Abstract
Background/objective
The purpose of this study was to report the outcomes of a clinical trial conducted in Japan to assess the safety and effectiveness of third-generation autologous chondrocyte implantation (ACI) using IK-01 (CaReS™), which does not require flap coverage, in the treatment of patients with focal cartilage injury of the knee.
Methods
This was an open label, exploratory clinical trial. Patients were enrolled between June 2012 and September 2016. The primary endpoint of the study was the International Knee Documentation Committee (IKDC) score at 52 weeks after implantation. The IKDC, Lysholm, and visual analog scale (VAS) scores were evaluated at the time of screening and at 4, 12, 24, 36, and 52 weeks after implantation. Improvements from the baseline scores were evaluated using the equation “(postoperative score) − (preoperative score).” Magnetic resonance imaging (MRI) was performed at 2, 12, 24, and 52 weeks after implantation, and MRI measurements were evaluated using T1 rho and T2 mapping.
Results
Nine patients were enrolled in this study and were examined for safety. Product quality did not satisfy the specification in one patient, and bacterial joint infection occurred in one patient. As a result, seven patients were included in the outcome analyses. The mean IKDC score significantly improved from 36.4 preoperatively to 74.1% at 52 weeks after implantation (p < 0.0001). The mean Lysholm and VAS scores also significantly improved from 39.6 to 57.4 to 89.6 and 22.9, respectively, after surgery. In the MRI evaluation, the T1 rho and T2 values of the implanted area were similar to those of the surrounding cartilage at 52 weeks after implantation.
Conclusions
Third generation ACI (IK-01) can be an effective treatment option for focal cartilage defects of the knee; however, surgeons must pay careful attention to the risk of postoperative joint infection.
Keywords: Autologous chondrocyte implantation, Third-generation, Knee
1. Introduction
Treatment of knee cartilage injury is challenging owing to the low healing capacity of the knee. The most common treatment is surgery, including marrow stimulation, osteochondral autograft, and autologous chondrocyte implantation (ACI). Since Brittberg et al. first reported the clinical outcomes of ACI,1 ACI has been recognized as a useful treatment option, especially for large cartilage defects.
In the first-generation ACI, a cell suspension is injected into the defect, which is then covered with the periosteum. However, limitations and disadvantages associated with its surgical technique have been reported,2,3 including leakage and non-uniform distribution of the injected cells, increased invasiveness due to the harvesting and hypertrophy of the periosteum, and the large skin incision required for transplantation. Thereafter, a type I/III collagen-based artificial flap was developed to reduce such disadvantages, especially those related to the periosteum. ACI techniques using a collagenous flap are commonly defined as the second-generation ACI.4,5 Meanwhile, the phenotypic chondrocyte change toward the fibroblastic phenotype during the monolayer culture was also a major concern in previous ACI techniques. Matrix-based chondrocyte implantation, the so-called third-generation ACI, has been developed to minimize the loss of the chondrocytic phenotype during the culture period. In third generation ACI, chondrocytes are cultured in a matrix after monolayer expansion or directly. The chondrocytes in the matrix are attached to the cartilage defect with fibrin glue or bioabsorbable sutures but without collagenous flap coverage.6, 7, 8 Owing to its simpler technique, matrix-based chondrocyte implantation has gained popularity, and favourable outcomes have been reported.9, 10, 11
CaReS™ is a third generation ACI product developed by Arthro Kinetics Biotechnology GmbH in Austria. In the CaReS system, isolated chondrocytes are cultured on a matrix based on type I collagen from rat tails for 2 weeks and then surgically attached to the cartilage defect with fibrin glue. Its unique characteristics are the three-dimensional culture of chondrocytes without a monolayer culture to decrease the change toward the fibroblastic phenotype.12 A previous basic study showed that chondrocytes had a more favourable phenotype and gene expression pattern in CaRes™ than other third generation ACI products.12 Another unique characteristic is the adjustable thickness of the product according to the thickness of the cartilage defect. In addition, good clinical outcomes have been reported for ACI using CaReS™.13, 14, 15 In Japan, only one type of third generation ACI (JACC™), an atelocollagen-based ACI with coverage using the periosteum or a collagenous membrane, can be clinically used under medical insurance coverage. Compared with JACC™, CaReS™ seems to be less invasive and technically easier to implant. However, CaReS™, which may have potential advantages over previous ACI techniques, has never been performed in Japan. To introduce another type of third generation ACI, which does not require flap coverage, we conducted a phase I/IIa clinical trial. This clinical trial aimed to evaluate the safety and effectiveness of third generation ACI using CaReS™ in Japan. Our hypothesis was that third generation ACI using CaReS™ would be safe and effective for the treatment of focal cartilage injury of the knee.
