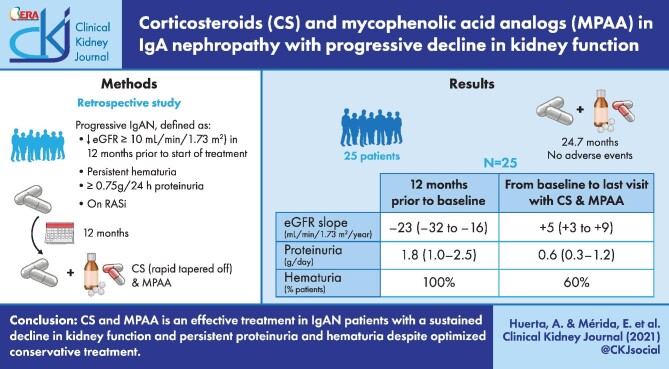
ABSTRACT
Background
A randomized controlled trial demonstrated a beneficial effect of corticosteroids (CS) + cyclophosphamide followed by azathioprine in progressive immunoglobulin A nephropathy (IgAN). Although treatment with CS and mycophenolic acid analogues (MPAAs) remains controversial in IgAN, there is no information about their effects in progressive IgAN.
Methods
Patients with progressive IgAN, defined by a decrease in estimated glomerular filtration rate (eGFR) of at least 10 mL/min/1.73 m2 in the 12 months prior to the start of treatment, proteinuria ≥0.75 g/24 h despite maximum tolerated doses of renin–angiotensin system blockers, and persistent haematuria who had received treatment with CS + MPAA were included in this retrospective study. The main outcome was the difference between the eGFR slope from the start of treatment with CS + MPAA to the last visit with this treatment with respect to the eGFR slope during the 12 months prior to the start of treatment.
Results
A total of 25 patients were included in the study. The mean duration of CS + MPAA treatment was 24.7 ± 15.2 months. In the 12 months prior to treatment the median rate of kidney function decline was 23 mL/min/1.73 m2/year [interquartile range (IQR) –32 to –16]. After the onset of treatment, the median eGFR slope was 5 mL/min/1.73 m2/year (IQR 3–9; P = 0.001 with respect to the 12 months prior to treatment). Proteinuria decreased from 1.8 g/day (IQR 1.0–2.5) at baseline to 0.6 g/day (IQR 0.3–1.2) at the end of treatment (P = 0.01) and haematuria disappeared in 40% of patients. There were no serious adverse effects requiring treatment discontinuation.
Conclusions
CS + MPAA is an effective treatment in IgAN patients with a sustained decline in kidney function accompanied by persistent proteinuria and haematuria despite optimized conservative treatment. Prospective studies are needed to confirm these results.
Keywords: GFR, haematuria, IgA nephropathy, immunosuppression, proteinuria
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Immunosuppressive treatment in immunoglobulin A nephropathy (IgAN) remains controversial. While corticosteroid (CS) treatment has shown favourable effects in several retrospective studies [1, 2] and in some randomized controlled trials (RCTs) [3–9], other large RCTs performed in recent years have been unable to conclusively demonstrate a beneficial influence of immunosuppressive treatments on the progression of the disease [10–12]. The numerous and serious side effects reported in these recent trials cast more doubts about the indication of immunosuppressive treatments in IgAN [11–13]. RCTs evaluating other drugs, such as rituximab, have also yielded negative results [14].
Some retrospective studies have suggested that mycophenolic acid analogues (MPAAs) might be effective in IgAN, slowing the decline of renal function and improving kidney histological lesions [15, 16]. However, RCTs performed with MPAAs have yielded conflicting results [17–23].
IgAN and rapid decline of kidney function constitute an uncommon type of disease with a very poor kidney prognosis. In some cases, the basis of the rapid loss of kidney function consists of the presence of cellular crescents in >50% of the glomeruli, and these cases are defined as crescentic IgAN. There are patients, however, in which the kidney function decline cannot be explained by the massive presence of crescents or by the concurrence of superimposed functional factors. In this type of progressive IgAN, an RCT demonstrated that the combined treatment of CS + cyclophosphamide followed by azathioprine was superior to conservative therapy [24]. However, in the STOP-IgAN trial, the same immunosuppressive scheme did not induce favourable effects on renal outcomes and was followed by numerous side effects [11].
