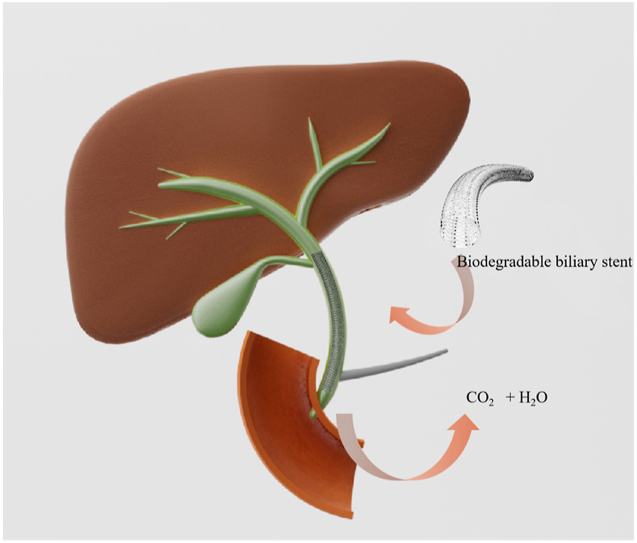
Abstract
Biliary stricture is defined as the reduction and narrowing of the bile duct lumen, which can be caused by many factors such as cancer and inflammation. Biliary stent placement can effectively alleviate benign and malignant biliary strictures. However, the commonly used plastic or metallic biliary stents are far from ideal and do not satisfy all clinical requirements,although several types of biodegradable biliary stents have been developed and used clinically. In this review, we summarized current development status of biodegradable stents with the emphasis on the stent materials. We also presented the future development trends based on the published literature.
Keywords: Biodegradable biliary stent, Biliary strictures, Polydioxanone, Poly (l-lactic acid), Drug-eluting stent
Graphical abstract
Highlights
-
•
Summary of current development status of bioresorbable biliary stents with the emphasis on the stent materials.
-
•
The future development trends based on the published literature.
-
•
The advantages of bioresorbable biliary stents compared with metallic and plastic biliary stents.
1. Introduction
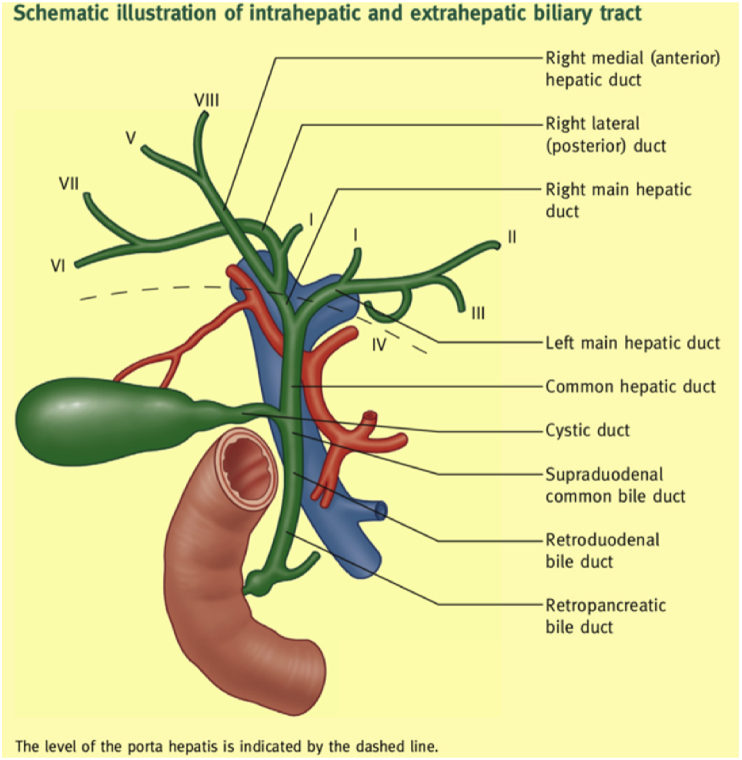
Bile ducts are long tubular structures that are responsible for transporting bile from the liver and gallbladder to the small intestine, as part of the digestive process. Multiple bile ducts form a branch shape, called biliary trees, which converge on the left and right hepatic ducts, collectively known as the common hepatic duct. The common hepatic duct descends and joins the cystic duct to form the common bile duct (CBD), which then continues to descend to meet the main pancreatic duct at the ampulla of Vater. The role of the Vater ampulla is to drain bile from the liver and gallbladder into the duodenum [1]. Hepatocytes continuously produce and secrete bile into the bile duct, and bile stored in the gallbladder during non-digestive periods. Contrastingly, bile is directly discharged from the gallbladder and the liver into the duodenum in large quantities to help with post-prandial digestion and decomposition of fat and fat-soluble vitamins during digestion. The anatomical structure of the biliary tract is shown in Fig. 1.
Fig. 1.
The anatomical structure of the biliary tract [1], with permission from Elsevier, 2021.
Narrowing of the bile duct lumen is called biliary stricture, which can be caused by factors such as intraoperative biliary injury, cancer, inflammation, and gallstone scarring [2]. Biliary strictures can be categorized as benign biliary stricture (BBS) or malignant biliary stricture (MBS). BBS is generally caused by injury to the bile duct or benign biliary disease, while MBS is generally the result of malignant bile duct tumors. In previous cases, MBS occurred in 72% of patients with biliary stricture. Orthotopic liver transplantation (OLT) and cholecystectomy accounted for approximately 80% of BBS in western countries [2]. Biliary strictures block the flow of bile from the liver and gallbladder to the small intestine, which results in abnormal digestion and can cause serious complications such as jaundice and cirrhosis.
Regarding biliary stricture treatments, although surgical treatment is immediately effective, it bestows considerable physical pain and psychological pressure onto the patient. Another commonly used treatment option is balloon dilatation, but it has a success rate of only 70%. Biliary stents are further treatment options, with the option between endoscopic retrograde cholangiopancreatography (ERCP), and percutaneous transhepatic cholangiogram (PTHC).
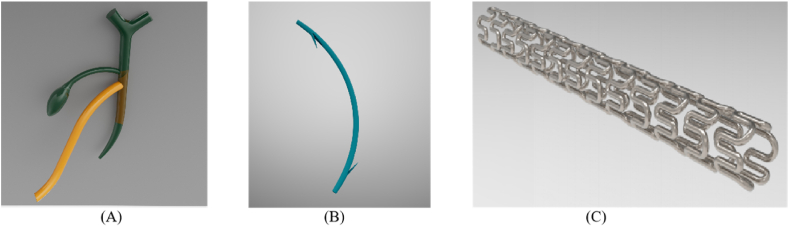
Biliary stents are usually used to restore the unidirectional flow of bile and help remodel the lumen when the bile duct is blocked or confined. At present, commonly used bile duct prostheses include T-shaped tubes and biliary stents made of plastic or metal, as shown in Fig. 2. The T-shaped tubes (Fig. 2(A)) are draining tubes that are inserted into the bile duct after open surgery to prevent stricture and to help with bile drainage [3]. However, the use of T-shaped tubes is associated with various complications, including lumen blockage, retrograde infection, and biliary leak following T-tube removal [4]. Alternatively, clinically available tubular plastic biliary stents (Fig. 2(B)) and metallic stents (Fig. 2(C)), although they are convenient to use, need to be surgically removed after benign biliary stricture (BBS) disappear or they will cause blockage of the bile ducts due to the accumulation of bile sludge inside the plastic tube or the tissue over growth inside the metallic stents due to the long term of foreign body irritation to the bile duct [5]. The stent remove process may also cause injure to the bile duct. The use of plastic stents may also have the possibility of causing acute cholangitis associated with common bile duct stones [6]. Overall, these typical issues associated with the use of none-degradable plastic and metal stents make them unsuitable for the treatment of benign bile duct strictures [7]. Table 1 listed some of the clinically available biliary stents.
Fig. 2.
Schematics of (A) T-tube; (B) plastic biliary stent; (C) metallic biliary stent. The diameter of human common bile duct is in the range of 2–8 mm [72].
