Abstract
Aims
To examine the association between benzodiazepine receptor agonist (BZRA) use and mortality in patients hospitalised for coronavirus disease 2019 (COVID-19).
Methods
A multicentre observational study was performed at Greater Paris University hospitals. The sample involved 14 381 patients hospitalised for COVID-19. A total of 686 (4.8%) inpatients received a BZRA at hospital admission at a mean daily diazepam-equivalent dose of 19.7 mg (standard deviation (s.d.) = 25.4). The study baseline was the date of admission, and the primary endpoint was death. We compared this endpoint between patients who received BZRAs and those who did not in time-to-event analyses adjusted for sociodemographic characteristics, medical comorbidities and other medications. The primary analysis was a Cox regression model with inverse probability weighting (IPW).
Results
Over a mean follow-up of 14.5 days (s.d. = 18.1), the primary endpoint occurred in 186 patients (27.1%) who received BZRAs and in 1134 patients (8.3%) who did not. There was a significant association between BZRA use and increased mortality both in the crude analysis (hazard ratio (HR) = 3.20; 95% confidence interval (CI) = 2.74–3.74; p < 0.01) and in the IPW analysis (HR = 1.61; 95% CI = 1.31–1.98, p < 0.01), with a significant dose-dependent relationship (HR = 1.55; 95% CI = 1.08–2.22; p = 0.02). This association remained significant in sensitivity analyses. Exploratory analyses indicate that most BZRAs may be associated with an increased mortality among patients hospitalised for COVID-19, except for diazepam, which may be associated with a reduced mortality compared with any other BZRA treatment.
Conclusions
BZRA use may be associated with an increased mortality among patients hospitalised for COVID-19, suggesting the potential benefit of decreasing dose or tapering off gradually these medications when possible.
Keywords: Benzodiazepine, COVID-19, mortality, SARS-CoV-2
Introduction
Global spread of the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has created an unprecedented infectious disease crisis worldwide (Chevance et al., 2020; Hoertel et al., 2020a, 2020b, 2021a). Benzodiazepine receptor agonists (BZRAs), including benzodiazepines and Z-drugs, potentiate the rapid neuroinhibitory effect of the neurotransmitter gamma-aminobutyric acid in the brain and spinal cord. These medications are commonly prescribed for anxiety and sleep problems and as muscle relaxants and pre-medications in anaesthesia, but are also effective for treating epilepsy, alcohol withdrawal syndrome and acute behavioural disturbance (Mallon et al., 2009; Hayhoe and Lee-Davey, 2018). Potential deleterious effects associated with these medications are well documented. Several studies (Belleville, 2010; Obiora et al., 2013; Weich et al., 2014; Nakafero et al., 2015; Palmaro et al., 2015; Parsaik et al., 2016), although not all (Rumble and Morgan, 1992; Kojima et al., 2000; Phillips and Mannino, 2005; Patorno et al., 2017), have found a significant association between BZRAs and increased all-cause mortality. BZRAs have also been associated with an increased risk of infection (Joya et al., 2009), including pneumonia (Obiora et al., 2013), asthma exacerbation (Nakafero et al., 2015) and respiratory depression (Roth, 2009). To our knowledge, there are no data on the association of BZRA use with mortality among patients hospitalised for coronavirus disease 2019 (COVID-19). Following a recent release by the US Food and Drug Administration (FDA) of a drug safety communication warning patients and health care providers about the risks of BZRA use (US Food and Drug Administration, 2020), it is important to investigate whether these medications are associated with an increased mortality among patients with COVID-19. If this was the case, a second unanswered question is whether all BZRAs or specific BZRA treatments are associated with this risk. This knowledge might help guide clinicians on the choice of medications for patients with COVID-19 with clinical indications for BZRAs.
In this report, we used data from an observational multicentre retrospective cohort study performed at Greater Paris University hospitals and examined the association between BZRAs and mortality. Our primary hypothesis was that BZRA use would be associated with an increased mortality among patients hospitalised for COVID-19 in time-to-event analyses adjusting for sociodemographic characteristics, medical comorbidities and other medications. Our secondary hypotheses were that this association would be dose-dependent with higher doses conferring greater risk, and at least partially explained by respiratory depression.
Methods
Setting and cohort assembly
A multicentre observational retrospective cohort study was conducted at 36 Assistance publique-Hôpitaux de Paris (AP-HP) hospitals (Hoertel et al., 2021c, 2021d, 2021e, 2021f, 2021g, 2021h; Sánchez-Rico et al., 2021). We included all adults aged 18 years or over who have been admitted to these medical centres from the beginning of the epidemic in France, i.e. 24th January until 1st May. COVID-19 was ascertained by a positive reverse-transcriptase-polymerase chain reaction (RT-PCR) test from analysis of nasopharyngeal or oropharyngeal swab specimens. This observational study using routinely collected data received approval from the Institutional Review Board of the AP-HP clinical data warehouse (decision CSE-20-20_COVID19, IRB00011591, 8 April 2020) and is part of a broader project aiming at examining the potential associations between psychotropic medications and COVID-19-related mortality, deposited in French at the ‘Entrepôt de Données de Santé’ (EDS) website (https://eds.aphp.fr/recherches-en-cours), which has led to several publications (Hoertel et al., 2021c, 2021d, 2021e, 2021f, 2021g, 2021h; Sánchez-Rico et al., 2021). AP-HP clinical Data Warehouse initiatives ensure patient information and informed consent regarding the different approved studies through a transparency portal in accordance with European Regulation on data protection and authorisation no. 1980120 from National Commission for Information Technology and Civil Liberties.
We used data from the AP-HP Health Data Warehouse (‘Entrepôt de Données de Santé (EDS)’). This warehouse contains all available clinical data on all inpatient visits for COVID-19 to any Greater Paris University hospital. The data included patient demographic characteristics, vital signs, laboratory test and RT-PCR test results, medication administration data, medication lists during current and past hospitalisations in AP-HP hospitals, current diagnoses, discharge disposition and death certificates.
