Highlights
-
•
Uveal melanoma is distinct from other melanomas.
-
•
Tebentafusp provided an overall survival benefit in HLA-A*02:01-positive patients with metastatic uveal melanoma.
-
•
With discrepancy between overall survival and progression-free survival, the result is an outlier in melanoma trials.
-
•
Confirmatory trials are needed for this compound.
Abstract
Uveal melanoma is distinct from other melanomas. In the advanced and metastatic stages, little to no improvement have been seen over time. Tebentafusp is a novel mechanism of action bispecific gp100 peptide-HLA-directed CD3 T-cell engager fusion protein (“-fusp”). Tebentafusp was granted full approval on January 25th 2022 in the setting of HLA-A*02:01-positive adult patients with unresectable or metastatic uveal melanoma. The approval was based on the overall survival advantage of tebentafusp over physician's choice therapy, in previously untreated uveal melanoma patients, based on the IMCgp100-202 trial. While we welcome positive results for this unmet need, three issues are raised by the trial. First, the control arm was restricted, precluding important options. Second, post-progression treatment was provided to a smaller fraction of patients than in real-life, which raises the question of whether overall survival was negatively impacted by limited care after the trial ended. Finally, the discrepancy between overall survival and progression-free survival benefit is an outlier in the context of previous melanoma trials. While it is clear that tebentafusp has an important role to play in this tumor type, the exact line is not yet well known. Confirmatory trials are needed for this compound.
Graphical abstract
The graphical abstract contains a reproduction of the wedjat eye, symbolizing the Eye of Horus, download from Wikipedia.org, image from Jeff Dahl, under CC BY-SA 4.0.
.
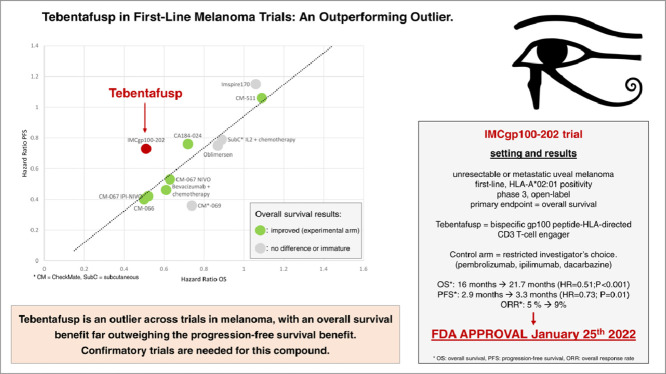
Tebentafusp is a bispecific gp100 peptide-HLA-directed CD3 T-cell engager, that was granted full approval on January 25th 2022 in the setting of HLA-A*02:01-positive adult patients with unresectable or metastatic uveal melanoma. After early stages development [1,2], the approval was based on the overall survival (OS) advantage of tebentafusp over physician's choice therapy, in previously untreated uveal melanoma patients, based on the IMCgp100-202, open-label, randomized, phase 3 trial (NCT03070392) [3].
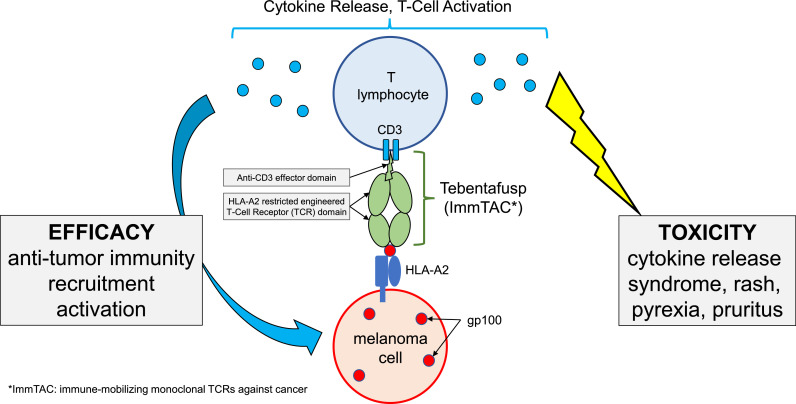
Tebentafusp is a compound from a new class of reagents, called ImmTACS, for “immune-mobilizing monoclonal TCRs against cancer (ImmTACs)” [4]. Tebentafusp is a fusion protein (“-fusp”) made of two parts. One side is a soluble affinity-enhanced T-cell receptor, targeting the gp100 protein, and the other is an anti-CD3 effector (activating anti-tumoral T cell response). Gp100 is expressed by melanoma cells, and the affinity of the T-cell receptor part of tebentafusp is restricted to gp100-HLA-A*02:01 complex recognition. When tebentafusp is attached to its HLA complex, this triggers anti-tumoral immunity through activation and recruitment of T lymphocytes (schematic illustration in Fig. 1) [5].
