Abstract
Efforts to discover antiviral drugs and diagnostic platforms have intensified to an unprecedented level since the outbreak of COVID-19. Nano-sized endosomal vesicles called exosomes have gained considerable attention from researchers due to their role in intracellular communication to regulate the biological activity of target cells through cargo proteins, nucleic acids, and lipids. According to recent studies, exosomes play a vital role in viral diseases including covid-19, with their interaction with the host immune system opening the door to effective antiviral treatments. Utilizing the intrinsic nature of exosomes, it is imperative to elucidate how exosomes exert their effect on the immune system or boost viral infectivity. Exosome biogenesis machinery is hijacked by viruses to initiate replication, spread infection, and evade the immune response. Exosomes, however, also participate in protective mechanisms by triggering the innate immune system. Besides that, exosomes released from the cells can carry a robust amount of information about the diseased state, serving as a potential biomarker for detecting viral diseases. This review describes how exosomes increase virus infectivity, act as immunomodulators, and function as a potential drug delivery carrier and diagnostic biomarker for diseases caused by HIV, Hepatitis, Ebola, and Epstein-Barr viruses. Furthermore, the review analyzes various applications of exosomes within the context of COVID-19, including its management.
Keywords: Exosomes, Viral infections, COVID-19, HIV, Immunity
1. Introduction
The course of infectious diseases resulting from viral or bacterial infection is changing due to human activities such as global travel, agriculture, deforestation, and consumption of exotic wildlife [1]. Many viral epidemics and pandemics are caused by outbreaks and a lack of knowledge about a viral pathogen, transmission patterns, and diagnosis [2]. In addition to self-resolving viral diseases, they can also cause treatable diseases or chronic diseases that can lead to death. Globally, viral infectious diseases contribute to high rates of mortality and morbidity. The last two decades have seen significant epidemic and pandemic outbreaks (Fig. 1 ) such as severe acute respiratory syndrome (SARS) in 2003, H5N1 in 2004, H1N1 in 2009, the Middle East respiratory syndrome (MERS) in 2012, Ebola in 2014, and the devastating Coronavirus disease (COVID-19) in 2019. A case fatality rate or risk (CFR) measures disease severity and represents the proportion of people dying from a disease. For example, acquired immunodeficiency syndrome (AIDS) by Human Immunodeficiency Virus (HIV) exhibits a CFR of 100% and is not curable, while Ebola has a CFR of 50% [3]. Hepatitis B virus (HBV) infects 250–350 million worldwide and causes 500,000–600,000 deaths annually [4]. A vaccine for SARS and MERS has not yet been developed [5], [6]. For Ebola, there is no licensed vaccine yet. However, several investigational Ebola vaccines are under clinical trials such as rVSV-ZEBOV [7] and cAd3-EBO [8]. The devastating effects of COVID-19 have led to an expedited search for affordable vaccines against it [9], [10], [11], [12].
Fig. 1.
Timeline of viral infections witnessed in the last two decades (2000–2020, and present). SARS-CoV: Severe Acute Respiratory Syndrome - Coronavirus; MERS-CoV: Middle East Respiratory Syndrome - Coronavirus, SARS-CoV-2: severe acute respiratory syndrome- Coronavirus-2 or Coronavirus responsible for COVID-19; H1N1: Swine flu virus and H5N1: Avian Influenza Virus.
1.1. Exosomes
Exosomes are extracellular vesicles secreted by eukaryotic cells and composed of proteins, DNA, mRNA, microRNA, long non-coding RNA, and circular RNA which participate in intercellular communication [13]. The recent findings have found that exosomes play a vital role in cell-cell signaling. Further, the presence of exosomes has been identified in several bodily fluids such as plasma, saliva, breast milk, cerebrospinal fluid, and amniotic fluid [14], [15], [16], [17], [18]. The presence of exosomes in the body fluids and the release of exosomes from the immune cells, cancer cells, and brain cells have attracted researchers from across the globe to explore the role of exosomes in diverse areas. The exosome's role in tumor detection and tumor therapy is notably investigated with hints of the modality being translated in the near future [19], [20], [21]. Studies suggesting exosomes derived from adipose-derived stem cells in alleviating oxidative stress and inflammation [22], and promoting ischemic repairment and angiogenesis have been reported [23], [24]. It has been suggested that the pivotal role of exosomes in the manifestation of the disease and applying the same in therapeutics and diagnostics can play a potential role in the prognosis and prevention of viral diseases. This review summarizes the dual role of exosomes in transmitting various viral diseases and their application in the management of the same diseases.
1.2. Exosomes: origin and biogenesis
Exosomes are lipid bilayer phospholipid membrane vesicles that originate from late-stage endosomes. The cell membrane invaginates into the cytoplasm to form primary or early endosomes, which further mature into late endosomes. Endosomes form multivesicular bodies (MVBs) when the limiting membrane folds inwards into intraluminal vesicles (ILVs). It is believed that these MVBs either fuse with lysozymes and get degraded or that they fuse with plasma membranes and release ILVs into the extracellular space as exosomes [25]. In the biogenesis of exosomes, the endosomal sorting complex required for transport (ESCRT) and the cytosolic protein complex play a crucial role in forming ILVs and sorting ubiquitinated proteins. Protein Alix TSG101 (Tumor susceptibility gene 101) and flotillin participate in exosome biogenesis and release, whereas annexins and Rab GTPase proteins aid in membrane transport and fusion (Fig. 2 ) [26]. The primary composition of exosomes is various tetraspanins such as CD9, CD63, CD81. These tetraspanins participate in cell penetration and fusion events and are widely identified as an exosomal marker apart from TSG101 protein [27]. The HSP70 surface protein receptor is essential for transporting exosomes to recipient cells and for identifying exosomes (Fig. 3 ).
Fig. 2.

General structure and composition of exosomes showing the phospholipid bilayer containing the tetraspanins (CD9, CD63, CD81), trans-membrane proteins, integrins, flotillin, numerous cholesterol molecules, and the internal hydrophilic compartment consisting of the genetic materials (RNA and DNA), heat shock proteins (HSP70 and HSP90), various amino acids and cellular metabolites.
Fig. 3.

Origin and biogenesis of exosomes. (1) Biogenesis of exosomes by endocytosis and formation of early sorting endosomes (ESE) by cell membrane invagination into cytoplasm (Cell 1) followed by maturation into the late sorting endosomes (LSE) and by invagination (cargo modification) leads to the generation of multivesicular bodies (MVB). (2) MVB fuses with plasma membranes releasing intraluminal vesicles as exosomes into the extracellular space. (3) Alternatively, the cell membrane budding can generate microvesicles, much larger than the exosomes. The exosomes in the extracellular matrix can be taken up by the neighboring cells by either the (4) Clathrin mediated endocytosis or (5) any of the pathways namely the phagocytosis, micropinocytosis, or by the formation of lipid rafts. Once inside the Cell 2, (6) the exosomes can assemble inside the ESE and adopt any of the three pathways (7) degradation following the fusion with the lysosomes, (8) degradation within the cytoplasm and the uptake by the endoplasmic reticulum (ER) or (9) form MVB which can further (10) release exosomes into the extracellular matrix. However, the released exosomes from Cell 2, may contain the components of the Cell 1, in addition to its own cellular components.
Exosomes change the phenotype of cells and alter the innate immunity of the host cell. When released into extracellular space, exosomes are influenced both by their size and surface by recipient cells [28]. The surface-expressed proteins determine the recognition and uptake by the acceptor cells. In most studies, exosomes are taken up by specific cells, whereas few studies report exosomes' uptake by non-specific ligand-receptor combinations. There are several uptake mechanisms reported for exosomes, including micropinocytosis, clathrin-dependent endocytosis, clathrin-independent endocytosis, and caveolin mediated uptake [29]. Based on their origin, exosomes carry diverse cargo that plays a vital role in the intercellular communication and transport of various biomolecules. Exosome composition reflects the origin and function of the exosome, as well as the pathological condition of the parent cell and its surroundings.
Exosomes play a crucial role in innate and acquired immunity (Fig. 4 ). In response to Tumor necrosis factor (TNFs), HLA-B- associated transcript 3 (BAT3), and Interleukin 15Ra (IL-15Ra) on the surface of the exosomes, mature dendritic cells (mDC) activated macrophages, further activate NK cells, enhancing their cytotoxic activity.
Fig. 4.
Role of dendritic cells derived exosomes in innate and acquired immunity. NK- natural killer cells, Treg -regulatory T cells, iDC-immature dendritic cells, MHCs- Major histocompatibility complex, BAT3- HLA-B-associated transcript 3, IL-Interleukin, TNF- Tumor necrosis factor.
Exosomes mediated antigen presentation to T cells can induce an immune and proinflammatory response. The mDC derived exosomes express the T cell co-stimulatory molecules and MHC class I and II, which can be an essential mechanism for antigen presentation. The proposed mechanisms for the antigen presentation by exosomes are cross-dressing pattern, cross-presentation, and direct exosome-induced T-cell activation [30], [31], [32]. In a cross-dressing pattern, DCs captures exosomes and directly present them to CD4+ or CD8+ T cells. The exosomes shuttle antigenic peptide-MHC complex to DCs populations. Cross presentation pattern involves DCs mediated presentation of antigenic protein/ peptides containing exosomes to the endogenous MHC class I and II molecules, followed by activating antigen-specific T-cells. In direct antigen presentation, DCs derived exosomes can directly activate CD4+ or CD8+ T cells, attributed to the expression of the T cell co-stimulatory molecules along with MHC class I and II on the exosomes [33].
Furthermore, exosomes are isolated from different plant sources, including juice, seeds, and leaves. Citrus lemon, ginger, grapefruit, Arabidopsis thaliana, coconut water, and carrots have all been found to contain exosomes [34], [35]. Plant-derived exosomes are involved in plant cell-to-cell communication and regulate the innate immunity of plants, like exosomes' role observed in the human body. Various medicinal plants possess antiviral properties [36], [37], [38], [39]. Sundaram et.al reported anti-bacterial activity of ginger-derived exosomes in chronic periodontitis against Porphyromonas gingivalis (P. gingivalis), an oral pathogenic bacteria which causes chronic periodontitis [37]. The ginger-derived exosomes like nanoparticles (GLEN) were selectively taken up by P. gingivalis pathogen and inhibited the growth of the pathogen. The GLENs significantly reduced the FimA (important component for attachment to host surfaces) expression and further inhibited the attachment of P. gingivalis to oral epithelial cells. Also, GLEN impacted the immune response by a decrease in the expression of bone resorptive cytokines (TNF-α, IL-6, and IL-8) and decreased the recruitment of macrophages and leukocytes.
1.3. Isolation of exosomes
Many types of isolation methods have been attempted for obtaining individual exosomes (Fig. 5 ), including ultracentrifugation, size-based techniques, such as ultrafiltration and size exclusion, immunoaffinity-based techniques, microfluidics-based techniques, and polymer precipitation methods [40]. In ultracentrifugation, exosomes are separated based on their density and size using centrifugal force. Ultracentrifugation is the standard technique used for exosome isolation with high throughput; however, it is also associated with several drawbacks, such as time-consuming results and machine-dependent outcomes. The unstable recovery rate and damage of exosomes upon repeated centrifugation are significant concerns.
Fig. 5.

Various methods available for the isolation of exosomes from samples of cell/ tissue culture fluids, plant extracts, and body fluids (plasma, urine, tears, semen). The methods include ultracentrifugation, density gradient centrifugation, ultrafiltration using various pore-sized membranes (MB), size exclusion chromatography, coprecipitation using precipitating agents (PAg), immunoaffinity using specific antibodies (Ab), and microfluidics.
The immunoisolation technique is based on chromatography, in which specific antibodies are immobilized on the stationary phase to bind with the target protein present on exosomes covalently. The immunoisolation technique can be used for high purity isolation for quantitative and qualitative studies of exosomes. The non-specific adsorption, high cost, low yield, and harsh operating conditions are several drawbacks of the immunoisolation technique. The polymer precipitation technique employs hydrophilic polymers such as PEG and decreases hydrophilicity of exosomes leading to precipitation of the exosomes followed by low-speed centrifugal force for isolation. The PEG-based kits available for rapid isolation and purification are expensive, and co-precipitation of contaminants such as lipoproteins is reported. Microfluidics is an emerging isolation technique to separate the exosomes based on biochemical composition, physical properties, and immunoaffinity [41]. Micro fluidics-based techniques can significantly reduce separation time, reagents, and volume however, low yield, low reproducibility, and high cost are hurdles for the technique. Above all, the ultracentrifuge technique is the gold standard for the isolation of exosomes.
2. Viral diseases and exosomes
The high expression of target cell-specific integrin and tetraspanin adhesion molecules also pave the way for targeting drug delivery [42]. Viruses transmit RNA or DNA in exosomes that activate the innate immune system. However, exosomes can also disrupt normal cellular physiology and cause disease. Hence the balance between the exosomes mediated infectivity and innate immune response decides the course of the disease and pathophysiological conditions. Exosome-based drug delivery can provide target-specific delivery as well as aid in boosting the immunogenic outcome. The potential proteins or viral RNA/ DNA present in exosomes and involved in promoting a diseased state can be targeted to impede further transmission. It is possible to identify and develop antiviral exosomes as a therapeutic for viral diseases [43]. Exosome charges carry specific RNAs corresponding to the pathophysiological condition of their source cells [44]. The presence of viral RNA, DNA, or virions is also detected [39], providing prognostic or diagnostic information. The information can be regularly monitored to assess the disease conditions in patients. By understanding how exosomes promote viral infectivity, clinical applications of exosomes can be developed. Many viral diseases have been linked to exosomes, such as AIDS, hepatitis, Ebola, COVID-19, and Epstein-Barr virus.
2.1. Coronavirus and exosomes
Coronavirus is (+) single-stranded RNA virus of family Coronaviridae and subfamily orthocoronavirinae responsible for causing infectious diseases such as Severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and COVID-19 [45]. The global fatality rate of SARS and MERS is 9.6% [46] and 37.1% [47], respectively.COVID-19 caused by SARS-CoV-2 affected more than 200 countries and is responsible for causing ~5,174,646 deaths globally till November 2021 [48]. SARS-Cov-2 S-protein is highly conserved among all coronaviruses, which aids in receptor recognition, viral attachment, and entry into host cells [49]. S protein facilitates the binding of the enveloped virus with angiotensin converting enzyme 2 (ACE2) expressed on the lower respiratory tract cells of the host [50]. People infected with SARS-CoV-2 disease can be symptomatic or asymptomatic. Symptoms can vary from mild to moderate respiratory infections. However, patients aged above 65 years as well as those with co-morbid conditions are at high risk of severe symptoms requiring mechanical ventilation and intensive care. Acute respiratory distress syndrome (ARDS), a joint immunopathological event, is the primary cause of coronavirus-related disease fatality [51].
2.1.1. Role in the transmission of Coronavirus infections
CD9 molecules are present in the exosomes and play a crucial role in the exosome biogenesis and cargo loading into exosomes by protein-protein interaction network in MVBs membrane [52]. Exosomes released from the virus-infected cells transport CD9 molecules which further facilitate the virus entry into the recipient cells. A study performed by Earnest et al., suggested that tetraspanin CD9 aids in MERS-Coronavirus entry and infection in the mouse lungs [53]. “TMPRSS (transmembrane serine protease 2)”, an exosomal protein is recently reported to facilitate the SARS-Cov entry in host cells via ACE2 receptor by cleavage of spike protein [54].
Klaud et al., reported that ERGIC-53, an ER-Golgi intermediate compartment marker and a cargo receptor required for glycoprotein transport, is associated with coronavirus entry in the host cell. The active secretion of ERGIC-53 from cells in viral particles and exosomes was observed, which further aided in viral infectivity either by acting as a co-receptor required for virus attachment to the host cell or by recruiting cellular proteins essential for virus assembly [55]. The exosomes released from SARS-Cov-2 infected cells help in transmitting the disease by utilizing the exosome's intercellular communication mechanism.
The secretion of ERGIC-53 in the exosome can increase the viral infection by exploiting the inherent transporting nature of exosomes. Gunasekaran et al., reported the active role of exosomes in chronic lung allograft rejection in patients suffering from respiratory viral infections from 17 viruses, including coronavirus. The coronavirus antigen and increased lung SAgs were detected in isolated exosomes from serum samples of patients. Further, immunizations of mice with exosomes, as mentioned above, amplified humoral and cellular immune responses towards lung SAgs and alloantigen, which increases the risk of developing chronic lung allograft dysfunction [56]. The above study suggested the potential of exosomes in the transfer of viral antigens of coronavirus in the body and aiding in augmenting infection. A recent study by Wang et al., suggested that exosomes can transfer ACE2 to the recipient cells, leading to internalization and susceptibility to the virus docking [57].
Barberis et al., studied the proteomic analysis of plasma-derived from COVID-19 patients and health control. The proteomic characterization found signature proteomic features in plasma derived from the COVID-19 patients and proteins related to the coagulation process, transport activity, complement activity, protease inhibitor, and defense/ immunity protein activity. The study suggested that SARS-CoV-2 uses the endocytosis route to spread the infection exosomes carrying viral materials [51]. Kwon et al., observed overexpressed SARS-Cov-2 genes in A549 lung epithelial cells, and the isolation of the EVs suggested the presence of viral RNA within them. Further, to study the uptake of EVs by cardiomyocytes, EVs were incubated with human induced pluripotent stem cells-derived cardiomyocytes (hiPSC-CMs) and hiPSC derived endothelial cells. The presence of EVs and viral RNA fragments were detected in both cells. Further, EVs mediated the increase of inflammation-related genes in the hiPSC-CMs upon uptake [58].
2.1.2. Role in therapy and diagnosis of Coronavirus infections
Researchers have conducted extensive research on exosomes, suggesting they play a dual role in infection transmission and infection prevention. Huang et al., reported the role of Interferon-Induced Transmembrane Proteins (IFITM1, IFITM2, and IFITM3) in impeding the entry and replication of SARS Coronavirus. Infectious agents such as influenza A, Marburg virus, and Epstein-Barr virus EBV are inhibited by IFITM, which are broad-spectrum antiviral proteins. The proposed possible explanation was a change in properties of late endosomes/ lysosomes or involvement of endocytic pathways. However, the exact mechanism of inhibition was not elucidated [59].
Feeley and his group showed that IFITM3 prevented influenza A virus infection by preventing the entry of viral components into the cytosol after endocytosis, thus preventing virus fusion [60], which may be involved in the inhibition of SARS coronavirus. Novk et al., proposed a possible pathway that leads to the IFITM3 mediated antiviral role of exosomes against the SARS Coronavirus highlighting the crucial role of exosomes. The authors observed that exosomes released from SARS-infected cells contain IFITM3 and ACE2. Alternatively, overexpressing IFITM3 or secreted Ace2 receptors end up neutralizing SARS coronavirus [61]. Nevertheless, further studies are necessary to confirm the presence of the ACE2 receptor on exosomes to support the proposed mechanism (Fig. 6 ). The isolation of exosomes exhibiting antiviral activity against coronavirus can be carried out to augment its activity or can be engineered as a potential therapy against coronavirus treatment.