Variables assessed
We obtained the following data for each patient at the time of the hospitalisation through electronic health records (Devlin et al., 2019; Jouffroy et al., 2020): sex; age, which was categorised into three classes based on age cutoffs (i.e. 18–50, 51–70, 71+) suggested by Williamson et al. (2020), who studied factors associated with COVID-19-related mortality using OpenSAFELY, a secure health analytics platform created on behalf of NHS England and covering about 40% of all patients in England; hospital, which was categorised into four classes following the administrative clustering of AP-HP hospitals in Paris and its suburbs based on their geographical location (i.e. AP-HP Centre – Paris University, Henri Mondor University Hospitals and at home hospitalisation; AP-HP Nord and Hôpitaux Universitaires Paris Seine-Saint-Denis; AP-HP Paris Saclay University and AP-HP Sorbonne University); obesity, which was defined as having a body mass index higher than 30 kg/m2 or an International Statistical Classification of Diseases and Related Health Problems (ICD-10) diagnosis code for obesity (E66.0, E66.1, E66.2, E66.8, E66.9); self-reported current smoking status; any medical condition associated with increased clinical severity related to COVID-19 and BZRA use (Gordon et al., 2020; Hur et al., 2020; Ruan et al., 2020; Williamson et al., 2020), based on ICD-10 diagnosis codes, including diabetes mellitus (E11), diseases of the circulatory system (I00–I99), diseases of the respiratory system (J00–J99), neoplasms (C00–D49), diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism (D5–D8), frontotemporal dementia (G31.0), peptic ulcer (K27), diseases of liver (K70–K95), hemiplegia or paraplegia (G81–G82), acute kidney failure or chronic kidney disease (N17–N19) and HIV (B20); any medication prescribed according to compassionate use or as part of a clinical trial (Haut Conseil de la Santé Publique, 2020) (e.g. hydroxychloroquine, azithromycin, remdesivir, tocilizumab, sarilumab or dexamethasone) and clinical markers of disease severity, including respiratory depression, defined by a respiratory rate <12 breaths/min or a resting peripheral capillary oxygen saturation in ambient air <90%, and any other clinical marker of disease severity, defined as having temperature >40°C or systolic blood pressure <100 mmHg or respiratory rate >24 breaths/min or plasma lactate levels higher than 2 mmol/l (Haut Conseil de la Santé Publique, 2020). For these last two variables, a third category for missing data was added. Additionally, to take into account possible confounding by indication bias for BZRAs, we recorded whether patients had any current psychiatric disorder (F00–F99) based on ICD-10 diagnosis codes, and whether they were prescribed any other psychotropic medication, including any antidepressant (Hoertel et al., 2021d), mood stabiliser (i.e. lithium or antiepileptic medications with mood stabilising effects) or antipsychotic medication (Hoertel et al., 2021f, 2021g).
All medical notes and prescriptions are computerised in Greater Paris University hospitals. Medications including their dosage, frequency, date and mode of administration were identified from medication administration data or scanned hand-written medical prescriptions, through two deep learning models based on bidirectional encoder representations from transformer (BERT) contextual embeddings (Devlin et al., 2019), one for the medications and another for their mode of administration. Contextualised word embeddings from BERT allow to project dense vector representations of words in lower dimensional spaces while maintaining semantic and contextual importance of a word in a numeric form (Devlin et al., 2019). This allows us to automatise the data collection, while significantly increasing its reliability (Devlin et al., 2019). The model was trained on the APmed corpus (Jouffroy et al., 2020), a previously annotated dataset for this task. Extracted medication names were then normalised to the anatomical therapeutic chemical terminology using approximate string matching.
BZRA use
Study baseline was defined as the date of hospital admission. To minimise the risk of immortal time bias, BZRA use was defined as receiving these medications at baseline. To be considered at baseline, BZRA use had to meet two conditions: (i) a first prescription of BZRA from baseline to up to 48 h from hospital admission and (ii) strictly before (in min) the exact moment of the end of the index hospitalisation or death. Patients with a BZRA prescription that did not meet these two conditions were excluded from the analysis. We chose this first condition to reduce the risk of immortal time bias that could result from the inclusion of patients who were first prescribed this medication lately and thus being by definition alive at that time, and we used 48 h delay because we considered that, in a context of overwhelmed hospital units during the COVID-19 peak incidence, all patients may not have received or been prescribed their usual medication regimens the first day of their hospital admission, or this treatment may not have been recorded at this time. We chose the second condition to ensure that BZRA exposure precedes the outcome or the end of the index hospitalisation.
Primary endpoint
The primary endpoint was the time from study baseline to death. Patients without an end-point event had their data censored on 1st May 2020.
Statistical analysis
We calculated frequencies of all baseline characteristics described above in patients receiving or not receiving BZRAs and compared them using standardised mean differences (SMDs). We considered SMDs >0.1 as reflecting substantial differences, a recommended threshold for declaring imbalance (Austin, 2009).
To examine the association between BZRA use and the endpoint of death, we performed Cox proportional-hazards regression models (Therneau and Grambsch, 2000). To help account for the non-randomised prescription of BZRAs and reduce the effects of confounding, the primary analysis used propensity score analysis with inverse probability weighting (IPW) (Robins et al., 2000; Geleris et al., 2020). The individual propensities for receiving any BZRA were estimated using a multivariable logistic regression model that included patient characteristics and other medications. In the IPW analyses, the predicted probabilities from the propensity-score models were used to calculate the stabilised IPW (Geleris et al., 2020). In the main analysis, the association between BZRA use and the endpoint was then estimated using an IPW Cox regression model. In the case of non-balanced covariates, an IPW multivariable Cox regression model adjusting for the non-balanced covariates was also performed. Kaplan–Meier curves were obtained using the IPW (Efron, 1981; Kassambara et al., 2020), and their pointwise 95% confidence intervals (CIs) were estimated using the non-parametric bootstrap method (Kassambara et al., 2020).
We conducted five sensitivity analyses to examine the robustness of the results from the main analysis. First, we performed a multivariable Cox regression model including as covariates the same variables as in the IPW analysis. Second, we used a univariate Cox regression model in a matched analytic sample using a 1 : 1 ratio, based on the same variables used for the IPW analysis and the multivariable Cox regression analysis. To reduce the effects of confounding, optimal matching was used to obtain the smallest average absolute distance across all clinical characteristics between the exposed patients and non-exposed matched controls. Third, to examine a potential indication bias of prescription of BZRAs in intensive care units (ICUs) as a possible treatment for palliative care or as an aid to oral intubation, we reproduced the main analyses after excluding all patients who had been hospitalised in ICUs. Fourth, we examined whether our findings were similar to models imputing missing data using multiple imputation (Stekhoven and Buehlmann, 2012) instead of excluding patients with any missing data as in the main analyses. Finally, to examine the potential influence of excluding patients who received a BZRA after 48 h from hospital admission while still accounting for a potential immortal time bias, we reproduced the main analysis while including all participants who received a BZRA at any time, and classifying BZRA use as a time-dependent variable (Therneau and Grambsch, 2000; Dekker et al., 2008). This analysis allows comparisons of the risk of occurrence of mortality between groups at each event time by re-evaluating study group based on whether participants had first received a BZRA by that time. Thus, patients enter the exposed group at the time of actual first initiation of the treatment. For example, a participant who was first prescribed a BZRA at 72 h from hospital admission is considered as non-exposed from baseline until 72 h, and as exposed from 72 h until the end of the study. For all analyses, a weighted Cox regression model was used when proportional hazards assumption was not met (Dunkler et al., 2018).
