Abstract
Transition-metal-catalyzed, coordination-assisted C(sp3)–H functionalization has revolutionized synthetic planning as, over the past few decades, the use of these directing groups has allowed for increased access to many strategic positions in organic molecules. Nonetheless, several challenges remain preeminent, such as the requirement of high temperatures, the difficulty in removing/converting directing groups, and, while many metals provide some reactivity, the difficulty in employing metals outside of palladium. This review aims to give a comprehensive overview of coordination-assisted, transition-metal-catalyzed, direct functionalization of nonactivated C(sp3)–H bonds by covering the literature since 2004 in order to demonstrate the current state-of-the-art methods as well as the current limitations. For clarity, this review has been divided into nine sections by the transition metal catalyst with subdivisions by the type of bond formation. Synthetic applications and reaction mechanism are discussed where appropriate.
Graphical Abstract

1. INTRODUCTION
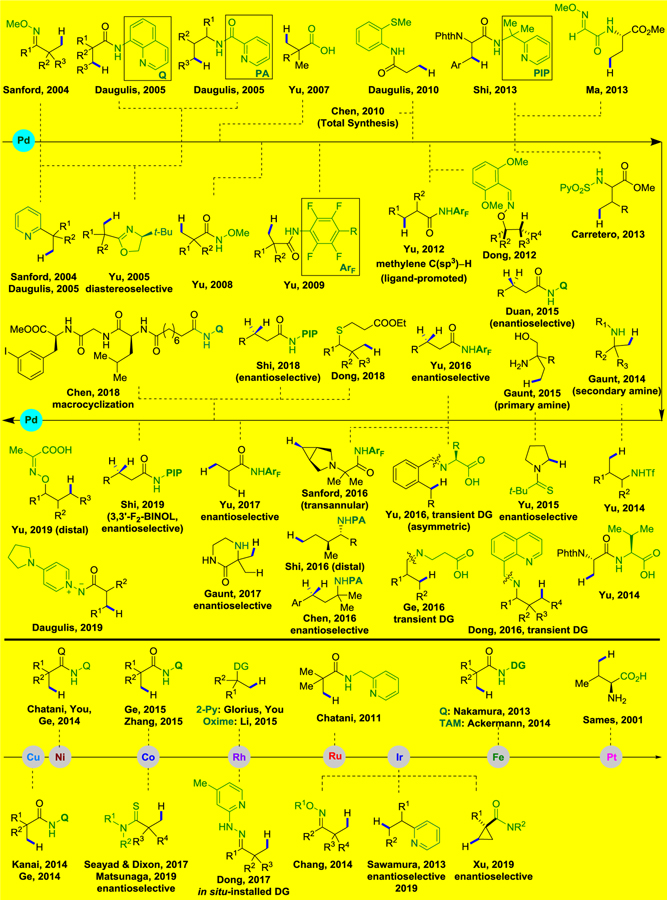
In recent years, tremendous progress has been made in building carbon–carbon and carbon–heteroatom linkages directly from carbon–hydrogen bonds.1–35 Furthermore, materials science and the pharmaceutical industry have benefited from the development of metal-catalyzed C–H bond activation/functionalization.36–44 Because sp2 and sp3 carbon–hydrogen (C–H) bonds are ubiquitous in organic compounds, differentiating among the various C–H bonds in a complex substrate is extremely difficult. In recent years, significant advances have been made to overcome this challenge. Directing groups (DGs) have featured prominently to enable reaction sequences that involve site-selective C–H activation/functionalization. Several pioneering examples of this strategy have been reported since the 1950s,45–48 such as cobalt-catalyzed ortho-carbonylation of aldimine by Murahashi,45 palladium-catalyzed ortho-halogenation of azobenzene by Fahey,46 ortho-C–H alkylation of phenol catalyzed by an cyclometalated ruthenium complex by Lewis,47 and ruthenium-catalyzed ortho-selective alkylation of aromatic ketones with olefins reported by Murai and co-workers.48
A multitude of functional groups, including amides, imines, azaheterocycles, amines, carboxylic acids, esters, ketones, oximes, aldehydes, and hydroxyl groups have been employed as DGs for catalytic C–H bond functionalizations. In addition, functional groups that contain sulfur, phosphorus, and silicon as well as those that demonstrate π-coordination have been developed as directing groups.
Compared to the significant progress in C(sp2)–H bond activation/functionalization, catalytic functionalization of C(sp3)–H bonds remains an enormous challenge due to the low acidity and the comparatively weak nature of the corresponding metal–alkyl bonds.49 Furthermore, compared to primary C(sp3)–H bonds, methylene C(sp3)–H bonds are more difficult to functionalize due to steric hindrance during the C–H metalation step.
Although earlier reviews have dealt with certain aspects of C(sp3)–H functionalization reactions, including the transition-metal-catalyzed arylation of unactivated C(sp3)–H bonds,8 alkyl C–H activation catalyzed by palladium,34 reactions assisted by specific directing groups (e.g. exo-type DG22 or amines30), and the cross-dehydrogenative coupling of C(sp3)–H bonds,50, a comprehensive overview of this cutting-edge area has not been summarized and numerous new advances have been made in the past several years. We reasoned that a review on coordination-assisted nonactivated C(sp3)–H functionalization reactions which covers the full breadth of transition metal catalysts would be a useful addition to the literature (Figure 1).
Figure 1.
Transition Metals in Periodic Table Used in Coordination-Assisted C(sp3)–H Activation and Covered by this Review Article.
This review will focus on transition-metal-catalyzed coordination-assisted functionalization reactions of nonactivated C(sp3)–H bonds, covering the literature published through March 2020 with any omissions being unintentional. Intramolecular C(sp3)–H functionalization methods that involve an initial oxidative addition event, for example with the metal adding into an Ar–X51–58 or Si–H59–66 bonds are outside of the scope of this review and will not be discussed except when illustrative of other key points. For clarity, this review has been divided into eight sections by metal type in which the discussion of C(sp3)–H bond functionalization is arranged according to the types of bonds that are formed. Moreover, both synthetic application and mechanism are discussed where appropriate. It is worth noting that this is the first comprehensive review that encompasses the large body of work in this field over the past 16 years (2004–March 2020) (Figure 2). We hope that this review will inspire new developments in coordination-assisted transition-metal-catalyzed nonactivated C(sp3)–H functionalization to overcome existing challenges and stimulate additional uses of these methods in the synthesis of natural products, pharmaceuticals, and other functionally significant molecules.
Figure 2.
Timeline of the Representative Discovery and Development of Coordination Assisted Transition Metal-Catalyzed C(sp3)–H Functionalization Reactions.
2. Palladium-Catalyzed, Coordination-Assisted C(sp3)–H Functionalization
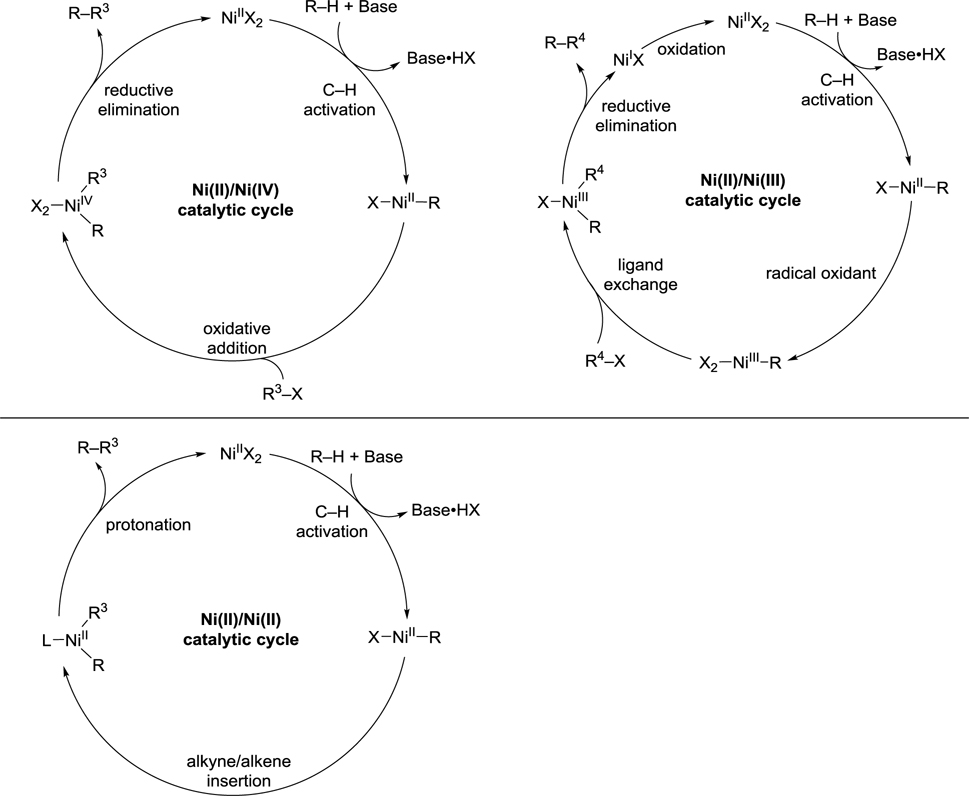
Palladium-catalyzed C–H functionalization reactions have been extensively investigated. These reactions can be subdivided based on the change of the oxidation state of the palladium catalyst during the catalytic cycle (Scheme 1).5,51–58, 67–70, For example, the Pd(0)/Pd(II) catalytic cycle that is well known in classical cross-coupling is also applicable in numerous C–H functionalization reactions,51–58 which will not be covered in this review. These reactions can also proceed through Pd(II)/Pd(0) and Pd(II)/Pd(IV) catalytic cycles.4,67,71 Bimetallic Pd(III)–Pd(III) intermediates have been proposed as the active palladium species in C–H functionalization reactions by the Sanford and the Ritter groups.68,69 C–H functionalization reactions can also proceed through a Pd(II)/Pd(II) catalytic cycle, in which the Pd–C bond is cleaved via direct electrophilic substitution without a change in oxidation state at the metal center.
Scheme 1.
Overview of Proposed Catalytic Cycles of Palladium-Catalyzed C–H Functionalization Reactions
2.1. Palladium-Catalyzed C(sp3)–H Functionalization Using Monodentate Directing Groups
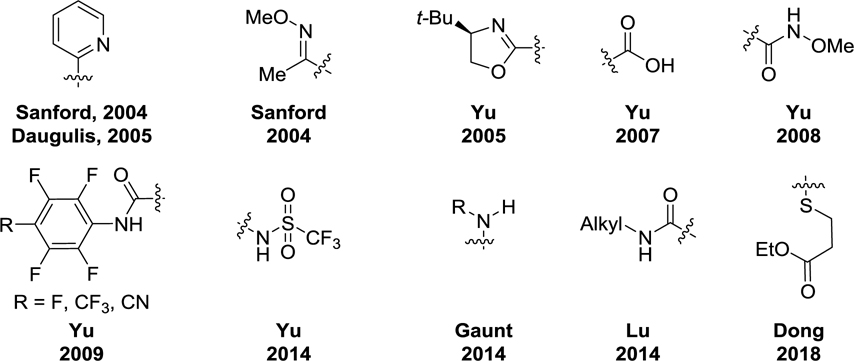
Most C(sp3)–H activation methods employ heteroatom-containing functional groups that coordinate to the metal and direct the metal’s insertion into the C–H bonds. In the last decade, a variety of monodentate DGs, including pyridines, oxazolines, oximes, amides, and amines, have been developed for C(sp3)–H activation (Figure 3).
Figure 3.
Representative Monodentate DGs for C(sp3)–H Activation
2.1.1. Carbon-Carbon Bond Formation
2.1.1.1. Arylation
Three pathways for the directed arylation of unactivated C(sp3)–H bonds with monodentate DGs are summarized in Scheme 2. Usually, cyclopalladated intermediate B is generated after C(sp3)–H activation. Subsequently, Pd(II)/Pd(0) or Pd(II)/Pd(IV) redox couples can operate. In the Pd(II)/Pd(0) pathway, Pd(II) intermediate C is produced by transmetalation with a nucleophilic coupling partner. Intermediate C then undergoes reductive elimination to afford the arylated product and Pd(0). In the Pd(II)/Pd(IV) pathway, after oxidative addition with an electrophilic coupling partner, a Pd(IV) intermediate E or G is generated. Pd(IV) intermediate E then undergoes reductive elimination to afford the arylated product and Pd(II). Alternatively, Pd(IV) intermediate G is converted to Pd(IV) intermediate H via C(sp2)–H palladation followed by reductive elimination to afford the arylated product and Pd(II).
Scheme 2.
Overview of Monodentate DG-assisted C(sp3)–H bond Arylation
2.1.1.1.1. Monodentate-Directing-Group-assisted C(sp3)–H Arylation via Pd(II)/Pd(IV) Catalysis
Monodentate nitrogen-based DGs have been recognized as one of the most efficient DG classes for transition-metal-catalyzed C–H functionalization since the pioneering investigations into stoichiometric cyclopalladation processes in the 1970s and 80s.72–75 In 2005, palladium-catalyzed, pyridine-directed C(sp3)–H bond arylation with 1-iodo-4-methylbenzene 2 was reported by the Daugulis group.76 Despite the high reaction temperature, long reaction times, and excess of aryl iodides were required in this reaction, the report is an early demonstration of C(sp3)–H arylation via a Pd(II)/Pd(IV) catalytic cycle (Scheme 3).
Scheme 3.
Pyridine-Directed C(sp3)–H Bond Arylation Reported by Daugulis (Daugulis et al., 2005).76
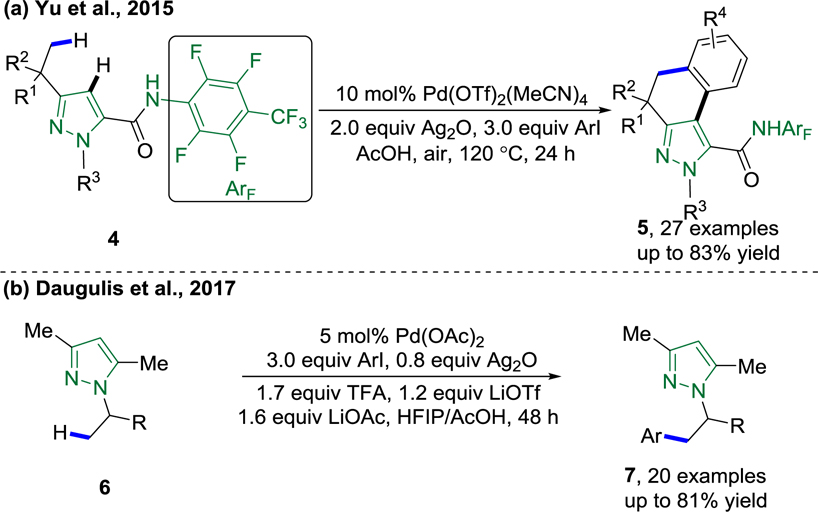
In 2015, Yu, Stamos and co-workers developed a cascade process involving pyrazole-directed C(sp3)–H bond arylation with aryl iodides followed by CONHArF (ArF = (4-CF3)-C6F4) -directed pyrazole C(sp2)–H bond and ortho-phenyl C–H bond activation/cyclization (Scheme 4a).77 Surprisingly, the pyrazole directed the arylation of the C(sp3)–H bond. Pd(OTf)2(MeCN)4 proved to be the most active palladium catalyst for this transformation. The catalytic system for this orchestrated cascade triple C–H activation tolerates a variety of functional groups with excellent selectivity and shortens the synthetic route to medicinally important benzo[e]indazole derivatives. In 2017, Daugulis and co-workers also demonstrated a pyrazole-directed C(sp3)–H bond arylation reaction, which was catalyzed by Pd(OAc)2 and employed silver(I) oxide as a halide-scavenger and base precursor (Scheme 4b).78 The pyrazole moiety in the product can be removed by ozonolysis to afford β-phenethylamines.
Scheme 4.
a) Pyrazole-Directed C(sp3)–H Bond Arylation Reported by Yu (Yu et al., 2015).77 b) Pyrazole-Directed C(sp3)–H Bond Arylation Reported by Daugulis (Daugulis et al., 2017).78
Inspired by the cyclometallation of 1-aminopyridine ylides with various transition metals (Pd, Rh, Pt), Daugulis and co-workers coupled a number of 1-aminopyridines onto aliphatic carboxylic acids to investigate C(sp3)–H bond functionalization with this family of directing groups (Scheme 5).79 The NaOTf additive was found to increase the yield of arylated product significantly when using hexafluoroisopropanol (HFIP) as the solvent at 90 °C. Secondary C(sp3)–H arylation was achieved using N-acetyl-L-phenylalanine (L1) as ligand. The discovery of 1-aminopyridine ylides expanded the arsenal of removable monodentate DGs for challenging C(sp3)–H activation reactions.
Scheme 5.
1-Aminopyridinium-Ylide-Directed β-C(sp3)–H Arylation (Daugulis et al., 2019).79
Through the electronic and steric modification of amine groups, Gaunt and co-workers developed a palladium-catalyzed C(sp3)–H arylation reaction of protected 1,2-amino alcohols (Scheme 6a).80 The reported reaction conditions include Pd(OAc)2 (15 mol%) with Ph2IOTf in the presence of sodium acetate in 1,2-dichloroethane at 70 °C. They succeeded in using both electron-rich and electron-deficient aryl iodide reagents, and various functional groups, such as bromo and fluoro, were tolerated. In 2019, Gaunt and co-workers observed that 2-halobenzoic acids could promote oxidative addition to a less hindered alkylamine-derived palladacycle (Scheme 6b). When 2-iodobenzoic acid (2 equiv) and AgOAc (1 equiv) were added to the solution of palladacycle 18 in CHCl3, a palladacycle 20 was identified.81 The authors propose that this seven-membered palladacycle forms through a concerted 1,2-arylpalladium migration and reductive elimination process triggered by decarboxylation. Free primary aliphatic amine-directed arylation of unactivated C(sp3)–H bonds with an acid additive has also been reported (Scheme 7).82,83
Scheme 6.
a) C(sp3)–H Arylation of Amino Alcohol Derivatives (Gaunt et al., 2015).80 b) C(sp3)–H Arylation of Alkylamines (Gaunt et al., 2019).81
Scheme 7.
a) Free-Amine-Directed, γ-C(sp3)–H Arylation of α-Amino Esters (Yao et al., 2019).82 b) Free-Amine-Directed δ-C(sp3)–H Arylation (Bannister et al., 2019).83
Other strongly coordinating monodentate DGs have also been developed. In 2016, Chen and co-workers reported an oxime-ether-directed, Pd(II)-catalyzed arylation of β-C(sp3)–H bonds using diaryliodonium salts as arylating reagents (Scheme 8).84 This technique allows the preparation of useful β-arylated oximes from ketones and aldehydes. After investigating different solvents, HFIP was found to accelerate the reaction.
Scheme 8.
Oxime-Directed β-Arylation of Oxime Ethers Using Diaryliodonium Salts (Chen et al., 2016).84
In 2018, the Dong group reported a thioether-directed, palladium-catalyzed γ-C(sp3)–H arylation reaction.85 The authors protected the free thiol through a Michael addition between the thiol substrate and ethyl acrylate, which maintained the ability of sulfur to coordinate to the Pd catalyst (Scheme 9). A variety of protecting groups have been tested under the standard reaction conditions. No desired product was obtained when thiols were protected as Michael adducts bearing pendant carboxylic acid or amide groups. Moreover, the corresponding tert-butyl ester was unstable under acidic conditions, and the methyl ester furnished the desired product in only low yield.
Scheme 9.
Thioether-Directed γ-C(sp3)–H Arylation (Dong et al., 2018).85
While nonactivated C(sp3)–H arylation assisted by strongly coordinating monodentate DGs containing nitrogen-, sulfur-, or phosphorus-coordination, has been well studied, the use of weakly coordinating DGs, such as carboxylates and ketones, remains a challenge.70 The Yu group established the first example of Pd(II)-catalyzed, carboxyl-directed arylation of β-C(sp3)–H bonds in simple aliphatic acids.86 However, this reaction suffered from low yields and a narrow substrate scope (Scheme 10). This reactivity of carboxylic acids was significantly promoted by sodium counterions. Subsequent studies support the proposal that the countercation binds to the carboxylate group in a κ2 coordination mode, which induces Pd(II) to coordinate with the unhindered oxygen lone pair in a κ1 fashion (39).87 C–H activation was triggered through a process analogous to the complex induced proximity effect (CIPE).88
Scheme 10.
Carboxyl-Directed Pd(II)-Catalyzed Arylation of β-C(sp3)–H Bonds (Yu et al., 2007).86
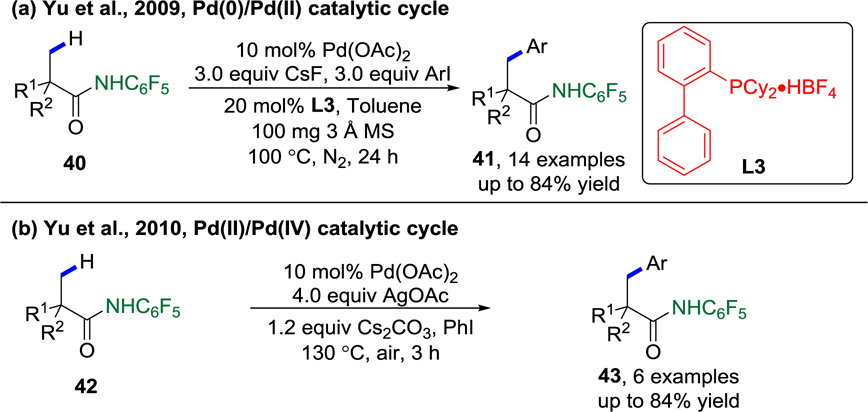
Due to the low yields in the first version of this Pd(II)-catalyzed, carboxylate-directed β-C(sp3)–H functionalization, the Yu group was motivated to develop a robust monodentate carboxamide DG that would demonstrate high reactivity, selectivity, and scope. In 2009, Yu and co-workers successfully introduced the CONHArF (ArF = C6F5) DG, which allowed the desired β-methyl C–H arylation reaction to proceed efficiently with Buchwald’s Cyclohexyl-JohnPhos ligand (L3) and CsF as the base (Scheme 11a).89 Various α-methylated amides were arylated in good to excellent yield. The authors observed that an acidic N–H bond in the DG is essential for reactivity. Although this protocol is believed to proceed through a Pd(0)/Pd(II) catalytic cycle, it represents the first introduction of the monodentate, weakly coordinating CONH–ArF (ArF = C6F5) DG for use in C(sp3)–H activation. Shortly after, the Yu lab successfully applied the same CONH–ArF DG in Pd(II)/Pd(IV) chemistry, which allowed the desired β-methyl C–H arylation reaction to proceed efficiently with Pd(OAc)2 as the catalyst, AgOAc as the additive, and Cs2CO3 as the base. Several carboxylic acid derivatives were arylated in moderate to good yields that were comparable to those obtained via the Pd(0)/Pd(II) catalytic cycle (Scheme 11b).90
Scheme 11.
The First Example of CONHArF (ArF = C6F5)-Directed Arylation of β-Methyl C(sp3)–H Bonds. a) via Pd(0)/Pd(II) catalytic cycle (Yu et al., 2009);89 b) (Yu et al., 2010).90
In 2012, the Yu laboratory described the first example of CONHArF (ArF = p-CF3C6F4)-directed, Pd(II)-catalyzed arylation of acyclic and cyclic β-methylene C(sp3)–H bonds, which was achieved through systematic screening of mutually repulsive 2,6-dialkoxypyridine and 2-alkoxyquinoline ligands.91 The reported reaction conditions are as follows: Pd(TFA)2 (10 mol%), L4 (20 mol%), ArI (3.0 equiv), Ag2CO3 (2.0 equiv) as the additive, K2HPO4 (1.2 equiv) as the base, hexane,110 °C, 24 h (Scheme 12a). In this system, the methylene C–H arylation of alicyclic substrates was also possible. In 2014 Yu and co-workers developed a one-pot synthesis of 4-aryl-2-quinolinones via ligand-promoted triple sequential C–H activation from the propionamide (Scheme 12b).92 The mechanism involves C(sp3)–H arylation, dehydrogenation, Heck-type addition of another aryl halide partner, and intramolecular C(sp2)–H amidation. After extensive screening, the optimized reaction conditions were reported to be with PdCl2 (10 mol%), using Ag2CO3 (3.0 equiv) as the additive and 2,5-lutidine L5 (20 mol%) as the ligand at 140 °C in t-Amyl-OH for 24 h. A wide variety of functional groups on the aryl iodide were well tolerated in the reaction. The installation of two different aryl groups saw the intramolecular amidation occur at the less hindered position.
Scheme 12.
a) The First Example of CONHArF (ArF = p-CF3C6F4)-Directed β-Methylene C(sp3)–H Arylation (Yu et al., 2012).91 b) Ligand-Promoted CONHArF (ArF = p-CF3C6F4)-Directed Triple C–H Activation Reactions (Yu et al., 2014).92
Later, the Yu group demonstrated the first example of ligand-controlled C(sp3)–H arylation of alanine derivatives that bear an p-CF3C6F4 amide auxiliary (Scheme 13).93 Monoarylation of the methyl C(sp3)–H bond with aryl iodides was achieved when a pyridine-based ligand (L6) was used. On the other hand, the arylation of the resulting benzylic methylene C(sp3)–H bond with different aryl iodides was enabled by a quinoline-based ligand (L7). Arylation of a phenylalanine derivative with aryl iodides promoted by quinoline-based ligand L7 were also investigated. In this system, various functional groups are well tolerated. Palladacycles 53 and 54 were synthesized via primary and secondary C(sp3)–H activation, respectively. Characterization of these complexes sheds light on the underlying mechanism of these transformations, offering a platform for further computational and kinetic studies. Palladacycles 53 and 54 were found to be viable precatalysts for primary and secondary C(sp3)–H arylation, respectively, with the addition of TFA to facilitate the dissociation of one of the pyridine ligands. Computational studies revealed that the nonactivated precatalyst was activated by the deprotonation of the amide with CsF.94 These studies also showed that the C(sp3)–H bond cleavage is the rate-limiting step.95 A reusable polymeric ligand platform was also designed to facilitate C(sp3)–H arylations of alanine by the Jones group in 2016.96
Scheme 13.
Ligand-promoted CONHArF (ArF = p-CF3C6F4)-Directed Arylation of Alanine and Phenylalanine-Derived Substrates (Yu et al., 2014).93
Ligand-accelerated, enantioselective, methylene C(sp3)–H bond activation was discovered by the Yu group in 2016 (Scheme 14a).97 The researchers observed that monodentate chiral ligands do not affect the stereochemistry of the palladium insertion step. Inspired by both quinoline-based ligands and chiral monoprotected amino acid (MPAA) ligands, chiral N-acetyl-protected aminoethyl quinoline (APAQ) ligands, which form six-membered chelates when bound to Pd, were found to enable Pd(II)-catalyzed enantioselective arylation of β-methylene C–H bonds of aliphatic amides with enantiomeric ratios reaching up to 96:4 and yields as high as 89%. Density functional theory (DFT) evidence suggests that the six-membered chelate can alleviate the steric repulsion between the quinoline group of the ligand and the ArF group of the substrate.98 A dimeric palladium species, which was further investigated by the Blackmond group, was proposed as the resting state but not as an active catalyst.99 A subsequent computational study by Bertrand, Yu, and co-workers explored three unprecedented amide-bridged Pd(II) dimers (58-60) and demonstrated that the quinoline ring of the APAQ ligands promotes catalytic activity (Scheme 14b).100 Dramatically reduced reactivity was observed when N-acetyl-protected aminoethylpyridine (APAPy) was used as a ligand, and Bertrand, Yu, and co-workers reasoned that the off-cycle Pd(II) dimer (58) with APAPy ligand is more stable, which slows the dissociation to the catalytically active species in solution.
Scheme 14.
Ligand-Accelerated Enantioselective Methylene C(sp3)–H bond Arylation (Yu et al., 2016; Bertrand et al., 2017).97,100
In 2017, the Yu group devised a protocol for the desymmetrization of the geminal methyl groups in an isopropyl moiety using chiral mono-N-protected aminomethyl oxazoline (MPAO) ligands (Scheme 15).101 Two challenging aspects of this desymmetrization include: (1) the differentiation between the α hydrogen atom and the α-methyl substituent, and (2) the long distance between the prochiral carbon and the ligated transition metal center. A bidentate, chiral, APAQ ligand was investigated first but gave almost no enantioselectivity, reflecting the different nature of the transition states for enantioselective methylene C–H activation and isopropyl desymmetrization. After extensive screening and ligand modification, chiral MPAO ligands were found to affect enantioselective C(sp3)–H activation of isopropyl groups. A wide range of aryl iodides compounds that contain electron-donating or electron-withdrawing groups are tolerated, producing desired products with enantiomeric ratios up to 98:2 using L11 as chiral ligand. By using an N-methoxyamide auxiliary as a directing group and replacing the N-acetyl moiety with an ortho-difluorobenzoyl group to make L12, Yu and coworkers maintained high enantioselectivity while improving the yield of 64, α,α-disubstituted α-amino acids containing chiral α-quaternary centers.
Scheme 15.
Asymmetric β-C(sp3)–H Arylation Provides Access to α-Chiral Centers (Yu et al., 2017).101
In 2018, Yu and co-workers prepared various chiral cyclobutanes via a Pd(II)-catalyzed enantioselective arylation of cyclobutyl carboxylic amides that bear α-hydrogen atoms using a chiral MPAO ligand (Scheme 16a, L13).102 A variety of aryl iodide compounds that contain electron-donating or electron-withdrawing functional groups afford the corresponding products in moderate to good yields and excellent enantioselectivities. In 2019, asymmetric C(sp3)–H arylation of cyclopropanes enabled by a N-protected aminosulfoxide ligand (L14) was reported by Colobert and co-workers. Stacking of π bonds between the ligand and the substrate leads to a nearly barrierless C–H activation process (Scheme 16b).103 Enantio- and diastereoselective C(sp3)–H arylation of cyclopentane, cyclohexane, and cycloheptane carboxylic acid derivatives directed by CONHArF (ArF = p-CF3C6F4) was further studied by Yu and co-workers (Scheme 16c).104 The proposed mechanism is shown in Scheme 17. C–H activation was ruled out as the stereo-determining step because the extent of stereoinduction was significantly influenced by substitution pattern of the aryl iodide. This observation is consistent with oxidative addition or reductive elimination being the stereodetermining step, given that oxidative addition potentially generates two diastereomeric PdIV intermediates (77, 78).
Scheme 16.
a) Enantioselective C(sp3)–H Arylation of Cyclobutyl Carboxylic Amides (Yu et al., 2018).102 b) Enantioselective C(sp3)–H Arylation of Cyclpropyl Carboxylic Amides (Colobert et al., 2019).103 c) Enantioselective C(sp3)–H Arylation Cycloalkyl Carboxylic Amides (Gooßen and Yu et al., 2020).104
Scheme 17.
Proposed Mechanism of Enantioselective C(sp3)–H Arylation Cycloalkyl Carboxylic Amides (Gooßen and Yu et al., 2020).104
The combination of asymmetric C(sp3)–H arylation with decarboxylative cross-coupling was developed by Baran and co-workers in 2019 (Scheme 18).105 A variety of enantiopure, trans-1,2-disubstituted building blocks were synthesized in this fashion.
Scheme 18.
Ligand-Accelerated Enantioselective Methylene C(sp3)–H bond Arylation Followed by Decarboxylative Cross-Coupling (Baran, Yu, and Schreiber et al., 2019).105
Cyclopalladation pathways that require six-membered alkyl palladacyles are generally both kinetically and thermodynamically disfavored when compared to analogous pathways that rely on the formation of five-membered alkyl palladacycles. As a result, reactions that require the formation of six-membered alkyl palladacycles remain underdeveloped. Inspired by their early research on ligand-enabled γ-olefination of amide derivatives, in 2016, Yu and co-workers designed a palladium-catalyzed, ligand-enabled, and CONHArF (ArF = p-CF3C6F4)-directed, γ-C(sp3)–H arylation of primary C–H bonds that are attached to quaternary carbon centers of aliphatic acid derivatives (Scheme 19).106 An extensive screening of various ligands was conducted, and tricyclic quinolines were found to be the most effective. Under standard conditions, this reaction can involve aryl or heteroaryl iodides that contain a range of functional groups and steric and electronic properties. The high diastereoselectivity (d.r. >20:1) was attributed to steric interactions between the methyl and the phthalimide groups in the six-membered palladacycle in which the trans-conformation is favored over the cis-conformation. Furthermore, carbamate formation followed by nucleophilic addition of LiSEt or LiOH/H2O2 can efficiently transform the amide auxiliary into the corresponding thioester or carboxylic acid, respectively.
Scheme 19.
Ligand-promoted CONHArF (ArF = p-CF3C6F4)-Directed Arylation of γ-C(sp3)–H Bonds (Yu et al., 2016).106
Palladium-catalyzed N-heterocyclic carbene (NHC) ligand-enabled C(sp3)–H arylation of piperidine and tetrahydropyran derivatives was developed by Yu and co-workers in 2016 (Scheme 20).107 Based on their earlier research that showed pyridine- and quinoline-type monodentate σ-donor ligands significantly accelerate C(sp3)–H functionalization of amide substrates, they sought to examine another class of σ-donor ligands, NHCs. After systematic screening of ligands, 4,5-dihydroimidazolium with sterically bulky N-alkyl groups (L21) was found to be the best choice for this transformation. Arylation of both the C3 and C4 C–H positions of piperidines and tetrahydropyrans were investigated. A wide range of functional groups such as halides, silyl ethers, and aldehydes were tolerated. The researchers proposed a mechanism whereby a Pd(II)/NHC species inserts into a C(sp3)–H bond to form an alkylpalladium(II) species that subsequently reacts with the aryl iodide.
Scheme 20.
N-Heterocyclic Carbene Ligand-Enabled CONHArF (ArF = p-CF3C6F4) -Directed C(sp3)–H Arylation (Yu et al., 2016).107
The readily installed and removable N-methoxyamide (CONHOMe) DG was used in Pd(II)/Pd(IV) catalysis by Yu and co-workers in 2015 (Scheme 20).108 Arylation of both primary and secondary C(sp3)–H bonds of amino acid scaffolds were accomplished. The monoarylation of primary C(sp3)–H bonds was promoted by a 2-picoline ligand, with a 2,6-lutidine ligand enabling the subsequent arylation of the secondary C(sp3)–H bonds in one pot. Notably, decomposition of the starting material was inhibited with acidic solvents such as 2,2,2-trifluoroethanol (TFE) or hexafluoro-2-propanol (HFIP). Under standard conditions, the reaction tolerates various substituted aryl iodide compounds that contain methyl, methoxy, and fluoro groups. Aryl iodide compounds that contain DGs such as acetamide, phosphonate, and hydroxyl groups were also tolerated reaction partners. Notably, aryl iodide compounds that contain dioxane and N-tosyl-protected indolyl also produce desired arylation product in moderate yields. Pyridyl and quinoline iodides afforded the desired product in lower yields, and 2,6-lutidine was identified as a more efficient ligand in these cases.
In 2018, Yu and co-workers developed a palladium-catalyzed, Weinreb-amide-directed β-methyl C(sp3)–H arylation reaction, which was enabled by commercially available 3-pyridinesulfonic acid (L22) as the ligand (Scheme 22a).109 Two challenges exist with this directing group compared to simple amides. First, the coordination of the carbonyl group on the DG with the Pd catalyst is weakened by the inductive effect of methoxy (102). Second, catalytic turnover can be decelerated by the unproductive bis-coordination of the catalyst to both the carbonyl group and methoxy (103). The ligand was computed to stabilize the substrate-bound Pd species, and this ligand effect was proposed to promote the dissociation of an acetate anion via electrostatic repulsion, which leads to the opening of a coordination site for C(sp3)–H bond cleavage. Also in 2018, Lu and co-workers disclosed a simple palladium-catalyzed, simple amide-directed arylation of β-methyl C(sp3)–H bonds, affording a wide range of β-aryl amides in good yields (Scheme 22b).110
Scheme 22.
a) Ligand-Enabled, Palladium-Catalyzed β-C(sp3)–H Arylation of Weinreb Amides (Yu et al., 2018).109 b) Simple Amides-Directed β-C(sp3)–H Arylation (Lu et al., 2018).110
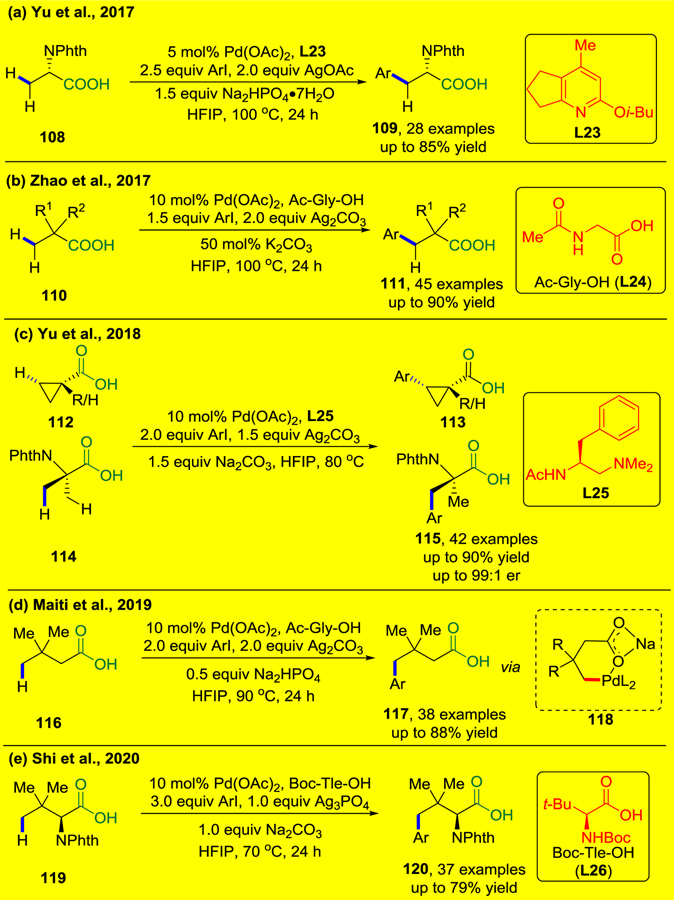
Although monodentate amide DGs that were developed by the Yu group (CONHR, R = 4-(CF3)C6F4, OMe) have demonstrated outstanding efficiency in directing C(sp3)–H activation, their applications are limited by the requirement of their installation and removal. In 2016, the Yu group reported two isolated examples of ligand-accelerated, free carboxylic-acid-directed reactions to allow enantioselective C(sp3)–H arylation of strained methylene C–H bonds (e.g. cyclopropane and cyclobutane) and an α-quaternary carbon center is necessary for the reactions to take place.97 Inspired by their previous work in ligand-accelerated catalysis, the Yu group has been reinvestigating the palladium-catalyzed, carboxylic-acid-directed C(sp3)–H functionalization through the design of new ligands.
In 2017, the Yu group developed a novel protocol using innate carboxylic acids as the DG, which was enabled by pyridine-type ligands (Scheme 24a).111 The base, Na2HPO4·7H2O, was proposed to deprotonate the acid substrates to allow Pd coordination. Unnatural amino acid building blocks can be efficiently prepared using this method, but heteroaryl iodides are incompatible with the reaction conditions. Contemporaneously, Zhao and co-workers established a palladium-catalyzed β-C(sp3)–H arylation of carboxylic acids and α-amino acids prompted by Ac-Gly-OH (L24) ligand (Scheme 24b). This reaction could be run at 10 gram-scale in good yield, demonstrating the practical utility of this reaction.112
Scheme 24.
a) Ligand-Enabled β-C–H Arylation of α-Amino Acids (Yu et al., 2017).111 b) Ligand-Enabled β-C(sp3)–H Arylation of Aliphatic Carboxylic Acids and α-Amino Acids (Zhao et al., 2017).112 c) Enantioselective β-C(sp3)–H Arylation of Substituted Cyclopropanecarboxylic Acids and 2-Aminoisobutyric Acid via PdII/PdIV (Yu et al., 2018).113 d) Carboxyl-Directed Pd(II)-Catalyzed γ-C(sp3)–H Arylation (Maiti et al., 2019).114 e) Carboxyl-directed γ-C(sp3)–H arylation of tert-Leucine and Peptides (Shi et al., 2020).115
In the subsequent year, the Yu group reported a new class of chiral ligands, mono-N-protected aminoethyl amines, that enable the enantioselective C–H activation of free carboxylic acids without the use of auxiliary DGs (Scheme 24c).113 One challenge in developing this reaction is that stereoselectivity is much harder to control due to the highly flexible metal–carboxylate intermediate when compared with metal–amide-DG complexes. After extensive screening, the N-acetyl-protected amino group (NHAc) was found to be crucial for achieving high enantioselectivity. A variety of chiral carboxylic acids were synthesised through enantioselective arylation of cyclopropanecarboxylic acid and N-phthaloyl-protected 2-aminoisobutyric acid using L25 as chiral ligand. This methodology was applied to the late-stage C(sp3)–H arylation of Itanapraced, a promising drug candidate for neurological disorders. In 2019, Maiti and co-workers expanded the palladium(II)-catalyzed arylation of free aliphatic acids to bring about reaction at the distal γ-C(sp3)–H bond using Ac-Gly-OH (L24) as ligand via a six-membered palladacycle intermediate (118, Scheme 24d).114 Mechanistic studies indicated that C–H activation irreversible and is the rate-determining step. The Shi group also reported the Pd(II)-catalyzed γ-C(sp3)–H arylation of tert-leucine and peptide derivatives thereof with the assistance of free carboxylic acid by using Boc-Tle-OH (L26) as the ligand (Scheme 23e).115 In this study, the weakly coordinating carboxylate DG outcompetes the strongly coordinating bidentate DG of the peptide backbone, providing the products of γ-C(sp3)–H arylation of the Tle residue exclusively.
Other weakly coordinating monodentate DGs have also been reported. The enantioselective arylation of cyclopropyl methylamines via a Pd(II)/Pd(IV) catalytic cycle with an MPAA ligand (L27) was developed by Yu and co-workers in 2015 (Scheme 25).116 The Pd(II)/Pd(0) catalytic cycle was ruled out because no desired product was detected without the addition of the silver salt. The Yu group succeeded in using aryl iodide compounds that contain electron-donating or electron-withdrawing substituents in the para, meta, and ortho positions as arylating reagents. The scalability of the reaction was also demonstrated in a 5-mmol-scale reaction between cyclopropylmethylamine and methyl-2-iodobenzoate, which produced the arylated product in 89% isolated yield and 98.6% ee. Notably, the MPAA ligand can override substrate-controlled diastereoselectivity in substrates that contain a chiral center.
Scheme 25.
Enantioselective C(sp3)–H Arylation of Cyclopropyl ethylamines via Pd(II)/Pd(IV) Catalytic Cycle (Yu et al., 2015).116
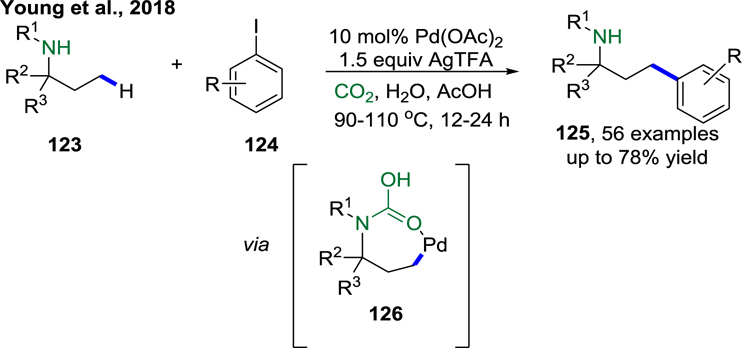
In 2018, Young and co-workers presented the first example of CO2-mediated, amine-directed C(sp3)–H arylation. CO2 was added in the form of dry ice and reacted reversibly with the amine to form a carbamic acid moiety. Both primary and secondary aliphatic amines can be arylated selectively at the γ-C–H positions (Scheme 26).117 Young and co-workers found that an α-tertiary center was not required for the primary amine substrates. A wide range of aryl iodides were tolerated and gave the desired products in good yields. The proposed role of CO2 as a transient DG that reacts in situ to form the corresponding carbamic acid was supported by an experiment in which the amine•CO2 adduct was prepared, subjected to the reaction conditions, and found to be catalytically competent. It was suggested that the concerted metalation/deprotonation step may be irreversible because no deuteration was observed when the reaction was performed in AcOD.
Scheme 26.
Carbon Dioxide-Mediated C(sp3)–H Arylation of Amine (Young et al., 2018).117
2.1.1.1.2. Monodentate-Directing-Group-Assisted C(sp3)–H Arylation via Pd(II)/Pd(0) Catalysis
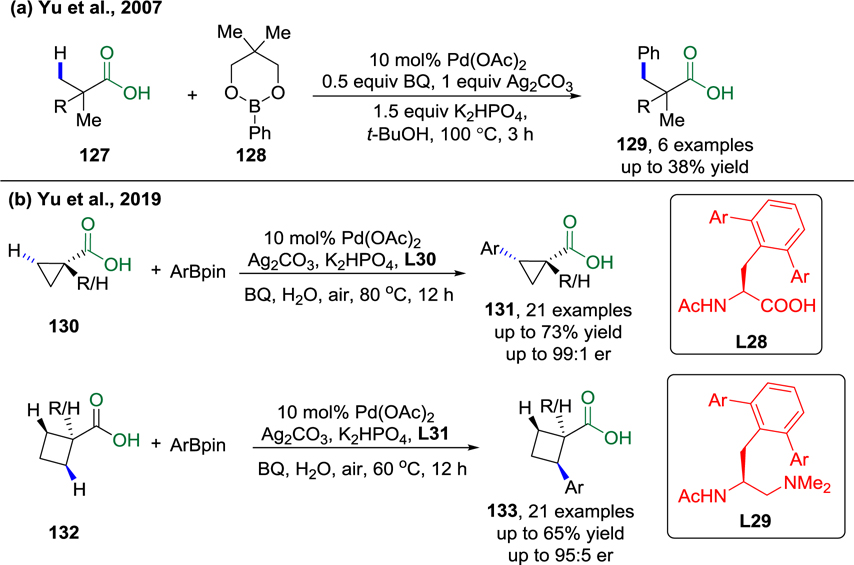
Organoboron reagents are widely used for traditional cross-coupling reactions. The integration of organoboron nucleophiles as coupling partners in C–H activation processes opens new possibilities in terms of the redox processes involved. In 2007, Yu and co-workers reported the first example of Pd(II)-catalyzed, carboxyl-directed arylation of β-C(sp3)–H bonds in simple aliphatic acids (Scheme 27a).86 In the work, arylboronic esters are employed as coupling partners. Recently, enantioselective C(sp3)–H arylation of cyclopropanecarboxylic acids and cyclobutanecarboxylic acids under Pd(II)/Pd(0) catalysis was also documented by the Yu group (Scheme 27b).118 Using either MPAA ligand (L28) or mono-N-protected aminoethyl amine (MPAAM) ligand (L29) was key to the success of this method. The enantioenriched arylated carboxylic acid products could be converted to cyclopropyl amines without loss of optical activity.
Scheme 27.
a) Carboxyl-Directed Pd(II)-Catalyzed Arylation of β-C(sp3)–H Bonds (Yu et al., 2007).86 b) Enantioselective C(sp3)–H Activation/Cross-Coupling Reactions of Free Carboxylic Acids via PdII/Pd0 (Yu et al., 2019).118
In 2008, Yu and co-workers developed a palladium-catalyzed arylation of C(sp3)–H bonds with aryl boronic acids by using CONHOMe as the DG (Scheme 28).119 Notably, CONHOMe groups can be transformed easily to esters, amides, or corresponding alkane fragments. Furthermore, the silver salt in this system can be replaced with air as a stoichiometric oxidant.
Scheme 28.
Methoxyamide-Directed Arylation of C(sp3)–H Bond with Arylboronic Acids (Yu et al., 2008).119
In 2011, the Yu group reported the enantioselective C–H activation reactions of cyclopropanes using a novel MPAA (L30) as the ligand. In this reaction, an acidic CONHArF (ArF = p-CNC6F4) group was employed as a weakly coordinating DG (Scheme 29a).120 The best ligand found for asymmetric induction in the C–H/R–BXn cross-coupling reactions was identified by extensive screening of chiral ligands with structurally diverse N-protecting groups and amino acid backbones. Various cyclopropanes substituted with alkyl and aryl groups at the α-position with respect to the amide DG can be tolerated, producing the desired products in up to 81% yield and 92% ee under mild conditions. However, this method is limited to the functionalization of relatively activated cyclopropyl C–H bonds.
Scheme 29.
a) Palladium-Catalyzed CONHArF (ArF = p-CNC6F4)-Directed Asymmetric Cyclopropane C–H Arylation (Yu et al., 2011)120 b) Palladium-Catalyzed CONHArF (ArF = p-CNC6F4)-Directed Asymmetric Cyclobutane C–H Arylation (Yu et al., 2014).121
In 2014, Yu and co-workers developed an enantioselective C–H arylation reaction of cyclobutanecarboxylic acid derivatives using arylboron reagents as arylating agents (Scheme 29b).121 The key to the success of this method was the discovery of a new class of chiral ligands, mono-N-protected α-amino-O-methylhydroxamic acids (L31), which are derived from MPAAs. However, substrates that contain α-hydrogen atoms give poor yields and enantioselectivities.
In 2015, a quinoline-based ligand was designed to promote cross-coupling reactions of β-C(sp3)–H bonds in carboxylic acid derivatives that bear a p-CF3C6F4 amide auxiliary with arylsilanes (Scheme 30).122 The optimized reaction conditions for the coupling of alanine-derived amide 141 with various organosilicon reagents were: ArSi(OEt)3 (2.0 equiv) Pd(OAc)2 (10 mol%), L32 (20 mol%), AgF (3.0 equiv) as additive in 1,4-dioxane (1.0 mL) at 110 °C for 8 h, followed by the addition of a second batch of organosilicon (2.0 equiv) and AgF (3.0 equiv) with subsequent heating for an additional 10 h. A variety of electron-rich and electron-poor triethoxyarylsilanes are tolerated in the arylation reaction. However, only 45% yield of the arylated product can be obtained when an amide derived from 2-methylpentanoic acid is subjected to the standard conditions. After extensive screening of ligands and bases, a variety of amides derived from aliphatic acids could be arylated in good yields by the use of L20 as ligand and KHCO3 as base.
Scheme 30.