Fig. 6.
Entry of HIV-1 virions in host cell and role of exosomes derived from HIV infected cells in transmission of the infection. (1–2) Entry of HIV-1 virus via viral envelop and binding to viral co-receptor (CXCR4 OR CCR5) in CD4 cells. (3–4) Co receptor mediated viral fusion and entry of viral content such as single stranded viral genomic RNA (gRNA), reverse transcriptase (RT), and integrase (IN) in cytoplasm. (5–8) Reverse transcription of gRNA into double stranded proviral copy of DNA (HIV-1 DNA) by HIV-1 reverse transcriptase followed by incorporation of proviral HIV-1 DNA copy into human genomic DNA (gDNA) by viral IN enzyme. (9–11) Proviral DNA transcribed by host cellular machinery into nascent viral RNA followed by translation into HIV-1 proteins such as Nef by host cell machinery followed by Assembly of viral proteins with nascent RNA including HIV TAR RNA sequences (vmiRTAR)into budding progeny virions. 12–13) Formation of immature viral progeny initiated by viral protease via cleavage from budding progeny followed by maturation of viral progeny within the extracellular milieu capable of propagating productive HIV-1 infection in neighboring cells. (14–16) Formation of endosomes via inward budding of the cellular plasm membrane followed by generation of cellular cytosol derived proteins along with viral proteins loaded exosomes which are released into the extracellular milieu by back fusion with plasma membrane. The exosomes containing viral proteins (HIV Nef and vmiRTAR) are capable of infection transmission via (17) transferring viral binding and entry receptors to HIV-1 susceptible cells and increasing the expression of viral binding sites on the target cell, (18) Nef-mediated internalization and degradation of CD4 molecules and Nef-mediated reduction of CD4 expression on the surface of exosomes and (19) suppression of Bim and Cdk9 expression and apoptosis by viral miRNA generated from vmiRTAR. Exosomes released from uninfected cells may be mediated by anti-HIV host factor Apobec3G enwrapped in exosomes (20) and exosomal CD4 binding to HIV-1 Env (21). (Reproduced from [50] under Creative Commons Attribution License).
Kate et al., developed S protein-containing exosome-based vaccines for the immunization against the SARS coronavirus. The highest antibodies were observed when S protein contained exosomes boosted with adenoviral vector vaccine compared to individual vaccines. The vaccine generated high levels of specific neutralizing antibodies against SARS and exhibited SARS-S-mediated entry [62]. Teng et al. observed that the exosomes released from SARS-CoV-2 infected lung epithelial cells are taken up by macrophages and induce apoptosis by activation of nuclear factor κB and inflammatory cytokines. Similarly, ginger exosome-like nanoparticles (GLEN) showed a therapeutic effect against SARS-CoV-2 [63].
China's Ruijin hospital is conducting a single-arm, open-label, combined interventional clinical trial testing the safety and efficacy of exosomes derived from allogeneic mesenchymal stem cells (MSCs-Exo) against COVID-19. Experimental data shows a beneficial effect on lung inflammation and other lung injury-related pathological issues in this study [64]. Hubei Shiyan Taihe hospital in China is conducting another clinical trial to determine if human umbilical cord mesenchymal stem cells (HUMSCs) can treat patients with lung injury following infection with COVID-19 [65]. Gunasekaran et al., reported that exosomes isolated from SARS infected cells augmented the humoral and cellular immune responses upon immunization of mice with exosomes mentioned above [56]. The discovery could be used as a key to developing vaccines against coronavirus to exploit exosome-induced immunity in virus-infected cells. Kumar et al., investigated the role of exosomes as antiviral protease inhibitor carriers, showing that exosomes bypass liver metabolism and deliver drugs to the targeted tissues [66]. Tsai et.al reported preclinical SARS-Cov-2 mRNA-loaded exosomes to achieve immunogenicity in mice. The study suggested that vaccination of mRNA-loaded exosomes led to dose-dependent development of anti-spike and anti-Nucleocapsid antibody response as well as antigen-specific CD4+ and CD8+ T cell response without any vaccine-related adverse effects [67]. Further, mRNA-loaded exosomes were more safer compared to the RNA-loaded lipid nanoparticles which led to severe cellular toxicity. The exosomes offer the advantage of specific tissue targeting which can be exploited for drug delivery. Fu et al. investigated the pseudotyping-based approach to load the EV membranes with receptor-binding domain (RBD) of the spike protein, a key domain in SAR-Cov-2 attachment, fusion, and cellular entry. The study suggested that the RBD tagged EVS can specifically recognize ACE2 receptors on the target cell required for the uptake. Further, upon cellular uptake by ACE2 receptors, siRNA encapsulated in RBD tagged EVs delivered into lung tissues inhibited pseudovirus SARS-Cov-2 infection in vivo. RBD-tagged EVs are a promising vehicle for delivering antiviral agents for SARS-Cov-2 treatment. However, a study employing authentic SARS-Cov-2 is required to confirm the findings [68].
Anand et al., suggested the convalescent plasma-derived exosomes (CPexo) for the covid-19 therapy. The hypothesis suggested the CPexo can offer potential therapeutic efficacy by acting as an immunotherapeutic, drug carrier, and diagnostic biomarker based on the literature suggesting effective and precise targeting of the pathogen by exosomes in preclinical studies [69]. Also, convalescent plasma is reported as a therapeutic approach for the Covid-19 [70], and human membrane or fluids has been FDA approved previously to encounter inflammation and tissue injury [69]. Mesenchymal stem cells (MSCs) derived exosomes have emerged as potential therapeutic candidates attributed to the features such as modulation of the immune response, reduce inflammation, ability to suppress viral infection attributed to the presence of specific cytokines. Several clinical trials associated with MSCs derived exosomes for the treatment of Covid-19 are ongoing in different parts of the world (Table 1 ). The completed and ongoing clinical trials of MSCs derived exosomes highlight the effectiveness in the treatment of SARS-Cov-19. However, detailed safety and efficacy need to be asses and long-term outcomes [71], [72].
Table 1.
Ongoing clinical trial employing Mesenchymal stem cells derived exosomes for the treatment of COVID-19.
| Place | Clinical trial number | Study title | Study type | Invention/treatment | Primary outcome measures |
|---|---|---|---|---|---|
| Ruijin hospital Shanghai Jiao Tong University school of medicine, Shanghai, China-200,025 | NCT 04276987 |
A Pilot Clinical Study on Aerosol Inhalation of the Exosomes Derived from Allogenic Adipose Mesenchymal Stem Cells in the Treatment of Severe Patients with Novel Coronavirus Pneumonia | Phase 1 30 participants |
Conventional treatment and 5 times aerosol inhalation of MSCs derived exosomes (2× 108 nano vesicles/3 ml from Day 1 to Day 5). |
1. Adverse reaction and severe adverse reaction up to 28 days after first treatment 2. Efficacy evaluation and clinical improvement within 28 days |
| TC Erciyes University, Turkey | NCT04389385 | Aerosol Inhalation of the Exosomes Derived from Allogenic COVID-19 T Cell in the Treatment of Early-Stage Novel Coronavirus Pneumonia | Phase 1 60 participants |
COVID-19 Specific T Cell-derived exosomes (CSTC-Exo). Inhaler CSTC-Exo treatment will be applied daily x 5 times (2.0 × 108 nano vesicle / 3 ml; on day 1 to day 5). |
1. Adverse reaction and severe adverse reaction up to 28 days after first treatment 2. Efficacy evaluation and clinical improvement within 28 days 3.Rate if recovery without mechanical ventilator |
| Organicell Regenerative Medicine, USA | NCT04384445 | A Phase I/II Randomized, Double Blinded, Placebo Trial to Evaluate the Safety and Potential Efficacy of Intravenous Infusion of Zofin for the Treatment of Moderate to SARS Related to COVID-19 Infection vs Placebo | Phase I/II Randomized, Double Blinded, Placebo Trial 20 Participants |
Zofin administered iv with 1 ml, containing 2–5 × 10^11 particles/mL in addition to the Standard Care. Placebo (saline) administered iv with 1 ml in addition to the Standard Care |
1.Incidence of any infusion related adverse effect within 60 days of treatment 2. Incidence of severe adverse effects within 60 days of treatment |
| Samara Regional Clinical Hospital V.D. Seredavin, Russia | NCT04491240 | The Protocol of Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Two-Sided Pneumonia | Phase I/II Randomized 30 participants |
All eligible study subjects randomized, double-blinded, to either the two treatment groups or placebo group. | 1.Safety assessment of non-serious and serious adverse effects till 30 days after clinic discharge 2. Safety assessment of non-serious and serious adverse effects during inhalation procedure |
| Direct Biologics, LLC, USA | NCT04493242 | Extracellular vesicle infusion treatment for Covid-19 associated ARDS (EXIT-COVID-19) | Phase II double-blinded, placebo-controlled randomized controlled trial 120 participants |
IV administration of bone marrow mesenchymal stem cell derived extracellular vesicles and normal saline | Seven-day efficacy assessment by measuring improvement in partial pressure of arterial oxygen to fraction of inspired oxygen ration |
| Hubei Shaiyan Taihe hospital, China | ChiCTR2000030484 | HUMSCs and Exosomes Treating Patients with Lung Injury following Novel Coronavirus Pneumonia (COVID-19) | 90 Participants | HUMSCs iv infusion and control group placebo (stem cell solvent) | PaO2 and FiO2 respiratory rate (without oxygen) The number and range of lesions indicated by CT and X-ray of lung Time for cough to become mild of absent |
| Wuxi fifth people's hospital, China | ChiCTR2000030261 | A study for the key technology of mesenchymal stem cells exosomes atomization in the treatment of novel coronavirus pneumonia (COVID-19) | 26 Participants | Aerosol inhalation of exosomes Control group: Blank |
Lung CT and Leukocytes and lymphocytes in blood routine |
Alteration of the protein content of exosomes in diseases condition is well reported in the literature which can be exploited as the diagnostic marker of the disease condition of the patient. Barberies et.al investigated the role of exosomes as a diagnostic marker in the SARS-CoV-19 infected patients. The study suggested that critical patients showed significantly high levels of C- reactive protein (CRP), alpha-1-acid glycoproteins (A1AG1and A1AG2), CXCL7, and lower levels of CCD34, C4BPA, and GELS compared to non-critical COVID-19 patients. It was observed that CRP protein was able to differentiate between positive patients and negative patients. Also, it discriminated between critical patients and non-critical patients [73]. Similarly, Balbi et al., derived the EVs from the participants who underwent a molecular test for SARS-CoV-2. The profiling of the exosome protein suggested the CD142, a platelet tissue factor (activates extrinsic pathway of blood coagulation cascade) as the highest discriminating surface protein. The CD142 showed increased activity in COVID-19 positive patients and also correlated with TNF-α serum levels [74]. A similar observation was made by Rosell et al., suggesting the increased platelet tissue factor associated with the severity and mortality of the COVID-19 [75]. This finding may pave the road for exploiting CD142 as a specific surface antigen for the prognosis of the disease. A study carried out by Grifoni et al., also suggested increased cytokine levels and presences of TNF, IL-6, and IL-1β [76]. The circulating exosomes can be a potential biomarker for the prognosis of COVID-19 infection.
2.2. Human Immunodeficiency Virus (HIV) and exosomes
Acquired Immunodeficiency Syndrome (AIDS) is a chronic immune system failure caused by the retrovirus human immunodeficiency virus (HIV). World Health Organization (WHO) data shows that the pandemic has infected 37.9 million people and caused more than 1 million globally. The only promising therapy for infection is highly active antiretroviral therapy (HAART), only to increase the life expectancy, and there is no cure. However, the recent clinical trials of antibodies, namely 3BNC117, a broadly neutralizing antibody, and monoclonal antibody 10–1074 developed for CD4 host cell and HIV viral envelope, respectively, have shown promising results by suppressing viremia in HIV-1 infected patients [77], [78].
2.2.1. Role in the transmission of HIV
The role of exosomes on the immune system and HIV infection and understanding the influence of HIV infection on exosome release has gained interest in the research community (Fig. 7 ). Gould et al., proposed a hypothesis suggesting that HIV hijacks the exosome machinery in T-cells and macrophages to increase the infectivity in other cells in a receptor-independent manner [79]. Researchers report a variety of studies that support this hypothesis [80], [81]. Immunoprecipitation studies revealed that the macrophages infected with HIV have a similar complement of cellular membrane proteins to viruses recovered from the extracellular medium, suggesting that the virus is initially released from the macrophages and bud into endosomal organelles and then released by fusion with the plasma membrane. These key findings suggested exploiting the preexisting exosomal pathway for retroviral budding and further transmitting the infection [80], [81]. However, studies contradicting the above results suggested plasma membrane as the primary site for the virus budding by sequestering intracellular plasma membrane domain and recruiting CD63 tetraspanin during virus assembly [82], [83], [84].
Fig. 7.
Exosomes secreted by HIV infected cells and acting as a carrier of HIV-1 protein Nef to bystander macrophages. (Reproduced from [57] available under Creative Commons Attribution License).
The function of TSG101, a subunit of endosomal sorting complex required for transport I (ESCRT-I), is linked to the formation of endosomes and MVBs [85]. Another study implicated that HIV-1 utilizes a similar subset of cellular component that of cytokinesis, i.e., TSG101 and Alix, an ESCRT-associated protein during membrane budding and implicating the direct budding of microvesicles from plasma membrane releasing budding viruses by exploiting TSG101 and Alix [86], [87]. Kadir et al., reported a study supporting the hypothesis of exploitation of exosome pathways by HIV. They reported exosomes/ MVBs mediated the transfer of virus and viral constituents from infected cells to uninfected cells, thus aiding in expediting HIV infection and dissemination. The incubation of an equal ratio of HIV-1 infected monocyte-derived macrophages (MDM) and uninfected MDM labeled with lipophilic dyes DiO and DiD, respectively were carried out. Upon incubation, the increased membrane exchange among infected and uninfected MDM was observed followed by the release of vesicles from infected and fusion with uninfected MDM within 4-6 min observed via video footage [88].
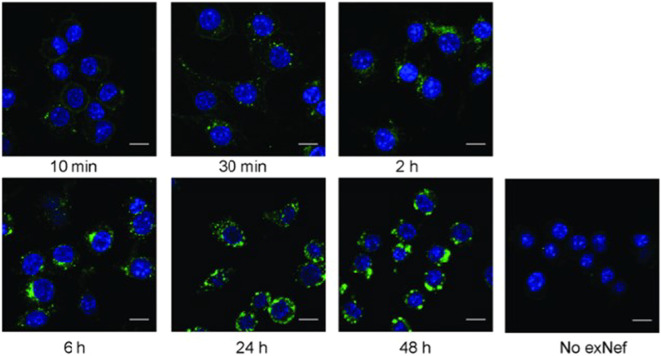
Negative regulatory factor (Nef) plays a crucial role in the infection and replication of HIV by exploiting host cell machinery and downregulation of important crucial antiviral immune responsive molecules such as MHC-I, MHC-II, CD4, and CD28 [89]. It is reported in various studies that Nef, a protein encoded by lentivirus, facilitates its release via exosomes by accelerating exosome release [90]. Mukkhamedova et al., observed that the exosomes isolated from the Nef expressing cells contained Nef. Further, incubation of Nef containing exosomes and macrophages led to a linear increase in exosomes uptake by macrophages. It was found that exosomes deliver Nef to the macrophages, which alters cellular functions and causes inflammation associated with HIV infection. Continuously incubating HIV-infected exosomes colored with a fluorescent dye (PKH67) inside RAW264.7 macrophages resulted in linear uptake of the exosomes inside the cells (Fig. 8 ). The study suggested rapid and quantitative uptake of exosomes by macrophages delivering NeF to the intracellular compartments [91]. This also results in HIV-associated co-morbidities. Moreover, its role in the augmentation of the membrane trafficking process and apoptosis of CD4+ T cells leads to adverse effects on the immune system [92]. Researchers have also reported that the exosomes released from infected cells can activate latent viral reservoirs and potentially suppress innate immunity [93], [94].
Fig. 8.
Dual role of exosomes in limiting and promoting the viral infections.
2.2.2. Role in therapeutics and diagnosis of HIV infections
The exosomes are reported to play a diverse role in the transmission and a preventive role against viral infection (Fig. 9 ). The CD8+ T lymphocytes-derived exosomes have shown the antiviral property by suppressing the HIV replication in vitro initiated by interferon-mediated signaling pathway [95], which can be further engineered for effective therapy against HIV. The RNA sequence in exosomes derived from HIV-infected cells has been isolated. Studies show the influential role of various miRNAs such as miR-28, miR-29a, miR-149, miR-150, miR-198, miR-223, miR-324, miR-378, and miR-382 protective role against HIV infection [96]. Narayanan and the group demonstrated the role of TAR RNA in exosomes derived from HIV-infected cells in inhibiting apoptosis by downregulating expressions of the Bim and Cdk9 pro-apoptotic protein [97]. The role of exosomes derived from various body fluids have protective action against HIV infection, such as breast milk-derived exosomes, which was seen to inhibit HIV-1 infection of dendritic cells [98] and semen derived exosomes inhibited RNA expression by inhibiting binding and recruitment of transcription factors such as NF-κB and Sp1 [99]. By identifying and purifying antiviral exosomes, the ability of intrinsic exosomes to interfere with HIV infection can be utilized to develop exosome-based anti-HIV therapies [100].
Fig. 9.

Possible protective mechanisms of exosomes in the treatment and prevention of SARS-CoV-2 (COVID-19) infections. In the absence of any limiting factors, the SARS-CoV-2 enters the lung cells via the coupling with Angiotensin Converting Enzyme (ACE2) receptors. Exosomes (1) can competitively bind to the ACE2 receptors on the cells thereby preventing the uptake of the SARS-CoV-2. Surface modification of the exosomes with anti-ACE2 antibodies (2) can lead to the binding of SARS-CoV-2 proteins to the exosomes thus preventing their interactions at the cell surface receptors. On the other hand, loading of the exosomes with anti-viral drugs can kill the SARS-CoV-2 virus outside the cell matrix (3) or after invasion into the lung cells (4).
