Abstract
The development of maintenance hemodialysis (HD) for end stage kidney disease patients is a success story that continues to save many lives. Nevertheless, intermittent renal replacement therapy is also a source of recurrent stress for patients. Conventional thrice weekly short HD is an imperfect treatment that only partially corrects uremic abnormalities, increases cardiovascular risk, and exacerbates disease burden. Altering cycles of fluid loading associated with cardiac stretching (interdialytic phase) and then fluid unloading (intradialytic phase) likely contribute to cardiac and vascular damage. This unphysiologic treatment profile combined with cyclic disturbances including osmotic and electrolytic shifts may contribute to morbidity in dialysis patients and augment the health burden of treatment. As such, HD patients are exposed to multiple stressors including cardiocirculatory, inflammatory, biologic, hypoxemic, and nutritional. This cascade of events can be termed the dialysis stress storm and sickness syndrome. Mitigating cardiovascular risk and morbidity associated with conventional intermittent HD appears to be a priority for improving patient experience and reducing disease burden. In this in-depth review, we summarize the hidden effects of intermittent HD therapy, and call for action to improve delivered HD and develop treatment schedules that are better tolerated and associated with fewer adverse effects.
Keywords: End stage kidney disease, Cardiovascular mortality, Dialytic morbidity, Circulatory stress, Biologic storm, Dialysis sickness, Personalized medicine
Core Tip: In this in-depth review, we summarize the hidden effects of intermittent hemodialysis (HD) therapy, namely, dialysis sickness and dialysis related morbidity. We call for action to improve delivered HD and develop treatment schedules that are better tolerated and associated with fewer adverse effects. The final aim is to reduce cardiovascular burden and improve patient outcomes.
INTRODUCTION
Conventional hemodialysis (HD) is a mature treatment that sustains life in almost 3 million patients with end stage kidney disease (ESKD) worldwide and provides a valuable bridging solution to kidney transplant[1-4]. However, by nature intermittent HD is an imperfect treatment that only partially corrects uremic abnormalities, increases cardiovascular risk, and is associated with a high disease burden[5-11]. The high treatment costs of renal replacement therapy represent in addition a significant health economic burden[12-14].
Recent evidence indicates that conventional high efficiency thrice-weekly intermittent HD schedules may be harmful to patients by provoking alternating cycles of fluid loading associated with cardiac stretching during the interdialytic period and fluid unloading that contribute to cardiac and vascular damage. This unphysiologic loading and unloading phenomenon combined with cyclical disturbances including osmotic and electrolytic shifts may contribute to dialytic morbidity and augment the health burden associated with the treatment of uremia[15-17].
Over past few years, several studies have emphasized the importance of ensuring optimal fluid volume and arterial pressure control, as well as adequately dosed and better tolerated dialysis therapy to improve patient outcomes[18]. The benefits of a dry weight first policy approach has been reinforced by interventional studies[19-21]. Fluid volume guidance has also been facilitated by means of supportive tools[22-24]. On the other hand, prospective clinical studies not only have documented that intermittent treatment might cause significant circulatory stress depending on treatment time and schedule[10,25-27], but have also shown that guided interdialytic and/or specific dialysis-based interventions might be able to reduce this risk[10,28,29].
However, few reports have focused on all aspects of dialysis patient management in a comprehensive way[30-32]. In this in-depth review, we summarize potential harmful effects of intermittent HD and propose solutions for achieving more cardioprotective and tolerable treatment.
INTERMITTENT EXTRACORPOREAL RENAL REPLACEMENT THERAPY IS THE SOURCE OF PERMANENT STRESS IN MAINTENANCE HD PATIENTS
Cardiocirculatory stress
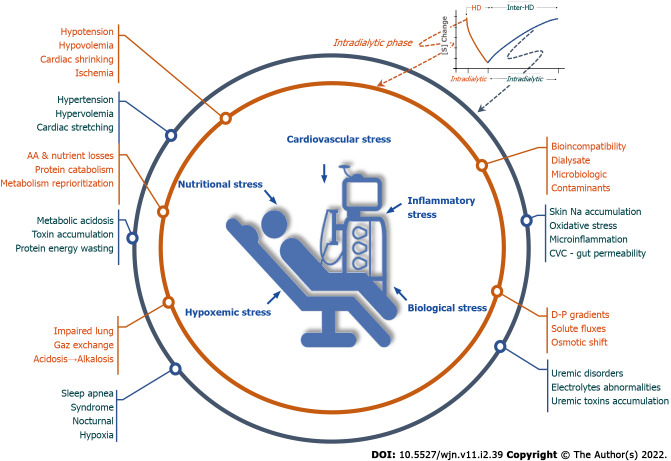
The ‘unphysiology’ of intermittent HD is recognized as a leading cause of dialysis intolerance and multiorgan morbidity[33,34]. This phenomenon was exacerbated by operational changes that resulted in shortening of dialysis treatment schedules and increasing dialysis efficiency[35]. As such, intermittent HD generates periodic changes in volume and blood pressure, osmotic shifts, and variation in circulating levels of compounds and electrolytes. Treatment-induced disturbances are in complete contrast with strictly regulated and stable conditions of the internal milieu in healthy subjects[32,36,37] (Figure 1).
Figure 1.
Intermittent extracorporeal renal replacement therapy is the source of permanent stress in hemodialysis patients. HD: Hemodialysis; CVC: Central venous catheter.
During the interdialytic period, anuric HD patients tend to accumulate sodium and fluid according to fluid and diet intake, leading to chronic fluid overload[38]. In this condition, fluid overload has two components: The first, resulting from cyclic changes imposed by intermittent treatment marked by weight gain and progressive increase of systemic arterial pressure and pulmonary arterial pressure with cardiac stretching occurring between two treatment sessions; and the second, which reflects chronic fluid overload that has accumulated over time, exposing patients to chronic cardiac stretching and structural cardiac remodeling[39] (Figure 1).
During the intradialytic period, sodium and fluid removal resulting from ultrafiltration (intradialytic weight loss) and the patient to dialysate sodium gradient contributes to reducing circulating blood volume and triggering an adaptative hemodynamic response[40,41]. In response to ultrafiltration provoking a reduction in blood volume and cardiac stroke volume, arterial pressure and tissue perfusion are maintained by an increase in vascular tone, mainly through vasoconstriction of alpha-adrenoceptor territories, and an increase of vascular refilling and in venous return[42,43]. Recent intradialytic imaging studies have shown that reductions in myocardial perfusion and contractility (myocardial stunning) are linked to ultrafiltration rate that happens even without ischemic cardiac disease[17,44,45]. Several observational studies have reported a strong association between mortality and high ultrafiltration rate or volume changes, drop in blood pressure, and end-organ ischaemic insult[10]. The systemic response is more complex than a simple reaction to hypovolemia, since it incompasses others factors such as vascular refilling capacity, thermal balance, electrolyte fluxes, nutrient losses, as well as the individual patient’s baseline cardiac reserve and neurohormonal stress responses[45,46]. Interesting, this response may be mitigated by various factors (e.g., age, gender, comorbidity, and medication) explaining individual or temporal variations in hemodynamic response[38,47]. The hemodynamic stress induced by dialysis must be considered as a potent disease modifier in highly susceptible patients[48] (Figure 1).
Whatever the exact contribution of these phenomena, dialysis-induced cyclical volemic changes (hyper- and hypo-volemia) provoke alternating cardiac loading and unloading. This volemia variation cycle is responsible for repetitive myocardial stretching, a mechanism that leads to release of inflammatory mediators and promotes cardiac fibrosis and arrhythmias[49,50] (Figure 1).
Inflammatory stress
Bio-incompatibility (or more specifically, hemo-incompatibility) of the extracorporeal blood circuit and its systemic effects is a well identified issue associated with several aspects of dialysis related morbidity[51,52]. In brief, the activation of a cascade of serum proteins and blood cells is induced upon contact with foreign material in the extracorporeal circuit[53,54], and endothelial damage may further induce a vascular endothelial breach[55]. This process is further modified by the geometry, design (e.g., blood air interface and dead space), and nature of blood tubing (e.g., type of polymer and plasticizer) or dialyzer membrane (e.g., cellulosic and synthetic), and may be amplified by microbial-derived products from dialysis fluid (e.g., lipopolysaccharide, endotoxins, and bacterial DNA)[56-59]. As a result, endothelial cells and circulating blood cells (e.g., platelets, leukocytes, and monocytes) are primed and activated to release pro-inflammatory mediators (e.g., platelet activating factor 4, beta-thromboglobulin, granulocytes proteinases, anaphylatoxins, and cytokines) and activate protein cascades (e.g., clotting cascades, complement activation, surface contact, and kallikrein-kinin system)[60-66]. Activation of the innate immune and coagulation systems amplifies and propagates this reaction[67]. Platelets and endothelial cell activation trigger coagulation, endothelial damage, vascular reactivity, and pulmonary trapping of cells. Mononuclear leukocyte activation results in the release of enzymes (e.g., granulocyte neutral proteinase and elastase)[60,68-70], and increases their reactivity and adhesiveness that may cause obstruction at the microcirculatory level. In the lungs, this may contribute to hypoxemia[71-73]. Activation of monocytes and macrophages induces release of proinflammatory cytokines [interleukin (IL)-1, IL-6, and tumor necrosis factor-α][74,75]. In addition, acute inflammatory reactions are amplified by oxidative stress in an amplifying loops contributing to a vicious circle[74]. Seminal studies performed in various HD settings (e.g., cellulosic vs synthetic dialyzers and contaminated vs ultrapure dialysate) have documented the importance of this “biologic storm” and provided evidence of its damaging effects (e.g., allergic reaction, lung dysfunction, thrombocytopenia, and inflammation)[67,76] (Figure 1).
Despite significant improvements in extracorporeal circuit biocompatibility and wide-spread use of ultrapure dialysis fluid, systemic hemobiological reactions periodically induced by extracorporeal treatment[77,78] are likely to contribute to a micro-inflammatory state in chronic HD patients that amplifies long-term deleterious effects[30,75,79] (Figure 1).
Biological stress
In the absence of significant kidney function, internal metabolic processes and dietary intake produce metabolites during the interdialytic phase that steadily accumulate over 48 h and lead to classical biologic uremic abnormalities[80]. During dialysis, biologic disorders are usually corrected, at least partially, within 4 h. Biologic gradients between the dialysate and blood may be large, resulting in high amplitude changes of body composition during each session[32,76,81,82]. This gradient stress may be easily quantitated by dialysate-blood gradient concentrations and time averaged deviations for various solutes that are exchanged during the dialysis session[81]. Solutes exchange in HD follows negative or positive gradients, knowing that solute gradient is conventionally defined as dialysate-plasma concentration difference. Uremic retention toxins (e.g., urea, creatinine, uric acid, potassium, and phosphate) are removed according to a negative gradient from blood to dialysate, while selected electrolytes (e.g., bicarbonate, calcium, and magnesium) or nutritional compounds (e.g., glucose) may move in the opposite direction. Unwanted removal of essential nutrients (e.g., amino acids, peptides, and water soluble vitamins such vitamin D) and albumin may occur, contributing to a nutritional stress. The description of biochemical changes during dialysis is beyond the scope of this review. Through this remark we emphasize the fact that dialysis patients are challenged by various and large osmotic changes due to movements of urea and uraemic metabolites, water shift from extra- to intra-cellular space, acid-base changes moving the patient from metabolic acidosis to mixed alkalosis, potassium swings from hyper- to hypo-kalemia, and divalent ion alterations moving from hyper- to hypo-phosphatemia and from hypo- to hyper-calcemia, while at the same time patients are losing amino acids and other important nutrients[83-86]. Clinical manifestations of these metabolic derangements range from none, through minor to severe symptoms (fatigue, headache, and cognitive impairment), with the most extreme manifestation being dialysis disequilibrium syndrome[87,88] (Figure 1).
Hypoxemic stress
During dialysis, in addition to circulatory stress and impaired tissue perfusion[89-91], hypoxemia may occur, which can be particularly marked in the early phase of a dialysis session, likely related to hemoincompatibility reactions inducing leukocyte trapping within the lungs. This observation suggests the occurrence of an additional respiratory stress resulting from impaired pulmonary gas exchange[92,93]. Prolonged intradialytic hypoxemia is likely to play an aggravating role in end organ damage by reducing further tissue oxygen delivery. We can speculate that this is a pathophysiologic link that explains the increased mortality observed in patients presenting with prolonged hypoxemia during HD[92] (Figure 1).