Ligand-Enabled C(sp3)–H Cross-Coupling with Arylsilanes (Yu et al., 2015).122
In 2014, monodentate triflimide-directed, palladium(II)-catalyzed coupling of γ-C(sp3)–H bonds with arylboron reagents enabled by an MPAA ligand was discovered by Yu and co-workers.123 Interestingly, the steric impact of the ligand on the reactivity of the catalytic system was observed when the D-enantiomer of N-acetyl-tert-leucine (D-Ac-Tle-OH, L33) was used. A broad range of arylboron reagents and a variety of alkyl amines were cross-coupled under the reaction conditions (Scheme 31a). Notably, arylboron reagents could be replaced with aryl iodide reagents as coupling partners, such that γ-C(sp3)–H arylation alternatively takes place via a Pd(II)/Pd(IV) catalytic cycle. To solve the problem that arises from removal of the triflyl DG within the substrates, Yu and co-workers developed a method that employs the readily removable nosyl protecting group for γ-C(sp3)–H arylation of alkyl amines, as enabled by a N-acetyl-protected aminomethyl oxazoline (APAO) ligand (Scheme 31b, L34).124
Scheme 31.
a) Triflamide-Directed γ-C(sp3)–H Bond Arylation (Yu et al., 2014).123 b) Ligand-Enabled γ-C(sp3)–H Arylation of Nosyl-Protected Amines (Yu et al., 2017).124 c) Enantioselective γ-C(sp3)–H Activation of Alkyl Amines via Pd(II)/Pd(0) Catalysis (Yu et al., 2018).125
In 2018, Yu and co-workers expanded the γ-C(sp3)–H arylation of cyclopropyl methylamines to acyclic alkylamines by using chiral APAO ligands via Pd(II)/Pd(0) catalysis (Scheme 31c).125 They observed that APAQ and MPAA ligands did not improve the yield and enantioselectivity significantly. On the other hand, the enantioselectivity was improved to 98% ee when N-acetyl-protected amino oxazoline (APAO) chiral ligands were used. The stereocenters on both the side chain and the oxazoline moiety of the ligand play key roles in chiral induction. The reaction conditions are: Pd(OAc)2 (10 mol%), ligand (15 mol%), ArBpin (2.0 equiv), Ag2CO3 (2.0 equiv) and H2O (5.5 equiv) as the additives and Na2CO3 (2.0 equiv) as the base in t-AmylOH at 80 °C for 12 h. Kinetic resolution of unsymmetrical amines via enantioselective γ-C(sp3)–H coupling has also been investigated and realized (Scheme 30c).
In 2015, Gaunt and co-workers developed a palladium-catalyzed C–H arylation of secondary aliphatic amines with arylboronic esters that is promoted by an MPAA ligand (Scheme 32a).126 A range of alicyclic amine derivatives, such as piperidine derivatives and compounds with azepine or morpholine scaffolds, all efficiently afforded the corresponding arylated products in good yields. The scope of arylboronic acid pinacol esters (ArBPin) was also investigated, and ArBPin compounds that have substituents at the meta and para positions of the aromatic ring afford the desired products in higher yields than ArBpin compounds that have substituents at the ortho position. They have also investigated the enantioselective C–H arylation of 155 with PhBPin by exploring a range of amino-acid ligands under similar reaction conditions. A 60% ee was obtained when MPAA L37 was used as chiral ligand. Recently, they developed a MPAA-promoted, palladium-catalyzed γ-C(sp3)–H arylation of tertiary alkylamines.127 Ac-Tle-OH (L38) lowered the energy of γ-C(sp3)–H activation relative to β-hydride elimination, which made the arylation reaction proceed smoothly with (hetero)arylboronic acids.
Scheme 32.
a) Palladium-Catalyzed β-C(sp3)–H Arylation of Secondary Amines (Gaunt et al., 2015).126 b) Palladium-Catalyzed γ-C(sp3)–H Arylation of Tertiary Alkylamines (Gaunt et al., 2020).127
Pd(TFA)2-catalyzed, thioamide-directed C(sp3)–H arylation of saturated azaheterocycles was developed by the Yu group in 2015.128 1,4-Benzoquinone (BQ) is essential for this transformation; its proposed role is in promoting reductive elimination. It was also observed that the terminal methyl groups of the thioamide are not functionalized and that Pd(OAc)2 affords only a trace amount of product. Both arylboronic acid and heteroarylboronic acids are tolerated under standard conditions, providing the corresponding products 161 in good to excellent yields (Scheme 33).
Scheme 33.
Thioamide-directed Amine α-functionalization via Palladium(II)-Catalyzed C(sp3)–H Arylation.128
In 2017, the Yu group demonstrated that chiral phosphoric acids are effective anionic ligands for enantioselective amine α-C(sp3)–H arylation (Scheme 34a).129 No desired arylation products were obtained when chiral MPAA ligands were employed. Chiral anionic phosphates were investigated due to the enhanced reactivity of Pd(TFA)2 compared to Pd(OAc)2, which indicates that the identity of the anionic ligand plays a significant role in this reaction. In the end, a bulky triisopropylbenzothioamide DG on the pyrrolidine and Pd2(dba)3 were observed to be the most suitable in terms of reactivity and enantiocontrol. Gong and co-workers also reported a thioamide-directed, palladium(II)-catalyzed enantioselective C(sp3)–H arylation that involves a chiral anionic Co(III) complex (164) and a chiral phosphoramidite ligand (L40) (Scheme 34b).130
Scheme 34.
a) Thioamide-directed Enantioselective Amine α-functionalization via Palladium(II)-Catalyzed C(sp3)–H Arylation: a) by Yu et al., 2017.129 b) by Gong et al., 2019.130
2.1.1.2. Alkenylation
While transition-metal-catalyzed olefination of C(sp2)–H bonds has been widely investigated, olefination of unactivated C(sp3)–H bonds remains rare. To enable olefination of unactivated C(sp3)–H bonds, strongly coordinating nitrogen-based, monodentate DGs have been employed. For example, in 2011, Sanford and co-workers reported a palladium-catalyzed, pyridine-directed C(sp3)–H alkenylation using air as the oxidant and AcOH as the solvent (Scheme 35a).131 In 2016, Yu and co-workers developed a pyrazole-directed C(sp3)–H olefination enabled by an MPAA ligand L41 (Scheme 35b).132
Scheme 35.
a) Pyridine-Directed C(sp3)–H Alkenylation (Sanford et al., 2011).131 b) Ligand-Promoted C(sp3)–H Olefination Directed by Pyrazoles (Yu et al., 2016).132
In 2015, Gaunt and co-workers developed a palladium-catalyzed C(sp3)–H alkenylation of amino alcohol derivatives.80 The conditions they found are: Pd(OAc)2 (10 mol%) and acrylate (3 equiv) in the presence of AgOAc and Li3PO4 in 1,2-dichloroethane at 120 °C. A wide range of pyrrolidines that contain a variety of useful functional groups were synthesized (Scheme 36a). In 2017, Gaunt and co-workers further developed a palladium-catalyzed C(sp3)–H alkenylation of morpholinones to synthesize various pyrrolidine moieties in good yields and excellent regio- and diastereoselectivities (Scheme 36b).133
Scheme 36.
a) Palladium-Catalyzed C(sp3)–H Alkenylation of Amino Alcohol Derivatives (Gaunt et al., 2015).80 b) Palladium-Catalyzed C(sp3)–H Alkenylation of Morpholinone Derivatives (Gaunt et al., 2015).133
Directed C(sp3)–H olefination with weakly coordinating DGs was pioneered by Yu and co-workers. In 2010, Yu and co-workers developed a Pd(II)-catalyzed, monodentate CONHArF (ArF = C6F5 and p-CF3C6F4)-directed C(sp3)–H alkenylation/1,4-conjugate addition with benzyl acrylate, which affords the corresponding lactam products in good to excellent yields.134 In accordance with their previous report, electron-withdrawing substituents (CF3, F, and NO2) on the N-aryl group improve the yield significantly. Cyclopropyl methylene C–H bonds and substrates that contain α-hydrogen atoms are also olefinated efficiently (Scheme 37).
Scheme 37.
Palladium-Catalyzed, CONHArF-Directed Alkenylation of β-C(sp3)–H Bonds (Yu et al., 2010).134
In 2014, Yu and co-workers detailed a ligand-promoted, Pd(II)-catalyzed γ-C(sp3)–H olefination/cyclization with CONHArF (ArF = p-CF3C6F4) as the DG (Scheme 38a).135 After extensive screening, a quinoline-based ligand (L20) was found to be crucial for realizing this transformation. Notably, this ligand can also be used to facilitate sequential γ-carbonylation followed by γ-olefination of pivalamide to give highly functionalized all-carbon quaternary centers. In 2016, the Yu group developed a Pd(II)-catalyzed γ-C(sp3)–H olefination of triflyl (Tf)- and 4-nitrobenzenesulfonyl (Ns)-protected amines in which the C(sp3)–H olefinated products undergo an in situ, aza-Wacker oxidative cyclization or an intramolecular conjugate addition to afford a variety of C-2 alkylated pyrrolidines (Scheme38b).136 3,4-Bis(trifluoromethyl)pyridine (L42) was identified to be the optimal ligand for Tf-protected α-amino esters, while 3-phenylquinoline (L43) was capable of promoting the γ-C(sp3)–H olefination of a range of Tf-protected alkyl amines. Furthermore, Ns-Tle-OMe (198) was olefinated with acrylates and styrenes using phenanthridine (L44) as the ligand. Interestingly, saturated pyrrolidines were obtained when methyl vinyl ketone, 1-phenylprop-2-en-1-one, and acrylonitrile were used as coupling partners.
Scheme 38.
a) Palladium-Catalyzed γ-C(sp3)–H Alkenylation of Aliphatic Carboxamides (Yu et al., 2014).135 b) Palladium-Catalyzed γ-C(sp3)–H Alkenylation of Aliphatic Amines(Yu et al., 2016).136
In 2017, Yu and co-workers showed that chiral MPAO ligand L45 was able to promote Pd(II)-catalyzed, enantioselective β-alkenylation of isobutyric amides (Scheme 39).101 The acidic CONHArF (ArF = p-CF3C6F4) was again used as an efficient DG to enable the synthesis of olefinated products in high enantioselectivities (up to 95:5 er).
Scheme 39.
Palladium-Catalyzed Enantioselective C(sp3)–H Alkenylation of Isobutyric Amides (Yu et al., 2017).101
All of the C(sp3)–H olefination reactions described above require a specific directing auxiliary group, and the majority of examples yield cyclized products, which may or may not be desirable depending on the envisioned application. In 2019, Yu and co-workers developed a Pd(II)-catalyzed C(sp3)–H olefination directed by the carbonyl group of native amides (Scheme 40).137 The electron-deficient pyridine-3-sulfonic acid ligand (L46) was found to be crucial for the conversion of secondary and tertiary amides to the corresponding olefinated products without the in situ cyclization.
Scheme 40.
Carbonyl-Directed β-C(sp3)–H Alkenylation of Native Amides (Yu et al., 2019).137
In 2018, the Yu group detailed the β-C(sp3)–H olefination of free carboxylic acids that is promoted by an N-acetyl-protected aminoethyl phenyl thioether ligand (L47).138 A broad range of free carboxylic acids undergo olefination followed by in situ 1,4-addition, providing the corresponding γ-lactone products that can be readily opened to give either the β-olefinated or γ-hydroxylated aliphatic acids (Scheme 41a). Recently, palladium(II)-catalyzed enantioselective C(sp3)–H alkenylation of free carboxylic acids with alkenyl-Bpin was also discovered by the Yu group.118 A variety of olefin-containing chiral acids were produced in high enantoselectivities (up to 98:2 er) using MPAA (L28) as chiral ligand, providing a valuable scaffold for organic synthesis and medicinal chemistry (Scheme 41b). Carboxylate-directed, palladium(II)-catalyzed γ-C(sp3)–H olefination of free carboxylic acids was also demonstrated by the van Gemmeren group in 2020.139 N-Ac-β-alanine (L48) was identified to be an efficient ligand (Scheme 41c). It was worth noting that N-Ac anthranilic acid derived ligands could also significantly promote this reaction.
Scheme 41.
a) Ligand-Enabled β-C(sp3)–H Olefination of Free Carboxylic Acids (Yu et al., 2018).138 b) Enantioselective C(sp3)–H Olefination of Free Carboxylic Acids (Yu et al., 2019).104 c) Ligand-Enabled γ-C(sp3)–H Olefination of Free Carboxylic Acids (van Gemmeren et al., 2020).139
2.1.1.3. Alkynylation
Alkyne functional groups are useful intermediates in organic synthesis owing to their ability to participate in many different types of reactions, such as cycloaddition and metathesis. While cross-coupling reactions between alkynes and aryl (pseudo)halides have been well developed, the alkynylation of unactivated C(sp3)–H bonds remained largely undeveloped until the past decade. In 2013, Yu and co-workers achieved the first C(sp3)–H alkynylation reaction of simple amides 215 with alkynyl bromides 216 directed by their own developed monodentate CONHArF (ArF = p-CF3C6F4) DG under Pd(0) catalysis (Scheme 42a).140 Although this reaction proceeded through a Pd(0)/Pd(II) catalytic cycle, these findings inspired their further studies of developing C(sp3)–H alkynylation using Pd(II) catalysis. In 2017, they successfully expanded this alkynylation protocol to a Pd(II)/Pd(IV) catalytic cycle by employing pyridine-based ligands. β- and γ-alkynylation of C(sp3)–H bonds of aliphatic amides using the same monodentate DG under palladium(II) catalysis was achieved. Pyridine-based ligand L49 was identified to be the optimal one and a variety of amides containing α-tertiary or α-quaternary carbon centers were competent under the optimized reaction conditions (Scheme 42b).141 In the same year, they developed an asymmetric version by desymmetrization of gem-dimethyl group. The Pd(II)-catalyzed enantioselective β-alkynylation of isobutyramide 220 bearing the same DG with alkynyl iodide 221 using MPAO L45 as chiral ligand was achieved, giving the product 222 in 94.5:5.5 er and 68% yield (Scheme 41c).101
Scheme 42.
a) CONHArF (ArF = p-CF3C6F4-Directed C(sp3)–H Alkynylation with an N-heterocyclic Carbene Ligand under Pd(0) Catalysis (Yu et al., 2013).140 b) CONHArF (ArF = p-CF3C6F4-Directed C(sp3)–H Alkynylation under Pd(II) Catalysis Enabledby Pyridine-Based Ligands (Yu et al., 2017).141 c) CONHArF (ArF = p-CF3C6F4-Directed Enantioselective C(sp3)–H Alkynylation of Isobutyriamide with a Chiral MPAO Ligand (Yu et al., 2017).101
2.1.1.4. Alkylation
Employing alkyl coupling partners (i.e., C(sp3)–organometallics or alkyl halides) in C(sp3)–H activation and in cross-coupling reactions generally is more challenging because the resulting alkyl-M intermediates are prone to undergo a rapid β-hydride elimination. The first protocol for palladium(II)-catalyzed C(sp3)–H alkylation with either methylboroxine or alkylboronic acids was developed by the Yu group in 2006 (Scheme 43).142 For the examples that involve methylboroxine, the authors propose that the methylboroxine assists in the C–H activation step by first coordinating with the pyridyl group and then chelating to Pd(OAc)2 to direct the C–H bond cleavage.
Scheme 43.
Pyridine-Directed C(sp3)–H Alkylation with Methylboroxine or Alkylboronic Acids (Yu et al., 2006).142
A ligand-promoted, palladium(II)-catalyzed C(sp3)–H alkylation of monodentate CONH-(p-CF3)C6F4 amides using alkyl iodides as the alkylating agents was reported by Yu and co-workers in 2014 (Scheme 44).143 The reaction conditions for this system are: Pd(TFA)2 (10 mol%) as the precatalyst, 9-methylacridine L50 (20 mol%) as ligand, and AgOPiv as the additive in 1,2-dichloroethane at 80 °C for 20 h. In this system, aliphatic amides that bear bulkier α-substituents give the alkylated products in higher yields. A 1-aminopyridinium-ylide-directed, palladium(II)-catalyzed ligand free C(sp3)–H alkylation using dibenzyl phosphate silver salt as the additive was reported by Daugulis in 2019, providing an alternative way to accomplish C(sp3)–H alkylation using palladium catalysis (Scheme 45).89
Scheme 44.
CONHArF (ArF = p-CF3C6F4)-Directed β-C(sp3)–H Alkylation with Alkyl Iodides (Yu et al., 2014).143
Scheme 45.
1-Aminopyridinium-Ylide-Directed β-C(sp3)–H Alkylation with Alkyl Iodides (Daugulis et al., 2019).89
2.1.1.5. Carbonylation
In 2010, Yu and co-workers described a novel palladium(II)-catalyzed β-C(sp3)–H carbonylation/cyclization reaction of N-arylamides under CO (1 atm) atmosphere (Scheme 46).144 The succinimide products could be hydrolysed with TFA/AcOH under reflux to give 2,2-dimethylsuccinic acids or treated with NaOMe in methanol at room temperature to open the cyclic imide at the less hindered carbonyl, providing the corresponding methyl carboxy amide. Other DGs, such as carboxylic acids, hydroxamic acids, oxazolines, and pyridines were investigated under the same conditions, and no desired product was obtained in these cases.
Scheme 46.
CONH–p-CF3C6F4-Directed Carbonylation of β-C(sp3)–H Bonds (Yu et al., 2010).144
Palladium-catalyzed direct C–H activation of free-NH aliphatic amines has been a longstanding challenge. Recently, the Gaunt group has made significant contributions to overcome these challenges. In 2014, Gaunt and co-workers reported a palladium-catalyzed C(sp3)–H carbonylation of hindered aliphatic amines via an unusual four-membered-palladacycle.145 A broad range of β-lactams were produced via palladium-catalyzed activation of the β-methyl C(sp3)–H bonds of secondary amine substrates (Scheme 47). The reaction conditions were found to be Pd(OAc)2 (10 mol%) with Cu(OAc)2 (10 mol%) as the oxidant in toluene at 120 °C under a CO/air mixture at atmospheric pressure for 22 h. A variety of secondary amines, including cyclic and acyclic amines are effective substrates, producing the resulting β-lactams in good yields.
Scheme 47.
Palladium-Catalyzed C–H Carbonylation of Aliphatic Amines (Gaunt et al., 2014).145
Shortly after, Gaunt and co-workers successfully realized a palladium-catalyzed C(sp3)–H carbonylation of amino alcohol derivatives.80 The reaction conditions are: Pd(OAc)2 (10 mol%) and AgOAc (2 equiv) in toluene at 100 °C with stirring under 6.25% CO in air at a slightly positive pressure. A wide range of pyrrolidinone products with a variety of useful functional groups were synthesized under the reaction conditions (Scheme 48a). Gaunt and co-workers observed that a strongly electron-withdrawing group adjacent to the amine lowers the efficiency of the transformation. Notably, a small amount of the α,β-unsaturated pyrrolidinone byproduct was observed in all cases. To simplify isolation, upon completion of the carbonylation reaction, the reaction vessel was charged with hydrogen gas, which converted the mixture to solely the saturated pyrrolidinone.
Scheme 48.
a) Palladium-Catalysed γ-Methyl C(sp3)–H Carbonylation of Amino Alcohol Derivatives (Gaunt et al., 2015).80 b) Palladium-Catalysed β-Methyl C(sp3)–H Carbonylation of Less Hindered Aliphatic Amines to β-Lactams (Gaunt et al., 2016).146 c) Palladium(II)-Catalyzed β-Methylene C(sp3)–H Carbonylation of Free Secondary Aliphatic Amines to trans-Disubstituted β-Lactams (Gaunt et al., 2017).147 d) Palladium(II)-Catalyzed β-Methylene C(sp3)–H Carbonylation of α-Tertiary Amines in the Presence of More Reactive β-Methyl C–H Bonds (Gaunt et al., 2017).148 e) Palladium(II)-Catalyzed γ-C(sp3)–H Carbonylation of Free(NH) Secondary Aliphatic Amines to trans-Disubstituted 2-Pyrrolidines (Gaunt et al., 2018).149
Although Pd(II)-catalyzed β-C(sp3)–H carbonylation of hindered amines have been achieved previously,80 the direct application of this protocol to more commonly encountered, less sterically hindered amine counterparts failed to give the desired products. To fill this gap, Gaunt et al. elegantly developed a general protocol to enable the palladium(II)-catalyzed C(sp3)–H carbonylation of unprotected, less hindered aliphatic amines in 2016 (Scheme 48b).146 The use of a bulky carboxylic acid ligand (1-adamantane carboxylic acid or 2,4,6-trimethylbenzoic acid), is key to this carbonylation reaction. The role of the sterically hindered carboxylate ligand was believed to be involved in ligand exchange on Pd(OAc)2, which led to the formation of a sterically biased palladium anhydride intermediate (242A) and steered the amine attack at the internal carbonyl of the anhydride intermediate to generate carbamoyl-Pd species 242B. Differing from the classical mechanism of C–H carbonylation, the insertion of CO to form the acylpalladium intermediate 242A is proposed to occur prior to C–H bond cleavage in this process.
Due to the increased steric interactions, Pd-catalyzed methylene C(sp3)–H carbonylation remains particularly challenging. In 2017, the unprecedent palladium(II)-catalyzed methylene C–H carbonylation of unprotected secondary aliphatic amines was successfully achieved by the Gaunt group, providing trans-disubstituted β-lactams 246 in excellent yields and selectivity (Scheme 48c).147 Interestingly, the concentration of CO can control the pathway of the C–H carbonylation process. Only the β-lactam product is obtained when the reaction is set up under a pure CO atmosphere, whereas the γ-lactam product could be observed when a CO/air mixture was used.
In 2017, the Gaunt group achieved the selective carbonylation of β-methylene C–H bonds of α-tertiary amines in the presence of conventionally more reactive and less hindered β-methyl C–H bonds (Scheme 48d).148 They hypothesized that the selectivity originated by the orientation of the activating C – H bond proximal to palladium to avoid the steric repulsion with a second ligated α-tertiary amine. As their continuous efforts towards the use of secondary aliphatic amines as valuable feedstocks in C–H activation strategy, they reported the highly diastereoselective synthesis of trans-4,5-disubstituted 2-pyrrolidines via a Pd(II)-catalyzed secondary-aliphatic-amine-directed γ-methyl C(sp3)–H carbonylation in 2018 (Scheme 48e).149
2.1.2. Carbon–Heteroatom Bond Formation
2.1.2.1. Carbon–Nitrogen Bond Formation
Because amines are very common in pharmaceuticals and natural products, it is attractive to develop versatile methods to forge C–N bonds through C(sp3)–H activation. In 2006, Che and co-workers reported a palladium-catalyzed, oxime-directed, cascade C(sp3)–H activation/nitrene insertion to afford amidated products in good yields with remarkable regio- and chemoselectivity (Scheme 49a).150 Amidation at secondary C(sp3)–H bonds was not observed. In 2009, palladium-catalyzed, amide-directed, intramolecular amidation of unactivated C(sp3)–H bonds was reported by Glorius and co-workers (Scheme 49b).151 Surprisingly, functionalization of the C(sp3)–H bond takes place over functionalization of the C(sp2)–H bond. When the N-acetyl group is replaced by N-formyl, -propionyl, or -isobutyryl, the corresponding products are formed in reduced yields. When N-pivaloyl, -benzoyl, or -trifluoroacetyl groups are used as DGs, no cyclization products are obtained. The reaction tolerates various functional groups, such as ethers, sulfones, carboxylic esters, thioethers, acetals, silanes, and ketones. However, the yield of the reaction dramatically drops when 5- and 6-substituted substrates are employed. In 2012, the Muñiz group reported a palladium-catalyzed intermolecular benzylic C(sp3)–H amidation with NFSI (Scheme 49c).152
Scheme 49.
a) Oxime-Directed Intermolecular C(sp3)–H Amidation via C(sp3)–H Activation/Nitrene Insertion (Yu et al., 2006).150 b) Amide-Directed Intramolecular Amidation of Unactivated C(sp3)–H Bonds (Glorius et al., 2009).151 c) Intermolecular Amidation of Benzylic C(sp3)–H Bonds (Muñiz, 2012).152 d) CONH–p-(CF3)C6F4-Directed Intermolecular β-C(sp3)–H Amination (Yu et al., 2015).153
In 2015, Yu and co-workers developed a CONH-(p-CF3)C6F4-directed, Pd(0)/PAr3-catalyzed intermolecular C(sp3)–H amination of simple aliphatic amides using an electron-deficient triarylphosphine ligand and O-benzoyl hydroxylmorpholine as the aminating reagent (Scheme 49d).153 The amination reaction is sensitive to the substitution pattern of the substrates, limiting its use to aliphatic amides that bear a quaternary α-carbon that contains at least one β-methyl group. The following observations support the proposed Pd(0)/Pd(II) cycle: (1) Pd(TFA)2 is not effective as a precatalayst for this transformation; however, excellent yields are obtained by using [{Pd-(allyl)Cl}2] or [Pd(dba)2]. (2) External oxidant is unnecessary when a Pd(0) catalyst is employed. (3) These reactions are inhibited by Ag(I) salts.
In 2014, the Gaunt group developed a palladium-catalyzed C(sp3)–H amination of aliphatic amines to give strained nitrogen heterocycles through a four-membered cyclopalladation pathway.145 A methyl group adjacent to unprotected secondary unactivated amines is selectively transformed into synthetically versatile aziridines (Scheme 50a). A range of nucleophiles can then open the aziridine to form highly substituted amine products in good yields. The same group evaluated the mechanism through reaction kinetics and theory, and acetic acid was found to promote the rate of the reaction by supressing the generation of the off-cycle bis-amine–palladium complex.154 In 2017, Gaunt and co-workers used chiral anionic BINOL-phosphoric acid ligands to realize an enantioselective version of the C(sp3)–H amination (Scheme 50b).155 By combining benziodoxole tosylate and AgOAc as oxidants, the Gaunt group extended the β-C–H amination to γ-C–H amination, which leads to the formation of azetidines (Scheme 50c).156 Based on their experimental findings and calculations, the γ-C–H amination was the net result of γ-C–OTs bond formation followed by the displacement by the amino group.
Scheme 50.
a) Palladium-Catalyzed Intramolecular β-C(sp3)–H Amination of Aliphatic Amines to Prepare Aziridines (Gaunt et al., 2014).145 b) Palladium-Catalyzed Enantioselective C(sp3)–H Amination of Aliphatic Amines (Gaunt et al., 2017).155 c) Palladium-Catalyzed Intramolecular γ-C(sp3)–H Amination to Azetidines (Gaunt et al., 2018).156
2.1.2.2. Carbon–Phosphorus Bond Formation
Phosphonation of benzylic C(sp3)–H bonds was reported by the Dong group in 2019.157 This is the only example of a palladium-catalyzed C(sp3)–H bond phosphonation reaction, and it is directed by pyridine. Only 8-methylquinoline derivatives reacted smoothly under the optimized conditions.
2.1.2.3. Carbon–Oxygen Bond Formation
In 2004, an efficient method for the Pd-catalyzed, oxime-directed oxygenation of unactivated methyl C(sp3)–H bonds using PhI(OAc)2 as oxidant was discovered by Sanford and co-workers (Scheme 52a).158 The reaction conditions are: Pd(OAc)2 (5 mol%) and PhI(OAc)2 (1.1 equiv) in AcOH/Ac2O (1:1) at 80–100 °C for 1.5–3.5 h. The reaction is thought to proceed through directed C(sp3)–H bond cleavage followed by oxidative addition of PhI(OAc)2 to form a Pd(IV) intermediate and then reductive elimination to form the C(sp3)–O bond and regenerate the Pd(II) catalyst. The regioselectivity of this transformation was examined by employing a series of aliphatic ketone O-methyl oximes. Notably, no β-hydride elimination was observed in any of these systems, which was attributed to the rigidity of the palladacycle intermediates. Under the reaction conditions, mono-oxygenation of primary β-C–H bonds occurs in preference to secondary β-C–H bonds or γ-C–H bonds. Interestingly, structurally rigid trans-decalone O-methyl oxime is oxygenated at an unactivated 2° C(sp3)–H bond with high diastereoselectivity. Based on this result, C–H activation and subsequent C(sp3)–O bond formation are postulated to proceed with high stereoselectivity. Pyridyl-directed oxygenation of unactivated C(sp3)–H bonds was also reported by the Sanford group in the same year.159 The oxime-directed oxygenation of C(sp3)–H bonds was later used by the Johnson group for the total synthesis of paspaline (Scheme 52b).160 In 2017, Mei and co-workers reported a palladium(II)-catalyzed C(sp3)–H oxygenation via electrochemical oxidation, which provides an alternative to conventional stoichiometric oxidants (Scheme 52c).161 Cyclic voltammogram (CV) of an oxazoline-containing, cyclometalated palladium(II) complex suggests that the anode could oxidize the alkyl palladium(II) intermediate to a high-valent Pd(III) or Pd(IV) species.
Scheme 52.
a) Palladium-Catalyzed Oxime-Directed Intermolecular C(sp3)–H Acetoxylation (Sanford et al., 2004).158 b) Application of C(sp3)–H oxygenation in the total synthesis of paspaline (Johnson et al., 2015).160 c) Palladium-Catalyzed C(sp3)–H Oxygenation via Electrochemical Oxidation (Mei et al., 2017).161
In 2005, Yu and co-workers reported the palladium-catalyzed oxazoline-directed acetoxylation of unactivated methyl C(sp3)–H bonds using MeCOOOtBu as oxidant and Ac2O as a promoter (Scheme 53).162 The diastereoselective C(sp3)–H acetoxylation was also achieved in moderate diastereoselectivity (up to 82% de) using a (S)-4-tert-butyloxazoline as DG. To avoid the oxidation of α-hydrogen atom to the nitrogen in the chiral oxazoline DG, lauroyl or benzoyl peroxide was used as the stoichiometric oxidant for the diastereoselective reaction. A 3:2 mixture of anti and syn trinuclear C(sp3)–Pd complexes was isolated by stirring substrate 275a with 1.5 equiv of Pd(OAc)2 in CH2Cl2 at 24 °C for 36 h. Remarkably, only the anti-isomer was obtained from chiral substrate 277a, which suggests that the stereocenter on the chiral oxazoline DG controls the geometry of the trinuclear complex. A palladium-catalyzed, Boc-directed acetoxylation of N-methylamines with IOAc as the oxidant has also been demonstrated by the Yu group.163
Scheme 53.
Palladium-catalyzed oxazoline-Directed C(sp3)–H Acetoxylation (Yu et al., 2005).162
In 2014, Lu and co-workers reported a palladium-catalyzed acyloxylation of unactivated C(sp3)–H bonds of simple amides with CF3CO2H/K2S2O8. A variety of amides were successfully converted to the corresponding β-acyloxy amides in good yields (Scheme 54).164 By using methanesulfonic anhydride (Ms2O), various β-mesyloxy amides could also be obtained. Furthermore, the β-mesyloxy amides could be easily transformed to β-fluoroamides and β-lactams.165
Scheme 54.
Palladium-Catalyzed β-C(sp3)–H Acyloxylation and Mesyloxylation of Simple Amides (Lu et al., 2014, 2017).164, 165
In 2015, Gaunt and co-workers reported a palladium-catalyzed C(sp3)–H acetoxylation of amino alcohol derivatives (Scheme 55a).80 The reaction conditions are: Pd(OAc)2 (5 mol%) and PhI(OAc)2 in a mixed solvent system of toluene and Ac2O at 60 °C. A broad range of substrates that contain alkyl chains with varying substitution patterns, protected hydroxyl groups, carbonyl moieties, and nitrogen-containing heterocycles were successfully acetoxylated under the standard conditions. In 2019, they reported the palladium-catalyzed C(sp3)–H acetoxylation of morpholinone derivatives (Scheme 55b).166 The above mentioned two examples were limited to the acetoxylation of secondary amines. While a Pd-catalyzed γ-C(sp3)–H acetoxylation of primary aliphatic amines was uncovered by Shi and co-workers in 2017 (Scheme 55c).167 Protonation of the primary amine with AcOH was found to accelerate the reaction.
Scheme 55.
a) Pd-Catalyzed C(sp3)–H Acetoxylation of Amino Alcohol Derivatives (Gaunt et al., 2015).80 b) Pd-Catalyzed C(sp3)–H Acetoxylation of Morpholinone Derivatives (Gaunt et al., 2019).166 c) Pd-Catalyzed C(sp3)–H Acetoxylation of Primary Amines (Shi et al., 2017).167
The formation of benzolactones via palladium-catalyzed, carboxylate-directed C(sp3)–H lactonization was disclosed by the Martin group in 2011 (Scheme 56a).168 This transformation is significantly promoted by a MPAA ligand, namely Ac-Leu-OH (L55). Silver carbonate was used as the stoichiometric oxidant and a silver benzoate salt was proposed as the reactive intermediate. Yu and co-workers further improved this process by the use of molecular oxygen as the sole oxidant in 2020 (Scheme 56b).169 3-Trifluoromethyl-2-pyridone (L56) was found to be a crucial ligand to promote the oxygenation reaction. Notably, this represents a rare example of C–H oxygenation via Pd(II)/Pd(0) catalysis.
Scheme 56.
a) Palladium-Catalyzed Carboxylate-Directed C(sp3)–H Lactonization using Silver Carbonate as Oxidant (Martin et al., 2011).168 b) Improved C(sp3)–H Lactonization using Molecular Oxygen as Oxidant (Yu et al., 2020).169
Palladium-catalyzed β-C(sp3)–H acetoxylation of free carboxylic acids was reported by the van Gemmeren group in 2019 (Scheme 57a).170 The inorganic base plays a key role in this reaction, with NaHFIP offering the best yield. A breakthrough of Pd-catalyzed β-C(sp3)–H lactonization of free alkyl aliphatic acids to synthesize the highly strained β-lactones was made by Yu and co-workers in 2020 (Scheme 57b).171 The reaction is catalyzed by Pd(CH3CN)2Cl2 (10 mol%) with α-methyl-N-acetyl β-alanine L57 (20 mol%) as the ligand, TBHP (in decane, 2.0 equiv) as the oxidant, and CsHCO3 (0.5 equiv) as the base in HFIP solvent. TBHP is proposed to promote the intramolecular C(sp3)–O reductive elimination from the Pd(IV) center to produce the β-lactone product due to the steric hindrance of t-BuO−. The highly strained β-lactones can be converted into a variety of functionalized alkyl aliphatic acids upon reaction with different nucleophiles. By switching the ligand from α-methyl-N-acetyl β-alanine L57 to the cyclopentane-based N-acetyl β-amino acid L58, Yu and co-workers were able to develop the palladium(II)-catalyzed β-C(sp3)–H acetoxylation of free carboxylic acids using Ac2O (Scheme 57c).172 When the reaction is performed without Ac2O under otherwise standard conditions, γ-, δ-, and ε-lactone products are obtained for the first time. Substrates containing α-hydrogen were also converted to lactones under the standard conditions. The authors proposed a Pd(II)/Pd(IV) catalytic cycle involving the following sequence of events. Aliphatic acid 301 undergoes palladium(II)-catalyzed β-C(sp3)–H activation to give palladacycle intermediate 302, which is oxidized by TBHP to form Pd(IV) intermediate 303. In the presence of Ac2O, intermediate 303 could undergoe ligand exchange to generate intermediate 304. Finally, β- acetoxylated product 305 is produced via reductive elimination. In the absence of Ac2O, the lacton product 298 is formed instead, as demonstrated in Scheme 57b.171
Scheme 57.
a) Palladium-Catalyzed β-C(sp3)–H Acetoxylation of Aliphatic Carboxylic Acids (van Gemmeren et al., 2019).170 b) Palladium-Catalyzed β-C(sp3)–H lactonization (Yu et al., 2020).171 c) Palladium-Catalyzed β-C(sp3)–H Acetoxylation of Aliphatic Carboxylic Acids with Ac2O (Yu et al., 2020).172
2.1.2.4. Carbon–Halogen Bond Formation
In 2005, Yu and co-workers reported the first palladium-catalyzed, oxazoline-directed, diastereoselective iodination of unactivated C–H bonds under mild conditions(Scheme 58a).173 The trinuclear palladium alkyl species 312 was obtained as a single isomer in 60% yield by stirring substrate 311 with Pd(OAc)2 (1 equiv) in CH2Cl2 at 24 °C for 36 h (Scheme 58b). The formation of new chiral centers was confirmed by treating 312 with trifluoroacetic acid, which allowed the isolation and X-ray analysis of 313 as the major syn-(S,S) complex. Moreover, they isolated PdI2 as a powder from the catalytic reaction mixture in quantitative yield. Based on these results, they rationalized that IOAc generated in situ from the reaction of AgOAc and I2 converts PdI2 into Pd(OAc)2 to close the catalytic cycle.174 They observed low reactivity and stereoselectivity when 4-isopropyl-oxazoline was used as the DG, and computational calculations support this finding by revealing that the catalyst resting state of 4-isopropyl-oxazoline is more stable than the catalyst resting state of 4-tert-butyl-oxazoline.175 In 2017, the Yu group reported that quinoline-type ligands could enable C(sp3)–H bromination and iodination of various aliphatic amides directed by CONH–p-CF3C6F4 (Scheme 58c).176 Moreover, in this same paper they also showed one example of a free-carboxylic-acid-directed C(sp3)–H bromination of pivalic acid.
Scheme 58.
a) Palladium-Catalyzed Oxazoline-Directed Monoiodination of Methyl C(sp3)–H Bonds (Yu et al., 2005).173 b) Charaacterization of a Chiral Trinuclear C(sp3)–Pd Complex and Computational Studies (Yu, Houk et al., 2012).175 c) Ligand-Enabled Pd(II)-Catalyzed C(sp3)–H Bromination and Iodination of Aliphatic Amides (Yu et al., 2017).176
In 2015, Yu and co-workers developed a ligand-enabled, Pd(II)-catalyzed C(sp3)–H fluorination reaction of α-amino acids utilizing Selectfluor as the fluorination reagent and monodentate CONH-(p-CF3)C6F4 as the directing group (Scheme 59).177 After extensive screening, the reaction was found to be sensitive to the amount of Selectfluor, Ag2CO3, and 1,4-dioxane. Ultimately, a wide range of unnatural, enantiopure, anti-β-fluoro-α-amino acids could be synthesized. They proposed a reaction mechanism in which the quinolone ligand (L60) coordinates to Pd(TFA)2 to form the Pd(II)•(L60)n intermediate A. Then, coordination of the substrate followed by C(sp3)–H activation affords intermediate 318. Next, the oxidative addition of Selectfluor to intermediate 318 delivers intermediate 320 with a fluorine ligand attached to the Pd(IV) center. Finally, C(sp3)–F reductive elimination from intermediate 320 affords the corresponding fluorinated product and regenerates the active Pd(II) species.
Scheme 59.
Pd-Catalyzed, CONH–p-CF3C6F4-Directed β-C(sp3)–H Fluorination of Amino Acid Derivatives (Yu et al., 2015).177
2.1.2.5. Carbon–Boron Bond Formation
In 2016, a ligand-promoted, palladium(II)-catalyzed C(sp3)–H borylation of carboxamides that bear the weakly coordinating, monodentate -NHArF (ArF = p-CF3C6F4) DG with bis(pinacolato)diboron as the borylating reagent was reported by Yu and co-workers (Scheme 60a).178 The reaction system is compatible with methyl C(sp3)–H bonds in both α-tertiary and α-quaternary carboxamides, as well as with methylene C(sp3)–H bonds in a variety of alicyclic carboxyamides. In order to demonstrate the practicality of this transformation, the borylated products were converted to various organic synthons through carbon–carbon and carbon–heteroatom bond formation. In the next year, Yu and co-workers successfully developed an enantioselective C(sp3)–H borylation of cyclobutane carboxamides via a Pd(II)/Pd(0) catalytic cycle using APAO ligand L19 as chiral ligand (Scheme 60b).179 Substrates that contain α-tertiary and α-quaternary carbon centers are compatible with this reaction. The use of APAO ligands is key to the success of this asymmetric borylation reaction.
Scheme 60.
a) Pd(II)-Catalyzed CONH–p-CF3C6F4-Directed β-C(sp3)–H Borylation of Aliphatic Carboxamides (Yu et al., 2016).178 b) Pd(II)-Catalyzed Enantioselective C(sp3)–H Borylation of Cyclobutane Carboxamides (Yu et al., 2017).179
2.2. Palladium-Catalyzed C(sp3)–H Functionalization Using Bidentate Directing Groups
Arguably, bidentate directing auxiliaries are the most widely used strategy for realizing transition-metal-catalyzed nonactivated C(sp3)–H bond functionalization reactions. After the pioneering work of Daugulis and co-workers,180,181 various types of DGs, including N,N-, N,S- and N,O-bidentate types,21,33,182,183 have been developed as shown in Figure 4. Due to the fact that bidentate, DG-coordinated, cyclopalladated intermediates are often thermodynamically stable, various electrophilic coupling partners can be employed to oxidize these intermediates to produce Pd(IV) species that can then undergo reductive elimination to form carbon-carbon and carbon-heteroatom bonds. The widespread use of bidentate DGs, especially 8-aminoquinoline and related DGs, in C(sp3)–H bond functionalization, has motivated the development of a number of robust methods for cleaving or transforming these DGs for various synthetic applications. . These methods have been thoroughly reviewed and thus will not be discussed in this here.184–187
Figure 4.
Representative Bidentate DGs for C(sp3)–H Activation.
2.2.1. Carbon-Carbon Bond Formation
2.2.1.1. Arylation
Two pathways for bidentate-DG-assisted, nonactivated C(sp3)–H bond arylation are outlined in Scheme 61. Generally, cyclopalladated intermediate B is generated after C(sp3)–H activation, which is then followed by two distinct catalytic cycles involving either a Pd(II)/Pd(IV) or a Pd(II)/Pd(0) redox couple. Reaction of the palladacycle B with electrophilic coupling partners leads to Pd(IV) intermediate C, which then undergoes reductive elimination to release arylated product D and Pd(II). The alternate pathway proceeds through transmetalation with a nucleophilic coupling partner to afford the Pd(II) intermediate E, and reductive elimination furnishes arylated product F and Pd(0), which needs to be re-oxidized to close the catalytic cycle. Thus far, only one example of bidentate-DG-assisted nonactivated C(sp3)–H bond arylation that is believed to proceed via a Pd(II)/Pd(0) pathway has been reported.
Scheme 61.
Overview of Bidentate-DG-Assisted C–H Bond Arylation
2.2.1.1.1. C(sp3)–H Arylation via Pd(II)/Pd(IV) Catalysis
While early work established that pyridine and various other related N-heterocycles were effective DGs in enabling C(sp3)–H bond functionalization,76 the products of such transformations have limited utility given that these heterocycles cannot be readily converted into other functional groups. Thus, the Daugulis group sought to develop a bidentate directing group that could enable these same transformations and be easily installed and removed. In 2005 they pioneeringly developed removable amide auxiliaries derived from 8-aminoquinoline (AQ) and picolinic acid (PA).180 It was proposed that the bidentate coordination of these auxiliaries to the metal center facilitates both the C–H activation and the oxidative addition steps by stabilizing the Pd(IV) intermediate species, which were suspected to be involved in the mechanism The 8-aminoquinoline group laid the foundation for the rapid development of a myriad of N,N-bidentate auxiliaries to facilitate diverse catalytic C–H activation methods.
The palladium(II)-catalyzed arylation of primary C(sp3)–H bonds and even more challenging secondary β-C(sp3)–H bonds of carboxamide substrates was demonstrated by employing the 8-aminoquinoline auxiliary to provide a route for accessing β-functionalized carboxylic acids upon cleavage of the 8-aminoquinoline group (Scheme 62a).180 The optimal conditions found were: Pd(OAc)2 (0.1 – 5 mol%) with AgOAc and an excess of an aryl iodide at 70–130°C. The Corey group used the 8-aminoquinoline DG to functionalize C(sp3)–H bonds in natural amino acid derivatives in 2006 (Scheme 62b).188 An unexpected arylation of the γ-C–H bond via a six-membered palladacycle was observed when isoleucine was used as the substrate, indicating that γ-C–H bond activation can occur when the β-C–H bond is hindered.
Scheme 62.
a) Pd-Catalyzed 8-Aminoquinoline-Directed C(sp3)–H Arylation of Aliphatic Carboxamides (Daugulis et al., 2005).180 b) Pd-Catalyzed 8-Aminoquinoline-Directed β-C(sp3)–H Arylation of Amino Acids (Corey et al., 2006).188
In 2010, Daugulis and co-workers expanded upon their preliminary study of 8-aminoquinoline-directed C(sp3)–H arylation in 2005, and developed a robust method for secondary C(sp3)–H arylation and alkylation directed by 8-aminoquinoline auxiliary (Scheme 63).181 In comparison to their previous study, in this work, they carried out a systematic investigation of the activities of different bidentate directing groups. They also developed a procedure that replaces the expensive silver salts with cesium salts. To obtain further insights into the coordination mode of the substrate with Pd(II), Daugulis and co-workers characterized the C–H cleavage intermediate 332. Palladacycle 332 was isolated in quantitative yield by combining the 8-aminoquinoline-derived pivalamide with palladium acetate in nitrile solvents at 60 °C for 3–4 hours (Scheme 63). Acetonitrile is labile and easily replaced by tert-butyl isonitrile to afford complex 332b in quantitative yield. Interestingly, complete H/D exchange of the aliphatic hydrogens was observed within minutes at room temperature when complex 332 was dissolved in CD3CO2D. At –35 °C, the first-order rate constant was measured to be k = 2.0 × 10−4 s−1. Reactions between complex 332 and either p-tolyl iodide or iodomethane were conducted. Both mono- and di-substituted products were afforded in addition to the recovered amide starting material. Through these mechanistic studies, Daugulis and co-workers propose a Pd(II)/Pd(IV) pathway that proceeds first through C–H activation and then through oxidative addition. Daugulis and co-workers were careful to note that they were unable to rule out the presence of an active Pd(III) dimeric species. However, in the strongly coordinating acetonitrile solvent, a dimeric Pd(III) species seems unlikely.
Scheme 63.
Pd-Catalyzed β-Methylene C(sp3)–H arylation of 8-Aminoquinoline Amides and Mechanistic Studies (Daugulis et al., 2010).181
Inspired by the ability of the 8-aminoquinoline DG to enable C–H functionalization at the β-methylene position, Chen and co-workers reported a benzo-ring construction under mild reaction conditions via Pd(II)-catalyzed intramolecular C(sp3)–H arylation of a tethered aryl iodide (Scheme 64a).189 After extensive screening of both aliphatic acids and benzoic acids, the addition of ortho-phenylbenzoic acid (o-PBA) was found to play a key role in the efficiency of the reaction and allows the transformation to proceed at room temperature. Also using o-PBA as the additive, Chen and co-workers reported a protocol for making peptide macrocycles via palladium-catalyzed, intramolecular C(sp3)–H arylation (Scheme 64b).190 The buttress from the cyclophane moiety makes the resulting peptide macrocycles more rigid and stable in their three-dimensional structure than flexible amides, esters or disulfide linkers. Non-aromatic amino acids could also be cyclized through attachment of a prosthetic iodoaryl substituent. Even relatively small peptide macrocycles could be prepared using this transformation, despite the resulting ring strain.
Scheme 64.
a) Pd-Catalyzed 8-Aminoquinoline-Directed Intramolecular Methylene β-C(sp3)–H Arylation (Chen et al., 2010).189 b) Synthesis of Peptide Macrocycles via Palladium-Catalyzed Intramolecular C(sp3)–H Arylation (Chen et al., 2018).190
The 8-aminoquinoline-directed, β-methylene C–H arylation was applied by the Chen group as a key C–C bond-forming step in the stereoselective synthesis of the bicyclic peptide celogentin C (Scheme 65a).191 The 8-aminoquinoline auxiliary could be effectively detached under mild conditions. In 2012, Baran and co-workers also achieved the concise total synthesis of the originally proposed structure of pipercyclobutanamide A by 8-aminoquinoline-directed, palladium-catalyzed sequential intermolecular C(sp3)–H arylation/alkenylation of the cyclobutanamide (Scheme 65b).192 In 2014, the Maimone group reported a total synthesis of podophyllotoxin through palladium-catalyzed C(sp3)–H arylation (Scheme 65c).193 The arylation product was observed by tuning the conformation of the substrate. The Chen group also achieved the total synthesis of mannopeptimycins using 8-aminoquinoline-directed β-methylene C–H arylation of Phth-Abu-AQ 348 to build the key L-MePhe 349 in 2016 (Scheme 65d).194 8-aminoquinoline-directed, cyclobutyl C(sp3)–H arylation strategy has also been used by Reisman and co-workers in the total synthesis of (+)-rumphellaone A (Scheme 64d).195 Collectively, these examples demonstrate the enabling nature of directed C(sp3)–H activation in complex molecule synthesis.
Scheme 65.
a) Synthesis of the Key Leu-Trp Intermediate of celogentin C via 8-Aminoquinoline-Directed Methylene C(sp3)–H Arylation (Chen et al., 2010).191 b) Total Synthesis of the Proposed Strucutre of Pipercyclobutanamide A via 8-Aminoquinoline-Directed Sequential C(sp3)–H Arylation and Olefination (Baran et al., 2012).192 c) Total Synthesis of Podophyllotoxin via 8-Aminoquinoline-Directed C(sp3)–H Arylation (Maimone et al., 2014).193 d) Synthesis of the Key L-MePhe Building Block of Mannopeptimycins via 8-Aminoquinoline-Directed Methylene C(sp3)–H Arylation (Chen et al., 2016).194 e) 8-Aminoquinoline-Directed Cyclobutyl C(sp3)–H Arylation as a Key Step in Total Synthesis of (+)-rumphellaone A (Reisman et al., 2019).195
In 2012, the diarylation of methyl groups and the diastereoselective monoarylation of methylene groups of amino acids was achieved by Daugulis and co-workers with the assistance of 8-aminoquinoline DG (Scheme 66).196 Notably, the 8-aminoquinoline DG can be removed with BF3•Et2O in MeOH at 100 °C to afford the corresponding methyl ester.
Scheme 66.
Pd(II)-Catalyzed β-C(sp3)–H Arylation of N-Phthaloyl-Protected α-Amino Acids (Daugulis et al., 2012).196
In 2014, the Chen group described the Pd(II)-catalyzed monoselective β-methyl C(sp3)–H arylation of alanine derivative without erosion of the α-stereocenter by employing the 8-aminoquinoline DG (Scheme 67a).197 By extending the reaction time to 2 days and reducing the reaction temperature to room temperature, Chen and co-workers were able to prevent a second arylation of the β-methyl group (Scheme 67a) that was observed by Daugulis and co-workers.178 Shortly after, the Shi group independently reported a 8-aminoquinoline-directed β-methyl C(sp3)–H monoarylation of alanine derivative that required a reaction length of only 2 h (Scheme 67b).198 A wide range of aryl iodides that bear synthetically useful functional groups can be used in this protocol, providing a general and practical synthetic access to a variety of β-aryl-amino acids. They successfully applied this protocol in the scalable formal synthesis of (−)-quinocarcin in 2019. The key building block, β–aryl-α-amino acid 359, was prepared in 82% yield on a 22.18 g scale with the retention of chirality (Scheme 67c).199 In 2016, an efficient synthesis of chiral β-aryl-α-hydroxy acids via palladium-catalyzed C(sp3)–H arylation of lactic acid through the assistance of 8-aminoquinoline DG with the retention of chirality was reported by the Shi group (Scheme 67d).200 After extensive screening, the best reaction conditions were Pd(OAc)2 (10 mol%) as catalyst and AgF (1.5 equiv) as the additive at 75 °C for 2 h. A range of aryl and heteroaryl iodides bearing various functional groups underwent the arylation successfully. The synthetic importance of these protocols was demonstrated by the synthesis of LY519818 and tesaglitazar, two potential agonists against peroxisome proliferator-activated receptors. In the above examples involving amino acids, the amine is protected by the phthalimido (Phth) group. In 2018, Kazmaier and co-workers reported the β-C(sp3)–H arylation of N-methylated amino acids and peptides using 8-aminoquinoline as DG (Scheme 67e).201 This method has been used to synthesize the key building blocks 365a and 365b, which were successfully applied in the total synthesis of abyssenine A and mucronine E.
