Abstract
Diffuse intrinsic pontine glioma (DIPG) and other H3K27M-mutated diffuse midline gliomas (DMGs) are universally lethal paediatric tumours of the central nervous system1. We have previously shown that the disialoganglioside GD2 is highly expressed on H3K27M-mutated glioma cells and have demonstrated promising preclinical efficacy of GD2-directed chimeric antigen receptor (CAR) T cells2, providing the rationale for a first-in-human phase I clinical trial (NCT04196413). Because CAR T cell-induced brainstem inflammation can result in obstructive hydrocephalus, increased intracranial pressure and dangerous tissue shifts, neurocritical care precautions were incorporated. Here we present the clinical experience from the first four patients with H3K27M-mutated DIPG or spinal cord DMG treated with GD2-CAR T cells at dose level 1 (1 × 106 GD2-CAR T cells per kg administered intravenously). Patients who exhibited clinical benefit were eligible for subsequent GD2-CAR T cell infusions administered intracerebroventricularly3. Toxicity was largely related to the location of the tumour and was reversible with intensive supportive care. On-target, off-tumour toxicity was not observed. Three of four patients exhibited clinical and radiographic improvement. Pro-inflammatory cytokine levels were increased in the plasma and cerebrospinal fluid. Transcriptomic analyses of 65,598 single cells from CAR T cell products and cerebrospinal fluid elucidate heterogeneity in response between participants and administration routes. These early results underscore the promise of this therapeutic approach for patients with H3K27M-mutated DIPG or spinal cord DMG.
Subject terms: CNS cancer, Paediatric research
A phase I dose-escalation trial of GD2-CAR T cells in children and young adults with diffuse midline gliomas to assess the feasibility of manufacturing, safety and tolerability, and to preliminarily assess efficacy.
Main
This phase I dose-escalation trial of autologous GD2-CAR T cells (containing a GD2 binding domain, a 4-1BB co-stimulatory domain and a CD3Ζ signalling domain) in children and young adults with pontine and spinal cord DMG characterized by a K27M mutation in genes encoding histone H3 (H3K27M) was designed with the primary objectives of assessing feasibility of manufacturing, safety and tolerability, and identifying the maximally tolerated dose or recommended phase II dose (Fig. 1a, b). Assessment of clinical activity was a secondary objective and identifying biomarkers of response was an exploratory objective. We anticipated the development of neurological symptoms related to CAR T cell-mediated inflammation in sites of central nervous system (CNS) disease2, which we have termed tumour inflammation-associated neurotoxicity (TIAN). To mitigate risks associated with TIAN, we excluded patients with bulky thalamic or cerebellar tumours, required placement of an Ommaya reservoir in patients with DIPG to monitor intracranial pressure (ICP), and instituted a TIAN toxicity management algorithm incorporating the removal of cerebrospinal fluid (CSF) via Ommaya, hypertonic saline, anti-cytokine agents and corticosteroids.
Fig. 1. Trial design and patients 1 and 2 with DIPG.
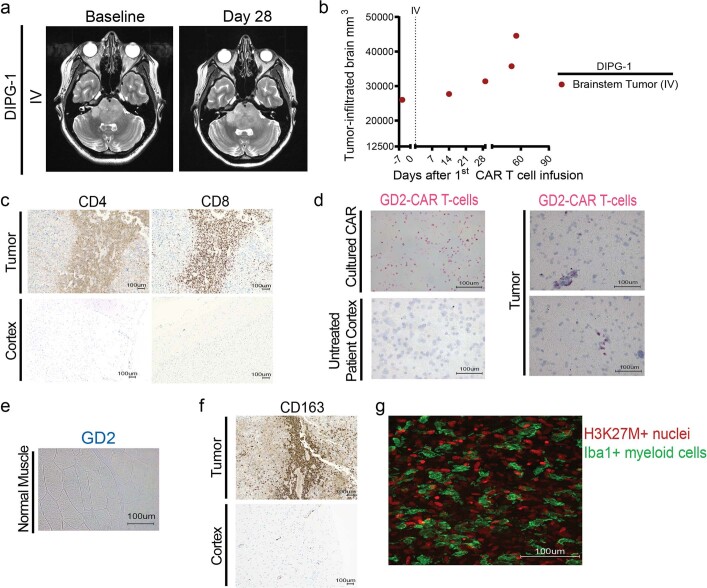
a, GD2–4-1BB–CD3Ζ CAR schematic. TM, transmembrane domain. b, Outline of clinical trial design. D0, day 0; LD, lymphodepleting chemotherapy. c, Post-mortem examination of participant 1 with DIPG (DIPG-1). From left to right: haemotoxylin and eosin staining; CD3 immunohistochemistry (brown); GD2-CAR mRNA puncta (pink; haematoxylin counterstain for all cells in blue); and GD2 antigen (blue) immunohistochemistry (H3K27M+ nuclei in brown). d, qPCR for GD2-CAR DNA from autopsy samples from DIPG-1 exhibit the presence of GD2-CAR in tumour-involved midbrain and pons, but not her uninvolved cortex or brain tissue from an untreated individual (control). Resected temporal lobe tumour from patient 1 with spinal DMG (spinal DMG-1) following i.v. infusion reveals the presence of GD2-CAR DNA. Data represent mean ± s.e.m., n = 3 technical replicates for each sample. e, MRI scan (axial T2, shown at the level of the lower pons) of participant 2 with DIPG (DIPG-2) before and 4 weeks after i.v. infusion (top row). An MRI scan before and 2 weeks following i.c.v. infusion is also shown (bottom row). A reduction in the tumour size is observed after each infusion (red arrows). f, Photographs of DIPG-2 demonstrate significant improvement in left facial strength 2 weeks after i.c.v. infusion. Photographs were obtained and published with informed consent. Schematics were created with BioRender.com.
Dose level 1
Beginning June 2020, four participants were enrolled on dose level 1 (DL1; 1 × 106 GD2-CAR T cells per kg administered intravenously; three participants with DIPG and one participant with spinal cord DMG; 5–25 years of age; one male and three female; Supplementary Table 1); all participants were more than 6 months from completion of standard radiotherapy. GD2-CAR T cells were successfully manufactured and met release criteria for all four patients (Supplementary Table 2). During cell manufacturing, patient 1 with spinal DMG experienced rapid tumour progression and was removed from the study but was treated at DL1 on a single-patient compassionate emergency investigational new drug application (eIND). Results are reported here with a data cut-off of March 2021.
Patient 1 with DIPG was a 14-year-old girl with H3K27M+ DIPG, with early radiographic signs of tumour progression, right sixth nerve palsy, left facial weakness, dysarthria, bilateral dysmetria and wide-based gait at the time of treatment. On day +6 following treatment with GD2-CAR T cells intravenously (i.v.), she experienced grade 1 cytokine release syndrome (CRS; 40.1 °C fever) together with worsening cranial nerve symptoms consistent with TIAN and was treated with tocilizumab (an IL-6 antagonist) and corticosteroids (Supplementary Table 3). On day +9, she experienced an acute episode of fever, hypertension, decreased responsiveness, hemiplegia and extensor posturing. Her Ommaya was immediately accessed; her ICP was elevated at 22 mmHg, 10 ml CSF was drained, and she returned to her neurological baseline within minutes. Anakinra (an IL-1R antagonist) was started. MRI demonstrated increased pontine oedema. She remained on corticosteroids, with transient improvement in her baseline dysarthria and dysmetria. One month post-infusion, increased disease (approximately 20% enlargement) was evident on MRI, followed by tumour progression resulting in death 3 months after infusion of GD2-CAR T cells, 3 months after radiographic progression was first noted and 13 months after diagnosis (Extended Data Fig. 1a, b). Post-mortem brain examination demonstrated substantial infiltration of lymphocytes in the tumour that is uncharacteristic for DIPG4; lymphocytic infiltration was not observed in areas of the normal brain (Fig 1c, Extended Data Fig. 1c). RNAscope identified cells expressing GD2-CAR mRNA transcript in the tumour, but not the unaffected cortex (Fig. 1c, Extended Data Fig. 1d). Similarly, the GD2-CAR transgene was detected by quantitative PCR (qPCR) of DNA from tumour tissue (Fig. 1d). GD2-antigen expression was substantially higher in the tumour than in the normal brain tissue (Fig. 1c, Extended Data Fig. 1e). Tumour microglial and other myeloid cell infiltration was prominent (Extended Data Fig. 1f, g).
Extended Data Fig. 1. Additional correlative findings and imaging for DIPG-1.
a, MRI images (axial T2) of participant 1 with DIPG (DIPG-1) prior to and Day+28 following GD2-CART infusion showed no improvement. b, Tumour volume change over time in DIPG-1. c, Post-mortem pons tumour tissue of DIPG-1 shows evidence of CD4+ and CD8+ T-cells by immunohistochemistry (brown). Unaffected cortex CD4+ staining depicts rare leptomeningeal vascular CD4+ cells and serves as an internal positive control. d, RNAscope probe against the GD2-CAR construct mRNA identifies GD2-CAR T cells (GD2-CAR mRNA puncta, pink). Positive control GD2-CAR T cells in tissue culture and negative control cortex from a DIPG patient not treated with CAR T cells were used to validate the RNAscope probe. GD2-CAR mRNA expression detected by RNAscope identified GD2-CAR T cells in tumour tissue from DIPG-1. GD2-CAR mRNA puncta = pink, hematoxylin counterstain for all cells = blue. e, Normal human muscle tissue immunostained for GD2 as negative control for GD2 antigen immunohistochemistry. f, CD163+ myeloid cells (brown) in tumor tissue of DIPG-1 by immunohistochemistry. Unaffected cortex CD163 immunostaining demonstrates microglial cells in their "resting" perivascular location. CD163+ reactive microglia/macrophages were not evident in the normal cortex of DIPG-1. g, Confocal microscopy of DIPG-1 tumour tissue obtained at autopsy demonstrates significant myeloid cells (Iba1+ cells, green) infiltrating the tumor (H3K27M+ cells, red). Scale bars = 100 micrometers.
Patient 2 with DIPG was a 21-year-old man with H3K27M+ DIPG exhibiting early signs of clinical and radiographic progression at the time of enrolment, with left sixth nerve palsy, left facial weakness, decreased left facial sensation, trismus with difficulty opening his mouth that limited large bites of food, right lower extremity sensory loss and wide-based gait with inability to tandem walk. On day +7 following i.v. GD2-CAR T cell administration, he developed grade 2 CRS (fever to 39.4 °C and hypotension responsive to fluids) and transient worsening of baseline deficits consistent with TIAN affecting pontine function, including transiently worsened trismus limiting oral intake to eating through a straw, transient right upper extremity sensory loss and mild left hearing loss lasting 2 days. MRI demonstrated increased T2/FLAIR signal in the left trigeminal nucleus (Extended Data Fig. 2a), which controls the muscles of mastication, correlating with the symptom of trismus. He was treated with tocilizumab and anakinra, but no corticosteroids (Supplementary Table 3). By the second week, he experienced marked improvement in trismus (near-normal mouth opening and no limitations to eating), improvement in facial symmetry, resolution of baseline sensory loss and improvement in gait with the ability to tandem walk (Supplementary Table 4). By 1 month following treatment with GD2-CAR T cells, he exhibited a near-normal neurological examination, with only a residual sixth nerve palsy. MRI demonstrated areas of improved T2/FLAIR signal and mildly decreased (approximately 17% smaller) tumour volume (Fig. 1e, Extended Data Fig. 2b).
Extended Data Fig. 2. Additional MRI findings.
a, Participant 2 with DIPG (DIPG-2) MRI images (axial T2) at the level of the mid-pons demonstrate T2 signal abnormality in the trigeminal nucleus (red arrow) that worsened (increased T2 signal, consistent with pseudoprogression) at Day+7 and progressively decreased on the Day+14 and Day+21 scans. Together with this T2 signal change, trigeminal function (muscles of mastication and left facial sensation) worsened around Day+7 and then progressively improved clinically. The size of the intra-tumoral cyst near the trigeminal nucleus decreased in size after treatment (red arrowhead) and the size and shape of the fourth ventricle normalized. b, Tumor volume change over time in DIPG-2. c, MRIs (axial T2) of cerebellum from participant 3 with DIPG (DIPG-3) shows increased disease in cerebellum over time, despite GD2-CAR T cell treatments. Disease in the cerebellar peduncle was relatively stable at 1 month post-IV infusion, but by the time of ICV infusion (new ICV baseline, bottom row) the cerebellar peduncle and cerebellar disease had increased, consistent with tumour progression at this site. d, Patient 1 with spinal cord DMG (Spinal DMG-1) MRI brain (axial T2) prior to and 21 days after ICV GD2-CAR T cell administration. Note the extensive spread of tumour throughout brain, which reduced following ICV treatment (for example, see arrows at the left inferior frontal lobe highlighting decreased infiltrative disease at Day 21).
