ABSTRACT
Background
Patients with stage 4/5 chronic kidney disease (CKD) suffer from various symptoms. The retention of uremic solutes is thought to be associated with those symptoms. However, there are relatively few rigorous studies on the potential links between uremic toxins and symptoms in patients with CKD.
Methods
The EQUAL study is an ongoing observational cohort study of non-dialyzed patients with stage 4/5 CKD. EQUAL patients from Germany, Poland, Sweden and the UK were included in the present study (n = 795). Data and symptom self-report questionnaires were collected between April 2012 and September 2020. Baseline uric acid and parathyroid hormone and 10 uremic toxins were quantified. We tested the association between uremic toxins and symptoms and adjusted P-values for multiple testing.
Results
Symptoms were more frequent in women than in men with stage 4/5 CKD, while levels of various uremic toxins were higher in men. Only trimethylamine N-oxide (TMAO; positive association with fatigue), p-cresyl sulfate (PCS) with constipation and 3-carboxy-4-methyl-5-propyl-2-furanpropionic acid (negative association with shortness of breath) demonstrated moderately strong associations with symptoms in adjusted analyses. The association of phenylacetylglutamine with shortness of breath was consistent in both sexes, although it only reached statistical significance in the full population. In contrast, TMAO (fatigue) and PCS and phenylacetylglutamine (constipation) were only associated with symptoms in men, who presented higher serum levels than women.
Conclusion
Only a limited number of toxins were associated with symptoms in persons with stage 4/5 CKD. Other uremic toxins, uremia-related factors or psychosocial factors not yet explored might contribute to symptom burden.
Keywords: CKD, elderly, symptoms, uremic toxins
INTRODUCTION
Patients with stage 4/5 chronic kidney disease (CKD) suffer from various symptoms, including anorexia, nausea, pruritus, fatigue, excessive daytime sleepiness, difficulty concentrating and pain [1]. This uremic symptom burden is known to increase with age [2]. In the EQUAL study, including elderly patients with advanced (stage 4/5) CKD, more than half of the patients reported fatigue, dry skin, bone or joint pain, loss of strength, muscle cramps, dry mouth, itching and decreased interest in sex, with a median of 12 (of 33) symptoms per patient [3]. The EQUAL investigators also reported a substantially higher symptom burden in women versus men and a marked impact of symptoms on the quality of life (QoL), recommending that this impact should have a more prominent role in clinical decision-making [3, 4].
As defined by the European Uremic Toxins Work Group [5], uremic toxins are harmful compounds that accumulate in the body during periods of renal function decline. Uremic toxins can be classified according to their molecular weight, water solubility and protein-binding status [5]. These toxins may constitute important non-traditional risk factors and are known to be associated with morbidity and mortality in patients with CKD [6]. The retention of uremic solutes is thought to be associated with the symptoms. Even though symptoms have clinical significance, there are relatively few rigorous studies on the potential links between uremic toxins and symptoms in patients with CKD. In one recent study, indoxyl sulfate (IS), p-cresol and uremic serum affected (either directly or indirectly) protease-activated receptor 2 expression in the skin of patients with CKD, suggesting that these compounds might have an important role in the pathogenesis of uremic pruritus [7]. In another study, total p-cresyl sulfate (PCS), but not IS, was independently associated with pruritus, after adjustment for known biomarkers [8]. Kynurenine, indole-3-acetic acid (IAA) and IS have been shown to alter skeletal muscle mitochondria in mice and might accentuate skeletal muscle fatigue, weakness and atrophy in CKD [9]. In a rat model, accumulation of IS led to behavioral alterations, including apathetic behavior, increased stress sensitivity and reduced locomotor and exploratory activity [10]. Moreover, the kynurenine pathway appears to be a key factor in promoting bone-aging phenotypes and bone breakdown and in interfering with stem cell function and osteogenesis; kynurenine might lead to detrimental effects in bone, with a lower bone mineral density and an elevated fracture risk [11, 12]. Several studies have demonstrated that trimethylamine N-oxide (TMAO) can promote atherosclerosis, thrombosis, heart failure, insulin resistance and kidney disease via tissue or cell type–specific reprogramming [13]. Hence TMAO might be involved in the genesis of certain symptoms. The observation that symptoms improve markedly or resolve completely after kidney transplantation has led to the long-standing view that symptoms are due, at least in part, to retained uremic toxins [14–16]. Since the levels of several uremic toxins such as IS, PCS and TMAO have been normalized or largely ameliorated after kidney transplantation [6], we were wondering whether these uremic toxins are responsible, at least in part, for the improvement of symptoms after kidney transplantation. The development and validation of a sensitive and robust assay for the simultaneous quantification of concentrations of 10 uremic toxins by our laboratory [17] allowed us to include these 10 uremic toxins in addition to urea, uric acid and parathyroid hormone (PTH) in this study.
In the present cross-sectional study, we sought to assess the relationship between levels of several uremic toxins and a range of symptoms in the EQUAL cohort of older patients with stage 4/5 CKD. We also sought to determine whether the levels of these uremic toxins might explain the sex difference in symptoms.
MATERIALS AND METHODS
Study design and population
The EQUAL study is an ongoing observational cohort study of non-dialyzed patients with stage 4/5 CKD receiving routine medical care in Germany, Italy, the Netherlands, Poland, Sweden and the UK. The main inclusion criteria were age ≥65 years and an incident estimated glomerular filtration rate (eGFR, as calculated using the Modification of Diet in Renal Disease equation) <20 mL/min/1.73 m². Patients were excluded if the decrease in eGFR resulted from an acute event or if they had previously been dialyzed or had received a kidney transplant. The study was approved by the appropriate independent ethics committees in each country. All the patients gave their written informed consent. The study has been described in detail elsewhere [18]. EQUAL patients from Germany, Poland, Sweden and the UK were included in the present study (n = 795).
Data collection
Clinical data (primary renal disease and cardiovascular risk factors including smoking status, body mass index, hemoglobin, blood pressure, cholesterol and diabetes mellitus), demographic data and laboratory data were collected between April 2012 and September 2020. Data on the following pre-existing cardiovascular comorbidities were also collected; cerebrovascular disease, peripheral vascular disease, myocardial infarction, angina pectoris, congestive heart failure, left ventricular hypertrophy, hypertension and cardiac arrhythmias. Data on the presence or absence of symptoms were obtained via self-report questionnaires at each visit. The list of symptoms was based on the Dialysis Symptom Index [19] (which comprises 30 symptoms), together with bleeding, weight loss and loss of strength. The overall symptom burden was scored as the total number of symptoms present and thus ranged from 0 to 33. The eGFR was calculated from the serum creatinine level (as standardized against isotope dilution mass spectrometry) using the Chronic Kidney Disease Epidemiology Collaboration equation. The albumin:creatinine ratio (ACR) was estimated via routine 24-h urine collection or (if 24-h data were not available) from a single sample. The primary kidney disease was coded according to the European Renal Association–European Dialysis and Transplantation Association's classification and grouped as glomerulonephritis, diabetes mellitus, tubulointerstitial disease, hypertension and miscellaneous kidney diseases. For the purposes of the current study, we only included patient data at baseline.
