Abstract
Purpose
To report a case of branch retinal artery occlusion (BRAO) followed by branch retinal vein occlusion (BRVO) and paracentral acute middle maculopathy (PAMM) in a patient with confirmed calciphylaxis.
Observations
A 52-year-old female with a history of BRAO in the right eye one-year prior presented with decreased vision and a new inferotemporal scotoma. Computed tomography angiography of the head and neck demonstrated vascular calcifications at the origin of both ophthalmic arteries, which were otherwise poorly visualized. Ophthalmic examination demonstrated retinal whitening superiorly with intraretinal hemorrhages inferiorly. Optical coherence tomography (OCT) demonstrated middle retinal hyperreflectivity and a mild epiretinal membrane. Fluorescein angiography (FFA) demonstrated delayed perfusion of superior retinal arcade. On further questioning, patient was found to have a history of IgA nephropathy with end-stage renal disease, secondary hyperparathyroidism and calciphylaxis. Calciphylaxis is a systemic disease, characterized by high levels of calcium and progressive calcification of the vascular medial layer leading to ischemia. Anterior ischemic optic neuropathy (AION) and crystalline retinopathy have been reported as ocular manifestations of calciphylaxis, however, there are very few reports on ophthalmic manifestations of calciphylaxis.
Conclusion and importance
Clinical manifestations of calciphylaxis are variable and a detailed clinical history is important to suspect calciphylaxis. Calciphylaxis should be considered in the differential diagnosis of BRAO, BRVO, PAMM or any ophthalmic vascular manifestation in patients with end-stage renal disease.
Keywords: Calciphylaxis, Hypercalcemia, Branch retinal artery occlusion, Branch retinal vein occlusion and paracentral acute middle maculopathy
Abbreviations: BRAO, Branch retinal artery occlusion; BRVO, Branch retinal vein occlusion; PAMM, Paracentral acute middle maculopathy
1. Introduction
Calciphylaxis is characterized by high levels of calcium deposition in small blood vessels, skin, and other organs.1 It most commonly occurs as a result of secondary hyperparathyroidism associated with end-stage renal disease (ESRD) and is known as calcific uremic arteriolopathy, and less commonly, there can be calciphylaxis from non-uremic causes.4 In uremic calciphylaxis, end-stage renal disease eventually leads to secondary hyperthyroidism causing abnormal calcium and phosphorus metabolism. This abnormality, in conjunction with inflammation and a hypercoagulable state, leads to vascular and extravascular calcification throughout the body and eventually to ischemia.5,6
The incidence of calciphylaxis has been estimated to be 3.49 per 1000 patient-years among patients with ESRD on hemodialysis, and the prognosis is poor with mortality rates of 30% at 6 months and 50% at 12 months.7 Calciphylaxis usually presents as severe painful skin lesions due to poor healing and are frequently complicated with infection and ulcers.4 Nonetheless, a variety of clinical presentations have been reported, including rhabdomyolysis, intravascular calciphylaxis mimicking endocarditis, temporal arteritis, pulmonary calcifications, and inflammatory breast cancer.8 Although considered rare, it has devastating consequences and it continues to be an unrecognized complication of ESRD.9
There are very few reports on the effect of calciphylaxis on the eye. Up to our knowledge, crystalline retinopathy, retinal vasculopathy, anterior ischemic optic neuropathy (AION) and ocular ischemic syndrome have been associated to calciphylaxis.”.10, 11, 12 Given that calciphylaxis is a systemic disease and is known to affect the eye vasculature, there is concern that other arteries around the eye may also be affected. We report a case of branch retinal artery occlusion (BRAO) followed by a branch retinal vein occlusion (BRVO) and paracentral acute middle maculopathy (PAMM) in a patient with a history of IgA nephropathy leading to end-stage renal disease and secondary hyperparathyroidism, with consequent calciphylaxis.