Clinical and radiographic improvement persisted until 2–3 months, when he experienced recrudescence of previous symptoms, including return of trismus, facial weakness and sensory loss, left hearing loss, increased weakness and sensory loss affecting the right side of his body, and gait instability that required wheelchair use for any distance beyond a few steps. He received a second dose of 30 × 106 GD2-CAR T cells administered intracerebroventricularly (i.c.v.) via Ommaya reservoir without lymphodepletion. He developed a fever (40 °C) within 24 h and approximately 48 h post-infusion developed acutely increased somnolence and new right third nerve palsy. His Ommaya reservoir was accessed, demonstrating elevated ICP of 34 mmHg; CSF was removed with immediate clinical improvement. Neuroimaging demonstrated obstructive hydrocephalus due to compression of the fourth ventricle by the expanded pons. The Ommaya was left accessed for continuous CSF drainage. Hypertonic saline, anakinra and systemic corticosteroids were administered (Supplementary Table 3). Hydrocephalus resolved without need for continued drainage of CSF after 2 days. Corticosteroids were discontinued after 4 days (on day +6). Two weeks post-i.c.v. infusion, his neurological examination had markedly improved, with near-normalization of facial strength (Fig. 1f) and sensation, improved trismus, hearing, right-sided weakness and dramatic improvement in gait that enabled independent walking for long distances (Supplementary Videos 1, 2, Supplementary Table 4). An MRI scan 2 weeks post-i.c.v. GD2-CAR T cell infusion demonstrated 27% reduction in tumour volume compared to his pre-i.c.v. infusion MRI (Fig. 1e, Extended Data Fig. 2b).
After the data cut-off of March 2021, he went on to receive three more i.c.v. infusions (five infusions total). Before the planned sixth infusion, he died due to an intratumoural haemorrhage in a known area of intratumoural vascular anomaly. Such intratumoural haemorrhages are relatively common in DIPG and risk increases with time from diagnosis5. He survived 26 months from diagnosis, 12 months after he began to exhibit radiographic and clinical progression, and 10 months after his first GD2-CAR T cell infusion.
Patient 3 with DIPG was a 5-year-old girl with H3K27M+ DIPG who enrolled before progression. She did not exhibit brainstem enlargement, only areas of infiltrative tumour evident as patchy abnormal T2/FLAIR signal in the left cerebral peduncle (corticospinal tract motor fibres) of the midbrain (Fig. 2a), anterior pons, medulla and left cerebellar peduncle. At baseline, she exhibited bilateral facial weakness, hypophonic voice, rightward tongue deviation, difficulty controlling oral secretions (drooling), chronic nausea, hypertonic right hemiparesis that limited use of the right arm and hand (her right hand was held in a flexed position; Fig. 2b), dysmetria and a hemiparetic, wide-based gait. On day 7 after i.v. administration of GD2-CAR T cells, she developed grade 1 CRS (fever of 40.4 °C) and transiently increased ataxia, consistent with TIAN. She was treated with tocilizumab and anakinra, but no corticosteroids (Supplementary Table 3). By 2 weeks post-infusion, her chronic nausea (present since diagnosis) had resolved, her right-sided motor function improved and she was using her right hand spontaneously in play, with improved ability to extend her fingers (Fig. 2b). By 1 month post-infusion, she was controlling oral secretions without drooling, taking bigger and stronger bites of food, speaking with a louder voice and showing improved facial strength that enabled a wide smile. These clinical improvements were accompanied by decreased T2/FLAIR MRI signal abnormality in the midbrain (Fig. 2a, c). By 6 weeks after i.v. GD2-CAR T cells, her balance, coordination and use of her right leg had improved, enabling her to ride a scooter using her right leg (Supplementary Table 4). With resolution of chronic nausea, her food intake improved; she increased from the sixth percentile to the thirtieth percentile for weight and grew 2 inches in height in the 2 months following i.v. GD2-CAR T cells.
Fig. 2. Patient 3 with DIPG and patient 1 with spinal DMG.
a, MRI images (axial T2) of participant 3 with DIPG (DIPG-3) showed a decrease in abnormal T2 signal (tumour) in the left cerebral peduncle corticospinal tract motor fibres (red arrows) 4 weeks following i.v. infusion that remained stably improved (MRI 2 weeks following i.c.v. infusion is also shown). b, The right hand of DIPG-3 at baseline, which exhibited poor strength, increased tone and was held in chronic flexion. Recovery of right-hand motor function was observed by 2 weeks after i.v. treatment, with increased movement and improved tone. c, Midbrain (left cerebral peduncle) tumour volume change over time in DIPG-3. d, Tumour volume change in the pons and medulla, and the middle cerebellar peduncles (MCP) and cerebellum over time in DIPG-3. e, Sagittal MRI images of patient 1 with spinal cord DMG (spinal DMG-1) show a decrease in the tumour (outlined in red) following i.v. treatment (blue arrow) and i.c.v. re-treatment (yellow arrow). f, Change over time in spinal cord tumour volume in spinal DMG-1. Grey shading indicates time period following i.c.v. infusion (c,d,f). g, Despite significant tumour reduction in f, a temporal lobe tumour (red arrow) in spinal DMG-1 did not respond (axial T2 MRI images). h, GD2 expression in the resected temporal lobe tumour from spinal DMG-1 was high and uniform by flow cytometry as compared to a fluorescence-minus-one control.
She was re-treated with 12.9 × 106 GD2-CAR T cells i.c.v. (equivalent to 1 × 106 per kg) without lymphodepletion 3 months after her i.v. infusion, when improvement in her clinical examination had plateaued and increased cerebellar peduncle disease was evident (Fig. 2d, Extended Data Fig. 2c). She experienced only mild headache. No anti-cytokine agents or steroids were used. Her clinical response was mixed. She demonstrated additional improvement in right-sided motor function (improved right ankle dorsiflexion and wrist extension, and further increased use of right hand) within 1 week of her second GD2-CAR T cell infusion (Supplementary Table 4). By contrast, her left-sided ataxia progressively worsened consistent with increased left cerebellar peduncle disease (Fig. 2d, Extended Data Fig. 2c).
After the data cut-off of March 2021, this participant received one more i.c.v. infusion but her tumour continued to progress. She died from tumour progression 20 months after diagnosis, 4 months after she had begun to exhibit tumour progression, and 7 months after her first GD2-CAR T cell infusion.
Patient 1 with spinal DMG was a 25-year-old woman with an H3K27M+ spinal cord DMG centred at thoracic levels T10–T12 who exhibited early signs of clinical and radiographic progression at the time of enrolment. After trial enrolment and during cell manufacturing, she experienced rapid tumour progression resulting in near-complete paraparesis, severe neuropathic pain, sensory loss below the T10 level, bladder dysfunction requiring urinary catheterization, and tumour spread to her temporal lobe. An urgent duraplasty was performed to relieve pressure in the spinal canal due to the rapidly expanding, tumour-infiltrated cord and to mitigate her severe neuropathic pain. Pain was moderately improved after duraplasty, but she still required a lidocaine drip and opiates for pain management. She no longer met criteria for GD2-CAR T cell infusion on-study due to rapid tumour progression with neurological deterioration and corticosteroid use before GD2-CAR T cell infusion and was removed from the protocol. She received GD2-CAR T cells (1 × 106 per kg) administered i.v. on an eIND. Beginning day +6, she exhibited grade 3 CRS (fever to 39.6 °C and hypotension requiring vasopressor support), which was treated with tocilizumab and corticosteroids for 3 days (days +8–11; Supplementary Table 3). Despite the trajectory of her rapid tumour progression before GD2-CAR T cell treatment, imaging demonstrated more than 90% reduction in volume of the spinal cord tumour by day +32 (Fig. 2e, f) accompanied by improved lower extremity motor and urinary function, resolution of neuropathic pain and discontinuation of all pain medications by 2 months post-infusion (Supplementary Table 4). By contrast, her brain metastasis did not exhibit improvement (Fig. 2g).
Both brain and spinal cord disease progressed by day +75 (Fig. 2e, f). The tumour had spread extensively throughout her brain (Extended Data Fig. 2d), and the bulkiest component in the medial temporal lobe was resected. Flow cytometry of the resected tissue demonstrated robust tumour cell GD2 expression (Fig. 2h) but few infiltrating T cells. Low levels of the GD2-CAR transgene were detected by qPCR in the resected tumour of the temporal lobe (Fig. 1d). With limited therapeutic options, she was re-treated with 50 × 106 GD2-CAR T cells administered i.c.v. following increased lymphodepletion, given the concern for immune rejection of the CAR T cells. The chosen dose for CAR T cells was within the range of cell doses administered i.c.v. on other brain tumour trials6,7. She was the first patient chronologically to receive GD2-CAR T cells i.c.v., and her course informed the i.c.v. strategy described above. Within 48 h, she developed persistent fevers (up to 39.9 °C) and grade 3 encephalopathy (grade 4 immune effector cell-associated neurotoxicity (ICANS)), associated with no MRI changes in the normal brain and diffuse slowing with triphasic waves on continuous EEG monitoring, consistent with a reversible toxic/metabolic/inflammatory encephalopathy (Extended Data Fig. 3). Given the extensive tumour invasion of brain structures including the thalami, hypothalamus, mammillary bodies, bilateral insula and bilateral frontal lobes, we were unable to distinguish between ICANS and TIAN as the primary cause for her encephalopathy. She was treated with anti-inflammatory therapy (anakinra, siltuximab (an IL-6 antagonist) and corticosteroids (i.v. and i.c.v.)) and oral dasatinib to dampen the activity of CAR T cells8 (Supplementary Table 3). Within 4 days, the encephalopathy resolved. Steroids were weaned and discontinued by day +24. Regression was again observed in her spinal tumour (more than 80% reduction over 3 weeks; Fig. 2e, f) as well as select areas of her brain disease (Extended Data Fig. 2d). Clinical improvement in spinal cord function was not observed after the second infusion as it was after the first (Supplementary Table 4). Both spinal and brain disease progressed further by 2 months following i.c.v. infusion and she received no further GD2-CAR T cell infusions. After the data cut-off of March 2021, she survived 20 months from diagnosis, 14 months from the beginning of tumour progression and 11 months after her first GD2-CAR T cell infusion.
Extended Data Fig. 3. Spinal DMG-1 EEG during episode of encephalopathy.
Electroencephalogram (EEG) demonstrates diffuse slowing with triphasic waves on Day 2-3 following ICV GD2-CAR T cell administration in Spinal DMG-1. Consistent with this EEG pattern that is often observed with reversible toxic/metabolic/inflammatory encephalopathy, mental status returned to baseline over the course of days.
Correlative findings
Serum and CSF samples were obtained routinely and at times of clinical intervention to assess markers of inflammation and correlates of response. Patient 1 with spinal DMG had CSF samples collected following only i.c.v. administration but not following i.v. administration, and limited CSF samples were obtained from patient 3 with DIPG due to her young age.
