Abstract
An integrated transgene-free multiplex gene-editing toolkit based on the Transgene Killer CRISPR technology greatly saves labor, time, and cost.
Dear Editor,
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9-based gene editing has been used to simultaneously generate mutations in multiple target genes in many plant species (Armario Najera et al., 2019; Bai et al., 2020; Luo et al., 2021). Utilization of ribozyme, endogenous tRNA, bacterium-derived Cpf1 endonuclease, and Csy4 ribonuclease to produce multiple guide RNAs (gRNAs) simplifies multiplex gene editing (Gao and Zhao, 2014; Xie et al., 2015; Wang et al., 2017; Ding et al., 2018; Xu et al., 2019; Zhang et al., 2021). However, multiplex gene editing is still labor-intensive and time-consuming. Editing efficiency of gRNAs depends on target sequences that differ in different genes (Ma et al., 2015; Miao et al., 2018). Consequently, a large number of transgenic plants are required for screening in a couple and even several generations to obtain homozygous mutations for all target genes. Further, due to the presence of the CRISPR/Cas9 transgenes, new mutations may be generated along with propagations in generations (Zhang et al., 2018). So far, several technologies have been developed to obtain transgene-free gene-edited plants, including examinations of a large population of plants generated by transiently expressed gene-editing components (Zhang et al., 2016; Chen et al., 2018), or segregations from stable gene-edited plants (Gao et al., 2016); however, screening and genotyping a large number of T0 or T1 plants are labor-intensive (He and Zhao, 2020). Transgenes can also be removed from gene-edited plants by crossing with the wild-type, but the removal is practically difficult in multiplex gene editing, considering the segregations of multiple loci including the CRISPR/Cas9 transgene and mutation loci in the F2 generation. Recently, the Transgene Killer CRISPR (TKC) technology was developed that relies on spatial–temporal expressions of suicide cassettes of p35S:: Cytoplasmic Male Sterility 2 (CMS2) and pREG2::BARNASE to kill all transgene-containing sperms and embryos, respectively (He et al., 2018). In this study, we developed a Customized Assembly and Simplified Editing (CASE) toolkit in rice (Oryza sativa) that combines TKC technology with multiplex gene editing. The toolkit provides an easy and efficient way to obtain transgene-free gene-edited plants for multiple genes in the T1 generation.
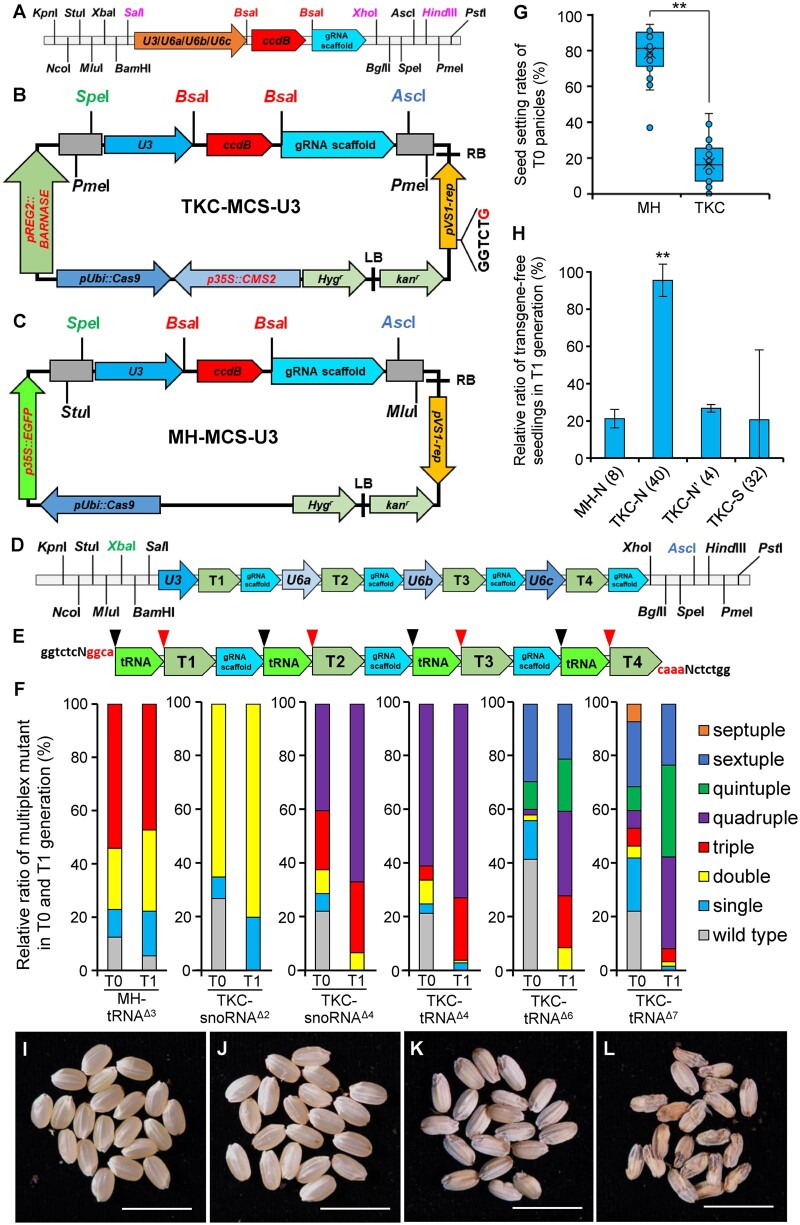
