Abstract
Pneumocystis carinii is the causative agent of P. carinii pneumonia (PCP), an opportunistic infection associated with AIDS and other immunosuppressed conditions. Although polyamine metabolism of this fungus has been shown to be a chemotherapeutic target, this metabolism has not been thoroughly investigated. Reported here is the effect of one polyamine analogue, N,N′-bis{3-[(phenylmethyl)amino]propyl}-1,7-diaminoheptane (BBS), on P. carinii. BBS inhibits the growth of P. carinii in culture, but at concentrations higher than those required to inhibit the growth of other pathogens. However, BBS is at least as active in an animal model of PCP as in other models of diseases studied. BBS causes some reduction in P. carinii polyamine content and polyamine biosynthetic enzyme activities, but the effect is less than that observed with other pathogens and very much less than the effect of the polyamine biosynthesis inhibitor dl-α-difluoromethylornithine. BBS enters P. carinii cells via a polyamine transporter, unlike all other cells that have been studied. P. carinii cells do not remove the benzyl groups of BBS, as is reported for mammalian cells. The most likely mode of action is displacement of natural polyamines. Overall, the activity of BBS provides further evidence that polyamines and polyamine metabolism are rational targets for the development of drugs to treat PCP. Because the details of BBS-P. carinii interaction differ from those of other cells studied, polyamine analogues may provide a highly specific treatment for PCP.
Polyamine metabolism has been demonstrated to be a chemotherapy target for Pneumocystis carinii pneumonia (PCP) caused by the fungus P. carinii, a common opportunistic pathogen associated with AIDS, cancer chemotherapy, treatment for rheumatic disease, and other immunosuppressed conditions (12). Although there are many compounds that can be used to interfere with polyamine metabolism, most of the clinical and much of the experimental animal data to date have been obtained with dl-α-difluoromethylornithine (DFMO; eflornithine), a compound which inhibits the critical polyamine biosynthetic enzyme ornithine decarboxylase (ODC). However, there are inhibitors of other polyamine biosynthetic steps as well as compounds which cause the acceleration of polyamine degradation or interfere with polyamine function (21). Among these is a series of bis-benzyl polyamine analogues synthesized by Merrell Dow Pharmaceuticals (now incorporated into Aventis). Here we report an examination of a bis-benzyl derivative, MDL-27695, N,N′-bis{3-[(phenylmethyl)amino]propyl}-1,7-diaminoheptane (BBS) [C6H5CH2NH(CH2)3NH(CH2)7NH(CH2)3NHCH2C6H5], for the ability to modulate the polyamine concentrations of P. carinii, to induce the polyamine catabolic enzyme spermine-spermidine acetyltransferase (SSAT), to block growth in vitro, and to treat an animal model of PCP. We found BBS to be active against P. carinii, thus demonstrating that polyamine analogues are a lead in the search for new compounds to treat PCP. Furthermore, the nature of the interaction of BBS with P. carinii is different than that with any other cell studied, suggesting that it is possible to design agents which would be highly specific for P. carinii.
MATERIALS AND METHODS
In vitro experiments.
Most in vitro experiments used P. carinii cells freshly isolated from infected rat lungs. To induce PCP, rats were pretreated with antibiotics to suppress other infections, immunosuppressed by adding dexamethasone to the drinking water, and inoculated by intratracheal instillation of a homogenate of a P. carinii-infected rat lung as previously described (14). After the development of pneumonia, the rats were sacrificed and the lungs were removed. Over a period of less than 90 min, the lungs were homogenized and P. carinii cells were isolated as previously detailed (16) and briefly described as follows. After mechanical homogenization in a phosphate-based buffer containing dithiothreitol, large debris was removed by 32 × g centrifugation, P. carinii cells were pelleted by 5,000 × g centrifugation, contaminating erythrocytes were removed by suspension in 0.85% NH4Cl, P. carinii cells were repelleted at 5,000 × g, host DNA was removed by incubation in DNase, and the cells were washed an additional three times in the phosphate-based buffer. Examination of Giemsa-stained smears revealed P. carinii cells only and absolutely no host cells or host cell nuclei. For all short-term in vitro experiments, the isolated P. carinii cells were suspended in Eagle minimal essential medium with Earle's salts (MEM) (GIBCO, Grand Island, N.Y.) and incubated at 37°C. For those experiments employing cultures of P. carinii, conditions were as previously described (17) except that S-adenosylmethionine was omitted.
HPLC methods.
