Abstract
Background
Electronic devices for blood pressure (BP) measurements require independent clinical validation as recommended by various authorities/societies, both in general and special populations such as pregnancy.
Objective
To assess the accuracy of the Withings BPM Connect device in pregnancy and pre-eclampsia according to the Universal Standard Validation Protocol.
Methods
The Withings BPM Connect device measures BP at the brachial level using the oscillometric method. The study was performed according to the so-called “modified AAMI/ESH/ISO (ISO 81060-2:2018) protocol” or the “Universal Standard Protocol”. The validation study included 45 pregnant women in the second and third gestational trimester: 15 with pre-eclampsia, 15 with gestational hypertension and 15 normotensives. Differences between mercury sphygmomanometer BP measurements (reference) and device BP values (test) and their standard deviation (SD) were calculated.
Results
The mean differences between the mercury standard and device BP values in pregnancy (n = 45) were −0.5 ± 5.7 mmHg for systolic BP (SBP) and −0.8 ± 3.8 mmHg for diastolic BP (DBP). In the preeclamptic patients (n = 15), the mean differences were 0.14 ± 5.5 mmHg for SBP and 0.39 ± 3.7 mmHg for DBP. These results fulfilled the protocol requirements (<5 ± 8 mmHg).
Conclusion
The Withings BPM Connect fulfills the validation protocol criteria in pregnancy and pre-eclampsia. Consequently, this device can be recommended for home BP measurements in this specific pregnancy population.
Keywords: blood pressure measurements, accuracy, validation, pregnancy, pre-eclampsia, home blood pressure, Withings
Introduction
Several non-mercury techniques measuring blood pressure (BP) have been developed to supplant the mercury-auscultatory method, such as the hybrid, aneroid and electronic devices using algorithms based principally on the oscillometric technique.1 These devices must undergo a validation study by experts in independent centers as recommended by guidelines and societies.2 Different protocols have been used to validate the accuracy of BP measuring devices such as the international protocol of the European Society of Hypertension (ESH),3 the British Hypertension Society (BHS) protocol,4 the Association for the Advancement of Medical Instrumentation (AAMI) protocol5 and the International Organization for Standardization (ISO).6 In 2018, members of the AAMI, ESH and ISO committees reached a consensus on an optimal validation standard, the AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018) which is now considered as the standard protocol for the validation of non-invasive blood pressure measuring devices.7 Elsewhere, international guidelines recommend submitting any new device for BP measurement to an independent clinical validation. Moreover, to allow new BP devices to be listed as “recommended accurate BP measuring devices” in certain scientific society websites, it is recommended to perform such independent clinical validation and publish its results.8
Over the last ten years, several devices have been validated, mostly in the general population.8 However, few studies have tested the accuracy of automated BP monitors in specific populations such as arrhythmia patients, pregnant women, etc.9,10 Pregnancy and pre-eclampsia have been acknowledged by experts as special populations.7 Indeed, pregnancy is a specific condition where vascular hemodynamic and arterial function and structure changes influence arterial signals and therefore BP determination. These hemodynamic changes are more pronounced in pre-eclampsia suggesting that automatic devices for BP measurements tend to underestimate BP in these conditions.11 Considering that hypertension in pregnancy is common and has been reported to occur in up to 10% of pregnancies;12 accurate BP measurements are of paramount importance in diagnosing and monitoring high-risk pregnant women.1 In this regard, the validation protocols recommend that BP devices designed to be used in pregnancy undergo specific validation of their accuracy in this special population. Only few devices have been shown to be accurate in pregnant women with and without pre-eclampsia.9–13 Therefore, after validating the Withings BPM Connect automatic device in the general population,14 the present study was designed to assess its accuracy in pregnant women including pre-eclampsia according to the Universal Standard Protocol.7
Methods
Ethics Committee
This prospective study using a medical device (Type IIA) was approved by the Ethics Committee of the Institute of Cardiology named after Levon Hovhannisyan (Yerevan, Armenia). Written informed consent was obtained from all included subjects. The study was performed according to the declaration of Helsinki and ICH good clinical practice.