The CASE toolkit consists of a set of four gRNA cloning vectors that contain either U3, U6a, U6b, or U6c small non-coding RNA (snoRNA) promoters (Figure 1A), and a TKC-MCS-U3 gene-editing backbone vector (Figure 1B). The former allowed one-step assembly of independent gRNAs to create customized combinations of gRNA cassettes with compatible restriction sites in flanking regions (Figure 1, A and D; Supplemental Figure S1; Ge et al., 2017). Then, the combinations of gRNA cassettes were transferred to the latter using restriction sites (Figure 1, B and D; Supplemental Figure S1). Constructs generated with this method were named TKC-snoRNAΔn in which “n” represents the number of target genes aimed. Alternatively, chemically synthesized gRNA cassettes, spaced with self-splicing tRNA, can be one-step assembled to the TKC-MCS-U3 backbone (Figure 1, B and E; Supplemental Figure S2). Constructs generated with this method were thus named TKC-tRNAΔn.
Figure 1.
The CASE toolkit for the creation of transgene-free multiplex null mutants in rice. A, The schematic diagram of cassettes of four gRNA cloning vectors (a commercial pMV vector, BGI Genomics, Beijing) containing rice snoRNA promoters of either U3, U6a, U6b, or U6c. Recognition sites of BsaI, flanking the ccdB gene, were used for the gRNA one-step assembly. Recognition sites in magenta words can be used to generate gRNA cassette combinations. ccdB, the ccdB lethal gene. B, The schematic diagram of the TKC-MCS-U3 backbone vector, modified from He et al. (2018). LB and RB, the left and right borders of the T-DNA. Hygr, hygromycin B-resistant gene. p35S::CMS2, rice CMS2 expressed under CaMV 35S promoter. pUbi::Cas9, Cas9 expressed under maize (Zea mays) Ubiquitin promoter. pREG2::BARNASE, bacterial BARNASE expressed under the Rice Embryo Globulin 2 promoter. The red “G” in pVS1-rep cassette represents a C-to-G substitution to eliminate the BsaI site. Kanr, kanamycin-resistant gene. C, The schematic diagram of the control MH-MCS-U3 backbone vector, modified from Ma et al. (2015), in which an enhanced Green Fluorescence Protein (eGFP) expressed under CaMV 35S promoter and a U3-driven gRNA expression cassette was added. D, A gRNA cassette series using snoRNA promoters of U3, U6a, U6b, and U6c, which were assembled from cassettes of gRNA cloning vectors shown in (A). XbaI and AscI were used in the following assembly of the gRNA cassette series to TKC-MCS-U3. T1, T2, T3, and T4 represent independent gRNAs. E, A chemically synthesized gRNA cassette containing four gRNAs spaced with tRNA. BsaI recognizes “ggtctc” (black) at both ends of the cassette, and produces “ggca” and “caaa” (red) sticky ends that will be used to deliver the gRNA cassette to TKC-MCS-U3 in one-step cloning. Black and red arrowheads marked the cleavage sites of RNases P and Z, respectively, at both ends of tRNAs. T1, T2, T3, and T4 represent individual gRNA. F, Analysis of multiplex gene-editing events generated using MH-tRNAΔ3, TKC-snoRNAΔ2, TKC-snoRNAΔ4, TKC-tRNAΔ4, TKC-tRNAΔ6, or TKC-tRNAΔ7 after Sanger sequencing. For T0 generation, all transgenic seedlings