The aim of this retrospective multicentre study was to evaluate the influence of a combined treatment of CS + MPAA in a predominantly Caucasian population with progressive IgAN, defined by strict criteria. Our results show a clear favourable effect of this immunosuppressive treatment, which was able to reverse the accelerated loss of kidney function that the patients showed before the onset of treatment.
MATERIALS AND METHODS
Patients
A total of 25 patients with progressive IgAN who had received treatment with CS + MPAA were included in this retrospective observational study. The criteria defining progressive IgAN were the following: a decrease in estimated glomerular filtration rate (eGFR) of at least 10 mL/min/1.73 m2 in the 12 months prior to the start of treatment and proteinuria ≥0.75 g/24 h despite maximum tolerated doses of renin–angiotensin system (RAS) blockers and haematuria [≥5 red blood cells (RBCs) per high power field (hpf)] at the beginning of treatment. Patients from the Hospitals Universitario 12 de Octubre, Madrid, Puerta de Hierro Majadahonda University Hospital, Madrid, and Británico, Buenos Aires, who met the criteria were included. The diagnosis of IgAN was established by kidney biopsy in all included patients. The mean interval between the performance of kidney biopsy and the onset of CS + MPAA treatment was 4.5 ± 11.9 months. In 21 patients, kidney biopsy was performed within the 12 months prior to treatment. Patients with crescentic IgAN, defined by the presence of crescents in >50% of glomeruli in the renal biopsy, were excluded, as well as those patients with a follow-up of <6 months from the start of treatment with CS + MPAA. Patients with diabetes, liver or systemic diseases, IgA vasculitis or any type of secondary IgAN were also excluded. In all cases, the existence of superimposed factors that justified the deterioration of kidney function were ruled out through a careful physical examination, review of potentially nephrotoxic drugs and careful examination of kidney biopsies, as well as kidney sonograms and other radiological studies in all cases before starting treatment.
Treatment
The immunosuppressive regimen consisted of a combination of CS + MPAA. The initial dose of CS consisted of oral prednisone (0.7–0.8 mg/kg/day, not exceeding 80 mg/day; initial mean dose 60 ± 19.7 mg/day) for 2–4 weeks and then tapered off over an additional 30 ± 0.3 weeks. A total of 19 patients received mycophenolate sodium and the remaining 6 received mycophenolate mofetil (MMF). The mean maximum dose of MMF, or its equivalent in mycophenolate sodium, was 1460 ± 518.8 mg/day and the mean duration of MPAA treatment was 24.7 ± 15.2 months. All patients received treatment with RAS blockers at the maximum tolerated doses throughout the follow-up. Prophylactic trimethoprim-sulfamethoxazole was prescribed to all patients.
Patient follow-up and data collection
Demographic and clinical data were extracted from the medical histories. The following data were systematically recorded at baseline (onset of CS + MPAA treatment), at months 12 and 6 before the start of treatment and at months 1, 3, 6, 12, 18 and 24 after the onset of treatment: systolic and diastolic blood pressure, weight, serum creatinine, eGFR [estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation], serum albumin, 24-h proteinuria and urinary sediment. These data were recorded every 12 months in those patients with a follow-up >24 months. The mean follow-up [the interval between baseline and the last outpatient visit, death or end-stage kidney disease (ESKD)] was 6.5 ± 4.1 years.
Definitions
A baseline was established as the time of CS + MPAA treatment onset. Follow-up was defined as the interval between baseline and the last outpatient visit, death or ESKD. ESKD was defined as an eGFR <15 mL/min/1.73 m2, with a need for chronic dialysis or renal transplantation. The eGFR was calculated by the CKD-EPI equation. Disappearance of haematuria was defined by the absence of haematuria or the presence of <5 RBCs/hpf in the urine sediment examination. When possible, kidney biopsies were re-evaluated and scored according to the Oxford classification of IgAN [25]: mesangial cellularity score, ≤0.5 (M0) and >0.5 (M1); the presence of endocapillary proliferation, absent (E0) and present (E1); segmental glomerulosclerosis, absent (S0) and present (S1); the severity of tubular atrophy/interstitial fibrosis, ≤25% (T0), 26–50% (T1) and >50% (T2); and cellular/fibrocellular crescents, absent (C0), 1–25% (C1) and 26–50% (C2).