Scheme 67.
a) Pd-Catalyzed Monoarylation of Alanine β-Methyl C(sp3)–H Bonds (Chen et al., 2014).197 b) Pd-Catalyzed β-Methyl C(sp3)–H Monoarylation of Alanine (Shi et al., 2014).198 c) Scalable Formal Synthesis of (−)-Quinocarcin via β-Methyl C(sp3)–H Monoarylation of Alanine (Shi et al., 2019).199 d) Pd-Catalyzed Monoarylation of Lactic Acid Directed by 8-Aminoquinoline (Shi et al., 2016).200 e) Pd-Catalyzed C(sp3)–H Arylation of N-Methyl Amino Acids and Peptides, and the Application in Total Synthesis of Abyssenine A and Mucronine E (Kazmaier et al., 2018).201
In 2013, palladium-catalyzed arylation of methylene C(sp3)–H bonds of cyclopropanes with the assistance of the 8-aminoquinoline DG was reported by the Babu group (Scheme 68a)202 and the Shuto group (Scheme 68b),203 respectively. Notably, the Shuto group also demonstrated several examples of arylation of tertiary cyclopropyl C(sp3)–H bonds.203 In the same year, the diarylation of cyclobutane was also developed by the Babu group (Scheme 68c).204 In 2016, the Chen group described a palladium-catalyzed direct arylation of methylene C(sp3)–H bonds of cyclopentane and cyclohexane carboxamides at room temperature (Scheme 68d).205
Scheme 68.
a) 8-Aminoquinoline-Directed Methylene C(sp3)–H Arylation of Cyclopropanes (Babu et al., 2013).202 b) 8-Aminoquinoline-Directed Tertiary C(sp3)–H Arylation of Cyclopropanes (Shuto et al., 2013).203 c) 8-Aminoquinoline-Directed Methylene C(sp3)–H Arylation of Cyclobutanes (Babu et al., 2013).204 d) 8-Aminoquinoline-Directed C(sp3)–H Arylation of Cyclopentanes and Cyclohexanes (Chen et al., 2016).205
In 2017, the Daugulis group reported a palladium-catalyzed, 8-aminoquinoline-directed β-C(sp3)–H arylation of phosphonamidates and phosphinic amides (Scheme 69a).206 Unlike the arylation of aliphatic carboxamides, only primary C(sp3)–H bonds could be functionalized in this protocol. Palladium-catalyzed, 8-aminoquinoline-directed arylation of γ-C(sp3)–H bonds via a six-membered cyclopalladation pathway was developed by the Maiti group in 2017 (Scheme 69b).207 In this case, the presence of substituents at the β-position is necessary to enable the activation of typically less favored distal position.
Scheme 69.
a) Pd-Catalyzed 8-Aminoquinoline-Directed C(sp3)–H Arylation of Phosphonamidates and Phosphinic Amides (Daugulis et al., 2017).206 b) Pd-Catalyzed 8-Aminoquinoline-Directed γ-C(sp3)–H Arylation (Maiti et al., 2017).207
While numerous reports have examined the use of aryl iodides as coupling partners in catalytic C(sp3) –H arylation, other aryl electrophiles have been less extensively studied. In 2014, the Zeng group reported an intermolecular arylation of unactivated C(sp3)–H bonds with aryl bromides with the assistance of the 8-aminoquinoline DG (Scheme 70a).208 The reaction conditions are: Pd(TFA)2 (5 mol%), K2CO3 (3.5 equiv) as the base, and PivOH (50 mol%) as the additive in t-AmylOH at 120 °C for 36 h. In this system, various functional groups are well tolerated.
Scheme 70.
a) 8-Aminoquinoline-Directed C(sp3)–H Arylation with Aryl Bromides (Zeng et al., 2014).208 b) 8-Aminoquinoline-Directed C(sp3)–H Arylation with Diarylhyperiodonium Triflates (Shi et al., 2014).209 c) 8-Aminoquinoline-Directed C(sp3)–H Arylation with (Diacetoxyiodo)arenes (Qin et al., 2015).210 d) 8-Aminoquinoline-Directed C(sp3)–H Arylation of a Lysine Derivative Using a Hypervalent Iodonium Reagent for the Total Synthesis of Streptide (Boger et al., 2019).211
In 2013, the palladium-catalyzed direct arylation of primary and secondary C(sp3)–H bonds with diarylhyperiodonium electrophiles with the assistance of 8-aminoquinoline DG was reported by the Shi group (Scheme 70b).209 Control experiments revealed that the reactivity of diarylhyperiodonium triflates is much higher than that of the corresponding aryl iodide under the same conditions. Moreover, the solubility of the counteranion of the diarylhyperiodonium salt was found to play a key role in the efficiency of this reaction. In 2015, the Qin group disclosed an unusual palladium-catalyzed direct arylation of secondary C(sp3)–H bonds with (diacetoxyiodo)arene reagents, which are usually used as oxygen electrophiles in C–H acetoxylation (Scheme 70c).210 In 2019, Boger and co-workers reported the secondary C(sp3)–H arylation of a lysine derivative using diarylhyperiodonium electrophiles with the assistance of 8-aminoquinoline DG to provide a key intermediate (377) in the total synthesis of Streptide (Scheme 70d).211
Saturated heterocycles are key building blocks in the synthesis of various bioactive compounds, including pharmaceuticals. The late-stage functionalization of these heterocycles could expand the structural diversity of screening libraries used in drug discovery. Palladium-catalyzed directed C(sp3)–H arylation of proline with the assistance of the 8-aminoquinoline DG was independently reported by the Bull and Zhang groups.212–214 Stereospecific 8-aminoquinoline-carboxamide-directed, palladium-catalyzed C(sp3)–H arylation has been developed for a variety of backbones, such as pyrrolidines and piperidines by the Bull and Kazmaier groups,215,216 tetrahydrofurans by the Babu group (Scheme 71),217 and pyroglutamic acid derivatives218 and azetidines219 by the Schreiber group (Scheme 72). In 2018, Messaoudi and co-workers reported a palladium(II)-catalyzed, diastereoselective C(sp3)–H activation of glycosyl carboxamides (Scheme 73).220 They observed high yields of the C(sp3)–H arylated products with β-glycosides. However, the α-glycosides under the same reaction conditions afforded arylated glucals that presumably arise from an AcOH elimination after the C(sp3)–H arylation.
Scheme 71.
8-Aminoquinoline-Directed C(sp3)–H Arylation of Aliphatic Heterocycles (Bull et al., 2014, 2016, 2018; Babu et al., 2015; Zhang et al., 2015; Kazmaier et al., 2016).212−217
Scheme 72.
8-Aminoquinoline-Directed C(sp3)–H Arylation of pyroglutamic acid derivatives and azetidines (Schreiber et al., 2017).218,219
Scheme 73.
Pd(II)-Catalyzed Diastereoselective C(sp3)–H Arylation of Glycosides (Messaoudi et al., 2018).220
The first example of γ-C(sp3)–H arylation of aliphatic amines using a strongly coordinating, pyridine-based N,N-bidentate DG was reported by Daugulis in 2005 (Scheme 74a).180 Arylation of both primary and secondary γ-C(sp3)–H bonds of aliphatic amine derivatives was achieved by employing a picolinamide (PA) auxiliary as the DG. Inspired by this study, a PA directed, palladium-catalyzed γ-C(sp3)–H arylation of aliphatic amines that could be run at substantially lower temperatures was reported by the Chen group in 2011 (Scheme 74b).221 One of the main improvements is the use of equivalents aryl iodides instead of the use as solvent. In this system, the conformation of the C–H bond relative to the DG was observed to influence the regio- and stereoselectivity of this reaction. One disadvantage of these procedures is that the amide group of the PA auxiliary is difficult to cleave. In order to solve this problem, a modified picolinic acid was prepared based on the hypothesis that a silyl-protected hydroxymethyl group at the ortho position of the PA group would allow the auxiliary to retain its directing ability but would also facilitate amide cleavage through intramolecular acyl transfer upon treatment with acid. The application of this protocol was demonstrated in the synthesis of (+)-obafluorin from a readily accessible threonine derivative. In 2014, the Chen group also reported a new pyridylmethylamine-based auxiliary group for the total synthesis of hibispeptin A via Pd-catalyzed C(sp3)–H arylation using a sterically hindered aryl iodide (Scheme74c).222 In 2018, the Jana group demonstrated that picolinamide DG could also enable the efficient synthesis of unsymmetrical γ-C(sp3)–H diarylation of amino acids (Scheme 74d).223 Ligands played a crucial role in promoting the reaction and tuning the mono- and unsymmetrical diarylations. They observed that bidentate 1,10-phenanthroline (L62) significantly improved the yield. Unsymmetrical diarylation was achieved by subjecting the monoarylated products to the second arylation conditions using quinoline-based ligand (L7).
Scheme 74.
a) Pd-Catalyzed Picolinamide-Directed γ-C(sp3)–H Arylation (Daugulis et al., 2005).180 b) Picolinamide-Directed C(sp3)–H Arylation and Formal Synthesis of (+)-Obafluorin (Chen et al., 2011).221 c) Total Synthesis of Hibispeptin A via Pd-catalyzed C(sp3)–H Arylation Directed by Pyridylmethylamine-Based Auxiliary (Chen et al., 2014).222 d) Picolinamide-Directed C(sp3)–H Arylation for the Formation of Unnatural Amino Acid Derivatives (Jana et al., 2018).223
In 2019, the Maiti and Paton groups reported a picolinamide-directed, palladium-catalyzed δ-C(sp3)–H arylation of aliphatic amines via a six-membered palladacycle intermediate.224 A propriately tuned ancillary ligands play a key role in this transformation. 4-Benzyloxy-2(1H)-pyridone (L63) was the best ligand for leucine, homoleucine, and analogous amine-derived substrates. Pyridine was identified as an efficient ligand to prevent the formation of diarylation byproducts when 2,4,4-trimethylpentan-2-amine (435) was employed as a substrate. Use of 1-hydroxy isoquinoline (L64) as ligand enabled more challenging heteroaryl iodides to serve as electrophilic coupling partners, . Moreover, iterative arylation of 2,4,4-trimethylpentan-2-amine was also demonstrated (435). Interestingly, a pyridine coordinated [5,6]-fused cyclopalladated intermediate (441) was isolated and characterized. A set of control experiments revelaled that intermediate 441 was a viable precatalyst for this transformation. DFT investigations indicated that the overall energy barrier was decreased significantly by employing the ligands due to their ability to break up the carboxylate-bridged trinuclear species that are otherwise stable in solution.
Although Chen and co-workers have developed an 8-aminoquinoline-directed, β-C(sp3)–H arylation to generate peptide macrocycles,185 the stereoselectivity of this protocol is limited. A subsequent report by the Chen group disclosed a PA-directed, γ-C(sp3)–H arylation strategy to prepare peptide macrocycles with higher yield and stereoselectivity. Peptide 444 that contain unprotected polar side chains can also cyclize well in aqueous solution to give the corresponding cyclic peptide 445 (Scheme 76).225
Scheme 76.
Synthesis of Peptide Macrocycles via Palladium-Catalyzed Picolinamide-Directed Intramolecular γ-C(sp3)–H Arylation (Chen et al., 2019).225
Palladium-catalyzed, picolinamide-directed γ-C(sp3)–H arylation of a variety of cycloalkyl amine derivatives, such as cyclopropyl methylamines,226 3-pinanamine,227 cycloalkylamines,228 rimantadine,229 and saturated bicyclic amines,230 have been developed, further demonstrating the robustness of picolinamide DG (Scheme 77).
Scheme 77.
Palladium Catalyzed Picolinamide-Directed C(sp3)–H Arylation of Aliphatic Carbocycles. a) Cyclopropyl Methylamines (Charette et al. 2013).226 b) 3-Pinanamine (Wang et al. 2014).227 c) Cycloalkylamines (Miyake et al. 2014).228 d) Rimantadine (Zhang et al. 2016).229 e) Saturated Bicyclic Amines (Sheppard et al. 2018).230
In 2016, the Maes group reported picolinamide-directed, palladium-catalyzed C5-arylation of 3-aminopiperidine (Scheme 78a).231 Upon use of catalytic 2,6-dimethylbenzoic acid as additive and a high reaction concentration, the yield of the products was improved significantly. In 2018, the Maulide group described the total synthesis of natural (–)-quinine and unnatural (+)-quinine using C(sp3) –H arylation as a key step. Remarkably, unnatural (+)-quinine was synthesised for the first time and biological activity was studied (Scheme 78b).232
Scheme 78.
a) Palladium-Catalyzed Picolinamide-Directed C(sp3)–H Arylation of 3-Aminopiperidine (Maes et al. 2016).231 b) Total Synthesis of Quinine and Analogues Using Pd-Catalyzed C(sp3)–H Arylation as a Key Step (Maulide et al. 2018).232
In 2013, the Shi group achieved the β-monoarylation of alanine using the newly developed 2-(pyridine-2-yl)isopropyl (PIP) DG.233 After extensive screening, the conditions were found to be Pd(OAc)2 (10 mol%), CuF2 (1.5 equiv), and DMPU (5.0 equiv) in acetone (0.1 M) at 100 °C for 24 h. A range of aryl iodides that contain different functional groups were found to be compatible with this protocol, and the desired products were furnished in good yields with complete stereoselectivity under the reaction conditions (Scheme 79a). It is noteworthy that the PIP DG can also be removed in high yield without loss of enantiopurity of the arylated products through a mild N-nitrosylation/hydrolysis sequence.
Scheme 79.
a) Pd-Catalyzed PIP-Directed β-C(sp3)–H Monoarylation of Bonds of Alanine (Shi et al., 2013).233 b) Pd-Catalyzed PIP-Directed Methylene C(sp3)–H Arylation with Aryl Bromides (Shi et al., 2014).234 c) PIP-Directed C(sp3)–H Arylation as a Key Step in the Total Synthesis of Aeruginosins 98B and 298A (Baudoin et al., 2015).235
The palladium-catalyzed direct arylation of unactivated methylene C(sp3)–H bonds with aryl bromide reagents with the assistance of a PIP DG was reported by the Shi group in 2014 (Scheme 79b).234 In this system, the reported reaction conditions are: Pd(OAc)2 (10 mol%), K2CO3 (2.5 equiv) as the base, and PivOH (20 mol%) as the additive in t-BuOH at 120 °C for 24 h. Both aryl iodide and bromide electrophiles are competent in this reaction as arylation reagents.
PIP-auxiliary-directed, palladium-catalyzed arylation of the C(sp3)–H bonds was used as a key step in the syntheses of aeruginosin 98B and aeruginosin 298A by the Baudoin group in 2015 (Scheme 79c).235 With PIP-amide as the DG in the presence of Pd(OAc)2 as the catalyst and K2CO3 as the base, 467 underwent C(sp3)–H arylation with O-benzyl-protected iodoarene 468 in CH3CN to provide 469a in 78% yield with complete regio- and stereoselectivity, even on gram scale. Cleavage of the PIP auxiliary under mild conditions with NOBF4 in pyridine at low temperature afforded the enantiopure carboxylic acid 470 in excellent yield. After several more steps, the aeruginosin family natural product syntheses were complete, with the aureginosin 298A being made on an unprecedentedly large scale (700 mg).
Due to the difficulty in removal of the amide-derived DGs in complexed molecules, Baran and co-workers embarked on recognizing a more readily removable DG that can be removed under mild conditions. As demonstrated above, picolinamide has been recognized as a powerful DG in C(sp3)–H functionalization. They rationalized that a picolinimide-based DG could meet this requirement, since imides are generally more labile to hydrolysis than amides. Based on these rationalization and after condition optimization, they successfully achieved the picolinimide-directed C(sp3)–H diarylation of cyclobutane, and the picolinimide DG was easily removed with ammonia and catalytic scandium(III) trifluoromethanesulfonate (Scheme 80a).236 Shortly after, 2-picolinimide was used by the Shi group as an efficient DG to assist the palladium-catalyzed C(sp3)–H arylation of various simple aliphatic carboxylic acids (Scheme 80b).237 The reaction was highly site-selective, favoring β-methyl C–H bonds for arylation over β-methylene, γ-methyl, benzene C–H and β-benzylic C–H bonds. Therefore, a sequential methyl and methylene C(sp3)–H arylation with different aryl iodides was achieved. Finally, the picolinimide can be easily cleaved by hydrolysis with LiOH/H2O2 at room temperature in a short time to give the corresponding free carboxylic acids or amides in high yields.
Scheme 80.
a) Picolinamide-Directed C(sp3)–H Arylation of Cyclobutane Reported by the Baran Group (Baran et al., 2014).236 b) Picolinamide-Directed C(sp3)–H Arylation Reported by the Shi Group (Shi et al., 2015).237
Due to the importance of the bidentate DG strategy in transition-metal-catalyzed C(sp3)–H bond activation reactions, a variety of other N,N-bidentate, monoanionic DGs have also been developed. These N,N-bidentate, monoanionic DGs are similar to PA or AQ in that they connect through a native nitrogen or carboxylic acid, but they are easier to remove. In 2013, the palladium-catalyzed monoarylation of primary γ-C(sp3)–H bonds of amino acid methyl esters that contain the easily detachable N-(2-pyridyl)sulfonyl DG was reported by Carretero and co-workers (Scheme 81a).238 An unprecedented C(sp3)–H arylation of dipeptides was also demonstrated with this method. Notably, the N-(2-pyridyl)sulfonyl auxiliary can be removed in one step when treated with Zn powder at 60 °C in a THF/NH4Cl (aq.) mixture for 16 h, giving the free primary amine in good yield. In 2013, a 2-methoxyiminoacetyl (MIA)-directed, palladium-catalyzed γ-arylation of substituted 2-aminobutanates was reported by Ma and co-workers (Scheme 81b).239 The MIA auxiliary could be removed easily by hydrolysis with 1N KOH in 1,4-dioxane at room temperature. It could also be readily transformed into a glycine moiety, which could be used in peptide synthesis. A removable oxazoline-carboxylate auxiliary was developed by the Shi group for the direct arylation of γ-methylene C(sp3)–H bonds of amine derivatives (Scheme 81c).240 A wide range of aryl iodide reagents with different substituents were compatible under the reaction conditions. However, low diastereoselectivity was observed in the arylation of aliphatic γ-C(sp3)–H bonds with substrates that bear chiral oxazoline-carboxylate auxiliaries.
Scheme 81.
a) N-(2-Pyridyl)sulfonyl-Amide-Directed γ-C(sp3)–H Arylation of Amino Acid Derivatives (Carretero et al., 2013).238 b) MIA-directed Palladium-Catalyzed γ-C(sp3)–H Arylation of Substituted 2-Aminobutanates (Ma et al., 2013).239 c) Palladium-Catalyzed Amino Oxazoline-Carboxylate Directed γ-Methylene C(sp3)–H Arylation (Shi et al., 2015).240
Easily accessible, modular 1,2,3-triazole-type bidentate DGs, which are readily available through Cu(I)-catalyzed 1,3-dipolar cycloadditions, were first introduced by the Ackermann group for iron-catalyzed C–H arylation in 2014.241 The modular properties enabled the diverse synthesis of various N-substituted 1,2,3-triazole DGs. In 2015, Ding and co-workers developed a palladium-catalyzed methyl C(sp3)–H arylation of alanine with the assistance of N-hexyl 1,2,3-triazole amide bidentate DG (Scheme 82a).242 In 2018, the Ackermann group reported the palladium-catalyzed methylene C(sp3)–H arylation of phenylalanine derivative (484) with N-benzyl 1,2,3-triazole amide bidentate DG (Scheme 82b).243 An unprecedented methyl C(sp3)–H arylation of the internal triazole peptides was also achieved using 1,2,3-triazole amide as both bidentate DG and peptide bond isosteres. The Ackermann group also used a triazole-assisted C(sp3)–H arylation strategy to introduce BODIPY fluorescent dyes into peptides (Scheme 82c)244 and cyclobutanes (Scheme 82d).245
Scheme 82.
a) Pd-catalyzed Triazole-Directed Methyl C(sp3)–H Arylation of Alanine C(sp3)–H (Ding et al., 2018).242 b) Triazole-Directed Primary and Secondary C(sp3)–H Arylation of Phenylalanine and Internal Isosteric Triazole Peptides (Ackermann et al., 2018).243 c) C(sp3)–H Arylation for BODIPY Labeling of Peptides (Ackermann et al., 2018).244 d) Methylene C(sp3)–H Arylation for BODIPY Labeling of Cyclobutanes (Ackermann et al., 2019).245
A type of easily available bidentate amino oxazoline DGs were developed by the Shi group in 2015. Pd-catalyzed secondary C(sp3)–H arylation of aliphatic carboxamides directed by this modifiable amino oxazoline DG was investigated. When a phenylalaninol-derived chiral amino oxazoline DG was used, diastereoselective benzylic C(sp3)–H arylation proceed in moderate yields with good diastereoselectivity (Scheme 83a).246 In 2015, a diastereoselective, β-methylene C(sp3)–H bond arylation of cyclopropanes using an isoleucine-derived bidentate DG was developed by the Hong group (Scheme 83b).247 They concluded that the diastereoselectivity was determined under Curtin–Hammett control in the oxidative addition step because C–H activation at either diastereotopic hydrogen is relatively facile.
Scheme 83.
a) Amino Oxazoline-Directed γ-Methylene C(sp3)–H Arylation (Shi et al., 2015).246 b) Isoleucine-NH2 Directed C(sp3)–H Arylation Reported by the Hong Group (Hong et al., 2015).247
Other N,N-bidentate auxiliaries for C(sp3)–H arylation of aliphatic carboxylic acids, such as 5-methylisoxazole-3-carboxamide (Scheme 84a, MICA),248 4-amino-2,1,3-benzothiadiazole (Scheme 84b),249 2-methyl-7-aminobenzoxazole (Scheme 84c),250 and 2-dimethylaminoethylamine (Scheme 84d),251 have also been reported.
Scheme 84.
a) Isoxazole-Carboxamide Directed γ-C(sp3)–H Bond Activation of α-Aminobutanoic Acid Derivatives (Liu et al., 2015).248 b) 4-Amino-2,1,3-Benzothiadiazole Directed β-C–H Arylation/Oxygenation of Carboxamides (Babu et al., 2016).249 c) 2-Methyl-7-Aminobenzoxazole Directed β-C(sp3)–H Arylation (Zhou et al., 2016).250 d) Aliphatic Diamine Directed β-C(sp3)–H Arylation (Yu et al., 2018).251
Generally, methods for the C–H functionalization of saturated azaheterocycles are dominated by functionalization of the highly activated C–H bonds α to nitrogen or the C–H bonds on exocyclic alkyl groups. In 2016, Sanford and co-workers developed a palladium-catalyzed transannular C–H arylation of a variety of alicyclic amines at sites remote from nitrogen (Scheme 85a).252 An amide derived from p-CF3C6F4NH2 was found to be the most effective bidentate DG for activation of transannular secondary C(sp3)–H bonds over C–H functionalization at the β-methyl sites. The conditions are as follows: Pd(OAc)2 (5 mol%) with CsOPiv (3.0 equiv) in t-AmylOH at 130 °C for 18 h. The scope of the reaction is broad, tolerating a myriad of functional groups on the aryl iodide coupling partner, such as bromides, unprotected hydroxyl groups, and aldehydes. However, under standard conditions, only 12% yield of the corresponding C–H arylated product 512 was obtained with piperidine substrate 511. The yield was improved to 44% by increasing the temperature and removing the solvent. Aminal formation is observed in the reaction for both the starting material and the product due to proximal C–H amination, which allowed them to isolate 55% of the desired product upon work-up with NaBH4. This method affords a variety of arylated alicyclic amines thatwould be challenging to prepare by traditional synthetic routes. Computational evidence suggests that excess ArI (30 equiv) is required in making the oxidative addition step favorable by thermodynamically overcoming the high energy requirements to form the C–H activation intermediate.253
Scheme 85.
a) CONHArF (ArF = p-CF3C6F4) -Directed Transannular C(sp3)–H Arylation (Sanford et al., 2016).252 b) CONHArF (ArF = p-CF3C6F4)-Directed Transannular C(sp3)–H Arylation of Azabicycloalkanes Enabled by Monodentate Nitrogen Ligand (Sanford et al., 2018).254
In 2018, the Sanford group was able to lower the reaction temperature and reduce the equivalents of aryl iodides by employing pyridine-carboxylate (L65) and quinoline-carboxylate (L66) ligands that slow the decomposition of the catalyst (Scheme 85b).254
As a complement to the extensively studied N,N-bidentate DGs, N,O-bidentate DGs have also been explored as they likely facilitate the oxidation of Pd(II) to Pd(IV) because of increased electron density at the Pd(II) center. In 2015, Song, Niu, and co-workers disclosed a palladium-catalyzed β-methyl C(sp3)–H arylation directed by a 2-aminopyridine-1-oxide moiety that proceeds without using silver salt additives as is required in comparable N,N-bidentate directed reactions (Scheme 86a).255 At nearly the same time, the Lu group reported a palladium-catalyzed pyridine-N-oxide-directed β-methylene C(sp3)–H arylation (Scheme 86b).256 In this case, the addition of AgOAc significantly promoted this transformation and enabled the arylation of more challenging methylene C(sp3)–H bonds.
Scheme 86.
Palladium-Catalyzed 2-Aminopyridine-1-oxide Directed C–H Arylation: a) Methyl C(sp3)–H Arylation (Song et al., 2015).255 b) Methylene C(sp3)–H Arylation (Lu et al., 2015).256
The Zhao group developed an oxalyl amide as an N,O-bidentate DG for γ-C(sp3)–H arylation of both aliphatic amines (Scheme 87a)257 and amino acids derived from the amine units (Scheme 87b).258 A wide array of functional groups are tolerated under the reaction conditions. Although a range of bidentate DGs have been developed for the effective C(sp3)–H activation of carboxylic acids, the established reactions generally proceeded from the formation of five- or six-membered palladacycles and few examples occurred via seven-membered palladacycles. In 2016, Zhao and co-workers developed a new N,O-bidentate DG, glycine dimethylamide, which was efficient for β-C(sp3)–H arylation of aliphatic carboxylic acids with ortho-substituted aryl iodides, a type of very challenging coupling partners for C–H arylation. Notably, the glycine dimethylamide bidentate DG could promote a subsequent intramolecular C(sp2)–H amination to prepare 2-quinolinones through seven-membered palladation (Scheme 87c).259 In 2018, Maiti and co-workers used 3-amino-1-methyl-1H-pyridin-2-one as an N,O-bidentate DG for both β- and γ-C(sp3)–H arylation of carboxylic acid derivatives (Scheme 87d).260 This protocol is efficient for direct arylation of both β-methylene and γ-methyl C(sp3)–H bonds.
Scheme 87.
Palladium-Catalyzed C(sp3)–H Arylation Directed by N,O-bidentate DG. a) Oxalyl Amide-Directed γ-C(sp3)–H Arylation of Amines (Zhao et al., 2015).257 b) Oxalyl Amide-Directed γ-C(sp3)–H Arylation of Amino Acids (Zhao et al., 2016).258 c) Glycine Dimethylamide-Directed β-C(sp3)–H Arylation of Aliphatic Carboxylic Acids (Zhao et al., 2016).259 d) 3-Amino-1-methyl-1H-pyridin-2-one-Directed C(sp3)–H Arylation of Carboxylic Acids (Maiti et al., 2018).260
Inspired by the discovery that mono-N-protected amino acid ligands promote Pd(II)-catalyzed C(sp3)–H activation through initial N,O-bidentate coordination, Yu and co-workers reasoned that native amino acids embedded in a peptide backbone could also bind to Pd(II) and facilitate activation of C(sp3)–H bonds in the adjacent amino acid unit. As envisaged, arylation of C(sp3)–H bonds at the N-terminus of various peptides could indeed be accomplished through native directivity without the need to install an external auxiliary (Scheme 88a).261 Moreover, tri- and tetrapeptides could also be arylated with a wide range of aryl iodides to afford peptides with modified phenylalanine residues, which demonstrates the applicability of Pd-catalyzed directed C(sp3)–H activation strategy in peptide synthesis and late-stage modifications.
Scheme 88.
a) Amino-Acid-Backbone-Directed C(sp3)–H Arylation (Yu et al., 2014).261 b) Amino-Acid-Directed C(sp3)–H Arylation of α-Hydroxy Acid Derivatives (Yu et al., 2015).262
The initial investigation of the reaction conditions with N-phthaloyl protected dipeptide as a substrate revealed that by using hexafluoroisopropyl alcohol (HFIP) as solvent, full conversion of the substrate was observed. Further screening indicated that by removing KF, higher selectivity for monoarylation of the dipeptide at the expense of yield was achieved. A wide range of aryl iodides that possess electron-donating or -withdrawing groups at the ortho, meta, or para position are competent coupling partners, and the desired products were isolated in good yields.
Various amino acids both at the C-terminus and N-terminus have also been surveyed. When a tripeptide was subjected to the optimized conditions, the presence of a carboxylic acid at the C-terminus inhibited the arylation and provided only 20% of the desired product. To remedy this problem, the benzyl or methyl ester starting material was used, and the yield increased greatly. Remarkably, selective arylation of tetrapeptides at the N-terminus has also been realized through the minor modification of the reaction conditions. Based on the above results, the Yu group explored the functionalization of O-protected α-hydroxy aliphatic acid derivatives by utilizing an amino acid DG (Scheme 88b).262 A wide range of β-aryl-α-hydroxy acids were obtained in moderate to good yields without unwanted epimerization of the stereocenters.
In 2017, the Albericio263 and Wang264 groups independently described the synthesis of macrocyclic peptides by means of amino acid-backbone-directed, intramolecular β-C(sp3)–H arylation (Scheme 89). A variety of cyclic peptides were prepared using this C(sp3)–H arylation protocol in moderate to good yields, demonstrating the power of C–H activation strategy.
Scheme 89.
Amino-Acid-Directed C(sp3)–H Arylation for Preparing Macrocyclic Peptides. a) (Albericio et al., 2017),263 and b) (Wang et al., 2017).264
In 2017, Yu and co-workers discovered that 2,2-dimethyl aminooxyacetic acid is a competent bidentate auxiliary for β-C(sp3)–H arylation of ketones via Pd(OAc)2 catalysis (Scheme 90a). This directing auxiliary addresses many of the limitations in catalytic β-C(sp3)–H arylation of ketones and derivatives thereof, such as limited ability to funtionalize methylene C(sp3)–H bonds and narrow functional group tolerance.265 During the DG optimization process, the Yu group found that the presence of gem-dimethyl substitution at the 2-position of the aminooxyacetic acid drastically increases the yield from 10% to 80%, which is attributed to the Thorpe−Ingold effect.233 The utility of this transformation was demonstrated by direct C(sp3)–H (hetero)arylation of santonin. Extension of this protocol to remote γ-C(sp3)–H functionalization of ketones, however, was unsuccessful. Based on the proposed transition state, Yu and colleagues reasoned that an X-type ligand that displaces the acetate could accelerate the C–H activation step of γ-C(sp3)–H functionalization. After extensive screening of 2-pyridone ligands, 2-pyridone (L67) bearing a nitro group at the 5-position was found to improve the yield to 70% in conjunction with the 2,2-dimethyl aminooxyacetic acid bidentate auxiliary (Scheme 90b).266 Yu and co-workers also developed a pyruvic acid-derived DG for activation of γ-C(sp3)–H bonds in preference to the β-C(sp3)–H bonds of aliphatic alcohol-derived substrates (Scheme 90c).267 The design principle for this DG was to destabilize the [5,5]-fused ring system compared to the alternative [5,6]-fused ring system by introducing a C–C double bond into the bicyclic structure, which increases ring strain in the former, thereby favoring six-membered cyclopalladation. Electron-deficient 2-pyridone ligands are required for this transformation as they stabilize the palladium catalyst and lower the transition-state energy for the C–H activation step. With the newly designed DG and optimized ligand (L68), arylation of both primary and secondary γ-C(sp3)–H bonds could be achieved on acyclic and cyclic alcohols even in the presence of β-C(sp3)–H bonds. A subsequent computational study reported by Dang and co-workers proposes an “outer-sphere” mechanism that favours six-membered cyclopalladation, which contrasts to the often considered “inner-sphere” process that proceeds through a five-membered cyclopalladium intermediate.268 By changing the [5,6]-fused palladacycle to the [6,5]-fused palladacycle using dichloro-substituted salicylic aldehydes as the DG, arylation of secondary β-C(sp3)–H bonds of aliphatic alcohols was realized by Yu and co-workers in 2020 (Scheme 90d).269 2-Pyridone ligands remain the best choice for this transformation and an electron-deficient pyridine (L69) was found to be the optimal.
Scheme 90.
a) Palladium-Catalyzed β-C(sp3)–H Arylation of Ketones Directed by 2,2-Dimethyl Aminooxyacetic Acid (Yu et al., 2017).265 b) Ligand-Enabled γ-C(sp3)–H Arlation of Ketones Directed by 2,2-Dimethyl Aminooxyacetic Acid (Yu et al., 2018).266 c) Ligand-Enabled γ-C(sp3)–H Arylation of Aliphatic Alcohols Directed by Pyruvic Acid-Derived DG (Yu et al., 2019).267 d) Ligand-Enabled β-C(sp3)–H Arylation of Aliphatic Alcohols Directed by Dichloro-Substituted Salicylic Aldehyde Derived DG (Yu et al., 2020).269
N-Pentafluorophenyl-pyruvamide-directed, palladium(II)-catalyzed primary β-C(sp3)–H arylation of aliphatic alcohols was reported by the Xu group in 2019 (Scheme 91a).270 Later, N-(3,5-ditrifluoromethylphenyl) pyruvamide was developed for both an intra- and intermolecular cross dehydrogenative-coupling (CDC) reaction between primary β-C(sp3)–H bonds of aliphatic alcohols and C(sp2)–H bonds of arenes (Scheme 92b).271
Scheme 91.
Palladium-Catalyzed β-C(sp3)–H Arylation of Alcohols (Xu et al., 2019).270,271
Scheme 92.
a) Pd-Catalyzed β-Methyl C(sp3)–H Monoarylation of 2-Methylthioanilide Amides (Daugulis et al., 2010).181 b) β-Methyl C(sp3)–H Monoarylation of N-Phthaloylalanine Derivatives (Daugulis et al., 2012).196 c) 2-Thiomethylamide Directed β-C(sp3)–H Arylation of Cyclopropanes (Babu et al., 2016).202 d) 2-Thiomethylamide Directed β-C(sp3)–H Arylation/Ring Opening of Cyclopropanes (Babu et al., 2016). 272
N,S-Bidentate DGs were pioneered by Daugulis and co-workers in 2010 when they observed that selective monoarylation of primary C(sp3)–H bonds in aliphatic acid substrates can be realized by using a 2-thiomethylthioaniline auxiliary, which provided improved selectivity in this reaction compared to 8-aminoquinoline (Scheme 92a).181 By employing the same DG, the monoarylation of the primary β-C(sp3)–H bond of N-phthaloylalanine derivatives under relatively mild conditions was demonstrated by Daugulis and co-workers in 2012 (Scheme 92b).196 Notably, the DG can be removed under Lewis acidic conditions with BF3·Et2O in MeOH at 100 °C to afford the corresponding methyl ester. The Babu group reported a palladium-catalyzed, 2-thiomethylaniline directed β-C(sp3)–H arylation of cyclopropane (Scheme 92c)202 which is followed by ring opening of cyclopropane when acetic acid was used as the co-solvent (Scheme 92d).272
Baran and co-workers reported concise total syntheses of piperarborenine B and piperarborenine D using N,S-bidentate-auxiliary directed, palladium-catalyzed C(sp3)–H arylation of cyclobutane as the key strategy (Scheme 93).273 By using 2-thiomethylaniline as the DG, it was found that 571 could be converted into the desired product 573 in gram scale using Pd(OAc)2 as the catalyst with the aid of PivOH and Ag2CO3 in HFIP at 90 °C for 36 h. Then, different pathways were utilized to access each of the desired natural products from intermediate 573. In the first reaction pathway, after epimerization of the ester moiety, a second C(sp3)–H arylation of 574 with 4-iodo-1,2-dimethoxybenzene (575) was performed under similar conditions to afford the arylated product 550 in 81% yield with complete stereoselectivity. In the second reaction pathway, after epimerization of the amide moiety, the second C(sp3)–H arylation of 578 with 575 was carried out under similar conditions to give the arylated product 579 in 46% yield also with complete stereoselectivity. Both the DG and the ester moiety were hydrolyzed, and condensation with dihydropyridone then completed the synthesis of piperarborenine D (577) and piperarborenine B (580), respectively. Based on the same strategy, the Baran group also achieved the synthesis and structural revision of several piperarborenine family natural products via C–H activation logic using 2-thiomethylaniline as the DG.236
Scheme 93.
2-Thiomethylaniline-Directed Sequential C(sp3)–H Arylation of Cyclobutane as a Key Step in the Total Synthesis of Piperarborenine Natural Products (Baran et al., 2011).273
The oxidized form of 2-(methylthio)aniline, 2-methylsulfinyl aniline (MSOA), can be employed as an auxiliary for β-methyl and methylene C(sp3)–H bond arylation with aryl iodides. In 2016, the Colobert group designed a chiral N,S-bidentate auxiliary for the palladium-catalyzed, diastereoselective C(sp3)–H arylation of cyclopropanes (Scheme 94a).274 Surprisingly, by treating starting material 581 with Pd(OAc)2 in acetonitrile with pyridine (2.2 equiv) an unprecedented, pyridine-stabilized, C(sp3)–H activated palladacycle intermediate 582 was obtained in a 60:40 d.r. with the sterically less demanding complex being slightly more prevalent. They hypothesized that this may explain the overall diastereoselectivity that improves when the bulkier sulfinyl auxiliary with the t-Bu group is used. In 2017, Colobert and co-workers used the same strategy to achieve the diastereoselective arylation of methylene C(sp3)–H bonds of linear aliphatic carboxamides with up to 9:1 diastereoselectivity (Scheme 94b).275 In the same year, MSOA was used as a DG for β-C(sp3)–H arylation of linear aliphatic amides with sterically hindered aryl iodides by He, Chen, et al. (Scheme 94c).276
Scheme 94.
Palladium-Catalyzed Chiral 2-Methylsulfinyl Aniline-Directed Diastereoselective C(sp3)–H Arylation of a) Cyclopropane (Colobert et al., 2016).274 b) Linear Aliphatic Carboxamides (Colobert et al., 2017).275 c) Palladium-Catalyzed 2-Sulfinylaniline-Directed C(sp3)–H Arylation with Sterically Hindered Aryl Iodides (He, Chen et al., 2017).276
Palladium-catalyzed enantioselective C(sp3)–H functionalization using strongly coordinating bidentate DGs remained largely undeveloped until the mid-2010s, largely due to that the racemic background reactions could easily occur in the absence of chiral ligands and the lack of available coordination sites around the metal to coordinate a chiral ligand. The pioneering example of a Pd-catalyzed, 8-aminoquinoline-directed, enantioselective methylene C(sp3)–H arylation of aliphatic carboxylic amides using phosphoric amide L70 as chiral ligand was reported by Duan in 2015 (Scheme 95a).277 The arylation of benzylic methylene C(sp3)–H bonds was acheived in good yields with good enantioselectivities (up to 91:9 er). However, when this protocol was applied to more challenging unbiased methylene C(sp3)–H bonds, both yield and ee value reduced dramatically (R = nPr, 68%, 26% ee; R = iPr, 20%, 28% ee). A series of mechanistic experiments indicated that the reaction rate is accelerated by the addition of chiral phosphoric amides or acids. They proposed that an enantioenriched intermediate 591 is formed by cleaving one of the two enantiotopic β-C–H bonds of 592 with the assistance of chiral phosphoric amide ligand L70. In 2018, the Chen group reported a Pd(0)-catalyzed, enantioselective benzylic methylene C(sp3)–H arylation of 3-arylpropanamides with the BINOL phosphoramidite (PIII) ligand L71 (Scheme 95b).278 This represents the first Pd(0)-catalyzed enantioselective C(sp3)–H arylation reaction employing bidentate DGs.
Scheme 95.
a) Pd(II)-Catalyzed 8-Aminoquinoline-Directed Enantioselective Arylation of Methylene C(sp3)–H Bonds Using Phosphoric Amide (Duan et al., 2015).277 b) Pd(0)-Catalyzed 8-Aminoquinoline-Directed Enantioselective Benzylic C–H Arylation Using Phosphoramidite (Chen et al., 2018).278
In 2016, Chen and co-workers reported an enantioselective, benzylic C–H arylation reaction directed by picolinamide (PA) (Scheme 96).279 The high enantioselectivity was enabled through the combination of the 2,2’-dihydroxy-1,1’-binaphthyl (BINOL) phosphoric acid ligand and Cs2CO3 under neat conditions. Both yield and enantioselectivity are significantly influenced by the alkali cation. A nonlinear effect with respect to the enantiopurity of the ligand was also observed. Based on these mechanistic investigations, the authors proposed the intermediacy of a Cs complex that contains two molecules of L73 in the stereo-determining C(sp3)–H palladation step.
Scheme 96.
Picolinamide-Directed Enantioselective Benzylic C(sp3)–H Arylation (Chen et al., 2016).279
While these examples demonstrated that significant progress has been made in the enantioselective functionalization of benzylic C(sp3)–H bonds, realizing an analogous process at completely unactivated methylene positions directed by strongly coordinating bidentate DGs remains challenging and exclusive. In 2018, the Shi group addressed this challenge by taking advantage of their own developed 2-(pyridine-2-yl)isopropyl (PIP) amine DG. They demonstrated the palladium(II)-catalyzed highly enantioselective arylation of unbiased β-methylene C(sp3)–H bonds by the combination of PIP bidentate DG with a non-C2-symmetric, monodentate chiral phosphoric acid (CPA) ligand (L74) (Scheme 97).280 Investigation of a variety of structurally diverse ligands revealed that fluorine-containing substituents could improve the enantioselectivity. Aryl bromide electrophiles that contain either electron-donating or electron-withdrawing substituents are tolerated, generating enantioenriched, β-arylated carboxylic acid derivatives in good to excellent yields. Notably, this is the first time that more readily available and relatively nonactivated aryl bromides were used as aryl reagents in Pd(II)-catalyzed enantioselective C–H arylation. Examination of various directing auxiliaries other than the PIP group revealed that replacement of the gem-dimethyl substituents with longer chains or adding substituents on the pyridyl ring results in lower yield and enantioselectivity. The linear correlation between ees of product and L74 indicated that only one chiral ligand was involved in the stereodetermining C–H cleavage step. They hypothesized that the high stereoinduction compared with other bidentate auxiliaries might be attributable to steric communication between the gem-dimethyl unit and the ligand.
Scheme 97.
Pd(II)-Catalyzed PIP-Directed Enantioselective Arylation of Unbiased Methylene C(sp3)–H Bonds with Aryl Bromides (Shi et al., 2018).280
2.2.1.2. Palladium-Catalyzed C(sp3)–H Arylation Using Transient DGs
While the introduction of a properly designed directing group can exhibit remarkable efficiency and chemoselectivity for C(sp3)–H activation reactions, as described above in this review, the covalently attached mono- or bidentate directing group must be used in stoichiometric amounts and necessitate concession steps for installation and removal. One approach to circumvent these limitations, as covered earlier, is through the use of a native chemical functional group in conjunction with an optimized catalyst and/or ligand. This approach, however, is neither universally successful nor universally applicable. Thus, an attractive complementary approach in which a well-designed “transient DG” can reversibly link to the substrate to direct the C–H functionalization and in situ deconstruction to release.281 Thus, these so called “transient DGs” could be used in catalytic amounts as their addition and removal happens during the reaction typically through passive means, such as condensation.282–287
In 2016, Yu and co-workers pioneerly reported a palladium-catalyzed C(sp3)–H arylation with catalytic amounts of a transient DG (Scheme 98a).288 Based on the development of the mono-N-protected amino acid ligands and the earlier use of amino acids as bidentate DGs in the C–H functionalization of peptides,261 they reasoned that catalytic quantities of the amino acid could be reversibly tethered to an aldehyde or ketone substrate via an imine linkage under the appropriate conditions. Once condensed, the imine moiety and the carboxylate could coordinate to the catalyst in a bidentate fashion to facilitate a subsequent C–H functionalization. The initially reported system uses glycine (40 mol%) as the transient DG, Pd(OAc)2 (10 mol%) as the catalyst, and AgTFA (1.5 equiv) as the additive in a 9:1 solvent mixture of AcOH and H2O at 90 °C for 36–48 h. In a control experiment, they found that when N-protected glycine was used, no conversion was detected. This result indicates that the formation of imine is essential for the reaction to proceed. Under the reaction conditions, the scope of aryl iodides and 2-methylbenzaldehyde was examined, and a variety of functional groups were found to be tolerated. The use of sterically hindered ortho-substituted aryl iodides leads to reduced yields. Aliphatic ketone substrates were investigated using the same strategy, and the optimal conditions for this transformation are as follows: glycine (50 mol%) and a 3:1 mixture of HFIP:AcOH as solvent at 110 °C for 36 h. A wide range of aryl iodides with electron-donating or -withdrawing substituents were well tolerated under the modified conditions. Notably, methylene C(sp3)–H bonds in cyclic substrates could also be activated, producing the corresponding syn products with excellent diastereoselectivity. γ-C(sp3)–H arylation was observed when β-C(sp3)–H bonds were absent in aliphatic ketone. Unfortunately, aliphatic aldehydes were incompatible with this protocol, due to the decomposition of the starting materials. Finally, benzaldehydes bearing methylene C(sp3)–H bonds were investigated using a chiral amino acid as transient DG to carry out enantioselective C–H arylation. By using L-tert-leucine in place of glycine, excellent enantioselectivity was achieved. Moreover, the yield of the arylated product was significantly improved by changing the ligand-to-Pd ratio from 4:1 to 2:1. Density functional theory calculations revealed that a six-membered transition state is favored for in the benzylic C(sp3)–H activation because of higher ring strain in the five-membered transition state for the alternative ortho-aryl C(sp2)–H activation process.289 The C(sp3)–H activation step was found to take place via a concerted metalation−deprotonation mechanism. Later, primary benzylic C(sp3)–H arylation using transient DGs has also been disclosed by Hu (Scheme 98b) 290 and Li (Scheme 98c).291
Scheme 98.
Pd-Catalyzed C(sp3)–H Arylation of 2-Alkylbenzaldehydes via Transient DG Strategy. a) Glycine and L-tert-Leucine as TDGs (Yu et al., 2016).288 b) Acetohydrazone as TDG (Hu et al., 2016).290 c) Semicarbazide as TDG (Li et al., 2020).291
In 2017, Pd-catalyzed β-methylene C(sp3)–H arylation of aliphatic ketones using α-benzyl β-alanine (TDG5) as a catalytic TDG was reported by the Yu group (Scheme 99a).292 β-Amino acid was used as a transient DG to generate a [5,6]-fused palladacycle, which promotes methylene C–H activation. In 2018, Wei and co-workers reported primary β-C(sp3)–H arylation of aliphatic ketones using 2-amino-N-isopropylacetamide (TDG6) as a transient DG (Scheme 99b).293 β- Methylene C(sp3)–H arylation of cyclic ketones was also amendable, giving the corresponding syn-products with excellent diastereoselectivity. The enantioselective β-methylene C(sp3)–H arylation of cyclobutyl ketones was achieved by the Yu group in 2020 (Scheme 99c). D-Valine (TDG7) was used as the chiral transient DG and 5-nitropyridone (L75) was used as the ligand.294 However, the substrate scope was limited to α-cyclobutyl ketones. When cyclopentyl ketone and linear aliphatic ketones were used, low enantioselectivity was obtained.
Scheme 99.
Pd-Catalyzed β-C(sp3)–H Arylation of Ketones via Transient DG Strategy. a) Aliphatic Methylene C(sp3)–H Arylation by α-Benzyl β-Alanine as TDG (Yu et al., 2017).292 b) Methyl and Cyclic Methylene C(sp3)–H Arylation by 2-Amino-N-isopropylacetamide as TDG (Wei et al., 2018).293 c) Enantioselective C(sp3)–H Arylation of Cyclobutyl Ketones by D-Valine as TDG (Yu et al., 2020).294
Palladium-catalyzed C(sp3)–H arylation of aliphatic aldehydes enabled by a transient DG was reported by Ge and co-workers in 2016 (Scheme 100a). 3-Aminopropanoic acid (TDG8) was discovered as a novel catalytic transient DG for β-C(sp3)–H arylation of aliphatic aldehydes.295 All of the arylation reactions of aliphatic aldehydes discussed thus far are limited to β-C(sp3)–H bonds. In 2020, Ge and co-workers developed the first example of γ-C(sp3)–H arylation of aliphatic aldehydes with L-phenylalanine (TDG9) as a transient DG and 3-nitro-5-(trifluoromethyl)pyridin-2-ol (L69) as a ligand (Scheme 100b).296 The Bull group also discovered examples C(sp3)–H arylation via transient DG strategy, including intramolecular C(sp3)–H arylation of tertiary aldehydes with 2-methoxyethan-1-amine (TDG10) (Scheme 101a)297 and diarylation of cyclohexanecarbaldehydes with t-Bu-amide (TDG11) (Scheme 101b).298
Scheme 100.
a) Site-selective β-C(sp3)–H Arylation of Aliphatic Aldehydes (Ge et al., 2016).295 b) Site-Selective γ-C(sp3)–H Arylation of Aliphatic Aldehydes (Ge et al., 2020).296
Scheme 101.
a) Intramolecular C(sp3)–H Arylation of Tertiary Aldehydes (Bull et al., 2019).297 b) Methylene β-C(sp3)–H Arylation of Cyclohexanecarbaldehydes (Bull et al., 2020).298
Meanwhile, the development of TDGs for C(sp3)–H functionalization of aliphatic amines has also been reported. In 2016, Dong and co-workers reported the use of an exo-imine-based bidentate DG generated in situ from the reaction of an aliphatic amine substrate with stoichiometric amounts of 8-formylquinoline (TDG12) for facilitating γ-C(sp3)–H functionalization (Scheme 102a).299 The pyridine base (L77) was employed to neutralize the HBF4 that is produced as the reaction proceeds. A γ-C(sp3)–H activated palladacycle (628) was isolated and characterized by X-ray crystallography, which sheds light on the mechanism of the reaction.
Scheme 102.
