Abstract
Enantioselective Cu-catalyzed C–O cross coupling reactions yielding atropisomeric resorcinol-bearing quinazolinones have been developed. Utilizing a new guanidinylated dimeric peptidic ligand, a set of products were generated in good yields with excellent stereocontrol. The transformation was readily scalable and a range of product derivatizations were performed.
Graphical Abstract
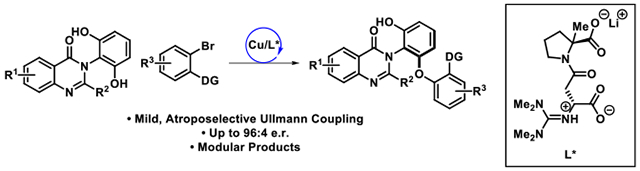
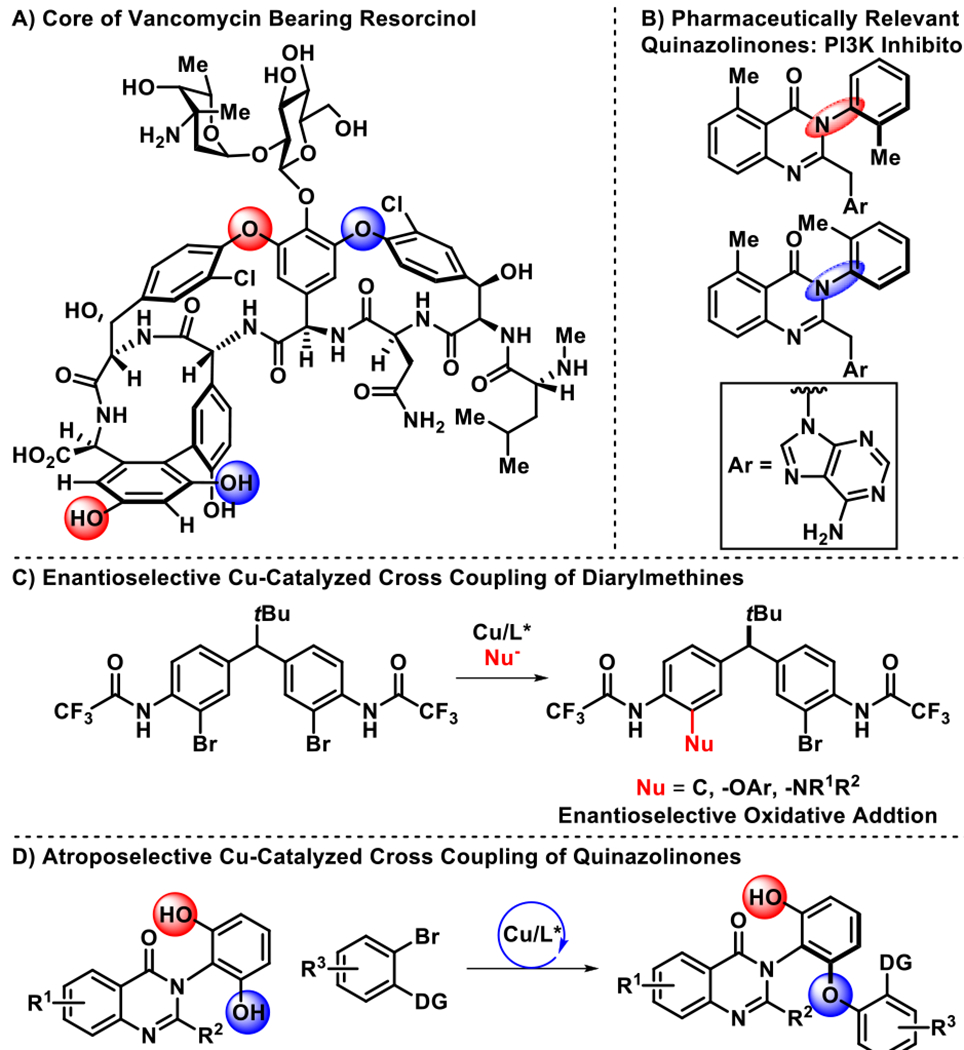
The selective functionalization of complex, multifunctional compounds is a frontier for both the fields of catalysis and medicinal chemistry, where there is a premium on (a) synthetic efficiency, (b) management of complex stereochemical issues, and (c) the creation of diversified scaffolds that interact selectively with complex biological targets.1 In this way, there is also a heuristic intersection between complex bioactive molecules like vancomycin, a potent antibiotic (Figure 1A) and enantiomerically pure scaffolds that exhibit isolable atropisomers (Figure 1B), wherein restricted rotation about a single bond defines functionally consequential stereogenicity.2 Research in our group began to address both of these challenges, with a particular emphasis on atroposelective halogenation.3 Metal-catalyzed cross coupling also creates powerful opportunities for scaffold diversification,4 and we have further examined these in the context of vancomycin and teicoplanin.5 In preliminary model studies of site-selective cross couplings, we also recently discovered a family of desymmetrization reactions based on peptidyl Cu-complexes (Figure 1C).6 These reactions built on previous pioneering studies of other Cu-catalyzed cross couplings.7–10 Our group reported highly enantioselective transformations for the privileged diarylmethane scaffold, but these studies do not provide a direct analogy to the challenges embedded within vancomycin, nor smaller molecules that bear atropisomeric axes. Moreover, these studies focused on site-selectivity within a bis(electrophilic) substrate. Accordingly, we wished to examine whether resorcinol-based functionality, which presents the site-selectivity challenge within a bis(nucleophilic) fragment, is amenable to enantioselective C–O bond-forming cross coupling with the peptidyl Cu-complexes we had developed.11 Herein, we describe unprecedented Cu-catalyzed atroposelective desymmetrizations of resorcinols within the biologically relevant quinazolinone scaffold (Figure 1D), grounding the viability of the approach for future examination within even more complicated structures.