2. Material and methods
This open-label exploratory study was aimed at assessing the safety and effectiveness of ACI using CaReS™ (the product code name was IK-01 in this clinical trial) in the treatment of focal cartilage injury of the knee. The study protocol was reviewed and approved by the Institutional Review Board of our hospital. Patients were enrolled between June 2012 and September 2016. This study was conducted according to the principles of the Declaration of Helsinki, the Japanese Pharmaceutical Affairs Law, and the ordinance of the Japanese Ministry of Health, Labour and Welfare. Inform ed consent was obtained from all the patients for experimentation with human subjects.
The inclusion criteria for this clinical trial were as follows: age ≥20 and ≤ 50 years, provision of informed consent, and International Knee Documentation Committee (IKDC) scores16 of <60 at the time of screening, a focal cartilage lesion (including osteochondritis dissecans [OCD]), International Cartilage Repair Society or Outerbridge grade III or IV,17,18 OCD grade III or IV by the Nelson classification on MRI,19 a cartilage lesion (after debridement) of ≥4 and ≤ 9 cm2 with a stable surrounding cartilage, and a microfracture or mosaic plasty-ineffective cartilage injury with an area of <4 cm2.
The exclusion criteria were as follows: patients with osteoarthritic lesions, multiple arthrosis in the same limb, >2 injured sites in the knee or a focal cartilage injury that needs to be treated in bilateral knees, gout and pseudogout, kissing lesion in the same knee, neuroparalysis in the same limb, and an injury requiring iliac bone grafting, and those who underwent meniscectomy or meniscal repair and ligament surgery within 8 weeks before screening.
2.1. Investigational product
Investigational products (IK-01) for the clinical trial were prepared at the Cell Processing Center (CPC) of either Arthro Kinetics Biotechnology in Krems, Austria (the first three cases) or the author's institution, Japan (the last six cases), both of which complied with Good Manufacturing Practice (GMP) for investigational products.
Cartilage tissues and blood samples were collected from each subject at our hospital and transported to the CPC. Cartilage cells were isolated through enzymatic digestion and were seeded in a type I collagen gel matrix derived from Caesarean Derived Sprague Dawley rats (Crl:CD(SD) rat, The Jackson Laboratory Japan, Inc., Yokohama, Japan). The cells were cultured for approximately 10 days, and the final products were transported to the hospital after the release tests. The transplantation was performed 14 days after the initial surgery for cartilage harvesting.
For quality control, the culture supernatant was examined for sterility and mycoplasma as in-process and release testing. The endotoxin test (<0.25EU/mL) was performed for the final supernatant as well. The final product for quality control was enzymatically digested to prepare the cell suspension, and the cell population doubling rate (>0.25/day) and cell viability (>80%) were measured. RT-PCR for the expression of type II collagen was also performed to ensure that the cell differentiation state was maintained in the gel. Only the final products that met the above quality specifications were released for transplantation. The final product of IK-01 was confirmed to be stable for more than four days at temperatures of 2–25 °C
2.2. Evaluation protocol
For the clinical outcome evaluation, IKDC, Lysholm,20 and visual analog scale (VAS) scores21 were evaluated at the time of screening and at 4, 12, 24, 36, and 52 weeks after implantation. The primary endpoint of this study was the IKDC score at 52 weeks after IK-01 implantation. Improvements from the baseline scores were evaluated using the equation “(postoperative score) − (preoperative score).” For objective assessments, magnetic resonance imaging (MRI) was performed at the time of screening and at 2, 12, 24, and 52 weeks after implantation.
