Summary
Background
In low-incidence countries, tuberculosis mainly affects migrants, mostly resulting from reactivation of latent tuberculosis infection (LTBI) acquired in high-incidence countries before migration. A nationwide primary care-based LTBI testing and treatment programme for migrants from high-incidence countries was therefore established in high tuberculosis incidence areas in England. We aimed to assess the effectiveness of this programme.
Methods
We did a retrospective, population-based cohort study of migrants who registered in primary care between Jan 1, 2011, and Dec 31, 2018, in 55 high-burden areas with programmatic LTBI testing and treatment. Eligible individuals were aged 16–35 years, born in a high-incidence country, and had entered England in the past 5 years. Individuals who tested interferon-γ release assay (IGRA)-negative were advised about symptoms of tuberculosis, whereas those who tested IGRA-positive were clinically assessed to rule out active tuberculosis and offered preventive therapy. The primary outcome was incident tuberculosis notified to the national Enhanced Tuberculosis Surveillance system.
Findings
Our cohort comprised 368 097 eligible individuals who had registered in primary care, of whom 37 268 (10·1%) were tested by the programme. 1446 incident cases of tuberculosis were identified: 166 cases in individuals who had IGRA testing (incidence 204 cases [95% CI 176–238] per 100 000 person-years) and 1280 in individuals without IGRA testing (82 cases [77–86] per 100 000 person-years). Overall, in our primary analysis including all diagnosed tuberculosis cases, a time-varying association was identified between LTBI testing and treatment and lower risk of incident tuberculosis (hazard ratio [HR] 0·76 [95% CI 0·63–0·91]) when compared with no testing. In stratified analysis by follow-up period, the intervention was associated with higher risk of tuberculosis diagnosis during the first 6 months of follow-up (9·93 [7·63–12·9) and a lower risk after 6 months (0·57 [0·41–0·79]). IGRA-positive individuals had higher risk of tuberculosis diagnosis than IGRA-negative individuals (31·9 [20·4–49·8]). Of 37 268 migrants who were tested, 6640 (17·8%) were IGRA-positive, of whom 1740 (26·2%) started preventive treatment. LTBI treatment lowered the risk of tuberculosis: of 135 incident cases in the IGRA-positive cohort, seven cases were diagnosed in the treated group (1·87 cases [95% CI 0·89–3·93] per 1000 person-years) and 128 cases were diagnosed in the untreated group (10·9 cases [9·16–12·9] per 1000 person-years; HR 0·14 [95% CI 0·06–0·32]).
Interpretation
A low proportion of eligible migrants were tested by the programme and a small proportion of those testing positive started treatment. Despite this, programmatic LTBI testing and treatment of individuals migrating to a low-incidence region is effective at diagnosing active tuberculosis earlier and lowers the long-term risk of progression to tuberculosis. Increasing programme participation and treatment rates for those testing positive could substantially impact national tuberculosis incidence.
Funding
National Institute for Health Research Health Protection Research Unit in Respiratory Infections.
Introduction
Tuberculosis incidence in high-income countries with a low incidence of the disease is driven disproportionately by cases in specific groups at high risk, particularly migrants from high-incidence countries.1, 2 In these individuals, tuberculosis usually develops within the first 2–5 years after migration, as a result of reactivation of latent tuberculosis infection (LTBI) acquired before migration.1, 3 Risk of progression from LTBI to active tuberculosis can be reduced with preventive treatment, and diagnosis and treatment of LTBI is a mainstay of tuberculosis control in low-incidence countries.3, 4 Systematic testing and treatment of migrants from high-incidence regions might therefore have a large impact on tuberculosis control and elimination.2, 5, 6
On the basis of the epidemiology of imported LTBI,7, 8 the risk of progression to active tuberculosis in migrants with a positive interferon-γ release assay (IGRA),9 health-economic analyses,3 and feasibility studies in primary care and potential screening pathways,10, 11 in England a primary care-based programme was launched in 2015 for systematic LTBI testing (by IGRA) and treatment for migrants from high-incidence countries residing in areas with the highest tuberculosis burden.12, 13 To our knowledge, this is a first, non-obligatory, national public health experiment that individuals can choose to enter if they wish. If successful, this programme would represent an important step towards tuberculosis elimination. Although underpinned by a large evidence base,3, 7, 8, 9, 10, 11 the programme has not been evaluated in a randomised controlled trial. In this cohort study, we aimed to pragmatically evaluate the effectiveness of this programme in the real world and to assess the risk of progression to tuberculosis in migrants with a positive IGRA and its mitigation by programmatic preventive treatment.
Research in context.
Evidence before this study
We searched PudMed for articles published in English between Jan 1, 2015, and July 31, 2021, about the effectiveness of programmatic testing and treatment for latent tuberculosis infection (LTBI) in migrants in high-income countries using the search terms “TB screening” AND “migrant”. Few studies have assessed the effectiveness of testing and treating migrants for LTBI in a programmatic setting. A systematic review on the effectiveness and cost-effectiveness of screening migrants for LTBI in the EU suggested that the effectiveness of LTBI programmes is limited by poorly predictive tests, long treatment durations, and a weak care cascade (ie, many individuals are lost to follow-up at each step). A population-based study provided indirect evidence that the reduction in tuberculosis notifications in the UK might be partly explained by the effectiveness of screening interventions, including LTBI testing, in non-EU migrants who had migrated in the past 5 years. England is the only country to have launched a nationwide LTBI testing and treatment programme for new entrants in primary care and its feasibility and effectiveness are of considerable international interest. Preliminary evidence from initial roll-out during the first pilot year of this interferon-γ release assay (IGRA)-based programme indicated a reduction in tuberculosis incidence among the small number of migrants who were initially tested.
Added value of this study
We assessed whether the UK programme was effective in averting active tuberculosis, the risk of progression to active tuberculosis in new entrants who test positive by IGRA, and the effectiveness of LTBI treatment to prevent active tuberculosis using the 3-month isoniazid and rifampicin regimen. We demonstrated that programmatic nationwide LTBI testing and treatment are feasible and effective to reduce the risk of tuberculosis in foreign-born individuals who had arrived in England in the previous 5 years. Programmatic LTBI testing and treatment is associated with earlier diagnosis of active tuberculosis and an overall lower risk of developing active tuberculosis over time. The risk of developing active tuberculosis was 31 times higher for IGRA-positive individuals than IGRA-negative individuals and LTBI treatment with a 3-month isoniazid and rifampicin regimen reduced this risk by 86%.
Implications of all the available evidence
Programmatic LTBI testing and treatment of new migrants to a low-incidence region is effective at diagnosing active tuberculosis earlier and reduces the long-term risk of progression to tuberculosis. National programmes such as this could therefore be important new public health interventions to enable tuberculosis elimination in low-incidence regions. Such programmes could be especially important to improve tuberculosis control at present considering the widespread disruption to tuberculosis services observed internationally since the start of the COVID-19 pandemic.
Methods
Study design and participants
We did a retrospective, population-based cohort study in individuals who registered in primary care between Jan 1, 2011, and Dec 31, 2018 in 55 high-burden (tuberculosis incidence >20 cases per 100 000 population per year) Clinical Commissioning Group areas of England where the LTBI testing and treatment programme was rolled out. Individuals were eligible to participate if they were aged 16–35 years, born in a high-incidence country (≥150 cases per 100 000 population per year or any country in sub-Saharan Africa), and had entered England within 5 years of the programme start date.12 Participants were excluded if they registered in an area without programmatic LTBI testing or their date of primary care registration was missing and they were not tested for LTBI. The cohort was divided into two groups; individuals who were tested for LTBI and those who were not. The group not tested for LTBI was used as the control group to assess overall programme effectiveness.