Figure 1.
Overview of Bioactive Molecules and Enantioselective Cu-Catalyzed Ullman Coupling
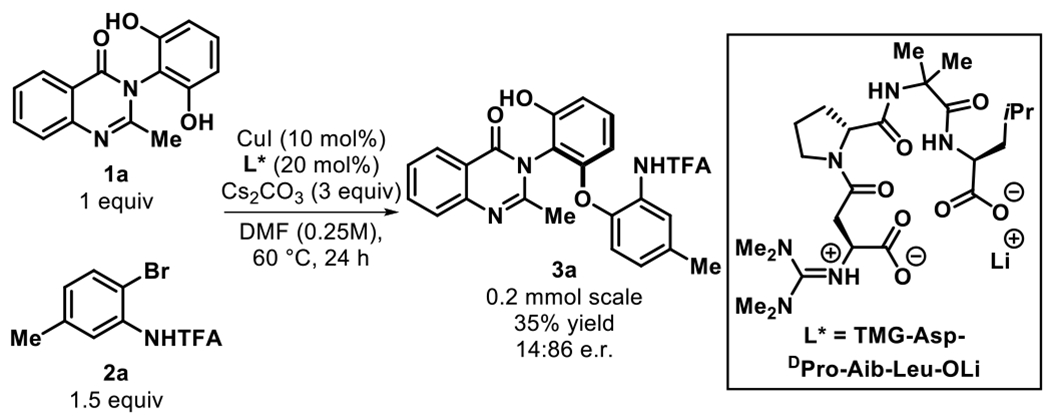
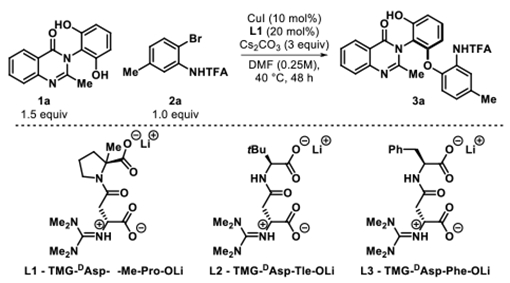
At the outset of our investigation, we selected the resorcinol bearing quinazolinone 1a for the optimization of the atroposelective Cu-catalyzed desymmetrization reaction. We first explored the use of tetrameric guanidinylated peptidic ligand L*, which was previously found to be successful in the C–O cross coupling of diarylmethanes.6b Using L*, quinazolinone 1a and arylbromide 2a were subjected to the reaction conditions shown in Figure 2 to give 3a in a promising 35% yield and 86:14 e.r. Arylbromide 2a was carefully selected to be the corresponding coupling partner as the trifluoroacetamide group serves as a directing group. After assessment of the reaction parameters, the optimal conditions were found to be CuI, truncated dimer L1, and Cs2CO3 in DMF at 40 °C for 48 h (59% yield, 95:5 e.r., Table 1, entry 1). To summarize our optimization efforts, a series of variations from the standard reaction conditions were performed to indicate their effects on the efficiency of the transformation (Table 1).12
Figure 2.
Cu-Catalyzed C–O Cross Coupling of Quinazolinone: Initial Hit
Table 1.