Targeting exosome-associated proteins in HIV infection, such as MHC-II, CCR5, CXCR4, TSG101, miRNA, mRNA, and ESCRT, therapeutic applications against HIV can be developed. Another approach can exploit exosomes' natural occurrence and inherent role in transporting mRNA and miRNA from one cell to another to deliver RNA-based therapeutics. A novel RNA delivery vehicle, such as exosomes, can address many shortcomings of conventional RNA delivery, including stability, high clearance, poor uptake, high phagocytosis and cytotoxicity rates, and biocompatibility issues [101]. Lattanzi et al., developed Nef mutant protein (Nefm) loaded exosomes as a vehicle of antigen cross-presentation against HIV-1. Nefm, a biologically inactive HIV-1 NeF, was loaded in the exosomes. It was observed that Nefm- exosomes enter the target cell less efficiently than virus-like particles (VLPs) but exhibit similar cross-presentation levels.
Further in vivo studies can open a potential road towards developing Nefm exosome-based vaccines against HIV-1 [102]. The therapeutic approach of loading anti-HIV RNA in exosomes or anti-HIV in anti-HIV exosomes can be a potential therapeutic modality. The addition of a specific targeting ligand could significantly improve the efficacy and targeting of therapy. In certain disease conditions, the levels of protein and EVs in body fluids increase. This phenomenon has been harvested as a noninvasive technique identifying the specific proteins acting as a biomarker of the disease. The role of exosomes as a biomarker in the prognosis and diagnosis of the disease can be potentially harvested in favor of the patient's treatment. It may be possible to utilize molecules in exosomes as a biomarker in the prognosis or diagnosis of HIV-1. Several HIV-1 related proteins were detected in the urine of an HIV-1 patient, including Gag, Tat, Vpu, Vpr, and Nef. Apart from this, neuron-derived exosomes (NDE) are a potential biomarker of cognitive impairment by HIV. HMGB1, NF-L, and Aβ proteins can be a potential biomarker in the prognosis of HIV-1 [101].
2.3. Hepatitis virus and exosomes
The hepatitis viruses (A, B, C, and E) are unrelated viruses that cause liver-related conditions (Fig. 10 ) such as acute and chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma [103]. HAV and HCA are RNA viruses of the family Picornaviridae and Flaviviridae, respectively. HBV is a DNA virus of the family Hepadnaviridae. In 2018, Shearer et al., reported the global prevalence of HBV to be 3.9%. [104]. Globally 142 million people suffer from chronic hepatitis C infection, and 399,000 people die from cirrhosis and hepatocellular carcinoma (HCC)[105].
Fig. 10.
Role of Exosomes in HBV progression and NK cell dysfunction. Exosomes released form the HBV infected cells hepatocytes aid in transfer of viral components to naïve hepatocytes and NK cells. The transfer of exosomes mediated HBV viral component in NK cells alters the cytolytic activity, cytokine production and cell proliferation by RIG-I expression and inactivating the NF-κB and p38 pathways. (Reproduced from Yang et al.,[91] available under Creative Commons Attribution License).
2.3.1. Role of exosomes in the transmission of hepatitis virus
The exploitation of exosomes by HBV during infection to increase infectivity and escape from the host's innate immune response by downregulating IL-12 is reported by Kouwaki et al., The HBV RNA, viral DNA, core protein, an envelope protein was detected in exosomes derived from infected hepatocytes to infect the hepatocytes [106]. Moreover, HBV increased the expression of immunosuppressive miRs such as miR-21 and miR-29a in exosomes attributed for the downregulation of IL-12 in macrophages, responsible for the activation of NK cells [107]. Yang et al., reported a viral component in exosomes isolated from chronic hepatitis B patients and the infective nature of isolated exosomes to naïve hepatocytes. The crucial finding was the role of exosomes in transferring HBV viral particles into NK cells followed by NK-cell dysfunction by downregulating activating NKp44 receptors and upregulating NKG2A, an inhibiting receptor (Fig. 10) [108]. Kapoor et al., observed that Hbx modulates the biogenesis of exosomes and exploits its intrinsic intracellular communication property to transfer the HBx viral load to further support the viral spread and pathogenesis [109]. A proposed mechanism of exosome-HBV infection and the disruption of the NK cell is presented in Fig. 11 .
Fig. 11.

Role of exosomes in transmission and infection by Hepatitis viruses. Hepatitis A, B, C and E viruses (HAV, HBV, HCV and HEV) enter hepatocytes (1) followed by the release of various viral components (2) with a few undergoing transcriptions in the nucleus of the cell. Once released, these components are moved into the extracellular matrix (3) being encapsulated inside the exosomes. These exosomes further infect neighboring hepatocytes (4), leading to the spread of infection, while they can evade the immune mechanism and travel to the liver aiding the progression of the Hepatitis infection (5). Alternatively, these exosomes can be ingested by the immune cells (ICs) such as the NK-cells, mast cells, and T-cells releasing another set of protective exosomes (6) which contain antiviral components eliciting host immune response against the invading viruses (7).
Exosomes secreted from HBV infected liver cells play a crucial role in downregulating tumor cell apoptosis by upregulating exosomal miR-21 and aiding in chemoresistance and pathogenesis of hepatic carcinoma. HBV-mediated exosome role in chemoresistance in hepatic carcinoma is mediated by upregulation of lysosome-associated membrane protein (Lamp2a), an essential protein of chaperone-mediated autophagy (CMA)[110]. The exosomes might also act as a reservoir of HBV DNA with the ability to reinfect the host with suppressed immunity in recovered patients [111]. These studies support the Trojan exosome hypothesis suggesting the exploitation of exosomes by the virus to augment the transmission and replication process further and escape the host's innate immune response.
Like HBV transmission, the role of exosomes in spreading the infection of HAV and HCV is well reported. The exosomes expressed from hepatitis virus-infected cells play a dual role, aiding in spreading viral infection by infecting naïve hepatocytes and protective role by activating innate immune signaling pathways. Various studies suggest that HCV can exploit exosomes by transferring HCV viral RNA in exosomes, impairing the dendritic cell and infecting the naïve hepatocytes [106], [112]. Whereas in HAV, not viral RNA but virions (eHCA) expressed in the exosomes play a vital role in spreading the infection to naïve hepatocytes [93]. The role of exosomes in neutralizing the anti-HCV antibodies to evade the innate immune response is also reported [113]. Gorji-bahari et al., suggested the role of the RAB5A gene in the regulation of HCC metastasis via regulating the pro-viral content of exosomes. The RAB5A inhibition can affect the HCC cell proliferation rate and migration capability [114].
2.3.2. Role in therapy and diagnosis of hepatitis
Apart from assisting in spreading the infection, exosomes in hepatitis infection also play a protective role by activating innate immunity signaling pathways. The activation of pDC (plasmacytoid dendritic cells), which secrets type I interferon against the viral infection, is mediated via the interaction of exosomes containing viral RNA/virions with pDCs during HAV and HCV viral infection [115]. The macrophages-derived exosomes deliver IFN-α expressed anti-HBV molecules via similar machinery pathways utilized by the virus during the invasion, such as micropinocytosis and clathrin-dependent pathways [116]. Another study reported pro-inflammatory substance IL-6 from monocytes mediated via exosomes released from HBV infected hepatocytes [117].
The detailed elucidation of the mechanistic pathway of antiviral exosomes can help develop the exosomal-based vaccine to boost the immune response towards the hepatitis viral infection. The immune-stimulatory role of the viral RNA and virions carrying exosomes can be taken advantage of in outweighing the role of spreading infection. The intrinsic nature of exosomes can be exploited for engineering exosomes as a delivery cargo of therapeutic molecules. Considering the active role of exosomes released from infected hepatocytes in activating innate and adaptive immune response by activating monocytes, dendritic cells, and NK-cells. Jesus et al., reported exosomes as an adjuvant for vaccination against HBV. However, an inadequate immune response against HBsAg was observed, with elevated IFN-α level and cellular immune response [118]. The inadequate response might be attributed to the co-administration of exosomes and antigen and not antigen-loaded exosomes, generating a better immune-stimulatory response. Various researches suggest the potential therapeutic role of exosomes in hepatocellular carcinoma treated with an emphasis on the immune modulation [119], [120], [121] and suggesting exosomes mediated inhibition of hepatic carcinoma cell proliferation [122], [123], [124]. The strategies can be based on exosomal miR-122 in gene therapy to increase the sensitivity of tumor cells towards chemotherapy [105] or exosomal miR- 335-5p delivery mediated HCC cell proliferation [119].
Pathophysiological complications alter the content of the exosomes, which can be utilized to gather information about the disease. The exosomes released from virally infected hepatocytes express viral RNA, core proteins, envelope protein, and viral DNA, which can be utilized for diagnostic purposes. The studies suggesting a role of the exosomes as a reservoir of HBV DNA are reported; in such cases, the conventional diagnostic measures can fail. Still, the exosome-based diagnosis technique can aid in detecting the chances of relapses [111]. Various individual groups report early-stage miRNA-based techniques along with exosomes in HCC. Early-stage detection of HCC was proposed based on a combination of exosomes with miRNA to overcome the inadequate diagnosis and poor sensitivity of Alpha-fetoprotein (AFP) and ultrasound screening. The combination of AFP further with miRNA-exosome improved sensitivity and efficiency of diagnosis [125]. Another study reported exosomal miR-718 as a biomarker in the detection of recurrence of HCC [126]. Another individual researcher reported a similar study for the diagnosis of HCC in comparison with chronic hepatitis [127]. The samples collected from the chronic hepatitis patients and measured for the serum exosomal microRNAs and serum circulating microRNAs suggested the significantly higher levels of miR-18a, miR-221, miR-222, and miR-224 when compared with chronic hepatitis B (CHB) and liver cirrhosis (LC) patients. The microRNA estimation can aid in distinguishing between HCC from CHB and LC.
2.4. Ebola Virus (EBOV) and exosomes
Ebola virus (EBOV) is an enveloped RNA virus of the family Filoviridae, it is the most virulent pathogen and has a high clinical fatality of 25–90% and an average CFR of 50%. The disease caused by EBOV, called Ebola virus disease, previously known as Ebola hemorrhagic fever, is fatal. In West Africa (2014–2016), the recent Ebola outbreak in Guinea, Liberia, and Sierra Leone led to 11,323 deaths out of 28,646 infected cases with ~46% CFR [6].
Ebola VP40 is an EBOV viral matrix protein responsible for viral assembly and virus budding in host cells [128]. Since 2016, Pleet et al., have worked extensively on EBOV and exosomes infection control [129], [130], [131]. The group reported exploiting the ESCRT pathway by EBOV, which plays a crucial role during viral budding in exosomes. The study suggested the EBOV VP40 mediates upregulation of extracellular CD63 and Alix, ESCRT proteins, which may increase the biogenesis of exosomes. Furthermore, the EBOV VP40 is secreted in exosomes and can induce apoptosis of uninfected recipient T-cells and bystander lymphopenia [129]. The data also suggest the role of exosomes secreted from the infected cell to deregulate the RNAi pathway (RNA interface) responsible for generating an innate antiviral response in the host.
2.4.1. Role of exosomes in the therapy and diagnosis of Ebola virus infections
Targeting the exosome biogenesis pathway can be a potential therapy against Ebola. Oxytetracycline, an FDA-approved exosome biogenesis down regulator, showed a positive effect on T-cells viability transfected with EBOV VP40. The improved T-cell viability can be explained by the downregulation of exosome release and the EBOV VP40 matrix protein responsible for cell apoptosis [129]. To confirm the results in vivo, the safety, and the benefits on the host cell and redox regulation of the whole system, further studies will be required. Numerous studies have suggested that exosomes may be used as a therapy for various infections [115] including HIV and hepatitis. The presence of EBOV VP40 matrix viral proteins and other glycoproteins (GP) in exosomes released from the infected cell is well established. Hence, their different role in immunomodulation can be investigated, which can be further exploited to develop vaccines and medicine against Ebola.
Anticoli et al., reported an exosome-based vaccine approach for virus infections such as Ebola and HIV-1. The platform was based on the cytotoxic T lymphocyte (CTL) immunization against viral infections [132]. Kyle et al., reported that Ebola has a considerable effect on lipid metabolism homeostasis based on the analysis of blood samples collected during the largest Ebola outbreak in West Africa from 2014 to 2016. The study suggested an increased level of few lipid classes in survivors, whereas few different lipids classes were increased in fatalities. Furthermore, it suggests that exosomes may play a role in lipid metabolism [133]. Several lipids were found to be high in the exosomes released by infected cells [134]. Lipid profiling can aid in monitoring the disease's state and recovery and identify biomarkers. Nanotraps (NT) is composed of cross-linked polymer particles that are functionalized and environment-friendly, allowing them to capture and retain a specific protein. The NT hydrogel particles, which can concentrate the exosomes to detect EBOV VP40 viral protein, can be a potential EBOV diagnostic tool [129].
2.5. Epstein-Barr Virus (EBV) and exosomes
Herpesvirus 4, also called EBV, is a double-stranded DNA disease that belongs to the herpesviridae family. The EBV is responsible for several lymphoid and epithelial malignancies such as infectious mononucleosis, Hodgkin's lymphoma, Non-Hodgkin's lymphoma, Burkitt's lymphoma, and post-transplant lymphoproliferative disease, gastric carcinoma, and nasopharyngeal carcinoma [135]. In addition, the EBV transforms resting B-cells into oncogenic lymphoblastoid cell lines and accounts for 1.8% of cancer-associated deaths globally [136]. In addition, it establishes various latent gene expression programmers in resting and proliferating cells known as latency 0, I, II, and III.
2.5.1. Exosomes in the transmission of EBV
Different studies have examined the role of virus-derived exosomes in modulating cancer development and progression in EBV infections. By affecting cell proliferation, transformation, and immune response invasion, viral miRNA plays an important role in the EBV life cycle and pathogenesis. To evade and adapt to host immune response, the EBV virus exploits host miRNA machinery [137]. Exosomes contain miRNA encoded by EBV that can cause T-cell inhibitory exosomes to be secreted during infection. The secretion of viral nuclear proteins and cell membrane proteins such as “Epstein-Barr nuclear antigen-2 (EBNA-2)” and latent membrane protein 1 (LMP-1) in EBV infected cells is reported in various studies [138], [139], [140]. LMP1 is an essential viral oncogene protein generally expressed in EBV-associated carcinogenesis in latency type II and III. LMP1 surface-bound exosomes modulate the host immune system by inhibiting T-cell activation and increasing natural killer cell toxicity [141]. Verweij et al., suggest that EBV utilizes the CD63-mediated endosomal-exosome pathway to downregulate NF-<UNK>B's chronic activation of NF-κB. As a result, LMP1 secreted into exosomes plays a crucial role in invading immune response and aids in the proliferation of EBV-infected cells [142]. An individual researcher also suggested that the LMP-1 internalized into exosomes to escape degradation induces upregulation and concentration of growth factor receptor and fibroblast growth factor-2 or receptor in exosomes, and modulates target cell phenotype [143], [144]. Another study by Nanbo et al., suggested that the LMP-1 mediates overexpression of the ICAM-1 intracellular adhesion molecule and led to the proliferation of target cells. The in vitro study also suggested caveolae-dependent endocytosis of exosomes in target cells and modulation of the phenotypic changes by unloading viral factors [145]. The exosomes released from EBV infected cells carry non-coding EBV RNAs and LMPs and are responsible for cellular transformation and innate antiviral immunity. These viral RNAs require further investigation to explore their role in the pathogenesis of EBV [146], [147]. EBV encoded small RNAs (EBERs), play a significant role in the activation of innate immunity by induction of DCs maturation and type-1 IFN and inflammatory cytokines via TLR3. However, EBERs are also associated with the immunopathologic disease caused by active EBV infection as well as activation of T of NK cells [147].
2.5.2. Exosomes in therapy and diagnosis of EBV infections
The exosomes derived from EBV infected cells express virus-specific nuclear proteins and cell membrane-specific proteins such as LMP-1 and EBNA-2. The exosomes' significant role in viral infection persistence and spread in the host makes it crucial in developing diagnostic or prognostic biomarkers and anticancer therapy. Houali et al., reported the LMP1 and BARF1 oncoproteins as a potential diagnostic marker based on the serum and saliva samples collected from the nasopharyngeal carcinoma patients (NPC). Saliva and serum body fluids contain exosomes that are effectively secreted and separated. Virus-derived oncoproteins were detected in NPCs, and they were associated with exosome-like vesicles [148]. Researchers identified the LMP-1-LMP-2a mRNA as a potential prognostic biomarker of extranodal NK/T-cell lymphoma, nasal type, which is distinct from other types of lymphomas [149]. Various studies have suggested a potential role of various proteins in prognosis and diagnosis are isolated from EBV-derived exosomes such as LMP-1, LMP-2a, BARF1, HIF1α, EBV DNA, EBV miRNA, EBV mRNA, and growth factors [150], [151], [152], [153].
The role of exosomes in modulating immune response and phenotype of cells aiding in evasion of virus from immune response and the formation and progression of cancer cells are well reported. In such a scenario, EBV-derived exosomes can be developed as a potential anticancer target to inhibit the proliferation and spreading of the infection [154]. Furthermore, the obstruction in the biogenesis and release of the exosomes can alleviate the down-regulated immune activity. The various oncogenic EBV-derived nuclear and cell membrane proteins such as LMP1, LMP2, BARF1, and EBNA-1, which play a vital role in spreading infection, are transported by exosomes to uninfected cells. Based on preclinical data on nasopharyngeal carcinoma patients, Cao et al., carried out clinical trials of safe and tolerable DNAzyme based adjuvant therapy on nasopharyngeal carcinoma patients. The study suggested a potential role of DNAzyme, a small catalytic DNA which downregulates the LMP1, for the treatment of nasopharyngeal carcinoma. The DNAzyme based adjuvant therapy during routine radiotherapy exhibited significant short-term tumor regression without severe side effects [155]. Various studies suggesting the oncogenic proteins of EBV as a potential anticancer target are under investigation [156], [157], [158].
Additionally, exosomes contribute to the development of antiviral immune responses as well as the spread of infection. Viral components trigger pattern recognition receptors (PPRs) and thereby stimulate the immune response of the host. Aziza and the group previously demonstrated that EBV encoded dUTPase activated TNF-*, IL1, IL-6, and IL-8 through the toll-like receptor 2 (TLR2) signaling pathway [159]. Due to an inflammatory response, impaired adaptive immune function, and increased cell proliferation, disease pathogenesis can occur.
Furthermore, EBV encoded dUTPase containing exosomes modulate the innate and adaptive immune response in human dendritic cells and mononuclear cells by B cell stimulatory action [160]. The significant role of dUTPase makes it a potential target for the development of antiviral therapeutics. Vallhov et al., investigated the specific targeting of exosomes derived from different cells and observed that the EBV transformed cells preferentially target B cells. The study suggested that EBV-derived LMP1 containing exosomes interact with B cells through EBV encoded glycoprotein gp350 and receptor CD21. Blocking this interaction blocks the EV uptake by B cells preventing the infection. The study suggested gp350 expressed exosomes effectively blocked EBV infection spread in vitro and emphasizes the potential of developing an exosome-based vaccine specifically targeting B cells for therapeutics [161].