Fig. 1.
Schematic Diagram Illustrating The Mechanism of Action of Tebentafusp.
Consequently, patients must have HLA-A*02:01 genotype positivity, which prevalence vary according to ethnicity. In a US cohort, HLA-A2 was found in in 35% of African-Americans and 50% of Caucasian. In those subjects, the subtype HLA-A*02:01 represented most of them, again with variation across ethnic groups (53% for Asian/Pacific Islander up to 96% in Caucasian) [6].
Uveal melanoma has a different biology than other melanomas, with almost no patients presenting tumoral BRAF activating mutation. The most common driver mutations are coding for the G proteins GNAQ and GNA11 [7]. Uveal melanoma, when progressing, preferentially metastasize to the liver, most probably due to specific molecular pathways [8]. In the advanced and metastatic stages, little to no improvement have been seen over decades, with median overall survival stagnating between 6 and 10 months over time [9]. Uveal melanoma present poorer response to checkpoint inhibitors as compared to cutaneous melanoma. This may be explained by a different tumor micro-environment (low CD8 T lymphocytes and PDL1 expression by tumor cells), yet immunotherapy strategies remain utilized and have demonstrated anti-tumoral activity in patients with uveal melanoma. Most treatment guidelines and strategies mirror those of cutaneous melanoma, although some recent trials have deliberately excluded uveal melanoma.
The IMCgp100-202 trial found an overall survival advantage of tebentafusp over physician's choice therapy. All 378 patients that were randomly assigned had metastatic uveal melanoma; stage at diagnosis was stage I in 16% of patient, stage II in 34%, stage III in 24%, stage IV in 8% (and missing in 18%). The experimental arm achieved 21.7 months median OS as compared to 16 months, a difference of 5.6 months (hazard ratio, HR=0.51; 95% CI 0.37 to 0.71; P<0.001). Progression-free survival (PFS) was also longer in tebentafusp treated patients with 3.3 months median PFS, compared to 2.9 median PFS in the control arm, a difference of 0.4 months (HR=0.73; 95% CI, 0.58 to 0.94; P=0.01).
Tebentafusp is administered on a weekly basis, intravenously. Toxicity may be reduced by progressive dose-escalation: tebentafusp was initiated at 20 μg, followed after eight days by 30 μg, and followed after one more week by 68 μg, before continuing on a weekly schedule. Tebentafusp led to cytokine release syndrome of any grade in 89% of patients (but of grade 3 or more in 1%). Other toxicities were mainly rash (83%, 18% of grade ≥ 3), pyrexia (76%, 4% of grade ≥ 3) pruritus (69%), chills (47%), nausea (43%) and fatigue (41%). Interestingly, most adverse events occurred during the first 4 weeks of dose-escalation, and decreased after this period. Adverse events leading to treatment discontinuation occurred in 2% of patients, with no treatment-reported deaths.
While we welcome positive results for this unmet need, there are three issues worth noting. First, the control arm was restricted, precluding important options. Second, post-progression treatment was provided to a smaller fraction of patients than in real-life, which raises the question of whether overall survival is negatively impacted by limited care after the trial ended. Finally, the discrepancy between OS and PFS benefit is an outlier in the context of previous melanoma trials.
First, the “investigator's choice” control arm was restricted to three options: single-agent pembrolizumab, single-agent ipilimumab, or dacarbazine. Pembrolizumab is an anti-PD1 monoclonal antibody, ipilimumab an anti-CLA4 monoclonal antibody: both are also known as “immune checkpoint inhibitors”. Dacarbazine is a cytotoxic chemotherapeutic agent, classified as an alkylating agent. A restricted “investigator's choice” is common in industry sponsored trials, and often blocks an important standard-of-care option [10]. This restriction may create substandard control arms and favor investigational compounds. In a retrospective study from Denmark, conducted in the immunotherapy era, most first-line patients with uveal melanoma were treated with checkpoint inhibitors, with 22% receiving ipilimumab in combination with nivolumab: yet, this combination was not allowed in the IMCgp100-202 trial. The dual checkpoint combination (ipilimumab plus nivolumab) has been studied both retrospectively and prospectively in uveal melanoma patients [11]. In a single arm phase 2 trial, the combination led to 11.5% overall response rate (ORR) with 12.7 months median OS. In contrast, the ORR with tebentafusp was 9%, and 5% in the control arm.