Table 1.
Clinically available biliary stents.
| Brand | Manufacturer | Brief description | Disadvantages | Reference |
|---|---|---|---|---|
| WallFlex™ Biliary RX Stents | Boston scientific | 1. Fully covered/partially covered/uncovered stent; | 1. High incidence of epithelial hyperplasia; 2. High incidence of Cholestasis; 3. Hard to remove; 4. Not ideal for patients with benign biliary strictures. |
[8] |
| 2. Self-expanding; | ||||
| 3. Guide wire: 0.035 inch; | ||||
| 4. The outer layer of the wire is nickel-titanium alloy and the inner core is platinum. | ||||
| 5. The coating is Permalume™ | ||||
| HANAROSTENT® Biliary | OLYMPUS | 1. Main material: nickel-titanium alloy | [86] | |
| 2. Uncovered stent; | ||||
| 3. Self-expanding; | ||||
| 4. Guide wire: 0.025 or 0.035 inch; | ||||
| 5. Hook-cross nitinol design; | ||||
| 6. Large flare-ends. | ||||
| Niti-S™ D Biliary Stent | Taewoong Medical | 1. Main material: nickel-titanium alloy; | [87] | |
| 2. Guide wire: 0.035 inch; | ||||
| DISCOVERY BILIARY™ | Endocor | 1. Main material: nitinol; | [88]. | |
| 2. Guide wire: 0.035 inch; | ||||
| 3. Self-expanding; | ||||
| 4. Open cell design; | ||||
| 5. Tantalum markers on the inner catheter. | ||||
| Self-Expanding Nitinol Stents | ENDO-FLEX | 1. Partially silicone coating/no coating; 2. Main material: nitinol; 3. Tantalum markers |
[89] | |
| ParaMount™ Mini GPS™ | Medtronic | 1. Main material: 316L stainless steel | [90] | |
| 2. Tantalum markers; | ||||
| 3. 6 F or 7 F guide catheter; | ||||
| 4. Balloon-expandable; | ||||
| Advanix™ Biliary Stent | Boston scientific | 1. Material: polypropylene (20%BiOCl) | 1. Limited sizes (diameter); 2. Need to be replaced frequently due to bile sludge accumulation; 3. Prone to cause acute cholangitis. |
[91] |
| 2. Thin wall design | ||||
| 3. Tapered stent tip | ||||
| 4. RX delivery system compatible | ||||
| PE 204 series | ENDO-FLEX | 1. Main material: polyethylene | [92] | |
| 2. Pigtail | ||||
| Cotton-Leung® Biliary Stent | Cook Medical | 1. Main material: polyethylene | [93] | |
| 2. Guide wire: 0.035 inch; | ||||
| 3. Tapered tip; | ||||
| 4. Proximal and distal flaps; | ||||
| 5. Gentle curved shape; |
As shown in Table 1, all the stents listed above are made of non-degradable plastics or metals. Those stents have to be removed surgically when they have completed their mission and are no longer needed. Therefore, they are far from ideal. In an ideal clinical scenario, a biliary stent should be able to disappear completely after its mission has completed. Therefore, a biodegradable stent becomes one of the choices.
Considering that most of the biliary stents used clinically are still none biodegradable stents which have to be removed by a secondary surgery, the use of biodegradable stents with better performances will provide a revolutionary treatment technique for biliary strictures. The choice of an appropriate biodegradable substrate material is the key for development of an excellent biliary stent.
2. Materials used in biodegradable biliary stent
In the light of the unmet clinical needs, an increasing number of researchers have started to focus on biodegradable biliary stents to overcome the shortcomings of none-biodegradable plastic and metal stents. Compared with plastic stents and metal stents which sometimes are difficult to remove due to tissue over-growth [9], biodegradable biliary stents do not need to be removed by performing a secondary surgery [10]. What's even more exciting is that biodegradable biliary stents can be used as drug delivery vehicles to treat diseases or to inhibit intimal hyperplasia [11].
Several types of biodegradable polymers and metals, i.e., Polydioxanone (PDX or PDO), Poly(l-lactic acid) (PLLA) based polymers and magnesium-based alloys with different biodegradation profiles, have been used in manufacturing biliary stents.
2.1. Polydioxanone (PDX)
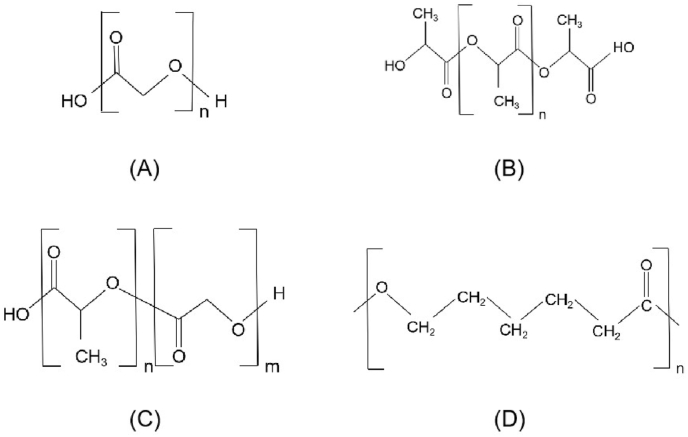
PDX or PDO is a biodegradable polymer with a chemical structure as shown below (Fig. 3(A)). Back in 1981, PDX was used in a FDA approved biodegradable suture [12]. In recent years, PDX is also being studied in the fields of tissue engineering and drug/gene-delivery application [13,14,[73], [74], [75]]. PDX degrades through a hydrolysis mechanism for the existence of ether bonds in its structure [15].
Fig. 3.
The chemical structure of (A) PDX; (B) PLLA; (C) PLGA; (D) PCL.
It was found that bile contributes significantly to the degradation of sutures as compared to other simulated body fluids, indicating that the speed of suture degradation was affected by not only the tissue environment, such as the temperature and the pH value (7.3) where the suture is located [17], but also the surrounding biological substances that the sutures came into contact with, such as phospholipids, phosphates, cholesterol, and other components contained in the bile. However, the hydrolysis of aliphatic ester bonds still is one of the main reasons responsible for the degradation of bioabsorbable sutures. Aside from hydrolysis degradation mechanism, enzymes in the bile may accelerate the degradation of biodegradable polymers. As a matter of fact, proteinase K and lipase PS have been used to accelerate the degradation of poly(l-lactide) (PLLA), poly(epsilon-caprolactone) (PCL) and their copolymers in vitro [66,84,85]. Enzymatic degradation is generally considered a "two-step" degradation mechanism: (1) the enzyme approaches the polymer surface; (2) the enzyme initiates hydrolysis of the polymer. Therefore, compared with other bodily fluids, bile may significantly accelerate the degradation of biodegradable polymers due to the presence of enzymes. Freudenberg et al. conducted an experiment in which a variety of absorbable sutures were incubated in 5 different body fluids, such as bile and pancreatic juice, to study the degree of degradation of the sutures over time [16]. The bile was collected through T-shaped tubes after bile duct surgery, and the pancreatic juice was obtained by abdominal drainage of patients with pancreatic fistula after pancreatic surgery. In Freudenberg's study, they compared sutures from nine brands. After 21 days of incubation in bile, all sutures were found to have lost most of their tensile strength, except for PDSII® (polydioxanone) which still retained 15 N. Similar to bile, pancreatic juice also accelerated the loss of tensile strength of the materials compared with the same pH buffer. Overall, the PDSII® sutures demonstrated the strongest resistance to degradation in both bile and pancreatic juice than the other sutures.
