Abstract
Introduction COVID-19 disease has caused a global health and economic crisis. The introduction of the different COVID-19 vaccines has resulted in a significant decrease in the morbidity and mortality associated with this disease. Adverse effects have been reported, including cardiological ones such as myocarditis or pericarditis after administration. Likewise, tyrosine kinase inhibitor drugs such as osimertinib used in lung cancer patients with epidermal growth factor receptor (EGFR) mutation are associated with heart failure or prolongation of the QT interval.
Case report 62-year-old woman diagnosed in September 2019 of lung adenocarcinoma stage IV with bilateral lung and lymph node involvement, carrier of an EGFR mutation (Ex19Del) on treatment with osimertinib. She attended emergency department for fever and hypotension 24 h after administration of the third dose of Moderna® COVID-19 vaccine in the context of acute myocarditis with evidence of severe left ventricular (LV) dysfunction in cardiogenic shock. She required vasoactive support, non-invasive mechanical ventilation, corticotherapy, immunoglobulins and subsequent ventricular support with Impella, with improvement of the clinical picture after 3 days. Cardiac magnetic resonance imaging (MRI) showed evidence of global myocardial oedema compatible with acute myocarditis. Coronary CT showed a lesion in the anterior descending coronary artery requiring revascularization. A few days later, she presented febrile symptoms with isolation of Staphylococcus aureus in the central line catheter and antibiotherapy with cloxacillin was started, with subsequent resolution of the infectious symptoms.
Conclusion This is an exceptional and controversial case of fulminant myocarditis probably related to the Modern COVID-19 vaccine in a patient diagnosed with metastatic lung adenocarcinoma on treatment with osimertinib. An increasing number of cases of myocarditis and pericarditis have been reported following vaccination with COVID-19 mRNA vaccines. In addition, retrospective data have shown an increased risk of QT prolongation and heart failure in patients treated with tyrosine kinase inhibitors. Hence, the need for close monitoring of cardiac function during treatment of these patients. Future studies will be necessary to evaluate unknown adverse reactions of these vaccines and their possible interaction with other antineoplastic drugs.
Keywords: Myocarditis, COVID-19 mRNA vaccine, Lung adenocarcinoma, Osimertinib
Introduction
Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) is the origin of the current COVID-19 pandemic with an increased risk of long-term cardiac sequelae in the infected population (Boukhris et al., 2020). The mechanism of infection of human cells is based on binding to the transmembrane enzyme angiotensin 2, mainly expressed in cells of the alveoli and cardiac tissue (Blanco-Melo et al., 2020). This damage results from an inappropriate or excessive immune response driven by T- and B-cell mediated mechanisms. Currently, the introduction of the different COVID-19 vaccines has resulted in a significant decrease in the morbidity and mortality associated with COVID-19 disease. All marketed vaccines have been proven to provide benefits that outweigh the potential associated risks among different age groups (Haas et al., 2021; Lopez Bernal et al., 2021). Some studies have reported cases of myocarditis in association with the different vaccines, mostly mild cases in young males after administration of the second dose of mRNA vaccines (Pfizer-BioNTech® and Moderna) (Salah and Mehta, 2021; Minocha et al., 2021). However, information on the characteristics and debut of COVID-19 vaccine-associated myocarditis is very limited.
On the other hand, epidermal growth factor receptor tyrosine kinase (anti-EGFR) inhibitors such as osimertinib have demonstrated efficacy as 1st-line therapy in patients with advanced non-small-cell lung cancer (NSCLC) with EGFR mutations (Ex19del/L858R) and T790M resistance mutations (Soria et al., 2018). Adverse effects after administration of these drugs such as heart failure or QT interval prolongation have been reported (Anand et al., 2019).
We present the case of a woman diagnosed with a lung adenocarcinoma stage IV on treatment with osimertinib who developed fulminant myocarditis after receiving the third dose of COVID-19 Moderna vaccine.
