Abstract
Background:
Prepectoral placement of tissue expanders(TE) for two-stage implant-based breast reconstruction potentially minimizes chest wall morbidity and postoperative pain. We explored 90-day clinical and health-related quality-of-life outcomes for prepectoral versus subpectoral TE breast reconstruction.
Methods:
We conducted a propensity score-matching analysis (nearest neighbor, 1:1 matching without replacement) of patients who underwent immediate prepectoral or subpectoral TE breast reconstruction between December 2017 and January 2019. Matched covariates included age, body mass index, race/ethnicity, smoking status, chemotherapy, radiotherapy, nipple-sparing mastectomy, and laterality of reconstruction. Outcomes of interest were perioperative analgesia use, 90-day postoperative patient-reported pain, complication rates, and BREAST-Q physical well-being of the chest(PWB-Chest) scores.
Results:
Of the initial cohort of 921patients, 238 were propensity-matched and included in the final analysis. The matched cohort had no differences in baseline characteristics. Postoperative ketorolac(p = 0.048) use was higher in the subpectoral group; there were no other significant differences in intraoperative and postoperative analgesia use. Prepectoral patients had lower pain on postoperative days 1–2 but no differences in days 3–10. BREAST-Q PWB-Chest scores did not differ. Prepectoral patients had higher rates of seroma than subpectoral patients(p < 0.001). Rates of TE loss did not differ.
Conclusions:
This matched analysis of 90-day complications found lower early postoperative pain in prepectoral TE patients but no longer-term patient-reported differences. Although prepectoral reconstruction patients experienced a higher rate of seroma, this did not translate to a difference in TE loss. Long-term analysis of clinical and patient-reported outcomes is needed to understand the full profile of the prepectoral technique.
INTRODUCTION
Prepectoral alloplastic breast reconstruction has become an accepted option for single and two-stage techniques.(1) Historically, subpectoral placement of the tissue expander has been the gold standard, given the increased vascularized soft tissue coverage; however, over the past decade plastic surgeons have revisited prepectoral breast reconstruction in an effort to reduce chest wall morbidity, perioperative narcotic use, and animation.(2) Some of the earliest alloplastic experience involved prepectoral placement,(3) which at that time was fraught with complications, including malposition, visibility, palpability, wrinkling, rippling, implant exposure, and skin breakdown.(2, 4, 5) Placing the prosthesis in the subpectoral plane addressed many of these early issues but presented its own set of challenges, including pain, animation deformity, and poor breast projection.(2, 6, 7)
Reported outcomes following prepectoral reconstruction compare favorably with subpectoral reconstruction, aiding in the prepectoral technique gaining clinical traction.(8–11) Although complications and outcomes are similar between these techniques,(12, 13) there is notable heterogeneity in reporting and assessment, including patient selection bias. There are also sizable patient level differences and inconsistencies in study time horizons between groups, both of which can impact study outcomes. Other studies report on small cohorts of patients, which can lead to underpowered results.(14–16)
Patient-reported outcomes (PROs) of subpectoral and prepectoral breast reconstruction with respect to pain and health-related quality of life (HR-QoL) vary and remain undetermined.(14, 17–19) The lack of manipulation and stretch of the pectoralis muscle would suggest that patients undergoing prepectoral breast reconstruction would have less pain. This hypothesis is supported by emerging data comparing patient-reported pain scores associated with placement of tissue expanders, with results trending toward less pain in the prepectoral group.(14, 18–22) PROs measured by the BREAST-Q, however, suggest comparable outcomes between subpectoral and prepectoral breast reconstruction patients, without significant differences.(2, 14, 17–19, 23) As prepectoral placement may impact both early HR-QoL and then later satisfaction, the timeframe and quality of these PRO studies must be considered. Reporting to date has not been consistent.
As with any new technique, it is essential that we use consistent and high-level methodologies to critically assess outcomes to have the best possible data to inform patient and surgeon decision-making, ideally incorporating both clinical outcomes and PROs. The purpose of our study was to perform a matched cohort analysis to examine perioperative pain, 90-day PROs, and 90-day complications following tissue expander placement in prepectoral and subpectoral alloplastic breast reconstruction patients. We hypothesized that, based on degree of muscle dissection and manipulation and degree of soft tissue coverage, prepectoral reconstruction patients would experience a trade-off between less pain and higher self-reported physical well-being of the chest scores and increased complication rates due to less vascularized soft tissue coverage of the tissue expander.
METHODS
Study Population
Following approval by our Institutional Review Board, we evaluated clinical outcomes and PROs, part of routine clinical care, for all patients undergoing postmastectomy immediate prepectoral or subpectoral implant-based reconstruction between December 2017 and January 2019 at an academic, National Cancer Institute–designated cancer center. Women aged 18 years and older undergoing two-stage reconstruction with placement of a tissue expander at the time of therapeutic or prophylactic mastectomy were included. Women who were younger than 18 years of age or who had preoperative radiotherapy were excluded. All patients underwent reconstruction at an ambulatory care facility, with 23-hour admissions. As part of standard pathways, patients were offered preoperative regional analgesic blocks (paravertebral blocks).
Patient Variables
The variables recorded for each patient included age, race/ethnicity, history of smoking, body mass index (BMI), history of psychiatric diagnosis (disorders defined as International Classification of Diseases, Tenth Revision: diagnosis codes such as ‘F%’ or International Classification of Diseases, Ninth Revision: diagnosis codes between ‘290’ and ‘319.99’), insurance status, marital status, chemotherapy status (i.e. neoadjuvant, adjuvant, none), radiotherapy status (i.e. preoperative, postoperative, none), whether mastectomy was nipple sparing, whether patient received axillary lymph node dissection, laterality of reconstruction, and acellular dermal matrix (ADM) use.