Atroposelective Cu-Catalyzed C–O Cross Coupling: Variation from the Standard Reaction Conditionsa
| |||
|---|---|---|---|
| Entry | Variation from “standard conditions” | Yield 2a (%)b,c | e.r. 3a |
| 1 | None | (59) | 95:5 |
| 2 | CuBr instead of CuI | 53 | 95:5 |
| 3 | Cu(MeCN)4PF6 instead of CuI | 51 | 93:7 |
| 4 | L2 instead of L1 | 22 | 85:15 |
| 5 | L3 instead of L1 | 26 | 92:8 |
| 6 | K2CO3 instead of Cs2CO3 | Not observed | - |
| 7 | K3PO4 instead of Cs2CO3 | Not observed | - |
| 8 | DMF/PhMe (1:1) instead of DMF | 38 | 95:5 |
| 9d | DMF/H2O instead of DMF | Not observed | - |
| 10 | 60 °C instead of 40 °C | 50 | 93:7 |
| 11 | ArCl instead of ArBr | Not observed | - |
Reactions run on 0.2 mmol scale.
Determined by 1H NMR analysis of the crude reaction mixtures using trimethyl benzene-1,3,5-tricarboxylate as internal standard.
Isolated yields in parenthesis.
5μL of H2O was added.
Utilizing other copper catalysts (CuBr and Cu(MeCN)4PF6) yielded 3a in comparable selectivity, but lower yields (53% and 51% respectively, Table 1, entries 2 and 3). Employing another sterically encumbered peptidic dimer ligand L2 gave the cross coupled product in significantly lower yield and selectivity (22% yield and 85:15 e.r., entry 4). Additionally, using another dimeric ligand L3 gave lower yield and slightly lower e.r. (entry 5). Interestingly, using K2CO3 or K3PO4 as the exogenous base did not generate any appreciable product, suggesting that the solubility and strength of the base plays a critical role in promoting the reaction (entries 6 and 7). A 1:1 mixture of DMF/PhMe, which was successful in our previous C–C cross coupling studies, led to 3a in lower yield but comparable e.r. (entry 8).6a Unlike the studies found by Ma and coworkers where H2O was found to be instrumental in providing high yield and enantioselectivity, the addition of water led to no observable product.8c Increasing the temperature to 60 °C led to lower yield due to nonproductive pathways such as protodemetalation of 2a (entry 10). Lastly, attempts to broaden our scope to include arylchlorides were ineffective as no product was observed (entry 11).
With the optimized conditions affording 3a in 59% yield and 95:5 e.r., we investigated the substrate scope of this reaction (Figure 3). Unsubstituted arylbromide 2b provided 3b in similar yield and excellent enantioselectivity (54% yield, 94:6 e.r.). The structure of 3b was unambiguously determined by single crystal X-ray crystallography.13 Electron deficient quinazolinone 1c was found to be effective in the reaction as it provided higher yields with comparable enantioselectivity (3c, 62% yield, 93:7 e.r.). Additionally, nitro-substituted arylbromide 2d which could be used as a future synthetic handle was tolerated in good yield and selectivity (3d, 51% yield, 91:9 e.r.). In the absence of an ortho-directing group, other halogen substituents are preserved in the transformation (3e). Notably, aza-quinazolinone (1f) was proficient in the reaction giving the respective cross coupled product 3f in 53% yield and 91:9 e.r. Electron rich arylbromides yielded the desired products in good yield and excellent enantioselectivity (3g and 3h). A limitation in this transformation is the tolerability of the arylbromides, wherein electron withdrawing substituents stunted the reactivity.14 Changing the −R2 group to a slightly larger group such as ethyl gave the product in comparable yield (3i). Unfortunately, other large groups including isopropyl, or benzyl were not tolerated in the reaction. Demonstrating the scalability of this transformation, model quinazolinone 1a (2mmol) underwent the title cross coupling to yield 3a in comparable yield and selectivity (54% yield and 94:6 e.r.). Interestingly, other nitrogen directing groups including acetyl or tosyl were incompatible with the transformation. We postulate that the trifluoromethyl acetamide group provides the appropriate pKa range necessary for the reaction.
Figure 3. Cu-Catalyzed C–O Cross Coupling: Substrate Scopea.