Siiki and his colleagues conducted multiple studies on braided PDX biliary stents (Ella-CS Ltd., Hradec Kralové, Czech Republic). PDX has been used in biomedical applications for over two decades and has a well-established biological safety profile. PDX degrades in approximately 3–5 months, and the resulting degradation products or intermediates are non-toxic to the body [18]. Siiki et al. followed 13 patients who underwent PDX biliary stent implantation by endoscopic retrograde cholangiography to treat the leakage of the cystic duct or benign biliary stricture. A subsequent evaluation of the effects of the stent implantation was carried out through liver function tests and magnetic resonance imaging. The follow-up results showed that the short-term and long-term biosafety and clinical feasibility of PDX biliary stent implantation, as well as the long-term patency, correlated well with expectations. At the median follow-up of 21 months, the clinical success rate of the biliary stent endoscopic implantation was 83%. However, 25% of the patients developed mild cholangitis during stent indwelling, while 17% of the patients developed restenosis. Factors such as slow degradation and rupturing of the stent may have caused intermittent obstruction of bile flow and resulted in acute cholangitis [19]. In their previous esophageal studies, PDX stent implantation were considered to be associated with secondary epithelial hyperplasia and stricture [20].
Other research teams also conducted animal experiments or clinical trials using PDX biliary stents. In one of these studies, Giménez et al. evaluated 13 patients with hepaticojejunostomy stenosis who had received PDX biliary stent implantation; the follow-up period was an average of 20 months [21]. With the exception of one case of stenosis recurrence and one case of cholangitis, 84.6% of the patients were asymptomatic during the follow-up period. Furthermore, Battistel et al. carried out a postoperative evaluation of 18 patients with PDX biliary stent (ELLA-CS, Hradec Kralove, Czech Republic) implantation due to stenosis following liver transplantation [22]. The findings demonstrated the safety and effectiveness of the PDX stents. In addition, Mauri et al. monitored 107 cases of PDX biliary stent implantation for two years and deduced that the patency rate of stenosis exceeded 80% [23]. The results reconfirmed the safety of the procedure as no major complications had occurred. Moreover, the risk of minor complications was low.
A fully biodegradable helical structured biliary stent ARCHIMEDES™ developed by Q3 Medical Devices Limited obtained CE certification in 2018 [24]. The biliary stents have been designed with three degradation rates (fast, medium, and slow) to meet various needs. In addition, BaSO4 is added into the stent material to make the stents radiopaque under X-rays. This variation in degradation rate was achieved by using different materials which are shown in Table 2 [21]. The materials used for the fast and medium degradation speed stent are mixtures which the main component is PDX. A single-center, prospective pilot study with 38 patients was conducted on this product [25]. A total of 53 stents were inserted over a guidewire through the working channel of a standard duodenoscope during ERCPs. Data from this study showed that ARCHIMEDES™ stents with all three degradation rates degraded as expected and presented excellent safety profile. Compared with the common plastic biliary stent, the ARCHIMEDES™ stent has better loadability, pushability and fluoroscopic visibility during stent placement. The short-term follow-up results indicated that stent migration occurred in 9.4% of these cases, similar to fully covered self-expandable metal stents (FCSEMS).
Table 2.
The material composition of the three different degradation rate stents of ARCHIMEDES™ [21].
| Degradation rate | Material composition |
|---|---|
| Fast (12 days) | PDX, polyethylene glycol (PEG) and barium sulphate |
| Medium (20 days) | PDX and barium sulphate |
| Slow (11 weeks) | Poly (lactide-co-caprolactone-co-trimethylene carbonate) and barium sulphate |
2.2. Polylactic acid (PLA) based polymers
As mentioned above, although the PDX biliary stent (ELLA-CS, Hradec Kralove, Czech Republic) has shown promising results in small-scale clinical trials, the mechanical properties of PDX are still poor and its Young's modulus is relatively low. These drawbacks could limit the application of PDX stents in complex biliary strictures. PLLA is a polyester which has a chemical structure shown above (Fig. 3(B)). PLLA degrades through the ester bonds hydrolysis [26]. More recently, PLLA has been used in fabrication of biodegradable bone screw, bone plate, cardiovascular stents, and tissue engineering scaffolds due to its biodegradable properties [[27], [28], [29], [30]]. PLLA stents have shown remarkable performances in vascular and non-vascular lumen, and therefore PLLA has gained increasing interests as a potential material for biliary stents [76].
Tian et al. studied the changes in mechanical properties, surface morphology, and physicochemical properties of PLLA monofilaments in bile during degradation [31]. The study used bile extracted from the bile ducts of pigs. The PLLA biliary stents were braided by using extruded and then solid-state drawn filament. PDX stents were used as control. Tensile test showed that the Young's modulus of PLLA monofilament is considerably higher than that of PDX suture, which indicates that PLLA monofilament have a stronger resistance to elastic deformation and provide better support to the stricture segments. In addition, the Young's modulus of the PLLA monofilament suture did not change much during the degradation process. It was also found that the chronic outward force of the PDX stent was much lower than the PLLA stent, which confirmed that the PLLA stent has a superior supporting effect on the narrow part of biliary tract than the PDX stent. The surface morphology of these stents demonstrated that the PLLA monofilament was clear of any obvious cracks. Through analysis of the mass loss, molecular weight, and crystallinity changes of PLLA monofilament during degradation, it was found that PLLA monofilament showed almost no mass loss, its molecular weight only decreased by 17.4%, and the crystallinity increased slightly. These observations could explain why the PLLA monofilament maintains superior mechanical properties during the degradation process in bile. The PLLA monofilament is quite stable throughout the process.
In another study, Meng et al. conducted a series of tests on the helical PLLA stent including animal experiments, the sludge attachment test, and the self-expansion test [5]. Experimental dogs underwent transection of the CBD to produce models of bile duct injury; 3 of these cases were sutured after implantation of stents, and 1 case was sutured without the use of stent. Examination of the 3 canine models showed that their -GT, ALP, and serum bilirubin values were not significantly different after stent implantation than before the transected common bile duct operation. Furthermore, these parameters were all at normal levels. The control canine model that had not received stent implantation had normal serum bilirubin level, but the ALP and -GT values had significantly increased. This canine model also showed symptoms of anorexia and jaundice. The cholangiogram from this canine model showed stenosis, but the symptoms disappeared following stent implantation. These experimental dogs were sacrificed after 3 months. It was found that these stents have no stenosis, filling defects, and cholestasis. Therefore, it was concluded that these stents supported the bile ducts well. Histopathological examination of the bile duct epithelium found no inflammation or endothelial hyperplasia; furthermore, the stents were easily removed.
Bile sludge formation on the stents is another clinical concern. The main components of bile sludge are calcium palmitate and bilirubin. The bile sludge attachment test was carried out by Meng et al. using bile taken from liver cancer patients [5]. In the study, polyethylene (PE) was used as the control sample. The PE samples and PLLA membranes were separately immersed in bile for 1, 2, 3, and 8 weeks. The scanning electron microscope (SEM) images showed that the PLLA sample adsorbed more bile sludge than the PE sample in the first two weeks of testing, but the bile sludge diminished from the third week due to the surface degradation of the PLLA sample. On a long-term basis, the bile sludge on the PLLA sample was significantly less than the bile sludge on the PE sample. These findings led to the proposal that PLLA has a self-cleaning effect in the bile duct. The results of the canine experiments also confirmed this hypothesis, as there was minimal bile sludge accumulation on the biliary stents of the tested subjects. These observations are most likely due to the free COOH groups at the end of the molecular chain of PLLA combined with Ca2+, which promotes the attachment of palmitate and bilirubin.
Poly(lactic-co-glycolic acid) (PLGA) is a kind of PLA-based polymers (Fig. 3(C)). The hydrolytic products of PLGA are lactic acid and glycolic acid, which can be removed by the body's normal metabolism. The in vitro degradation experiment of PLGA stent (molar ratio LA/GA = 80/20) in bile and related tests carried out by Xu et al. showed that the PLGA stent can stay in the body for 14–21 days, and its mechanical strength can be maintained for about 4 days [32]. The study carried out by Freudenberg et al. also demonstrated that PLGA stents have fast degradation rates in both bile and pancreatic juice [16]. The PLGA stent can provide short-term support for bile ducts. As a co-polymer, the degradation rate of PLGA can be increased by changing the ration of lactic acid and glycolic acid [33].