Finally, we performed additional exploratory analyses. First, we searched for a potential dose-dependent relationship by testing the association between the daily dose of BZRA received during the first day of prescription (converted into diazepam-equivalent dose (Ashton, 2002) and dichotomised at the mean value) and the endpoint, adjusted for the same covariates used in the main analysis, among patients who received a BZRA at baseline. Second, we examined whether respiratory depression or other clinical markers of disease severity may at least partly explain this association by adjusting successively for these variables. Finally, we examined the relationships between each BZRA and mortality using the same statistical approach as described for the main analysis.
For all associations, we performed residual analyses to assess the fit of the data, checked assumptions, including proportional hazards assumption using proportional hazards tests and diagnostics based on weighted residuals (Grambsch and Therneau, 1994; Therneau and Grambsch, 2000), and examined the potential influence of outliers. To improve the quality of result reporting, we followed the recommendations of The Strengthening the Reporting of Observational Studies in Epidemiology Initiative. Because our main hypothesis focused on the association between BZRA use and mortality, and was tested in a single model in the main analysis, statistical significance was fixed at two-sided p-value <0.05. As described above, we planned to perform additional exploratory analyses only if a significant association was found in the main analysis. All analyses were conducted in R software version 2.4.3 (R Project for Statistical Computing).
Results
Characteristics of the cohort
Of the 17 131 patients with a positive COVID-19 RT-PCR test who had been hospitalised for COVID-19, 1963 patients (11.5%) were excluded because of missing data or their young age (i.e. <18 years old). In addition, of the 1473 patients who received a BZRA at any time during the visit, 787 were excluded because they were prescribed it more than 48 h after hospital admission. Of the remaining 14 381 adult patients, 686 (4.8%) received a BZRA in the first 48 h of hospitalisation at a mean diazepam-equivalent dose of 19.7 mg per day (standard deviation (s.d.) = 25.4, median = 10.0, lower quartile = 5.0, higher quartile = 22.9, range = 1.85–240.0 mg) (online Supplementary Fig. S1). Of these 686 patients, 41.1% (N = 282) had a prescription of BZRA during a prior hospitalisation at AP-HP in the past 6 months, and 36.4% (N = 183) received at least two BZRA medications. The median delay from hospital admission to first prescription of BZRA was 0.94 days (s.d. = 0.55).
Over a mean follow-up of 14.5 days (median = 7 days; interquartile range (IQR) = 24; s.d. = 18.1), 1320 patients (9.2%) had an end-point event at the time of data cutoff on 1st May. Among patients who received a BZRA, the mean follow-up was 12.9 days (IQR = 13; s.d. = 13.1; median = 8 days), while it was of 14.5 days (IQR = 24; s.d. = 18.3; median = 7 days) in those who did not.
Associations between baseline characteristics and the endpoint are shown in online Supplementary Table S1. The distributions of patient characteristics according to BZRA use are shown in Table 1. In the full sample, BZRA use significantly differed according to all characteristics except for sex ratio, and the direction of the associations indicated older age and greater medical severity of patients receiving any BZRA. After applying the propensity score weights, these differences were substantially reduced. In the matched analytic sample, there were no significant differences in any characteristic (Table 1).
Table 1.
Characteristics of adult patients hospitalised for COVID-19 receiving or not receiving a BZRA at hospital admission (N = 14 381)
| Exposed to any BZRA (N = 686) | Not exposed to any BZRA (N = 13 695) | Non-exposed matched group (N = 686) | Exposed to any BZRA v. not exposed | Exposed to any BZRA v. not exposed | Exposed to any BZRA v. non-exposed matched group | |
|---|---|---|---|---|---|---|
| Crude analysis | Analysis weighted by IPW | Matched analytic sample analysis | ||||
| N (%) | N (%) | N (%) | SMD | SMD | SMD | |
| Age | 0.893 | 0.174 | 0.029 | |||
| 18–50 years | 70 (10.2%) | 5669 (41.4%) | 65 (9.4%) | |||
| 51–70 years | 192 (28.0%) | 4395 (32.1%) | 198 (28.9%) | |||
| More than 70 years | 424 (61.8%) | 3631 (26.5%) | 423 (61.7%) | |||
| Sex | 0.047 | 0.095 | 0.108 | |||
| Women | 348 (50.7%) | 7270 (53.1%) | 311 (45.3%) | |||
| Men | 338 (49.3%) | 6425 (46.9%) | 375 (54.7%) | |||
| Hospital | 0.387 | 0.050 | 0.063 | |||
| AP-HP Centre – Paris University, Henri Mondor University Hospitals and at home hospitalisation | 206 (30.0%) | 6585 (48.1%) | 212 (30.9%) | |||
| AP-HP Nord and Hôpitaux Universitaires Paris Seine-Saint-Denis | 222 (32.4%) | 3685 (26.9%) | 227 (33.1%) | |||
| AP-HP Paris Saclay University | 122 (17.8%) | 1575 (11.5%) | 106 (15.5%) | |||
| AP-HP Sorbonne University | 136 (19.8%) | 1850 (13.5%) | 141 (20.6%) | |||
| Obesitya | 0.186 | 0.074 | 0.079 | |||
| Yes | 135 (19.7%) | 1758 (12.8%) | 114 (16.6%) | |||
| No | 551 (80.3%) | 11 937 (87.2%) | 572 (83.4%) | |||
| Smokingb | 0.263 | 0.074 | 0.065 | |||
| Yes | 112 (16.3%) | 1072 (7.83%) | 96 (14.0%) | |||
| No | 574 (83.7%) | 12 623 (92.2%) | 590 (86.0%) | |||
| Any medical conditionc | 0.711 | 0.058 | 0.027 | |||
| Yes | 393 (57.3%) | 3336 (24.4%) | 402 (58.6%) | |||
| No | 293 (42.7%) | 10 359 (75.6%) | 284 (41.4%) | |||
| Medication according to compassionate use or as part of a clinical triald | 0.371 | 0.103 | 0.017 | |||
| Yes | 170 (24.8%) | 1483 (10.8%) | 165 (24.1%) | |||
| No | 516 (75.2%) | 12 212 (89.2%) | 521 (75.9%) | |||
| Any current psychiatric disordere | 0.582 | 0.052 | 0.007 | |||
| Yes | 154 (22.4%) | 498 (3.6%) | 152 (22.2%) | |||
| No | 532 (77.6%) | 13 197 (96.4%) | 534 (77.8%) | |||
| Any antidepressant | 1.045 | 0.185 | 0.075 | |||
| Yes | 285 (41.5%) | 411 (3.0%) | 260 (37.9%) | |||
| No | 401 (58.5%) | 13 284 (97.0%) | 426 (62.1%) | |||
| Any mood stabiliser medicationf | 0.617 | 0.404 | 0.011 | |||
| Yes | 143 (20.8%) | 284 (2.1%) | 140 (20.4%) | |||
| No | 543 (79.2%) | 13 411 (97.9%) | 546 (79.6%) | |||
| Any antipsychotic medication | 0.714 | 0.305 | 0.014 | |||
| Yes | 163 (23.8%) | 198 (1.4%) | 159 (23.2%) | |||
| No | 523 (76.2%) | 13 497 (98.6%) | 527 (76.8%) |
SMD, standardised mean difference.