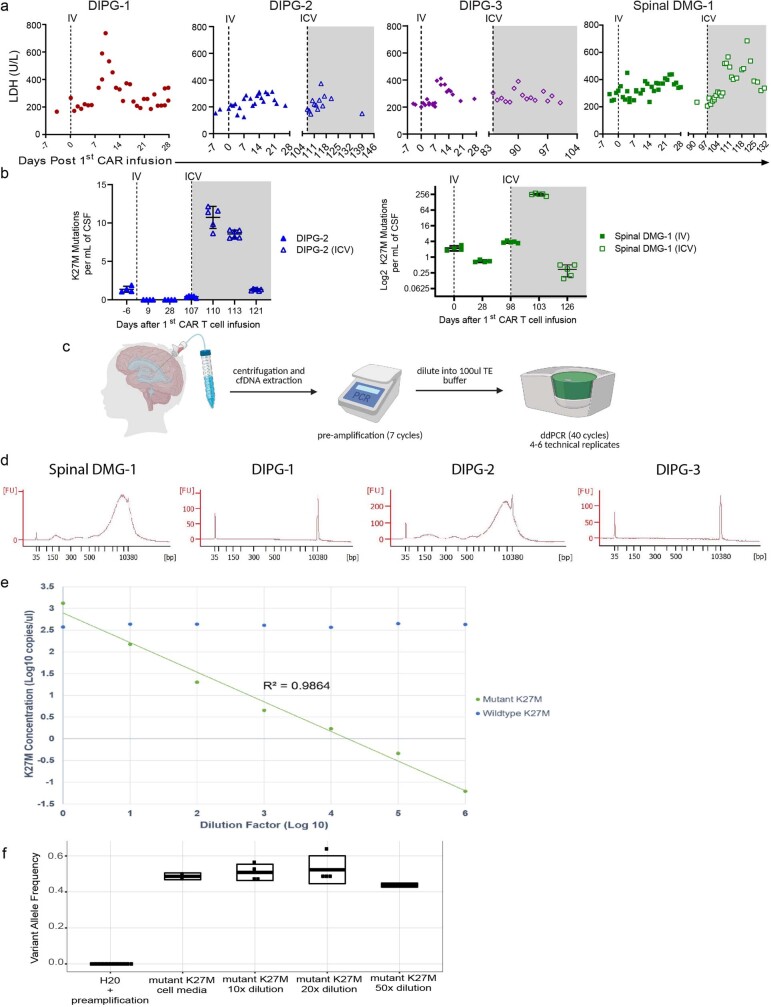
Correlating with tumour response, serum levels of lactate dehydrogenase increased in all patients after GD2-CAR T cell treatment and tracked with evidence of inflammation (Extended Data Fig. 4a). Using digital droplet PCR for the tumour-specific H3K27M mutation, cell-free tumour DNA was detected in CSF in two of four patients (Extended Data Fig. 4b–f). CSF cell-free tumour DNA was elevated during peak inflammation post-i.c.v. treatment, a pattern expected with tumour cell killing9. Additional sampling may further elucidate the kinetics of tumour cell killing in future studies.
Extended Data Fig. 4. Peripheral blood LDH kinetics, CSF cell-free tumor DNA (ctDNA) findings, and ctDNA validation.
a, Elevated Lactate Dehydrogenase (LDH) levels approximately 7–14 days following GD2-CAR T cell infusions (either IV or ICV) in N = 4 patients. Filled dot=IV, open dot=ICV. b, Cell-free tumor DNA (ctDNA) from patient CSF was evaluated using digital droplet PCR for the H3K27M mutation in the H3F3A gene. N=at least 4 technical replicates represented as Log2 of mutations per mL CSF ± SEM. Each point equals one technical replicate at the indicated timepoint; Filled dot=IV, open dot=ICV. DIPG-2 and Spinal DMG-1 demonstrated increasing cfDNA levels directly following GD2-CAR T cell ICV infusion (p < 0.0001 calculated by t-test). All available CSF samples from patients with adequate DNA extraction are represented here. DIPG-1 and DIPG-3 exhibited below the limit of detection levels and are not shown (limit of detection = 1 mutation per mL). c, Workflow for cell-free tumor DNA (ctDNA) assay. Cell-free DNA (cfDNA) was extracted from 1–4ml of CSF and subjected to 7 cycles pre-amplification followed by 40 cycles of digital droplet PCR (ddPCR). Schematic created with BioRender.com. d, Representative bioanalyzer traces of patients demonstrate adequate cfDNA quantities in Spinal DMG-1 and DIPG-2 without detectable cfDNA in DIPG-1 and DIPG-3 (i.e. there was not enough total cell-free DNA to run the assay). e, Validation of ddPCR assay through serial dilution of mutant H3K27M gBlock (IDT) against a fixed background of wild type H3K27M gBlock demonstrates linear mutant H3K27M detection. Sample concentrations (copies/ul) and dilution factors are plotted at Log10 scale. The QuantaSoft Pro Software calculates the starting concentration of each target DNA molecule by modeling as a Poisson distribution; the formula used for Poisson modeling is: Copies per droplet = -ln(1-p) where p = fraction of positive droplets. f, Inclusion of negative and positive controls for each ddPCR assay was performed alongside patient samples. Pre-amplified water served as a negative control for every run. Conditioned medium from H3K27M-mutated DIPG cell cultures was used as a positive control. Serial dilutions of H3K27M-mutated DIPG cell culture medium demonstrate a reproducible variant allele frequency (VAF) of the positive control.
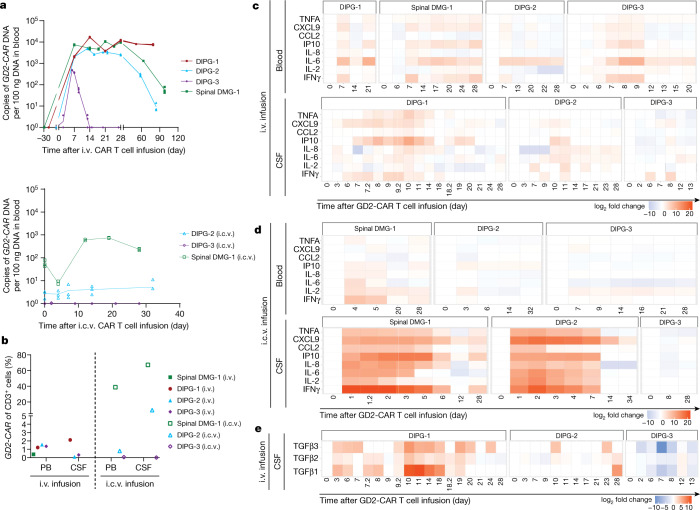
GD2-CAR T cell expansion and persistence was monitored using GD2-CAR transgene qPCR and flow cytometry of cell-surface CAR expression. GD2-CAR T cell expansion in blood assessed by qPCR was similar in magnitude to expansion of highly active CAR T cells for haematological malignancies10–12 (Fig. 3a). Flow cytometry-based GD2-CAR T cell detection in blood was limited, potentially due to decreased surface expression of GD2-CAR after activation13. GD2-CAR T cells were detected in CSF by flow cytometry, particularly following i.c.v. administration (Fig. 3b, Extended Data Fig. 6b).
Fig. 3. CAR T cell kinetics and cytokine production.
a, qPCR for GD2-CARDNA illustrates kinetics of CAR T cell expansion and persistence in the peripheral blood following i.v. (top) and i.c.v. (bottom) administration. Each point represents one technical replicate; n = 3 technical replicates per timepoint per patient, 4 patients (i.v.), 3 patients (i.c.v.). b, Flow cytometry of GD2-CAR demonstrated significantly higher proportion of CAR T cells in the CSF following i.c.v. GD2-CAR T cell infusion than following i.v. infusion at peak inflammation timepoints. Each dot represents one patient. n = 4 patients (i.v.), 3 patients (i.c.v.). c, d, Pro-inflammatory cytokines in the blood and CSF after i.v. infusion (c), n = 4 patients, and after i.c.v. infusion (d), n = 3 patients. Note that the expression of IFNγ in the CSF of spinal DMG-1 on D1–3 after i.c.v. infusion was above the upper limit of detection of the assay. e, Levels of the immunosuppressive cytokines TGFβ1, TGFβ2 and TGFβ3 in the CSF after i.v. infusion. n = 3 patients. Heatmaps in c–e were generated from Luminex multiplex cytokine analysis of patient blood plasma and CSF in technical duplicates. Average pg ml−1 results are represented by log2 fold change from D0 timepoint.
Extended Data Fig. 6. Single cell RNA-sequencing of GD2-CAR T cells in CSF and from manufactured product.
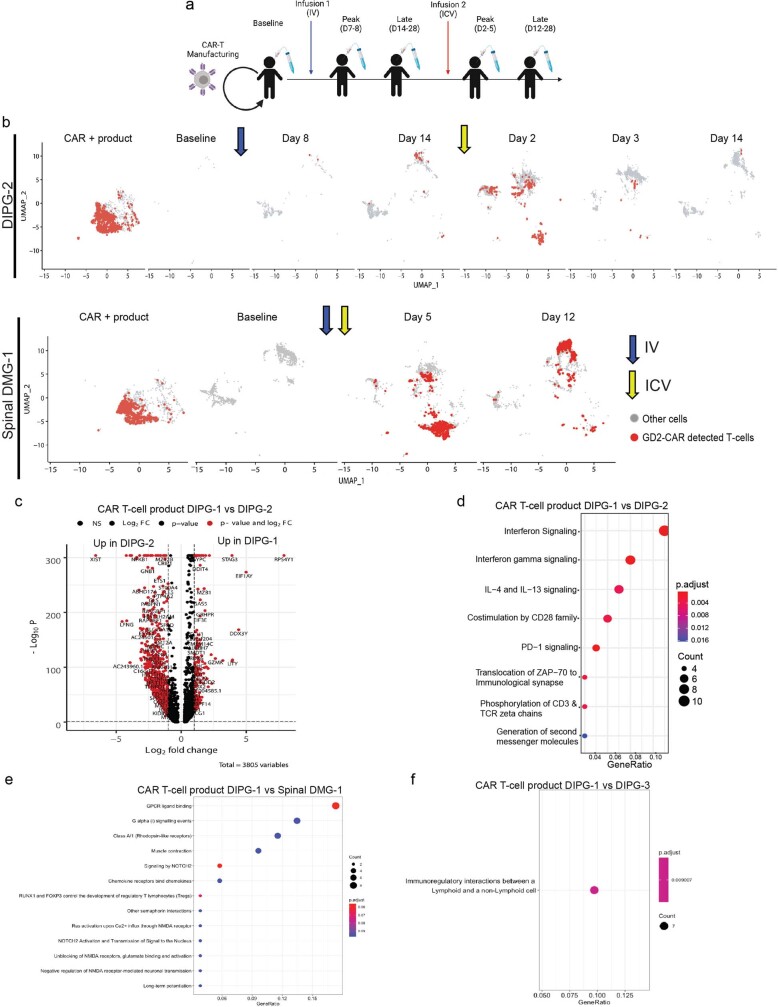
a, Schematic of single cell RNA sequencing (scRNA-seq) via 10X Genomics platform, conducted on sorted GD2-CAR+ and CAR- infusion products, as well as CSF samples at indicated timepoints throughout treatment. 65,598 single cells were sequenced, including 20,000 single cells from the CAR+ fraction of patient products and 25,598 cells obtained from patient CSF (shown in Fig. 4). Schematic created with BioRender.com. b, GD2-CAR+ T cells were identified in post-treatment CSF samples of DIPG-2 and Spinal DMG-1 following GD2-CAR T cell administration. T cells with detected GD2-CAR expression are represented as red dots. A single UMAP was generated with flow-sorted GD2-CAR T cell product and cells from the CSF then individually visually represented based on day of the sample. Note alterations in GD2-CAR T cell profile over time. CSF studies were not obtained for Spinal DMG-1 following IV infusion. CSF studies were also limited in DIPG-3: Following ICV GD2-CAR T cell administration, DIPG-3 did not require CSF drainage during the period of peak inflammation and given her young age, elective CSF collection was more limited. Therefore, the only post-ICV timepoint for CSF collection from DIPG-3 was Day 14, at which point there were not enough cells in CSF to conduct scRNAseq. Blue arrow=IV infusion, Yellow arrow=ICV infusion. c, Volcano plot representing CAR T cell product of DIPG-1 compared to DIPG-2. d–f, Gene ontology term analysis of the most significantly enriched pathways in DIPG-1CAR+ product relative to DIPG-2 (d), Spinal DMG-1 (e), and DIPG-3 (f) CAR+ product prior to CAR T cell administration. The y-axis shows the enhanced gene ontology terms, while the x-axis (“GeneRatio”) corresponds to the overlap between the up-regulated genes and genes associated with the given gene ontology terms in the dataset. Color of the circle corresponds to the p-value significance of the pathway enrichment, relative to all other genes in the dataset. Size of the circle corresponds to the relative number of matching up-regulated genes in the given gene ontology term.
In blood, cytokines implicated in CRS, including IL-6, were higher following i.v. than i.c.v. administration, consistent with the higher grade CRS observed after i.v. administration. In CSF, the levels of pro-inflammatory cytokines, including IFNγ, TNF, IL-2 and IL-6, were higher following i.c.v. administration than following i.v. administration (Fig. 3c, d, Extended Data Fig. 5a–c). Patient 1 with DIPG exhibited increased levels of immunosuppressive cytokines, including TGFβ, in the CSF (Fig. 4e, Extended Data Fig. 5d) and prominent myeloid populations in tumour tissue (Extended Data Fig. 1f, g), which may correlate with her lack of response. Cytokine analyses confirmed that anakinra (modified recombinant IL-1RA) crossed the blood–brain barrier, with detectable levels of IL-1RA found in both the blood and the CSF following i.v. administration of anakinra (Extended Data Fig. 5e).