Study Design
The study was carried out according to the “AAMI/ESH/ISO (ISO-81060-2:2018) Universal protocol”.7 The recommendations for performing and reporting validation studies were strictly followed.7
Study Population
According to the validation protocol, 45 women in 2nd and 3rd trimesters of pregnancy were included, 15 of whom had pre-eclampsia defined as elevated systolic BP (SBP) of at least 140 mmHg and/or diastolic BP (DBP) of at least 90 mmHg with proteinuria, 15 had gestational hypertension (new onset in pregnancy with SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg without proteinuria), and 15 were normotensive.7 The other inclusion criteria were patients older than 18 years of age and arm circumference between 22 cm and 42 cm in accordance with the instructions for use of the Withings BPM Connect device. Exclusion criteria were arrhythmia, poor quality of Korotkoff sounds, absence of Korotkoff K5 sounds, patient unable to give consent or properly understand protocol information, patient wearing an implantable electrical medical device, patient with an open wound and/or damaged skin in the upper arms, the latter being in accordance with the instructions for use of the Withings BPM Connect device.
Procedures and Measurements
Tested Device
The WITHINGS BPM CONNECT device is a digital automatic device for home BP measurement at the brachial level (Figure 1). The device measures BP using the oscillometric method during cuff inflation, with a pressure range of 0–285 mmHg (systolic: 60–230 mmHg, diastolic: 40–130 mmHg) and pulse rate range of 40–180 beats/min. At the end of BP determination, a fast cuff deflation is performed using a release valve. The device includes:
- A cuff assembly, for BP measurement in arm circumference 22–42 cm including:
- Soft cuff of 55 cm length and 15 cm width with inflatable bladder of 23 cm length and 12.5 cm width,
- Velcro pads to secure the cuff around the subject’s arm.
- A main unit mounted on the outer side of the cuff including:
- Pneumatic circuit: a pump, a release valve, a pressure sensor, analog to digital converter, memory, Wi-Fi, and Bluetooth components,
- Li-ion rechargeable battery and a micro-USB charging plug,
- LED screen and a trigger button.
Figure 1.
The Withings BPM Connect device.
Devices used in this study were equipped with the version 5c (electronic) and 4a (mechanical) hardware, and FW1401 firmware. Further details regarding the device are provided in the user-manual. For the present study, 3 BPM Connect devices were provided by WITHINGS SA France, one of which was randomly chosen to perform the study.
Reference Blood Pressure Measurements – Mercury Sphygmomanometer
The validation team consisted of three investigators, namely two observers and one supervisor trained in accurate BP measurements. BP was measured by the two observers blinded to each other’s result using: 2 parallel connected mercury sphygmomanometers (KDM®, Germany), calibrated prior to study initiation, and a “Y” connected teaching stethoscope (3MTM Littmann®, United States), after which BP was measured by the supervisor using the tested device. Agreement between the 2 observers was verified by the supervisor to ensure that the difference between their measurements was < 4 mmHg for SBP and DBP. In case of disagreement between the 2 observers, additional pairs of measurements were performed with a maximum of 8 pairs of BP determinations after which the subject was excluded. Korotkoff sound (K5) was used for reference diastolic BP.
The circumference of the arm was measured to ensure that the reference cuff-size being used was adequate for the subject. Three cuffs with inflatable bladder dimensions 9×18 cm, 12×24 cm and 15×32 cm respectively were used such that the length reached 75–100% of the midarm circumference and width at 37–50%.