During the interdialytic phase, sleep apnea syndrome (SAS) and nocturnal hypoxemia have emerged as important additional cardiovascular risk factors in HD patients[80]. SAS marked by repetitive pause of breathing during sleep resulting in hypoxemia and hypercapnia is highly prevalent in HD patients[80,94]. In addition, SAS is associated with profound changes in cardiac loading conditions, lung arterial pressure, and autonomic activation, all factors that have been associated with significant cardiovascular morbidity such as left ventricular hypertrophy or arrhythmias and sudden cardiac death[95-98]. Although uremic abnormalities contribute to the development of SAS, the role of fluid overload exacerbating upper airways obstruction should not be neglected as recently pointed out by a study exploring fluid displacement into nuchal and peripharyngeal soft tissues in healthy subjects[99]. It is therefore tempting to speculate that chronic fluid overload is partly responsible for an edema of upper airway especially during sleep while in the supine position, thereby contributing to the occurrence of SAS (Figure 1).
In brief, whatever mechanisms are associated with impaired pulmonary gas exchange in HD patients, occurring either during intradialytic or interdialytic phases, prolonged periods of hypoxemia are likely to represent an additional stressor[34] (Figure 1).
Nutritional stress
Loss of muscle mass is common in HD patients and represents one of the most important predictors of mortality[100,101]. Sarcopenia is the main component of the protein-energy wasting syndrome that results from complex uremic abnormalities and the adverse effects of HD treatment[102-104] (Figure 1).
On one hand, acute studies assessing muscle and whole body protein turnover conducted in stable patients have consistently demonstrated an imbalance in protein synthesis and degradation during HD sessions[105-108]. It has been also shown that losses of amino acids during HD, ranging between 8 and 10 g per session, contributed significantly to the net protein catabolism[85,109-111]. Interestingly, this amino acid loss leads to reprioritization of protein metabolism during HD sessions. Amino acid loss during HD stimulates muscle and liver protein catabolism in order to preserve plasma and intra-cellular amino acid concentrations. Furthermore, amino acid utilization for protein synthesis either by the liver or muscle is impaired in HD patients, mainly through activation of cytokine pathways (IL-6) rather than because of amino acid depletion[112-114]. Remarkably, amino acid repletion by IV administration during HD tends to increase muscle protein synthesis but does not decrease muscle protein breakdown[115]. It is also interesting to note that dextrose depletion (when dextrose-free dialysate is used)[116] and other aspects of HD including type of membrane (cellulosic vs synthetic)[117,118] and dialysate microbiologic purity[119,120] may modulate this muscle protein catabolism phenomenon[121] (Figure 1).
On the other hand, long-term precise nutritional studies conducted in stable patients under strict metabolic conditions have shown that HD-induced imbalance in protein metabolism[122,123] might be compensated for by dietary protein and caloric supplements[124,125]. As shown, the net negative protein metabolic imbalance observed on dialysis days might be compensated for by increasing dietary protein and caloric intake (about 25%) during non-dialysis days, leading to a neutral protein and caloric balance on a weekly basis[124,126]. However, in practice, this can be hard to achieve.
In brief, intermittent HD treatment is associated with repetitive nutritional stress conditions due to reprioritization of protein metabolism within the muscle and liver (Figure 1).
Dialysis sickness and dialysis related morbidity
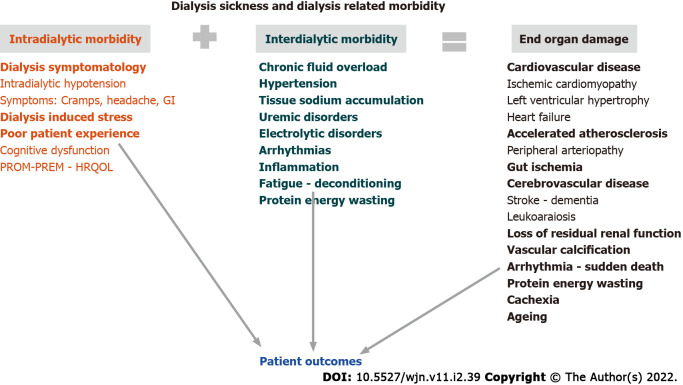
Dialysis sickness (DS) refers to the concept that inter-, peri-, and intra-dialytic morbidity resulting from the hemodynamic, inflammatory, biological, hypoxemic, and nutritional stresses discussed above, and can result in the long-term in end organ damage as summarized in Figure 2.
Figure 2.
Dialysis Related Pathology linked to patient outcomes. GI: Glycaemic index; PROM: Patient reported outcomes measures; PREM: Patient reported experience measures; HRQOL: Health-related quality of life.
Dialysis-related morbidity (intra- and peri-dialytic symptomatology) has a negative impact on patients’ perception and on their quality of life (QoL)[16,48,93,127,128]. This can be measured by scoring scales according to patient reported outcomes measures (PROM) or patient reported experience measures (PREM)[129-131]. Intra- and inter-dialytic symptoms that include hypotensive episodes, cramps, headache, fatigue, pruritus, and sleep disorders are the most frequently reported[132]. PROMs, PREMs, and most domains of health related QoL are significantly reduced in patients treated by conventional HD and tend to be improved by daily or extended treatment schedules[133-135]. Furthermore, dialysis symptom burden has been shown to be associated with increased mortality and hospitalization risks. Indeed, these clinical performance indicators are strongly recommended to assess dialysis adequacy and patient experience[129,136-139] (Figure 1).
End organ damage results from exposure to hemodynamic and pulmonary stressors leading to poor tissue perfusion and oxygen delivery, which are further aggravated by biological and cytokine “storms”. Multifactorial and repetitive systemic stressors induced by intermittent HD treatment are likely to have harmful long-term effects on the function and structural modeling of vital organs (e.g., cardiac stunning, leukoaraiosis, gut ischemia, and hepato-splanchnic changes). Some of these cardiovascular effects are enhanced by chronic low-grade inflammation acting on endothelial dysfunction and contributing to poor outcomes[10,28,140-142]. The combination of cardiocirculatory stress, hypovolemia, and electrolyte changes occurring during HD sessions creates pro-arrhythmogenic conditions that may contribute to clinically significant cardiac arrhythmias during the interdialytic phase[143-147]. Cardiac structural changes following myocardial stunning and remodeling in response to cyclical dialysis-induced phenomenon, such as fibrotic scarring and loss of segmental contractile function with irregular electrical conductivity, are plausibly increasing the risk of sudden cardiac death[44,146,148-151]. These findings mimick the intense physiologic demands endured by healthy subjects under extreme conditions[152]. In order to mitigate dialysis-induced organ damage, we propose that conventional HD treatment schedule may be adapted and personalized, as a new treatment paradigm.
CALL FOR DESIGNING AND APPLYING A MORE CARDIOVASCULAR PROTECTIVE HD TREATMENT
Optimizing hemodynamic management
The inevitable sodium and fluid accumulation that occurs during the interdialytic phase in anuric HD patients is responsible for chronic extracellular fluid overload with its adverse effects[153,154]. Hypertension is part of this constellation of disorders being recognized as the leading cause of cardiac and vascular disease in HD patients[19,20]. Management of fluid volume has been identified as a specific cardiovascular risk factor: On one hand, persistence of chronic fluid overload is independently associated with increased cardiovascular risk[155]; on the other hand, overly-rapid fluid volume reduction (i.e., ultrafiltration rate) and hypovolemia are also associated with an increased risk of cardiovascular mortality[10,156] (Figure 3).
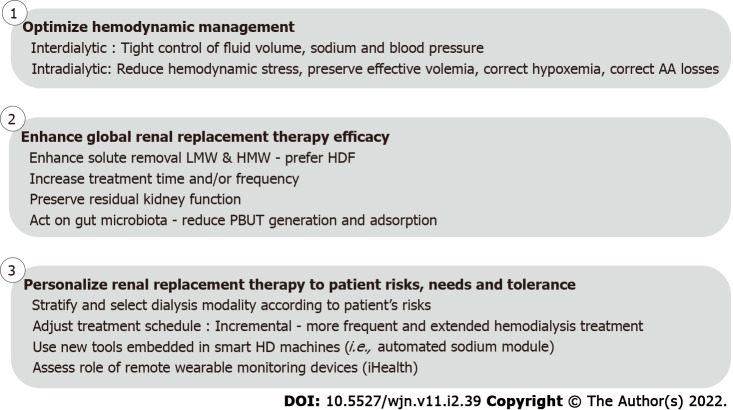
Figure 3.
Action plan to design and implement a more cardioprotective renal replacement treatment in order to improve patient outcomes. HD: Hemodialysis; PBUT: Protein bound uremic toxins; LMW: Low-molecular-weight; HMW: High-molecular-weight; HDF: On-line hemodiafiltration.
In other words, sodium and fluid volume homeostasis and blood pressure need to be managed more precisely during the interdialytic phase to achieve suitable targets. Additionally, hemodynamic stress secondary to volume contraction should be mitigated during dialysis by the use of appropriate tools and adjustment of the treatment schedule. Better monitoring of blood pressure and hemodynamics that are applicable to the clinical setting are also needed. This is a fundamental challenge of intermittent HD (Figure 3).
Improving sodium, fluid volume, and pressure management during the interdialytic phase: Salt and fluid management of the dialysis patient represents a major challenge for clinicians. A combined approach is needed that includes clinical management (a dry weight probing policy, e.g., ultrafiltration, dialysate sodium prescription, and diet education) supported by assessment tools (e.g., multifrequency bioimpedance and lung ultrasound)[157], cardiac biomarkers [e.g., B-type natriuretic peptide (BNP) and NTproBNP], HD technical options (e.g., sodium control module), and algorithms (e.g., artificial intelligence) using advanced analytics in the future[38,158] (Figure 3).
Reducing hemodynamic stress induced by HD: Intradialytic morbidity (i.e., fatigue, headache, cramps, hypotension, and alteration of cognitive function) is largely dependent on fluid removal (i.e., ultrafiltration) and dialysis efficiency (i.e., osmotic and solute concentration changes, and electrolytes shifts). The intensity and frequency of these symptoms also depend on patient characteristics (e.g., age, gender, and anthropometrics), metabolism, and body composition, and on the HD treatment schedule (e.g., treatment time and frequency). It is well recognized that longer and more frequent dialysis treatment schedules are better tolerated with reduced circulatory stress and slower osmotic and electrolytic changes, as compared to short and less frequent dialysis schedules[159,160]. In that respect, ultrafiltration rate, reflecting fluid volume removed per time unit, is a well-recognized cardiac risk factor in dialysis patients that also associates with mortality risk[40]. In addition, it reflects the fact that biochemical gradients and solute fluxes are reduced per time unit, as well as osmotic changes and water shifts occurring within the central nervous system (Figure 3).
In a stepwise approach, increasing treatment time and/or dialysis frequency should ideally represent the first and most rational step to reduce risks associated with ultrafiltration rate and osmotic changes in non-compliant or fragile patients[161]. As a next step, modulating patients’ hemodynamic responses through various tools embedded in the HD machine is another appealing option[162]. Monitoring blood volume during dialysis sessions is useful to identify critical volemia, to estimate remaining fluid in the interstitium, or to quantify vascular refilling capacity[163], but it is not sufficient to manage patient hemodynamic response[164]. Instead, surveillance of central venous oxygen saturation (ScvO2) in patients with central venous catheters may indicate critical changes in organ perfusion before they result in clinical symptomatology. Interestingly, the decline in ScvO2 during dialysis has been correlated to ultrafiltration volume[165,166]. With arterio-venous fistula, near infrared spectroscopy, a non-invasive method, could be of interest to estimate tissue oxygenation[167]. Feedback controlled ultrafiltration system relying on blood volume changes has improved hemodynamic stability in selected studies, but so far has not improved patient outcomes and intradialytic morbidity[168,169]. Some studies have shown that using dialysate sodium and ultrafiltration profiling, with or without blood volume monitoring, may preserve intradialytic hemodynamic status but at the expense of an increased risk of subclinical salt loading, thirst, high interdialytic weight gain, and chronic fluid overload[170]. Adjusting dialysis thermal balance to preserve peripheral vascular resistance and cardiac output is also a simple strategy to improve hemodynamic tolerance that has been proven effective in several studies[171]. The main objective is to deliver isothermic or better, hypothermic dialysis, to prevent thermal gain during a dialysis session which is associated with an inappropriate hemodynamic response (vasodilation, tachycardia, and drop in ejection fraction)[172]. Hypothermic HD could be manually achieved by setting dialysate temperature 0.5-1 °C below the patient’s core temperature. Automated thermal control of dialysis sessions requires the use of an online blood temperature monitor that can control precisely the thermal balance of patients to a preset target[173]. Both approaches reduce hypotension incidence (Figure 3).
Another important component of intradialytic morbidity relates to biochemical stress as reflected by the magnitude of dialysate-plasma solute gradient, a major determinant of solute fluxes[170,174-176]. Reducing instantaneous solute fluxes while keeping solute mass removal constant during dialysis session may be an interesting approach to reduce intradialytic morbidity. This issue could be easily addressed by reducing blood flow and increasing treatment time and/or frequency to slow instantaneous solute fluxes. This is a usual practice in Japan but it is not the most popular nor the most appealing in Western countries[177]. Another approach within the current short dialysis treatment schedule would be to continuously adjust flow parameters to reduce instantaneous solute fluxes while keeping solute mass transfer constant. Advanced technology will facilitate such an approach in the future, relying on microsensors positioned on dialysate side, feeding specific algorithms, and then providing feedback control to the HD monitor to adjust relative flows and gradients (Figure 3).
