Abstract
N6-methyladenosine (m6A) is the most abundant internal modification in eukaryotic messenger RNA. Although the role of m6A has been demonstrated in many biological processes, including embryonic development, flowering time control, microspore generation, fruit ripening, and stress responses, its contribution to other aspects of plant development still needs to be explored. Herein, we show the potential link between m6A deposition and the expansion of tomato (Solanum lycopersicum) fruits through parallel m6A-immunoprecipitation-sequencing (m6A-seq) and RNA-seq analyses. We found that global m6A levels increased during tomato fruit expansion from immature green to mature green stage. m6A-seq revealed that thousands of protein-coding genes are m6A-modified mainly in the 3ʹ-untranslated regions. m6A-seq and RNA-seq analyses showed a positive association between m6A methylation and mRNA abundance. In particular, a large number of fruit expansion-related genes involved in hormone responses and endoreduplication were m6A modified and expressed more actively than the non-m6A-modified genes, suggesting a potential role of m6A modification in tomato fruit expansion. Importantly, altering m6A levels by direct injection of 3-deazaneplanocin A (DA; m6A writer inhibitor) or meclofenamic acid (MA; m6A eraser inhibitor) into tomato fruits suppressed fruit expansion; however, injection of exogenous DA or MA accelerated or delayed fruit ripening, respectively. Collectively, these results suggest a dynamic role of m6A methylation in the expansion and ripening of tomato fruits.
Global mRNA m6A levels increase during tomato fruit expansion from immature green to mature green stages, and expansion and ripening of tomato fruits require dynamic modification of m6A.
Introduction
RNA molecules are heavily modified co-transcriptionally and posttranscriptionally in all living organisms. To date, over 150 different chemical modifications of cellular RNAs have been identified (Cantara et al., 2011; Boccaletto et al., 2018). In particular, recent advents in high-throughput sequencing technologies have uncovered N6-methyladenosine (m6A) as the most abundant, dynamic, and reversible mRNA modification in eukaryotes (Liu and Pan, 2016; Covelo-Morales et al., 2018). The installation and removal of m6A marks are catalyzed by methyltransferases (referred to as “writers”) and demethylases (referred to as “erasers”), respectively (Meyer and Jaffrey, 2017; Hu et al., 2019; Zheng et al., 2020). Dynamic and reversible m6A modifications in plants are modulated by writer components, including methyltransferase A (MTA), MTB, FKBP12-INTERACTING PROTEIN 37, VIRILIZER, and an E3 ubiquitin ligase HAKAI (Zhong et al., 2008; Bodi et al., 2012; Růžička et al., 2017; Zhang et al., 2019), and eraser proteins, such as Alpha-Ketoglutarate-Dependent Dioxygenase homolog 9B (ALKBH9B) and ALKBH10B (Duan et al., 2017; Martínez-Pérez et al., 2017). An increasing body of evidence has demonstrated the importance of m6A RNA methylation in controlling mRNA metabolism, including RNA processing, stability, splicing, nuclear-to-cytoplasmic export, and translation control (Wang et al., 2014; Meyer et al., 2015; Wang et al., 2015; Xiao et al., 2016; Coots et al., 2017; Shi et al., 2017; Luo et al., 2020), which are all crucial for various biological processes in plants, such as embryogenesis, floral transition, development of trichomes, leaves and roots, sporogenesis, fruit ripening, and abiotic stress responses (Zhong et al., 2008; Bodi et al., 2012; Duan et al., 2017; Růžička et al., 2017; Arribas-Hernández et al., 2018; Scutenaire et al., 2018; Wei et al., 2018; Zhang et al., 2019; Arribas-Hernández et al., 2020; Kim et al., 2020; Hu et al., 2021; Shao et al., 2021; Song et al., 2021). Although these findings have demonstrated the vital roles of m6A methylation in plant development at different development stages, the functional roles of m6A modification in the development of crops are largely unknown.
Tomato (Solanum lycopersicum) is a widely cultivated horticultural crop and serves as a valuable model plant to study fruit-related traits, including fruit expansion, quality, and ripening. Being an important part of human diet, the quality of tomato fruits, which depends on the developmental process of expansion and ripening, draws consistent attention. Fruit expansion determines the fruit size, whereas ripening impacts fruit nutritional value and shelf life (Zhou et al., 2019). A previous study has demonstrated that epigenetic 5-methylcytosine DNA methylation plays an important role in the regulation of tomato fruit ripening; disruption of DNA demethylase gene SlDML2 leads to global DNA hypermethylation and remarkable inhibition of fruit ripening (Lang et al., 2017). Moreover, a recent study has shown that disruption of SlALKBH2, an m6A RNA demethylase in tomato, is associated with increased stability of SlDML2 transcripts and delayed fruit ripening, highlighting a molecular link between DNA methylation and m6A RNA methylation during fruit ripening (Zhou et al., 2019). In addition, a recent study has demonstrated that m6A methylation regulates strawberry fruit ripening in an abscisic acid (ABA)-dependent manner (Zhou et al., 2021). Although these studies suggest the crucial role of epitranscriptomic RNA m6A methylation in regulating the fruit ripening process, the importance of m6A methylation in the expansion of fruits, including tomato, has never been explored.
To understand the role of mRNA m6A methylation in the expansion of tomato fruits, we performed transcriptome-wide m6A RNA immunoprecipitation-sequencing (m6A-seq) and RNA-seq of tomato fruits at different expansion stages and assessed the relation between m6A modification and the abundance of RNA transcripts related to cell expansion, hormone response, and endoreduplication. Herein, we investigated the ways by which m6A methylation affected gene expression at different developmental stages and suggested a link between m6A modification and tomato fruit expansion.
Results
Global m6A level increases during the expansion of tomato fruits
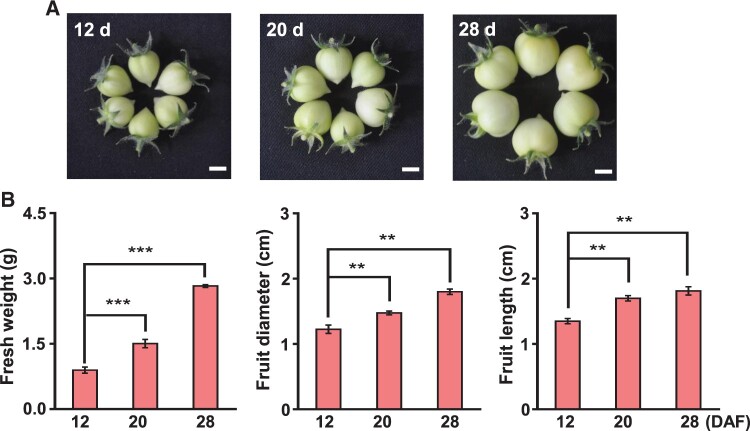
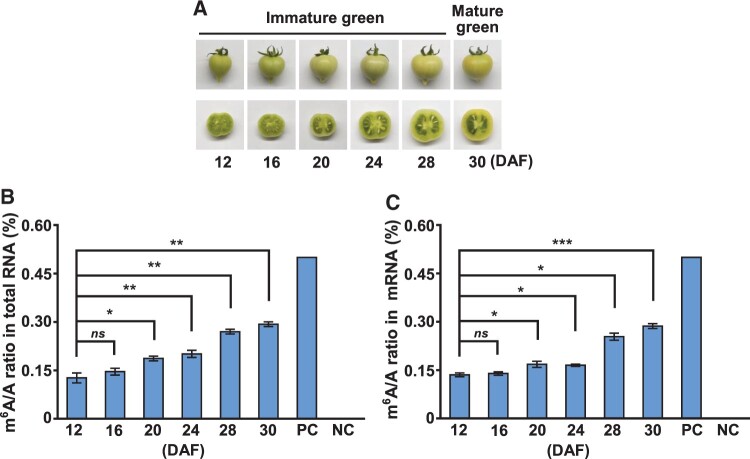
Considering the results from a previous report on the decrease in m6A methylation during the ripening of tomato fruits (Zhou et al., 2019), we first aimed to investigate how m6A levels are altered during the expansion stages of tomato fruits. We selected three time points, 12, 20, and 28 d after flowering (DAF), which showed clear differences in the fruit expansion (Figure 1A); it is evident that the fresh weight, fruit diameter, and fruit length of the tomatoes increased during the expansion stages (Figure 1B). Next, we analyzed the m6A levels in tomato fruits during different expansion stages from immature green to mature green stages (Figure 2A) and found that m6A levels in both total RNA and mRNA increased considerably in an expansion stage-dependent manner (Figure 2, B and C). Considering that m6A modification in rRNA can also affect developmental programs in animals (Ignatova et al., 2020), we measured the m6A levels in rRNA but found no significant changes in rRNA m6A levels in tomato fruits during different expansion stages (Supplemental Figure S1), suggesting that rRNA m6A methylation is not associated with tomato fruit expansion. These dynamic changes in m6A levels during the different developmental stages suggest that m6A modification in mRNA contributes positively to the expansion of tomato fruits.