Case report
A 62-year-old woman with no past history of interest. She was diagnosed in September 2019 with a lung adenocarcinoma stage IV with bilateral lung and lymph node involvement. Carrier of an EGFR mutation (Ex19Del). She started treatment with osimertinib at 80 mg/day. In November 2019, she was admitted for grade III cardiac, hepatic and pulmonary toxicity (pericarditis, pneumonitis and alteration of the hepatic profile) requiring treatment with NSAIDs and colchicine and readjustment of osimertinib to 40 mg/day. He continues to be monitored by Cardiology department with adequate tolerance and preserved left ventricular ejection fraction (LVEF). In February 2021, during a re-evaluation study, a liver lesion was found with partial response in the rest of the sites and, after discussing the case in a multidisciplinary committee, she underwent laparoscopic non-anatomical resection of segment III of the liver. She continued treatment with osimertinib at 40 mg/day. In November 2021, due to blurred vision, a cranial computed axial tomography (CAT) scan was performed with evidence of a selar lesion that suggested a metastatic lesion by MRI. Pending assessment of local therapeutic approach.
This patient consulted for fever (39.0 °C) at home, intense asthenia and hypotension (50/30 mmHg). Examination revealed sinus tachycardia (150 bpm) and skin pallor. In a directed anamnesis, she mentioned vaccination the previous day with a third dose of vaccine (Moderna) against COVID-19 and influenza and the onset of symptoms that morning. Intensive fluidtherapy was started with no clinical improvement. Urgent laboratory tests showed slight alterations in renal and hepatic function parameters and no elevation of acute phase reactants. An electrocardiogram (ECG) was requested with a finding of sinus tachycardia with no signs of acute ischaemia. Myocardial damage markers (MDM), troponin I high sensibility 12.487,6 pg/mL (1st determination). 8.147,2 pg/mL (2nd determination) (Table 1 ).
Table 1.
Analytical control on arrival at the emergency department.
| Parameters | Value | Reference range |
|---|---|---|
| Chemistry | ||
| Haemoglobin | 13,6 g/dL | 12–15 |