2.3. MRI evaluation
The cartilage repair status was evaluated with fast spin-echo proton density-weighted imaging and T1 rho and T2 mapping in sagittal images. The relative ratios of T1 rho and T2 mapping values were calculated as the ratio of the implanted area to the surrounding cartilage area.
The FS-PW MR images were evaluated using the previously reported scoring system,22 which consists of eight parameters: signal intensity, graft infill, border integration, surface contour, structure, subchondral lamina, subchondral bone, and effusion. Each parameter was rated from 1 to 4 points as poor (1), fair (2), good (3), and excellent (4). The mean total score was used for overall assessment of morphological repair status. The scoring was performed by reviewers blinded to the patient information.
2.4. Surgical procedures
Standard medial and lateral para-patellar tendon portals were created for arthroscopic observations. The size of the cartilage injury sites was measured using a scaled probe. Cartilage (≥0.4 mg) was harvested from the proximal non-weight-bearing area of the trochlear in the patellofemoral joint. Chondrocytes were isolated from the cartilage fragment and cultured on rat tail-derived type I collagen for 2 weeks and then transplanted into the cartilage defect.
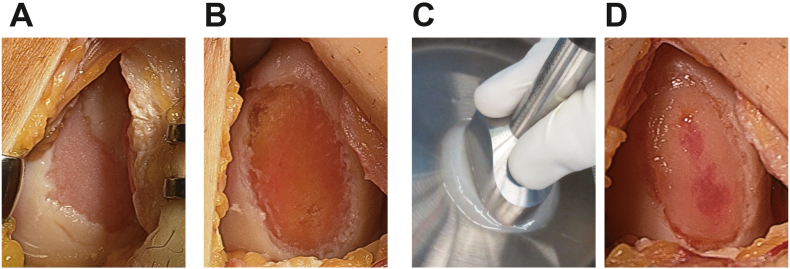
First, the injured site was debrided to remove fibrous tissue (Fig. 1A and B). The surrounding damaged cartilage was then removed to create a sufficiently stable shoulder using a special moulder. The gel-like chondrocyte matrix was molded into a size 2 mm wider than the injured site using a special moulder (Fig. 1C). Before implantation, a drop of fibrin glue (KM Biologics Co., Ltd., Kumamoto, Japan) was applied to the defect, and then the chondrocyte gel was placed in the defect. More drops of fibrin glue were applied on the border between the injured site and the surrounding cartilage to secure fixation (Fig. 1D). Then, the operated limb was held to ensure that the implanted site was parallel to the ground to ensure that the gel was evenly flattened for at least 10 min until the implanted gel stably fitted the site. After confirming that the gel was stable at the site by gently moving the knee from extension to flexion several times, the hardness of the implanted chondrocyte gel was measured. After saline irrigation, the wound was closed layer by layer.
Fig. 1.
Procedures for IK-01 implantation.
(A) Before implantation (B) After debridement of the injured site. (C) The gel-like chondrocyte matrix is molded out using a special molder. (D) Immediately after implantation.
Second-look arthroscopy was performed 52 weeks after implantation to confirm healing status. As an additional assessment, the hardness of the implanted and surrounding cartilages was measured at the time of implantation and second-look arthroscopy using an indenter device (Supplemental Material).
A same pre- and post-operative prophylactic antibiotic regime was used in all the patients: 1.0 g of Cephalosporin was administered intravenously 30 min before surgery and every 8 h after surgery. On the postoperative second and third day, 1.0 g of Cefazolin was administered two times per day.
2.5. Rehabilitation
A knee brace was applied for 1 week. Progressive ROM exercises were started 1 week after implantation. Partial weight bearing was started 4 weeks after implantation for cartilage injuries in the weight-bearing area, including the medial and lateral femoral condyles. Full weight bearing was permitted 6 weeks after the implantation.
2.6. Adverse events
All information on the adverse events that occurred during the period from cartilage harvest to 52 weeks after implantation was collected. Surgery-related adverse events were analysed further.