Procedures
A pilot programme started in January, 2014 and was rolled out to all 55 high-burden Clinical Commissioning Group areas by Jan 30, 2015.12 IGRA was used to identify tuberculosis infection, which was then investigated to determine whether it represented active tuberculosis disease or LTBI; investigation for active tuberculosis in individuals who tested IGRA-positive was thus part of the screening pathway. Eligible individuals were offered LTBI testing by IGRA in primary care, allowing for alternative suitable settings according to local circumstances.13 IGRA-negative individuals were advised to be vigilant for symptoms of tuberculosis, and IGRA-positive individuals had clinical assessment including chest radiography, followed by respiratory samples for microscopy and culture if radiographic appearances suggested tuberculosis, to rule out active tuberculosis before referral to local secondary care tuberculosis services for preventive therapy, usually 3 months treatment with isoniazid and rifampicin.13, 14 Individuals identified with active tuberculosis were referred to their local tuberculosis service for standard treatment.13, 14
We used a validated probabilistic linkage method15 to create a cohort of all foreign-born individuals who registered in primary care in England from Jan 1, 2011, to Dec 31, 2018 by linking three different datasets (appendix p 4). This cohort represents the total number of individuals eligible for the programme; the number invited to participate by their general practitioners was not available. A detailed description of data sources, linkage methods, and all variables used in the analysis is provided in the appendix (p 4).
All databases were stored, processed, and analysed at the UK Health Security Agency (Colindale, UK). Under the UK Health and Social Care Act 2012, the UK Health Security Agency has authority to hold and analyse national surveillance data for public health evaluation purposes. By participating in the pre-entry screening and LTBI testing programmes, individuals consented for their data to be used by the UK Health Security Agency and the National Health Service for monitoring and evaluation and due to the nature of the study the requirement for ethical approval was waived.
Outcomes
The primary outcome measure was new, incident cases of active tuberculosis at any anatomical site, either bacteriologically or clinically diagnosed, notified to the national enhanced tuberculosis surveillance system.16
Statistical analysis
In the primary analysis, we assumed that all individuals with tuberculosis could be identified through the screening pathway and accordingly included all cases diagnosed during the study period; however, cases notified within 21 days of LTBI treatment start date were assumed to be prevalent (not incident) and excluded from analyses,17 as were cases notified before roll-out of the programme on Jan 1, 2014. We then did a sensitivity analysis to assess the potential for overestimating the effectiveness of the programme or of LTBI treatment by inclusion of all tuberculosis cases in the analysis. The sensitivity analysis therefore assumed all cases diagnosed within 60 days and 90 days of primary care registration or IGRA testing to be prevalent (not incident) and these cases were excluded from analysis. Cohort time started on IGRA testing date for the analysis of overall programme effectiveness; for individuals who were not tested, start time was the date of primary care registration plus 99 days (the median time between primary care registration and IGRA testing for individuals who underwent IGRA testing). For analysis of treatment effectiveness, cohort time started at treatment start date; for individuals who did not start treatment, cohort time started on the date of IGRA testing plus 52 days (the median time between IGRA testing and treatment start). Cohort time ended at death, development of active tuberculosis, or the end of the study (Nov 30, 2019), whichever occurred first. We used log-rank tests to assess differences between Kaplan-Meier survival curves for time to tuberculosis diagnosis according to LTBI testing, IGRA positivity, and LTBI treatment. Cox proportional-hazards models were used to assess the effect of the same variables on time to tuberculosis diagnosis after adjustment for covariates. For the survival analysis, univariate models were first fitted for each covariate; we then assessed effect interactions and modifications between the main outcomes and all covariates, after which a multivariable model was fitted including all covariates and significant effect modifications. We did sensitivity analyses to account for the imputation method using complete case analysis, for treatment completion using only participants with confirmed date of treatment completion, and excluding tuberculosis cases diagnosed within 60 and 90 days of primary care registration or LTBI testing. A detailed description of the statistical analysis is provided in the appendix (p 5).
There are different possible mechanisms that can give rise to variations in the hazard of tuberculosis diagnosis over time in the cohort of individuals who received LTBI testing compared with those who did not. First, by virtue of repeated clinical follow-up, any incident tuberculosis in the tested cohort is likely to be diagnosed and notified more rapidly than in the non-tested cohort. Second, preventive therapy would tend to reduce the incidence of tuberculosis among individuals who are tested and treated. The first mechanism would be expected to lead to a short-term increased hazard of tuberculosis diagnosis, while the second mechanism would cause an overall decrease. To capture the combined effect of these two mechanisms, we estimated the overall effect of LTBI testing and treatment while allowing for a time-varying effect.18, 19 This analytical approach considers the two different benefits of the programme: identification of prevalent cases of active tuberculosis through screening and prevention of progression from LTBI to active disease by treatment. We then repeated the analysis stratified by time of follow-up to assess whether the hazard ratio (HR) varied during follow-up. Missing values were imputed by multiple imputation by chained equations (appendix p 5).20, 21 We used Stata statistical software (version 15.1) for statistical analyses. The adjusted number needed to treat (NNT) was calculated using the R package stdReg with R Open software (version 3.5.3).22 All p values were two-tailed and values of less than 0·05 were considered to indicate a statistically significant difference.
Role of the funding source
The funder of the study had no role in study design, data collection, analysis, interpretation, or writing of the manuscript.
Results
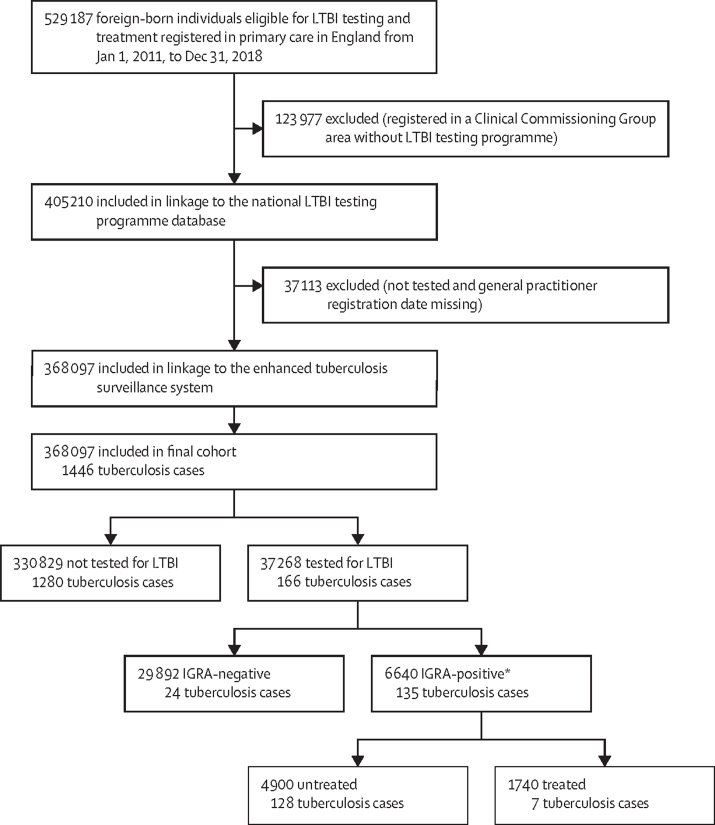
The cohort comprised 368 097 eligible individuals, of whom 37 268 (10·12%) had IGRA testing and 330 829 (89·88%) did not (figure 1). Among the 37 268 individuals who had IGRA testing, 29 892 (80·21%) were IGRA-negative and 6640 (17·82%) IGRA-positive. Of the 6640 individuals who tested positive, 1740 (26·20%) started preventive treatment and 4900 (73·80%) did not (figure 1, table 1). The baseline characteristics of study participants are summarised in table 1.