were analyzed, and for T1 generation, seedlings germinated from normal-looking seeds (TKC-N for TKC-MCS-U3 vectors, shown in (H)) were randomly selected. All decoding results of T1 generation are shown in Supplemental Table S4. G, Box plot diagrams showing the seed setting rates of T0 transgenic plants generated using MH-MCS-U3- and TKC-MCS-U3-based constructs. Upper and lower limits, center line, upper and lower quartiles indicate maximum and minimum, median, first, and third values, respectively. Asterisks indicate significant differences between samples, n = 28 (two-tailed Student’s t test, **P ˂ 0.01). H, Frequencies of transgene-free in T1 seedlings generated using MH-MCS-U3- and TKC-MCS-U3-based constructs. MH-N indicates T1 seedlings germinated from normal-looking seeds (see (I)) in MH-MCS-U3-based gene editing. TKC-N/Nʹ and TKC-S indicate T1 seedlings germinated from normal-looking (see (J)) and shrunken seeds (see (K)) from TKC-MCS-U3-based gene editing, respectively. Note that four TKC-Nʹ lines showed an unexpected low transgene-free ratio. Numbers (n) in brackets indicate numbers of independent T0 lines. Primers used for transgene detection are listed in Supplemental Table S1. Group data were expressed as means ± Standard deviation (sd) (two-tailed Student’s t test, **P ˂ 0.01 compared with the MH-N (8)). I–L, T1 seeds from MH-MCS-U3-based gene editing are normal-looking (I), whereas those from TKC-MCS-U3-based consisted of three types: normal-looking (J), shrunken (K), and aborted (L). Bar = 1 cm.
To examine the gene-editing efficiency of the CASE toolkit, constructs were generated to target five combinations of genes in the CLAVATA3/EMBRYO SURROUNDING REGION (CLE) family (Goad et al., 2017), including TKC-snoRNAΔ2, TKC-snoRNAΔ4, TKC-tRNAΔ4, TKC-tRNAΔ6, and TKC-tRNAΔ7 (Figure 1F; Supplemental Figure S3). A conventional triple gene-editing construct of MH-tRNAΔ3 was used as a control, in which three chemically synthesized gRNAs, spaced with tRNAs, were one-step assembled to the MH-MCS-U3 backbone (Figure 1F; Supplemental Figures S2 and S3; Ma et al., 2015). After Agrobacterium tumefaciens-mediated transformations in rice, transgene-positive transformants were identified in the T0 generation through polymerase chain reaction (PCR) detections of backbone vectors (Supplemental Figure S4; Supplemental Table S1), and editing of target genes in individual transgenic seedlings was analyzed by Sanger sequencing and decoded in the CRISPR-GE website (Xie et al., 2017). The gene-editing events were further analyzed in the T1 generation (Supplemental Tables S1–S4). Effective multiplex gene editing was observed in T0 and T1 generations when either the TKC-MCS-U3 or MH-MCS-U3 constructs were used (Figure 1F), suggesting that the CASE toolkit is effective in multiplex gene editing.