Extended Data Fig. 5. Absolute values of pro-inflammatory and suppressive cytokines in pg/mL and anakinra levels measured by IL1-Ra in CSF and serum.
a, Time-course of anti-inflammatory agents (tocilizumab, anakinra and corticosteroids) administered to patients following CAR T cell infusions represented graphically to provide context for interpreting cytokine levels at various timepoints. b–d, The same data in absolute values that is represented in heatmap form in Fig. 4. Each data point represents the pg/ml value of the indicated cytokine at the timepoint following GD2-CAR T cell infusion. b, Pro-inflammatory cytokine levels in blood and cerebrospinal fluid (CSF) following intravenous (IV) administration. N = 4 patients (blood) and 3 patients (CSF). c, Pro-inflammatory cytokine levels in blood and cerebrospinal fluid (CSF) following intra-cerebroventricular (ICV) administration. N = 3 patients. d, Immune-suppressive TGFβ cytokine levels in the CSF following IV CAR T cell administration. N = 3 patients. e, Peak serum and CSF IL1-Ra levels during anakinra treatment. Anakinra is recombinant IL1-Ra. CSF levels of anakinra, which can cross the blood-brain barrier, were approximately one tenth those of serum levels. Data generated by Luminex multi-plex cytokine analysis of patient blood plasma and CSF samples. N = 3 patients. b–e, Each data point represents the mean of two technical replicates. Red=DIPG-1, Blue=DIPG-2, Purple= DIPG-3, Green=Spinal DMG-1. Filled dot=IV, open dot=ICV.
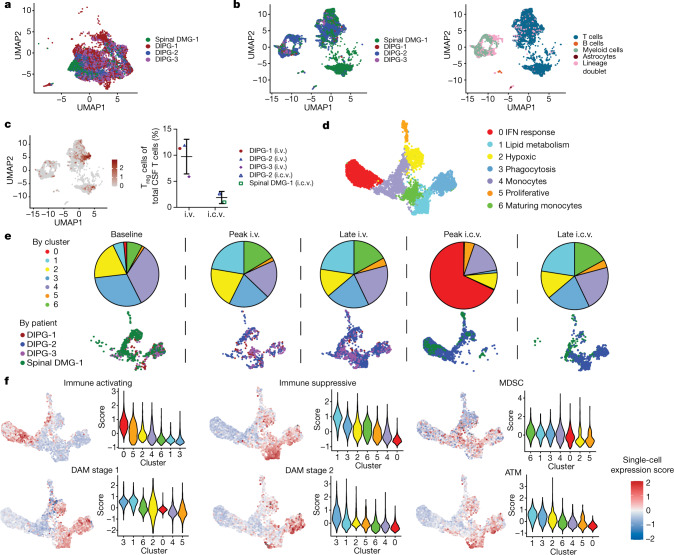
Fig. 4. Single-cell transcriptomic analyses identifies distinct myeloid subpopulations.
a, UMAP representation identifies cellular populations within GD2-CAR+ flow-sorted T cell products. n = 20,000 single cells (5,000 GD2-CAR+ cells from each of the 4 patient products). b, UMAP representation identifies cellular populations within CSF samples from patients. CSF cells by patient (left) and CSF cells by cell type (right) are shown. n = 25,598 single cells. c, UMAP highlighting FOXP3+ regulatory T (Treg) cells (left). Treg cell population (defined by CD4, FOXP3 and CD25 expression) identified in CSF samples at peak inflammation timepoints following i.v. or i.c.v. administration (right). n = 523 Treg cells were analysed from a total of 17,699 CSF T cells from 3 participants after i.v. and 2 participants after i.c.v. administration. With a Bayesian model-based single-cell compositional data analysis (scCODA) framework, the log fold change was 0.6, the inclusion probability was 0.75 and the false discovery rate was less than 0.05. d, CSF sample single-cell RNA sequencing was filtered to isolate myeloid cells. Clustering was conducted after data integration by Harmony. n = 6,497 myeloid cells. e, Pie charts represent myeloid cluster proportions at different timepoints. A single UMAP was generated with myeloid cells from the CSF, and then individually visually represented based on timepoint of the sample, coloured by patient. Note the alterations in the presence of myeloid clusters over time. f, Expression signature of ‘immune activating’, ‘immune suppressive’, ‘disease-associated myeloid (DAM) stage 1’, ‘DAM stage 2’, ‘myeloid-derived suppressor cell (MDSC)’ and ‘axon tract-associated microglial (ATM)’ cell states and associated representation within clusters based on single-cell expression scores (Z score).
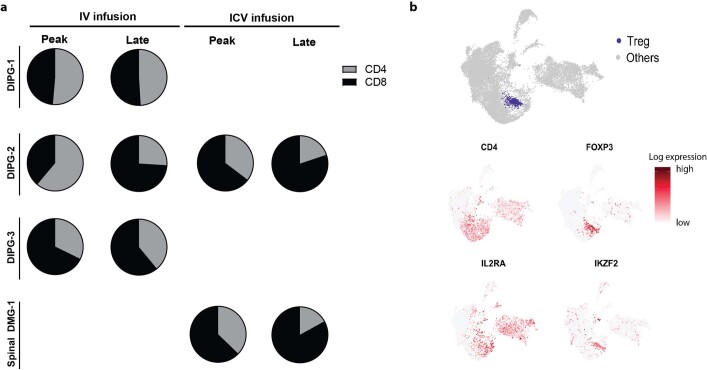
Single-cell RNA sequencing was performed on cells isolated from CSF (Extended Data Fig. 6a) as well as flow-sorted GD2-CAR-positive and GD2-CAR-negative T cells from the manufactured products (Fig. 4a, b). We analysed 65,598 single cells: 25,598 cells from CSF and 40,000 cells from manufactured products. The cellular composition of CSF included lymphocytes, myeloid cells, rare astrocytes and B cells (Fig. 4b). We observed a population of lineage doublets, raising the possibility of phagocytosis of T cells by myeloid cells (Fig. 4b). We observed more GD2-CAR T cells in CSF at times of peak inflammation, and more CSF GD2-CAR T cells after i.c.v. versus i.v. administration for patient 2 with DIPG, the only patient in whom this comparison was possible (Extended Data Fig. 6b). By single-cell RNA sequencing, we did not identify GD2-CAR T cells in the CSF of patients 1 and 3 with DIPG. Comparing manufactured cell products for patient 1 with DIPG, who did not have a response, to patient 2 with DIPG, who exhibited radiographic and clinical benefit, revealed numerous transcriptional differences. These included baseline interferon and PD1 signalling activation pathways in patient 1 with DIPG, potentially indicating increased tonic signalling associated with reduced GD2-CAR T cell activity14 (Extended Data Fig. 6c, d). Comparisons of other products are shown in Extended Data Fig. 6e, f. Assessing all lymphocytes from the CSF of all participants revealed that the T cell CD4:CD8 ratios in CSF differed over time between patients (Extended Data Fig. 7a). Increased numbers of regulatory T (Treg) cells were found in patient CSF after i.v. GD2-CAR T cell administration compared to i.c.v. administration (Fig. 4c, Extended Data Fig. 7b).
Extended Data Fig. 7. CD4:CD8 Ratios of T cells throughout treatment and gene ontology term analysis of CAR T cell products prior to CAR T cell treatment.
a, Pie charts represent CSF T cell CD4:CD8 ratios from patients at different timepoints following CAR T cell administration. b, Top panel: CSF T cell UMAP projections highlighting Tregs in scRNA-seq data. The Treg cell cluster is highlighted in purple, while all other T cells are colored grey. Bottom panel: UMAP projections highlighting Tregs in scRNA-seq data by expressions of canonical marker genes of Tregs (CD4, FOXP3, CD25, and IKZF2).
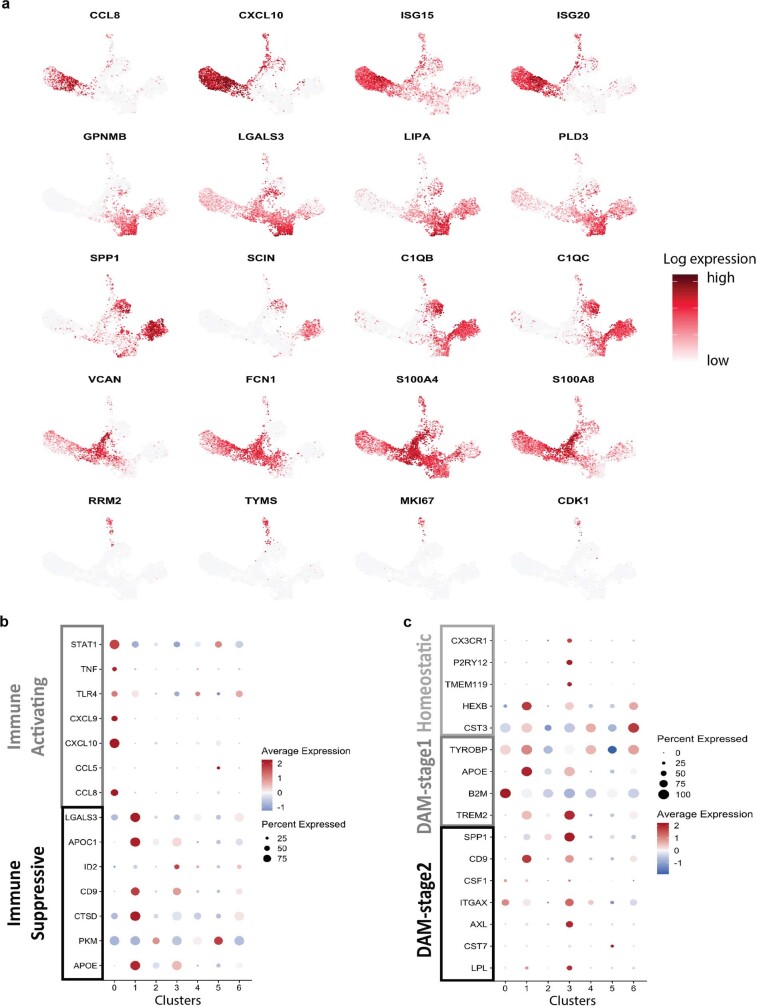
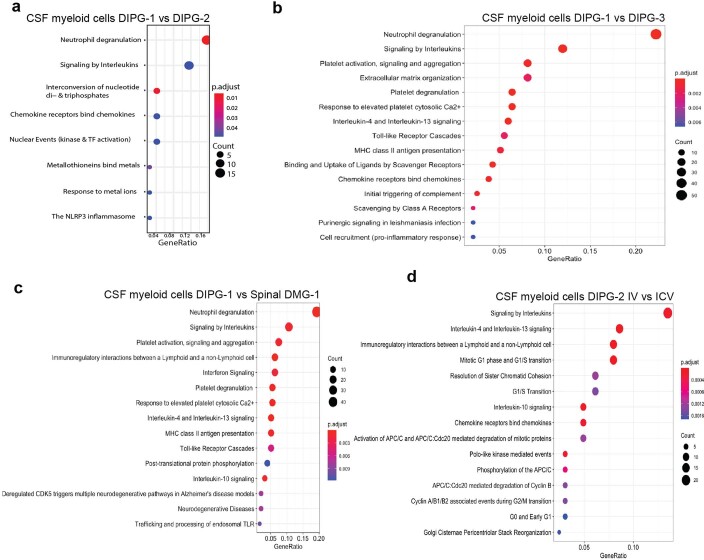
Examination of all CSF myeloid cells from all patients after each administration route revealed seven distinct clusters of cells, including monocytes, microglia and macrophages expressing various functional signatures, and proliferating myeloid cells (Fig. 4d, Extended Data Fig. 8a, Supplementary Table 5). We identified a myeloid population characterized by interferon response, present primarily at peak inflammation timepoints after i.c.v. administration (Fig. 4d, e). This cluster exhibited a distinctly immune-activating signature (Fig. 4f, Extended Data Fig. 8b), concordant with the pro-inflammatory CSF cytokine profile observed after i.c.v. administration (Fig. 3c, d). By contrast, i.v. administration and late timepoints had CSF myeloid cell subpopulations that expressed prominent phagocytosis and lipid metabolism gene programmes. These cells also exhibited a strong immune-suppressive profile, which aligned with transcriptional signatures of disease-associated microglia15, myeloid-derived suppressor cell and axon tract-associated microglia16 cell states (Fig. 4e, f, Extended Data Fig. 8). Comparison of the CSF myeloid cell fraction in patient 1 with DIPG to other patients at the time of peak inflammation highlighted differentially increased interleukin, chemokine and neutrophil degranulation processes (Extended Data Fig. 9a–c). Similarly, comparison of the CSF myeloid cell fraction in patient 2 with DIPG at peak inflammation following i.v. versus i.c.v. administration revealed increased myeloid activation processes following i.v. administration (Extended Data Fig. 9d).
