Abstract
Exophiala is a rare fungus that can cause fatal infections in patients who are immunocompromised. This case describes a 55-year-old post-renal transplant patient undergoing active treatment for multiple myeloma who presented to the emergency room with complaints of cough and dyspnea. She was found to have a cavitary lesion in the left upper lobe of her lung, and direct sampling via bronchoscopy revealed an invasive fungal infection with an Exophiala species. Despite initial improvement on antifungal therapy, her clinical course deteriorated requiring pneumonectomy and formation of brain abscess. This case emphasizes the challenge of invasive fungal infections and the importance of broad differentials in patients who are immunocompromised.
Keywords: Renal transplant, Exophiala, Pneumonia, Brain abscess, Immunosuppressed, Multiple myeloma, Pneumonectomy
Introduction
Well known to medical literature is the susceptibility of post-transplant patients to opportunistic fungal infections including Pneumocystis jirovecii, Cryptococcus, Coccidioides, and Aspergillus. Less common fungi include those of the Exophiala species, which is a variety of black yeasts that can be isolated from soil, sauna facilities or dishwashers [1], [2]. Fungi of the Exophiala genus most commonly cause skin and subcutaneous infections and less commonly brain abscesses and pneumonia [3], [4]. The following case presents a renal transplant patient undergoing active treatment for malignancy who developed a cavitary lung infection secondary to Exophiala, subsequently leading to pneumonectomy, brain abscess, and a fatal clinical course. These findings have not been previously described in the medical literature.
Case description
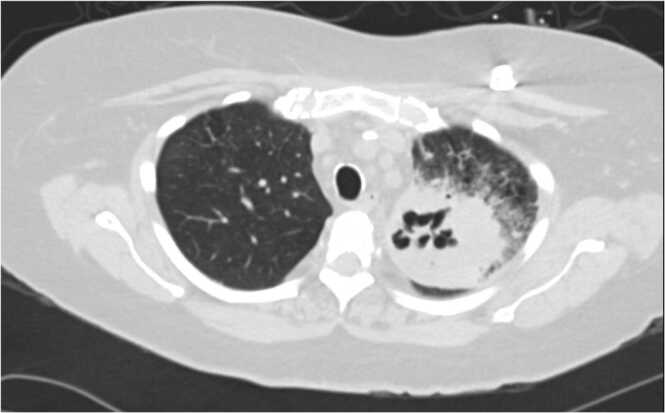
This patient is a 55-year-old woman who presented to the hospital with complaints of cough, dyspnea, and weakness for 2 weeks. She was recently treated with doxycycline for community acquired pneumonia as an outpatient. She had a past medical history of hypertension, recurrent urinary tract infections with extended spectrum beta-lactamase (ESBL) producing organisms, multiple myeloma with serum light chain disease status post autologous stem cell transplant times two, status post port, and end stage renal disease status post deceased donor renal transplant 8 months prior. She had previously achieved remission of her myeloma early on in disease course with two cycles of induction bortezomib, thalidomide, dexamethasone and autologous stem cell transplant but then required cyclophosphamide, bortezomib, and dexamethasone when recurrence was noted a few years later. She then had her second autologous transplant and was started on chronic lenalidomide. Second line ixazomib was added to lenalidomide due to upward trending light chains and she has remained on this maintenance combination therapy for the last 3 years. Current medications included ixazomib 2.3 mg weekly, lenalidomide 10 mg daily, mycophenolate 540 mg twice daily, prednisone 5 mg daily, and tacrolimus 11 mg twice daily. She completed 6 months of valganciclovir prophylaxis however her prophylactic trimethoprim-sulfamethoxazole was stopped early due to hyperkalemia. She had no history of recent travel, exposure to animals, or gardening. At presentation, she was tachycardic with an oxygen saturation of 93% on room air and crackles bilaterally on lung exam. Labs were significant for an acute kidney injury with elevated creatinine of 2.32 mg/dL, a mild leukocytosis of 11.3 × 109/L, and a urinalysis demonstrating pyuria with detectable leukocyte esterase. Chest radiograph showed persistent left upper lobe opacity as compared to the one 2 weeks prior. Computed tomography (CT) further characterized the opacity as a large 7-centimeter x 6-centimeter left upper lobe cavitary lesion with adjacent interstitial thickening and ground glass opacities (Fig. 1).
Fig. 1.
Cavitary lung lesion on left side.
The patient was admitted on vancomycin 15 mg/kg every 24 h and cefepime 2 g every 12 h, and Infectious Disease and Pulmonology were consulted. Blood cultures to rule out bacteremia were obtained, as well as routine respiratory and acid-fast cultures from the sputum. She underwent bronchoscopy with bronchoalveolar lavage (BAL) and fungal stain from the procedure was positive for few fungal hyphae, morphologically similar to aspergillus per pathology and patient was placed on 200 mg twice daily voriconazole empirically pending fungal cultures. Aspergillus antigen in serum was undetectable. Mycobacterium tuberculosis polymerase chain reaction (PCR) from BAL sample was negative. Blood cultures and urine cultures returned positive for ESBL Klebsiella pneumoniae. CT of the abdomen and pelvis to rule out deeper seeded infection or alternative etiology of bacteremia was negative. Given history of recurrent ESBL urinary tract infections, the genitourinary tract was likely source. She clinically improved and was discharged to complete a 14-day course of meropenem and voriconazole 200 mg twice daily.