3. Exosomes in personalized medicine
A patient's exosomes contain a wealth of information about their individual health conditions. To achieve an effective outcome, individualized or customized medicine is necessary due to distinct genetics, environment, and personal habits that affect individual health. As a result, EVs have been successfully applied to the diagnosis and prognosis of cancer. In terms of early diagnosis and personalized treatment, liquid biopsy-based on EVs has proved to be beneficial [162]. Various studies suggest the potential of exosome genomics in deciding the therapy. Kato et al., presented significant findings suggesting the presence of a higher level of p-glycoprotein in docetaxel-resistant patients' exosomes than docetaxel non-resistant patient exosomes. In order to overcome docetaxel resistance caused by the multidrug resistance protein 1 (MDR1) gene, taxane can be used to treat castration-resistant prostate cancer [163]. Personalized medicine is plagued by poor diagnostics and biomarkers. The exosomes based adaptive therapy brings together patients response-dependent treatment plans in personalized medicine [162].
The non-invasiveness, easy accessibility, and cell-specific origin of exosomes make them favorable as diagnostic biomarkers. In combination with existing diagnostic tools, the exosomes can further improve the accuracy and sensitivity of diagnostic markers. The exosome DNA represents the whole genomic DNA and mutations present in the origin cells, which can be further employed to predict diseases induced by mutations [164].
Piffoux and his colleagues reviewed the allogeneic exosomes and personalized cancer immunotherapy [165]. There has been considerable attention paid to the physical triggering of exosomes for theragnostic by inducing high exosome loading, secretion, and therapeutic effectiveness. With bioengineered exosomes, cellular targeted delivery can be devised, and their biodistribution and interaction can be controlled. Small size, lipidic bilayer, and indigenous nature provide low immunogenicity, high uptake and prevent phagocytosis. In addition, exosomes possess a protective nature, which makes them potential carriers in therapy. As a result, exosomes can be used in personalized medicine to treat various viral infections and virus-mediated cancers.
4. Exosomes and critical challenges
To utilize the exosome's endogenous nature of specific cell-to-cell communication in therapeutics such as protein and peptide delivery, RNA delivery, gene delivery, vaccination, and immunotherapy is underway. Techniques for isolating and purifying exosomes pose the biggest challenge in exosome-based applications. The application of techniques depends on the application area; however, the recovery and specificity vary from technique to technique. There is a need for standardized techniques for the isolation and purification of exosomes. The techniques that would be specific, viable, easy to replicate, high throughput, and high recovery. The endogenous nature of the exosomes provides in vivo stability in circulation and prevents them from phagocytosis and macrophages mediated degradation. However, the half-life of exosomes in vivo is approximately 2–20 min [166]. The already present endogenous content limits the exogenous loading of the drug reducing the loading efficiency.
Various methods have been reported for exosome drug loadings, such as preloading and post-loading; however, there is a need to readdress them to achieve optimal performance. During the large-scale production of exosomes, the parameters like scalability, variability, purity, potency, and reproducibility of the produced exosomes raise serious concerns. The large-scale production and translation to clinics will be costly. The role of exosomes needs to be studied in-depth for a better understanding of harnessing exosomes in biomedical applications [167].
The drug-loaded and tagged exosomes might behave differently in vivo. The pharmacokinetics (PK) and pharmacodynamics of the therapeutic exosomes may vary from the already existing exosomes. Long-term safety and toxicity studies are needed to investigate exosomes' effect upon in vivo exposure thoroughly. The in vitro models can be employed to investigate the effect of drug-loaded exosomes on recipient cells, uptake mechanism, intracellular and extracellular behavior, and drug unloading mechanisms. Further in vivo methods and physiologically based pharmacokinetic models can investigate the PK of therapeutic exosomes [168]. The development of artificial exosomes is an excellent alternative for translational medicine and is capable of addressing the above-mentioned vital challenges and producing homogenous exosomes. The development of artificial exosomes is underway and holds a promising future in exosome-based biomedical applications.
5. Conclusion and prospects
In order to address the unmet need in the treatment and prognosis of viral diseases, the dual role of exosomes in transmitting various diseases and enhancing the innate immunity of host cells can be exploited. Exosomes have significant potential as a therapeutic, prognostic, and diagnostic biomarker for various diseases. Barriers to overcome include isolation and purification techniques, biochemical compositions, and in-depth details of the uptake mechanism. The exosome-based delivery platform paves the way for safer and more efficient delivery of siRNA, miRNA, and proteins with little toxicity and immunogenicity. In patients exposed to chronic conditions, the prognosis of the disease may help increase life expectancy. Even though exosomes possess tremendous potential, their translation to clinics is hampered by various barriers at both isolation and production levels. The storage and stability of exosomes are also critical aspects that must be addressed. According to origin and health conditions, exosome composition differs, determining its function after releasing in extracellular space. The dual role of exosomes further complicates the isolation and application of the exosomes in theranostics. There is a need for extensive research to unravel the exosomes' biogenesis and differentiate between exosomes that aid in disease transmission and exosomes that set off the innate immunity of the host.
6. Introduction
The course of infectious diseases resulting from viral or bacterial infection is changing due to human activities such as global travel, agriculture, deforestation, and consumption of exotic wildlife [1]. Many viral epidemics and pandemics are caused by outbreaks and a lack of knowledge about a viral pathogen, transmission patterns, and diagnosis [2]. In addition to self-resolving viral diseases, they can also cause treatable diseases or chronic diseases that can lead to death. Globally, viral infectious diseases contribute to high rates of mortality and morbidity. The last two decades have seen significant epidemic and pandemic outbreaks (Fig. 1) such as severe acute respiratory syndrome (SARS) in 2003, H5N1 in 2004, H1N1 in 2009, the Middle East respiratory syndrome (MERS) in 2012, Ebola in 2014, and the devastating Coronavirus disease (COVID-19) in 2019. A case fatality rate or risk (CFR) measures disease severity and represents the proportion of people dying from a disease. For example, acquired immunodeficiency syndrome (AIDS) by Human Immunodeficiency Virus (HIV) exhibits a CFR of 100% and is not curable, while Ebola has a CFR of 50% [3]. Hepatitis B virus (HBV) infects 250–350 million worldwide and causes 500,000–600,000 deaths annually [4]. A vaccine for SARS and MERS has not yet been developed [5], [6]. For Ebola, there is no licensed vaccine yet. However, several investigational Ebola vaccines are under clinical trials such as rVSV-ZEBOV [7] and cAd3-EBO [8]. The devastating effects of COVID-19 have led to an expedited search for affordable vaccines against it [9], [10], [11], [12].
6.1. Exosomes
Exosomes are extracellular vesicles secreted by eukaryotic cells and composed of proteins, DNA, mRNA, microRNA, long non-coding RNA, and circular RNA which participate in intercellular communication [13]. The recent findings have found that exosomes play a vital role in cell-cell signaling. Further, the presence of exosomes has been identified in several bodily fluids such as plasma, saliva, breast milk, cerebrospinal fluid, and amniotic fluid [14], [15], [16], [17], [18]. The presence of exosomes in the body fluids and the release of exosomes from the immune cells, cancer cells, and brain cells have attracted researchers from across the globe to explore the role of exosomes in diverse areas. The exosome's role in tumor detection and tumor therapy is notably investigated with hints of the modality being translated in the near future [19], [20], [21]. Studies suggesting exosomes derived from adipose-derived stem cells in alleviating oxidative stress and inflammation [22], and promoting ischemic repairment and angiogenesis have been reported [23], [24]. It has been suggested that the pivotal role of exosomes in the manifestation of the disease and applying the same in therapeutics and diagnostics can play a potential role in the prognosis and prevention of viral diseases. This review summarizes the dual role of exosomes in transmitting various viral diseases and their application in the management of the same diseases.
6.2. Exosomes: origin and biogenesis
Exosomes are lipid bilayer phospholipid membrane vesicles that originate from late-stage endosomes. The cell membrane invaginates into the cytoplasm to form primary or early endosomes, which further mature into late endosomes. Endosomes form multivesicular bodies (MVBs) when the limiting membrane folds inwards into intraluminal vesicles (ILVs). It is believed that these MVBs either fuse with lysozymes and get degraded or that they fuse with plasma membranes and release ILVs into the extracellular space as exosomes [25]. In the biogenesis of exosomes, the endosomal sorting complex required for transport (ESCRT) and the cytosolic protein complex play a crucial role in forming ILVs and sorting ubiquitinated proteins. Protein Alix TSG101 (Tumor susceptibility gene 101) and flotillin participate in exosome biogenesis and release, whereas annexins and Rab GTPase proteins aid in membrane transport and fusion (Fig. 2) [26]. The primary composition of exosomes is various tetraspanins such as CD9, CD63, CD81. These tetraspanins participate in cell penetration and fusion events and are widely identified as an exosomal marker apart from TSG101 protein [27]. The HSP70 surface protein receptor is essential for transporting exosomes to recipient cells and for identifying exosomes (Fig. 3).
Exosomes change the phenotype of cells and alter the innate immunity of the host cell. When released into extracellular space, exosomes are influenced both by their size and surface by recipient cells [28]. The surface-expressed proteins determine the recognition and uptake by the acceptor cells. In most studies, exosomes are taken up by specific cells, whereas few studies report exosomes' uptake by non-specific ligand-receptor combinations. There are several uptake mechanisms reported for exosomes, including micropinocytosis, clathrin-dependent endocytosis, clathrin-independent endocytosis, and caveolin mediated uptake [29]. Based on their origin, exosomes carry diverse cargo that plays a vital role in the intercellular communication and transport of various biomolecules. Exosome composition reflects the origin and function of the exosome, as well as the pathological condition of the parent cell and its surroundings.
Exosomes play a crucial role in innate and acquired immunity (Fig. 4). In response to Tumor necrosis factor (TNFs), HLA-B- associated transcript 3 (BAT3), and Interleukin 15Ra (IL-15Ra) on the surface of the exosomes, mature dendritic cells (mDC) activated macrophages, further activate NK cells, enhancing their cytotoxic activity.
Exosomes mediated antigen presentation to T cells can induce an immune and proinflammatory response. The mDC derived exosomes express the T cell co-stimulatory molecules and MHC class I and II, which can be an essential mechanism for antigen presentation. The proposed mechanisms for the antigen presentation by exosomes are cross-dressing pattern, cross-presentation, and direct exosome-induced T-cell activation [30], [31], [32]. In a cross-dressing pattern, DCs captures exosomes and directly present them to CD4+ or CD8+ T cells. The exosomes shuttle antigenic peptide-MHC complex to DCs populations. Cross presentation pattern involves DCs mediated presentation of antigenic protein/ peptides containing exosomes to the endogenous MHC class I and II molecules, followed by activating antigen-specific T-cells. In direct antigen presentation, DCs derived exosomes can directly activate CD4+ or CD8+ T cells, attributed to the expression of the T cell co-stimulatory molecules along with MHC class I and II on the exosomes [33].
Furthermore, exosomes are isolated from different plant sources, including juice, seeds, and leaves. Citrus lemon, ginger, grapefruit, Arabidopsis thaliana, coconut water, and carrots have all been found to contain exosomes [34], [35]. Plant-derived exosomes are involved in plant cell-to-cell communication and regulate the innate immunity of plants, like exosomes' role observed in the human body. Various medicinal plants possess antiviral properties [36], [37], [38], [39]. Sundaram et.al reported anti-bacterial activity of ginger-derived exosomes in chronic periodontitis against Porphyromonas gingivalis (P. gingivalis), an oral pathogenic bacteria which causes chronic periodontitis [37]. The ginger-derived exosomes like nanoparticles (GLEN) were selectively taken up by P. gingivalis pathogen and inhibited the growth of the pathogen. The GLENs significantly reduced the FimA (important component for attachment to host surfaces) expression and further inhibited the attachment of P. gingivalis to oral epithelial cells. Also, GLEN impacted the immune response by a decrease in the expression of bone resorptive cytokines (TNF-α, IL-6, and IL-8) and decreased the recruitment of macrophages and leukocytes.
6.3. Isolation of exosomes
Many types of isolation methods have been attempted for obtaining individual exosomes (Fig. 5), including ultracentrifugation, size-based techniques, such as ultrafiltration and size exclusion, immunoaffinity-based techniques, microfluidics-based techniques, and polymer precipitation methods [40]. In ultracentrifugation, exosomes are separated based on their density and size using centrifugal force. Ultracentrifugation is the standard technique used for exosome isolation with high throughput; however, it is also associated with several drawbacks, such as time-consuming results and machine-dependent outcomes. The unstable recovery rate and damage of exosomes upon repeated centrifugation are significant concerns.
The immunoisolation technique is based on chromatography, in which specific antibodies are immobilized on the stationary phase to bind with the target protein present on exosomes covalently. The immunoisolation technique can be used for high purity isolation for quantitative and qualitative studies of exosomes. The non-specific adsorption, high cost, low yield, and harsh operating conditions are several drawbacks of the immunoisolation technique. The polymer precipitation technique employs hydrophilic polymers such as PEG and decreases hydrophilicity of exosomes leading to precipitation of the exosomes followed by low-speed centrifugal force for isolation. The PEG-based kits available for rapid isolation and purification are expensive, and co-precipitation of contaminants such as lipoproteins is reported. Microfluidics is an emerging isolation technique to separate the exosomes based on biochemical composition, physical properties, and immunoaffinity [41]. Micro fluidics-based techniques can significantly reduce separation time, reagents, and volume however, low yield, low reproducibility, and high cost are hurdles for the technique. Above all, the ultracentrifuge technique is the gold standard for the isolation of exosomes.
7. Viral diseases and exosomes
The high expression of target cell-specific integrin and tetraspanin adhesion molecules also pave the way for targeting drug delivery [42]. Viruses transmit RNA or DNA in exosomes that activate the innate immune system. However, exosomes can also disrupt normal cellular physiology and cause disease. Hence the balance between the exosomes mediated infectivity and innate immune response decides the course of the disease and pathophysiological conditions. Exosome-based drug delivery can provide target-specific delivery as well as aid in boosting the immunogenic outcome. The potential proteins or viral RNA/ DNA present in exosomes and involved in promoting a diseased state can be targeted to impede further transmission. It is possible to identify and develop antiviral exosomes as a therapeutic for viral diseases [43]. Exosome charges carry specific RNAs corresponding to the pathophysiological condition of their source cells [44]. The presence of viral RNA, DNA, or virions is also detected [39], providing prognostic or diagnostic information. The information can be regularly monitored to assess the disease conditions in patients. By understanding how exosomes promote viral infectivity, clinical applications of exosomes can be developed. Many viral diseases have been linked to exosomes, such as AIDS, hepatitis, Ebola, COVID-19, and Epstein-Barr virus.
7.1. Coronavirus and exosomes
Coronavirus is (+) single-stranded RNA virus of family Coronaviridae and subfamily orthocoronavirinae responsible for causing infectious diseases such as Severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and COVID-19 [45]. The global fatality rate of SARS and MERS is 9.6% [46] and 37.1% [47], respectively.COVID-19 caused by SARS-CoV-2 affected more than 200 countries and is responsible for causing ~5,174,646 deaths globally till November 2021 [48]. SARS-Cov-2 S-protein is highly conserved among all coronaviruses, which aids in receptor recognition, viral attachment, and entry into host cells [49]. S protein facilitates the binding of the enveloped virus with angiotensin converting enzyme 2 (ACE2) expressed on the lower respiratory tract cells of the host [50]. People infected with SARS-CoV-2 disease can be symptomatic or asymptomatic. Symptoms can vary from mild to moderate respiratory infections. However, patients aged above 65 years as well as those with co-morbid conditions are at high risk of severe symptoms requiring mechanical ventilation and intensive care. Acute respiratory distress syndrome (ARDS), a joint immunopathological event, is the primary cause of coronavirus-related disease fatality [51].
7.1.1. Role in the transmission of Coronavirus infections
CD9 molecules are present in the exosomes and play a crucial role in the exosome biogenesis and cargo loading into exosomes by protein-protein interaction network in MVBs membrane [52]. Exosomes released from the virus-infected cells transport CD9 molecules which further facilitate the virus entry into the recipient cells. A study performed by Earnest et al., suggested that tetraspanin CD9 aids in MERS-Coronavirus entry and infection in the mouse lungs [53]. “TMPRSS (transmembrane serine protease 2)”, an exosomal protein is recently reported to facilitate the SARS-Cov entry in host cells via ACE2 receptor by cleavage of spike protein [54].
Klaud et al., reported that ERGIC-53, an ER-Golgi intermediate compartment marker and a cargo receptor required for glycoprotein transport, is associated with coronavirus entry in the host cell. The active secretion of ERGIC-53 from cells in viral particles and exosomes was observed, which further aided in viral infectivity either by acting as a co-receptor required for virus attachment to the host cell or by recruiting cellular proteins essential for virus assembly [55]. The exosomes released from SARS-Cov-2 infected cells help in transmitting the disease by utilizing the exosome's intercellular communication mechanism.
The secretion of ERGIC-53 in the exosome can increase the viral infection by exploiting the inherent transporting nature of exosomes. Gunasekaran et al., reported the active role of exosomes in chronic lung allograft rejection in patients suffering from respiratory viral infections from 17 viruses, including coronavirus. The coronavirus antigen and increased lung SAgs were detected in isolated exosomes from serum samples of patients. Further, immunizations of mice with exosomes, as mentioned above, amplified humoral and cellular immune responses towards lung SAgs and alloantigen, which increases the risk of developing chronic lung allograft dysfunction [56]. The above study suggested the potential of exosomes in the transfer of viral antigens of coronavirus in the body and aiding in augmenting infection. A recent study by Wang et al., suggested that exosomes can transfer ACE2 to the recipient cells, leading to internalization and susceptibility to the virus docking [57].
Barberis et al., studied the proteomic analysis of plasma-derived from COVID-19 patients and health control. The proteomic characterization found signature proteomic features in plasma derived from the COVID-19 patients and proteins related to the coagulation process, transport activity, complement activity, protease inhibitor, and defense/ immunity protein activity. The study suggested that SARS-CoV-2 uses the endocytosis route to spread the infection exosomes carrying viral materials [51]. Kwon et al., observed overexpressed SARS-Cov-2 genes in A549 lung epithelial cells, and the isolation of the EVs suggested the presence of viral RNA within them. Further, to study the uptake of EVs by cardiomyocytes, EVs were incubated with human induced pluripotent stem cells-derived cardiomyocytes (hiPSC-CMs) and hiPSC derived endothelial cells. The presence of EVs and viral RNA fragments were detected in both cells. Further, EVs mediated the increase of inflammation-related genes in the hiPSC-CMs upon uptake [58].