2. Case report
A 52-year-old female with a history of BRAO in the right eye one-year prior was evaluated in the emergency department for acute onset of blurry vision in the right eye and new inferotemporal scotoma for 6 hours. Marked anisocoria was noted on initial examination, however, no other focal neurological deficit was detected. Extended stroke code was activated and ophthalmology was consulted. Computed tomography angiography (CTA) of the head and neck did not show hemorrhage, infarct, or any other acute findings. However, there was evidence of advanced intracranial atherosclerosis with extensive internal carotid artery calcifications and mild luminal irregularities. In particular, there were vascular calcifications at the origin of the ophthalmic arteries, which otherwise showed poor contrast opacification and flow-related enhancement (Fig. 1). Ophthalmic evaluation demonstrated multiple intraretinal hemorrhages along the inferior arcade with tortuous veins concerning for BRVO. Stroke work up demonstrated hemoglobin A1c of 4.7%, total cholesterol 144 (Ref: <200 mg/dL), LDL 84 (Ref: <130mg/dL), HDL 47 (Ref: >40 mg/dL) and transthoracic echocardiogram with no severe aortic obstruction and normal left ventricle size with normal systolic function. The patient was discharged on strict return precautions after 24 hours of observation and was instructed to continue on Apixaban.
Fig. 1.
Noncontrast (A) and contrast-enhanced angiographic (B) computed tomography (CT) images of the head show vascular calcifications at the origin of the ophthalmic arteries (red arrows). Additional contrast-enhanced CT angiographic image (C) obtained more inferiorly demonstrates poor flow-related enhancement within the proximal ophthalmic arteries (green arrows). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Her medical history was significant for IgA nephropathy diagnosed thirteen years prior that led to ESRD and hemodialysis dependency. After three years of diagnosed nephropathy, the patient was diagnosed with hypercalcemia complicated by calcinosis cutis, tumoral calcinosis causing severe shoulder pain with extensive periarticular calcifications, and mitral and aortic valve stenosis requiring replacement of both valves. Given borderline elevated parathyroid hormone (PTH) and suspected secondary/tertiary hyperparathyroidism, a subtotal parathyroidectomy was performed. Nonetheless, a few weeks after the parathyroidectomy was performed, the patient was readmitted to vascular surgery with right toe dry chronic gangrene. A guillotine amputation of right lower extremity was performed, and histopathologic evaluation was highly concerning for calciphylaxis (Fig. 2). Interestingly, despite successful removal of parathyroid glands and normal levels of PTH, elevated calcium levels persisted. Complete hypercalcemia workup demonstrated increased parathyroid hormone related peptide (PTHrp), negative myeloma studies and no vitamin D toxicity. She was therefore diagnosed with severe calciphylaxis and hypercalcemia of unknown origin. In the acute setting, she received calcitonin and had ongoing treatment with phosphate binders, sodium thiophosphate and low calcium dialysate. Given calciphylaxis and a history of mitral and aortic valve repair, she was started on chronic anticoagulation. Nonetheless, one year after the amputation, she suffered pulseless electrical activity arrest during an infected catheter exchange, requiring urgent transcatheter aortic valve replacement for critical aortic stenosis without any complications and has not required any additional surgical interventions.
Fig. 2.