To verify the autonomous transgene removal of the CASE toolkit, we examined seed setting rates, and analyzed the existence of transgenes in T1 plants by PCR. In MH-MCS-U3-based triple gene editing (abbreviated as MH), the seed setting rate in T0 panicles was 78.68% ± 13.21% (Figure 1G), all T1 seeds examined were normal looking (Figure 1I), and in T1 seeds produced from eight independent lines the ratio of transgene-free seeds was calculated as 21.43% ± 4.98% (abbreviated as MH-N; Figure 1H; Supplemental Table S2). In contrast, T0 plants from the TKC-MCS-U3-based multiplex gene editing (abbreviated as TKC) displayed a significantly lower seed setting rate of 16.94% ± 11.91% (Figure 1G), and T1 seeds produced consisted of three types: normal-looking (Figure 1J), shrunken (Figure 1K), and aborted (Figure 1L). Among 533 T1 seedlings germinated from normal-looking seeds (abbreviated as TKC-N/N’) of 44 independent T0 lines from five TKC-MCS-U3-based multiplex gene editing events, 40 showed a significantly higher transgene-free ratio of 95.37% ± 8.67% (Figure 1H; Supplemental Table S2), and the other four lines showed an unexpected low transgene-free ratio of 26.73% ± 2.02% similar to that of MH-MCS-U3-based gene editing, which are most likely caused by defected suicide cassettes of p35S::CMS2 and/or pREG2::BARNASE (Figure 1H; Supplemental Table S2). Meanwhile, 209 T1 seedlings germinated from shrunken seeds (abbreviated as TKC-S) of 32 independent T0 lines showed a substantially lower transgene-free ratio of 20.57% ± 37.54% similar to that of MH-MCS-U3-based gene editing (Figure 1H; Supplemental Table S2). All aborted seeds from the TKC-MCS-U3-based multiplex gene editing failed to germinate (Figure 1L). It is plausible that those aborted and most shrunken seeds carried the TKC-MCS-U3 transgenes (Figure 1H), which has not been discussed previously (He et al., 2018). Therefore, the embryo suicide cassette pREG2::BARNASE and the microgametophyte suicide cassette p35S::CMS2 provide a double security for the removal of the transgene in TKC-based gene editing, and the low seed setting rates together with the high ratios of shrunken and aborted seeds can serve as indicators for the transgene self-removal of the CASE toolkit.
After the identification of transgene-free plants in the T1 generation, 15 or more T1 individuals from progenies of those 40 high transgene-free TKC-N lines generated using TKC-snoRNAΔ2, TKC-snoRNAΔ4, TKC-tRNAΔ4, TKC-tRNAΔ6, or TKC-tRNAΔ7 were randomly chosen for Sanger sequencing, of which 80%, 66.67%, 68.70%, 19.14%, and 0% were double, quadruple, quadruple, sextuple, and septuple null mutants, respectively (Table 1; Supplemental Tables S3 and S4). As a control, among 31 T1 individuals from the MH-tRNAΔ3 transformation, only 10.03% were transgene-free triple null mutants (Table 1; Supplemental Table S3). In the septuple gene editing using TKC-tRNAΔ7, the efficiency of sextuple gene editing (22.95%) in the T1 generation was comparable to that in the sextuple gene editing (20.73%) (Figure 1F; Supplemental Table S3). However, we failed to identify septuple mutants (Figure 1F; Table 1), which may be caused either by low gene-editing efficiency of OsCLE508 gRNA, or embryo lethality of the septuple mutant (Supplemental Figure S3; Supplemental Table S3).
Table 1.
Ratios of transgene-free multiplex null mutants detected in the T1 generation
| Gene Editing Events | Ratio of Transgene-Free Multiplex Null Mutants (%) | Number of Plants Required |
|---|---|---|
| MH-tRNAΔ3 | 10.03 | ≥22 |
| TKC-snoRNAΔ2 | 80.00 | ≥2 |
| TKC-snoRNAΔ4 | 66.67 | ≥3 |
| TKC-tRNAΔ4 | 68.70 | ≥2 |
| TKC-tRNAΔ6 | 19.14 | ≥11 |
| TKC-tRNAΔ7 | 0.00 | – |
In summary, the CASE toolkit provides an easy and effective way to obtain transgene-free multiplex gene-editing plants in T1 generation (Figure 1F). We calculate that, when double to sextuple gene editing are executed, after the identification of gene editing in the T0 generation, only 2–11 T1 plants are needed to obtain transgene-free null mutants with a >90% probability (Table 1). Thus, the CASE toolkit has considerable potential for the creation of massive transgene-free multiplex knockout null mutants in rice, and even in other plant species as well.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. The schematic diagram of customized combinations of multiple gRNA cassettes driven by rice snoRNA promoters U3, U6a, U6b, and U6c and assembly to the TKC-MCS-U3 backbone vector.