Defined as having a body-mass index higher than 30 kg/m2 or an ICD-10 diagnosis code for obesity (E66.0, E66.1, E66.2, E66.8, E66.9).
Current smoking status was self-reported.
Assessed using ICD-10 diagnosis codes for diabetes mellitus (E11), diseases of the circulatory system (I00–I99), diseases of the respiratory system (J00–J99), neoplasms (C00-D49), diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism (D5–D8), frontotemporal dementia (G31.0), peptic ulcer (K27), diseases of liver (K70–K95), hemiplegia or paraplegia (G81–G82), acute kidney failure or chronic kidney disease (N17–N19) and HIV (B20).
Any medication prescribed as part of a clinical trial or according to compassionate use (e.g. hydroxychloroquine, azithromycin, remdesivir, tocilizumab, sarilumab or dexamethasone).
Assessed using ICD-10 diagnosis codes (F00–F99).
Included lithium and antiepileptic medications with mood stabilising properties.
SMD > 0.1 indicates substantial difference.
Study endpoint
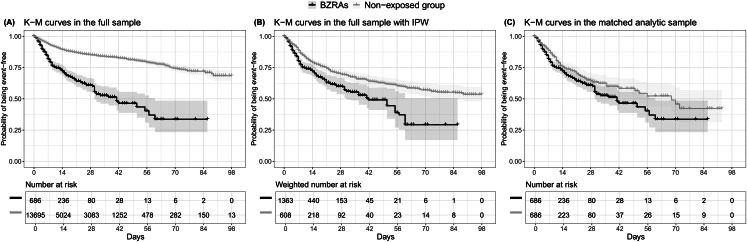
The endpoint of death occurred in 186 patients (27.1%) who received a BZRA at baseline and 1134 patients (8.3%) who did not. The crude, unadjusted analysis (hazard ratio (HR) = 3.20; 95% CI = 2.74–3.74; p < 0.001), the primary analysis with IPW (HR = 1.61; 95% CI = 1.31–1.98; p < 0.001) and the multivariable IPW Cox regression adjusting for unbalanced covariates (HR = 1.56; 95% CI = 1.29–1.89; p < 0.001) showed a significant association between BZRA use and increased mortality (Fig. 1; Table 2).
Fig. 1.
Kaplan–Meier curves for mortality in the full sample crude analysis (N = 14 381) (A), in the full sample analysis with IPW (N = 14 381) (B), and in the matched analytic sample using a 1 : 1 ratio (N = 1372) (C), according to BZRA use at baseline, among adult patients hospitalised for COVID-19. The shaded areas represent pointwise 95% CIs. Numbers at risk in panel B are weighted.
Table 2.
Association between BZRA use at baseline and mortality among adult patients hospitalised for COVID-19
| Number of events/number of patients | Crude Cox regression analysis | Multivariable Cox regression analysis | Analysis weighted by IPW | Analysis weighted by IPW adjusted for unbalanced covariatesa | Number of events/number of patients in the matched groups | Univariate Cox regression in a 1 : 1 ratio matched analytic sample | Cox regression in a 1 : 1 ratio matched analytic sample adjusted for unbalanced covariatesb | |
|---|---|---|---|---|---|---|---|---|
| N/N (%) | HR (95% CI; p-value) | HR (95% CI; p-value) | HR (95% CI; p-value) | HR (95% CI; p-value) | N/N (%) | HR (95% CI; p-value) | HR (95% CI; p-value) | |
| No BZRA | 1134/13 695 (8.3%) | Ref. | Ref. | Ref. | Ref. | 143/686 (20.8%) | Ref. | Ref. |
| Any BZRA | 186/686 (27.1%) | 3.20 (2.74–3.74; <0.001*) | 1.94 (1.45–2.59; <0.001*) | 1.61 (1.31–1.98; <0.001*) | 1.56 (1.29–1.89; <0.001*) | 186/686 (27.1%) | 1.34 (1.08–1.67; 0.009*) | 1.36 (1.09–1.69; 0.006*) |
BZRA, benzodiazepine receptor agonist; HR, hazard ratio; CI, confidence interval.
*Two-sided p-value is significant (p < 0.05).
Adjusted for age, medication according to compassionate use or as part of a clinical trial, any mood stabiliser medication and any antipsychotic medication.
Adjusted for sex.
In sensitivity analyses, the multivariable Cox regression model in the full sample also indicated a significant association (HR = 1.94; 95% CI = 1.45–2.59; p < 0.001), as did the univariate Cox regression model in a matched analytic sample using a 1 : 1 ratio (HR = 1.34; 95% CI = 1.08–1.67; p = 0.009) (Table 2). Similarly, the primary analysis using imputed data yielded significant results (online Supplementary Table S2), as did that considering BZRA use as a time-dependent variable and including all patients who received a BZRA at any time during the visit (online Supplementary Table S3). The exclusion from the analyses of the patients who had been admitted to ICUs did not alter the significance of the association (online Supplementary Table S4).
