decades of life [9–11]. Furthermore, following an initial diagnosis of AAV, patients remain at risk of developing new onset hypertension and diabetes, with the severity of renal impairment, anaemia and anti-myeloperoxidase (MPO) ANCA serotype as key associated factors [12]. Remarkably, whilst smoking is a well-recognized risk factor for CVD, it has also been shown to have protective properties in the context of autoimmune diseases, including AAV [13]. The potential pathogenetic role of nicotine inhibiting T-cell function and the role of carbon monoxide in vascular tone are possible explanations for the protective effects despite the direct endothelial damage caused [13].
Compounding the impact of these more traditional CV considerations is the potential limited uptake of management options, with data suggesting that only a third of patients are on lipid-lowering therapy and a similar proportion remain with suboptimal blood pressure control [9]. Despite these factors, conventional risk prediction models such as the Framingham tool are thought to underestimate the level of CV risk in the AAV population, with a disease-specific model demonstrating superior predictive accuracy within 5 years of diagnosis [7, 10]. In line with this, an increased cumulative incidence of CVD following the initial diagnosis of AAV has been observed and a positive correlation with the Birmingham Vasculitis Activity Score (BVAS) strengthens support for AAV-related risk factors [7, 11].
Disease activity, endothelial dysfunction and accelerated atherosclerosis
Small vessel vasculitis is characterized by destructive inflammation and necrosis of vessel walls. Neutrophil activation within the vessel wall and pro-inflammatory cytokines result in endothelial injury, inflammation, blood vessel occlusion and ischaemia leading to end-organ damage [4, 14]. Endothelial cell necrosis and detachment from the basement membrane not only provide a potential biomarker of disease activity [14], but the necrotic debris may itself have detrimental effects away from areas of inflammation [15]. The process of endothelial dysfunction also leads to the formation of endothelial-derived microparticles, which are complex structures derived from activated platelets and dead endothelial cells [16]. These microparticles have been associated with unstable plaque formation and are known to mediate adverse CV events [16].
Due to the degree of inflammation in AAV patients at presentation, it is plausible that endothelial dysfunction is the most prevalent in the acute phase, and the risk of accelerated atherosclerosis is highest in the first few months of diagnosis [17]. This is also borne out in the study by Massicotte-Azarniouch et al. [8]. Bai et al. [11] undertook a retrospective study of 504 patients, identifying BVAS as an independent predictor of CV-related mortality in AAV, indicating the degree of disease activity is likely to be associated with amplified levels of endothelial dysfunction and an increased risk of CV events [11]. More recently, Wu et al. [18] demonstrated that a C-reactive protein monomer (mCRP), which is responsible for activating platelets, complement and pro-inflammatory cytokines, was associated with CVD in AAV patients. This strengthens the link between AAV and CVD independent of conventional risk factors. Furthermore, successful remission-induction therapy can restore endothelial function, potentially minimizing CV risk with early disease control [19]. This has also been demonstrated with an improvement in any resulting arterial stiffness and impairment of vasodilation, which are an early indicator of atheromatous disease [20, 21]. Accordingly, the impact of arterial stiffness has been shown to be an independent predictor of mortality in multiple patient groups, and its presence in the cerebral microvasculature of patients with AAV as a consequence of disease has been confirmed [22, 23]. This risk appears to persist into remission [24].
A further consequence of inflammation-driven endothelial dysfunction is platelet activation as a further pathway towards a hypercoagulable, pro-thrombotic state [25, 26]. Here, we focus mainly on arterial disease in AAV, but multiple studies have also shown a significant association between AAV and an increased incidence of venous thromboembolic (VTE) events [26, 27]. It is currently unclear whether pathways leading to venous and arterial disease are largely identical, overlapping or different, and further research is required to tease out similarities and differences. When considering atherosclerotic plaque rupture, increased platelet activation with thrombocytosis, which is often seen in active AAV, may increase the likelihood of platelet aggregation with subsequent thrombus formation [28, 29]. Furthermore, antiphospholipid antibodies of various specificities and positive functional tests for lupus anticoagulant are prevalent in a subgroup of AAV patients [30]. Why some patients with AAV form these antibodies while many others do not remains poorly understood, together with the long-term implications of having these antibodies. Whilst the VTE risk associated with antiphospholipid antibodies and lupus anticoagulant is well known, they are also associated with accelerated atherosclerosis, further contributing to CV risks [31]. In keeping with this, Tabakovic et al. [32] identified a stroke incidence of 9.7/1000 person years in the AAV population, with the highest risk within the first year and an increased platelet count at AAV diagnosis as an independent risk factor [32]. Whilst prospective studies have not been undertaken looking at anticoagulation or antiplatelet therapy in AAV, this suggests there may be a role and further research work into the potential benefit is needed.