PLLA shows excellent mechanical properties and stability during the degradation in bile, and the resistance to attachment of sludge make it a serious contender. PLGA has only recently been considered for biliary stent preparation, and more studies need to be conducted to evaluate its feasibility.
2.3. Polycaprolactone (PCL) and related materials
Polycaprolactone (PCL) is a promising, absorbable material that has been used in the manufacture of bone implants and cardiovascular stents [34,35,71]. The good biocompatibility, non-toxicity, and biodegradability make it an ideal biomaterial [70]. PCL is a linear polyester (Fig. 3(D)) which has an ultra-low glass transition temperature (Tg = −62 °C) and a low melting point (Tm = 57 °C). While bringing excellent mechanical properties, the low melting point also brings many application limitations to PCL, resulting it rarely used alone [34].
PCL is used mainly as the coating material for metallic or PDX biliary stents [36,[77], [78], [79]]. Chang et al. studied the in vitro degradation behavior of PCL film in bile. The PCL film incubated in bile did not show huge difference compared with the pristine PCL film. This phenomenon may be caused by the coverage of the emulsifier and emulsifying fats emulsified fat of bile on the surface of the PCL, which hinders the biodegradation of PCL [37].
PCL can work with other materials to function as a biliary stent. Jang et al. assembled a 3D-printed paclitaxel-PCL stent with a bare metal nitinol stent to form a drug-eluting stent with two layers [69]. Guerra et al., produced a PCL/PLA composite stent that exhibited the properties of PLA and PCL respectively at different stages [34].
PCL can form copolymers with other monomers to obtain performance-enhanced materials. Furthermore, the biodegradable polymer in the slow degradation biliary stents ARCHIMEDES™ is poly (lactide-co-caprolactone-co-trimethylene carbonate). Poly (lactide-co-caprolactone-co-trimethylene carbonate) is a terpolymer of lactic acid, caprolactone and trimethylene carbonate. The degradation rate of PLLA is relatively quicker compared with PCL and poly (trimethylene carbonate) (PTMC). PTMC is very soft at 40–60 [38]. The degradation of PTMC is through a surface erosion mechanism, and its degradation in aqueous solution is extremely slow. When copolymerized with polyester materials such as PLLA and PCL, the structural stability and degradation of PTMC will be significantly improved. The copolymerization of these three materials makes them complementary to each other, forming a biodegradable material with adjustable degradation rate and tailored mechanical properties. There are also many studies showed that the poly(lactide-co--caprolactone) can improve the properties of each single material [39].
Although there are few publications on the use of PCL and its related materials as the materials for making biliary stents, the excellent degradation and mechanical properties of PCL-based copolymers make it a good candidate for future applications.
2.4. Biodegradable magnesium alloys
In addition to polymer materials, biodegradable magnesium alloys are considered potential materials for biodegradable biliary stents due to their ecstatic performance in cardiovascular field [40].
A study on the corrosion resistance and degradation behavior of pure magnesium (Mg) and magnesium alloy WE43 in human bile demonstrated that WE43 alloy is one of the strong candidate materials for the biliary stent. They found that both pure magnesium and WE43 alloys showed slower degradation rates in bile than in other body fluids, possibly due to the adhesion and aggregation of Mg (H2PO4) 2 on the surface. Compared with pure Mg, WE43 alloy has even slower degradation rate and corrosion rate in human bile, which may be caused by more Mg (H2PO4)2 aggregation on the surface of WE43 alloy [41]. In addition, some researchers have recently showed through in vivo and in vitro experiments that the degradation products of Mg could significantly inhibit the activities of bile duct-related cancer cells, providing a strong support for the use of biodegradable magnesium-based alloy stents for the treatment of biliary stricture [42].
Recently, UNITY-B, developed by Q3 Medical Devices Limited for treating biliary strictures, has obtained CE certification. UNITY-B is a balloon expandable biodegradable stent made from magnesium alloy-MgNdMn21 with a polymer coating on the surface. A clinical study on the safety and effectiveness of this product had also shown an excellent success rate as high as 94.4% [43]. However, its long term and real-world clinical application results still need to be seen.
2.5. Comparison of the materials used in biliary stent
At present, polymers and metals are the most studied biodegradable materials. As discussed before, most research has focused on materials that are well established in the vascular or non-vascular fields. To choose the proper materials, the degradation time of materials, surgical operation (direct placement or ERCP) and functional requirements (drainage or support) should be taken into consideration. Two CE certified products, ARCHIMEDES™and UNITY-B, are made of PDX and magnesium alloy respectively. However, these materials degrade relatively quick and lost their mechanical strength in a few weeks after implantation, therefore, their safety and efficacy are still needed to be further confirmed in real world clinical applications. On the other hand, PLLA seems to exhibit superior mechanical properties than PDX and may be the ideal choice when longer mechanical support is required. There are relatively few studies on biodegradable PCL and PLGA, which means there are lots of space for future exploration.
As summarized above, both biodegradable polymers and metals are used in making biliary stents with different characteristics. The comparison of the biodegradable materials used in biliary stent is shown below in Table 3.
Table 3.
Comparison of the materials used in biliary stent.
| Material | Degradation product |
Degradation time in bile | Mechanical properties retention time in bile | Animal test | Clinical trial | Brand (suture/stent) | Reference |
|---|---|---|---|---|---|---|---|
|
PLGA (80/20) |
lactic acid, glycolic acid |
2–3 weeks | 4 days | safe and effective (canine model) |
/ | Polysorb®,Vicryl®,Vicryl rap® | [16,32] |
| PGA | glycolic acid | 2 months | 1 week | / | / | / | [44,45] |
| PDX | glyoxylic acid, glycine | 3–5 months | 3 months | safe and effective (porcine model) |
safe and effective | ARCHIMEDES™, ELLA-CS® | [18,45] |
| PLLA | lactic acid | >9 months | >8 weeks | safe and effective (canine model) |
/ | / | [46] |
| PCL | hydroxycaproic acid | >70 days | / | / | / | / | [37,47,48] |
|
WE43 (Mg) |
Mg(H2PO4)2 | / | >60 days | / | safe and effective | UNITY-B™ | [41,43] |
PLGA: poly l-lactide-co-glycolide; PGA: poly(glyoxylic acid); PDX: Polydioxanone; PLLA: Poly(l-lactic acid); PCL: Polycaprolactone; Mg: magnesium.
3. Future trends
3.1. Drug-eluting biodegradable biliary stents
When the bile duct becomes injured, local fibroblasts proliferate abnormally to the site and a large amount of collagen is synthesized, which can cause stenosis [49]. Therefore, drugs that can inhibit the proliferation of fibroblasts and regulate collagen metabolism could potentially prevent and treat biliary stricture. Paclitaxel (PTX) is an FDA-approved anti-tumor drug. It is commonly used in clinical settings to treat breast cancer, esophageal cancer, rectal cancer, and other malignant diseases [50]. It also has been used as an antiproliferation drug on cardiovascular stents to prevent restenosis [51]. Several reports have shown reduced anastomotic fibrosis and inhibited biliary stricture as a result of PTX administration. Wang studied the inhibitory effect of paclitaxel-N-succinyl hydroxyethyl chitosan (PTX-Suc-HECTS) sustained-release film on bile duct scarring in rabbits and found that early local application of PTX-Suc-HECTS effectively prevented the abnormal proliferation of fibroblasts, which consequently restricted scar stenosis after bile duct suture [52]. Jang et al. also obtained promising results following the safety evaluation of a drug delivery system in the porcine biliary tract, whereby a metal stent covered with paclitaxel membrane (MSCPM-III) was implanted. But they also reported that there was no significant difference in stent patency and patient survival between the MSCPM group and the control group [53,54]. In addition to PTX, there are other drugs that could potentially inhibit biliary stricture, such as gemcitabine, rapamycin, and mitomycin-C. Gemcitabine is the standard chemotherapy drug used to treat advanced pancreatic cancer and cholangiocarcinoma. The degradation time of gemcitabine controlled-release polyurethane (PU)/polytetrafluoroethylene (PTFE) film is approximately 30 days [55]. Whilst the safety of gemcitabine in porcine bile ducts has been confirmed [56], there is currently a lack of relevant evidence from human clinical studies. Xiao et al. developed a metallic biliary stent coated with gemcitabine (GEM) plus cisplatin (CIS). This stent has been initially confirmed to have continuous local drug release and effective anti-tumor properties. The combination of GEM and CIS provides an excellent performance beyond the single GEM or CIS drug. The release of GEM played the leading role at the early stage, while the release of CIS subsequently ensured the maintenance of long-term anti-tumor effects [57]. Rapamycin is an anti-proliferation immunosuppressant. Chong et al. have found that rapamycin can inhibit the formation of stenosis in the rabbit urethra, possibly by inhibiting the proliferation of fibroblasts and/or collagen expression [58]. It is reported that rapamycin significantly inhibited hepatic fibrosis [59]. Mitomycin-C is an antibiotic chemotherapeutic agent, which inhibits RNA synthesis, as well as the rapid proliferation of fibroblasts and epithelial cells. Subsequently, protein expression is diminished and the incidence of scarring reduces [60].