Second, 43% of the IMCgp100-202 trial’ patients received a subsequent systemic therapy; lower than in real-life settings: this may reflect a poor access to disease modifying therapies in a trial conducted globally. In a retrospective analysis from a nationwide database conducted in Denmark, 54% of first-line treated patients received a second line treatment [12]. Interestingly, the subgroup analysis of IMCgp100-202 showed no benefit from tebentafusp in patients treated with ipilimumab in the control arm, suggesting that ipilimumab, at some point of the treatment course, may play an important role. Among control arm patients treated with pembrolizumab and receiving subsequent therapy, 72% were treated with another checkpoint inhibitor. We would have expected this rate to be higher, bearing in mind that other than ipilimumab-based options (alone or in combination) are very limited at progression.
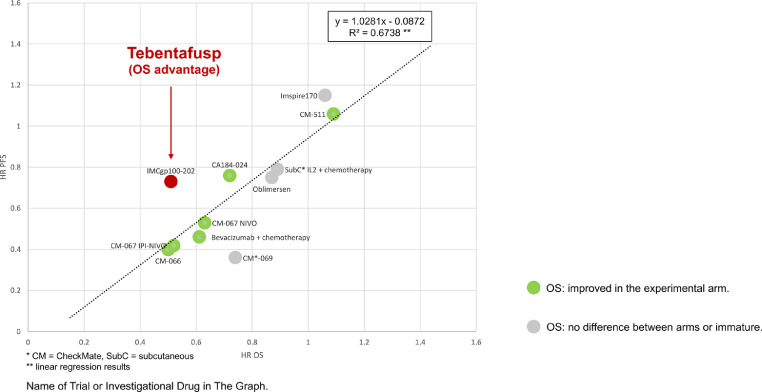
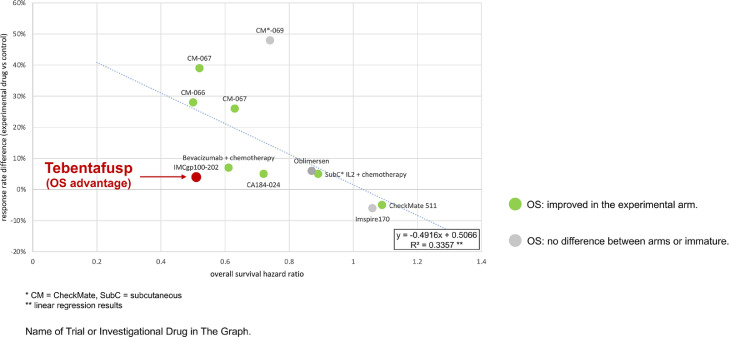
Third, the overall survival (OS) benefit is far larger than, and disproportionate to the PFS benefit in terms of hazard ratios (HR). When the added time of median PFS was 0.4 months in patients receiving tebentafusp, the added length in median OS was 5.7 months. This was also seen in a subgroup analysis demonstrating a significant improvement in median OS among patients without disease control according to RECIST v1.1 (6.5 in the control arm vs. 15.3 months with tebentafusp (HR 0.43, 95% CI 0.27–0.68)). Also, the ORRs were poor in both arm (9% with tebentafusp, 5% in the control arm). Previously, checkpoint inhibitors demonstrated less correlation between ORR and OS than conventional chemotherapy [13]. We compare this trial to others, mathematically confirming that tebentafusp is an outlier across trials in melanoma, with an OS benefit far outweighing the PFS benefit (Fig. 2) and the ORR advantage (Fig. 3).
Fig. 2.
Hazard Ratios (HR) For Overall Survival (OS) And Progression-free-survival (PFS) In Selected Randomized Trials In First-Line Advanced Or Metastatic Melanoma.
Fig. 3.
Hazard Ratio (HR) For Overall Survival (OS) and Response Rates Differences In Selected Randomized Trials In First-Line Advanced Or Metastatic Melanoma.
Tebentafusp has been heralded as an advance in this unique and neglected subtype of melanoma, and, indeed, we hope it is. Yet, overall survival gains far outstrip the PFS advance. Use of subsequent therapies is less than even real-world settings, and the control arm notably precluded treatment with ipilimumab plus nivolumab, the most active regimen, whose overall response rate surpasses the investigational arm. While it is clear that tebentafusp has an important role to play in this tumor type, the exact line is not yet well known. Confirmatory trials are needed for this compound.
Funding
This project was funded by Arnold Ventures, LLC through a grant paid to the University of California, San Francisco.