7.1.2. Role in therapy and diagnosis of Coronavirus infections
Researchers have conducted extensive research on exosomes, suggesting they play a dual role in infection transmission and infection prevention. Huang et al., reported the role of Interferon-Induced Transmembrane Proteins (IFITM1, IFITM2, and IFITM3) in impeding the entry and replication of SARS Coronavirus. Infectious agents such as influenza A, Marburg virus, and Epstein-Barr virus EBV are inhibited by IFITM, which are broad-spectrum antiviral proteins. The proposed possible explanation was a change in properties of late endosomes/ lysosomes or involvement of endocytic pathways. However, the exact mechanism of inhibition was not elucidated [59].
Feeley and his group showed that IFITM3 prevented influenza A virus infection by preventing the entry of viral components into the cytosol after endocytosis, thus preventing virus fusion [60], which may be involved in the inhibition of SARS coronavirus. Novk et al., proposed a possible pathway that leads to the IFITM3 mediated antiviral role of exosomes against the SARS Coronavirus highlighting the crucial role of exosomes. The authors observed that exosomes released from SARS-infected cells contain IFITM3 and ACE2. Alternatively, overexpressing IFITM3 or secreted Ace2 receptors end up neutralizing SARS coronavirus [61]. Nevertheless, further studies are necessary to confirm the presence of the ACE2 receptor on exosomes to support the proposed mechanism (Fig. 6). The isolation of exosomes exhibiting antiviral activity against coronavirus can be carried out to augment its activity or can be engineered as a potential therapy against coronavirus treatment.
Kate et al., developed S protein-containing exosome-based vaccines for the immunization against the SARS coronavirus. The highest antibodies were observed when S protein contained exosomes boosted with adenoviral vector vaccine compared to individual vaccines. The vaccine generated high levels of specific neutralizing antibodies against SARS and exhibited SARS-S-mediated entry [62]. Teng et al. observed that the exosomes released from SARS-CoV-2 infected lung epithelial cells are taken up by macrophages and induce apoptosis by activation of nuclear factor κB and inflammatory cytokines. Similarly, ginger exosome-like nanoparticles (GLEN) showed a therapeutic effect against SARS-CoV-2 [63].
China's Ruijin hospital is conducting a single-arm, open-label, combined interventional clinical trial testing the safety and efficacy of exosomes derived from allogeneic mesenchymal stem cells (MSCs-Exo) against COVID-19. Experimental data shows a beneficial effect on lung inflammation and other lung injury-related pathological issues in this study [64]. Hubei Shiyan Taihe hospital in China is conducting another clinical trial to determine if human umbilical cord mesenchymal stem cells (HUMSCs) can treat patients with lung injury following infection with COVID-19 [65]. Gunasekaran et al., reported that exosomes isolated from SARS infected cells augmented the humoral and cellular immune responses upon immunization of mice with exosomes mentioned above [56]. The discovery could be used as a key to developing vaccines against coronavirus to exploit exosome-induced immunity in virus-infected cells. Kumar et al., investigated the role of exosomes as antiviral protease inhibitor carriers, showing that exosomes bypass liver metabolism and deliver drugs to the targeted tissues [66]. Tsai et.al reported preclinical SARS-Cov-2 mRNA-loaded exosomes to achieve immunogenicity in mice. The study suggested that vaccination of mRNA-loaded exosomes led to dose-dependent development of anti-spike and anti-Nucleocapsid antibody response as well as antigen-specific CD4+ and CD8+ T cell response without any vaccine-related adverse effects [67]. Further, mRNA-loaded exosomes were more safer compared to the RNA-loaded lipid nanoparticles which led to severe cellular toxicity. The exosomes offer the advantage of specific tissue targeting which can be exploited for drug delivery. Fu et al. investigated the pseudotyping-based approach to load the EV membranes with receptor-binding domain (RBD) of the spike protein, a key domain in SAR-Cov-2 attachment, fusion, and cellular entry. The study suggested that the RBD tagged EVS can specifically recognize ACE2 receptors on the target cell required for the uptake. Further, upon cellular uptake by ACE2 receptors, siRNA encapsulated in RBD tagged EVs delivered into lung tissues inhibited pseudovirus SARS-Cov-2 infection in vivo. RBD-tagged EVs are a promising vehicle for delivering antiviral agents for SARS-Cov-2 treatment. However, a study employing authentic SARS-Cov-2 is required to confirm the findings [68].
Anand et al., suggested the convalescent plasma-derived exosomes (CPexo) for the covid-19 therapy. The hypothesis suggested the CPexo can offer potential therapeutic efficacy by acting as an immunotherapeutic, drug carrier, and diagnostic biomarker based on the literature suggesting effective and precise targeting of the pathogen by exosomes in preclinical studies [69]. Also, convalescent plasma is reported as a therapeutic approach for the Covid-19 [70], and human membrane or fluids has been FDA approved previously to encounter inflammation and tissue injury [69]. Mesenchymal stem cells (MSCs) derived exosomes have emerged as potential therapeutic candidates attributed to the features such as modulation of the immune response, reduce inflammation, ability to suppress viral infection attributed to the presence of specific cytokines. Several clinical trials associated with MSCs derived exosomes for the treatment of Covid-19 are ongoing in different parts of the world (Table 1). The completed and ongoing clinical trials of MSCs derived exosomes highlight the effectiveness in the treatment of SARS-Cov-19. However, detailed safety and efficacy need to be asses and long-term outcomes [71], [72].
Alteration of the protein content of exosomes in diseases condition is well reported in the literature which can be exploited as the diagnostic marker of the disease condition of the patient. Barberies et.al investigated the role of exosomes as a diagnostic marker in the SARS-CoV-19 infected patients. The study suggested that critical patients showed significantly high levels of C- reactive protein (CRP), alpha-1-acid glycoproteins (A1AG1and A1AG2), CXCL7, and lower levels of CCD34, C4BPA, and GELS compared to non-critical COVID-19 patients. It was observed that CRP protein was able to differentiate between positive patients and negative patients. Also, it discriminated between critical patients and non-critical patients [73]. Similarly, Balbi et al., derived the EVs from the participants who underwent a molecular test for SARS-CoV-2. The profiling of the exosome protein suggested the CD142, a platelet tissue factor (activates extrinsic pathway of blood coagulation cascade) as the highest discriminating surface protein. The CD142 showed increased activity in COVID-19 positive patients and also correlated with TNF-α serum levels [74]. A similar observation was made by Rosell et al., suggesting the increased platelet tissue factor associated with the severity and mortality of the COVID-19 [75]. This finding may pave the road for exploiting CD142 as a specific surface antigen for the prognosis of the disease. A study carried out by Grifoni et al., also suggested increased cytokine levels and presences of TNF, IL-6, and IL-1β [76]. The circulating exosomes can be a potential biomarker for the prognosis of COVID-19 infection.
7.2. Human Immunodeficiency Virus (HIV) and exosomes
Acquired Immunodeficiency Syndrome (AIDS) is a chronic immune system failure caused by the retrovirus human immunodeficiency virus (HIV). World Health Organization (WHO) data shows that the pandemic has infected 37.9 million people and caused more than 1 million globally. The only promising therapy for infection is highly active antiretroviral therapy (HAART), only to increase the life expectancy, and there is no cure. However, the recent clinical trials of antibodies, namely 3BNC117, a broadly neutralizing antibody, and monoclonal antibody 10–1074 developed for CD4 host cell and HIV viral envelope, respectively, have shown promising results by suppressing viremia in HIV-1 infected patients [77], [78].
7.2.1. Role in the transmission of HIV
The role of exosomes on the immune system and HIV infection and understanding the influence of HIV infection on exosome release has gained interest in the research community (Fig. 7). Gould et al., proposed a hypothesis suggesting that HIV hijacks the exosome machinery in T-cells and macrophages to increase the infectivity in other cells in a receptor-independent manner [79]. Researchers report a variety of studies that support this hypothesis [80], [81]. Immunoprecipitation studies revealed that the macrophages infected with HIV have a similar complement of cellular membrane proteins to viruses recovered from the extracellular medium, suggesting that the virus is initially released from the macrophages and bud into endosomal organelles and then released by fusion with the plasma membrane. These key findings suggested exploiting the preexisting exosomal pathway for retroviral budding and further transmitting the infection [80], [81]. However, studies contradicting the above results suggested plasma membrane as the primary site for the virus budding by sequestering intracellular plasma membrane domain and recruiting CD63 tetraspanin during virus assembly [82], [83], [84].
The function of TSG101, a subunit of endosomal sorting complex required for transport I (ESCRT-I), is linked to the formation of endosomes and MVBs [85]. Another study implicated that HIV-1 utilizes a similar subset of cellular component that of cytokinesis, i.e., TSG101 and Alix, an ESCRT-associated protein during membrane budding and implicating the direct budding of microvesicles from plasma membrane releasing budding viruses by exploiting TSG101 and Alix [86], [87]. Kadir et al., reported a study supporting the hypothesis of exploitation of exosome pathways by HIV. They reported exosomes/ MVBs mediated the transfer of virus and viral constituents from infected cells to uninfected cells, thus aiding in expediting HIV infection and dissemination. The incubation of an equal ratio of HIV-1 infected monocyte-derived macrophages (MDM) and uninfected MDM labeled with lipophilic dyes DiO and DiD, respectively were carried out. Upon incubation, the increased membrane exchange among infected and uninfected MDM was observed followed by the release of vesicles from infected and fusion with uninfected MDM within 4-6 min observed via video footage.
Negative regulatory factor (Nef) plays a crucial role in the infection and replication of HIV by exploiting host cell machinery and downregulation of important crucial antiviral immune responsive molecules such as MHC-I, MHC-II, CD4, and CD28 [89]. It is reported in various studies that Nef, a protein encoded by lentivirus, facilitates its release via exosomes by accelerating exosome release [90]. Mukkhamedova et al., observed that the exosomes isolated from the Nef expressing cells contained Nef. Further, incubation of Nef containing exosomes and macrophages led to a linear increase in exosomes uptake by macrophages. It was found that exosomes deliver Nef to the macrophages, which alters cellular functions and causes inflammation associated with HIV infection. Continuously incubating HIV-infected exosomes colored with a fluorescent dye (PKH67) inside RAW264.7 macrophages resulted in linear uptake of the exosomes inside the cells (Fig. 8). The study suggested rapid and quantitative uptake of exosomes by macrophages delivering NeF to the intracellular compartments [91]. This also results in HIV-associated co-morbidities. Moreover, its role in the augmentation of the membrane trafficking process and apoptosis of CD4+ T cells leads to adverse effects on the immune system [92]. Researchers have also reported that the exosomes released from infected cells can activate latent viral reservoirs and potentially suppress innate immunity [93], [94].
7.2.2. Role in therapeutics and diagnosis of HIV infections
The exosomes are reported to play a diverse role in the transmission and a preventive role against viral infection (Fig. 9). The CD8+ T lymphocytes-derived exosomes have shown the antiviral property by suppressing the HIV replication in vitro initiated by interferon-mediated signaling pathway [95], which can be further engineered for effective therapy against HIV. The RNA sequence in exosomes derived from HIV-infected cells has been isolated. Studies show the influential role of various miRNAs such as miR-28, miR-29a, miR-149, miR-150, miR-198, miR-223, miR-324, miR-378, and miR-382 protective role against HIV infection [96]. Narayanan and the group demonstrated the role of TAR RNA in exosomes derived from HIV-infected cells in inhibiting apoptosis by downregulating expressions of the Bim and Cdk9 pro-apoptotic protein [97]. The role of exosomes derived from various body fluids have protective action against HIV infection, such as breast milk-derived exosomes, which was seen to inhibit HIV-1 infection of dendritic cells [98] and semen derived exosomes inhibited RNA expression by inhibiting binding and recruitment of transcription factors such as NF-κB and Sp1 [99]. By identifying and purifying antiviral exosomes, the ability of intrinsic exosomes to interfere with HIV infection can be utilized to develop exosome-based anti-HIV therapies [100].
Targeting exosome-associated proteins in HIV infection, such as MHC-II, CCR5, CXCR4, TSG101, miRNA, mRNA, and ESCRT, therapeutic applications against HIV can be developed. Another approach can exploit exosomes' natural occurrence and inherent role in transporting mRNA and miRNA from one cell to another to deliver RNA-based therapeutics. A novel RNA delivery vehicle, such as exosomes, can address many shortcomings of conventional RNA delivery, including stability, high clearance, poor uptake, high phagocytosis and cytotoxicity rates, and biocompatibility issues [101]. Lattanzi et al., developed Nef mutant protein (Nefm) loaded exosomes as a vehicle of antigen cross-presentation against HIV-1. Nefm, a biologically inactive HIV-1 NeF, was loaded in the exosomes. It was observed that Nefm- exosomes enter the target cell less efficiently than virus-like particles (VLPs) but exhibit similar cross-presentation levels.
Further in vivo studies can open a potential road towards developing Nefm exosome-based vaccines against HIV-1 [102]. The therapeutic approach of loading anti-HIV RNA in exosomes or anti-HIV in anti-HIV exosomes can be a potential therapeutic modality. The addition of a specific targeting ligand could significantly improve the efficacy and targeting of therapy. In certain disease conditions, the levels of protein and EVs in body fluids increase. This phenomenon has been harvested as a noninvasive technique identifying the specific proteins acting as a biomarker of the disease. The role of exosomes as a biomarker in the prognosis and diagnosis of the disease can be potentially harvested in favor of the patient's treatment. It may be possible to utilize molecules in exosomes as a biomarker in the prognosis or diagnosis of HIV-1. Several HIV-1 related proteins were detected in the urine of an HIV-1 patient, including Gag, Tat, Vpu, Vpr, and Nef. Apart from this, neuron-derived exosomes (NDE) are a potential biomarker of cognitive impairment by HIV. HMGB1, NF-L, and Aβ proteins can be a potential biomarker in the prognosis of HIV-1 [101].
7.3. Hepatitis virus and exosomes
The hepatitis viruses (A, B, C, and E) are unrelated viruses that cause liver-related conditions (Fig. 10) such as acute and chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma [103]. HAV and HCA are RNA viruses of the family Picornaviridae and Flaviviridae, respectively. HBV is a DNA virus of the family Hepadnaviridae. In 2018, Shearer et al., reported the global prevalence of HBV to be 3.9%. [104]. Globally 142 million people suffer from chronic hepatitis C infection, and 399,000 people die from cirrhosis and hepatocellular carcinoma (HCC)[105].
7.3.1. Role of exosomes in the transmission of hepatitis virus
The exploitation of exosomes by HBV during infection to increase infectivity and escape from the host's innate immune response by downregulating IL-12 is reported by Kouwaki et al., The HBV RNA, viral DNA, core protein, an envelope protein was detected in exosomes derived from infected hepatocytes to infect the hepatocytes [106]. Moreover, HBV increased the expression of immunosuppressive miRs such as miR-21 and miR-29a in exosomes attributed for the downregulation of IL-12 in macrophages, responsible for the activation of NK cells [107]. Yang et al., reported a viral component in exosomes isolated from chronic hepatitis B patients and the infective nature of isolated exosomes to naïve hepatocytes. The crucial finding was the role of exosomes in transferring HBV viral particles into NK cells followed by NK-cell dysfunction by downregulating activating NKp44 receptors and upregulating NKG2A, an inhibiting receptor (Fig. 10) [108]. Kapoor et al., observed that Hbx modulates the biogenesis of exosomes and exploits its intrinsic intracellular communication property to transfer the HBx viral load to further support the viral spread and pathogenesis [109]. A proposed mechanism of exosome-HBV infection and the disruption of the NK cell is presented in Fig. 11 .
Exosomes secreted from HBV infected liver cells play a crucial role in downregulating tumor cell apoptosis by upregulating exosomal miR-21 and aiding in chemoresistance and pathogenesis of hepatic carcinoma. HBV-mediated exosome role in chemoresistance in hepatic carcinoma is mediated by upregulation of lysosome-associated membrane protein (Lamp2a), an essential protein of chaperone-mediated autophagy (CMA)[110]. The exosomes might also act as a reservoir of HBV DNA with the ability to reinfect the host with suppressed immunity in recovered patients [111]. These studies support the Trojan exosome hypothesis suggesting the exploitation of exosomes by the virus to augment the transmission and replication process further and escape the host's innate immune response.
Like HBV transmission, the role of exosomes in spreading the infection of HAV and HCV is well reported. The exosomes expressed from hepatitis virus-infected cells play a dual role, aiding in spreading viral infection by infecting naïve hepatocytes and protective role by activating innate immune signaling pathways. Various studies suggest that HCV can exploit exosomes by transferring HCV viral RNA in exosomes, impairing the dendritic cell and infecting the naïve hepatocytes [106], [112]. Whereas in HAV, not viral RNA but virions (eHCA) expressed in the exosomes play a vital role in spreading the infection to naïve hepatocytes [93]. The role of exosomes in neutralizing the anti-HCV antibodies to evade the innate immune response is also reported [113]. Gorji-bahari et al., suggested the role of the RAB5A gene in the regulation of HCC metastasis via regulating the pro-viral content of exosomes. The RAB5A inhibition can affect the HCC cell proliferation rate and migration capability [114].
7.3.2. Role in therapy and diagnosis of hepatitis
Apart from assisting in spreading the infection, exosomes in hepatitis infection also play a protective role by activating innate immunity signaling pathways. The activation of pDC (plasmacytoid dendritic cells), which secrets type I interferon against the viral infection, is mediated via the interaction of exosomes containing viral RNA/virions with pDCs during HAV and HCV viral infection [115]. The macrophages-derived exosomes deliver IFN-α expressed anti-HBV molecules via similar machinery pathways utilized by the virus during the invasion, such as micropinocytosis and clathrin-dependent pathways [116]. Another study reported pro-inflammatory substance IL-6 from monocytes mediated via exosomes released from HBV infected hepatocytes [117].
The detailed elucidation of the mechanistic pathway of antiviral exosomes can help develop the exosomal-based vaccine to boost the immune response towards the hepatitis viral infection. The immune-stimulatory role of the viral RNA and virions carrying exosomes can be taken advantage of in outweighing the role of spreading infection. The intrinsic nature of exosomes can be exploited for engineering exosomes as a delivery cargo of therapeutic molecules. Considering the active role of exosomes released from infected hepatocytes in activating innate and adaptive immune response by activating monocytes, dendritic cells, and NK-cells. Jesus et al., reported exosomes as an adjuvant for vaccination against HBV. However, an inadequate immune response against HBsAg was observed, with elevated IFN-α level and cellular immune response [118]. The inadequate response might be attributed to the co-administration of exosomes and antigen and not antigen-loaded exosomes, generating a better immune-stimulatory response. Various researches suggest the potential therapeutic role of exosomes in hepatocellular carcinoma treated with an emphasis on the immune modulation [119], [120], [121] and suggesting exosomes mediated inhibition of hepatic carcinoma cell proliferation [122], [123], [124]. The strategies can be based on exosomal miR-122 in gene therapy to increase the sensitivity of tumor cells towards chemotherapy [105] or exosomal miR- 335-5p delivery mediated HCC cell proliferation [119].