Waters AccQ.Fluor reagent was used for precolumn derivatization of polyamines and related compounds as previously described for high-performance liquid chromatography (HPLC) analysis of polyamines (15). For all HPLC separations, the hardware, software, column, and mobile phase gradient components were as previously described for polyamine analysis (15) except that the elution gradient was modified for the analysis of BBS as follows. The gradient begins with 100% eluent A (140 mM acetate, 17 mM triethanolamine, pH 5.05) with a linear change to 60% eluent A–40% acetonitrile over 10 min. This is followed by a shallow linear gradient from 60% eluent A–40% acetonitrile to 55% eluent A–45% acetonitrile over 10 min. All other conditions were as previously reported. The BBS retention time is 13.82 min. Quantitation, reproducibility, linearity, recovery, and stability of derivatized BBS were demonstrated (data not shown). Analysis of the debenzylated BBS (MDL-26752) uses the same conditions except that the second gradient is extended to 12 min. SSAT analysis was as previously described (13). Extracts of P. carinii cells for HPLC analysis were prepared by sedimentation of cells by microcentrifugation, resuspension in eluent A, sonication, and clarification by centrifugation as previously described (15).
In vivo activity.
For testing the activity of BBS in vivo, the animal model was as described earlier (8) but was different than the model used for harvesting P. carinii cells from the lungs. Prior to the initiation of treatment protocols, spontaneous PCP was induced in rats by immunosuppression with twice weekly cortisone acetate injections (25 mg kg of body weight−1 subcutaneously [s.c.]) combined with an 8% protein diet for 5 weeks. The cortisone protocol was maintained throughout the 3-week treatment period, but normal rat chow was substituted for the low-protein diet. Oxytetracycline (250 mg liter−1) was always included in the drinking water to suppress bacterial infections. Treatment was given over 3 weeks and consisted of three daily intraperitoneal (i.p.) injections of 5 mg of BBS kg−1 and/or 3% DFMO added to the drinking water. At the end of the treatment period, the animals were sacrificed and the lungs were removed and homogenized with a fixed ratio of homogenization buffer volume-to-lung weight. Microscope slide smears were made from the homogenate and stained with cresyl echt violet, and the intensity of PCP was measured by counting the total number of cysts in a preset number of microscope fields as previously described (8).
RESULTS
In vitro activity of BBS.
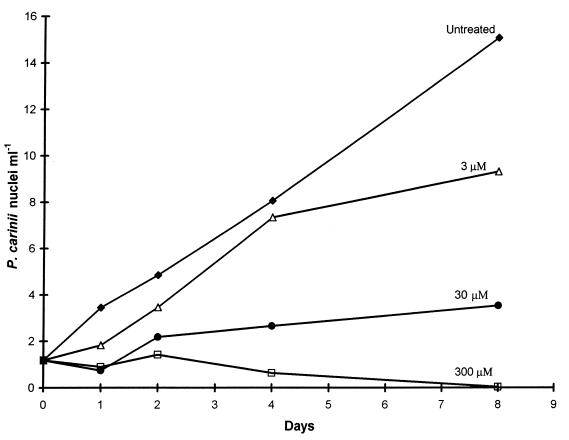
Before studying the effect of BBS on the polyamine metabolism of P. carinii, it was necessary to know if BBS had any effect on P. carinii cells and at what concentration. Cultures of P. carinii were exposed to 3.0, 30, and 300 μM BBS. Growth was followed for 8 days, and the data are presented in Fig. 1. At the end, 3.0 and 30 μM BBS had reduced growth by 38 and 77%, respectively, and 300 μM BBS had eliminated P. carinii cells from the culture.
FIG. 1.
Effect of BBS on growth of P. carinii in culture. Cultures were inoculated in parallel on day 0, and determining the increase in the number of P. carinii nuclei followed a previously described DNA assay method (17). The concentrations of BBS used for each culture are above the individual curves.
Uptake of BBS.
