Abstract
Artemisinin and its derivatives are important new antimalarials which are now used widely in Southeast Asia. Clinically relevant artemisinin resistance has not yet been reported but is likely to occur. In order to understand how the malaria parasite might become resistant to this drug, we studied artemisinin resistance in the murine malaria parasite Plasmodium yoelii. The artemisinin-resistant strain (ART), which is approximately fourfold less sensitive to artemisinin than the sensitive NS strain, accumulated 43% less radiolabeled drug in vitro (P < 0.01). Within the parasite, the drug appeared to react with the same parasite proteins in both strains. The translationally controlled tumor protein, one of the artemisinin target proteins, did not differ between the strains. No DNA sequence difference was found, but the resistant strain was found to express 2.5-fold-more protein than the sensitive strain (P < 0.01). Thus, the phenotype of artemisinin resistance in P. yoelii appears to be multifactorial.
Drug-resistant malaria is a major worldwide public health problem. In Southeast Asia, for example, Plasmodium falciparum strains have become resistant to all of the classical antimalarials (12). Fortunately, these strains are still susceptible to the artemisinin derivatives; derivatives such as artemether and artesunate are now widely used in this region (11).
Artemisinin was originally isolated from Artemisia annua, an herb used as an ancient Chinese herbal remedy. All of the artemisinin compounds contain stable endoperoxide bridges. Evidence from a variety of labs suggests that the antimalarial activity of artemisinin is dependent on the cleavage of the endoperoxide by intraparasitic heme. The cleaved endoperoxide ultimately becomes a carbon-centered free radical which then functions as an alkylating agent, reacting with both heme and parasite proteins (but not DNA) (7, 11, 14). In P. falciparum, one of the principal alkylation targets is the translationally controlled tumor protein (TCTP) homolog (4). Some intraparasitic TCTP is situated in the membrane surrounding the heme-rich food vacuole (5), where heme could catalyze the formation of drug-protein adducts.
Clinically relevant resistance to artemisinin derivatives has not yet been reported, although P. falciparum isolates may vary two- to fourfold in their in vitro sensitivities to these drugs (2, 3, 18). However, since the drugs are being widely used, artemisinin resistance is likely to develop in the near future. Recently, a strain of the murine malaria parasite Plasmodium yoelii with unstable resistance to artemisinin was obtained (13). Here we attempted to characterize the mechanisms of resistance in this strain.
MATERIALS AND METHODS
Parasites.
Plasmodium yoelii strains ART and NS (13) were passaged and grown in male ICR mice (Harlan, Indianapolis, Ind.) by intraperitoneal injection of 106 parasitized erythrocytes. Each mouse infected with the resistant strain was given a subcutaneous injection of artemisinin (150 mg/kg of body weight in sesame oil). When parasitemias were >20%, mice were anesthetized with ketamine and exsanguinated by cardiac puncture with a heparinized syringe. The blood was diluted with RPMI 1640 (Gibco BRL, Gaithersburg, Md.) and washed twice. All reagents were obtained from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise noted.
Uptake of radiolabeled drug and reaction with parasite proteins.
Freshly obtained infected erythrocytes were suspended into RPMI 1640 to a hematocrit of 10%. Each incubation used infected cells from a single mouse; infected erythrocytes from four ART- and six NS-infected mice were used. To each suspension, 0.65 μCi of [10-3H]dihydroartemisinin (1.8 Ci/mmol; Moravek Biochemical, Brea, Calif.) ([3H]DHA) was added per ml. The mixture was incubated in a 37°C water bath for 3 h. After the cells were washed twice with RPMI 1640, the parasites were isolated by saponin lysis (8) and stored at −80°C. After thawing, 5 to 10 μl was removed and added to 30 μl of 10 mM Tris-HCl (pH 7.2). The samples were then sonicated for 5 s followed by centrifugation at 20,000 × g for 5 s at room temperature. The pellets were discarded. Protein concentrations in the supernatants were determined with the Bio-Rad microassay (6). In some experiments, the pellets were first solubilized in 1% sodium dodecyl sulfate (SDS) at 4°C for 15 min followed by centrifugation for 30 min at 14,000 × g. To measure radioactivity, aliquots of parasite homogenate (5 μl) were added to 5 ml of scintillation fluid (ScintiVerse BD). Counts were measured in a Beckman LS7000 scintillation counter. To identify radiolabeled protein bands, the same parasite homogenates (20 μg) were run on 10% NuPAGE gels (Novex, San Diego, Calif.) at 200 V for 30 min. After staining of the gels with Coomassie blue, the gels were treated with En3Hance (NEN Life Science Products, Boston, Mass.), dried, and exposed to X-Omat XAR2 autoradiography film (Eastman Kodak Co., Rochester, N.Y.) for 60 days at −80°C. Prints of the autoradiograms were made on electrophoresis duplicating paper (Eastman Kodak Co.).