Histopathologic sections of right below knee amputation specimen demonstrates (A) Adipose tissue from the foci of calcium deposition and fibrosis (arrows). (Hematoxylin-eosin; Original magnification X20) (B) Calcium deposition (asterisk) and hemosiderin-laden macrophages (arrow), indicating prior bleeding into the soft tissue. (Hematoxylin-eosin; Original magnification X100) (C) Foreign body giant cell reaction (arrow heads) to calcium deposition (arrows). (Hematoxylin-eosin; Original magnification X100) (D) Foreign body giant cell reaction (black arrow), oxalate deposition (red arrows), and calcium deposition (arrowhead). (Hematoxylin-eosin; Original magnification X200). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
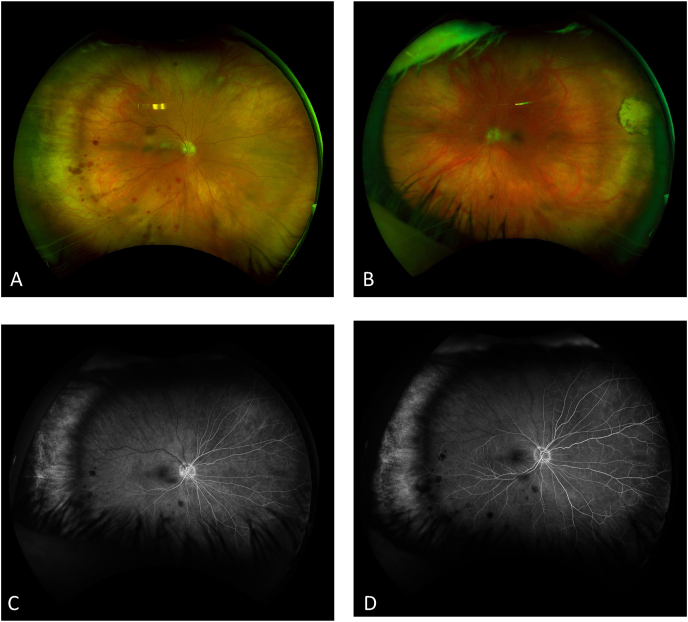
Two weeks after her exam in the emergency department, her best corrected visual acuity (BCVA) decreased to 20/80 in the right eye (baseline 20/20, 6 months prior) and remained 20/20 in the left eye. The intraocular pressure was 17 mmHg and 13 mmHg in the right and left eye, respectively. Slit lamp examination of the anterior segment was unremarkable. Fundus examination demonstrated a cup-to-disc ratio of 0.1 bilaterally and optic disc drusen bilaterally. Optic nerve drusen, macular epiretinal membrane with superior retinal whitening, and peripheral dot hemorrhages were present in the right eye. (Fig. 3A). Fluorescein angiography (FFA) showed delayed perfusion of superior retinal arcade artery at 1 min but no emboli or neovascularization. (Fig. 3B and C). Interestingly, optical coherence tomography (OCT) imaging of the right eye showed middle retinal hyperreflectivity suggestive of PAMM and a mild epiretinal membrane with no subretinal fluid or cystoid macular edema (Fig. 4). Of note, OCT from previous BRAO episode had no hyperreflectivity. The only abnormality seen in the fellow eye was the optic disc drusen. A diagnosis of BRVO with PAMM and a history of BRAO of the right eye was made and a dose of 0.5mg of Ranibizumab, an anti–vascular endothelial growth factor (anti-VEGF), was administered in the right eye.
Fig. 3.
(A) Fundus photograph of the right eye demonstrating disc drusen, dot hemorrhages inferiorly in the periphery, with no neovascularization and inner retinal whitening throughout the superior macula. (B) Fundus photograph of left eye demonstrating disc drusen and temporal chorioretinal scar without vessel abnormalities. (C) Fluorescein angiography at 25 seconds and 57 seconds (D) demonstrating delayed perfusion of superior retinal arcade artery to 1 minute with no disc edema, emboli or neovascularization.
Fig. 4.
Near-infrared reflectance image (A) and optical coherence tomography (OCT) (B) of the right eye one year prior when BRAO was diagnosed. Near-infrared reflectance image (C) and OCT (D) at presentation of BRVO demonstrating a band-like hyperreflectivity at the level of the inner nuclear layer (INL) with thickening (asterisk) and mild epiretinal membrane with no subretinal fluid.
3. Discussion
Combined BRVO and BRAO is not common and usually occurs in the presence of systemic diseases, including diabetes, dyslipidemia, systemic lupus, and hyperhomocysteinemia.13 In this case of retinal artery and vein occlusion, the clinical presentation was consistent with systemic disease pathogenesis associated with calciphylaxis. The microangiopathy and vascular occlusion seen in calciphylaxis are due to progressive calcification of the media of blood vessels as well as fibrosis underneath the intima.4 Although, the exact mechanism by which this happens remains unknown, it is believed to result from an upregulation of osteogenesis and downregulation of vascular calcification inhibition.12 In patients with ESRD, deposition of crystalline hydroxyapatite (a calcium-phosphate precipitate) is caused by upregulation of bone morphogenetic proteins, while downregulating vascular calcification inhibitors.14 The resulting calcification of the vessel media leads to obstruction or severe decrease in blood flow.4
Small arterioles in the subcutaneous fat and dermis are also known to be the target for calciphylaxis. In a case of calciphylaxis-related cases of AION, ischemia due to obstruction or severely reduced blood flow involving the short posterior ciliary arteries led to the vision loss.15 In crystalline retinopathy and vasculopathy, it is thought that the visual loss was a result of thrombogenic microangiopathy secondary to crystalline deposition within the retinal vasculature.11 We believe the BRAO could have been secondary to calcium deposition in the medial wall of the artery, a calcific embolus, an embolic event due to severe aortic stenosis, or a combination of all of the above. On the other hand, considering BRVO usually occurs at arteriovenous crossing, where thickening of the arterial wall can compress the vein, it is possible that the calcium deposition within the artery predisposes for BRVO by mechanical compression of the vein. Evidence of vascular calcification of ophthalmic arteries bilaterally on CT supports the theory of vascular calcification seen in this patient.