2.7. Statistical analyses
A sample t-test was used to assess improvements in clinical scores at 52 weeks from baseline. The normality of data was assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. For the assessment of differences in the IKDC and Lysholm scores among the evaluation timings, the Kruskal-Wallis test with post-hoc adjustment by Bonferroni was used. For the assessment of VAS, one-way analysis of variance with post-hoc Tukey's HSD was used. Differences were considered statistically significant at p values < 0.05. All tests were performed using SPSS software v26.0 (IBM Software Group, Chicago, IL, USA).
3. Results
Nine patients were enrolled in this study and examined for the safety analysis. One patient could not receive the implantation because the quality of the cultured cells did not satisfy the quality specifications. One patient had an infection after the implantation and was thus excluded from the analysis 5 days after implantation. As a result, seven patients were included in the outcome analyses. The patients’ demographic characteristics are shown in Table 1.
Table 1.
Patient demographic and clinical outcomes.
| Age | Gender | Injury situation | Previous surgery | Location | ICRS grade | Area (cm2) | Depth (mm) | Time from Injury to surgery (months) | Time from previous surgery to index surgery (months) | IKDC (Preop.) (52 W) | Lysholm (Preop.) (52 W) | VAS (Preop.) (52 W) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 27 | Female | Traffic accident | Debridement | Patella | III | 4.5 | 3.0 | 26 | 22 | 37 | 42 | 84 |
| 83 | 93 | 49 | ||||||||||
| 42 | Female | ADL | Drilling | LFC | III | 2.0 | 3.0 | 7 | 5.5 | 24 | 26 | 52 |
| 69 | 89 | 0 | ||||||||||
| 24 | Male | ADL | None | Trochlea | IV | 4.5 | 3.0 | 6 | 38 | 42 | 69 | |
| 76 | 97 | 21 | ||||||||||
| 47 | Female | Sports | None | MFC | IV | 4.5 | 2.0 | 9 | 46 | 41 | 21 | |
| 71 | 88 | 15 | ||||||||||
| 45 | Male | ADL | Drilling | MFC | IV | 4.5 | 2.0 | 31 | 24 | 41 | 41 | 16 |
| 83 | 83 | 39 | ||||||||||
| 43 | Male | Sports | Diagnostic | MFC | IV | 4.5 | 3.0 | 12 | 9 | 32 | 42 | 100 |
| Arthroscopy | 61 | 79 | 36 | |||||||||
| 44 | Male | Work | Drilling | MFC | IV | 4.5 | 2.0 | 9 | 8 | 37 | 39 | 60 |
| 76 | 100 | 0 |
ADL, activity in daily life. LFC, lateral femoral condyle, MFC, medial femoral condyle.
52 W, 52 weeks.
3.1. Primary endpoint
The mean IKDC score was significantly improved from 36.4 preoperatively to 74.1 at 52 weeks after implantation (p < 0.001). The mean improvement in IKDC score at 52 weeks was 37.7 (95% CI, 30.36–45.07; Table 2).
Table 2.
Improvements in clinical outcomes at 52 weeks.
| Clinical score | Preop.Mean | 52 weeks.Mean | Improvement (Post -Pre) | Improvement 95% CI |
t-test (p-value) | |
|---|---|---|---|---|---|---|
| Lower bound | Upperbound | |||||
| IKDC | 36.4 | 74.1 | 37.7 | 30.36 | 45.07 | P < 0.01 |
| Lysholm | 39.0 | 89.6 | 50.6 | 41.80 | 59.34 | P = 0.02 |
| VAS | 57.4 | 22.9 | −34.6 | −64.13 | −5.02 | p = 0.03 |
CI, confidence interval; IKDC, the International Knee Documentation Committee Score; VAS, visual analog scale.
3.2. Clinical outcomes
The IKDC and Lysholm scores gradually increased over the 52 weeks after implantation. The IKDC and Lysholm scores significantly increased at 36 weeks while VAS significantly decreased at 24 weeks after implantation compared with preoperative scores (Fig. 2, Supplemental tables). The mean improvements in the Lysholm, and VAS scores at 52 weeks are shown in Table 2.
Fig. 2.
Serial changes in the IKDC, Lysholm, and VAS scores for 52 weeks after implantation. ∗ and ∗∗ indicate statistically significant difference compared with preoperative score. ∗P < 0.05, ∗∗P < 0.01.