Pathophysiological complications alter the content of the exosomes, which can be utilized to gather information about the disease. The exosomes released from virally infected hepatocytes express viral RNA, core proteins, envelope protein, and viral DNA, which can be utilized for diagnostic purposes. The studies suggesting a role of the exosomes as a reservoir of HBV DNA are reported; in such cases, the conventional diagnostic measures can fail. Still, the exosome-based diagnosis technique can aid in detecting the chances of relapses [111]. Various individual groups report early-stage miRNA-based techniques along with exosomes in HCC. Early-stage detection of HCC was proposed based on a combination of exosomes with miRNA to overcome the inadequate diagnosis and poor sensitivity of Alpha-fetoprotein (AFP) and ultrasound screening. The combination of AFP further with miRNA-exosome improved sensitivity and efficiency of diagnosis [125]. Another study reported exosomal miR-718 as a biomarker in the detection of recurrence of HCC [126]. Another individual researcher reported a similar study for the diagnosis of HCC in comparison with chronic hepatitis [127]. The samples collected from the chronic hepatitis patients and measured for the serum exosomal microRNAs and serum circulating microRNAs suggested the significantly higher levels of miR-18a, miR-221, miR-222, and miR-224 when compared with chronic hepatitis B (CHB) and liver cirrhosis (LC) patients. The microRNA estimation can aid in distinguishing between HCC from CHB and LC.
7.4. Ebola Virus (EBOV) and exosomes
Ebola virus (EBOV) is an enveloped RNA virus of the family Filoviridae, it is the most virulent pathogen and has a high clinical fatality of 25–90% and an average CFR of 50%. The disease caused by EBOV, called Ebola virus disease, previously known as Ebola hemorrhagic fever, is fatal. In West Africa (2014–2016), the recent Ebola outbreak in Guinea, Liberia, and Sierra Leone led to 11,323 deaths out of 28,646 infected cases with ~46% CFR [6].
Ebola VP40 is an EBOV viral matrix protein responsible for viral assembly and virus budding in host cells [128]. Since 2016, Pleet et al., have worked extensively on EBOV and exosomes infection control [129], [130], [131]. The group reported exploiting the ESCRT pathway by EBOV, which plays a crucial role during viral budding in exosomes. The study suggested the EBOV VP40 mediates upregulation of extracellular CD63 and Alix, ESCRT proteins, which may increase the biogenesis of exosomes. Furthermore, the EBOV VP40 is secreted in exosomes and can induce apoptosis of uninfected recipient T-cells and bystander lymphopenia [129]. The data also suggest the role of exosomes secreted from the infected cell to deregulate the RNAi pathway (RNA interface) responsible for generating an innate antiviral response in the host.
7.4.1. Role of exosomes in the therapy and diagnosis of Ebola virus infections
Targeting the exosome biogenesis pathway can be a potential therapy against Ebola. Oxytetracycline, an FDA-approved exosome biogenesis down regulator, showed a positive effect on T-cells viability transfected with EBOV VP40. The improved T-cell viability can be explained by the downregulation of exosome release and the EBOV VP40 matrix protein responsible for cell apoptosis [129]. To confirm the results in vivo, the safety, and the benefits on the host cell and redox regulation of the whole system, further studies will be required. Numerous studies have suggested that exosomes may be used as a therapy for various infections [115] including HIV and hepatitis. The presence of EBOV VP40 matrix viral proteins and other glycoproteins (GP) in exosomes released from the infected cell is well established. Hence, their different role in immunomodulation can be investigated, which can be further exploited to develop vaccines and medicine against Ebola.
Anticoli et al., reported an exosome-based vaccine approach for virus infections such as Ebola and HIV-1. The platform was based on the cytotoxic T lymphocyte (CTL) immunization against viral infections [132]. Kyle et al., reported that Ebola has a considerable effect on lipid metabolism homeostasis based on the analysis of blood samples collected during the largest Ebola outbreak in West Africa from 2014 to 2016. The study suggested an increased level of few lipid classes in survivors, whereas few different lipids classes were increased in fatalities. Furthermore, it suggests that exosomes may play a role in lipid metabolism [133]. Several lipids were found to be high in the exosomes released by infected cells [134]. Lipid profiling can aid in monitoring the disease's state and recovery and identify biomarkers. Nanotraps (NT) is composed of cross-linked polymer particles that are functionalized and environment-friendly, allowing them to capture and retain a specific protein. The NT hydrogel particles, which can concentrate the exosomes to detect EBOV VP40 viral protein, can be a potential EBOV diagnostic tool [129].
7.5. Epstein-Barr Virus (EBV) and exosomes
Herpesvirus 4, also called EBV, is a double-stranded DNA disease that belongs to the herpesviridae family. The EBV is responsible for several lymphoid and epithelial malignancies such as infectious mononucleosis, Hodgkin's lymphoma, Non-Hodgkin's lymphoma, Burkitt's lymphoma, and post-transplant lymphoproliferative disease, gastric carcinoma, and nasopharyngeal carcinoma [135]. In addition, the EBV transforms resting B-cells into oncogenic lymphoblastoid cell lines and accounts for 1.8% of cancer-associated deaths globally [136]. In addition, it establishes various latent gene expression programmers in resting and proliferating cells known as latency 0, I, II, and III.
7.5.1. Exosomes in the transmission of EBV
Different studies have examined the role of virus-derived exosomes in modulating cancer development and progression in EBV infections. By affecting cell proliferation, transformation, and immune response invasion, viral miRNA plays an important role in the EBV life cycle and pathogenesis. To evade and adapt to host immune response, the EBV virus exploits host miRNA machinery [137]. Exosomes contain miRNA encoded by EBV that can cause T-cell inhibitory exosomes to be secreted during infection. The secretion of viral nuclear proteins and cell membrane proteins such as “Epstein-Barr nuclear antigen-2 (EBNA-2)” and latent membrane protein 1 (LMP-1) in EBV infected cells is reported in various studies [138], [139], [140]. LMP1 is an essential viral oncogene protein generally expressed in EBV-associated carcinogenesis in latency type II and III. LMP1 surface-bound exosomes modulate the host immune system by inhibiting T-cell activation and increasing natural killer cell toxicity [141]. Verweij et al., suggest that EBV utilizes the CD63-mediated endosomal-exosome pathway to downregulate NF-<UNK>B's chronic activation of NF-κB. As a result, LMP1 secreted into exosomes plays a crucial role in invading immune response and aids in the proliferation of EBV-infected cells [142]. An individual researcher also suggested that the LMP-1 internalized into exosomes to escape degradation induces upregulation and concentration of growth factor receptor and fibroblast growth factor-2 or receptor in exosomes, and modulates target cell phenotype [143], [144]. Another study by Nanbo et al., suggested that the LMP-1 mediates overexpression of the ICAM-1 intracellular adhesion molecule and led to the proliferation of target cells. The in vitro study also suggested caveolae-dependent endocytosis of exosomes in target cells and modulation of the phenotypic changes by unloading viral factors [145]. The exosomes released from EBV infected cells carry non-coding EBV RNAs and LMPs and are responsible for cellular transformation and innate antiviral immunity. These viral RNAs require further investigation to explore their role in the pathogenesis of EBV [146], [147]. EBV encoded small RNAs (EBERs), play a significant role in the activation of innate immunity by induction of DCs maturation and type-1 IFN and inflammatory cytokines via TLR3. However, EBERs are also associated with the immunopathologic disease caused by active EBV infection as well as activation of T of NK cells [147].
7.5.2. Exosomes in therapy and diagnosis of EBV infections
The exosomes derived from EBV infected cells express virus-specific nuclear proteins and cell membrane-specific proteins such as LMP-1 and EBNA-2. The exosomes' significant role in viral infection persistence and spread in the host makes it crucial in developing diagnostic or prognostic biomarkers and anticancer therapy. Houali et al., reported the LMP1 and BARF1 oncoproteins as a potential diagnostic marker based on the serum and saliva samples collected from the nasopharyngeal carcinoma patients (NPC). Saliva and serum body fluids contain exosomes that are effectively secreted and separated. Virus-derived oncoproteins were detected in NPCs, and they were associated with exosome-like vesicles [148]. Researchers identified the LMP-1-LMP-2a mRNA as a potential prognostic biomarker of extranodal NK/T-cell lymphoma, nasal type, which is distinct from other types of lymphomas [149]. Various studies have suggested a potential role of various proteins in prognosis and diagnosis are isolated from EBV-derived exosomes such as LMP-1, LMP-2a, BARF1, HIF1α, EBV DNA, EBV miRNA, EBV mRNA, and growth factors [150], [151], [152], [153].
The role of exosomes in modulating immune response and phenotype of cells aiding in evasion of virus from immune response and the formation and progression of cancer cells are well reported. In such a scenario, EBV-derived exosomes can be developed as a potential anticancer target to inhibit the proliferation and spreading of the infection [154]. Furthermore, the obstruction in the biogenesis and release of the exosomes can alleviate the down-regulated immune activity. The various oncogenic EBV-derived nuclear and cell membrane proteins such as LMP1, LMP2, BARF1, and EBNA-1, which play a vital role in spreading infection, are transported by exosomes to uninfected cells. Based on preclinical data on nasopharyngeal carcinoma patients, Cao et al., carried out clinical trials of safe and tolerable DNAzyme based adjuvant therapy on nasopharyngeal carcinoma patients. The study suggested a potential role of DNAzyme, a small catalytic DNA which downregulates the LMP1, for the treatment of nasopharyngeal carcinoma. The DNAzyme based adjuvant therapy during routine radiotherapy exhibited significant short-term tumor regression without severe side effects [155]. Various studies suggesting the oncogenic proteins of EBV as a potential anticancer target are under investigation [156], [157], [158].
Additionally, exosomes contribute to the development of antiviral immune responses as well as the spread of infection. Viral components trigger pattern recognition receptors (PPRs) and thereby stimulate the immune response of the host. Aziza and the group previously demonstrated that EBV encoded dUTPase activated TNF-*, IL1, IL-6, and IL-8 through the toll-like receptor 2 (TLR2) signaling pathway [159]. Due to an inflammatory response, impaired adaptive immune function, and increased cell proliferation, disease pathogenesis can occur.
Furthermore, EBV encoded dUTPase containing exosomes modulate the innate and adaptive immune response in human dendritic cells and mononuclear cells by B cell stimulatory action [160]. The significant role of dUTPase makes it a potential target for the development of antiviral therapeutics. Vallhov et al., investigated the specific targeting of exosomes derived from different cells and observed that the EBV transformed cells preferentially target B cells. The study suggested that EBV-derived LMP1 containing exosomes interact with B cells through EBV encoded glycoprotein gp350 and receptor CD21. Blocking this interaction blocks the EV uptake by B cells preventing the infection. The study suggested gp350 expressed exosomes effectively blocked EBV infection spread in vitro and emphasizes the potential of developing an exosome-based vaccine specifically targeting B cells for therapeutics [161].
8. Exosomes in personalized medicine
A patient's exosomes contain a wealth of information about their individual health conditions. To achieve an effective outcome, individualized or customized medicine is necessary due to distinct genetics, environment, and personal habits that affect individual health. As a result, EVs have been successfully applied to the diagnosis and prognosis of cancer. In terms of early diagnosis and personalized treatment, liquid biopsy-based on EVs has proved to be beneficial [162]. Various studies suggest the potential of exosome genomics in deciding the therapy. Kato et al., presented significant findings suggesting the presence of a higher level of p-glycoprotein in docetaxel-resistant patients' exosomes than docetaxel non-resistant patient exosomes. In order to overcome docetaxel resistance caused by the multidrug resistance protein 1 (MDR1) gene, taxane can be used to treat castration-resistant prostate cancer [163]. Personalized medicine is plagued by poor diagnostics and biomarkers. The exosomes based adaptive therapy brings together patients response-dependent treatment plans in personalized medicine [162].
The non-invasiveness, easy accessibility, and cell-specific origin of exosomes make them favorable as diagnostic biomarkers. In combination with existing diagnostic tools, the exosomes can further improve the accuracy and sensitivity of diagnostic markers. The exosome DNA represents the whole genomic DNA and mutations present in the origin cells, which can be further employed to predict diseases induced by mutations [164].
Piffoux and his colleagues reviewed the allogeneic exosomes and personalized cancer immunotherapy [165]. There has been considerable attention paid to the physical triggering of exosomes for theragnostic by inducing high exosome loading, secretion, and therapeutic effectiveness. With bioengineered exosomes, cellular targeted delivery can be devised, and their biodistribution and interaction can be controlled. Small size, lipidic bilayer, and indigenous nature provide low immunogenicity, high uptake and prevent phagocytosis. In addition, exosomes possess a protective nature, which makes them potential carriers in therapy. As a result, exosomes can be used in personalized medicine to treat various viral infections and virus-mediated cancers.
9. Exosomes and critical challenges
To utilize the exosome's endogenous nature of specific cell-to-cell communication in therapeutics such as protein and peptide delivery, RNA delivery, gene delivery, vaccination, and immunotherapy is underway. Techniques for isolating and purifying exosomes pose the biggest challenge in exosome-based applications. The application of techniques depends on the application area; however, the recovery and specificity vary from technique to technique. There is a need for standardized techniques for the isolation and purification of exosomes. The techniques that would be specific, viable, easy to replicate, high throughput, and high recovery. The endogenous nature of the exosomes provides in vivo stability in circulation and prevents them from phagocytosis and macrophages mediated degradation. However, the half-life of exosomes in vivo is approximately 2–20 min [166]. The already present endogenous content limits the exogenous loading of the drug reducing the loading efficiency.
Various methods have been reported for exosome drug loadings, such as preloading and post-loading; however, there is a need to readdress them to achieve optimal performance. During the large-scale production of exosomes, the parameters like scalability, variability, purity, potency, and reproducibility of the produced exosomes raise serious concerns. The large-scale production and translation to clinics will be costly. The role of exosomes needs to be studied in-depth for a better understanding of harnessing exosomes in biomedical applications [167].
The drug-loaded and tagged exosomes might behave differently in vivo. The pharmacokinetics (PK) and pharmacodynamics of the therapeutic exosomes may vary from the already existing exosomes. Long-term safety and toxicity studies are needed to investigate exosomes' effect upon in vivo exposure thoroughly. The in vitro models can be employed to investigate the effect of drug-loaded exosomes on recipient cells, uptake mechanism, intracellular and extracellular behavior, and drug unloading mechanisms. Further in vivo methods and physiologically based pharmacokinetic models can investigate the PK of therapeutic exosomes [168]. The development of artificial exosomes is an excellent alternative for translational medicine and is capable of addressing the above-mentioned vital challenges and producing homogenous exosomes. The development of artificial exosomes is underway and holds a promising future in exosome-based biomedical applications.
10. Conclusion and prospects
In order to address the unmet need in the treatment and prognosis of viral diseases, the dual role of exosomes in transmitting various diseases and enhancing the innate immunity of host cells can be exploited. Exosomes have significant potential as a therapeutic, prognostic, and diagnostic biomarker for various diseases. Barriers to overcome include isolation and purification techniques, biochemical compositions, and in-depth details of the uptake mechanism. The exosome-based delivery platform paves the way for safer and more efficient delivery of siRNA, miRNA, and proteins with little toxicity and immunogenicity. In patients exposed to chronic conditions, the prognosis of the disease may help increase life expectancy. Even though exosomes possess tremendous potential, their translation to clinics is hampered by various barriers at both isolation and production levels. The storage and stability of exosomes are also critical aspects that must be addressed. According to origin and health conditions, exosome composition differs, determining its function after releasing in extracellular space. The dual role of exosomes further complicates the isolation and application of the exosomes in theranostics. There is a need for extensive research to unravel the exosomes' biogenesis and differentiate between exosomes that aid in disease transmission and exosomes that set off the innate immunity of the host.
CRediT authorship contribution statement
Pinal Chaudhari: Conceptualization, Writing – original draft, Writing – review & editing. Vivek Ghate: Conceptualization, Visualization, Writing – review & editing. Madhavan Nampoothiri: Writing – review & editing, Supervision. Shaila Lewis: Conceptualization, Supervision, Project administration, Writing – review & editing.
Declaration of Competing Interest
None.
References
- 1.Pekosz A., Glass G.E. Maryland Medicine : MM : A Publication of MEDCHI, the Maryland State Medical Society. Vol. 9. 2008. Emerging viral diseases; p. 11. [Google Scholar]
- 2.Mourya D., Yadav P., Ullas P., Bhardwaj S., Sahay R., Chadha M. Indian Journal of Medical Research. Vol. 149. Wolters Kluwer Medknow Publications; 2019. Emerging/re-emerging viral diseases & new viruses on the Indian horizon [Internet] pp. 447–467. cited 2021 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.2020. Ebola Virus Disease [Internet] cited 2020 Apr 28. [Google Scholar]
- 4.Shepard C.W., Simard E.P., Finelli L., Fiore A.E., Bell B.P. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol. Rev. 2006;28(1):112–125. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- 5.WHO . WHO; 2010. Chapter 3: HIV/AIDS: Confronting a Killer. [Google Scholar]
- 6.2020. Ebola Virus Disease [Internet] cited 2020 Apr 26. [Google Scholar]
- 7.Experimental Ebola Vaccine Safe, Prompts Immune Response | National Institutes of Health (NIH) [Internet]. [cited 2022 Mar 7].
- 8.Researching Ebola in Africa | NIH: National Institute of Allergy and Infectious Diseases [Internet]. [cited 2022 Mar 7].