Additional analyses showed a significant dose-dependent relationship between baseline daily BZRA dose and the endpoint (HR = 1.55; 95% CI = 1.08–2.22; p = 0.017), based on the primary IPW analysis. Additional adjustments for respiratory depression, any other clinical markers of disease severity, or both, resulted in still significant associations, which were of similar magnitude to that observed in the primary analysis (online Supplementary Table S5). We found that all individual BZRAs were significantly associated with an increased mortality, except for diazepam (online Supplementary Table S6). Following adjustments, there were significant associations of any BZRA other than diazepam and midazolam with an increased mortality, as compared to not receiving BZRAs (online Supplementary Table S6). Finally, compared with any other BZRA treatment, diazepam use was significantly associated with a reduced mortality in the crude analysis (HR = 0.44; 95% CI = 0.23–0.86; p = 0.017), in the primary analysis (HR = 0.31; 95% CI = 0.13–0.74; p = 0.008), and in the sensitivity analyses (online Supplementary Fig. S2; Tables S6–S8).
Discussion
In this multicentre retrospective observational study involving a large sample of patients hospitalised for COVID-19, we found that BZRA use was significantly and substantially associated with an increased mortality, independently of patient characteristics and other medications, and with a significant dose-dependent relationship. This association remained significant in multiple sensitivity analyses. Exploratory analyses suggested that most BZRAs could be associated with this risk in these patients, except for diazepam, which may be associated with a reduced mortality compared with any other BZRA treatment.
We found that BZRA use was significantly associated with an increased mortality among patients hospitalised for COVID-19. This association might be explained by several mechanisms. First, although the risk of respiratory depression associated with benzodiazepines in the general population is debated and was not found to play a substantial role in our study, it might be relevant among elderly patients with COVID-19 and in those with pre-existing comorbidities, such as chronic obstructive pulmonary disease (Ostuzzi et al., 2020). Second, benzodiazepines may be associated with an increased risk of secondary infections, and particularly pneumonia, in patients with COVID-19 (Sun et al., 2019). Finally, in patients with COVID-19 and known risk factors for delirium (e.g. old age, dementia and multiple comorbidities), BZRA use may favour the occurrence of this condition (Ostuzzi et al., 2020), which is associated with unfavourable COVID-19 disease prognosis (Chen et al., 2020). These results suggest the need to carefully reevaluate the indication of BZRAs and decrease in dose or taper these medications when possible in patients with COVID-19 (Hayhoe and Lee-Davey, 2018).
Exploratory analyses indicate that most BZRAs may be associated with an increased mortality among patients hospitalised for COVID-19, except for diazepam. This exception could be in line with preclinical findings. Prior studies have indicated a central role of acid sphingomyelinase/ceramide system in SARS-CoV-2 infections (Carpinteiro et al., 2020, 2021; Hoertel et al., 2021b; Kornhuber et al., 2021) and that the formation of ceramide-enriched membrane platforms mediates the entry of the virus into epithelial cells (Kornhuber et al., 2021). Furthermore, prior work indicated that plasma levels of ceramides are significantly and substantially associated with COVID-19 clinical severity and inflammation markers in patients with COVID-19 (Marín-Corral et al., 2021; Torretta et al., 2021). Finally, prior observational studies reported that taking a FIASMA medication upon hospital admission is associated with a reduced likelihood of intubation or death (Darquennes et al., 2021; Hoertel et al., 2021c, 2021h). Although most BZRAs do not influence cellular ceramide levels, diazepam has been shown to interact with the acyl coenzyme A binding protein, also known as ‘diazepam binding inhibitor’, a protein that contributes to ceramide synthesis (Guidotti et al., 1983; Ferreira et al., 2017). This interaction may result in a reduced activity of the ceramide synthase pathway and, finally, reduced cellular ceramide concentration, which might possibly explain the reduced adverse effects of diazepam on COVID-19 compared to other BZRAs. However, this result should be interpreted with caution and other studies are required to confirm this finding and this potential underlying mechanism.
Our study has several limitations. First, there are two possible major inherent biases in observational studies: unmeasured confounding and confounding by indication. We tried to minimise the effects of confounding in several different ways. First, we used an analysis with IPW to minimise the effects of confounding by indication (Robins et al., 2000; Geleris et al., 2020). Second, we performed multiple sensitivity analyses, which showed similar results. Finally, although some amount of unmeasured confounding may remain, our analyses adjusted for numerous potential confounders. Other limitations include missing data for some baseline characteristic variables (i.e. 11.5%), which might be explained by the overwhelming of all hospital units during the COVID-19 peak incidence, and different results might have been observed during a lower COVID-19 incidence period. However, imputation of missing data did not alter the significance of our results. Second, BZRA use was defined as receiving these medications within the first 48 h from hospital admission and before the end of the index hospitalisation or death. We used this delay because we considered that, in a context of overwhelmed hospital units during the COVID-19 peak incidence, all patients may not have received or been prescribed their usual medication regimens the first day of their hospital admission, or this treatment may not have been recorded at this time. This delay may have introduced an immortal time bias. However, this bias is likely to have biased the results towards the null hypothesis, leading to potentially underestimate the strength of the association, and similar results were found when considering BZRA use as a time-dependent variable. Third, although this study is part of a broader project to examine potential associations between the use of psychotropic medications and COVID-19-related mortality, deposited at the EDS website (https://eds.aphp.fr/recherches-en-cours), the specific protocol of the present study has not been deposited in a public domain. Therefore, no comparison can be done between the current analyses with a pre-specified protocol, and inflation of type I error might have occurred due to multiple testing across psychotropic medication classes and molecules. However, in these exploratory analyses, we are primarily interested in results to generate new hypotheses. In this context, it may be more important to explore leads that might ultimately turn out to be incorrect rather than miss potentially important findings (Rothman, 2014). Also, we performed several sensitivity analyses that yielded similar results. Fourth, inflation of type I error might have occurred in secondary analyses due to multiple testing. Fifth, our study cannot establish a causal relationship between BZRA use and increased mortality (Le Strat and Hoertel, 2011). Finally, despite the multicentre design, our results may not be generalisable to outpatients or other regions.
Our findings indicate that BZRA use may be associated with an increased mortality among patients hospitalised for COVID-19 and suggest a potential benefit of decreasing dose or tapering off gradually these medications when possible in these patients.