In summary, one should consider that fluid volume removal and solute fluxes (dependent in part on blood-dialysate concentration gradients) are potentially modifiable factors of the dialysis prescription (Figure 3).
Enhancing renal care efficacy
The limited efficiency of contemporary HD in restoring the internal milieu composition and in controlling circulating levels of middle and large molecular sized uremic toxins, has stimulated use of convective-based therapies (e.g., hemodiafiltration) and more porous membranes (i.e., high cut-off)[36]. Therefore, the so-called ‘residual syndrome’, reflecting incomplete removal of uremic toxins, is another potential contributor to patient morbidity and mortality[178,179] (Figure 3).
Enhancing treatment efficiency by combining high efficiency hemodiafiltration and extended treatment time has been shown in recent studies to be able to address most remaining issues in adults. In brief, extended on-line hemodiafiltration (HDF) treatment has been associated with tight control of fluid volume and blood pressure without antihypertensive medications, normalization of phosphate levels while phosphate binders were stopped, correction of anemia while erythropoietic stimulating agent consumption was reduced by 50%, and a significant improvement of nutritional status and physical activity[180,181]. Interestingly, in a pediatric population, extended HDF has been also shown to improve intermediary outcomes (i.e., fluid volume, blood pressure, inflammation, phosphate, and nutrition), to reduce cardiovascular disease progression, and to promote catch-up growth[182-184] (Figure 3).
Preserving residual kidney function is an important feature in dialysis patients since it is associated with a reduced disease and treatment burden and mortality[185-187]. Fluid volume and blood pressure control are usually better achieved with less dietary restriction[188]. Circulating levels of uremic toxins are significantly reduced, particularly for middle and large molecular weight substances but also for protein-bound uremic toxins[189]. In brief, all dialysis conditions, but particularly those ensuring a better hemodynamic stability, should be considered to prevent the repetitive ischemic kidney insults during HD[190] (Figure 3).
Acting on the gut to reduce protein-bound uremic toxin production has been recently suggested as a potential way of reducing circulating levels of protein bound uremic toxins (PBUT) such as indoxyl sulfate and paracresyl sulfate[191]. A few studies have confirmed positive effects of this option using either probiotics or adsorbers (AST120) administered orally in reducing plasma PBUT concentrations[192,193]. Unfortunately, published interventional studies have not confirmed potential long-term clinical benefits on patient outcomes[194] but further studies with better design and greater statistical power are warranted (Figure 3).
Personalizing renal replacement treatment schedule
Treatment schedule adaptation: A ‘one–size–fits-all’ approach is unlikely to work, and this should be kept in mind for optimizing renal replacement therapies in the future. Accordingly, dialysis prescription including treatment schedule (time and frequency), modality, dose, and efficiency[134,195,196], and electrolyte prescription should be tailored to patient profile, needs, and tolerance[197,198]. Furthermore, treatment prescription should be adapted over time to an individual patient’s results in a personalized way to follow patient metabolic changes, treatment tolerance, and symptoms. Dialysis prescription should return to physiologic principles; it should not be the patient who must adapt to a fixed treatment, but the treatment should fit to the patient needs and tolerance instead.
In this context, the treatment schedules offered to patients should be expanded and become more flexible. It is not our intent to develop this concept further but to highlight recent interesting findings (Figure 3).
Incremental dialysis is an interesting concept that deserves more attention in particular in incident ESKD patients and in emerging countries[199]. It relies on the fact that HD acts as a complement to residual kidney function. In other words, the number of dialysis sessions and/or treatment time per week is inversely related to the glomerular filtration rate. Recent comprehensive reviews have addressed this issue to which we refer the interested reader for more details on clinical benefits and implementation[200]. In brief, incremental dialysis has the capacity to facilitate treatment implementation in new patients by reducing treatment burden, but also potentially to mitigate a shortage of renal replacement therapy resources in low and middle income countries (Figure 3).
Extended HD schedules (i.e., long and nocturnal dialysis, alternate day dialysis, and daily HD) appear particularly attractive in terms of improving outcomes[181]. Extended treatment schedules must be viewed from two aspects: On one hand, outcomes are favorable including with kidney transplant[195,201-204]; on the other hand, they increase treatment burden and cost, except if home HD is chosen[205]. In this context, to solve both logisitical and cost issues, it is therefore proposed to develop extended treatment schedules at home or in self-care facilities[206] (Figure 3).
Use of new tools for monitoring and adapting treatment prescription: A whole body bioimpedance cardiography (BIC) non-invasive device has been assessed in HD patients. BIC has interesting features to measure the hemodynamic response to fluid removal (e.g., cardiac output and total peripheral vascular resistance) during dialysis. Based on these findings, it has been suggested that dialysis patients might be clustered into various categories defined as low or high cardiac output, low or high total peripheral vascular resistance, or normal hemodynamics[207,208]. BIC has the potential to support physicians to individualize dialysis treatment, although this would need to be tested in interventional studies[208]. Approaches using BIC warrant further studies to validate measurements and explore impact on patient outcomes[209] (Figure 3).
More recently, lung ultrasononography (LUS) has been proposed as a point-of-care tool to complete physical examination[24,210,211]. Lung ultrasound is a noninvasive method to estimate extravascular lung water easily mastered by nephrologists that help to quantify lung congestion by counting B-lines per lung area unit (Comet line scoring). The “Lung water by ultrasound guided treatment to prevent death and cardiovascular complications in high risk ESRD patients with cardiomyopathy” study has shown the clinical value of LUS in the management of HD patients at high cardiovascular risk[212,213] (Figure 3).
A further tool to reduce intradialytic hemodynamic stress is the development of wearable non-pervasive methods for continuous blood pressure monitoring. This would allow detection of subtle changes in blood pressure to prompt interventions such as reduction of ultrafiltration rate to prevent hypotension. Recent work using additional pressure sensors placed on dialysis lines to derive blood pressure without the need for additional equipment attached to the patient, shows promise in this regard[214,215]. Considering the high cardiac mortality risk of HD patients (10 to 100 times greater than the general population)[216], it appears of utmost importance to pay closer attention to cardiovascular monitoring to ensure early and appropriate intervention for improving outcomes[49]. Interestingly, new remote technologies or so-called connected iHealth devices offer convenient new tools for monitoring high risk HD patients during the interdialytic period in a fully automated and ambulatory mode[217]. Detection of clinical significant arrhythmias would be one important functionality, as shown in recent studies[146,218] (Figure 3).
FUTURE DEVELOPMENT OF HD AND RENAL REPLACEMENT THERAPY
In order to reduce dialysis associated morbidity and to improve patient experience, three main approaches should be proposed and explored.
Designing and adapting HD treatment schedule to individual patient needs, tolerance, and risks
Aside from the introduction of more flexible treatment schedules, recent studies have also shown the potential interest of stratifying patients according to their risks at short or medium term outcomes[219,220]. A better understanding of patient risks could help physicians to prescribe more appropriate and individualized therapy. Also, scoring systems could be tested as supports to alter specific treatment prescription features in an attempt to reduce early mortality of ESKD patients transitioning to dialysis.
Using automated systems embedded in intelligent dialysis machines
The technology relies on the combination of patient biologic sensors coupled to a feedback control loop and governed by adaptive algorithms embedded in the dialysis machine. The first example is the sodium control module that has been assessed and validated in clinical trials[72,221]. Using continuous conductivity cell measurements on inlet and outlet dialysate flow, an embedded algorithm controls plasma sodium concentration changes (i.e., tonicity) and allows precise monitoring of plasma sodium concentration and sodium mass removal occurring within dialysis session. Interestingly, sodium mass transfer and plasma tonicity rely on an automated and self-adapting function that follows medical prescription setting. Further outcome based studies are needed to establish clinical benefits to patients and the device’s clinical added value[222].
Combined use of connected iHealth devices, advanced analytics, and artificial intelligence will be able to support medical decision making and to predict future outcome
Personalized medicine relying on iHealth trackers, advanced analytics, and artificial intelligence (artificial neuronal networks and machine learning) may allow identification of patients at increased risk. In this respect, the use of such tools will be able to support physician decision-making for individual patients to select the most appropriate treatment modality or suitable technical approach (i.e., ultrafiltration rate and dialysate sodium) to reduce cardiovascular burden[223,224]. Furthermore, iHealth trackers and machine learning support may also be applied to continuous vital signs monitoring and other intra-dialytic hemodynamic variables. The ultimate goal is to detect or predict the occurrence of future clinical events with sufficient precision and time to intervene. Such iHealth trackers seem particularly attractive to monitor arrythmias and maybe to help prevent sudden cardiac death[217]. In brief, the paradigm of precision medicine appears particularly relevant to renal replacement therapy for designing a personalized, more effective, better tolerated, and more acceptable HD treatment[225].
CONCLUSION
In this in-depth review, we have summarized factors that are implicated in the cardiovascular and multi-organ morbidity associated with conventional short intermittent HD treatment schedules. Hidden risks result mainly from the conjunction of two main phenomena: First, the intermittent nature of the treatment that is responsible for an unphysiologic profile (illustrated by peaks and troughs reflecting fluctuation of internal milieu composition) and a multifactorial systemic stress; second, the incomplete correction of uremic metabolic abnormalities that may be summarized as “residual syndrome”. Such systemic stress induced by HD treatment is likely implicated in the poor dialysis tolerance and end-organ injury contributing to the DS syndrome. We summarize this cascade of events as the dialysis stress storm and sickness syndrome (D4S) and propose that D4S may act as a negative disease modifier of patient outcome.
Mitigating cardiovascular burden in HD requires further concerted actions to change the treatment paradigm. Such an approach will have multiple targets that should ideally include optimizing hemodynamic management both during the inter- and intra-dialytic phase, enhancing renal replacement therapy efficacy, and personalizing treatment schedule with use of new monitoring tools.
Footnotes
Conflict-of-interest statement: Canaud B is acting as scientist consultant for FMC. No conflict of interest exists for other authors.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Francophone Society of Nephrology, Dialysis and Transplant; ERA - EDTA; ISN; American Society of Nephrology.
Peer-review started: March 26, 2021
First decision: October 17, 2021
Article in press: March 23, 2022
Specialty type: Urology and nephrology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Patoulias D, Greece S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
Contributor Information
Bernard Canaud, Global Medical Office, Fresenius Medical Care, Bad Homburg 61352, Germany; Department of Nephrology, Montpellier University, Montpellier 34000, France. bernard.canaud@fmc-ag.com.
Jeroen P Kooman, Department of Internal Medicine, Maastricht University, Maastricht 6229 HX, Netherlands.
Nicholas M Selby, Centre for Kidney Research and Innovation, Academic Unit for Translational Medical Sciences, School of Medicine, University of Nottingham, Derby DE22 3DT, United Kingdom.
Maarten Taal, Centre for Kidney Research and Innovation, Academic Unit for Translational Medical Sciences, School of Medicine, University of Nottingham, Derby DE22 3DT, United Kingdom.
Andreas Maierhofer, Global Research Development, Fresenius Medical Care, Schweinfurt 97424, Germany.
Pascal Kopperschmidt, Global Research Development, Fresenius Medical Care, Schweinfurt 97424, Germany.
Susan Francis, Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham NG7 2RD, United Kingdom.
Allan Collins, Global Medical Office, Fresenius Medical Care, Bad Homburg 61352, Germany.
Peter Kotanko, Renal Research Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10065, United States.