MPO is an enzyme that is mainly found in neutrophils and is involved in microbicidal activity as well as the formation of reactive oxidant species that can result in tissue damage. Localization of elevated MPO and their catalytic activity has been found at sites of atherosclerotic lesions, with a known positive association between MPO levels and CV risk in the chronic kidney disease population [33]. Increased expression of MPO as the autoantigen in anti-MPO AAV may contribute to the presence of accelerated atherosclerosis in patients and could account for the finding by Monti et al. [12] of the anti-MPO serotype as an additional risk factor for CVD. Roth Flach et al. [33] support the notion of early MPO inhibition in plaque stabilization and preventing worsening atherosclerotic lesions.
Immunosuppressive therapy and CV risk
Alongside cytotoxic and B-cell depleting therapy, glucocorticoids (GCs) remain central to current remission-induction and remission-maintenance treatment strategies. Due to the relapsing–remitting nature of the disease, AAV patients are at risk of repeated treatment courses and a high cumulative GC exposure. The adverse effects of GC therapy have been recognized for decades, in particular their effects on the CV system [34, 35]. The term GC toxicity and its measurement by way of the glucocorticoid toxicity index (GTI) is recognized as an assessment tool through which clinicians can measure GC-associated morbidity [36, 37]. Interestingly, four of the nine composite GTI domains relate to adverse effects on the CV system: body mass index, glucose tolerance, blood pressure and lipid profile [36]. This highlights the burden of GC on CV risk.
GCs have been shown to cause increases in blood pressure by way of mineralocorticoid receptor overstimulation, sodium retention and increased peripheral vascular resistance [35]. Mebrahtu et al. [38] showed increased rates of hypertension with higher cumulative doses of oral GCs, particularly amongst vasculitis cohorts. Changes to a patient's lipid profile through increased total cholesterol and triglycerides lead to atheromatous disease, which in addition to weight gain and glucose intolerance all contribute to elevated cardiometabolic risk in patients [35, 38]. This may be further amplified by the impact of rituximab, used as an induction, remission and maintenance treatments in AAV. Rituximab has been shown to increase cholesterol, triglyceride and lipid levels, but the overall risk on CVD is offset by its improvements in endothelial function and improvements in disease activity [39]. Despite this, the effects of immunosuppression treatment are evident by the burden of CV events in those receiving them and need to be considered when treating patients.
Multiple studies have shown the risk of CVD, including heart failure, arrhythmias, atherosclerotic disease and MI amongst those with auto-inflammatory disease with a dose-dependent effect of GC [34, 40]. Massicotte-Azarniouch et al. [8] demonstrated that CV events were highest in the first 3 months from diagnosis. This may be explained by the high burden of disease activity, but the impact of GC exposure also needs to be considered. Somewhat surprisingly, a recent study showed no impact of GC exposure on CV risk [41]. Although the Plasma Exchange and Glucocorticoids for Treatment of ANCA Associated Vasculitis (PEXIVAS) trial has made great strides in establishing a role for decreased-dose oral GCs as part of standard remission-induction therapy, many patients are still exposed to a high cumulative oral and intravenous dose within the first 3 months. When considering the evidence base for the current dose of intravenous methylprednisolone that is typically used, scope remains for the evaluation of its current role to help mitigate the risks of treatment-related damage.
Should we screen for occult cardiac disease and atheroma in AAV?