Collection and analysis of uremic toxins
Upon enrollment of the patients, serum samples were collected and immediately frozen. The samples were thawed immediately prior to analysis. Uric acid, urea and PTH were assayed in onsite biochemistry laboratories, using standard autoanalyzer techniques. The following 10 uremic toxins were assayed in serum samples using a validated ultra high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) technique: 3-carboxy-4-methyl-5-propyl-2-furanpropionic acid (CMPF), hippuric acid, IAA, IS, kynurenic acid, kynurenine, p-cresyl glucuronide (PCG), PCS, phenylacetylglutamine (PAG) and TMAO [17]. To determine total concentrations, serum samples were first precipitated with methanol. The supernatant was evaporated with nitrogen and then reconstituted in 80 µL of water. The assay's limit of quantification was between 10 and 50 ng/mL, depending on the compound. The intra- and inter-assay variabilities (evaluated at three different concentrations: 150, 8000 and 40 000 ng/mL) for the 10 compounds were all <13%.
Statistical analysis
The patient characteristics were reported overall and by sex as the mean [standard deviation (SD)] for normally distributed continuous variables, the median [interquartile range (IQR)] for skewed continuous variables and the frequency (percentage) for categorical variables. Skewed variables were log-transformed to obtain a more normal distribution. Outliers in uremic toxin measurements were identified and capped using the IQR method, which defines an outlier as a value above the 75th percentile or below the 25th percentile by a factor of 1.5 times the IQR. Logistic regression was used to model the association between each biomarker and the odds of a patient reporting the presence of a symptom at baseline. Symptoms were selected from the Dialysis Symptom Index by prioritizing those with an overall prevalence >25%. In addition to individual symptoms, we tested the association between uremic toxins and groups of symptoms (Supplementary data, Table S1). Effect sizes were reported as the standardized odds ratio for a 1 SD increment in the biomarker level. Sex-specific effects were assessed by including an interaction term for patient sex and the biomarker of interest. Uremic toxins were standardized and centered to improve the model's fit and enable the comparison of effect sizes from one biomarker to another. The models were adjusted for confounders defined a priori [20]. To reduce the probability of observing a significant effect by chance as a result of multiple testing, the threshold for statistical significance was set to P < 0.01. In addition, we adjusted P-values for multiple testing using the Benjamini–Hochberg procedure for false discovery rates [21]. Missing values in covariates were imputed. All analyses were performed with SAS software (version 9.4; SAS Institute, Cary, NC, USA) and R software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient characteristics
Demographic and clinical characteristics at baseline are summarized for all 795 patients and by sex in Table 1 and for uremic toxin concentrations in Table 2. The eGFR was lower in men than in women, which might explain (at least in part) the higher levels of uremic toxins observed in men. The overall prevalence of symptoms at inclusion was high (54.1%; Table 3) with a median number of 13 (IQR 8–18) symptoms: 14 (IQR 9–19) in women and 12 (IQR 8–17) in men (P < 0.001). All symptoms were more frequent in women than in men (except for decreased interest in sex and difficulty becoming sexually aroused).
Table 1.
Patient characteristics at baseline, by sex
| Characteristics | Overall (N = 795) | Female (n = 285) | Male (n = 510) | P-value |
|---|---|---|---|---|
| Age (years), mean (SD) | 76.16 (6.68) | 76.32 (6.91) | 76.07 (6.56) | 0.61 |
| Primary renal disease, n (%) | ||||
| Glomerular disease | 91 (11.4) | 25 (8.8) | 66 (12.9) | 0.02 |
| Tubulo-interstitial disease | 79 ( 9.9) | 40 (14.0) | 39 ( 7.6) | |
| Diabetes | 159 (20.0) | 49 (17.2) | 110 (21.6) | |
| Hypertension | 276 (34.7) | 102 (35.8) | 174 (34.1) | |
| Miscellaneous renal disorders | 190 (23.9) | 69 (24.2) | 121 (23.7) | |
| Weight (kg), mean (SD) | 81.47 (16.93) | 74.76 (16.17) | 85.22 (16.18) | <0.001 |
| Height (cm), mean (SD) | 168.64 (9.81) | 159.67 (7.22) | 173.66 (7.13) | <0.001 |
| BMI (kg/m2), mean (SD) | 28.60 (5.39) | 29.26 (6.12) | 28.23 (4.90) | 0.01 |
| SGA, n (%) | 0.41 | |||
| 2 | 6 ( 0.8) | 3 ( 1.1) | 3 ( 0.6) | |
| 3 | 10 ( 1.3) | 3 ( 1.1) | 7 ( 1.4) | |
| 4 | 58 ( 7.3) | 27 ( 9.5) | 31 ( 6.1) | |
| 5 | 166 (20.9) | 55 (19.3) | 111 (21.8) | |
| 6 | 269 (33.8) | 101 (35.4) | 168 (32.9) | |
| 7 | 286 (36.0) | 96 (33.7) | 190 (37.3) | |
| eGFR (mL/min/1.73 m2), mean (SD) | 17.70 (5.42) | 18.47 (5.44) | 17.28 (5.37) | 0.003 |
| ACR, median (IQR) | 38.85 (7.36, 173.48) | 31.90 (4.84, 165.32) | 46.45 (9.04, 179.32) | 0.04 |
| Systolic blood pressure (mmHg), mean (SD) | 146.49 (22.22) | 146.35 (23.46) | 146.57 (21.53) | 0.90 |
| Diastolic blood pressure (mmHg), mean (SD) | 74.72 (11.43) | 74.07 (11.63) | 75.08 (11.32) | 0.23 |
| Smoking status, n (%) | <0.001 | |||
| Current smoker | 59 ( 7.4) | 23 ( 8.1) | 36 ( 7.1) | |
| Ex-smoker | 410 (51.6) | 111 (38.9) | 299 (58.6) | |
| Never | 312 (39.2) | 146 (51.2) | 166 (32.5) | |
| Cholesterol (mmol/L), mean (SD) | 4.67 (1.35) | 4.98 (1.44) | 4.49 (1.27) | <0.001 |
| Hb (g/dL), mean (SD) | 11.6 (1.5) | 11.5 (1.5) | 11.6 (1.6) | 0.25 |
| Calcium (mmol/L), mean (SD) | 2.28 (0.16) | 2.32 (0.15) | 2.26 (0.15) | <0.001 |
| PO4 (mmol/L), mean (SD) | 1.31 (0.33) | 1.30 (0.30) | 1.31 (0.35) | 0.84 |
| Albumin (g/dL), mean (SD) | 37.56 (5.48) | 37.73 (5.33) | 37.46 (5.57) | 0.51 |
| Potassium (mmol/L), mean (SD) | 4.59 (0.60) | 4.55 (0.60) | 4.61 (0.60) | 0.17 |
| Sodium (mmol/L), mean (SD) | 140.36 (3.24) | 140.16 (3.47) | 140.47 (3.11) | 0.20 |
| Bicarbonate (mmol/L), mean (SD) | 22.48 (3.86) | 23.28 (4.01) | 22.03 (3.71) | <0.001 |
| Charlson comorbidity score, mean (SD) | 7.10 (1.92) | 6.85 (1.81) | 7.24 (1.97) | 0.01 |
| Hypertension, n (%) | 698 (87.8) | 248 (87.0) | 450 (88.2) | 0.70 |
| Diabetes, n (%) | 318 (40.0) | 101 (35.4) | 217 (42.5) | 0.06 |
| Cerebrovascular disease, n (%) | 121 (15.2) | 41 (14.4) | 80 (15.7) | 0.70 |
| Peripheral vascular disease, n (%) | 104 (13.1) | 28 ( 9.8) | 76 (14.9) | 0.05 |
| Chronic heart failure, n (%) | 142 (17.9) | 50 (17.5) | 92 (18.0) | 0.94 |
| Myocardial infarction, n (%) | 139 (17.5) | 37 (13.0) | 102 (20.0) | 0.02 |
| Left ventricular hypertrophy, n (%) | 141 (17.7) | 41 (14.4) | 100 (19.6) | 0.08 |
| Atrial fibrillation, n (%) | 148 (18.6) | 51 (17.9) | 97 (19.0) | 0.77 |
| Country, n (%) | ||||
| Germany | 133 (16.7) | 55 (19.3) | 78 (15.3) | 0.04 |
| Poland | 69 ( 8.7) | 22 ( 7.7) | 47 ( 9.2) | |
| Sweden | 286 (36.0) | 86 (30.2) | 200 (39.2) | |
| UK | 307 (38.6) | 122 (42.8) | 185 (36.3) |
SGA: subjective global assessment; Hb: hemoglobin; PO4: serum phosphate; BMI: body mass index.
