Abstract
The ongoing pandemic resulting from severe acute respiratory syndrome—caused by coronavirus 2 (SARS-CoV-2)—has posed a multitude of healthcare challenges of unprecedented proportions. Intestinal enterocytes have the highest expression of angiotensin-converting enzyme-2 (ACE2), which functions as the key receptor for SARS-CoV-2 entry into cells. As such, particular interest has been accorded to SARS-CoV-2 and how it manifests within the gastrointestinal system. The acute and chronic alimentary clinical implications of infection are yet to be fully elucidated, however, the gastrointestinal consequences from non-SARS-CoV-2 viral GI tract infections, coupled with the generalized nature of late sequelae following COVID-19 disease, would predict that motility disorders are likely to be seen in these patients. Determination of the chronic effects of COVID-19 disease, herein defined as GI disease which is persistent or recurrent more than 3 months following recovery from the acute respiratory illness, will require comprehensive investigations comprising combined endoscopic- and motility-based evaluation. It will be fascinating to ascertain whether the specific post-COVID-19 phenotype is hypotonic or hypertonic in nature and to identify the most vulnerable target portions of the gut. A specific biological hypothesis is that motility disorders may result from SARS-CoV-2-induced angiotensin-converting enzyme 2 (ACE2) depletion. Since SARS-CoV-2 is known to exhibit direct neuronal tropism, the potential also exists for the development of neurogenic motility disorders. This review aims to explore some of the potential pathophysiologic mechanisms underlying motility dysfunction as it relates to ACE2 and thereby aims to provide the foundation for mechanism-based potential therapeutic options.
Keywords: Intestinal motility, Gastrointestinal microbiome, SARS-CoV-2, ACE2
Introduction
SARS-COV-2 is the causative agent of the ongoing global coronavirus disease 2019 (COVID-19) pandemic. The adverse impact on human health and the consequence of the acute phase is already prodigious but unfortunately still accelerating in scope. Assuming herd immunity is achievable because of the cumulative effects of ongoing vaccination and unmitigated infection, the focus will turn toward the chronic health effects of SARS-COV-2, which are predicted to be as highly varied as the primary disease. The chronic secondary effects of SARS-COV-2 are yet to be revealed. Primary SARS-COV-2 infection most commonly affects the respiratory tract [1] which, in susceptible individuals, can result in lethal acute respiratory distress syndrome [2]. Beyond lung involvement, COVID-19 is now recognized to result in multi-organ dysfunction, rendering the scope of scientific inquiry to include the highly varied, concurrent extra-pulmonary manifestations. This review will focus on disorders of GI tract motility that persist or recur more than 3 months following recovery from acute COVID-19 disease and is considered a manifestation of so-called “long-COVID.”
Gastrointestinal manifestations are common in COVID-19 disease with a recent study of 318 hospitalized patients reporting 61% of patients having at least one important GI tract symptom [3]. After convalescence from a SARS-CoV-2 infection, the lasting physiologic implications within the gastrointestinal tract are yet to be elucidated. Post-infectious dysmotility disorders such as irritable bowel syndrome (PI-IBS) and gastroparesis have been demonstrated in several studies [4–6]. The gastrointestinal consequences from non-SARS-CoV-2 viral GI tract infections, coupled with the generalized nature of late sequelae following COVID-19 disease, would predict that motility disorders are likely to be seen in these patients and have chronic effects past the acute infection [7]. Dysregulation of gut motility has been associated with symptoms including but not limited to, diarrhea, nausea, vomiting, and abdominal pain [8]. It is noteworthy that persistence of SARS-CoV-2 RNA was found in fecal specimens in 23% of the patients after negative results were seen in respiratory samples, providing a potential explanation for persistent GI tract manifestations [9].
Determination of the chronic effects of COVID-19 disease will require comprehensive investigations comprising combined endoscopic- and motility-based evaluations. It is uncertain as to whether the specific post-COVID-19 phenotype is hypotonic or hypertonic in nature, and the most vulnerable target portions of the gut require investigation. A specific biological hypothesis is that motility disorders may result from SARS-CoV-2-induced angiotensin-converting enzyme 2 (ACE2) depletion. Since SARS-CoV-2 is known to exhibit direct neuronal tropism, the potential also exists for the development of neurogenic motility disorders. Correlation of motility status and ACE2 expression determined by biopsy will be critical to help inform clinically important pathological pathways and the most appropriate treatment approaches.
ACE2 Dysregulation in COVID-19
ACE2 is a type I integral membrane protein with carboxypeptidase activity that cleaves the carboxyl-terminal amino acid phenylalanine from angiotensin II to produce the peptide angiotensin 1–7, which exhibits potent anti-inflammatory and vasodilator effects [10]. The interaction between the receptor-binding domain (RBD) of the viral spike protein with ACE2 initiates the host entry process, which requires proteolytic cleavage by the host receptor transmembrane protease serine 2 (TMPRSS2) [11]. Continuous infection of host target cells by SARS-CoV-2 facilitates ACE2 down-regulation and ultimately leads to chronic ACE2 deficiency [12] (Fig. 1). The pulmonary phase of the SARS-CoV-2 infection targets alveolar epithelial cells in both the upper and lower respiratory tracts composed of type 1 and 2 pneumocytes, which express high levels of ACE2.