3.2. 3D printed biliary stents
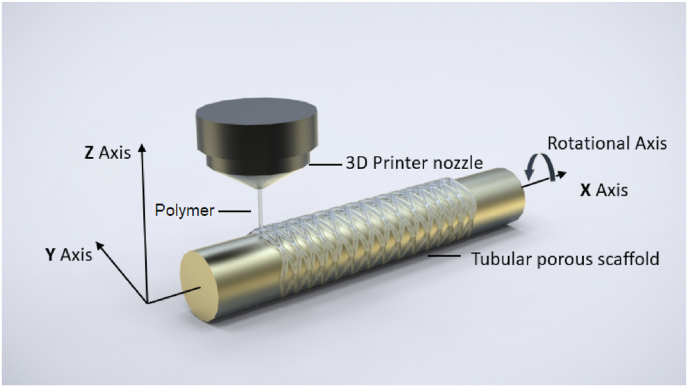
Additive manufacturing or 3D printing has been used in fabrication of various tissue engineering scaffolds with controlled pore size and porous structures [61,62]. 3D printing combines computer-aided design, material processing and computer aided manufacturing technology, converts digital model files into machine-recognizable code through software and numerical control system to “print” or make 3D objects. Compared with laser cutting technology, which is commonly used to prepare cardiovascular stents, 3D printing has the outstanding capability to print scaffolds with complex structures. The choice of materials for 3D printing can range from polymers and composites to metal and alloys. Among many types of 3D printing technologies, such as fused deposition modelling (FDM), inkjet printing, stereolithography (SLA) [63], a 3D 4-axial printing (3D4P) technology has shown promise in producing bioresorbable PLLA coronary stents [64]. Unlike other traditional 3D printing technologies which are based on a layer by layer fabrication fashion, the 3D4P technology is a technology that prints materials on a rotation mandrel (Fig. 4) and has been used in producing bioresorbable scaffolds for trachea tissue engineering [65]. One of the major advantages of this 3D4P technology is that it has an extremely high efficiency in producing porous tubular scaffolds because it greatly reduces the number of layers needed to produce a tubular scaffold. 3D4P also has been used to print biodegradable peripheral stents using novel biodegradable copolymers, such as Poly(TMC‐b‐(LLA‐ran‐GA)) [66,67]. It is therefore anticipated that 3D4P will be a promising fabrication technology for developing biliary stents.
Fig. 4.
An illustration of 3D4P technology.
3.3. Tissue engineered biliary stents
Tissue engineering principles have been applied to develop better tubular stents to improve their integration with host tissue and obtained a nature living tissue surface. Recently, a review has summarized the literature on the application of tissue-engineered endothelialised stents to reduce stent thrombosis of coronary and peripheral stents after implantation [80], indicating that tissue engineering approach is a possible solution to tubular structure tissue regeneration.
For biliary stents, Boyer et al. explored a bioengineered approach in which they used 3D printed scaffold impregnated with stem cells as bio-synthetic biliary stents aimed at preventing biofilm formation and stents obstruction [68]. Biofilm formation is one of the major causes of biliary ‘sludge’ formation that ultimately leads to the loss of stent patency. In a proof-of-concept research, a poly(vinyl alcohol) (PVA) stent was 3D printed and crosslinked with glutaraldehyde. Then the stents were infused with collagen, human placental mesenchymal stem cells (PMSCs), and cholangiocytes. Through swelling analysis and a series of cell experiments, the feasibility of bio-integrating biliary stents was confirmed. Although PVA is non-degradable, this biosynthesized living biliary stent brings a new concept in the development of ideal biliary stents.
Other biodegradable materials have been used in developing tissue-engineered bile ducts scaffolds as a treatment of biliary diseases, such as postoperative biliary tract injury, stricture and biliary leakage [83]. A copolymer of PLLA and PCL scaffold was made and reinforced with polyglycolic acid (PGA) fibers. The scaffolds were designed to be absorbed in the body within six to eight weeks [81,82]. One hour after seeding the scaffold with bone marrow cells, the artificial duct was implanted as a bypass graft into the extrahepatic bile duct in each of the pigs from which bone marrow cells had been collected. The graft site was harvested six months after implantation and subjected to macroscopy and histology analysis. Histology study showed that the neo-bile duct was almost similar to the native common bile duct in morphology. The study results showed that the tubular artificial bile duct, a substitute for extrahepatic bile duct, could carry bile to the duodenum without any leakage in the peritoneal.
Both PCL and PLGA polymers were used to fabricate bilayered scaffolds for seeding with human bone marrow-derived mesenchymal stem cells (hMSCs), cell seeded scaffolds were then transplanted into 18 pigs for evaluation its efficacy on bile duct repairing, respectively. During the animal study, there were no signs of bile duct narrowing and cholestasis in all experimental animals. At the end of 6 months, the cell seeded scaffolds showed superior repairing effect on the bile duct injury as compared to the blank PCL/PLGA scaffolds [83].
Autologous tissue, instead of cells, can also be used together with a novel biodegradable polymer stents to regenerate bile duct. A biodegradable copolymer poly [sebacic acid-co-(1,3-propanediol)-co-(1,2-propanediol) was used to fabricate a novel biodegradable biliary stents. The stents were used to reconstruct minipig's common bile duct (CBD) by duct to duct anastomosis and covered with a vascularized greater omentum. No severe CBD dilation and stricture were observed at both months 1 and 3. There were also no sign of necrosis, bile duct stricture, bile leakage or abdominal abscess was found at month 3 postoperatively [94].
Overall, tissue engineering approach showed promising preliminary results. However, it is still in a very early stage and its long term safety and efficacy still remain to be seen.
4. Conclusions
The clinical use of biodegradable stents has shown promising results. However, this is just the beginning of the biodegradable stent era, and improvements are still needed for currently available stents on the markets in terms of material degradation rate and mechanical support. An additional desirable property of the stents is their ability to locally deliver anti-tumor or anti-inflammatory therapeutic agents that can treat and restore the diseased biliary ducts to their normal state quickly, e.g. stents that can elute therapeutic reagents from their surface coating (similar to drug eluting cardiovascular stents) or from their bulk stent material. As a more efficient fabrication technology, 3D printing may produce a more affordable device for both a patient and a health care system.
In summary, the development of biodegradable biliary stent is still in its infancy stage. It is expected that more innovative technologies will be applied in developing new biodegradable biliary stents. These technologies may include new biodegradable materials, novel stent structure designs, advanced stent fabrication methods and drug delivery technologies.
