Abstract
Introduction and importance
Holoprosencephaly is a rare brain malformation consisting of impaired midline cleavage of the embryonic forebrain presenting with variable features of craniofacial dysmorphism. It affects 1 in 10,000 live births occurring more in females than males. We present a case of alobar HPE and aim to raise awareness on the importance of early prenatal detection and counselling.
Case presentation
We present a case of 3200-gram female baby, born by spontaneous vaginal delivery with APGAR scores of 5 and 6 in the first and fifth minute of life respectively. On admission, the baby was lethargic, had central and peripheral cyanosis, hypothermic with temperature of 32.1 °C, respiratory rate of 65 breaths/min, heart rate of 135 beats/min and oxygen saturation of 94% with an oropharyngeal airway and on oxygen support via a face mask. She had microcephaly, hypotelorism, and a small nose with a single imperforate nostril. She was diagnosed to have alobar holoprosencephaly with cebocephaly. A computed tomography scan of the brain revealed a cephalohematoma in the vertex and an intranasal soft tissue density lesion blocking the entrance measuring approximately 10 × 8.5 mm. Absence of the corpus callosum and septum pellucidum with a resulting monoventricle formed from the lateral ventricles, the fusion of the thalami and a sizeable arachnoid cyst involving the left cerebellar hemisphere were evident. She was started on IV antibiotics and IV fluids. Non-invasive airway management was opted for by the ENT team based on the condition of the baby. She succumbed to death 6 days post admission due to severe respiratory failure.
Clinical discussion
The types of HPE are alobar, semi lobar, lobar and interhemispheric variants. Alobar HPE is the most severe form and is incompatible with life. Clinical presentation entails facial dysmorphism with features of hypotelorism, microcephaly and a blind ended nostril. Alobar and semilobar HPE can reliably be diagnosed with ultrasound during the first and second trimesters of pregnancy. Absence of choroid plexus and fused cortex are pathognomonic characteristic on ultrasound and CT scan respectively.
Conclusion
Alobar holoprosencephaly is a rare brain malformation which is incompatible with life.
Prenatal ultrasound screening of the foetus brain is essential and reliable in making a diagnosis. Absence of the “butterfly” sign in the foetal brain ultrasonography should raise a high index of suspicion for brain malformation with unfavourable outcome. Legal medical termination of pregnancy may serve as an early intervention.
Keywords: Holoprosencephaly, Cebocephaly, Proboscis, Case report
Highlights
-
•
Holoprosencephaly is a rare midline forebrain malformation.
-
•
The alobar holoprosencephaly type is incompatible with life.
-
•
Ultrasonography is reliable in making the diagnosis.
-
•
Early pregnancy decisions favour compatible outcomes.
1. Introduction
Holoprosencephaly (HPE) is the most common midline structural anomaly of the forebrain affecting anterior neural features resulting in incomplete separation of the forebrain into two discrete hemispheres during the third and fourth week of gestation. It affects 1 in 10,000 live births occurring more in females than males [1], [2], [3], [4]. HPE occurs when there is a failure of complete separation of the two hemispheres and a failure of transverse cleavage into diencephalon and telencephalon. The aetiology of HPE is varied with most cases being sporadic. Chromosomal abnormalities affect 25% to 50% of cases with HPE and are associated with trisomy 13, 18 and triploidy [3], [5], [6]. We present a case of alobar HPE with cebocephaly in a neonate born to an otherwise healthy woman referred to our tertiary hospital from a primary care facility. It is to our knowledge that a case of semilobar HPE has been described [7] but there is no record of alobar HPE in Tanzania. With this case we intend to raise awareness of early prenatal screening and emphasise on meticulous analysis of early signs of congenital anomalies in obstetrics ultrasound scans. This case report has been reported in line with the SCARE criteria [8].
2. Presentation of case
We present the case of a 6 h old female baby who was referred to our centre from a primary health facility. She was delivered at term via spontaneous vaginal delivery and weighed 3200 g. She was reported by the referring nurse to have an APGAR score of 5 and 6 in the first and fifth minute respectively. Immediately post-delivery she developed difficulty in breathing associated with severe chest indrawing and bluish discolouration of the whole body was observed. Oxygen supplement via a face mask was attempted without success hence manual bag ventilation was initiated while the baby was being transferred to our tertiary facility. She was the first born to her parents. Her mother is 19 years old; a stay-at-home mother and the father is 25 years old motorcycle taxi driver. They both did not report any history of a similar condition or congenital abnormalities in their lineages.