γ-C(sp3)–H Arylation of Free Amines Enabled by Transient DG strategy. In Situ Formed exo-Imine-Based Bidentate DG (Dong et al., 2016).299
In 2016, 2-hydroxynicotinaldehyde (TDG13) was reported as a catalytic transient DG for γ-C(sp3)–H arylation of aliphatic amines by the Yu group (Scheme 103a).300 It is noteworthy that when the reaction was conducted in 2 mmol scale, the loading of the transient DG could be lowered to 4%, showing the high efficiency and robustness of this protocol. Thus far, C(sp3)–H activation of amine derivatives largely limited to the functionalization of γ-position through a kinetically favoured five-membered palladacycle intermediate. The Yu group successfully overcome these limitations to enable the switchable γ-C(sp3)–H arylation of free amines using a transient DG. This transformation was then extended to γ-methylene and δ-methyl C(sp3)–H bonds in 2018 through the development of new ligands and transient DGs (Scheme 103b).301 The driving force for the switchable selectivity might be attributable to the preferred formation of less strained [5,6]-bicyclicpalladacycle containing double bond rather than a [5,5]-bicyclicpalladacycle.267 Specifically, the 6-chloro-substituted hydroxybenzaldehyde (TDG14) favors a six-membered coordination with palladium(II) catalyst to enable γ-C(sp3)–H cleavage, forming a [6,5]-bicyclicpalladacycle intermediate with less strain. While 2-methoxy-substituted glyoxylic acid (TDG15) favors a five-membered coordination with palladium(II) catalyst to enable δ-C(sp3)–H cleavage, forming a [5,6]-bicyclicpalladacycle intermediate forms a five-membered palladium complex. Notably, a broad range of medicinally important heteroaryl iodides were compatible with this protocol. The use of quinolone-based ligands significantly improved reaction efficiency. When using the combination of hydroxybenzaldehyde (TDG13) and pyridine ligand (L76), even typically unreactive heteroaryl bromides could be used as coupling partners in the heteroarylation of cyclohexylamine (633a).
Scheme 103.
a) Pd-Catalyzed γ-C(sp3)–H Arylation of Free Amines Using 2-Hydroxynicotinaldehyde as TDG (Yu et al., 2016).300 b) Ligand-Enabled Pd-Catalyzed γ- and δ- C(sp3)–H (Hetero)arylation of Amines with TDGs (Yu et al., 2018).301
In 2017, the Pd(OAc)2-catalyzed C(sp3)–H arylation of primary aliphatic amines enabled by a transient DG was reported by Ge and co-workers. Glyoxylic acid (TDG16) was developed as a novel catalytic transient DG for γ-C(sp3)–H arylation of primary aliphatic amines (Scheme 104a).302 By treating the tert-amylamine with glyoxylic acid (1 equiv), Pd(OAc)2 (1 equiv), and pyridine (1 equiv) in HFIP at 100 °C, the cyclopalladated intermediate 640 was obtained. The palladacycle 640 could be transformed to the corresponding arylation product 638 under the standard conditions. A Pd(II)−Ag(I) heterodimeric complex was observed by Dang and co-workers, which emphasized the importance of silver(I) carboxylate additive in lowering the activation barrier of the C(sp3)–H activation transition state (Scheme 104b).303
Scheme 104.
a) C(sp3)–H Arylation of Free Amines Using Glyoxylic Acid as TDG (Ge et al., 2017).302 b) [5,5]-Fused Palladacycle Characterized by the Dang Group (Dang et al., 2019).303
In 2017, the Murakami group used a sterically modified salicylaldehyde (TDG17) that could be conveniently condensed onto an aliphatic amine substrate to promote γ-C(sp3)–H arylation and then be fully removed under acidic conditions after the reaction is complete (Scheme 105a).304 While the Murakami group uses stoichiometric loadings of the modified salicylaldehyde TDG, they also demonstrate that a loading of 20 mol% also provides their desired product, albeit in diminished yield but with around 3 turnovers of the TDG, suggesting that dual catalytic cycles with the Pd and TDG could be envisioned. In 2018, the Bull group reported a primary γ-C(sp3)–H arylation of free amines using an alkyl acetal (2,2-dimethoxyethoxy)benzene (TDG18) as a transient DG in catalytic amount (Scheme 105b).305 γ-C(sp3)–H arylation of free amino esters employing 2-hydroxynicotinaldehyde (TDG13) as a DG with an acid workup using SOCl2 in EtOH to recover all of the products was reported by Kamenecka and co-workers in 2018 (Scheme 105c).306
Scheme 105.
a) Sterically Modified Salicylaldehyde (Murakami et al., 2017).304 b) Alkyl Acetal (2,2-Dimethoxyethoxy)benzene (Bull et al., 2018).305 c) 2-Hydroxynicotinaldehyde (Kamenecka et al., 2018).306
2.2.1.1.2. C(sp3)–H Arylation via Pd(II)/Pd(0) Catalysis
Palladium-catalyzed, bidentate DG directed C(sp3)–H arylation via Pd(II)/Pd(0) catalytic systems are relatively undeveloped. In 2018, Li and co-workers designed a weak N,O-bidentate DG for β-C(sp3)–H arylation through a Pd(II)/Pd(0) catalytic cycle enabled by APAO ligand (L37) (Scheme 106).307 Preliminary results on enantioselective β-C(sp3)–H arylation via gem-dimethyl desymmetrization of isobutyramide has also been demonstrated by using L37 as chiral ligand, giving monoarylated product in 43% yield with 73:27 er.
Scheme 106.
Palladium-Catalyzed N,O-Bidentate DG Directed β-C(sp3)–H Arylation with Aryltrifluoroborates.307
2.2.1.2. Alkenylation
A bidentate-DG-directed, palladium-catalyzed γ-C(sp3)–H alkenylation was reported by Chen and co-workers in 2011 in the functionalization of aliphatic amine derivatives (Scheme 107a).221 The optimized reaction conditions were as follows: alkenyl iodide (1.5 equiv), Pd(OAc)2 (10 mol%), and AgOAc (1.5 equiv) in t-BuOH at 110 oC for 20 h. The addition of benzoquinone led to a slight increase in yield. In 2015, Shi and co-workers used alkenyl bromide compounds as coupling partners in a C(sp3)–H alkenylation reaction with picolinimide DG (Scheme 107b).237 Even in the presence of primary β-C(sp3)–H bonds, alkenylation took place exclusively at the secondary C(sp3)–H bonds on the cyclic ring system. Both electron-rich and electron-deficient alkenyl bromides gave the corresponding products in modest yields.
Scheme 107.
a) Picolinamide-Directed C(sp3)–H Alkenylation (Chen et al., 2011).221 b) Picolinimide-Directed C(sp3)–H Alkenylation (Shi et al., 2015).237
In 2012, the Baran group reported the use of an aminoquinoline-directed, palladium-catalyzed C(sp3)–H alkenylation method to introduce an alkene moiety onto a cyclobutane core, thereby preparing a key intermediate in the synthesis of pipercyclobutanamide A (Scheme 108a).192 In 2014, the synthesis of β-olefinated α-amino acid was reported by the Chen group via a palladium(II)-catalyzed, aminoquinoline-carboxamide-directed γ-C(sp3)–H alkenylation (Scheme 108b).308 After extensive screening, the combination of 3 equiv AgOAc and 2 equiv TFA at room temperature in dioxane was found to give the desired products in good yields. Switching the solvent to a biphasic solvent system of TCE/H2O (1:1) and elevating the reaction temperature to 65 °C led to improved yields. Alkenyl iodide electrophiles that bear various functional groups could be coupled to the alanine-derived substrate in a stereoretentive-retention fashion at room temperature. Moreover, the olefinated products could be transformed to γ-lactams in excellent yield and with high diastereoselectivity (d.r.>15:1) upon treatment with 1.2 equiv of 4-dimethylaminopyridine (DMAP) in MeOH at room temperature for 4 h.
Scheme 108.
8-Aminoquinoline-Directed C(sp3)–H Olefination a) As a Key Step in the Total Synthesis of Pipercyclobutanamide A (Baran et al., 2012).192 b) Phthaloyl Alanine with Alkenyl Iodide Reagents (Chen et al., 2014).308 c) Acyclic Aliphatic Amides (Rao et al., 2015).309 d) As a Key Step in the Total Synthesis of (+)-Psiguadial B (Reisman et al., 2016).310 e) Cyclopropanes (Shuto et al., 2019).311
In 2015, a palladium-catalyzed, β-methylene C(sp3)–H alkenylation of acyclic aliphatic amides was developed by Rao and co-workers (Scheme 108c).309 A range of alkenyl iodides, such as 3-iodo-α, β-unsaturated ketones and (E)-β-aryl vinyl iodides, worked well under the optimized conditions. They also discovered that the product distribution was significantly affected by solvent effects. While toluene afforded solely the β-C(sp3)–H alkenylation product, an additional δ-C(sp2)–H alkenylation was observed when the reaction was performed in MeCN. In addition, Z/E isomerization occurred when (Z)-(2-iodovinyl)-benzene was employed as the alkene reagent, potentially via an allyl-type Pd(IV) intermediate. In 2016, the Reisman group employed 8-aminoquinoline-directed, palladium(II)-catalyzed C(sp3)–H alkenylation as a key step in the total synthesis of (+)-Psiguadial B (Scheme 108d).310 Palladium(II)-catalyzed C(sp3)–H alkenylation of cyclopropanes directed by 8-aminoquinoline DG was also reported by the Shuto group in 2019 (Scheme 108e).311 In 2018, the Yu group reported a preliminary result on γ-C(sp3)–H alkenylation of ketones by using the previously discussed 2,2-dimethyl aminooxyacetic acid auxiliary (Scheme 109).263
Scheme 109.
γ-C(sp3)–H Alkenylation of Aliphatic Ketones with Alkenyl Iodide (Yu et al., 2015).263
In 2020, both the Liu and the Ackermann groups took advantage of C(sp3)–H alkenylation transformations to develop methods for palladium(II)-catalyzed β-C(sp3)–H glycosylation of amino acids and peptides. 8-Aminoquinoline was employed by Liu and co-workers as a DG to enable the glycosylation of various N-phthaloyl α-amino acids (Scheme 110a).312 The Ackermann group used triazolyldimethylmethyl (TAM) as their main DG and was also able to employ 8-aminoquinoline as a DG under slightly altered conditions to achieve the glycosylation of various N-phthaloyl α-amino acids (Scheme 110b).313 Besides, an unprecedented late-stage glycosylation of internal peptides directed by TAM was also achieved. The silver salt Ag2CO3 was used by both groups. Notably, this is the first time glycals were used as coupling partners for β-C(sp3)–H functionalization of amino acids and peptides.
Scheme 110.
Bidentate DG-Directed C(sp3)–H Glycosylation with 1-Iodoglycals a) N-Phthaloyl α-Amino Acids (Liu et al., 2020).312 b) N-Phthaloyl α-Amino Acids and Internal Peptides (Ackermann et al., 2020).313
2.2.1.3. Alkynylation
In 2011, the Chatani group demonstrated the first palladium(II)-catalyzed alkynylation of unactivated C(sp3)–H bonds (Scheme 111a).314 A variety of aliphatic carboxylic amide derivatives were successfully alkynylated with a TIPS-protected bromoalkyne electrophile directed by 8-aminoquinoline DG. In 2014, one example of palladium(II)-catalyzed, 8-aminoquinoline-directed β-C(sp3)–H alkynylation of N-phthaloyl alanine was reported by the Chen group (Scheme 111b).308 In 2015, an example of palladium(II)-catalyzed diastereoselective C(sp3)–H alkynylation of cyclopropane was reported by the Hong group using an isoleucine-derived amide chiral bidentate DG (Scheme 111c).247 The Shi group reported in 2016 a comprehensive study of a silver-free C(sp3)–H alkynylation of N-phthaloyl amino acids and aliphatic acids directed by 1,2,3-triazole amine (TAM) (Scheme 111d).315 In 2017, Yu and co-workers reported a palladium(II)-catalyzed C(sp3)–H alkynylation of oligopeptides with various sterically bulky alkynyl bromides (Scheme 111e).316 The addition of tetrabutylammonium acetate (NBu4OAc) dramatically increased the yield. It was proposed that the acetate anion in NBu4OAc enables the C–H activation while the quaternary ammonium cation stabilizes the high-valent palladium intermediate during the C–H activation step.
Scheme 111.
Palladium-Catalyzed β-C(sp3)–H Alkynylation a) Aliphatic Amides with 8-Aminoquinoline DG (Chatani et al., 2011).314 b) N-phthaloyl alanine with 8-Aminoquinoline DG (Chen et al., 2014).308 c) Diastereoselective Alkynylation of Cyclopropane With an Isoleucine-Derived Amide Chiral DG (Hong et al., 2015.247 d) N-Phthaloyl Amino Acids and Aliphatic Acids Directed by 1,2,3-Triazole Amide (Shi et al., 2016).315 e) Alanine and Oligopeptides (Yu et al., 2017).316
In 2018, the Balaraman group reported a palladium-catalyzed, amide-directedγ-C(sp3)–H alkynylation of aliphatic amines.317 It is noteworthy that a variety of N,N-bidentate DGs related to picolinamide bearing pyrazine, quinoline, or isoquinoline heterocycles, were found to be effective in promoting the reaction. Both cyclic and linear amine substrates could be successfully alkynylated in good yields (Scheme 112).
Scheme 112.
Palladium-Catalyzed γ-C(sp3)–H Alkynylation of Aliphatic Amines (Balaraman et al., 2018).317
In 2019, the first Pd-catalyzed enantioselective alkynylation of unbiased methylene C(sp3)–H bonds was reported by Shi and co-workers using 3,3′-fluorinated-BINOL as the chiral ligand and PIP as DG (Scheme 113).318 A broad range of aliphatic amides were well tolerated, giving the alkynylated products in good yields with high enantioselectivities (up to 96% ee). Control experiments revealed that the use of the 8-aminoquinoline DG in place of PIP-NH2 led to lower yield and enantioselectivity under identical conditions (36%, 48% ee). A positive nonlinear effect revealed that the stereodetermining C–H palladation step may be influenced by multiple ligands. A dramatic ligand acceleration was observed, which might attribute to the unprecedented high stereocontrol.
Scheme 113.
Pd(II)-Catalyzed Enantioselective Alkynylation of Unbiased Methylene C(sp3)–H Bonds (Shi et al., 2019).318
2.2.1.4. Alkylation
Compared to aryl halides, alkyl halides are resistant to oxidative addition and susceptible to base-induced elimination. Provided that they are able to undergo oxidative addition, the resulting alkylmetal intermediates are prone to β-hydride elimination. Thus, the alkylation of C(sp3)–H bonds is an inherently challenging family of reactions to develop, and progress on this front is slow. Pioneering work on bidentate-DG-directed palladium-catalyzed C(sp3)–H bond alkylation was reported by Daugulis and co-workers in 2010 (Scheme 114a).181 Two examples of alkylation of β-methyl C(sp3)–H bonds of aminoquinolyl propanamide (707) were demonstrated with branched and linear primary alkyl iodides as coupling partners (e.g. i-butyl iodide and n-octyl iodide). 8-Aminoquinoline-directed alkylation of amino-acid-derived substrates was also developed by the Daugulis group in 2012.196
Scheme 114.
8-Aminoquinoline-Directed C(sp3)–H Alkylation a) A Pioneering Study (Daugulis et al., 2010).181 b) Methylene C(sp3)–H Alkylation with α-Haloacetates and Methyl Iodide (Chen et al., 2013).319 c) C(sp3)–H Alkylation of N-Phthaloyl α-Amino Acids (Shi et al., 2013).320
Subsequently, the Chen group expanded upon this Pd-catalyzed alkylation to functionalize unactivated β-methylene C(sp3)–H bonds of aminoquinolyl aliphatic carboxamides with α-haloacetate and methyl iodide (Scheme 114b).319 This protocol was applied to the alkylation of β-methylene position of N-phthaloyl α-amino acids with 2 equiv of α-haloacetate or MeI in good to excellent yields and high diastereoselectivities. Deuterium-labelled methyl iodide was also employed to afford the isotopically enriched amino acids.
The Shi group independently reported the Pd-catalyzed C(sp3)–H alkylation of N-phthaloyl α-amino acids directed by 8-aminoquinoline under mild conditions (Scheme 114c).320 The alkylation of N-phthaloyl alanine with a wide range of primary alkyl iodides that encompass various length alkyl chains and synthetically useful functional groups was achieved, affording the desired alkylation products in good to high yields. Notably, this reaction also represented the first example of Pd-catalyzed alkylation of C(sp3)–H bonds with alkyl bromide reagents, which are less expensive and more readily available than alkyl iodide reagents. As expected, the β−2°-C(sp3)–H bonds of various amino acid derivatives were also alkylated with α-iodoacetate esters and α-bromoacetate esters. The γ-alkylation of C(sp3)–H bonds could be achieved when no reactive β-C–H bonds are present. Finally, the significance of this palladium-catalyzed alkylation is further demonstrated by the synthesis of β-branched α-amino acids via several sequential functionalizations of C(sp3)–H bonds.
Thus far, unactivated methylene C(sp3)–H alkylation was limited to the use of α-haloacetates and methyl iodide without β-hydrogen atom. To overcome this limitations, the Shi group reported a palladium-catalyzed alkylation of unactivated methylene C(sp3)–H bonds with an expanded scope of alkyl iodide reagents in 2014 (Scheme 115a).321 Screening of various additives showed that the use of silver salts together with 4-Cl-C6H4SO2NH2 and NaOCN significantly improved the reaction yield. Linear and branched primary alkyl iodide coupling partners that bear β-hydrogen atoms worked smoothly under the optimized reaction conditions. In addition, 2-amino-butyric acid (Abu), norvaline (Nva), lysine (Lys), ornithine (Orn), and phenylalanine (Phe) derivatives were all compatible with the alkylation protocol. Furthermore, alkylation of the γ-methyl C(sp3)–H bond of isoleucine was achieved. Importantly, a series of β, β-heterodialkylated α-amino acids could be prepared by sequential C(sp3)–H functionalization of alanine. Additionally, both alkylation/arylation and arylation/alkylation sequences could be achieved to provide a variety of β-alkyl-β-aryl α-amino acids in good yields. In 2016, an efficient method for the synthesis of chiral β-alkyl-α-hydroxy acids via palladium-catalyzed C(sp3)–H alkylation of lactic acid with the assistance of an 8-aminoquinoline DG with retention of chirality was also reported by the Shi group (Scheme 115b).322
Scheme 115.
8-Aminoquinoline-Directed C(sp3)–H Alkylation with Various β-Hydrogen Containing Alkyl Iodides. a) Methylene C(sp3)–H Alkylation of N-Phthaloyl α-Amino Acids (Shi et al., 2014).321 b) Methyl C(sp3)–H Alkylation of Lactic Acid (Shi et al., 2016).322
Palladium-catalyzed, 8-aminoquinoline-assisted, site-selective cyanomethylation of unactivated C(sp3)–H bonds with acetonitrile was reported by Ge and co-workers in 2016 (Scheme 116a).323 The authors propose that this reaction proceeds through a Pd(II) species in which the Pd is coordinated to the DG, which can then perform an irreversible C(sp3)–H bond activation under basic conditions to create a cyclometalated Pd(II) complex. This complex then undergoes transmetallation with a cyanomethyl Cu(II) species that is likely the result of a Ag-promoted reaction with the acetonitrile. Upon reductive elimination, the product is produced and the Pd(0) is reoxidized by Cu(II) and/or Ag(I) to the Pd(II) species. In 2017, the Yang group developed a procedure for synthesizing chiral γ-phosphono-α-amino acids via 8-aminoquinoline directed C(sp3)–H alkylation of α-amino acids (Scheme 116b).324 Pd-catalyzed C(sp3)–H alkylation of cyclopropanes directed by 8-aminoquinoline was developed by the Shuto group (Scheme 117).309,325
Scheme 116.
a) Cyanomethylation of Unactivated Methylene C(sp3)–H Bonds with Acetonitrile (Ge et al., 2017).323 b) Methylene C(sp3)–H Alkylation of Amino Acids with (Iodomethyl)phosphonate (Yang et al., 2017).324
Scheme 117.
8-Aminoquinoline-Directed C(sp3)–H Alkylation of Cyclopropane (Shuto et al., 2016, 2019).311,325
After achieving ortho-C(sp2)–H alkylation with picolinamide-protected benzylamines using alkyl halide reagents,326 the Chen group reported a picolinamide-directed, Pd-catalyzed alkylation of unactivated methyl C(sp3)–H bonds with alkyl iodides in 2013 (Scheme 118a).327 After extensive screening, a catalytic amount of dibenzyl phosphate (BnO)2PO2H was found to promote the C–H alkylation reaction, and they proposed that it might act as a solid-to-solution phase-transfer catalyst (PTC) for Ag2CO3. In the presence of excess methyl iodide, exo- or endo-norbornene substrates undergo an initial addition of a methyl group at the γ-C(sp3)–H that is followed by two sequential δ-C(sp3)–H activations at the newly formed methylene position to give an isopropyl group in 77% and 88% yield, respectively. In 2018, Shi and co-workers reported a Pd(II)-catalysed selective δ-methyol C(sp3)–H alkylation of amino acids and peptides in the presence of γ-methyl C(sp3)–H bonds using maleimides as alkylating reagents (Scheme 118b).328 The authors invoke the Curtin-Hammett principle to suggest that both the five- and six-membered palladacycles are reversibly formed in this reaction and that the reaction proceeds faster through the thermodynamically less disfavoured six-membered palladacycle intermediate.
Scheme 118.
a) Picolinamide-Directed Alkylation of Unactivated Methyl C(sp3)–H Bonds with Alkyl Iodides (Chen et al., 2013).327 b) Remote Selective δ-C(sp3)–H Alkylation of Amino Acids and Peptides with Maleimides (Shi et al., 2018).328
2.2.1.5. Carbonylation
An N,O-bidentate DG-enabled, palladium-catalyzed γ-C(sp3)–H carbonylation of aliphatic amines was reported by Zhao and co-workers in 2015 (Scheme 119a).329 Oxalyl amide was used as an efficient DG and 3-(trifluoromethyl)benzoic acid was found to be most effective additive. Notably, amines bearing two β-substituents are more reactive compared to those bearing only one β-methyl group, providing the corresponding pyrrolidines in good to excellent yields. The oxalyl amide DG can be readily removed under basic conditions at 50 °C.
Scheme 119.
Pd-Catalyzed γ-C(sp3)–H Carbonylation. a) Oxalyl Amide DG (Zhao et al., 2015).329 b) Picolinamide DG (Wang et al., 2015).330 c) N-(2-pyridyl)sulfonamide DG (Carretero et al., 2016).331
Another example of palladium-catalyzed γ-C(sp3)–H carbonylation of aliphatic amines was reported by Wang and co-workers in the same year using picolinamide as the DG (Scheme 119b).330 The success of this reaction relied on the judicious choice of TEMPO as a proper oxidant because classic oxidants, such as Cu(II), Ag(I), PhI(OAc)2, DDQ, NFSI, CAN, and K2S2O8, were found to be ineffective in this transformation. A range of commercial α-amino acids and amino alcohols were converted into the corresponding γ-lactams in good yield. Finally, a concise total synthesis of rac-pregabalin was acheived using this γ-C(sp3)–H carbonylation protocol as the key step. In 2016, the Carretero group also reported a carbonylative cyclization of amines via Pd(II)-catalyzed γ-C(sp3)–H activation with sub-stoichiometric Mo(CO)6 as the CO source directed by N-(2-pyridyl)sulfonyl auxiliary (Scheme 119c).331 α- and β-amino acid derivatives could also be used, affording the corresponding γ-lactams in good yields.
The C(sp3)–H carbonylation of alcohol derivatives enabled by a DG containing an N-acetyl-protected aminoethyl pyridine scaffold was first reported by the Yu group in 2019 (Scheme 116).332 They proposed that the hemilabile ether was crucial for the transformation, in which it dissociated after C–H activation step to allow the binding of carbon monoxide. Apart from the γ-C(sp3)–H carbonylation, an unprecedented δ-C(sp3)–H carbonylation was also achieved, giving the desired product (751) in 33% yield.
Up until now, palladium-catalyzed C(sp3)–H carbonylation was limited to the functionalization of primary C–H bonds. The first Pd(II)-catalyzed alkoxycarbonylation of more unactivated secondary C(sp3)–H bonds was acheived by the Shi group in 2016 with readily available and operationally simple alkyl chloroformates (Scheme 121a).333 In their initial efforts to use carbon monoxide as the carbonylation source, they found that the corresponding CO-ligand palladacycle complex derived from N-phthaloyl phenylalanine bearing the 8-aminoquinoline DG was very stable under a variety of conditions, likely due to the difficulty of CO migratory insertion as well as the challenging CO reductive elimination. Thus, alkyl chloroformates were used to oxidize the CH3CN-bound palladacycle via a Pd(II)/Pd(IV) redox process, which then triggers a facile reductive elimination to generate the carbonylated product. The methylene C(sp3)–H alkoxycarbonylation of a broad range of N-phthaloyl amino acids were achieved. The relative stability of CO-ligand palladacycle complexes has been extensively studied by Shi, Hong, Wang and co-workers in 2019 (Scheme 121b).334 Based on computational study, they found that the Gibbs Free Energy (ΔG) for ligand exchange with four different electrophiles is significantly higher with the CO-coordinated palladacycle than with the palladacycles coordinated with CH3CN, DMSO, and pyridine (> 3.2 kcal/mol). It was also observed that the CO-coordinated palladacycle is extremely stable under a variety of conditions.
Scheme 121.
a) Palladium-Catalyzed Alkoxycarbonylation of Unactivated β-Methylene C(sp3)–H Bonds with Alkyl Chloroformates (Shi et al., 2016).333 b) Synthesis of Stable CO-Coordinated Palladacycles and Mechanistic Studies (Wang et al., 2019)334
2.2.2. Carbon–Heteroatom Bond Formation
2.2.2.1. Carbon–Oxygen Bond Formation
The first C(sp3)–H acetoxylation of protected amino acids was reported by the Corey group in 2006 (Scheme 122a).188 A number of N-phthaloylamino acids with various carboxylate-based directing auxiliaries, such as N-methoxyamide, Weinreb amide, oxazoline, picolinamide (PA), pyridin-2-ylmethanamine, and 8-aminoquinoline, were investigated. Promising results were only observed when the 8-aminoquinoline group was used. Screening of various additives showed that the use of Mn(OAc)2 led to critical improvement of the yield. Corey and co-workers postulated that the stereochemistry of the β-functionalization can be understood in terms of a preference for forming the sterically favored trans-palladacycle intermediate (764). In 2012, the Daugulis group also described a method for C–H acetoxylation of amino acid derivatives using PhI(OAc)2 as the oxidant and Ac2O as the solvent.196 In 2015, an improved synthesis of anti-β-hydroxy-α-amino acids via Pd(II)-catalyzed arylation and/or alkylation of C(sp3)–H bonds followed by β-acetoxylation was developed by Shi and co-workers.335
Scheme 122.
a) 8-Aminoquinoline-Directed β-Methylene C(sp3)–H Acetoxylation of N-Phthaloylamino Acids (Corey et al., 2006).188 b) Picolinamide-Directed γ-Methyl C(sp3)–H Alkoxylation (Chen et al., 2012).336
In 2012, the alkoxylation of unactivated C(sp3)–H bonds with broad scope of PA DG-containing substrates was reported by the Chen group (Scheme 122b).336 A wide range of primary alcohols, such as MeOH, n-PrOH, BnOH, CF3CH2OH, and n-octyl alcohol, can be used, giving the desired products in good to excellent yields. In addition, secondary alcohols, such as i-PrOH and cyclohexanol provided the corresponding products in good yields after prolonged reaction time. Even t-BuOH afforded the tert-butyl ether product in 63% yield. Aliphatic amine substrates bearing primary γ-C(sp3)–H bonds with or without α-substituents can be methoxylated under the standard reaction conditions.
In 2012, Sahoo and co-workers described a process for selective acetoxylation of unactivated β-C(sp3)–H bonds using PhI(OAc)2 as the oxidant with the assistance of S-methyl-S-2-pyridylsulfoximine (MPyS) at room temperature (Scheme 123a).337 Control experiments indicated that the carboxylic acid solvent participated in C–O bond formation, while the PhI(OAc)2 oxidant turned over the catalytic cycle. Thus, a variety of carboxylic acids CD3CO2H, EtCO2H, n-PrCO2H, and iso-PrCO2H could be incorporated into the oxygenated products. Finally, the DG can be easily removed by simple acid hydrolysis and can be recycled after recovery, which improves the practical utility of this method. Over the next few years, a number of additional directing groups were employed in successful C(sp3)–H acetoxylation. In 2013, 2-N-pyridine-1,2,3-triazole-4-amide-directed, methyl C(sp3)–H acetoxylation was reported by the Shi group (Scheme 123b).338 In 2014, palladium-catalyzed γ-C(sp3)–H acetoxylation of N-alkylpicolinamide substrates using PhI(OAc)2 oxidant was developed by the Chen group (Scheme 123c).339 In 2015, palladium-catalyzed oxygenation of C(sp3)–H bonds with the assistance of oxalyl amide DG was reported by the Zhao group (Scheme 123d).340
Scheme 123.
a) MPyS-N-Amide-Directed Primary β-C(sp3)–H Acetoxylation (Sahoo et al., 2012).337 b) 1,2,3-Triazole-4-Amide Directed Methyl C(sp3)–H Acetoxylation (Shi et al., 2013).338 c) Picolinamide-Directed γ-C(sp3)–H Acetoxylation (Chen et al., 2014).339 d) Oxalyl-Amide-Directed γ-C(sp3)–H Acetoxylation (Zhao et al., 2014).340
Methylene C(sp3)–H bond alkoxylation, which is more challenging compared to the methyl C(sp3)–H bond alkoxylation due to high steric hindrance, was achieved by the Shi group in 2013 (Scheme 124).341 Inspired by the early efforts of Daugulis, the authors designed and reported the first use of a novel 2-(pyridine-2-yl)isopropyl (PIP) DG, which proved most efficient for this transformation. Optimization revealed that the use of recrystallized PhI(OAc)2 (from HOAc and hexane) significantly improved the yield. This reaction protocol was very sensitive to steric hindrance; substrates with linear chains gave high yields, while substrates bearing sterically demanding groups at the α position failed. A large number of alcohols, such as linear or branched primary alcohols, were found to be compatible with the reaction protocol. Additionally, secondary alcohols could be applied in the alkoxylation reaction, albeit in slightly lower yields. Later, C–H alkoxylation of unactivated methylene using cyclic hypervalent iodine (I3+) oxidants with the aid of 8-aminoquinoline was reported by the Rao group.342–344
Scheme 124.
PIP-Directed Unactivated Methylene C(sp3)–H Alkoxylation of (Shi et al., 2013).341
Palladium-catalyzed β-C(sp3)–H acetoxylation of tripeptides via N,N-bidentate coordination of peptide backbone was demonstrated by Yu and co-workers in 2014 (Scheme 125).261 Currently, the acetoxylation of tripeptides N-terminus is limited to alanine.
Scheme 125.
Amino-Acid-Directed C(sp3)–H β-Acetoxylation of Dipeptides (Yu et al., 2014).261
In 2012, Dong and co-workers developed a Pd-catalyzed, oxime-directed functionalization of unactivated β-C(sp3)–H bonds via a five-membered exo-palladacycle (Scheme 126a).345 The substrate scope of primary, secondary, and tertiary alcohols derivatives was examined, and the corresponding vicinal dioxygenated products were isolated in good yields. Notably, in substrates that lack methyl groups at the β-positions, C–H activation can take place at methylene positions. Additionally, the methine tertiary C–H bonds of norborneol-derived substrates could also be oxidized. In 2015, the Dong group further expanded this protocol to an analogous intramolecular version for the synthesis of cyclic ethers (Scheme 126b).346 In the same year, the Dong group developed a Pd-catalyzed, 8-quinolinecarboxaldehyde-oxime-directed sulfonyloxylation of an nonactivated C(sp3)–H bond at the β-position, providing a convenient and efficient approach for the synthesis of β-sulfonyloxylated alcohols (Scheme 126c).347 Remarkably, both methyl and unactivated methylene C–H bonds could be sulfonyloxylated in moderate yields. The tosyl group could be used as a late-stage intermediate to prepare complex molecules via nucleophilic substitution (SN2) reactions. In 2016, Dong and co-workers extended this strategy to a hydrazone-based DG strategy for the β-C(sp3)–H oxidation of aliphatic amines (Scheme 126d).348 Inspired by these precedents, the C(sp3)–H acetoxylation of alcohol derivatives that bear a monoanionic N,N-bidentate DG was disclosed by Xu and co-workers in 2019 (Scheme 126e).270 The potential application of this reaction was demonstrated by removing the DG and Ac group to generate 1,2-diols in good yields.
Scheme 126.
exo-Oxime-type DG Enabled -Directed C(sp3)–H Oxygenation. a) Intermolecular Acetoxylation (Dong et al., 2012).345 b) Intramolecular Etherification (Dong et al., 2012).346 c) Tosyloxylation (Dong et al., 2015).347 d) β-Acetoxylation of Aliphatic Amines (Dong et al., 2016).348 e) Monoanionic-N,N-bidentate DG (Xu et al., 2019).270
All the aforementioned bidentate DGs proceed through five- or six-membered metallacycle intermediates. Transition-metal-catalyzed C(sp3)–H activation via four-membered metallacycle intermediates is less developed. Two notable examples covered earlier were monodentate directed, Pd-catalyzed C(sp3)–H carbonylation and Pd-catalyzed C(sp3)–H activation of aliphatic amines, both reported by Gaunt in 2014.145 In 2015, the Zhang group reported 1-aminoanthraquinone-directed, α-C(sp3)–H acetoxylation of aliphatic carboxamides via a four-membered palladacycle intermediate (Scheme 127a).349 In 2020, the Hartwig group reported primary β-C(sp3)–H acetoxylation of aliphatic amines via a four-membered palladacycle by using salicaldimine as the DG (Scheme 127b).350 However, isolation of the four-membered palladacycle was unsuccessfully. DFT calculations indicated that formation of the four-membered palladacycle is endergonic, in contrast to formation of the corresponding five-membered palladacycle, which is exergonic. These results explain the challenge of isolating the former.
Scheme 127.
Palladium-Catalyzed Intermolecular Acetoxylation of C(sp3)–H Bonds via a Four-Membered Palladacycle (Zhang et al., 2015; Hartwig et al., 2020).349,350
2.2.2.2. Carbon–Nitrogen Bond Formation
In 2012, the Chen group reported a Pd-catalyzed, picolinamide-directed, and PhI(OAc)2-mediated intramolecular amination of unactivated γ- and δ-C(sp3)–H bonds of amine substrates, leading to the formation of azetidines and pyrrolidines (Scheme 128a).351 Chen and co-workers found that the selective intramolecular amination of primary δ-C(sp3)–H bonds in leucine-derived substrate in the presence a sterically less accessible tertiary γ-C(sp3)–H bond was achieved, giving the pyrrolidine product. In addition, no pyrrolidine products were isolated in the cyclization reactions of substrates that bear primary C(sp3)–H groups at both γ and δ positions, which can be explained by preferential formation of the kinetically favored five-membered palladacycle over the six-membered palladacycle. In order to improve the synthetic utility of this protocol, a modified picolinic acid (PAre) was introduced as an auxiliary since PAre can be removed under mild conditions, such as in 1M HCl at room temperature for 24 h, unlike PA, which traditionally requires significantly harsher conditions. Contemporaneously, the Daugulis group reported a similar method to achieve the same transformation (Scheme 128b).352 In 2013, Shi and co-workers reported an example of intramolecular amination of primary C(sp3)–H bonds with a triazole-derived DG (Scheme 128c).338 An oxalyl-amide-assisted, intramolecular δ-C(sp3)–H/N–H cyclization was used in the synthesis of pyrrolidones was reported by Zhao and co-workers in 2014 (Scheme 124d).353
Scheme 128.
a-b) Picolinamide-Directed Intramolecular Amination of Unactivated C(sp3)–H Bonds (Chen et al., 2012; Daugulis et al., 2012).349,352 c) 1,2,3-Triazole-4-Amide Directed Intramolecular γ-(sp3)–H Amination (Shi et al., 2013).338 d) Oxalyl-Amide-Directed Intramolecular δ-(sp3)–H Amination (Zhao et al., 2014).353
In 2013, Chen and co-workers developed a method for the synthesis of pyrrolidones via the Pd-catalyzed, carboxamide-directed, intramolecular amidation of unactivated γ-methyl C(sp3)–H bonds (Scheme 129a).354 When the reaction was attempted with a substrate that required formation of the kinetically more favorable five-membered palladacycle intermediate, no desired lactam product was observed, likely due to the ring strain inherent in four-membered β-lactams. Chen and co-workers, therefore, proceeded to explore a starting material that could access the kinetically unfavorable six-membered palladacycle intermediate that did indeed react readily with PhI(OAc)2 to afford the γ-lactam products with high selectivity. Notably, this intramolecular γ-C(sp3)–H amination reaction could be applied in conjunction with other aminoquinoline-directed C(sp3)–H functionalization reactions. However, the cleavage of the DG is difficult with respect to this intramolecular C–H lactamization method. After evaluating modified auxiliary groups, 8-amino-5-methoxyquinoline (MQ) was found to be installed readily on carboxylic acid substrates and removed smoothly in the presence of ceric ammonium nitrate (3 equiv) in CH3CN/H2O at room temperature for 5 h.
Scheme 129.
a) Synthesis of Pyrrolidones via the Pd-Catalyzed Intramolecular γ-Methyl C(sp3)–H Amidation (Chen et al., 2013).354 b) Synthesis of β-Lactams via PIP-Directed Intramolecular β-Methylene C(sp3)–H Amidation (Shi et al., 2013).233 c) 8-Aminoquinoline-Directed Intramolecular β-C(sp3)–H Amidation for the Synthesis of β-Lactams (Wu et al., 2014).356
The Shi group developed a robust protocol for the stereoselective synthesis of α-amino-β-lactams through Pd(II)-catalyzed sequential C(sp3)–H monoarylation/amidation (Scheme 129b).233 After extensive screening, the optimized reaction conditions were reported as Pd(OAc)2 (10 mol%), NaIO3 (0.3 mmol) as the oxidant, and Ac2O (1.5 mmol) as the additive in MeCN (3 mL) under N2 atmosphere at 70 °C for 48 h. The N,N-bidentate PIP amide DG was found to be effective both in controlling selectivity in the monoarylation step and in enhancing reactivity in the amination step. In 2017, they reported an improved synthesis of α-amino-β-lactams by employing MQ as a removable bidentate DG.355
In 2014, the Wu group also developed an efficient method to synthesize β-lactams with high regioselectivity through a Pd-catalyzed, intramolecular β-C(sp3)–H amination of 8-aminoquinoline carboxamides (Scheme 129c).356 They explored numerous aryl iodides with electron-withdrawing substituents and found that C6F5I could act as an efficient oxidant to enable the desired C–N reductive elimination rather than C–C reductive elimination, leading to the formation of desired β-lactams in high yield and selectivity. In 2014, during the endeavors to the synthesis of aryltetralin lignan natural products, the Maimone group was also able to isolate β-lactams produced by palladium-catalyzed intramolecular amidation of benzylic C(sp3)–H bonds using electron-deficient aryl iodides as oxidants.193
In 2020, Chen and co-workers demonstrated the synthesis of chiral β-lactams via Pd-catalyzed, 8-aminoquinoline-directed, enantioselective benzylic methylene C(sp3)–H amidation using 3,3’-fluorinated-BINOL (L78) as a chiral ligand (Scheme 130a).357 A sterically hindered aryl iodide oxidant was found to promote C−N reductive elimination. Unfortunately, the amidation of aliphatic secondary C(sp3)–H bonds using various 5-substituted 8-aminoquinoline DGs failed to give any promising results. At the same time, the Shi group successfully reported the palladium-catalyzed, enantioselective amidation of both benzylic and aliphatic secondary β-C(sp3)–H bonds using PIP as bidentate DG (Scheme 130b).358 The C–N reductive elimination product is the main product observed when using electron-deficient aryl iodide compounds,which is proposed to inhibit the undesired C–C reductive elimination. The amidation products via β-benzylic methylene C(sp3)–H bonds activation were obtained in high yields and ees in the presence of a 3,3’-dichlorinated-BINOL ligand (L82). By switching 3,3’-dichlorinated-BINOL to (S)-3,3’-fluorinated-H8-BINOL, chiral β-lactams via aliphatic secondary C(sp3)–H amidation were obtained in good yields and ees (L83).
Scheme 130.
Synthesis of Chiral β-Lactams a) Via 5-Subsituted 8-Amioquinoline-Directed Enantioselective Benzylic C(sp3)–H Amidation (Chen et al., 2020).357 b) Via PIP-Directed Enantioselective Amidation of Both Benzyic and Unbiased Methylene C(sp3)–H Bonds (Shi et al., 2020).358
In 2015, a Pd(II)-catalyzed, intermolecular amination of unactivated methyl C(sp3)–H bonds using 2-(methylthio)aniline as a bidentate DG was reported by the Qin group (Scheme 131a).359 The reaction conditions were reported as PdCl2 (10 mol%) as catalyst with Cs2CO3 (2 equiv) as base in benzene at 110 °C for 24–34 h. Kinetic isotope experiments revealed that the β-C(sp3)–H bond cleavage step was the turnover-limiting step. In 2017, the Zhang group reported the 2-(pyridin-2-yl)isopropyl (PIP)-directed, intermolecular amination of methylene C(sp3)–H bonds with diisopropylazodiformate (DIAD) as the amination reagent (Scheme 131b).360 In the same year, Liu and co-workers reported an intermolecular methyl C(sp3)–H sulfonamidation of aliphatic alcohol derivatives with N-fluorobenzenesulfonimide (NFSI) (Scheme 131c).361
Scheme 131.
a) Intermolecular Methyl C(sp3)–H Amination Directed by 2-Thiomethylaniline (Qin et al., 2015).359 b) Intermolecular Amination of Unactivated Methylene C(sp3)–H Bonds Directed by PIP (Zhang et al., 2017).360 c) Intermolecular Sulfonamidation of Methyl C(sp3)–H Bonds of Aliphatic Alcohols (Liu et al., 2017).361
2.2.2.3. Carbon–Halogen Bond Formation
In 2014, the Sahoo group reported a palladium-catalyzed halogenation of unactivated primary C(sp3)–H bonds with the assistance of the S-methyl-S-2-pyridylsulfoximine DG (Scheme 132a).362 N-Bromophthalimide and N-chlorophthalimide were used as halogen sources. However, only primary C(sp3)–H bonds that are adjacent to quaternary carbon centers could be transformed into C–Cl and C–Br bonds. In 2017, Yu and co-workers developed an aminooxyacetic-acid-auxiliary-directed β-C(sp3)–H iodination of ketones catalyzed by Pd(TFA)2 (Scheme 132b).363 A wide range of ketones underwent β-iodination in good to excellent yields. Interestingly, a C–H insertion intermediate was isolated, which provided evidence for the L,X-type coordination mode of the oxime-carboxylic acid DG. In 2020, the Polyzos group reported a creative method for the halogenation of β-C(sp3)–H bonds with alkyl halides (Scheme 133).364 By visible light excitation of the palladacycle, an electron transfer to the alkyl halide produces a palladium(III) intermediate, which can either undergo disproportionation or be oxidised by Oxone to generate a palladium(IV) complex. Reductive elimination from the palladium(IV) complex affords the halogenation product in moderate to good yields.
Scheme 132.
a) Sulfoximine-Directed Bromination and Chlorination of Primary β-C(sp3)–H Bonds (Sahoo et al., 2014).362 b) β-C(sp3)–H Iodination of Ketones (Yu et al., 2017).363
Scheme 133.
5-Chloro-8-Aminoquinoline-Directed Light-Induced β-C(sp3)–H Halogenations (Polyzos et al., 2020).364
In 2015, the first bidentate-DG-directed, palladium-catalyzed C(sp3)–H fluorination of aliphatic carboxamides to synthesize β-fluoro α-amino acids was reported by the Shi group (Scheme 134).365 A broad range of substituted phenylalanine derivatives were fluorinated using Selectfluor as the F+ source.. Gratifyingly, when 2-methylbenzoic anhydride (L84) was used as an additive, fluorination of aliphatic methylene C(sp3)–H bonds was also achieved. A wide range of protected natural and unnatural amino acid derivatives containing the PIP DG were compatible with this fluorination protocol, providing the desired products in moderate yields. Notably, a gram-scale reaction was performed, and the fluorinated product 852 was obtained in 69% yield. To further demonstrate the synthetic utility of this fluorination method, removal of the PIP DG was carried out, and the corresponding products were obtained in good yields with retention of configuration. Palladacycle intermediate 855 was isolated in 86% yield when 851 was allowed to react with 1 equiv Pd(OAc)2 in DCM at room temperature overnight. A stoichiometric reaction of 855 with 1.05 equiv of Selectfluor in d3-MeCN was also conducted, which led to the formation of 856 in 75% yield within 5–10 minute. Treatment of 856 with an aqueous solution of NH4Cl and Na2S provided the fluorinated product 852 in 86% yield with retention of configuration, consistent with direct C−F reductive elimination from the palladium center. Finally, they found that 855 was a viable precatalyst for the C–H fluorination of 851. Bidentate-DG-directed, palladium-catalyzed methylene C(sp3)–H fluorination of alcohol derivatives was also observed by the Dong group in 2015.347
Scheme 134.
PIP-Directed Fluorination of Unactivated Methylene C(sp3)–H Bonds of α-Amino Acids (Shi et al., 2015).365
Soon afterwards, the Ge group also developed a highly site-selective, PIP-directed, palladium-catalyzed C(sp3)–H fluorination of aliphatic acid and α-amino acid derivatives (Scheme 135a).366 The reaction conditions reported on 0.30 mmol scale were: Pd(OAc)2 (10 mol%), Selectfluor (2.5 equiv), Fe(OAc)2 (0.3 equiv), Ag2CO3 (2.0 equiv), and 300 μL i-PrCN in 1,2-DCE (3.0 mL) at 150 °C for 14 h. The substrate scope was investigated, and the authors noted a large preference for functionalization of the unactivated β-C(sp3)–H bond over the relatively reactive benzylic γ-C(sp2)–H bond. After the fluorination reaction, the PIP auxiliary could be removed, and the desired products were isolated in good yields. Contemporaneously, 8-aminoquinoline-directed, Pd(II)-catalyzed fluorination of unactivated methylene and methyl C(sp3)–H bonds at the β-position of carboxylic acids was developed by the Xu group (Scheme 135b).367 Huang and co-workers later observed a significant difference between 8-aminoquinoline and 8-aminoquinoxaline DGs for C(sp3)–H fluorination reactions, with the latter providing the desired product in greater yield (Scheme 135c).368 Experimental and computational studies revealed that the Pd(II)/Pd(IV) oxidation step was significantly affected by changes of the DG structure. In 2018, Xu and co-workers developed a method for the β-C(sp3)–H fluorination of simple aliphatic alcohol derivatives using a novel exo-type DG (Scheme 136).369 Both methyl C(sp3)–H bonds and methylene C(sp3)–H bonds could be functionalized in this transformation.
Scheme 135.
Palladium-Catalyzed Fluorination of Unactivated Methylene C(sp3)–H Bonds of Aliphatic Carboxamides. a) With PIP DG (Ge et al., 2015).366 b) With 8-Aminoquinoline DG (Xu et al., 2015).367 c) With 8-Aminoquinoxaline DG (Huang et al., 2016).368
Scheme 136.
Palladium-Catalyzed C(sp3)–H Fluorination of Alcohol Derivatives (Xu et al., 2018).369
In 2018, the Yu group discovered that a bulky amino amide substrate could be used as a catalytic, transient DG for palladium(II)-catalyzed, enantioselective C(sp3)–H fluorination (Scheme 137).370 When simple glycine was used as the transient DG, the corresponding C(sp3)–H acetoxylation product was obtained as the major product. The authors had to consider how to promote the desired reductive elimination step, so they tested a series of amino acid transient DGs and found that selectivity for formation of the fluorinated product increased with increasing steric bulk on the side chain. They further found that switching to an amino amide transient DG further improved fluorination selectivity, with the N,N-diethyl amide proving optimal. Deuterium incorporation experiments indicated that the C(sp3)–H insertion process is irreversible. The Yu group proposed that the C(sp3)−F bond formation proceeds via an inner-sphere reductive elimination pathway with retention of configuration while the C(sp3)−O bond formation occurs through an SN2-type mechanism, consistent with the observation that the fluorinated and acetoxylated products have opposite absolute stereochemical configuration, as determined by X-ray crystallography.
Scheme 137.
Pd-Catalyzed Enantioselective C(sp3)–H Fluorination Using a Transient DG (Yu et al., 2018).370
2.2.2.5. Carbon–Sulfur and –Selenium Bond Formation
Although sulfonylation through a Pd(II)/ Pd(IV) catalytic cycle was demonstrated early by the Dong group, this type of reaction is generally limited to C(sp2)–H bonds.371,372 In 2015, Shi and co-workers reported a Pd-catalyzed sulfonylation of unactivated C(sp3)–H bonds with sodium sulfinates, which provides a method for the synthesis of diverse aryl alkyl sulfones (Scheme 138a).373 MesCO2H, a sterically bulky benzoic acid, was the best carboxylic acid additive for this protocol. A wide range of sodium arylsulfinates that bear electron-donating or -withdrawing groups were tolerated and gave the desired sulfonylated products in good yields. Finally, the potential utility of this C(sp3)–H sulfonylation protocol was demonstrated by removal of the 8-aminoquinoline auxiliary in a representative product and through late-stage sulfonylation of β-citronellol, (−)-santonin, and cholic acid.
Scheme 138.
Palladium-Catalyzed, 8-Aminoquinoline-Directed C(sp3)–H Chalcogenation. a) β-C(sp3)–H Sulfonylation (Shi et al., 2015).373 b) β-C(sp3)–H Trifluoromethylthiolation (Besset et al., 2015).374 c) γ-C(sp3)–H Chalcogenation (Maiti et al., 2018).375
Among sulfur-containing functional groups, the trifluoromethylthiol functional group has recently attracted attention in medicinal chemistry, and in 2015, the first example of a palladium-catalyzed trifluoromethylthiolation of unactivated C(sp3)–H bonds was disclosed by Besset and co-workers (Scheme 138b).374 However, the highest yield of the desired product was 53%. In 2018, the Maiti group reported an 8-aminoquinoline-directed, γ-C(sp3)–H chalcogenation of α-amino acids using disulfides or diselenides enabled by 2-chloroquinoline (L85) ligand (Scheme 138c).375
2.2.2.6. Carbon–Boron, –Silicon, and –Germanium Bond Formation
In 2014, Shi and co-workers reported the direct transformation of unactivated primary γ-C(sp3)–H bonds of amino acid derivatives into C(sp3)−B bonds using B2pin2 as the borylating reagent with the assistance of picolinamide as DG (Scheme 139).376 Notably, oxygen was the sole oxidant in this transformation. A variety of amine substrates, such as amino acids and alkyl amines were converted to desired alkyl boron species in good yields.
Scheme 139.