Despite initial improvement, she returned to the hospital two weeks later with fever and dyspnea. Original fungal culture from the first admission resulted as an Exophiala species. Voriconazole was stopped at this time due to suspected drug toxicity and she was started on liposomal amphotericin B 3 mg/kg every 24 h. Itraconazole was later added to liposomal amphotericin B due to worsening of her cavitary fungal pneumonia. Although she was treated with combination antifungal therapy, she continued to decline with progression to near complete opacification of left lung requiring a left pneumonectomy. Repeat blood cultures again became positive for ESBL Klebsiella pneumoniae, and her implanted port was removed as a suspected source of recurrent bacteremia. She then developed confusion and hallucinations. Magnetic resonance imaging with and without contrast of the brain revealed a 1.4 cm ring enhancing abscess in the right parietal lobe and a Chiari 1 malformation. Neurosurgery was consulted for possible brain biopsy but she was deemed too high risk for surgical intervention. Lumbar puncture to rule out etiology was unable to be obtained due to elevated risk for herniation. The remainder of her hospital course was complicated by further respiratory failure and stroke with brain herniation. Patient passed away shortly afterwards.
Discussion
Exophiala infection is a rare diagnosis but is being identified more frequently, likely related to increases in immunosuppression related to solid organ transplantation [1]. Infection is commonly limited to cutaneous involvement, but disseminated disease can occur and affect the lungs, digestive tract, and central nervous system [5]. Review of the literature suggests that most reported cases of Exophiala pneumonia are present in patients who are immunocompromised or have underlying lung disease, such as cystic fibrosis or chronic bronchiectasis [5], [3]. Additionally, the published cases of Exophiala pneumonia were mild and had complete resolution of disease with oral itraconazole [5], [3]. Our case is unique in that we discuss an immunocompromised patient who developed a cavitary pneumonia due to Exophiala ultimately requiring pneumonectomy despite coverage with two antifungal agents. The patient’s clinical course was then complicated by developing brain abscess and death. While extremely rare, it is possible that our patient developed disseminated disease. However, it remained unclear whether disease was truly disseminated, as we could not prove the brain abscess to be fungal. Since there was a known concomitant ESBL Klebsiella bacteremia, the etiology of the brain abscess could have been bacterial.
Due to its rarity, there are no established guidelines for treatment of Exophiala infection, though successful treatment has been described in the literature with azole antifungals in conjunction with surgical resection [6]. Prognosis is poor in disseminated disease even with treatment, approaching mortality rates of around 70% [1]. The most common strains isolated include Dermatitis, Xenobiotica, and Oligosperma, but Exophiala organisms are difficult to speciate based on morphology alone and molecular markers are often required to distinguish between species [4]. Our lab was not able to make that distinction. In vitro studies of antifungal susceptibility have shown that most species are susceptible to itraconazole, amphotericin B, voriconazole, and posaconazole [4]. Susceptibilities for our patient had to be processed at an outside lab facility, and unfortunately, we never received the final report. Although studies have demonstrated that outcomes of combination therapy with an azole and amphotericin B is similar to that of azole monotherapy, combination therapy is still widely used, as was the case for this patient [2]. The detection of Exophiala infection in immunosuppressed patients requires a high degree of suspicion due to its rarity. Even though disseminated Exophiala carries a poor prognosis, it must be considered in these patients to avoid treatment delays and reduce mortality.
CRediT authorship contribution statement
Adrienne M Gonzales DO, Kim Minh Le DO, Patrick Shin MD, MaryAnn P Tran equally contributed to literature review and writing of the abstract, introduction, case description, and discussion.
Ethical approval
N/a.
Consent
Obtained.
Sources of funding
N/A.
Author Statement
As stated we have no competing interesting for this paper and appropriate consent was granted for publication. Thank you to the editors for accepting our case for publication and allowing us to present it in this journal. Though the outcome was unfortunate, we believe this is a very unique case and are excited to present to you the details in hopes that it can serve as a reference for those clinicans who might come across a similar case.
Conflicts of interest
None.
Footnotes
There are no conflicts of interest for any of the authors listed on this paper. Appropriate consent has been obtained to publish this case report.
References
- 1.Revankar S.G., Patterson J.E., Sutton D.A., Pullen R., Rinaldi M.G. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin Infect Dis. 2002;34(4):467–476. doi: 10.1086/338636. [DOI] [PubMed] [Google Scholar]
- 2.Gao L., Sun Y., He C., Zeng T., Li M. Synergistic effects of tacrolimus and azoles against Exophiala dermatitidis. Antimicrob Agents Chemother. 2017;61(12) doi: 10.1128/AAC.00948-17. [e00948-17. Published 2017 Nov 22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen Y., Stead W. Exophiala pneumonia presenting with a cough productive of black Sputum. Case Rep Infect Dis. 2015;2015:1–3. doi: 10.1155/2015/821049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng J.S., Sutton D.A., Fothergill A.W., Rinaldi M.G., Harrak M.J., de Hoog G.S. Spectrum of clinically relevant Exophiala species in the United States. J Clin Microbiol. 2007;45(11):3713–3720. doi: 10.1128/jcm.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukai Y., Nureki S., Hata M., Shigenaga T., Tokimatsu I., Miyazaki E., et al. Exophiala dermatitidis pneumonia successfully treated with long-term itraconazole therapy. J Infect Chemother. 2014;20(7):446–449. doi: 10.1016/j.jiac.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Palmisano A., Morio F., Le Pape P., Degli Antoni A.M., Ricci R., Zucchi A., et al. Multifocal phaeohyphomycosis caused Byexophiala xenobiotica in a kidney transplant recipient. Transpl Infect Dis. 2015;17(2):297–302. doi: 10.1111/tid.12350. [DOI] [PubMed] [Google Scholar]