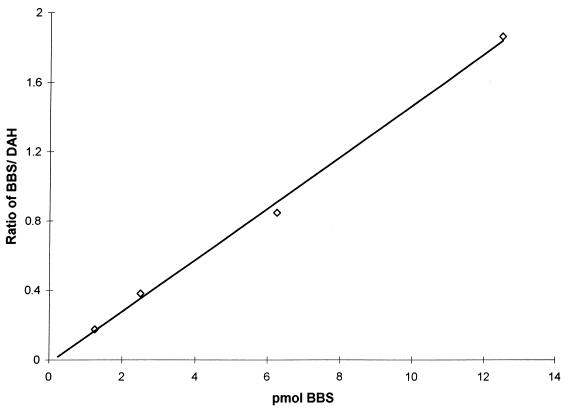
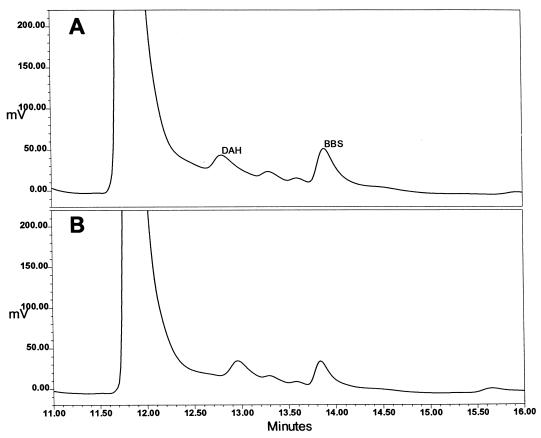
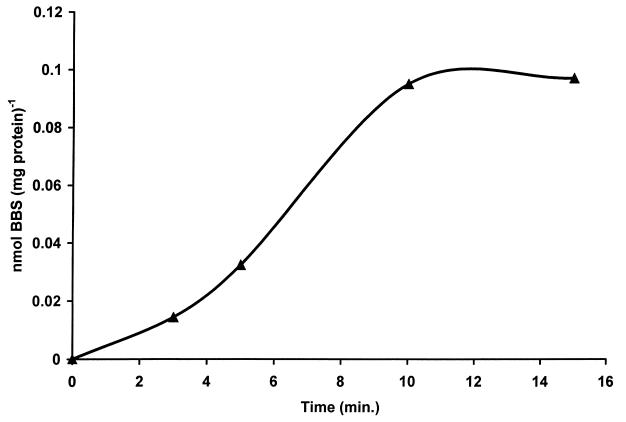
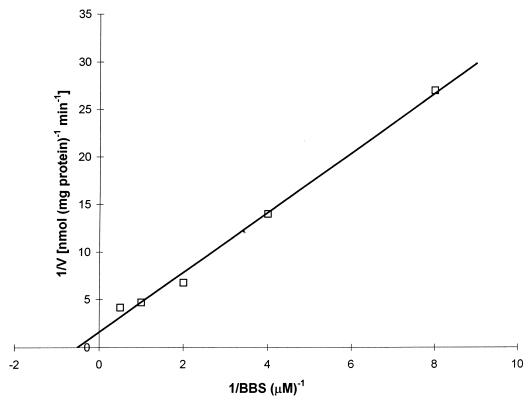
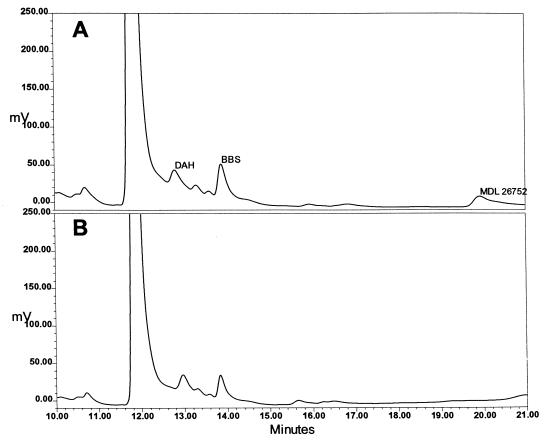
Figure 2 presents a standard curve produced by the HPLC method developed for the analysis of BBS content; there is a linear relationship between the ratio of the area of the BBS peak to the area of the internal standard peak and the amount of BBS added to the sample. Figure 3 presents chromatograms of extracts of P. carinii. Figure 3B is an analysis of an extract of cells that had been incubated for 24 h with 100 μM BBS. Figure 3A is the same except that 2 pmol of BBS were added to the injected sample to reinforce the BBS peak and confirm the identification. The chromatograms show that the addition of BBS to the P. carinii extract yielded no additional peak but reinforced the peak identified as BBS. When P. carinii cells were incubated with 1.0 mM BBS, the analogue accumulated within the cells, reaching a maximum concentration of 0.95 nmol mg protein−1 at 10 min, as shown in Fig. 4. BBS uptake was completely inhibited when 2.0 mM spermidine was included in the incubation medium. Uptake was reduced by 85% when the cells were incubated with BBS at 4°C. From the slope and intercept of a Lineweaver-Burk plot (Fig. 5), Km and Vmax values for BBS transport were calculated to be 1.98 μM and 0.63 nmol mg protein−1 min−1, respectively. These results demonstrate that BBS enters P. carinii cells via a transporter, one that can also transport spermidine.