Procedure for BP Measurements and Data Collection
The validation procedure began with the patient seated comfortably and relaxed for at least 5 minutes, the back and the arm supported with the middle of the upper arm at heart level, legs uncrossed and feet flat on the floor. BP measurements were performed according to the “same arm, sequential measurements” method on the left arm supported at heart level as described in the AAMI/ESH/ISO Universal Standard.7 Measurements by the tested device were performed on the same arm supported at heart level as recommended by the manufacturer. As required, nine consecutive BP measurements at 1 minute intervals were performed in each participant using the mercury sphygmomanometers (5 times: R0, R1, R2, R3, R4) and the tested device (4 times: T0, T1, T2, T3) beginning with the standard mercury sphygmomanometer. The first auscultatory and first device measurements represented the recruitment pressures (R0 and T0) and were not used in the accuracy assessment of the test device.
Statistical Analysis
The AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018) requirements were strictly followed. Results were analyzed and presented according to validation protocol requirements.7 Statistical analysis was performed using specific analysis software established by the International Society of Vascular Health (ISVH). Each reference BP measurements (R1, R2, R3, R4) represented the mean of the simultaneous readings by the two observers. Each test device measurement was compared against the mean of the previous and next reference BP readings (eg, T1 versus the mean of R1-R2). Differences were calculated by subtracting the reference BP measurements from the test device measurements. The mean BP difference (test versus reference device) and its SD, ie Criterion 1 of the AAMI/ESH/ISO protocol, was calculated for SBP and DBP measurements. Standardized Bland-Altman scatter plots were used to illustrate device–observer differences versus mean device and observer values for all pairs of comparisons.
Results
Study Population
Fifty-nine patients were preselected according to the inclusion/exclusion criteria and the required phenotype: 47 were initially recruited with 2 being excluded due to completed BP range. A total of 45 women in the 2nd and 3rd trimesters of pregnancy were ultimately included: 15 with pre-eclampsia, 15 with gestational hypertension and 15 normotensives.
The clinical characteristics of the participants in the study are summarized in Table 1. Results show that their characteristics were in accordance with the requirements of the validation protocol.
Table 1.
Characteristics of the Study Participants (n=45)
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 31 ± 5 | 21–42 |
| Arm circumference (cm) | 28.8 ± 2.6 | 23.5–35.5 |
| Entry SBP R0 (mmHg) | 135 ± 21 | 89–164 |
| Entry DBP R0 (mmHg) | 89 ± 14 | 59–111 |
| Normotensive women | 15 | |
| Hypertensive women | 15 | |
| Pre-eclampsia women | 15 | |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
BP Measurements
The mean BP differences between the simultaneous observers’ measurements were 0.3 ± 2.3/0.2 ± 2.1 mmHg (systolic/diastolic, (range −4 to 4 mmHg)).
Results of the validation analysis are shown in Table 2. For criterion 1, the mean differences between the tested device and reference BP values (tested device minus reference BP values) were −0.5 ± 5.7 mmHg for SBP and −0.8 ± 3.8 mmHg for DBP. Hence, the observed values fulfilled the requirements of the (AAMI/ESH/ISO)” – (ISO 81060-2:2018) Universal protocol whereby the difference must be 5 mmHg or less, and its standard deviation (SD) 8 mmHg or less for SBP and DBP.7 Criterion 2 is not applicable in special populations as indicated in the AAMI/ESH/ISO – (ISO 81060-2:2018) Universal protocol.2,7 The above results having fulfilled the requirements of the (AAMI/ESH/ISO)” – (ISO 81060-2:2018) Universal protocol, the tested device was therefore qualified as having successfully “PASSED” the validation.
Table 2.
Validation Study Results According to Protocol Requirements (Criterion 1)
| Pass Requirement | SBP | DBP | |
|---|---|---|---|
| Criterion 1 (135 BP pairs) | |||
| Mean BP difference (mmHg) | ≤ 5 | −0.5 | −0.8 |
| SD (mmHg) | ≤ 8 | 5.7 | 3.8 |
| Pass | Pass | ||
| Result | Pass | ||
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
Analysis according to the subgroups of patients is depicted in Table 3. For criterion 1, the mean differences between the tested device and reference BP values are shown for each subgroup of patients: normotensives, hypertensives, and preeclampsia. All differences in each subgroup for both SBP and DBP remained within the required standard of the protocol (< 5 ± 8 mmHg).