Table 2.
Uremic toxin levels, by sex
| Biomarker | Overall (N = 795) | Female (n = 285) | Male (n = 510) | P-value |
|---|---|---|---|---|
| TMAO (ng/mL), median (IQR) | 1538 (972–3014) | 1254 (855–2282) | 1706 (1061–3228) | <0.001 |
| Kynurenine (ng/mL), median (IQR) | 580 (460–719) | 553 (441–700) | 598 (470–736) | 0.014 |
| Hippuric acid (ng/mL), median (IQR) | 2680 (1246–5178) | 2020 (939–4230) | 2884 (1650–5534) | <0.001 |
| PAG (ng/mL), median (IQR) | 3478 (1936–6020) | 2992 (1701–5070) | 3694 (2036–6489) | 0.002 |
| IS (ng/mL), median (IQR) | 3917 (2204–7615) | 3440 (1797–5708) | 4352 (2448–8516) | <0.001 |
| Kynurenic acid (ng/mL), median (IQR) | 50 (41–65) | 45 (37–56) | 54 (43–69) | <0.001 |
| PCG (ng/mL), median (IQR) | 128 (72–271) | 127 (72–246) | 130 (72–285) | 0.65 |
| PCS (ng/mL), median (IQR) | 24 441 (16 196–36 951) | 21 682 (14 386–32 698) | 27 438 (17 106–40 276) | <0.001 |
| IAA (ng/mL), median (IQR) | 451 (320–694) | 406 (297–662) | 477 (338–702) | 0.004 |
| CMPF (ng/mL), median (IQR) | 2451 (870–5090) | 2189 (657–4372) | 2525 (1115–5264) | 0.012 |
| Uric acid (µmol/L), median (IQR) | 443 (375–537) | 434 (360–539) | 446 (381–537) | 0.012 |
| PTH (pmol/L), median (IQR) | 16 (9–24) | 14 (8–21) | 17 (10–27) | 0.003 |
| Urea (mmol/L), median (IQR) | 18 (15–23) | 17 (14–22) | 19 (16–23) | <0.001 |
For the references uremic solutes, please consult the database at https://database.uremic-toxins.org/home.php.
Table 3.
Prevalence of symptoms at baseline, by sex
| Symptoms | Overall | Women | Men | P-value |
|---|---|---|---|---|
| Constipation | 33.5 | 41.9 | 28.8 | <0.001 |
| Decreased appetite | 29.3 | 34.2 | 26.6 | 0.03 |
| Muscle cramps | 57.1 | 59.5 | 55.8 | 0.35 |
| Swelling in legs | 56.5 | 63.7 | 52.4 | 0.003 |
| Shortness of breath | 46.8 | 52.5 | 43.6 | 0.02 |
| Dizziness | 37 | 40.8 | 34.8 | 0.11 |
| Restless legs | 32.3 | 41.5 | 27.2 | <0.001 |
| Tingling in feet | 34.5 | 40.5 | 31.1 | 0.01 |
| Fatigue | 76.2 | 79.9 | 74.2 | 0.082 |
| Cough | 38.6 | 38.7 | 38.6 | 0.99 |
| Dry mouth | 55.8 | 62.3 | 52.3 | 0.01 |
| Bone or joint pain | 58.6 | 69.0 | 52.8 | <0.001 |
| Headache | 25.7 | 34.9 | 20.5 | <0.001 |
| Muscle soreness | 35.6 | 42.6 | 31.7 | 0.003 |
| Dry skin | 57.9 | 69.4 | 51.5 | <0.001 |
| Itching | 54.6 | 55.6 | 54.0 | 0.71 |
| Trouble falling asleep | 40.3 | 49.3 | 35.2 | <0.001 |
| Trouble staying asleep | 54.1 | 61.6 | 49.9 | 0.002 |
| Decreased interest in sex | 58.9 | 50.7 | 63.4 | 0.001 |
| Difficulty becoming sexually aroused | 57.9 | 45.4 | 64.8 | <0.001 |
| Loss of strength | 64.3 | 67.6 | 62.4 | 0.17 |
Significant differences between women and men are in bold.
Associations between uremic toxin levels and symptoms
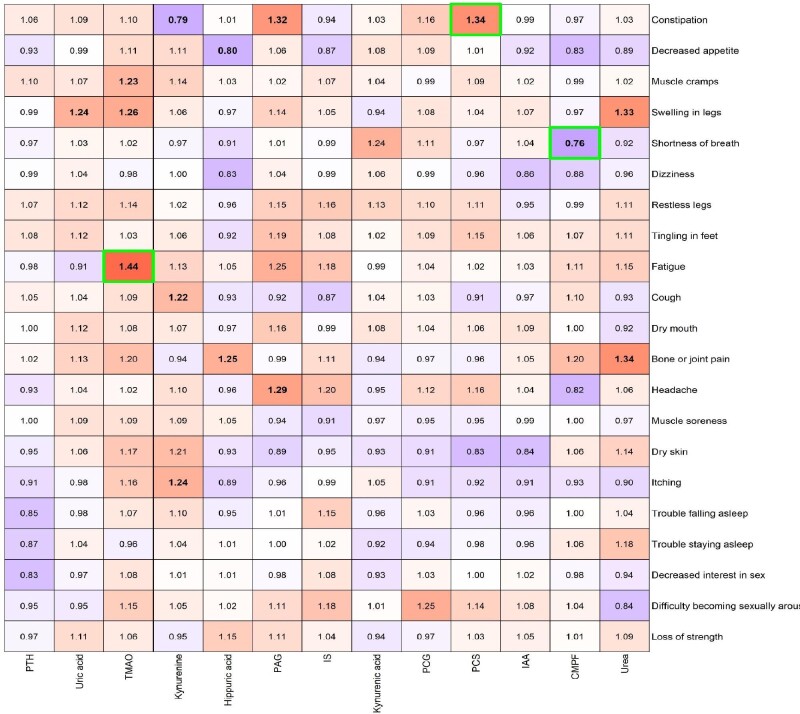
Symptoms were selected from the Dialysis Symptom Index by prioritizing those with an overall prevalence >25%. For the cohort as a whole, the associations between uremic toxin levels and symptoms adjusted for age, primary renal disease, Charlson comorbidity index, ACR and eGFR are presented in Figure 1 (Supplementary data, Tables S2 and S3). Uric acid and PCS were positively associated with swelling in the legs and constipation, respectively, and CMPF was inversely associated with shortness of breath. Hippuric acid was inversely associated with decreased appetite and positively associated with bone or joint pain. PAG was positively associated with both constipation and headache. TMAO was positively associated with muscle cramps, swelling in the legs and fatigue. Kynurenine was inversely associated with constipation and positively associated with cough and itching. Urea was positively associated with both swelling in the legs and bone or joint pain. PTH, IS, IAA, kynurenic acid and PCG were not associated with any of the selected 21 symptoms. While studying symptom groups, uric acid was found to be associated with symptoms related to fluid overload and TMAO was found to be associated with musculoskeletal symptoms (Supplementary data, Table S4). After P-value adjustment for multiple testing, the associations TMAO–fatigue, PCS–constipation and CMPF–shortness of breath remained statistically significant. Associations between baseline levels of uremic toxins and longitudinal symptoms measured provided similar results (data not shown).