FIG. 2.
Standard curve for analysis of BBS. The assay method was as described in Materials and Methods. The ordinate scale is the ratio of the area under the curve of the BBS peak to the area under the curve of the peak of the internal standard, 1,7-diaminoheptane (DAH) (r2 > 0.99).
FIG. 3.
Detection of BBS in an extract of P. carinii. (A) Chromatogram of an extract of P. carinii which had been incubated with BBS and to which extra BBS was added. (B) Chromatogram of the same P. carinii extract but with no additional BBS added. The internal standard peak is of 1,7-diaminoheptane (DAH). The reinforcement of the BBS peak confirms its identity. The ordinate is the output of the fluorescence detector expressed in millivolts, as reported by the Waters Millennium software used for chromatography control and analysis.
FIG. 4.
Uptake of BBS by P. carinii. The accumulation of BBS is expressed relative to the amount of P. carinii protein. Exposure was done by adding 1.0 mM BBS to cells in culture medium. After the exposure times indicated, extracts were prepared for BBS analysis as described in Materials and Methods.
FIG. 5.
Lineweaver-Burk plot of BBS transport. The ordinate is the reciprocal of the rate of BBS transport expressed as nanomoles of BBS taken up (milligram protein)−1 minute−1. The abscissa is the reciprocal of the BBS concentration in the incubating medium. For all concentrations of BBS, transport was measured over a 10-min period.
Effect of BBS on intracellular polyamine concentrations.
P. carinii cells isolated from rat lungs were divided into four equal aliquots and suspended in RPMI 1640 tissue culture media (GIBCO); serum was omitted to avoid the variable polyamine content of serum. One aliquot was extracted immediately for polyamine analysis. Two aliquots were incubated at 31°C with 300 μM BBS; one of these was extracted for polyamine analysis after 3 h and the other after 12 h. The final aliquot was incubated at 31°C for 12 h without BBS. The data presented in Table 1 show that BBS had a moderate effect on P. carinii polyamine content. A 3- and a 12-h exposure to 100 μM BBS caused reductions of 8 and 22%, 11 and 31%, and 7 and 24% of putrescine, spermidine, and spermine, respectively. For comparison, we previously showed that 3- and 12-h exposures to 1 mM DFMO caused reductions of 88 and 94%, 85 and 96%, and 83 and 90% of putrescine, spermidine, and spermine, respectively (13).
TABLE 1.
Effect of exposure to 100 μM BBS in vitro
| Time of exposure to 100 μM BBS (h) | Polyamine content (pmol mg protein−1)a(n = 3)
|
||
|---|---|---|---|
| Putrescine | Spermidine | Spermine | |
| 0 | 686 ± 121 | 645 ± 311 | 376 ± 97 |
| 3 | 637 ± 45 | 625 ± 120 | 353 ± 39 |
| 12 | 535 ± 66 | 445 ± 94 | 286 ± 22 |
| 12 (untreated) | 628 ± 88 | 670 ± 94 | 399 ± 82 |
The data are means with standard deviations.
Effect of BBS on SSAT and ODC activities.
SSAT and ODC activities of cells that had been cultured with and without BBS were measured. Without exposure to BBS, the specific activity of SSAT was 1.62 ± 0.8 (n = 3) pmol of N1-acetylspermidine produced min−1 mg protein−1 and the mean specific activity of ODC was 787 (n = 2; 772 and 801) pmol of putrescine produced min−1 mg protein−1. After exposing cells to 100 μM BBS for 24 h, SSAT activity was 1.87 ± 0.11 (n = 3) pmol min−1 mg protein−1 and mean ODC activity was 610 (n = 2; 603 and 616) pmol of putrescine produced min−1 mg protein−1. The slight increase in SSAT activity and decrease in ODC activity are not considered significant. The direct effect of BBS on ODC in lysates of P. carinii was also measured. The addition of 30 μM BBS to a P. carinii cell lysate caused ODC activity to decrease from 789 to 580 pmol of putrescine produced min−1 mg protein−1. Although the 22% reduction in ODC activity caused by the exposure of intact cells and the 26% reduction caused by addition to a cell lysate are minor effects, they do parallel the 22, 31, and 24% reductions in the content of putrescine, spermidine, and spermine, respectively, in cells exposed to 100 μM BBS for 12 h, as calculated from the data in Table 1.