Table 3.
Blood Pressure Differences According to Subgroup of Patients
| Pregnancy Status | Number | Mean SBP Difference ± SD (mmHg) | Mean DBP Difference ± SD (mmHg) |
|---|---|---|---|
| Normotensive | 15 | 0.40 ± 5.64 | −1.54 ± 3.11 |
| Hypertensive | 15 | −1.99 ± 5.89 | −1.13 ± 4.30 |
| Pre-eclampsia | 15 | 0.14 ± 5.50 | 0.39 ± 3.68 |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
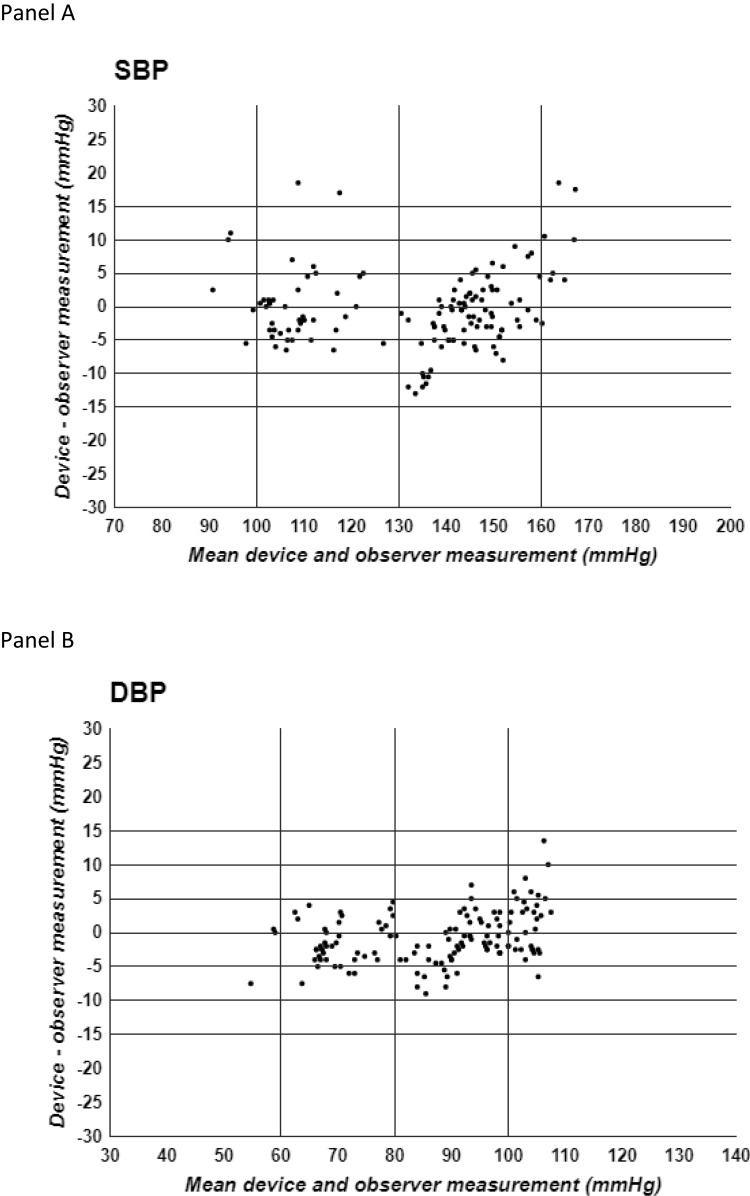
Standardized Bland-Altman scatter plots of the differences in test-reference BP values against their mean values are illustrated in Figure 2 for SBP (panel A) and DBP (panel B).
Figure 2.
Standardized Bland-Altman scatter plots of the Test-Reference BP differences against their mean values. Panel A SBP = Systolic blood pressure, Panel B DBP = Diastolic blood pressure.