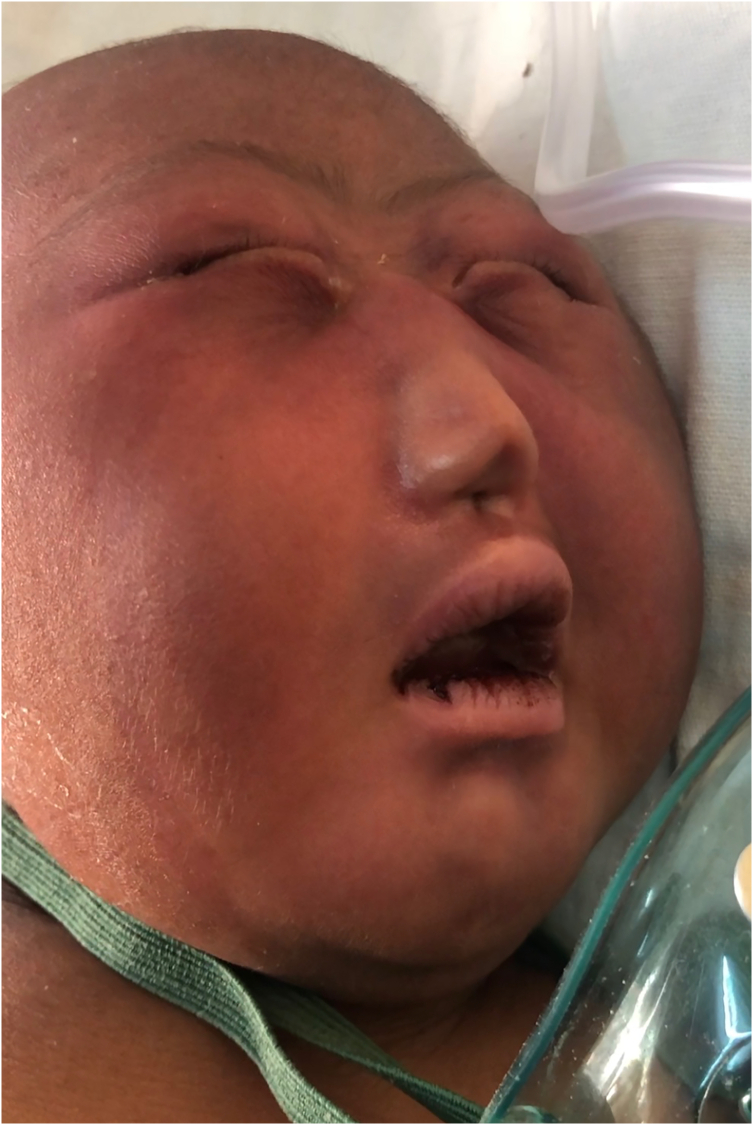
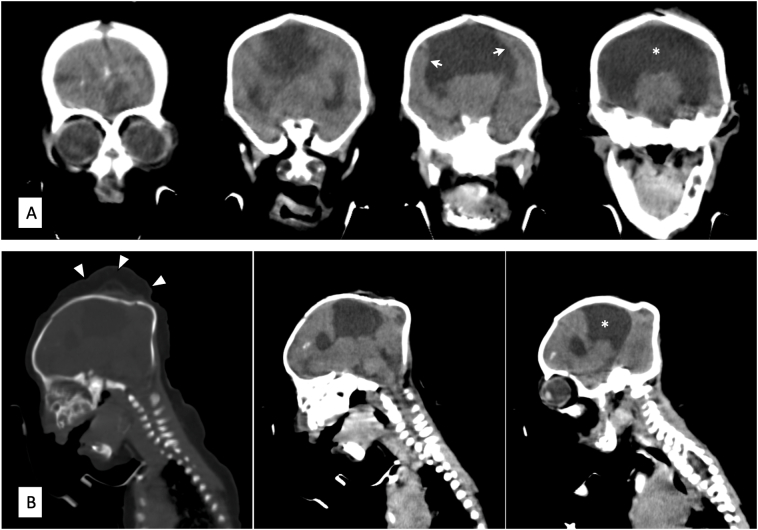
On admission, the baby was lethargic, had central and peripheral cyanosis, hypothermic. She had abnormal facial feature, small head, hypotelorism, a small nose with a single imperforate nostril (Fig. 1). She had unstable vital signs of severe hypothermia 32.1 °C, heart rate of 132 beats per minute and respiratory rate of 65 breaths per minute. Oxygen saturation was 94% with an oropharyngeal airway and on oxygen support via face mask. On systemic examination, sucking, startle, grasping and Moro primitive reflexes were absent. Afterwards the baby was started on intravenous dextrose 10% at 128 mL for 24 h alongside intravenous antibiotics Ampicillin and Gentamycin. The ENT team was consulted due to concern over airway compromise and given the prognosis associated with this anomaly non-surgical intervention was preferred hence oral airway was kept patent. Preliminary laboratory blood analysis showed an otherwise normal full blood count parameters, blood urea of 6.11 mmol/L. serum creatinine of 68 μmol/L, total protein of 53.2 g/dL and serum calcium level of 2.19 mmol/L. A multiplanar non-contrast computerized tomography scan of the brain (Fig. 2) revealed the head and nose to be small with a cephalohematoma in the vertex and an intranasal soft tissue density lesion blocking the entrance measuring approximately 10 × 8.5 mm. Absence of the corpus callosum and septum pellucidum with a resulting monoventricle formed from the lateral ventricles, the fusion of the thalami and a sizeable arachnoid cyst involving the left cerebellar hemisphere. Scattered calcifications were seen in the frontal lobes. All these investigations had to be exempted by the hospital social welfare due to parents' socio-economic status. The baby continued to be nursed in the neonatal ward receiving IV antibiotics and IV fluids. After 6 days of hospital stay, the baby passed away due to severe respiratory failure.
Fig. 1.
Cebocephaly: Picture of neonate showing hypotelorism, microcephaly and a proboscis with a single blind-ending nostril.
Fig. 2.
Non-contrast computed tomographic scans of the brain in coronal (A) and sagittal (B) views showing the fusion of the lateral ventricles to form a monoventricle (asterisk), fused cortex (white arrows) and cephalohematoma of the vertex (white arrowheads).
3. Discussion