Extended Data Fig. 8. Expression of cell cluster-specific marker genes and myeloid signature genes.
a, CSF myeloid cell UMAP projections highlighting myeloid cell clusters in scRNA-seq data by top differentially expressed genes of each cell cluster. b, c, Expression of myeloid transcriptional signature genes (y-axis) of identified myeloid cell clusters (x-axis). Dot sizes represent the percentage of cells expressing the gene in the given cluster. Color scale shows scaled average expression. Genes of immune activating and suppressive (b), homeostatic, and DAM stage 1 and 2 signatures (c) are plotted.
Extended Data Fig. 9. Gene ontology term analysis of myeloid cells from CSF following GD2-CAR T cell treatment.
a–c, Gene ontology term analysis demonstrates the most significantly activated pathways in the myeloid fraction of DIPG-1 compared to DIPG-2 (a), DIPG-3 (b), and Spinal DMG-1 (c). d, Gene ontology term analysis demonstrates the most significantly enriched pathways in the myeloid fraction of DIPG-2 CSF sample on Day 8 of IV CAR T administration relative to the myeloid fraction of DIPG-2 CSF sample on Day 2 of ICV CAR T cell administration (time points of peak inflammation following IV or ICV administration for this patient). The y-axis shows the enhanced gene ontology terms, while the x-axis (“GeneRatio”) corresponds to the overlap between the up-regulated genes and genes associated with the given gene ontology term in the dataset. Color of the circle corresponds to the p-value significance of the pathway enrichment, relative to all other genes in the dataset. Size of the circle corresponds to the relative number of matching up-regulated genes in the given gene ontology term. Only myeloid cells in CSF are included in this analysis.
Together, these correlative studies provide further evidence of GD2-CAR T cell activity against H3K27M+ DIPG and spinal cord DMG and begin to elucidate the heterogeneity observed in antitumour responses.
Discussion
H3K27M+ DIPG and spinal cord DMGs are extremely aggressive, universally fatal tumours with few therapeutic options. The average life expectancy is 10 months from diagnosis, and the 5-year survival is less than 1%17. Palliative radiotherapy is the only established treatment, and neither cytotoxic nor targeted pharmacological approaches have demonstrated improved prognosis to date18. Implementation of immunotherapy as treatment for tumours in these precarious neuroanatomical locations is both promising and dangerous. This phase I study was carefully designed to manage potential consequences of inflammation-induced swelling of an already expanded brainstem. The toxicities associated with GD2-CAR T cell infusions were manageable and reversible with intensive supportive care in the inpatient setting. CRS and ICANS were similar to that described with other CAR T cell therapies19,20, but patients also developed signs and symptoms consistent with CAR T cell-mediated inflammation in sites of CNS disease, which we have termed TIAN. TIAN most often manifested as transient worsening of existing deficits but also resulted in episodes of increased ICP secondary to brainstem oedema and consequent obstructive hydrocephalus, which would have been life-threatening unless immediately and appropriately managed. Our toxicity management algorithm incorporated several pre-emptive measures that enabled safe delivery of this potent therapy. As increasingly efficacious CAR T cell and other immunotherapies are deployed for CNS tumours, TIAN is likely to emerge as an important axis of toxicity in neuro-immuno-oncology.
Two categories of TIAN were evident in these patients: the first category of TIAN relates to ICP and intracranial space constraints secondary to inflammation-induced tissue oedema and/or obstruction of CSF flow. This can require urgent and/or emergent management utilizing principles of neurocritical care. The second category of TIAN relates to primary dysfunction of brain or spinal cord structures affected by inflammation, is typically transient, frequently manifests as worsening of pre-existing symptoms and can often be managed conservatively unless the neurological dysfunction involves critical functions such as respiratory drive.
Although patients developed symptoms of on-tumour neurotoxicity, they did not manifest any signs or symptoms of on-target, off-tumour toxicity. GD2 is expressed on normal neural tissues, including the brain and peripheral nerves, and treatment with the US Food and Drug Administration-approved anti-GD2 antibody dinutuximab for neuroblastoma is associated with transient painful neuropathy in most children21,22. None of these four patients developed painful neuropathies or any other clinical or radiographic indication of on-target, off-tumour toxicity. This study adds to the growing evidence that GD2 can be safely targeted with CAR T cells23–26, and is consistent with evidence that CAR T cells require high antigen density for full effector function10,13,27,28. Indeed, autopsy tissue from patient 1 with DIPG confirmed robustly differential GD2 expression levels and T cell infiltration between the tumour and the uninvolved brain.
Although the clinical experience reported here is early and the number of participants treated to date is small, three of four patients derived radiographic and clinical benefit after i.v. administration of GD2-CAR T cells. The improvement observed in neurological function highlights the extent to which this diffusely infiltrating tumour chiefly integrates with and disrupts—rather than destroys—neural circuits29, underscoring the potential that a tumour cell-specific therapy offers for functional recovery. i.c.v. administration of a second dose of GD2-CAR T cells provided additional radiographic and/or clinical benefit in three of three patients treated. In comparison to i.v. infusions, i.c.v. administrations were associated with less systemic toxicity such as CRS, and correlated with enhanced levels of pro-inflammatory cytokines and reduced immunosuppressive cell populations in CSF. The promising early experience with GD2-CAR T cells for DIPG and spinal cord DMG described here sets the stage for further optimization of this approach for this historically lethal CNS cancer. This clinical trial will continue to treat patients with H3K27M+ DIPG and spinal DMG using GD2-CAR T cell therapy to determine optimal dose, route and schedule, and to determine efficacy.
Methods
Clinical trial design
This phase I dose-escalation trial of GD2-CAR T cells in children and young adults with pontine and spinal H3K27M+ DMG uses a 3 + 3 design with the primary objectives of assessing feasibility of manufacturing, safety and tolerability, and identifying the maximally tolerated dose or recommended phase II dose. Assessment of clinical activity is the secondary objective and identifying correlative biomarkers of response is an exploratory objective. Patients were eligible for enrolment if they had a pathologically confirmed diagnosis of H3K27M-mutated DIPG or spinal cord DMG, had completed standard radiotherapy and were not receiving corticosteroid therapy (additional eligibility and exclusion criteria below).
Toxicity management
In preclinical studies, a fraction of the mice exhibited brainstem oedema as a result of on-tumour inflammation that led to obstructive hydrocephalus due to the neuroanatomical location of these tumours2. We anticipated the development of neurological symptoms related to CAR T cell-mediated inflammation in sites of CNS disease, which we have termed TIAN. To mitigate the risks associated with TIAN, we excluded patients with bulky thalamic or cerebellar tumours, required placement of an Ommaya reservoir in patients with DIPG, conducted both routine and symptom-prompted ICP measurements and instituted a toxicity management plan that included TIAN management (CSF removal via Ommaya, hypertonic saline and/or corticosteroids) and CRS management using anti-cytokine agents (tocilizumab, an IL-6R antagonist, and anakinra, an IL-1R antagonist) and corticosteroids. In addition, the CAR construct incorporated an iCasp9-inducible suicide switch, which could be activated with rimiducid in the event of life-threatening toxicity that is not manageable with the approaches and agents discussed above30.
Oversight and informed consent
The clinical study and all amendments were approved by the Stanford University Institutional Review Board. An external data and safety monitoring board (DSMB) reviewed the protocol, amendments and all important patient events and outcomes. Before enrolment in the clinical trial, patients and/or their parents provided written, informed consent, and minor assent was obtained when appropriate. In addition, patients and/or their parents provided written, informed consent for use of photographs and videos that may be used in scientific presentations and publications in print and electronic formats.
CAR T cell manufacturing
Autologous peripheral blood mononuclear cells (PBMCs) were collected via apheresis and GD2-CAR T cells were manufactured using the closed-system CliniMACs Prodigy (Miltenyi). In brief, CD4 and CD8 T cells were selected and transduced with one bicistronic retroviral vector encoding an iCasp9 domain (Bellicum Pharmaceuticals, Inc.), and a GD2–4-1BB–CD3Ζ CAR (Fig. 1a) containing the 14g2a scFv under control of an MSCV promoter. GD2-CAR T cells were expanded for 7 days in the presence of recombinant human IL-7 and IL-15, plus the addition of the tyrosine kinase inhibitor dasatinib on days 3 and 5 to improve T cell fitness31.
CAR T cell administration
Lymphodepleting chemotherapy (cyclophosphamide 500 mg m2 daily and fludarabine 25 mg m2 daily on days −4, −3 and −2) was administered followed by i.v. CAR T cell infusion on day 0 (Fig. 1b). Patients were monitored closely, including routine ICP measurements, in the inpatient setting through toxicity resolution. For patients receiving second infusions, CAR T cells were infused i.c.v. via the Ommaya. The first patient to be treated with i.c.v. administered CAR T cells (patient 1 with spinal DMG) received a flat dose of 50 million CAR T cells after increased lymphodepleting chemotherapy (cyclophosphamide 600 mg m2 daily and fludarabine 30 mg m2 daily on days −5, −4, −3 and −2) under a single-patient eIND (see below); subsequent patients received 30 million CAR T cells or the equivalent of their weight-based dose at DL1, whichever was lower, i.c.v. without lymphodepletion.
Clinical trial eligibility and exclusion criteria
Inclusion criteria
- Disease status
-
(i)Dose-escalation phase and DIPG expansion cohort: tissue diagnosis of H3K27M-mutated DIPG with radiographically evident tumour restricted to the brainstem, or
-
(ii)Dose-escalation phase and spinal DMG expansion cohort: tissue diagnosis of H3K27M-mutated DMG of the spinal cord.
-
(i)
Age: greater than or equal to 2 years of age and less than or equal to 30 years of age.
- Previous therapy:
-
(i)At least 4 weeks following completion of front-line radiation therapy.
-
(ii)At least 3 weeks post-chemotherapy or five half-lives, whichever is shorter, must have elapsed since any previous systemic therapy, except for systemic inhibitory/stimulatory immune checkpoint therapy, which requires 3 months.
-
(i)
Performance status: participants over 16 years of age, Karnofsky ≥ 60% or Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; participants 16 years of age or younger, Lansky scale ≥ 60%. Participants who are unable to walk because of paralysis, but who are up in a wheelchair, will be considered ambulatory for the purpose of assessing the performance score.
- Normal organ and marrow function (supportive care is allowed per institutional standards, that is, filgrastim, transfusion):
-
(i)Absolute neutrophil count (ANC) ≥ 1,000 per µl
-
(ii)Platelet count ≥ 100,000 per µl
-
(iii)Absolute lymphocyte count ≥ 150 per µl
-
(iv)Haemoglobin ≥ 8 g dl−1
-
(v)Adequate renal, hepatic, pulmonary and cardiac function defined as:
-
(vi)Creatinine within institutional norms for age that is, ≤2 mg dl−1 in adults or according to the table below in children younger than 18 years of age) or creatinine clearance (as estimated by Cockcroft Gault equation) ≥60 ml min−1
-
(vii)Serum alanine transaminase/aspartate transaminase (ALT/AST) ≤ 3.0 upper limit of normal (ULN;grade 1)
-
(viii)Total bilirubin ≤ 1.5 mg dl−1, except in participants with Gilbert’s syndrome
-
(ix)Cardiac ejection fraction ≥ 45%, no evidence of physiologically significant pericardial effusion as determined by an echocardiogram, and no clinically significant electrocardiogram findings
-
(x)Baseline oxygen saturation > 92% on room air
-
(i)
Female individuals of childbearing potential must have a negative serum or urine pregnancy test (female individuals who have undergone surgical sterilization are not considered to be of childbearing potential).
Contraception: participants of childbearing or child-fathering potential must be willing to practice birth control from the time of enrolment on this study and for 4 months after receiving the preparative lymphodepletion regimen or for as long as GD2-CAR T cells are detectable in peripheral blood or CSF.
Ability to give informed consent. Paediatric participants will be included in age appropriate discussion and written assent will be obtained for those 7 years of age or older, when appropriate.
Exclusion criteria
Bulky tumour involvement of the cerebellar vermis or hemispheres (pontocerebellar peduncle involvement is allowed), thalamic lesions that in the investigator’s assessment place the participant at unacceptable risk for herniation.
Clinically significant swallowing dysfunction as judged by clinical assessment.
Current systemic corticosteroid therapy.
Previous CAR therapy.
Uncontrolled fungal, bacterial, viral or other infection. Previously diagnosed infection for which the patient continues to receive antimicrobial therapy is permitted if responding to treatment and clinically stable.
Ongoing infection with HIV or hepatitis B virus (HBsAg positive) or hepatitis C virus (anti-HCV positive). A history of hepatitis B or hepatitis C is permitted if the viral load is undetectable per qPCR and/or nucleic acid testing.