| Leukocytes | 9.02 × 10 <3 | 3.8–11 × 10<3 |
| Neutrophils | 4.96 × 10<3 | 1.8-7 × 10<3 |
| Platelets | 234 × 10<3 | 140–450 |
| Blood urea nitrogen | 30 mg/dL | 19–49 |
| Creatinine | 1,16 mg/dL | 0,51–0,95 |
| Calcium | 9,7 mg/dL | 8,7–10,4 |
| Sodium | 138 mmol/L | 136–145 |
| Potassium | 3,8 mmol/L | 3,4–5,1 |
| Alanine aminotransferase (ALT/GPT) | 71 U/L | 10–49 |
| Aspartate aminotransferase (AST/GOT) | 102 U/L | <31 |
| Lactate dehydrogenase (LDH) | 263 U/L | 120–246 |
| Gamma-glutamyl transferase (GGT) | 227 U/L | <38 |
| Alkaline phosphatase | 358 U/L | 46–116 |
| C-Reactive protein | 3,33 mg/dL | 0–0,5 |
| Procalcitonin | 0.35 ng/mL | <0,5 |
| CARDIAC MARKERS | ||
| Troponin I high sensibility | 12.487,6 pg/mL | <34 |
| NT-proBNP | 8.069 pg/mL | <300 |
| URINE | ||
| pH | 8,5 | 5,5-6,5 |
| Nitrites | Negative | Negative |
| Leukocytes | Negative | Negative |
| Red blood cells | 6,6 u/L | 0-30 |
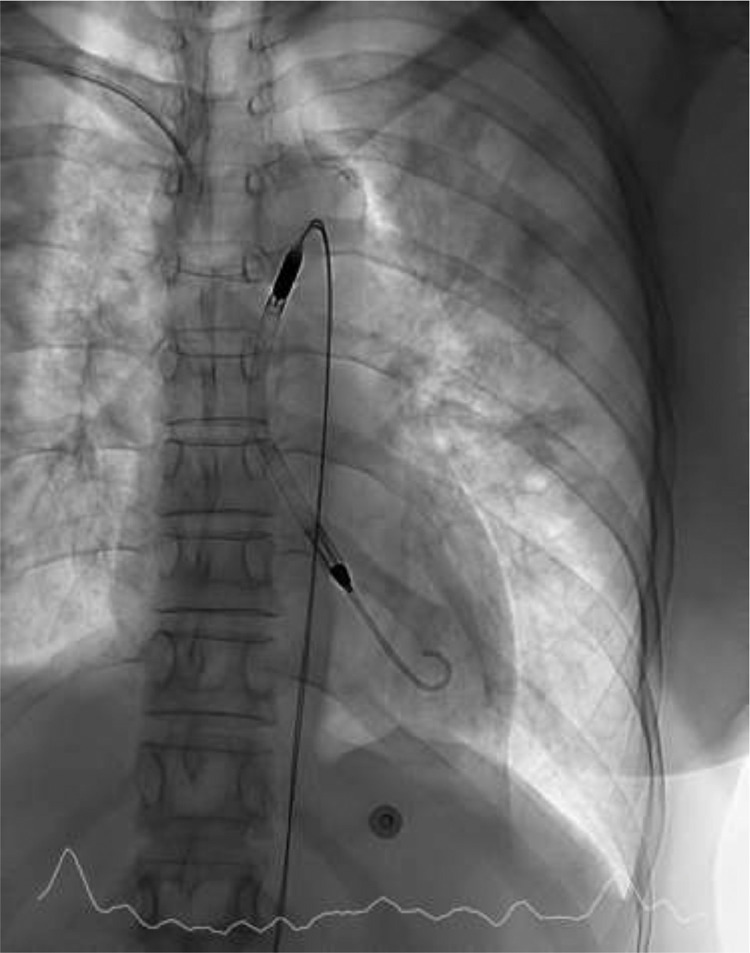
Urgent assessment by the Cardiology department was requested, with transthoracic echocardiography (TTE) showing a dilated LV with severely depressed LVEF, diffuse hypokinesia and significant pulmonary congestion. The overall picture was suggestive of fulminant myocarditis with severe left ventricular dysfunction in cardiogenic shock. Vasoactive and inotropic support was started with dobutamine (DBT) and noradrenaline (NA) at high doses and non-invasive mechanical ventilation was started with BiPAP. Suspicion of myocarditis led to treatment with glucocorticoids and immunoglobulins. Ventricular assist device (Impella) implantation was offered due to a normofunctioning right ventricle and respiratory stability, but the patient and family initially refused, although they later accepted its placement (Fig. 1 ). The patient presented progressive improvement in ventricular function, which allowed for a reduction in the supply of inotropic agents and removal of the Impella after 72 h of support.
Fig. 1.
Ventricular assist device Impella placement under fluoroscopy.
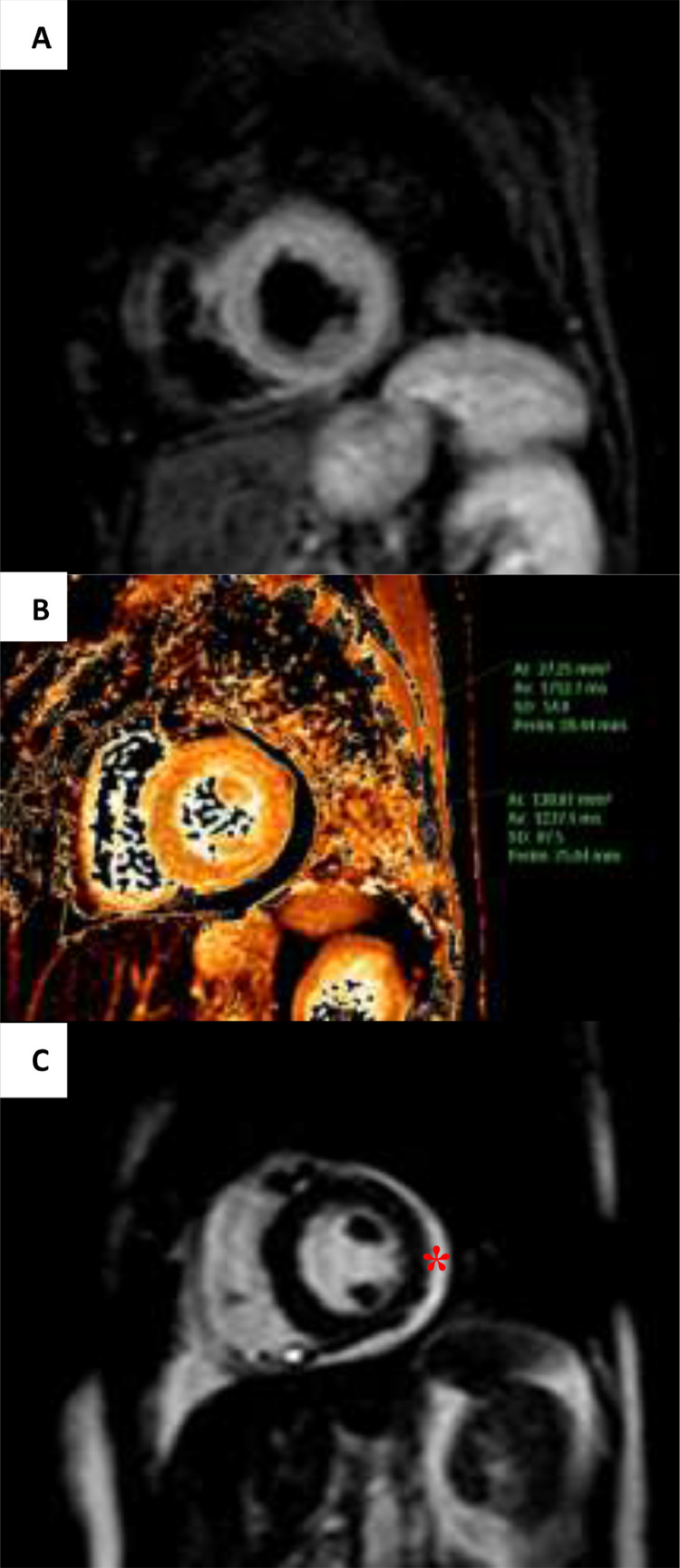
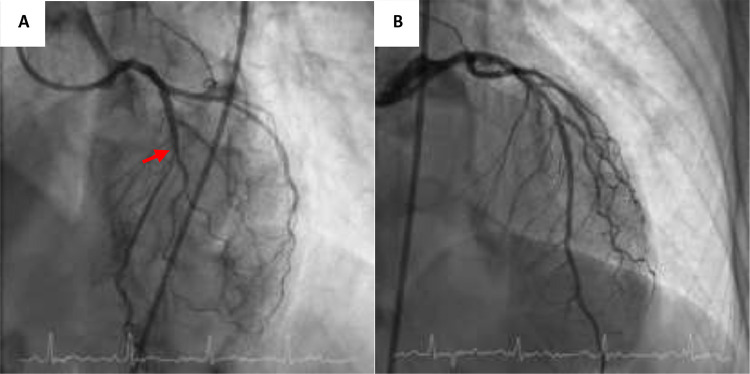