Outcomes
The main outcome of the study was the difference between the eGFR slope (expressed in mL/min/1.73 m2/year) from the start of treatment with CS + MPAA to the last visit with this treatment with respect to the eGFR slope during the 12 months prior to the start of CS + MPAA treatment.
Secondary outcomes were the change in proteinuria and the change in the prevalence of haematuria from the start of treatment with CS + MPAA to the last visit with this treatment, the difference between the eGFR slope from the start of treatment with CS + MPAA to the last visit with this treatment with respect to the eGFR slope after treatment withdrawal and the adverse events related to CS + MPAA treatment.
Statistical analysis
Quantitative data are shown as mean ± standard deviation or median and IQR according to the normal distribution. Qualitative data are shown as frequency and percentage. We used linear models to estimate the slope of eGFR. The comparisons of the variables before and after the treatment with MPAA were performed with non-parametric tests for paired samples. Comparisons between groups were made with the Wilcoxon paired test or McNemar test, as appropriate. P-values <0.05 were considered significant. We performed statistical analysis with Stata version 14 software (StataCorp, College Station, TX, USA).
RESULTS
Baseline characteristics
Baseline demographics and clinical and laboratory characteristics of the 25 patients included in the study are shown in Table 1. All patients were treated with the maximum tolerated doses of RAS blockers for at least 12 months before the start of treatment with CS + MPAA. The median eGFR at baseline was 48.7 mL/min/1.73 m2 (IQR 34–67), median proteinuria was 1.8 g/day (IQR 1.0–2.5), and all patients presented with microhaematuria. A total of 21 kidney biopsies had been performed within the 12 months prior to the start of treatment and all of them were re-evaluated according to the Oxford classification. Mesangial hypercellularity was found in 52% of the patients (11/21), endocapillary hypercellularity in 52% (11/21), segmental glomerulosclerosis in 62% (13/21), tubular atrophy/interstitial fibrosis >25% in 9% (2/21) and crescents in 62% (13/21). The mean number of glomeruli showing crescents was 12 ± 12.6% (range 0–40) and they were cellular in all cases. Coexistence of mesangial and endocapillary hypercellularity was observed in nine patients. The absence of mesangial or endocapillary hypercellularity was found in seven patients, but three of them presented cellular crescents in 23.3 ± 15.3% of the glomeruli.
Table 1.
Baseline characteristics of patients (N = 25)
| Characteristics | Values |
|---|---|
| Age (years), mean ± SD | 38.6 ± 17.7 |
| Male, n (%) | 16 (64.0) |
| Ethnic origin, n | |
| White | 22 |
| Asiatic | 2 |
| Latin American | 1 |
| Systolic blood pressure (mmHg), mean ± SD | 129.3 ± 14.8 |
| Diastolic blood pressure (mmHg), mean ± SD | 76.6 ± 10.1 |
| Serum creatinine (mg/dL), mean ± SD | 1.7 ± 0.4 |
| eGFR (mL/min/1.73 m2), median (IQR) | 48.7 (34–67) |
| Proteinuria (g/day), median (IQR) | 1.8 (1–2.5) |
| Haematuria, n (%) | 25 (100) |
| Renal biopsy, n (%)a | |
| M1 | 11 (52.4) |
| E1 | 11 (52.4) |
| S1 | 13 (61.9) |
| T1 | 1 (4.8) |
| T2 | 1 (4.8) |
| C1 | 10 (47.6) |
| C2 | 3 (14.3) |
M1, mesangial hypercellularity; E1, endocapillary hypercellularity; S1, segmental glomerulosclerosis; T1: tubular atrophy/interstitial fibrosis >25%; T2: tubular atrophy/interstitial fibrosis >25%.
Available in 21 patients.
Main outcome
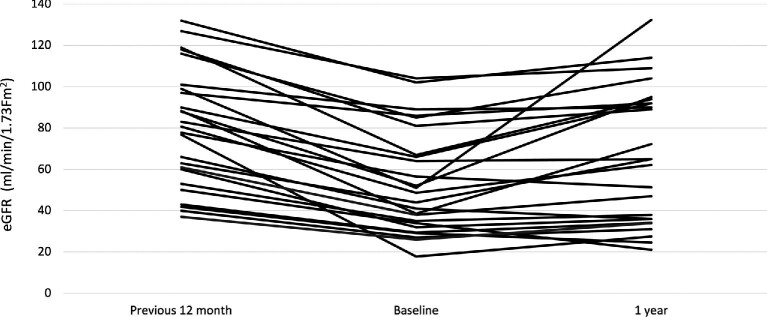
As shown in Table 2, a significant change in the slope of eGFR was observed after the onset of CS + MPAA treatment. During the 12 months prior to treatment, the median rate of kidney function decline was –23 mL/min/1.73 m2/year (IQR –32 to –16). After the onset of treatment, this kidney function decline was reversed. The eGFR slope between baseline and the last visit with CS + MPAA treatment was 5 mL/min/1.73 m2/year (IQR 3–9; P = 0.001) with respect to the 12 months prior to treatment. The 2-year eGFR slope after the onset of treatment was 3.5 mL/min/1.73 m2/year (IQR 1–8).