CRediT authorship contribution statement
Timothée Olivier: Conceptualization, Writing – original draft, Writing – review & editing. Vinay Prasad: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
Vinay Prasad's Disclosures: Research funding: Arnold Ventures; Royalties: Johns Hopkins Press, Medscape; Honoraria: Grand Rounds/lectures from universities, medical centers, non-profits, and professional societies; Consulting: UnitedHealthcare; Speaking fees: Evicore; Other: Plenary Session podcast has Patreon backers. Timothée Olivier have no financial nor non-financial conflicts of interest to report.
References
- 1.Middleton M.R., McAlpine C., Woodcock V.K., et al. Tebentafusp, A TCR/Anti-CD3 bispecific fusion protein targeting gp100, potently activated antitumor immune responses in patients with metastatic melanoma. Clin. Cancer Res. 2020;26(22):5869–5878. doi: 10.1158/1078-0432.CCR-20-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacco J.J., Carvajal R.D., Butler M.O., Shoushtari A.N., Hassel J.C. 64MO-A phase (ph) II, multi-center study of the safety and efficacy of tebentafusp (tebe) (IMCgp100) in patients (pts) with metastatic uveal melanoma (mUM) Ann. Oncol. 2020;31(7):S1441–S1451. https://oncologypro.esmo.org/meeting-resources/esmo-immuno-oncology-virtual-congress-2020/a-phase-ph-ii-multi-center-study-of-the-safety-and-efficacy-of-tebentafusp-tebe-imcgp100-in-patients-pts-with-metastatic-uveal-melanoma-mum Suppl. [Google Scholar]
- 3.Nathan P., Hassel J.C., Rutkowski P., et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl. J. Med. 2021;385(13):1196–1206. doi: 10.1056/NEJMoa2103485. [DOI] [PubMed] [Google Scholar]
- 4.Liddy N., Bossi G., Adams K.J., et al. Monoclonal TCR-redirected tumor cell killing. Nat. Med. 2012;18(6):980–987. doi: 10.1038/nm.2764. [DOI] [PubMed] [Google Scholar]
- 5.Bossi G., Buisson S., Oates J., Jakobsen B.K., Hassan N.J. ImmTAC-redirected tumour cell killing induces and potentiates antigen cross-presentation by dendritic cells. Cancer Immunol. Immunother. 2014;63(5):437–448. doi: 10.1007/s00262-014-1525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis J.M., Henson V., Slack R., Ng J., Hartzman R.J., Katovich Hurley C. Frequencies of HLA-A2 alleles in five U.S. population groups. Predominance Of A*02011 and identification of HLA-A*0231. Hum. Immunol. 2000;61(3):334–340. doi: 10.1016/s0198-8859(99)00155-x. [DOI] [PubMed] [Google Scholar]
- 7.Chattopadhyay C., Kim D.W., Gombos D.S., et al. Uveal melanoma: from diagnosis to treatment and the science in between. Cancer. 2016;122(15):2299–2312. doi: 10.1002/cncr.29727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsantoulis P., Delorenzi M., Bièche I., et al. Prospective validation in epithelial tumors of a gene expression predictor of liver metastasis derived from uveal melanoma. Sci. Rep. 2019;9(1):17178. doi: 10.1038/s41598-019-52841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Vidal C., Fernandez-Diaz D., Fernandez-Marta B., et al. Treatment of metastatic uveal melanoma: systematic review. Cancers. 2020;12(9):2557. doi: 10.3390/cancers12092557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivier T., Haslam A., Prasad V. Reporting of physicians’ or investigators’ choice of treatment in oncology randomized clinical trials. JAMA Netw. Open. 2022;5(1) doi: 10.1001/jamanetworkopen.2021.44770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piulats J.M., Espinosa E., de la C., Merino L., et al. Nivolumab plus ipilimumab for treatment-naïve metastatic uveal melanoma: an open-label, multicenter, phase II trial by the Spanish multidisciplinary melanoma group (GEM-1402) JCO. 2021;39(6):586–598. doi: 10.1200/JCO.20.00550. [DOI] [PubMed] [Google Scholar]
- 12.Bol K.F., Ellebaek E., Hoejberg L., et al. Real-world impact of immune checkpoint inhibitors in metastatic uveal melanoma. Cancers. 2019;11(10):1489. doi: 10.3390/cancers11101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roviello G., Andre F., Venturini S., et al. Response rate as a potential surrogate for survival and efficacy in patients treated with novel immune checkpoint inhibitors: a meta-regression of randomised prospective studies. Eur. J. Cancer. 2017;86:257–265. doi: 10.1016/j.ejca.2017.09.018. [DOI] [PubMed] [Google Scholar]