DNA amplification and sequencing.
Erythrocytes were pelleted and washed three times with phosphate-buffered saline (PBS) and disrupted with lysis buffer (10 mM Tris-HCl [pH 7.5], 10 mM EDTA, 1% SDS) at 60°C for 15 min. The lysed sample was treated with RNase (Boehringer Mannheim, Indianapolis, Ind.) (final concentration, 50 μg/ml) at 37°C for 1 h. Following RNase treatment, the sample was incubated overnight at 37°C with proteinase K. Protein was removed by two extractions with phenol-chloroform-isoamyl alcohol (25:24:1). DNA was precipitated with sodium acetate (final concentration, 0.3 M), washed in 70% ethanol, and reprecipitated in TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]).
PCR.
Primers selected for this study were based on the P. falciparum TCTP sequence. Two pairs of 5′- and 3′-end primers (both pairs added in a 1:1 ratio) were used in the amplification procedure because of variability in the codons' third position. The two 5′ primers were PyA1 (5′-ATGAAAGTATTTAAAGATGTATTTAC) and PYA2 (5′-ATGAAAGTTTTCAAAGACGTTTTCAC), and the two 3′ primers were PyB1 (5′-TTTTTCTTCAAATAATCCATC) and PyB2 (5′-TTTTTCTTCGAAAAGACCGTC). These primers were also used for sequencing the fragments. Primer synthesis and DNA sequencing were done at the Biomedical Research Core Facilities on the University of Michigan campus. In a total volume of 100 μl, 10 μl of a 10× PCR buffer (500 mM KCl, 100 mM Tris-HCl [pH 8.3]) was combined with 2 mM MgCl2, 2.5 U of Ampli-Taq (Perkin-Elmer, Foster City, Calif.), 200 μM deoxynucleoside triphosphates (Boehringer Mannheim), a 0.25 μM concentration of each primer, and 5 μg of DNA template. Amplification of DNA for the PCR was done in a PTC-100 (MJ Research, Watertown, Mass.). A 48 to 38°C touchdown program was used; this program begins with a 3-min hot start at 94°C followed by the first cycle, which includes a 92°C denaturation step for 30 s, a 48°C annealing step for 1 min, and a 72°C extension step for 1 min. This is repeated for 10 cycles except that the annealing temperature decreases 1°C each cycle. There are an additional 20 cycles using 38°C as the annealing temperature. An 8-min extension at 72°C follows the last cycle. The reaction products were purified on a 1% low-melting-point agarose gel (Gibco BRL) and extracted using a gel extraction kit (QIAquick; Qiagen, Chatsworth, Calif.). Sequence alignments were performed on MacVector version 5.0 (International Biotechnologies, New Haven, Conn.).
Immunoblotting.
Western blots were run for both resistant and sensitive strains to compare and quantify the pattern and intensity of the parasite TCTP reaction with antibody made against recombinant P. falciparum TCTP (6). Parasites were isolated from the freshly exsanguinated blood by saponin lysis (6) and stored at −80°C. For each experiment, samples were thawed, suspended in 10 mM Tris-HCl (pH 7.0), and gently sonicated for 10 s with a Branson sonifier. Electrophoresis was performed on 10% NuPAGE gels to determine if protein band intensity or pattern differed between the resistant and sensitive strains. Parasite protein (5 μg) was loaded into each well and 20 μl of prestained molecular weight standards (Seeblue; Novex) was added to a single lane. The gel was run at 200 V for 30 min and placed into a 0.025% Coomassie R-250 stain for at least 2 h followed by destaining until the background was clear. Protein was transferred to either nitrocellulose or polyvinylidene difluoride at 25 V for 1 h using the Novex transfer system. The membranes were blocked overnight at 4°C in 3% nonfat dry milk in Tris-buffered saline (20 mM Tris-HCl [pH 7.6], 137 mM NaCl) with 0.1% Tween 20 followed by a washing in the same buffer. The membranes were then incubated in anti-TCTP at a concentration of 1:15,000 for 60 min at room temperature. Following extensive washing in the buffer, the membranes were used in one of two Western blotting protocols. The nitrocellulose membranes were incubated with horseradish peroxidase-conjugated rabbit anti-goat antibody (Dako Corp., Carpinteria, Calif.) at a concentration of 1:1,500 for 1 h at room temperature. Following extensive washing with the buffer, the membranes were developed by the enhanced chemiluminescence technique (Amersham Pharmacia Biotech) and exposed to autoradiography film. The polyvinylidene difluoride membranes were incubated with alkaline phosphatase-linked anti-rabbit antibody at a concentration of 1:10,000 for 1 h at room temperature. Following a washing, these membranes were developed by the enhanced chemifluorescence (ECF) technique (Amersham Pharmacia Biotech). The membrane was scanned with the blue filter using the Molecular Dynamics (Sunnyvale, Calif.) Storm FluorImager system. The bands from the ECF were quantified to determine if there was a difference in the density of the reaction between the resistant and sensitive strains.