Acknowledgements
The authors acknowledge the EDS APHP COVID consortium integrating the APHP Health Data Warehouse team as well as all the APHP staff and volunteers who contributed to the implementation of the EDS-COVID database and operating solutions for this database. Collaborators of the EDS APHP COVID consortium: Pierre-Yves Ancel, Alain Bauchet, Nathanaël Beeker, Vincent Benoit, Mélodie Bernaux, Ali Bellamine, Romain Bey, Aurélie Bourmaud, Stéphane Breant, Anita Burgun, Fabrice Carrat, Charlotte Caucheteux, Julien Champ, Sylvie Cormont, Christel Daniel, Julien Dubiel, Catherine Ducloas, Loic Esteve, Marie Frank, Nicolas Garcelon, Alexandre Gramfort, Nicolas Griffon, Olivier Grisel, Martin Guilbaud, Claire Hassen-Khodja, François Hemery, Martin Hilka, Anne Sophie Jannot, Jerome Lambert, Richard Layese, Judith Leblanc, Léo Lebouter, Guillaume Lemaitre, Damien Leprovost, Ivan Lerner, Kankoe Levi Sallah, Aurélien Maire, Marie-France Mamzer, Patricia Martel, Arthur Mensch, Thomas Moreau, Antoine Neuraz, Nina Orlova, Nicolas Paris, Bastien Rance, Hélène Ravera, Antoine Rozes, Elisa Salamanca, Arnaud Sandrin, Patricia Serre, Xavier Tannier, Jean-Marc Treluyer, Damien Van Gysel, Gaël Varoquaux, Jill Jen Vie, Maxime Wack, Perceval Wajsburt, Demian Wassermann and Eric Zapletal.
Availability of data and materials
Data from the AP-HP Health Data Warehouse can be obtained upon request at https://eds.aphp.fr//.
Author contributions
Study protocol: NH, MS-R, FL. Conceptualisation: NH, MS-R, FL, NB. Data curation: MS-R, RV, NB, AN. Formal analysis: NH, MS-R, RV. Methodology: NH, MS-R. Writing – original draft: NH. Writing – review and editing: NH, MS-R, FL, RV, NB, AN, CL, GA, EG, JK, CB, MO, JMA, MA, CC, PM.
Financial support
This work did not receive any external funding.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S2045796021000743.
click here to view supplementary material
Conflict of interest
NH, MS-R, MA, EG, JK, AC and FL are inventors of a patent application related to methods of treating COVID-19, filled by Assistance Publique – Hôpitaux de Paris in France. Other authors declare no conflict of interest related to this work.
References
- Ashton CH (2002) Benzodiazepines: how they work and how to withdraw. The Ashton Manual, August.
- Austin PC (2009) Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Communications in Statistics-Simulation and Computation 38, 1228–1234. [Google Scholar]
- Belleville G (2010) Mortality hazard associated with anxiolytic and hypnotic drug use in the national population health survey. The Canadian Journal of Psychiatry 55, 558–567. [DOI] [PubMed] [Google Scholar]
- Carpinteiro A, Edwards MJ, Hoffmann M, Kochs G, Gripp B, Weigang S, Adams C, Carpinteiro E, Gulbins A, Keitsch S, Sehl C, Soddemann M, Wilker B, Kamler M, Bertsch T, Lang KS, Patel S, Wilson GC, Walter S, Hengel H, Pöhlmann S, Lang PA, Kornhuber J, Becker KA, Ahmad SA, Fassbender K and Gulbins E (2020) Pharmacological inhibition of acid sphingomyelinase prevents uptake of SARS-CoV-2 by epithelial cells. Cell Reports Medicine 1, 100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpinteiro A, Gripp B, Hoffmann M, Pöhlmann S, Hoertel N, Edwards MJ, Kamler M, Kornhuber J, Becker KA and Gulbins E (2021) Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells. Journal of Biological Chemistry 296, 100701. doi: 10.1016/j.jbc.2021.100701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J and Ning Q (2020) Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 368, m1091. doi: 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevance A, Gourion D, Hoertel N, Llorca P-M, Thomas P, Bocher R, Moro M-R, Laprévote V, Benyamina A, Fossati P, Masson M, Leaune E, Leboyer M and Gaillard R (2020) Ensuring mental health care during the SARS-CoV-2 epidemic in France: a narrative review. L'Encephale 46, 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darquennes G, Le Corre P, Le Moine O and Loas G (2021) Association between functional inhibitors of acid sphingomyelinase (FIASMAs) and reduced risk of death in COVID-19 patients: a retrospective cohort study. Pharmaceuticals 14, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker FW, de Mutsert R, van Dijk PC, Zoccali C and Jager KJ (2008) Survival analysis: time-dependent effects and time-varying risk factors. Kidney International 74, 994–997. [DOI] [PubMed] [Google Scholar]
- Devlin J, Chang M-W, Lee K and Toutanova K (2019) BERT: pre-training of deep bidirectional transformers for language understanding. arXiv. doi: 1810.04805
- Dunkler D, Ploner M, Schemper M and Heinze G (2018) Weighted Cox regression using the R package coxphw. Journal of Statistical Software 84, 1–26.30450020 [Google Scholar]
- Efron B (1981) Nonparametric standard errors and confidence intervals. Canadian Journal of Statistics 9, 139–158. [Google Scholar]
- Ferreira NS, Engelsby H, Neess D, Kelly SL, Volpert G, Merrill AH, Futerman AH and Færgeman NJ (2017) Regulation of very-long acyl chain ceramide synthesis by acyl-CoA-binding protein. Journal of Biological Chemistry 292, 7588–7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Labella A, Manson DK, Kubin C, Barr RG, Sobieszczyk ME and Schluger NW (2020) Observational study of hydroxychloroquine in hospitalized patients with COVID-19. New England Journal of Medicine 382, 2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, O'Meara MJ, Rezelj V, Guo JZ, Swaney DL, Tummino TA, Hüttenhain R, Kaake RM, Richards A, Tutuncuoglu B, Foussard H, Batra J, Haas K, Modak M, Kim M, Haas P, Polacco BJ, Braberg H, Fabius JM, Eckhardt M, Soucheray M, Bennett MJ, Cakir M, McGregor, MJ, Li Q, Meyer B, Roesch F, Vallet T, Mac Kain A, Miorin L, Moreno E, Naing Z, Zar Chi Z, Yuan P, Shiming S, Ying Z, Ziyang Z, Shen W, Kirby IT, Melnyk JE, Chorba JS, Lou K, Dai SA, Barrio-Hernandez I, Memon D, Hernandez-Armenta C, Lyu J, Mathy CJP, Perica T, Pilla KB, Ganesan SJ, Saltzberg DJ, Rakesh R, Xi Liu, Rosenthal SB, Calviello L, Venkataramanan S, Liboy-Lugo J, Lin Y, Huang X, Liu Y, Wankowicz SA, Bohn M, Safari M, Ugur FS, Koh C, Savar NS, Tran QD, Shengjuler D, Fletcher SJ, O'Neal MC, Cai Y, Chang JCJ, Broadhurst DJ, Klippsten S, Sharp PP, Wenzell NA, Kuzuoglu-Ozturk D, Wang H, Trenker R, Young JM, Cavero DA, Hiatt J, Roth TL, Rathore U, Subramanian A, Noack J, Hubert M, Stroud RM, Frankel AD, Rosenberg OS, Verba KA, Agard DA, Ott M, Emerman M, Jura N, von Zastrow M, Verdin E, Ashworth A, Schwartz O, d'Enfert C, Mukherjee S, Jacobson M, Malik HS, Fujimori DG, Ideker T, Craik CS, Floor SN, Fraser JS, Gross JD, Sali A, Roth BL, Ruggero D, Taunton J, Kortemme T, Beltrao P, Vignuzzi M, García-Sastre A, Shokat KM, Shoichet BK and Krogan NJ (2020) A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583, 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grambsch PM and Therneau TM (1994) Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81, 515–526. [Google Scholar]
- Guidotti A, Forchetti CM, Corda MG, Konkel D, Bennett CD and Costa E (1983) Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proceedings of the National Academy of Sciences 80, 3531–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut Conseil de la Santé Publique (2020) Statement on the management at home or in a care facility of suspected or confirmed Covid-19 patients. 8 April 2020. Available at https://www.hcsp.fr (accessed 5 May 2021).