Picolinamide-Directed C(sp3)–H Borylation (Shi et al., 2014).376
While studying the Pd-catalyzed silylation of ortho-C(sp2)–H bonds with hexamethyldisilane in 2014, Kanai and co-workers also explored a limited substrate scope in which Pd-catalyzed the silylation of unactivated C(sp3)–H bonds, albeit with the highest yield under the reported reaction conditions being only 39% (Scheme 140a).377 In 2016, the Shi group reported an 8-aminoquinoline-directed, palladium(II)-catalyzed β-C(sp3)–H silylation of amino acids (Scheme 140b).378 Both primary and secondary β-C(sp3)–H could be silylated in moderate to good yield. Contemporaneously, Zhang and co-workers reported the silylation of unactivated β-C(sp3)–H bonds (Scheme 140c).379 In 2018, Liu and co-workers extended the reaction conditions developed by the Shi group378 to the synthesis of β-germyl-α-amino acids (Scheme 140d).380 In 2019, the Shi group described a procedure for γ-C(sp3)–H silylation of peptides using picolinamide (PA) as the DG (Scheme 140e).381 A variety of α-amino acids, α-amino alcohols, and oligopeptides were transformed to the corresponding silylated products in good yields. In 2017, Maiti and co-workers developed reaction conditions for the silylation and germylation of unactivated γ-C(sp3)–H bonds of aliphatic acids via six-membered palladacycles through the blocking of β-positions (Scheme 141).382
Scheme 140.
a−c) 8-Aminoquinoline-Directed C(sp3)–H Silylation (Kanai et al., 2014;377 Shi et al., 2016;378 Zhang et al., 2016379). D) 8-Aminoquinoline-Directed C(sp3)–H Germylation (Liu et al., 2018).380 e) Picolinamide-Directed C(sp3)–H Silylation of Peptides (Shi et al., 2019).381
Scheme 141.
8-Aminoquinoline-Directed γ-C(sp3)–H Silylation and Germylation (Maiti et al., 2017).382
3. Nickel-Catalyzed Coordination-Assisted Functionalization of C(sp3)–H Bonds.
While palladium has proven to be a privileged metal in C–H functionalization, its expense and scarcity have driven the development of earth-abundant nickel catalysts as competent alternatives in certain cross-coupling reactions. Compared to palladium, nickel offers increased access to alkyl coupling partners because it is frequently able to engage in single electron transfer (SET) processes and it undergoes a sluggish β-hydride elimination from alkylnickel species.383–390 Three general catalytic cycles for involving different redox processes have been proposed for nickel-catalyzed C(sp3)–H activation reactions (Scheme 142). In particular, both Ni(II)/Ni(IV) and Ni(II)/Ni(III) catalytic cycles have been invoked in a number of reports on C(sp3)–H arylation and alkylation, whereas a Ni(II)/Ni(II) catalytic cycle is thought to be operative in reaction involving C(sp3)–H activation followed by alkyne/alkene insertion. The first example of Ni-catalyzed, coordination-assisted functionalization of C–H bonds using an N,N-bidentate DG involved the oxidative annulation of aromatic amides with internal alkynes for the synthesis of isoquinolones as reported by the Chatani group in 2011.391 Since then, tremendous progress has been made in the field of Ni-catalyzed C(sp2)–H bond functionalization using N,N-bidentate DGs.392–395
Scheme 142.
Catalytic Cycles of Nickel-Catalyzed C(sp3)–H Functionalization Reactions
Nickel catalyzed C(sp3)–H bond activation reactions usually require high temperatures, and to the best of our knowledge, 8-aminoquinoline is the only N,N-bidentate DG that has been used for Ni-catalyzed C(sp3)–H bond functionalization. Nevertheless, the first nickel(II)-catalyzed β-arylation of unactivated C–H bonds of aliphatic amides was reported by the Chatani group in 2014, using the 8-aminoquinoline moiety as the bidentate DG and iodoarenes as coupling partners (Scheme 143a).396 Ni(OTf)2 was applied as the catalyst with the aid of a sterically bulky carboxylic acid (MesCO2H) and a base (Na2CO3), which are proposed to facilitate a concerted metalation-deprotonation step during the C–H cleavage step. It is worth noting that the reaction proceeds smoothly with either Ni(OAc)2 or Ni(COD)2 as the precatalyst.
Scheme 143.
Nickel-Catalyzed, 8-Aminoquinoline-Directed β-C(sp3)–H Arylation of Aliphatic Amides. a) With Aryl Iodides (Chatani et al., 2014).374 b) With Diaryliodonium Triflate Salts (Chatani et al., 2014).397 c) With Aryl Iodides or Bromides (You et al., 2014).398 d) With Bromothiophenes (Qiu et al., 2015).399 e) Via C(sp3)–H/C(sp2)–H Cross-CouplingWith Heteroarenes (Yin et al., 2017).400
The Chatani group also demonstrated the first example of a Ni(II)-catalyzed arylation of C(sp3)–H bonds in aliphatic amides with diaryliodonium salts (Scheme 143b).397 In this case, the desired products were isolated in good yields even in the absence of a carboxylic acid additive. Since diaryliodonium salts could undergo decomposition under heat or basic conditions to generate aryl iodides or aryl triflate, a control experiment was conducted to confirm that diaryliodonium salts are the main arylation reagent (76% yield) in the system; 4-MeOC6H4I (37% yield) might have a small contribution, while 4-MeOC6H4OTf (0% yield) has no contribution to this arylation process.
A similar system for the nickel(II)-catalyzed arylation of C(sp3)–H bonds in aliphatic amides using 8-aminoquinoline as the bidentate DG was independently achieved by the You group (Scheme 143c).398 Ni(OTf)2 was used as the catalyst precursor, Na2CO3 (2 equiv) was the base, and PivOH (20 mol%) was the sterically bulky carboxylic acid. Moreover, using PPh3 (20 mol%) and DMSO (3.5 equiv) as additives was found to be essential to promote the cross-coupling in high yields when run in 1,4-dioxane at 160 °C for 24−36 h. Both aryl iodides and bromides were successful arylating reagents. In this system, various functional groups, such as Ac, CO2Me, CONEt2, CN, and CHO, were well tolerated. In 2015, the nickel-catalyzed directed C(sp3)–H arylation of aliphatic amides with bromothiophenes was reported by the Qiu group.399 In 2017, Yin and co-workers reported a Ni(OTf)2-catalyzed C(sp3)–H/C(sp2)–H cross-coupling of thiophenes directed by 8-aminoquinoline.400
Liu and co-workers recently explored the mechanism of Ni-catalyzed C(sp3)–H activation through DFT calculations.401 Compared to the analogous palladacycle intermediate formed after oxidative addition, the nickelacycle intermediate formed via C(sp3)–H activation is thermodynamically less favorable, which makes the C–H metalation process generally reversible. The mechanism for subsequent elementary steps involving the nickelacycle depends on the coupling partners, and both Ni(III) and Ni(IV) intermediates were computed to be viable. A computational study by Sunoj and co-workers also shows that the oxidative insertion step is rate-limiting.402 Interestingly, experimental evidence reported by Love, Schafer, and co-workers showed that the rate-determining step in a model substrate in which C(sp3)–H activation occurred alpha to an amide nitrogen was the C(sp3)–H activation.403 The Love group also made a seminal contribution by isolating the first example of an 8-aminoquinoline-directed nickelacycle intermediate formed via C(sp3)–H activation (Scheme 140). A similar nickelacycle intermediate was synthesized by Sanford and co-workers through decarbonylation.404
The first example of nickel(II)-catalyzed β-alkylation of aliphatic amides with linear alkyl halides (iodides, bromides, and chlorides) with the assistance of the 8-aminoquinoline DG was reported by the Ge group in 2014 (Scheme 145).405 In this case, Ni(acac)2 was employed as the precatalyst with 1,2-bis(diphenylphosphino)benzene (dppbz) (10 mol%) as the ligand. However, this transformation gave exclusive alkylation at the β-methyl C–H bonds in preference to β-methylene, β-benzylic, γ-methyl, and γ-benzene C–H bonds, thereby limiting use of the method to aliphatic amides bearing a quaternary α-carbon and containing at least one β-methyl group. Notably, the addition of TEMPO resulted in decreased yield of this reaction and the observation of an alkyl–TEMPO adduct, suggesting the formation of a carbon-centered radical via alkyl–X homolysis. These results also supports a plausible Ni(II)/Ni(III) cycle and excludes the Ni(II)/Ni(IV) cycle that was described earlier for the arylation and alkylation of C(sp3)–H and C(sp2)–H bonds respectively.
Scheme 145.
Nickel-Catalyzed β-C(sp3)–H Alkylation of Aliphatic Amides (Ge et al 2014).405
In 2015, You and co-workers used 2,2-disubstituted propanamides containing the bidentate 8-aminoquinoline auxiliary in nickel-catlayzed coupling with either symmetrical or unsymmetrical alkynes to give a range of alkenylated products (Scheme 146a).406 Ni(II) complexes were the preferred precatalysts, and the optimized reaction conditions were: Ni(OAc)2 (30 mol%) and PPh3 (60 mol%) in a mixture of i-PrOH and toluene (1:5) at 170 °C for 24 h. The system was also highly site-selective, favoring C–H bond alkenylation at β-methyl positions over β-methylene, γ-methyl, or aryl sites. Deuterium-labelling experiments indicated that the hydrogen source in protonolysis might come from the generated AcOH in the reaction system. Moreover, the KIE value was found to be kH/kD = 1.0, indicating that the cleavage of the methyl C–H bond with a nickel species was not involved in the turnover-determining step. Notably, treatment of the alkenylated products with PCC in CH2Cl2 at 100 °C triggered an oxidative O-cyclization process, ultimately leading to cleavage of the 8-aminoquinoline moiety to furnish γ-butyrolactones in moderate to high yield.
Scheme 146.
a) Nickel-Catalyzed β-C(sp3)–H Alkenylation of Aliphatic Amides with Alkynes (You et al., 2015).406 b) Nickel-Catalyzed Insertion of Alkynes and Electron-Deficient Alkenes into β-C(sp3)–H Bonds (Maiti et al., 2015).407
The Maiti group reported a similar system for the nickel(II)-catalyzed insertion of alkynes into unactivated C(sp3)–H bonds using 8-aminoquinoline as the bidentate auxiliary (Scheme 146b).407 The optimized reaction conditions were: Ni(OAc)2·4H2O (10 mol%) in DMF at 140 °C for 24 h. The authors succeeded in using both symmetrical and unsymmetrical internal alkynes. Terminal alkynes are also compatible coupling partners, affording the desired product with excellent regio- and stereoselectivity, albeit in low yields. After minor modifications to the reaction conditions, electron-deficient olefins were also found to be compatible, providing access to C(sp3)–H alkylated products.
Another nickel-catalyzed protocol to affect alkenylation of unactivated C(sp3)–H bonds was reported by the Shi group. In this method, styrenyl iodides were used as electrophiles with aliphatic carboxamides that contain a bidentate 8-aminoquinoline DG (Scheme 147).408 After extensive screening, the optimized reaction conditions were determined to be with Ni(acac)2 (10 mol%) as precatayst, BINOL (40 mol%) as a ligand, and Li2CO3 (2 equiv) and KTFA (2 equiv) as inorganic bases in DMSO at 140 °C for 8 h. Both electron-deficient and electron-rich (E)-β-iodostyrenes reacted smoothly with a wide variety of carboxamides that bear both linear and cyclic chains to give exclusively the (E)-isomers 889 in moderate to good yields.
Scheme 147.
Nickel-Catalyzed β-C(sp3)–H Alkenylation of Aliphatic Amides with Styrenyl Iodides (Shi et al., 2015).408
In 2016, Zhang and co-workers developed a method to access a range of γ-lactams from terminal alkynes and aliphatic amides via nickel catalyzed C(sp3)–H bond activation.409 Shi and co-workers reported an 8-aminoquinoline-directed C(sp3)–H alkynylation of aliphatic amides with terminal alkynes (Scheme 144b).410 While NiBr2 was found to be the best nickel catalyst, the addition of Me2S·CuBr co-catalyst and DavePhos ligand greatly enhance reactivity.
Scheme 144.
Nickelacycle Intermediate via C(sp3)–H Activation (Love, Schafer et al., 2018).403
The Ge group reported a Ni(dme)2I2-catalyzed, intramolecular, dehydrogenative cyclization of aliphatic amides via C(sp3)–H amination with TEMPO (3 equiv) as the single-electron oxidant (Scheme 149a).411 The presence of K2HPO4 (2 equiv) as the base and a catalytic amount of TBAI (10 mol%) promoted the dehydrogenative cyclization in a solvent mixture of butyronitrile and benzonitrile in a 3:2 ratio at 150 °C for 24 h. Under these reaction conditions, reaction at the β-methyl group took place selectively in the presence of γ-methyl, β-methylene, and aromatic C–H bonds. Ge and co-workers achieved a direct carbonylation of C(sp2)–H and C(sp3)–H bonds with DMF as the CO source (Scheme 149b).412 With a novel Ni/Cu synergistic catalysis system, succinimide derivatives were prepared from the corresponding aromatic or aliphatic amides under oxygen atomosphere. The authors observed a preference for functionalizing the methyl group over methylene groups, including relatively reactive benzyl positions, when aliphatic amides were used as substrates.
Scheme 149.
a) Nickel-Catalyzed Intramolecular Amidation of Aliphatic Amides (Ge et al., 2014).411 b) Nickel-Catalyzed β-C(sp3)–H Carbonylation of Aliphatic Amides with DMF (Ge et al., 2015).412
Nickel-catalyzed thiolation of β-methyl C(sp3)–H bonds of aliphatic carboxamides with disulfides was independently reported by the Shi, Zhang and Yin groups in 2015.413–416 The Shi group demonstrated nickel-catalyzed thiolation of β-methyl C(sp3)–H bonds of a broad range of aliphatic carboxamides with various disulfides (Scheme 150a).413 The reaction conditions were determined to be with (dppp)NiCl2 (10 mol%) as the precatalyst, BINOL (20 mol%) as a ligand, KTFA (2 equiv) as a base and Ag2O (1 equiv) in DMSO (1 mL) at 140 °C for 12 h. A wide variety of aliphatic acid derivatives and disulfides are tolerated in the reaction. An approach for thiolation of C(sp3)–H bonds that necessitates only Ni(OTf)2 (20 mol%) and LiOt-Bu (5 equiv) in DMF (0.5M) at 120 °C along with the disulfide or thiol was also developed (Scheme 150b).414
Scheme 150.
Nickel-Catalyzed β-C(sp3)–H Thiolation of Aliphatic Amides. a) Shi B.-F. et al., 2015.413 b) Shi X. et al., 2015.414 c) Zhang et al., 2015.415 d) Yin et al., 2015.416 e) Nickel-Catalyzed C(sp3)–H Aminoxylation of Aliphatic Amides (You, et al., 2017).417
The Zhang group demonstrated the thioetherification of β-C(sp3)–H bonds of aliphatic amides by using Ni(OTf)2 (10 mol%) as the catalyst, Ac-Gly-OH (20 mol%) as the ligand, Na2CO3 (2 equiv) as the base, and TBAI (4 equiv) as the additive in DMF at 140 °C for 12 h (Scheme 150c).415 1,2-diphenyl diselenide was also found to be reactive under the standard conditions. The nickel-catalyzed thiolation of C(sp3)–H bonds in aliphatic amides using 8-aminoquinoline as the bidentate auxiliary DG was also described by the Yin group in 2015 (Scheme 150d).416 In 2017, You and co-workers developed a protocol to prepare various N-alkoxyamine derivatives via nickel-catalyzed aminoxylation of an unactivated C(sp3)–H bond using stable nitroxyl radicals (Scheme 146e).417
4. Copper-Catalyzed, Coordination-Assisted Functionalization of C(sp3)–H Bonds.
Copper salts have been widely used to facilitate carbon–carbon and carbon–heteroatom bond formation in organic reactions. Use of copper as a catalyst offers practical advantages due to its abundance, lower cost and non-toxic nature.418 While copper-promoted C(sp3)–H functionalization through outer-sphere mechanisms, such as bioinspired oxidations/oxygenations and those involving radical or Cu-nitrene species, have been well-studied,418–423 copper-promoted inner-sphere C(sp3)–H bond activation reactions are rare.3,424–426 A typical catalytic cycle for inner sphere, Cu-promoted C(sp3)–H activation is proposed to proceed as follows (Scheme 151): the initial C(sp3)–H bond activation occurs through cyclocupration to form an alkyl–Cu(II) species 954, which is oxidized either by another Cu salt or a silver salt, to form an alkyl–Cu(III) species 955. Often, ligand exchange will then occur, followed by reductive elimination to give the final produt 960 with concomitant generation of a Cu(I) species, which must be reoxidized to Cu(II), often with a silver salt (Scheme 147, path I).428–435,437 In some circumstances, Cu(II) is thought to coordinate to the DG first to form 957, which can be oxidized to Cu(III), and then perform C(sp3)–H bond activation to give an alkyl–Cu(III) species 959 (Scheme 151, path II).427,436 One difficulty in using copper to functionalize C(sp3)–H bonds is that accessing the Cu(III) species is energetically challenging and often requires high temperature and high loading of copper salts. Thus far, copper-mediated C(sp3)–H activation reactions are limited to systems using the 8-aminoquinoline bidentate DG, and the most successful examples are intramolecular amidation and intermolecular acetoxylation reactions.
Scheme 151.
Plausible Catalytic Cycles for Copper-Catalyzed C(sp3)–H Functionalization Reactions
In 2014, the Kuninobu and Kanai groups (Scheme 152a)427 as well as the Ge group (Scheme 152b)428 independently reported examples of Cu-catalyzed intramolecular amidation of C(sp3)–H bonds, which provided efficient methods for the synthesis of β-lactams. The protocol reported by the Kanai group was catalyzed by Cu(OAc)2 with Ag2CO3 as oxidant, while CuCl and duroquinone were employed as the catalyst and oxidant, respectively, by the Ge group. In 2015, You and co-workers described a more economical and practical protocol that uses oxygen as the sole oxidant in the production of β-lactam compounds (Scheme 152c).429 An intermolecular version was disclosed by Qin and co-workers in 2016 (Scheme 152d).430 The potential utility of this method was further demonstrated in the synthesis of CNS disorder modulator HY-2901.
Scheme 152.
Cu-Catalyzed Intramolecular β-C(sp3)–H Amidation. a) Kanai et al., 2014.427 b) Ge et al., 2014.428 c) You et al., 2017.429 d) Cu-Promoted Intermolecular β-C(sp3)–H Amination (Qin et al., 2016).430
Copper-catalyzed, 8-aminoquinoline-directed β-methyl C(sp3)–H acyloxylation of aliphatic amides was reported by Ge and co-workers in 2014 (Scheme 153a).431 Cu(OAc)2 (50 mol%) was employed as catalyst, and AgOAc (3 equiv) was added as an acetate source and oxidant. However, the reaction must be run at a high temperature (170 °C). At the same time, Kanai and co-workers reported a copper-mediated C(sp3)–H acetoxylation of unactivated methyl groups using the 8-aminoquinoline DG. The use of 1.0 equivalent of Cu(OAc)2 as catalyst and 5.0 equivalents of AgOAc as the oxidant was a requisite (Scheme 153b).432 Copper-catalyzed, TBAB-accelerated C(sp3)–H acyloxylation with carboxylic acids was demonstrated by Zhang and co-workers (Scheme 153c).433
Scheme 153.
a) Cu-Catalzyed β-C(sp3)–H Acetoxylation of Aliphatic Amides (Ge et al., 2014).431 b) Cu-Mediated β-C(sp3)–H Acetoxylation of Aliphatic Amides (Kanai et al., 2014).432 c) Copper-Catalyzed C(sp3)–H Acyloxylation (Zhang et al., 2019).433 d) Cu-Mediated β-C(sp3)–H Aryloxylation and Vinyloxylation of Aliphatic Amides with Organosilanes (Zhang et al., 2015).434
In the same year, Zhang and co-workers reported a copper-mediated oxidation of β-C(sp3)–H bonds of propionamides with organosilanes (Scheme 153d).434 They observed that the desired products could not be isolated when phenol was employed under the standard conditions, which excluded a pathway involving C−O coupling of the C(sp3)–H bond with phenol generated from trimethoxy(phenyl)silane. O18-labeling experiments showed that Cu(OAc)2 served as the source of the oxygen in the aryloxylation product. The proposed catalytic cycle starts with C(sp3)–H bond activation and oxidation to an alkyl–Cu(III) species that then undergoes C–O reductive elimination between the alkyl group and an acetate ligand to provide the newly formed ester oxygen and a Cu(I) species ligated to the DG. Oxidation of Cu(I) to Cu(II), presumably with Ag2O and HOAc, then allows the acetate ligand to hydrolize to give a Cu(II) species bound to the DG and a terminal alcohol arising from the acetate ligand. Oxidation via disproportionation and subsequent transmetalation is followed by reductive elimination of the alcohol and the aryl or alkenyl group to provide the desired products. An oxidative demethylation of propionamides that might proceed via a similar mechanism of initial C(sp3)–H acetoxylation followed by subsequent oxidative decarboxylation was reported by the Fu group.435
In 2015, the Ge group demonstrated that polyfluoroarenes were effective coupling partners in the copper-promoted, cross-dehydrogenative coupling of unactivated C(sp3)–H bonds of aliphatic amides (Scheme 154a).436 It was found that pyridine was essential for this transformation and di-tert-butyl peroxide was the best oxidant. However, stoichiometric Cu(OAc)2 is needed in this reaction. Deuterium-labeling experiments show that an arylcopper intermediate may be involved in the C(sp3)–H bond cleavage step of the amide and that cyclometalation of the amide is not the turnover-limiting step.
Scheme 154.
a) Cu-Mediated β-C(sp3)–H Cross-Dehydrogenative Coupling of Aliphatic Amides with Polyfluoroarenes (Ge et al., 2015).436 b) Copper-Catalyzed C(sp3)–H Alkynylation/Annulation of Aliphatic Amides and Alkynyl Carboxylic Acids or Terminal Alkynes (Zhang et al., 2016).437
In 2016, Zhang and co-workers demonstrated a copper-catalyzed C(sp3)–H alkynylation/annulation reaction between aliphatic amides and alkynyl carboxylic acids or terminal alkynes (Scheme 154b).437 The authors postulated that the decarboxylation process may be promoted by the copper or silver salts to form phenylacetylide species.
Ge and co-workers observed a Cu(OAc)2-promoted carbonylation of β-C(sp3)–H bonds with nitromethane as the carbonyl source (Scheme 155).438 They posit that the expected product arises from C(sp3)–H bond activation of the MeNO2 followed by reductive elimination at the β-position to provide the standard reductive elimination product 995. The nitro-group then coordinates with Lewis acid to provide the iminium ion 996, which then undergoes an intramolecular cyclization with the amide nitrogen of the DG to form 997. Lewis-acid-promoted loss of oxygen results in the formation of a nitroso group that is subsequently eliminated in the formation of a cyclic imine 999. Upon treatment with water and subsequent oxidation, the desired product 1001 is generated.
Scheme 155.
Cu-Mediated β-C(sp3)–H Carbonylation of Aliphatic Amides with Nitromethane (Ge et al., 2016).438
5. Cobalt-Catalyzed Coordination-Assisted Functionalization of C(sp3)–H Bonds.
Co(III)-catalyzed functionalization of electronically activated C(sp3)–H bonds, such as benzylic C(sp3)–H bonds in 8-methylquinolines, has been well studied.439–446 In all these cases, Co(III) is proposed to first coordinate to the nitrogen of the quinoline before performing benzylic C(sp3)–H bond insertion, which allows a number of possible elementary steps to take place: alkene439–442 or alkyne443 coordination followed by migratory insertion, transmetalation followed by reductive elimination,444 carbene formation followed by migratory insertion,445 or nitrene formation via decarboxylative ring opening followed by migratory insertion.446 However, the use of cobalt catalysts in the transformations of nonactivated C(sp3)–H bonds is relatively underdeveloped.447–450
The first example of Co(III)-catalyzed nonactivated C(sp3)–H activation was not reported utill 2015. Ge and co-workers disclosed a cobalt-catalyzed intramolecular amination of nonactivated β-C(sp3)–H bonds of aliphatic amides directed by 8-aminoquinoline (Scheme 156).451 A wide range of 2,2-disubstituted propanamides bearing either linear or cyclic substituents at the α-position were successfully functionalized catalyzed by Co(OAc)2 in the presence of Ag2CO3 and PhCO2Na, providing a rapid acess of various β-lactams. Direct intermolecular amination of C(sp3)–H bonds catalyzed by Co(acac)3 (20 mol%) was also reported in the same communication. Radical intermediates were shown not to be involved in the catalytic cycle for the formation of β-lactams. However, in the case of γ-lactams, the addition of TEMPO dramatically reduces the yield, suggesting that a single electron oxidation produces an alkyl radical species that leads to the γ-lactams.
Scheme 156.
Cobalt-Catalyzed β-C(sp3)–H Intra- and Intermolecular Amidation of Aliphatic Amides (Ge et al., 2015).451
In 2017, Seayad, Dixon, and co-workers reported a thioamide-directed, cobalt-catalyzed amidation of C(sp3)–H bonds with (hetero)aryl-, and alkyl-substituted dioxazolones (Scheme 157a).452 A wide range of thioamides with N-containing heterocycles, such as piperazine, morpholine, and tetrahydroisoquinoline, were all tolerated and afforded the corresponding products in good yields. In 2019, the Matsunaga group uncovered a Cp*Co/chiral carboxylic acid (CCA) system for the enantioselective C(sp3)–H amidation of thioamides (Scheme 157b).453 Imide-protected amino acids were screened and CCA-1 showed high enantioselectivity. The reactivity and selectivity were further improved by combining sterically hindered cobalt catalyst (1013) with the MS13X zeolite. In order to overcome the limited structural diversity of imide-protected amino acids, chiral 2-aryl ferrocene carboxylic acids (CCA-2) were developed as a type of efficient chiral ligands that also displayed good reactivity and enantioselectivity by the Matsunaga group.454 Recently, Loh and co-workers demonstrated a Cp*CoIII-catalyzed, thioamide-directed amination of C(sp3)–H bonds using anthranils as the amine electrophiles.455
Scheme 157.
a) Thioamide-Directed Cobalt(III)-Catalyzed C(sp3)–H Amidation (Seayad et al., 2017).452 b) Enantioselective C(sp3)–H Amidation of Thioamides Catalyzed by a Cobalt(III)/Chiral Carboxylic Acid (Matsunaga et al., 2019).453 c) Enantioselective C(sp3)–H Amidation of Thioamides with Chiral 2-Aryl Ferrocene Carboxylic Acid Ligand (Matsunaga et al., 2019).454 d) Cobalt(III)-Catalyzed C(sp3)–H Amination with Anthranils (Loh et al., 2017).455
Co(acac)2-catalyzed C(sp3)–H carbonylative cyclization of aliphatic amides was independently reported by the Lei,456 Sundararaju,457 and Gaunt458 groups (Scheme 158). Ag2CO3 is found to be an excellent oxidant for this type of carbonylation reaction.
Scheme 158.
8-Aminoquinoline-Directed, Cobalt-Catalyzed Carbonylation of Unactivated C(sp3)–H bonds of Aliphatic Amides. [a) Lei et al., 2017;456 b) Sundararaju et al., 2017;457 c) Gaunt et al., 2017 458].
Zhang and co-workers demonstrated a cobalt-catalyzed C(sp3)–H alkynyation/cyclization of aliphatic amides and terminal alkynes (Scheme 159).459 Aromatic amides bearing either electron-withdrawing or electron-donating groups are also compatible under similar reaction conditions. Control experiments indicated that a catalytic amount of Ag2CO3 (10 mol %) and stoichiometric TBAI were required for this cyclization.
Scheme 159.
Cobalt-Catalyzed β-C(sp3)–H Alkynylation/Cyclization of Aliphatic Amides with Alkynyl Terminal Alkynes (Zhang et al., 2015).459
In 2017, the Shi group reported a Co(OAc)2·4H2O-catalyzed, 8-aminoquinoline-directed multiple C(sp3)–H activation strategy for the synthesis of bicyclo[n.1.0]alkanes (Scheme 160).460 This reaction is proposed start with the coordination of the Co(II) species with 8-aminoquinoline and the alkene, which is in situ oxidized to the active Co(III) species 1033, followed by methyl C(sp3)–H activation and alkene insertion to form intermediate 1035. The second methylene C(sp3)–H activation of intermediate 1035 then occurs to give cobaltacycle intermediate 1036. Subsequent reductive elimination and decomplexation leads to the formation of bicyclo[n.1.0]alkane prfemoduct. Reoxidation of the Co(I) species with Ag(I) will regenerate the active Co(III) species to close the catalytic cycle.
Scheme 160.
Synthesis of Bicyclo[n.1.0]alkanes by a Cobalt-Catalyzed Twofold C(sp3)–H Activation Cascade (Shi et al., 2017).460
6. Rhodium-Catalyzed Coordination-Assisted Functionalization of C(sp3)–H Bonds.
Compared to Rh-catalyzed C(sp2)–H functionalization, Rh-catalyzed activation of C(sp3)–H bonds has received much less attention.6,9,461–463 The functionalization of electronically activated C(sp3)–H bonds, such as allylic,464 benzylic, 465–473 and those adjacent to a heteroatoms,474–478 have been investigated, but these systems are outside the scope of this review. Meanwhile, Rh-catalyzed activation of unactivated C(sp3)–H bonds is more challenging and still relatively rare.
The Cp*Rh(III)-catalyzed β-arylation of unactivated C(sp3)–H bonds of 2-alkylpyridine derivatives was reported by the Glorius group in 2015 (Scheme 161a).479 In this case, [Cp*Rh(CH3CN)3](SbF6)2 (5.0 mol%) combined with Ag2O (2.5 equiv), and triphenylboroxin (2.0 equiv) was effective for generating the desired product. Arylation of the C(sp3)–H bond of 8-benzylquinoline was found to proceed at a lower temperature (80 °C) with a lower loading of the triarylboroxine and the oxidant. In this case, the products are attractive due to the biological properties of functionalized quinolines. In 2017, Zhao and co-workers achieved a Rh(III)-catalyzed, trimethylpyrazole-directed arylation of unactivated C(sp3)–H bonds (Scheme 161b).480 A wide range of functional groups were tolerated under the optimized conditions.
Scheme 161.
a) Rh(III)-Catalyzed β-C(sp3)–H Arylation of 2-Alkylpyridine Derivatives (Glorius et al., 2015).479 b) Trimethylpyrazole-Directed Rh(III)-Catalyzed Arylation of Unactivated C(sp3)–H Bonds (Zhao et al., 2017).480
In 2015, the You group reported a Rh(III)-catalyzed, pyridine-directed amination of unactivated C(sp3)–H bonds using sulfonamides as the nitrogen coupling partner at room temperature (Scheme 162a).481 [Cp*Rh(CH3CN)3](SbF6)2 and PhI(OAc)2 were found to be the best catalyst/oxidant combination for this transformation. NaOAc (30 mol%) was required to improve the yield of the product. A variety of 2-ethylpyridine derivatives were suitable substrates for this transformation, and the method was compatible with different sulfonamides, such as aryl sulfonamides possessing an electron-withdrawing group on the aromatic ring and alkyl sulfonamides. Furthermore, cyclohexane ketoximes and (E)-2-methylcyclohexanone O-methyl oxime were viable substrate classes under the reaction conditions. Based on control experiments, the authors postulated that a nitrene intermediate is likely involved in the sulfonamidation process.
Scheme 162.
Rh(III)-Catalyzed β-C(sp3)–H Amidation Reactions. a) With Sulfonamides Directed by Pyridine and Oxime (You et al., 2015).481 b) With 3-Substituted 1,4,2-Dioxazol-5-Ones Directed by Oxime (Li et al., 2015).482 c) Amidation of 1-Arylethan-1-ol Oxime With 3-Substituted 1,4,2-Dioxazol-5-Ones Directed by Oxime (Xu et al., 2018).483 d) With Arylsulfonamides Directed by 3,5-Dimethylpyrazole Amides (Wang et al., 2019).484
Rh(III)-catalyzed amidation of unactivated C(sp3)–H bonds of ketoximies was reported by the Li group in 2015 with [{RhCp*Cl2}2]/AgSbF6 as the catalyst and AgOAc (8 mol%) as the additive (Scheme 162b).482 3-Substituted 1,4,2-dioxazol-5-ones reagents were used as nitrogen electrophiles, and a variety of substituents at the 3-postion were tolerated, including aryl groups bearing electron-donating or electron-withdrawing groups at different positions. Compatible substrates include 8-methylquinoline, the oxime ether of L-(−)-carvone, and even aliphatic cyclic and acyclic ketoximes. Deuterium-labelling experiments revealed that the C–H activation step is irreversible and thus the turnover-limiting step in the catalytic cycle. In 2018, the Xu group expanded the scope of Rh(III)-catalyzed amidation to 1-arylethan-1-ol oxime substrates (Scheme 162c).483 Notably, C(sp3)–H bonds were functionalized in preference to potentially more reactive C(sp2)–H bonds. In 2019, the Wang group developed a Rh(III)-catalyzed bidentate-DG-directed amidation reaction with arylsulfonamides (162d).484
Rh(I)-catalyzed alkenylation of primary C(sp3)–H bonds of ketone derivatives with alkynes was documented by Dong and co-workers in 2017 (Scheme 163).485 A hydrazone derived from 2-hydrazinyl-4-methylpyridine was used as the DG and was installed in situ. The alkenylation products were converted into corresponding alkylated ketones by reduction with H2 over Pd/C. The hydrazone auxiliary could also be removed in one pot through the addition of aqueous HCl. A mechanism involving oxidative addition of Rh(I) to the C(sp3)–H bond to form a Rh(III)(Alkyl)(H) intermediate was proposed for this transformation based on the isolation of an analogous Ir(III)(Alkyl)(H) complex.
Scheme 163.
Rh(I)-catalyzed β-C(sp3)–H alkenylation of ketones using an in situ-installed DG (Dong et al., 2017).485
7. Iridium-Catalyzed Coordination-Assisted Functionalization of C(sp3)–H Bonds.
It is well known that iridium complexes react with a broad range of directing-group-containing compounds to form iridacycles via C–H bond activation.486 However, early reports of iridium-catalyzed C(sp3)–H activation were limited to C–H bonds adjacent to heteroatoms,487–492 which is outside the scope of this review as this class of C–H bonds are uniquely activated.
Pyridine has been used as a DG to achieve the Ir-catalyzed borylation of 2-alkylpyridines (Scheme 164a–c). In 2013, the Sato group discovered an Ir(I)-catalyzed, pyridine-directed triple C(sp3)–H borylation in which pyridine DGs bearing electron-donating functional groups at the 4-position gave the desired products in high yield.493 Ir-catalyzed primary and secondary C(sp3)–H borylation of 2-alkylpyridines using silica-supported monophosphine–Ir catalysts was reported by the Sawamura group in 2013 (Scheme 163b).494 The silica-supported monophosphine ligands show significant ligand-accelerating effect in this C(sp3)–H borylation transformation, and is crucial for the borylation of methylene C(sp3)–H bonds. In the same year, Sawamura and co-workers also developed a novel type of polystyrene(PS)-phosphane covalently bound hybrid for Ir-catalyzed C(sp3)–H borylation of 2-pentylpyridine.495 Enantioselective borylation of secondary C(sp3)–H bonds with an Ir(I) catalyst was reported by Sawamura and co-workers in 2019.496 The extended aromatic environment on the BINOL-based monophosphite ligand (L87) increased the enantioselectivity significantly over atropisomeric BINOL-based monophosphite ligands. DFT calculations suggested the the monophosphite-Ir-tris(boryl) complex forms a narrow chiral reaction pocket.
Scheme 164.
Ir(I)-Catalyzed Pyridine Directed C(sp3)–H Borylation. a) Triple Methyl C(sp3)–H Borylation (Sato et al., 2013).493 b) Using Silica-Supported Monophosphine–Ir Catalysts (Sawamura et al., 2013).494 c) Enantioselective Methylene C(sp3)–H Borylation Using BINOL-Based Monophosphite Ligand (Sawamura et al., 2019).496 d) Ir(Ⅰ)-Catalyzed Amide Directed Enantioselective C(sp3)–H Borylation of Cyclopropanes with Chiral Bidentate Boryl Ligand (Xu et al., 2019).498
In 2019, the group of Xu elegantly developed a new type of chiral bidentate boryl ligands (CBLs) containing a pyridyl group, a chiral boryl moiety, and a sterically bulky aryl, which successfully enabled the iridium-catalyzed asymmetric C(sp2)–H borylation of diarylmethylamines.497 Inspired by this promising result, they further extended this mode to Ir(I)-catalyzed, enantioselective C(sp3)–H borylation of cyclopropanes using CBL (L88) as a chiral ligand (Scheme 164d).498 A variety of cyclopropanecarboxamides with α-quaternary carbon centers are well tolerated, providing β-borylated products with high enantioselectivities (up to 96% ee). The resulting borylated products could be used as versatile precursors for subsequent stereospecific transformations and the synthesis of a bioactive compound, Levomilnacipran, was achieved.
Ir(III)-catalyzed C–H amidation of methyl C(sp3)–H bonds of ketoximes with sulfonyl or acyl azides under mild conditions was reported by the Chang group in 2014 (Scheme 165a).499 A range of α-methyl cyclic ketoximes and acyclic ketoximes reacted with acyl or sulfonyl azides to afford the corresponding products in moderate to excellent yields. This amidation protocol occurred exclusively at the methyl C(sp3)–H bond without functionalizing methylene C(sp3)–H bonds. As is evident the broad substrate scope and mild conditions of this procedure, it is effective for late-stage functionalization of natural products and complex synthetic compounds, such as a derivative of santonin.
Scheme 165.
Ir(III)-Catalyzed C(sp3)–H Amidation. a) Ketoximes with Sulfonyl or Acyl Azides (Chang et al., 2014).499 b) Alcohol-Derived Oximes (Chang et al., 2014).500 c) Ketoximes with Azidoformates (Chang et al., 2016).501 d) Picolinamide-Directed Methylene C(sp3)–H Sulfonylamidation (Zeng et al., 2016).502
The same group later extended this mode of reactivity to synthesize various 1,2-amino alcohols from alcohol-derived substrates (Scheme 165b).500 Azidoformates have also been investigated by the Chang group as nitrogen coupling partners in the synthesis of primary alkylamines (Scheme 165c).501 An Ir(III)-catalyzed, bidentate-auxiliary-directed intermolecular sulfonylamidation of methylene C(sp3)–H bonds with azides was disclosed by the Zeng group in 2016 (Scheme 165d).502
In 2015, [(Cp*IrCl2)2]-catalyzed C(sp3)–H arylation of ketoximes with diaryliodonium triflate salts was reported by the Shi group (Scheme 166).503 Reaction optimization revealed that the addition of PivOH and 4 Å MS resulted in a significantly improved yield. This protocol was demonstrated to serve as an efficient tool for the synthesis of C(β)-arylated lanostane and for the late-stage C(sp3)–H arylation of oleanolic acid.
Scheme 166.
Ir(III)-Catalyzed C(sp3)–H Arylation of Ketoximes with Diaryliodonium Salts (Shi et al., 2015).503
In 2020, the Echavarren group reported an Ir(III)-catalyzed primary C(sp3)–H alkynylation of ketones and alcohols directed by oximes using a bromo-alkyne (1.2 equiv), catalyzed by [Cp*IrCl2]2 (7 mol%), with substoichiometric amounts of AgSbF6(30 mol%) and LiOAc (30 mol%), with Ag2CO3 (1 equiv) in 1,2-DCE. (Scheme 167a).504 Under the same reaction conditions, pyridine-, pyrazole-, and pyrazine-directed primary C(sp3)–H alkynylation was also achieved. 2-Acylimidazol-directed, Ir(III)-catalyzed primary C(sp3)–H alkynylation using alkynyl bromides was reported by the Chatani group in 2020 (Scheme 167b).505 During the course of mechanistic studies, a catalytically competent six-membered iridacycle was successfully isolated and characterized.
Scheme 167.
a) Ir(III)-Catalyzed β-C(sp3)–H Alkynylation Directed by Oximes (Echavarren et al., 2020).504 b) Ir(III)-Catalyzed C(sp3)–H Alkynylation of 2-Acylimidazoles (Chatani et al., 2020).505
8. Iron-Catalyzed Coordination-Assisted Functionalization of C(sp3)–H Bonds.
Iron is the second-most abundant metal in the earth’s crust after aluminium and is inexpensive compared to the precious metals often used in catalysis.506 Moreover, unlike palladium, iron can adopt oxidation states from −2 to +5 (and in rare cases +6). High-oxidation state iron catalysts (+3, +4, or even +5) are most commonly used in nonactivated C(sp3)–H bond functionalization reactions. In terms of reactivity, iron-catalyzed C(sp3)–H bonds functionalization is currently limited to C−C bond-forming reactions, such as arylation and alkylation.507,508
In 2013, Nakamura and co-workers reported the first 8-aminoquinoline-directed C(sp3)–H arylation of aliphatic carboxamides catalyzed by Fe(acac)3 (Scheme 168a).509 Arylzinc reagents were employed as nucleophiles in combination with 1,2-bis(diphenylphosphino)benzene (dppbz) as ligand and 1,2-dichloro-2-methylpropane (DCIB) as the organic oxidant. This reaction is sensitive to the choice of ligand and shows a complete preference for C–H bond activation of the methyl group over a potentially competitive benzylic site. They also found that the arylation reaction was very sensitive to the structure of the substrate. All of the reactive substrates have an α-quaternary carbon center while those containing either a wider or small CH3−C−C(=O) bond angle (e.g. 1-methylcyclobutanecarboxamide and 1-methylcyclopropanecarboxamide) are unreactive. KIE studies and the observed selectivity and reactivity indicate that the reaction proceed through the formation of a metallacycle intermediate via rate-determining C–H cleavage rather than a radical pathway. In 2015, the direct methylation of a C(sp3)–H bond with an AlMe3/Fe(III) system was also reported by the Nakamura group (Scheme 168b).510 In 2017, the Nakamura group then expanded this methodology by using aryl-, heteroaryl-, and alkenylboron reagents as coupling partners (Scheme 168c).511
Scheme 168.
Iron-Catalyzed 8-Aminoquinoline-Directed β-C(sp3)–H Functionalization of Aliphatic Amides. a) Arylation with Arylmagnesium Bromides (Nakamura et al., 2013).509 b) Methylation with Trimethylaluminum (Nakamura et al., 2015).510 c) Arylation and Alkenylation of C(sp3)–H Bonds with Organoborates (Nakamura et al., 2017).511 Iron-Catalyzed Triazole-Directed β-C(sp3)–H Functionalization of Aliphatic Amides. d) Arylation with Arylmagnesium Bromides (Ackermann et al., 2014).241 e) Alkylation with Alkylmagnesium Bromides (Ackermann et al., 2015).512
The triazolyldimethylmethyl (TAM) moiety was used as a directing group by the Ackermann group for the iron-catalyzed arylation and methylation of unactivated C(sp3)–H bonds (Scheme 168d–e).241,512 The high selectivity for functionalization of the methyl C(sp3)–H bond over the weaker benzylic C(sp3)–H bond is indicative of an organometallic C–H metalation mechanism.
9. Ruthenium-Catalyzed Coordination-Assisted Functionalization of C(sp3)–H Bonds.
Ruthenium catalysts are among the earliest catalysts employed for C–H bond activation.48,513–515 Ruthenium-catalyzed direct functionalization of electronically biased C(sp3)–H bonds, such as those at benzylic positions or adjacent to heteroatoms, has been well studied and is outside of the scope of this review.516–526 Beyond examples with activated C–H bonds, the number of C(sp3)–H activation reactions catalyzed by ruthenium is rare and only two examples have been reported thus far. In 2011, the Chatani group developed the first Ru(0)-catalyzed carbonylation of unactivated β-C(sp3)–H bonds of aliphatic amides to give the corresponding succinimides (Scheme 169).527 The presence of the 2-pyridinylmethylamino moiety is required as an N,N-bidentate ligand, as evidenced formation of a dinuclear ruthenium complex when the substrate is combined with Ru3(CO)12. Although the reaction still proceeds when water is removed, the efficiency of the reaction decreases significantly. Interestingly, in the absence of ethylene, no carbonylation product could be detected. The authors postulated that ethylene functions as a hydrogen acceptor. Moreover, long reaction time (5 days) is needed.
Scheme 169.
Ru(0)-Catalyzed β-C(sp3)–H Carbonylation of Aliphatic Amides (Chatani et al., 2011).527
A second example of Ru(0)-catalyzed functionalization of unactivated β-C(sp3)–H bonds was reported by You and co-workers in 2016.528 They described a Ru3(CO)12-catalyzed, pyridine-directed silylation of unactivated C(sp3)–H bonds (Scheme 170). A wide range of 2-alkylpyridines were converted to the corresponding silylated products in modest to excellent yields.
Scheme 170.
Ru(0)-Catalyzed Intermolecular Direct Silylation of Unreactive C(sp3)–H Bonds (You et al., 2016).528
10. Platinum-Catalyzed Coordination-Assisted Functionalization of C(sp3)–H Bonds.
Pt catalysts have a rich history in activation of C(sp3)–H bonds via an electrophilic mechanism, dating back to pioneering work by Shilov.529,530 Pt salts had also been shown to coordinate with amino acids.531 In 2001, Sames and co-workers reported hydroxylation of α-amino acids using K2PtCl4 as a catalyst and CuCl2 as a stoichiometric oxidant (Scheme 171).532 Hydroxylation of various aliphatic substrates, including n-butylamine and n-pentanoic acid, have also been observed. A coordination-directed mechanism was proposed based on the regioselectivity of reactions with α-amino acids.
Scheme 171.
Pt(II)-Catalyzed Intramolecular Hydroxylation of Unreactive C(sp3)–H Bonds (Sames et al., 2001).532
11. Conclusions and Future Outlook
Transition-metal-catalyzed, coordination-assisted C(sp3)–H functionalization has grown rapidly as a research area over the last few decades. Continued developments in this field hold great potential to revolutionize synthetic planning and execution. As illustrated, the proliferation of different metal catalysts, the implementation of new strategies, and the expansion of directing groups (including native functional groups, auxiliaries, and transient groups), has opened the door to functionalization of more strategic C–H bonds in organic molecules. This flexibility allows chemists to envision a greater number of C(sp3)–H functionalization disconnections in synthetic planning, both in target- and diversity-oriented pursuits.
This progress notwithstanding, numerous challenges remain towards the long-term vision of being able to functionalize any desired C(sp3)–H bond within a molecule of interest. This includes the direct use of chemical feedstocks without introduction of stoichiometric auxiliaries, the ability to control site- and stereoselectivity through catalyst and ligand design, and the improvement of catalyst turnover. We anticipate that efforts devoted towards these ends will stimulate more exciting advances at the nexus of organic synthesis, organometallic chemistry, and homogenous catalysis in the coming era.
Figure 5.
Bidentate Transient DGs for C(sp3)–H Activation.
Scheme 21.
Methoxyamide-Directed Arylation of Methyl β-C–H Arylation of α-Amino Acids (Yu et al., 2015).108
Scheme 23.
Palladium-Catalyzed Asymmetric β-C(sp3)–H Arylation of Free Carboxylic Acids (Yu et al., 2016).97
Scheme 51.
Palladium-Catalyzed Phosphonation of C(sp3)–H Bonds (Dong et al., 2019).157
Scheme 75.
Palladium-Catalyzed Picolinamide-Directed δ-C(sp3)–H Arylation (Maiti et al., 2019).224
Scheme 120.
Palladium-Catalyzed γ-C(sp3)–H Carbonylation of Aliphatic Alcohols (Yu et al., 2019).332
Scheme 148.
Nickel-Catalyzed Oxidative Coupling of Unactivated C(sp3)–H Bonds in Aliphatic Amides with Terminal Alkynes. a) Alkynylation/Cyclization To Access γ-Lactams (Zhang et al., 2016).409 b) Alkynylation (Shi et al., 2017).410
ACKNOWLEDGMENT
This work is supported by the Natural Science Foundation of China (21925109, 21772170 for B.F.S.), Outstanding Young Talents of Zhejiang Province High-level Personnel of Special Support (ZJWR0108 for B.F.S.), Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University (B.F.S.), the National Institute of Health (5R35GM125052-04 for K.M.E.), National Science Foundation Graduate Research Fellowship (NSF/DGE-1842471 for A.M.R.), and the Schimmel Family Endowed Fellowship (C.Z.R.). Dedicated to the 100th anniversary of Chemistry at Nankai University.
ABBREVIATIONS
- Å
Ångstrom
- Ac
acetyl
- acac
acetylacetonate
- APAQ
N-acetyl-protected aminoethyl quinoline
- AQ
8-aminoquinoline
- Ar
aryl
- atm
atmosphere
- BDE
bond dissociation energy
- Bn
benzyl
- Boc
tert-butyloxycarbonyl
- BQ
1,4-benzoquinone
- Bu
butyl
- C
Celsius
- CAN
ceric ammonium nitrate
- Cbz
carboxylbenzyl
- Co
cobalt
- cod
1,5-cyclooctadiene
- CPA
chiral phosphoric acid
- Cp*
pentamethyl cyclopentadienyl
- Cu
copper
- dba
dibenzylideneacetone
- DCE
1,2-dichloroethane
- DCM
dichloromethane
- DDQ
2,3-Dichloro-5,6-dicyano-p-benzoquinone
- DFT
density functional theory
- DG
Directing Group
- 2,5-DHBQ
2,5-dihydroxy-p-benzoquinone
- DMF
N,N-dimethylformamide
- DMSO
dimethyl sulfoxide
- de
diastereomeric excess
- DMPU
N,N′-dimethylpropyleneurea
- dr
diastereomeric ratio
- ee
enantiomeric excess
- equiv
equivalent
- Et
ethyl
- Fe
iron
- FG
Functional Group
- HFIP
hexafluoroisopropanol
- Ir
Iridium
- L
neutral ligand
- MIA
2-Methoxyiminoacetyl
- MPAA
mono-N-protected amino acid
- MPAO
mono-N-protected aminomethyl oxazoline
- NFSI
N-fluorobenzenesulfonimide
- NHC
N-heterocyclic carbenes
- Ni
nickel
- Ns
nosyl
- p
para
- PA
picolinamide
- Pd
palladium
- Ph
phenyl
- Phe
phenylalanine
- phen
phenanthroline
- Phth
phthalimido
- Pin
pinacolato
- PIP
2-pyridylisopropylamine
- Piv
pivaloyl
- Pt
Platinum
- r.t
room temperature
- Rh
rhodium
- Ru
ruthenium
- t
tert
- TBAI
tetra-n-butylammonium iodide
- TBDPS
tert-butyldiphenylsilyl
- TBHP
tert-Butyl hydroperoxide
- TDG
transient directing group
- TEMPO
(2,2,6,6-tetramethylpiperidin-1-yl)oxyl
- Tf
trifluoromethylsulfonyl
- TFA
trifluoroacetic acid
- THF
tetrahydrofuran
- TIPS
triisopropylsilyl
- Tle
tert-leucine
- TM
Transition Metal
- TOAB
tetra-n-octylammonium bromide
- Tol
p-tolyl, 4-methylphenyl
- X
halogen or anionic ligand
Biographies
Bin Liu was born in Shangrao, China. He received his B.Sc. from Nanchang University in Nanchang, Jiangxi in 2009. He earned his Ph.D. in 2014 under the supervision of Prof. Bing-Feng Shi at Zhejiang University, where his research focused on the nitrogen containing functional group directed C–H functionalization. In 2016, he joined the laboratory of Prof. Garret. M. Miyake at University of Colorado Boulder as a postdoctoral researcher, focusing on visible light promoted reactions based on electron donor-acceptor complex and synthesis of brush copolymers. In 2017, He moved to the Colorado State University with the Miyake group. In 2021, he joined the College of Chemistry at Nanchang University as a Professor.