Supplemental Figure S2. The schematic diagram of one-step assembly of single gRNA, or chemically synthesized gRNA cassettes containing four successive gRNAs spaced with tRNAs, to the TKC-MCS-U3 backbone vector.
Supplemental Figure S3. Schematic diagrams of positions and sequences of gRNAs for multiplex gene editing in this study.
Supplemental Figure S4. Detections of transgene in T0 and T1 generations in multiplex gene editing through PCR analyses.
Supplemental Table S1. Primers used in this study.
Supplemental Table S2. Transgene-free ratios analyses in T1 generation.
Supplemental Table S3. Multiplex null mutant analyses in T1 generation.
Supplemental Table S4. Mutations identified in the T1 generation.
Supplementary Material
Acknowledgments
We thank Prof. Yao-Guang Liu (South China Agricultural University) for sharing the pYLCRISPR/Cas9Pubi-H (Addgene #66187) backbone vector, pYLsgRNA-OsU3m (Addgene #66193), pYLsgRNA-OsU6a (Addgene #66194), pYLsgRNA-OsU6b (Addgene #66196), and pYLsgRNA-OsU6c (Addgene #66197) cloning vectors (Addgene #66197), Prof. Le-Qing Qu (Institute of Botany, Chinese Academy of Sciences), Prof. Yong-Xiu Liu (Institute of Botany, Chinese Academy of Sciences), and Prof. Guo-Zheng Qin (Institute of Botany, Chinese Academy of Sciences) for generous sharing of their experimental instruments, Yan-Ping Han (Institute of Botany, Chinese Academy of Sciences) for her excellent work of rice callus transformation, Dr. Saddam Saqib (Mohi-ud-din Islami University AJ&K, Pakistan), and Dr. Wajid Zaman (Lushan Botanical Garden, Chinese Academy of Sciences, China) for their comments on the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31871455), the National Transgenic Science and Technology Program (2019ZX08010-001), and the Youth Innovation Promotion Association of the Chinese Academy of Sciences.
Conflict of interest statement. None declared.
Contributor Information
Jin-Lei Liu, Key Laboratory of Plant Molecular Physiology, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; College of Life Sciences, University of Chinese Academy of Sciences, Beijing 100049, China.
Meng-Meng Chen, Key Laboratory of Plant Molecular Physiology, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; College of Life Sciences, University of Chinese Academy of Sciences, Beijing 100049, China.
Wen-Qiang Chen, Key Laboratory of Plant Molecular Physiology, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; College of Life Sciences, University of Chinese Academy of Sciences, Beijing 100049, China.
Chun-Ming Liu, Key Laboratory of Plant Molecular Physiology, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; College of Life Sciences, University of Chinese Academy of Sciences, Beijing 100049, China; School of Advanced Agricultural Sciences, Peking University, Beijing 100871, China.
Yubing He, State Key Laboratory of Crop Genetics and Germplasm Enhancement, Nanjing Agricultural University, Nanjing 210095, China; Collaborative Innovation Center for Modern Crop Production Co-sponsored by Province and Ministry, Nanjing Agricultural University, Nanjing 210095, China; Excellence and Innovation Center, Jiangsu Academy of Agricultural Science, Nanjing 210014, China.
Xiu-Fen Song, Key Laboratory of Plant Molecular Physiology, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; College of Life Sciences, University of Chinese Academy of Sciences, Beijing 100049, China.
Y.H. and X.F.S. conceived the research plans; J.L.L. performed most of the experiments; M.M.C., W.Q.C., and X.F.S. conducted some experiments; J.L.L., X.F.S., Y.H., and C.M.L. wrote the article with contributions from all of the authors.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Xiu-Fen Song (xfsong@ibcas.ac.cn).