Figure 1.
Heat map of the ORs for the association between uremic toxins and symptoms, adjusted for age, primary renal disease, Charlson comorbidity score, ACR and eGFR (Supplementary data, Figure S1: unadjusted heat map). Bold ORs indicate statistical significance (P < 0.01) and green borders indicate statistical significance after adjustment for multiple testing.
Sex-specific associations between uremic toxin levels and symptoms
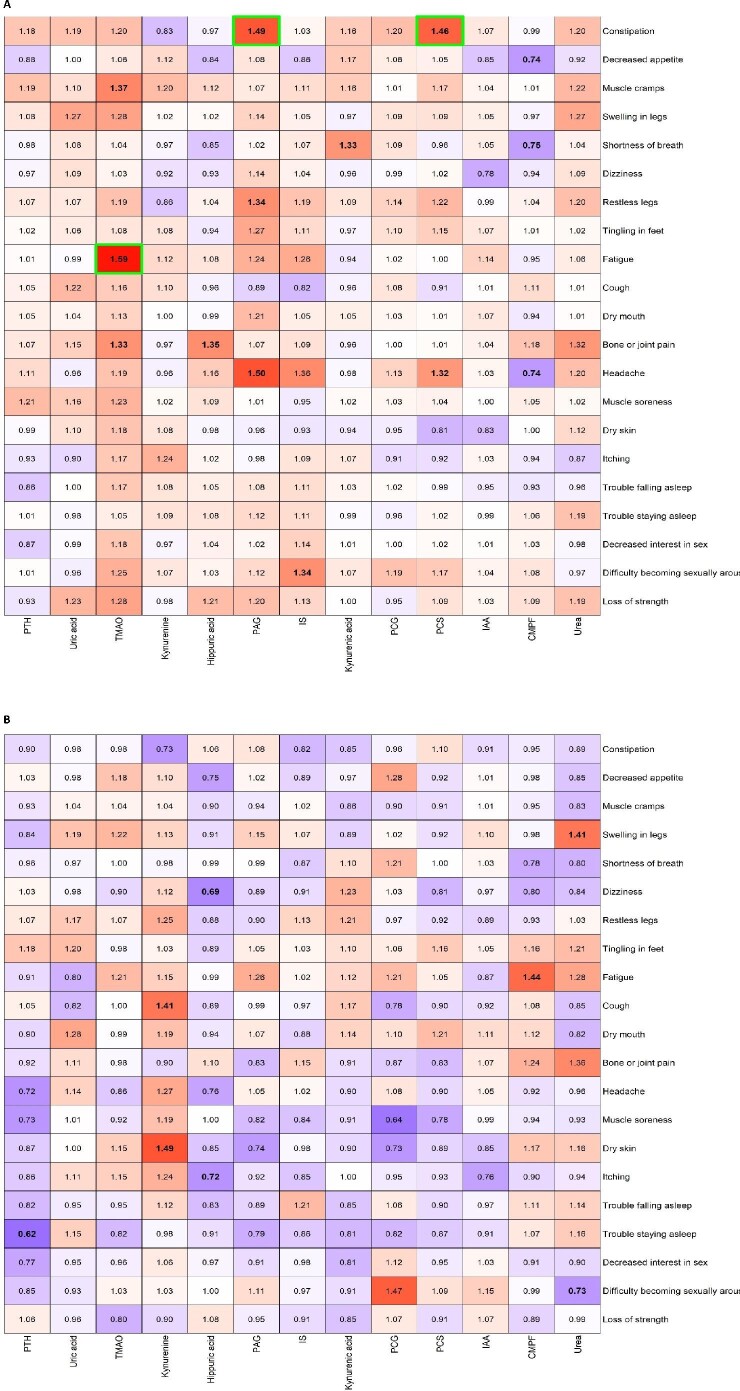
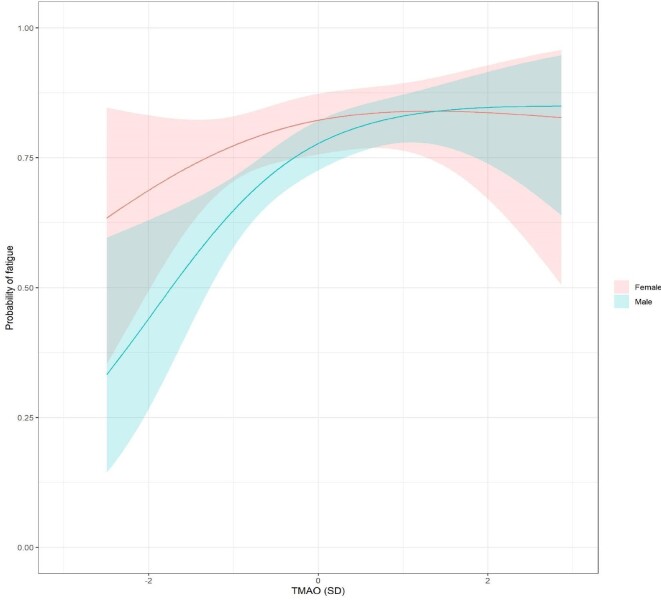
The sex-specific associations between uremic toxins and symptoms adjusted for age, primary renal disease, Charlson comorbidity index, ACR and eGFR are presented in Figure 2 (Supplementary data, Tables S5 and S6). PCS was positively associated with constipation and headache, but only in men. Similarly, PAG was positively associated with constipation, headache and restless legs only in men, as was TMAO for muscle cramps, fatigue (Figure 3) and bone or joint pain. On the other hand, kynurenine was positively associated with cough and dry skin only in women. CMPF was inversely associated with decreased appetite, shortness of breath and headache in men and positively associated with fatigue in women. Hippuric acid was inversely associated with itching and dizziness in women and positively associated with bone or joint pain in men. PTH was inversely associated with trouble staying asleep in women and IS and kynurenic acid were positively associated with difficulty in becoming sexually aroused and shortness of breath in men, respectively. In women, urea was positively associated with swelling of the legs and inversely associated with difficulty becoming sexually aroused. IAA, uric acid and PCG were not associated with any symptoms. Studying symptom groups, TMAO was only associated with musculoskeletal symptoms and symptoms related to sexuality in men (Supplementary data, Table S7). After P-value adjustment for multiple testing, only the associations in men between TMAO–fatigue, PAG–constipation and PCS–constipation remained statistically significant. The sex-specific associations between eGFR and symptoms and eGFR and uremic toxins are provided in Supplementary data, Table S8.