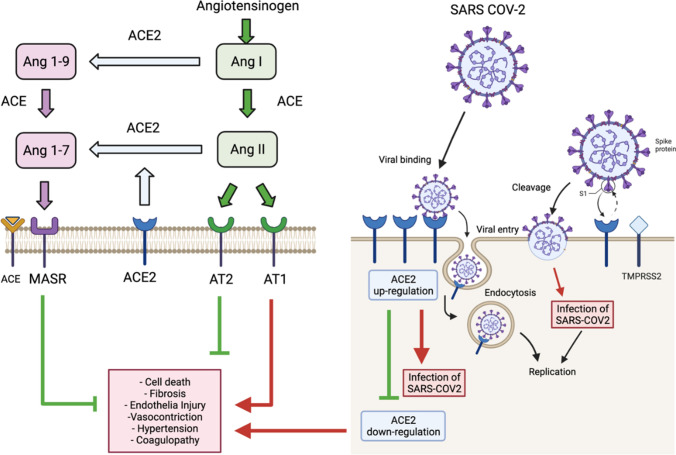
Fig. 1.
The right side of the figure illustrates the SARS-CoV-2 host cell entry process and how it is mediated by binding of the receptor-binding domain (RBD) viral S1 spike protein to the cellular angiotensin-converting enzyme 2 (ACE2) functional receptor. This requires proteolytic cleavage by the host receptor transmembrane protease serine 2 (TMPRSS2). Then, the viral complex is endocytosed with subsequent viral replication that causes a multitude of effects (1b) including down-regulation of cell surface ACE2 and chronic ACE2 deficiency. The left side of the figure is a functional schematic of the renin-angiotensin system. Renin helps convert precursor angiotensinogen to angiotensin I (ANG I) that is subsequently converted to ANG II by angiotensin-converting enzyme (ACE). ANG II has two major receptor isoforms differential expressed throughout the body: AT1R and AT2R. AT1R is the most studied and best understood angiotensin receptor. Through binding ANG II type 1 receptor (AT1R), ANG II stimulates fibrosis, cell death, endothelial cell injury, coagulopathy, and vasoconstriction. Conversely, the ANG II type 2 receptor (AT2R) appears to counterbalance effects of AT1R and stimulates vasodilation, anti-fibrosis, and tissue repair. ANG I and ANG II can be metabolized via the carboxypeptidase ACE2 to form Ang-(1–9) and Ang-(1–7), respectively. Ang-(1–7) then binds to the Mas receptor (Mas-R) exerting protective antifibrotic, anti-inflammatory effects, along with stimulating nitric oxide release and vasodilation
The highest expression of ACE2 occurs in the small intestine particularly in the ileal enterochromaffin cells (ECs) with other locations being the upper esophagus, rectum ECs, as well as epithelial cells in the stomach and colonocytes [13]. In vitro laboratory analysis has shown that the enterocyte lineages are infectable when exposed to SARS-CoV-2 virus. Furthermore, a strong interferon expression from these infected cells has been observed consequent to infection [14]. ACE2 has also been shown to have a renin–angiotensin system (RAS)-independent function related to intestinal amino acid regulation, and in intestinal microbiome homeostasis [15].
Interestingly, ACE2 expression is increased in the terminal ileum and the colon in IBD patients compared to healthy individuals [16]. This is more evident in Crohn’s disease (CD) compared to ulcerative colitis (UC). This was even true for the non-inflamed region of the colon pointing toward underlying pathophysiology at the genetic level. It is noteworthy that IBD features elevation in ACE2 and trypsin-like proteases, especially in the ileum and colon, which provides a facilitated point of entry for SARS-CoV-2 [17]. On the contrary, higher soluble ACE2 levels are found in IBD patients, which compete with the SARS-CoV-2 virus, thus providing plausible compensatory protection [18, 19]. Nevertheless, IBD is neither a statistically protective nor predisposing factor for SARS-CoV-2 infection [16, 20, 21].
ACE2 and Gastrointestinal Motility
Burgeoning evidence suggests that the renin–angiotensin–aldosterone system (RAS) modulates gastrointestinal function in both humans and animals [18, 22]. The RAS system was mechanistically thought to exhibits its effects solely through the endocrine systemic effects, but more recent data indicate that most organs, including the gastrointestinal tract, express all of the required components for local production and action of RAS, suggestive of additional paracrine/autocrine functions [22]. Several intermediate mediators in the RAS cascade exhibit a multitude of complex downstream effects. The ACE/Ang II/AT1R has literature-validated adverse physiologic effects including vasoconstriction, inflammation, and/or increased oxidative stress [23]. Increasing attention has been accorded to the RAS system as the understanding of its modulatory role within the gastrointestinal tract continues to evolve. ACE2 is an integral component of the RAS system and converts high-affinity angiotensin (Ang) II to Ang (1–7) [24] leading to Mas receptor activation which counters the effects of Ang II. The therapeutic benefit attributed to ACE inhibitors and AT1R blockers (ARBs) is theorized to be the shunting RAS substrates toward Ang (1–7) production and Mas receptor activation [25]. In the context of ACE2 deficiency, RAS substrate homeostasis is altered to increase Ang II production and reduce Ang (1–7)/mas receptor activation systemically [26]. The MAS1 oncogene [MAS receptor (MasR)] is a G protein-coupled receptor, which binds the angiotensin-II metabolite angiotensin (1–7) (Ang1–7). Activation of the MasR axis preserves the homeostatic milieu and is conducive to normal gastric motility. How SARS-CoV2 infection and subsequent pathway disequilibrium influence gastrointestinal motility may be dictated by several pathophysiologic mechanisms affecting RAS signaling.