Our limited ability to accurately assess CV involvement at presentation is likely part of the problem and limits our grasp of CV risk in AAV. Novel biomarkers in CVD are becoming more accessible, but remain imperfect due to limitations on their reliability and accuracy [42]. Many patients undergo routine cardiac investigations at presentation, inclusive of Electrocardiogram (ECGs) and echocardiograms, but these are not always reliable for identifying CV involvement [43]. Cardiac magnetic resonance imaging (MRI) with late gadolinium enhancement has been shown to identify subtle myocardial abnormalities caused by active AAV, which are otherwise not identified on routine investigations. Giollo et al. [44] used cardiac MRI in patients with granulomatosis with polyangiitis and identified evidence of myocardial fibrosis and abnormal left ventricular features, compared with healthy controls. Although few studies have been undertaken, overall they suggest that the use of cardiac MRI in AAV may be used as a means of identifying those at risk and to support monitoring and treatment plans [43]. However, even with sophisticated imaging differentiating between pre-existent cardiac disease and cardiac involvement by AAV remains notoriously difficult in clinical practice. The same applies to imaging of atheroma and studies of the vascular wall. Increased intima media thickness of the carotid arteries has been described using duplex ultrasound [23], but the interpretation of such findings remains difficult in an individual patient. For now, we remain sceptical of whether earlier, more aggressive or more detailed imaging confers significant advantages in patients with AAV over and above current standards of care. Table 1 provides a summary of further avenues of research.
Table 1.
Areas of uncertainty, suggested avenues of research and proposed study models
| Research question | Proposed research model |
|---|---|
| Risk prediction model for determining those with AAV at highest risk of CVD | Prospective and retrospective cohorts can be used to inform on a binary outcome and predictive variables for adverse CV events in AAV. Using regression coefficients to predict CV risk and outcomes in AAV |
| Association between GTI and CV events | Retrospective cohort study comparing GTI scores and relationship with CV outcomes |
| Methods to identify early atheromatous disease in AAV | Prospective cohort study looking at the use of imaging to reliably identify early atheromatous disease and correlate with CV events and outcomes |
| Antiphospholipid antibodies and CV risk | Prospective, long-term cohort study, identifying those with antiphospholipid antibodies and observing the incidence of CV events between patients with and without antibodies |
| Impact of aspirin in AAV to reduce CV events | Randomized control trial looking at CV outcomes such as MI, stroke and death from CVD |
| Timing of strategies to address CV risk | Randomized controlled trial comparing a strategy of addressing CV risk early on versus addressing it later, i.e. when in remission |
| More aggressive imaging to identify occult cardiac and vascular disease | Randomized controlled trial comparing a strategy of early imaging versus standard of care |
| The role of ACE inhibitors/ARBs and DRI in improving CV events and mortality | Randomized controlled trial looking at effect of ACE inhibitors/ARBs and DRIs on CV events and mortality |
How can we improve CV risk in AAV?
One important message from the study by Massicotte-Azarniouch et al. [8] is the finding that the CV risk peaks as early as 3 months after diagnosis. This observation should challenge widespread practice to wait for remission before considering CV risk in any detail and prompt us to consider and address CV risk much earlier. It is highly likely that steroid exposure plays a major role in CV risk and early reduction of GC dose, and the use of steroid sparing agents such as C5a receptor antagonists could be considered [37]. In the absence of specific CV risk scores for this particular population, formal assessment of risk should probably include the use of scores established in the general population. This will act as a prompt to clinicians in those patients who would already be at high risk even if they had not developed AAV. Kronbichler et al. [27] have argued that such a formal assessment should occur periodically, e.g. every 6 months. Such risk assessment should perhaps be documented in clinic letters, particularly where formal letter templates facilitate such an approach, i.e. in a dedicated vasculitis clinic environment. We also feel that many patients with AAV may not be aware of their CV risk, particularly during periods of active disease when both the clinician's and the patient's focus of attention is around the choice of treatment, side effects and prognosis, but not on CV risk. It is also tempting to think (but unproven) that dedicated vasculitis clinics may have clear advantages in reducing CV risk, e.g. by adhering to a more structured approach or through the fact that physicians who treat AAV infrequently will be