Figure 2.
Heat map of ORs for the association between uremic toxins and symptoms in (A) men and (B) women, adjusted for age, primary renal disease, Charlson comorbidity score at baseline, ACR and eGFR (Supplementary data, Figure S2: unadjusted heat maps). Bold ORs indicate statistical significance (P < 0.01) and green borders indicate statistical significance after adjustment for multiple testing.
Figure 3.
The sex-specific effect of TMAO on the probability of reporting fatigue.
DISCUSSION
The present study assesses, for the first time to our knowledge, the potential overall and sex-specific associations between uremic toxins and symptoms in a large cohort of older patients with stage 4/5 CKD. We found that only a limited number of toxins were associated with symptoms in persons with stage 4/5 CKD. Other uremic toxins, uremia-related factors or psychosocial factors not yet explored might contribute to symptom burden. Our hypothesis that a higher level of uremic toxins in women or a greater sex-specific effect of uremic toxins in women might explain the higher frequency of symptoms in women compared with men was not confirmed, at least for the uremic toxins under investigation, as we found no clear pattern between the sexes.
Only TMAO (positive association with fatigue), PCS with constipation and CMPF (negative association with shortness of breath) demonstrated moderately strong associations with symptoms in adjusted analysis.
Unfortunately there is no clear answer if sex influences TMAO concentrations. Some studies indicate a relationship between sex and metabolite concentration in healthy individuals, while others do not [22]. A correlation between increased TMAO levels and neurological disorders has also been hypothesized, but the role of TMAO in the central nervous system has not been fully explored. Due to the importance of TMAO as a mediator of inflammatory processes, the possible participation of this compound in the etiology of neurological disorders is presumed [22]. This neurological alteration could be one explanation for the association between TMAO and fatigue observed in the present study.
Gut-derived uremic toxins such as PCS cause oxidative stress and proinflammatory responses, which may contribute to gut epithelial integrity and could generate constipation in CKD patients [23]. However, it has been shown that constipation may lead to production of PCS in nondiabetic nondialysis CKD patients [24].
It has been demonstrated that the effects of CMPF appear to contribute to the increase in the free fraction of furosemide during hemodialysis [25]. Therefore we could consider that the increase CMPF levels in CKD patients may be a compensatory mechanism to overcome shortness of breath in these patients.
Our results did not confirm the associations between uremic toxins and symptoms described previously in preclinical studies and in CKD cohorts [7–9, 11, 12]. This discrepancy may be partly due to the differences in the study population (e.g. Asian patients versus European patients) and/or to the fact that in our study we did not evaluate the free levels of some uremic toxins (e.g. IS and PCS). Free and bound toxins may have different clinical impacts in terms of symptoms, which might explain the lack of an overall association. Indeed, it has been reported that free PCS (but not total PCS, i.e. free + protein-bound) is associated with cardiovascular outcomes in nondialyzed patients with CKD [26, 27]. We previously reported that cognitive impairment was independently associated with high serum levels of PTH and uric acid but not IS or PCS [28]. However, we did not observe a significant association between serum PTH and uric acid levels and symptoms in the present study. The fact that serum PTH determination was assayed in onsite biochemistry laboratories, which may have different assay types, would explain the present negative results. Recently, metabolomic analysis of hemodialysis patients did not identify any solutes associated with pruritus [29]. Furthermore, in the Choices for Health Outcomes in Caring for ESKD (CHOICE) study, no clinical or laboratory parameter was strongly associated with multiple symptoms [30]. The potential roles of other uremic toxins not examined in the present study remain to be explored. Finally, limitations of the present study include the investigator-selected symptom groupings, which may not be optimal and the lack of exploration of the potential side effects of drugs used in CKD patients, which could be one of multiple factors inducing the symptoms.
Most symptoms were more frequent in women than in men, despite higher uremic toxin levels in men. However, the absence of significant associations between uremic toxins and symptoms in the present study may suggest that other factors related to low kidney function are involved in this sex difference. For example, anemia may be associated with fatigue. However, in this study, hemoglobin levels were similar in men and women. Furthermore, heart failure may induce shortness of breath. Again, there was no difference between men and women in the prevalence of chronic heart failure. We reported previously that uremic mice and nonuremic mice do not differ significantly in terms of anxiety [31]. It has been reported that anxiety and depressive symptoms increase over time in dialysis patients from families with conflictual relationships [32]. It may be that the self-administered questionnaires used here did not capture all the symptoms and missed certain environmental and psychosocial factors, such as poor family relationships, potentially explaining the variation in symptoms.
Indeed, other psychosocial differences between men and women may better explain the higher symptom burden found in women. First, it has been shown that men are more likely to deny perceived signs of physical weakness due to sociocultural norms (such as masculinity) [33]. Second, men and women apply different coping strategies to deal with the disease burden, which may affect the perception and reporting of symptoms [34,35]. Last, women seem to be more sensitive to symptoms compared with men [36], and this difference persists even after excluding gynecologic and reproductive symptoms [37].
CONCLUSION
In conclusion, symptoms are frequent in patients (especially in women) with stage 4/5 CKD and we observed high levels of different uremic toxins, particularly in men. However, we did not observe any consistent patterns between uremic toxins and symptoms in the cohort as a whole or by sex. Other uremic toxins or uremia-related factors such as depression, gastroparesis and medications can contribute to symptom burden. In addition, various psychosocial factors may explain the overall and sex-specific burden of symptoms, but these are still to be determined.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank all the patients and health professionals for participating in the EQUAL study.