Figure 1.
Study design and participants
LTBI=latent tuberculosis infection. IGRA=interferon-γ release assay. *Participants with indeterminate or no available IGRA results were excluded.
Table 1.
Baseline characteristics of study participants (n=368 097)
| Tested for LTBI (n=37 268) | Not tested for LTBI (n=330 829) | |
|---|---|---|
| Age, years | ||
| 16–25 | 16 396/37 051 (44·0%) | 180 334 (54·5%) |
| 26–35 | 20 655/37 051 (55·4%) | 150 495 (45·5%) |
| Sex | ||
| Female | 16 518/36 579 (45·2%) | 162 190/330 823 (49·0%) |
| Male | 20 061/36 579 (54·8%) | 168 633/330 823 (51·0%) |
| ETS region of origin | ||
| Africa | 4297/22 546 (19·1%) | 90 668 (27·4%) |
| The Americas or Europe | 306/22 546 (0·8%) | 10 153 (3·1%) |
| East and southeast Asia | 606/22 546 (2·7%) | 25 787 (7·8%) |
| South Asia | 17 337/22 546 (76·9%) | 204 221 (61·7%) |
| Tuberculosis incidence in country of origin (cases per 100 000)* | ||
| 40–149 | 2138/22 546 (9·5%) | 46 456 (14·0%) |
| 150–349 | 19 757/22 546 (87·6%) | 258 595 (78·2%) |
| >350 | 651/22 546 (2·9%) | 25 778 (7·8%) |
| Tuberculosis incidence in area of residence (cases per 100 000)† | ||
| ≤9·2 | 3132/33 789 (9·3%) | 35 936/319 090 (11·3%) |
| 9·3–31·6 | 14 016/33 789 (41·5%) | 203 003/319 090 (63·6%) |
| >31·6 | 16 641/33 789 (49·2%) | 80 151/319 090 (25·1%) |
| Deprivation index | ||
| 1–3 deciles (most deprived) | 21 579/35 948 (60·0%) | 161 651/323 764 (49·9%) |
| 4–6 deciles | 11 791/35 948 (32·8%) | 120 217/323 764 (37·1%) |
| 7–10 deciles (least deprived) | 2578/35 948 (7·2%) | 41896/323 764 (12·9%) |
| Year of arrival in England or primary care registration | ||
| 2011–12 | 1882/22 546 (8·3%) | 76 342 (23·1%) |
| 2013–14 | 2610/22 546 (11·6%) | 58 853 (17·8%) |
| 2015–16 | 6910/22 546 (30·1%) | 84 178 (25·4%) |
| 2017–18 | 11 144/22 546 (49·4%) | 111 456 (33·7%) |
| Pre-entry screening for active tuberculosis | ||
| Yes | 7704 (20·7%) | 80 641 (24·4%) |
| No | 29 564 (20·7%) | 250 188 (75·6%) |
| IGRA for latent tuberculosis | ||
| Positive | 6640 (17·8%) | NA |
| Negative | 29 892 (80·2%) | NA |
| Indeterminate | 353 (0·9%) | NA |
| No result available | 383 (1·0%) | NA |
| LTBI treatment | ||
| Yes | 1740 (26·2%) | NA |
| No | 4900 (73·8%) | NA |
Data are n (%) or n/N (%). ETS=enhanced tuberculosis surveillance system. IGRA= interferon-γ release assay. LTBI=latent tuberculosis infection. NA=not applicable.
WHO estimated tuberculosis incidence.13
The UK Health Security Agency estimated tuberculosis incidence in Clinical Commissioning Group area of residence.
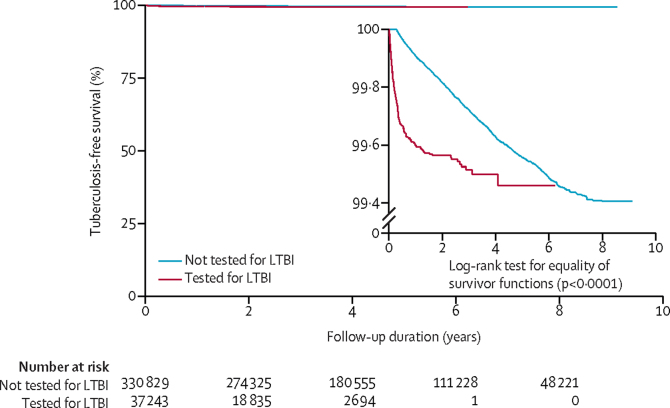
A total of 1446 tuberculosis cases were identified in the entire cohort during the study period (figure 1), giving an incidence of 88 cases (95% CI 83–92) per 100 000 person-years in a total follow-up time of 1 638 236 person-years, with a median follow-up time of 3·9 years (IQR 2·29–6·60) per person. In individuals who had IGRA testing, 166 tuberculosis cases were identified (incidence 204 cases [95% CI 176–238] per 100 000 person-years, and among individuals without IGRA testing, 1280 cases were identified (incidence 82 cases [95% CI 77–86] per 100 000 person-years (figure 1, table 2). The unadjusted Kaplan-Meier curve indicated that individuals who received an IGRA test had an increased risk of tuberculosis diagnosis compared with those who had no IGRA test (figure 2); however, the curves converged showing that the increased risk was not sustained as time accrued, indicating that the hazard of tuberculosis diagnosis is not proportional—ie, the HR changes over time.19 For this reason, we estimated the overall effect (ie, the average effect during the overall follow-up time) of LTBI testing and treatment over time allowing for a time-varying effect.18, 19 This analysis showed that overall LTBI testing and treatment is associated with lower risk of tuberculosis diagnosis compared with no testing (HR 0·76 [95% CI 0·63–0·91]; table 2). A stratified analysis showed that the intervention was associated with higher risk of tuberculosis diagnosis during the first 6 months of follow-up (9·93 [7·63–12·9]), but after 6 months, the intervention was associated with a significantly lower risk (0·57 [0·41–0·79]; table 2). Of the 1446 tuberculosis cases, 234 were diagnosed during the first 6 months: 124 cases among individuals with IGRA testing (incidence 4·65 cases [95% CI 3·90–5·54] per one person-year) and 110 cases among individuals without testing (incidence 2·59 cases [95% CI 2·15–3·13] per one person-year). 1212 tuberculosis cases were diagnosed subsequently: 42 in individuals with IGRA testing (incidence 51·8 cases [95% CI 38·3–70·1] per 100 000 person-years) and 1170 cases among individuals without testing (incidence 75·1 cases [95% CI 70·9–79·5] per 100 000 person-years; table 2).
Table 2.