Pawlik and colleagues determined Ang (1–7) to have a dose-dependent protective effect in the esophagi of rat models exposed to gastric refluxate through stimulation of MasR. MasR activation following Ang(1–7) administration in the rat esophageal reflux model induces protective circulatory and anti-inflammatory effects [27]. A similar study by Magierowski et al. concluded that Ang (1–7) provided gastric protection in rats exposed to water immersion and restraint stress (WRS)-induced ulcerogenesis through an increase in gastric blood flow (mediated by endogenous prostaglandins and sensory neuropeptides) and via inhibition of pro-inflammatory markers such as inducible Nitric Oxide Synthase (iNOS), interleukin- 1 β (IL-1β) and TNF-α [28]. Conversely, Ang II worsened WRS ulcerogenesis.
Metabolic pathways may also be affected by SARS-CoV-2-induced ACE2 deficiency. MasR knockout (MasR-KO) c57BL/6 mice evince changes characteristic of metabolic syndrome [29]. Specifically, MasR-KO showed variations in body morphology, a lower glucose tolerance, impaired insulin sensitivity, and increases in their fasting blood glucose [29]. Symptoms of gastric dysmotility are often described in patients with diabetes mellitus [30]. Approximately 75% of diabetic patients report nausea, bloating, diarrhea, or constipation contingent on the duration of abnormal glucose homeostasis [31].
Ang II also has a direct relationship with electrolyte and fluid dynamics within the duodenum. Bicarbonate secretion is stimulated via Ang II acting on AT1 and AT2 in the duodenum [32] and is responsible for intestinal pH homeostasis required for pancreatic enzyme activation, micelle formation, and fat absorption [33]. Additionally, secreted bicarbonate is required for normal expansion and solubility of intestinal mucus [34]. During SARS-CoV-2 infection, ACE2 is down-regulated, effectively increasing Ang II production [35]. This could potentiate bicarbonate secretion, decrease mucosal viscosity, and accelerate gastrointestinal transit times within the small bowel and may explain the diarrheal phenotype commonly seen with acute infections [35]. Further, SARS-CoV-2 binding to ACE2 likely leads to the dysregulation of nutrient transport. ACE2 regulates sodium-dependent amino acid and glucose transporters in the brush border of enterocytes which regulates the absorption of nutrients and maintains osmotic and electrolyte balance [35, 36]. ACE2 mediated dysregulation of sodium-dependent glucose transporter (SGLT1 or SLC5A1) at the intestinal epithelium known to play a pathogenetic role in diabetes mellitus (DM) will prompt studies of gylcemic control during SARS-CoV-2 infection in patients with DM [36]. Prior inflammatory conditions which disrupt the multilayered intestinal mucosal system may allow for GI entry of the SARS-Co-2 using ACE2 and its resultant replication plausible [35], and this remains an urgent question in the field.
Ang II, in conjunction with the enteric sympathetic nervous symptom, acts to modulate sodium and water absorption within the jejunum and ileum [37–39]. In rat studies, low doses of Ang II administration to the jejunum resulted in sodium and water absorption via AT2R stimulation. Conversely, at higher doses, sodium and water absorption was inhibited through AT1R [40]. The increased endoluminal sodium and water concentration potentially portend an ATR1-mediated hypermotile phenotype. Conversely, other studies have demonstrated that Ang II and Ang III potentially increase sodium and water absorption through stimulation of sympathetic neurons acting on adrenergic receptors located on intestinal epithelial cells [22], suggesting that the net effect on sodium and water consequent to COVID disease may be context-dependent and tuned by the equilibrium of Ang II/ATR1/ATR2/Ang (1–7) signaling.
Separate lines of investigation suggest alimentary dysbiosis as a mechanism of gastrointestinal dysmotility. Here again, ACE2 plays a vital role in gut microbiota regulation [41]. Studies have demonstrated that ACE2 associates with a neutral amino acid transporter B0AT1 on the small intestine brush border membrane [42, 43]. ACE2-knockout mice exhibited decreased levels of serum amino acids, impaired uptake of amino acids, namely tryptophan, decreased expression of antimicrobial peptides, and alteration of the intestinal microbiota which was recovered by tryptophan administration [15]. The KO mice also had an increased susceptibility to developing severe colitis when challenged with intestinal irritants dextran sodium sulfate and trinitrobenzene sulfonic acid [15].