Metabolism of BBS.
BBS behaves as a prodrug in some cells, the active form being the result of debenzylation by polyamine oxidase to produce NH2(CH2)3NH(CH2)7NH(CH2)3NH2(MDL-26752) (3). To examine this possibility for P. carinii, an HPLC assay for MDL-26752 was developed and used to determine if this compound is produced in P. carinii cells exposed to BBS. Figure 6 presents chromatograms of an extract of P. carinii cells which had been exposed to 100 μM BBS for 24 h. For chromatogram A, 2 pmol of authentic MDL-26752 and 2 pmol of BBS were added to demonstrate the ability to detect these compounds. Chromatogram B was obtained with the P. carinii extract alone, showing that there was no MDL-26752 produced in cells incubated with BBS. Two additional experiments were performed to confirm the conclusion that MDL-26752 is not the active compound. In the first experiment, 30 μM MDL-26752 was added to a culture to determine if this compound has anti-P. carinii activity. Figure 7 shows the growth of P. carinii cells exposed to 30 μM BBS or 30 μM MDL-26752 and control cells. BBS suppressed growth, but MDL-26752 did not. Since the polyamine oxidase of HTC cells was found to be responsible for the conversion of BBS to MDL-26752 (3), the second experiment was to determine if an extract of P. carinii with known polyamine oxidase activity had the ability to perform this conversion. Preparation of the extract and conditions of the assay were the same as used previously for polyamine oxidase (13). No HPLC-detectable MDL-26752 was produced (data not shown).
FIG. 6.
Detection of the debenzylated metabolic product of BBS, MDL-26752. (A) Shown is an extract of P. carinii that had been incubated with 100 μM BBS for 24 h and to which 2.0 pmol of MDL-26752 and 2.0 pmol of BBS had been added. (B) The same extract without the added MDL-26752 or BBS. The ordinate is the output of the fluorescence detector expressed in millivolts as reported by the Waters Millennium software used for chromatography control and analysis.
FIG. 7.
Lack of activity of the BBS derivative MDL-26752. Growth of cultures of P. carinii with no additive, with 30 μM BBS, and with 30 μM MDL 26752 was followed.
In vivo activity of BBS.
BBS was active in a rat model of PCP; the animal model and the method used for the evaluation of efficacy were as previously described (8). A daily dosage of 15 mg of BBS kg−1 (administered as three daily doses of 5 mg of BBS kg−1) for 3 weeks caused a 97% suppression in cyst count after 3 weeks of treatment (44 ± 18 cysts per 250 microscopic fields of lung homogenate smear [n = 7] versus the cyst count of the control [1,451 ± 762; n = 7]). The effect was roughly equivalent to that of 3% (wt/vol) DFMO added to the drinking water (2,220 mg/kg/day) for 3 weeks (49 ± 31 cysts per 250 microscopic fields of lung homogenate smear [n = 7], 3.4% of the cyst count of the control). Although the mean number of cysts was further reduced by the combination of BBS and DFMO in drinking water (26 ± 4 cysts per 250 microscopic fields of lung homogenate smear [n = 7], 1.8% of the cyst count of the control), the difference cannot be accepted as significant due to the variance in the counts of the controls.
DISCUSSION
The legitimacy of polyamines as a therapeutic target for PCP was previously demonstrated by the activity of the polyamine precursor analogue DFMO in vitro (10), in an animal model (9), and in the clinic (20). The results reported here show that a polyamine analogue, BBS, is also active against PCP and provide information about the interaction of this analogue and P. carinii. The choice of BBS for the study was based on previous reports of activity against Plasmodium, the malaria parasite, about 10 years ago (7) and subsequent reports of activity against the protozoans Leishmania donovani (1) and Trypanosoma cruzi (11), as well as the filarial parasite Brugia pahangi (19). A summary of previous results with BBS and other parasites is presented below to provide perspective for the results presented here. This synopsis is complex because the approaches taken and parameters measured were not consistent.