Table 2.
Change in eGFR slope before and after CS + MPAA treatment
| 12 months prior to baseline (n = 25) | From baseline to the last visit with CS + MPAA treatment (n = 25) | From baseline to the last visit with CS + MPAA treatment (n = 21) | From the last visit with CS + MPAA treatment to end of follow-up (n = 21) | |
|---|---|---|---|---|
| 4 | ||||
| eGFR slope (mL/min/1.73 m2/year) | –23 (–32 to –16) | 5 (3–9)* | 4 (3–8) | –2.1 (–6.4 to –0.6)**, *** |
*P = 0.001 and **P = 0.001 compared with the eGFR slope between baseline and the last visit with CS + MPAA treatment and ***P = 0.001 compared with the eGFR slope in the 12 months prior to baseline.
Only six patients (24%) continued to show a decline in kidney function after the start of treatment, although this decline was significantly slower [–7.1 mL/min/1.73 m2/year (IQR –8.6 to –2.9)] compared with [–16 mL/min/1.73 m2/year (IQR –25 to –12.7) in the 12 months prior to treatment; P = 0.001]. There were no differences in age, gender, baseline kidney function, baseline proteinuria or in the biopsy score (IgAN Oxford classification) between these 6 patients and the remaining 19 with a positive change in the eGFR slope.
The evolution of eGFR before and after CS + MPAA treatment is shown in Table 3. The individual changes in the eGFR slope before and after treatment are shown in Figure 1.
Table 3.
Evolution of eGFR, proteinuria and haematuria in all patients (N = 25)
| Characteristics | –12 months | Baseline | 1 month | 3 months | 6 months | 12 months | Last visit with CS + MPAA treatment |
|---|---|---|---|---|---|---|---|
| eGFR (mL/min/1.73 m2), median (IQR) | 80.7 (60–99)*** | 48.7 (34–67) | 49.4 (36.8–75.0) | 58.0 (40.5–84.0)* | 59.8 (36.1–92.5)** | 65.0 (36.0–92.0)** | 64.0 (33.4–90.0)* |
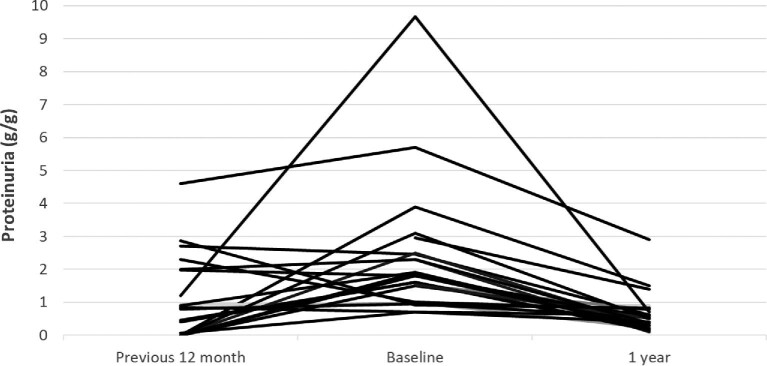
| Proteinuria (g/day), median (IQR) | 0.8 (0–2.0) | 1.8 (1.0–2.5) | 1.4 (0.9–2.5) | 1.0 (0.6–1.3)*** | 0.6 (0.4–1.1)*** | 0.5 (0.3–0.8)*** | 0.6 (0.3–1.2)*** |
| Patients with haematuria, n (%) | 25 (100) | 25 (100) | 25 (100) | 22 (88.0) | 16 (64.0)* | 15 (60.0)*** | 15 (60.0)*** |
*P < 0.05, **P < 0.01 and ***P < 0.001 with respect to baseline values.