Multivariable Cox regression model analysis of factors associated with time to tuberculosis diagnosis
| Tuberculosis cases per person-years of follow-up* | Incidence per 100 000 person-years (95% CI) | HR (95% CI)† | p value | ||
|---|---|---|---|---|---|
| Model 1 | |||||
| Age, years | |||||
| 16–25 | 773/946 153 | 81 (76–87) | 0·85 (0·76–0·94) | 0·003 | |
| 26–35 | 671/691 635 | 97 (89–104) | 1 (ref) | .. | |
| Sex | |||||
| Female | 826/829 167 | 99 (93–106) | 1 (ref) | .. | |
| Male | 618/807 300 | 76 (70–82) | 0·77 (0·69–0·86) | <0·0001 | |
| ETS region of origin | |||||
| Africa | 389/423 387 | 91 (83–101) | 1 (ref) | .. | |
| The Americas or Europe | 0/30 893 | 0 | 0 | .. | |
| East and southeast Asia | 49/98 843 | 49 (37–65) | 0·60 (0·45–0·82) | 0·001 | |
| South Asia | 931/1 051 901 | 88 (83–94) | 1·16 (0·97–1·39) | 0·089 | |
| Tuberculosis incidence in country of origin (cases per 100 000)‡ | |||||
| 40–149 | 192/196 674 | 97 (84–112) | 1 (ref) | .. | |
| 150–349 | 1105/1 297 876 | 85 (80–90) | 0·62 (0·50–0·77) | <0·0001 | |
| >350 | 72/110 474 | 65 (51–82) | 0·63 (0·47–0·84) | 0·002 | |
| Tuberculosis incidence in area of residence (cases per 100 000)§ | |||||
| ≤9·2 | 118/146 624 | 80 (67–96) | 1 (ref) | .. | |
| 9·3–31·6 | 855/970 862 | 88 (82–94) | 1·05 (0·86–1·30) | 0·583 | |
| >31·6 | 428/460 358 | 92 (84–102) | 1·16 (0·93–1·45) | 0·161 | |
| Deprivation index | |||||
| 1–3 deciles (most deprived) | 788/821 584 | 95 (89–102) | 1 (ref) | .. | |
| 4–6 deciles | 495/583 213 | 84 (77–92) | 0·89 (0·79–1·00) | 0·063 | |
| 7–10 deciles (least deprived) | 128/196 727 | 65 (54–77) | 0·73 (0·60–0·89) | 0·002 | |
| Year of arrival in England or primary care registration | |||||
| 2011–12 | 477/629 979 | 75 (69–82) | 1 (ref) | .. | |
| 2013–14 | 340/368 240 | 92 (83–102) | 1·21 (1·03–1·42) | 0·018 | |
| 2015–16 | 374/362 069 | 103 (93–114) | 1·26 (1·07–1·48) | 0·004 | |
| 2017–18 | 178/244 736 | 72 (62–84) | 0·78 (0·63–0·96) | 0·024 | |
| Pre-entry screening for active tuberculosis | |||||
| Yes | 322/374 036 | 86 (77–96) | 1·27 (1·03–1·56) | 0·022 | |
| No | 1124/1 264 200 | 88 (83–94) | 1 (ref) | .. | |
| Pre-entry screening: year of arrival in England or primary care registration¶ | |||||
| 2011–12 | .. | .. | 1 (ref) | .. | |
| 2013–14 | .. | .. | 0·92 (0·69–1·23) | 0·599 | |
| 2015–16 | .. | .. | 0·89 (0·67–1·18) | 0·430 | |
| 2017–18 | .. | .. | 0·43 (0·29–0·63) | <0·0001 | |
| Time-variant LTBI testing and treatment | |||||
| Yes | 166/80 995 | 204 (176–238) | 0·76 (0·63–0·91) | 0·003 | |
| No | 1280/1 557 241 | 82 (77–86) | 1 (ref) | .. | |
| LTBI testing and treatment by follow-up period | |||||
| Model 2 (<6 months follow-up)‖ | |||||
| Yes | 124/26·6 | 4·65 (3·90–5·54) | 9·93 (7·63–12·9) | <0·001 | |
| No | 110/42·3 | 2·59 (2·15–3·13) | 1 (ref) | .. | |
| Model 3 (>6 months follow-up)** | |||||
| Yes | 42/80 969 | 51·8 (38·3–70·1) | 0·57 (0·41–0·79) | 0·001 | |
| No | 1170/1 557 198 | 75·1 (70·9–79·5) | 1 (ref) | .. | |
| Model 4 (1-year follow-up)§ | |||||
| Yes | 18/76 490 | 23·5 (14·8–37·3) | 0·36 (0·22–0·58) | <0·001 | |
| No | 977/1 555 582 | 62·8 (58·9–66·8) | 1 (ref) | .. | |
| Model 5 (2-year follow up)§ | |||||
| Yes | 8/57 548 | 13·9 (6·95–27·8) | 0·36 (0·18–0·73) | 0·005 | |
| No | 698/1 474 486 | 47·3 (43·9–50·9) | 1 (ref) | .. | |
| Model 6 (3-year follow-up)§ | |||||
| Yes | 2/30 296 | 6·6 (1·65–26·4) | 0·29 (0·07–1·18) | 0·085 | |
| No | 464/1 352 527 | 34·3 (31·3–37·5) | 1 (ref) | .. | |
HR=hazard ratio. ETS=enhanced tuberculosis surveillance system. LTBI=latent TB infection.
Included only participants with no missing data for that characteristic.
HR (95% CI) and p value estimates were derived from the total cohort of migrants, excluding those who developed tuberculosis 21 days after starting treatment (n=368 077), after multiple imputation for missing values by chained equations model.
WHO estimated tuberculosis incidence.13
The UK Health Security Agency estimated tuberculosis incidence in Clinical Commissioning Group area of residence.
Effect modification is shown in the multiplicative scale using pre-entry active tuberculosis screening (yes) as the baseline group.
Models 2–6 include the same covariates as model 1 with the addition of the time-variant effect of LTBI testing and treatment.
Rate per one person-year (95% CI).
Figure 2.
Tuberculosis-free survival among migrants tested for LTBI and those not tested for LTBI
The unadjusted Kaplan-Meier curve was derived using all study participants, excluding those who developed tuberculosis 21 days after starting treatment (n=368 077). The inset shows the same data on an enlarged y-axis. LTBI=latent tuberculosis infection.
In the sensitivity analysis, after excluding tuberculosis cases diagnosed within 60 and 90 days of primary care registration or LTBI testing, the overall effectiveness of the LTBI testing and treatment programme was lower, but the association between LTBI testing and a lower risk of tuberculosis diagnosis remained significant (HR 0·82 [95% CI 0·69–0·99] when excluding individuals diagnosed within 60 days; 0·81 [0·68–0·96] when excluding individuals diagnosed within 90 days; appendix pp 14–16). The intervention remained associated with higher risk of tuberculosis diagnosis during the first 6 months of follow-up (11·14 [8·11–15·30] when excluding patients diagnosed with tuberculosis within 60 days; 15·72 [10·30–23·90] when excluding individuals diagnosed with tuberculosis within 90 days), followed by a significantly lower risk after 6 months (0·55 [0·38–0·77] when excluding individuals diagnosed with tuberculosis within 60 days; 0·56 [0·39–0·79] when excluding individuals diagnosed with tuberculosis within 90 days; appendix pp 14, 16).
The effect of pre-entry screening for active tuberculosis was included in the analysis as a potential confounder.23, 24 Pre-entry screening was associated with a higher risk of tuberculosis diagnosis than no pre-entry screening (HR 1·27 [95% CI 1·03–1·56]; table 2). No interaction was identified (ie, the effect of pre-entry screening on LTBI testing and treatment did not change, depending on the value of the pre-entry screening) between pre-entry screening and LTBI testing and treatment (data not shown).25
Pre-entry screening for active tuberculosis was rolled out in 2012 and LTBI testing and treatment from 2014 onwards.13, 23 We therefore assessed whether the effects of the two interventions were modified by year of entry into England or of primary care registration. No effect modification was identified between LTBI testing and treatment and cohort year (data not shown), but the effect of pre-entry screening differed by cohort year: the risk of developing tuberculosis was lower in individuals who had pre-entry screening in the cohort year 2017–18 than the cohort year 2011–12 (HR 0·43 [95% CI 0·29–0·63]), whereas no effect was observed in earlier years (table 2; appendix pp 9, 14). The fact that no effect was observed in earlier cohort years is likely to be explained by the exclusion of individuals who developed tuberculosis before January, 2014 when the LTBI programme roll-out began. Hence, the protective effect of pre-entry screening for active tuberculosis in the present study would only be observed in the later cohort years. All associations remained significant after accounting for area-level variation with random effects (appendix p 17).