BBS at concentrations ranging from 1 to 10 μM was active against many parasites in vitro, but much higher concentrations were required for an effect on P. carinii. An in vitro MIC50 of 3.0 μM was reported for the human malaria parasite Plasmodium falciparum (4). For L. donovani mammal-infective amastigotes in vitro, 1.0 μM BBS eliminated 77 to 100% of them from mouse peritoneal macrophages (2). For the vector-infective promastigotes of L. donovani, 10, 20, and 30 μM BBS caused 45, 50, and 62% inhibitions of growth, respectively, compared to the growth of untreated controls (18). Exposure of mammal-infective T. cruzi trypomastigotes to 1 μM BBS prior to adding to mammalian cells caused a 61% reduction in the number of infected mammalian cells and a 69% reduction in the mean number of parasites per infected cell compared to untreated control trypomastigotes (11). When T. cruzi was already growing in mammalian cells, a 24-h exposure to 1 μM BBS caused a 41% reduction in parasite multiplication, compared to untreated controls. In experiments with B. pahangi isolated from infected animals and maintained in vitro, the addition of 1 μM BBS was associated with a 90% loss in viability in 6 days and 5 and 10 μM BBS were associated with a 100% viability loss in 5 and 3 days, respectively; in untreated control worms, there was only a 30% loss in viability over 6 days (19). The data presented in Fig. 1 show that P. carinii is less sensitive to BBS in vitro, with 300 mM BBS required to eliminate P. carinii from culture.
In contrast to the relative insensitivity of P. carinii to BBS in vitro, an animal model of PCP was at least as sensitive as any other disease model tested. In a Plasmodium berghei mouse model of malaria, a dose of 15 mg of BBS kg−1 given i.p. once on the day of infection and three times a day (total of 45 mg kg−1 day−1) for the subsequent 3 days cured 3 of 14 animals in one experiment and 15 of 20 in another (4). Combining this treatment with 3% DFMO in the drinking water increased the cure rates to 14 of 14 and 18 of 20. In animals allowed to develop intense parasitemia before beginning treatment, the same combination of BBS and DFMO administered for 2 weeks cured 15 of 20 (7). For L. donovani-infected mice, 45 mg of BBS kg−1 in three divided doses for 5 days reduced the parasite burden by 83, 96, and 90% in the liver, spleen, and bone marrow, respectively (1). For L. donovani-infected hamsters, this treatment reduced liver parasites by 99.9% (1). There are no in vivo data for either T. cruzi or B. pahangi. The rat model of PCP responded more sensitively than either the malaria or the leishmaniasis model. A dose of 5 mg of BBS kg−1 given three times per day (total of 15 mg kg−1 day−1) caused a 97% reduction in the number of cysts compared to that of untreated controls. DFMO given as a 3% solution in the drinking water also caused a 97% reduction in the number of cysts. The combination of the same doses of these two drugs seemed to cause a further reduction in the number of cysts, but the difference was not significant due to the variance in the response rate to DFMO alone. The longer treatment period used for PCP complicates this comparison to responses of other infections, but a 3-week treatment protocol is standard for P. carinii, while models of both malaria and leishmaniasis respond more quickly to other drugs as well.
Previous investigators have suggested that BBS acts via inhibition of polyamine synthesis in some cases and by displacement of natural polyamines in others. For Plasmodium, the interaction of BBS was not studied directly due to the intracellular nature of this parasite and because methods of growing P. falciparum cells outside erythrocytes had not been developed at the time of that study (6). However, based on the more rapid inhibition of radiolabeled hypoxanthine than leucine incorporation, they proposed that BBS acts on DNA by displacing the natural polyamines (6). HTC cells, a rat hepatoma cell line, were used for a general study of the effect of BBS on polyamine metabolism (3). HTC cells actively transport BBS but use a transporter system separate from that for polyamines. Exposure of HTC cells to 1 μM BBS resulted in repression of the polyamine biosynthetic enzymes ODC and S-adenosylmethionine decarboxylase (AdoMetDC) by approximately 50% at 8 h. Although ODC activity had recovered slightly and AdoMetDC had essentially fully recovered after 24 h of continuous exposure, the content of putrescine, spermidine, and spermine had declined by 86, 50, and 24%, respectively. In HTC cells, BBS is a prodrug with the active compound being the debenzylated free amine derivative NH2(CH2)3NH(CH2)7NH(CH2)3NH2(MDL-26752); this conversion is mediated by polyamine oxidase (5). The implication of these studies was that BBS may be a prodrug for Plasmodium as well. In lysates of the promastigote (i.e., vector) form of L. donovani, 10 μM BBS inhibited ODC by 36% and AdoMetDC by 58%, but a 12-h exposure of intact promastigotes to 10 μM BBS caused an almost threefold increase in intracellular putrescine and an almost fourfold increase in spermidine (18). Upon continued exposure, these elevated concentrations declined until, at 48 h, they were close to the pretreatment concentrations. A low concentration of BBS (5 μM) actually antagonized the ability of DFMO to reduce the polyamine content of L. donovani promastigotes in vitro, a curious and seemingly contradictory observation. However, 50 μM BBS inhibited promastigote incorporation of uracil, thymidine, and methionine by 35, 18, and 66%, respectively; the effect on methionine incorporation occurred quicker and was greater. The conclusion was that BBS works against L. donovani by interfering with macromolecular synthesis rather than interfering with polyamine metabolism; presumably this interference is by displacement of natural polyamines (18). Other workers found that BBS is not converted to the debenzylated form by L. donovani cells, indicating that BBS is the active compound (1). BBS is also not metabolized by B. pahangi, and the killing effect was suggested to be due to direct interference with polyamine function (19). Brugia does not use a polyamine transporter since polyamines do not block BBS uptake; BBS does block polyamine uptake, but this is likely a secondary effect.