Determining the potential chronicity of a post-infectious dysmotility disorder is another important and evolving topic of consideration. The emergence of a novel lifelong disorder is not only distressing at the patient level but would further challenge the allocation of health care resources. Interestingly, studies have demonstrated that patients who have tested positive for SARS-CoV-2 may continue to shed viral RNA in stool samples [44]. Furthermore, Xiao et al. noted the presence of both fecal viral RNA and virus positive stool despite negative respiratory specimens [9]. Evidence also exists of active viral replication within the gastrointestinal tract [45] potentially leading to continued gastrointestinal infection beyond the respiratory phase. Gastrointestinal viral persistence may predispose to chronic down-regulation of ACE2 and ongoing symptoms. A recent study by Al-Aly et al. performed a cohort analysis investigating the 6-month outcomes of incident diagnosis between non-hospitalized SARS-CoV-2 patients and those unaffected by the virus. Multiple disorders affecting almost every organ system, including the gastrointestinal tract, was revealed by elevated hazard ratios (HR) greater than 1 per 1000 SARS-CoV-2 patients at 6 months, as follows: esophageal disorders (6.90 (4.58, 9.07)), gastrointestinal disorders (3.58 (2.15, 4.88)), dysphagia (HR 2.83 (1.79, 3.76)), and abdominal pain (5.73 (3.7, 7.62)). Moreover, there was noted to be increased use of laxatives (9.22 (6.99, 11.31)), antiemetics (9.22 (6.99, 11.31)), histamine antagonists (4.83 (3.63, 5.91)), other antacids (1.07 (0.62, 1.42)), and antidiarrheal agents (2.87 (1.70, 3.91)) [46]. Further longitudinal studies are required to characterize the post-COVID-19 GI tract disease burden.
SARS-CoV-2 Neurotropism
Documented neuronal tropism of the SARS-CoV-2 virus may account for the varied central neurogenic disorders observed in COVID-19 infection [47]. This also raises the possibility that direct enteric neuron invasion of the SARS-CoV-2 virus and suggests a neurogenic basis for COVID-19 related GI tract dysmotility although the data on this point is very fragmentary at present.
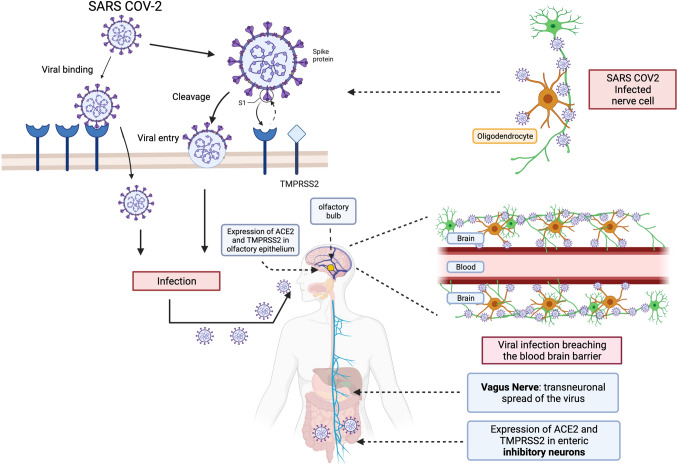
There exist a variety of potential mechanisms of potential SARS-CoV-2 entry routes into the human brain based on ACE2 and cell entry protease expression in the olfactory epithelium, myelin-forming cells, enteric inhibitory neurons, and vascular endothelium [48]. The presence of the viral entry receptors on the cells of the olfactory epithelium and central and enteric nervous system provides a possible viral entry mechanism for neural invasion by SARS-CoV-2 through the olfactory and transvagal routes [48]. Specifically, synaptic junctions of ACE2- and protease-rich inhibitory enteric neurons may allow retrograde spread of the virus in the central nervous system. It has also been proposed that SARS-CoV-2 ingress and transneuronal spread may occur through the olfactory nerves or via a hematogenous route after breaching the blood brain barrier (BBB) [48]. Thus, there are several alternative potential pathways which permit SARS-CoV-2 entry into the central nervous system, although the specific anatomical regions involved remain to be clarified (Fig. 2). It will be translationally important to determine if direct neurogenic invasion in the gut during SARS-CoV-2 infection results in motility disorders independently from the adverse effects of ACE2-mediated alterations in metabolism and inflammatory signaling.
Fig. 2.
Neural invasion of SARS-CoV-2 can occur via olfactory epithelium, enteric inhibitory neurons, or hematogenous spread after breaching the blood brain barrier. Both ACE2 and cell entry protease (TMPRSS2) aid in viral entry and are expressed in cells including olfactory epithelium, myelin-forming cells, enteric inhibitory neurons, and vascular endothelium. Therefore, enteric neural synaptic junctions containing ACE2 and related proteases may support retrograde spread of SARS-CoV-2 in the central nervous system and contribute to central neurogenic disorders observed in COVID-19 infection
Summary of Mechanisms of SARS-CoV-2-Induced Motility Phenotype
Collectively, these studies provide several putative pathophysiological mechanisms through which SARS-CoV-2 may affect GI tract tonus, apart from direct viral cytopathy, including RAS dysfunction, intestinal dysbiosis, and possible secondary enteric neuronal virus invasion. The central role of ACE2 seems compelling but remains speculative at this point pending careful motility-based investigations in post-COVID-19 patient cohorts. Many fascinating and clinical crucial questions remain unanswered such as whether ACE2 deficiency is reversible, and if chronic ACE2 deficiency is a pathogenetic driver of so-called “long-COVID-19” multi-organ disorders.
Treatment Overview
This review invokes the rationale for mechanism-based treatments for post-COVID-19 motility disorders, specifically focused on restoring protective levels of ACE2. Additional treatments address the neurogenic component of post-COVID-19 motility disorders with innovative but mainly unproven therapeutics ranging from probiotics, autonomic modulators to neurostimulation.