The established types of HPE in decreasing order of severity are alobar, semi lobar, lobar and interhemispheric variants [1], [9]. Alobar HPE, the most severe form presents in approximately two-thirds of individuals is characterized by a single ventricle, absence of corpus callosum and fused thalami with cebocephaly, a severe facial dysmorphism [10], [11]. It entails hypotelorism, microcephaly and a proboscis with a single blind-ending nostril [6]. All these above-mentioned features were found in our patient after a CT scan of the brain was done. Other less severe abnormalities include single maxillary central incisor and midline cleft lip and palate. The fused cortex in a computerized tomography can appear in one of three basic shapes: pancake where cerebral tissue is confined to the anterior basicranium; cup where cerebral tissue lines variable amounts of the anterior cranium with a dorsal cyst present posteriorly; and ball where a complete rim of tissue surrounding the monoventricle without dorsal cyst is noted [2].
HPE can reliably be diagnosed with ultrasound through the first and second trimesters of pregnancy [9]. Alobar and semilobar HPE are predominantly diagnosed in the first trimester. Our patient attended Antenatal Care Clinic in a primary health centre but she never did an ultrasound during pregnancy due to financial constraints. A characteristic absence of choroid plexus “butterfly” appearance of the brain is a pathognomonic sign of HPE [2], [9]. Alobar HPE is incompatible with life, hence early and thorough sonography may provide a means to the early intervention by proper counselling for possible termination of the pregnancy [1]. In our patient, since ultrasound was not done during pregnancy the option of pregnancy termination was not considered. Many legal concerns beyond the scope of this discussion regarding medical abortion exist in our country.
4. Conclusion
The importance of ultrasonography screening for the detection of congenital anomalies is emphasised in our report. Prenatal ultrasound screening of the foetus brain is essential and reliable in making a diagnosis. Absence of the “butterfly” sign in the foetal brain ultrasonography should raise a high index of suspicion for brain malformation. In most resource-limited settings, ultrasonography is widely accessible. However, primary care practitioners lack the knowledge of existence and or detection of in utero anomalies. Nevertheless, controversies of abortion policies in many countries like Tanzania hinder efficiency in the efforts of reducing child and maternal mortalities particularly neonatal mortalities of precedent causes like holoprosencephaly.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Data availability
We have not shared patient's hospital records as they contain personal identification information.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Ethical approval
Institutional Ethical approval not required for reporting individual cases and or case series.
Funding
No financial support for the research, authorship, and/or publication of this article was received for this article.