The ability of spermidine to block the uptake of BBS by P. carinii cells indicates that, in contrast to HTC cells and B. pahangi cells, BBS enters P. carinii cells by a polyamine transporter. The addition of 30 μM BBS to P. carinii lysates has little direct effect on polyamine biosynthesis enzymes, in contrast to effects on L. donovani. Compared to HTC cells and L. donovani cells, BBS has little effect on P. carinii polyamine content.
The results presented here demonstrate that BBS, a polyamine analogue, is active against P. carinii in culture and is therapeutic in an animal model of PCP. Because BBS did not greatly perturb the polyamine content of P. carinii, our working hypothesis for the effect of BBS on P. carinii is similar to the explanation given for other cells; i.e., BBS interferes with polyamine function—perhaps by displacing the natural polyamines from critical sites in the cell. The data suggest, but do not prove, that DFMO enhances the effect of BBS. Such an enhancement is consistent with our working hypothesis since DFMO-induced reduction of natural polyamines would be expected to enhance the ability of BBS to compete with and displace the remaining natural polyamines.
Because biochemical aspects of the interaction of BBS with P. carinii differ from that of all other cell types reported, it is possible that polyamine analogues represent a highly specific lead for therapy for PCP. The higher activity of BBS in vivo than in vitro relative to the effects on other cells could be due to the longer in vivo treatment period, pharmacokinetic factors, or production of an as yet undetected metabolite by the host that is effective against P. carinii. Pharmacodynamic factors also could be important considering that nutrient supply and other external conditions could change internal conditions enough to affect the interaction of BBS and the internal target in P. carinii. Finally, P. carinii may be more sensitive than the other organisms to perturbation in polyamine function. Overall, the vulnerability of P. carinii cells to polyamine analogues and to inhibitors of polyamine biosynthesis demonstrate that polyamines are an excellent target for drug development to treat PCP.
ACKNOWLEDGMENTS
This work was supported in part by U.S. Public Health Service grants 1RO1-AI27685, 1RO1-AI33825, 1RO1-AI41947, and 2T32-A107382.