Figure 1.
Phenotypes of tomato fruits during the expansion stages. A, Photographs of fresh fruits were taken at 12, 20, and 28 DAF. Scale bar = 1 cm (B). The fresh weight, diameter, and length of the fruits were measured on the indicated days. Data represent means ± standard deviation of three biological replicates (n = 6), and asterisks indicate significant differences (Student’s t test, **P < 0.01, ***P < 0.001).
Figure 2.
The global m6A levels in total RNA and mRNA during tomato fruit expansion stages. A, Photographs of the fruits at different expansion stages of tomato. The levels of m6A in (B) total RNA and (C) mRNA at different stages of fruit expansion are shown in (A). Data represent means ± standard deviation of three biological replicates (n = 4), and asterisks indicate significant differences (Student’s t test, *P < 0.05, **P < 0.01, ***P < 0.001). ns, not significant. PC, positive control. NC, negative control.
Important features of m6A profile during the expansion of tomato fruits
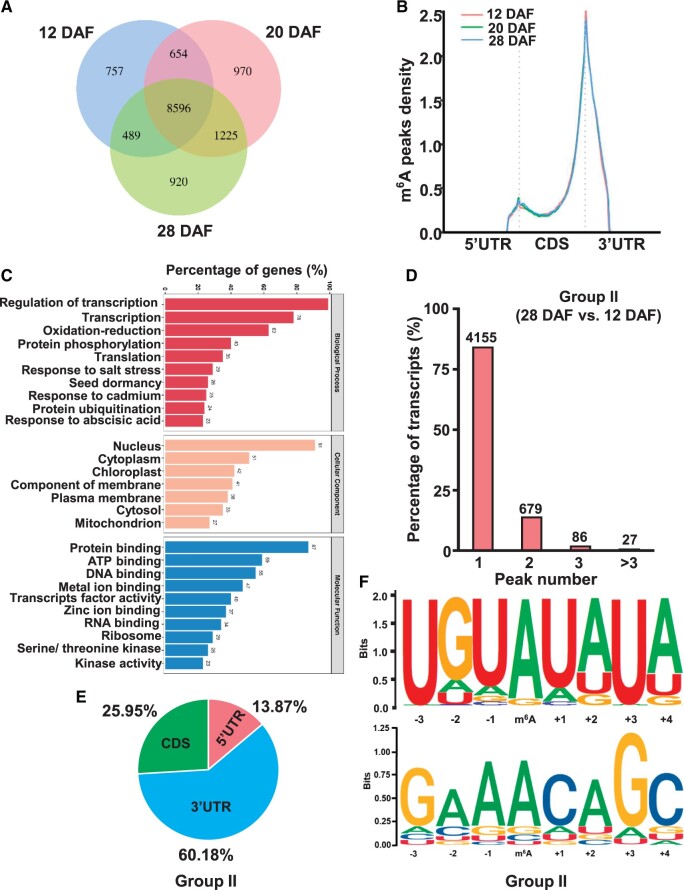
To understand the association between m6A modification and fruit expansion, we conducted m6A-seq and RNA-seq for the tomato fruits collected 12, 20, and 28 DAF. We generated a total of 65‒74, 64‒64, and 80‒84 million reads for the tomato fruits at 12, 20, and 28 DAF, respectively, among which 53‒59, 51‒52, and 64‒67 million distinct reads, respectively, were uniquely aligned to the Tomato Genome SL3.0 (Supplemental Table S1). Transcriptome-wide location of m6A modification was identified using an algorithm as described previously (Meng et al., 2014). The confident m6A peaks obtained from two biological replicates which showed a high Pearson’s correlation coefficient were used for subsequent bioinformatics analyses (Supplemental Figure S2). The validity of our m6A-seq data was further established by m6A-IP-qPCR analysis of six randomly selected m6A-containing transcripts (Supplemental Figure S3). Overall, we identified a total of 15,720, 16,547, and 16,381 m6A peaks in the tomato fruit at 12, 20, and 28 DAF, respectively (Supplemental Data Set S1), using m6A-seq. The majority of protein-coding transcripts (>66%) contained a single m6A peak (Supplemental Figure S4A). Consistent with previous studies conducted in other plants and mammals, the distribution of m6A peaks in protein-coding mRNA in all groups was enriched mainly in the 3ʹ-untranslated region (UTR) and relatively less in the 5ʹ-UTR and coding sequences (CDS) (Supplemental Figure S4B). Gene ontology (GO) analysis of the biological function of m6A-modified genes revealed that m6A-containing genes were involved in various cellular functions, including protein metabolism, DNA and RNA processing, and transcription regulation (Supplemental Figure S4C).
Next, to analyze the differences of m6A methylome between different stages of fruit expansion, we compared all m6A peaks identified in each developmental stage and found that most of the m6A peaks (8,596 peaks) were common in all samples, whereas 757, 970, and 920 m6A peaks were unique to tomato fruits at 12, 20, and 28 DAF, respectively (Figure 3A). To further evaluate the reliability of our m6A-seq data, the m6A-containing transcripts identified in the immature green tomato fruits in this study were compared with those identified in the immature green tomato fruits by Zhou et al. (2019). Although the tomato cultivars used in our study and the previous study by Zhou et al. (Micro-Tom versus Ailsa Craig) are different, and the developmental stages investigated in the two studies (28 DAF versus 39 DAF) are not identical, ∼83% (7,471/9,047) of the m6A-containing transcripts overlapped each other (Supplemental Figure S5), indicating that our m6A-seq data are reliable. GO analysis of the overlapped m6A-containing transcripts showed that they are involved in various cellular functions, including transcription regulation, protein metabolism, DNA and RNA processing, and chloroplast function (Figure 3B). Analysis of the density of m6A peaks in all samples revealed similar m6A distribution patterns with m6A mainly enriched in the 3ʹ-UTR (Figure 3C). To obtain a deeper view of changes in m6A landscape during fruit expansion, stages of fruit expansion were divided into the following three comparison groups: Group I, 20 DAF versus 12 DAF; Group II, 28 DAF versus 12 DAF; Group III, 28 DAF versus 20 DAF. We found that differential m6A peaks in all comparison groups are mainly modified at one site in the 3ʹ-UTR (Figure 3, D and E; Supplemental Figure S6, A and B). Motif analysis of m6A peaks in all comparison groups resulted in the identification of URUAY (R = A/G, Y = U/A) sequence motif, which is a plant-specific motif previously identified in tomato, maize, rice, and Arabidopsis (Wei et al., 2018; Zhou et al., 2019; Miao et al., 2020, Luo et al., 2020; Hu et al., 2021). Interestingly, the RRACH motif found in animals (Dominissini et al., 2012, 2013; Linder et al., 2015), as well as plants, were identified in comparison Groups II and III, but not in Group I (Figure 3F; Supplemental Figure S6C).