Andrew M. Romine was born in the Philadelphia area. He received his B.S. in chemistry and business, economics, and management from the California Institute of Technology in 2016 performing research in the laboratories of Prof. G. Jeffrey Snyder (2013) and Prof. Robert H. Grubbs (2015–2016). During his undergraduate, he also attended a summer research program at Institut Català d’Investigació Química in 2014 performing research in the laboratory of Prof.Vladimir V. Grushin. Andrew is currently an NSF fellow and fifth-year doctoral student at Scripps Research working in the laboratory of Prof. Keary M. Engle.
Camille Z. Rubel received a B.S. degree in chemistry and a minor in gender and women’s studies from UC Berkeley where she carried out undergraduate research under the supervision of Prof. John Hartwig. She is currently a graduate student in the group of Prof. Keary Engle at the Scripps Research Institute, and her research is supported by the Schimmel Family Endowed Fellowship.
Keary M. Engle grew up in West Michigan and received his undergraduate education at University of Michigan (B.S., 2007) during which time he carried out research in the laboratory of Prof. Adam J. Matzger. Following a one-year stint as a Fulbright Scholar at the Max-Plank-Institute für Kohlenforschung with Prof. Manfred T. Reetz, he completed graduate studies at the Scripps Research Institute with Prof. Jin-Quan Yu (Ph.D., 2013) and the University of Oxford with Profs. Véronique Gouverneur and John M. Brown (D.Phil., 2013). After two years as an NIH Postdoctoral Fellow at California Institute of Technology with Prof. Robert H Grubbs, he began his independent career as an Assistant Professor at the Scripps Research Institute in 2015 and was promoted to Professor in 2020.
Bing-Feng Shi grew up in Shandong, China and received his B.S. degree from Nankai University in 2001 and a Ph.D. degree from the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences under the guidance of Professor Biao Yu in 2006. Following time as a postdoctoral fellow at the University of California, San Diego (2006–2007), he moved to The Scripps Research Institute working with Professor Jin-Quan Yu as a research associate. In 2010, he joined the Department of Chemistry at Zhejiang University as a professor. His research focus is transition metal-catalyzed C–H functionalization and the synthetic applications.
Footnotes
Notes
The authors declare no competing financial interest
REFERENCES
- (1).Labinger JA; Bercaw JE Understanding and Exploiting C–H Bond Activation. Nature 2002, 417, 507–514. [DOI] [PubMed] [Google Scholar]
- (2).Kakiuchi F; Murai S Catalytic C–H/Olefin Coupling. Acc. Chem. Res. 2002, 35, 826–834. [DOI] [PubMed] [Google Scholar]
- (3).Daugulis O; Do H-Q; Shabashov D Palladium- and Copper-Catalyzed Arylation of Carbon-Hydrogen Bonds. Acc. Chem. Res. 2009, 42, 1074–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Chen X; Engle KM; Wang D-H; Yu J-Q Palladium(II)-CataIyzed C–H Activation/C–C Cross–Coupling Reactions: Versatility and Practicality. Angew. Chem. Int. Ed. 2009, 48, 5094–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Lyons TW; Sanford MS Palladium-Catalyzed Ligand-Directed C–H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Ackermann L Carboxylate-Assisted Transition-Metal-Catalyzed C–H Bond Functionalizations: Mechanism and Scope. Chem. Rev. 2011, 111,1315–1345. [DOI] [PubMed] [Google Scholar]
- (7).Colby DA; Bergman RG; Ellman JA Rhodium-Catalyzed C−C Bond Formation via Heteroatom-Directed C–H Bond Activation. Chem. Rev. 2010, 110, 624–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Baudoin O Transition Metal-Catalyzed Arylation of Unactivated C(sp3)–H Bonds. Chem. Soc. Rev. 2011, 40, 4902–4911. [DOI] [PubMed] [Google Scholar]
- (9).Song G; Wang F; Li X C−C, C−O and C−N Bond Formation via Rhodium(III)-Catalyzed Oxidative C–H Activation. Chem. Soc. Rev. 2012, 41, 3651–3678. [DOI] [PubMed] [Google Scholar]
- (10).Zheng C; You S-L Recent Development of Direct Asymmetric Functionalization of Inert C–H Bonds. RSC Adv. 2014, 4, 6173–6214. [Google Scholar]
- (11).Liu C; Yuan J; Gao M; Tang S; Li W; Shi R; Lei A Oxidative Coupling between Two Hydrocarbons: An Update of Recent C–H Functionalizations. Chem. Rev. 2015, 115, 12138–12204. [DOI] [PubMed] [Google Scholar]
- (12).Yang Y; Lan J; You J Oxidative C–H/C–H Coupling Reactions between Two (Hetero)Arenes. Chem. Rev. 2017, 117, 8787–8863. [DOI] [PubMed] [Google Scholar]
- (13).Chen Z; Rong M-Y; Nie J; Zhu X-F; Shi B-F; Ma J-A Catalytic Alkylation of Unactivated C(sp3)–H Bonds for C(sp3)–C(sp3) Bond Formation. Chem. Soc. Rev. 2019, 48, 4921–4942. [DOI] [PubMed] [Google Scholar]
- (14).Zhang Q; Shi B-F From Reactivity and Regioselectivity to Stereoselectivity: An Odyssey of Designing PIP Amine and Related Directing Groups for C–H Activation. Chin. J. Chem. 2019, 37, 647–656. [Google Scholar]
- (15).Park Y; Kim Y; Chang S Transition Metal-Catalyzed C–H Amination: Scope, Mechanism, and Applications. Chem. Rev. 2017, 117, 9247–9301. [DOI] [PubMed] [Google Scholar]
- (16).Dong Z; Ren Z; Thompson SJ; Xu Y; Dong G Transition-Metal-Catalyzed C–H Alkylation Using Alkenes. Chem. Rev. 2017, 117, 9333–9403 [DOI] [PubMed] [Google Scholar]
- (17).Mkhalid IAI; Barnard JH; Marder TB; Murphy JM; Hartwig JF C–H Activation for the Construction of C−B Bonds. Chem. Rev. 2010, 110, 890–931. [DOI] [PubMed] [Google Scholar]
- (18).Gandeepan P; Müller T; Zell D; Cera G; Warratz S; Ackermann L 3d Transition Metals for C–H Activation. Chem. Rev. 2019, 119, 2192–2452. [DOI] [PubMed] [Google Scholar]
- (19).Zhang M; Zhang Y; Jie X; Zhao H; Li G; Su W Recent Advances in Directed C–H Functionalizations Using Monodentate Nitrogen-Based Directing Groups. Org. Chem. Front. 2014, 1, 843–895. [Google Scholar]
- (20).Chen Z; Wang B; Zhang J; Yu W; Liu Z; Zhang Y Transition Metal-Catalyzed C–H Bond Functionalizations by the Use of Diverse Directing Groups. Org. Chem. Front 2015, 2, 1107–1295. [Google Scholar]
- (21).Daugulis O; Roane J; Tran LD Bidentate, Monoanionic Auxiliary-Directed Functionalization of Carbon-Hydrogen Bonds. Acc. Chem. Res. 2015, 48, 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Xu Y; Dong G sp3 C–H Activation via Exo-Type Directing Groups. Chem. Sci. 2018, 9, 1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).He G; Wang B; Nack WA; Chen G Syntheses and Transformations of α-Amino Acids via Palladium-Catalyzed Auxiliary-Directed sp3 C–H Functionalization. Acc. Chem. Res. 2016, 49, 635–645. [DOI] [PubMed] [Google Scholar]
- (24).Hummel JR; Boerth JA; Ellman JA Transition-Metal-Catalyzed C–H Bond Addition to Carbonyls, Imines, and Related Polarized π Bonds. Chem. Rev. 2017, 117, 9163–9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Newton CG; Wang S-G; Oliveira CC; Cramer N Catalytic Enantioselective Transformations Involving C–H Bond Cleavage by Transition-Metal Complexes. Chem. Rev. 2017, 117, 8908–8976. [DOI] [PubMed] [Google Scholar]
- (26).Chu JCK; Rovis T Complementary Strategies for Directed C(sp3)–H Functionalization: A Comparison of Transition-Metal-Catalyzed Activation, Hydrogen Atom Transfer, and Carbene/Nitrene Transfer. Angew. Chem. Int. Ed. 2018, 57, 62–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Saint-Denis TG; Zhu R-Y; Chen G; Wu Q-F; Yu J-Q Enantioselective C(sp3)–H Bond Activation by Chiral Transition Metal Catalysts. Science 2018, 359, eaao4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Sambiagio C; Schönbauer D; Blieck R; Dao-Huy T; Pototschnig G; Schaaf P; Wiesinger T; Zia MF; Wencel-Delord J; Besset T; et al. Comprehensive Overview of Directing Groups Applied in Metal-Catalysed C–H Functionalisation Chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Abrams DJ; Provencher PA; Sorensen EJ Recent Applications of C–H Functionalization in Complex Natural Product Synthesis. Chem. Soc. Rev. 2018, 47, 8925–8967. [DOI] [PubMed] [Google Scholar]
- (30).He C; Whitehurst WG; Gaunt MJ Palladium-Catalyzed C(sp3)–H Bond Functionalization of Aliphatic Amines. Chem 2019, 5, 1031–1058. [Google Scholar]
- (31).Mishra AA; Subhedar D; Bhanage BM Nickel, Cobalt and Palladium Catalysed C–H Functionalization of Un-Activated C(sp3)–H Bond. Chem. Rec 2019, 19, 1829–1857. [DOI] [PubMed] [Google Scholar]
- (32).Yoshino T; Matsunaga S Cobalt-Catalyzed C(sp3)–H Functionalization Reactions. Asian J. Org. Chem. 2018, 7, 1193–1205. [Google Scholar]
- (33).Rej S; Ano Y; Chatani N Bidentate Directing Groups: An Efficient Tool in C–H Bond Functionalization Chemistry for the Expedient Construction of C–C Bonds. Chem. Rev. 2020, 120, 1788–1887. [DOI] [PubMed] [Google Scholar]
- (34).He J; Wasa M; Chan KSL; Shao Q; Yu J-Q Palladium-Catalyzed Transformations of Alkyl C–H Bonds. Chem. Rev. 2017, 117, 8754–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Wang J; Dong G Palladium/Norbornene Cooperative Catalysis. Chem. Rev. 2019, 119, 7478–7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).McMurray L; O’Hara F; Gaunt MJ Recent Developments in Natural Product Synthesis Using Metal-Catalysed C–H Bond Functionalisation. Chem. Soc. Rev. 2011, 40, 1885–1898. [DOI] [PubMed] [Google Scholar]
- (37).Gutekunst WR; Baran PS C–H Functionalization Logic in Total Synthesis Chem. Soc. Rev. 2011, 40, 1976–1991. [DOI] [PubMed] [Google Scholar]
- (38).Yamaguchi J; Yamaguchi AD; Itami K C–H Bond Functionalization: Emerging Synthetic Tools for Natural Products and Pharmaceuticals. Angew. Chem. Int. Ed. 2012, 51, 8960–9009. [DOI] [PubMed] [Google Scholar]
- (39).Chen DY-K; Youn SW C–H Activation: A Complementary Tool in the Total Synthesis of Complex Natural Products. Chem. - Eur. J 2012, 18, 9452–9474. [DOI] [PubMed] [Google Scholar]
- (40).Noisier AFM; Brimble MA C–H Functionalization in the Synthesis of Amino Acids and Peptides. Chem. Rev. 2014, 114, 8775–8806. [DOI] [PubMed] [Google Scholar]
- (41).Cernak T; Dykstra KD; Tyagarajan S; Vachal P; Krska SW The Medicinal Chemist’s Toolbox for Late Stage Functionalization of Drug-Like Molecules. Chem. Soc. Rev. 2016, 45, 546–576. [DOI] [PubMed] [Google Scholar]
- (42).Wencel-Delord J; Glorius F C–H Bond Activation Enables the Rapid Construction and Late-Stage Diversification of Functional Molecules. Nat. Chem. 2013, 5, 369–375. [DOI] [PubMed] [Google Scholar]
- (43).Okamoto K; Zhang J; Housekeeper JB; Marder SR; Luscombe CK C–H Arylation Reaction: Atom Efficient and Greener Syntheses of π-Conjugated Small Molecules and Macromolecules for Organic Electronic Materials. Macromolecules 2013, 46, 8059–8078. [Google Scholar]
- (44).Segawa Y; Maekawa T; Itami K Synthesis of Extended π-Systems through C–H Activation. Angew. Chem. Int. Ed. 2015, 54, 66–81. [DOI] [PubMed] [Google Scholar]
- (45).Murahashi S Synthesis of Phthalimidines from Schiff Bases and Carbon Monoxide. J. Am. Chem. Soc. 1955, 77, 6403–6404. [Google Scholar]
- (46).Fahey DR The Coordination-Catalyzed ortho-Halogenation of Azobenzene. J. Organomet. Chem. 1971, 27, 283–292. [Google Scholar]
- (47).Lewis LN; Smith JF Catalytic C−C Bond Formation via ortho-Metalated Complexes. J. Am. Chem. Soc. 1986, 108, 2728–2735. [Google Scholar]
- (48).Murai S; Kakiuchi F; Sekine S; Tanaka Y; Kamatani A; Sonoda M; Chatani N Efficient Catalytic Addition of Aromatic Carbon-Hydrogen Bonds to Olefins. Nature 1993, 366, 529–531. [Google Scholar]
- (49).Siegbahn PEM Trends of Metal-Carbon Bond Strengths in Transition Metal Complexes. J. Phys. Chem. 1995, 99, 12723–12729. [Google Scholar]
- (50).Yeung CS; Dong VM Catalytic Dehydrogenative Cross-Coupling: Forming Carbon-Carbon Bonds by Oxidizing Two Carbon-Hydrogen Bonds. Chem. Rev. 2011, 111, 1215–1292. [DOI] [PubMed] [Google Scholar]
- (51).Baudoin O; Herrbach A; Guéritte F The Palladium-Catalyzed C–H Activation of Benzylic Gem-Dialkyl Groups. Angew. Chem. Int. Ed. 2003, 42, 5736–5740. [DOI] [PubMed] [Google Scholar]
- (52).Hitce J; Retailleau P; Baudoin O Palladium-Catalyzed Intramolecular C(sp3)–H Functionalization: Catalyst Development and Synthetic Applications. Chem.- Eur. J. 2007, 13, 792–799. [DOI] [PubMed] [Google Scholar]
- (53).Lafrance M; Gorelsky SI; Fagnou K High-Yielding Palladium-Catalyzed Intramolecular Alkane Arylation: Reaction Development and Mechanistic Studies. J. Am. Chem. Soc. 2007, 129, 14570–14571. [DOI] [PubMed] [Google Scholar]
- (54).Chaumontet M; Piccardi R; Audic N; Hitce J; Peglion JL; Clot E; Baudoin O Synthesis of Benzocyclobutenes by Palladium-Catalyzed C–H Activation of Methyl Groups: Method and Mechanistic Study. J. Am. Chem. Soc. 2008, 130, 15157–15166. [DOI] [PubMed] [Google Scholar]
- (55).Rousseaux S; Davi M; Sofack-Kreutzer J; Pierre C; Kefalidis CE; Clot E; Fagnou K; Baudoin O Intramolecular Palladium-Catalyzed Alkane C–H Arylation from Aryl Chlorides. J. Am. Chem. Soc. 2010, 132, 10706–10716. [DOI] [PubMed] [Google Scholar]
- (56).Nakanishi M; Katayev D; Besnard C; Kündig EP Fused Indolines by Palladium-Catalyzed Asymmetric C–C Coupling Involving an Unactivated Methylene Group. Angew. Chem. Int. Ed. 2011, 50, 7438–7441. [DOI] [PubMed] [Google Scholar]
- (57).Saget T; Lemouzy SJ; Cramer N Chiral Monodentate Phosphines and Bulky Carboxylic Acids: Cooperative Effects in Palladium-Catalyzed Enantioselective C(sp3)–H Functionalization. Angew. Chem. Int. Ed. 2012, 51, 2238–2242. [DOI] [PubMed] [Google Scholar]
- (58).Yan J-X; Li H; Liu X-W; Shi J-L; Wang X; Shi Z-J Palladium-Catalyzed C(sp3)–H Activation: A Facile Method for the Synthesis of 3,4-Dihydroquinolinone Derivatives. Angew. Chem. Int. Ed. 2014, 53, 4945–4949. [DOI] [PubMed] [Google Scholar]
- (59).Kuninobu Y; Nakahara T; Takeshima H; Takai K Rhodium-Catalyzed Intramolecular Silylation of Unactivated C(sp3)–H Bonds. Org. Lett. 2013, 15, 426–428. [DOI] [PubMed] [Google Scholar]
- (60).Lee T; Hartwig JF Rhodium-Catalyzed Enantioselective Silylation of Cyclopropyl C–H Bonds. Angew. Chem. Int. Ed. 2016, 55, 8723–8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Karmel C; Li B; Hartwig JF Rhodium-Catalyzed Regioselective Silylation of Alkyl C–H Bonds for the Synthesis of 1,4-Diols. J. Am. Chem. Soc. 2018, 140, 1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Simmons EM; Hartwig JF Catalytic Functionalization of Unactivated Primary C–H Bonds Directed by an Alcohol. Nature 2012, 483, 70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Li B; Driess M; Hartwig JF Iridium-Catalyzed Regioselective Silylation of Secondary Alkyl C–H Bonds for the Synthesis of 1,3-Diols. J. Am. Chem. Soc. 2014, 136, 6586–6589. [DOI] [PubMed] [Google Scholar]
- (64).Ghavtadze N; Melkonyan FS; Gulevich AV; Huang C; Gevorgyan V Conversion of 1-Alkenes into 1,4-Diols through an Auxiliary-Mediated Formal Homoallylic C–H Oxidation. Nat. Chem 2014, 6, 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Larsen MA; Cho SH; Hartwig JF Iridium-Catalyzed, Hydrosilyl-Directed Borylation of Unactivated Alkyl C–H Bonds. J. Am. Chem. Soc. 2016, 138, 762–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Su B; Hartwig JF Ir-Catalyzed Enantioselective, Intramolecular Silylation of Methyl C–H Bonds. J. Am. Chem. Soc. 2017, 139, 12137–12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Muñiz K High-Oxidation-State Palladium Catalysis: New Reactivity for Organic Synthesis. Angew. Chem. Int. Ed. 2009, 48, 9412–9423. [DOI] [PubMed] [Google Scholar]
- (68).Deprez NR; Sanford MS Synthetic and Mechanistic Studies of Pd-Catalyzed C–H Arylation with Diaryliodonium Salts: Evidence for a Bimetallic High Oxidation State Pd Intermediate. J. Am. Chem. Soc. 2009, 131, 11234–11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Powers DC; Geibel MAL; Klein JEMN; Ritter T Bimetallic Palladium Catalysis: Direct Observation of Pd(III)–Pd(III) Intermediates. J. Am. Chem. Soc. 2009, 131, 17050–17051. [DOI] [PubMed] [Google Scholar]
- (70).Engle KM; Mei T-S; Wasa M; Yu J-Q Weak Coordination as a Powerful Means for Developing Broadly Useful C–H Functionalization Reactions. Acc. Chem. Res. 2012, 45, 788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Xu L-M; Li B-J; Yang Z; Shi Z-J Organopalladium(IV) Chemistry. Chem. Soc. Rev. 2010, 39, 712–733. [DOI] [PubMed] [Google Scholar]
- (72).Hartwell GE; Lawrence RV; Smas MJ The Formation of Palladium(II)- and Platinum(II)-Carbon Bonds by Proton Abstraction from Benzo[h]quinoline and 8-Methylquinoline. J. Chem. Soc. D, 1970, 912. [Google Scholar]
- (73).Constable AG; McDonald WS; Sawkins LC; Shaw BL Palladation of Dimethylhydrazones, Oximes, and Oxime O-Allyl Ethers: Crystal Structure of [Pd3(ON = CPriPh)6]. J. Chem. Soc., Chem. Commun. 1978, 1061–1062. [Google Scholar]
- (74).Ryabov AD Cyclopalladated Complexes in Organic Synthesis. Synthesis 1985, 1985, 233–252. [Google Scholar]
- (75).Fuchita Y; Hiraki K; Uchiyama T Metallation of Aliphatic Carbon Atoms. Part 1. Synthesis and Characterization of the Cyclopalladated Complexes of 2-Neopentylpyridine. J. Chem. Soc., Dalt. Trans 1983, 897–899. [Google Scholar]
- (76).Shabashov D; Daugulis O Catalytic Coupling of C–H and C–I Bonds Using Pyridine as a Directing Group. Org. Lett. 2005, 3657–3659. [DOI] [PubMed] [Google Scholar]
- (77).Yang W; Ye S; Fanning D; Coon T; Schmidt Y; Krenitsky P; Stamos D; Yu J-Q Orchestrated Triple C–H Activation Reactions Using Two Directing Groups: Rapid Assembly of Complex Pyrazoles. Angew. Chem. Int. Ed. 2015, 54, 2501–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Gulia N; Daugulis O Palladium-Catalyzed Pyrazole-Directed sp3 C–H Bond Arylation for the Synthesis of β-Phenethylamines. Angew. Chem. Int. Ed. 2017, 56, 3630–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Le KKA; Nguyen H; Daugulis O 1-Aminopyridinium Ylides as Monodentate Directing Groups for sp3 C–H Bond Functionalization. J. Am. Chem. Soc. 2019, 141, 14728–14735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Calleja J; Pla D; Gorman TW; Domingo V; Haffemayer B; Gaunt MJ A Steric Tethering Approach Enables Palladium-Catalysed C–H Activation of Primary Amino Alcohols. Nat. Chem. 2015, 7, 1009–1016. [DOI] [PubMed] [Google Scholar]
- (81).Whitehurst WG; Blackwell JH; Hermann GN; Gaunt MJ Carboxylate-Assisted Oxidative Addition to Aminoalkyl PdII Complexes: C(sp3)–H Arylation of Alkylamines by Distinct PdII/PdIV Pathway. Angew. Chem. Int. Ed. 2019, 58, 9054–9059. [DOI] [PubMed] [Google Scholar]
- (82).Pramanick PK; Zhou Z; Hou Z-L; Yao B Free Amino Group-Directed γ-C(sp3)–H Arylation of α-Amino Esters with Diaryliodonium Triflates by Palladium Catalysis. J. Org. Chem. 2019, 84, 5684–5694. [DOI] [PubMed] [Google Scholar]
- (83).Lin H; Pan X; Barsamian AL; Kamenecka TM; Bannister TD Native Directed Site-Selective δ-C(sp3)–H and δ-C(sp2)–H Arylation of Primary Amines. ACS Catal. 2019, 9, 4887–4891. [Google Scholar]
- (84).Peng J; Chen C; Xi C β-Arylation of Oxime Ethers Using Diaryliodonium Salts through Activation of Inert C(sp3)–H Bonds Using a Palladium Catalyst. Chem. Sci. 2016, 7, 1383–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Jin L; Wang J; Dong G Palladium-Catalyzed γ-C(sp3)–H Arylation of Thiols by a Detachable Protecting/Directing Group. Angew. Chem. Int. Ed. 2018, 57, 12352–12355. [DOI] [PubMed] [Google Scholar]
- (86).Giri R; Maugel N; Li J-J; Wang D-H; Breazzano SP; Saunders LB; Yu J-Q Palladium-Catalyzed Methylation and Arylation of sp2 and sp3 C–H Bonds in Simple Carboxylic Acids. J. Am. Chem. Soc. 2007, 129, 3510–3511. [DOI] [PubMed] [Google Scholar]
- (87).Giri R; Yu J-Q Synthesis of 1,2- and 1,3-Dicarboxylic Acids via Pd(II)-Catalyzed Carboxylation of Aryl and Vinyl C–H Bonds. J. Am. Chem. Soc. 2008, 130, 14082–14083. [DOI] [PubMed] [Google Scholar]
- (88).Whisler MC; MacNeil S; Snieckus V; Beak P Beyond Thermodynamic Acidity: A Perspective on the Complex-Induced Proximity Effect (CIPE) in Deprotonation Reactions. Angew. Chem., Int. Ed 2004, 43, 2206–2225. [DOI] [PubMed] [Google Scholar]
- (89).Wasa M; Engle KM; Yu J-Q Pd(0)/PR3-Catalyzed Intermolecular Arylation of sp3 C–H Bonds. J. Am. Chem. Soc. 2009, 131, 9886–9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Wasa M; Yu J-Q Amide-directed Arylation of sp3 C–H Bonds using Pd(II) and Pd(0) Catalysts. Tetrahdron 2010, 66, 4811–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Wasa M; Chan KSL; Zhang X-G; He J; Miura M; Yu J-Q Ligand-Enabled Methylene C(sp3)–H Bond Activation with a Pd(II) Catalyst. J. Am. Chem. Soc. 2012, 134, 18570–18572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Deng Y; Gong W; He J; Yu J-Q Ligand-Enabled Triple C–H Activation Reactions: One-Pot Synthesis of Diverse 4-Aryl-2-Quinolinones from Propionamides. Angew. Chem. Int. Ed. 2014, 53, 6692–6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).He J; Li S; Deng Y; Fu H; Laforteza BN; Spangler JE; Homs A; Yu J-Q Ligand-Controlled C(sp3)–H Arylation and Olefination in Synthesis of Unnatural Chiral α-Amino Acids. Science 2014, 343, 1216–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Jiang J; Yu J-Q; Morokuma K Mechanism and Stereoselectivity of Directed C(sp3)–H Activation and Arylation Catalyzed by Pd(II) with Pyridine Ligand and Trifluoroacetate: A Computational Study. ACS Catal 2015, 5, 3648–3661. [Google Scholar]
- (95).Dang Y; Qu S; Nelson JW; Pham HD; Wang Z-X; Wang X The Mechanism of a Ligand-Promoted C(sp3)–H Activation and Arylation Reaction via Palladium Catalysis: Theoretical Demonstration of a Pd(II)/Pd(IV) Redox Manifold. J. Am. Chem. Soc. 2015, 137, 2006–2014. [DOI] [PubMed] [Google Scholar]
- (96).Lee L-C; He J; Yu J-Q; Jones CW Functionalized Polymer-Supported Pyridine Ligands for Palladium-Catalyzed C(sp3)–H Arylation. ACS Catal. 2016, 6, 5245–5250. [Google Scholar]
- (97).Chen G; Gong W; Zhuang Z; Andrä MS; Chen Y-Q; Hong X; Yang Y-F; Liu T; Houk KN; Yu J-Q Ligand-Accelerated Enantioselective Methylene C(sp3)–H Bond Activation. Science 2016, 353, 1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Yang Y-F; Chen G; Hong X; Yu J-Q; Houk KN The Origins of Dramatic Differences in Five-Membered vs Six-Membered Chelation of Pd(II) on Efficiency of C(sp3)–H Bond Activation. J. Am. Chem. Soc. 2017, 139, 8514–8521. [DOI] [PubMed] [Google Scholar]
- (99).Hill DE; Pei Q-L; Zhang E-X; Gage JR; Yu J-Q; Blackmond DG A General Protocol for Addressing Speciation of the Active Catalyst Applied to Ligand-Accelerated Enantioselective C(sp3)–H Bond Arylation. ACS Catal. 2018, 8, 1528–1531. [Google Scholar]
- (100).Romero EA; Chen G; Gembicky M; Jazzar R; Yu J-Q; Bertrand G Understanding the Activity and Enantioselectivity of Acetyl-Protected Aminoethyl Quinoline Ligands in Palladium-Catalyzed β-C(sp3)–H Bond Arylation Reactions. J. Am. Chem. Soc. 2019, 141, 16726–16733. [DOI] [PubMed] [Google Scholar]
- (101).Wu Q-F; Shen P-X; He J; Wang X-B; Zhang F; Shao Q; Zhu R-Y; Mapelli C; Qiao JX; Poss MA; Yu J-Q Formation of α-Chiral Centers by Asymmetric β-C(sp3)–H Arylation, Alkenylation, and Alkynylation. Science 2017, 355, 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Wu Q-F; Wang X-B; Shen P-X; Yu J-Q Enantioselective C–H Arylation and Vinylation of Cyclobutyl Carboxylic Amides. ACS Catal. 2018, 8, 2577–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Jerhaoui S; Djukic JP; Wencel-Delord J; Colobert F Asymmetric, Nearly Barrierless C(sp3)–H Activation Promoted by Easily-Accessible N-Protected Aminosulfoxides as New Chiral Ligands. ACS Catal. 2019, 9, 2532–2542. [Google Scholar]
- (104).Andrä MS; Schifferer L; Pollok CH; Merten C; Gooßen LJ; Yu J-Q Enantio- and Diastereoswitchable C–H Arylation of Methylene Groups in Cycloalkanes. Chem.- Eur. J 2019, 25, 8503–8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Shang M; Feu KS; Vantourout JC; Barton LM; Osswald HL; Kato N; Gagaring K; McNamara CW; Chen G; Hu L; et al. Modular, Stereocontrolled Cβ–H/Cα–C Activation of Alkyl Carboxylic Acids. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 8721–8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Li S; Zhu R-Y; Xiao K-J; Yu J-Q Ligand-Enabled Arylation of γ-C–H Bonds. Angew. Chem. Int. Ed. 2016, 55, 4317–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Ye S; Yang W; Coon T; Fanning D; Neubert T; Stamos D; Yu J-Q N-Heterocyclic Carbene Ligand-Enabled C(sp3)–H Arylation of Piperidine and Tetrahydropyran Derivatives. Chem.- Eur. J 2016, 22, 4748–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Chen G; Shigenari T; Jain P; Zhang Z; Jin Z; He J; Li S; Mapelli C; Miller MM; Poss MA; et al. Ligand-Enabled β-C–H Arylation of α-Amino Acids Using a Simple and Practical Auxiliary. J. Am. Chem. Soc. 2015, 137, 3338–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Park H; Chekshin N; Shen P-X; Yu J-Q Ligand-Enabled, Palladium-Catalyzed β-C(sp3)–H Arylation of Weinreb Amides. ACS Catal. 2018, 8, 9292–9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Zhao R; Lu W Palladium-Catalyzed β-Arylation of Amide via Primary sp3 C–H Activation. Organometallics 2018, 37, 2188–2192. [Google Scholar]
- (111).Chen G; Zhuang Z; Li G-C; Saint-Denis TG; Hsiao Y; Joe CL; Yu J-Q Ligand-Enabled β-C–H Arylation of α-Amino Acids Without Installing Exogenous Directing Groups. Angew. Chem. Int. Ed. 2017, 56, 1506–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Zhu Y; Chen X; Yuan C; Li G; Zhang J; Zhao Y Pd-Catalysed Ligand-Enabled Carboxylate-Directed Highly Regioselective Arylation of Aliphatic Acids. Nat. Commun. 2017, 8, 14904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Shen P-X; Hu L; Shao Q; Hong K; Yu J-Q Pd(II)-Catalyzed Enantioselective C(sp3)–H Arylation of Free Carboxylic Acids. J. Am. Chem. Soc. 2018, 140, 6545–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Dolui P; Das J; Chandrashekar HB; Anjana SS; Maiti D Ligand-Enabled PdII-Catalyzed Iterative γ-C(sp3)–H Arylation of Free Aliphatic Acid. Angew. Chem. Int. Ed. 2019, 58, 13773–13777. [DOI] [PubMed] [Google Scholar]
- (115).Liu L; Liu Y-H; Shi B-F Synthesis of Amino Acids and Peptides with Bulky Side Chains: Via Ligand-Enabled Carboxylate-Directed γ-C(sp3)–H Arylation. Chem. Sci. 2020, 11, 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Chan KSL; Fu H-Y; Yu J-Q Palladium(II)-Catalyzed Highly Enantioselective C–H Arylation of Cyclopropylmethylamines. J. Am. Chem. Soc. 2015, 137, 2042–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Kapoor M; Liu D; Young MC Carbon Dioxide-Mediated C(sp3)–H Arylation of Amine Substrates. J. Am. Chem. Soc. 2018, 140, 6818–6822. [DOI] [PubMed] [Google Scholar]
- (118).Hu L; Shen P-X; Shao Q; Hong K; Qiao JX; Yu J-Q Pd(II)-Catalyzed Enantioselective C(sp3)–H Activation/Cross-Coupling Reactions of Free Carboxylic Acids. Angew. Chem. Int. Ed. 2019, 58, 2134–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Wang D-H; Wasa M; Giri R; Yu J-Q Pd(II)-Catalyzed Cross-Coupling of sp3 C–H Bonds with sp2 and sp3 Boronic Acids Using Air as the Oxidant. J. Am. Chem. Soc. 2008, 130, 7190–7191. [DOI] [PubMed] [Google Scholar]
- (120).Wasa M; Engle KM; Lin DW; Yoo EJ; Yu J-Q Pd(II)-Catalyzed Enantioselective C–H Activation of Cyclopropanes. J. Am. Chem. Soc. 2011, 133, 19598–19601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Xiao K-J; Lin DW; Miura M; Zhu R-Y; Gong W; Wasa M; Yu J-Q Palladium(II)-Catalyzed Enantioselective C(sp3)–H Activation Using a Chiral Hydroxamic Acid Ligand. J. Am. Chem. Soc. 2014, 136, 8138–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).He J; Takise R; Fu H; Yu J-Q Ligand-Enabled Cross-Coupling of C(sp3)–H Bonds with Arylsilanes. J. Am. Chem. Soc. 2015, 137, 4618–4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Chan KSL; Wasa M; Chu L; Laforteza BN; Miura M; Yu J-Q Ligand-Enabled Cross-Coupling of C(sp3)–H Bonds with Arylboron Reagents via Pd(II)/Pd(0) Catalysis. Nat. Chem 2014, 6, 146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Shao Q; He J; Wu Q-F; Yu J-Q Ligand-Enabled γ-C(sp3)–H Cross-Coupling of Nosyl-Protected Amines with Aryl- and Alkylboron Reagents. ACS Catal. 2017, 7, 7777–7782. [Google Scholar]
- (125).Shao Q; Wu Q-F; He J; Yu J-Q Enantioselective γ-C(sp3)–H Activation of Alkyl Amines via Pd(II)/Pd(0) Catalysis. J. Am. Chem. Soc. 2018, 140, 5322–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).He C; Gaunt MJ Ligand-Enabled Catalytic C–H Arylation of Aliphatic Amines by a Four-Membered-Ring Cyclopalladation Pathway. Angew. Chem. Int. Ed. 2015, 54, 15840–15844. [DOI] [PubMed] [Google Scholar]
- (127).Rodrigalvarez J; Nappi M; Azuma H; Flodén NJ; Burns ME; Gaunt MJ Catalytic C(sp3)–H Bond Activation in Tertiary Alkylamines. Nat. Chem. 2020, 12, 76–81. [DOI] [PubMed] [Google Scholar]
- (128).Spangler JE; Kobayashi Y; Verma P; Wang D-H; Yu J-Q α-Arylation of Saturated Azacycles and N-Methylamines via Palladium(II)-Catalyzed C(sp3)–H Coupling. J. Am. Chem. Soc. 2015, 137, 11876–11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Jain P; Verma P; Xia G; Yu J-Q Enantioselective Amine α-Functionalization via Palladium-Catalysed C–H Arylation of Thioamides. Nat. Chem 2017, 9, 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Jiang H-J; Zhong X-M; Yu J; Zhang Y; Zhang X; Wu Y-D; Gong L-Z Assembling a Hybrid Pd Catalyst from a Chiral Anionic CoIII Complex and Ligand for Asymmetric C(sp3)–H Functionalization. Angew. Chem. Int. Ed. 2019, 58, 1803–1807. [DOI] [PubMed] [Google Scholar]
- (131).Stowers KJ; Fortner KC; Sanford MS Aerobic Pd-Catalyzed sp3 C–H Olefination: A Route to Both N-Heterocyclic Scaffolds and Alkenes. J. Am. Chem. Soc. 2011, 133, 6541–6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Yang W; Ye S; Schmidt Y; Stamos D; Yu J-Q Ligand-Promoted C(sp3)–H Olefination En Route to Multi-Functionalized Pyrazoles. Chem. - Eur. J 2016, 22, 7059–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).He C; Gaunt MJ Ligand-Assisted Palladium-Catalyzed C–H Alkenylation of Aliphatic Amines for the Synthesis of Functionalized Pyrrolidines. Chem. Sci. 2017, 8, 3586–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Wasa M; Engle KM; Yu J-Q Pd(II)-Catalyzed Olefination of sp3 C–H Bonds. J. Am. Chem. Soc. 2010, 132, 3680–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Li S; Chen G; Feng C-G; Gong W; Yu J-Q Ligand-Enabled γ-C–H Olefination and Carbonylation: Construction of β-Quaternary Carbon Centers. J. Am. Chem. Soc. 2014, 136, 5267–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Jiang H; He J; Liu T; Yu J-Q Ligand-Enabled γ-C(sp3)–H Olefination of Amines: En Route to Pyrrolidines. J. Am. Chem. Soc. 2016, 138, 2055–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Park H; Li Y; Yu J-Q Utilizing Carbonyl Coordination of Native Amides for Palladium-Catalyzed C(sp3)–H Olefination. Angew. Chem. Int. Ed. 2019, 58, 11424–11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Zhuang Z; Yu C-B; Chen G; Wu Q-F; Hsiao Y; Joe CL; Qiao JX; Poss MA; Yu J-Q Ligand-Enabled β-C(sp3)–H Olefination of Free Carboxylic Acids. J. Am. Chem. Soc. 2018, 140, 10363–10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Ghosh KK; Uttry A; Mondal A; Ghiringhelli F; Wedi P; van Gemmeren M Ligand-Enabled γ-C(sp3)–H Olefination of Free Carboxylic Acids. Angew. Chem. Int. Ed. 2020, 59, 12848–12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).He J; Wasa M; Chan KSL; Yu J-Q Palladium(0)-Catalyzed Alkynylation of C(sp3)–H Bonds. J. Am. Chem. Soc. 2013, 135, 3387–3390. [DOI] [PubMed] [Google Scholar]
- (141).Fu H; Shen P-X; He J; Zhang F; Li S; Wang P; Liu T; Yu J-Q Ligand-Enabled Alkynylation of C(sp3)–H Bonds with Palladium(II) Catalysts. Angew. Chem. Int. Ed. 2017, 129, 1899–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Chen X; Goodhue CE; Yu J-Q Palladium-Catalyzed Alkylation of sp2 and sp3 C–H Bonds with Methylboroxine and Alkylboronic Acids: Two Distinct C–H Activation Pathways. J. Am. Chem. Soc. 2006, 128, 12634–12635. [DOI] [PubMed] [Google Scholar]
- (143).Zhu R-Y; He J; Wang X-C; Yu J-Q Ligand-Promoted Alkylation of C(sp3)–H and C(sp2)–H Bonds. J. Am. Chem. Soc. 2014, 136, 13194–13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Yoo EJ; Wasa M; Yu J-Q Pd(II)-Catalyzed Carbonylation of C(sp3)–H Bonds: A New Entry to 1,4-Dicarbonyl Compounds. J. Am. Chem. Soc. 2010, 132, 17378–17380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).McNally A; Haffemayer B; Collins BSL; Gaunt MJ Palladium-Catalysed C–H Activation of Aliphatic Amines to Give Strained Nitrogen Heterocycles. Nature 2014, 510, 129–133. [DOI] [PubMed] [Google Scholar]
- (146).Willcox D; Chappell BGN; Hogg KF; Calleja J; Smalley AP; Gaunt MJ A General Catalytic β-C–H Carbonylation of Aliphatic Amines to β-Lactams. Science 2016, 354, 851–857. [DOI] [PubMed] [Google Scholar]
- (147).Cabrera-Pardo JR; Trowbridge A; Nappi M; Ozaki K; Gaunt MJ Selective Palladium(II)-Catalyzed Carbonylation of Methylene β-C–H Bonds in Aliphatic Amines. Angew. Chem. Int. Ed. 2017, 56, 11958–11962. [DOI] [PubMed] [Google Scholar]
- (148).Hogg KF; Trowbridge A; Alvarez-Pérez A; Gaunt MJ The α-Tertiary Amine Motif Drives Remarkable Selectivity for Pd-Catalyzed Carbonylation of β-Methylene C–H Bonds. Chem. Sci. 2017, 8, 8198–8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Png ZM; Cabrera-Pardo JR; Peiró Cadahía J; Gaunt MJ Diastereoselective C–H Carbonylative Annulation of Aliphatic Amines: A Rapid Route to Functionalized γ-Lactams. Chem. Sci. 2018, 9, 7628–7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Thu H-Y; Yu W-Y; Che C-M Intermolecular Amidation of Unactivated sp2 and sp3 C–H Bonds via Palladium-Catalyzed Cascade C–H Activation/Nitrene Insertion. J. Am. Chem. Soc. 2006, 128, 9048–9049. [DOI] [PubMed] [Google Scholar]
- (151).Neumann JJ; Rakshit S; Droge T; Glorius F Palladium-Catalyzed Amidation of Unactivated C(sp3)–H Bonds: From Anilines to Indolines. Angew. Chem. Int. Ed. 2009, 48, 6892–6895. [DOI] [PubMed] [Google Scholar]
- (152).Iglesias Á; Álvarez R; De-Lera ÁR; Muñiz K Palladium-Catalyzed Intermolecular C(sp3)–H Amidation. Angew. Chem. Int. Ed. 2012, 51, 2225–2228. [DOI] [PubMed] [Google Scholar]
- (153).He J; Shigenari T; Yu J-Q Palladium(0)/PAr3-Catalyzed Intermolecular Amination of C(sp3)–H Bonds: Synthesis of β-Amino Acids. Angew. Chem. Int. Ed. 2015, 54, 6545–6549. [DOI] [PubMed] [Google Scholar]
- (154).Smalley AP; Gaunt MJ Mechanistic Insights into the Palladium-Catalyzed Aziridination of Aliphatic Amines by C–H Activation. J. Am. Chem. Soc. 2015, 137, 10632–10641. [DOI] [PubMed] [Google Scholar]
- (155).Smalley AP; Cuthbertson JD; Gaunt MJ Palladium-Catalyzed Enantioselective C–H Activation of Aliphatic Amines Using Chiral Anionic BINOL-Phosphoric Acid Ligands. J. Am. Chem. Soc. 2017, 139, 1412–1415. [DOI] [PubMed] [Google Scholar]
- (156).Nappi M; He C; Whitehurst WG; Chappell BGN; Gaunt MJ Selective Reductive Elimination at Alkyl Palladium(IV) by Dissociative Ligand Ionization: Catalytic C(sp3)–H Amination to Azetidines. Angew. Chem. Int. Ed. 2018, 57, 3178–3182. [DOI] [PubMed] [Google Scholar]
- (157).Chen L; Zhou Z; Zhang S; Li X; Ma X; Dong J Palladium(II)-Catalyzed Oxidative C(sp3)−P Bond Formation: Via C(sp3)–H Bond Activation. Chem. Commun. 2019, 55, 13693–13696. [DOI] [PubMed] [Google Scholar]
- (158).Desai LV; Hull KL; Sanford MS Palladium-Catalyzed Oxygenation of Unactivated sp3 C–H Bonds. J. Am. Chem. Soc. 2004, 126, 9542–9543. [DOI] [PubMed] [Google Scholar]
- (159).Dick AR; Hull KL; Sanford MS A Highly Selective Catalytic Method for the Oxidative Functionalization of C–H Bonds. J. Am. Chem. Soc. 2004, 126, 2300–2301. [DOI] [PubMed] [Google Scholar]
- (160).Sharpe RJ; Johnson JS A Global and Local Desymmetrization Approach to the Synthesis of Steroidal Alkaloids: Stereocontrolled Total Synthesis of Paspaline. J. Am. Chem. Soc. 2015, 137, 4968–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Yang Q-L; Li Y-Q; Ma C; Fang P; Zhang X-J; Mei T-S Palladium-Catalyzed C(sp3)–H Oxygenation via Electrochemical Oxidation. J. Am. Chem. Soc. 2017, 139, 3293–3298. [DOI] [PubMed] [Google Scholar]
- (162).Giri R; Liang J; Lei J-G; Li J-J; Wang D-H; Chen X; Naggar IC; Guo C; Foxman BM; Yu J-Q Pd-Catalyzed Stereoselective Oxidation of Methyl Groups by Inexpensive Oxidants under Mild Conditions: A Dual Role for Carboxylic Anhydrides in Catalytic C–H Bond Oxidation. Angew. Chem. Int. Ed. 2005, 44, 7420–7424. [DOI] [PubMed] [Google Scholar]
- (163).Wang D-H; Hao X-S; Wu D-F; Yu J-Q Palladium-Catalyzed Oxidation of Boc-Protected N-Methylamines with IOAc as the Oxidant: A Boc-Directed sp3 C–H Bond Activation. Org. Lett. 2006, 8, 3387–3390. [DOI] [PubMed] [Google Scholar]
- (164).Zhou L; Lu W Palladium-Catalyzed β-Acyloxylation of Simple Amide via sp3 C–H Activation. Org. Lett. 2014, 16, 508–511. [DOI] [PubMed] [Google Scholar]
- (165).Zhao R; Lu W Palladium-Catalyzed β-Mesylation of Simple Amide via Primary sp3 C–H Activation. Org. Lett. 2017, 19, 1768–1771. [DOI] [PubMed] [Google Scholar]
- (166).Buettner CS; Willcox D; Chappell BGN; Gaunt MJ Mechanistic Investigation into the C(sp3)–H Acetoxylation of Morpholinones. Chem. Sci. 2019, 10, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Chen K; Wang D; Li Z-W; Liu Z; Pan F; Zhang Y-F; Shi Z-J Palladium Catalyzed C(sp3)–H Acetoxylation of Aliphatic Primary Amines to γ-Amino Alcohol Derivatives. Org. Chem. Front. 2017, 4, 2097–2101. [Google Scholar]
- (168).Novák P; Correa A; Gallardo-Donaire J; Martin R Synergistic Palladium-Catalyzed C(sp3)–H Activation/C(sp3)−O Bond Formation: A Direct, Step-Economical Route to Benzolactones. Angew. Chem. Int. Ed. 2011, 50, 12236–12239. [DOI] [PubMed] [Google Scholar]
- (169).Qian S; Li Z-Q; Li M; Wisniewski SR; Qiao JX; Richter JM; Ewing WR; Eastgate MD; Chen JS; Yu J-Q Ligand-Enabled Pd(II)-Catalyzed C(sp3)–H Lactonization Using Molecular Oxygen as Oxidant. Org. Lett. 2020, 22, 3960–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Ghosh KK; Uttry A; Koldemir A; Ong M; Van Gemmeren M Direct β-C(sp3)–H Acetoxylation of Aliphatic Carboxylic Acids. Org. Lett. 2019, 21, 7154–7157. [DOI] [PubMed] [Google Scholar]
- (171).Zhuang Z; Yu J-Q Lactonization as a General Route to β-C(sp3)–H Functionalization. Nature 2020, 577, 656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Zhuang Z; Herron AN; Fan Z; Yu J-Q Ligand-Enabled Monoselective β- C(sp3)–H Acyloxylation of Free Carboxylic Acids Using a Practical Oxidant. J. Am. Chem. Soc. 2020, 142, 6769–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Giri R; Chen X; Yu J-Q Palladium-Catalyzed Asymmetric Iodination of Unactivated C–H Bonds under Mild Conditions. Angew. Chem. Int. Ed. 2005, 44, 2112–2115. [DOI] [PubMed] [Google Scholar]
- (174).Giri R; Wasa M; Breazzano SP; Yu J-Q Converting gem-Dimethyl Groups into Cyclopropanes via Pd-Catalyzed Sequential C–H Activation and Radical Cyclization. Org. Lett. 2006, 8, 5685–5688. [DOI] [PubMed] [Google Scholar]
- (175).Giri R; Lan Y; Liu P; Houk KN; Yu J-Q Understanding Reactivity and Stereoselectivity in Palladium-Catalyzed Diastereoselective sp3 C–H Bond Activation: Intermediate Characterization and Computational Studies. J. Am. Chem. Soc. 2012, 134, 14118–14126. [DOI] [PubMed] [Google Scholar]