3.3. MRI evaluation
The T1 rho and T2 mapping values of the implanted cartilage gradually decreased to values similar to those of the surrounding cartilage at 52 weeks after surgery. The mean total MRI value was also gradually increased (Table 3).
Table 3.
MRI evaluation.
| T1 rho value (ms) |
|||
|---|---|---|---|
| Implant site | Adjacent area | Ratio Implant/Adjacent | |
| 2W | 157.4 ± 89.0 | 102.6 ± 56.6 | 1.53 ± 0.48 |
| 12W | 82.2 ± 50.0 | 58.6 ± 23.1 | 1.41 ± 0.58 |
| 24W | 60.2 ± 18.4 | 57.1 ± 24.7 | 1.11 ± 0.30 |
| 52W |
80.0 ± 46.4 |
81.0 ± 47.3 |
1.00 ± 0.20 |
| T2 value (ms) | |||
| Implant site |
Adjacent area |
Ratio Implant/Adjacent |
|
| 2W | 186.2 ± 141.5 | 68.3 ± 16.0 | 3.00 ± 3.01 |
| 12W | 92.0 ± 55.9 | 59.7 ± 12.9 | 1.57 ± 1.03 |
| 24W | 85.1 ± 17.0 | 57.6 ± 12.2 | 1.54 ± 0.47 |
| 52W | 60.4 ± 7.3 | 54.2 ± 6.5 | 1.14 ± 0.28 |
| Total score of the morphological repair status | |
|---|---|
| 2W | 1.8 ± 0.4 |
| 12W | 2.7 ± 0.6 |
| 24W | 2.9 ± 0.7 |
| 52W | 3.1 ± 0.6 |
Data are expressed as mean ± standard deviation.
3.4. Arthroscopic evaluation
A typical case is shown in Fig. 3. A complete integration with the surrounding cartilage was observed in six of the seven patients. Partial detachment and incomplete integration were observed in one patient, but most parts of the repaired cartilage firmly attached to the site, requiring no surgical treatment.
Fig. 3.
A 44-year-old Male patient. (A) Arthroscopic views of the medial femoral condyle at the time of cartilage harvest. (B) Arthroscopic views of the implanted site 52 weeks after implantation. (C) Magnetic resonance images at baseline, 4, 12, 26 and 52 weeks after implantation. A cartilage injury associate with marked high signal change in the bone marrow area due to drilling, which was performed 8 months earlier, were observed in the medial condyle. The bone marrow lesion was mostly reduced and the injured site was repaired by a slightly overgrown cartilage-like tissue integrated with adjacent area at 26 weeks and 52 weeks after implantation.
3.5. Adverse events
Sixty-two events occurred during the study period, of which 60 were minor events, one was moderate, and one was severe. The most frequent events were wound complications (18 cases in seven patients), followed by joint effusion (six cases in five patients). The moderate event was delayed wound healing, and the severe event was septic arthritis. In the patient with a severe adverse event, methicillin-resistant Staphylococcus aureus (MRSA) was detected in a joint fluid specimen from the seventh patient. The implanted cartilage was removed 5 days after implantation.
4. Discussion
The most significant finding in this clinical trial was that objectively and effectively satisfactory outcomes were confirmed after third generation ACI (IK-01).
Previous basic studies have reported promising results for CaReS™, showing favourable conditions for maintaining the chondrocytic phenotype. Muller-Rath et al. reported that primary human chondrocytes seeded on CaRes™ were viable after 6 weeks of culture and maintained the chondrocytic phenotype 3 weeks after implantation in a nude mouse model.23 Albrecht et al. compared chondrocyte-related gene expression patterns among matrix-associated ACI products, including Hyalograft C™, MACI™, CaReS™, and Novocart 3D™, which are commercially available in European countries at the time of the examination. CaReS™ showed the highest COL2 and aggrecan expression levels and COL2/COL1 ratio, which suggests its superior ability to maintain the chondrocytic phenotype compared to other products.12 Based on the results, Schneider et al.14 conducted a prospective multicentre study of ACI using CaReS™ to treat knee cartilage injuries and reported significantly improved mean IKDC score from 42.4 preoperatively to 70.5 at the final follow-up in 116 patients. Similarly, the IKDC score in our study improved from 36.4 preoperatively to 74.1 at 52 weeks after implantation, confirming the effectiveness of CaReS™ treatment for knee cartilage injuries.