Therapeutics Directed to ACE2
The administration of recombinant human ACE2 (rhACE2) in various animal disease models has been shown to have favorable results [49]. In conditions with an imbalance in the renin–angiotensin–aldosterone system, treatment with exogenous ACE2 in mice led to the prevention of deleterious effects associated with Ang 1–8 such as hypertension, oxidative stress, and tubulointerstitial fibrosis [50, 51]. Intravenous injections of rhACE2 in healthy human subjects suppressed Ang 1–8 levels for at least 24 h and multiple injections were well-tolerated without significant adverse effects or toxicity [52]. Similarly, increasing effective levels of ACE2 through exogenous rhACE2 may represent a possible therapeutic intervention in patients with post-COVID-19 motility disorders that result from chronic ACE2 depletion. There are several clinical trials involving rhACE2 that are currently underway. One study by Apeiron Biologics is investigating rhACE2 as a treatment for patients with the acute phase of COVID-19 to block viral entry and decrease replication (ClinicalTrials.Gov; NCT04335136). Another trial by GlaxoSmithKline is exploring the effects of rhACE2 on patients with acute lung injury (ClinicalTrials.Gov; NCT01597635). Recombinant ACE2 is also being studied as a potential treatment for pulmonary arterial hypertension (ClinicalTrials.Gov; NCT01884051).
ACE2 Activators
Several compounds have been discovered which enhance the activation of endogenous ACE2, namely 1-[[2-(dimethylamino) ethyl] amino]-4-(hydroxymethyl)-7-[[(4-methyl phenyl) sulfonyl] oxy]-9H–xanthone9 (XNT), diminazene (DMZ), and resorcinolnaphthalein [53, 54]. These compounds have mainly been studied for their cardiopulmonary protective effects in rodents. For instance, DMZ prevented the development of pulmonary arterial hypertension (PAH) in hypoxia, monocrotaline, and bleomycin models [55]. XNT was shown to reduce blood pressure and reverse cardiorenal fibrosis in spontaneously hypertensive rats [53, 56]. In the monocrotaline-induced PAH rat model, resorcinolnaphthalein prevented hemodynamics by improving vasorelaxation and attenuating anti-inflammatory cytokines [57, 58]. There have not been any studies evaluating the role of ACE2 activators in patients with post-COVID-19 motility disorders. While some of these compounds have undesired side effects, such as renal, hepatic, and cerebral toxicity with chronic use of DMZ, these molecules could serve as a starting point for the development of safer, more tolerable drugs [59].
Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers
As SARS-CoV-2 utilizes the ACE2 receptor for entry into human cells, the role of angiotensin-converting enzyme inhibitors (ACEI’s) and angiotensin receptor blockers (ARB’s) in COVID-19 has been posited. ACEI’s upregulate the ACE2 receptor which could theoretically enhance viral entry into human cells [60]. ARB’s, on the other hand, could theoretically exert a protective effect through the blockade of the ACE2 receptor [60]. ACEI’s and ARB’s have also been shown to have a protective effect on acute lung injury induced by oleic acid in animal models [61]. Several studies have concluded that ACEI’s and ARB exposure was not associated with a higher risk of COVID-19 infection and, in fact, led to lower mortality when compared to patients on non-ACEI’s/ARB’s antihypertensive drugs [62–65]. The current guidelines recommend against the discontinuation of ACEI’s and ARB’s in COVID-19 patients.
Probiotics
Intestinal dysbiosis has been implicated in many common diseases such as obesity, type 2 diabetes, hypertension, and congenital heart disease [66, 67]. In hospitalized COVID-19 patients, intestinal dysbiosis was noted with decreased probiotics such as Lactobacillus and Bifidobacterium [68]. Several studies have demonstrated the communication between intestinal bacteria and the nervous system known as the microbiome-gut-brain axis [69–73]. Neufeld et. al investigated the mechanism by which intestinal bacteria alters the nervous system and found that communication occurred via afferent sensory neurons [74]. Probiotics have been shown to have antioxidant properties, reduce blood pressure, favorably modify cholesterol concentrations and release ACE-inhibiting peptides [75–77]. Probiotics may in part exert their effects by modulating the autonomic nervous system. One study showed that intraduodenal injection of Lactobacillus johnsonii resulted in reduced renal sympathetic nerve activity and increased gastric vagal nerve activity [78].
A study managing COVID-19 with oral bacteriotherapy in addition to standard treatment showed remission of gastrointestinal symptoms for nearly all patients compared to less than half of the control [79]. In addition, it decreased the risk of respiratory failure and ICU admission. Additionally, research has been done in an effort to block residual ACE2 receptor proteins by various probiotics [80]. The binding energies or certain probiotics were shown to be high enough to block the protease residues on the catalytic site on spike proteins, making them therapeutic for COVID-19 [80].
Thus, the beneficial effects of probiotics in post-COVID-19 motility disorders may accrue from restoration of the gut microbiosis and/or through potentiation of vagal nerve activity, although evidence in support of probiotic therapy is so far limited.
Neurostimulation
Vagal nerve stimulation may represent a possible therapeutic intervention in modulating the immune system. In patients who have evidence of autonomic dysfunction as a sequela of SARS-CoV-2 infection, vagal nerve stimulation assists in the downregulation of secreted pro-inflammatory chemokines such as interleukin-1ß, TNF, and IL-8 [81]. The attenuation of these chemokines may result in downstream alleviation in autonomic dysfunction and symptoms. Clinical trials involving noninvasive vagal nerve stimulation in COVID-19 patients are currently ongoing [82–85].