Figure 3.
Changes of m6A methylome during tomato fruit expansion stages. A, Venn diagrams showing the overlap of m6A peaks in tomato fruits at 12, 20, and 28 DAF. B, GO enrichment analysis of common m6A-modified genes shown in (A). C, Distribution of m6A peaks in transcript segments divided into 5ʹ-UTR, coding sequence, and 3ʹ-UTR. D, Proportions of the m6A-modified transcripts containing different number of m6A peaks in comparison Group II (28 DAF versus 12 DAF). E, A Pie chart depicting the fraction of m6A peaks in different gene segments. F, Sequence motifs identified using HOMER in m6A peaks.
Association between m6A methylation and the expression of fruit expansion-related genes
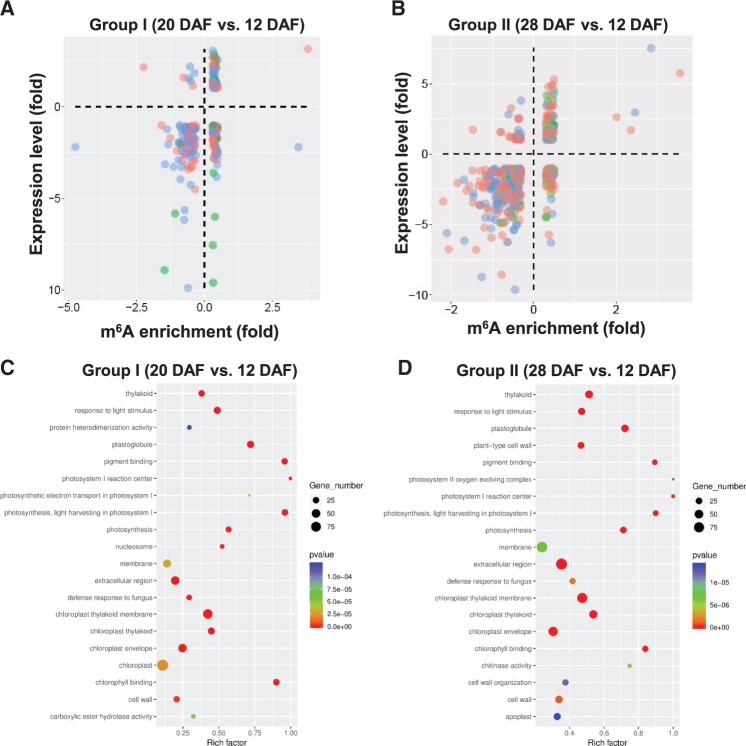
m6A modification has been shown to affect either negatively or positively the level of transcripts depending on developmental stages or environmental conditions (Shen et al., 2016; Hu et al., 2021). In tomato, m6A methylation is negatively associated with mRNA abundance during ripening (Zhou et al., 2019). To evaluate the relation between m6A modification and transcript levels during the expansion stages of tomato fruits, we performed RNA-seq analysis of the same samples used for m6A-seq. Comparison of the differentially expressed genes (DEGs; fold change >2; P < 0.05; Supplemental Data Set S2) with altered m6A levels (fold change >2; P < 0.05) in different comparison groups revealed that ∼70% mRNAs in Group I, 78% mRNAs in Group II, and 81% mRNAs in Group III with increased or decreased m6A levels tended to enhance or reduce transcript levels, respectively, whereas only ∼30% in Group I, 22% in Group II, and 19% in Group III with increased or decreased m6A levels tended to negatively affect transcript levels (Figure 4, A and B; Supplemental Figure S7A). These results suggest an overall positive association between m6A modification and transcript levels during the expansion of tomato fruits. This highlights the multifaceted effects of m6A modification on gene expression during the different developmental stages in tomato fruits. To understand the function of m6A-modified DEGs, we performed GO analysis and found that a majority of the m6A-modified DEGs were involved in cellular functions related to photosynthesis, chlorophyll-binding, and cell wall organization (Figure 4, C and D; Supplemental Figure S7B).
Figure 4.
Association between m6A methylation and gene expression. A and B, Association between m6A modification and the expression of m6A-containing genes in Groups I and II. C and D, GO analysis of the m6A-containing DEGs identified in comparison Groups I and II.
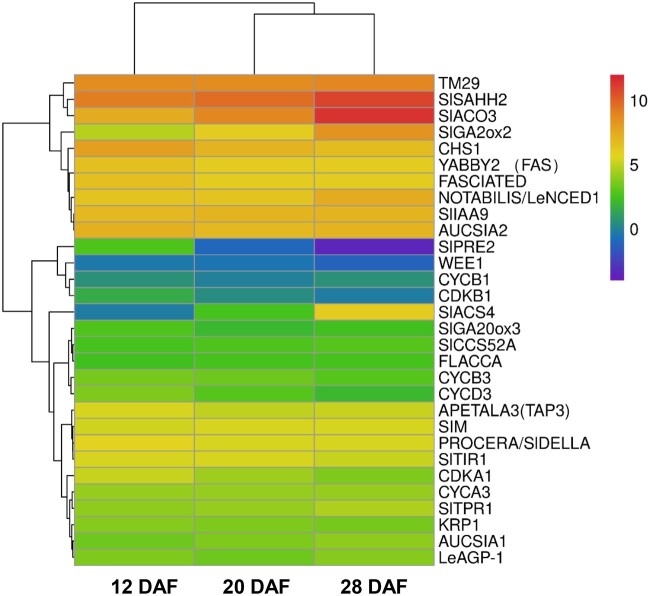
To further reveal the role of m6A modification in the regulation of tomato fruit expansion, we analyzed the m6A patterns in the genes related to fruit expansion (Supplemental Table S2). Many genes that induce or repress fruit expansion contain m6A marks at one or all stages of fruit expansion (Supplemental Table S2). Moreover, RNA-seq analysis revealed that genes with m6A methylation were expressed more actively than genes without m6A modification (Figure 5; Supplemental Figure S8). Notably, a large number of genes involved in auxin and gibberellic acid signaling and endoreduplication, as well as several genes related to cell expansion and pericarp thickness contain m6A modification.
Figure 5.
Expression levels of m6A-modified genes associated with tomato fruit expansion. Total RNA was extracted from tomato fruits at 12, 20, and 28 DAF and was subjected to RNA-seq analysis. The Heat Map represents average read depth obtained from two biological replicates.