Outcomes of Interest
Intraoperative and postoperative clinical data on analgesic use were recorded for each patient. Variables included intraoperative intravenous administration of ketorolac and narcotics (i.e. fentanyl, hydromorphone, morphine; measured in morphine milligram equivalents), total visit intravenous narcotics administered (from surgery to discharge), postoperative ketorolac administration, and paravertebral block use. Patient-reported postoperative pain scores were recorded during the 10-day period following reconstruction. Patients were asked to complete a novel, patient-driven daily online assessment (19 questions) about the quality of their recovery, which included questions about nausea/vomiting, fatigue, anxiety, and pain. Postoperative pain scores were reported for the question: “What is the severity of your pain at its worst?” Patients could respond with “none” (0), “mild” (1), “moderate” (2), “severe” (3), or “very severe” (4). Complication data for each patient were recorded for the 90-day postoperative period and included mastectomy skin flap necrosis, nipple necrosis, breast hematoma, breast seroma, breast cellulitis (superficial), deep tissue expander infection, exposed tissue expander, leaking/ruptured tissue expander, tissue expander removal, and readmission within 90 days. Cellulitis was defined as a superficial cutaneous infection requiring treatment with antibiotics. A deep infection was defined as culture positive fluid around the tissue expander. All complications were calculated by breast, except for readmissions, as readmission is a patient-centric outcome in contrast to a laterality-specific variable.
Breast reconstruction–related PROs were assessed using the reconstruction module of the BREAST-Q and focused on physical well-being of the chest and upper body. Patients were administered the BREAST-Q preoperatively and postoperatively as part of routine clinical care. During expansion, patients were only asked to complete the physical wellbeing of the chest quality of life domain. Values for BREAST-Q were converted to summary scores ranging from 0 to 100, with higher scores correlating with superior patient satisfaction or better quality of life. A minimal clinically important difference of 4 points on the Q-Score was considered clinically important(24). Time points of interest included 2-week, 6-week, and 3-month scores.
The primary outcomes of interest were intraoperative and postoperative clinical data on analgesic use and patient-reported HR-QoL as measured through pain scores and BREAST-Q domain scores. Secondary outcomes of interest were procedure complication rates.
Propensity Score Matching
To balance possible confounders between the two cohorts, we performed a propensity score–matched analysis with one prepectoral patient matched to one subpectoral patient using nearest-neighbor (1:1) matching without replacement. Matched covariates included age, BMI, race/ethnicity, smoking history, timing of chemotherapy, postoperative radiotherapy, nipple-sparing mastectomy status, axillary lymph node dissection status (as a proxy for postoperative radiotherapy), and laterality of reconstruction. The distribution of the matched cohorts was assessed by jitter plot graphical analysis.
Statistical Analysis
We used a two-sided Student t-test (continuous variables) and Pearson chi-square test or Fisher exact test (categorical variables) to compare baseline demographics between unmatched prepectoral and subpectoral cohorts and to compare differences after matching. Intraoperative and postoperative analgesia data were reported as mean (standard deviation [SD]) and median (interquartile range[IQR]) for continuous variables and counts and frequencies for categorical variables. Differences in analgesics were assessed with Pearson chi-square or Mann-Whitney U test (continuous variables). Average pain scores for all patients with reported data were recorded. Differences at each timepoint were analyzed using a Mann-Whitney U test. Differences in complications were assessed with Pearson chi-square test or Fisher exact test. BREAST-Q domain scores were reported as mean (SD) and median (IQR) for both cohorts where differences were assessed with a Mann-Whitney U test. A subgroup analysis was conducted to assess the differences in scores between prepectoral and subpectoral patients by laterality of reconstruction. Differences in scores were assessed with a Mann-Whitney U test. All tests with a p value of < 0.05 were considered statistically significant. All statistical analyses were performed using R statistical software (version 3.6.2, packages: tidyverse, readxl, MatchIt).
RESULTS
A total of 921 breast reconstruction patients were initially included: 802 underwent subpectoral tissue expander placement and 119 underwent prepectoral tissue expander placement.
Demographics: Unmatched Cohort
In the unmatched cohort, the average age was 48.3 years (SD: 10.9) and the average BMI was 25.2 kg/m2 (SD: 5.8; Table 1). Most were white, Hispanic or not Hispanic (n = 712, 77.3%), never smokers (n = 609, 66.1%), and married (n = 630, 68.4%). The majority had no chemotherapy (n = 487, 52.9%) and no radiotherapy (n = 761, 82.6%). Most had bilateral reconstructions (n = 551, 59.8%) and skin-sparing mastectomy (n = 786, 85.3%). A greater proportion of prepectoral patients had no chemotherapy (p < 0.001) and no radiotherapy (p = 0.002) compared with subpectoral patients.