References
- Armario Najera V, Twyman RM, Christou P, Zhu C (2019) Applications of multiplex genome editing in higher plants. Curr Opin Biotechnol 59: 93–102 [DOI] [PubMed] [Google Scholar]
- Bai M, Yuan J, Kuang H, Gong P, Li S, Zhang Z, Liu B, Sun J, Yang M, Yang L, et al. (2020) Generation of a multiplex mutagenesis population via pooled CRISPR-Cas9 in soya bean. Plant Biotechnol J 18: 721–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Li W, Katin-Grazzini L, Ding J, Gu X, Li Y, Gu T, Wang R, Lin X, Deng Z, et al. (2018) A method for the production and expedient screening of CRISPR/Cas9-mediated non-transgenic mutant plants. Hortic Res 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Chen K, Chen Y, Li H, Xie K (2018) Engineering introns to express RNA guides for Cas9- and Cpf1-mediated multiplex genome editing. Mol Plant 11: 542–552 [DOI] [PubMed] [Google Scholar]
- Gao X, Chen J, Dai X, Zhang D, Zhao Y (2016) An effective strategy for reliably isolating heritable and Cas9-free Arabidopsis mutants generated by CRISPR/Cas9-mediated genome editing. Plant Physiol 171: 1794–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhao Y (2014) Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing. J Integr Plant Biol 56: 343–349 [DOI] [PubMed] [Google Scholar]
- Goad DM, Zhu C, Kellogg EA (2017) Comprehensive identification and clustering of CLV3/ESR-related (CLE) genes in plants finds groups with potentially shared function. New Phytol 216: 605–616 [DOI] [PubMed] [Google Scholar]
- Ge Z, Bergonci T, Zhao Y, Zou Y, Du S, Liu MC, Luo X, Ruan H, García-Valencia LE, Zhong S, et al. (2017) Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 358: 1596–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zhao Y (2020) Technological breakthroughs in generating transgene-free and genetically stable CRISPR-edited plants. aBIOTECH 1: 88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zhu M, Wang L, Wu J, Wang Q, Wang R, Zhao Y (2018) Programmed self-elimination of the CRISPR/Cas9 construct greatly accelerates the isolation of edited and transgene-free rice plants. Mol Plant 11: 1210–1213 [DOI] [PubMed] [Google Scholar]
- Luo J, Li S, Xu J, Yan L, Ma Y, Xia L (2021) Pyramiding favorable alleles in an elite wheat variety in one generation by CRISPR-Cas9-mediated multiplex gene-editing. Mol Plant 14: 847–850 [DOI] [PubMed] [Google Scholar]
- Ma X, Zhang Q, Zhu Q, Liu W, Chen Y, Qiu R, Wang B, Yang Z, Li H, Lin Y, et al. (2015) A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant 8: 1274–1284 [DOI] [PubMed] [Google Scholar]
- Miao C, Xiao L, Hua K, Zou C, Zhao Y, Bressan RA, Zhu JK (2018) Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc Natl Acad Sci USA 115: 6058–6063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Mao Y, Lu Y, Tao X, Zhu JK (2017) Multiplex gene editing in rice using the CRISPR-Cpf1 system. Mol Plant 10: 1011–1013 [DOI] [PubMed] [Google Scholar]
- Xie K, Minkenberg B, Yang Y (2015) Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc Natl Acad Sci USA 112: 3570–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ma X, Zhu Q, Zeng D, Li G, Liu YG (2017) CRISPR-GE: A convenient software toolkit for CRISPR-based genome editing. Mol Plant 10: 1246–1249 [DOI] [PubMed] [Google Scholar]
- Xu R, Qin R, Li H, Li J, Yang J, Wei P (2019) Enhanced genome editing in rice using single transcript unit CRISPR-LbCpf1 systems. Plant Biotechnol J 17: 553–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Xing HL, Wang ZP, Zhang HY, Yang F, Wang XC, Chen QJ (2018) Potential high-frequency off-target mutagenesis induced by CRISPR/Cas9 in Arabidopsis and its prevention. Plant Mol Biol 96: 445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liang Z, Zong Y, Wang Y, Liu J, Chen K, Qiu JL, Gao C (2016) Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat Commun 7: 12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ren Q, Tang X, Liu S, Malzahn AA, Zhou J, Wang J, Yin D, Pan C, Yuan M, et al. (2021) Expanding the scope of plant genome engineering with Cas12a orthologs and highly multiplexable editing systems. Nat Commun 12: 1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.