Altered m6A modification by direct injection of writer or eraser inhibitor represses the expansion of tomato fruits
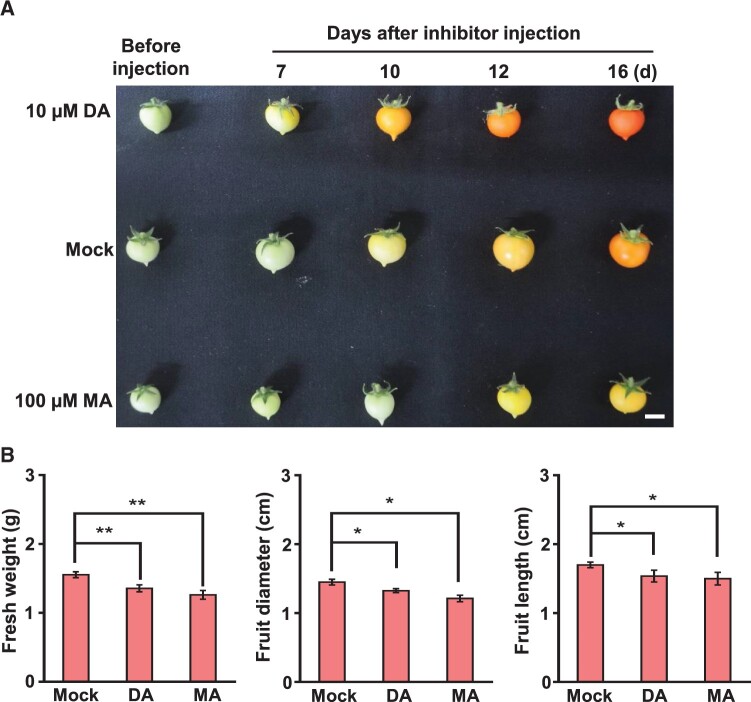
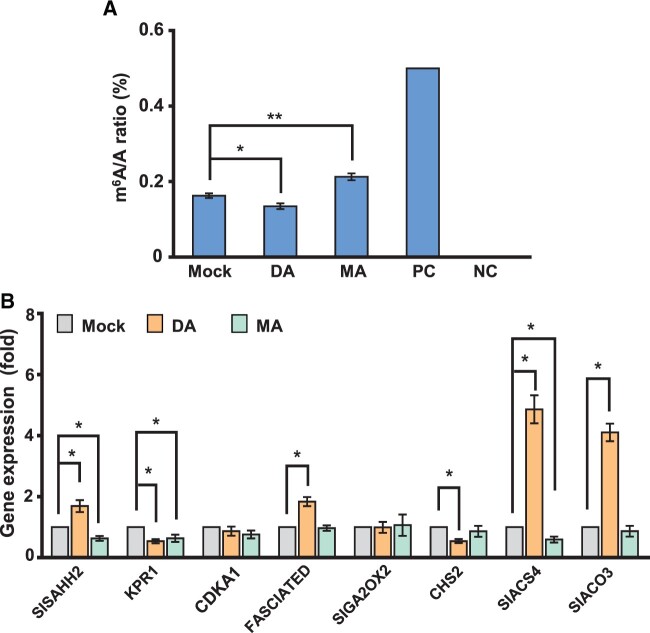
To further evaluate the effect of m6A modification on fruit development, we employed a method of direct injection of m6A writer or eraser inhibitor into tomato fruits (Supplemental Figure S9). The inhibitors used in this study were 3-deazaneplanocin A (DA) and Meclofenamic acid (MA). DA acts as an S-adenosylhomocysteine synthesis inhibitor and is known to inhibit m6A methylation in animal cells (Fustin et al., 2013, 2018), whereas MA is a well-characterized inhibitor of the fat mass and obesity-associated protein, an m6A demethylase in animals (Huang et al., 2015). We injected DA or MA solution into the tomato fruits at 12 DAF and measured the size of tomato fruits 7 d later. We found that the injected fruits were smaller than those with mock treatment; the fresh weight, diameter, and length of the inhibitor-injected fruits decreased (Figure 6, A and B), indicating that DA or MA treatment inhibits tomato fruit expansion. Contrary to the inhibitory effects of DA and MA on tomato fruit expansion, DA or MA treatment accelerated or delayed fruit ripening, respectively (Figure 6A). To verify whether these changes in fruit development were due to altered m6A modification by the inhibitors, we analyzed m6A levels in tomato fruits subjected to DA or MA treatment. We found that DA and MA treatment decreased and increased m6A levels in RNA, respectively, compared with mock treatment (Figure 7A), suggesting that changes in the expansion and ripening of tomato fruits are because of altered m6A levels modulated by inhibitor treatment. These results are in line with a previous report that suggested that m6A levels gradually decreased during the ripening stage of tomato fruits and that increased m6A levels because of m6A demethylase inhibition were associated with delayed fruit ripening (Zhou et al., 2019). Considering that DA and MA are not specific inhibitors to the methylation and demethylation in RNA, we evaluated whether DA and MA affect methylation in DNA. The results showed that the levels of 6mA and 5mC in DNA were not significantly altered by DA and MA treatment (Supplemental Figure S10), suggesting that DA and MA do not influence DNA methylation during the expansion and ripening of tomato fruits. To further elucidate how altered m6A methylation affects fruit expansion, the expression levels of genes involved in fruit expansion were analyzed. Notably, transcript levels of several m6A-modified genes were altered after DA or MA treatment, whereas the levels of non-m6A-modified genes were not affected by inhibitor treatment (Figure 7B; Supplemental Figure S11). Collectively, our results point to the importance of m6A methylation in regulating the expansion of tomato fruits as well as the ripening of tomato fruits.
Figure 6.
Effects of direct injection of m6A writer or eraser inhibitor on tomato fruit development. A, Phenotypes of the tomato fruits before and after injection of DA or MA. Scale bar = 1 cm. B, The fresh weight, diameter, and length of the tomato fruits were measured 7 d after injection. Data represent means ± standard deviation of three biological replicates (n = 6), and asterisks indicate significant differences (Student t test, *P < 0.05, **P < 0.01).
Figure 7.
Effects of m6A writer or eraser inhibitor on global m6A modification and the expression of m6A-modified tomato fruit expansion-related genes. A, The levels of m6A in total RNA in tomato fruits 7 d after DA or MA injection. B, Transcript levels of tomato fruit expansion-related genes at 7 d after inhibitor injection were determined via RT-qPCR. Data represent means ± standard deviation of three biological replicates (n = 6), and asterisks indicate significant differences (Student’s t test, *P < 0.05, **P < 0.01).
Discussion
Despite the increasing understanding of the crucial roles of RNA m6A methylation in various biological processes, the importance of m6A methylation in the expansion of fruits, including tomato, has never been explored. In this study, we showed a close association between m6A modification and the expansion of tomato fruits. Notably, overall m6A levels increase during tomato fruit expansion (Figure 2), which is in contrast to that seen in tomato fruit ripening (Zhou et al., 2019). As m6A methylation is a dynamic and reversible process with regulatory functions (Yue et al., 2015; Liu and Pan, 2016; Meyer and Jaffrey, 2017; Hu et al., 2019; Zheng et al., 2020), differential m6A methylation patterns during the different stages of fruit development suggest that the expression and/or activity of m6A writers and erasers are modulated depending on the developmental stages of fruits. Through parallel m6A-seq and RNA-seq analyses of tomato fruits at different expansion stages, we demonstrated that a positive association exists between m6A modification and the expression levels of the genes related to cell expansion, hormone signaling, and endoreduplication, which play a crucial role in the expansion of tomato fruits.
The m6A methylome identified in tomato fruits at different expansion stages contains far more m6A-modified genes than those identified in tomato fruits during ripening (Zhou et al., 2019), but it has a similar number of genes compared with those in other plants species, including Arabidopsis and maize (Shen et al., 2016; Luo et al., 2020; Hu et al., 2021). In this study, m6A-seq revealed the presence of m6A marks predominantly in the 3ʹ-UTR of mRNAs in tomato fruits during all expansion stages (Figure 3), which is in line with the findings of other studies that have demonstrated the biased deposition of m6A marks near the stop codon and in the 3ʹ-UTR in plants under normal and stress conditions (Shen et al., 2016; Zhang et al., 2019; Zhou et al., 2019; Luo et al., 2020; Hu et al., 2021), These results suggest that although the abundance of m6A could vary across species or depending on environmental stimuli, the deposition of m6A marks primarily in the 3ʹ-UTR of mRNAs is an evolutionarily conserved feature. Notably, as the major m6A motif, we identified conserved URUAY (R = A/G, Y = U/A) sequence (Figure 3; Supplementary Figure S6), which is a previously identified plant-specific motif (Wei et al., 2018; Zhou et al., 2019; Luo et al., 2020; Miao et al., 2020; Hu et al., 2021). In addition, RRACH-like motifs, which were identified in animals (Dominissini et al., 2012, 2013; Meyer et al., 2012; Linder et al., 2015) and two plant species (Arabidopsis and maize; Duan et al., 2017; Miao et al., 2020) were also identified (Figure 3, Supplemental Figure S6). To determine the precise m6A motifs in plants, it is highly desirable to employ single-nucleotide resolution mapping of m6A, which has been successfully used in human and mouse mRNAs (Linder et al., 2015). Moreover, as all the transcripts that contain these conserved motifs are not methylated, it is important to determine how certain transcripts are selected for methylation by m6A writers. Several cellular factors, including transcription factors, histone marks, and microRNAs, have been shown to affect de novo m6A deposition at specific loci in animal transcripts (Chen et al., 2015; Shi et al., 2019; Huang et al., 2020); similarly, it will be interesting to determine the underlying mechanisms by which specific m6A motifs are selected for methylation in diverse plant species depending on the developmental and environmental cues.