Table 1:
Demographics (Unmatched)
| Total Cohort | Subpectoral | Prepectoral | p value* | |
|---|---|---|---|---|
| n = 921 | n = 802 | n = 119 | ||
|
| ||||
| Age, mean years (SD) | 48.3 (10.9) | 48 (10.7) | 50.3 (11.7) | 0.052 |
|
| ||||
| Race, n (%) | 0.994 | |||
|
| ||||
| White, Hispanic or not Hispanic | 712 (77.3) | 620 (77.3) | 92 (77.3) | |
| Black, Hispanic or not Hispanic | 73 (7.9) | 63 (7.9) | 10 (8.4) | |
| Asian | 86 (9.3) | 75 (9.4) | 11 (9.2) | |
| Other/Unknown | 50 (5.4) | 44 (5.5) | 6 (5) | |
|
| ||||
| Smoking, n (%) | 0.313 | |||
|
| ||||
| Never Smoker | 609 (66.1) | 523 (65.2) | 86 (72.3) | |
| Previous | 262 (28.5) | 234 (29.2) | 28 (23.5) | |
| Current | 50 (5.4) | 45 (5.6) | 5 (4.2) | |
|
| ||||
| Hypertension, n (%) | 171 (18.6) | 149 (18.6) | 22 (18.5) | 0.981 |
|
| ||||
| Diabetes, n (%) | 49 (5.3) | 42 (5.2) | 7 (5.9) | 0.770 |
|
| ||||
| BMI, mean kg/m2 (SD) | 25.2 (5.8) | 26.2 (5.7) | 26.4 (5.9) | 0.625 |
|
| ||||
| Marital Status, n (%) | 0.126 | |||
|
| ||||
| Single | 187 (20.3) | 170 (21.2) | 17 (14.3) | |
| Married | 630 (68.4) | 548 (68.3) | 82 (68.9) | |
| Life/Domestic Partner | 6 (0.7) | 5 (0.6) | 1 (0.8) | |
| Separated | 12 (1.3) | 11 (1.4) | 1 (0.8) | |
| Divorced | 68 (7.4) | 55 (6.9) | 13 (10.9) | |
| Widowed | 18 (2.0) | 13 (1.6) | 5 (4.2) | |
|
| ||||
| Chemotherapy, n (%) | < 0.001 | |||
|
| ||||
| Neoadjuvant | 204 (22.2) | 184 (22.9) | 20 (16.8) | |
| Adjuvant | 230 (25.0) | 214 (26.7) | 16 (13.5) | |
| None | 487 (52.9) | 404 (50.4) | 83 (69.8) | |
|
| ||||
| Radiotherapy, n (%) | 0.002 | |||
|
| ||||
| Postoperative | 160 (17.4) | 151 (18.8) | 9 (7.6) | |
| None | 761 (82.6) | 651 (81.2) | 110 (92.4) | |
|
| ||||
| Laterality, n (%) | 0.215 | |||
|
| ||||
| Unilateral | 370 (40.2) | 316 (39.4) | 54 (45.4) | |
| Bilateral | 551 (59.8) | 486 (60.6) | 65 (54.6) | |
|
| ||||
| NSM, n (%) | 0.069 | |||
|
| ||||
| Yes | 135 (14.7) | 111 (13.8) | 24 (20.2) | |
| None | 786 (85.3) | 691 (86.2) | 95 (79.8) | |
|
| ||||
| Positioning, n (%) | - | |||
|
| ||||
| Total Submuscular | 209 (22.7) | 209 (26.1) | - | |
| Submuscular with ADM | 593 (64.4) | 593 (73.9) | - | |
|
| ||||
| ADM Use, n (%) | - | |||
|
| ||||
| Yes | 305 (33.1) | 209 (26.1) | 96 (80.7) | |
| None | 616 (66.9) | 593 (73.9) | 23 (19.3) | |
SD, standard deviation; n, number of patients; BMI, body mass index; NSM, nipple-sparing mastectomy; ADM, acellular dermal matrix.
“-“ indicates no value applicable or no p value calculated
p value calculated with Student t-test (continuous variables) or chi-square test (categorical variables)
Demographics: Matched Cohort
After matching and assessing the distribution of propensity scores between patients, a total of 238 patients were included in the final analysis: 119 prepectoral patients and 119 subpectoral patients (Table 2). There were no statistical differences between these cohorts on all matched and unmatched variables.
Table 2:
Demographics (Matched)
| Total Cohort | Subpectoral | Prepectoral | p value* | |
|---|---|---|---|---|
| n = 238 | n = 119 | n = 119 | ||
|
| ||||
| Age, mean years (SD) | 50.5 (11.5) | 50.7 (11.3) | 50.3 (11.7) | 0.783 |
|
| ||||
| Race, n (%) | 0.956 | |||
|
| ||||
| White, Hispanic or not Hispanic | 184 (77.3) | 92 (77.3) | 92 (77.3) | |
| Black, Hispanic or not Hispanic | 22 (9.2) | 12 (10.1) | 10 (8.4) | |
| Asian | 21 (8.8) | 10 (8.4) | 11 (9.2) | |
| Other/Unknown | 11 (4.6) | 5 (4.2) | 6 (5.0) | |
|
| ||||
| Smoking, n (%) | 0.428 | |||
|
| ||||
| Never Smoker | 179 (75.2) | 93 (78.2) | 86 (72.3) | |
| Previous | 48 (20.2) | 20 (16.8) | 28 (23.5) | |
| Current | 11 (4.6) | 6 (5) | 5 (4.2) | |
|
| ||||
| Hypertension, n (%) | 53 (22.3) | 31 (26.1) | 22 (18.5) | 0.161 |
|
| ||||
| Diabetes, n (%) | 14 (5.9) | 7 (5.9) | 7 (5.9) | 1 |
|
| ||||
| BMI, mean kg/m2 (SD) | 26.8 (5.9) | 27.1 (6) | 26.4 (5.9) | 0.408 |
|
| ||||
| Marital Status, n (%) | 0.104 | |||
|
| ||||
| Single | 46 (19.3) | 29 (24.4) | 17 (14.3) | |
| Married | 163 (68.5) | 81 (68.1) | 82 (68.9) | |
| Life/Domestic Partner | 1 (0.4) | 0 (0) | 1 (0.8) | |
| Separated | 2 (0.8) | 1 (0.8) | 1 (0.8) | |
| Divorced | 19 (8) | 6 (5.0) | 13 (10.9) | |
| Widowed | 7 (2.9) | 2 (1.7) | 5 (4.2) | |
|
| ||||
| Chemotherapy, n (%) | 0.434 | |||
|
| ||||
| Neoadjuvant | 35 (14.7) | 15 (12.6) | 20 (16.8) | |
| Adjuvant | 38 (16) | 22 (18.5) | 16 (13.5) | |
| None | 165 (69.3) | 82 (68.9) | 83 (69.8) | |
|
| ||||
| Radiotherapy, n (%) | 1 | |||
|
| ||||
| Postoperative | 18 (7.6) | 9 (7.6) | 9 (7.6) | |
| None | 220 (92.4) | 110 (92.4) | 110 (92.4) | |
|
| ||||
| Laterality, n (%) | 0.516 | |||
|
| ||||
| Unilateral | 113 (47.5) | 59 (49.6) | 54 (45.4) | |
| Bilateral | 125 (52.5) | 60 (50.4) | 65 (54.6) | |
|
| ||||
| NSM, n (%) | 0.743 | |||
|
| ||||
| Yes | 46 (19.3) | 22 (18.5) | 24 (20.2) | |
| None | 192 (80.7) | 97 (81.5) | 95 (79.8) | |
|
| ||||
| Positioning, n (%) | - | |||
|
| ||||
| Total Submuscular | 86 (36.1) | 86 (72.3) | - | |
| Submuscular with ADM | 33 (13.9) | 33 (27.7) | - | |
|
| ||||
| ADM Use, n (%) | - | |||
|
| ||||
| Yes | 129 (54.2) | 33 (27.7) | 96 (80.7) | |
| None | 109 (45.8) | 86 (72.3) | 23 (19.3) | |
SD, standard deviation; n, number of patients; BMI, body mass index; NSM, nipple-sparing mastectomy; ADM, acellular dermal matrix.