Clinically significant systemic illness or medical condition (for example, significant cardiac, pulmonary, hepatic or other organ dysfunction), that in the judgement of the principal investigator is likely to interfere with assessment of safety or efficacy of the investigational regimen and its requirements.
In the investigator’s judgement, the participant is unlikely to complete all protocol-required study visits or procedures, including follow-up visits, or comply with the study requirements for participation.
Known sensitivity or allergy to any agents and/or reagents used in this study.
Primary immunodeficiency or history of autoimmune disease (for example, Crohns, rheumatoid arthritis and systemic lupus) requiring systemic immunosuppression and/or systemic disease-modifying agents within the past 2 years.
Response assessment
Radiographic responses were evaluated by gadolinium-enhanced MRI scans of the brain and/or spinal cord. Because DMGs are diffusely infiltrative of CNS structures and therefore are difficult to measure, volumetric tumour segmentation of T2/FLAIR signal abnormality was performed by a neuroradiologist (K.W.Y.) to measure radiographic change.
Clinical response was assessed by neurological examination. To quantify clinical change, a clinical improvement score (CIS) was calculated. The CIS represents an unweighted quantification of the neurological examination and is conducted by a neurologist who examines the participant before infusion and then at designated timepoints after therapy. For each neurological symptom or sign, one point is assigned. Improvement in a given symptom or sign from pre-infusion baseline add one point to the score, no change adds zero and one point is subtracted for symptom or sign worsening. The reported score is a sum of the positive and negative points. For example, a participant with improved right upper extremity strength and improved left sixth nerve palsy would receive a score of +2. A participant with improved right upper and lower extremity strength, improved left sixth nerve palsy but worsened left facial sensation would also receive a score of +2 (+3 − 1 = +2). The assessment cannot be conducted within 7 days of corticosteroid administration. Sample forms are available in Supplementary Tables 6, 7.
Cell-free DNA rare mutation detection
Patient CSF samples were collected at pre-determined and trigger timepoints throughout the treatment course. To gently separate the cellular compartment, samples were spun within 1 h of collection at 1,800g for 10 min at 4 °C followed by 20,000g for 10 min. Supernatant was frozen at −80 °C. DNA extraction was performed on 0.5–3 ml CSF supernatant aliquots using the QIAmp Circulating Nucleic Acids Kit (Qiagen) into 25 µl extraction buffer. All samples were assessed for cell-free DNA concentration by nanodrop and fragment size distribution with the BioAnalyzer (Agilent).
Forward and reverse primers and wild-type or mutant probes were designed to optimally detect the H3F3A K27M mutation through digital droplet polymerase chain reaction (ddPCR), and a dilution series of mutant H3K27M g-block against a wild-type H3 background was performed to validate linearity and confirm limit of detection. Seven PCR cycles for pre-amplification were performed on 10 µl of extracted cell-free DNA with Q5 Hot Start High-Fidelity Master Mix (New England Biolabs) for each timepoint. Preamplified reactions were immediately placed on ice after the final extension and diluted with 100 μl TE buffer, pH 8.0, to inactivate the Q5 polymerase. Samples were used immediately for ddPCR (40 cycles; Bio-Rad) and run as 4–6 technical replicates. The raw spectral data were analysed using the Bio-Rad software to plot the signal for mutant and wild-type alleles. All runs contained positive and negative controls. Results were analysed in R to plot variant allele frequency (VAF) as well as mutation count per volume over time.
qPCR measurement of in vivo GD2-CAR expansion
Patient blood and tumour tissue samples were processed and mononuclear cells were viably cryopreserved. DNA was extracted from whole blood (2 × 106–5 × 106 PBMCs) using QIAmp DNA Blood Mini Kit (51306, Qiagen) at baseline and multiple timepoints following CAR administration and from tumour tissue samples using DNeasy 96 Blood & Tissue Kit (69582, Qiagen). CAR presence was measured by qPCR using the primer and probe sequences provided as below and in Supplementary Table 8. For the standard curve, a custom Minigene plasmid (IDT) was designed containing a partial GD2–4-1BB–CD3Ζ sequence and a partial albumin sequence, which served as a control for normalization (Supplementary Table 8). The standard curve contained a tenfold serial dilution of plasmid between 5 × 108 and 5 × 100 copies. Both plasmid and patient DNA from each timepoint were run in triplicate, with each reaction containing 5 µl of DNA (50 ng total), 200 nM forward and reverse albumin primers (or 300 nM forward and reverse GD2–4-1BB–CD3Ζ primers), 150 nM probe suspended in 10 µl of TaqMan Fast Universal PCR Master Mix (2X), no AmpErase UNG (Thermo Fisher Scientific) and 24.5 µl (albumin) or 22.5 µl (GD2–4-1BB–CD3Ζ) of TE buffer (AM9935, Invitrogen). The Thermo Fisher Scientific QuantStudio 6 Pro Real-time qPCR Instrument was used for qPCR with 20 µl per reaction. The quality metrics for all qPCR standard curve results were R2 > 0.95 and efficiency was 70–110%.
Albumin results from plate normalized to average albumin, then GD2-CAR copy number (copies per 50 ng DNA) adjusted to albumin and modified to copies per 100 ng DNA by the following equation: copy number (copies per 100 ng DNA) = 2 × (GD2-CAR copy number × (albumin copy number/average albumin)). qPCR reagents included albumin probe (5’-/56-FAM/CCTGTCATG/ZEN/CCCACACAAATCTCTCC/3IABkFQ/-3’), albumin forward primer (5’-GCTGTCATCTCTTGTGGGCTGT-3’), albumin reverse primer (5’-ACTCATGGGAGCTGCTGGTTC-3’), GD2-CAR FAM probe (5’-/56-FAM/TCATGTTGT/ZEN/AGCCGGTGA AGGAGC/3IABkFQ/-3’), GD2-CAR forward primer (5’-CTCTGTGATGATCTCCTGCAA-3’), GD2-CAR reverse primer (5’-CGATCCATTCCAGGCTCTT-3’), and GD2 Albumin Minigene Plasmid (does not include proprietary backbone sequence; 5’-GCTGGCCTTTTGCTCACAAGCTTGGGGTTGCTGTCATCTCTTGTGGGCTGTAATC ATCGTCTAGGCTTAAGAGTAATATTGCAAAACCTGTCATGCCCACACAAATCTCT CCCTGGCATTGTTGTCTTTGCAGATGTCAGTGAAAGAGAACCAGCAGCTCCCATG AGTCCCAAGCTATGTTCTTTCCTGCGTTTCTCTGGTGGAACCTGGCGCCTCTGTG ATGATCTCCTGCAAGGCCAGCGGCA GCTCCTTCACCGGCTACAACATGAACTGGG TGCGCCAGAACATCGGCAAGAGCCTGGAATGGATCGGCGCCATCGACCCCTACTACGGCGGCACCAGCT).
Real-time flow cytometry assay
A high-dimensional (Hi-D) immuno-phenoptyping flow cytometry panel was designed for immune profiling of CAR T cells in real time. PBMCs were isolated from fresh whole blood by gradient centrifugation on ficoll (Ficoll-Paque Plus, GE Healthcare, Sigma-Aldrich). Two to five million PBMCs were stained with fixable live/dead aqua (Invitrogen) amine-reactive viability stain. Cells were then preincubated with Fc block (trustain, BioLegend) for 5 min, then stained at room temperature with the following fluorochrome-conjugated mAb in a 15-colour, 17-parameter staining combination (Supplementary Table 9).
CAR-tranduced T cells were used as positive control included in daily staining experiments. Stained and fixed cells were acquired on a LSR (BD BioSciences) five-laser (blue 488 nm, violet 405 nm, UV laser 355 nm, red 640 nm and green 532 nm lasers) analyser. At least 106 cells were acquired unless restricted by the number of cells isolated from 8 ml of whole blood or when acquiring isolated cells from CSF. The assay limit of detection for cells calculated as 1 in 104 of total acquired PBMCs. Representative gating is shown in Extended Data Fig. 10a.
Extended Data Fig. 10. Representative gating for flow cytometry of patient samples.
a, Representative gating of a patient CSF sample by flow cytometry (identical gating was used for PBMC obtained from blood). b, Gating of tumour cells by flow cytometry from temporal lobe resection tissue from Spinal DMG-1.
Luminex cytokines
Patient blood and CSF samples were collected at pre-determined and trigger timepoints throughout treatment. Samples were spun at 250g for 6 min. Supernatant was frozen at −80 °C until batched for assessment. Cytokine assessment was performed by the Immunoassay Team-Human Immune Monitoring Center at Stanford University. Panels include Luminex-EMD Millipore HIMC H80 (panel 1 is Milliplex HCYTA-60K-PX48; panel 2 is Milliplex HCP2MAG-62K-PX23; panel 3 includes the Milliplex HSP1MAG-63K-06 and HADCYMAG-61K-03 (resistin, leptin and HGF) to generate a 9 plex) and TGFβ (TGFBMAG-64K-03). Kits were purchased from EMD Millipore Corporation and used according to the manufacturer’s recommendations with modifications described. The assay setup followed recommended protocol. In brief, samples were diluted threefold (panels 1 and 2) or tenfold (panel 3). Of the diluted sample, 25 µl was mixed with antibody-linked magnetic beads in a 96-well plate and incubated overnight at 4 °C with shaking. Cold and room temperature incubation steps were performed on an orbital shaker at 500–600 rpm. Plates were washed twice with wash buffer in a Biotek ELx405 washer (BioTek Instruments). Following 1-h incubation at room temperature with biotinylated detection antibody, streptavidin–phycoerythrin (PE) was added for 30 min with shaking. Plates were washed as described above and PBS was added to wells for reading in the Luminex FlexMap3D Instrument with a lower bound of 50 beads per sample per cytokine. Custom Assay Chex control beads were purchased and added to all wells (Radix Biosolutions). All wells collected met quality control metrics with a bead count >50. Data are represented in pg ml−1 based on standard curves or heat maps of fold change from baseline timepoint. All samples were run in technical duplicate.
Single-cell RNA sequencing
Single-cell RNA sequencing (scRNA-seq) was performed using 5′ v2 Single Cell Immune Profiling technology (10X Genomics) according to the manufacturer’s protocol. In brief, cells from CSF samples collected at indicated timepoints before and after CAR T cell administration, as well as sorted CAR+ and CAR− cells from the CAR T cell infusion products, were counted, resuspended to 700–1,200 cells per µl, and captured using Single Cell Chip A on the 10x Chromium Controller (10X Genomics) to generate gel bead-in emulsions (GEMs). Reverse transcription inside GEMs was performed using a C1000 Touch Thermal Cycler (Bio-Rad). Barcoded complementary DNA (cDNA) was recovered through post-GEM-RT cleanup and PCR amplification. Recovered cDNA was amplified and used to construct 5′ whole-transcriptome libraries. Quality of cDNA and each library was assessed using Agilent 2100 Bioanalyzer. The libraries were indexed using a Chromium i7 Sample Index Kit, pooled and sequenced on NovaSeq 6000 System (Illumina) by Novogene.
Raw sequencing data were processed using the Cell Ranger software version 6.0.0 or higher (10X Genomics). Sequencer’s base call files (BCLs) were demultiplexed into FASTQ files using the cellranger mkfastq pipeline. To optimize CAR sequence detection in the scRNA-seq data, we probed the FASTQ files using SeqKit32 in 50-base-pair segments from the GD2-CAR plasmid sequence. Once we identified the segments with the most hits, we created a custom reference by appending these segments to the GRCh38 5.0.0 human genome reference with the cellranger mkref pipeline. FASTQ files were aligned to this custom reference using the cellranger count pipeline.
Unique molecular identifier (UMI) count matrices from Cell Ranger were analysed using Seurat33. Dead cells and cell debris with more than 15% of UMI counts mapping to mitochondrial genes or less than 300 genes detected were excluded from the analysis. Cell doublets containing more than 10,000 genes or more than 50,000 UMI counts were also excluded. Next, scRNA-seq data were subsampled to 5,000 cells per sample, and all data were normalized together to the sequencing depth using the SCTransform pipeline34. Data between patients were integrated using reciprocal principal component analysis (RPCA). PCA was performed using all variable genes with the exception of TCR and BCR genes to prevent clonotypes from driving the final layout. In addition, a set of curated genes relevant to T cell function or cell-type identification were included into the list of variable genes. UMAP embedding was performed using the first 50 principal components. Cell types were assigned to all cells based on canonical lineage marker expression. Cell-type doublets (‘lineage doublets’) were identified as cells expressing markers of more than one lineage: T cells (CD2, CD3E, CD3D, CD3G, CD247, CD7 and GD2-CAR), B cells (CD19, CD22 and PAX5), microglia/myeloid cells (CD14, CD68, CD163, CSF1R and AIF1) or astrocytes (GFAP). Differential expression analysis was performed on SCT counts modelled as a negative binomial distribution using the FindMarkers pipeline.