The impact of mRNA m6A modification on gene expression is multifaceted. In mammals, m6A modification found mainly in the 3ʹ-UTR of mRNA is negatively associated with gene expression (Yue et al., 2018). Several studies in Arabidopsis have demonstrated a negative link between m6A modification and gene expression under normal conditions but a positive association under salt stress conditions (Anderson et al., 2018; Hu et al., 2021). Contrary to a previous study that showed an overall negative link between m6A modification and gene expression during the ripening of tomato fruits (Zhou et al., 2019), our results demonstrated an overall positive association between m6A modification and mRNA abundance during the expansion of tomato fruits (Figure 4). This opposite association between m6A methylation and gene expression during the different developmental stages of tomato fruits raises an intriguing question of how m6A modification differentially affects mRNA abundance depending on the developmental process of tomato fruits. Interestingly, a recent study has demonstrated an overall positive link between m6A modification and gene expression during the ripening of strawberry fruits (Zhou et al., 2021). Several studies have demonstrated that specific RNA-binding proteins (referred to as “readers”) recognize and bind m6A marks and play a vital role in determining mRNA fate (Hu et al., 2019; Huang et al., 2020; Shao et al., 2021). In particular, several YT521-B homology (YTH) domain-containing proteins as m6A readers have been identified to regulate either negatively or positively mRNA stability in animals and plants (Du et al., 2016; Shi et al., 2017; Huang et al., 2018; Wei et al., 2018; Baquero-Perez et al., 2019; Song et al., 2021). Considering the fact that many RNA-binding proteins, including YTH proteins, K-homology proteins, CCHC zinc-finger proteins, and RNA-recognition motif proteins, have been identified as potential m6A readers in mammalian cells (Edupuganti et al., 2017), it will be worthy to identify additional m6A readers that are expressed during the expansion or ripening stages of tomato fruits and determine their roles in regulating the stability and abundance of RNA transcripts during tomato fruit development.
The growth of fruits depends on two cellular processes—cell division and cell expansion. Cell division occurs during the early staged after fruit setting, whereas fruit expansion occurring during the later stages is mainly due to cell expansion, which is influenced by phytohormones, such as auxin and gibberellic acid and endoreduplication. We found that in tomato fruit, a large portion of genes involved in hormone signaling pathways and endoreduplication are m6A-modified (Supplemental Table S2). Analysis of RNA-seq data further revealed that m6A-modified genes are expressed at a much higher level than non-m6A-modified genes (Figure 5; Supplemental Figure S8). These results demonstrated the important role of m6A modification in fruit expansion through the regulation of hormones and/or endoreduplication. Previously reported m6A-seq data have also shown m6A marks on a large number of genes involved in hormone signaling in Arabidopsis and maize (Shen et al., 2016; Luo et al., 2020; Hu et al., 2021). Moreover, recent reports have demonstrated that a decrease in m6A levels due to loss-of-function of an m6A writer component influences the expression of many genes involved in hormone signaling pathways (Shen et al., 2016; Hu et al., 2021). Notably, the phenotypes of Arabidopsis and rice m6A writer mutants mimic those of hormone-deficit mutant phenotypes, such as dwarfism, small size, and multiple branches (Růžička et al., 2017; Zhang et al., 2019). These observations suggest a close link between m6A modification and the regulation of hormone signaling, which contributes to fruit development, as well as the normal growth of plants.
Decreased m6A modification via knockout or knockdown of m6A writer genes causes severe defects in plant growth and development (Zhong et al., 2008; Shen et al., 2016; Růžička et al., 2017; Zhang et al., 2019); therefore, it is impractical to evaluate the effect of m6A modification on fruit development by using a transgenic approach. Instead, direct injection of m6A writer inhibitor, DA (Fustin et al., 2013, 2018), or eraser inhibitor, MA (Huang et al., 2015), into the tomato fruits employed in this study is a valuable alternative to evaluate the effect of m6A modification on fruit development. Interestingly, both exogenous m6A writer and eraser inhibitors suppressed the expansion of tomato fruits, whereas writer inhibitor treatment and eraser inhibitor treatment accelerated and delayed fruit ripening, respectively (Figure 6). The delayed ripening of tomato fruits by m6A eraser inhibitor treatment and a concomitant increase in m6A levels observed in this study is in line with the previous report that has shown an increase in m6A levels by knockout of m6A demethylase causes delayed fruit ripening (Zhou et al., 2019). These observations indicate that the direct inhibitor injection approach can be used to mimic transgenic approaches. Although the mechanisms by which both m6A writer and eraser inhibitors suppress tomato fruit expansion are not known, we postulate that the maintenance of proper m6A homeostasis at different developmental stages is critical for fruit development. Notably, the expression of several m6A-modified genes involved in fruit expansion, such as KRP1, FASCIATED, SIACS4, and SIACO3, was modulated by either the m6A writer or eraser inhibitor treatment, whereas that of non-m6A-modified genes was not altered (Figure 7; Supplemental Figure S11), suggesting that m6A modification indeed affects the expression of genes involved in fruit growth. Kip-related Protein 1 (KPR1), as a cyclin-dependent kinase inhibitor, has been shown to participate in the control of endoreduplication (Bisbis et al., 2006), which is a critical process to determine cell size in developing tomato fruits. The number of locules is another important factors contributing to fruit size. FASCIATED is one of the vital loci that negatively affect carpel number and ultimate locule number in tomato fruits (Barrero and Tanksley, 2004). SIACS4 and SIACO3 are two key genes involved in ethylene biosynthesis, which affects fruit development by modulating the levels of auxin-signaling repressor gene, such as SIIAA9 (Wang et al., 2009). The decreased KRP1 levels and the increased FASCIATED, SIACS4, and SIACO3 levels observed in the tomato fruits after injection of m6A writer or eraser inhibitor (Figure 7) suggest an association between m6A levels and the levels of transcripts involved in fruit development. Moreover, GO analysis for all m6A-containing transcripts in developing tomato fruits showed an enrichment of genes related to chloroplast function, such as chloroplast biogenesis, photosynthesis, and pigment binding (Figures 3 and 4; Supplemental Figures S4 and S7), which is similar to a previous report demonstrating that a large number of transcripts with m6A marks are associated with chloroplast function in Arabidopsis (Luo et al., 2014). Considering that chloroplast biogenesis and function and photosynthesis are critical for the accumulation of starch and nutritional components during early fruit development (Nadakuduti et al., 2014), our findings support the association between m6A modification and chloroplast function during tomato fruit development. Understanding the molecular mechanisms connecting m6A modification to fruit expansion and ripening warrants further research.