To determine the isoelectric point of the TCTP homolog, 25 μg of parasite protein was placed into the well of an isoelectric focusing (IEF) gel and run by following the manufacturer's specifications (Novex). Twenty microliters each of IEF standards (Bio-Rad, Hercules, Calif.) was also run on the gel. The protein was transferred to nitrocellulose and immunoblotted as described above except that the primary antibody was used at a 1:10,000 dilution. The standards were stained, after the blot was developed, with 0.025% Coomassie R-250.
Statistics.
Standard deviations and significance (as determined by the Student t test) were calculated using Microsoft Excel (Macintosh version 5.0).
Nucleotide sequence accession number.
The sequence of the P. yoelii gene has been given GenBank accession number AF124820.
RESULTS
Artemisinin-resistant P. yoelii accumulated significantly less drug than artemisinin-sensitive parasites (P < 0.01). Resistant parasites accumulated 43% less [3H]DHA than sensitive parasites (54.3 versus 94.3 nmol/mg of protein [Table 1]). Preparations of ART and NS parasites were harvested at approximately the same day of infection and at approximately the same levels of parasitemia. The preparations showed no significant difference in stage distribution (percent trophozoites). The [3H]DHA in both strains was not membrane associated, since treatment of the lysates with SDS did not increase measurable counts (data not shown).
TABLE 1.
[3H]DHA accumulation in artemisinin-sensitive and -resistant parasites
| Mouse | Trophozoites (%) | Parasitemia (%) | Day of sacrificea | [3H]DHA uptakeb |
|---|---|---|---|---|
| NS | ||||
| 22 | 8.2 | 34.4 | 9 | 127.4 |
| 30 | 8.4 | 25.7 | 5 | 96.3 |
| 31 | 10.7 | 23.6 | 5 | 90.8 |
| 40 | 2.1 | 16.4 | 9 | 95.9 |
| 41 | 8.0 | 17.1 | 9 | 98.8 |
| 57 | 6.1 | 16.3 | 8 | 56.8 |
| Mean ± SD | 7.3 ± 2.9 | 22.3 ± 7.2 | 7.5 ± 2.0 | 94.3 ± 22.7c |
| ART | ||||
| 28 | 10.3 | 36.5 | 8 | 58.4 |
| 38 | 20.4 | 35.6 | 10 | 40.4 |
| 45 | 9.5 | 19.0 | 7 | 56.8 |
| 46 | 7.4 | 17.8 | 7 | 61.4 |
| Mean ± SD | 11.9 ± 5.8 | 27.2 ± 10.2 | 8 ± 1.4 | 54.3 ± 9.3 |
Day postinfection.
Measured in nanomoles per milligram of protein.
[3H]DHA uptake is significantly greater in the NS group than the ART group (P < 0.01).