even more focused on managing active disease and not CV risk. Another obvious advantage of such a dedicated environment is to provide suitable patient information in a targeted manner and to those who require it. Formally assessing and documenting the risk in clinic letters may serve as a starting point for discussing CV risk with them and their families. Based on current evidence, optimization of blood pressure control and diabetes is clearly an imperative factor in improving CV outcomes and reducing early atherosclerosis [5]. Inhibition of the renin–angiotensin–aldosterone system (RAAS) with angiotensin–converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are also potential targets for improving CV risk factors in AAV patients. The use of ACE inhibitors and ARBs for CV risk reduction is multifactorial and the result of reduced arterial stiffness, systemic inflammation and endothelial dysfunction [45]. It is recognized that proteinuria in AAV is associated with poor renal outcomes and the use of RAAS inhibition should be utilized for its synergistic improvements in blood pressure, proteinuria and CV risk [46]. When considering this, the use of direct renin inhibition (DRI) may offer an additional benefit by reducing alternative complement pathway activation to aid disease control [47]. Of note, patients with AAV were excluded from most recent trials of SGLT-2 inhibitors, and studies in this population are therefore eagerly awaited [48]. The situation around statins is slightly more complex, not least because they influence key inflammatory mechanisms in vasculitis [49, 50]. Recent studies suggest that lipid-lowering treatment is underutilized in AAV [5]. For now, statin treatment should probably be regarded as standard unless there are good reasons to avoid these drugs. A French trial of statin treatment in AAV is currently underway (STATVAS, NCT02117453) and should inform practice in this regard. The role of antiplatelet therapy is even less clear and not routinely recommended. We feel that in view of current evidence, primary prevention with antiplatelet therapy should be considered on a case-by-case basis, tailoring treatment based on patients' bleeding risk profile. Further research and randomized control trials looking at the use of antiplatelet drugs are needed to answer the risk–benefit questions surrounding systemic anticoagulation in AAV. A summary of recommendations for further research is provided in Table 1, with recommendations for clinical practice in Table 2.
Table 2.
Suggestions to improve CV risk in AAV
| Consider CV risk at baseline and use established scores for risk stratification, e.g. GTI |
| Re-assess CV risk periodically, e.g. every 3 months |
| Document CV risk and risk factors in clinic letters |
| Consider therapeutic regimes with less steroid exposure in patients with high CV risk |
| Address CV risk early in the diagnosis and do not delay until remission is achieved |
| Educate patients on their individual CV risk |
| Aim for smoking cessation in current smokers |
| Manage traditional risk factors such as blood pressure, diabetes and hyperlipidaemia |
| Include CV risk in departmental algorithms and guidelines |
CONCLUSION
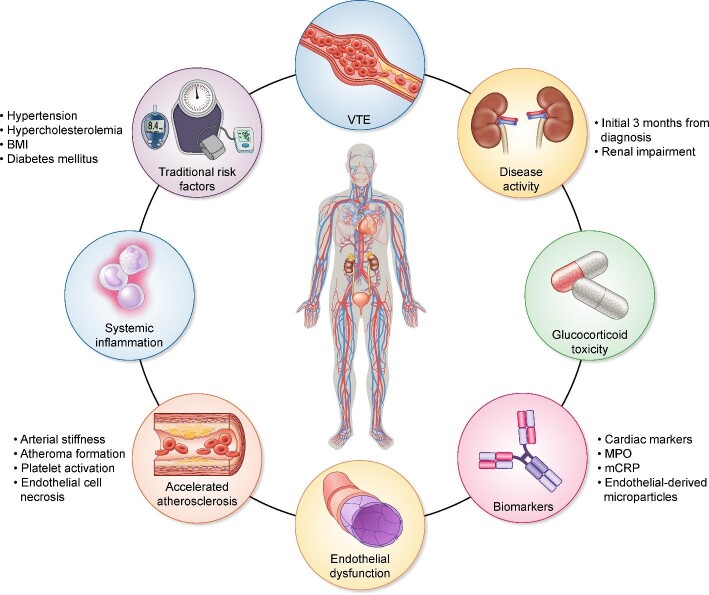
AAV is strongly associated with increased CVD, including MI, cerebrovascular disease and cardiac arrhythmias [7, 8]. The underlying pathophysiology is complex (Figure 1), multifaceted and not fully understood. Accelerated atherosclerotic disease may occur as a direct consequence of disease activity and endothelial damage, treatment-related toxicity or due to other currently unknown factors. The study by Massicotte-Azarniouch et al. [8] corroborates the significant risk for these patients, provides important new information about the timing of such risk and informs further research. The study by Massicotte-Azarniouch et al. [8] also challenges common practice to only focus on CV risk once remission has been achieved. One of the main findings of their study is to demonstrate peak risk early on, suggesting that strategies to address this risk should be employed much earlier. A key question is whether different therapeutic regimes for achieving and maintaining remission confer the same CV risk or not. It is tempting to think that approaches that work in the general population, such as lipid-lowering treatment, smoking cessation and blood pressure control, should also work in AAV. However, good quality evidence in this specific group of patients remains sparse. Further studies should improve our understanding of the underlying mechanisms, delineate the influence of different treatment regimens and provide a more robust evidence base for treatment aimed at reducing CV risk. The last decade or so of research has provided us with less toxic but equally efficient treatment regimens for AAV, especially in elderly patients and those with comorbidity. Further research and a pragmatic approach to address CV risk should now be employed together to address CV risk and thereby ensure that we are not missing a beat in this vulnerable group of patients.