FIGURE 1:
Individual changes in the eGFR before and after the onset of CS + MPAA treatment.
Secondary outcomes
During the 12 months prior to CS + MPAA treatment the median proteinuria increased from 0.8 g/day (IQR 0–2.0) to 1.8 (1.0–2.5) (P = 0.01). After the start of treatment, proteinuria showed a significant decrease (Figure 2). At the time of the last visit with CS + MPAA treatment, proteinuria had decreased to 0.6 g/day (IQR 0.3–1.2; P < 0.001) (Table 3).
FIGURE 2:
Individual changes in proteinuria before and after the onset of CS + MPAA treatment.
As shown in Table 3, the proportion of patients with haematuria decreased after the introduction of CS + MPAA treatment, and at the last visit, haematuria had disappeared in 10 patients (40%). After a mean follow-up of 24.7 ± 15.2 months, treatment with CS + MPAA was discontinued in 21 patients. The mean follow-up after treatment withdrawal was 44.3 ± 30.4 months. As shown in Table 2, there was a significant decline in kidney function after treatment discontinuation [eGFR slope –2.1 mL/min/1.73 m2/year (IQR –6.4 to –0.6) compared with 4 mL/min/1.73 m2/year (IQR 3–8)] in the period in which these patients were treated with CS + MPAA. Nevertheless, this decline in kidney function after treatment discontinuation was significantly slower than kidney function decline in the 12 months prior to treatment (Table 2). No significant changes in proteinuria were detected and the reappearance of haematuria was observed in two patients (Table 4).
Table 4.
Changes in kidney function, proteinuria and haematuria after the withdrawal of CS + MPAA treatment (N = 21)
| Characteristics | Last visit with CS + MPAA treatment | End of follow-up |
|---|---|---|
| eGFR (mL/min/1.73 m2), median (IQR) | 64.0 (38.4–90.0) | 54.0 (19.0–80.0)* |
| Proteinuria (g/day), median (IQR) | 0.6 (0.3–1.1) | 0.7 (0.3–1.5) |
| Patients with haematuria, n (%) | 12 (57.1) | 14 (66.7) |
*P = 0.002.
Adverse events
There were no serious adverse effects that caused treatment discontinuation. Infectious complications occurred in three patients, gastrointestinal disturbances in two patients, haematological complications in two patients and transient hyperglycaemia in one patient.
DISCUSSION
In this retrospective multicentre study, we report the efficacy of combined treatment with CS + MPAA in patients with progressive IgAN defined by strict criteria: all the patients showed a progressive deterioration of kidney function ≥10 mL/min/1.73 m2 in the 12 months prior to treatment, proteinuria ≥0.75 g/day despite the optimization of RAS blocker treatment [26, 27] and conservative measures and persistent microhaematuria. The combination of CS + MPAA was able to reverse the decline in kidney function, with the eGFR slope becoming positive in most cases. Likewise, proteinuria decreased significantly and, in an important proportion of patients (40%), haematuria disappeared.
The use of immunosuppressive treatments in IgAN continues to be surrounded by persistent controversy. Although CS has been shown to be beneficial in several retrospective studies and in some prospective trials [1–9], it induces numerous side effects that ultimately counteract these favourable effects [10–13]. An RCT showed similar favourable effects and fewer adverse events of a regimen combining low-dose CS + MMF compared with a full-dose CS regimen, thus suggesting the potential of MPAAs as CS-sparing agents [19].
Regarding the treatment with MPAA in IgAN, the results are also controversial. Some RCTs failed to demonstrate favourable effects of MPAA compared with conservative treatment in regard to proteinuria decrease or preservation of kidney function in Caucasian cohorts [21–23]. It is important to underline that most patients included in these studies had stable kidney function, and in one of them, patients with advanced deterioration of kidney function were included, which could have influenced the results of the trial [22]. In contrast, in another trial carried out in China, MMF reduced proteinuria by >50% in a significantly greater proportion of IgAN patients than the control group [17] and an extended follow-up of this study showed a favourable effect of MMF treatment on kidney function outcomes [18]. A systematic review of RCTs evaluating MPAA in IgAN concluded that a relatively short course of MMF might be beneficial in treating the disease, although the need for high-quality, well-designed RCTs with a large sample size was evident [20].