Future Perspectives
There are currently hundreds of clinical trials underway to evaluate the therapeutic efficacy of novel, mechanism-based approaches to address the protean sequelae of SARS-CoV-2 infection, several of which have been highlighted in this review. Only a limited number, however, have been designed to specifically address acute and chronic disorders caused by SARS-CoV-2 affecting the GI tract. A review of evolving data suggests that the role of ACE2 depletion can serve as mechanistic model to help inform more comprehensive investigations and therapies, both systemically and with specific focus directed to the GI tract. Addressing this challenging imperative will be required to control the devastating global burden of COVID-19.
Declarations
Conflict of interest
None of the authors have conflicts of interest to declare, financial or otherwise. Authors confirm that the manuscript is original and that no aspect has been published elsewhere. Authors also have read and approved the final version of the manuscript prior to submission. The manuscript was drafted in accordance with each institution’s IRB ethical publication policies.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grant MC, Geoghegan L, Arbyn M et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15:e0234765. [DOI] [PMC free article] [PubMed]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a Report of 72314 cases from the Chinese Center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Redd WD, Zhou JC, Hathorn KE et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020;159:765–767 e762. [DOI] [PMC free article] [PubMed]
- 4.Klem F, Wadhwa A, Prokop LJ et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology. 2017;152:1042–1054 e1041. [DOI] [PMC free article] [PubMed]
- 5.Zanini B, Ricci C, Bandera F, et al. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am J Gastroenterol. 2012;107:891–899. doi: 10.1038/ajg.2012.102. [DOI] [PubMed] [Google Scholar]
- 6.Thorn AR. Not just another case of nausea and vomiting: a review of postinfectious gastroparesis. J Am Acad Nurse Pract. 2010;22:125–133. doi: 10.1111/j.1745-7599.2009.00485.x. [DOI] [PubMed] [Google Scholar]
- 7.Leung TYM, Chan AYL, Chan EW, et al. Short- and potential long-term adverse health outcomes of COVID-19: a rapid review. Emerg Microbes Infect. 2020;9:2190–2199. doi: 10.1080/22221751.2020.1825914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmulson M, Ghoshal UC, Barbara G. Managing the inevitable surge of Post-COVID-19 functional gastrointestinal disorders. Am J Gastroenterol. 2021;116:4–7. doi: 10.14309/ajg.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 9.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833 e1833. [DOI] [PMC free article] [PubMed]
- 10.Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.RES.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 11.Glowacka I, Bertram S, Muller MA, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamers MM, Beumer J, van der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neurath MF. COVID-19 and immunomodulation in IBD. Gut. 2020;69:1335–1342. doi: 10.1136/gutjnl-2020-321269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbara G, Grover M, Bercik P et al. Rome foundation working team report on post-infection irritable bowel syndrome. Gastroenterology. 2019;156:46–58 e47. [DOI] [PMC free article] [PubMed]
- 18.Garg M, Royce SG, Tikellis C, et al. Imbalance of the renin-angiotensin system may contribute to inflammation and fibrosis in IBD: a novel therapeutic target? Gut. 2020;69:841–851. doi: 10.1136/gutjnl-2019-318512. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim IM, Abdelmalek DH, Elshahat ME, Elfiky AA. COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect. 2020;80:554–562. doi: 10.1016/j.jinf.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins PDR, Ng S, Danese S, Rao K. The risk of SARS-CoV-2 in immunosuppressed IBD patients. Crohns Colitis 360. 2020;2:otaa026. [DOI] [PMC free article] [PubMed]
- 21.Monteleone G, Ardizzone S. Are patients with inflammatory bowel disease at increased risk for Covid-19 infection? J Crohns Colitis. 2020;14:1334–1336. doi: 10.1093/ecco-jcc/jjaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg M, Angus PW, Burrell LM, Herath C, Gibson PR, Lubel JS. Review article: the pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment Pharmacol Ther. 2012;35:414–428. doi: 10.1111/j.1365-2036.2011.04971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohara K, Brosnihan KB, Ferrario CM. Angiotensin(1–7) in the spontaneously hypertensive rat. Peptides. 1993;14:883–891. doi: 10.1016/0196-9781(93)90063-M. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawlik MW, Kwiecien S, Pajdo R et al. Esophagoprotective activity of angiotensin-(1-7) in experimental model of acute reflux esophagitis. Evidence for the role of nitric oxide, sensory nerves, hypoxia-inducible factor-1alpha and proinflammatory cytokines. J Physiol Pharmacol. 2014;65:809–822. [PubMed]
- 28.Magierowski M, Jasnos K, Pawlik M et al. Role of angiotensin-(1-7) in gastroprotection against stress-induced ulcerogenesis. The involvement of mas receptor, nitric oxide, prostaglandins, and sensory neuropeptides. J Pharmacol Exp Ther. 2013;347:717–726. [DOI] [PubMed]