128 IGRA-positive individuals who did not start treatment developed tuberculosis (incidence 1197 cases [95% CI 1000–1420] per 100 000 person-years), and 24 IGRA-negative individuals developed tuberculosis (incidence 37 cases [95% CI 24–55] per 100 000 person-years; HR 31·9 [95% CI 20·4–49·8]; table 3; appendix pp 10, 18). The risk of tuberculosis was lower in the sensitivity analysis when tuberculosis cases diagnosed within 60 and 90 days of primary care registration or LTBI testing were excluded (HR 18·6 [11·6–29·9] when excluding individuals diagnosed within 60 days; 14·6 [8·97–23·7] when excluding individuals diagnosed within 90 days; appendix pp 19–20). All associations remained significant after accounting for area-level variation with random effects (appendix p 21).
Table 3.
Multivariable Cox regression model analysis of time to tuberculosis diagnosis according to IGRA status
| Tuberculosis cases per person-years of follow-up* | Incidence per 100 000 person-years (95% CI) | HR (95% CI)† | p value | |
|---|---|---|---|---|
| IGRA status | ||||
| Positive | 128/10 684 | 1190 (1000–1420) | 31·9 (20·4–49·8) | <0·0001 |
| Negative | 24/64 537 | 37 (24–55) | 1 (ref) | .. |
| Age, years | ||||
| 16–25 | 62/33 747 | 183 (143–235) | 1·04 (0·74–1·46) | 0·795 |
| 26–35 | 97/42 849 | 226 (185–276) | 1 (ref) | .. |
| Sex | ||||
| Female | 94/33 899 | 277 (226–339) | 1 (ref) | .. |
| Male | 63/41 010 | 153 (120–196) | 0·65 (0·46–0·90) | 0·010 |
| ETS region of origin | ||||
| Africa | 24/7598 | 315 (211–471) | 1 (ref) | .. |
| The Americas or Europe | 0/614 | 0 | 0 | .. |
| East and southeast Asia | 2/1045 | 191 (478–764) | 0·78 (0·17–3·53) | 0·750 |
| South Asia | 58/35 394 | 163 (126–211) | 0·84 (0·42–1·69) | 0·616 |
| Tuberculosis incidence in country of origin (cases per 100 000)‡ | ||||
| 40–149 | 12/3856 | 311 (176–547) | 1 (ref) | .. |
| 150–349 | 67/39 690 | 168 (132–214) | 0·69 (0·33–1·45) | 0·305 |
| >350 | 5/1106 | 451 (188–108) | 0·81 (0·27–2·36) | 0·694 |
| Tuberculosis incidence in area of residence (cases per 100 000)§ | ||||
| ≤9·2 | 8/5668 | 141 (70–282) | 1 (ref) | .. |
| 9·3–31·6 | 61/25 554 | 238 (185–306) | 1·77 (0·83–3·78) | 0·135 |
| >31·6 | 69/37 733 | 182 (144–231) | 1·05 (0·49–2·24) | 0·885 |
| Deprivation index | ||||
| 1–3 deciles (most deprived) | 91/45 531 | 199 (162–245) | 1 (ref) | .. |
| 4–6 deciles | 50/23 543 | 212 (160–280) | 1·06 (0·73–1·54) | 0·750 |
| 7–10 deciles (least deprived) | 11/4965 | 221 (122–400) | 1·25 (0·66–2·36) | 0·486 |
| Year of arrival in England or primary care registration | ||||
| 2011–12 | 12/4684 | 256 (145–451) | 1 (ref) | .. |
| 2013–14 | 16/6923 | 231 (141–377) | 1·51 (0·77–2·97) | 0·224 |
| 2015–16 | 32/17 123 | 186 (132–264) | 0·74 (0·40–1·37) | 0·346 |
| 2017–18 | 24/15 921 | 150 (101–224) | 0·21 (0·10–0·44) | <0·0001 |
IGRA=interferon-γ release assay. HR=hazard ratio. ETS=enhanced tuberculosis surveillance system. LTBI=latent tuberculosis infection.
Included only participants with no missing information for that characteristic.
HR (95% CI) and p value estimates were derived from the total cohort of migrants with a positive IGRA who did not start LTBI treatment, after multiple imputation for missing values by chained equations model (n=34 792); migrants with indeterminate IGRA results or with no results were excluded.
WHO estimated tuberculosis incidence.13
UK Health Security Agency estimated tuberculosis incidence in Clinical Commissioning Group area of residence.
135 tuberculosis cases were diagnosed in the IGRA-positive cohort during 15 470 person-years follow-up, with median follow-up of 2·1 years (IQR 1·45–5·31) per person. In the group who started LTBI treatment, seven individuals developed active tuberculosis (incidence 187 cases [95% CI 89–393] per 100 000 person-years) compared with 128 individuals in the untreated group (incidence 1090 cases [95% CI 916–1290] per 100 000 person-years). Thus, LTBI treatment was associated with a substantially lower risk of developing active tuberculosis (HR 0·14 [95% CI 0·06–0·32]; table 4; appendix p 10). This effect remained after accounting for area-level variation with random effects (appendix p 23). The confirmed date of treatment completion was available for 988 (56·7%) of 1740 individuals who started treatment. A sensitivity analysis including only individuals who started treatment yielded a higher value for treatment effectiveness (HR 0·08 [95% CI 0·02–0·26]; appendix pp 23–24). Thus, the programme would remain effective even if a substantial proportion of individuals had poor compliance. The effectiveness of treatment was lower, but remained significant, in the sensitivity analysis when tuberculosis cases diagnosed within 60 and 90 days of primary care registration or LTBI testing were excluded (0·23 [0·09–0·57] for 60 days; 0·25 [0·09–0·67] for 90 days; appendix pp 25–26).
Table 4.
Multivariable Cox regression model analysis of time to tuberculosis diagnosis according to treatment status
| Tuberculosis cases per person-years of follow-up* | Incidence per 100 000 person-years (95% CI) | HR (95% CI)† | p value | |
|---|---|---|---|---|
| Treatment | ||||
| Yes | 7/3728 | 187 (89–393) | 0·14 (0·06–0·32) | <0·0001 |
| No | 128/11742 | 1090 (916–1296) | 1·00 | .. |
| Age, years | ||||
| 16–25 | 50/4892 | 1020 (774–1340) | 1·27 (0·88–1·83) | 0·192 |
| 26–35 | 83/10218 | 812 (655–1007) | 1·00 | .. |
| Sex | ||||
| Female | 80/7747 | 1032 (829–1280) | 1·00 | .. |
| Male | 53/7415 | 714 (546–935) | 0·71 (0·50–1·01) | 0·059 |
| ETS region of origin | ||||
| Africa | 21/2323 | 903 (589–1380) | 1·00 | .. |
| The Americas or Europe | 0/68·51 | 0 | 0 | .. |
| East and southeast Asia | 2/258 | 772 (193–3080) | 0·85 (0·17–4·12) | 0·847 |
| South Asia | 52/6845 | 759 (578–996) | 0·87 (0·45–1·66) | 0·655 |
| Tuberculosis incidence in country of origin (cases per 100 000)‡ | ||||
| 40–149 | 9/988 | 910 (473–1750) | 1·00 | .. |
| 150–349 | 61/8157 | 747 (581–961) | 0·73 (0·42–1·26) | 0·257 |
| >350 | 5/351 | 1420 (592–3410) | 0·93 (0·27–3·19) | 0·915 |
| Tuberculosis incidence in area of residence (cases per 100 000)§ | ||||
| ≤9·2 | 8/1176 | 680 (340–1360) | 1·00 | .. |
| 9·3–31·6 | 54/5461 | 988 (757–1290) | 1·72 (0·80–3·69) | 0·163 |
| >31·6 | 53/7373 | 718 (549–940) | 0·66 (0·31–1·43) | 0·300 |
| Deprivation index | ||||
| 1–3 deciles (most deprived) | 80/9246 | 865 (694–1070) | 1·00 | .. |
| 4–6 deciles | 39/4574 | 852 (622–1160) | 1·12 (0·74–1·69) | 0·564 |
| 7–10 deciles (least deprived) | 9/858 | 1040 (545–2010) | 1·14 (0·56–2·34) | 0·708 |
| Year of arrival in England or primary care registration | ||||
| 2011–12 | 12/978 | 1220 (696–2150) | 1·00 | .. |
| 2013–14 | 15/1438 | 1042 (628–1730) | 1·07 (0·54–2·10) | 0·833 |
| 2015–16 | 29/3844 | 754 (524–1080) | 0·90 (0·48–1·68) | 0·744 |
| 2017–18 | 19/3235 | 587 (374–920) | 1·00 (0·46–2·15) | 0·996 |
HR=hazard ratio. ETS=enhanced tuberculosis surveillance system.