- Hayhoe B and Lee-Davey J (2018) Tackling benzodiazepine misuse: the time to take decisive action has come. BMJ 362, k3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N, Blachier M, Blanco C, Olfson M, Massetti M, Limosin F and Leleu H (2020a) Facing the COVID-19 epidemic in NYC: a stochastic agent-based model of various intervention strategies. medRxiv. doi: 10.1101/2020.04.23.20076885 [DOI] [Google Scholar]
- Hoertel N, Blachier M, Blanco C, Olfson M, Massetti M, Sánchez-Rico M, Limosin F and Leleu H (2020b) A stochastic agent-based model of the SARS-CoV-2 epidemic in France. Nature Medicine 26, 1417–1421. [DOI] [PubMed] [Google Scholar]
- Hoertel N, Blachier M, Sánchez-Rico M, Limosin F and Leleu H (2021a) Impact of the timing and adherence to face mask use on the course of the COVID-19 epidemic in France. Journal of Travel Medicine 28, taab016. Published online 2021 Jan 28. doi: 10.1093/jtm/taab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N, Sánchez-Rico M, Cougoule C, Gulbins E, Kornhuber J, Carpinteiro A, Becker KA, Reiersen AM, Lenze EJ, Seftel D, Lemogne C and Limosin F (2021b) Repurposing antidepressants inhibiting the sphingomyelinase acid/ceramide system against COVID-19: current evidence and potential mechanisms. Molecular Psychiatry 26, 1417–1421. doi: 10.1038/s41380-021-01254-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N, Sánchez-Rico M, Gulbins E, Kornhuber J, Carpinteiro A, Abellán M, de la Muela P, Vernet R, Beeker N, Neuraz A, Delcuze A, Alvarado JM, Meneton P and Limosin F (2021c) Association between psychotropic medications functionally inhibiting acid sphingomyelinase and reduced risk of intubation or death among individuals with mental disorder and severe COVID-19: an observational study. MedRxiv. doi: 10.1101/2021.02.18.21251997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N, Sánchez-Rico M, Vernet R, Beeker N, Jannot A-S, Neuraz A, Salamanca E, Paris N, Daniel C, Gramfort A, Lemaitre G, Bernaux M, Bellamine A, Lemogne C, Airagnes G, Burgun A, Limosin F and On behalf of AP-HP/Universities/INSERM COVID-19 Research Collaboration and AP-HP COVID CDR Initiative (2021d) Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Molecular Psychiatry 26, 5199–5212. doi: 10.1038/s41380-021-01021-4 [DOI] [PubMed] [Google Scholar]
- Hoertel N, Sánchez-Rico M, Vernet R, Beeker N, Neuraz A, Alvarado JM, Daniel C, Paris N, Gramfort A, Lemaitre G, Salamanca E, Bernaux M, Bellamine A, Burgun A and Limosin F (2021e) Dexamethasone use and mortality in hospitalized patients with coronavirus disease 2019: a multicenter retrospective observational study. British Journal of Clinical Pharmacology 87, 3766–3775. doi: 10.1111/bcp.14784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N, Sánchez-Rico M, Vernet R, Jannot A-S, Neuraz A, Blanco C, Lemogne C, Airagnes G, Paris N, Daniel C, Gramfort A, Lemaitre G, Bernaux M, Bellamine A, Beeker N and Limosin F (2021f) Observational study of chlorpromazine in hospitalized patients with COVID-19. Clinical Drug Investigation 41, 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N, Sánchez-Rico M, Vernet R, Jannot A-S, Neuraz A, Blanco C, Lemogne C, Airagnes G, Paris N, Daniel C, Gramfort A, Lemaitre G, Bernaux M, Bellamine A, Beeker N, Limosin F and on behalf of the AP-HP/Universities/INSERM COVID-19 research collaboration and AP-HP COVID CDR Initiative (2021g) Observational study of haloperidol in hospitalized patients with COVID-19. PLoS ONE 16, e0247122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N, Sánchez-Rico M, Gulbins E, Kornhuber J, Carpinteiro A, Lenze AJ, Reiersen AM, Abellán M, de la Muela P, Vernet R, Blanco C, Cougoule C, Beeker N, Neuraz A, Gorwood P, Alvarado JM, Meneton P and Limosin F (2021h) Association between FIASMAs and reduced risk of intubation or death in individuals hospitalized for severe COVID-19: an observational multicenter study. Clinical Pharmacology & Therapeutics, cpt.2317. doi: 10.1002/cpt.2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur K, Price CP, Gray EL, Gulati RK, Maksimoski M, Racette SD, Schneider AL and Khanwalkar AR (2020) Factors associated With intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngology–Head and Neck Surgery 163, 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouffroy J, Feldman SF, Lerner I, Rance B, Neuraz A and Burgun A (2020) MedExt: combining expert knowledge and deep learning for medication extraction from French clinical texts. [DOI] [PMC free article] [PubMed]
- Joya FL, Kripke DF, Loving RT, Dawson A and Kline LE (2009) Meta-analyses of hypnotics and infections: eszopiclone, ramelteon, zaleplon, and zolpidem. Journal of Clinical Sleep Medicine: JCSM 5, 377–383. [PMC free article] [PubMed] [Google Scholar]
- Kassambara A, Kosinski M and Biecek P (2020) survminer: Drawing Survival Curves using ‘ggplot2’. Available at https://CRAN.R-project.org/package=survminer.