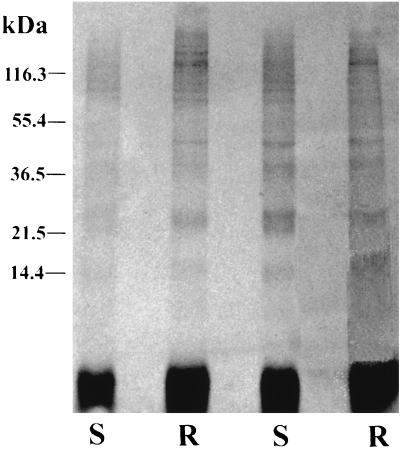
We next tested whether [3H]DHA might react with proteins differently in the two strains. Extracts were made from resistant and sensitive parasites exposed to [3H]DHA, analyzed by SDS gel electrophoresis, and exposed to film. Bands were seen at 14, 22.5, 36.5, 42, and >150 kDa in both strains (Fig. 1). The autoradiograms resemble those previously reported for [3H]DHA-treated P. falciparum (with bands at 25, 32, 42, 50, 65, and >200 kDa) (1). Bands of similar molecular masses are labeled in each of the four lanes, but no consistent differences in band intensities were seen between the two strains.
FIG. 1.
SDS gel autoradiogram of [3H]DHA-treated P. yoelii ART (artemisinin resistant [R]) and NS (artemisinin sensitive [S]).
We then tested whether artemisinin resistance might be associated with a change in the P. yoelii TCTP sequence or expression levels. The P. yoelii gene was amplified by PCR using primers based on the P. falciparum gene and sequenced. The P. yoelii and P. falciparum TCTPs were identical in 88% of the amino acids and similar in 97% (Fig. 2). The TCTP nucleotide sequence was identical in both the ART and NS strains.
FIG. 2.
Alignment of TCTP sequences from P. yoelii and P. falciparum (GenBank accession number AA549873).
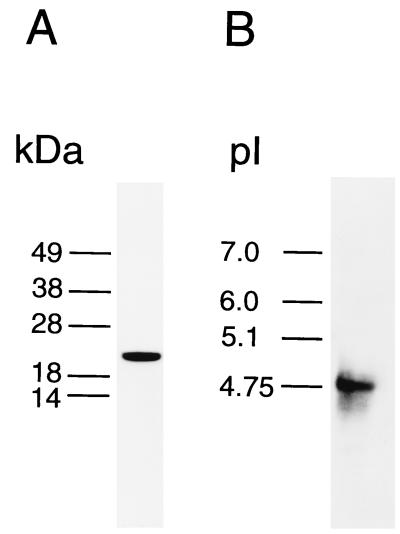
We then studied the P. yoelii TCTP using antisera prepared against recombinant P. falciparum TCTP. The molecular mass of P. yoelii TCTP, as estimated from the immunoblots, was 22 kDa (Fig. 3A). The pI for TCTP was estimated to be about 4.8 (Fig. 3B). These numbers are similar to those found for the P. falciparum protein.
FIG. 3.
Immunoblotting of P. yoelii gels using anti-TCTP. SDS (A) and IEF (B) gels were run on homogenates of P. yoelii strain NS, immunoblotted with anti-TCTP antibody, and visualized by enhanced chemiluminescence.
Artemisinin-resistant parasites contained significantly more TCTP than sensitive parasites. Resistant and sensitive isolates were analyzed by SDS gel electrophoresis, transferred to membranes, and immunoblotted with anti-TCTP. As can be seen in Table 2, there were no significant differences between the preparations in terms of stage (percent trophozoites) or in terms of parasitemia and day of sacrifice of the host mouse. The intensities of the TCTP bands were quantitated with the fluorimager. The mean densities for the resistant and sensitive strains as calculated using the ECF protocol differed 2.5-fold (3,344 and 1,375, respectively [Table 2]). This difference was significant at a P value of <0.01.
TABLE 2.
TCTP levels in artemisinin-sensitive and -resistant parasites
| Mouse | Trophozoites (%) | Parasitemia (%) | Day of sacrificea | TCTP valueb |
|---|---|---|---|---|
| NS | ||||
| 3 | 1.5 | 18.8 | 7 | 1,682 |
| 4 | 45.0 | 45.0 | 7 | 117 |
| 9 | 1.7 | 18.0 | 7 | 848 |
| 10 | 3.5 | 33.5 | 7 | 2,490 |
| 12 | 4.4 | 16.3 | 7 | 608 |
| 37 | 21.6 | 48.4 | 7 | 2,505 |
| Mean ± SD | 13.0 ± 17.4 | 30.0 ± 14.4 | 7 ± 0 | 1,375 ± 1,006c |
| ART | ||||
| 7 | 6.3 | 39.8 | 7 | 5,612 |
| 13 | 5.8 | 27.8 | 7 | 4,366 |
| 14 | 5.4 | 39.1 | 7 | 3,654 |
| 15 | 7.6 | 19.0 | 7 | 3,759 |
| 16 | 16.4 | 55.5 | 7 | 1,797 |
| 17 | 7.9 | 38.6 | 7 | 1,915 |
| 35 | 11.6 | 59.1 | 12 | 2,985 |
| 62 | 15.6 | 23.1 | 6 | 2,664 |
| Mean ± SD | 9.6 ± 4.4 | 37.8 ± 14.4 | 7.5 ± 1.9 | 3,344 ± 1,281 |
Day postinfection.