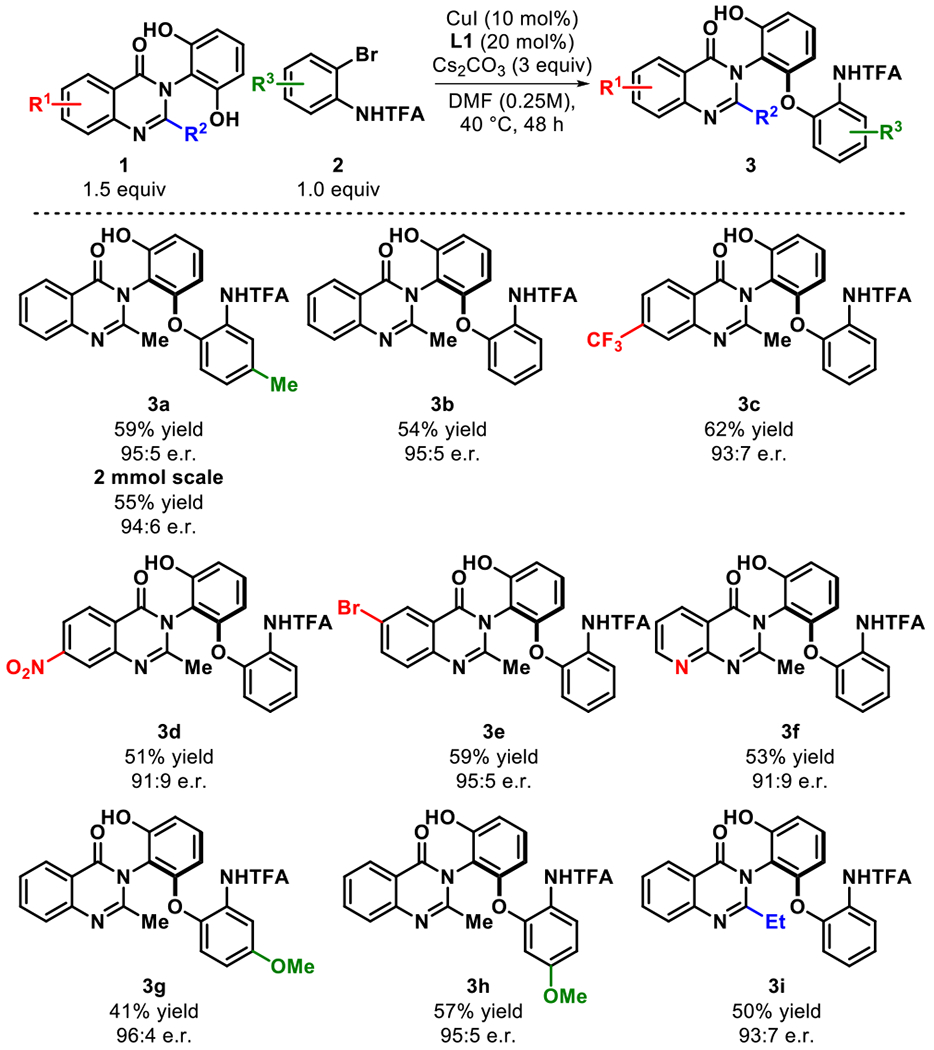
a Reactions run on 0.2 mmol scale. Isolated yields.
Finally, product derivatization studies were undertaken to assess the synthetic utility of the enantioenriched quinazolinones using product 3a (Figure 4). Utilizing the remaining hydroxyl group, a SNAr reaction with ethyl 2-chloropyrimidine-5-carboxylate furnished 4 in excellent yield and retention of stereochemistry. Additionally, exploiting the electron rich nature of the resorcinol, we were able to access dibrominated 5 in 90% yield and 95:5 e.r. Deprotection of the trifluoromethyl acetamide, which served as a directing group in our asymmetric reaction, was achieved in excellent yield (6, 92% yield, 92:8 e.r.). The pendant hydroxyl group on 3a could also be removed via reductive coupling in moderate yield while retaining the enantioselectivity (7).
Figure 4. Derivatization of Quinazolinone 3aa.
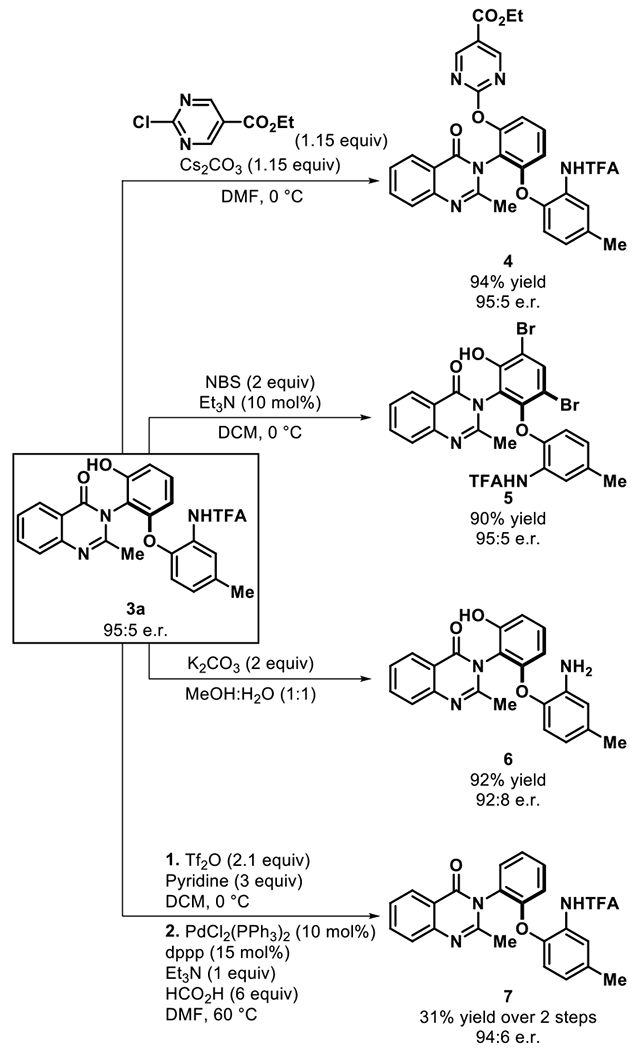
a Reactions run on 0.1 mmol scale. Isolated yields.