The main inclusion critereon in most of the RCTs performed in IgAN has consisted of the presence of proteinuria >1 g/day despite optimized supportive treatment. Histological lesions as scored by the Oxford classification of IgAN have a significant influence on the final outcomes of the disease, according to several studies [25, 28–31]. In this regard, MMF treatment significantly reduced the mean percentage of glomeruli showing endocapillary hypercellularity and cellular/fibrocellular crescents, as well as mesangial IgA deposition, in a group of patients in whom kidney biopsy was repeated after MMF treatment [16]. On the other hand, some recent studies have shown that the persistence and amount of haematuria significantly influences kidney outcomes and that the disappearance of haematuria improves kidney survival [32, 33]. The coexistence of active histological lesions, persistent proteinuria and haematuria and a progressive decline of kidney function presents a profile of IgAN patients with a high probability of rapidly reaching kidney failure. Nevertheless, neither histological lesions nor the presence of haematuria or declining kidney function has traditionally been used as a selection critereon in IgAN published trials. The addition of such histological and clinical criteria besides the presence of persistent proteinuria despite conservative treatment could likely help to identify those patients who may benefit from immunosuppressive treatments. A study that included a small number of cases with IgAN and evidence of clinical and histological aggressiveness (acute inflammatory histologic changes, renal failure and severe proteinuria and haematuria) showed that a combined treatment of high-dose CS + MMF induced a clear improvement in renal function accompanied by a significant decrease in the amount of proteinuria and haematuria [15].
Few studies on the treatment of progressive IgAN have been reported. An RCT demonstrated the efficacy of CS + cyclophosphamide, followed by CS + azathioprine, in patients with progressive deterioration of kidney function [24]. According to the results of our study, the combination of CS + MPAA could be an alternative to cyclophosphamide/azathioprine in this type of patient, inducing less toxicity in the short and long terms. The rationale for this dual CS + MPAA approach was based mainly on the anti-inflammatory effects of CS plus the antiproliferative and CS-sparing effects of MPAA. Although the Oxford classification has not been validated to indicate any therapeutic regimen, the proliferative histological lesions encountered in our patients, which were associated with elevated proteinuria, microhaematuria and a decline in kidney function, were indicators that immunosuppression would be of benefit. The presence of mesangial or endocapillary hypercellularity, as well as glomerular crescents, in the renal biopsies of our patients, together with the absence of chronic lesions, should be underlined: no fibrotic crescents were observed and only two patients presented interstitial fibrosis. CS + MPAA treatment was well tolerated, with no adverse effects that required treatment discontinuation.
Another aspect of our study that deserves mention is the evolution of kidney function after the discontinuation of immunosuppressive treatment. A significant worsening of the eGFR slope compared with the treatment period was observed, but this worsening was remarkably slower than the rapid decline in kidney function that all patients presented before treatment. This change in trend suggests a favourable effect of the immunosuppressive treatment on the glomerular lesions, which persists for many months or even years after its withdrawal. This legacy effect of immunosuppression has been reported in previous studies that have analysed the impact of MPAA treatment and other immunosuppressive regimens in IgAN patients [15–17, 34].
The main limitations of our study are inherent in its retrospective character, in addition to the relatively small number of patients and the lack of a non-immunosuppressed control group. On the other hand, our study has several strengths, including the strict homogeneous inclusion criteria, the careful evaluation to exclude superimposed factors that could have accelerated the loss of renal function and the long and well-recorded follow-up.
In summary, the combination of CS + MPAA is an effective and well-tolerated treatment in patients with progressive IgAN, defined by a sustained decline in kidney function accompanied by persistent proteinuria and haematuria despite optimized conservative treatment. In our experience, such treatment was able to reverse the rapid loss of renal function and this favourable effect was largely maintained after treatment discontinuation. However, prospective controlled studies are needed to confirm these results.
ACKNOWLEDGEMENTS
The work in this study was supported by the Instituto de Salud Carlos III/Fondo Europeo de Desarrollo Regional (grants PI16/01685 and PI19/01624 to M.P.), the Red de Investigación Renal (RD12/0021/0029 and RETYC 16/009 to M.P.) and the Autonomous Region of Madrid (S2017/BMD-3673 to M.P.).
Contributor Information
Ana Huerta, Department of Nephrology, Hospital Universitario Puerta del Hierro Majadahonda, Madrid, Spain; REDInREN ISCIII 016/009, Madrid, Spain.