“-“ indicated no value applicable or no p value calculated
p value calculated with Student t-test (continuous variables) or chi-square test (categorical variables)
Intraoperative and Postoperative Analgesia
No significant differences in intraoperative total milligram morphine equivalents (MME) administered or frequency of ketorolac use were noted between prepectoral and subpectoral matched patients (Table 3). A significantly higher proportion of subpectoral patients received ketorolac (p = 0.048) postoperatively, but a non-significantly higher proportion of prepectoral patients received intraoperative ketorolac. There were no significant differences in total MME administered postoperatively or frequency of paravertebral block use between the two groups.
Table 3:
Intraoperative and Postoperative Analgesia
| Total Cohort | Subpectoral | Prepectoral | p value* | |
|---|---|---|---|---|
| n = 238 | n = 119 | n = 119 | ||
|
| ||||
| Intraoperative Period | ||||
|
| ||||
| Total MME | 0.613 | |||
|
| ||||
| Mean (SD) | 30.4 (18.6) | 30.5 (17.5) | 30.3 (19.6) | |
| Median (IQR) | 25.0 (20.0–40.0) | 30.0 (18.5–40.0) | 20.0 (20.0–40.0) | |
|
| ||||
| Ketorolac, n (%) | 0.233 | |||
|
| ||||
| Yes | 101 (42.4) | 46 (38.7) | 55 (46.2) | |
| No | 137 (57.6) | 73 (61.3) | 64 (53.8) | |
|
| ||||
| Postoperative Period | ||||
|
| ||||
| Visit Total MME | 0.383 | |||
|
| ||||
| Mean (SD) | 58.9 (42.5) | 57.4 (32.3) | 60.5 (50.8) | |
| Median (IQR) | 50.0 (33.3–70.8) | 50.0 (35.0–75.0) | 47.5 (31.3–67.8) | |
|
| ||||
| Ketorolac, n (%) | 0.048 | |||
|
| ||||
| Yes | 23 (9.7) | 16 (13.4) | 7 (5.9) | |
| No | 215 (90.3) | 103 (86.6) | 112 (94.1) | |
|
| ||||
| PVB Use, n (%) | 0.876 | |||
|
| ||||
| Yes | 186 (78.2) | 94 (79.0) | 92 (77.3) | |
| No | 52 (21.8) | 25 (21.0) | 27 (22.7) | |
SD, standard deviation; IQR, interquartile range; n, count; MME, morphine milligram equivalents; PVP, paravertebral block.
p value calculated using chi-square test (categorical variables) or Mann-Whitney test (continuous variables).
Patient-Reported Pain Scores
For both groups, severity of pain was mild overall in the first 10 postoperative days (Table 4). On days 1–2, the subpectoral group had significantly higher average pain scores than the prepectoral group (p = 0.042). However, by days 3–4 and through the remainder of the early postoperative period, no significant differences were noted in patient-reported pain (p > 0.05 at all-time points; Figure 1).
Table 4:
Patient-Reported Postoperative Pain Scores
| Subpectoral | Postoperative Day Range | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1–2 | 3–4 | 5–6 | 7–8 | 9–10 | ||||||
|
| ||||||||||
| n | Sum of Scores | n | Sum of Scores | n | Sum of Scores | n | Sum of Scores | n | Sum of Scores | |
| None | 3 | 0 | 6 | 0 | 5 | 0 | 6 | 0 | 9 | 0 |
| Mild | 5 | 5 | 28 | 28 | 24 | 24 | 31 | 31 | 24 | 24 |
| Moderate | 21 | 42 | 23 | 46 | 22 | 44 | 13 | 26 | 12 | 24 |
| Severe | 3 | 9 | 5 | 15 | 1 | 3 | 3 | 9 | 5 | 15 |
| Very Severe | - | - | - | - | - | - | - | - | - | - |
| No Response | 87 | - | 57 | - | 67 | - | 66 | - | 69 | - |
|
| ||||||||||
| Average Score for All Patients with Scores | 1.75 | 1.44 | 1.37 | 1.25 | 1.26 | |||||
| Prepectoral | Postoperative Day Range | |||||||||
|
| ||||||||||
| 1–2 | 3–4 | 5–6 | 7–8 | 9–10 | ||||||
|
| ||||||||||
| n | Sum of Scores | n | Sum of Scores | n | Sum of Scores | n | Sum of Scores | n | Sum of Scores | |
|
| ||||||||||
| None | 3 | 0 | 8 | 0 | 14 | 0 | 9 | 0 | 15 | 0 |
| Mild | 16 | 16 | 37 | 37 | 39 | 39 | 43 | 43 | 37 | 37 |
| Moderate | 14 | 28 | 21 | 42 | 22 | 44 | 21 | 42 | 14 | 28 |
| Severe | 2 | 6 | 6 | 18 | 1 | 3 | 2 | 6 | - | - |
| Very Severe | - | - | - | - | - | - | - | - | - | |
| No Response | 84 | - | 47 | - | 43 | - | 44 | - | 53 | - |
|
| ||||||||||
| Average Score for All Patients with Scores | 1.43 | 1.35 | 1.13 | 1.21 | 0.98 | |||||
|
| ||||||||||
| p value | 0.042 | 0.453 | 0.067 | 0.917 | 0.114 | |||||
n: number of patients
Scores ranged from none to very severe where none = 0, mild = 1, moderate = 2, severe = 3, and very severe = 4. Patients with no recorded pain score for that day did not contribute to the analysis. For each postoperative day range, patients contributed their most recent score. The scores were summed and averaged among patients with a score.