APPENDIX
The EQUAL study investigators: Andreas Schneider, Anke Torp, Beate Iwig, Boris Perras, Christian Marx, Christiane Drechsler, Christof Blaser, Christoph Wanner, Claudia Emde, Detlef Krieter, Dunja Fuchs, Ellen Irmler, Eva Platen, Hans Schmidt-Gürtler, Hendrik Schlee, Holger Naujoks, Ines Schlee, Sabine Cäsar, Joachim Beige, Jochen Röthele, Justyna Mazur, Kai Hahn, Katja Blouin, Katrin Neumeier, Kirsten Anding-Rost, Lothar Schramm, Monika Hopf, Nadja Wuttke, Nikolaus Frischmuth, Pawlos Ichtiaris, Petra Kirste, Petra Schulz, Sabine Aign, Sandra Biribauer, Sherin Manan, Silke Röser, Stefan Heidenreich, Stephanie Palm, Susanne Schwedler, Sylke Delrieux, Sylvia Renker, Sylvia Schättel, Theresa Stephan, Thomas Schmiedeke, Thomas Weinreich, Til Leimbach, Torsten Stövesand, Udo Bahner, Wolfgang Seeger, Adamasco Cupisti, Adelia Sagliocca, Alberto Ferraro, Alessandra Mele, Alessandro Naticchia, Alex Còsaro, Andrea Ranghino, Andrea Stucchi, Angelo Pignataro, Antonella De Blasio, Antonello Pani, Aris Tsalouichos, Antonio Bellasi, Biagio Raffaele Di Iorio, Alessandra Butti, Cataldo Abaterusso, Chiara Somma, Claudia D'alessandro, Claudia Torino, Claudia Zullo, Claudio Pozzi, Daniela Bergamo, Daniele Ciurlino, Daria Motta, Domenico Russo, Enrico Favaro, Federica Vigotti, Ferruccio Ansali, Ferruccio Conte, Francesca Cianciotta, Francesca Giacchino, Francesco Cappellaio, Francesco Pizzarelli, Gaetano Greco, Gaetana Porto, Giada Bigatti, Giancarlo Marinangeli, Gianfranca Cabiddu, Giordano Fumagalli, Giorgia Caloro, Giorgina Piccoli, Giovanbattista Capasso, Giovanni Gambaro, Giuliana Tognarelli, Giuseppe Bonforte, Giuseppe Conte, Giuseppe Toscano, Goffredo Del Rosso, Irene Capizzi, Ivano Baragetti, Lamberto Oldrizzi, Loreto Gesualdo, Luigi Biancone, Manuela Magnano, Marco Ricardi, Maria Di Bari, Maria Laudato, Maria Luisa Sirico, Martina Ferraresi, Michele Provenzano, Moreno Malaguti, Nicola Palmieri, Paola Murrone, Pietro Cirillo, Pietro Dattolo, Pina Acampora, Rita Nigro, Roberto Boero, Roberto Scarpioni, Rosa Sicoli, Rosella Malandra, Silvana Savoldi, Silvio Bertoli, Silvio Borrelli, Stefania Maxia, Stefano Maffei, Stefano Mangano, Teresa Cicchetti, Tiziana Rappa, Valentina Palazzo, Walter De Simone, Anita Schrander, Bastiaan van Dam, Carl Siegert, Carlo Gaillard, Charles Beerenhout, Cornelis Verburgh, Cynthia Janmaat, Ellen Hoogeveen, Ewout Hoorn, Friedo Dekker, Johannes Boots, Henk Boom, Jan-Willem Eijgenraam, Jeroen Kooman, Joris Rotmans, Kitty Jager, Liffert Vogt, Maarten Raasveld, Marc Vervloet, Marjolijn van Buren, Merel van Diepen, Nicholas Chesnaye, Paul Leurs, Pauline Voskamp, Peter Blankestijn, Sadie van Esch, Siska Boorsma, Stefan Berger, Constantijn Konings, Zeynep Aydin, Aleksandra Musiała, Anna Szymczak, Ewelina Olczyk, Hanna Augustyniak-Bartosik, Ilona Miśkowiec-Wiśniewska, Jacek Manitius, Joanna Pondel, Kamila Jędrzejak, Katarzyna Nowańska, Łukasz Nowak, Maciej Szymczak, Magdalena Durlik, Szyszkowska Dorota, Teresa Nieszporek, Zbigniew Heleniak, Andreas Jonsson, Anna-Lena Blom, Björn Rogland, Carin Wallquist, Denes Vargas, Emöke Dimény, Fredrik Sundelin, Fredrik Uhlin, Gunilla Welander, Isabel Bascaran Hernandez, Knut-Christian Gröntoft, Maria Stendahl, Maria Svensson, Marie Evans, Olof Heimburger, Pavlos Kashioulis, Stefan Melander, Tora Almquist, Ulrika Jensen, Alistair Woodman, Anna McKeever, Asad Ullah, Barbara McLaren, Camille Harron, Carla Barrett, Charlotte O'Toole, Christina Summersgill, Colin Geddes, Deborah Glowski, Deborah McGlynn, Dympna Sands, Fergus Caskey, Geena Roy, Gillian Hirst, Hayley King, Helen McNally, Houda Masri-Senghor, Hugh Murtagh, Hugh Rayner, Jane Turner, Joanne Wilcox, Jocelyn Berdeprado, Jonathan Wong, Joyce Banda, Kirsteen Jones, Lesley Haydock, Lily Wilkinson, Margaret Carmody, Maria Weetman, Martin Joinson, Mary Dutton, Michael Matthews, Neal Morgan, Nina Bleakley, Paul Cockwell, Paul Roderick, Phil Mason, Philip Kalra, Rincy Sajith, Sally Chapman, Santee Navjee, Sarah Crosbie, Sharon Brown, Sheila Tickle, Suresh Mathavakkannan, Ying Kuan
Contributor Information
Ziad A Massy, Centre for Research in Epidemiology and Population Health, INSERM UMRS 1018, Team5, Paris, France; University Versailles-Saint Quentin, University Paris-Saclay, Villejuif 91190, Paris, France; Department of Nephrology, CHU Ambroise Paré, APHP, 92104 Boulogne Billancourt Cedex, Paris, France.
Nicholas C Chesnaye, ERA Registry, Department of Medical Informatics, Academic Medical Center, University of Amsterdam, Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
Islam Amine Larabi, Laboratory of Pharmacology and Toxicology, CHU, Raymond Poincare, Garches, and INSERM U‑1173, UFR des Sciences de la Santé Simone Veil, Montigny le Bretonneux, Université de Versailles-Saint-Quentin-en-Yvelines, Versailles, France.
Friedo W Dekker, Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, The Netherlands.
Marie Evans, Renal Unit, Department of Clinical Intervention and Technology, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden.
Fergus J Caskey, Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
Claudia Torino, IFC-CNR, Clinical Epidemiology and Pathophysiology of Renal Diseases and Hypertension, Reggio Calabria, Italy.
Gaetana Porto, G.O.M., Bianchi Melacrino Morelli, Reggio Calabria, Italy.
Maciej Szymczak, Department of Nephrology and Transplantation Medicine, Wroclaw Medical University, Wroclaw, Poland.
Christiane Drechsler, Division of Nephrology, University Hospital of Würzburg, Würzburg, Germany.
Christoph Wanner, Division of Nephrology, University Hospital of Würzburg, Würzburg, Germany.
Kitty J Jager, ERA Registry, Department of Medical Informatics, Academic Medical Center, University of Amsterdam, Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
Jean Claude Alvarez, Laboratory of Pharmacology and Toxicology, CHU, Raymond Poincare, Garches, and INSERM U‑1173, UFR des Sciences de la Santé Simone Veil, Montigny le Bretonneux, Université de Versailles-Saint-Quentin-en-Yvelines, Versailles, France.