Declaration of competing interest
None.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Mahadevan V. Vol. 38. Elsevier Ltd; 2020. pp. 432–436. (Anatomy of the Gallbladder and Bile Ducts', Surgery (United Kingdom)). 8. [DOI] [Google Scholar]
- 2.Altman A., Zangan S. Benign biliary strictures. Semin. Intervent. Radiol. 2016;33:297–306. doi: 10.1055/s-0036-1592325. (04) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y., et al. Repair of a common bile duct defect with a decellularized ureteral graft. World J. Gastroenterol. 2016;22(48):10575–10583. doi: 10.3748/wjg.v22.i48.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronnekleiv-Kelly S., Cho C. vol. 2. Biliary Tract and Pancreas; 2017. pp. 537–548. (Bile Duct Exploration and Biliary-Enteric Anastomosis. Blumgart's Surgery of the Liver). Set. [Google Scholar]
- 5.Lam R., Muniraj T. Fully covered metal biliary stents: a review of the literature. World J. Gastroenterol. 2021;27(38):6357–6373. doi: 10.3748/wjg.v27.i38.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohn S., Park J., Kim K., Kim T. Complications and management of forgotten long-term biliary stents. World J. Gastroenterol. 2017;23(4):622. doi: 10.3748/wjg.v23.i4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng B., et al. Study of biodegradable and self-expandable PLLA helical biliary stent in vivo and in vitro. J. Mater. Sci. Mater. Med. 2006;17(7):611–617. doi: 10.1007/s10856-006-9223-9. [DOI] [PubMed] [Google Scholar]
- 8.www.bostonscientific.com. 2021. WallFlex™ Biliary RX Stents.https://www.bostonscientific.com/en-US/products/stents--gastrointestinal/wallflex-biliary-rx-stents.html [online] Available at: [Google Scholar]
- 9.Irani S., et al. Endoscopic treatment of benign biliary strictures using covered self-expandable metal stents (CSEMS) Dig. Dis. Sci. 2014;59(1):152–160. doi: 10.1007/s10620-013-2859-7. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzo-Zúñiga V., et al. Biodegradable stents in gastrointestinal endoscopy. World J. Gastroenterol. 2014;20(9):2212–2217. doi: 10.3748/wjg.v20.i9.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siiki A., Sand J., Laukkarinen J. A systematic review of biodegradable biliary stents: promising biocompatibility without stent removal. Eur. J. Gastroenterol. Hepatol. 2018;30(8):813–818. doi: 10.1097/MEG.0000000000001167. [DOI] [PubMed] [Google Scholar]
- 12.Bartholomew R. PDS (polydioxanone suture): a new synthetic absorbable suture in cataract surgery. Ophthalmologica. 1981;183(2):81–85. doi: 10.1159/000309144. [DOI] [PubMed] [Google Scholar]
- 13.Saska S., Pilatti L., Silva E., Nagasawa M., Câmara D., Lizier N., Finger E., Dyszkiewicz Konwińska M., Kempisty B., Tunchel S., Blay A., Shibli J. Polydioxanone-based membranes for bone regeneration. Polymers. 2021;13(11):1685. doi: 10.3390/polym13111685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goonoo N., Jeetah R., Bhaw-Luximon A., Jhurry D. Polydioxanone-based bio-materials for tissue engineering and drug/gene delivery applications. Eur. J. Pharm. Biopharm. 2015;97:371–391. doi: 10.1016/j.ejpb.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Sabino M., González S., Márquez L., Feijoo J. Study of the hydrolytic degradation of polydioxanone PPDX. Polym. Degrad. Stabil. 2000;69(2):209–216. [Google Scholar]
- 16.Freudenberg S., et al. Biodegradation of absorbable sutures in body fluids and pH buffers. Eur. Surg. Res. 2004;36(6):376–385. doi: 10.1159/000081648. [DOI] [PubMed] [Google Scholar]
- 17.Tomihata K., Suzuki M., Ikada Y. The pH dependence of monofilament sutures on hydrolytic degradation. J. Biomed. Mater. Res. 2001;58(5):511–518. doi: 10.1002/jbm.1048. [DOI] [PubMed] [Google Scholar]
- 18.Grolich T., et al. Self-expandable biodegradable biliary stents in porcine model. J. Surg. Res. 2015;193(2):606–612. doi: 10.1016/j.jss.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Siiki A., et al. A pilot study of endoscopically inserted biodegradable biliary stents in the treatment of benign biliary strictures and cystic duct leaks. Am. Soc. Gastrointest. Endosc. 2018;87(4):1132–1137. doi: 10.1016/j.gie.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 20.Hair C.S., Devonshire D.A. Severe hyperplastic tissue stenosis of a novel biodegradable esophageal stent and subsequent successful management with high-pressure balloon dilation. Endoscopy. 2010;42(SUPPL. 2):132–133. doi: 10.1055/s-0029-1244011. [DOI] [PubMed] [Google Scholar]
- 21.Giménez M.E., et al. The Management of Hepaticojejunostomy Strictures. 2016;29(2):112–116. doi: 10.1590/0102-6720201600020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Battistel M., et al. Vol. 43. Springer US; 2020. pp. 749–755. (Biodegradable Biliary Stents for Percutaneous Treatment of Post-liver Transplantation Refractory Benign Biliary Anastomotic Strictures', CardioVascular and Interventional Radiology). 5. [DOI] [PubMed] [Google Scholar]
- 23.Mauri G., et al. Biodegradable biliary stent implantation in the treatment of benign bilioplastic-refractory biliary strictures: preliminary experience. Eur. Radiol. 2013;23(12):3304–3310. doi: 10.1007/s00330-013-2947-2. [DOI] [PubMed] [Google Scholar]
- 24.Stent P., Mangiardi E.K. 2018. GI PRODUCTS Amg International GmbH Receives European CE Mark Approval for ARCHIMEDES Biodegradable Biliary and Pancreatic Stent Novel Biodegradable Stent Technology Approved for Sale in Europe. [Google Scholar]
- 25.Anderloni A., et al. New biliary and pancreatic biodegradable stent placement: a single-center, prospective, pilot study (with video) Am. Soc. Gastrointest. Endosc. 2020;92(2):405–411. doi: 10.1016/j.gie.2020.02.049. [DOI] [PubMed] [Google Scholar]
- 26.Weir N., Buchanan F., Orr J., Dickson G. Degradation of poly-L-lactide. Part 1: in vitro and in vivo physiological temperature degradation. Proc. IME H J. Eng. Med. 2004;218(5):307–319. doi: 10.1243/0954411041932782. [DOI] [PubMed] [Google Scholar]
- 27.Liu G., Fu M., Li F., Fu W., Zhao Z., Xia H., Niu Y. Tissue-engineered PLLA/gelatine nanofibrous scaffold promoting the phenotypic expression of epithelial and smooth muscle cells for urethral reconstruction. Mater. Sci. Eng. C. 2020;111:110810. doi: 10.1016/j.msec.2020.110810. [DOI] [PubMed] [Google Scholar]
- 28.Shuai C., Yang W., Feng P., Peng S., Pan H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact Mater. 2021;6(2):490–502. doi: 10.1016/j.bioactmat.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shuai C., Zan J., Qi F., Wang G., Liu Z., Yang Y., Peng S. nMgO-incorporated PLLA bone scaffolds: enhanced crystallinity and neutralized acidic products. Mater. Des. 2019;174:107801. [Google Scholar]
- 30.Yin T., Du R., Wang Y., Huang J., Ge S., Huang Y., Tan Y., Liu Q., Chen Z., Feng H., Du J., Wang Y., Wang G. Bioactive Materials; 2021. Two-stage Degradation and Novel Functional Endothelium Characteristics of a 3-D Printed Bioresorbable Scaffold. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian Y., et al. A poly(L-lactic acid) monofilament with high mechanical properties for application in biodegradable biliary stents. J. Appl. Polym. Sci. 2021;138(2) doi: 10.1002/app.49656. [DOI] [Google Scholar]