In summary, this study uncovered important features of m6A methylome associated with the expansion of tomato fruits. Through parallel m6A-seq and RNA-seq analyses at three different stages of expansion, we found a positive association between m6A modification and mRNA abundance during fruit expansion, which is contradictory to what was observed during tomato fruit ripening. Importantly, we found that many cell expansion-related genes involved in hormone signaling and endoreduplication exhibit increased m6A methylation and that m6A-modified genes are generally expressed more actively than non-m6A-modified genes. Considering that a recent study has proposed a link between DNA methylation and m6A RNA methylation during fruit ripening (Zhou et al., 2019), it would be interesting to further determine the importance of DNA methylation as well as RNA methylation in the expansion and ripening of tomato fruits. Given that direct injection of m6A writer or eraser inhibitor into tomato fruits can alter global m6A levels and gene expression levels, thus affecting the expansion and ripening of tomato fruits, it would be a practical approach to evaluate the effect of m6A modification in fruit development. This study not only improves our understanding but also provides valuable background information required for further mechanistic studies on the role of m6A methylation in fruit development and ripening, which can be utilized to engineer epitranscriptomic RNA modifications for crop improvement.
Materials and methods
Plant materials and growth conditions
Micro-Tom tomato (S. lycopersicum) was used in this study. Micro-Tom seeds were surface sterilized with 70% (v/v) ethanol for 2 min, followed by 1% (w/v) sodium hypochlorite application for 10 min, and then rinsed with distilled water. For seed germination, seeds in distilled water were incubated at 28°C in the dark with gentle agitation for 2‒3 d. The germinated seeds were transferred to soil consisting of vermiculite, peat moss, and perlite (in a 3:1:1 ratio) and grown in a growth room at 23 ± 2°C under long-day conditions (16-h-light/8-h-dark cycle). The plants were watered every 3 d. For measuring m6A levels, fruit samples were collected at 12, 16, 20, 24, 28, and 30 DAF. For phenotype analysis and m6A-seq and RNA-seq, the fruit samples were collected at 12, 20, and 28 DAF.
Measurement of m6A/A ratio
Total RNA was extracted from fruit samples using a Plant RNeasy extraction kit (Qiagen, Valencia, CA, USA), and mRNA was isolated from the total RNA using a poly(A) Spin mRNA Isolation Kit (New England Biolabs, Ipswich, MA, USA) according to the manufacturer’s instructions. The m6A level was determined using an EpiQuik m6A RNA Methylation Quantification Kit (EPIGENTEK, Farmingdale, NY, USA) as described previously (Hu et al., 2021). Briefly, total RNA or mRNA were bound to strip wells containing an m6A-specific antibody. After washing the wells, capture and detection antibody solutions were added, and the signals were quantified colorimetrically by reading the absorbance at 450 nm. The m6A levels in the samples were calculated using a standard curve. The 6mA and 5mC levels in DNA were determined using a MethylFlash m6A DNA Methylation ELISA Kit and MethylFlash Global DNA Methylation (5-mC) ELISA Easy Kit (EPIGENTEK, Farmingdale, NY, USA), respectively, according to the manufacturer’s instructions.
m6A-MeRIP-seq and RNA-seq
All experiments regarding RNA preparation and RNA-seq were performed as described previously (Hu et al., 2021). Briefly, the poly(A) mRNAs were fragmented into ∼100-nucleotide (nt)-long oligonucleotides using divalent cations, and the cleaved RNA fragments were subjected to m6A-immunoprecipitation by incubating with an m6A-specific antibody (Synaptic Systems, Goettingen, Germany) in IP buffer (50 mM Tris–HCl, 750 mM NaCl, 0.5% (w/v) Igepal CA-630, and 0.5 μg μL−1 BSA) at 4°C for 24 h. The eluted m6A-containing immunoprecipitated fragments (IP fractions) and nonimmunoprecipitated fragments (input control) were used to construct a cDNA library according to a strand-specific library preparation protocol. The average insert size for the paired-end libraries was ∼100 ± 50 bp. RNA sequencing was performed at LC-BIO Bio-tech Ltd (Hangzhou, China) using the Illumina Novaseq 6000 platform.
Analysis of m6A-seq and RNA-seq data
m6A-seq and RNA-seq data were analyzed as described previously (Hu et al., 2021). Briefly, Cutadapt (Martin, 2011) and Perl script in house were used to remove the reads that contained adaptor contamination, low-quality bases, and undetermined bases, after which sequence quality was verified using fastp. HISAT2 (Langmead and Salzberg, 2012) was used to map the reads to the genome of S. lycopersicum (version 3.2) with default parameters. Mapped reads of IP and input libraries were used for peak calling in the R package exomePeak (Meng et al., 2014), which identifies m6A peaks with bed or bam format that can be adapted for visualization on the IGV software (http://www.igv.org/). The called peaks were annotated by intersection with gene architecture using the R package ChIPseeker (Yu et al., 2015). StringTie (Pertea et al., 2015) was used to analyze expression levels of all mRNAs from input libraries by calculating Fragments Per Kilobase of transcript per Million (= total _exon_fragments/mapped_reads (millions) × exon_length (kb). The differentially expressed mRNAs were selected with log2 (fold change) >1 or log2 (fold change) less than −1 and P < 0.05 using R package edgeR (Robinson et al., 2010). The HOMER software-4.10/bin/findMotifs Genome.pl script (http://homer.ucsd.edu/homer/motif/) was used to detect conserved sequence motifs followed by localization of the motifs with respect to peak summits using Perl scripts. GO analysis of m6A-modified genes was performed using the OmicStudio tools (https://www.omicstudio.cn/tool).
RT-qPCR and m6A-IP-qPCR
m6A-IP-qPCR was performed as previously described (Dominissini et al., 2013) with minor modifications (Hu et al., 2021). Briefly, the same mRNA used for m6A-MeRIP-seq has fragmented into ∼300-nt-long fragments in an RNA fragmentation buffer (10 mM Tris–HCl, pH 7.0, 10 mM ZnCl2). The fragmented mRNA was subjected to m6A-IP using an m6A-specific antibody as described above. The input RNAs and immunoprecipitated RNAs were reverse-transcribed using reverse transcriptase with random hexamer primers. The levels of each transcript were determined by quantitative real-time PCR conducted in a Rotor-Gene Q thermal cycler (Qiagen) using an SYBR Green PCR kit (Qiagen) and gene-specific primers listed in Supplemental Table S3 as previously described (Park et al., 2020). The PCR cycle threshold value for the immunoprecipitated sample was initially normalized against that of ACTIN, which has no internal m6A modification and serves as an internal control and then normalized against that of the input RNAs.
Direct injection of inhibitor solution into tomato fruits
The m6A writer or eraser inhibitor solution was directly injected into tomato fruits as previously described (Orzaez et al., 2006) with minor modifications. Briefly, tomato fruits at 12 DAF of the immature green stage were infiltrated using a 1-mL syringe with a 0.5-mm diameter needle. The needle was injected up to 3‒4 mm into the fruit through the peduncle. Approximately 50 µL of inhibitor solution was injected each try, and the progress of the injection was monitored by observing a slight change in color in the infiltrated area and appearance of water drops through a hydathode. Once the entire fruit surface was infiltrated, several drops of infiltration solution began to run off the hydathode at the tip of the sepals. A total of 100 µL of 10 µM DA or 100 µM MA were introduced into the fruits via two successive injections at a 2-d interval.
Accession numbers
The MeRIP-seq and RNA-seq data used in this study have been deposited in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus database under accession number GSE178904.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. The m6A levels in rRNA during tomato fruit expansion stages.
Supplemental Figure S2. Pearson correlation analysis of input and immunoprecipitation reads between sequence profiles from two biological replicates of tomato fruits at 12, 20, and 28 DAF.
Supplemental Figure S3. Validation of m6A enrichment for six randomly selected m6A-modified genes.
Supplemental Figure S4. The conserved feature of m6A methylome in tomato fruits at different expansion stages.
Supplemental Figure S5. Venn diagram showing the overlap of m6A peaks in tomato fruits at immature green stage.
Supplemental Figure S6. Comparison of m6A methylome in tomato fruits at different expansion stages.
Supplemental Figure S7. Association between m6A methylation and gene expression in comparison Group III.