- (176).Zhu R-Y; Saint-Denis TG; Shao Y; He J; Sieber JD; Senanayake CH; Yu J-Q Ligand-Enabled Pd(II)-Catalyzed Bromination and Iodination of C(sp3)–H Bonds. J. Am. Chem. Soc. 2017, 139, 5724–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Zhu R-Y; Tanaka K; Li G-C; He J; Fu H-Y; Li S-H; Yu J-Q Ligand-Enabled Stereoselective β-C(sp3)–H Fluorination: Synthesis of Unnatural Enantiopure Anti-β-Fluoro-α-Amino Acids. J. Am. Chem. Soc. 2015, 137, 7067–7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).He J; Jiang H; Takise R; Zhu R-Y; Chen G; Dai H-X; Dhar TGM; Shi J; Zhang H; Cheng PTW; et al. Ligand-Promoted Borylation of C(sp3)–H Bonds with Palladium(II) Catalysts. Angew. Chem. Int. Ed. 2016, 55, 785–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).He J; Shao Q; Wu Q; Yu J-Q Pd(II)-Catalyzed Enantioselective C(sp3)–H Borylation. J. Am. Chem. Soc. 2017, 139, 3344–3347. [DOI] [PubMed] [Google Scholar]
- (180).Zaitsev VG; Shabashov D; Daugulis O Highly Regioselective Arylation of sp3 C–H Bonds Catalyzed by Palladium Acetate. J. Am. Chem. Soc. 2005, 127, 13154–13155. [DOI] [PubMed] [Google Scholar]
- (181).Shabashov D; Daugulis O Auxiliary-Assisted Palladium-Catalyzed Arylation and Alkylation of sp2 and sp3 Carbon-Hydrogen Bonds. J. Am. Chem. Soc. 2010, 132, 3965–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Rouquet G; Chatani N Catalytic Functionalization of C(sp2)–H and C(sp3)–H Bonds by Using Bidentate Directing Groups. Angew. Chem. Int. Ed. 2013, 52, 11726–11743. [DOI] [PubMed] [Google Scholar]
- (183).Tang H; Huang X-R; Yao J; Chen H Understanding the Effects of Bidentate Directing Groups: A Unified Rationale for sp2 and sp3 C–H Bond Activations. J. Org. Chem. 2015, 80, 4672–4682. [DOI] [PubMed] [Google Scholar]
- (184).Berger M; Chauhan R; Rodrigues CAB; Maulide N Bridging C–H Activation: Mild and Versatile Cleavage of the 8-Aminoquinoline Directing Group. Chem. - Eur. J 2016, 22, 16805–16808. [DOI] [PubMed] [Google Scholar]
- (185).Deguchi T; Xin H-L; Morimoto H; Ohshima T Direct Catalytic Alcoholysis of Unactivated 8-Aminoquinoline Amides. ACS Catal. 2017, 7, 3157–3161. [Google Scholar]
- (186).Fitzgerald LS; O’Duill ML A Guide to Directing Group Removal: 8-Aminoquinoline. Chem. - Eur. J 2021, 27, 8411–8436. [DOI] [PubMed] [Google Scholar]
- (187).Carvalho RL; Almeida RG; Murali K; Machado LA; Pedrosa LF; Dolui P; Maiti D; Júnior ENS Removal and Modification of Girecting Groups Used in Metal-Catalyzed C–H Functionalization: the Magical Step of Conversion into ‘Conventional’ Functional Groups. Org. Biomol. Chem. 2021, 19, 525–547. [DOI] [PubMed] [Google Scholar]
- (188).Reddy BVS; Reddy LR; Corey EJ Novel Acetoxylation and C–C Coupling Reactions at Unactivated Positions in α-Amino Acid Derivatives. Org Lett. 2006, 8, 3391–3394. [DOI] [PubMed] [Google Scholar]
- (189).Feng Y; Wang Y; Landgraf B; Liu S; Chen G Facile Benzo-Ring Construction via Palladium-Catalyzed Functionalization of Unactivated sp3 C–H Bonds under Mild Reaction Conditions. Org. Lett. 2010, 12, 3414–3417. [DOI] [PubMed] [Google Scholar]
- (190).Zhang X; Lu G; Sun M; Mahankali M; Ma Y; Zhang M; Hua W; Hu Y; Wang Q; Chen J; et al. A General Strategy for Synthesis of Cyclophane-Braced Peptide Macrocycles via Palladium-Catalysed Intramolecular sp3 C–H Arylation. Nat. Chem. 2018, 10, 540–548. [DOI] [PubMed] [Google Scholar]
- (191).Feng Y; Chen G Total Synthesis of Celogentin C by Stereoselective C–H Activation. Angew. Chem. Int. Ed. 2010, 49, 958–961. [DOI] [PubMed] [Google Scholar]
- (192).Gutekunst WR; Gianatassio R; Baran PS Sequential C(sp3)–H Arylation and Olefination: Total Synthesis of the Proposed Structure of Pipercyclobutanamide A. Angew. Chem. Int. Ed. 2012, 51, 7507–7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (193).Ting CP; Maimone TJ C–H Bond Arylation in the Synthesis of Aryltetralin Lignans: A Short Total Synthesis of Podophyllotoxin. Angew. Chem., Int. Ed. 2014, 53, 3115–3119. [DOI] [PubMed] [Google Scholar]
- (194).Wang B; Liu Y; Jiao R; Feng Y; Li Q; Chen C; Liu L; He G; Chen G Total Synthesis of Mannopeptimycins α and β. J. Am. Chem. Soc. 2016, 138, 3926–3932. [DOI] [PubMed] [Google Scholar]
- (195).Beck JC; Lacker CR; Chapman LM; Reisman SE A Modular Approach to Prepare Enantioenriched Cyclobutanes: Synthesis of (+)-Rumphellaone A. Chem. Sci. 2019, 10, 2315–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Tran LD; Daugulis O Nonnatural Amino Acid Synthesis by Using Carbon-Hydrogen Bond Functionalization Methodology. Angew. Chem. Int. Ed. 2012, 51, 5188–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Wang B; Nack WA; He G; Zhang S-Y; Chen G Palladium-Catalyzed Trifluoroacetate-Promoted Mono-Arylation of the β-Methyl Group of Alanine at Room Temperature: Synthesis of β-Arylated α-Amino Acids through Sequential C–H Functionalization. Chem. Sci. 2014, 5, 3952–3957. [Google Scholar]
- (198).Chen K; Zhang S-Q; Xu J-W; Hu F; Shi B-F A General and Practical Palladium-Catalyzed Monoarylation of β-Methyl C(sp3)–H of Alanine. Chem. Commun. 2014, 50, 13924–13927. [DOI] [PubMed] [Google Scholar]
- (199).Fang S-L; Jiang M-X; Zhang S; Wu Y-J; Shi B-F Scalable Formal Synthesis of (−)-Quinocarcin. Org. Lett. 2019, 21, 4609–4613. [DOI] [PubMed] [Google Scholar]
- (200).Chen K; Li X; Zhang S-Q; Shi B-F Palladium-Catalyzed C(sp3)–H Arylation of Lactic Acid: Efficient Synthesis of Chiral β-Aryl-α-Hydroxy Acids. Org. Chem. Front. 2016, 3, 204–208. [Google Scholar]
- (201).Kinsinger T; Kazmaier U C–H Functionalization of N-Methylated Amino Acids and Peptides as Tool in Natural Product Synthesis: Synthesis of Abyssenine A and Mucronine E. Org. Lett. 2018, 20, 7726–7730. [DOI] [PubMed] [Google Scholar]
- (202).Parella R; Gopalakrishnan B; Babu SA Auxiliary-Enabled Pd-Catalyzed Direct Arylation of Methylene C(sp3)–H Bond of Cyclopropanes: Highly Diastereoselective Assembling of Di- and Trisubstituted Cyclopropanecarboxamides. Org. Lett. 2013, 15, 3238–3241. [DOI] [PubMed] [Google Scholar]
- (203).Hoshiya N; Kobayashi T; Arisawa M; Shuto S Palladium-Catalyzed Arylation of Cyclopropanes via Directing Group-Mediated C(sp3)–H Bond Activation to Construct Quaternary Carbon Centers: Synthesis of cis- and trans-1,1,2-Trisubstituted Chiral Cyclopropanes. Org. Lett. 2013, 15, 6202–6205. [DOI] [PubMed] [Google Scholar]
- (204).Parella R; Gopalakrishnan B; Babu SA Direct Bis-Arylation of Cyclobutanecarboxamide via Double C–H Activation: An Auxiliary-Aided Diastereoselective Pd-Catalyzed Access to Trisubstituted Cyclobutane Scaffolds Having Three Contiguous Stereocenters and an All-cis Stereochemistry. J. Org. Chem. 2013, 78, 11911–11934. [DOI] [PubMed] [Google Scholar]
- (205).Nack WA; Wang B; Wu X; Jiao R; He G; Chen G Palladium-Catalyzed Arylation of β-Methylene C(sp3)–H Bonds at Room Temperature: Desymmetrization of Simple Cycloalkyl Carboxylic Acids. Org. Chem. Front. 2016, 3, 561–564. [Google Scholar]
- (206).Nguyen TT; Daugulis O Palladium-Catalyzed, Aminoquinoline-Directed Arylation of Phosphonamidate and Phosphinic Amide sp3 C–H Bonds. Chem. Commun. 2017, 53, 4609–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Dey A; Pimparkar S; Deb A; Guin S; Maiti D Chelation-Assisted Palladium-Catalyzed γ-Arylation of Aliphatic Carboxylic Acid Derivatives. Adv. Synth. Catal. 2017, 359, 1301–1307. [Google Scholar]
- (208).Wei Y; Tang H; Cong X; Rao B; Wu C; Zeng X Pd(II)-Catalyzed Intermolecular Arylation of Unactivated C(sp3)–H Bonds with Aryl Bromides Enabled by 8-Aminoquinoline Auxiliary. Org. Lett. 2014, 16, 2248–2251. [DOI] [PubMed] [Google Scholar]
- (209).Pan F; Shen P-X; Zhang L-S; Wang X; Shi Z-J Direct Arylation of Primary and Secondary sp3 C–H Bonds with Diarylhyperiodonium Salts via Pd Catalysis. Org. Lett. 2013, 15, 4758–4761. [DOI] [PubMed] [Google Scholar]
- (210).Gou Q; Zhang Z-F; Liu Z-C; Qin J Palladium-Catalyzed Cs2CO3-Promoted Arylation of Unactivated C(sp3)–H Bonds by (Diacetoxyiodo)Arenes: Shifting the Reactivity of (Diacetoxyiodo)Arenes from Acetoxylation to Arylation. J. Org. Chem. 2015, 80, 3176–3186. [DOI] [PubMed] [Google Scholar]
- (211).Isley NA; Endo Y; Wu ZC; Covington BC; Bushin LB; Seyedsayamdost MR; Boger DL Total Synthesis and Stereochemical Assignment of Streptide. J. Am. Chem. Soc. 2019, 141, 17361–17369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (212).Affron DP; Davis OA; Bull JA Regio- and Stereospecific Synthesis of C-3 Functionalized Proline Derivatives by Palladium Catalyzed Directed C(sp3)–H Arylation. Org. Lett. 2014, 16, 4956–4959. [DOI] [PubMed] [Google Scholar]
- (213).Feng R; Wang B; Liu Y; Liu Z; Zhang Y Efficient Synthesis of cis-3-Substituted Prolines by Bidentate-Assisted Palladium Catalysis. Eur. J. Org. Chem. 2015, 2015, 142–151. [Google Scholar]
- (214).Affron DP; Bull JA Palladium-Catalyzed Directed C(sp3)–H Arylation of Saturated Heterocycles at C-3 Using a Concise Optimization Approach. Eur. J. Org. Chem. 2016, 2016, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (215).Antermite D; Affron DP; Bull JA Regio- and Stereoselective Palladium-Catalyzed C(sp3)–H Arylation of Pyrrolidines and Piperidines with C(3) Directing Groups. Org. Lett. 2018, 20, 3948–3952. [DOI] [PubMed] [Google Scholar]
- (216).Mondal B; Roy B; Kazmaier U Stereoselective Peptide Modifications via β-C(sp3)–H Arylations. J. Org. Chem. 2016, 81, 11646–11655. [DOI] [PubMed] [Google Scholar]
- (217).Parella R; Babu SA Regio- and Stereoselective Pd-Catalyzed Direct Arylation of Unactivated sp3 C(3)–H Bonds of Tetrahydrofuran and 1,4-Benzodioxane Systems. J. Org. Chem. 2015, 80, 2339–2355. [DOI] [PubMed] [Google Scholar]
- (218).Verho O; Maetani M; Melillo B; Zoller J; Schreiber SL Stereospecific Palladium-Catalyzed C–H Arylation of Pyroglutamic Acid Derivatives at the C3 Position Enabled by 8-Aminoquinoline as a Directing Group. Org. Lett. 2017, 19, 4424–4427. [DOI] [PubMed] [Google Scholar]
- (219).Maetani M; Zoller J; Melillo B; Verho O; Kato N; Pu J; Comer E; Schreiber SL Synthesis of a Bicyclic Azetidine with in vivo Antimalarial Activity Enabled by Stereospecific, Directed C(sp3)–H Arylation. J. Am. Chem. Soc. 2017, 139, 11300–11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (220).Probst N; Grelier G; Dahaoui S; Alami M; Gandon V; Messaoudi S Palladium(II)-Catalyzed Diastereoselective 2,3-trans C(sp3)–H Arylation of Glycosides. ACS Catal. 2018, 8, 7781–7786. [Google Scholar]
- (221).He G; Chen G A Practical Strategy for the Structural Diversification of Aliphatic Scaffolds through the Palladium-Catalyzed Picolinamide-Directed Remote Functionalization of Unactivated C(sp3)–H Bonds. Angew. Chem. Int. Ed. 2011, 50, 5192–5196. [DOI] [PubMed] [Google Scholar]
- (222).He G; Zhang SY; Nack WA; Pearson R; Rabb-Lynch J; Chen G Total Synthesis of Hibispeptin A via Pd-Catalyzed C(sp3)–H Arylation with Sterically Hindered Aryl Iodides. Org. Lett. 2014, 16, 6488–6491. [DOI] [PubMed] [Google Scholar]
- (223).Das S; Bairy G; Jana R Ligand-Promoted γ-C(sp3)–H Arylation and Unsymmetrical Diarylation to Access Unnatural Amino Acid Derivatives. Org. Lett. 2018, 20, 2667–2671. [DOI] [PubMed] [Google Scholar]
- (224).Guin S; Dolui P; Zhang X; Paul S; Singh VK; Pradhan S; Chandrashekar HB; Anjana SS; Paton RS; Maiti D Iterative Arylation of Amino Acids and Aliphatic Amines via δ-C(sp3)–H Activation: Experimental and Computational Exploration. Angew. Chem. Int. Ed. 2019, 58, 5633–5638. [DOI] [PubMed] [Google Scholar]
- (225).Li B; Li X; Han B; Chen Z; Zhang X; He G; Chen G Construction of Natural-Product-Like Cyclophane-Braced Peptide Macrocycles via sp3 C–H Arylation. J. Am. Chem. Soc. 2019, 141, 9401–9407. [DOI] [PubMed] [Google Scholar]
- (226).Roman DS; Charette AB C–H Functionalization of Cyclopropanes: A Practical Approach Employing a Picolinamide Auxiliary. Org. Lett. 2013, 15, 4394–4397. [DOI] [PubMed] [Google Scholar]
- (227).Cui W; Chen S; Wu JQ; Zhao X; Hu W; Wang H Palladium-Catalyzed Remote C(sp3)–H Arylation of 3-Pinanamine. Org. Lett. 2014, 16, 4288–4291. [DOI] [PubMed] [Google Scholar]
- (228).Seki A; Takahashi Y; Miyake T Synthesis of cis-3-Arylated Cycloalkylamines through Palladium-Catalyzed Methylene sp3 Carbon-Hydrogen Bond Activation. Tetrahedron Lett. 2014, 55, 2838–2841. [Google Scholar]
- (229).Fan Z; Shu S; Ni J; Yao Q; Zhang A Ligand-Promoted Pd(II)-Catalyzed Functionalization of Unactivated C(sp3)–H Bond: Regio- and Stereoselective Synthesis of Arylated Rimantadine Derivatives. ACS Catal. 2016, 6, 769–774. [Google Scholar]
- (230).Coomber CE; Benhamou L; Bučar DK; Smith PD; Porter MJ; Sheppard TD Silver-Free Palladium-Catalyzed C(sp3)–H Arylation of Saturated Bicyclic Amine Scaffolds. J. Org. Chem. 2018, 83, 2495–2503. [DOI] [PubMed] [Google Scholar]
- (231).Van Steijvoort BF; Kaval N; Kulago AA; Maes BUW Remote Functionalization: Palladium-Catalyzed C5(sp3)-H Arylation of 1-Boc-3-Aminopiperidine through the Use of a Bidentate Directing Group. ACS Catal. 2016, 6, 4486–4490. [Google Scholar]
- (232).O’Donovan DH; Aillard P; Berger M; de la Torre A; Petkova D; Knittl-Frank C; Geerdink D; Kaiser M; Maulide N C–H Activation Enables a Concise Total Synthesis of Quinine and Analogues with Enhanced Antimalarial Activity. Angew. Chem. Int. Ed. 2018, 57, 10737–10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (233).Zhang Q; Chen K; Rao W; Zhang Y; Chen F-J; Shi B-F Stereoselective Synthesis of Chiral α-Amino-β-Lactams through Palladium(II)-Catalyzed Sequential Monoarylation/Amidation of C(sp3)–H Bonds. Angew. Chem. Int. Ed. 2013, 52, 13588–13592. [DOI] [PubMed] [Google Scholar]
- (234).Zhang Q; Yin X-S; Zhao S; Fang S-L; Shi B-F Pd(II)-Catalyzed Arylation of Unactivated Methylene C(sp3)–H Bonds with Aryl Halides Using a Removable Auxiliary. Chem. Commun. 2014, 50, 8353–8355. [DOI] [PubMed] [Google Scholar]
- (235).Dailler D; Danoun G; Baudoin O A General and Scalable Synthesis of Aeruginosin Marine Natural Products Based on Two Strategic C(sp3)–H Activation Reactions. Angew. Chem. Int. Ed. 2015, 54, 4919–4922. [DOI] [PubMed] [Google Scholar]
- (236).Gutekunst WR; Baran PS Applications of C–H Functionalization Logic to Cyclobutane Synthesis. J. Org. Chem. 2014, 79, 2430–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (237).Zhang Y-F; Zhao H-W; Wang H; Wei J-B; Shi Z-J Readily Removable Directing Group Assisted Chemo- and Regioselective C(sp3)–H Activation by Palladium Catalysis. Angew. Chem. Int. Ed. 2015, 54, 13686–13690. [DOI] [PubMed] [Google Scholar]
- (238).Rodríguez N; Romero-Revilla JA; Fernández-Ibáñez MÁ; Carretero JC Palladium-Catalyzed N-(2-Pyridyl)Sulfonyl-Directed C(sp3)–H γ-Arylation of Amino Acid Derivatives. Chem. Sci. 2013, 4, 175–179. [Google Scholar]
- (239).Fan M; Ma D Palladium-Catalyzed Direct Functionalization of 2-Aminobutanoic Acid Derivatives: Application of a Convenient and Versatile Auxiliary. Angew. Chem. Int. Ed. 2013, 52, 12152–12155. [DOI] [PubMed] [Google Scholar]
- (240).Ling P-X; Fang S-L; Yin X-S; Chen K; Sun B-Z; Shi B-F Palladium-Catalyzed Arylation of Unactivated γ-Methylene C(sp3)–H and δ-C–H Bonds with an Oxazoline-Carboxylate Auxiliary. Chem. - Eur. J 2015, 21, 17503–17507. [DOI] [PubMed] [Google Scholar]
- (241).Gu Q; Al Mamari HH; Graczyk K; Diers E; Ackermann L Iron-Catalyzed C(sp2)–H and C(sp3)–H Arylation by Triazole Assistance. Angew. Chem. Int. Ed. 2014, 53, 3868–3871. [DOI] [PubMed] [Google Scholar]
- (242).Zhang G; Xie X; Zhu J; Li S; Ding C; Ding P Pd(II)-Catalyzed C(sp3)–H Arylation of Amino Acid Derivatives with Click-Triazoles as Removable Directing Groups. Org. Biomol. Chem. 2015, 13, 5444–5449. [DOI] [PubMed] [Google Scholar]
- (243).Bauer M; Wang W; Lorion MM; Dong C; Ackermann L Internal Peptide Late-Stage Diversification: Peptide-Isosteric Triazoles for Primary and Secondary C(sp3)–H Activation. Angew. Chem. Int. Ed. 2018, 130, 209–213. [DOI] [PubMed] [Google Scholar]
- (244).Wang W; Lorion MM; Martinazzoli O; Ackermann L BODIPY Peptide Labeling by Late-Stage C(sp3)–H Activation. Angew. Chem. Int. Ed. 2018, 57, 10554–10558. [DOI] [PubMed] [Google Scholar]
- (245).Virelli M; Wang W; Kuniyil R; Wu J; Zanoni G; Fernandez A; Scott J; Vendrell M; Ackermann L BODIPY-Labeled Cyclobutanes by Secondary C(sp3)–H Arylations for Live-Cell Imaging. Chem. - Eur. J 2019, 25, 12712–12718. [DOI] [PubMed] [Google Scholar]
- (246).Chen K; Li Z-W; Shen P-X; Zhao H-W; Shi Z-J Development of Modifiable Bidentate Amino Oxazoline Directing Group for Pd-Catalyzed Arylation of Secondary C–H Bonds. Chem. - Eur. J. 2015, 21, 7389–7393. [DOI] [PubMed] [Google Scholar]
- (247).Kim J; Sim M; Kim N; Hong S Asymmetric C–H Functionalization of Cyclopropanes Using an Isoleucine-NH2 Bidentate Directing Group. Chem. Sci. 2015, 6, 3611–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (248).Pasunooti KK; Banerjee B; Yap T; Jiang Y; Liu C-F Auxiliary-Directed Pd-Catalyzed γ-C(sp3)–H Bond Activation of α-Aminobutanoic Acid Derivatives. Org. Lett. 2015, 17, 6094–6097. [DOI] [PubMed] [Google Scholar]
- (249).Reddy C; Bisht N; Parella R; Babu SA 4-Amino-2,1,3-Benzothiadiazole as a Removable Bidentate Directing Group for the Pd(II)-Catalyzed Arylation/Oxygenation of sp2/sp3 β-C–H Bonds of Carboxamides. J. Org. Chem. 2016, 81, 12143–12168. [DOI] [PubMed] [Google Scholar]
- (250).Luo F; Yang J; Li Z; Xiang H; Zhou X Design and Synthesis of 2-Methyl-7-Aminobenzoxazole as Auxiliary in the Palladium(II)-Catalyzed Arylation of a beta-Positioned C(sp3)–H Bond. Adv. Synth. Catal. 2016, 358, 887–893. [Google Scholar]
- (251).Lou J; Wang Q; He Y; Yu Z A Simple Aliphatic Diamine Auxiliary for Palladium-Catalyzed Arylation of Unactivated β-C(sp3)–H Bonds. Adv. Synth. Catal. 2018, 360, 4571–4584. [Google Scholar]
- (252).Topczewski JJ; Cabrera PJ; Saper NI; Sanford MS Palladium-Catalysed Transannular C–H Functionalization of Alicyclic Amines. Nature 2016, 531, 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (253).Dewyer AL; Zimmerman PM Simulated Mechanism for Palladium-Catalyzed, Directed γ-Arylation of Piperidine. ACS Catal. 2017, 7, 5466–5477. [Google Scholar]
- (254).Cabrera PJ; Lee M; Sanford MS Second-Generation Palladium Catalyst System for Transannular C–H Functionalization of Azabicycloalkanes. J. Am. Chem. Soc. 2018, 140, 5599–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (255).Zhang S-K; Yang X-Y; Zhao X-M; Li P-X; Niu J-L; Song M-P Palladium-Catalyzed Selective β-Arylation of Aliphatic Amides Using a Removable N,O-Bidentate Auxiliary. Organometallics 2015, 34, 4331–4339. [Google Scholar]
- (256).Liu J; Xie Y; Zeng W; Lin D; Deng Y; Lu X Pd(II)-Catalyzed Pyridine N-Oxides Directed Arylation of Unactivated C(sp3)–H Bonds. J. Org. Chem. 2015, 80, 4618–4626. [DOI] [PubMed] [Google Scholar]
- (257).Han J; Zheng Y; Wang C; Zhu Y; Shi D-Q; Zeng R; Huang Z-B; Zhao Y Palladium-Catalyzed Oxalyl Amide-Directed γ-Arylation of Aliphatic Amines. J. Org. Chem. 2015, 80, 9297–9306. [DOI] [PubMed] [Google Scholar]
- (258).Han J; Zheng Y; Wang C; Zhu Y; Huang Z-B; Shi D-Q; Zeng R; Zhao Y Pd-Catalyzed Coupling of γ-C(sp3)–H Bonds of Oxalyl Amide-Protected Amino Acids with Heteroaryl and Aryl Iodides. J. Org. Chem. 2016, 81, 5681–5689. [DOI] [PubMed] [Google Scholar]
- (259).Guan M; Pang Y; Zhang J; Zhao Y Pd-Catalyzed Sequential β-C(sp3)–H Arylation and Intramolecular Amination of δ-C(sp2)–H Bonds for Synthesis of Quinolinones: via an N,O-Bidentate Directing Group. Chem. Commun. 2016, 52, 7043–7046. [DOI] [PubMed] [Google Scholar]
- (260).Pati TK; Debnath S; Kundu M; Khamrai U; Maiti DK 3-Amino-1-Methyl-1H-Pyridin-2-One-Directed PdII Catalysis: C(sp3)–H Activated Diverse Arylation Reaction. Org. Lett. 2018, 20, 4062–4066. [DOI] [PubMed] [Google Scholar]
- (261).Gong W; Zhang G; Liu T; Giri R; Yu J-Q Site-Selective C(sp3)–H Functionalization of Di-, Tri-, and Tetrapeptides at the N-Terminus. J. Am. Chem. Soc. 2014, 136, 16940–16946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (262).Toba T; Hu Y; Tran AT; Yu J-Q β-C(sp3)–H Arylation of α-Hydroxy Acid Derivatives Utilizing Amino Acid as a Directing Group. Org. Lett. 2015, 17, 5966–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (263).Noisier AFM; García J; Ionuţ IA; Albericio F Stapled Peptides by Late-Stage C(sp3)–H Activation. Angew. Chem. Int. Ed. 2017, 56, 314–318. [DOI] [PubMed] [Google Scholar]
- (264).Tang J; He Y; Chen H; Sheng W; Wang H Synthesis of Bioactive and Stabilized Cyclic Peptides by Macrocyclization Using C(sp3)–H Activation. Chem. Sci. 2017, 8, 4565–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (265).Zhu R-Y; Liu L-Y; Park HS; Hong K; Wu Y; Senanayake CH; Yu J-Q Versatile Alkylation of (Hetero)Aryl Iodides with Ketones via β-C(sp3)–H Activation. J. Am. Chem. Soc. 2017, 139, 16080–16083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (266).Zhu R-Y; Li Z-Q; Park HS; Senanayake CH; Yu J-Q Ligand-Enabled γ-C(sp3)–H Activation of Ketones. J. Am. Chem. Soc. 2018, 140, 3564–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (267).Xia G; Weng J; Liu L; Verma P; Li Z; Yu J-Q Reversing Conventional Site-Selectivity in C(sp3)–H Bond Activation. Nat. Chem 2019, 11, 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (268).Jin X; Xu H; Zhao N; Li R; Dang Y Origins of Unconventional γ site Selectivity in Palladium-Catalyzed C(sp3)–H Activation and Arylation of Aliphatic Alcohols. Org. Lett. 2020, 22, 1464–1468. [DOI] [PubMed] [Google Scholar]
- (269).Xia G; Zhuang Z; Liu L; Schreiber SL; Melillo B; Yu J-Q Ligand-Enabled β-Methylene C(sp3)–H Arylation of Masked Aliphatic Alcohols. Angew. Chem. Int. Ed. 2020, 59, 7783–7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (270).Wang B-X; Mao Y-J; Hao H-Y; Wu Q-Z; Zhou K; Lou S-J; Xu D-Q Pd-Catalysed Selective C(sp3)–H Arylation and Acetoxylation of Alcohols. Chem. Commun. 2019, 55, 7049–7052. [DOI] [PubMed] [Google Scholar]
- (271).Hao H-Y; Mao Y-J; Xu Z-Y; Lou S-J; Xu D-Q Selective Cross-Dehydrogenative C(sp3)–H Arylation with Arenes. Org. Lett. 2020, 22, 2396–2402. [DOI] [PubMed] [Google Scholar]
- (272).Gopalakrishnan B; Mohan S; Parella R; Babu SA Diastereoselective Pd(II)-Catalyzed sp3 C–H Arylation Followed by Ring Opening of Cyclopropanecarboxamides: Construction of Anti β-Acyloxy Carboxamide Derivatives. J. Org. Chem. 2016, 81, 8988–9005. [DOI] [PubMed] [Google Scholar]
- (273).Gutekunst WR; Baran PS Total Synthesis and Structural Revision of the Piperarborenines via Sequential Cyclobutane C–H Arylation. J. Am. Chem. Soc. 2011, 133, 19076–19079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (274).Jerhaoui S; Chahdoura F; Rose C; Djukic JP; Wencel-Delord J; Colobert F Enantiopure Sulfinyl Aniline as a Removable and Recyclable Chiral Auxiliary for Asymmetric C(sp3)–H Bond Activation. Chem. - Eur. J 2016, 22, 17397–17406. [DOI] [PubMed] [Google Scholar]
- (275).Jerhaoui S; Djukic JP; Wencel-Delord J; Colobert F Stereoselective Sulfinyl Aniline-Promoted Pd-Catalyzed C–H Arylation and Acetoxylation of Aliphatic Amides. Chem. - Eur. J 2017, 23, 15594–15600. [DOI] [PubMed] [Google Scholar]
- (276).Mu D; Gao F; Chen G; He G Palladium-Catalyzed β-C–H Arylation of Alkyl Carboxamides with Sterically Hindered Aryl Iodides Using Ortho-Sulfinyl Aniline Auxiliaries. ACS Catal. 2017, 7, 1880–1885. [Google Scholar]
- (277).Yan S-B; Zhang S; Duan W-L Palladium-Catalyzed Asymmetric Arylation of C(sp3)–H Bonds of Aliphatic Amides: Controlling Enantioselectivity Using Chiral Phosphoric Amides/Acids. Org. Lett. 2015, 17, 2458–2461. [DOI] [PubMed] [Google Scholar]
- (278).Tong H-R; Zheng S; Li X; Deng Z; Wang H; He G; Peng Q; Chen G Pd(0)-Catalyzed Bidentate Auxiliary Directed Enantioselective Benzylic C–H Arylation of 3-Arylpropanamides Using the BINOL Phosphoramidite Ligand. ACS Catal. 2018, 8, 11502–11512. [Google Scholar]
- (279).Wang H; Tong H-R; He G; Chen G An Enantioselective Bidentate Auxiliary Directed Palladium-Catalyzed Benzylic C–H Arylation of Amines Using a BINOL Phosphate Ligand. Angew. Chem. Int. Ed. 2016, 55, 15387–15391. [DOI] [PubMed] [Google Scholar]
- (280).Yan S-Y; Han Y-Q; Yao Q-J; Nie X-L; Liu L; Shi B-F Palladium(II)-Catalyzed Enantioselective Arylation of Unbiased Methylene C(sp3)–H Bonds Enabled by a 2-Pyridinylisopropyl Auxiliary and Chiral Phosphoric Acids. Angew. Chem. Int. Ed. 2018, 57, 9093–9097. [DOI] [PubMed] [Google Scholar]
- (281).Young JP; Park J-W; Jun C-H Metal-Organic Cooperative Catalysis in C–H and C–C Bond Activation and Its Concurrent Recovery. Acc. Chem. Res. 2008, 41, 222–234. [DOI] [PubMed] [Google Scholar]
- (282).Rousseau G; Breit B Removable Directing Groups in Organic Synthesis and Catalysis. Angew. Chem. Int. Ed. 2011, 50, 2450–2494. [DOI] [PubMed] [Google Scholar]
- (283).Qin Y; Zhu L; Luo S Organocatalysis in Inert C–H Bond Functionalization. Chem. Rev. 2017, 117, 9433–9520. [DOI] [PubMed] [Google Scholar]
- (284).Gandeepan P; Ackermann L Transient Directing Groups for Transformative C–H Activation by Synergistic Metal Catalysis. Chem 2018, 4, 199–222. [Google Scholar]
- (285).Liao G; Zhang T; Lin Z-K; Shi B-F Transition Metal-Catalyzed Enantioselective C–H Functionalization via Chiral Transient Directing Group Strategies. Angew. Chem. Int. Ed. 2020, 59, 19773–19786. [DOI] [PubMed] [Google Scholar]
- (286).Mo F; Dong G Regioselective Ketone α-Alkylation with Simple Olefins via Dual Activation. Science 2014, 345, 68–72. [DOI] [PubMed] [Google Scholar]
- (287).Kim D-S; Park W-J; Jun C-H Metal−Organic Cooperative Catalysis in C–H and C−C Bond Activation. Chem. Rev. 2017, 117, 8977–9015. [DOI] [PubMed] [Google Scholar]
- (288).Zhang F-L; Hong K; Li T-J; Park H; Yu J-Q Functionalization of C(sp3)–H Bonds Using a Transient Directing Group. Science 2016, 351, 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (289).Liu W; Zheng J; Liu Z; Hu W; Wang X; Dang Y How Does Palladium-Amino Acid Cooperative Catalysis Enable Regio- and Stereoselective C(sp3)–H Functionalization in Aldehydes and Ketones? A DFT Mechanistic Study. ACS Catal. 2018, 8, 7698–7709. [Google Scholar]
- (290).Ma F; Lei M; Hu L Acetohydrazone: A Transient Directing Group for Arylation of Unactivated C(sp3)–H Bonds. Org. Lett. 2016, 18, 2708–2711. [DOI] [PubMed] [Google Scholar]
- (291).Wen F; Li Z Semicarbazide: A Transient Directing Group for C(sp3)–H Arylation of 2-Methylbenzaldehydes. Adv. Synth. Catal. 2020, 362, 133–138. [Google Scholar]
- (292).Hong K; Park H; Yu J-Q Methylene C(sp3)–H Arylation of Aliphatic Ketones Using a Transient Directing Group. ACS Catal. 2017, 7, 6938–6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (293).Wang J; Dong C; Wu L; Xu M; Lin J; Wei K Palladium-Catalyzed β-C–H Arylation of Ketones Using Amino Amide as a Transient Directing Group: Applications to Synthesis of Phenanthridinone Alkaloids. Adv. Synth. Catal. 2018, 360, 3709–3715. [Google Scholar]
- (294).Xiao L-J; Hong K; Luo F; Hu L; Ewing WR; Yeung K-S; Yu J-Q PdII-Catalyzed Enantioselective C(sp3)–H Arylation of Cyclobutyl Ketones Using a Chiral Transient Directing Group. Angew. Chem. Int. Ed. 2020, 59, 9594–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (295).Yang K; Li Q; Liu Y; Li G; Ge H Catalytic C–H Arylation of Aliphatic Aldehydes Enabled by a Transient Ligand. J. Am. Chem. Soc. 2016, 138, 12775–12778. [DOI] [PubMed] [Google Scholar]
- (296).Li B; Lawrence B; Li G; Ge H Ligand-Controlled Direct γ-C–H Arylation of Aldehydes. Angew. Chem. Int. Ed. 2020, 59, 3078–3082. [DOI] [PubMed] [Google Scholar]
- (297).St John-Campbell S; Bull JA Intramolecular Palladium(II)/(IV) Catalysed C(sp3)–H Arylation of Tertiary Aldehydes Using a Transient Imine Directing Group. Chem. Commun. 2019, 55, 9172–9175. [DOI] [PubMed] [Google Scholar]
- (298).St John-Campbell S; White AJP; Bull JA Methylene C(sp3)–H β, β′-Diarylation of Cyclohexanecarbaldehydes Promoted by a Transient Directing Group and Pyridone Ligand. Org. Lett. 2020, 22, 1807–1812. [DOI] [PubMed] [Google Scholar]
- (299).Xu Y; Young MC; Wang C; Magness DM; Dong G Catalytic C(sp3)–H Arylation of Free Primary Amines with an exo Directing Group Generated In Situ. Angew. Chem. Int. Ed. 2016, 55, 9084–9087. [DOI] [PubMed] [Google Scholar]
- (300).Wu Y; Chen Y-Q; Liu T; Eastgate MD; Yu J-Q Pd-Catalyzed γ-C(sp3)–H Arylation of Free Amines Using a Transient Directing Group. J. Am. Chem. Soc. 2016, 138, 14554–14557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (301).Chen Y-Q; Wang Z; Wu Y; Wisniewski SR; Qiao JX; Ewing WR; Eastgate MD; Yu J-Q Overcoming the Limitations of γ- and δ-C–H Arylation of Amines through Ligand Development. J. Am. Chem. Soc. 2018, 140, 17884–17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (302).Liu Y; Ge H Site-Selective C–H Arylation of Primary Aliphatic Amines Enabled by a Catalytic Transient Directing Group. Nat. Chem. 2017, 9, 26–32. [Google Scholar]
- (303).Feng W; Wang T; Liu D; Wang X; Dang Y Mechanism of the Palladium-Catalyzed C(sp3)–H Arylation of Aliphatic Amines: Unraveling the Crucial Role of Silver(I) Additives. ACS Catal. 2019, 9, 6672–6680. [Google Scholar]
- (304).Yada A; Liao W; Sato Y; Murakami M Buttressing Salicylaldehydes: A Multipurpose Directing Group for C(sp3)–H Bond Activation. Angew. Chem. Int. Ed. 2017, 56, 1073–1076. [DOI] [PubMed] [Google Scholar]
- (305).St John-Campbell S; Ou AK; Bull JA Palladium-Catalyzed C(sp3)–H Arylation of Primary Amines Using a Catalytic Alkyl Acetal to Form a Transient Directing Group. Chem. - Eur. J 2018, 24, 17838–17843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (306).Lin H; Wang C; Bannister TD; Kamenecka TM Site-Selective γ-C(sp3)–H and γ-C(sp2)–H Arylation of Free Amino Esters Promoted by a Catalytic Transient Directing Group. Chem. - Eur. J 2018, 24, 9535–9541. [DOI] [PubMed] [Google Scholar]
- (307).Cai Z; Li S; Gao Y; Fu L; Li G Weak, Bidentate Chelating Group Assisted Cross-Coupling of C(sp3)–H Bonds in Aliphatic Acid Derivatives with Aryltrifluoroborates. Chem. Commun. 2018, 54, 12766–12769. [DOI] [PubMed] [Google Scholar]
- (308).Wang B; Lu C; Zhang S-Y; He G; Nack WA; Chen G Palladium-Catalyzed Stereoretentive Olefination of Unactivated C(sp3)–H Bonds with Vinyl Iodides at Room Temperature: Synthesis of β-Vinyl α-Amino Acids. Org. Lett. 2014, 16, 6260–6263. [DOI] [PubMed] [Google Scholar]
- (309).Shan G; Huang G; Rao Y Palladium-Catalyzed Unactivated β-Methylene C(sp3)–H Bond Alkenylation of Aliphatic Amides and Its Application in a Sequential C(sp3)–H /C(sp2)–H Bond Alkenylation. Org. Biomol. Chem. 2015, 13, 697–701. [DOI] [PubMed] [Google Scholar]
- (310).Chapman LM; Beck JC; Wu L; Reisman SE Enantioselective Total Synthesis of (+)-Psiguadial B. J. Am. Chem. Soc. 2016, 138, 9803–9806. [DOI] [PubMed] [Google Scholar]
- (311).Minami T; Fukuda K; Hoshiya N; Fukuda H; Watanabe M; Shuto S Synthesis of Enantiomerically Pure 1,2,3-Trisubstituted Cyclopropane Nucleosides Using Pd-Catalyzed Substitution via Directing Group-Mediated C(sp3)–H Activation as a Key Step. Org. Lett. 2019, 21, 656–659. [DOI] [PubMed] [Google Scholar]
- (312).Liu Y; Wang Y; Dai W; Huang W; Li Y; Liu H Palladium-Catalysed C(sp3)–H Glycosylation for the Synthesis of C-Alkyl Glycoamino Acids. Angew. Chem. Int. Ed. 2020, 132, 3491–3494. [DOI] [PubMed] [Google Scholar]
- (313).Wu J; Kaplaneris N; Ni S; Kaltenhäuser F; Ackermann L Late-Stage C(sp2)–H and C(sp3)–H Glycosylation of C-Aryl/Alkyl Glycopeptides: Mechanistic Insights and Fluorescence Labeling. Chem. Sci. 2020, 11, 6521–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (314).Ano Y; Tobisu M; Chatani N Palladium-Catalyzed Direct Ethynylation of C(sp3)–H Bonds in Aliphatic Carboxylic Acid Derivatives. J. Am. Chem. Soc. 2011, 133, 12984–12986. [DOI] [PubMed] [Google Scholar]
- (315).Ye X; Xu C; Wojtas L; Akhmedov NG; Chen H; Shi X Silver-Free Palladium-Catalyzed sp3 and sp2 C–H Alkynylation Promoted by a 1,2,3-Triazole Amine Directing Group. Org. Lett. 2016, 18, 2970–2973. [DOI] [PubMed] [Google Scholar]
- (316).Liu T; Qiao JX; Poss MA; Yu J-Q Palladium(II)-Catalyzed Site-Selective C(sp3)–H Alkynylation of Oligopeptides: A Linchpin Approach for Oligopeptide-Drug Conjugation. Angew. Chem. Int. Ed. 2017, 56, 10924–10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (317).Landge VG; Parveen A; Nandakumar A; Balaraman E Pd(II)-Catalyzed Gamma-C(sp3)–H Alkynylation of Amides: Selective Functionalization of R Chains of Amides R1C(O)NHR. Chem. Commun. 2018, 54, 7483–7486. [DOI] [PubMed] [Google Scholar]
- (318).Han Y-Q; Ding Y; Zhou T; Yan S-Y; Song H; Shi B-F Pd(II)-Catalyzed Enantioselective Alkynylation of Unbiased Methylene C(sp3)–H Bonds Using 3,3′-Fluorinated-BINOL as a Chiral Ligand. J. Am. Chem. Soc. 2019, 141, 4558–4563. [DOI] [PubMed] [Google Scholar]
- (319).Zhang S-Y; Li Q; He G; Nack WA; Chen G Stereoselective Synthesis of β-Alkylated α-Amino Acids via Palladium-Catalyzed Alkylation of Unactivated Methylene C(sp3)–H Bonds with Primary Alkyl Halides. J. Am. Chem. Soc. 2013, 135, 12135–12141. [DOI] [PubMed] [Google Scholar]
- (320).Chen K; Hu F; Zhang S-Q; Shi B-F Pd(II)-Catalyzed Alkylation of Unactivated C(sp3)–H Bonds: Efficient Synthesis of Optically Active Unnatural α-Amino Acids. Chem. Sci. 2013, 4, 3906–3911. [Google Scholar]
- (321).Chen K; Shi B-F Sulfonamide-Promoted Palladium(II)-Catalyzed Alkylation of Unactivated Methylene C(sp3)–H Bonds with Alkyl Iodides. Angew. Chem. Int. Ed. 2014, 53, 11950–11954. [DOI] [PubMed] [Google Scholar]
- (322).Chen K; Li X; Zhang S-Q; Shi B-F Synthesis of Chiral α-Hydroxy Acids via Palladium-Catalyzed C(sp3)–H Alkylation of Lactic Acid. Chem. Commun. 2016, 52, 1915–1918. [DOI] [PubMed] [Google Scholar]
- (323).Liu Y; Yang K; Ge H Palladium-Catalyzed Ligand-Promoted Site-Selective Cyanomethylation of Unactivated C(sp3)–H Bonds with Acetonitrile. Chem. Sci. 2016, 7, 2804–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (324).Yang Q; Yang S-D Highly Efficient and Divergent Construction of Chiral γ-Phosphono-α-Amino Acids via Palladium-Catalyzed Alkylation of Unactivated C(sp3)–H Bonds. ACS Catal. 2017, 7, 5220–5224. [Google Scholar]
- (325).Hoshiya N; Takenaka K; Shuto S; Uenishi J Pd(II)-Catalyzed Alkylation of Tertiary Carbon via Directing-Group-Mediated C(sp3)–H Activation: Synthesis of Chiral 1,1,2-Trialkyl Substituted Cyclopropanes. Org. Lett. 2016, 18, 48–51. [DOI] [PubMed] [Google Scholar]
- (326).Zhao Y; Chen G Palladium-Catalyzed Alkylation of Ortho-C(sp2)–H Bonds of Benzylamide Substrates with Alkyl Halides. Org. Lett. 2011, 13, 4850–4853. [DOI] [PubMed] [Google Scholar]
- (327).Zhang S-Y; He G; Nack WA; Zhao Y; Li Q; Chen G Palladium-Catalyzed Picolinamide-Directed Alkylation of Unactivated C(sp3)–H Bonds with Alkyl Iodides. J. Am. Chem. Soc. 2013, 135, 2124–2127. [DOI] [PubMed] [Google Scholar]
- (328).Zhan B-B; Li Y; Xu J-W; Nie X-L; Fan J; Jin L; Shi B-F Site-Selective δ-C(sp3)–H Alkylation of Amino Acids and Peptides with Maleimides via a Six-Membered Palladacycle. Angew. Chem. Int. Ed. 2018, 57, 5858–5862. [DOI] [PubMed] [Google Scholar]
- (329).Wang C; Zhang L; Chen C; Han J; Yao Y; Zhao Y Oxalyl Amide Assisted Palladium-Catalyzed Synthesis of Pyrrolidones via Carbonylation of γ- C(sp3)–H Bonds of Aliphatic Amine Substrates. Chem. Sci. 2015, 6, 4610–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (330).Wang P-L; Li Y; Wu Y; Li C; Lan Q; Wang X-S Pd-Catalyzed C(sp3)–H Carbonylation of Alkylamines: A Powerful Route to γ-Lactams and γ-Amino Acids. Org. Lett. 2015, 17, 3698–3701. [DOI] [PubMed] [Google Scholar]
- (331).Hernando E; Villalva J; Martínez ÁM; Alonso I; Rodríguez N; Gómez Arrayás R; Carretero JC Palladium-Catalyzed Carbonylative Cyclization of Amines via γ-C(sp3)–H Activation: Late-Stage Diversification of Amino Acids and Peptides. ACS Catal. 2016, 6, 6868–6882. [Google Scholar]
- (332).Tanaka K; Ewing WR; Yu J-Q Hemilabile Benzyl Ether Enables γ- C(sp3)–H Carbonylation and Olefination of Alcohols. J. Am. Chem. Soc. 2019, 141, 15494–15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (333).Liao G; Yin X-S; Chen K; Zhang Q; Zhang S-Q; Shi B-F Stereoselective Alkoxycarbonylation of Unactivated C(sp3)–H Bonds with Alkyl Chloroformates via Pd(II)/Pd(IV) Catalysis. Nat. Commun. 2016, 7, 12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (334).Jiang Y; Zhang S-Q; Cao F; Zou J-X; Yu J-L; Shi B-F; Hong X; Wang Z Unexpected Stability of CO-Coordinated Palladacycle in Bidentate Auxiliary Directed C(sp3)–H Bond Activation: A Combined Experimental and Computational Study. Organometallics 2019, 38, 2022–2030. [Google Scholar]
- (335).Chen K; Zhang S-Q; Jiang H-Z; Xu J-W; Shi B-F Practical Synthesis of anti-ß-Hydroxy-α-Amino Acids by PdII-Catalyzed Sequential C(sp3)–H Functionalization. Chem. - Eur. J 2015, 21, 3264–3270. [DOI] [PubMed] [Google Scholar]
- (336).Zhang S-Y; He G; Zhao Y; Wright K; Nack WA; Chen G Efficient Alkyl Ether Synthesis via Palladium-Catalyzed, Picolinamide-Directed Alkoxylation of Unactivated C(sp3)–H and C(sp2)–H Bonds at Remote Positions. J. Am. Chem. Soc. 2012, 134, 7313–7316. [DOI] [PubMed] [Google Scholar]
- (337).Rit RK; Yadav MR; Sahoo AK Pd(II)-Catalyzed Primary-C(sp3)–H Acyloxylation at Room Temperature. Org. Lett. 2012, 14, 3724–3727. [DOI] [PubMed] [Google Scholar]
- (338).Ye X; He Z; Ahmed T; Weise K; Akhmedov NG; Petersen JL; Shi X 1,2,3-Triazoles as Versatile Directing Group for Selective sp2 and sp3 C–H Activation: Cyclization vs Substitution. Chem. Sci. 2013, 4, 3712–3716. [Google Scholar]
- (339).Li Q; Zhang SY; He G; Nack WA; Chen G Palladium-Catalyzed Picolinamide-Directed Acetoxylation of Unactivated γ-C(sp3)–H Bonds of Alkylamines. Adv. Synth. Catal. 2014, 356, 1544–1548. [Google Scholar]
- (340).Liu P; Han J; Chen C-P; Shi D-Q; Zhao Y-S Palladium-Catalyzed Oxygenation of C(sp2)–H and C(sp3)–H Bonds under the Assistance of Oxalyl Amide. RSC Adv. 2015, 5, 28430–28434. [Google Scholar]
- (341).Chen F-J; Zhao S; Kochi A; Chen K; Zhang Q; Zhang S-Q; Shi B-F Pd(II)-Catalyzed Alkoxylation of Unactivated C(sp3)–H and C(sp2)–H Bonds Using a Removable Directing Group: Efficient Synthesis of Alkyl Ethers. Chem. Sci. 2013, 4, 4187–4192. [Google Scholar]
- (342).Shan G; Yang X; Zong Y; Rao Y An Efficient Palladium-Catalyzed C–H Alkoxylation of Unactivated Methylene and Methyl Groups with Cyclic Hypervalent Iodine (I3+) Oxidants. Angew. Chem. Int. Ed. 2013, 52, 13606–13610. [DOI] [PubMed] [Google Scholar]
- (343).Zong Y; Rao Y Developing Pd(II) Catalyzed Double sp3 C–H Alkoxylation for Synthesis of Symmetric and Unsymmetric Acetals. Org. Lett. 2014, 16, 5278–5281. [DOI] [PubMed] [Google Scholar]
- (344).Hu J; Lan T; Sun Y; Chen H; Yao J; Rao Y Unactivated C(sp3)–H Hydroxylation through Palladium Catalysis with H2O as the Oxygen Source. Chem. Commun. 2015, 51, 14929–14932. [DOI] [PubMed] [Google Scholar]
- (345).Ren Z; Mo F; Dong G Catalytic Functionalization of Unactivated sp3 C–H Bonds via exo-Directing Groups: Synthesis of Chemically Differentiated 1,2-Diols. J. Am. Chem. Soc. 2012, 134, 16991–16994. [DOI] [PubMed] [Google Scholar]