Histology is the gold standard for diagnosis. The hallmarks of calciphylaxis include luminal narrowing secondary to medial calcification. Giant cells may be present as a result of foreign body reaction, however, there is a lack of true granulomatous inflammation.16 Chehade et al. reported a case of AION and calciphylaxis and the histopathology of a temporal artery biopsy demonstrated a small muscular artery with circumferential calcification and luminal narrowing without granulomatous inflammation, consistent with calciphylaxis.10 In our case, histopathologic analysis after the below knee amputation demonstrated ulceration, necrosis, giant cell formation calcium and calcium oxalate deposition which are all compatible with calciphylaxis in this clinical context, although, calcification within the walls of the arteries was not definitively identified. Disc drusen in our patient is further evidence of calcification in the eye. Although, there is no clear association between calciphylaxis and disc drusen, its presence correlates with the tendency to calcify seen in this disease process and disc drusen have been associated with retinal vascular abnormalities and retinal vein occlusion.18, 19, 20
Treatment for calciphylaxis is multimodal including wound management, pain control and nutrition.4 Intravenous sodium thiosulfate is widely used as it is a reducing agent capable of forming soluble complexes with calcium and phosphorus, however, there are no prospective studies on this medication.17 The most common ophthalmologic complication of calciphylaxis is known to be AION and in the majority of cases this presents with a pale, swollen disc with evidence of choroidal and retinal ischemia on fundus FFA and elevated erythrocyte sedimentation rate (ESR), in cases of renal disease, which could mimic giant cell arteritis.10 Nonetheless, the spectrum of calciphylaxis is broad and crystalline retinopathy, ocular ischemic syndrome and retinal arterial or venous occlusion should all raise concern for this rare entity.12 Given the limited information of the effects of calciphylaxis in the eye and the devastating ocular complications, calciphylaxis should be in the differential diagnosis of any ophthalmic vascular complication in patients with ESRD. Further studies are warranted to establish the effect of calciphylaxis in the eye and to uncover possible treatments to preserve vision.
4. Conclusion
Calciphylaxis is a rare disease characterized by high levels of calcium deposition in blood vessels, skin, and other organs as result of secondary hyperparathyroidism associated with ESRD.1,4 Progressive calcification of the media of blood vessels leads to vascular occlusion and decreased blood flow.4 Clinical manifestations are variable and there are only a few cases of ophthalmic involvement with AION as the most common ocular manifestation.10 A detailed clinical history is important to suspect calciphylaxis and should be considered in the differential diagnosis of vascular occlusions in patients with ESRD.
Patient consent
Written consent to publish this case has been obtained.
Funding
VBM is supported by NIH grants [R01 EY031952, R01 EY030151, R01NS98950, R01EY031360, and P30EY026877], Research to Prevent Blindness (RPB), New York, NY, and Stanford Center for Optic Disc Drusen. The funding organizations had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
None reported.
Acknowledgements
None.