FIGURE 1:
Pathogenic factors influencing CVD in AAV. BMI, body mass index.
FUNDING
No funding was required.
CONFLICT OF INTEREST STATEMENT
A.W. is a member of the CKJ editorial board. The results presented in this paper have not been published previously in whole or part.
Contributor Information
Lauren Floyd, Department of Nephrology, Lancashire Teaching Hospitals NHS Foundation Trust, Preston, UK.
Adam D Morris, Department of Nephrology, Lancashire Teaching Hospitals NHS Foundation Trust, Preston, UK.
Alexander Woywodt, Department of Nephrology, Lancashire Teaching Hospitals NHS Foundation Trust, Preston, UK.
Ajay Dhaygude, Department of Nephrology, Lancashire Teaching Hospitals NHS Foundation Trust, Preston, UK.
REFERENCES
- 1. Dregan A, Charlton J, Chowienczyk P et al. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation 2014; 130: 837–844 [DOI] [PubMed] [Google Scholar]
- 2. Roman MJ, Shanker B-A, Davis A et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med 2003; 349: 2399–2406 [DOI] [PubMed] [Google Scholar]
- 3. Faurschou M, Mellemkjaer L, Sorensen IJ et al. Increased morbidity from ischemic heart disease in patients with Wegener's granulomatosis. Arthritis Rheum 2009; 60: 1187–1192 [DOI] [PubMed] [Google Scholar]
- 4. Robson J, Doll H, Suppiah R et al. Damage in the ANCA-associated vasculitides: long-term data from the European Vasculitis Study Group (EUVAS) therapeutic trials. Ann Rheum Dis 2013; 74: 177–184 [DOI] [PubMed] [Google Scholar]
- 5. Bramlage CP, Kröplin J, Wallbach M et al. Management of cardiovascular risk factors in patients with ANCA-associated vasculitis. J Eval Clin Pract 2017; 23: 747–754 [DOI] [PubMed] [Google Scholar]
- 6. Flossmann O, Berden A, De Groot K et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011; 70: 488–494 [DOI] [PubMed] [Google Scholar]
- 7. Houben E, Penne EL, Voskuyl AE et al. Cardiovascular events in anti-neutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis of observational studies. Rheumatology (Oxford) 2018; 57: 555–562 [DOI] [PubMed] [Google Scholar]
- 8. Massicotte-Azarniouch D, Petrcich W, Walsh M et al. Association of anti-neutrophil cytoplasmic antibody-associated vasculitis and cardiovascular events: a population-based cohort study. Clin Kidney J 2021: sfab229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roubille C, Coffy A, Rincheval N et al. Ten-year analysis of the risk of severe outcomes related to low-dose glucocorticoids in early rheumatoid arthritis. Rheumatology (Oxford) 2021; 60: 3738–3746 [DOI] [PubMed] [Google Scholar]
- 10. Suppiah R, Judge A, Batra R et al. A model to predict cardiovascular events in patients with newly diagnosed Wegener's granulomatosis and microscopic polyangiitis. Arthritis Care Res (Hoboken) 2011; 63: 588–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bai Y-H, Li Z-Y, Chang D-Y et al. The BVAS is an independent predictor of cardiovascular events and cardiovascular disease-related mortality in patients with ANCA-associated vasculitis: a study of 504 cases in a single Chinese center. Semin Arthritis Rheum 2018; 47: 524–529 [DOI] [PubMed] [Google Scholar]
- 12. Monti S, Robson J, Klersy C et al. Early development of new cardiovascular risk factors in the systemic vasculitides. Clin Exp Rheumatol 2020; 38: 126–134 [PubMed] [Google Scholar]
- 13. Haubitz M, Woywodt A, De Groot K et al. Smoking habits in patients diagnosed with ANCA associated small vessel vasculitis. Ann Rheum Dis 2005; 64: 1500–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woywodt A, Streiber F, de Groot K et al. Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet 2003; 361: 206–210 [DOI] [PubMed] [Google Scholar]