Eva Mérida, REDInREN ISCIII 016/009, Madrid, Spain; Department of Nephrology, Hospital Universitario Doce de Octubre, Madrid, Spain.
Laura Medina, Department of Nephrology, Hospital Universitario Infanta Leonor, Madrid, Spain.
Maria Fernandez, Department of Nephrology, Hospital Universitario Doce de Octubre, Madrid, Spain.
Eduardo Gutierrez, REDInREN ISCIII 016/009, Madrid, Spain; Department of Nephrology, Hospital Universitario Doce de Octubre, Madrid, Spain.
Eduardo Hernandez, REDInREN ISCIII 016/009, Madrid, Spain; Department of Nephrology, Hospital Universitario Doce de Octubre, Madrid, Spain.
Paula López-Sánchez, Department of Nephrology, Hospital Universitario Puerta del Hierro Majadahonda, Madrid, Spain.
Angel Sevillano, REDInREN ISCIII 016/009, Madrid, Spain; Department of Nephrology, Hospital Universitario Doce de Octubre, Madrid, Spain.
Jose Portolés, Department of Nephrology, Hospital Universitario Puerta del Hierro Majadahonda, Madrid, Spain; REDInREN ISCIII 016/009, Madrid, Spain.
Hernan Trimarchi, Department of Nephrology, Hospital Británico de Buenos Aires, Buenos Aires, Argentina.
Manuel Praga, REDInREN ISCIII 016/009, Madrid, Spain; Research Institute, Hospital Universitario 12 de Octubre, Madrid, Spain; Department of Medicine, Complutense University, Madrid, Spain.
CONFLICT OF INTEREST STATEMENT
H.T. is a member of the CKJ editorial board.
REFERENCES
- 1. Kobayashi Y, Hiki Y, Kokubo T et al. Steroid therapy during the early stage of progressive IgA nephropathy. A 10-year follow-up study. Nephron 1996; 72: 237–242 [DOI] [PubMed] [Google Scholar]
- 2. Tesar V, Troyanov S, Bellur S et al. VALIGA study of the ERA-EDTA immunonephrology working group. Corticosteroids in IgA nephropathy: a retrospective analysis from the VALIGA study. J Am Soc Nephrol 2015; 26: 2248–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Julian BA, Barker C. Alternate-day prednisone therapy in IgA nephropathy. Preliminary analysis of a prospective, randomized, controlled trial. Contrib Nephrol 1993; 104: 198–206 [PubMed] [Google Scholar]
- 4. Shoji T, Nakanishi I, Suzuki A et al. Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. Am J Kidney Dis 2000; 35: 194–201 [DOI] [PubMed] [Google Scholar]
- 5. Katafuchi R, Ikeda K, Mizumasa T et al. Controlled, prospective trial of steroid treatment in IgA nephropathy: a limitation of low-dose prednisolone therapy. Am J Kidney Dis 2003; 41: 972–983 [DOI] [PubMed] [Google Scholar]
- 6. Pozzi C, Andrulli S, Del Vecchio L et al. Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol 2004; 15: 157–163 [DOI] [PubMed] [Google Scholar]
- 7. Horita Y, Tadokoro M, Taura K et al. Prednisolone co-administered with losartan confers renoprotection in patients with IgA nephropathy. Ren Fail 2007; 29: 441–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manno C, Torres DD, Rossini M et al. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant 2009; 24: 3694–3701 [DOI] [PubMed] [Google Scholar]
- 9. Lv J, Zhang H, Chen Y et al. Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: a randomized controlled trial. Am J Kidney Dis 2009; 53: 26–32 [DOI] [PubMed] [Google Scholar]
- 10. Pozzi C, Andrulli S, Pani A et al. Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. J Am Soc Nephrol 2010; 21: 1783–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rauen T, Eitner F, Fitzner C et al. STOP-IgAN investigators. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 2015; 373: 2225–2236 [DOI] [PubMed] [Google Scholar]
- 12. Pozzi C, Andrulli S, Pani A et al. IgA nephropathy with severe chronic renal failure: a randomized controlled trial of corticosteroids and azathioprine. J Nephrol 2013; 26: 86–93 [DOI] [PubMed] [Google Scholar]