- 29.Oliveira LP, Guimaraes VHD, Oliveira JR et al. Genetic deletion of the angiotensin-(1-7) receptor Mas leads to alterations in gut villi length modulating TLR4/PI3K/AKT and produces microbiome dysbiosis. Neuropeptides. 2020;82:102056. [DOI] [PubMed]
- 30.Wang H, Zhao K, Shi N, Niu Q, Liu C, Chen Y. Electroacupuncture regularizes gastric contraction and reduces apoptosis of interstitial cells of Cajal in diabetic rats. Front Physiol. 2021;12:560738. [DOI] [PMC free article] [PubMed]
- 31.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–1996. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 32.Johansson B, Holm M, Ewert S, Casselbrant A, Pettersson A, Fandriks L. Angiotensin II type 2 receptor-mediated duodenal mucosal alkaline secretion in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1254–1260. doi: 10.1152/ajpgi.2001.280.6.G1254. [DOI] [PubMed] [Google Scholar]
- 33.Dorsey J, Gonska T. Bacterial overgrowth, dysbiosis, inflammation, and dysmotility in the Cystic Fibrosis intestine. J Cyst Fibros. 2017;16:S14–S23. doi: 10.1016/j.jcf.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest. 2009;119:2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar A, Faiq MA, Pareek V et al. Relevance of SARS-CoV-2 related factors ACE2 and TMPRSS2 expressions in gastrointestinal tissue with pathogenesis of digestive symptoms, diabetes-associated mortality, and disease recurrence in COVID-19 patients. Med Hypotheses. 2020;144:110271. [DOI] [PMC free article] [PubMed]
- 36.Broer S, Fairweather SJ. Amino acid transport across the mammalian intestine. Compr Physiol. 2018;9:343–373. doi: 10.1002/cphy.c170041. [DOI] [PubMed] [Google Scholar]
- 37.Levens NR. Response of isolated rat jejunum to angiotensin peptides. Am J Physiol. 1986;251:G559–566. doi: 10.1152/ajpgi.1986.251.4.G559. [DOI] [PubMed] [Google Scholar]
- 38.Levens NR. Response of rat jejunum to changes in sodium and volume balance. Am J Physiol. 1986;251:G413–420. doi: 10.1152/ajpgi.1986.251.3.G413. [DOI] [PubMed] [Google Scholar]
- 39.Levens NR, Peach MJ, Carey RM. Interactions between angiotensin peptides and the sympathetic nervous system mediating intestinal sodium and water absorption in the rat. J Clin Invest. 1981;67:1197–1207. doi: 10.1172/JCI110135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin XH, Wang ZQ, Siragy HM, Guerrant RL, Carey RM. Regulation of jejunal sodium and water absorption by angiotensin subtype receptors. Am J Physiol. 1998;275:R515–523. doi: 10.1152/ajpregu.1998.275.2.R515. [DOI] [PubMed] [Google Scholar]
- 41.Viana SD, Nunes S, Reis F. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities - Role of gut microbiota dysbiosis. Ageing Res Rev. 2020;62:101123. [DOI] [PMC free article] [PubMed]
- 42.Camargo SM, Singer D, Makrides V, et al. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136:872–882. doi: 10.1053/j.gastro.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kowalczuk S, Bröer A, Tietze N, Vanslambrouck JM, Rasko JE, Bröer S. A protein complex in the brush-border membrane explains a Hartnup disorder allele. Faseb J. 2008;22:2880–2887. doi: 10.1096/fj.08-107300. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- 45.Qian Q, Fan L, Liu W et al. Direct evidence of active SARS-CoV-2 replication in the intestine. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 46.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021. [DOI] [PubMed]
- 47.DosSantos MF, Devalle S, Aran V, et al. Neuromechanisms of SARS-CoV-2: a review. Front Neuroanat. 2020;14:37. doi: 10.3389/fnana.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar A, Pareek V, Prasoon P, et al. Possible routes of SARS-CoV-2 invasion in brain: in context of neurological symptoms in COVID-19 patients. J Neurosci Res. 2020;98:2376–2383. doi: 10.1002/jnr.24717. [DOI] [PubMed] [Google Scholar]
- 49.Ferreira AJ, Santos RA, Bradford CN, et al. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension. 2010;55:207–213. doi: 10.1161/HYPERTENSIONAHA.109.140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong J, Basu R, Guo D et al. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–728. [DOI] [PubMed]
- 51.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haschke M, Schuster M, Poglitsch M, et al. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin Pharmacokinet. 2013;52:783–792. doi: 10.1007/s40262-013-0072-7. [DOI] [PubMed] [Google Scholar]
- 53.Hernández Prada JA, Ferreira AJ, Katovich MJ, et al. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension. 2008;51:1312–1317. doi: 10.1161/HYPERTENSIONAHA.107.108944. [DOI] [PubMed] [Google Scholar]
- 54.Kulemina LV, Ostrov DA. Prediction of off-target effects on angiotensin-converting enzyme 2. J Biomol Screen. 2011;16:878–885. doi: 10.1177/1087057111413919. [DOI] [PubMed] [Google Scholar]
- 55.Shenoy V, Gjymishka A, Jarajapu YP, et al. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am J Respir Crit Care Med. 2013;187:648–657. doi: 10.1164/rccm.201205-0880OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferreira AJ, Shenoy V, Yamazato Y, et al. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:1048–1054. doi: 10.1164/rccm.200811-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li G, Liu Y, Zhu Y, et al. ACE2 activation confers endothelial protection and attenuates neointimal lesions in prevention of severe pulmonary arterial hypertension in rats. Lung. 2013;191:327–336. doi: 10.1007/s00408-013-9470-8. [DOI] [PubMed] [Google Scholar]