EQUAL study investigators:
Andreas Schneider, Anke Torp, Beate Iwig, Boris Perras, Christian Marx, Christiane Drechsler, Christof Blaser, Christoph Wanner, Claudia Emde, Detlef Krieter, Dunja Fuchs, Ellen Irmler, Eva Platen, Hans Schmidt-Gürtler, Hendrik Schlee, Holger Naujoks, Ines Schlee, Sabine Cäsar, Joachim Beige, Jochen Röthele, Justyna Mazur, Kai Hahn, Katja Blouin, Katrin Neumeier, Kirsten Anding-Rost, Lothar Schramm, Monika Hopf, Nadja Wuttke, Nikolaus Frischmuth, Pawlos Ichtiaris, Petra Kirste, Petra Schulz, Sabine Aign, Sandra Biribauer, Sherin Manan, Silke Röser, Stefan Heidenreich, Stephanie Palm, Susanne Schwedler, Sylke Delrieux, Sylvia Renker, Sylvia Schättel, Theresa Stephan, Thomas Schmiedeke, Thomas Weinreich, Til Leimbach, Torsten Stövesand, Udo Bahner, Wolfgang Seeger, Adamasco Cupisti, Adelia Sagliocca, Alberto Ferraro, Alessandra Mele, Alessandro Naticchia, Alex Còsaro, Andrea Ranghino, Andrea Stucchi, Angelo Pignataro, Antonella De Blasio, Antonello Pani, Aris Tsalouichos, Antonio Bellasi, Biagio Raffaele Di Iorio, Alessandra Butti, Cataldo Abaterusso, Chiara Somma, Claudia D'alessandro, Claudia Torino, Claudia Zullo, Claudio Pozzi, Daniela Bergamo, Daniele Ciurlino, Daria Motta, Domenico Russo, Enrico Favaro, Federica Vigotti, Ferruccio Ansali, Ferruccio Conte, Francesca Cianciotta, Francesca Giacchino, Francesco Cappellaio, Francesco Pizzarelli, Gaetano Greco, Gaetana Porto, Giada Bigatti, Giancarlo Marinangeli, Gianfranca Cabiddu, Giordano Fumagalli, Giorgia Caloro, Giorgina Piccoli, Giovanbattista Capasso, Giovanni Gambaro, Giuliana Tognarelli, Giuseppe Bonforte, Giuseppe Conte, Giuseppe Toscano, Goffredo Del Rosso, Irene Capizzi, Ivano Baragetti, Lamberto Oldrizzi, Loreto Gesualdo, Luigi Biancone, Manuela Magnano, Marco Ricardi, Maria Di Bari, Maria Laudato, Maria Luisa Sirico, Martina Ferraresi, Michele Provenzano, Moreno Malaguti, Nicola Palmieri, Paola Murrone, Pietro Cirillo, Pietro Dattolo, Pina Acampora, Rita Nigro, Roberto Boero, Roberto Scarpioni, Rosa Sicoli, Rosella Malandra, Silvana Savoldi, Silvio Bertoli, Silvio Borrelli, Stefania Maxia, Stefano Maffei, Stefano Mangano, Teresa Cicchetti, Tiziana Rappa, Valentina Palazzo, Walter De Simone, Anita Schrander, Bastiaan van Dam, Carl Siegert, Carlo Gaillard, Charles Beerenhout, Cornelis Verburgh, Cynthia Janmaat, Ellen Hoogeveen, Ewout Hoorn, Friedo Dekker, Johannes Boots, Henk Boom, Jan-Willem Eijgenraam, Jeroen Kooman, Joris Rotmans, Kitty Jager, Liffert Vogt, Maarten Raasveld, Marc Vervloet, Marjolijn van Buren, Merel van Diepen, Nicholas Chesnaye, Paul Leurs, Pauline Voskamp, Peter Blankestijn, Sadie van Esch, Siska Boorsma, Stefan Berger, Constantijn Konings, Zeynep Aydin, Aleksandra Musiała, Anna Szymczak, Ewelina Olczyk, Hanna Augustyniak-Bartosik, Ilona Miśkowiec-Wiśniewska, Jacek Manitius, Joanna Pondel, Kamila Jędrzejak, Katarzyna Nowańska, Łukasz Nowak, Maciej Szymczak, Magdalena Durlik, Szyszkowska Dorota, Teresa Nieszporek, Zbigniew Heleniak, Andreas Jonsson, Anna-Lena Blom, Björn Rogland, Carin Wallquist, Denes Vargas, Emöke Dimény, Fredrik Sundelin, Fredrik Uhlin, Gunilla Welander, Isabel Bascaran Hernandez, Knut-Christian Gröntoft, Maria Stendahl, Maria Svensson, Marie Evans, Olof Heimburger, Pavlos Kashioulis, Stefan Melander, Tora Almquist, Ulrika Jensen, Alistair Woodman, Anna McKeever, Asad Ullah, Barbara McLaren, Camille Harron, Carla Barrett, Charlotte O'Toole, Christina Summersgill, Colin Geddes, Deborah Glowski, Deborah McGlynn, Dympna Sands, Fergus Caskey, Geena Roy, Gillian Hirst, Hayley King, Helen McNally, Houda Masri-Senghor, Hugh Murtagh, Hugh Rayner, Jane Turner, Joanne Wilcox, Jocelyn Berdeprado, Jonathan Wong, Joyce Banda, Kirsteen Jones, Lesley Haydock, Lily Wilkinson, Margaret Carmody, Maria Weetman, Martin Joinson, Mary Dutton, Michael Matthews, Neal Morgan, Nina Bleakley, Paul Cockwell, Paul Roderick, Phil Mason, Philip Kalra, Rincy Sajith, Sally Chapman, Santee Navjee, Sarah Crosbie, Sharon Brown, Sheila Tickle, Suresh Mathavakkannan, and Ying Kuan
FUNDING
Main funding was received from the European Renal Association and contributions from the Swedish Medical Association, the Stockholm County Council ALF Medicine and Center for Innovative Research, the Italian Society of Nephrology, the Dutch Kidney Foundation (SB 142), the Young Investigators grant in Germany and the National Institute for Health Research in the UK. The results presented in this article have not been published previously in whole or part, except in abstract format
AUTHORS’ CONTRIBUTIONS
Z.M., J.C.A., I.A.L., N.C. and K.J. made substantial contributions in conception and design, acquisition of data and analysis and interpretation of data. Z.M., N.C., K.J., J.C.A., I.A., F.W.D., M.E., F.J.C., C.T., G.T., M.S., C.D. and C.W. were involved in drafting the article or revising it critically for important intellectual content. Final approval of the version to be published was provided by Z.M., N.C., K.J., J.C.A., I.A., F.W.D., M.E., F.J.C., C.T., G.T., M.S., C.D. and C.W. Agreement to be accountable for all aspects of the work is provided by Z.M., N.C., K.J., J.C.A., I.A., F.W.D., M.E., F.J.C., C.T., G.T., M.S., C.D. and C.W.
CONFLICT OF INTEREST STATEMENT
Z.M. reports public funding, grants to charities, travel and accommodation support from Amgen, outside the present work; public funding, travel and accommodation support from Sanofi-Genzyme and grants from the French government, MSD, GlaxoSmithKline, Lilly, FMC, Baxter, Outsuka and AstraZeneca. M.E. reports no conflicts of interest in relation to this publication. M.E. reports payment for advisory boards and lectures by Astellas Pharma, Vifor Pharma and AstraZeneca, outside the present work; and institutional grants from AstraZeneca and Astellas Pharma. C.W. reports honoraria for consultancy and lecturing from Amicus, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Sanofi-Genzyme and Takeda, outside the present work. K.J. reports grants from the European Renal Association while conducting the study. F.W.D., C.T., A.I.L. and J.C.A. report no conflicts of interest.