Together with the results of our assessments, these observations suggest that an acceptable maturation of the implanted CaReS™ can be obtained 1 or 2 years after implantation.
In Japan, Ochi et al. developed an atelocollagen-associated ACI (JACC™) and reported its clinical outcomes for the first time.24 Then, a multicentre clinical trial revealed good 2-year clinical outcomes of the atelocollagen-associated ACI.25 On the basis of these favourable outcomes, JACC™ was pharmaceutically approved as the first ACI in Japan by the Japanese Ministry of Health, Labour and Welfare in 2012. Compared with JACC™, CaReS™ is technically easier and less invasive to implant, as CaReS™ can be attached to the cartilage defect with a fibrin glue and does not require a periosteal or collagenous flap. In addition, uniform implantation with the same height as the surrounding cartilage is possible only in the CaReS™ system.
In a randomized controlled study, Barlett et al. reported that the clinical, arthroscopic, and histological outcomes of ACI using a type I/III collagen artificial flap (the second-generation ACI) and MACI™ were similar.26 Meanwhile, Zeifang et al. reported results of their randomized controlled study to compare the clinical outcomes after the first-generation ACI using periosteal coverage with those after the third generation ACI using Bioseed-C™ at 12 and 24 months after surgery. The results showed no significant differences between the two systems in most of the clinical scores, but the improvement in Lysholm score was better in the first-generation ACI.27 However, the difference was minimal, and the reason why only the difference in Lysholm score was significant is unclear. Currently, it remains to be clarified whether a clinically important difference may exist between the ACI generations or surgical techniques.28 If the outcomes are similar, the third generation ACI seems to be a favourable option considering its technical simplicity and reduced invasiveness as compared with the lower-generation ACI. Indeed, good mid-to long-term clinical outcomes have also been reported for third generation ACI.11,29,30 ACI using CaReS™ has been widely performed in European countries. However, after the pharmaceutical regulation was renewed, Arthro Kinetics Biotechnology abandoned the reaccreditation owing to the cost of the required clinical trial and stopped the production of CaReS™. Currently, the production technology of CaReS™ has been transferred to a Japanese company, and a multicentre clinical trial based on the results of the present clinical trial is ongoing.
Regarding the adverse events that occurred in this clinical trial, bacterial joint infection with MRSA occurred in one patient, resulting in the removal of the implanted graft. As the absence of bacteria was checked in the final step of the production process, contamination during the culture period was not likely to be the cause of the infection. Rather, the infection seemed to have occurred during the surgical procedure or was caused by skin bacteria. Wood et al. comprehensively examined the adverse events after ACI and reported 497 adverse events after ACI in 294 patients. Among these events, surgical site infections occurred in 18 patients, joint infections in 11 (3.7%), and superficial wound infections in 7 (2.4%).31 Although the infection rate of ACI was low, great care should be taken during surgery to prevent infection, which may cause serious joint damage.
5. Limitations
This clinical trial had several limitations. First, the number of patients evaluated was small, and more cases should be analysed. However, the present preliminary clinical trial aimed to confirm safety and effectiveness, which have already been reported in European countries. In addition, the relatively consistent favourable outcomes in our study patients provided sufficient preliminary information. Second, no control group was included to prove the superiority over the conventional treatments, including bone marrow stimulation techniques or atelocollagen-associated ACI. Therefore, randomized controlled studies are warranted.
Despite these limitations, this clinical trial confirmed the safety and effectiveness of IK-01 through multiple assessments and warrants a pivotal and randomized clinical trial for pharmaceutical approval in Japan.
6. Conclusions
Third generation ACI (IK-01) may be an effective treatment option for focal cartilage defects of the knee, but surgeons must pay careful attention to joint infections after surgery.
Funding
This research did not receive any specific grant from funding agencies in th e public, commercial, or not-for-profit sectors, and no material support of an y kind was received.