- 13. Rauen T, Fitzner C, Eitner F et al. Effects of two immunosuppressive treatment protocols for IgA nephropathy. J Am Soc Nephrol 2018; 29: 317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lafayette RA, Canetta PA, Rovin BH et al. A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol 2017; 28: 1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roccatello D, Rossi D, Marletto F et al. Long-term effects of methylprednisolone pulses and mycophenolate mofetil in IgA nephropathy patients at risk of progression. J Nephrol 2012; 25: 198–203 [DOI] [PubMed] [Google Scholar]
- 16. Beckwith H, Medjeral-Thomas N, Galliford J et al. Mycophenolate mofetil therapy in immunoglobulin a nephropathy: histological changes after treatment. Nephrol Dial Transplant 2017; 32: i123–i128 [DOI] [PubMed] [Google Scholar]
- 17. Tang S, Leung JC, Chan LY et al. Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int 2005; 68: 802–812 [DOI] [PubMed] [Google Scholar]
- 18. Tang SC, Tang AW, Wong SS et al. Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int 2010; 77: 543–549 [DOI] [PubMed] [Google Scholar]
- 19. Hou JH, Le WB, Chen N et al. Mycophenolate mofetil combined with prednisone versus full-dose prednisone in IgA nephropathy with active proliferative lesions: a randomized controlled trial. Am J Kidney Dis 2017; 69: 788–795 [DOI] [PubMed] [Google Scholar]
- 20. Chen Y, Li Y, Yang S et al. Efficacy and safety of mycophenolate mofetil treatment in IgA nephropathy: a systematic review. BMC Nephrol 2014; 15: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maes BD, Oyen R, Claes K et al. Mycophenolate mofetil in IgA nephropathy: results of a 3-year prospective placebo-controlled randomized study. Kidney Int 2004; 65: 1842–1849 [DOI] [PubMed] [Google Scholar]
- 22. Frisch G, Lin J, Rosenstock J et al. Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double-blind randomized controlled trial. Nephrol Dial Transplant 2005; 20: 2139–2145 [DOI] [PubMed] [Google Scholar]
- 23. Hogg RJ, Bay RC, Jennette JC et al. Randomized controlled trial of mycophenolate mofetil in children, adolescents, and adults with IgA nephropathy. Am J Kidney Dis 2015; 66: 783–791 [DOI] [PubMed] [Google Scholar]
- 24. Ballardie FW, Roberts ISD. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol 2002; 13: 142–148 [DOI] [PubMed] [Google Scholar]
- 25. Cattran DC, Coppo R, Cook HT et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009; 76: 534–545 [DOI] [PubMed] [Google Scholar]
- 26. Praga M, Gutiérrez E, González E et al. Treatment of IgA nephropathy with ACE inhibitors: a randomized and controlled trial. J Am Soc Nephrol 2003; 14: 1578–1583 [DOI] [PubMed] [Google Scholar]
- 27. Reich HN, Troyanov S, Scholey JW et al. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 2007; 18: 3177–3183 [DOI] [PubMed] [Google Scholar]
- 28. Bellur SS, Troyanov S, Cook HT et al. Immunostaining findings in IgA nephropathy: correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol Dial Transplant 2011; 26: 2533–2536 [DOI] [PubMed] [Google Scholar]
- 29. Herzenberg AM, Fogo AB, Reich HN et al. Validation of the Oxford classification of IgA nephropathy. Kidney Int 2011; 80: 310–317 [DOI] [PubMed] [Google Scholar]
- 30. Barbour SJ, Espino-Hernandez G, Reich HN et al. The MEST score provides earlier risk prediction in lgA nephropathy. Kidney Int 2016; 89: 167–175 [DOI] [PubMed] [Google Scholar]
- 31. Trimarchi H, Barratt J, Cattran DC et al. Oxford classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 2017; 91: 1014–1021 [DOI] [PubMed] [Google Scholar]
- 32. Sevillano AM, Gutiérrez E, Yuste C et al. Remission of hematuria improves renal survival in IgA nephropathy. J Am Soc Nephrol 2017; 28: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu GZ, Guo L, Dong JF et al. Persistent hematuria and kidney disease progression in IgA nephropathy: a cohort study. Am J Kidney Dis 2020; 76: 90–99 [DOI] [PubMed] [Google Scholar]
- 34. Coppo R. Is a legacy effect possible in IgA nephropathy?. Nephrol Dial Transplant 2013; 28: 1657–1662 [DOI] [PubMed] [Google Scholar]