REFERENCES
- 1.Baumann R J, Hanson W L, McCann P P, Sjoerdsma A, Bitonti A J. Suppression of both antimony-susceptible and antimony-resistant Leishmania donovani by a bis(benzyl)polyamine analog. Antimicrob Agents Chemother. 1990;34:722–727. doi: 10.1128/aac.34.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann R J, McCann P P, Bitonti A J. Suppression of Leishmania donovani by oral administration of a bis(benzyl)polyamine analog. Antimicrob Agents Chemother. 1991;35:1403–1407. doi: 10.1128/aac.35.7.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitonti A J, Bush T L, McCann P P. Regulation of polyamine biosynthesis in rat hepatoma (HTC) cells by a bisbenzyl polyamine analogue. Biochem J. 1989;257:769–774. doi: 10.1042/bj2570769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitonti A J, Dumont J A, Bush T L, Edwards M L, Stemerick D M, McCann P P, Sjoerdsma A. Bis(benzyl)polyamine analogs inhibit the growth of chloroquine-resistant human malaria parasites (Plasmodium falciparum) in vitro and in combination with alpha-difluoromethylornithine cure murine malaria. Proc Natl Acad Sci USA. 1989;86:651–655. doi: 10.1073/pnas.86.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bitonti A J, Dumont J A, Bush T L, Stemerick D M, Edwards M L, McCann P P. Bis(benzyl)polyamine analogs as novel substrates for polyamine oxidase. J Biol Chem. 1990;265:382–388. [PubMed] [Google Scholar]
- 6.Bitonti A J, Dumont J A, McCann P P. Uptake of antimalarial bis(benzyl)polyamine analogs by human erythrocytes. Biochem Pharmacol. 1989;38:3638–3642. doi: 10.1016/0006-2952(89)90138-x. [DOI] [PubMed] [Google Scholar]
- 7.Bitonti A J, McCann P P, Sjoerdsma A. The effects of polyamine analogues on malaria parasites in vitro and in vivo. Adv Exp Med Biol. 1988;250:717–726. doi: 10.1007/978-1-4684-5637-0_63. [DOI] [PubMed] [Google Scholar]
- 8.Clarkson A B, Jr, Sarić M, Grady R W. Deferoxamine and eflornithine (dl-α-difluoromethylornithine) in a rat model of Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1990;34:1833–1835. doi: 10.1128/aac.34.9.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarkson A B, Jr, Williams D E, Rosenberg C. Efficacy of dl-α-difluoromethylornithine in a rat model of Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1988;32:1158–1163. doi: 10.1128/aac.32.8.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cushion M T, Stanforth D, Linke M J, Walzer P D. Method of testing the susceptibility of Pneumocystis carinii to antimicrobial agents in vitro. Antimicrob Agents Chemother. 1985;28:796–801. doi: 10.1128/aac.28.6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majumder S, Kierszenbaum F. Inhibition of host cell invasion and intracellular replication of Trypanosoma cruzi by N,N′-bis(benzyl)-substituted polyamine analogs. Antimicrob Agents Chemother. 1993;37:2235–2238. doi: 10.1128/aac.37.10.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marton L J, Pegg A E. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 13.Merali S. Pneumocystis carinii polyamine catabolism. J Biol Chem. 1999;274:21017–21022. doi: 10.1074/jbc.274.30.21017. [DOI] [PubMed] [Google Scholar]
- 14.Merali S, Chin K, Del Angel L, Grady R W, Armstrong M, Clarkson A B., Jr Clinically achievable plasma deferoxamine concentrations are therapeutic in a rat model of Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1995;39:2023–2026. doi: 10.1128/aac.39.9.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merali S, Clarkson A B., Jr Polyamine analysis using N-hydroxysuccinimidyl-6-aminoquinoyl carbamate for pre-column derivatization. J Chromatogr B. 1996;675:321–326. doi: 10.1016/0378-4347(95)00363-0. [DOI] [PubMed] [Google Scholar]
- 16.Merali S, Clarkson A B., Jr Polyamine content of Pneumocystis carinii and response to the ornithine decarboxylase inhibitor dl-α-difluoromethylornithine. Antimicrob Agents Chemother. 1996;40:973–978. doi: 10.1128/aac.40.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merali S, Frevert U, Williams J H, Chin K, Bryan R, Clarkson A B. Continuous axenic cultivation of Pneumocystis carinii. Proc Natl Acad Sci USA. 1999;96:2402–2407. doi: 10.1073/pnas.96.5.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhopadhyay R, Madhubala R. Effects of bis(benzyl)polyamine analogs on Leishmania donovani promastigotes. Exp Parasitol. 1995;81:39–46. doi: 10.1006/expr.1995.1090. [DOI] [PubMed] [Google Scholar]
- 19.Muller S, Luchow A, McCann P P, Walter R D. Effect of bis(benzyl)polyamine derivatives on polyamine transport and survival of Brugia pahangi. Parasitol Res. 1991;77:612–615. doi: 10.1007/BF00931024. [DOI] [PubMed] [Google Scholar]
- 20.Smith D E, Davies S, Smithson J, Harding I, Gazzard B G. Eflornithine versus cotrimoxazole in the treatment of Pneumocystis carinii pneumonia in AIDS patients. AIDS. 1992;6:1489–1493. doi: 10.1097/00002030-199212000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Woster P M. S-adenosylmethionine decarboxylase and spermine/spermidine N1-acetyltransferase-emerging targets for rational inhibitor design. In: Casero R, editor. Polyamines: regulation and molecular interaction. R. G. Austin, Tex: Landes Company; 1995. [Google Scholar]