Supplemental Figure S8. Expression levels of non-m6A-modified genes associated with tomato fruit expansion.
Supplemental Figure S9. Exogenous application of m6A writer or eraser inhibitor into tomato fruits.
Supplemental Figure S10. Effects of m6A writer or eraser inhibitor on methylation in DNA.
Supplemental Figure S11. Effects of m6A writer or eraser inhibitor on the expression of non-m6A-modified tomato fruit expansion-related genes.
Supplemental Table S1. A summary of m6A-seq results of tomato fruits at 12, 20, and 28 DAF.
Supplemental Table S2. List of genes related to tomato fruit expansion.
Supplemental Table S3. List of primer pairs used in this study.
Supplemental Data Set S1. Genomic loci, fold enrichment, and associated genes for each m6A peak in tomato fruits at 12, 20, and 28 DAF.
Supplemental Data Set S2. Differential m6A peaks and gene expression in comparison Groups I, II, and III.
Funding
This work was supported by a grant from the New Breeding Technologies Development Program (PJ01478301), Rural Development Administration, Republic of Korea, by a grant from the Mid-career Researcher Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (NRF-2021R1A2C1004187), Republic of Korea, and by the Priority Academic Program Development of Jiangsu Higher Education Institutions (Grant No. PAPD).
Conflict of interest statement. The authors declare no conflict of interest.
Supplementary Material
Contributor Information
Jianzhong Hu, Jiangsu Key Laboratory of Phylogenomics and Comparative Genomics, School of Life Sciences, Jiangsu Normal University, Xuzhou, 221116, China; Department of Applied Biology, College of Agriculture and Life Sciences, Chonnam National University, Gwangju, 61186, Korea.
Jing Cai, Department of Applied Biology, College of Agriculture and Life Sciences, Chonnam National University, Gwangju, 61186, Korea.
Amara Umme, Department of Applied Biology, College of Agriculture and Life Sciences, Chonnam National University, Gwangju, 61186, Korea.
Yao Chen, Jiangsu Key Laboratory of Phylogenomics and Comparative Genomics, School of Life Sciences, Jiangsu Normal University, Xuzhou, 221116, China.
Tao Xu, Jiangsu Key Laboratory of Phylogenomics and Comparative Genomics, School of Life Sciences, Jiangsu Normal University, Xuzhou, 221116, China.
Hunseung Kang, Jiangsu Key Laboratory of Phylogenomics and Comparative Genomics, School of Life Sciences, Jiangsu Normal University, Xuzhou, 221116, China; Department of Applied Biology, College of Agriculture and Life Sciences, Chonnam National University, Gwangju, 61186, Korea.
H.K. and T.X. planned and designed the experiments. J.H., J.C., A.U., and Y.C. performed experiments. J.H., T.X., and H.K. analyzed the data. H.K. and J.H. wrote the manuscript. All authors read and approved the final draft of the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is Hunseung Kang (hskang@jnu.ac.kr).
References
- Anderson SJ, Kramer MC, Gosai SJ, Yu X, Vandivier LE, Nelson ADL, Anderson ZD, Beilstein MA, Fray RG, Lyons E, et al. (2018) N6-methyladenosine inhibits local ribonucleolytic cleavage to stabilize mRNAs in Arabidopsis. Cell Rep 25: 1146–1157 [DOI] [PubMed] [Google Scholar]
- Arribas-Hernández L, Bressendorff S, Hansen MH, Poulsen C, Erdmann S, Brodersen P (2018) An m6A-YTH module controls developmental timing and morphogenesis in Arabidopsis. Plant Cell 30: 952‒967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribas-Hernández L, Simonini S, Hansen MH, Paredes EB, Bressendorff S, Dong Y, Østergaard L, Brodersen P (2020) Recurrent requirement for the m6A-ECT2/ECT3/ECT4 axis in the control of cell proliferation during plant organogenesis. Development 147: dev189134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquero-Perez B, Antanaviciute A, Yonchev ID, Carr IM, Wilson SA, Whitehouse A (2019) The Tudor SND1 protein is an m6A RNA reader essential for replication of Kaposi’s sarcoma-associated herpesvirus. eLife 8: e47261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero LS, Tanksley SD (2004) Evaluating the genetic basis of multiple-locule fruit in a broad cross section of tomato cultivars. Theor Appl Genet 109: 669–679 [DOI] [PubMed] [Google Scholar]
- Bisbis B, Delmas F, Joubès J, Sicard A, Hernould M, Inzé D, Mouras A, Chevalier C (2006) Cyclin-dependent kinase (CDK) inhibitors regulate the CDK-cyclin complex activities in endoreduplicating cells of developing tomato fruit. J Biol Chem 281: 7374‒7383 [DOI] [PubMed] [Google Scholar]
- Boccaletto P, Machnicka MA, Purta E, Piatkowski P, Baginski B, Wirecki TK, de Crécy-Lagard V, Ross R, Limbach PA, Kotter A, et al. 2018. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res 46: D303‒D307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodi Z, Zhong S, Mehra S, Song J, Graham N, Li H, May S, Fray RG (2012) Adenosine methylation in Arabidopsis mRNA is associated with the 3ʹend and reduced levels cause developmental defects. Front Plant Sci 3: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantara WA, Crain PF, Rozenski J, McCloskey JA, Harris KA, Zhang X, Vendeix FAP, Fabris D, Agris PF (2011) The RNA modification database RNAMDB: 2011 update. Nucleic Acids Res 39: D195‒D201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Hao YJ, Zhang Y, Li MM, Wang M, Han W, Wu Y, Lv Y, Hao J, Wang L, et al. (2015) m6A RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell Stem Cell 16: 289–301 [DOI] [PubMed] [Google Scholar]
- Coots RA, Liu XM, Mao Y, Dong L, Zhou J, Wan J, Zhang X, Qian SB (2017) m6A facilitates eIF4F-independent mRNA translation. Mol Cell 68: 504–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covelo-Molares H, Bartosovic M, Vanacova S (2018). RNA methylation in nuclear pre-mRNA processing. WIREs RNA 9: e1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. (2012). Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485: 201–206 [DOI] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Salmon-Divon M, Amariglio N, Rechavi G (2013) Transcriptome-wide mapping of N6-methyladenosine by m6A-seq based on immunocapturing and massively parallel sequencing. Nat Protocol 8: 176. [DOI] [PubMed] [Google Scholar]
- Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, Ma JB, Wu L (2016) YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4–NOT deadenylase complex. Nat Commun 7: 12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan HC, Wei LH, Zhang C, Wang Y, Chen L, Lu Z, Chen PR, He C, Jia G (2017) ALKBH10B is an RNA N6-methyladenosine demethylase affecting Arabidopsis floral transition. Plant Cell 29: 2995–3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edupuganti RR, Geiger S, Lindeboom RGH, Shi H, Hsu PJ, Lu Z, Wang SY, Baltissen MPA, Jansen PWTC, Rossa M, et al. (2017) N6-methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nat Struct Mol Biol 24: 870–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fustin JM, Doi M, Yamaguchi Y, Hida H, Nishimura S, Yoshida M, Hida H, Nishimura S, Yoshida M, Isagawa T, et al. (2013) RNA methylation-dependent RNA processing controls the speed of the circadian clock. Cell 155: 793–806 [DOI] [PubMed] [Google Scholar]
- Fustin JM, Kojima R, Itoh K, Chang HY, Ye S, Zhuang B, Oji A, Gibo S, Narasimamurthy R, Virshup D, et al. (2018) Two Ck1δ transcripts regulated by m6A methylation code for two antagonistic kinases in the control of the circadian clock. Proc Natl Acad Sci USA 115: 5980–5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Cai J, Park SJ, Lee K, Li Y, Chen Y, Yun JY, Xu T, Kang H (2021) N6‐methyladenosine mRNA methylation is important for salt stress tolerance in Arabidopsis. Plant J 106: 1759–1775 [DOI] [PubMed] [Google Scholar]