p value calculated with Mann-Whitney test.
Figure 1: Patient-Reported Postoperative Pain Scores.
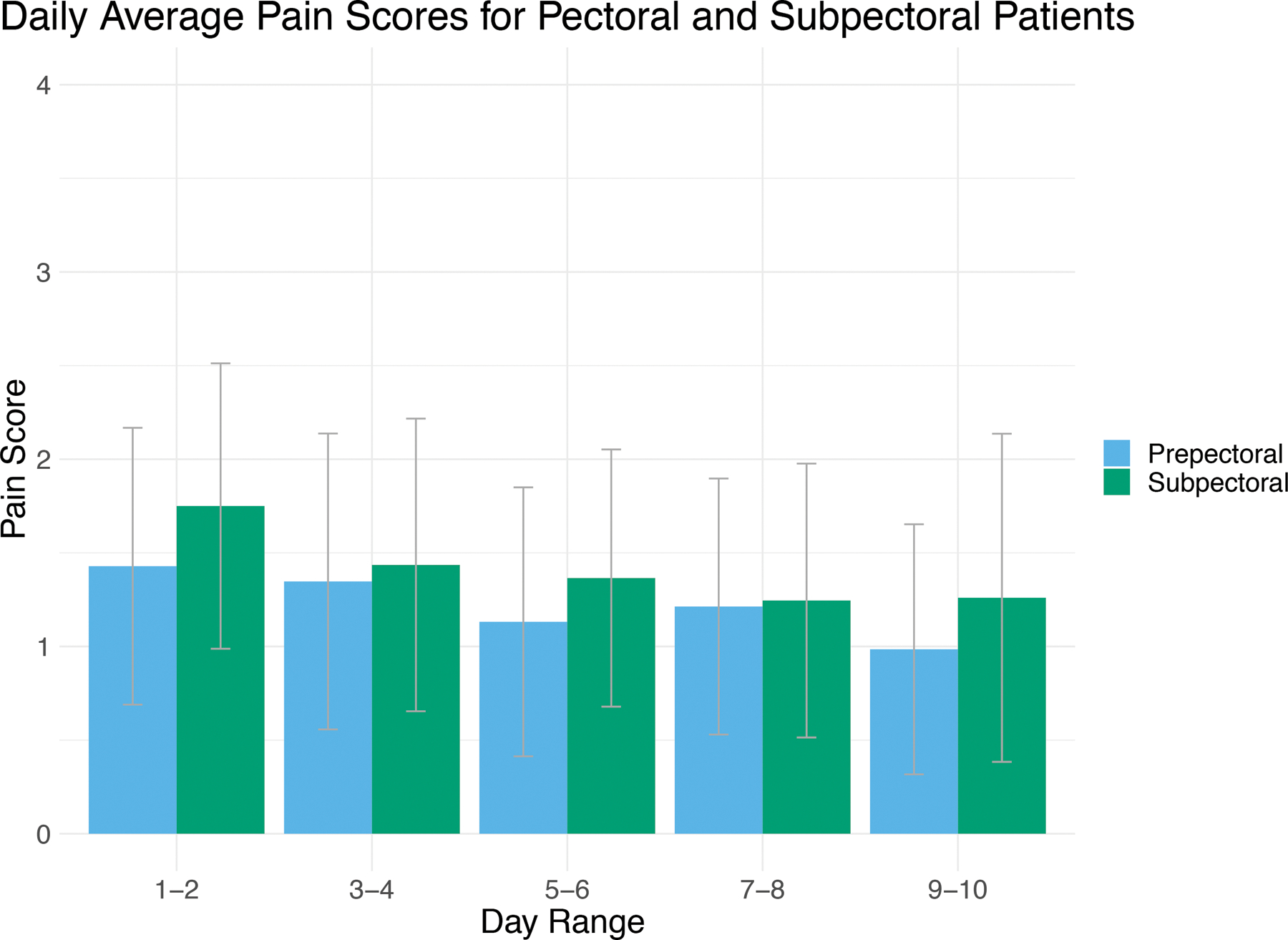
Patient response to question: “What is the severity of your pain at its worst?” Patients could respond with “none” (0), “mild” (1), “moderate” (2), “severe” (3), and “very severe” (4). Scores for each modality were averaged to give a composite representation of the cohort in a two-day period. Overall, patients experienced mild to moderate pain over the first 10 days
BREAST-Q Scores
There were no significant differences in postoperative physical well-being of the chest BREAST-Q scores between cohorts at all time points (Table 5). In a subgroup analysis comparing unilateral prepectoral versus subpectoral patients, there were no significant differences in scores at any time point. Scores trended toward improvement over the postoperative period.