Relative band intensity as measured by ECF.
TCTP levels are significantly lower in the NS group than the ART group (P < 0.01).
DISCUSSION
This study was a first attempt to understand the mechanisms by which malaria parasites become resistant to artemisinin and its derivatives using artemisinin-resistant and -sensitive strains of P. yoelii. We tested the hypotheses that artemisinin resistance might be associated with either (i) decreased drug accumulation or (ii) an alteration in TCTP, a possible drug target. Resistant parasites were found to accumulate significantly less drug than sensitive parasites. TCTP had the same sequence in both strains. However, the resistant strain expressed about 2.5 times as much TCTP as the sensitive strain. Thus, artemisinin resistance in P. yoelii appears to be multifactorial.
Attempts to select for artemisinin-resistant strains of the human malaria parasite P. falciparum were unsuccessful both in our lab (unpublished results) and in others (Dennis Kyle, personal communication). Clinical artemisinin-resistant isolates are also not available. Accordingly, we chose to study artemisinin resistance in the P. yoelii ART strain. There were two drawbacks, however, to working with this model (13). First, the strain exhibits a variable degree of resistance, ranging from 4- to 27-fold. (At the time of the study, the ART strain was 4-fold more resistant than the NS strain [unpublished results].) Second, its resistance phenotype is unstable. It must be passaged in mice administered artemisinin at the time of passage or else it reverts to a sensitive phenotype. Fortunately, this latter dose of drug is unlikely to have affected the parasites used in this study. The half-life of artemisinin is <12 h (11), and infected erythrocytes were harvested from mice 7 to 8 days after inoculation, so the parasites would have undergone >7 full replication cycles between the time of drug exposure and harvesting.
We previously suggested that TCTP might be a drug target for artemisinin because P. falciparum TCTP reacts with [3H]DHA both in vitro and in intact infected erythrocytes (4). The overexpression of TCTP in artemisinin-resistant parasites is consistent with this hypothesis. Overexpression of other drug targets, such as dihydrofolate reductase and ornithine decarboxylase, has been shown to be associated with drug resistance in other parasites, such as Leishmania (16). However, the difference in TCTP expression observed here might be a secondary effect unrelated to the mechanism of resistance. Proof of the involvement of TCTP in the mechanism of action of artemisinin will require either (i) construction of TCTP-deficient malaria strains or (ii) demonstration that artemisinin treatment affects TCTP function. Unfortunately, the function of TCTP is currently unknown, although several functions have been suggested (9, 10, 15, 17).
TCTP of P. yoelii is very similar to that of P. falciparum. The molecular masses are similar (22 for P. yoelii versus 25 kDa for P. falciparum), as are the pIs (4.8 for P. yoelii versus 4.8 to 4.9 for P. falciparum). A total of 88% of the amino acids were identical, and the P. yoelii protein reacts well with antibody prepared against recombinant P. falciparum TCTP. Thus, studies on the function of P. yoelii TCTP should shed light on the function of TCTP in human malaria parasites.
The artemisinin derivatives are very important new antimalarials that are now being used widely. It is only a matter of time before clinically important artemisinin resistance is observed. The results of this study should offer clues to the mechanism of artemisinin resistance in human parasites once it arises.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant AI-26848 (to S.R.M.).
We thank Paul Hossler, Rosemary Rochford, and Xing-Qing Pan for their help.