- Hu J, Manduzio S, Kang H (2019) Epitranscriptomic RNA methylation in plant development and abiotic stress responses. Front Plant Sci 10: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Weng H, Chen J (2020) The biogenesis and precise control of RNA m6A methylation. Trends Genet 36: 44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, Mesquita A, Liu C, Yuan CL, et al. (2018). Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol 20: 285‒295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yan J, Li Q, Li J, Gong S, Zhou H, Gan J, Jiang H, Jia GF, Luo C, Yang CG (2015) Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res 43: 373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatova VV, Stolz P, Kaiser S, Gustafsson TH, Lastres PR, Sanz-Moreno A, Cho YL, Amarie OV, Aguilar-Pimentel A, Klein-Rodewald T, et al. (2020) The rRNA m6A methyltransferase METTL5 is involved in pluripotency and developmental programs. Genes Dev 34: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Shim S, Lee H, Seo PJ (2020) m6A mRNA modification as a new layer of gene regulation in plants. J Plant Biol 63: 97–106 [Google Scholar]
- Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang Z, Wang Y, Tang K, Tang D, Datsenka T, Cheng J, Zhang Y, Handa AK, Zhu JK (2017) Critical roles of DNA demethylation in the activation of ripening-induced genes and inhibition of ripening-repressed genes in tomato fruit. Proc Natl Acad Sci USA 114: E4511–4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR (2015) Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods 12: 767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Pan T (2016) N6-methyladenosine–encoded epitranscriptomics. Nat Struct Mol Biol 23: 98–102 [DOI] [PubMed] [Google Scholar]
- Luo JH, Wang Y, Wang M, Zhang LY, Peng HR, Peng HR, Jia GF, He Y (2020) Natural variation in RNA m6A methylation and its relationship with translational status. Plant Physiol 182: 332–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo GZ, MacQueen A, Zheng G, Duan H, Dore LC, Lu Z, Liu J, Chen K, Jia GF, Bergelson J, et al. (2014) Unique features of the m6A methylome in Arabidopsis thaliana. Nat Commun 5: 5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M (2011) CUTADAPT removes adapter sequences from high-throughput sequencing reads. EMBnet J 17: 1 [Google Scholar]
- Martínez-Pérez M, Aparicio F, López-Gresa MP, Bellés JM, Sánchez-Navarro JA, Pallás V (2017). Arabidopsis m6A demethylase activity modulates viral infection of a plant virus and the m6A abundance in its genomic RNAs. Proc Natl Acad Sci USA 114: 10755–10760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Lu Z, Liu H, Zhang L, Zhang S, Chen Y, Rao MK, Huang Y (2014) A protocol for RNA methylation differential analysis with MeRIP-Seq data and exomePeak R/Bioconductor package. Methods 69: 274–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, Jaffrey SR (2015) 5′UTR m6A promotes cap-independent translation. Cell 163: 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Jaffrey SR (2017) Rethinking m6A readers, writers, and erasers. Annu Rev Cell Dev Biol 33: 319–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z, Zhang T, Qi Y, Song J, Han Z, Ma C (2020) Evolution of the RNA N6-methyladenosine methylome mediated by genomic duplication. Plant Physiol 182: 345–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadakuduti S, Holdsworth WL, Klein CL, Barry CS (2014) KNOX genes influence a gradient of fruit chloroplast development through regulation of GOLDEN2-LIKE expression in tomato. Plant J 78: 1022–1033 [DOI] [PubMed] [Google Scholar]
- Orzaez D, Mirabel S, Wieland WH, Granell A (2006) Agroinjection of tomato fruits. A tool for rapid functional analysis of transgenes directly in fruit. Plant Physiol 140: 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Lee HJ, Lee K, Kang H (2020) A La-related protein LaRP6a delays flowering of Arabidopsis thaliana by upregulating FLC transcript levels. J Plant Biol 63: 369‒378 [Google Scholar]
- Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL (2015) StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotech 33: 290–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růžička K, Zhang M, Campilho A, Bodi Z, Kashif M, Saleh M, Eeckhout D, Sedeer ES, Li HY, Zhong S, et al. (2017). Identification of factors required for m6A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol 215: 157–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scutenaire J, Deragon JM, Jean V, Benhamed M, Raynaud C, Favory JJ, Merret R, Bousquet-Antonelli C (2018) The YTH domain protein ECT2 is an m6A reader required for normal trichome branching in Arabidopsis. Plant Cell 30: 986–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Wong CE, Shen L, Yu H (2021) N6-methyladenosine modification underlies messenger RNA metabolism and plant development. Curr Opin Plant Biol 63: 102047. [DOI] [PubMed] [Google Scholar]
- Shen L, Liang Z, Gu X, Chen Y, Teo ZWN, Hou X, Cai WM, Dedon PC, Liu L, Yu H (2016) N6-methyladenosine RNA modification regulates shoot stem cell fate in Arabidopsis. Dev Cell 38: 186–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C (2017) YTHDF3 facilitates translation and decay of N6-methyladenosine modified RNA. Cell Res 27: 315–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Wei J, He C (2019) Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol Cell 74: 640–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Yang J, Wang C, Lu Q, Shi L, Tayier S, Jia G (2021). Arabidopsis N6-methyladenosine reader CPSF30-L recognizes FUE signals to control polyadenylation site choice in liquid-like nuclear bodies. Mol Plant 14: 571–587 [DOI] [PubMed] [Google Scholar]
- Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. (2014) N6-methyladenosine dependent regulation of messenger RNA stability. Nature 505: 117–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Schauer N, Usadel B, Frasse P, Zouine M, Hernould M, Latché A, Pech JC, Fernie AR, Bouzayen M (2009) Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell 21: 1428–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Fu Y, Parisien M, Dai Q, Jia G, et al. (2015). N6-methyladenosine modulates messenger RNA translation efficiency. Cell 161: 1388–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LH, Song P, Wang Y, Lu Z, Tang Q, Yu Q, Xiao Y, Zhang X, Duan HC, Jia G (2018) The m6A reader ECT2 controls trichome morphology by affecting mRNA stability in Arabidopsis. Plant Cell 30: 968–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, et al. (2016) Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol Cell 61: 507–519 [DOI] [PubMed] [Google Scholar]
- Yu G, Wang LG, He QY (2015) ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31: 2382–2383 [DOI] [PubMed] [Google Scholar]
- Yue Y, Liu J, He C (2015) RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev 29: 1343–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, Cheng T, Gao M, Shu X, Ma H, et al. (2018) VIRMA mediates preferential m6A mRNA methylation in 3ʹUTR and near stop codon and associates with alternative polyadenylation. Cell Discov 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhang YC, Liao JY, Yu Y, Zhou YF, Feng YZ, Feng YZ, Yang YW, Lei MQ, Bai M, et al. (2019) The subunit of RNA N6-methyladenosine methyltransferase OsFIP regulates early degeneration of microspores in rice. PLoS Genet 15: e1008120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng HX, Sun X, Zhang XS, Sui N (2020) m6A editing: new tool to improve crop quality? Trends Plant Sci 25: 859‒867 [DOI] [PubMed] [Google Scholar]
- Zhong S, Li H, Bodi Z, Button J, Vespa L, Herzog M, Fray RG (2008) MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell 20: 1278–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Tian S, Qin G (2019) RNA methylomes reveal the m6A-mediated regulation of DNA demethylase gene SlDML2 in tomato fruit ripening. Genome Biol 20: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Tang R, Li X, Tian S, Li B, Qin G (2021) N6-methyladenosine RNA modification regulates strawberry fruit ripening in an ABA-dependent manner. Genome Biol 22: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.