Discussion
This study is the first to provide information on the accuracy of the WITHINGS BPM Connect device from WITHINGS SA for BP measurement at the arm (brachial) level in patients with arm circumference 22–42 cm in pregnancy and pre-eclampsia. This validation study was performed according to the recent Universal Standard Protocol.7 Results showed that the Withings BPM Connect achieved the protocol requirements criteria in pregnancy and pre-eclampsia. Several points of the present study warrant further consideration with regard to their interpretation.
Oscillometric Devices
Many of the new generation of these devices have added benefits such as automatic repeated BP measurements, memory storage, and communicability. Despite these advantages, there are several persistent concerns with these devices, including, 1) the accuracy of BP measurements: this is of concern in the general population and even more so in special populations such as children, arrhythmia patients, etc. where questionable accuracy of some of these devices have been reported;11,13 2) the inter-individual variability of oscillometric BP measurements: in some patients, BP measurements obtained using the oscillometric methods show substantial variability and/or high disparity, in comparison to the auscultatory method. The reasons of such discrepancy and variability remain unclear. Therefore, it is recommended to verify the accuracy of automatic oscillometric BP measurement devices at the individual level prior to its clinical application.
Special Populations
The AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018)7 recommends clinical validation of automatic devices for BP measurements in the general population but also in other special populations. The following instances are regarded as special populations: children, pregnancy, pre-eclampsia, arm circumference > 42 cm, arrhythmia, and atrial fibrillation. This study focused on pregnant women in whom substantial hemodynamic changes occur. Some of these hemodynamic changes modify the pulse wave characteristics and consequently the oscillogram, thereby ultimately affecting the accuracy of the device.15 Given that the present study was undertaken according to a strict validation protocol in pregnancy, any extrapolation of these results to other populations would be inappropriate and arbitrary.
Compliance with the Validation Protocol
The present study was performed according to the latest universal validation protocol. In a systematic review9 of the accuracy of BP measurement devices in pregnancy, authors analyzed 18 studies in which brachial home BP devices were examined. The authors reported that the devices passed validation in 13 studies, 3 of which had no protocol violation, 7 had at least 1 minor violation, 1 had at least 1 major violation and 2 had major and minor violations. In the present study, special attention was made to perform the study according to the validation protocol requirements without any major or minor violation.
Previous Studies
The above-mentioned systematic review9 reported that 13/18 studies (9 devices) showed successful validation. However, some of the devices passed the validation in one study and failed in another, even if performed according to the same validation protocol. Such contradictory results raise the question on the reproducibility of the validation studies. Therefore, it would appear advisable to duplicate validation studies in at least two different expert centers (eg 2 studies). For instance, one clinical validation, if successful, would seemingly be sufficient to approve the device and consider its registration on the list of “approved” devices of several institutions and scientific societies.7,8 The present study is the first to assess the accuracy of the Withings BPM Connect in pregnancy and pre-eclampsia, the results of which corroborate the findings of a previous validation study of the same device performed in the general population.14
Conclusion
The Withings BPM Connect fulfils the validation requirements of the AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018) for both SBP and DBP measurements in pregnancy and pre-eclampsia. Consequently, this device can be recommended for home BP measurements and clinical use in pregnancy.
Acknowledgments
Our heartfelt thanks to all of the patients who took part in the study. Our grateful thanks to the Foundation-Medical Research Institutes® (F-MRI) and the International Society of Vascular Health for their support. The study was supported by Withings® SA France, the International Society of Vascular Health (ISVH®) France and the Foundation-Medical Research Institutes (F-MRI)® Switzerland.
Disclosure
J Topouchian, Z Hakobyan, P Zelveian, H Gharibyan and R Asmar have conducted validation studies for various manufacturers. Investigators received honorarium for this validation study. J Topouchian reports personal fees from FMRI. R Asmar reports personal honorarium from Withings, during the conduct of the study. J Asmar declares no conflicts of interest.