Table 5:
Patient-Reported Outcome Scores – BREAST-Q Physical Well-Being of the Chest
| Physical Well-Being of the Chest | |||||||
|---|---|---|---|---|---|---|---|
| Method of Reconstruction | Postoperative: 2 Weeks |
p * | Postoperative: 6 Weeks | p * | Postoperative: 3 Months | p * | |
| Prepectoral | Responses, n | 36 | 0.297 | 49 | 0.914 | 61 | 0.686 |
| Mean (SD) | 64.4 (17.5) | 69.4 (19.0) | 70.7 (19.7) | ||||
| Median (IQR) | 64 (50–72) | 72 (55–80) | 72 (60–85) | ||||
| Subpectoral | Responses, n | 26 | 44 | 43 | |||
| Mean (SD) | 58.6 (21.6) | 69.5 (17.8) | 69.7 (17.2) | ||||
| Median (IQR) | 57.5 (41.3–75) | 72 (50–80) | 72 (60–85) | ||||
| Unilateral Reconstruction | |||||||
| Method of Reconstruction | Postoperative: 2 Weeks | p * | Postoperative: 6 Weeks | p * | Postoperative: 3 Months | p * | |
| Prepectoral | Responses, n | 19 | 0.274 | 20 | 0.15 | 26 | 0.598 |
| Mean (SD) | 64.8 (15.2) | 59.9 (15.9) | 68.0 (18.2) | ||||
| Median (IQR) | 64 (50–72) | 64 (50–69) | 72 (50–79) | ||||
| Subpectoral | Responses, n | 8 | 20 | 21 | |||
| Mean (SD) | 55.4 (15.5) | 70.1 (19.1) | 70.9 (19.2) | ||||
| Median (IQR) | 57.5 (49–65) | 68 (60–77) | 72 (60–85) | ||||
| Bilateral Reconstruction | |||||||
| Method of Reconstruction | Postoperative: 2 Weeks | p * | Postoperative: 6 Weeks | p * | Postoperative: 3 Months | p * | |
| Prepectoral | Responses, n | 17 | 0.608 | 29 | 0.138 | 35 | 0.343 |
| Mean (SD) | 64.0 (20.2) | 75.9 (18.4) | 72.7 (20.7) | ||||
| Median (IQR) | 64 (55–76) | 80 (68–85) | 72 (64–88.5) | ||||
| Subpectoral | Responses, n | 18 | 24 | 22 | |||
| Mean (SD) | 60.1 (24.1) | 69.1 (17.0) | 68.6 (15.4) | ||||
| Median (IQR) | 59.5 (41.3–76) | 76 (60–80) | 72 (60–79) | ||||
SD, standard deviation; IQR, interquartile range.
p value calculated using Mann Whitney test.
Complications
Matched prepectoral and subpectoral patients had similar complication profiles, with no statistically significant differences for all complications except for breast seroma (Table 6). Prepectoral patients experienced higher rates of breast seroma per reconstructed breast (n = 31, 16.9%) compared with subpectoral patients (16.9% vs. 3.4%, p < 0.001). No differences were noted in 90-day reconstructive failure (4.4% vs. 3.4%, p = 0.62).
Table 6:
Postoperative Complications
| Subpectoral (breast n = 179) | Prepectoral (breast n = 184) | p value* | |
|---|---|---|---|
| Mastectomy Flap Necrosis, n (%) | 13 (7.3) | 12 (6.5) | 0.781 |
| Nipple Necrosis, n (%) | 2 (1.1) | 3 (1.6) | 1.000 |
| Breast Hematoma, n (%) | 6 (3.4) | 4 (2.2) | 0.538 |
| Breast Seroma, n (%) | 6 (3.4) | 31 (16.9) | < 0.001 |
| Breast Cellulitis, n (%) | 4 (2.2) | 10 (5.4) | 0.113 |
| Infected TE, n (%) | 2 (1.1) | 6 (3.3) | 0.284 |
| Exposed TE, n (%) | 3 (1.7) | 0 (0) | 0.119 |
| TE Removal, n (%) | 6 (3.4) | 8 (4.4) | 0.622 |
| Readmission, n (%) ** | 13 (10.9) | 14 (11.8) | 0.900 |
SD, standard deviation; breast n, number of breasts; TE, tissue expander; n, number of patients.
p value calculated with chi-square test or Fisher’s exact test.
Percentages are calculated with subgroup sample size (119 patients) as denominator.
DISCUSSION
Our propensity-matched cohort study of 238 patients who underwent prepectoral or subpectoral tissue expander placement for two-stage alloplastic breast reconstruction demonstrates comparable patient-reported pain, outcomes, and complications at 90 days. To date, this is the largest study of its kind and the first to utilize the propensity-matching technique, which provides reliable and homogenous groups to compare these breast reconstruction techniques, using the plane of tissue expander placement as the main variable between cohorts. Importantly, the time horizon was consistent at 90 days across the study cohorts. Our study addresses the early postoperative profile of this technique and supports its continued use and future investigation of the long-term implications of this plane of reconstruction.
Perioperative pain and subsequent pain control are critical aspects of any surgery, particularly during a nationwide opioid epidemic. The prepectoral and subpectoral groups had similar pain scores during the first postoperative week, though early scores slightly favored prepectoral reconstruction patients. These data concur with recent studies showing similar pain within the first 1–2 postoperative weeks following subpectoral or prepectoral tissue expander placement(14, 22) but differ from smaller studies that report less pain in prepectoral reconstruction patients.(19, 21, 25) Studies that include a mix of patients receiving both direct-to-implant and tissue expander reconstruction have also reported increased pain in the subpectoral group.(18, 20) Our data represent a more homogenous group than previously published in the literature and describe comparable early postoperative pain experienced by patients undergoing reconstruction with immediate prepectoral or subpectoral tissue expanders. All reconstructions were performed over the same time period, with a consistent approach to postoperative pain, which may explain apparent similarities in postoperative opioid administration. The main variable of difference was plane of expander placement. Pain data were obtained via a novel online PRO measure administered daily. We did not assess postoperative day 0 pain score data during the outpatient stay and relied instead on analgesic administration, as this is a more controlled variable and subject to less bias than recorded postoperative pain scores in the recovery area. These results suggest that early postoperative pain may be more related to the mastectomy itself than the plane of tissue expander placement in immediate reconstruction.