During admission, cardiac MRI was performed, compatible with acute myocarditis with evidence of global myocardial oedema and mild pericardial effusion (Fig. 2 ). Also, we performed a coronary CT scan, showing a significant lesion in the anterior descending artery, which was confirmed by invasive coronary angiography and revascularized with a drug-eluting stent (Fig. 3 ). A few days later, the patient presented febrile symptoms with elevated acute phase reactants and isolation of methicillin-sensitive Staphylococcus aureus in the central catheter, which was removed. TTE was performed to look for signs of infective endocarditis, with inconclusive results. She was started on antibiotics with cloxacillin for 15 days with good clinical evolution and was discharged from hospital on a course of corticotherapy, colchicine and antibiotherapy.
Fig. 2.
Myocarditis features showed by cardiac magnetic resonance at the short axis level of papillay muscles. (A) Hyperintense myocardial signal in the T2 weighted-Short Tau Inversion recovery sequence. (B) Increased native T1-weighted relaxation time (1232 ms, regional normal value 995 ± 36 ms). (C) Mild pericardial effusion (asterisk).
Fig. 3.
(A) Significant lesion at the level of the middle left anterior descending coronary artery (red arrow). (B) Left descending coronary artery after successful percutaneous treatment.
Discussion
The COVID-19 pandemic has caused a global health and economic crisis. Although initially reported as a respiratory tract disease, a wide range of organ complications (gastrointestinal, neurological, thromboembolic, immunological and cardiovascular) have now been described (Boukhris et al., 2020). Myocarditis is known to be due to inflammation of the myocardium and can be caused by infectious diseases; mainly viruses (especially coxsackievirus), adenovirus, parvovirus B19 or human herpesvirus 6; among others, although bacteria and protozoa have also been described as causative agents (Rose, 2016). Cardiotropic virus myocarditis represents the main cause of myocarditis in developing countries and of hospitalized cases in our geographical area. Histopathological evidence of myocarditis is found in approximately 10% of post-mortem examinations.