Further analysis of patient 1 with DIPG and other participant CSF samples was performed using the R-based ‘Seurat’ package35 to assess transcriptional differences preceding and during CAR T cell administration. First, the large Seurat object was subset into various smaller objects to enable multiple comparisons across different DIPG CSF samples (see Fig. 4). Seurat objects subsequently underwent scaling and dimensionality reduction based on the UMAP algorithm36. Myeloid fractions of the dataset were identified using the ‘AddModuleScore’ function in Seurat to isolate cells with relatively high expression of myeloid genes (AIF1, CSF1R, CX3CR1, CD14, CD68 and CD163), compared to their expression of B cell-related (CD19, CD22 and PAX5) and T cell-related (CD2, CD3E, CD3D, CD3G, CD247 and CD7) genes. Scoring of cells based on disease-associated myeloid (DAM) or myeloid-derived suppressor cell (MDSC) gene expression was implemented using the ‘AddModuleScore’ and ‘FeaturePlot’ functions in Seurat. DAM genes included SPP1, GPNMB, IGF1, CLEC7A, LPL, CD9, CD63, LGALS3, FABP5, ITGAX, APOE and TYROBP, based on previous noteworthy characterizations of myeloid cells in disease15,16,37,38. MDSC genes included CD33, CD14, CD15 and IL4RA39. Volcano plot analysis was conducted using the R-based ‘EnhancedVolcano’ package40 . Gene ontology (GO) analysis was performed using the R-based ‘ReactomePA’ package41.
Single-cell expression scores were computed in a similar way as previously described42. Given a set of genes (Gj) for a gene set (for example, a DAM signature), a score, SCj(i), which quantifies the scaled expression (Z-score) of Gj for each cell i, was computed as the average scaled expression (Er) of the genes in Gj, compared to the average scaled expression of a control gene set Gcont: SC (i) = average[Er(G, i)] − average[Er(Gcont, i)]. For each gene of the gene set, its control gene set contains 100 genes with the most similar aggregated expression level to that gene. Therefore, the control gene set represents a 100-folder larger but comparable distribution of expression levels to that of the considered gene set.
Graph-based clustering with data integration was adapted to identify cellular clusters. We selected highly variable genes (HVGs) using the FindVariableFeatures function in Seurat and used the scaled expression values of these HVGs for PCA. To disentangle sample-specific biological variations from cell subpopulation-specific variations and integrate multiple samples, we applied a linear adjustment method called Harmony to the first 30 principle components with default parameters to generate a corrected embedding43. We chose the first 20 Harmony corrected dimensions for constructing UMAP embeddings by RunUMAP in Seurat and clustered cells by the Louvain algorithm based FindClusters in Seurat. Cells that were from different samples, but expressed similar gene programmes, were well mixed. We next identified differentially expressed genes (DEGs) by FindAllMarkers in Seurat and tested genes that were detected in a minimum of 30% of the cells within each cluster and that showed at least a 0.5-fold mean log difference. We utilized Wilcoxon rank-sum test with Bonferroni correction for multiple testing and only kept genes with adjusted P < 0.05. Cell clusters were annotated with manual inspection of their top DEGs. In addition, we tested for enrichment of described gene sets (GO biological processes) and compared expression programmes of each cell cluster with those of a published glioblastoma-associated myeloid cell dataset44.
Histology, immunohistochemistry, immunofluorescence and RNAscope
Immunohistochemistry (other than for GD2 and H3K27M) was performed on formalin-fixed paraffin-embedded tissue sections per standard protocols including deparaffinization, antigen retrieval, incubation with primary antibody, and detection per the manufacturers’ instructions. The following antibodies were used: CD3 (790–4341, Ventana; rabbit polyclonal, prediluted), CD4 (NCL-CD4-368, Leica (Novocastra); mouse monoclonal, 1:40 dilution), CD8 (M7103, Dako; monoclonal mouse, 1:400 dilution) and CD163 (760–4437, Ventana; monoclonal mouse, prediluted). Stains for CD3 and CD163 were performed on a Ventana BenchMark Ultra automated stainer using CC1 antigen retrieval. Stains for CD4 and CD8 were performed on a Leica Bond automated stainer using ER2 antigen retrieval.
For H3K27M and GD2 immunohistochemistry, primary tumour samples from patients were transferred to cryomolds and embedded in optimal-cutting temperature (OCT) compound (TissueTek). Cryosections (10 µm) were generated on a cryostat (Leica). Tissue was fixed with 4% PFA at 4 °C for 20 min then washed and endogenous peroxidase activity was neutralized (Bloxall, Vector Laboratories; 10 min at room temperature) before permeabilization (0.3% Triton X-100, TBS, 15 min at room temperature) and blocking (5% horse serum, Vector Laboratories; 20 min at room temperature). Sequential double-staining immunohistochemistry was conducted for H3K27M (ab190631, Abcam; 1:1,000, 1 h at room temperature) and GD2 (14g2a, BD; 1:500, 1 h at room temperature). H3K27M was developed with a peroxidase secondary (ImmPRESS VR anti-rabbit IgG, Vector Laboratories; 30 min at room temperature) and DAB substrate (BD). After quenching the DAB substrate development in TBS and staining with the 14g2a primary antibody, the GD2 signal was developed using a polymer-based alkaline phosphatase secondary antibody (ImmPRESS AP anti-mouse IgG, Vector Laboratories; 30 min at room temperature) and blue alkaline phosphatase substrate (Vector Blue AP substrate kit, Vector Laboratories; 5 min at room temperature). Alkaline phosphatase development was quenched in TBS, and samples were mounted and imaged by light microscopy (Zeiss Axio Imager M2).
For immunofluorescence, primary tumour samples from patients were fixed in 4% PFA overnight and then transferred to 30% sucrose until samples sunk (3–4 days). Serial 40-µm cryosections were generated on an automatic freezing microtome (HM450, Thermo Fisher) then incubated with 3% normal donkey serum in 0.3% Triton X-100 in TBS blocking solution at 1 h at room temperature. Sections were stained overnight at 4 °C in primary antibody. Antibodies used were mouse anti-H3K27M (ab190631, Abcam; 1:1000) and rabbit anti-IBA1 (019–19741, Wako; 1:500) diluted in 1% normal donkey serum in 0.3% Triton X-100 in TBS blocking solution. Sections were incubated in secondary antibody conjugated with either 594 or 488 for 2 h at room temperature (715-585-150 and 711-545-152, Jackson Immunoresearch; 1:500). Samples were mounted with ProLong Gold mounting medium (P36930, Life Technologies) and were imaged using confocal microscopy (LSM710, Zeiss). For RNAscope in situ hybridization to visualize the GD2-CAR construct, primary tumor samples from patients were transferred to cryomolds and embedded in optimal-cutting temperature (OCT) compound (TissueTek). 10-µm cryosections were generated on a cryostat (Leica). Slides were fixed with cold 4% PFA for 15 minutes at 4 oC and then dehydrated in increasing ethanol rinses. To prepare for staining, tissue was pretreated with RNAscope hydrogen peroxide for 10 min at room temperature, then treated with RNAscope protease IV and incubated for 30 min at room temperature (ACD 322381). Tissue was then stained following the kit protocol for RNAscope 2.5 HD Duplex Assay (ACD, 322500). To stain control cells for GD2-CAR RNAscope, GD2-CAR T cells were thawed from frozen and prepared for staining according to the RNAscope Multiplex v2 Assay (ACD document MK-50 010). In short, cells were seeded in growth medium on chamber slides for 24 h and then fixed with 4% PFA for 30 min at room temperature. Cells were then dehydrated with ethanal and stored in 100% ethanol at –20 oC until staining. On the day of staining, cells were rehydrated with ethanol rinses and then pretreated with RNAscope hydrogen peroxide for 10 min at room temperature followed by RNAscope protease III diluted 1:15 with PBS for 10 min at room temperature (ACD 322381) and then stained alongside the tissue samples. A custom probe was designed to target the GD2-CARconstruct using the following sequence:AUGCUGCUGCUCGUGACAUCUCUGCUGCUGUGCGAGCUGCCCCACCCCGCCUUUCUGCUGAUCCCCGAUAUCCUGCUGACCCAGACCCCUCUGAGCCUGCCUGUGUCUCUGGGCGAUCAGGCCAGCAUCAGCUGCAGAUCCAGCCAGAGCCUGGUGCACCGGAACGGCAACACCUACCUGCACUGGUAUCUGCAGAAGCCCGGCCAGAGCCCCAAGCUGCUGAUUCACAAGGUGUCCAACCGGUUCAGCGGCGUGCCCGACAGAUUUUCUGGCAGCGGCUCCGGCACCGACUUCACCCUGAAGAUCAGCCGGGUGGAAGCCGAGGACCUGGGCGUGUACUUCUGCAGCCAGUCCACCCACGUGCCCCCCCUGACAUUUGGCGCCGGAACAAAGCUGGAACUGAAGGGCAGCACAAGCGGCAGCGGCAAGCCUGGAUCUGGCGAGGGAAGCACCAAGGGCGAAGUGAAGCUGCAGCAGAGCGGCCCCUCUCUGGUGGAACCUGGCGCCUCUGUGAUGAUCUCCUGCAAGGCCAGCGGCAGCUCCUUCACCGGCUACAACAUGAACUGGGUGCGCCAGAACAUCGGCAAGAGCCUGGAAUGGAUCGGCGCCAUCGACCCCUACUACGGCGGCACCAGCUACAACCAGAAGUUCAAGGGCAGAGCCACCCUGACCGUGGACAAGAGCAGCUCCACCGCCUACAUGCACCUGAAGUCCCUGACCAGCGAGGACAGCGCCGUGUACUACUGCGUGUCCGGCAUGGAAUACUGGGGCCAGGGCACAAGCGUGACCGUGUCCUCUGCGGCCGCAACCACGACGCCAGCGCCGCGACCACCAACACCGGCGCCCACCAUCGCGUCGCAGCCCCUGUCCCUGCGCCCAGAGGCGUGCCGGCCAGCGGCGGGGGGCGCAGUGCACACGAGGGGGCUGGACUUCGCCUGUGAUAUCUACAUCUGGGCGCCCUUGGCCGGGACUUGUGGGGUCCUUCUCCUGUCACUGGUUAUCACCCUUUACUGCAAACGGGGCAGAAAGAAACUCCUGUAUAUAUCAAACAACCAUUUAUGAGACCAGUACAAACUACUCAAGAGGAAAUGGCUGUAGCUGCCGAUUUCCAGAAGAAGAAGAAGGAGGAUGUAACUGAGAGUGAAGUUCAGCAGGAGCGCAGACGCCCCCGCGUACCAGCAGGGCCAGAACCAGCUCUAUAACGAGCUCAAUCUAGGACGAAGAGAGGAGUACGAUGUUUUGGACAAGAGACGUGGCCGGGACCCUAGAUGGGGGGAAAGCCGAGAAGGAAGAACCCUCAGAAGGCCUGUACAAUGAACUGCAGAAAGAUAAGAUGGCGGAGGCCUACAGUGAGAUUGGGAUGAAAGGCGAGCGCCGGAGGGGCAAGGGGCACGAUGGCCUUUACCAGGGUCUCAGUACAGCCACCAAGGACACCUACGACGCCCUUCACAUGCAGGCCCUGCCCCCUCGC. After probe hybridization, amplification and detection according to the kit manual, slides were counterstained with 50% hematoxylin for 30 sec, followed by 0.02% ammonia water for blueing. Slides were dried at 60 °C for 15 min, then mounted and imaged by light microscopy (Zeiss Axio Imager M2).
Flow cytometry of resected tumour tissue
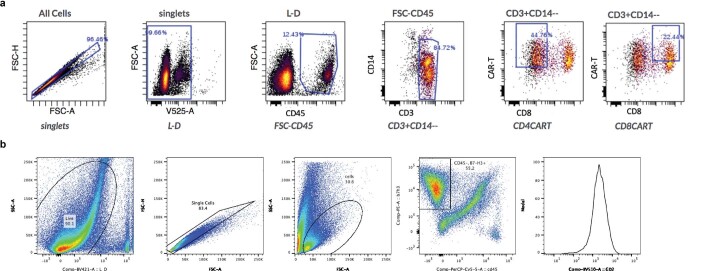
Tumour resection material was digested into single-cell suspensions as previously described45. Single-cell suspensions were stained with the following antibodies (Supplementary Table 10) and gating was performed using fluorescence minus one controls (FMO) on a BD LSRFortessa. DIPG cells were gated to evaluate GD2 expression in the CD45−, B7-H3+ population. The gating strategy is depicted in Extended Data Fig. 10b.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-022-04489-4.