References
- 1.Thomas B, Wulf S, Bikbov B, Perico N, Cortinovis M, Courville de Vaccaro K, Flaxman A, Peterson H, Delossantos A, Haring D, Mehrotra R, Himmelfarb J, Remuzzi G, Murray C, Naghavi M. Maintenance Dialysis throughout the World in Years 1990 and 2010. J Am Soc Nephrol. 2015;26:2621–2633. doi: 10.1681/ASN.2014101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 3.Jain D, Haddad DB, Goel N. Choice of dialysis modality prior to kidney transplantation: Does it matter? World J Nephrol. 2019;8:1–10. doi: 10.5527/wjn.v8.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16:573–585. doi: 10.1038/s41581-020-0315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson BM, Akizawa T, Jager KJ, Kerr PG, Saran R, Pisoni RL. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet. 2016;388:294–306. doi: 10.1016/S0140-6736(16)30448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopes AA, Bragg-Gresham JL, Satayathum S, McCullough K, Pifer T, Goodkin DA, Mapes DL, Young EW, Wolfe RA, Held PJ, Port FK Worldwide Dialysis Outcomes and Practice Patterns Study Committee. Health-related quality of life and associated outcomes among hemodialysis patients of different ethnicities in the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2003;41:605–615. doi: 10.1053/ajkd.2003.50122. [DOI] [PubMed] [Google Scholar]
- 7.Couillerot-Peyrondet AL, Sambuc C, Sainsaulieu Y, Couchoud C, Bongiovanni-Delarozière I. A comprehensive approach to assess the costs of renal replacement therapy for end-stage renal disease in France: the importance of age, diabetes status, and clinical events. Eur J Health Econ. 2017;18:459–469. doi: 10.1007/s10198-016-0801-6. [DOI] [PubMed] [Google Scholar]
- 8.Rayner HC, Pisoni RL, Bommer J, Canaud B, Hecking E, Locatelli F, Piera L, Bragg-Gresham JL, Feldman HI, Goodkin DA, Gillespie B, Wolfe RA, Held PJ, Port FK. Mortality and hospitalization in haemodialysis patients in five European countries: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2004;19:108–120. doi: 10.1093/ndt/gfg483. [DOI] [PubMed] [Google Scholar]
- 9.USRDS . Annual Data Report. In: Chapter 5: Mortality. United States: NIDDK, 2018; 2: 411-426. [Google Scholar]
- 10.McIntyre CW. Recurrent circulatory stress: the dark side of dialysis. Semin Dial. 2010;23:449–451. doi: 10.1111/j.1525-139X.2010.00782.x. [DOI] [PubMed] [Google Scholar]
- 11.McIntyre C, Crowley L. Dying to Feel Better: The Central Role of Dialysis-Induced Tissue Hypoxia. Clin J Am Soc Nephrol. 2016;11:549–551. doi: 10.2215/CJN.01380216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson JK, Neovius M, Jacobson SH, Elinder CG, Hylander B. Healthcare costs in chronic kidney disease and renal replacement therapy: a population-based cohort study in Sweden. BMJ Open. 2016;6:e012062. doi: 10.1136/bmjopen-2016-012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanholder R, Davenport A, Hannedouche T, Kooman J, Kribben A, Lameire N, Lonnemann G, Magner P, Mendelssohn D, Saggi SJ, Shaffer RN, Moe SM, Van Biesen W, van der Sande F, Mehrotra R Dialysis Advisory Group of American Society of Nephrology. Reimbursement of dialysis: a comparison of seven countries. J Am Soc Nephrol. 2012;23:1291–1298. doi: 10.1681/ASN.2011111094. [DOI] [PubMed] [Google Scholar]
- 14.Vanholder R, Annemans L, Brown E, Gansevoort R, Gout-Zwart JJ, Lameire N, Morton RL, Oberbauer R, Postma MJ, Tonelli M, Biesen WV, Zoccali C European Kidney Health Alliance. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol. 2017;13:393–409. doi: 10.1038/nrneph.2017.63. [DOI] [PubMed] [Google Scholar]
- 15.Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–1220. doi: 10.1111/j.1523-1755.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 16.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol. 2015;26:724–734. doi: 10.1681/ASN.2014020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assimon MM, Wang L, Flythe JE. Cumulative Exposure to Frequent Intradialytic Hypotension Associates With New-Onset Dementia Among Elderly Hemodialysis Patients. Kidney Int Rep. 2019;4:603–606. doi: 10.1016/j.ekir.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maduell F, Ramos R, Varas J, Martin-Malo A, Molina M, Pérez-Garcia R, Marcelli D, Moreso F, Aljama P, Merello JI. Hemodialysis patients receiving a greater Kt dose than recommended have reduced mortality and hospitalization risk. Kidney Int. 2016;90:1332–1341. doi: 10.1016/j.kint.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal R, Flynn J, Pogue V, Rahman M, Reisin E, Weir MR. Assessment and management of hypertension in patients on dialysis. J Am Soc Nephrol. 2014;25:1630–1646. doi: 10.1681/ASN.2013060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal R, Weir MR. Dry-weight: a concept revisited in an effort to avoid medication-directed approaches for blood pressure control in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:1255–1260. doi: 10.2215/CJN.01760210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal R, Alborzi P, Satyan S, Light RP. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension. 2009;53:500–507. doi: 10.1161/HYPERTENSIONAHA.108.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moissl U, Arias-Guillén M, Wabel P, Fontseré N, Carrera M, Campistol JM, Maduell F. Bioimpedance-guided fluid management in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8:1575–1582. doi: 10.2215/CJN.12411212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wabel P, Chamney P, Moissl U, Jirka T. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 2009;27:75–80. doi: 10.1159/000167013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoccali C. Lung Ultrasound in the Management of Fluid Volume in Dialysis Patients: Potential Usefulness. Semin Dial. 2017;30:6–9. doi: 10.1111/sdi.12559. [DOI] [PubMed] [Google Scholar]
- 25.McIntyre CW. Effects of hemodialysis on cardiac function. Kidney Int. 2009;76:371–375. doi: 10.1038/ki.2009.207. [DOI] [PubMed] [Google Scholar]
- 26.Assa S, Hummel YM, Voors AA, Kuipers J, Westerhuis R, Groen H, Bakker SJ, Muller Kobold AC, van Oeveren W, Struck J, de Jong PE, Franssen CF. Hemodialysis-induced regional left ventricular systolic dysfunction and inflammation: a cross-sectional study. Am J Kidney Dis. 2014;64:265–273. doi: 10.1053/j.ajkd.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Buchanan C, Mohammed A, Cox E, Köhler K, Canaud B, Taal MW, Selby NM, Francis S, McIntyre CW. Intradialytic Cardiac Magnetic Resonance Imaging to Assess Cardiovascular Responses in a Short-Term Trial of Hemodiafiltration and Hemodialysis. J Am Soc Nephrol. 2017;28:1269–1277. doi: 10.1681/ASN.2016060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, Camici PG. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3:19–26. doi: 10.2215/CJN.03170707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eldehni MT, Odudu A, McIntyre CW. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol. 2015;26:957–965. doi: 10.1681/ASN.2013101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freemont AJ. The pathology of dialysis. Semin Dial. 2002;15:227–231. doi: 10.1046/j.1525-139x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 31.Cambi V, Arisi L, Bignardi L, Garini G, Rossi E, Savazzi G, Migone L. Preliminary results obtained with short dialysis schedules. Ateneo Parmense Acta Biomed. 1975;46:349–358. [PubMed] [Google Scholar]
- 32.Kjellstrand CM, Evans RL, Petersen RJ, Shideman JR, von Hartitzsch B, Buselmeier TJ. The "unphysiology" of dialysis: a major cause of dialysis side effects? Kidney Int Suppl. 1975:30–34. [PubMed] [Google Scholar]
- 33.Kjellstrand CM, Evans RL, Petersen RJ, Shideman JR, Von Hartitzsch B, Buselmeier TJ. The "unphysiology" of dialysis: a major cause of dialysis side effects? Hemodial Int. 2004;8:24–29. doi: 10.1111/j.1492-7535.2004.00083.x. [DOI] [PubMed] [Google Scholar]
- 34.Canaud B, Kooman JP, Selby NM, Taal MW, Francis S, Maierhofer A, Kopperschmidt P, Collins A, Kotanko P. Dialysis-Induced Cardiovascular and Multiorgan Morbidity. Kidney Int Rep. 2020;5:1856–1869. doi: 10.1016/j.ekir.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cambi V, Savazzi G, Arisi L, Bignardi L, Bruschi G, Rossi E, Migone L. Short dialysis schedules (SDS)--finally ready to become routine? Proc Eur Dial Transplant Assoc. 1975;11:112–120. [PubMed] [Google Scholar]
- 36.Ledebo I. Does convective dialysis therapy applied daily approach renal blood purification? Kidney Int Suppl. 2001;78:S286–S291. doi: 10.1046/j.1523-1755.2001.59780286.x. [DOI] [PubMed] [Google Scholar]
- 37.Modell H, Cliff W, Michael J, McFarland J, Wenderoth MP, Wright A. A physiologist's view of homeostasis. Adv Physiol Educ. 2015;39:259–266. doi: 10.1152/advan.00107.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canaud B, Chazot C, Koomans J, Collins A. Fluid and hemodynamic management in hemodialysis patients: challenges and opportunities. J Bras Nefrol. 2019;41:550–559. doi: 10.1590/2175-8239-JBN-2019-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kjellström B, Braunschweig F, Löfberg E, Fux T, Grandjean PA, Linde C. Changes in right ventricular pressures between hemodialysis sessions recorded by an implantable hemodynamic monitor. Am J Cardiol. 2009;103:119–123. doi: 10.1016/j.amjcard.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 40.Flythe JE, Brunelli SM. The risks of high ultrafiltration rate in chronic hemodialysis: implications for patient care. Semin Dial. 2011;24:259–265. doi: 10.1111/j.1525-139X.2011.00854.x. [DOI] [PubMed] [Google Scholar]
- 41.Flythe JE, Assimon MM, Wang L. Ultrafiltration Rate Scaling in Hemodialysis Patients. Semin Dial. 2017;30:282–283. doi: 10.1111/sdi.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levin NW, de Abreu MHFG, Borges LE, Tavares Filho HA, Sarwar R, Gupta S, Hafeez T, Lev S, Williams C. Hemodynamic response to fluid removal during hemodialysis: categorization of causes of intradialytic hypotension. Nephrol Dial Transplant. 2018;33:1643–1649. doi: 10.1093/ndt/gfy048. [DOI] [PubMed] [Google Scholar]
- 43.McGuire S, Horton EJ, Renshaw D, Jimenez A, Krishnan N, McGregor G. Hemodynamic Instability during Dialysis: The Potential Role of Intradialytic Exercise. Biomed Res Int. 2018;2018:8276912. doi: 10.1155/2018/8276912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baldamus CA, Ernst W, Frei U, Koch KM. Sympathetic and hemodynamic response to volume removal during different forms of renal replacement therapy. Nephron. 1982;31:324–332. doi: 10.1159/000182675. [DOI] [PubMed] [Google Scholar]
- 46.Baldamus CA, Ernst W, Kachel HG, Lysaght M, Koch KM. Hemodynamics in hemofiltration. Contrib Nephrol. 1982;32:56–60. doi: 10.1159/000406905. [DOI] [PubMed] [Google Scholar]
- 47.Jefferies HJ, Virk B, Schiller B, Moran J, McIntyre CW. Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning) Clin J Am Soc Nephrol. 2011;6:1326–1332. doi: 10.2215/CJN.05200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chou JA, Kalantar-Zadeh K, Mathew AT. A brief review of intradialytic hypotension with a focus on survival. Semin Dial. 2017;30:473–480. doi: 10.1111/sdi.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chirakarnjanakorn S, Navaneethan SD, Francis GS, Tang WH. Cardiovascular impact in patients undergoing maintenance hemodialysis: Clinical management considerations. Int J Cardiol. 2017;232:12–23. doi: 10.1016/j.ijcard.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herum KM, Choppe J, Kumar A, Engler AJ, McCulloch AD. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol Biol Cell. 2017;28:1871–1882. doi: 10.1091/mbc.E17-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mollahosseini A, Abdelrasoul A, Shoker A. A critical review of recent advances in hemodialysis membranes hemocompatibility and guidelines for future development. Mater Chem Phys. 2020;248:122911. [Google Scholar]