- 9.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al Kaabi N., Zhang Y., Xia S., Yang Y., Al Qahtani M.M., Abdulrazzaq N., et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J.H., Marks F., Clemens J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat. Med. 2021;27(2):205–211. doi: 10.1038/s41591-021-01230-y. [DOI] [PubMed] [Google Scholar]
- 12.Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai J., Su Y., Zhong S., Cong L., Liu B., Yang J. Signal Transduction and Targeted Therapy. Vol. 5. Springer Nature; 2020. Exosomes: key players in cancer and potential therapeutic strategy [Internet] pp. 1–10. cited 2021 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller S., Rupp C., Stoeck A., Runz S., Fogel M., Lugert S., et al. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007;72(9):1095–1102. doi: 10.1038/sj.ki.5002486. [DOI] [PubMed] [Google Scholar]
- 15.Madison M.N., Jones P.H., Okeoma C.M. Exosomes in human semen restrict HIV-1 transmission by vaginal cells and block intravaginal replication of LP-BM5 murine AIDS virus complex. Virology. 2015;482:189–201. doi: 10.1016/j.virol.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yagi Y., Ohkubo T., Kawaji H., Machida A., Miyata H., Goda S., et al. Next-generation sequencing-based small RNA profiling of cerebrospinal fluid exosomes. Neurosci. Lett. 2017;636:48–57. doi: 10.1016/j.neulet.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 17.Qin W., Tsukasaki Y., Dasgupta S., Mukhopadhyay N., Ikebe M., Sauter E.R. Exosomes in human breast milk promote EMT. Clin. Cancer Res. 2016;22(17):4517–4524. doi: 10.1158/1078-0432.CCR-16-0135. [DOI] [PubMed] [Google Scholar]
- 18.Machida T., Tomofuji T., Ekuni D., Maruyama T., Yoneda T., Kawabata Y., et al. MicroRNAs in salivary exosome as potential biomarkers of aging. Int. J. Mol. Sci. 2015;16(9):21294–21309. doi: 10.3390/ijms160921294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian C., Xiao Y., Wang J., Li Y., Li S., Wei B., et al. Rapid exosomes concentration and in situ detection of exosomal microRNA on agarose-based microfluidic chip. Sensors Actuators B Chem. 2021;333 [Google Scholar]
- 20.Wang J., Ma P., Kim D.H., Liu B.F., Demirci U. Nano Today. Vol. 37. Elsevier B.V.; 2021. Towards microfluidic-based exosome isolation and detection for tumor therapy; p. 101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu W., Hurley J., Roberts D., Chakrabortty S., Enderle D., Noerholm M., et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann. Oncol. 2021;32(4):466–477. doi: 10.1016/j.annonc.2021.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen K., Jia Y., Wang X., Zhang J., Liu K., Wang J., et al. Exosomes from adipose-derived stem cells alleviate the inflammation and oxidative stress via regulating Nrf2/HO-1 axis in macrophages. Free Radic. Biol. Med. 2021;165:54–66. doi: 10.1016/j.freeradbiomed.2021.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z., Zhao Y., Zhao Y., Zhang Y., Yao X., Hang R. Exosomes secreted by macrophages upon copper ion stimulation can promote angiogenesis. Mater. Sci. Eng .C. 2021;123 doi: 10.1016/j.msec.2021.111981. [DOI] [PubMed] [Google Scholar]
- 24.Huang C., Luo W., Wang Q., Ye Y., Fan J., Lin L., et al. Human mesenchymal stem cells promote ischemic repairment and angiogenesis of diabetic foot through exosome miRNA-21-5p. Stem Cell Res. 2021;52 doi: 10.1016/j.scr.2021.102235. [DOI] [PubMed] [Google Scholar]
- 25.Bebelman M.P., Smit M.J., Pegtel D.M., Baglio S.R. Pharmacology and Therapeutics. Vol. 188. Elsevier Inc.; 2018. Biogenesis and function of extracellular vesicles in cancer; pp. 1–11. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Liu Y., Liu H., Tang W.H. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9(1):1–18. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vlassov A.V., Magdaleno S., Setterquist R., Conrad R. Biochimica et Biophysica Acta - General Subjects. Elsevier; 2012. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials; pp. 940–948. [DOI] [PubMed] [Google Scholar]
- 28.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Nature Cell Biology. Vol. 21. Nature Publishing Group; 2019. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication; pp. 9–17. [DOI] [PubMed] [Google Scholar]
- 29.Mulcahy L.A., Pink R.C., Carter D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell Vesicles. 2014;3(1):24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anel A., Gallego-Lleyda A., de Miguel D., Naval J., Martínez-Lostao L. Role of exosomes in the regulation of T-cell mediated immune responses and in autoimmune disease. Cells. 2019;8(2):154. doi: 10.3390/cells8020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindenbergh M.F., Stoorvogel W. Annual Review of Immunology. Vol. 36. Annual Reviews Inc.; 2018. Antigen presentation by extracellular vesicles from professional antigen-presenting cells; pp. 435–459. [DOI] [PubMed] [Google Scholar]
- 32.MFS Lindenbergh, Wubbolts R., EGF Borg, Veld E.M., Boes M., Stoorvogel W. Dendritic cells release exosomes together with phagocytosed pathogen; potential implications for the role of exosomes in antigen presentation. J. Extracell Vesicles. 2020;9(1) doi: 10.1080/20013078.2020.1798606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schorey J.S., Cheng Y., Singh P.P., Smith V.L. Exosomes and other extracellular vesicles in host–pathogen interactions. EMBO Rep. 2015;16(1):24–43. doi: 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Z., Yu S., Li M., Gui X., Li P. Isolation of exosome-like nanoparticles and analysis of MicroRNAs derived from coconut water based on small RNA high-throughput sequencing. J. Agric. Food Chem. 2018;66(11):2749–2757. doi: 10.1021/acs.jafc.7b05614. [DOI] [PubMed] [Google Scholar]
- 35.Rome S. Biological properties of plant-derived extracellular vesicles. Food Funct. 2019;10(2):529–538. doi: 10.1039/c8fo02295j. [DOI] [PubMed] [Google Scholar]
- 36.Mukhtar M., Arshad M., Ahmad M., Pomerantz R.J., Wigdahl B., Parveen Z. Antiviral potentials of medicinal plants. Virus Res. 2008;131:111–120. doi: 10.1016/j.virusres.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundaram K., Miller D.P., Kumar A., Teng Y., Sayed M., Mu J., et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of porphyromonas gingivalis. iScience. 2019;21:308–327. doi: 10.1016/j.isci.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang M., Viennois E., Xu C., Merlin D. Tissue Barriers. Vol. 4. Taylor and Francis Inc; 2016. Plant derived edible nanoparticles as a new therapeutic approach against diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akuma P., Okagu O.D., Udenigwe C.C. Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Front. Sustain. Food Syst. 2019;3:23. [Google Scholar]
- 40.Yang X.X., Sun C., Wang L., Guo X.L. Journal of Controlled Release. Vol. 308. Elsevier B.V.; 2019. New insight into isolation, identification techniques and medical applications of exosomes [Internet] pp. 119–129. cited 2020 May 31. [DOI] [PubMed] [Google Scholar]
- 41.Davies R.T., Kim J., Jang S.C., Choi E.J., Gho Y.S., Park J. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab. Chip. 2012;12(24):5202–5210. doi: 10.1039/c2lc41006k. [DOI] [PubMed] [Google Scholar]
- 42.Zöller M. Nature Reviews Cancer. Vol. 9. 2009. Tetraspanins: push and pull in suppressing and promoting metastasis [Internet] pp. 40–55. cited 2020 Apr 26. [DOI] [PubMed] [Google Scholar]
- 43.Popowski K.D., Dinh P.C., George A., Lutz H., Cheng K. Exosome therapeutics for COVID-19 and respiratory viruses. View. 2021;2(3):20200186. doi: 10.1002/VIW.20200186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harding C.V., Heuser J.E., Stahl P.D. Journal of Cell Biology. Vol. 200. The Rockefeller University Press; 2013. Exosomes: looking back three decades and into the future; pp. 367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69(4):635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.2020. WHO EMRO | MERS Situation Update, January 2020 | MERS-CoV | Epidemic and Pandemic Diseases [Internet] [Google Scholar]
- 47.Chan-Yeung M., Xu R.H. Respirology. Vol. 8. Wiley-Blackwell; 2003. SARS: epidemiology [internet] p. S9. cited 2021 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mortality Analyses - Johns Hopkins Coronavirus Resource Center [Internet]. [cited 2021 Nov 26].
- 49.Huang Y., Yang C., Feng Xu X., Xu W., Wen Liu S. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020;41(9):1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Astuti I., Ysrafil. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab. Syndr. 2020;14(4):407. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barberis E., Vanella V.V., Falasca M., Caneapero V., Cappellano G., Raineri D., et al. Circulating exosomes are strongly involved in SARS-CoV-2 infection. Front. Mol. Biosci. 2021;8:1. doi: 10.3389/fmolb.2021.632290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bebelman M.P., Smit M.J., Pegtel D.M., Baglio S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018;188:1–11. doi: 10.1016/j.pharmthera.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 53.Earnest J.T., Hantak M.P., Li K., McCray P.B., Perlman S., Gallagher T. The tetraspanin CD9 facilitates MERS-coronavirus entry by scaffolding host cell receptors and proteases. PLoS Pathog. 2017;13(7) doi: 10.1371/journal.ppat.1006546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klaus J.P., Eisenhauer P., Russo J., Mason A.B., Do D., King B., et al. The intracellular cargo receptor ERGIC-53 is required for the production of infectious arenavirus, coronavirus, and filovirus particles. Cell Host Microbe. 2013;14(5):522–534. doi: 10.1016/j.chom.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gunasekaran M., Bansal S., Ravichandran R., Sharma M., Perincheri S., Rodriguez F., et al. Respiratory viral infection in lung transplantation induces exosomes that trigger chronic rejection. J. Heart Lung Transplant. 2020;39(4):379–388. doi: 10.1016/j.healun.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J., Chen S., Bihl J. Exosome-mediated transfer of ACE2 (Angiotensin-converting enzyme 2) from endothelial progenitor cells promotes survival and function of endothelial cell. Oxidative Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/4213541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kwon Y., Nukala S.B., Srivastava S., Miyamoto H., Ismail N.I., Jousma J., et al. Detection of viral RNA fragments in human iPSC cardiomyocytes following treatment with extracellular vesicles from SARS-CoV-2 coding sequence overexpressing lung epithelial cells. Stem Cell Res. Ther. 2020;11(1):1–5. doi: 10.1186/s13287-020-02033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang I.C., Bailey C.C., Weyer J.L., Radoshitzky S.R., Becker M.M., Chiang J.J., et al. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza a virus. PLoS Pathog. 2011;7(1) doi: 10.1371/journal.ppat.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feeley E.M., Sims J.S., John S.P., Chin C.R., Pertel T., Chen L.M., et al. IFITM3 inhibits influenza a virus infection by preventing cytosolic entry. PLoS Pathog. 2011;7(10) doi: 10.1371/journal.ppat.1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Novak J.A. Exosomes: Antiviral agents in the human lung. Master’s theses. 2013 https://ecommons.luc.edu/cgi/viewcontent.cgi?article=2466&context=luc_theses [Google Scholar]
- 62.Kuate S., Cinatl J., Doerr H.W., Überla K. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology. 2007;362(1):26–37. doi: 10.1016/j.virol.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teng Y., Xu F., Zhang X., Mu J., Sayed M., Hu X., et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol. Ther. 2021;29(8):2424–2440. doi: 10.1016/j.ymthe.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.A pilot clinical study on inhalation of mesenchymal stem cells exosomes treating severe novel coronavirus pneumonia. Case Med. Res. 2020:1–10. [Google Scholar]
- 65.HUMSCs and exosomes treating patients with lung injury following novel coronavirus pneumonia (COVID-19) ChiCTR2000030484. Chin. Clin. Trial Regist. 2020:1–4. [Google Scholar]
- 66.Kumar S., Zhi K., Mukherji A., Gerth K. Repurposing antiviral protease inhibitors using extracellular vesicles for potential therapy of COVID-19 [Internet] Viruses. MDPI AG. 2020;12 doi: 10.3390/v12050486. [cited 2021 May 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsai S.J., Atai N.A., Cacciottolo M., Nice J., Salehi A., Guo C., et al. Exosome-mediated mRNA delivery in vivo is safe and can be used to induce SARS-CoV-2 immunity. J. Biol. Chem. 2021;297(5) doi: 10.1016/j.jbc.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fu Y., Xiong S. Tagged extracellular vesicles with the RBD of the viral spike protein for delivery of antiviral agents against SARS-COV-2 infection. J. Control. Release. 2021;335(March):584–595. doi: 10.1016/j.jconrel.2021.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anand K., Vadivalagan C., Joseph J.S., Singh S.K., Gulati M., Shahbaaz M., et al. A novel nano therapeutic using convalescent plasma derived exosomal (CPExo) for COVID-19: a combined hyperactive immune modulation and diagnostics. Chem. Biol. Interact. 2021;344(May) doi: 10.1016/j.cbi.2021.109497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dauletova M., Hafsan H., Mahhengam N., Zekiy A.O., Ahmadi M., Siahmansouri H. Mesenchymal stem cell alongside exosomes as a novel cell-based therapy for COVID-19: a review study. Clin. Immunol. 2021;226(March) doi: 10.1016/j.clim.2021.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rezakhani L., Kelishadrokhi A.F., Soleimanizadeh A., Rahmati S. Mesenchymal stem cell (MSC)-derived exosomes as a cell-free therapy for patients infected with COVID-19: real opportunities and range of promises. Chem. Phys. Lipids. 2021;234 doi: 10.1016/j.chemphyslip.2020.105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barberis E., Vanella V.V., Falasca M., Caneapero V., Cappellano G., Raineri D., et al. Circulating exosomes are strongly involved in SARS-CoV-2 infection. Front. Mol. Biosci. 2021;8:29. doi: 10.3389/fmolb.2021.632290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Balbi C., Burrello J., Bolis S., Lazzarini E., Biemmi V., Pianezzi E., et al. Circulating extracellular vesicles are endowed with enhanced procoagulant activity in SARS-CoV-2 infection. EBioMedicine. 2021;67 doi: 10.1016/j.ebiom.2021.103369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosell A., Havervall S., Von Meijenfeldt F., Hisada Y., Aguilera K., Grover S.P., et al. Patients with COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality—brief report. Arterioscler. Thromb. Vasc. Biol. 2021;41(2):878. doi: 10.1161/ATVBAHA.120.315547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caskey M., Schoofs T., Gruell H., Settler A., Karagounis T., Kreider E.F., et al. Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nat. Med. 2017;23(2):185–191. doi: 10.1038/nm.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caskey M., Klein F., Lorenzi J.C.C., Seaman M.S., West A.P., Buckley N., et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522(7557):487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gould S.J., Booth A.M., JEK Hildreth. Proceedings of the National Academy of Sciences of the United States of America. Vol. 100. National Academy of Sciences; 2003. The Trojan exosome hypothesis; pp. 10592–10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kramer B., Pelchen-Matthews A., Deneka M., Garcia E., Piguet V., Marsh M. HIV interaction with endosomes in macrophages and dendritic cells. Blood Cells Mol. Dis. 2005;35(2):136–142. doi: 10.1016/j.bcmd.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen D.G., Booth A., Gould S.J., Hildreth J.E.K. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J. Biol. Chem. 2003;278(52):52347–52354. doi: 10.1074/jbc.M309009200. [DOI] [PubMed] [Google Scholar]
- 82.S Welsch , OT Keppler , A Habermann , I Allespach , J Krijnse-Locker , H-G Krä . HIV-1 Buds Predominantly at the Plasma Membrane of Primary Human Macrophages. [DOI] [PMC free article] [PubMed]
- 83.Deneka M., Pelchen-Matthews A., Byland R., Ruiz-Mateos E., Marsh M. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J. Cell Biol. 2007;177(2):329–341. doi: 10.1083/jcb.200609050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nkwe D.O., Pelchen-Matthews A., Burden J.J., Collinson L.M., Marsh M. The intracellular plasma membrane-connected compartment in the assembly of HIV-1 in human macrophages. BMC Biol. 2016;14:50. doi: 10.1186/s12915-016-0272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Willms E., Johansson H.J., Mäger I., Lee Y., Blomberg K.E.M., Sadik M., et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016;6(1):1–12. doi: 10.1038/srep22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carlton J.G., Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science (80- ) 2007;316(5833):1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 87.Nabhan J.F., Hu R., Oh R.S., Cohen S.N., Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. U. S. A. 2012;109(11):4146–4151. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kadiu I., Narayanasamy P., Dash P.K., Zhang W., Gendelman H.E. Biochemical and biologic characterization of exosomes and microvesicles as facilitators of HIV-1 infection in macrophages. J. Immunol. 2012;189(2):744–754. doi: 10.4049/jimmunol.1102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Das S.R., Jameel S. Biology of the HIV nef protein. Indian J. Med. Res. 2005;121:315–332. [PubMed] [Google Scholar]
- 90.Campbell T.D., Khan M., Huang M.-B., Bond V.C., Powell M.D. 2008. HIV-1 Nef Protein Is Secreted Into Vesicles That Can Fuse with Target Cells and Virions. [PMC free article] [PubMed] [Google Scholar]