Included only participants with no missing information for that characteristic.
HR (95% CI) and p value estimates were derived from the total cohort of migrants with a positive interferon-γ release assay after multiple imputation for missing values by chained equations model excluding those who developed tuberculosis 21 days after starting treatment (n=6620).
WHO estimated tuberculosis incidence.13
UK Health Security Agency estimated tuberculosis incidence in Clinical Commissioning Group area of residence.
The adjusted NNT was estimated using only participants with no missing data (n=3812). The NNT, with adjustment for all covariates,22 defined as the average number of untreated IGRA-positive individuals who would have had to start LTBI treatment at baseline to prevent one case of tuberculosis before 2 years was 32·5 (95% CI 17·0–48·0; table 4) and before 3 years was 26 (7·0–45·0). In the sensitivity analysis, the adjusted NNTs were 33·7 (19·0–48·4) when excluding individuals diagnosed within 60 days and 34·8 (19·8–49·8) when excluding individuals diagnosed within 90 days (appendix pp 25–26).
Data on treatment regimens were available for 1600 (92%) of the 1740 treated individuals; of these 1564 (97·8%) were treated with rifampicin and isoniazid for 3 months, 34 (2·1%) were treated with isoniazid for 6 months, and two (0·1%) were treated with rifampicin for 4 months. 74 (4·6%) of 1600 individuals reported adverse reactions, including 11 (0·7%) with hepatotoxicity, of whom five were treated with rifampicin and isoniazid for 3 months (five [0·3%] of 1564 patients on this regimen) and six were treated with isoniazid for 6 months (six [17·6%] of 34 patients on this regimen; appendix p 29).
Discussion
The LTBI testing and treatment programme can reduce the risk of being diagnosed with tuberculosis in newly arrived foreign-born individuals in England. Programmatic LTBI testing and treatment is associated with earlier tuberculosis diagnosis and an overall lower risk of tuberculosis. IGRA-positive individuals have a 31-times higher risk of developing active tuberculosis than IGRA-negative individuals and LTBI treatment reduced this risk by 86%. We identified key limitations of the programme. These include a low proportion of eligible migrants who were tested and low uptake of preventive treatment in those testing IGRA-positive. Addressing these limitations would improve the population-level effectiveness of the programme and thereby reduce national tuberculosis incidence.
Our results show that after an initial increase in tuberculosis incidence in the tested group, the intervention reduces the risk of developing tuberculosis in the longer term. The initial increase in tuberculosis incidence is explained by the mandatory clinical and radiographical assessment for active tuberculosis in IGRA-positive individuals.13, 26 For this reason, our primary analysis included all tuberculosis cases because we assumed that they all could be identified through the screening pathway. However, cases diagnosed early after primary care registration or LTBI testing might instead be asymptomatic prevalent cases,27 detected by programmatic IGRA testing followed by chest radiography if positive. We addressed this through sensitivity analyses that denoted all tuberculosis cases identified within 60 or 90 days as prevalent, thus excluding them from evaluation of the programme's effectiveness in preventing tuberculosis. This further confirmed the programme's effectiveness in preventing progression to tuberculosis and its effect on increasing early case detection of active tuberculosis. Active case finding is a potentially effective, risk-group-based, health-service-initiated screening intervention to detect and treat active tuberculosis.26, 28 Our results suggest that programmatic LTBI testing could be used as an active case finding intervention for this key high-risk group.29 The intervention risk stratifies newly arrived foreign-born individuals, who have higher risk of developing tuberculosis compared with the general population.13, 29 In this model, the confirmatory diagnostic investigation is systematically triggered by a positive IGRA result.13 Importantly, earlier diagnosis of active tuberculosis through the LTBI testing programme is likely to reduce tuberculosis transmission.26, 28, 30
The LTBI testing and treatment programme was underpinned by modelling suggesting that it could cost-effectively avert future cases of tuberculosis.3 Tuberculosis incidence in England has significantly reduced since 2013, with the lowest incidence recorded in 2018 (8·3 cases per 100 000 population).29 This evaluation provides strong evidence that the pre-entry active tuberculosis screening and LTBI testing and treatment programmes are contributing to this reduction, consistent with preliminary data.24, 31 As England progresses towards tuberculosis elimination,29 the proportion of cases arising in groups not included in the programme, such as migrants resident for more than 5 years or older than 35 years, will increase.29 Therefore the LTBI programme will require periodic adjustment to address the epidemiological transition brought about, in part, by its success.
We estimated the risk of tuberculosis stratified by IGRA results in the largest programmatic cohort of foreign-born individuals with the longest follow-up to date, and demonstrate the utility of IGRAs for large-scale risk stratification in a routine setting. Our estimate of tuberculosis incidence among IGRA-positive migrants is similar to that reported in a meta-analysis published in 2020.32 The risk estimate in IGRA-positive individuals compared with IGRA-negative individuals is consistent with a previous estimate in a UK-based cohort of migrants.33
We present evidence of effectiveness of a 3-month rifampicin and isoniazid treatment regimen for LTBI, consistent with a 2017 meta-analysis of studies done in people with HIV, and children and adults with silicosis.34 We furthermore observed a low risk of hepatotoxicity, confirming that this regimen is safe and effective.
We provide a programmatic NNT estimate of relevance for national and international policy makers. Our estimates of NNT were similar to other studies in the UK and Norway,35, 36 and lower than modelling-based estimates for the USA.37, 38 The high NNT raises important issues because LTBI treatment might be given with no benefit, while exposing individuals to potential adverse events, even if this risk is low.1 WHO guidelines recommend LTBI treatment in risk groups based on direct and surrogate evidence of increased risk and, since tuberculosis is contagious, the disease has public health consequences beyond the individual patients.39 Low-incidence countries have set a goal to eliminate tuberculosis,2 but this is not possible without testing and treating LTBI in migrants.4 Our evaluation provides evidence to guide policy makers, clinicians, and patients to make informed decisions regarding LTBI treatment, weighing the individual risks and benefits against the overall public health benefit.1 These considerations should inform LTBI testing and treatment programmes, and consideration of the priorities of migrant communities.40, 41, 42
The programme is provided free to all migrants from countries with high tuberculosis incidence staying in England for at least 6 months, with the only prerequisite being registration for primary care.41, 43 However, primary care registration among migrants is lower than in UK-born individuals and undocumented migrants do not register.24, 41, 43 These individuals are therefore not reached by the programme and thus were not included in our cohort; improving rates of primary care registration among migrants would probably enhance the impact of the programme at the national level and could result in other individual-level and public health benefits. To our knowledge, our study is the first to quantify the losses at individual steps of the LTBI cascade of care for foreign-born individuals in a nationwide programmatic setting. Although only around one in four IGRA-positive individuals started LTBI treatment, the programme was effective. Quantifying the proportion of migrants lost at each stage of the care cascade, and understanding the reasons for this, are important to enable improvements in programme participation. In other settings, migrants are less likely than other groups to complete LTBI screening and start and complete treatment.44 Reasons for not completing testing and treatment include language barriers, self-perceived low risk of LTBI or active tuberculosis, stigma, mistrust, and fear of deportation or immigration status.41, 44 Addressing such barriers will be pivotal for further increasing the effectiveness of the programme.