Guarantor
Desderius Celestine Chussi.
Research registration number
N/A.
CRediT authorship contribution statement
Ibukun John Ariyo, Deborah N Mchaile, Desderius Celestine Chussi: conceptualized and wrote the first draft of the manuscript. Ibukun John Ariyo, Michael Kayuza, Marco Magwizi: collected and summarized patient's data. Desderius Celestine Chussi, Deborah N Mchaile: revised and wrote the final version of the manuscript. All authors read and accepted the final version for submission.
Declaration of competing interest
The authors declare no conflict of interest.
References
- 1.Raam M.S., Solomon B.D., Muenke M. Holoprosencephaly: a guide to diagnosis and clinical management. Indian Pediatr. 2011;48:457–466. doi: 10.1007/s13312-011-0078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callahan J., Harmon C., Aleshire J., Hickey B., Jones B. Alobar holoprosencephaly with cebocephaly. J. Diagn. Med. Sonogr. 2017;33:39–42. doi: 10.1177/8756479316664477. [DOI] [Google Scholar]
- 3.Gondré-Lewis M.C., Gboluaje T., Reid S.N., Lin S., Wang P., Green W., Diogo R., Fidélia-Lambert M.N., Herman M.M. The human brain and face: mechanisms of cranial, neurological and facial development revealed through malformations of holoprosencephaly, cyclopia and aberrations in chromosome 18. J. Anat. 2015;227:255–267. doi: 10.1111/joa.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C.P., Su T.H., Chern S.R., Su J.W., Lee C.C., Wang W. Alobar holoprosencephaly, cebocephaly, and micropenis in a Klinefelter fetus of a diabetic mother. Taiwan. J Obstet. Gynecol. 2012;51:630–634. doi: 10.1016/j.tjog.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Yamada S. Embryonic holoprosencephaly: pathology and phenotypic variability. Congenit. Anom. (Kyoto) 2006;46:164–171. doi: 10.1111/j.1741-4520.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- 6.Lo H.F., Hong M., Krauss R.S. Concepts in multifactorial etiology of developmental disorders: gene-gene and gene-environment interactions in holoprosencephaly. Front. CellDev. Biol. 2021;9:1–10. doi: 10.3389/fcell.2021.795194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pallangyo P., Lyimo F., Nicholaus P., Makungu H., Mtolera M., Mawenya I. Semilobar holoprosencephaly in a 12-month-old baby boy born to a primigravida patient with type 1 diabetes mellitus: a case report. J. Med. Case Rep. 2016;10:4–9. doi: 10.1186/s13256-016-1141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A., Beamish A.J., Noureldin A., Rao A., Vasudevan B., Challacombe B., Perakath B., Kirshtein B., Ekser B., Pramesh C.S., Laskin D.M., Machado-Aranda D., Miguel D., Pagano D., Millham F.H., Roy G., Kadioglu H., Nixon I.J., Mukherjee I., McCaul J.A., Ngu J.Chi-Yong, Albrecht J., Rivas J.G., Raveendran K., Derbyshire L., Ather M.H., Thorat M.A., Valmasoni M., Bashashati M., Chalkoo M., Teo N.Z., Raison N., Muensterer O.J., Bradley P.J., Goel P., Pai P.S., Afifi R.Y., Rosin R.D., Coppola R., Klappenbach R., Wynn R., Wilde R.L.De, Surani S., Giordano S., Massarut S., Raja S.G., Basu S., Enam S.A., Manning T.G., Cross T., Karanth V.K., Kasivisvanathan V., Mei Z. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Monteagudo A. Holoprosencephaly. Am. J. Obstet. Gynecol. 2020;223:B13–B16. doi: 10.1016/j.ajog.2020.08.178. [DOI] [PubMed] [Google Scholar]
- 10.Sikakulya F.K., Kiyaka S.M., Masereka R., Ssebuufu R. Alobar holoprosencephaly with cebocephaly in a neonate born to an HIV-positive mother in Eastern Uganda. Case Rep. Otolaryngol. 2021;2021:1–4. doi: 10.1155/2021/7282283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanz-Cortes M., Raga F., Bonilla-Musoles F. 3D sonographic prenatal diagnosis of lobar holoprosencephaly associated with cebocephaly. Assessment and diagnosis with multiplanar reconstruction [4] Prenat. Diagn. 2007;27:585–586. doi: 10.1002/pd.1743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We have not shared patient's hospital records as they contain personal identification information.