Analogous to the reports by the Ma group using ionic ligands,15 the Cu-catalyzed cross coupling reaction likely proceeds through the generation of bidentate Cu-based catalyst A (Figure 5). Then, deprotonation of 2 by Cs2CO3 gives the trifluoroacetimidate 2’, which directs the oxidative addition leading to the formation of B. Afterwards, atroposelective coordination would give C and deprotonation of one hydroxyl group would give D. Then product-forming reductive elimination releases 3 and regenerates the active catalyst A. An alternative order of events, demonstrated by Hartwig and coworkers for related reactions with neutral ligands on the Cu-center,16 might also be considered and has not been experimentally excluded.
Figure 5.
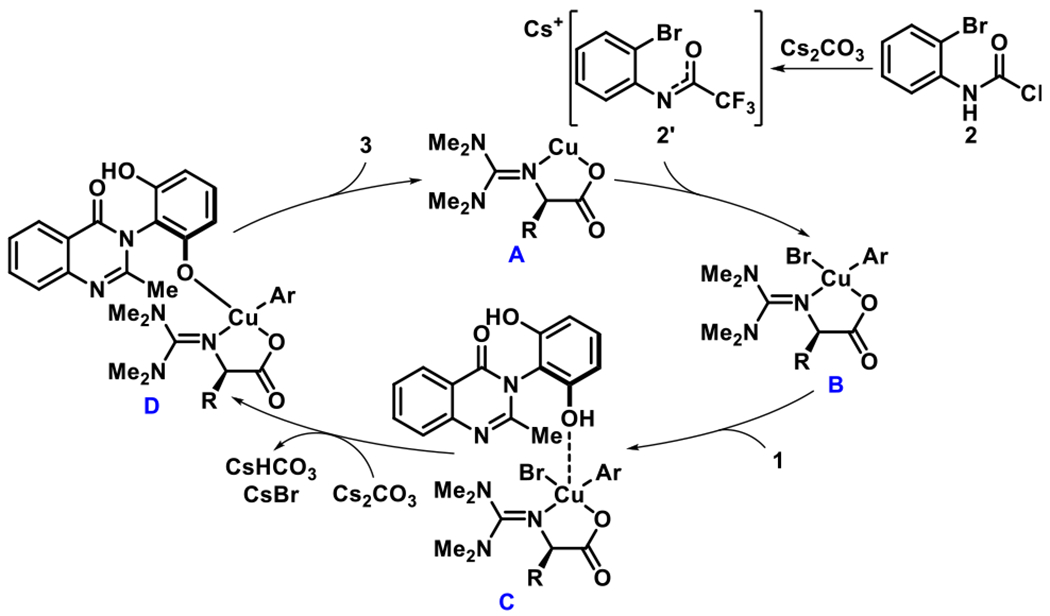
Postulated Mechanism for the Cu-Catalyzed C–O Cross Coupling
In conclusion, we have developed an atroposelective Cu-catalyzed C–O cross coupling reaction utilizing a guanidinylated peptidic ligand to form functionalized quinazolinones. The reaction was found to tolerate a range of functional groups including other halogens and heterocycles in good yield and excellent enantioselectivity. To demonstrate the synthetic utility of the products, we performed a diverse set of derivatizations. These findings set the stage for late stage functionalizations in highly complex molecular environments, which we are now actively investigating.
Supplementary Material
ACKNOWLEDGMENT
This research was supported by the National Institutes of Health (NIHGMS R35 132092). H.Y. thanks the Natural Sciences and Engineering Research Council (NSERC) for financial support. We thank Dr. Brandon Q. Mercado (Yale University) for single-crystal X-ray analysis of 3a. We thank Dr. Yuk Cheung (Chris) Chan (Yale University), Dr. Zebediah Girvin (Yale University), Dr. Elizabeth Stone (Yale University), Dr. Omar Beleh (Yale University), Dr. Aaron Featherston (Yale University) and Professor Margaret Hilton (West Virginia University) for insightful discussion during the project.