Our matched data show comparable early postoperative HR-QoL following prepectoral or subpectoral tissue expander placement, which addresses movement and pain in the upper extremities and chest.(14, 17–19) Physical well-being of the chest scores during the first 90 days following surgery for the cohorts overall and in the subgroup analysis by laterality were similar between the two groups. These data support two recent studies showing no difference in PROs between prepectoral and subpectoral reconstruction patients up to three months after immediate reconstruction with tissue expanders.(14, 19) Differences in PROs between prepectoral and subpectoral reconstruction are only reported in heterogenous studies that include both direct-to-implant and tissue expander reconstruction.(17, 18) Overall, the preponderance of data, including our study, support similar PROs and satisfaction following either subpectoral or prepectoral alloplastic reconstruction. These early postoperative data do not address the likelihood of animation deformity due to subpectoral placement nor do they address rippling or implant visibility in prepectoral placement, which may occur later in the recovery period following exchange to the permanent implant.
Our data demonstrate that immediate prepectoral and subpectoral tissue expander placement have a similar complication profile in the first 90 postoperative days. The only statistically significant difference was an increased rate of seroma in the prepectoral group, likely due to a higher use of ADMs and an early learning curve relating to drain management. Increased seroma rates in prepectoral reconstruction have been noted.(25) Our practice for prepectoral reconstruction drain removal has now become more conservative, requiring output to be less than 30 cc for two consecutive days prior to removal. Our seroma rate decreased following this adjustment in postoperative care.
Our data generally concur with other studies, showing similar complication rates for hematoma, seroma, wound dehiscence, mastectomy skin flap necrosis, and infection between the two groups.(9, 14–16, 19, 22) The highest quality study to date used a matched cohort of 40 prepectoral and 40 subpectoral reconstruction patients to compare outcomes and found no difference in complications.(16) However, the time horizon or length of follow-up with an alloplastic device was not provided. Importantly, significant differences were noted in the ultimate form of reconstruction, with a higher proportion of prepectoral reconstruction patients undergoing delayed autologous reconstruction, which can present a bias in length of follow-up with prosthetic devices in the prepectoral cohort. Our study takes the matching concept a step further, matching on more variables to create cohorts where the main difference is plane of reconstruction.
Although we present high-level evidence comparing prepectoral and subpectoral breast reconstructions, our analysis has several notable limitations . First, this cohort of patients is from our early experience with prepectoral breast reconstruction and our analysis is retrospective, though utilizing a prospectively maintained database. We attempted to strengthen the retrospective aspect of this study by using propensity matching to control for confounding variables. Performing a propensity score–matched analysis potentially decreases the selection bias introduced by retrospective studies by balancing possible confounders upfront. Yet the methodology itself has limitations. If any variable of importance has not been matched, the study results may not reflect the true association between the groups.(26) We addressed this limitation to the extent possible by using prior research to guide the choice of matched variables. Further, though appropriately powered to detect moderate differences, the study is underpowered to detect small differences in complications between cohorts. Regarding pain, we only evaluated analgesia consumption in the hospital and not as an outpatient. The breakdown between total subpectoral and subpectoral with ADM should also be noted, as we have treated these two groups as equal based on a randomized, controlled study at our institution showing no difference in postoperative pain.(27) Regarding PROs, not all patients completed the PRO measures administered postoperatively (subpectoral at 24% vs. prepectoral at 42%), which may bias the results. We also only examined physical well-being of the chest and only evaluated outcomes through 90 days postoperatively. The sensitivity of the BREAST-Q physical well-being of the chest domain to detect differences in plane of expander placement is unknown. Given the 90 day study time horizon, implant related outcomes (rippling, animation) were not assessed however certainly warrant further study. Additionally, even with propensity matching there is a selection bias favoring the prepectoral reconstruction cohort. Decisions on plane of expander placement were made intraoperatively based on operative findings following the completion of the mastectomy. Our data are from an urban, academic cancer center in the northeastern United States and, as such, these findings may not be generalizable. Longer-term studies, including a PRO analysis following the final implant placement, are warranted to better understand the outcome profile of prepectoral breast reconstruction. Ideally, this would be assessed through a randomized clinical trial, given that, to date, outcomes between the two modalities appear to be similar. Such a trial would afford the best possible evidence for adjusting current practice patterns and surgical technique.
Conclusions
With the adoption of any new surgical technique, it is important to understand performance compared with the standard of care. In a matched analysis of early, 90-day complications, patients with prepectoral tissue expander reported lower early postoperative pain but no differences in physical well-being of the chest scores. Prepectoral reconstruction patients experienced a higher rate of breast seroma, though this did not translate to a difference in expander loss. Continued long-term analysis of clinical outcomes and PROs is warranted to understand the full profile of this technique.
Acknowledgments
Financial Disclosure: Joseph Dayan is a consultant for Stryker. Dr. Mehrara has an investigator-initiated award from PureTech Corp. and serves as an advisor to the company. All other authors declare that they have no competing interests. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Accepted for presentation at the 99th Annual Meeting of the American Association of Plastic Surgeons, May 15–18 2021, Miami, Florida.
REFERENCES
- 1.American Society of Plastic Surgery. 2018. Plastic Surgery Statistics Report. Available at: https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf. Accessed 9/15/2020.