- 52.Wagner S, Zschätzsch S, Erlenkoetter A, Rauber L, Stauss-Grabo M, Gauly A. Hemocompatibility of Polysulfone Hemodialyzers - Exploratory Studies on Impact of Treatment Modality and Dialyzer Characteristics. Kidney360. 2020;1:25–35. doi: 10.34067/KID.0000342019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber M, Steinle H, Golombek S, Hann L, Schlensak C, Wendel HP, Avci-Adali M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front Bioeng Biotechnol. 2018;6:99. doi: 10.3389/fbioe.2018.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doyle AJ, Hunt BJ. Current Understanding of How Extracorporeal Membrane Oxygenators Activate Haemostasis and Other Blood Components. Front Med (Lausanne) 2018;5:352. doi: 10.3389/fmed.2018.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martens RJH, Broers NJH, Canaud B, Christiaans MHL, Cornelis T, Gauly A, Hermans MMH, Konings CJAM, van der Sande FM, Scheijen JLJM, Stifft F, Wirtz JJJM, Kooman JP, Schalkwijk CG. Relations of advanced glycation endproducts and dicarbonyls with endothelial dysfunction and low-grade inflammation in individuals with end-stage renal disease in the transition to renal replacement therapy: A cross-sectional observational study. PLoS One. 2019;14:e0221058. doi: 10.1371/journal.pone.0221058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaefer RM, Heidland A, Hörl WH. Effect of dialyzer geometry on granulocyte and complement activation. Am J Nephrol. 1987;7:121–126. doi: 10.1159/000167446. [DOI] [PubMed] [Google Scholar]
- 57.Taylor JE, McLaren M, Mactier RA, Henderson IS, Stewart WK, Belch JJ. Effect of dialyzer geometry during hemodialysis with cuprophane membranes. Kidney Int. 1992;42:442–447. doi: 10.1038/ki.1992.307. [DOI] [PubMed] [Google Scholar]
- 58.Cheung AK. Biocompatibility of hemodialysis membranes. J Am Soc Nephrol. 1990;1:150–161. doi: 10.1681/ASN.V12150. [DOI] [PubMed] [Google Scholar]
- 59.Schindler R, Beck W, Deppisch R, Aussieker M, Wilde A, Göhl H, Frei U. Short bacterial DNA fragments: detection in dialysate and induction of cytokines. J Am Soc Nephrol. 2004;15:3207–3214. doi: 10.1097/01.ASN.0000145049.94888.26. [DOI] [PubMed] [Google Scholar]
- 60.Hörl WH, Jochum M, Heidland A, Fritz H. Release of granulocyte proteinases during hemodialysis. Am J Nephrol. 1983;3:213–217. doi: 10.1159/000166713. [DOI] [PubMed] [Google Scholar]
- 61.Muñoz de Bustillo E, Alvarez Chiva V. Leukocyte--endothelial cell interactions in haemodialysis-induced neutropenia. Nephrol Dial Transplant. 1996;11:572–574. doi: 10.1093/oxfordjournals.ndt.a027343. [DOI] [PubMed] [Google Scholar]
- 62.Windus DW, Atkinson R, Santoro S. The effects of hemodialysis on platelet activation with new and reprocessed regenerated cellulose dialyzers. Am J Kidney Dis. 1996;27:387–393. doi: 10.1016/s0272-6386(96)90362-5. [DOI] [PubMed] [Google Scholar]
- 63.Schoorl M, Schoorl M, Nubé MJ, Bartels PC. Platelet depletion, platelet activation and coagulation during treatment with hemodialysis. Scand J Clin Lab Invest. 2011;71:240–247. doi: 10.3109/00365513.2011.558106. [DOI] [PubMed] [Google Scholar]
- 64.Coppo R, Amore A, Cirina P, Scelfo B, Giacchino F, Comune L, Atti M, Renaux JL. Bradykinin and nitric oxide generation by dialysis membranes can be blunted by alkaline rinsing solutions. Kidney Int. 2000;58:881–888. doi: 10.1046/j.1523-1755.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- 65.Krishnan A, Vogler EA, Sullenger BA, Becker RC. The effect of surface contact activation and temperature on plasma coagulation with an RNA aptamer directed against factor IXa. J Thromb Thrombolysis. 2013;35:48–56. doi: 10.1007/s11239-012-0778-7. [DOI] [PubMed] [Google Scholar]
- 66.Marney AM, Ma J, Luther JM, Ikizler TA, Brown NJ. Endogenous bradykinin contributes to increased plasminogen activator inhibitor 1 antigen following hemodialysis. J Am Soc Nephrol. 2009;20:2246–2252. doi: 10.1681/ASN.2009050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hakim RM. Clinical implications of hemodialysis membrane biocompatibility. Kidney Int. 1993;44:484–494. doi: 10.1038/ki.1993.272. [DOI] [PubMed] [Google Scholar]
- 68.Heidland A, Hörl WH, Heller N, Heine H, Neumann S, Heidbreder E. Proteolytic enzymes and catabolism: enhanced release of granulocyte proteinases in uremic intoxication and during hemodialysis. Kidney Int Suppl. 1983;16:S27–S36. [PubMed] [Google Scholar]
- 69.Hörl WH, Feinstein EI, Wanner C, Frischmuth N, Gösele A, Massry SG. Plasma levels of main granulocyte components during hemodialysis. Comparison of new and reused dialyzers. Am J Nephrol. 1990;10:53–57. doi: 10.1159/000168054. [DOI] [PubMed] [Google Scholar]
- 70.Schaefer RM, Heidland A, Hörl WH. Release of leukocyte elastase during hemodialysis. Effect of different dialysis membranes. Contrib Nephrol. 1985;46:109–117. [PubMed] [Google Scholar]
- 71.Craddock PR, Fehr J, Dalmasso AP, Brighan KL, Jacob HS. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977;59:879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ságová M, Wojke R, Maierhofer A, Gross M, Canaud B, Gauly A. Automated individualization of dialysate sodium concentration reduces intradialytic plasma sodium changes in hemodialysis. Artif Organs. 2019;43:1002–1013. doi: 10.1111/aor.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hörl WH, Schaefer RM, Heidland A. Effect of different dialyzers on proteinases and proteinase inhibitors during hemodialysis. Am J Nephrol. 1985;5:320–326. doi: 10.1159/000166956. [DOI] [PubMed] [Google Scholar]
- 74.Morena M, Delbosc S, Dupuy AM, Canaud B, Cristol JP. Overproduction of reactive oxygen species in end-stage renal disease patients: a potential component of hemodialysis-associated inflammation. Hemodial Int. 2005;9:37–46. doi: 10.1111/j.1492-7535.2005.01116.x. [DOI] [PubMed] [Google Scholar]
- 75.Cobo G, Lindholm B, Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant. 2018;33:iii35–iii40. doi: 10.1093/ndt/gfy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saha M, Allon M. Diagnosis, Treatment, and Prevention of Hemodialysis Emergencies. Clin J Am Soc Nephrol. 2017;12:357–369. doi: 10.2215/CJN.05260516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koga Y, Fujieda H, Meguro H, Ueno Y, Aoki T, Miwa K, Kainoh M. Biocompatibility of Polysulfone Hemodialysis Membranes and Its Mechanisms: Involvement of Fibrinogen and Its Integrin Receptors in Activation of Platelets and Neutrophils. Artif Organs. 2018;42:E246–E258. doi: 10.1111/aor.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kohlová M, Amorim CG, Araújo A, Santos-Silva A, Solich P, Montenegro MCBSM. The biocompatibility and bioactivity of hemodialysis membranes: their impact in end-stage renal disease. J Artif Organs. 2019;22:14–28. doi: 10.1007/s10047-018-1059-9. [DOI] [PubMed] [Google Scholar]
- 79.Eswari JS, Naik S. A critical analysis on various technologies and functionalized materials for manufacturing dialysis membranes. Mater Sci Energy Technol. 2020;3:116–126. [Google Scholar]
- 80.Chan CT. Sleep apnea with intermittent hemodialysis: time for a wake-up call! J Am Soc Nephrol. 2006;17:3279–3280. doi: 10.1681/ASN.2006101110. [DOI] [PubMed] [Google Scholar]
- 81.Lopot F, Válek A. Mathematical Concept of Dialysis Unphysiology. Home Hemodial Int (1997) 1998;2:18–21. doi: 10.1111/hdi.1998.2.1.18. [DOI] [PubMed] [Google Scholar]
- 82.Lopot F, Nejedlý B, Sulková S. Physiology in daily hemodialysis in terms of the time average concentration/time average deviation concept. Hemodial Int. 2004;8:39–44. doi: 10.1111/j.1492-7535.2004.00073.x. [DOI] [PubMed] [Google Scholar]
- 83.Burmeister JE, Scapini A, da Rosa Miltersteiner D, da Costa MG, Campos BM. Glucose-added dialysis fluid prevents asymptomatic hypoglycaemia in regular haemodialysis. Nephrol Dial Transplant. 2007;22:1184–1189. doi: 10.1093/ndt/gfl710. [DOI] [PubMed] [Google Scholar]
- 84.Abe M, Kalantar-Zadeh K. Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Nat Rev Nephrol. 2015;11:302–313. doi: 10.1038/nrneph.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chazot C, Shahmir E, Matias B, Laidlaw S, Kopple JD. Dialytic nutrition: provision of amino acids in dialysate during hemodialysis. Kidney Int. 1997;52:1663–1670. doi: 10.1038/ki.1997.500. [DOI] [PubMed] [Google Scholar]
- 86.Raimann JG, Kruse A, Thijssen S, Kuntsevich V, Dabel P, Bachar M, Diaz-Buxo JA, Levin NW, Kotanko P. Metabolic effects of dialyzate glucose in chronic hemodialysis: results from a prospective, randomized crossover trial. Nephrol Dial Transplant. 2012;27:1559–1568. doi: 10.1093/ndt/gfr520. [DOI] [PubMed] [Google Scholar]
- 87.Sahani MM, Daoud TM, Sam R, Andrews J, Cheng YL, Kjellstrand CM, Ing TS. Dialysis Disequilibrium Syndrome Revisited. Hemodial Int. 2001;5:92–96. doi: 10.1111/hdi.2001.5.1.92. [DOI] [PubMed] [Google Scholar]
- 88.Zepeda-Orozco D, Quigley R. Dialysis disequilibrium syndrome. Pediatr Nephrol. 2012;27:2205–2211. doi: 10.1007/s00467-012-2199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romaldini H, Rodriguez-Roisin R, Lopez FA, Ziegler TW, Bencowitz HZ, Wagner PD. The mechanisms of arterial hypoxemia during hemodialysis. Am Rev Respir Dis. 1984;129:780–784. doi: 10.1164/arrd.1984.129.5.780. [DOI] [PubMed] [Google Scholar]
- 90.Cardoso M, Vinay P, Vinet B, Léveillée M, Prud'homme M, Téjédor A, Courteau M, Gougoux M, St-Louis G, Lapierre L, Piette Y. Hypoxemia during hemodialysis: a critical review of the facts. Am J Kidney Dis. 1988;11:281–297. doi: 10.1016/s0272-6386(88)80133-1. [DOI] [PubMed] [Google Scholar]
- 91.Campos I, Chan L, Zhang H, Deziel S, Vaughn C, Meyring-Wösten A, Kotanko P. Intradialytic Hypoxemia in Chronic Hemodialysis Patients. Blood Purif. 2016;41:177–187. doi: 10.1159/000441271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meyring-Wösten A, Zhang H, Ye X, Fuertinger DH, Chan L, Kappel F, Artemyev M, Ginsberg N, Wang Y, Thijssen S, Kotanko P. Intradialytic Hypoxemia and Clinical Outcomes in Patients on Hemodialysis. Clin J Am Soc Nephrol. 2016;11:616–625. doi: 10.2215/CJN.08510815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chou JA, Streja E, Nguyen DV, Rhee CM, Obi Y, Inrig JK, Amin A, Kovesdy CP, Sim JJ, Kalantar-Zadeh K. Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. Nephrol Dial Transplant. 2018;33:149–159. doi: 10.1093/ndt/gfx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Forni Ogna V, Ogna A, Pruijm M, Bassi I, Zuercher E, Halabi G, Phan O, Bullani R, Teta D, Gauthier T, Cherpillod A, Mathieu C, Mihalache A, Cornette F, Haba-Rubio J, Burnier M, Heinzer R. Prevalence and Diagnostic Approach to Sleep Apnea in Hemodialysis Patients: A Population Study. Biomed Res Int. 2015;2015:103686. doi: 10.1155/2015/103686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kerns ES, Kim ED, Meoni LA, Sozio SM, Jaar BG, Estrella MM, Parekh RS, Bourjeily G. Obstructive Sleep Apnea Increases Sudden Cardiac Death in Incident Hemodialysis Patients. Am J Nephrol. 2018;48:147–156. doi: 10.1159/000489963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ito K, Ookawara S, Fueki M, Imai S, Hattori T, Kiryu S, Sugai Y, Wada N, Shindo M, Ohnishi Y, Iino N, Tabei K, Morishita Y. Sleep apnea syndrome caused lowering of cerebral oxygenation in a hemodialysis patient: a case report and literature review. Ren Replace Ther. 2018;4:54. [Google Scholar]
- 97.Zoccali C, Mallamaci F, Tripepi G. Nocturnal hypoxemia predicts incident cardiovascular complications in dialysis patients. J Am Soc Nephrol. 2002;13:729–733. doi: 10.1681/ASN.V133729. [DOI] [PubMed] [Google Scholar]