REFERENCES
- 1. Murphy EL, Murtagh FEM, Carey I et al. Understanding symptoms in patients with advanced chronic kidney disease managed without dialysis: use of a short patient-completed assessment tool. Nephron Clin Pract 2009; 111: c74–c80 [DOI] [PubMed] [Google Scholar]
- 2. Eliasen M, Jørgensen T, Schröder A et al. Somatic symptom profiles in the general population: a latent class analysis in a Danish population-based health survey. Clin Epidemiol 2017; 23: 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van de Luijtgaarden MWM, Caskey FJ, Wanner C et al. Uraemic symptom burden and clinical condition in women and men of ≥65 years of age with advanced chronic kidney disease: results from the EQUAL study. Nephrol Dial Transplant 2019; 34: 1189–1196 [DOI] [PubMed] [Google Scholar]
- 4. Voskamp PWM, Van Diepen M, Evans M et al. The impact of symptoms on health-related quality of life in elderly pre-dialysis patients: effect and importance in the EQUAL study. Nephrol Dial Transplant 2019; 34: 1707–1715 [DOI] [PubMed] [Google Scholar]
- 5. Vanholder R, De Smet R, Glorieux G et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int 2003; 63: 1934–1943 [DOI] [PubMed] [Google Scholar]
- 6. Liabeuf S, Cheddani L, Massy ZA. Uremic toxins and clinical outcomes: the impact of kidney transplantation. Toxins (Basel) 2018; 10: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim SJ, Zhang X, Cho SB et al. Uremic solutes of indoxyl sulfate and p-cresol enhance protease-activated receptor-2 expression in vitro and in vivo in keratinocytes. Hum Exp Toxicol 2021; 40: 113–123 [DOI] [PubMed] [Google Scholar]
- 8. Wang CP, Lu YC, Tsai IT et al. Increased levels of total p-cresylsulfate are associated with pruritus in patients with chronic kidney disease. Dermatology 2016; 232: 363–370 [DOI] [PubMed] [Google Scholar]
- 9. Thome T, Salyers ZR, Kumar RA et al. Uremic metabolites impair skeletal muscle mitochondrial energetics through disruption of the electron transport system and matrix dehydrogenase activity. Am J Physiol Cell Physiol 2019; 317: C701–C713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karbowska M, Hermanowicz JM, Tankiewicz-Kwedlo A et al. Neurobehavioral effects of uremic toxin–indoxyl sulfate in the rat model. Sci Rep 2020; 10: 9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anaya JM, Bollag WB, Hamrick MW et al. The role of tryptophan metabolites in musculoskeletal stem cell aging. Int J Mol Sci 2020; 21: 6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mor A, Pawlak K, Kalaska B et al. Modulation of the paracrine kynurenic system in bone as a new regulator of osteoblastogenesis and bone mineral status in an animal model of chronic kidney disease treated with lp533401. Int J Mol Sci 2020; 21: 5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown JM, Hazen SL. Microbial modulation of cardiovascular disease. Nat Rev Microbiol 2018; 16: 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kovacs AZ, Molnar MZ, Szeifert L et al. Sleep disorders, depressive symptoms and health-related quality of life—a cross-sectional comparison between kidney transplant recipients and waitlisted patients on maintenance dialysis. Nephrol Dial Transplant 2011; 26: 1058–1065 [DOI] [PubMed] [Google Scholar]
- 15. Molnar MZ, Novak M, Ambrus C et al. Restless legs syndrome in patients after renal transplantation. Am J Kidney Dis 2005; 45: 388–396 [DOI] [PubMed] [Google Scholar]
- 16. Azar SA, Hatefi R, Talebi M. Evaluation of effect of renal transplantation in treatment of restless legs syndrome. Transplant Proc 2007; 39: 1132–1133 [DOI] [PubMed] [Google Scholar]
- 17. Fabresse N, Uteem I, Lamy E et al. Quantification of free and protein bound uremic toxins in human serum by LC-MS/MS: comparison of rapid equilibrium dialysis and ultrafiltration. Clin Chim Acta 2020; 507: 228–235 [DOI] [PubMed] [Google Scholar]
- 18. Jager KJ, Ocak G, Drechsler C et al. The EQUAL study: a European study in chronic kidney disease stage 4 patients. Nephrol Dial Transplant 2012; 27: 27–31 [DOI] [PubMed] [Google Scholar]
- 19. Weisbord SD, Fried LF, Arnold RM et al. Development of a symptom assessment instrument for chronic hemodialysis patients: the dialysis symptom index. J Pain Symptom Manage 2004; 27: 226–240 [DOI] [PubMed] [Google Scholar]
- 20. Jager K, Zoccali C, MacLeod A et al. Confounding: what it is and how to deal with it. Kidney Int 2008; 73: 256–260 [DOI] [PubMed] [Google Scholar]
- 21. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995; 57: 289–300 [Google Scholar]
- 22. Gątarek P, Kałużna-Czaplińska J. Trimethylamine N-oxide (TMAO) in human health. EXCLI J 2021; 20: 301–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ikee R, Sasaki N, Yasuda T et al. Chronic kidney disease, gut dysbiosis, and constipation: a burdensome triplet. Microorganisms 2020; 8: 1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramos CI, Armani RG, Canziani ME et al. Bowel habits and the association with uremic toxins in non-dialysis-dependent chronic kidney disease patients. J Ren Nutr 2020; 30: 31–35 [DOI] [PubMed] [Google Scholar]
- 25. Takamura N, Maruyama T, Otagiri M. Effects of uremic toxins and fatty acids on serum protein binding of furosemide: possible mechanism of the binding defect in uremia. Clin Chem 1997; 43: 2274–2280 [PubMed] [Google Scholar]
- 26. Liabeuf S, Barreto D V, Barreto FC et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 2010; 25: 1183–1191 [DOI] [PubMed] [Google Scholar]
- 27. Glorieux G, Vanholder R, Van Biesen W et al. Free p-cresyl sulfate shows the highest association with cardiovascular outcome in chronic kidney disease. Nephrol Dial Transplant 2021; 36: 998–1005 [DOI] [PubMed] [Google Scholar]
- 28. Puy L, Bugnicourt JM, Liabeuf S et al. Cognitive impairments and dysexecutive behavioral disorders in chronic kidney disease. J Neuropsychiatry Clin Neurosci 2018; 30: 310–317 [DOI] [PubMed] [Google Scholar]
- 29. Bolanos CG, Pham NM, Mair RD et al. Metabolomic analysis of uremic pruritus in patients on hemodialysis. PLoS One 2021; 16: e0246765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rhee EP, Guallar E, Hwang S et al. Prevalence and persistence of uremic symptoms in incident dialysis patients. Kidney360 2020; 1: 86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chillon JM, Brazier F, Bouquet P et al. Neurological disorders in a murine model of chronic renal failure. Toxins (Basel) 2013; 6: 180–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Untas A, Rascle N, Idier L et al. Family relations, mental health and adherence to nutritional guidelines in patients facing dialysis initiation. Psychol Health 2012; 27: 753–766 [DOI] [PubMed] [Google Scholar]
- 33. Courtenay WH. Constructions of masculinity and their influence on men's well-being: a theory of gender and health. Soc Sci Med 2000; 50: 1385–1401 [DOI] [PubMed] [Google Scholar]
- 34. Kristofferzon ML, Lindqvist R, Nilsson A. Relationships between coping, coping resources and quality of life in patients with chronic illness: a pilot study. Scand J Caring Sci 2011; 25: 476–483 [DOI] [PubMed] [Google Scholar]
- 35. Gemmell LA, Terhorst L, Jhamb M et al. Gender and racial differences in stress, coping, and health-related quality of life in chronic kidney disease. J Pain Symptom Manage 2016; 52: 806–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Voskamp PWM, van Diepen M, Evans M et al. The impact of symptoms on health-related quality of life in elderly pre-dialysis patients: effect and importance in the EQUAL study. Nephrol Dial Transplant 2019; 34: 1707–1715 [DOI] [PubMed] [Google Scholar]
- 37. Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in men and women. J Gen Intern Med 2001; 16: 266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.