References
- 1.Stergiou G, Palatini P, Parati G, et al. Consensus document – 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39(7):1293–1302. doi: 10.1097/HJH.0000000000002843 [DOI] [PubMed] [Google Scholar]
- 2.Stergiou G, Palatini P, Asmar R, et al.; on behalf of the European Society of Hypertension Working Group on Blood Pressure Monitoring. Recommendations and practical guidance for performing and reporting validation studies according to the universal standard for the validation of blood pressure measuring devices by the association for the advancement of medical instrumentation/European society of hypertension/International organization for standardization (AAMI/ESH/ISO). J Hypertens. 2019;37:459–466. doi: 10.1097/HJH.0000000000002039 [DOI] [PubMed] [Google Scholar]
- 3.O’Brien E, Atkins N, Stergiou G, et al.; Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010;15(1):23–38. doi: 10.1097/MBP.0b013e3283360e98 [DOI] [PubMed] [Google Scholar]
- 4.O’Brien E, Petrie J, Littler WA, et al. The British Hypertension Society Protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993;11(suppl 2):S43–S62. [DOI] [PubMed] [Google Scholar]
- 5.Association for the Advancement of Medical Instrumentation. ANSI/AAMI/ISO 11137-2:2013; 2013.
- 6.International Organization for Standardization. Noninvasive sphygmomanometers: part 2: clinical investigation of intermittent automated measurement type. ISO 81060-2:2018. Available from: https://www.iso.org/standard/73339.html. Accessed July 8, 2021.
- 7.Stergiou GS, Alpert B, Mieke S, et al. A universal standard for the validation of blood pressure measuring devices: association for the advancement of medical instrumentation/European society of hypertension/International organization for standardization (AAMI/ESH/ISO) collaboration statement. J Hypertens. 2018;36(3):472–478. doi: 10.1097/HJH.0000000000001634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stergiou G, O’Brien E, Myers M, Palatini P, Parati G; STRIDE BP Scientific Advisory Board. Stride BP: an International initiative for accurate blood pressure measurement. J Hypertens. 2020;38:395–399. doi: 10.1097/HJH.0000000000002289 [DOI] [PubMed] [Google Scholar]
- 9.Bello NA, Wooley JJ, Cleary KL, et al. Accuracy of blood pressure measurement devices in pregnancy. A systematic review of validation studies. Hypertension. 2018;71:326–335. doi: 10.1161/HYPERTENSIONAHA.117.10295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topouchian J, Hakobyan Z, Asmar J, Gurgenian S, Zelveian P, Asmar R. Accuracy of the Omron M3 comfort and the Omron Evolv for self-blood pressure measurements in pregnancy and preeclampsia – validation according to the universal standard protocol. Vasc Health Risk Manag. 2018;14:189–197. doi: 10.2147/VHRM.S165524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo C, Taylor RS, Gamble G, McCowan L, North RA. Use of automated home blood pressure monitoring in pregnancy: is it safe? Am J Obstet Gynecol. 2002;187(5):1321–1328. doi: 10.1067/mob.2002.126847 [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. ISBN: 9789241548335. Available from: https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/9789241548335/en. Accessed January 24, 2022. [PubMed]
- 13.Pomini F, Scavo M, Ferrazzani S, De Carolis S, Caruso A, Mancuso S. There is poor agreement between manual auscultatory and automated oscillometric methods for the measurement of blood pressure in normotensive pregnant women. J Matern Fetal Med. 2001;10:398–403. doi: 10.1080/714052781 [DOI] [PubMed] [Google Scholar]
- 14.Topouchian J, Zelveian P, Hakobyan Z, Gharibyan H, Asmar R. Accuracy of the Withings BPM connect device for self-blood pressure measurements in general population – validation according to the association for the advancement of medical instrumentation/European society of hypertension/International organization for standardization universal standard. In press 2022. [DOI] [PMC free article] [PubMed]
- 15.De Greeff A, Shennan A. Blood pressure measuring devices: ubiquitous, essential but imprecise. Expert Rev Med Devices. 2008;5:573–579. doi: 10.1586/17434440.5.5.573 [DOI] [PubMed] [Google Scholar]