- 98.Zoccali C, Benedetto FA, Tripepi G, Cambareri F, Panuccio V, Candela V, Mallamaci F, Enia G, Labate C, Tassone F. Nocturnal hypoxemia, night-day arterial pressure changes and left ventricular geometry in dialysis patients. Kidney Int. 1998;53:1078–1084. doi: 10.1111/j.1523-1755.1998.00853.x. [DOI] [PubMed] [Google Scholar]
- 99.Chiu KL, Ryan CM, Shiota S, Ruttanaumpawan P, Arzt M, Haight JS, Chan CT, Floras JS, Bradley TD. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am J Respir Crit Care Med. 2006;174:1378–1383. doi: 10.1164/rccm.200607-927OC. [DOI] [PubMed] [Google Scholar]
- 100.Stenvinkel P, Carrero JJ, von Walden F, Ikizler TA, Nader GA. Muscle wasting in end-stage renal disease promulgates premature death: established, emerging and potential novel treatment strategies. Nephrol Dial Transplant. 2016;31:1070–1077. doi: 10.1093/ndt/gfv122. [DOI] [PubMed] [Google Scholar]
- 101.Canaud B, Ye X, Usvyat L, Kooman J, van der Sande F, Raimann J, Wang Y, Kotanko P. Clinical and predictive value of simplified creatinine index used as muscle mass surrogate in end-stage kidney disease haemodialysis patients-results from the international MONitoring Dialysis Outcome initiative. Nephrol Dial Transplant. 2020;35:2161–2171. doi: 10.1093/ndt/gfaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimbürger O, Bárány P, Suliman ME, Lindholm B, Stenvinkel P, Qureshi AR. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr. 2008;27:557–564. doi: 10.1016/j.clnu.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 103.Sabatino A, Cuppari L, Stenvinkel P, Lindholm B, Avesani CM. Sarcopenia in chronic kidney disease: what have we learned so far? J Nephrol. 2021;34:1347–1372. doi: 10.1007/s40620-020-00840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kopple JD, Massry SG, Kalantar-Zadeh K. Nutritional Management of Renal Disease. In: Workeneh B, Mitch WE. The Influence of Kidney Disease on Protein and Amino Acid Metabolism. Amsterdam: Elsevier, 2013: 1-16. [Google Scholar]
- 105.Garibotto G, Russo R, Sofia A, Sala MR, Robaudo C, Moscatelli P, Deferrari G, Tizianello A. Skeletal muscle protein synthesis and degradation in patients with chronic renal failure. Kidney Int. 1994;45:1432–1439. doi: 10.1038/ki.1994.187. [DOI] [PubMed] [Google Scholar]
- 106.Lim VS, Ikizler TA, Raj DS, Flanigan MJ. Does hemodialysis increase protein breakdown? J Am Soc Nephrol. 2005;16:862–868. doi: 10.1681/ASN.2004080624. [DOI] [PubMed] [Google Scholar]
- 107.Lim VS, Kopple JD. Protein metabolism in patients with chronic renal failure: role of uremia and dialysis. Kidney Int. 2000;58:1–10. doi: 10.1046/j.1523-1755.2000.00135.x. [DOI] [PubMed] [Google Scholar]
- 108.Almushayt SJ, Hussain S, Wilkinson DJ, Selby NM. A Systematic Review of the Acute Effects of Hemodialysis on Skeletal Muscle Perfusion, Metabolism, and Function. Kidney Int Rep. 2020;5:307–317. doi: 10.1016/j.ekir.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gil HW, Yang JO, Lee EY, Lee EM, Choi JS, Hong SY. The effect of dialysis membrane flux on amino acid loss in hemodialysis patients. J Korean Med Sci. 2007;22:598–603. doi: 10.3346/jkms.2007.22.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hynote ED, McCamish MA, Depner TA, Davis PA. Amino acid losses during hemodialysis: effects of high-solute flux and parenteral nutrition in acute renal failure. JPEN J Parenter Enteral Nutr. 1995;19:15–21. doi: 10.1177/014860719501900115. [DOI] [PubMed] [Google Scholar]
- 111.Hendriks FK, Smeets JSJ, Broers NJH, van Kranenburg JMX, van der Sande FM, Kooman JP, van Loon LJC. End-Stage Renal Disease Patients Lose a Substantial Amount of Amino Acids during Hemodialysis. J Nutr. 2020;150:1160–1166. doi: 10.1093/jn/nxaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Raj DS, Moseley P, Dominic EA, Onime A, Tzamaloukas AH, Boyd A, Shah VO, Glew R, Wolfe R, Ferrando A. Interleukin-6 modulates hepatic and muscle protein synthesis during hemodialysis. Kidney Int. 2008;73:1054–1061. doi: 10.1038/ki.2008.21. [DOI] [PubMed] [Google Scholar]
- 113.van Hall G. Cytokines: muscle protein and amino acid metabolism. Curr Opin Clin Nutr Metab Care. 2012;15:85–91. doi: 10.1097/MCO.0b013e32834e6ea2. [DOI] [PubMed] [Google Scholar]
- 114.van Hall G, Steensberg A, Fischer C, Keller C, Møller K, Moseley P, Pedersen BK. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab. 2008;93:2851–2858. doi: 10.1210/jc.2007-2223. [DOI] [PubMed] [Google Scholar]
- 115.Raj DS, Adeniyi O, Dominic EA, Boivin MA, McClelland S, Tzamaloukas AH, Morgan N, Gonzales L, Wolfe R, Ferrando A. Amino acid repletion does not decrease muscle protein catabolism during hemodialysis. Am J Physiol Endocrinol Metab. 2007;292:E1534–E1542. doi: 10.1152/ajpendo.00599.2006. [DOI] [PubMed] [Google Scholar]
- 116.Gutierrez A, Bergström J, Alvestrand A. Hemodialysis-associated protein catabolism with and without glucose in the dialysis fluid. Kidney Int. 1994;46:814–822. doi: 10.1038/ki.1994.337. [DOI] [PubMed] [Google Scholar]
- 117.Gutierrez A. Protein catabolism in maintenance haemodialysis: the influence of the dialysis membrane. Nephrol Dial Transplant. 1996;11 Suppl 2:108–111. doi: 10.1093/ndt/11.supp2.108. [DOI] [PubMed] [Google Scholar]
- 118.Gutierrez A, Alvestrand A, Wahren J, Bergström J. Effect of in vivo contact between blood and dialysis membranes on protein catabolism in humans. Kidney Int. 1990;38:487–494. doi: 10.1038/ki.1990.230. [DOI] [PubMed] [Google Scholar]
- 119.Susantitaphong P, Riella C, Jaber BL. Effect of ultrapure dialysate on markers of inflammation, oxidative stress, nutrition and anemia parameters: a meta-analysis. Nephrol Dial Transplant. 2013;28:438–446. doi: 10.1093/ndt/gfs514. [DOI] [PubMed] [Google Scholar]
- 120.Ikizler TA, Cano NJ, Franch H, Fouque D, Himmelfarb J, Kalantar-Zadeh K, Kuhlmann MK, Stenvinkel P, TerWee P, Teta D, Wang AY, Wanner C International Society of Renal Nutrition and Metabolism. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013;84:1096–1107. doi: 10.1038/ki.2013.147. [DOI] [PubMed] [Google Scholar]
- 121.Stegmayr B. Dialysis Procedures Alter Metabolic Conditions. Nutrients. 2017;9 doi: 10.3390/nu9060548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Farrell PC, Hone PW. Dialysis-induced catabolism. Am J Clin Nutr. 1980;33:1417–1422. doi: 10.1093/ajcn/33.7.1417. [DOI] [PubMed] [Google Scholar]
- 123.Ikizler TA, Wingard RL, Sun M, Harvell J, Parker RA, Hakim RM. Increased energy expenditure in hemodialysis patients. J Am Soc Nephrol. 1996;7:2646–2653. doi: 10.1681/ASN.V7122646. [DOI] [PubMed] [Google Scholar]
- 124.Borah MF, Schoenfeld PY, Gotch FA, Sargent JA, Wolfsen M, Humphreys MH. Nitrogen balance during intermittent dialysis therapy of uremia. Kidney Int. 1978;14:491–500. doi: 10.1038/ki.1978.154. [DOI] [PubMed] [Google Scholar]
- 125.Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985;27:58–65. doi: 10.1038/ki.1985.10. [DOI] [PubMed] [Google Scholar]
- 126.Slomowitz LA, Monteon FJ, Grosvenor M, Laidlaw SA, Kopple JD. Effect of energy intake on nutritional status in maintenance hemodialysis patients. Kidney Int. 1989;35:704–711. doi: 10.1038/ki.1989.42. [DOI] [PubMed] [Google Scholar]
- 127.Johnson S, Crane PB, Neil J, Christiano C. Coping with Intradialytic Events and Stress Associated with Hemodialysis. Nephrol Nurs J. 2019;46:13–21. [PubMed] [Google Scholar]
- 128.Kuipers J, Oosterhuis JK, Paans W, Krijnen WP, Gaillard CAJM, Westerhuis R, Franssen CFM. Association between quality of life and various aspects of intradialytic hypotension including patient-reported intradialytic symptom score. BMC Nephrol. 2019;20:164. doi: 10.1186/s12882-019-1366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van der Willik EM, Meuleman Y, Prantl K, van Rijn G, Bos WJW, van Ittersum FJ, Bart HAJ, Hemmelder MH, Dekker FW. Patient-reported outcome measures: selection of a valid questionnaire for routine symptom assessment in patients with advanced chronic kidney disease - a four-phase mixed methods study. BMC Nephrol. 2019;20:344. doi: 10.1186/s12882-019-1521-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Loon IN, Bots ML, Boereboom FTJ, Grooteman MPC, Blankestijn PJ, van den Dorpel MA, Nubé MJ, Ter Wee PM, Verhaar MC, Hamaker ME. Quality of life as indicator of poor outcome in hemodialysis: relation with mortality in different age groups. BMC Nephrol. 2017;18:217. doi: 10.1186/s12882-017-0621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nair D, Finkelstein FO. Toward Developing a Patient-Reported Outcome Measure for Fatigue in Hemodialysis. Am J Kidney Dis. 2019;74:151–154. doi: 10.1053/j.ajkd.2019.03.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Flythe JE, Hilliard T, Castillo G, Ikeler K, Orazi J, Abdel-Rahman E, Pai AB, Rivara MB, St Peter WL, Weisbord SD, Wilkie C, Mehrotra R. Symptom Prioritization among Adults Receiving In-Center Hemodialysis: A Mixed Methods Study. Clin J Am Soc Nephrol. 2018;13:735–745. doi: 10.2215/CJN.10850917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Finkelstein FO, Finkelstein SH. Time to Rethink Our Approach to Patient-Reported Outcome Measures for ESRD. Clin J Am Soc Nephrol. 2017;12:1885–1888. doi: 10.2215/CJN.04850517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Finkelstein FO, Schiller B, Daoui R, Gehr TW, Kraus MA, Lea J, Lee Y, Miller BW, Sinsakul M, Jaber BL. At-home short daily hemodialysis improves the long-term health-related quality of life. Kidney Int. 2012;82:561–569. doi: 10.1038/ki.2012.168. [DOI] [PubMed] [Google Scholar]
- 135.Kliger AS, Finkelstein FO. Can we improve the quality of life for dialysis patients? Am J Kidney Dis. 2009;54:993–995. doi: 10.1053/j.ajkd.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 136.Jaar BG, Chang A, Plantinga L. Can we improve quality of life of patients on dialysis? Clin J Am Soc Nephrol. 2013;8:1–4. doi: 10.2215/CJN.11861112. [DOI] [PubMed] [Google Scholar]
- 137.Jaber BL, Lee Y, Collins AJ, Hull AR, Kraus MA, McCarthy J, Miller BW, Spry L, Finkelstein FO FREEDOM Study Group. Effect of daily hemodialysis on depressive symptoms and postdialysis recovery time: interim report from the FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study. Am J Kidney Dis. 2010;56:531–539. doi: 10.1053/j.ajkd.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 138.Mapes DL, Bragg-Gresham JL, Bommer J, Fukuhara S, McKevitt P, Wikström B, Lopes AA. Health-related quality of life in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44:54–60. doi: 10.1053/j.ajkd.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 139.Mapes DL, Lopes AA, Satayathum S, McCullough KP, Goodkin DA, Locatelli F, Fukuhara S, Young EW, Kurokawa K, Saito A, Bommer J, Wolfe RA, Held PJ, Port FK. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003;64:339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 140.Odudu A, Eldehni MT, McCann GP, McIntyre CW. Randomized Controlled Trial of Individualized Dialysate Cooling for Cardiac Protection in Hemodialysis Patients. Clin J Am Soc Nephrol. 2015;10:1408–1417. doi: 10.2215/CJN.00200115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McIntyre CW, Goldsmith DJ. Ischemic brain injury in hemodialysis patients: which is more dangerous, hypertension or intradialytic hypotension? Kidney Int. 2015;87:1109–1115. doi: 10.1038/ki.2015.62. [DOI] [PubMed] [Google Scholar]
- 142.Grant CJ, Huang SS, McIntyre CW. Hepato-splanchnic circulatory stress: An important effect of hemodialysis. Semin Dial. 2019;32:237–242. doi: 10.1111/sdi.12782. [DOI] [PubMed] [Google Scholar]