- 15. Kirsch T, Woywodt A, Beese M et al. Engulfment of apoptotic cells by microvascular endothelial cells induces proinflammatory responses. Blood 2006; 109: 2854–2862 [DOI] [PubMed] [Google Scholar]
- 16. Mallat Z, Benamer H, Hugel B et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 2000; 101: 841–843 [DOI] [PubMed] [Google Scholar]
- 17. Clifford AH, Cohen Tervaert JW. Cardiovascular events and the role of accelerated atherosclerosis in systemic vasculitis. Atherosclerosis 2021; 325: 8–15 [DOI] [PubMed] [Google Scholar]
- 18. Wu K-L, Liang Q-H, Huang B-T et al. The plasma level of mCRP is linked to cardiovascular disease in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Res Ther 2020; 22: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raza K, Thambyrajah J, Townend JN et al. Suppression of inflammation in primary systemic vasculitis restores vascular endothelial function: lessons for atherosclerotic disease? Circulation 2000; 102: 1470–1472 [DOI] [PubMed] [Google Scholar]
- 20. Booth AD, Wallace S, McEniery CM et al. Inflammation and arterial stiffness in systemic vasculitis: a model of vascular inflammation. Arthritis Rheum 2004; 50: 581–588 [DOI] [PubMed] [Google Scholar]
- 21. Filer AD, Gardner-Medwin JM, Thambyrajah J et al. Diffuse endothelial dysfunction is common to ANCA associated systemic vasculitis and polyarteritis nodosa. Ann Rheum Dis 2003; 62: 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guerin AP, Blacher J, Pannier B et al. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 2001; 103: 987–992 [DOI] [PubMed] [Google Scholar]
- 23. González-Suárez I, Ríos-Blanco JJ, Arpa J. Accelerated atherosclerosis in ANCA-associated vasculitis. Acta Neurol Scand 2017; 136: 688–693 [DOI] [PubMed] [Google Scholar]
- 24. Nienhuis HLA, de Leeuw K, Smit AJ et al. Enhanced endothelium-dependent microvascular responses in patients with Wegener's granulomatosis. J Rheumatol 2007; 34: 1875–1881 [PubMed] [Google Scholar]
- 25. Kambas K, Chrysanthopoulou A, Vassilopoulos D et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis 2014; 73: 1854–1863 [DOI] [PubMed] [Google Scholar]
- 26. Isaacs B, Gapud EJ, Antiochos B et al. Venous thrombotic events in ANCA-associated vasculitis: incidence and risk factors. Kidney360 2020; 1: 258–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kronbichler A, Leierer J, Gauckler P et al. Comorbidities in ANCA-associated vasculitis. Rheumatology (Oxford) 2020; 59: iii79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martel C, Cointe S, Maurice P et al. Requirements for membrane attack complex formation and anaphylatoxins binding to collagen-activated platelets. PLoS One 2011; 6: e18812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Willeke P, Kümpers P, Schlüter B et al. Platelet counts as a biomarker in ANCA-associated vasculitis. Scand J Rheumatol 2015; 44: 302–308 [DOI] [PubMed] [Google Scholar]
- 30. Rees JD, Lança S, Marques PV et al. Prevalence of the antiphospholipid syndrome in primary systemic vasculitis. Ann Rheum Dis 2006; 65: 109–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jordan N, D'cruz DP. Association of lupus anticoagulant with long-term damage accrual in antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Care Res (Hoboken) 2016; 68: 711–715 [DOI] [PubMed] [Google Scholar]