REFERENCES
- 1.Asawamahasakda W, Ittarat I, Pu Y M, Ziffer H, Meshnick S R. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob Agents Chemother. 1994;38:1854–1858. doi: 10.1128/aac.38.8.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basco L K, Le Bras J. In vitro activity of artemisinin derivatives against African isolates and clones of Plasmodium falciparum. Am J Trop Med Hyg. 1993;49:301–307. doi: 10.4269/ajtmh.1993.49.301. [DOI] [PubMed] [Google Scholar]
- 3.Basco L K, Le Bras J. In vitro susceptibility of Cambodian isolates of Plasmodium falciparum to halofantrine, pyronaridine and artemisinin derivatives. Ann Trop Med Parasitol. 1994;88:137–144. doi: 10.1080/00034983.1994.11812851. [DOI] [PubMed] [Google Scholar]
- 4.Bhisutthibhan J, Pan X Q, Hossler P A, Walker D J, Yowell C A, Carlton J, Dame J B, Meshnick S R. The Plasmodium falciparum translationally controlled tumor protein homolog and its reaction with the antimalarial drug artemisinin. J Biol Chem. 1998;273:16192–16198. doi: 10.1074/jbc.273.26.16192. [DOI] [PubMed] [Google Scholar]
- 5.Bhisutthibhan J, Philbert M A, Fujioka M, Aikawa M, Meshnick S R. The Plasmodium falciparum translationally controlled tumor protein: subcellular localization and calcium binding. Eur J Cell Biol. 1999;78:665–670. doi: 10.1016/S0171-9335(99)80052-1. [DOI] [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Cumming J N, Ploypradith P, Posner G H. Antimalarial activity of artemisinin (qinghaosu) and related trioxanes: mechanism(s) of action. Adv Pharmacol. 1997;37:253–297. doi: 10.1016/s1054-3589(08)60952-7. [DOI] [PubMed] [Google Scholar]
- 8.Fairfield A S, Meshnick S R, Eaton J W. Malaria parasites adopt host cell superoxide dismutase. Science. 1983;221:764–766. doi: 10.1126/science.6348944. [DOI] [PubMed] [Google Scholar]
- 9.Gachet Y, Lee M, Sawitzki B, Tournier S, Poulton T, Bommer U A. Intracellular colocalisation of the translationally controlled protein P23 with cytoskeletal structures. Biochem Soc Trans. 1997;25:269S. doi: 10.1042/bst025269s. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald S M. Histamine-releasing factors. Curr Opin Immunol. 1996;8:778–783. doi: 10.1016/s0952-7915(96)80004-5. [DOI] [PubMed] [Google Scholar]
- 11.Meshnick S R, Taylor T E, Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol Rev. 1996;60:301–315. doi: 10.1128/mr.60.2.301-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters W. Drug resistance in malaria parasites of animals and man. Adv Parasitol. 1998;41:1–62. doi: 10.1016/s0065-308x(08)60421-2. [DOI] [PubMed] [Google Scholar]
- 13.Peters W, Robinson B L. The chemotherapy of rodent malaria. LVI. Studies on the development of resistance to natural and synthetic endoperoxides. Ann Trop Med Parasitol. 1999;93:325–339. doi: 10.1080/00034989958320. [DOI] [PubMed] [Google Scholar]
- 14.Robert A, Meunier B. Is alkylation the main mechanism of action of the antimalarial drug artemisinin? Chem Soc Rev. 1998;27:273–279. [Google Scholar]
- 15.Sanchez J C, Schaller D, Ravier F, Golaz O, Jaccoud S, Belet M, Wilkins M R, James R, Deshusses J, Hochstrasser D. Translationally controlled tumor protein: a protein identified in several nontumoral cells including erythrocytes. Electrophoresis. 1997;18:150–155. doi: 10.1002/elps.1150180127. [DOI] [PubMed] [Google Scholar]
- 16.Segovia M. Leishmania gene amplification: a mechanism of drug resistance. Ann Trop Med Parasitol. 1994;88:123–130. doi: 10.1080/00034983.1994.11812849. [DOI] [PubMed] [Google Scholar]
- 17.Sturzenbaum S R, Kille P, Morgan A J. Identification of heavy metal induced changes in the expression patterns of the translationally controlled tumour protein (TCTP) in the earthworm Lumbricus rubellus. Biochim Biophys Acta. 1998;1398:294–304. doi: 10.1016/s0167-4781(98)00077-3. [DOI] [PubMed] [Google Scholar]
- 18.Wongsrichanalai C, Nguyen T D, Trieu N T, Wimonwattrawatee T, Sookto P, Heppner D G, Kawamoto F. In vitro susceptibility of Plasmodium falciparum isolates in Vietnam to artemisinin derivatives and other antimalarials. Acta Trop. 1997;63:151–158. doi: 10.1016/s0001-706x(96)00618-3. [DOI] [PubMed] [Google Scholar]