- 143.Karaboyas A, Zee J, Brunelli SM, Usvyat LA, Weiner DE, Maddux FW, Nissenson AR, Jadoul M, Locatelli F, Winkelmayer WC, Port FK, Robinson BM, Tentori F. Dialysate Potassium, Serum Potassium, Mortality, and Arrhythmia Events in Hemodialysis: Results From the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2017;69:266–277. doi: 10.1053/j.ajkd.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pun PH, Middleton JP. Dialysate Potassium, Dialysate Magnesium, and Hemodialysis Risk. J Am Soc Nephrol. 2017;28:3441–3451. doi: 10.1681/ASN.2017060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rhee CM, Chou JA, Kalantar-Zadeh K. Dialysis Prescription and Sudden Death. Semin Nephrol. 2018;38:570–581. doi: 10.1016/j.semnephrol.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Charytan DM, Foley R, McCullough PA, Rogers JD, Zimetbaum P, Herzog CA, Tumlin JA MiD Investigators and Committees. Arrhythmia and Sudden Death in Hemodialysis Patients: Protocol and Baseline Characteristics of the Monitoring in Dialysis Study. Clin J Am Soc Nephrol. 2016;11:721–734. doi: 10.2215/CJN.09350915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Roy-Chaudhury P, Tumlin JA, Koplan BA, Costea AI, Kher V, Williamson D, Pokhariyal S, Charytan DM MiD investigators and committees. Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int. 2018;93:941–951. doi: 10.1016/j.kint.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 148.Gorcsan J 3rd, Haugaa KH. Ventricular Arrhythmias and Reduced Echocardiographic Inferior Wall Strain: Is Regional Function an Important Risk Marker? Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.005900. [DOI] [PubMed] [Google Scholar]
- 149.Kalra PA, Green D, Poulikakos D. Arrhythmia in hemodialysis patients and its relation to sudden death. Kidney Int. 2018;93:781–783. doi: 10.1016/j.kint.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 150.Nguyen MN, Kiriazis H, Gao XM, Du XJ. Cardiac Fibrosis and Arrhythmogenesis. Compr Physiol. 2017;7:1009–1049. doi: 10.1002/cphy.c160046. [DOI] [PubMed] [Google Scholar]
- 151.Nguyen TP, Qu Z, Weiss JN. Cardiac fibrosis and arrhythmogenesis: the road to repair is paved with perils. J Mol Cell Cardiol. 2014;70:83–91. doi: 10.1016/j.yjmcc.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kooman JP, Katzarski K, van der Sande FM, Leunissen KM, Kotanko P. Hemodialysis: A model for extreme physiology in a vulnerable patient population. Semin Dial. 2018;31:500–506. doi: 10.1111/sdi.12704. [DOI] [PubMed] [Google Scholar]
- 153.Dekker MJE, Kooman JP. Fluid status assessment in hemodialysis patients and the association with outcome: review of recent literature. Curr Opin Nephrol Hypertens. 2018;27:188–193. doi: 10.1097/MNH.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 154.Dekker MJE, van der Sande FM, van den Berghe F, Leunissen KML, Kooman JP. Fluid Overload and Inflammation Axis. Blood Purif. 2018;45:159–165. doi: 10.1159/000485153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zoccali C, Moissl U, Chazot C, Mallamaci F, Tripepi G, Arkossy O, Wabel P, Stuard S. Chronic Fluid Overload and Mortality in ESRD. J Am Soc Nephrol. 2017;28:2491–2497. doi: 10.1681/ASN.2016121341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.London GM. Ultrafiltration intensification for achievement of dry weight and hypertension control is not always the therapeutic gold standard. J Nephrol. 2011;24:395–397. doi: 10.5301/jn.5000006. [DOI] [PubMed] [Google Scholar]
- 157.van der Sande FM, van de Wal-Visscher ER, Stuard S, Moissl U, Kooman JP. Using Bioimpedance Spectroscopy to Assess Volume Status in Dialysis Patients. Blood Purif. 2020;49:178–184. doi: 10.1159/000504079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pinter J, Chazot C, Stuard S, Moissl U, Canaud B. Sodium, volume and pressure control in haemodialysis patients for improved cardiovascular outcomes. Nephrol Dial Transplant. 2020;35:ii23–ii30. doi: 10.1093/ndt/gfaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tentori F, Zhang J, Li Y, Karaboyas A, Kerr P, Saran R, Bommer J, Port F, Akiba T, Pisoni R, Robinson B. Longer dialysis session length is associated with better intermediate outcomes and survival among patients on in-center three times per week hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2012;27:4180–4188. doi: 10.1093/ndt/gfs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fotheringham J, Sajjad A, Stel VS, McCullough K, Karaboyas A, Wilkie M, Bieber B, Robinson BM, Massy ZA, Jager KJ. The association between longer haemodialysis treatment times and hospitalization and mortality after the two-day break in individuals receiving three times a week haemodialysis. Nephrol Dial Transplant. 2019;34:1577–1584. doi: 10.1093/ndt/gfz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.van der Sande FM, Dekker MJ, Leunissen KML, Kooman JP. Novel Insights into the Pathogenesis and Prevention of Intradialytic Hypotension. Blood Purif. 2018;45:230–235. doi: 10.1159/000485160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Locatelli F, Buoncristiani U, Canaud B, Köhler H, Petitclerc T, Zucchelli P. Haemodialysis with on-line monitoring equipment: tools or toys? Nephrol Dial Transplant. 2005;20:22–33. doi: 10.1093/ndt/gfh555. [DOI] [PubMed] [Google Scholar]
- 163.Sinha AD, Light RP, Agarwal R. Relative plasma volume monitoring during hemodialysis AIDS the assessment of dry weight. Hypertension. 2010;55:305–311. doi: 10.1161/HYPERTENSIONAHA.109.143974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Leung KCW, Quinn RR, Ravani P, Duff H, MacRae JM. Randomized Crossover Trial of Blood Volume Monitoring-Guided Ultrafiltration Biofeedback to Reduce Intradialytic Hypotensive Episodes with Hemodialysis. Clin J Am Soc Nephrol. 2017;12:1831–1840. doi: 10.2215/CJN.01030117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang H, Chan L, Meyring-Wösten A, Campos I, Preciado P, Kooman JP, van der Sande FM, Fuertinger D, Thijssen S, Kotanko P. Association between intradialytic central venous oxygen saturation and ultrafiltration volume in chronic hemodialysis patients. Nephrol Dial Transplant. 2018;33:1636–1642. doi: 10.1093/ndt/gfx271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Harrison LE, Selby NM, McIntyre CW. Central venous oxygen saturation: a potential new marker for circulatory stress in haemodialysis patients? Nephron Clin Pract. 2014;128:57–60. doi: 10.1159/000362557. [DOI] [PubMed] [Google Scholar]
- 167.Polinder-Bos HA, Elting JWJ, Aries MJ, García DV, Willemsen AT, van Laar PJ, Kuipers J, Krijnen WP, Slart RH, Luurtsema G, Westerhuis R, Gansevoort RT, Gaillard CA, Franssen CF. Changes in cerebral oxygenation and cerebral blood flow during hemodialysis - A simultaneous near-infrared spectroscopy and positron emission tomography study. J Cereb Blood Flow Metab. 2020;40:328–340. doi: 10.1177/0271678X18818652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Santoro A, Mancini E, Paolini F, Cavicchioli G, Bosetto A, Zucchelli P. Blood volume regulation during hemodialysis. Am J Kidney Dis. 1998;32:739–748. doi: 10.1016/s0272-6386(98)70128-3. [DOI] [PubMed] [Google Scholar]
- 169.Beaubien-Souligny W, Denault A, Robillard P, Desjardins G. The Role of Point-of-Care Ultrasound Monitoring in Cardiac Surgical Patients With Acute Kidney Injury. J Cardiothorac Vasc Anesth. 2019;33:2781–2796. doi: 10.1053/j.jvca.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 170.Trinh E, Weber C. The Dialysis Sodium Gradient: A Modifiable Risk Factor for Fluid Overload. Nephron Extra. 2017;7:10–17. doi: 10.1159/000453674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Selby NM, Burton JO, Chesterton LJ, McIntyre CW. Dialysis-induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol. 2006;1:1216–1225. doi: 10.2215/CJN.02010606. [DOI] [PubMed] [Google Scholar]
- 172.Schneditz D. Temperature and thermal balance in hemodialysis. Semin Dial. 2001;14:357–364. doi: 10.1046/j.1525-139x.2001.00088.x. [DOI] [PubMed] [Google Scholar]
- 173.Maggiore Q, Pizzarelli F, Santoro A, Panzetta G, Bonforte G, Hannedouche T, Alvarez de Lara MA, Tsouras I, Loureiro A, Ponce P, Sulkovà S, Van Roost G, Brink H, Kwan JT Study Group of Thermal Balance and Vascular Stability. The effects of control of thermal balance on vascular stability in hemodialysis patients: results of the European randomized clinical trial. Am J Kidney Dis. 2002;40:280–290. doi: 10.1053/ajkd.2002.34506. [DOI] [PubMed] [Google Scholar]
- 174.Brunelli SM, Spiegel DM, Du Mond C, Oestreicher N, Winkelmayer WC, Kovesdy CP. Serum-to-dialysate potassium gradient and its association with short-term outcomes in hemodialysis patients. Nephrol Dial Transplant. 2018;33:1207–1214. doi: 10.1093/ndt/gfx241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hecking M, Karaboyas A, Saran R, Sen A, Inaba M, Rayner H, Hörl WH, Pisoni RL, Robinson BM, Sunder-Plassmann G, Port FK. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clin J Am Soc Nephrol. 2012;7:92–100. doi: 10.2215/CJN.05440611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Basile C, Rossi L, Lomonte C. Dialysate bicarbonate concentration: Too much of a good thing? Semin Dial. 2018;31:576–582. doi: 10.1111/sdi.12716. [DOI] [PubMed] [Google Scholar]
- 177.Nitta K, Masakane I, Hanafusa N, Taniguchi M, Hasegawa T, Nakai S, Goto S, Wada A, Hamano T, Hoshino J, Joki N, Abe M, Yamamoto K Hidetomo Nakamoto on behalf of Japanese Society for Dialysis Therapy Renal Data Registry Committee. Annual dialysis data report 2017, JSDT Renal Data Registry. Ren Replace Ther. 2019;5:53. [Google Scholar]
- 178.Depner TA. Uremic toxicity: urea and beyond. Semin Dial. 2001;14:246–251. doi: 10.1046/j.1525-139x.2001.00072.x. [DOI] [PubMed] [Google Scholar]
- 179.Meyer TW, Hostetter TH. Approaches to uremia. J Am Soc Nephrol. 2014;25:2151–2158. doi: 10.1681/ASN.2013121264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Maduell F, Arias M, Durán CE, Vera M, Fontseré N, Azqueta M, Rico N, Pérez N, Sentis A, Elena M, Rodriguez N, Arcal C, Bergadá E, Cases A, Bedini JL, Campistol JM. Nocturnal, every-other-day, online haemodiafiltration: an effective therapeutic alternative. Nephrol Dial Transplant. 2012;27:1619–1631. doi: 10.1093/ndt/gfr491. [DOI] [PubMed] [Google Scholar]
- 181.Maduell F, Ojeda R, Arias-Guillen M, Rossi F, Fontseré N, Vera M, Rico N, Gonzalez LN, Piñeiro G, Jiménez-Hernández M, Rodas L, Bedini JL. Eight-Year Experience with Nocturnal, Every-Other-Day, Online Haemodiafiltration. Nephron. 2016;133:98–110. doi: 10.1159/000446970. [DOI] [PubMed] [Google Scholar]
- 182.Ağbaş A, Canpolat N, Çalışkan S, Yılmaz A, Ekmekçi H, Mayes M, Aitkenhead H, Schaefer F, Sever L, Shroff R. Hemodiafiltration is associated with reduced inflammation, oxidative stress and improved endothelial risk profile compared to high-flux hemodialysis in children. PLoS One. 2018;13:e0198320. doi: 10.1371/journal.pone.0198320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shroff R, Smith C, Ranchin B, Bayazit AK, Stefanidis CJ, Askiti V, Azukaitis K, Canpolat N, Ağbaş A, Aitkenhead H, Anarat A, Aoun B, Aofolaju D, Bakkaloglu SA, Bhowruth D, Borzych-Dużałka D, Bulut IK, Büscher R, Deanfield J, Dempster C, Duzova A, Habbig S, Hayes W, Hegde S, Krid S, Licht C, Litwin M, Mayes M, Mir S, Nemec R, Obrycki L, Paglialonga F, Picca S, Samaille C, Shenoy M, Sinha MD, Spasojevic B, Stronach L, Vidal E, Vondrák K, Yilmaz A, Zaloszyc A, Fischbach M, Schmitt CP, Schaefer F. Effects of Hemodiafiltration versus Conventional Hemodialysis in Children with ESKD: The HDF, Heart and Height Study. J Am Soc Nephrol. 2019;30:678–691. doi: 10.1681/ASN.2018100990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fischbach M, Terzic J, Menouer S, Dheu C, Seuge L, Zalosczic A. Daily on line haemodiafiltration promotes catch-up growth in children on chronic dialysis. Nephrol Dial Transplant. 2010;25:867–873. doi: 10.1093/ndt/gfp565. [DOI] [PubMed] [Google Scholar]
- 185.Li T, Wilcox CS, Lipkowitz MS, Gordon-Cappitelli J, Dragoi S. Rationale and Strategies for Preserving Residual Kidney Function in Dialysis Patients. Am J Nephrol. 2019;50:411–421. doi: 10.1159/000503805. [DOI] [PubMed] [Google Scholar]