- 32. Tabakovic D, Smith R, Jayne D MA. Incidence rate, predictors and outcome of Stroke in patients with ANCA associated vasculitis – a population-based study [abstract]. Arthritis Rheumatol 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roth Flach RJ, Su C, Bollinger E et al. Myeloperoxidase inhibition in mice alters atherosclerotic lesion composition. PLoS One 2019; 14: e0214150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pujades-Rodriguez M, Morgan AW, Cubbon RM et al. Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: a population-based cohort study. PLoS Med 2020; 17: e1003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ng MKC, Celermajer DS. Glucocorticoid treatment and cardiovascular disease. Heart 2004; 90: 829–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miloslavsky EM, Naden RP, Bijlsma JWJ et al. Development of a Glucocorticoid Toxicity Index (GTI) using multicriteria decision analysis. Ann Rheum Dis 2017; 76: 543–546 [DOI] [PubMed] [Google Scholar]
- 37. Floyd L, Morris A, Joshi M et al. Glucocorticoid therapy in ANCA vasculitis: using the glucocorticoid toxicity index as an outcome measure. Kidney360 2021; 2: 1002–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mebrahtu TF, Morgan AW, West RM et al. Oral glucocorticoids and incidence of hypertension in people with chronic inflammatory diseases: a population-based cohort study. Can Med Assoc J 2020; 192: E295–E301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hsue PY, Scherzer R, Grunfeld C et al. Depletion of B-cells with rituximab improves endothelial function and reduces inflammation among individuals with rheumatoid arthritis. J Am Heart Assoc 2021; 3: e001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Souverein PC, Berard A, Van Staa TP et al. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart 2004; 90: 859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roubille C, Henriquez S, Mercuzot C et al. Impact of cardiovascular risk factors on the occurrence of cardiovascular events in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides. J Clin Med 2021; 10: 2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vasan RS. Biomarkers of cardiovascular disease. Circulation 2006; 113: 2335–2362 [DOI] [PubMed] [Google Scholar]
- 43. Greulich S, Mayr A, Kitterer D et al. T1 and T2 mapping for evaluation of myocardial involvement in patients with ANCA-associated vasculitides. J Cardiovasc Magn Reson 2017; 19: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giollo A, Dumitru RB, Swoboda PP et al. Cardiac magnetic resonance imaging for the detection of myocardial involvement in granulomatosis with polyangiitis. Int J Cardiovasc Imaging 2021; 37: 1053–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ruilope LM, Redón J, Schmieder R. Cardiovascular risk reduction by reversing endothelial dysfunction: ARBs, ACE inhibitors, or both? Expectations from the ONTARGET Trial Programme. Vasc Health Risk Manag 2007; 3: 1–9 [PMC free article] [PubMed] [Google Scholar]
- 46. Neumann I, Kain R, Regele H et al. Histological and clinical predictors of early and late renal outcome in ANCA-associated vasculitis. Nephrol Dial Transplant 2005; 20: 96–104 [DOI] [PubMed] [Google Scholar]
- 47. Békássy ZD, Kristoffersson A-C, Rebetz J et al. Aliskiren inhibits renin-mediated complement activation. Kidney Int 2018; 94: 689–700 [DOI] [PubMed] [Google Scholar]
- 48. Säemann M, Kronbichler A. Call for action in ANCA-associated vasculitis and lupus nephritis: promises and challenges of SGLT-2 inhibitors. Ann Rheum Dis 2021: annrheumdis-2021–221474 [DOI] [PubMed] [Google Scholar]
- 49. Hölschermann H, Schuster D, Parviz B et al. Statins prevent NF-kappaB transactivation independently of the IKK-pathway in human endothelial cells. Atherosclerosis 2006; 185: 240–245 [DOI] [PubMed] [Google Scholar]
- 50. Choi M, Schreiber A, Eulenberg-Gustavus C et al. Endothelial NF-kB blockade abrogates ANCA-induced GN. J Am Soc Nephrol 2017; 28: 3191–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]