- 32.Xu X., et al. Feasibility of biodegradable PLGA common bile duct stents: an in vitro and in vivo study. J. Mater. Sci. Mater. Med. 2009;20(5):1167–1173. doi: 10.1007/s10856-008-3672-2. [DOI] [PubMed] [Google Scholar]
- 33.Zeng C., Liu L., Zhu H., Teng G. The exploration of a novel biodegradable drug-eluting biliary stent: preliminary work. Cardiovasc. Intervent. Radiol. 2021;44(10):1633–1642. doi: 10.1007/s00270-021-02892-4. [DOI] [PubMed] [Google Scholar]
- 34.Guerra A.J., et al. 3D-printed PCL/PLA composite stents: towards a new solution to cardiovascular problems. Materials. 2018;11(9):1–13. doi: 10.3390/ma11091679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu T., et al. Development of 3D-printed sulfated chitosan modified bioresorbable stents for coronary artery disease. Front. Bioeng. Biotechnol. 2020;8(May):1–12. doi: 10.3389/fbioe.2020.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim D.H., et al. Preclinical evaluation of sorafenib-eluting stent for suppression of human cholangiocarcinoma cells. Int. J. Nanomed. 2013;8:1697–1711. doi: 10.2147/IJN.S43508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang H.M., et al. Vol. 313. Elsevier B.V.; 2014. pp. 828–833. (Ex Vivo Evaluation of Biodegradable Poly(ε-Caprolactone) Films in Digestive Fluids', Applied Surface Science). [DOI] [Google Scholar]
- 38.Yang L., Li J., Li M., Gu Z. The in vitro and in vivo degradation of cross-linked poly(trimethylene carbonate)-based networks. Polymers. 2016;8(4):151. doi: 10.3390/polym8040151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J., et al. Study on degradation of ureteral stent coated with Polycaprolactone lactide. J. Phys. Conf. 2021;1732:012150. doi: 10.1088/1742-6596/1732/1/012150. [DOI] [Google Scholar]
- 40.Aljihmani L., Alic L., Boudjemline Y., Hijazi Z., Mansoor B., Serpedin E., Qaraqe K. Magnesium-based bioresorbable stent materials: review of reviews. J Bio- and Tribo-Corrosion. 2019;5(1) [Google Scholar]
- 41.Liu Y., Zheng S., Li N., Guo H., Zheng Y., Peng J. Study on the in vitro degradation behavior of pure Mg and WE43 in human bile for 60 days for future usage in biliary. Mater. Lett. 2016;179:100–103. [Google Scholar]
- 42.Peng H., Fan K., Zan R., Gong Z., Sun W., Sun Y., Wang W., Jiang H., lou J., Ni J., Suo T., Zhang X. Degradable magnesium implants inhibit gallbladder cancer. Acta Biomater. 2021;128:514–522. doi: 10.1016/j.actbio.2021.04.051. [DOI] [PubMed] [Google Scholar]
- 43.Ltd Q. Receives European CE Mark Approval for UNITY-B Biodegradable Stent; 2021. Amg International GmbH, a Q3 Medical Devices Ltd Company.https://www.prnewswire.com/news-releases/amg-international-gmbh-a-q3-medical-devices-ltd-company-receives-european-ce-mark-approval-for-unity-b-biodegradable-stent-301298110.html [online] Prnewswire.com. Available at. [Google Scholar]
- 44.Jenjob R., et al. Recent trend in applications of polymer materials to stents. Gastrointest Intervent. 2015;4(2):83–88. doi: 10.18528/gii150022. [DOI] [Google Scholar]
- 45.Kwon C., et al. Digestive Endoscopy; 2021. Mechanical Properties and Degradation Process of Biliary Self‐expandable Biodegradable Stents; pp. 1–12. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto K., et al. Experimental study of poly-L-lactic acid biodegradable stents in normal canine bile ducts. Cardiovasc. Intervent. Radiol. 2011;34(3):601–608. doi: 10.1007/s00270-010-0045-2. [DOI] [PubMed] [Google Scholar]
- 47.Sun H., et al. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 2006;27(9):1735–1740. doi: 10.1016/j.biomaterials.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Woodruff M., Hutmacher D. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010;35(10):1217–1256. [Google Scholar]
- 49.Kim J.Y., et al. Vol. 8. Elsevier Ltd; 2017. pp. 250–263. (Anti-fibrotic Effects of Synthetic Oligodeoxynucleotide for TGF-Β1 and Smad in an Animal Model of Liver Cirrhosis', Molecular Therapy - Nucleic Acids). September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sohn J.S., et al. Polymer prodrug approaches applied to paclitaxel. Polym. Chem. 2010;1(6):778–791. doi: 10.1039/b9py00351g. [DOI] [Google Scholar]
- 51.Hong M.K., Lee S.Y. Differential effects of drug-coated balloon angioplasty for in-stent restenosis. J. Am. Coll. Cardiol. 2020;75(21):2679–2681. doi: 10.1016/j.jacc.2020.04.005. Elsevier. [DOI] [PubMed] [Google Scholar]
- 52.Wang Tao. Kunming Medical University; 2018. Paclitaxel-modified Chitosan Hydrogel Membrane Preparation, Properties and Inhibit Rabbit Bile Duct Scar.https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CDFDLAST2019&filename=1018289149.nh Ph.D. thesis. [Google Scholar]
- 53.Jang S.I., et al. Safety evaluation of paclitaxel-eluting biliary metal stent with sodium caprate in porcine biliary tract. Gut and Liver. 2019;13(4):471–478. doi: 10.5009/gnl18454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang S.I., et al. Efficacy of a metallic stent covered with a paclitaxel-incorporated membrane versus a covered metal stent for malignant biliary obstruction: a prospective comparative study. Dig. Dis. Sci. 2013;58(3):865–871. doi: 10.1007/s10620-012-2472-1. [DOI] [PubMed] [Google Scholar]
- 55.Lee J.W., Yang S.G., Na K. Gemcitabine-releasing polymeric films for covered self-expandable metallic stent in treatment of gastrointestinal cancer. Int. J. Pharm. 2012;427(2):276–283. doi: 10.1016/j.ijpharm.2012.02.016. Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- 56.Chung M.J., et al. Safety evaluation of self-expanding metallic biliary stents eluting gemcitabine in a porcine model. J. Gastroenterol. Hepatol. 2012;27(2):261–267. doi: 10.1111/j.1440-1746.2011.06866.x. [DOI] [PubMed] [Google Scholar]
- 57.Xiao J.B., et al. Feasibility and efficacy evaluation of metallic biliary stents eluting gemcitabine and cisplatin for extrahepatic cholangiocarcinoma. World J. Gastroenterol. 2020;26(31):4589–4606. doi: 10.3748/WJG.V26.I31.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chong T., et al. Rapamycin inhibits formation of urethral stricture in rabbits. J. Pharmacol. Exp. Therapeut. 2011;338(1):47–52. doi: 10.1124/jpet.110.178624. [DOI] [PubMed] [Google Scholar]
- 59.Biecker E., et al. Long-term treatment of bile duct-ligated rats with rapamycin (sirolimus) significantly attenuates liver fibrosis: analysis of the underlying mechanisms. J. Pharmacol. Exp. Therapeut. 2005;313(3):952–961. doi: 10.1124/jpet.104.079616. [DOI] [PubMed] [Google Scholar]
- 60.Szabó D., et al. Antifibrotic effect of mitomycin-C on human vocal cord fibroblasts. Laryngoscope. 2019;129(7):E255–E262. doi: 10.1002/lary.27657. [DOI] [PubMed] [Google Scholar]
- 61.Mondschein R.J., et al. Polymer Structure-Property Requirements for Stereolithographic 3D Printing of Soft Tissue Engineering scaffolds. Biomaterials. 2017;140:170–188. doi: 10.1016/j.biomaterials.2017.06.005. Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- 62.Wen Y., et al. 3D printed porous ceramic scaffolds for bone tissue engineering: a review. Biomater. Sci. 2017;5(9):1690–1698. doi: 10.1039/c7bm00315c. [DOI] [PubMed] [Google Scholar]
- 63.Ngo T., Kashani A., Imbalzano G., Nguyen K., Hui D. Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Compos. B Eng. 2018;143:172–196. [Google Scholar]