- 91.Mukhamedova N., Hoang A., Dragoljevic D., Dubrovsky L., Pushkarsky T., Low H., et al. Exosomes containing HIV protein nef reorganize lipid rafts potentiating inflammatory response in bystander cells. PLoS Pathog. 2019;15(7) doi: 10.1371/journal.ppat.1007907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muratori C., Cavallin L.E., Krätzel K., Tinari A., De Milito A., Fais S., et al. Massive secretion by T cells is caused by HIV nef in infected cells and by nef transfer to bystander cells. Cell Host Microbe. 2009;6(3):218–230. doi: 10.1016/j.chom.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 93.Arenaccio C., Chiozzini C., Columba-Cabezas S., Manfredi F., Federico M. Cell activation and HIV-1 replication in unstimulated CD4+ T lymphocytes ingesting exosomes from cells expressing defective HIV-1. Retrovirology. 2014;11(1):46. doi: 10.1186/1742-4690-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nazimek K., Ptak W., Nowak B., Ptak M., Askenase P.W., Bryniarski K. Macrophages play an essential role in antigen-specific immune suppression mediated by T CD8+ cell-derived exosomes. Immunology. 2015;146(1):23–32. doi: 10.1111/imm.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tumne A., Prasad V.S., Chen Y., Stolz D.B., Saha K., Ratner D.M., et al. Noncytotoxic suppression of human immunodeficiency virus type 1 transcription by exosomes secreted from CD8+ T cells. J. Virol. 2009;83(9):4354–4364. doi: 10.1128/JVI.02629-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ellwanger J.H., Veit T.D., Chies J.A.B. Exosomes in HIV infection: a review and critical look. Infect. Genet. Evol. 2017;53:146–154. doi: 10.1016/j.meegid.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 97.Narayanan A., Iordanskiy S., Das R., Van Duyne R., Santos S., Jaworski E., et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J. Biol. Chem. 2013;288(27):20014–20033. doi: 10.1074/jbc.M112.438895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Näslund T.I., Paquin-Proulx D., Paredes P.T., Vallhov H., Sandberg J.K., Gabrielsson S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS. 2014;28(2):171–180. doi: 10.1097/QAD.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 99.JL Welch , H Kaddour , PM Schlievert , JT Stapleton , CMOkeoma . Semen exosomes promote transcriptional silencing of HIV-1 by disrupting NF-kB/Sp1/Tat circuitry 1 2 Running title: Negative Regulation of HIV-1 Transcription by Semen exosomes The abstract contains 231 words. The text contains 6,510 words. Downloaded from. J Virol. 2018. [DOI] [PMC free article] [PubMed]
- 100.Rezaie J., Aslan C., Ahmadi M., Zolbanin N.M., Kashanchi F., Jafari R. Cell and Bioscience. Vol. 11. BioMed Central Ltd; 2021. The versatile role of exosomes in human retroviral infections: from immunopathogenesis to clinical application [Internet] pp. 1–15. cited 2021 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pang S.-W., Teow S.-Y. Exosomes. Elsevier; 2020. Emerging therapeutic roles of exosomes in HIV-1 infection; pp. 147–178. [Google Scholar]
- 102.Lattanzi L., Federico M. A strategy of antigen incorporation into exosomes: comparing cross-presentation levels of antigens delivered by engineered exosomes and by lentiviral virus-like particles. Vaccine. 2012;30(50):7229–7237. doi: 10.1016/j.vaccine.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 103.Liang T.J. Hepatitis B: the virus and disease. Hepatology. 2009;49(SUPPL. 5):S13. doi: 10.1002/hep.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Razavi-Shearer D., Gamkrelidze I., Nguyen M.H., Chen D.S., Van Damme P., Abbas Z., et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol. Hepatol. 2018;3(6):383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 105.2016. Hepatitis C [Internet] cited 2020 Apr 26. [Google Scholar]
- 106.Tamai K., Shiina M., Tanaka N., Nakano T., Yamamoto A., Kondo Y., et al. Regulation of hepatitis C virus secretion by the hrs-dependent exosomal pathway. Virology. 2012;422(2):377–385. doi: 10.1016/j.virol.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 107.Kouwaki T., Fukushima Y., Daito T., Sanada T., Yamamoto N., Mifsud E.J., et al. Extracellular vesicles including exosomes regulate innate immune responses to hepatitis B virus infection. Front. Immunol. 2016;7(AUG) doi: 10.3389/fimmu.2016.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang Y., Han Q., Hou Z., Zhang C., Tian Z., Zhang J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol. Immunol. 2017;14(5):465–475. doi: 10.1038/cmi.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kapoor N.R., Chadha R., Kumar S., Choedon T., Reddy V.S., Kumar V. The HBx gene of hepatitis B virus can influence hepatic microenvironment via exosomes by transferring its mRNA and protein. Virus Res. 2017;240(April):166–174. doi: 10.1016/j.virusres.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 110.Liu D.X., Li P.P., Guo J.P., Li L.L., Guo B., Bin Jiao H., et al. Exosomes derived from HBV-associated liver cancer promote chemoresistance by upregulating chaperone-mediated autophagy. Oncol. Lett. 2019;17(1):323–331. doi: 10.3892/ol.2018.9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sukriti S., Choudhary M.C., Maras J.S., Sharma S., Thangariyal S., Singh A., et al. Extracellular vesicles from hepatitis B patients serve as reservoir of hepatitis B virus DNA. J. Viral Hepat. 2019;26(1):211–214. doi: 10.1111/jvh.12995. [DOI] [PubMed] [Google Scholar]
- 112.Ramakrishnaiah V., Thumann C., Fofana I., Habersetzer F., Pan Q., De Ruiter P.E., et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc. Natl. Acad. Sci. U. S. A. 2013;110(32):13109–13113. doi: 10.1073/pnas.1221899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang J., Yao Y., Chen X., Wu J., Gu T., Tang X. Host derived exosomes-pathogens interactions: potential functions of exosomes in pathogen infection. Biomed. Pharmacother. 2018;108(September):1451–1459. doi: 10.1016/j.biopha.2018.09.174. [DOI] [PubMed] [Google Scholar]
- 114.Gorji-bahri G., Moghimi H.R., Hashemi A. RAB5A effect on metastasis of hepatocellular carcinoma cell line via altering the pro-invasive content of exosomes. Exp. Mol. Pathol. 2021;120 doi: 10.1016/j.yexmp.2021.104632. [DOI] [PubMed] [Google Scholar]
- 115.Dreux M., Garaigorta U., Boyd B., Décembre E., Chung J., Whitten-Bauer C., et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12(4):558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yao Z., Qiao Y., Li X., Chen J., Ding J., Bai L., et al. Exosomes exploit the virus entry machinery and pathway to transmit alpha interferon-induced antiviral activity. J. Virol. 2018;92(24) doi: 10.1128/JVI.01578-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jia X., Chen J., Megger D.A., Zhang X., Kozlowski M., Zhang L., et al. Label-free proteomic analysis of exosomes derived from inducible hepatitis B virus-replicating HepAD38 cell line. Mol. Cell. Proteomics. 2017;16(4):S144–S160. doi: 10.1074/mcp.M116.063503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jesus S., Soares E., Cruz M.T., Borges O. Exosomes as adjuvants for the recombinant hepatitis B antigen: first report. Eur. J. Pharm. Biopharm. 2018;133:1–11. doi: 10.1016/j.ejpb.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 119.Wang F., Li L., Piontek K., Sakaguchi M., Selaru F.M. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology. 2018;67(3):940–954. doi: 10.1002/hep.29586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Clayton A., Harris C.L., Court J., Mason M.D., Morgan B.P. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur. J. Immunol. 2003;33(2):522–531. doi: 10.1002/immu.200310028. [DOI] [PubMed] [Google Scholar]
- 121.Takahashi K., Yan I.K., Wood J., Haga H., Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol. Cancer Res. 2014;12(10):1377–1387. doi: 10.1158/1541-7786.MCR-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aucher A., Rudnicka D., Davis D.M. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J. Immunol. 2013;191(12):6250–6260. doi: 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fonsato V., Collino F., Herrera M.B., Cavallari C., Deregibus M.C., Cisterna B., et al. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor MicroRNAs. Stem Cells. 2012;30(9):1985–1998. doi: 10.1002/stem.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lv L.H., Le Wan Y., Lin Y., Zhang W., Yang M., Li G.N., et al. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J. Biol. Chem. 2012;287(19):15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu W.H., Ren L.N., Wang X., Wang T., Zhang N., Gao Y., et al. Combination of exosomes and circulating microRNAs may serve as a promising tumor marker complementary to alpha-fetoprotein for early-stage hepatocellular carcinoma diagnosis in rats. J. Cancer Res. Clin. Oncol. 2015;141(10):1767–1778. doi: 10.1007/s00432-015-1943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sugimachi K., Matsumura T., Hirata H., Uchi R., Ueda M., Ueo H., et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br. J. Cancer. 2015;112(3):532–538. doi: 10.1038/bjc.2014.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sohn W., Kim J., Kang S.H., Yang S.R., Cho J.Y., Cho H.C., et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp. Mol. Med. 2015;47(9) doi: 10.1038/emm.2015.68. e184-e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Soni S.P., Stahelin R.V. The ebola virus matrix protein VP40 selectively induces vesiculation from phosphatidylserine-enriched membranes. J. Biol. Chem. 2014;289(48):33590–33597. doi: 10.1074/jbc.M114.586396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pleet M.L., Mathiesen A., DeMarino C., Akpamagbo Y.A., Barclay R.A., Schwab A., et al. Ebola VP40 in exosomes can cause immune cell dysfunction. Front. Microbiol. 2016;7(NOV) doi: 10.3389/fmicb.2016.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pleet M.L., Erickson J., DeMarino C., Barclay R.A., Cowen M., Lepene B., et al. Ebola virus VP40 modulates cell cycle and biogenesis of extracellular vesicles. J. Infect. Dis. 2018;218(suppl_5):S365–S387. doi: 10.1093/infdis/jiy472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pleet M.L., DeMarino C., Stonier S.W., Dye J.M., Jacobson S., Aman M.J., et al. Vol. 11. Viruses; MDPI AG: 2019. Extracellular vesicles and ebola virus: a new mechanism of immune evasion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Anticoli S., Manfredi F., Chiozzini C., Arenaccio C., Olivetta E., Ferrantelli F., et al. An exosome-based vaccine platform imparts cytotoxic T lymphocyte immunity against viral antigens. Biotechnol. J. 2018;13(4) doi: 10.1002/biot.201700443. [DOI] [PubMed] [Google Scholar]
- 133.Kyle J.E., Burnum-Johnson K.E., Wendler J.P., Eisfeld A.J., Halfmann P.J., Watanabe T. 2018. Plasma Lipidome Reveals Critical Illness and Recovery From Human Ebola Virus Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hosseini H.M., Fooladi A.A.I., Nourani M.R., Ghanezadeh F. The role of exosomes in infectious diseases. Inflamm. Allergy Drug Targets. 2013;12(1):29–37. doi: 10.2174/1871528111312010005. [DOI] [PubMed] [Google Scholar]
- 135.Walling D.M., Hudnall S.D., Yen-Moore A. Mucocutaneous Manifestations of Viral Diseases. CRC Press; 2002. Epstein-Barr virus; pp. 145–172. [Google Scholar]
- 136.Luftig M.A., Stanfield B.A. F1000Research. Vol. 6. Faculty of 1000 Ltd; 2017. Recent advances in understanding Epstein-Barr virus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pegtel D.M., van de Garde M.D.B., Middeldorp J.M. Viral miRNAs exploiting the endosomal–exosomal pathway for intercellular cross-talk and immune evasion. Biochim. Biophys. Acta. 2011;1809(11–12):715–721. doi: 10.1016/j.bbagrm.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 138.Vazirabadi G., Geiger T.R., Coffin W.F., Martin J.M. Epstein-Barr virus latent membrane protein-1 (LMP-1) and lytic-1 LMP-1 localization in plasma membrane-derived extracellular vesicles and intracellular virions. J. Gen. Virol. 2003;84:1997–2008. doi: 10.1099/vir.0.19156-0. [DOI] [PubMed] [Google Scholar]
- 139.Tang Y., Luo C., Cheng A., Lu S., Xu J., Fu T., et al. Expression of latent membrane proteins in Epstein-Barr virus-transformed lymphocytes in vitro. Mol. Med. Rep. 2014;10(2):1117–1121. doi: 10.3892/mmr.2014.2313. [DOI] [PubMed] [Google Scholar]
- 140.Rasul A.E., Nagy N., Sohlberg E., Ádori M., Claesson H.E., Klein G., et al. Simultaneous detection of the two main proliferation driving EBV encoded proteins, EBNA-2 and LMP-1 in single B cells. J. Immunol. Methods. 2012;385(1–2):60–70. doi: 10.1016/j.jim.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 141.Zhao M., Nanbo A., Sun L., Lin Z. Microorganisms. Vol. 7. MDPI AG; 2019. Extracellular vesicles in Epstein-Barr virus’ life cycle and pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Verweij F.J., van Eijndhoven M.A.J., Hopmans E.S., Vendrig T., Wurdinger T., Cahir-McFarland E., et al. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-κB activation. EMBO J. 2011;30(11):2115–2129. doi: 10.1038/emboj.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meckes D.G., Shair K.H.Y., Marquitz A.R., Kung C.P., Edwards R.H., Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc. Natl. Acad. Sci. U. S. A. 2010;107(47):20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ceccarelli S., Visco V., Raffa S., Wakisaka N., Pagano J.S., Torrisi M.R. Epstein-barr virus latent membrane protein 1 promotes concentration in multivesicular bodies of fibroblast growth factor 2 and its release through exosomes. Int. J. Cancer. 2007;121(7):1494–1506. doi: 10.1002/ijc.22844. [DOI] [PubMed] [Google Scholar]
- 145.Nanbo A., Kawanishi E., Yoshida R., Yoshiyama H. Exosomes derived from epstein-barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J. Virol. 2013;87(18):10334–10347. doi: 10.1128/JVI.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Canitano A., Venturi G., Borghi M., Ammendolia M.G., Fais S. Exosomes released in vitro from epstein-barr virus (EBV)-infected cells contain EBV-encoded latent phase mRNAs. Cancer Lett. 2013;337(2):193–199. doi: 10.1016/j.canlet.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 147.Iwakiri D., Takada K. Advances in Cancer Research. Vol. 107. Adv Cancer Res; 2010. Role of EBERs in the pathogenesis of EBV infection; pp. 119–136. [DOI] [PubMed] [Google Scholar]
- 148.Houali K., Wang X., Shimizu Y., Djennaoui D., Nicholls J., Fiorini S., et al. A new diagnostic marker for secreted epstein-barr virus encoded LMP1 and BARF1 oncoproteins in the serum and saliva of patients with nasopharyngeal carcinoma. Clin. Cancer Res. 2007;13(17):4993–5000. doi: 10.1158/1078-0432.CCR-06-2945. [DOI] [PubMed] [Google Scholar]
- 149.Mao Y., Zhang D.-W., Zhu H., Lin H., Xiong L., Cao Q., et al. LMP1 and LMP2A are potential prognostic markers of extranodal NK/T-cell lymphoma, nasal type (ENKTL) Diagn. Pathol. 2012;7(1):178. doi: 10.1186/1746-1596-7-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Teow S.Y., Liew K., ASB Khoo, Peh S.C. International Journal of Biological Sciences. Vol. 13. Ivyspring International Publisher; 2017. Pathogenic role of exosomes in epstein-barr virus (EBV)-associated cancers; pp. 1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stevens S.J.C., Verkuijlen S.A.W.M., Hariwiyanto B., Harijadi Fachiroh J., Paramita D.K., et al. Diagnostic value of measuring epstein-barr virus (EBV) DNA load and carcinoma-specific viral mRNA in relation to anti-EBV immunoglobulin a (IgA) and IgG antibody levels in blood of nasopharyngeal carcinoma patients from Indonesia. J. Clin. Microbiol. 2005;43(7):3066–3073. doi: 10.1128/JCM.43.7.3066-3073.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang G., Zong J., Lin S., Verhoeven R.J.A., Tong S., Chen Y., et al. Circulating epstein-barr virus microRNAs miR-BART7 and miR-BART13 as biomarkers for nasopharyngeal carcinoma diagnosis and treatment. Int. J. Cancer. 2015;136(5):E301–E312. doi: 10.1002/ijc.29206. [DOI] [PubMed] [Google Scholar]
- 153.De Paoli P., Pratesi C., Bortolin M.T. The Epstein Barr virus DNA levels as a tumor marker in EBV-associated cancers. J. Cancer Res. Clin. Oncol. 2007;133:809–815. doi: 10.1007/s00432-007-0281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marleau A.M., Chen C.-S., Joyce J.A., Tullis R.H. Exosome removal as a therapeutic adjuvant in cancer. J. Transl. Med. 2012;10(1):134. doi: 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cao Y., Yang L., Jiang W., Wang X., Liao W., Tan G., et al. Therapeutic evaluation of epstein-barr virus-encoded latent membrane Protein-1 targeted DNAzyme for treating of nasopharyngeal carcinomas. Mol. Ther. 2014;22(2):371–377. doi: 10.1038/mt.2013.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ma B., Hui E.P., King A., To K.F., Mo F., Leung S.F., et al. A phase II study of patients with metastatic or locoregionally recurrent nasopharyngeal carcinoma and evaluation of plasma epstein-barr virus DNA as a biomarker of efficacy. Cancer Chemother. Pharmacol. 2008;62(1):59–64. doi: 10.1007/s00280-007-0575-8. [DOI] [PubMed] [Google Scholar]
- 157.Stoker S.D., Novalić Z., Wildeman M.A., Huitema A.D.R., Verkuijlen S.A.W.M., Juwana H., et al. Epstein-barr virus-targeted therapy in nasopharyngeal carcinoma. J. Cancer Res. Clin. Oncol. 2015;141(10):1845–1857. doi: 10.1007/s00432-015-1969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee H.G., Kim H., Kim E.J., Park P.G., Dong S.M., Choi T.H., et al. Targeted therapy for epstein-barr virus-associated gastric carcinoma using low-dose gemcitabine-induced lytic activation. Oncotarget. 2015;6(31):31018–31029. doi: 10.18632/oncotarget.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ariza M.-E., Glaser R., Kaumaya P.T.P., Jones C., Williams M.V. The EBV-encoded dUTPase activates NF-κB through the TLR2 and MyD88-dependent signaling pathway. J. Immunol. 2009;182(2):851–859. doi: 10.4049/jimmunol.182.2.851. [DOI] [PubMed] [Google Scholar]
- 160.Ariza M.E., Rivailler P., Glaser R., Chen M., Williams M.V. Epstein-Barr virus encoded dUTPase containing exosomes modulate innate and adaptive immune responses in human dendritic cells and peripheral blood mononuclear cells. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0069827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Urbanelli L., Buratta S., Tancini B., Sagini K., Delo F., Porcellati S., et al. The role of extracellular vesicles in viral infection and transmission. Vaccines. 2019;7(3):1–20. doi: 10.3390/vaccines7030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Braig Z. Biomedical Journal. Elsevier B.V; 2019. Personalized medicine: from diagnostic to adaptive. [Google Scholar]
- 163.Kato T., Mizutani K., Kameyama K., Kawakami K., Fujita Y., Nakane K., et al. Serum exosomal P-glycoprotein is a potential marker to diagnose docetaxel resistance and select a taxoid for patients with prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2015;33(9) doi: 10.1016/j.urolonc.2015.04.019. 385.e15-385.e20. [DOI] [PubMed] [Google Scholar]
- 164.Thakur B.K., Zhang H., Becker A., Matei I., Huang Y., Costa-Silva B. Cell Research. Vol. 24. Nature Publishing Group; 2014. Double-stranded DNA in exosomes: a novel biomarker in cancer detection; pp. 766–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Piffoux M., Nicolás-Boluda A., Mulens-Arias V., Richard S., Rahmi G., Gazeau F. Advanced Drug Delivery Reviews. Vol. 138. Elsevier B.V.; 2019. Extracellular vesicles for personalized medicine: the input of physically triggered production, loading and theranostic properties [Internet] pp. 247–258. cited 2020 Jun 4. [DOI] [PubMed] [Google Scholar]
- 166.Wiklander O.P.B., Nordin J.Z., O’Loughlin A., Gustafsson Y., Corso G., Mäger I., et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell Vesicles. 2015;2015(4):1–13. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Meng W., He C., Hao Y., Wang L., Li L., Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585–598. doi: 10.1080/10717544.2020.1748758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bowers E.C., Hassanin A.A., Ramos K.S. Toxicology in Vitro. Vol. 64. Elsevier Ltd; 2020. In vitro models of exosome biology and toxicology: new frontiers in biomedical research; p. 104462. [DOI] [PMC free article] [PubMed] [Google Scholar]