Supplementary information
This file contains the Clinical Trial Protocol.
This file contains Supplementary Tables 1-10.
Baseline Gait in participant with DIPG 2 (DIPG-2) prior to intracerebroventricular GD2-CAR T cell infusion. Gait exam on the day of admission, prior to CAR T cell infusion (D0). Gait is wide-based and mildly unstable, with decreased right arm swing and right lower extremity circumduction. He takes several (6) steps to turn. He walks back across the room on toes, then turns again with multiple steps and attempts to perform tandem walking but is unable. Videos obtained and published with informed consent.
Gait in participant with DIPG 2 (DIPG-2) at two weeks following intracerebroventricular GD2-CAR T cell infusion. On Day +14 after ICV GD2-CAR T cell infusion, gait is markedly improved, with a narrow base, increased speed, improved right circumduction and improved arm swing. Note improved (two steps) turning. Videos obtained and published with informed consent.
Acknowledgements
We are grateful to our courageous patients and their families; A. Quinones-Hinojosa, K. Jaeckle, H. Tran, D. Poon, M. Casazza, D. Brown, S. Herrera, R. Taiwo, R. Jamiolkowski, A. Wu, S. Vaca, D. Herrick, D. Purger, D. Hong, A. Cluster, D. Limbrick, E. Mayne, C. Tesi-Rocha, C. Wusthoff, S. Lee, F. Baumer, J. Klotz, T. Nguyen and many others on the clinical teams for collaboration in the care of these patients; J. Senesac and Bellicum for providing study-enabling materials in the form of the viral vector and the AP1903 supply; J. S. Tamaresis for statistical support in the trial design; Y. Pan for help with figure formatting; S. Marsh and B. Stevens for input on myeloid cell transcriptional signatures; N. Lepori-Bui, T. Tutt and A.-L. Gramstrup Petersen for their expertise in cell formulation; and the Stanford Human Immune Monitoring Core (HIMC) for performing cytokine assays. This work was supported by the National Institutes of Health R01 CA263500-01 (C.L.M. and M.M.), the Parker Institute for Cancer Immunotherapy (C.L.M. and R.G.M.), CureSearch (C.L.M., M.M. and R.G.M.), a St. Baldrick’s Foundation - Stand Up 2 Cancer Pediatric Cancer Dream Team translational research grant (SU2CAACR-DT1113; C.L.M., R.G.M., S.R. and M.M.), the Stella S. Jones Foundation (M.M.), The V Foundation (R.G.M.), NIH Director’s Pioneer Award (DP1NS111132; M.M.), NIH Director’s New Innovator Award (DP2-CA272092; R.G.M.), ChadTough Defeat DIPG (J. Mahdi), Waxman Family Research Fund (A.C.G.) and the Virginia and D.K. Ludwig Fund for Cancer Research (C.L.M. and M.M.). The St. Baldrick’s Foundation partners with Stand Up 2 Cancer, a programme of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Extended data figures and tables
Source data
Author contributions
M.M. is the principal investigator of the trial. C.L.M. is the Investigational New Drug (IND) holder. C.W.M., R.G.M., C.L.M. and M.M. conceived the project. S.M., E.E. and C.B. oversaw regulatory affairs. S.M., E.E., M.M., C.L.M., C.W.M., R.G.M., S. Partap, C.J.C., L.R., T.T.C., K.E.W. and G.G. planned, designed and/or wrote the clinical trial, amendments and treatment protocols. K.E.W. and M.M. designed the clinical improvement scale. S. Patel, H.C. and S.A.F. performed process development for cellular manufacturing and supervised cellular manufacturing. R.G.M., S.R., L.M.S., R.M.R., V.B, J. Moon, A.R., E.H.N., C.B., J. Mahdi, C.E., S.G., L.R., T.T.C., S. Partap, P.G.F., C.J.C., G.G., K.L.D., C.L.M. and M.M. participated in patient care. K.W.Y. read radiographs and performed volumetric analysis of MRI scans. I.J.C., M.C.R., C.B., J. Moon., M.K. and M.F. participated in the collection and/or processing of patient samples. A.C.G., V.B., R.G.M., Z.E., W.R., S.K. and B.S. performed correlative studies. A.C.G. performed GD2 tissue staining and designed and carried out RNAscope in situ hybridization. V.B. designed and carried out the ctDNA assay. S.R., L.J., R.M., Z.G., A.Y.M., S.M.G. and M.G.F. analysed scRNA-seq data. S. Prabhu., S.R., V.B., A.C.G., R.G.M., M.M. and C.L.M. analysed other correlative studies. A.M.S.T. and H.V. performed the brain autopsy for patient 1 with DIPG and performed neuropathological examination and immunohistochemical analyses on the autopsy tissue. R.G.M., S.R., M.M. and C.L.M. prepared figures and wrote the manuscript. All authors reviewed and edited the manuscript. M.M. and C.L.M. supervised all aspects of the work.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All raw data are provided in the source data files. The single-cell RNA sequencing data have been uploaded to the Gene Expression Omnibus (GSE186802). Source data are provided with this paper.
Competing interests
Stanford University is in the process of applying for a patent application covering treatment of H3K27M-mutated gliomas with GD2-CAR T cells that lists M.M., C.L.M., R.G.M and C.W.M. as inventors. C.L.M. is a cofounder and holds equity in Lyell Immunopharma and Syncopation Life Sciences, which are developing CAR-based therapies, Red Tree Venture Capital, Ensme and Mammoth and consults for Lyell, Syncopation, Red Tree, NeoImmune Tech, Apricity, Nektar, Immatics, Ensoma and Mammoth. R.G.M. is a cofounder of and holds equity in Syncopation Life Sciences; he is also a consultant for Lyell Immunopharma, Syncopation Life Sciences, NKarta, Gamma Delta Therapeutics, Aptorum Group, Illumina Radiopharmaceuticals, ImmunAI, Arovella Therapeutics and Zai Lab. M.M. is on the scientific advisory board for Cygnal Therapeutics.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Robbie G. Majzner, Sneha Ramakrishna
These authors jointly supervised this work: Crystal L. Mackall, Michelle Monje
Contributor Information
Crystal L. Mackall, Email: cmackall@stanford.edu
Michelle Monje, Email: mmonje@stanford.edu.
Extended data
is available for this paper at 10.1038/s41586-022-04489-4.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-022-04489-4.
References
- 1.Louis DN, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Mount CW, et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas. Nat. Med. 2018;24:572–579. doi: 10.1038/s41591-018-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Theruvath J, et al. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat. Med. 2020;26:712–719. doi: 10.1038/s41591-020-0821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin GL, et al. Non-inflammatory tumor microenvironment of diffuse intrinsic pontine glioma. Acta Neuropathol. Commun. 2018;6:51. doi: 10.1186/s40478-018-0553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broniscer A, et al. Intratumoral hemorrhage among children with newly diagnosed, diffuse brainstem glioma. Cancer. 2006;106:1364–1371. doi: 10.1002/cncr.21749. [DOI] [PubMed] [Google Scholar]
- 6.Brown CE, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed N, et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3:1094–1101. doi: 10.1001/jamaoncol.2017.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber EW, et al. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv. 2019;3:711–717. doi: 10.1182/bloodadvances.2018028720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stallard S, et al. CSF H3F3A K27M circulating tumor DNA copy number quantifies tumor growth and in vitro treatment response. Acta Neuropathol. Commun. 2018;6:80. doi: 10.1186/s40478-018-0580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry TJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DW, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker AJ, et al. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol. Ther. 2017;25:2189–2201. doi: 10.1016/j.ymthe.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long AH, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keren-Shaul H, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–1290.e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Hammond TR, et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50:253–271.e6. doi: 10.1016/j.immuni.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooney T, et al. Contemporary survival endpoints: an International Diffuse Intrinsic Pontine Glioma Registry study. Neuro-Oncol. 2017;19:1279–1280. doi: 10.1093/neuonc/nox107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robison NJ, Kieran MW. Diffuse intrinsic pontine glioma: a reassessment. J. Neurooncol. 2014;119:7–15. doi: 10.1007/s11060-014-1448-8. [DOI] [PubMed] [Google Scholar]
- 19.Lee DW, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 20.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marconi S, et al. Expression of gangliosides on glial and neuronal cells in normal and pathological adult human brain. J. Neuroimmunol. 2005;170:115–121. doi: 10.1016/j.jneuroim.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Yu AL, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louis CU, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pule MA, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straathof K, et al. Antitumor activity without on-target off-tumor toxicity of GD2-chimeric antigen receptor T cells in patients with neuroblastoma. Sci. Transl. Med. 2020;12:eabd6169. doi: 10.1126/scitranslmed.abd6169. [DOI] [PubMed] [Google Scholar]
- 26.Heczey A, et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat. Med. 2020;26:1686–1690. doi: 10.1038/s41591-020-1074-2. [DOI] [PubMed] [Google Scholar]
- 27.Majzner RG, et al. Tuning the antigen density requirement for CAR T-cell activity. Cancer Discov. 2020;10:702–723. doi: 10.1158/2159-8290.CD-19-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majzner RG, et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin. Cancer Res. 2019;25:2560–2574. doi: 10.1158/1078-0432.CCR-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkatesh HS, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573:539–545. doi: 10.1038/s41586-019-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Stasi A, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber EW, et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science. 2021;372:eaba1786. doi: 10.1126/science.aba1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen W, Le S, Li Y, Hu F. SeqKit: a cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS ONE. 2016;11:e0163962. doi: 10.1371/journal.pone.0163962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuart T, et al. Comprehensive integration of single-cell data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hafemeister C, Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019;20:296. doi: 10.1186/s13059-019-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao Y, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becht E, et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 2019;37:38–44. doi: 10.1038/nbt.4314. [DOI] [PubMed] [Google Scholar]
- 37.Krasemann S, et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47:566–581.e9. doi: 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q, et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron. 2019;101:207–223.e10. doi: 10.1016/j.neuron.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrusiewicz, K., Colwell, N. A. & Heimberger, A. B. in Translational Immunotherapy of Brain Tumors (ed. Sampson, J. H.) 63–82 (2017).
- 40.Blighe, K., Rana, S. & Lewis, M. EnhancedVolcano: Publication-ready volcano plots with enhanced colouring and labeling. R package version 1.12.0. GitHubhttps://github.com/kevinblighe/EnhancedVolcano (2021).
- 41.Yu G, He QY. ReactomePA: an R/Bioconductor package for reactome pathway analysis and visualization. Mol. Biosyst. 2016;12:477–479. doi: 10.1039/c5mb00663e. [DOI] [PubMed] [Google Scholar]
- 42.Gojo J, et al. Single-cell RNA-seq reveals cellular hierarchies and impaired developmental trajectories in pediatric ependymoma. Cancer Cell. 2020;38:44–59.e9. doi: 10.1016/j.ccell.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korsunsky I, et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods. 2019;16:1289–1296. doi: 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pombo Antunes AR, et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat. Neurosci. 2021;24:595–610. doi: 10.1038/s41593-020-00789-y. [DOI] [PubMed] [Google Scholar]
- 45.Lin GL, Monje M. A protocol for rapid post-mortem cell culture of diffuse intrinsic pontine glioma (DIPG) J. Vis. Exp. 2017;121:55360. doi: 10.3791/55360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains the Clinical Trial Protocol.
This file contains Supplementary Tables 1-10.
Baseline Gait in participant with DIPG 2 (DIPG-2) prior to intracerebroventricular GD2-CAR T cell infusion. Gait exam on the day of admission, prior to CAR T cell infusion (D0). Gait is wide-based and mildly unstable, with decreased right arm swing and right lower extremity circumduction. He takes several (6) steps to turn. He walks back across the room on toes, then turns again with multiple steps and attempts to perform tandem walking but is unable. Videos obtained and published with informed consent.
Gait in participant with DIPG 2 (DIPG-2) at two weeks following intracerebroventricular GD2-CAR T cell infusion. On Day +14 after ICV GD2-CAR T cell infusion, gait is markedly improved, with a narrow base, increased speed, improved right circumduction and improved arm swing. Note improved (two steps) turning. Videos obtained and published with informed consent.
Data Availability Statement
All raw data are provided in the source data files. The single-cell RNA sequencing data have been uploaded to the Gene Expression Omnibus (GSE186802). Source data are provided with this paper.