- Kojima M, Wakai K, Kawamura T, Tamakoshi A, Aoki R, Lin Y, Nakayama T, Horibe H, Aoki N and Ohno Y (2000) Sleep patterns and total mortality: a 12-year follow-up study in Japan. Journal of Epidemiology 10, 87–93. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Hoertel N and Gulbins E (2021) The acid sphingomyelinase/ceramide system in COVID-19. Molecular Psychiatry. doi: 10.1038/s41380-021-01309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Strat Y and Hoertel N (2011) Correlation is no causation: gymnasium proliferation and the risk of obesity. Addiction (Abingdon, England) 106, 1871–1872. [DOI] [PubMed] [Google Scholar]
- Mallon L, Broman J-E and Hetta J (2009) Is usage of hypnotics associated with mortality? Sleep medicine 10, 279–286. [DOI] [PubMed] [Google Scholar]
- Marín-Corral J, Rodríguez-Morató J, Gomez-Gomez A, Pascual-Guardia S, Muñoz-Bermúdez R, Salazar-Degracia A, Pérez-Terán P, Restrepo MI, Khymenets O, Haro N, Masclans JR and Pozo OJ (2021) Metabolic signatures associated with severity in hospitalized COVID-19 patients. International Journal of Molecular Sciences 22, 4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakafero G, Sanders RD, Nguyen-Van-Tam JS and Myles PR (2015) Association between benzodiazepine use and exacerbations and mortality in patients with asthma: a matched case-control and survival analysis using the United Kingdom Clinical Practice Research Datalink. Pharmacoepidemiology and Drug Safety 24, 793–802. [DOI] [PubMed] [Google Scholar]
- Obiora E, Hubbard R, Sanders RD and Myles PR (2013) The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax 68, 163–170. [DOI] [PubMed] [Google Scholar]
- Ostuzzi G, Papola D, Gastaldon C, Schoretsanitis G, Bertolini F, Amaddeo F, Cuomo A, Emsley R, Fagiolini A, Imperadore G, Kishimoto T, Michencigh G, Nosé M, Purgato M, Dursun S, Stubbs B, Taylor D, Thornicroft G, Ward PB, Hiemke C, Correll CU and Barbui C (2020) Safety of psychotropic medications in people with COVID-19: evidence review and practical recommendations. BMC Medicine 18, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmaro A, Dupouy J and Lapeyre-Mestre M (2015) Benzodiazepines and risk of death: results from two large cohort studies in France and UK. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology 25, 1566–1577. [DOI] [PubMed] [Google Scholar]
- Parsaik AK, Mascarenhas SS, Khosh-Chashm D, Hashmi A, John V, Okusaga O and Singh B (2016) Mortality associated with anxiolytic and hypnotic drugs – a systematic review and meta-analysis. The Australian and New Zealand Journal of Psychiatry 50, 520–533. [DOI] [PubMed] [Google Scholar]
- Patorno E, Glynn RJ, Levin R, Lee MP and Huybrechts KF (2017) Benzodiazepines and risk of all cause mortality in adults: cohort study. BMJ 358, j2941. doi: 10.1136/bmj.j2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B and Mannino DM (2005) Does insomnia kill? Sleep 28, 965–971. [DOI] [PubMed] [Google Scholar]
- Robins JM, Hernán MÁ and Brumback B (2000) Marginal structural models and causal inference in epidemiology. Epidemiology (Cambridge, Mass.) 11, 550–560. [DOI] [PubMed] [Google Scholar]
- Roth T (2009) Hypnotic use for insomnia management in chronic obstructive pulmonary disease. Sleep Medicine 10, 19–25. [DOI] [PubMed] [Google Scholar]
- Rothman KJ (2014) Six persistent research misconceptions. Journal of General Internal Medicine 29, 1060–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q, Yang K, Wang W, Jiang L and Song J (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine 46, 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumble R and Morgan K (1992) Hypnotics, sleep, and mortality in elderly people. Journal of the American Geriatrics Society 40, 787–791. [DOI] [PubMed] [Google Scholar]
- Sánchez-Rico M, Limosin F, Vernet R, Beeker N, Neuraz A, Blanco C, Olfson M, Lemogne C, Meneton P, Daniel C, Paris N, Gramfort A, Lemaitre G, De La Muela P, Salamanca E, Bernaux M, Bellamine A, Burgun A and Hoertel N, on Behalf Of Ap-Hp/Université de Paris/Inserm Covid-Research Collaboration/Ap-Hp Covid Cdr Initiative/Entrepôt de Données de Santé Ap-Hp Consortium (2021) Hydroxyzine use and mortality in patients hospitalized for COVID-19: A multicenter observational study. Journal of Clinical Medicine 10, 5891. doi: 10.3390/jcm10245891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stekhoven DJ and Buehlmann P (2012) MissForest – non-parametric missing value imputation for mixed-type data. Bioinformatics (Oxford, England) 28, 112–118. [DOI] [PubMed] [Google Scholar]
- Sun G, Zhang L, Zhang L, Wu Z and Hu D (2019) Benzodiazepines or related drugs and risk of pneumonia: a systematic review and meta-analysis. International Journal of Geriatric Psychiatry 34, 513–521. [DOI] [PubMed] [Google Scholar]
- Therneau TM and Grambsch PM (2000) Modeling Survival Data: Extending the Cox Model. Springer. New York. [Google Scholar]
- Torretta E, Garziano M, Poliseno M, Capitanio D, Biasin M, Santantonio TA, Clerici M, Lo Caputo S, Trabattoni D and Gelfi C (2021) Severity of COVID-19 patients predicted by serum sphingolipids signature. International Journal of Molecular Sciences 22, 10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration (2020) FDA requiring Boxed Warning updated to improve safe use of benzodiazepine drug class. 23 September 2020. Available at https://www.fda.gov/drugs/drug-safety-and-availability/fda-requiring-boxed-warning-updated-improve-safe-use-benzodiazepine-drug-class (accessed 27 December 2020).
- Weich S, Pearce HL, Croft P, Singh S, Crome I, Bashford J and Frisher M (2014) Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. BMJ 348, g1996. doi: 10.1136/bmj.g1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong A, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans S, Smeeth L and Goldacre B (2020) Factors associated with COVID-19-related death using OpenSAFELY. Nature 584, 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S2045796021000743.
click here to view supplementary material
Data Availability Statement
Data from the AP-HP Health Data Warehouse can be obtained upon request at https://eds.aphp.fr//.