References
- 1.Milas M., Bush R.L., Lin P., et al. Calciphylaxis and nonhealing wounds: the role of the vascular surgeon in a multidisciplinary treatment. J Vasc Surg. 2003;37(3):501–507. doi: 10.1067/mva.2003.70. [DOI] [PubMed] [Google Scholar]
- 4.Nigwekar S.U., Kroshinsky D., Nazarian R.M., et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66(1):133–146. doi: 10.1053/j.ajkd.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baby D., Upadhyay M., Joseph M.D., et al. Calciphylaxis and its diagnosis: a review. J Fam Med Prim Care. 2019;8(9):2763–2767. doi: 10.4103/jfmpc.jfmpc_588_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slough S., Servilla K.S., Harford A.M., Konstantinov K.N., Harris A., Tzamaloukas A.H. Association between calciphylaxis and inflammation in two patients on chronic dialysis. Adv Perit Dial Conf. 2006;22:171–174. [PubMed] [Google Scholar]
- 7.Nigwekar S.U. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26(4):276–281. doi: 10.1097/MNH.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanna R.M., Nabil J.R., Lopez E.A., Corry D.B., Wilson J. Multiple sites of calciphylaxis in a patient with chronic renal failure. Saudi J Kidney Dis Transpl. 2015;26(2):344–348. doi: 10.4103/1319-2442.152506. [DOI] [PubMed] [Google Scholar]
- 9.Trost O., Kadlub N., Trouilloud P., Malka G., Danino A. [Calciphylaxis: a severe but unrecognized complication in end-stage renal disease patients. A review of 2 cases] Ann Chir Plast Esthet. 2005;50(6):746–750. doi: 10.1016/j.anplas.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Chehade L.K., Hissaria P., Simon S. Calciphylaxis: when a bird is not a duck. Can J Ophthalmol. 2020;55(2):e66–e69. doi: 10.1016/j.jcjo.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Naysan J., Dansingani K.K., Balaratnasingam C., et al. Crystalline retinopathy and retinal vasculopathy in calcific uremic arteriolopathy (calciphylaxis) Retin Cases Brief Rep. 2018;12(4):331–335. doi: 10.1097/ICB.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 12.Cherayil N.R., Scoles D., Moran A.M., Elder D.E., Tamhankar M.A. Gazing into the crystal ball: calciphylaxis causing striking retinal vascular calcification, ocular ischemic syndrome, crystalline retinopathy, and ischemic optic neuropathy. J Neuro Ophthalmol. 2021;41(2):e212–e214. doi: 10.1097/WNO.0000000000001090. [DOI] [PubMed] [Google Scholar]
- 13.Sengupta S., Pan U. Combined branch retinal vein and branch retinal artery occlusion - clinical features, systemic associations, and outcomes. Indian J Ophthalmol. 2017;65(3):238–241. doi: 10.4103/ijo.IJO_340_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nigwekar S.U., Thadhani R., Brandenburg V.M. Calciphylaxis. N Engl J Med. 2018;378(18):1704–1714. doi: 10.1056/NEJMra1505292. [DOI] [PubMed] [Google Scholar]
- 15.Huerva V., Sanchez M.C., Ascaso F.J., Craver L., Fernandez E. Calciphylaxis and bilateral optic neuropathy. J Fr Ophtalmol. 2011;34(9) doi: 10.1016/j.jfo.2011.02.010. 651 e651-654. [DOI] [PubMed] [Google Scholar]
- 16.Chang J.J. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32(5):205–215. doi: 10.1097/01.ASW.0000554443.14002.13. [DOI] [PubMed] [Google Scholar]
- 17.Nigwekar S.U., Brunelli S.M., Meade D., Wang W., Hymes J., Lacson E., Jr. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8(7):1162–1170. doi: 10.2215/CJN.09880912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallagher M.J., Clearkin L.G. Drug or drusen? Central retinal vein occlusion in a young healthy woman with disc drusen. Eye (Lond). Jun. 2000;14(Pt 3A):401–402. doi: 10.1038/eye.2000.103. [DOI] [PubMed] [Google Scholar]
- 19.Richmond P.P., Orth D.H. Branch retinal vein occlusion associated with optic nerve drusen: a case report. Ophthalmic Surg. 1989;20(1):38–41. [PubMed] [Google Scholar]
- 20.Borruat F.X., Sanders M.D. [Vascular anomalies and complications of optic nerve drusen] Klin Monbl Augenheilkd. 1996;208(5):294–296. doi: 10.1055/s-2008-1035219. [DOI] [PubMed] [Google Scholar]