Footnotes
The authors declare no competing financial interests.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures, optimization, characterization and X-Ray data (PDF)
X-ray data for 3b (CIF)
FAIR Data is available as Supporting Information for Publication and includes the primary NMR FID files for compounds L1, 1a-1f, 3a-3i, 4-7
REFERENCES
- (1).(a) Busacca CA; Fandrick DR; Song JJ; Senanayake CH The Growing Impact of Catalysis in the Pharmaceutical Industry. Adv. Synth. Catal 2011, 353 , 1825. [Google Scholar]; (b) Caille S; Cui S; Faul MM; Mennen SM; Tedrow JS; Walker SD Molecular Complexity as a Driver for Chemical Process Innovation in the Pharmaceutical Industry. J. Org. Chem 2019, 84, 4583. [DOI] [PubMed] [Google Scholar]
- (2).(a) Clayden J; Moran WJ; Edwards PJ; LaPlante SR The Challenge of Atropisomerism in Drug Discovery. Angew. Chem. Int. Ed 2009, 48, 6398. [DOI] [PubMed] [Google Scholar]; (b) Evarts JB; Ulrich RG Atropisomers of 2-purinyl-3-tolyl-quinazolinone derivatives and methods of use. U.S. Patent 20100249155, September 30, 2010.; (c) LaPlante SR; Fader LD; Fandrick KR; Fandrick DR; Hucke O; Kemper R; Miller SPF; Edwards PJ Assessing Atropisomer Axial Chirality in Drug Discovery and Development. J. Med. Chem 2011, 54, 7005. [DOI] [PubMed] [Google Scholar]; (d) Toenjes ST; Gustafson JL Atropisomerism in medicinal chemistry: challenges and opportunities. Future Med. Chem 2018, 10, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Glunz PW Recent encounters with atropisomerism in drug discovery. Bioorganic Med. Chem. Lett 2018, 28, 53. [DOI] [PubMed] [Google Scholar]; (f) Metrano AJ ; Miller SJ . Peptide-Based Catalysts Reach the Outer Sphere through Remote Desymmetrization and Atroposelectivity. Acc. Chem. Res 2019, 52, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).(a) Gustafson JL; Lim D; Miller SJ Dynamic Kinetic Resolution of Biaryl Atropisomers via Peptide-Catalyzed Asymmetric Bromination. Science, 2010, 328, 1251. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pathak TP; Miller SJ Site-Selective Bromination of Vancomycin. J. Am. Chem. Soc 2012, 134, 6120. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Beleh OM; Miller E; Toste FD; Miller SJ Catalytic Dynamic Kinetic Resolutions in Tandem to Construct Two-Axis Terphenyl Atropisomers. J. Am. Chem. Soc 2020, 142, 16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).For selected reviews and examples on late stage functionalization and references therein, see:; (a) Karimov RR; Sharma A; Hartwig JF Late Stage Azidation of Complex Molecules Late Stage Azidation of Complex Molecules. ACS. Cent. Sci 2016, 2, 715. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shugrue CR; Miller SJ Applications of Nonenzymatic Catalysts to the Alteration of Natural Products. Chem. Rev 2017, 117, 11894. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) White MC; Zhao J Aliphatic C–H Oxidations for Late-Stage Functionalization. J. Am. Chem. Soc 2018, 140, 13988. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hong B; Luo T; Lei X Late-Stage Diversification of Natural Products. ACS Cent. Sci 2020, 6, 622. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Mu T; Wei B; Zhu D; Yu B Site-selective C-H hydroxylation of pentacyclic triterpenoids directed by transient chiral pyridine-imino groups. Nat. Commun 2020, 11. 4371. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Börgel J; Ritter T Late-Stage Functionalization. Chem. 2020, 6, 1877. [Google Scholar]; (g) Feng K; Quevedo RE; Kohrt JT; Oderinde MS; Reilly U; White MC. Late-stage oxidative C(sp3)–H methylation. Nature, 2020, 580, 621. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Kim KE; Kim AN; McCormick CJ; Stoltz BM Late-Stage Diversification: A Motivating Force in Organic Synthesis. J. Am. Chem. Soc 2021, 143, 16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Pathak TP; Miller SJM Chemical tailoring of teicoplanin with site-selective reactions. J. Am. Chem. Soc 2013, 135, 8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) Kim B; Chinn AJ; Fandrick DR; Senanayake CH; Singer RA; Miller SJ Distal Stereocontrol Using Guanidinylated Peptides as Multifunctional Ligands: Desymmetrization of Diarylmethanes via Ullman Cross-Coupling. J. Am. Chem. Soc 2016, 138, 7939. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chinn AJ; Kim B; Kwon Y; Miller SJ Enantioselective Intermolecular C–O Bond Formation in the Desymmetrization of Diarylmethines Employing a Guanidinylated Peptide-Based Catalyst. J. Am. Chem. Soc 2017, 139, 18107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kwon Y; Chinn AJ; Kim B; Miller SJ Divergent Control of Point and Axial Stereogenicity: Catalytic Enantioselective C-N Bond-Forming Cross-Coupling and Catalyst-Controlled Atroposelective Cyclodehydration. Angew. Chem. Int. Ed 2018, 57, 6251. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hwang J; Mercado BQ; Miller SJ Chirality-matched catalyst-controlled macrocyclization reactions. PNAS, 2021, 118, e2113122118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).For selected reviews on Cu-catalyzed cross coupling and references therein, see:; (a) Evano G; Blanchard N; Toumi M, Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis. Chem. Rev 2008, 108, 3054. [DOI] [PubMed] [Google Scholar]; (b) Ma D; Cai Q Copper/Amino Acid Catalyzed Cross-Couplings of Aryl and Vinyl Halides with Nucleophiles. Acc. Chem. Res 2008, 41, 1450. [DOI] [PubMed] [Google Scholar]; (c) Cherney AH; Kadunce NT; Reisman SE Enantioselective and Enantiospecific Transition-Metal-Catalyzed Cross-Coupling Reactions of Organometallic Reagents To Construct C–C Bonds. Chem. Rev 2015, 115, 9587. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Bhunia S; Pawar GG; Kumar SV; Jiang Y; Ma D Selected Copper-Based Reactions for C-N, C-O, C-S, and C-C Bond Formation. Angew. Chem. Int. Ed 2017, 56, 16136. [DOI] [PubMed] [Google Scholar]; (e) Cheng JK; Xiang S-H; Li S; Ye L; Tan B; Recent Advances in Catalytic Asymmetric Construction of Atropisomers. Chem. Rev 2021, 121, 4805. [DOI] [PubMed] [Google Scholar]; (f) Palani V; Perea MA; Sarpong R Site-Selective Cross-Coupling of Polyhalogenated Arenes and Heteroarenes with Identical Halogen Groups. Chem. Rev 2021. ASAP. DOI: 10.1021/acs.chemrev.1c00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).For selected examples on Cu-catalyzed cross coupling, see:; (a) Marcoux J-F; Doye S; Buchwald SL A General Copper-Catalyzed Synthesis of Diaryl Ethers. J. Am. Chem. Soc 1997, 119, 10539. [Google Scholar]; (b) Shafir A; Buchwald SL Highly Selective Room-Temperature Copper-Catalyzed C–N Coupling Reactions J. Am. Chem. Soc 2006, 128, 8742. [DOI] [PubMed] [Google Scholar]; (c) Xie X; Chen Y; Ma D Enantioselective Arylation of 2-Methylacetoacetates Catalyzed by CuI/trans-4-Hydroxy-L-proline at Low Reaction Temperatures. J. Am. Chem. Soc 2006, 128, 16050. [DOI] [PubMed] [Google Scholar]; (d) Lv X; Bao W A β-Keto Ester as a Novel, Efficient, and Versatile Ligand for Copper(I)-Catalyzed C–N, C–O, and C–S Coupling Reactions. J. Org. Chem 2007, 72, 3863. [DOI] [PubMed] [Google Scholar]; (e) Fan M; Zhou W; Jiang Y; Ma D CuI/Oxalamide Catalyzed Couplings of (Hetero)aryl Chlorides and Phenols for Diaryl Ether Formation. Angew. Chem. Int. Ed 2016, 55, 6211. [DOI] [PubMed] [Google Scholar]; (f) Zhai Y; Chen X; Zhou W; Fan M; Lai Y; Ma D Copper-Catalyzed Diaryl Ether Formation from (Hetero)aryl Halides at Low Catalytic Loadings. J. Org. Chem 2017, 82, 4964. [DOI] [PubMed] [Google Scholar]; (g) Chen Z; Jiang Y; Zhang L; Guo Y; Ma D Oxalic Diamides and tert-Butoxide: Two Types of Ligands Enabling Practical Access to Alkyl Aryl Ethers via Cu-Catalyzed Coupling Reaction. J. Am. Chem. Soc 2019, 141, 3541. [DOI] [PubMed] [Google Scholar]; (h) Frey J; Malekafzali A; Delso I; Choppin S; Colobert F; Wencel-Delord J Enantioselective Synthesis of N–C Axially Chiral Compounds by Cu-Catalyzed Atroposelective Aryl Amination. Angew. Chem. Int. Ed 2020, 59, 8844. [DOI] [PubMed] [Google Scholar]; (i) Ray R; Hartwig JF Oxalohydrazide Ligands for Copper-Catalyzed C–O Coupling Reactions with High Turnover