- 2.Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral implant-based breast reconstruction: rationale, indications, and preliminary results. Plast Reconstr Surg 2017;139:287–294. [DOI] [PubMed] [Google Scholar]
- 3.Snyderman RK, Guthrie RH Reconstruction of the female breast following radical mastectomy. Plast Reconstr Surg 1971;47:565–567. [DOI] [PubMed] [Google Scholar]
- 4.Artz JS, Dinner MI, Foglietti MA, Sampliner J Breast reconstruction utilizing subcutaneous tissue expansion followed by polyurethane-covered silicone implants: a 6-year experience. Plast Reconstr Surg 1991;88:635–641. [PubMed] [Google Scholar]
- 5.Gruber RP, Kahn RA, Lash H, Maser MR, Apfelberg DB, Laub DR Breast reconstruction following mastectomy: a comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg 1981;67:312–317. [DOI] [PubMed] [Google Scholar]
- 6.Gabriel A, Sigalove S, Sigalove NM, et al. Prepectoral revision breast reconstruction for treatment of implant-associated animation deformity: a review of 102 reconstructions. Aesthet Surg J 2018;38:519–526. [DOI] [PubMed] [Google Scholar]
- 7.Spear SL, Schwartz J, Dayan JH, Clemens MW Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg 2009;33:44–48. [DOI] [PubMed] [Google Scholar]
- 8.Nahabedian MY Current approaches to prepectoral breast reconstruction. Plast Reconstr Surg 2018;142:871–880. [DOI] [PubMed] [Google Scholar]
- 9.Nahabedian MY, Cocilovo C Two-stage prosthetic breast reconstruction: a comparison between prepectoral and partial subpectoral techniques. Plast Reconstr Surg 2017;140:22S–30S. [DOI] [PubMed] [Google Scholar]
- 10.Sbitany H, Piper M, Lentz R Prepectoral breast reconstruction: a safe alternative to submuscular prosthetic reconstruction following nipple-sparing mastectomy. Plast Reconstr Surg 2017;140:432–443. [DOI] [PubMed] [Google Scholar]
- 11.Woo A, Harless C, Jacobson SR Revisiting an old place: single-surgeon experience on post-mastectomy subcutaneous implant-based breast reconstruction. Breast J 2017;23:545–553. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Su Y, Xiu B, et al. Comparison of prepectoral and subpectoral breast reconstruction after mastectomies: a systematic review and meta analysis. Eur J Surg Oncol 2019;45:1542–1550. [DOI] [PubMed] [Google Scholar]
- 13.Wagner RD, Braun TL, Zhu H, Winocour S A systematic review of complications in prepectoral breast reconstruction. J Plast Reconstr Aesthet Surg 2019;72:1051–1059. [DOI] [PubMed] [Google Scholar]
- 14.Baker BG, Irri R, MacCallum V, Chattopadhyay R, Murphy J, Harvey JR A prospective comparison of short-term outcomes of subpectoral and prepectoral strattice-based immediate breast reconstruction. Plast Reconstr Surg 2018;141:1077–1084. [DOI] [PubMed] [Google Scholar]
- 15.Bettinger LN, Waters LM, Reese SW, Kutner SE, Jacobs DI Comparative study of prepectoral and subpectoral expander-based breast reconstruction and Clavien IIIb score outcomes. Plast Reconstr Surg Glob Open 2017;5:e1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Momeni A, Remington AC, Wan DC, Nguyen D, Gurtner GC A matched-pair analysis of prepectoral with subpectoral breast reconstruction: is there a difference in postoperative complication rate? Plast Reconstr Surg 2019;144:801–807. [DOI] [PubMed] [Google Scholar]
- 17.Bernini M, Calabrese C, Cecconi L, et al. Subcutaneous direct-to-implant breast reconstruction: surgical, functional, and aesthetic results after long-term follow-up. Plast Reconstr Surg Glob Open 2015;3:e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cattelani L, Polotto S, Arcuri MF, Pedrazzi G, Linguadoca C, Bonati E One-step prepectoral breast reconstruction with dermal matrix-covered implant compared to submuscular implantation: functional and cost evaluation. Clin Breast Cancer 2018;18:e703–e711. [DOI] [PubMed] [Google Scholar]
- 19.Walia GS, Aston J, Bello R, et al. Prepectoral versus subpectoral tissue expander placement: a clinical and quality of life outcomes study. Plast Reconstr Surg Glob Open 2018;6:e1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Copeland-Halperin LR, Yemc L, Emery E, et al. Evaluating postoperative narcotic use in prepectoral versus dual-plane breast reconstruction following mastectomy. Plast Reconstr Surg Glob Open 2019;7:e2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaeffer CV, Dassoulas KR, Thuman J, Campbell CA Early functional outcomes after prepectoral breast reconstruction: a case-matched cohort study. Ann Plast Surg 2019;82:S399–S403. [DOI] [PubMed] [Google Scholar]
- 22.Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg 2016;69:e77–86. [DOI] [PubMed] [Google Scholar]
- 23.Casella D, Di Taranto G, Marcasciano M, et al. Subcutaneous expanders and synthetic mesh for breast reconstruction: long-term and patient-reported BREAST-Q outcomes of a single-center prospective study. J Plast Reconstr Aesthet Surg 2019;72:805–812. [DOI] [PubMed] [Google Scholar]
- 24.Voineskos SH, Klassen AF, Cano SJ, Pusic AL, Gibbons CJ Giving Meaning to Differences in BREAST-Q Scores: Minimal Important Difference for Breast Reconstruction Patients. Plast Reconstr Surg 2020;145:11e–20e. [DOI] [PubMed] [Google Scholar]
- 25.Kraenzlin F, Darrach H, Khavanin N, et al. Tissue expander-based breast reconstruction in the prepectoral versus subpectoral plane: an analysis of short-term outcomes. Ann Plast Surg Published online June 17, 2020. [DOI] [PubMed] [Google Scholar]
- 26.Austin PC, Mamdani MM, Stukel TA, Anderson GM, Tu JV The use of the propensity score for estimating treatment effects: administrative versus clinical data. Stat Med 2005;24:1563–1578. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy CM, Lee CN, Halvorson EG, et al. The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast Reconstr Surg 2012;130:57S–66S. [DOI] [PMC free article] [PubMed] [Google Scholar]