- (346).Thompson SJ; Thach DQ; Dong G Cyclic Ether Synthesis via Palladium-Catalyzed Directed Dehydrogenative Annulation at Unactivated Terminal Positions. J. Am. Chem. Soc. 2015, 137, 11586–11589. [DOI] [PubMed] [Google Scholar]
- (347).Xu Y; Yan G; Ren Z; Dong G Diverse sp3 C–H Functionalization through Alcohol β-Sulfonyloxylation. Nat. Chem. 2015, 7, 829–834. [DOI] [PubMed] [Google Scholar]
- (348).Huang Z; Wang C; Dong G A Hydrazone-Based exo-Directing-Group Strategy for β C–H Oxidation of Aliphatic Amines. Angew. Chem. Int. Ed. 2016, 55, 5299–5303. [DOI] [PubMed] [Google Scholar]
- (349).Wang M; Yang Y; Fan Z; Cheng Z; Zhu W; Zhang A Pd-Catalyzed α-Selective C(sp3)–H Acetoxylation of Amides through an Unusual Cyclopalladation Mechanism. Chem. Commun. 2015, 51, 3219–3222. [DOI] [PubMed] [Google Scholar]
- (350).Su B; Bunescu A; Qiu Y; Zuend SJ; Ernst M; Hartwig JF Palladium-Catalyzed Oxidation of β-C(sp3)–H Bonds of Primary Alkylamines through a Rare Four-Membered Palladacycle Intermediate. J. Am. Chem. Soc. 2020, 142, 7912–7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (351).He G; Zhao Y; Zhang S; Lu C; Chen G Highly Efficient Syntheses of Azetidines, Pyrrolidines, and Indolines via Palladium Catalyzed Intramolecular Amination of C(sp3)–H and C(sp2)–H Bonds at γ and δ Positions. J. Am. Chem. Soc. 2012, 134, 3–6. [DOI] [PubMed] [Google Scholar]
- (352).Nadres ET; Daugulis O Heterocycle Synthesis via Direct C–H/N–H Coupling. J. Am. Chem. Soc. 2012, 134, 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (353).Wang C; Chen C; Zhang J; Han J; Wang Q; Guo K; Liu P; Guan M; Yao Y; Zhao Y Easily Accessible Auxiliary for Palladium-Catalyzed Intramolecular Amination of C(sp2)–H and C(sp3)–H Bonds at δ- And ε-Positions. Angew. Chem. Int. Ed. 2014, 53, 9884–9888. [DOI] [PubMed] [Google Scholar]
- (354).He G; Zhang SY; Nack WA; Li Q; Chen G Use of a Readily Removable Auxiliary Group for the Synthesis of Pyrrolidones by the Palladium-Catalyzed Intramolecular Amination of Unactivated γ-C(sp3)–H Bonds. Angew. Chem. Int. Ed. 2013, 52, 11124–11128. [DOI] [PubMed] [Google Scholar]
- (355).Ling P-X; Fang S-L; Yin X-S; Zhang Q; Chen K; Shi B-F Palladium-Catalyzed Sequential Monoarylation/Amidation of C(sp3)–H Bonds: Stereoselective Synthesis of α-Amino-β-Lactams and anti-α,β-Diamino Acid. Chem. Commun. 2017, 53, 6351–6354. [DOI] [PubMed] [Google Scholar]
- (356).Sun W-W; Cao P; Mei R-Q; Li Y; Ma Y-L; Wu B Palladium-Catalyzed Unactivated C(sp3)–H Bond Activation and Intramolecular Amination of Carboxamides: A New Approach to β-Lactams. Org. Lett. 2014, 16, 480–483. [DOI] [PubMed] [Google Scholar]
- (357).Tong H-R; Zheng W; Lv X; He G; Liu P; Chen G Asymmetric Synthesis of β-Lactam via Palladium-Catalyzed Enantioselective Intramolecular C(sp3)–H Amidation. ACS Catal. 2020, 10, 114–120. [Google Scholar]
- (358).Zhou T; Jiang M-X; Yang X; Yue Q; Han Y-Q; Ding Y; Shi B-F Synthesis of Chiral β-Lactams by Pd-Catalyzed Enantioselective Amidation of Methylene C(sp3)–H Bonds. Chin. J. Chem. 2020, 38, 242–246. [Google Scholar]
- (359).Gou Q; Liu G; Liu Z-N; Qin J PdII-Catalyzed Intermolecular Amination of Unactivated C(sp3)–H Bonds. Chem. - Eur. J 2015, 21, 15491–15495. [DOI] [PubMed] [Google Scholar]
- (360).Bai H-Y; Ma Z-G; Yi M; Lin J-B; Zhang S-Y Palladium-Catalyzed Direct Intermolecular Amination of Unactivated Methylene C(sp3)–H Bonds with Azodiformates via Bidentate-Chelation Assistance. ACS Catal 2017, 7, 2042–2046. [Google Scholar]
- (361).Dong Y; Liu G Auxiliary-Assisted Palladium-Catalyzed Direct C(sp3)–H Sulfonamidation to Afford 1,2-Amino Alcohol Derivatives. J. Org. Chem. 2017, 82, 3864–3872. [DOI] [PubMed] [Google Scholar]
- (362).Rit RK; Yadav MR; Ghosh K; Shankar M; Sahoo AK Sulfoximine Assisted Pd(II)-Catalyzed Bromination and Chlorination of Primary β-C(sp3)–H Bond. Org. Lett. 2014, 16, 5258–5261. [DOI] [PubMed] [Google Scholar]
- (363).Zhu R-Y; Liu L-Y; Yu J-Q Highly Versatile β-C(sp3)–H Iodination of Ketones Using a Practical Auxiliary. J. Am. Chem. Soc. 2017, 139, 12394–12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (364).Czyz ML; Weragoda GK; Horngren TH; Connell TU; Gomez D; O’Hair RAJ; Polyzos A Photoexcited Pd(II) Auxiliaries Enable Light-Induced Control in C(sp3)–H Bond Functionalisation. Chem. Sci. 2020, 11, 2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (365).Zhang Q; Yin X-S; Chen K; Zhang S-Q; Shi B-F Stereoselective Synthesis of Chiral β-Fluoro α-Amino Acids via Pd(II)-Catalyzed Fluorination of Unactivated Methylene C(sp3)–H Bonds: Scope and Mechanistic Studies. J. Am. Chem. Soc. 2015, 137, 8219–8226. [DOI] [PubMed] [Google Scholar]
- (366).Miao J; Yang K; Kurek M; Ge H Palladium-Catalyzed Site-Selective Fluorination of Unactivated C(sp3)–H Bonds. Org. Lett 2015, 17, 3738–3741. [DOI] [PubMed] [Google Scholar]
- (367).Zhu Q; Ji D; Liang T; Wang X; Xu Y Efficient Palladium-Catalyzed C–H Fluorination of C(sp3)–H Bonds: Synthesis of β-Fluorinated Carboxylic Acids. Org. Lett. 2015, 17, 3798–3801. [DOI] [PubMed] [Google Scholar]
- (368).Sun H; Zhang Y; Chen P; Wu Y-D; Zhang X; Huang Y Ligand-Assisted Palladium(II)/(IV) Oxidation for sp3 C–H Fluorination. Adv. Synth. Catal. 2016, 358, 1946–1957. [Google Scholar]
- (369).Mao Y-J; Lou S-J; Hao H-Y; Xu D-Q Selective C(sp3)–H and C(sp2)–H Fluorination of Alcohols Using Practical Auxiliaries. Angew. Chem. Int. Ed. 2018, 57, 14085–14089. [DOI] [PubMed] [Google Scholar]
- (370).Park H; Verma P; Hong K; Yu J-Q Controlling Pd(IV) Reductive Elimination Pathways Enables Pd(II)-Catalysed Enantioselective C(sp3)–H Fluorination. Nat. Chem 2018, 10, 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (371).Zhao X; Dimitrijević E; Dong VM Palladium-Catalyzed C–H Bond Functionalization with Arylsulfonyl Chlorides. J. Am. Chem. Soc. 2009, 131, 3466–3467. [DOI] [PubMed] [Google Scholar]
- (372).Zhao X; Dong VM Carbon-Sulfur Reductive Elimination from Palladium(IV) Sulfinate Complexes. Angew. Chem. Int. Ed. 2011, 123, 962–964. [DOI] [PubMed] [Google Scholar]
- (373).Rao W-H; Zhan B-B; Chen K; Ling P-X; Zhang Z-Z; Shi B-F Pd(II)-Catalyzed Direct Sulfonylation of Unactivated C(sp3)–H Bonds with Sodium Sulfinates. Org. Lett. 2015, 17, 3552–3555. [DOI] [PubMed] [Google Scholar]
- (374).Xiong H-Y; Besset T; Cahard D; Pannecoucke X Palladium(II)-Catalyzed Directed Trifluoromethylthiolation of Unactivated C(sp3)–H Bonds. J. Org. Chem. 2015, 80, 4204–4212. [DOI] [PubMed] [Google Scholar]
- (375).Guin S; Deb A; Dolui P; Chakraborty S; Singh VK; Maiti D Promoting Highly Diastereoselective γ-C–H Chalcogenation of α-Amino Acids and Aliphatic Carboxylic Acids. ACS Catal 2018, 8, 2664–2669. [Google Scholar]
- (376).Zhang L-S; Chen G; Wang X; Guo Q-Y; Zhang X-S; Pan F; Chen K; Shi Z-J Direct Borylation of Primary C–H Bonds in Functionalized Molecules by Palladium Catalysis. Angew. Chem. Int. Ed. 2014, 53, 3899–3903. [DOI] [PubMed] [Google Scholar]
- (377).Kanyiva KS; Kuninobu Y; Kanai M Palladium-Catalyzed Direct C–H Silylation and Germanylation of Benzamides and Carboxamides. Org. Lett. 2014, 16, 1968–1971. [DOI] [PubMed] [Google Scholar]
- (378).Liu Y-J; Liu Y-H; Zhang Z-Z; Yan S-Y; Chen K; Shi B-F Divergent and Stereoselective Synthesis of β-Silyl-α-Amino Acids through Palladium-Catalyzed Intermolecular Silylation of Unactivated Primary and Secondary C–H Bonds. Angew. Chem. Int. Ed. 2016, 55, 13859–13862. [DOI] [PubMed] [Google Scholar]
- (379).Pan J-L; Li Q-Z; Zhang T-Y; Hou S-H; Kang J-C; Zhang S-Y Palladium-Catalyzed Direct Intermolecular Silylation of Remote Unactivated C(sp3)–H Bonds. Chem. Commun. 2016, 52, 13151–13154. [DOI] [PubMed] [Google Scholar]
- (380).Zhou Z-X; Rao W-H; Zeng M-H; Liu Y-J Facile Synthesis of Unnatural β-Germyl-α-Amino Amides: Via Pd(II)-Catalyzed Primary and Secondary C(sp3)–H Bond Germylation. Chem. Commun. 2018, 54, 14136–14139. [DOI] [PubMed] [Google Scholar]
- (381).Zhan B-B; Fan J; Jin L; Shi B-F Divergent Synthesis of Silicon-Containing Peptides via Pd-Catalyzed Post-Assembly γ-C(sp3)–H Silylation. ACS Catal. 2019, 9, 3298–3303. [Google Scholar]
- (382).Deb A; Singh S; Seth K; Pimparkar S; Bhaskararao B; Guin S; Sunoj RB; Maiti D Experimental and Computational Studies on Remote γ-C(sp3)–H Silylation and Germanylation of Aliphatic Carboxamides. ACS Catal. 2017, 7, 8171–8175. [Google Scholar]
- (383).Rosen BM; Quasdorf KW; Wilson DA; Zhang N; Resmerita AM; Garg NK; Percec V Nickel-Catalyzed Cross-Couplings Involving Carbon-Oxygen Bonds. Chem. Rev. 2011, 111, 1346–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (384).Tasker SZ; Standley EA; Jamison TF Recent Advances in Homogeneous Nickel Catalysis. Nature 2014, 509, 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (385).Diccianni JB; Diao T Mechanisms of Nickel-Catalyzed Cross-Coupling Reactions. Trends Chem. 2019, 1, 830–844. [Google Scholar]
- (386).Kleiman JP; Dubeck M The Preparation of Cyclopentadienyl [o-(Phenylazo)Phenyl] Nickel. J. Am. Chem. Soc. 1963, 85, 1544–1545. [Google Scholar]
- (387).Misal Castro LC; Chatani N Nickel Catalysts/N,N′-Bidentate Directing Groups: An Excellent Partnership in Directed C–H Activation Reactions. Chem. Lett. 2015, 44, 410–421. [Google Scholar]
- (388).Zhan B-B; Liu B; Hu F; Shi B-F Recent Progress on Nickel-Catalyzed Direct Functionalization of Unactivated C–H Bonds. Chin. Sci. Bull. 2015, 60, 2907–2917. [Google Scholar]
- (389).Khake SM; Chatani N Chelation-Assisted Nickel-Catalyzed C–H Functionalizations. Trends Chem. 2019, 1, 524–539. [Google Scholar]
- (390).Liu Y-H; Xia Y-N; Shi B-F Ni-Catalyzed Chelation-Assisted Direct Functionalization of Inert C–H Bonds. Chin. J. Chem. 2020, 38, 635–662. [Google Scholar]
- (391).Shiota H; Ano Y; Aihara Y; Fukumoto Y; Chatani N Nickel-Catalyzed Chelation-Assisted Transformations Involving Ortho C–H Bond Activation: Regioselective Oxidative Cycloaddition of Aromatic Amides to Alkynes. J. Am. Chem. Soc. 2011, 133, 14952–14955. [DOI] [PubMed] [Google Scholar]
- (392).Song W; Ackermann L Nickel-Catalyzed Alkyne Annulation by Anilines: Versatile Indole Synthesis by C–H/N–H Functionalization. Chem. Commun. 2013, 49, 6638–6640. [DOI] [PubMed] [Google Scholar]
- (393).Aihara Y; Chatani N Nickel-Catalyzed Direct Alkylation of C–H Bonds in Benzamides and Acrylamides with Functionalized Alkyl Halides via Bidentate-Chelation Assistance. J. Am. Chem. Soc. 2013, 135, 5308–5311. [DOI] [PubMed] [Google Scholar]
- (394).Yan S-Y; Liu Y-J; Liu B; Liu Y-H; Shi B-F Nickel-Catalyzed Thiolation of Unactivated Aryl C–H Bonds: Efficient Access to Diverse Aryl Sulfides. Chem. Commun. 2015, 51, 4069–4072. [DOI] [PubMed] [Google Scholar]
- (395).Yi J; Yang L; Xia C; Li F Nickel-Catalyzed Alkynylation of a C(sp2)–H Bond Directed by an 8-Aminoquinoline Moiety. J. Org. Chem. 2015, 80, 6213–6221. [DOI] [PubMed] [Google Scholar]
- (396).Aihara Y; Chatani N Nickel-Catalyzed Direct Arylation of C(sp3)–H Bonds in Aliphatic Amides via Bidentate-Chelation Assistance. J. Am. Chem. Soc. 2014, 136, 898–901. [DOI] [PubMed] [Google Scholar]
- (397).Iyanaga M; Aihara Y; Chatani N Direct Arylation of C(sp3)–H Bonds in Aliphatic Amides with Diaryliodonium Salts in the Presence of a Nickel Catalyst. J. Org. Chem. 2014, 79, 11933–11939. [DOI] [PubMed] [Google Scholar]
- (398).Li M; Dong J; Huang X; Li K; Wu Q; Song F; You J Nickel-Catalyzed Chelation-Assisted Direct Arylation of Unactivated C(sp3)–H Bonds with Aryl Halides. Chem. Commun. 2014, 50, 3944–3946. [DOI] [PubMed] [Google Scholar]
- (399).Wang X; Zhu L; Chen S; Xu X; Au CT; Qiu R Nickel-Catalyzed Direct C(sp3) –H Arylation of Aliphatic Amides with Thiophenes. Org. Lett. 2015, 17, 5228–5231. [DOI] [PubMed] [Google Scholar]
- (400).Wang X; Xie P; Qiu R; Zhu L; Liu T; Li Y; Iwasaki T; Au C-T; Xu X; Xia Y; Yin S-F; Kambe N Nickel-Catalysed Direct Alkylation of Thiophenes via Double C(sp3)–H/C(sp2)–H Bond Cleavage: The Importance of KH2PO4. Chem. Commun. 2017, 53, 8316–8319. [DOI] [PubMed] [Google Scholar]
- (401).Omer HM; Liu P Computational Study of Ni-Catalyzed C–H Functionalization: Factors That Control the Competition of Oxidative Addition and Radical Pathways. J. Am. Chem. Soc. 2017, 139, 9909–9920. [DOI] [PubMed] [Google Scholar]
- (402).Singh S; Surya K; Sunoj RB Aliphatic C(sp3)–H Bond Activation Using Nickel Catalysis: Mechanistic Insights on Regioselective Arylation. J. Org. Chem. 2017, 82, 9619–9626. [DOI] [PubMed] [Google Scholar]
- (403).Beattie DD; Grunwald AC; Perse T; Schafer LL; Love JA Understanding Ni(II)-Mediated C(sp3)–H Activation: Tertiary Ureas as Model Substrates. J. Am. Chem. Soc. 2018, 140, 12602–12610. [DOI] [PubMed] [Google Scholar]
- (404).Roy P; Bour JR; Kampf JW; Sanford MS Catalytically Relevant Intermediates in the Ni-Catalyzed C(sp2)–H and C(sp3)–H Functionalization of Aminoquinoline Substrates. J. Am. Chem. Soc. 2019, 141, 17382–17387 [DOI] [PubMed] [Google Scholar]
- (405).Wu X; Zhao Y; Ge H Nickel-Catalyzed Site-Selective Alkylation of Unactivated C(sp3)–H Bonds. J. Am. Chem. Soc. 2014, 136, 1789–1792. [DOI] [PubMed] [Google Scholar]
- (406).Li M; Yang Y; Zhou D; Wan D; You J Nickel-Catalyzed Addition-Type Alkenylation of Unactivated, Aliphatic C–H Bonds with Alkynes: A Concise Route to Polysubstituted γ-Butyrolactones. Org. Lett. 2015, 17, 2546–2549. [DOI] [PubMed] [Google Scholar]
- (407).Maity S; Agasti S; Earsad AM; Hazra A; Maiti D Nickel-Catalyzed Insertion of Alkynes and Electron-Deficient Olefins into Unactivated sp3 C–H Bonds. Chem. - Eur. J 2015, 21, 11320–11324. [DOI] [PubMed] [Google Scholar]
- (408).Liu Y-J; Zhang Z-Z; Yan S-Y; Liu Y-H; Shi B-F Ni(II)/BINOL-Catalyzed Alkenylation of Unactivated C(sp3)–H Bonds. Chem. Commun. 2015, 51, 7899–7902. [DOI] [PubMed] [Google Scholar]
- (409).Lin C; Zhang J; Chen Z; Liu Y; Liu Z; Zhang Y An Approach to Five-Membered Lactams from Aliphatic Amides and Terminal Acetylenes by Nickel Catalysis. Adv. Synth. Catal. 2016, 358, 1778–1793. [Google Scholar]
- (410).Luo F-X; Cao Z-C; Zhao H-W; Wang D; Zhang Y-F; Xu X; Shi Z-J Nickel-Catalyzed Oxidative Coupling of Unactivated C(sp3)–H Bonds in Aliphatic Amides with Terminal Alkynes. Organometallics 2017, 36, 18–21. [Google Scholar]
- (411).Wu X; Zhao Y; Ge H Nickel-Catalyzed Site-Selective Amidation of Unactivated C(sp3)–H Bonds. Chem. - Eur. J 2014, 20, 9530–9533. [DOI] [PubMed] [Google Scholar]
- (412).Wu X; Zhao Y; Ge H Direct Aerobic Carbonylation of C(sp2)–H and C(sp3)–H Bonds through Ni/Cu Synergistic Catalysis with DMF as the Carbonyl Source. J. Am. Chem. Soc. 2015, 137, 4924–4927. [DOI] [PubMed] [Google Scholar]
- (413).Yan S-Y; Liu Y-J; Liu B; Liu Y-H; Zhang Z-Z; Shi B-F Nickel-Catalyzed Direct Thiolation of Unactivated C(sp3)–H Bonds with Disulfides. Chem. Commun. 2015, 51, 7341–7344. [DOI] [PubMed] [Google Scholar]
- (414).Ye X; Petersen JL; Shi X Nickel-Catalyzed Directed Sulfenylation of sp2 and sp3 C–H Bonds. Chem. Commun. 2015, 51, 7863–7866. [DOI] [PubMed] [Google Scholar]
- (415).Lin C; Yu W; Yao J; Wang B; Liu Z; Zhang Y Nickel-Catalyzed Direct Thioetherification of β-C(sp3)–H Bonds of Aliphatic Amides. Org. Lett. 2015, 17, 1340–1343. [DOI] [PubMed] [Google Scholar]
- (416).Wang X; Qiu R; Yan C; Reddy VP; Zhu L; Xu X; Yin S-F Nickel-Catalyzed Direct Thiolation of C(sp3)–H Bonds in Aliphatic Amides. Org. Lett. 2015, 17, 1970–1973. [DOI] [PubMed] [Google Scholar]
- (417).Wang C; Zhang L; You J Nickel-Catalyzed Aminoxylation of Inert Aliphatic C(sp3)–H Bonds with Stable Nitroxyl Radicals under Air: One-Pot Route to α-Formyl Acid Derivatives. Org. Lett. 2017, 19, 1690–1693. [DOI] [PubMed] [Google Scholar]
- (418).Trammell R; Rajabimoghadam K; Garcia-Bosch I Copper-Promoted Functionalization of Organic Molecules: From Biologically Relevant Cu/O2 Model Systems to Organometallic Transformations. Chem. Rev. 2019, 119, 2954–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (419).Andrus MB; Lashley JC Copper Catalyzed Allylic Oxidation with Peresters. Tetrahedron 2002, 58, 845–866. [Google Scholar]
- (420).Doyle MP; Duffy R; Ratnikov M; Zhou L Catalytic Carbene Insertion into C–H Bonds. Chem. Rev. 2010, 110, 704–724. [DOI] [PubMed] [Google Scholar]
- (421).Gephart RT; Warren TH Copper-Catalyzed sp3 C–H Amination. Organometallics 2012, 31, 7728–7752. [Google Scholar]
- (422).Guo X-X; Gu D-W; Wu Z; Zhang W Copper-Catalyzed C–H Functionalization Reactions: Efficient Synthesis of Heterocycles. Chem. Rev. 2015, 115, 1622–1651. [DOI] [PubMed] [Google Scholar]
- (423).Wang F; Chen P; Liu G Copper-Catalyzed Radical Relay for Asymmetric Radical Transformations. Acc. Chem. Res. 2018, 51, 2036–2046. [DOI] [PubMed] [Google Scholar]
- (424).Rao W-H; Shi B-F Recent Advances in Copper-Mediated Chelation-Assisted Functionalization of Unactivated C–H Bonds. Org. Chem. Front. 2016, 3, 1028–1047. [Google Scholar]
- (425).Shang M; Sun S-Z; Wang H-L; Wang M-M; Dai H-X Recent Progress on Copper-Mediated Directing-Group-Assisted C(sp2)–H Activation. Synthesis 2016, 48, 4381–4399. [Google Scholar]
- (426).Liu J; Chen G; Tan Z Copper-Catalyzed or -Mediated C–H Bond Functionalizations Assisted by Bidentate Directing Groups. Adv. Synth. Catal. 2016, 358, 1174–1194. [Google Scholar]
- (427).Wang Z; Ni J; Kuninobu Y; Kanai M Copper-Catalyzed Intramolecular C(sp3)–H and C(sp2)–H Amidation by Oxidative Cyclization. Angew. Chem. Int. Ed. 2014, 53, 3496–3499. [DOI] [PubMed] [Google Scholar]
- (428).Wu X; Zhao Y; Zhang G; Ge H Copper-Catalyzed Site-Selective Intramolecular Amidation of Unactivated C(sp3)–H Bonds. Angew. Chem. Int. Ed. 2014, 53, 3706–3710. [DOI] [PubMed] [Google Scholar]
- (429).Wang C; Yang Y; Qin D; He Z; You J Copper-Catalyzed Intramolecular Dehydrogenative Amidation of Unactivated C(sp3)–H Bonds Using O2 as the Sole Oxidant. J. Org. Chem. 2015, 80, 8424–8429. [DOI] [PubMed] [Google Scholar]
- (430).Gou Q; Yang Y-W; Liu Z-N; Qin J Intermolecular Amination of Unactivated C(sp3)–H Bonds with Cyclic Alkylamines: Formation of C(sp3)–N Bonds through Copper/Oxygen-Mediated C(sp3)–H/N–H Activation. Chem. - Eur. J 2016, 22, 16057–16061. [DOI] [PubMed] [Google Scholar]
- (431).Wu X; Zhao Y; Ge H Copper-Promoted Site-Selective Acyloxylation of Unactivated C(sp3)–H Bonds. Chem. Asian J 2014, 9, 2736–2739. [DOI] [PubMed] [Google Scholar]
- (432).Wang Z; Kuninobu Y; Kanai M Copper-Mediated Direct C(sp3)–H and C(sp2)–H Acetoxylation. Org. Lett. 2014, 16, 4790–4793. [DOI] [PubMed] [Google Scholar]
- (433).Wang F; Li X; Li Z; Zhou S; Zhang W Copper Salts/TBAB-Catalyzed Chemo- and Regioselective β-C(sp3)–H Acyloxylation of Aliphatic Amides. ACS Omega 2019, 4, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (434).Zhang J; Chen H; Wang B; Liu Z; Zhang Y Copper-Mediated Aryloxylation and Vinyloxylation of β-C(sp3)–H Bond of Propionamides with Organosilanes. Org. Lett. 2015, 17, 2768–2771. [DOI] [PubMed] [Google Scholar]
- (435).Liu J-H; Cui M; Lu X-Y; Zhang Z-Q; Xiao B; Fu Y Unprecedented Copper-Mediated Oxidative Demethylation of Propionamides via Bidentate-Chelation Assistance. Chem. Commun. 2016, 52, 1242–1245. [DOI] [PubMed] [Google Scholar]
- (436).Wu X; Zhao Y; Ge H Pyridine-Enabled Copper-Promoted Cross Dehydrogenative Coupling of C(sp2)–H and Unactivated C(sp3)–H Bonds. Chem. Sci. 2015, 6, 5978–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (437).Zhang J; Li D; Chen H; Wang B; Liu Z; Zhang Y Alkynylation and Annulation of Aliphatic Amides with Alkynyl Carboxylic Acids: Efficient Synthesis of Pyrrolidones. Adv. Synth. Catal. 2016, 358, 792–807. [Google Scholar]
- (438).Wu X; Miao J; Li Y; Li G; Ge H Copper-Promoted Site-Selective Carbonylation of sp3 and sp2 C–H Bonds with Nitromethane. Chem. Sci. 2016, 7, 5260–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (439).Chen X-X; Ren J-T; Xu J-L; Xie H; Sun W; Li Y-M; Sun M Cobalt (III)-Catalyzed 1, 4-Addition of C (sp3)–H Bonds to Maleimides. Synlett 2018, 29, 1601–1606. [Google Scholar]
- (440).Li N; Wang Y; Kong L; Chang J; Li X Cobalt(III)/Rhodium(III)-Catalyzed Regio- and Stereoselective Allylation of 8-methylquinoline via sp3 CH Activation. Adv. Synth. Catal. 2019, 361, 3880–3885. [Google Scholar]
- (441).Kumar R; Kumar R; Chandra D; Sharma U Cp*Co(III)-Catalyzed Alkylation of Primary and Secondary C(sp3)–H Bonds of 8-Alkylquinolines with Maleimides. J. Org. Chem. 2019, 84, 1542–1552. [DOI] [PubMed] [Google Scholar]
- (442).Tan H; Khan R; Xu D; Zhou Y; Zhang X; Shi G; Fan B Cobalt-Catalyzed Ring-Opening Addition of Azabenzonorbornadienes via C(sp3)–H Bond Activation of 8- Methylquinoline. Chem. Commun. 2020, 56, 12570–12573. [DOI] [PubMed] [Google Scholar]
- (443).Sen M; Emayavaramban B; Barsu N; Premkumar JR; Sundararaju B Cp*Co(III)-Catalyzed C(sp3)–H Bond Activation: A Highly Stereoselective and Regioselective Alkenylation of 8- Methylquinoline with Alkynes. ACS Catal. 2016, 6, 2792–2796. [Google Scholar]
- (444).Friis SD; Johansson MJ; Ackermann L Cobalt-Catalysed C–H Methylation for Late-Stage Drug Diversification. Nat. Chem 2020, 12, 511–519. [DOI] [PubMed] [Google Scholar]
- (445).Yan SY; Ling PX; Shi BF Cobalt (III)-Catalyzed Alkylation of Primary C(sp3)–H Bonds with Diazo Compounds. Adv. Synth. Catal. 2017, 359, 2912–2917. [Google Scholar]
- (446).Barsu N; Rahman MA; Sen M; Sundararaju B Cp*CoIII-Catalyzed C(sp3)–H Bond Amidation of 8-Methylquinoline. Chem. - Eur. J 2016, 22, 9135–9138. [DOI] [PubMed] [Google Scholar]
- (447).Moselage M; Li J; Ackermann L Cobalt-Catalyzed C–H Activation. ACS Catal. 2016, 6, 498–525. [Google Scholar]
- (448).Wei D; Zhu X; Niu J-L; Song MP High-Valent-Cobalt-Catalyzed C–H Functionalization Based on Concerted Metalation-Deprotonation and Single Electron-Transfer Mechanisms. ChemCatChem 2016, 8, 1242–1263. [Google Scholar]
- (449).Yoshino T; Matsunaga S (Pentamethylcyclopentadienyl)Cobalt(III)-Catalyzed C–H Bond Functionalization: From Discovery to Unique Reactivity and Selectivity. Adv. Synth. Catal. 2017, 359, 1245–1262. [Google Scholar]
- (450).Baccalini A; Vergura S; Dolui P; Zanoni G; Maiti D Recent Advances in Cobalt-Catalysed C–H Functionalizations. Org. Biomol. Chem. 2019, 17, 10119–10141. [DOI] [PubMed] [Google Scholar]
- (451).Wu X; Yang K; Zhao Y; Sun H; Li G; Ge H Cobalt-Catalysed Site-Selective Intra-and Intermolecular Dehydrogenative Amination of Unactivated sp3 Carbons. Nat. Commun. 2015, 6, 6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (452).Tan PW; Mak AM; Sullivan MB; Dixon DJ; Seayad J Thioamide-Directed Cobalt(III)-Catalyzed Selective Amidation of C(sp3)–H Bonds. Angew. Chem. Int. Ed. 2017, 56, 16550–16554. [DOI] [PubMed] [Google Scholar]
- (453).Fukagawa S; Kato Y; Tanaka R; Kojima M; Yoshino T; Matsunaga S Enantioselective C(sp3)–H Amidation of Thioamides Catalyzed by a CobaltIII /Chiral Carboxylic Acid Hybrid System. Angew. Chem. Int. Ed. 2019, 58, 1153–1157. [DOI] [PubMed] [Google Scholar]
- (454).Sekine D; Ikeda K; Fukagawa S; Kojima M; Yoshino T; Matsunaga S Chiral 2-Aryl Ferrocene Carboxylic Acids for the Catalytic Asymmetric C(sp3)–H Activation of Thioamides. Organometallics 2019, 38, 3921–3926. [Google Scholar]
- (455).Liu R-H; Shan Q-C; Hu X-H; Loh T-P Site-Selective C(sp3)–H Amination of Thioamide with Anthranils under Cp∗CoIII Catalysis. Chem. Commun. 2019, 55, 5519–5522. [DOI] [PubMed] [Google Scholar]
- (456).Zeng L; Tang S; Wang D; Deng Y; Chen J-L; Lee J-F; Lei A Cobalt-Catalyzed Intramolecular Oxidative C(sp3)–H/N–H Carbonylation of Aliphatic Amides. Org. Lett. 2017, 19, 2170–2173. [DOI] [PubMed] [Google Scholar]
- (457).Barsu N; Bolli SK; Sundararaju B Cobalt Catalyzed Carbonylation of Unactivated C(sp3)–H Bonds. Chem. Sci. 2017, 8, 2431–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (458).Williamson P; Galván A; Gaunt MJ Cobalt-Catalysed C–H Carbonylative Cyclisation of Aliphatic Amides. Chem. Sci. 2017, 8, 2588–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (459).Zhang J; Chen H; Lin C; Liu Z; Wang C; Zhang Y Cobalt-Catalyzed Cyclization of Aliphatic Amides and Terminal Alkynes with Silver-Cocatalyst. J. Am. Chem. Soc. 2015, 137, 12990–12996. [DOI] [PubMed] [Google Scholar]
- (460).Zhang Z-Z; Han Y-Q; Zhan B-B; Wang S; Shi B-F Synthesis of Bicyclo[n.1.0]Alkanes by a Cobalt-Catalyzed Multiple C(sp3)–H Activation Strategy. Angew. Chem. Int. Ed. 2017, 56, 13145–13149. [DOI] [PubMed] [Google Scholar]
- (461).Patureau F;Wencel-Delord WJ; Glorius F Cp*Rh-Catalyzed C–H Activations: Versatile Dehydrogenative Cross-Couplings of C–H Positions with Olefins, Alkynes, and Arenes. Aldrichimica Acta 2012, 45, 31–41. [Google Scholar]
- (462).Satoh T; Miura M Oxidative Coupling of Aromatic Substrates with Alkynes and Alkenes under Rhodium Catalysis. Chem. - Eur. J 2010, 16, 11212–11222. [DOI] [PubMed] [Google Scholar]
- (463).Rej S; Chatani N Rhodium-Catalyzed C(sp2)− or C(sp3)–H Bond Functionalization Assisted by Removable Directing Groups. Angew. Chem. Int. Ed. 2019, 58, 8304–8329. [DOI] [PubMed] [Google Scholar]
- (464).Rakshit S; Patureau FW; Glorius F Pyrrole Synthesis via Allylic sp3 C–H Activation of Enamines Followed by Intermolecular Coupling with Unactivated Alkynes. J. Am. Chem. Soc. 2010, 132, 9585–9587. [DOI] [PubMed] [Google Scholar]
- (465).Huang X; You J Rhodium(III)-Catalyzed C(sp3)–H Amidation of 8-Methylquinolines with Amides at Room Temperature. Chem. Lett. 2015, 44, 1685–1687. [Google Scholar]
- (466).Kim JH; Greßies S; Boultadakis-Arapinis M; Daniliuc C; Glorius F Rh(I)/NHC*-Catalyzed Site- and Enantioselective Functionalization of C(sp3)–H Bonds toward Chiral Triarylmethanes. ACS Catal. 2016, 6, 7652–7656. [Google Scholar]
- (467).Liu B; Hu P; Zhou X; Bai D; Chang J; Li X Cp∗Rh(III)-Catalyzed Mild Addition of C(sp3)–H Bonds to α,β-Unsaturated Aldehydes and Ketones. Org. Lett. 2017, 19, 2086–2089. [DOI] [PubMed] [Google Scholar]
- (468).Kong L; Zhou X; Xu Y; Li X Rhodium(III)-Catalyzed Acylation of C(sp3)–H Bonds with Cyclopropenones. Org. Lett. 2017, 19, 3644–3647. [DOI] [PubMed] [Google Scholar]
- (469).Kong L; Liu B; Zhou X; Wang F; Li X Rhodium(III)-Catalyzed Regio- and Stereoselective Benzylic α-Fluoroalkenylation with Gem-Difluorostyrenes. Chem. Commun. 2017, 53, 10326–10329. [DOI] [PubMed] [Google Scholar]
- (470).Liu B; Zhou T; Li B; Xu S; Song H; Wang B Rhodium(III)-Catalyzed Alkenylation Reactions of 8-Methylquinolines with Alkynes by C(sp3)–H Activation. Angew. Chem. Int. Ed. 2014, 53, 4191–4195. [DOI] [PubMed] [Google Scholar]
- (471).Wang N; Li R; Li L; Xu S; Song H; Wang B Rhodium(III)-Catalyzed Intermolecular Amidation with Azides via C(sp3)–H Functionalization. J. Org. Chem. 2014, 79, 5379–5385. [DOI] [PubMed] [Google Scholar]
- (472).Hu X-H; Yang X-F; Loh T-P Chelation-Assisted Rhodium-Catalyzed Direct Amidation with Amidobenziodoxolones: C(sp2)–H, C(sp3)–H, and Late-Stage Functionalizations. ACS Catal. 2016, 6, 5930–5934. [Google Scholar]
- (473).Fukagawa S; Kojima M; Yoshino T; Matsunaga S Catalytic Enantioselective Methylene C(sp3)–H Amidation of 8-Alkylquinolines Using a Cp*RhIII/Chiral Carboxylic Acid System. Angew. Chem. Int. Ed. 2019, 58, 18154–18158. [DOI] [PubMed] [Google Scholar]
- (474).Kawamorita S; Miyazaki T; Iwai T; Ohmiya H; Sawamura M Rh-Catalyzed Borylation of N-Adjacent C(sp3)–H Bonds with a Silica-Supported Triarylphosphine Ligand. J. Am. Chem. Soc. 2012, 134, 12924–12927. [DOI] [PubMed] [Google Scholar]
- (475).Zhou B; Chen Z; Yang Y; Ai W; Tang H; Wu Y; Zhu W; Li Y Redox-Neutral Rhodium-Catalyzed C–H Functionalization of Arylamine N-Oxides with Diazo Compounds: Primary C(sp3)–H/C(sp2)–H Activation and Oxygen-Atom Transfer. Angew. Chem. Int. Ed. 2015, 54, 12121–12126. [DOI] [PubMed] [Google Scholar]
- (476).Greßies S; Klauck FJR; Kim JH; Daniliuc CG; Glorius F Ligand-Enabled Enantioselective C(sp3)–H Activation of Tetrahydroquinolines and Saturated Aza-Heterocycles by RhI. Angew. Chem. Int. Ed. 2018, 57, 9950–9954. [DOI] [PubMed] [Google Scholar]
- (477).Reyes RL; Harada T; Taniguchi T; Monde K; Iwai T; Sawamura M Enantioselective Rh- or Ir-Catalyzed Directed C(sp3)–H Borylation with Phosphoramidite Chiral Ligands. Chem. Lett. 2017, 46, 1747–1750. [Google Scholar]
- (478).Reyes RL; Sato M; Iwai T; Sawamura M Asymmetric Synthesis of α-Aminoboronates via Rhodium-Catalyzed Enantioselective C(sp3)–H Borylation. J. Am. Chem. Soc. 2020, 142, 589–597. [DOI] [PubMed] [Google Scholar]
- (479).Wang X; Yu D-G; Glorius F Cp*RhIII-Catalyzed Arylation of C(sp3)–H Bonds. Angew. Chem. Int. Ed. 2015, 54, 10280–10283. [DOI] [PubMed] [Google Scholar]
- (480).Yuan C; Tu G; Zhao Y Rhodium(III)-Catalyzed Selective Monoarylation of β or γ-C(sp3)–H Bonds Assisted by a Trimethylpyrazole Group. Org. Lett. 2017, 19, 356–359. [DOI] [PubMed] [Google Scholar]
- (481).Huang X; Wang Y; Lan J; You J Rhodium(III)-Catalyzed Activation of –H Bonds and Subsequent Intermolecular Amidation at Room Temperature. Angew. Chem. Int. Ed. 2015, 54, 9404–9408. [DOI] [PubMed] [Google Scholar]
- (482).Wang H; Tang G; Li X Rhodium(III)-Catalyzed Amidation of Unactivated C(sp3)–H Bonds. Angew. Chem. Int. Ed. 2015, 54, 13049–13052. [DOI] [PubMed] [Google Scholar]
- (483).Dong Y; Chen J; Xu H Rhodium(III)-Catalyzed Directed Amidation of Unactivated C(sp3)–H Bonds to Afford 1,2-Amino Alcohol Derivatives. Chem. Commun. 2018, 54, 11096–11099. [DOI] [PubMed] [Google Scholar]
- (484).Wang Y; Liu H; Li B; Wang B Rhodium(III)-Catalyzed Intermolecular Unactivated Secondary C(sp3)–H Bond Amidation Directed by 3,5-Dimethylpyrazole. Adv. Synth. Catal. 2019, 361, 1564–1569. [Google Scholar]
- (485).Xu Y; Young MC; Dong G Catalytic Coupling between Unactivated Aliphatic C–H Bonds and Alkynes via a Metal–Hydride Pathway. J. Am. Chem. Soc. 2017, 139, 5716–5719. [DOI] [PubMed] [Google Scholar]
- (486).Han Y-F; Jin G-X Cyclometalated [Cp*M(ĈX)] (M = Ir, Rh; X = N, C, O, P) Complexes. Chem. Soc. Rev. 2014, 43, 2799–2823. [DOI] [PubMed] [Google Scholar]
- (487).DeBoef B; Pastine SJ; Sames D Cross-Coupling of sp3 C–H Bonds and Alkenes: Catalytic Cyclization of Alkene-Amide Substrates. J. Am. Chem. Soc. 2004, 126, 6556–6557. [DOI] [PubMed] [Google Scholar]
- (488).Tsuchikama K; Kasagawa M; Endo K; Shibata T Cationic Ir(I)-Catalyzed sp3 C–H Bond Alkenylation of Amides with Alkynes. Organic Letters. 2009, 11, 1821–1823. [DOI] [PubMed] [Google Scholar]
- (489).Pan S; Endo K; Shibata T Ir(I)-Catalyzed Enantioselective Secondary sp3 C–H Bond Activation of 2-(Alkylamino)Pyridines with Alkenes. Org. Lett. 2011, 13, 4692–4695. [DOI] [PubMed] [Google Scholar]
- (490).Yamauchi D; Nishimura T; Yorimitsu H Hydroxoiridium-Catalyzed Hydroalkylation of Terminal Alkenes with Ureas by C(sp3)–H Bond Activation. Angew. Chem. Int. Ed. 2017, 56, 7200–7204. [DOI] [PubMed] [Google Scholar]
- (491).Tran AT; Yu J-Q Practical Alkoxythiocarbonyl Auxiliaries for Iridium(I)-Catalyzed C–H Alkylation of Azacycles. Angew. Chem. Int. Ed. 2017, 56, 10530–10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (492).Verma P; Richter JM; Chekshin N; Qiao JX; Yu J-Q Iridium(I)-Catalyzed α-C(sp3)–H Alkylation of Saturated Azacycles. J. Am. Chem. Soc. 2020, 142, 5117–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (493).Mita T; Ikeda Y; Michigami K; Sato Y Iridium-Catalyzed Triple C(sp3)–H Borylations: Construction of Triborylated sp3-Carbon Centers. Chem. Commun. 2013, 49, 5601–5603. [DOI] [PubMed] [Google Scholar]
- (494).Kawamorita S; Murakami R; Iwai T; Sawamura M Synthesis of Primary and Secondary Alkylboronates through Site-Selective C(sp3)–H Activation with Silica-Supported Monophosphine-Ir Catalysts. J. Am. Chem. Soc. 2013, 135, 2947–2950. [DOI] [PubMed] [Google Scholar]
- (495).Iwai T; Harada T; Hara K; Sawamura M Threefold Cross-Linked Polystyrene-Triphenylphosphane Hybrids: Mono-P-Ligating Behavior and Catalytic Applications for Aryl Chloride Cross-Coupling and C(sp3)–H Borylation. Angew. Chem. Int. Ed. 2013, 52, 12322–12326. [DOI] [PubMed] [Google Scholar]
- (496).Reyes RL; Iwai T; Maeda S; Sawamura M Iridium-Catalyzed Asymmetric Borylation of Unactivated Methylene C(sp3)–H Bonds. J. Am. Chem. Soc. 2019, 141, 6817–6821. [DOI] [PubMed] [Google Scholar]
- (497).Zou X; Zhao H; Li Y; Gao Q; Ke Z; Xu S Chiral Bidentate Boryl Ligand Enabled Iridium-Catalyzed Asymmetric C(sp2)–H Borylation of Diarylmethylamines. J. Am. Chem. Soc. 2019, 141, 5334–5342. [DOI] [PubMed] [Google Scholar]
- (498).Shi Y; Gao Q; Xu S Chiral Bidentate Boryl Ligand Enabled Iridium-Catalyzed Enantioselective C(sp3)–H Borylation of Cyclopropanes. J. Am. Chem. Soc. 2019, 141, 10599–10604. [DOI] [PubMed] [Google Scholar]
- (499).Kang T; Kim Y; Lee D; Wang Z; Chang S Iridium-Catalyzed Intermolecular Amidation of sp3 C–H Bonds: Late-Stage Functionalization of an Unactivated Methyl Group. J. Am. Chem. Soc. 2014, 136, 4141–4144. [DOI] [PubMed] [Google Scholar]
- (500).Kang T; Kim H; Kim JG; Chang S Synthesis of 1,2-Amino Alcohols via Catalytic C–H Amidation of sp3 Methyl C–H Bonds. Chem. Commun. 2014, 50, 12073–12075. [DOI] [PubMed] [Google Scholar]
- (501).Kim H; Park G; Park J; Chang S A Facile Access to Primary Alkylamines and Anilines via Ir(III)-Catalyzed C–H Amidation Using Azidoformates. ACS Catal. 2016, 6, 5922–5929. [Google Scholar]
- (502).Xiao X; Hou C; Zhang Z; Ke Z; Lan J; Jiang H; Zeng W Iridium(III)-Catalyzed Regioselective Intermolecular Unactivated Secondary C(sp3)–H Bond Amidation. Angew. Chem. Int. Ed. 2016, 55, 11897–11901. [DOI] [PubMed] [Google Scholar]
- (503).Gao P; Guo W; Xue J; Zhao Y; Yuan Y; Xia Y; Shi Z Iridium(III)-Catalyzed Direct Arylation of C–H Bonds with Diaryliodonium Salts. J. Am. Chem. Soc. 2015, 137, 12231–12240. [DOI] [PubMed] [Google Scholar]
- (504).Tan E; Zanini M; Echavarren AM Iridium-Catalyzed β-Alkynylation of Aliphatic Oximes as Masked Carbonyl Compounds and Alcohols. Angew. Chem. Int. Ed. 2020, 59, 10470–10473. [DOI] [PubMed] [Google Scholar]
- (505).Mahato SK; Chatani N The Iridium(III)-Catalyzed Direct C(sp2)– and C(sp3)–H Alkynylation of 2-Acylimidazoles with Various Alkynyl Bromides: Understanding the Full Catalytic Cycle. ACS Catal. 2020, 10, 5173–5178. [Google Scholar]
- (506).Bauer I; Knölker HJ Iron Catalysis in Organic Synthesis. Chem. Rev. 2015, 115, 3170–3387. [DOI] [PubMed] [Google Scholar]
- (507).Sun C-L; Li B-J; Shi Z-J Direct C–H Transformation via Iron Catalysis. Chem. Rev. 2011, 111, 1293–1314. [DOI] [PubMed] [Google Scholar]
- (508).Shang R; Ilies L; Nakamura E Iron-Catalyzed C–H Bond Activation. Chem. Rev. 2017, 117, 9086–9139. [DOI] [PubMed] [Google Scholar]
- (509).Shang R; Ilies L; Matsumoto A; Nakamura E β-Arylation of Carboxamides via Iron-Catalyzed C(sp3)–H Bond Activation. J. Am. Chem. Soc. 2013, 135, 6030–6032. [DOI] [PubMed] [Google Scholar]
- (510).Shang R; Ilies L; Nakamura E Iron-Catalyzed Directed C(sp2)–H and C(sp3)–H Functionalization with Trimethylaluminum. J. Am. Chem. Soc. 2015, 137, 7660–7663. [DOI] [PubMed] [Google Scholar]
- (511).Ilies L; Itabashi Y; Shang R; Nakamura E Iron/Zinc-Co-Catalyzed Directed Arylation and Alkenylation of C(sp3)–H Bonds with Organoborates. ACS Catal. 2017, 7, 89–92. [Google Scholar]
- (512).Graczyk K; Haven T; Ackermann L Iron-Catalyzed C(sp2)–H and C(sp3)–H Methylations of Amides and Anilides. Chem. - Eur. J 2015, 21, 8812–8815. [DOI] [PubMed] [Google Scholar]
- (513).Arockiam PB; Bruneau C; Dixneuf PH Ruthenium(II)-Catalyzed C–H Bond Activation and Functionalization. Chem. Rev. 2012, 112, 5879–5918. [DOI] [PubMed] [Google Scholar]
- (514).Nareddy P; Jordan F; Szostak M Recent Developments in Ruthenium-Catalyzed C–H Arylation: Array of Mechanistic Manifolds. ACS Catal. 2017, 7, 5721–5745. [Google Scholar]
- (515).Bruneau C sp3 C–H Bond Functionalization with Ruthenium Catalysts. Top. Organomet. Chem. 2014, 48, 195–236. [Google Scholar]
- (516).Liu B; Li B; Wang B Ru(II)-Catalyzed Amidation Reactions of 8-Methylquinolines with Azides via C(sp3)–H Activation. Chem. Commun. 2015, 51, 16334–16337. [DOI] [PubMed] [Google Scholar]
- (517).Chatani N; Asaumi T; Yorimitsu S; Ikeda T; Kakiuchi F; Murai S Ru3 (CO)12 -Catalyzed Coupling Reaction of sp3 C–H Bonds Adjacent to a Nitrogen Atom in Alkylamines with Alkenes. J. Am. Chem. Soc. 2001, 123, 10935–10941. [DOI] [PubMed] [Google Scholar]
- (518).Pastine SJ; Gribkov DV; Sames D sp3 C–H Bond Arylation Directed by Amidine Protecting Group: α-Arylation of Pyrrolidines and Piperidines. J. Am. Chem. Soc. 2006, 128, 14220–14221. [DOI] [PubMed] [Google Scholar]
- (519).Prokopcová H; Bergman SD; Aelvoet K; Smout V; Herrebout W; Van Der Veken B; Meerpoel L; Maes BUW C-2 Arylation of Piperidines through Directed Transition-Metal-Catalyzed sp3 C–H Activation. Chem. - Eur. J 2010, 16, 13063–13067. [DOI] [PubMed] [Google Scholar]
- (520).Jun C-H; Hwang D-C; Na S-J Chelation-Assisted Alkylation of Benzylamine Derivatives by Ru0 Catalyst. Chem. Commun. 1998, 1405–1406. [Google Scholar]
- (521).Dastbaravardeh N; Schnürch M; Mihovilovic MD Ruthenium(II)-Catalyzed sp3 C–H Bond Arylation of Benzylic Amines Using Aryl Halides. Org. Lett. 2012, 14, 3792–3795. [DOI] [PubMed] [Google Scholar]
- (522).Dastbaravardeh N; Schnürch M; Mihovilovic MD Ruthenium(0)-Catalyzed sp3 C–H Bond Arylation of Benzylic Amines Using Arylboronates. Org. Lett. 2012, 14, 1930–1933. [DOI] [PubMed] [Google Scholar]
- (523).Kumar NYP; Jeyachandran R; Ackermann L C(sp3)–H Bond Arylations Catalyzed by Well-Defined [Ru(O2CMes)2(p-Cymene)]. J. Org. Chem. 2013, 78, 4145–4152. [DOI] [PubMed] [Google Scholar]
- (524).Schinkel M; Wang L; Bielefeld K; Ackermann L Ruthenium(II)-Catalyzed C(sp3)–H α-Alkylation of Pyrrolidines. Org. Lett. 2014, 16, 1876–1879. [DOI] [PubMed] [Google Scholar]
- (525).Bergman SD; Storr TE; Prokopcová H; Aelvoet K; Diels G; Meerpoel L; Maes BUW The Role of the Alcohol and Carboxylic Acid in Directed Ruthenium-Catalyzed C(sp3)–H α-Alkylation of Cyclic Amines. Chem. - Eur. J 2012, 18, 10393–10398. [DOI] [PubMed] [Google Scholar]
- (526).Kulago AA; Van Steijvoort BF; Mitchell EA; Meerpoel L; Maes BUW Directed Ruthenium-Catalyzed C(sp3)–H α-Alkylation of Cyclic Amines Using Dioxolane-Protected Alkenones. Adv. Synth. Catal. 2014, 356, 1610–1618. [Google Scholar]
- (527).Hasegawa N; Charra V; Inoue S; Fukumoto Y; Chatani N Highly Regioselective Carbonylation of Unactivated C(sp3)–H Bonds by Ruthenium Carbonyl. J. Am. Chem. Soc. 2011, 133, 8070–8073. [DOI] [PubMed] [Google Scholar]
- (528).Li W; Huang X; You J Ruthenium-Catalyzed Intermolecular Direct Silylation of Unreactive C(sp3)–H Bonds. Org. Lett. 2016, 18, 666–668. [DOI] [PubMed] [Google Scholar]
- (529).Shilov AE; Shul’pin GB ActiVation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes; Kluwer Academic Publishers: Dordrecht, 2000. [Google Scholar]
- (530).Lersch M; Tilset M Mechanistic Aspects of C–H Activation by Pt Complexes. Chem. Rev. 2005, 105, 2471–2526. [DOI] [PubMed] [Google Scholar]
- (531).Appleton TG Donor Atom Preferences in Complexes of Platinum and Palladium with Amino Acids and Related Molecules. Coord. Chem. Rev. 1997, 166, 313–359. [Google Scholar]
- (532).Dangel BD; Johnson JA; Sames D Selective Functionalization of Amino Acids in Water: A Synthetic Method via Catalytic C–H Bond Activation J. Am. Chem. Soc. 2001, 123, 8149–8150. [DOI] [PubMed] [Google Scholar]