- 58.Li G, Xu YL, Ling F, et al. Angiotensin-converting enzyme 2 activation protects against pulmonary arterial hypertension through improving early endothelial function and mediating cytokines levels. Chin Med J (Engl). 2012;125:1381–1388. [PubMed] [Google Scholar]
- 59.Peregrine AS, Mamman M. Pharmacology of diminazene: a review. Acta Trop. 1993;54:185–203. doi: 10.1016/0001-706X(93)90092-P. [DOI] [PubMed] [Google Scholar]
- 60.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 61.He X, Han B, Mura M, et al. Angiotensin-converting enzyme inhibitor captopril prevents oleic acid-induced severe acute lung injury in rats. Shock. 2007;28:106–111. doi: 10.1097/SHK.0b013e3180310f3a. [DOI] [PubMed] [Google Scholar]
- 62.Morales DR, Conover MM, You SC, et al. Renin–angiotensin system blockers and susceptibility to COVID-19: an international, open science, cohort analysis. Lancet Digit Health. 2021;3:e98–e114. doi: 10.1016/S2589-7500(20)30289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernández-Ruiz I. RAAS inhibitors do not increase the risk of COVID-19. Nat Rev Cardiol. 2020;17:383. doi: 10.1038/s41569-020-0401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cannata F, Chiarito M, Reimers B, et al. Continuation versus discontinuation of ACE inhibitors or angiotensin II receptor blockers in COVID-19: effects on blood pressure control and mortality. Eur Heart J Cardiovasc Pharmacother. 2020;6:412–414. doi: 10.1093/ehjcvp/pvaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1020–1026. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miele L, Giorgio V, Alberelli MA, De Candia E, Gasbarrini A, Grieco A. Impact of gut microbiota on obesity, diabetes, and cardiovascular disease risk. Curr Cardiol Rep. 2015;17:120. doi: 10.1007/s11886-015-0671-z. [DOI] [PubMed] [Google Scholar]
- 67.Salomon J, Ericsson A, Price A et al. Dysbiosis and intestinal barrier dysfunction in pediatric congenital heart disease is exacerbated following cardiopulmonary bypass. JACC Basic Transl Sci. 2021. [DOI] [PMC free article] [PubMed]
- 68.Xu K, Cai H, Shen Y, et al. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun. 2010;24:9–16. doi: 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 70.Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil. 2012;24:405–413. doi: 10.1111/j.1365-2982.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- 71.Cryan JF, O'Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 72.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McVey Neufeld KA, Mao YK, Bienenstock J, Foster JA, Kunze WA. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil. 2013;25:183–e188. doi: 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- 75.Ait-Belgnaoui A, Durand H, Cartier C, et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, Xu N, Xi A, Ahmed Z, Zhang B, Bai X. Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl Microbiol Biotechnol. 2009;84:341–347. doi: 10.1007/s00253-009-2012-x. [DOI] [PubMed] [Google Scholar]
- 77.Ramchandran L, Shah NP. Proteolytic profiles and angiotensin-I converting enzyme and alpha-glucosidase inhibitory activities of selected lactic acid bacteria. J Food Sci. 2008;73:M75–81. doi: 10.1111/j.1750-3841.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- 78.Tanida M, Yamano T, Maeda K, Okumura N, Fukushima Y, Nagai K. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci Lett. 2005;389:109–114. doi: 10.1016/j.neulet.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 79.d'Ettorre G, Ceccarelli G, Marazzato M, et al. Challenges in the Management of SARS-CoV2 Infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front Med (Lausanne). 2020;7:389. doi: 10.3389/fmed.2020.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anwar F, Altayb HN, Al-Abbasi FA, Al-Malki AL, Kamal MA, Kumar V. Antiviral effects of probiotic metabolites on COVID-19. J Biomol Struct Dyn. 2021;39:4175–4184. doi: 10.1080/07391102.2020.1775123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lerman I, Hauger R, Sorkin L, et al. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: a randomized, Blinded, Healthy Control Pilot Trial. Neuromodulation. 2016;19:283–290. doi: 10.1111/ner.12398. [DOI] [PubMed] [Google Scholar]
- 82.Azabou E, Bao G, Bounab R, Heming N, Annane D. Vagus Nerve stimulation: a potential adjunct therapy for COVID-19. Front Med (Lausanne). 2021;8:625836. [DOI] [PMC free article] [PubMed]
- 83.Kaniusas E, Szeles JC, Kampusch S, et al. Non-invasive auricular vagus nerve stimulation as a potential treatment for Covid19-originated acute respiratory distress syndrome. Front Physiol. 2020;11:890. doi: 10.3389/fphys.2020.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bara GA, de Ridder D, Maciaczyk J. Can neuromodulation support the fight against the COVID19 pandemic? Transcutaneous non-invasive vagal nerve stimulation as a potential targeted treatment of fulminant acute respiratory distress syndrome. Med Hypotheses. 2020;143:110093. [DOI] [PMC free article] [PubMed]
- 85.Baptista AF, Baltar A, Okano AH et al. Applications of non-invasive neuromodulation for the management of disorders related to COVID-19. Front Neurol. 2020;11:573718. [DOI] [PMC free article] [PubMed]