Coronaviruses (severe acute respiratory syndrome, middle east respiratory syndrome coronavirus (MERS-CoV, 2012) and the current SARS-CoV-2) are known to cause myocarditis (Rezkalla and Kloner, 2021). We report the case of a 62-year-old woman diagnosed with metastatic lung adenocarcinoma who presented with acute myocarditis after the third dose of the Modern vaccine against COVID-19.
Vaccines allow the immune system to develop neutralising antibodies that provide protection against infection. Using different technology platforms (RNA vaccines, replication-incompetent vector vaccines, recombinant protein vaccines and inactivated vaccines), the currently marketed COVID-19 mRNA (Pfizer-BioNTech and Moderna) and adenovirus (Janssen® and AstraZeneca®) vaccines have been developed. Adverse reactions have been reported following administration; localised at the injection site with pain and redness, and systemic adverse effects such as fever, fatigue, headache and myalgia. Serious adverse reactions such as anaphylaxis have been reported following administration of Pfizer-BioNTech and Moderna's COVID-19 vaccine or thromboembolic events following AstraZeneca's vaccine (Greinacher et al., 2021).
There is an increasing number of cases of myocarditis and pericarditis following vaccination with mRNA COVID-19. Most cases occur in adolescent boys, usually within a few days after vaccination with COVID-19 and after the second dose (Salah and Mehta, 2021; Minocha et al., 2021). In Israel, 110 cases of myocarditis were identified after administration of two doses of the Pfizer-BioNTech vaccine and 90% of the cases were in males (Vogel and Couzin-Frankel, 2021).
The 62-year-old female patient presented clinical signs of fever and hypotension less than 24 h after administration of the 3rd dose of the mRNA vaccine Moderna COVID-19. The clinical and imaging diagnosis of acute myocarditis with evidence of cardiogenic shock was confirmed. A thorough study of other possible causes or aetiological agents that could justify the clinical picture as a concurrent infectious process was carried out, however, no analytical alterations or clear infectious focus were found. In the differential diagnosis, we studied the possible drug interaction/toxicity with the agent TKi (osimertinib) used daily since the diagnosis of lung cancer in October 2019. As previously mentioned, the dose adjustment was required from November of that year due to digestive, cardiological (pericarditis) and pulmonary toxicity. Since then, adequate tolerance has been monitored by cardiology with preserved LVEF and no other incidents reported.
In the literature, retrospective data showed that osimertinib treatment increases the risk of QT prolongation, heart failure and atrial fibrillation (Anand et al., 2019). Cardiotoxicity, defined as a decrease in LVEF by at least 10% and below 50%, was observed in 5% of cases and the median time to complications was 5.5 months (Mok et al., 2017). QT prolongation has been reported as the most common cardiological adverse effect in 10% of patients receiving osimertinib (Soria et al., 2018). A meta-analysis puts the rate at around 2% (Yi et al., 2019). This represents a major clinical problem, requiring discontinuation of treatment due to the excessive risk of serious, life-threatening cardiac arrhythmias. While atrial or ventricular arrhythmias and evidence of heart failure have been described in association with administration of this anti-EGFR drug, myocarditis is much rarer (Oyakawa et al., 2017). The onset of cardiological symptoms is usually within a few weeks of starting treatment.
However, considering the time lag between vaccine administration and sudden onset of symptoms, and the prolonged time (2 years) on osimertinib treatment with good tolerance after dose titration, makes Modern mRNA vaccine COVID-19 more likely as a probable causative agent or trigger for acute symptoms.
Although cardiological side effects due to osimertinib treatment have been described as listed above, no cases of myocarditis following COVID-19 vaccination have yet been reported in this population. Aetiopathologically, there would be an increased risk for the development of myocarditis in vaccinated patients treated with these tyrosine kinase agents, hence the exceptionality of this case. It would be necessary to establish close monitoring of cardiac function parameters, with measurement of NT-proBNP and troponins pre- and post-vaccination, serial echocardiography and to establish specific recommendations for vaccination (COVID19 and influenza) in this subpopulation that do not currently exist.