Our study had some limitations. We did not attempt to exclude incident cases acquired from transmission within England; however, there would have been few such cases in our cohort considering the local epidemiology of tuberculosis transmission.45 Our analysis did not account for the presence of comorbidities, such as HIV or diabetes, which increase risk of tuberculosis, but this would not affect our main findings or implications for low-incidence countries. Our results might be affected by missing information; however, we accounted for the potential bias introduced by missing values by using a multiple imputation model assuming the data was missing at random. We then did sensitivity analysis using only participants with complete information and the results did not vary. The risk of verification, incorporation, and overdiagnosis bias was minimised by using record linkage to establish the final disease status; assignment was based on a quality-assured surveillance system that captures all notifications and de-notifications.16 The UK Health Security Agency did not have permission to obtain data on individuals who opted not to be tested, hence we could not specifically quantify programme uptake. Our linkage method did not allow us to establish whether the tuberculosis diagnosis was the result of a programmatic positive IGRA test; therefore, we cannot ascertain whether cases identified in the first 6 months were diagnosed as a result of programmatic LTBI testing. Moreover, we could not identify IGRA-positive individuals who were not candidates for treatment (eg, those who might have already been treated). Although clinical assessment of IGRA-positive individuals was mostly done in secondary care, in some areas, such as east London, this was done in primary care. We could not account for the resulting potential heterogeneity in the clinical pathways for exclusion of active tuberculosis. Within our cohort, we could not determine which migrants might have been refugees or asylum seekers. Since our cohort did not include undocumented migrants, our evaluation did not assess the effect of LTBI testing and treatment in this population. It is possible that individuals diagnosed in the early follow-up period differed from those who remained tuberculosis-free during this period, which could introduce selection bias.46 In our cohort, the only pathway to treatment was through programmatic LTBI testing; thus, no untested individual had treatment and for this reason we could not apply standard statistical methods to account for selection bias, and cannot therefore rule out the presence of such bias in the estimate of overall programme effectiveness.46 However, LTBI treatment was effective at averting tuberculosis in IGRA-positive individuals, suggesting that treatment effectiveness, and not selection bias, was the main contributor to overall programme effectiveness.
In conclusion, this study shows that programmatic LTBI testing and treatment of new migrants is feasible and effective in a nationwide routine setting, lowering the risk of developing tuberculosis and facilitating earlier diagnosis of tuberculosis. The programme would be substantially more effective if a higher proportion of eligible migrants participated, and in particular, if those who test IGRA-positive started treatment. National programmes such as this could therefore be important public health interventions to enable tuberculosis elimination in low-incidence regions.
Data sharing
The study protocol and STATA files (containing the commands used) can be made available upon request. However, the de-identified individual participant data that underlie the results reported in this article are the property of the UK Health Security Agency and cannot be made available. Please contact the corresponding author for further information.
Declaration of interests
AL is named inventor on patents pertaining to T-cell-based diagnosis, including current and second-generation IGRA technologies and flow-cytometric diagnosis and prognosis of tuberculosis infection and disease; some of these patents were assigned by the University of Oxford (Oxford, UK) to Oxford Immunotec, resulting in the T-SPOT.TB test with royalty entitlements for the University of Oxford and AL. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This research was funded by the National Institute for Health Research (NIHR) Health Protection Research Unit in Respiratory Infections at Imperial College London (London, UK) in partnership with the UK Health Security Agency (London, UK). The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, the Department of Health, or the UK Health Security Agency. AL was supported by the NIHR Imperial Biomedical Research Centre. We thank Nirmalan Arinaminpathy whose constructive feedback greatly contributed to the final version of this manuscript and Umar Niazi for his assistance in the calculation of the adjusted number needed to treat.
Contributors
LCB-A and AL conceived and designed the study. MCM and ODC gathered, processed, and cleaned the data. AM, A-MO and LCB-A did the record linkage. LCB-A, RJH, and SMC analysed the data. LCB-A and AL had full access to all the data in the study. LCB-A wrote the first draft of the manuscript followed by iterative revision with AL. All authors substantially contributed to discussion of content and reviewed and edited the manuscript before submission. All authors were involved in the decision to submit and agreed to publish the paper.
Supplementary Material
References
- 1.Campbell JR, Dowdy D, Schwartzman K. Treatment of latent infection to achieve tuberculosis elimination in low-incidence countries. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45:928–952. doi: 10.1183/09031936.00214014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pareek M, Watson JP, Ormerod LP, et al. Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis. 2011;11:435–444. doi: 10.1016/S1473-3099(11)70069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosales-Klintz S, Bruchfeld J, Haas W, et al. Guidance for programmatic management of latent tuberculosis infection in the European Union/European Economic Area. Eur Respir J. 2019;53 doi: 10.1183/13993003.02077-2018. [DOI] [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention and Control Programmatic management of latent tuberculosis infection in the European Union. 2018. https://www.ecdc.europa.eu/sites/default/files/documents/October-2018-Programmatic-management-LTBI-EU.pdf
- 6.WHO . World Health Organization; Geneva: 2015. Guidelines on the management of latent tuberculosis infection.https://apps.who.int/iris/bitstream/handle/10665/136471/9789241548908_eng.pdf;jsessionid=A098AA6028BDE499F4AAED67C3D7F4DE?sequence=1 [PubMed] [Google Scholar]
- 7.Pareek M, Baussano I, Abubakar I, Dye C, Lalvani A. Evaluation of immigrant tuberculosis screening in industrialized countries. Emerg Infect Dis. 2012;18:1422–1429. doi: 10.3201/eid1809.120128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pareek M, Abubakar I, White PJ, Garnett GP, Lalvani A. Tuberculosis screening of migrants to low-burden nations: insights from evaluation of UK practice. Eur Respir J. 2011;37:1175–1182. doi: 10.1183/09031936.00105810. [DOI] [PubMed] [Google Scholar]
- 9.Abubakar I, Lalvani A, Southern J, et al. Two interferon gamma release assays for predicting active tuberculosis: the UK PREDICT TB prognostic test study. Health Technol Assess. 2018;22:1–96. doi: 10.3310/hta22560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalvani A, Pareek M. Immigrant screening for TB: a missed opportunity to improve TB control in the United Kingdom. Pathog Glob Health. 2012;106:5–7. doi: 10.1179/204777312X13305103762501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pareek M, Bond M, Shorey J, et al. Community-based evaluation of immigrant tuberculosis screening using interferon γ release assays and tuberculin skin testing: observational study and economic analysis. Thorax. 2013;68:230–239. doi: 10.1136/thoraxjnl-2011-201542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Public Health England Collaborative tuberculosis strategy for England 2015–2020. 2015. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/403231/Collaborative_TB_Strategy_for_England_2015_2020_.pdf