- 186.Wang AY. Preserving Residual Kidney Function in Hemodialysis Patients-Back in the Spotlight. J Am Soc Nephrol. 2016;27:3504–3507. doi: 10.1681/ASN.2016060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Obi Y, Rhee CM, Mathew AT, Shah G, Streja E, Brunelli SM, Kovesdy CP, Mehrotra R, Kalantar-Zadeh K. Residual Kidney Function Decline and Mortality in Incident Hemodialysis Patients. J Am Soc Nephrol. 2016;27:3758–3768. doi: 10.1681/ASN.2015101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Krediet RT. Preservation of Residual Kidney Function and Urine Volume in Patients on Dialysis. Clin J Am Soc Nephrol. 2017;12:377–379. doi: 10.2215/CJN.00330117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Snauwaert E, Holvoet E, Van Biesen W, Raes A, Glorieux G, Vande Walle J, Roels S, Vanholder R, Askiti V, Azukaitis K, Bayazit A, Canpolat N, Fischbach M, Godefroid N, Krid S, Litwin M, Obrycki L, Paglialonga F, Ranchin B, Samaille C, Schaefer F, Schmitt CP, Spasojevic B, Stefanidis CJ, Van Dyck M, Van Hoeck K, Collard L, Eloot S, Shroff R. Uremic Toxin Concentrations are Related to Residual Kidney Function in the Pediatric Hemodialysis Population. Toxins (Basel) 2019;11 doi: 10.3390/toxins11040235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Marants R, Qirjazi E, Grant CJ, Lee TY, McIntyre CW. Renal Perfusion during Hemodialysis: Intradialytic Blood Flow Decline and Effects of Dialysate Cooling. J Am Soc Nephrol. 2019;30:1086–1095. doi: 10.1681/ASN.2018121194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Graboski AL, Redinbo MR. Gut-Derived Protein-Bound Uremic Toxins. Toxins (Basel) 2020;12 doi: 10.3390/toxins12090590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yamamoto S, Kazama JJ, Omori K, Matsuo K, Takahashi Y, Kawamura K, Matsuto T, Watanabe H, Maruyama T, Narita I. Continuous Reduction of Protein-Bound Uraemic Toxins with Improved Oxidative Stress by Using the Oral Charcoal Adsorbent AST-120 in Haemodialysis Patients. Sci Rep. 2015;5:14381. doi: 10.1038/srep14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mafra D, Borges NA, Lindholm B, Shiels PG, Evenepoel P, Stenvinkel P. Food as medicine: targeting the uraemic phenotype in chronic kidney disease. Nat Rev Nephrol. 2021;17:153–171. doi: 10.1038/s41581-020-00345-8. [DOI] [PubMed] [Google Scholar]
- 194.Schulman G, Berl T, Beck GJ, Remuzzi G, Ritz E, Arita K, Kato A, Shimizu M. Randomized Placebo-Controlled EPPIC Trials of AST-120 in CKD. J Am Soc Nephrol. 2015;26:1732–1746. doi: 10.1681/ASN.2014010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kjellstrand CM, Buoncristiani U, Ting G, Traeger J, Piccoli GB, Sibai-Galland R, Young BA, Blagg CR. Short daily haemodialysis: survival in 415 patients treated for 1006 patient-years. Nephrol Dial Transplant. 2008;23:3283–3289. doi: 10.1093/ndt/gfn210. [DOI] [PubMed] [Google Scholar]
- 196.Laville M, Fouque D. Nutritional aspects in hemodialysis. Kidney Int Suppl. 2000;76:S133–S139. doi: 10.1046/j.1523-1755.2000.07617.x. [DOI] [PubMed] [Google Scholar]
- 197.Peters SA, Bots ML, Canaud B, Davenport A, Grooteman MP, Kircelli F, Locatelli F, Maduell F, Morena M, Nubé MJ, Ok E, Torres F, Woodward M, Blankestijn PJ HDF Pooling Project Investigators. Haemodiafiltration and mortality in end-stage kidney disease patients: a pooled individual participant data analysis from four randomized controlled trials. Nephrol Dial Transplant. 2016;31:978–984. doi: 10.1093/ndt/gfv349. [DOI] [PubMed] [Google Scholar]
- 198.Davenport A, Peters SA, Bots ML, Canaud B, Grooteman MP, Asci G, Locatelli F, Maduell F, Morena M, Nubé MJ, Ok E, Torres F, Woodward M, Blankestijn PJ HDF Pooling Project Investigators. Higher convection volume exchange with online hemodiafiltration is associated with survival advantage for dialysis patients: the effect of adjustment for body size. Kidney Int. 2016;89:193–199. doi: 10.1038/ki.2015.264. [DOI] [PubMed] [Google Scholar]
- 199.Wong J, Vilar E, Davenport A, Farrington K. Incremental haemodialysis. Nephrol Dial Transplant. 2015;30:1639–1648. doi: 10.1093/ndt/gfv231. [DOI] [PubMed] [Google Scholar]
- 200.Garofalo C, Borrelli S, De Stefano T, Provenzano M, Andreucci M, Cabiddu G, La Milia V, Vizzardi V, Sandrini M, Cancarini G, Cupisti A, Bellizzi V, Russo R, Chiodini P, Minutolo R, Conte G, De Nicola L. Incremental dialysis in ESRD: systematic review and meta-analysis. J Nephrol. 2019;32:823–836. doi: 10.1007/s40620-018-00577-9. [DOI] [PubMed] [Google Scholar]
- 201.Susantitaphong P, Koulouridis I, Balk EM, Madias NE, Jaber BL. Effect of frequent or extended hemodialysis on cardiovascular parameters: a meta-analysis. Am J Kidney Dis. 2012;59:689–699. doi: 10.1053/j.ajkd.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A, Mahallati H, Hemmelgarn BR, Manns BJ. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298:1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 203.Koh TJK. Nocturnal hemodialysis: improved quality of life and patient outcomes. Int J Nephrol Renovasc Dis. 2019;12:59–68. doi: 10.2147/IJNRD.S165919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.FHN Trial Group. Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wilk AS, Lea JP. How Extended Hemodialysis Treatment Time Can Affect Patient Quality of Life. Clin J Am Soc Nephrol. 2019;14:1687–1689. doi: 10.2215/CJN.12241019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Trinh E, Chan CT. The Rise, Fall, and Resurgence of Home Hemodialysis. Semin Dial. 2017;30:174–180. doi: 10.1111/sdi.12572. [DOI] [PubMed] [Google Scholar]
- 207.Doenyas-Barak K, de Abreu MHFG, Borges LE, Tavares Filho HA, Yunlin F, Yurong Z, Levin NW, Kaufman AM, Efrati S, Pereg D, Litovchik I, Fuchs S, Minha S. Non-invasive hemodynamic profiling of patients undergoing hemodialysis - a multicenter observational cohort study. BMC Nephrol. 2019;20:347. doi: 10.1186/s12882-019-1542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Feng Y, Zou Y, Zheng Y, Levin NW, Wang L. The value of non-invasive measurement of cardiac output and total peripheral resistance to categorize significant changes of intradialytic blood pressure: a prospective study. BMC Nephrol. 2018;19:310. doi: 10.1186/s12882-018-1087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kolb J, Kitzler TM, Tauber T, Morris N, Skrabal F, Kotanko P. Proto-dialytic cardiac function relates to intra-dialytic morbid events. Nephrol Dial Transplant. 2011;26:1645–1651. doi: 10.1093/ndt/gfq599. [DOI] [PubMed] [Google Scholar]
- 210.Ekinci C, Karabork M, Siriopol D, Dincer N, Covic A, Kanbay M. Effects of Volume Overload and Current Techniques for the Assessment of Fluid Status in Patients with Renal Disease. Blood Purif. 2018;46:34–47. doi: 10.1159/000487702. [DOI] [PubMed] [Google Scholar]
- 211.Torino C, Gargani L, Sicari R, Letachowicz K, Ekart R, Fliser D, Covic A, Siamopoulos K, Stavroulopoulos A, Massy ZA, Fiaccadori E, Caiazza A, Bachelet T, Slotki I, Martinez-Castelao A, Coudert-Krier MJ, Rossignol P, Gueler F, Hannedouche T, Panichi V, Wiecek A, Pontoriero G, Sarafidis P, Klinger M, Hojs R, Seiler-Mussler S, Lizzi F, Siriopol D, Balafa O, Shavit L, Tripepi R, Mallamaci F, Tripepi G, Picano E, London GM, Zoccali C. The Agreement between Auscultation and Lung Ultrasound in Hemodialysis Patients: The LUST Study. Clin J Am Soc Nephrol. 2016;11:2005–2011. doi: 10.2215/CJN.03890416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Loutradis C, Papadopoulos CE, Sachpekidis V, Ekart R, Krunic B, Karpetas A, Bikos A, Tsouchnikas I, Mitsopoulos E, Papagianni A, Zoccali C, Sarafidis P. Lung Ultrasound-Guided Dry Weight Assessment and Echocardiographic Measures in Hypertensive Hemodialysis Patients: A Randomized Controlled Study. Am J Kidney Dis. 2020;75:11–20. doi: 10.1053/j.ajkd.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 213.Torino C, Tripepi R, Loutradis C, Sarafidis P, Tripepi G, Mallamaci F, Zoccali C. Can the assessment of ultrasound lung water in haemodialysis patients be simplified? Nephrol Dial Transplant. 2021;36:2321–2326. doi: 10.1093/ndt/gfaa285. [DOI] [PubMed] [Google Scholar]
- 214.Stewart J, Stewart P, Walker T, Horner DV, Lucas B, White K, Muggleton A, Morris M, Selby NM, Taal MW. A Feasibility Study of Non-Invasive Continuous Estimation of Brachial Pressure Derived From Arterial and Venous Lines During Dialysis. IEEE J Transl Eng Health Med. 2021;9:2700209. doi: 10.1109/JTEHM.2020.3035988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Stewart J, Stewart P, Walker T, Viramontes-Hörner D, Lucas B, White K, Taal MW, Selby NM, Morris M. An iterative run-to-run learning model to derive continuous brachial pressure estimates from arterial and venous lines during dialysis treatment. Biomed Signal Proces. 2021;65:102346. [Google Scholar]
- 216.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 217.Kooman JP, Wieringa FP, Han M, Chaudhuri S, van der Sande FM, Usvyat LA, Kotanko P. Wearable health devices and personal area networks: can they improve outcomes in haemodialysis patients? Nephrol Dial Transplant. 2020;35:ii43–ii50. doi: 10.1093/ndt/gfaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Villarroel M, Jorge J, Meredith D, Sutherland S, Pugh C, Tarassenko L. Non-contact vital-sign monitoring of patients undergoing haemodialysis treatment. Sci Rep. 2020;10:18529. doi: 10.1038/s41598-020-75152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Floege J, Gillespie IA, Kronenberg F, Anker SD, Gioni I, Richards S, Pisoni RL, Robinson BM, Marcelli D, Froissart M, Eckardt KU. Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int. 2015;87:996–1008. doi: 10.1038/ki.2014.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Couchoud CG, Beuscart JB, Aldigier JC, Brunet PJ, Moranne OP REIN registry. Development of a risk stratification algorithm to improve patient-centered care and decision making for incident elderly patients with end-stage renal disease. Kidney Int. 2015;88:1178–1186. doi: 10.1038/ki.2015.245. [DOI] [PubMed] [Google Scholar]
- 221.Kuhlmann U, Maierhofer A, Canaud B, Hoyer J, Gross M. Zero Diffusive Sodium Balance in Hemodialysis Provided by an Algorithm-Based Electrolyte Balancing Controller: A Proof of Principle Clinical Study. Artif Organs. 2019;43:150–158. doi: 10.1111/aor.13328. [DOI] [PubMed] [Google Scholar]
- 222.Canaud B, Kooman J, Selby NM, Taal M, Francis S, Kopperschmidt P, Maierhofer A, Kotanko P, Titze J. Sodium and water handling during hemodialysis: new pathophysiologic insights and management approaches for improving outcomes in end-stage kidney disease. Kidney Int. 2019;95:296–309. doi: 10.1016/j.kint.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 223.Bucalo ML, Barbieri C, Roca S, Ion Titapiccolo J, Ros Romero MS, Ramos R, Albaladejo M, Manzano D, Mari F, Molina M. The anaemia control model: Does it help nephrologists in therapeutic decision-making in the management of anaemia? Nefrologia (Engl Ed) 2018;38:491–502. doi: 10.1016/j.nefro.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 224.Barbieri C, Cattinelli I, Neri L, Mari F, Ramos R, Brancaccio D, Canaud B, Stuard S. Development of an Artificial Intelligence Model to Guide the Management of Blood Pressure, Fluid Volume, and Dialysis Dose in End-Stage Kidney Disease Patients: Proof of Concept and First Clinical Assessment. Kidney Dis (Basel) 2019;5:28–33. doi: 10.1159/000493479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Canaud B, Collins A, Maddux F. The renal replacement therapy landscape in 2030: reducing the global cardiovascular burden in dialysis patients. Nephrol Dial Transplant. 2020;35:ii51–ii57. doi: 10.1093/ndt/gfaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]