Numbers. Angew. Chem. Int. Ed 2021, 60, 8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).For selected examples on enantioslective, intramolecular Cu-catalyzed reactions, see:; (a) Zhou F; Guo J; Liu J; Ding K; Yu S; Cai Q Copper-Catalyzed Desymmetric Intramolecular Ullmann C–N Coupling: An Enantioselective Preparation of Indolines. J. Am. Chem. Soc 2012, 134, 14326. [DOI] [PubMed] [Google Scholar]; (b) Yang W; Long Y; Zhang S; Zeng Y; Cai Q Copper-Catalyzed Enantioselective Intramolecular N-Arylation, an Efficient Method for Kinetic Resolutions. Org. Lett 2013, 15, 3598. [DOI] [PubMed] [Google Scholar]; (c) Zhou F; Cheng G-J; Yang W; Long Y; Zhang S; Wu Y-D; Zhang X; Cai Q Enantioselective formation of cyano-bearing all-carbon quaternary stereocenters: desymmetrization by copper-catalyzed N-arylation. Angew. Chem. Int. Ed 2014, 53, 9555. [DOI] [PubMed] [Google Scholar]; (d) Yang W; Liu Y; Zhang S; Cai Q Copper-Catalyzed Intramolecular Desymmetric Aryl C-O Coupling for the Enantioselective Construction of Chiral Dihydrobenzofurans and Dihydrobenzopyrans. Angew. Chem. Int. Ed 2015, 54, 8805. [DOI] [PubMed] [Google Scholar]; (e) Liu J; Tian Y; Shi J; Zhang S; Cai Q An Enantioselective Synthesis of Spirobilactams through Copper-Catalyzed Intramolecular Double N-Arylation and Phase Separation. Angew. Chem. Int. Ed 2015, 54, 10917. [DOI] [PubMed] [Google Scholar]; (f) Fan X; Zhang X; Li C; Gu Z Enantioselective Atropisomeric Anilides Synthesis via Cu-Catalyzed Intramolecular Adjacent C–N Coupling. ACS Catal. 2019, 9, 2286. [Google Scholar]
- (10).For selected examples of site-selective Cu-catalyzed C–O cross coupling reactions, see:; (a) Dimakos V; Garrett GE; Taylor MS Site-Selective, Copper-Mediated O-Arylation of Carbohydrate Derivatives. J. Am. Chem. Soc 2017, 139, 15515. [DOI] [PubMed] [Google Scholar]; (b) Shang W; Mou Z-D; Tang H; Zhang X; Liu J; Fu Z; Niu D Site-Selective O-Arylation of Glycosides. Angew. Chem. Int. Ed 2018, 57, 314. [DOI] [PubMed] [Google Scholar]
- (11).For selected examples of enantioselective quinazolinone synthesis, see:; (a) Hirai M; Terada S; Yoshida H; Ebine K; Hirata T; Kitagawa O Catalytic Enantioselective Synthesis of N–C Axially Chiral Mebroqualone and Its Derivatives through Reductive Asymmetric Desymmetrization. Org. Lett 2016, 18 5700. [DOI] [PubMed] [Google Scholar]; (b) Wang Y-B; Zheng S-C; Hu Y-M; Tan B Brønsted acid-catalysed enantioselective construction of axially chiral arylquinazolinones. Nat. Commun 2017, 8, 15489. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Teng F; Yu T; Peng Y; Hu W; Hu H; He Y; Luo S; Zhu Q Palladium-Catalyzed Atroposelective Coupling—Cyclization of 2-Isocyanobenzamides to Construct Axially Chiral 2-Aryl- and 2,3-Diarylquinazolinones. J. Am. Chem. Soc 2021, 143, 2722. [DOI] [PubMed] [Google Scholar]
- (12).Protodemetalation of the arylbromide was observed, alongside decomposition of the quinazolinone.
- (13).Refer to SI X-ray crystallographic data. CCDC Number: 2129281.
- (14).Aryliodides are also tolerated in the reaction; however, electron deficient aryliodide equivalents were also ineffective in the transformation.
- (15).For selected reviews and references therein, see:; (a) Copper-Mediated Cross-Coupling Reactions. Evano G, Blanchard N, Wiley, Hoboken, 2013 [Google Scholar]; (b) Alicia C, Xavi R, The role of organometallic copper(III) complexes in homogeneous catalysis. Chem. Sci 2013, 4, 2301. [Google Scholar]
- (16).(a) Tye JW; Weng Z; Johns AM; Incarvito CD; Hartwig JF Copper Complexes of Anionic Nitrogen Ligands in the Amidation and Imidation of Aryl Halides. J. Am. Chem. Soc 2008, 130, 9971. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tye JW; Weng Z; Giri R; Hartwig JF Copper(I) Phenoxide Complexes in the Etherification of Aryl Halides. Angew. Chem. Int. Ed 2010, 49, 2185. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Huang Z; Hartwig JF Copper(I) Enolate Complexes in α-Arylation Reactions: Synthesis, Reactivity, and Mechanism. Angew. Chem. Int. Ed 2011, 51, 1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