- 64.Zhao J., et al. Drug loaded nanoparticle coating on totally bioresorbable PLLA stents to prevent in-stent restenosis. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106(1):88–95. doi: 10.1002/jbm.b.33794. [DOI] [PubMed] [Google Scholar]
- 65.Gao M., et al. Tissue-engineered trachea from a 3d-printed scaffold enhances whole-segment tracheal repair. Sci. Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-05518-3. Springer US. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen X., et al. Biodegradable poly(trimethylene carbonate-b-(L-lactide-ran-glycolide)) terpolymers with tailored molecular structure and advanced performance. Polym. Adv. Technol. 2018;29(6):1684–1696. doi: 10.1002/pat.4272. [DOI] [Google Scholar]
- 67.Fan T., et al. Effect of segment structures on the hydrolytic degradation behaviors of totally degradable poly(L-lactic acid)-based copolymers. J. Appl. Polym. Sci. 2019;136(33):28–30. doi: 10.1002/app.47887. [DOI] [Google Scholar]
- 68.Boyer C.J., et al. 3D printing for bio-synthetic biliary stents. Bioengineering. 2019;6(1) doi: 10.3390/bioengineering6010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jang B., Jeong J., Ji S., Im D., Lee M., Park S., Park W. Advanced stent applications of material extrusion 3D printing for palliative treatment of unresectable malignant hilar biliary obstruction. Mater. Des. 2020;195:109005. [Google Scholar]
- 70.Qiu T., Jiang W., Yan P., Jiao L., Wang X. Development of 3D-printed sulfated chitosan modified bioresorbable stents for coronary artery disease. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dwivedi R., Kumar S., Pandey R., Mahajan A., Nandana D., Katti D., Mehrotra D. Polycaprolactone as biomaterial for bone scaffolds: review of literature. J Oral Biol Craniofac Res. 2020;10(1):381–388. doi: 10.1016/j.jobcr.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lal N. JOURNAL OF CLINICAL AND DIAGNOSTIC RESEARCH; 2014. Ultrasonographic Measurement of Normal Common Bile Duct Diameter and its Correlation with Age, Sex and Anthropometry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cont L., Grant D., Scotchford C., Todea M., Popa C. Composite PLA scaffolds reinforced with PDO fibers for tissue engineering. J. Biomater. Appl. 2011;27(6):707–716. doi: 10.1177/0885328211423792. [DOI] [PubMed] [Google Scholar]
- 74.Padmakumar S., Menon D. Nanofibrous polydioxanone depots for prolonged intraperitoneal paclitaxel delivery. Current Drug Delivery. 2019;16(7):654–662. doi: 10.2174/1567201816666190816102949. [DOI] [PubMed] [Google Scholar]
- 75.Bottino M., Albuquerque M., Azabi A., Münchow E., Spolnik K., Nör J., Edwards P. A novel patient‐specific three‐dimensional drug delivery construct for regenerative endodontics. J. Biomed. Mater. Res. B Appl. Biomater. 2018;107(5):1576–1586. doi: 10.1002/jbm.b.34250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao G., Li X., Tian Y., Wu G., Zhang Y., Jiang W., Yang J., Ni Z. Poly(l-lactic acid) monofilaments for biodegradable braided self-expanding stent. J. Mater. Sci. 2021;56(21):12383–12393. [Google Scholar]
- 77.Zhu Y., Hu C., Li B., Yang H., Cheng Y., Cui W. A highly flexible paclitaxel-loaded poly(ε-caprolactone) electrospun fibrous-membrane-covered stent for benign cardia stricture. Acta Biomater. 2013;9(9):8328–8336. doi: 10.1016/j.actbio.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y., Chen K., Liu H., Shao Y., Chu C., Xue F., Bai J. A study of a biodegradable braided Mg stent for biliary reconstruction. J. Mater. Sci. 2020;55(36):17170–17182. [Google Scholar]
- 79.Chen M., Liang P., Chang K., Huang J., Chang Y., Chang F., Wong J., Lin F. Prototype of biliary drug-eluting stent with photodynamic and chemotherapy using electrospinning. Biomed. Eng. Online. 2014;13(1) doi: 10.1186/1475-925X-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsukada J., Mela P., Jinzaki M., Tsukada H., Schmitz-Rode T., Vogt F. Stem Cell Reviews and Reports; 2021. Development of in Vitro Endothelialised Stents - Review - [DOI] [PubMed] [Google Scholar]
- 81.Miyazawa M., Aikawa M., Okada K., Toshimitsu Y., Okamoto K., Koyama I., Ikada Y. Regeneration of extrahepatic bile ducts by tissue engineering with a bioabsorbable polymer. J. Artif. Organs. 2011;15(1):26–31. doi: 10.1007/s10047-011-0590-8. [DOI] [PubMed] [Google Scholar]
- 82.Watanabe M., Shin'oka T., Tohyama S., Hibino N., Konuma T., Matsumura G., Kosaka Y., Ishida T., Imai Y., Yamakawa M., Ikada Y., Morita S. Tissue-engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Eng. 2001;7(4):429–439. doi: 10.1089/10763270152436481. [DOI] [PubMed] [Google Scholar]
- 83.Zong C., Wang M., Yang F., Chen G., Chen J., Tang Z., Liu Q., Gao C., Ma L., Wang J. A novel therapy strategy for bile duct repair using tissue engineering technique: PCL/PLGA bilayered scaffold with hMSCs. J. Regen. Med. Tissue Eng. 2015;11(4):966–976. doi: 10.1002/term.1996. [DOI] [PubMed] [Google Scholar]
- 84.Zeng J., Chen X., Liang Q., Xu X., Jing X. Enzymatic degradation of poly(L-lactide) and poly(-caprolactone) electrospun fibers. Macromol. Biosci. 2004;4(12):1118–1125. doi: 10.1002/mabi.200400092. [DOI] [PubMed] [Google Scholar]
- 85.Bazgir M., Zhang W., Zhang X., Elies J., Saeinasab M., Coates P., Youseffi M., Sefat F. Fabrication and characterization of PCL/PLGA coaxial and bilayer fibrous scaffolds for tissue engineering. Materials. 2021;14(21):6295. doi: 10.3390/ma14216295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Medical.olympusamerica.com . 2021. HANAROSTENT® Biliary | Olympus America | Medical.https://medical.olympusamerica.com/products/hanarostent-biliary [online] Available at. [Google Scholar]
- 87.Taewoongusa.com . 2021. Niti-S™ D Biliary Stent | Taewoong Medical.https://taewoongusa.com/products/niti-s-d-biliary-stent/ [online] Available at: [Google Scholar]
- 88.Endocor.com . 2021. Endocor GmbH: Pioneering Innovation for Life™: SERENITY Biliary™.http://www.endocor.com/products/serenity-biliarytm/ [online] Available at. [Google Scholar]
- 89.Endo-flex de. 2021. Biliary SEMS | ENDO-FLEX GmbH.https://www.endo-flex.de/en/products/detailpage/biliary-sems/ [online] Available at: [Google Scholar]
- 90.Europe.medtronic.com . 2021. Peripheral Stents - ParaMount Mini GPS.https://europe.medtronic.com/xd-en/healthcare-professionals/products/cardiovascular/peripheral-stents/paramount.html [online] Available at. [Google Scholar]
- 91.www.bostonscientific.com. 2021. Advanix™ Biliary Stent with NaviFlex™ RX Delivery System.https://www.bostonscientific.com/en-US/products/stents--gastrointestinal/advanix-biliary-stent-with-naviflex-rx-delivery-system.html [online] Available at. [Google Scholar]
- 92.Medicalexpo.com . 2021. PE 204 Series - Biliary Stent by Endo-Flex | MedicalExpo.https://www.medicalexpo.com/prod/endo-flex/product-78692-491012.html online] Available at: [Google Scholar]
- 93.Cookmedical.com . 2021. Cotton-Leung® Biliary Stent Only | Cook Medical.https://www.cookmedical.com/products/esc_clso_webds/ [online] Available at. [Google Scholar]
- 94.Liang Yue-Long, et al. Repair of bile duct defect with degradable stent and autologous tissue in a porcine model. World J. Gastroenterol. 2012;18(37):5205–5210. doi: 10.3748/wjg.v18.i37.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]