- 13.Public Health England Latent TB testing and treatment for migrants: a practical guide for commissioners and practitioners. 2015. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/442192/030615_LTBI_testing_and_treatment_for_migrants_1.pdf
- 14.National Institute for Health and Care Excellence Tuberculosis. NICE guideline. Jan 13, 2016. https://www.nice.org.uk/guidance/ng33/resources/tuberculosis-pdf-1837390683589 [PubMed]
- 15.Aldridge RW, Shaji K, Hayward AC, Abubakar I. Accuracy of probabilistic linkage using the enhanced matching system for public health and epidemiological studies. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Public Health England Guidance on notifying tuberculosis (TB) cases. 2014. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/360263/Guidance_on_Notifying_Tuberculosis__TB__cases.pdf
- 17.Abubakar I, Drobniewski F, Southern J, et al. Prognostic value of interferon-γ release assays and tuberculin skin test in predicting the development of active tuberculosis (UK PREDICT TB): a prospective cohort study. Lancet Infect Dis. 2018;18:1077–1087. doi: 10.1016/S1473-3099(18)30355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleves M, Gutierrez RG, Gould W, Marchenko YV. In: An introduction to survival analysis using Stata. Cleves M, Gutierrez RG, Gould W, Marchenko YV, editors. Stata Press; College Station, TX: 2010. Model building using stcox; pp. 189–196. [Google Scholar]
- 19.Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med. 2018;6:121. doi: 10.21037/atm.2018.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin DB. John Wiley; New York, NY: 1987. Multiple imputation for nonresponse in surveys. [Google Scholar]
- 21.He Y. Missing data analysis using multiple imputation: getting to the heart of the matter. Circ Cardiovasc Qual Outcomes. 2010;3:98–105. doi: 10.1161/CIRCOUTCOMES.109.875658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sjölander A. Estimation of causal effect measures with the R-package stdReg. Eur J Epidemiol. 2018;33:847–858. doi: 10.1007/s10654-018-0375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aldridge RW, Zenner D, White PJ, et al. Prevalence of and risk factors for active tuberculosis in migrants screened before entry to the UK: a population-based cross-sectional study. Lancet Infect Dis. 2016;16:962–970. doi: 10.1016/S1473-3099(16)00072-4. [DOI] [PubMed] [Google Scholar]
- 24.Berrocal-Almanza LC, Harris R, Lalor MK, et al. Effectiveness of pre-entry active tuberculosis and post-entry latent tuberculosis screening in new entrants to the UK: a retrospective, population-based cohort study. Lancet Infect Dis. 2019;19:1191–1201. doi: 10.1016/S1473-3099(19)30260-9. [DOI] [PubMed] [Google Scholar]
- 25.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41:514–520. doi: 10.1093/ije/dyr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho J, Fox GJ, Marais BJ. Passive case finding for tuberculosis is not enough. Int J Mycobacteriol. 2016;5:374–378. doi: 10.1016/j.ijmyco.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Kendall EA, Shrestha S, Dowdy DW. The epidemiological importance of subclinical tuberculosis. A critical reappraisal. Am J Respir Crit Care Med. 2021;203:168–174. doi: 10.1164/rccm.202006-2394PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lönnroth K, Corbett E, Golub J, et al. Systematic screening for active tuberculosis: rationale, definitions and key considerations. Int J Tuberc Lung Dis. 2013;17:289–298. doi: 10.5588/ijtld.12.0797. [DOI] [PubMed] [Google Scholar]
- 29.Public Health England Tuberculosis in England report. 2019. https://webarchive.nationalarchives.gov.uk/ukgwa/20190801150347/https://www.gov.uk/government/publications/tuberculosis-in-england-annual-report
- 30.Azman AS, Golub JE, Dowdy DW. How much is tuberculosis screening worth? Estimating the value of active case finding for tuberculosis in South Africa, China, and India. BMC Med. 2014;12:216. doi: 10.1186/s12916-014-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas HL, Harris RJ, Muzyamba MC, et al. Reduction in tuberculosis incidence in the UK from 2011 to 2015: a population-based study. Thorax. 2018;73:769–775. doi: 10.1136/thoraxjnl-2017-211074. [DOI] [PubMed] [Google Scholar]
- 32.Campbell JR, Winters N, Menzies D. Absolute risk of tuberculosis among untreated populations with a positive tuberculin skin test or interferon-gamma release assay result: systematic review and meta-analysis. BMJ. 2020;368:m549. doi: 10.1136/bmj.m549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zenner D, Loutet MG, Harris R, Wilson S, Ormerod LP. Evaluating 17 years of latent tuberculosis infection screening in north-west England: a retrospective cohort study of reactivation. Eur Respir J. 2017;50 doi: 10.1183/13993003.02505-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zenner D, Beer N, Harris RJ, Lipman MC, Stagg HR, van der Werf MJ. Treatment of latent tuberculosis infection: an updated network meta-analysis. Ann Intern Med. 2017;167:248–255. doi: 10.7326/M17-0609. [DOI] [PubMed] [Google Scholar]
- 35.Kruijshaar ME, Abubakar I, Stagg HR, Pedrazzoli D, Lipman M. Migration and tuberculosis in the UK: targeting screening for latent infection to those at greatest risk of disease. Thorax. 2013;68:1172–1174. doi: 10.1136/thoraxjnl-2013-203254. [DOI] [PubMed] [Google Scholar]
- 36.Winje BA, Grøneng GM, White RA, Akre P, Aavitsland P, Heldal E. Immigrant screening for latent tuberculosis infection: numbers needed to test and treat, a Norwegian population-based cohort study. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-023412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose DN. Benefits of screening for latent Mycobacterium tuberculosis infection. Arch Intern Med. 2000;160:1513–1521. doi: 10.1001/archinte.160.10.1513. [DOI] [PubMed] [Google Scholar]
- 38.Tasillo A, Salomon JA, Trikalinos TA, Horsburgh CR, Jr, Marks SM, Linas BP. Cost-effectiveness of testing and treatment for latent tuberculosis infection in residents born outside the United States with and without medical comorbidities in a simulation model. JAMA Intern Med. 2017;177:1755–1764. doi: 10.1001/jamainternmed.2017.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lönnroth K, Glaziou P, Weil D, Floyd K, Uplekar M, Raviglione M. Beyond UHC: monitoring health and social protection coverage in the context of tuberculosis care and prevention. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denholm JT, Matteelli A, Reis A. Latent tuberculous infection: ethical considerations in formulating public health policy. Int J Tuberc Lung Dis. 2015;19:137–140. doi: 10.5588/ijtld.14.0543. [DOI] [PubMed] [Google Scholar]
- 41.Berrocal-Almanza LC, Botticello J, Piotrowski H, et al. Engaging with civil society to improve access to LTBI screening for new migrants in England: a qualitative study. Int J Tuberc Lung Dis. 2019;23:563–570. doi: 10.5588/ijtld.18.0230. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell EMH, Heumann SG, Sprague L, Tesfaye DH, Van Dam A, Spruijt I. ‘Reservoir of infection' or ‘fount of knowledge’? Forging equal partnerships and shifting power to address LTBI. Int J Tuberc Lung Dis. 2019;23:527–528. doi: 10.5588/ijtld.19.0193. [DOI] [PubMed] [Google Scholar]
- 43.Stagg HR, Jones J, Bickler G, Abubakar I. Poor uptake of primary healthcare registration among recent entrants to the UK: a retrospective cohort study. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:1269–1278. doi: 10.1016/S1473-3099(16)30216-X. [DOI] [PubMed] [Google Scholar]
- 45.Davidson JA, Thomas HL, Maguire H, et al. Understanding tuberculosis transmission in the United Kingdom: findings from 6 years of mycobacterial interspersed repetitive unit-variable number tandem repeats strain typing, 2010–2015. Am J Epidemiol. 2018;187:2233–2242. doi: 10.1093/aje/kwy119. [DOI] [PubMed] [Google Scholar]
- 46.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol and STATA files (containing the commands used) can be made available upon request. However, the de-identified individual participant data that underlie the results reported in this article are the property of the UK Health Security Agency and cannot be made available. Please contact the corresponding author for further information.