Conclusion
This represents an exceptional and controversial case of fulminant myocarditis in probable association with Modern COVID-19 vaccine in a patient diagnosed with metastatic lung adenocarcinoma on osimertinib therapy. COVID-19 disease has systemic involvement at several levels. The recent introduction of vaccines has shown a decrease in morbidity and mortality due to this virus but an increase in yet unknown adverse effects, including cardiological alterations. With respect to the anti-EGFR drugs used in lung cancer, there are known alterations and toxicities related to their administration, hence the need for careful monitoring of cardiac function during treatment with these antineoplastic agents. Future studies will be necessary to evaluate unknown adverse reactions of these vaccines and their possible interaction with other drugs.
Patient consent statement
Consent to publish: The patient in this report provided consent for the anonymous publication of her experiences. Written informed consent for publication of their case was obtained from the patient. All authors of this report are greatly obliged to the Editorial Board for the publication of this report.
CRediT authorship contribution statement
Eduardo Terán Brage: Conceptualization, Methodology, Investigation, Writing – original draft. Jonnathan Roldán Ruíz: Investigation, Writing – original draft. Javier González Martín: Resources, Visualization. Juan Diego Oviedo Rodríguez: Resources, Visualization. Rosario Vidal Tocino: Writing – review & editing, Supervision. Sara Rodríguez Diego: Writing – review & editing, Visualization, Project administration. Pedro Luis Sánchez Hernández: Project administration. Lorena Bellido Hernández: Project administration. Emilio Fonseca Sánchez: Project administration.
Declaration of Competing Interest
None.
References
- Boukhris M., Hillani A., Moroni F., Annabi M.S., Addad F., Ribeiro M.H., et al. Cardiovascular implications of the COVID-19 pandemic: a global perspective. Can. J. Cardiol. 2020;36(7):1068–1080. doi: 10.1016/j.cjca.2020.05.018. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045. doi: 10.1016/j.cell.2020.04.026. May 28e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Bernal J., Andrews N., Gower C., Robertson C., Stowe J., Tessier E., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salah H.M., Mehta J.L. COVID-19 vaccine and myocarditis. Am. J. Cardiol. 2021;157:146–148. doi: 10.1016/j.amjcard.2021.07.009. Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minocha P.K., Better D., Singh R.K., Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J. Pediatr. 2021;238:321–323. doi: 10.1016/j.jpeds.2021.06.035. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria J.C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. Jan 11. [DOI] [PubMed] [Google Scholar]
- Anand K., Ensor J., Trachtenberg B., Bernicker E.H. Osimertinib-induced cardiotoxicity: a retrospective review of the FDA adverse events reporting system (FAERS) J. Am. Coll. Cardiol. CardioOncol. 2019;1:172–178. doi: 10.1016/j.jaccao.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N.R. Viral myocarditis. Curr. Opin. Rheumatol. 2016;28(4):383–389. doi: 10.1097/BOR.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezkalla S.H., Kloner R.A. Viral myocarditis: 1917–2020: from the influenza A to the COVID-19 pandemics. Trends Cardiovasc. Med. 2021;31(3):163–169. doi: 10.1016/j.tcm.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G., Couzin-Frankel J. Israel reports link between rare cases of heart inflammation and COVID-19 vaccination in young men. Science. 2021 https://www.sciencemag.org/news/2021/06/israel-reports-link-between-rare-cases-heart-inflammation-and-covid-19-vaccination [Google Scholar]
- Mok T.S., Wu Y.L., Ahn M.J., Garassino M.C., Kim H.R., Ramalingam S.S., et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674. Feb 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L., Fan J., Qian R., Luo P., Zhang J. Efficacy and safety of osimertinib in treating EGFR-mutated advanced NSCLC: a meta-analysis. Int. J. Cancer. 2019;145(1):284–294. doi: 10.1002/ijc.32097. Jul 1doi: 10.1002/ijc.32097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyakawa T., Nakashima K., Naito T. Cardiac dysfunction caused by osimertinib. J. Thorac. Oncol. 2017;12(10):e159–e160. doi: 10.1016/j.jtho.2017.05.016. Oct. [DOI] [PubMed] [Google Scholar]