Declaration of competing interest
None.
Acknowledgements
We would like to thank Junko Yamasaki in Kobe University Hospital Clinical & Translational Research Center, and Yoshiyuki Takahashi and Mayumi Miyata sin Institute of Biomedical Research and Innovation Hospital for their assistance to conduct the clinical trial. We appreciate Yohei Kawakami, Yuichiro Nishizawa, Kyohei Nishida, Takao Inokuchi, Shunsuke Kirizuki, Tokio Matsuzaki, Naoki Nakano for their research assistance and medical supports. We also would like to acknowledge Editage for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.asmart.2022.03.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 2.Goyal D., Goyal A., Keyhani S., Lee E.H., Hui J.H. Evidence-based status of second- and third-generation autologous chondrocyte implantation over first generation: a systematic review of level I and II studies. Arthroscopy. 2013;29:1872–1878. doi: 10.1016/j.arthro.2013.07.271. [DOI] [PubMed] [Google Scholar]
- 3.Peterson L., Minas T., Brittberg M., Nilsson A., Sjogren-Jansson E., Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Haddo O., Mahroof S., Higgs D., et al. The use of chondrogide membrane in autologous chondrocyte implantation. Knee. 2004;11:51–55. doi: 10.1016/S0968-0160(03)00041-3. [DOI] [PubMed] [Google Scholar]
- 5.Briggs T.W., Mahroof S., David L.A., Flannelly J., Pringle J., Bayliss M. Histological evaluation of chondral defects after autologous chondrocyte implantation of the knee. J Bone Joint Surg Br. 2003;85:1077–1083. doi: 10.1302/0301-620x.85b7.13672. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett W., Gooding C.R., Carrington R.W., Skinner J.A., Briggs T.W., Bentley G. Autologous chondrocyte implantation at the knee using a bilayer collagen membrane with bone graft. A preliminary report. J Bone Joint Surg Br. 2005;87:330–332. doi: 10.1302/0301-620x.87b3.15552. [DOI] [PubMed] [Google Scholar]
- 7.Behrens P., Bitter T., Kurz B., Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)--5-year follow-up. Knee. 2006;13:194–202. doi: 10.1016/j.knee.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Steinwachs M. New technique for cell-seeded collagen-matrix-supported autologous chondrocyte transplantation. Arthroscopy. 2009;25:208–211. doi: 10.1016/j.arthro.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Niemeyer P., Schubert T., Grebe M., Hoburg A. Matrix-associated chondrocyte implantation is associated with fewer reoperations than microfracture: results of a population-representative, matched-pair claims data analysis for cartilage defects of the knee. Orthop J Sports Med. 2019;7 doi: 10.1177/2325967119877847. 2325967119877847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebert J.R., Fallon M., Wood D.J., Janes G.C. A prospective clinical and radiological evaluation at 5 Years after arthroscopic matrix-induced autologous chondrocyte implantation. Am J Sports Med. 2017;45:59–69. doi: 10.1177/0363546516663493. [DOI] [PubMed] [Google Scholar]
- 11.Schuette H.B., Kraeutler M.J., McCarty E.C. Matrix-assisted autologous chondrocyte transplantation in the knee: a systematic review of mid- to long-term clinical outcomes. Orthop J Sports Med. 2017;5 doi: 10.1177/2325967117709250. 2325967117709250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albrecht C., Tichy B., Nurnberger S., et al. Gene expression and cell differentiation in matrix-associated chondrocyte transplantation grafts: a comparative study. Osteoarthritis Cartilage. 2011;19:1219–1227. doi: 10.1016/j.joca.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Welsch G.H., Mamisch T.C., Zak L., et al. Evaluation of cartilage repair tissue after matrix-associated autologous chondrocyte transplantation using a hyaluronic-based or a collagen-based scaffold with morphological MOCART scoring and biochemical T2 mapping: preliminary results. Am J Sports Med. 2010;38:934–942. doi: 10.1177/0363546509354971. [DOI] [PubMed] [Google Scholar]
- 14.Schneider U., Rackwitz L., Andereya S., et al. A prospective multicenter study on the outcome of type I collagen hydrogel-based autologous chondrocyte implantation (CaReS) for the repair of articular cartilage defects in the knee. Am J Sports Med. 2011;39:2558–2565. doi: 10.1177/0363546511423369. [DOI] [PubMed] [Google Scholar]
- 15.Petri M., Broese M., Simon A., et al. CaReS (MACT) versus microfracture in treating symptomatic patellofemoral cartilage defects: a retrospective matched-pair analysis. J Orthop Sci. 2013;18:38–44. doi: 10.1007/s00776-012-0305-x. [DOI] [PubMed] [Google Scholar]
- 16.Irrgang J.J., Anderson A.F., Boland A.L., et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29:600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 17.Brittberg M., Winalski C.S. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 18.Outerbridge R.E. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752–757. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 19.Nelson D.W., DiPaola J., Colville M., Schmidgall J. Osteochondritis dissecans of the talus and knee: prospective comparison of MR and arthroscopic classifications. J Comput Assist Tomogr. 1990;14:804–808. doi: 10.1097/00004728-199009000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Lysholm J., Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150–154. doi: 10.1177/036354658201000306. [DOI] [PubMed] [Google Scholar]
- 21.Aitken R.C. Measurement of feelings using visual analogue scales. Proc Roy Soc Med. 1969;62:989–993. doi: 10.1177/003591576906201005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson W.B., Fick D., Wood D.J., Linklater J.M., Zheng M.H., Ackland T.R. MRI and clinical evaluation of collagen-covered autologous chondrocyte implantation (CACI) at two years. Knee. 2007;14:117–127. doi: 10.1016/j.knee.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Muller-Rath R., Gavenis K., Andereya S., Mumme T., Schmidt-Rohlfing B., Schneider U. A novel rat tail collagen type-I gel for the cultivation of human articular chondrocytes in low cell density. Int J Artif Organs. 2007;30:1057–1067. doi: 10.1177/039139880703001205. [DOI] [PubMed] [Google Scholar]
- 24.Ochi M., Uchio Y., Kawasaki K., Wakitani S., Iwasa J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br. 2002;84:571–578. doi: 10.1302/0301-620x.84b4.11947. [DOI] [PubMed] [Google Scholar]
- 25.Tohyama H., Yasuda K., Minami A., et al. Atelocollagen-associated autologous chondrocyte implantation for the repair of chondral defects of the knee: a prospective multicenter clinical trial in Japan. J Orthop Sci. 2009;14:579–588. doi: 10.1007/s00776-009-1384-1. [DOI] [PubMed] [Google Scholar]
- 26.Bartlett W., Skinner J.A., Gooding C.R., et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 27.Zeifang F., Oberle D., Nierhoff C., Richter W., Moradi B., Schmitt H. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38:924–933. doi: 10.1177/0363546509351499. [DOI] [PubMed] [Google Scholar]
- 28.Everhart J.S., Jiang E.X., Poland S.G., Du A., Flanigan D.C. Failures, reoperations, and improvement in knee symptoms following matrix-assisted autologous chondrocyte transplantation: a meta-analysis of prospective comparative trials. Cartilage. 2021;13(1_suppl):1022S–1035S. doi: 10.1177/1947603519870861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreuz P.C., Kalkreuth R.H., Niemeyer P., Uhl M., Erggelet C. Long-term clinical and MRI results of matrix-assisted autologous chondrocyte implantation for articular cartilage defects of the knee. Cartilage. 2019;10:305–313. doi: 10.1177/1947603518756463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aldrian S., Zak L., Wondrasch B., et al. Clinical and radiological long-term outcomes after matrix-induced autologous chondrocyte transplantation: a prospective follow-up at a minimum of 10 years. Am J Sports Med. 2014;42:2680–2688. doi: 10.1177/0363546514548160. [DOI] [PubMed] [Google Scholar]
- 31.Wood J.J., Malek M.A., Frassica F.J., et al. Autologous cultured chondrocytes: adverse events reported to the United States Food and Drug Administration. J Bone Joint Surg Am. 2006;88:503–507. doi: 10.2106/JBJS.E.00103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.