Abstract
The increasing incidence of life-threatening fungal infections has driven the search for new, broad-spectrum fungicidal agents that can be used for treatment and prophylaxis in immunocompromised patients. Natural-product inhibitors of cell wall (1,3)-β-d-glucan synthase such as lipopeptide pneumocandins and echinocandins as well as the glycolipid papulacandins have been evaluated as potential therapeutics for the last two decades. As a result, MK-0991 (caspofungin acetate; Cancidas), a semisynthetic analogue of pneumocandin Bo, is being developed as a broad-spectrum parenteral agent for the treatment of aspergillosis and candidiasis. This and other lipopeptide antifungal agents have limited oral bioavailability. Thus, we have sought new chemical structures with the mode of action of lipopeptide antifungal agents but with the potential for oral absorption. Results of natural-product screening by a series of newly developed methods has led to the identification of four acidic terpenoid (1,3)-β-d-glucan synthase inhibitors. Of the four compounds, the in vitro antifungal activity of one, enfumafungin, is comparable to that of L-733560, a close analogue of MK-0991. Like the lipopeptides, enfumafungin specifically inhibits glucan synthesis in whole cells and in (1,3)-β-d-glucan synthase assays, alters the morphologies of yeasts and molds, and produces a unique response in Saccharomyces cerevisiae strains with point mutations in FKS1, the gene which encodes the large subunit of glucan synthase.
During the last two decades, antifungal therapy for serious disseminated infections has relied on the use of agents that either disrupt membrane function by binding preferentially to ergosterol or inhibit the biosynthesis of this fungal sterol. Amphotericin B, an ergosterol-binding polyene, is a potent, broad-spectrum, fungicidal agent that must be used cautiously due to dose-limiting nephrotoxicity (17). Newer lipid formulations of amphotericin B have reduced some of the side effects and increased the total dose that can be administered (22). Members of the azole class of antifungal agents, which are inhibitors of lanosterol demethylase, a late step in ergosterol biosynthesis, have improved safety profiles, encouraging their widespread use (44, 47). The expanded use of the azoles has led to the appearance of strains that have acquired resistance or that are intrinsically resistant to these agents (43). Many of the strains are cross-resistant to all azoles, emphasizing the need for additional therapies with entirely different modes of action.
Members of a new class of antifungal agents, the (1,3)-β-d-glucan synthase inhibitors, have recently shown promising activity in the clinic for the treatment of life-threatening infections due to Candida and will be evaluated for their activities for the treatment of aspergillosis (A. Arathoon, E. Gotuzzo, L. Noriega, J. Andrade, Y. S. Kim, C. A. Sable, and M. DeStefano, Abstr. 99th Annu. Meet. Infect. Dis. Soc. Am., 1998; C. A. Sable, A. Villanouva, E. Arathon, E. Gotuzzo, G. Tuscato, D. Uip, L. Noriega, C. Rivera, E. Rojas, V. Taylor, R. Berman, G. B. Calandra, and J. Chodakewitz, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. S-74, 1997). The members of the new group of antifungal agents are the lipopeptides MK-0991 (caspofungin acetate; Cancidas), LY303366, and FK463 and are generally known as the echinocandins and pneumocandins (9, 29, 57). They have fungicidal activity and are effective against the growing list of azole-resistant Candida strains. The agents inhibit fungal cell wall synthesis, a target unique to lower eukaryotes, and thus have excellent therapeutic ratios. As a result of the development of these antifungal agents, inhibition of fungal cell wall glucan synthesis has been validated as an effective method for the treatment of fungal infections (9, 29, 57).
Although the (1,3)-β-d-glucan synthase inhibitors provide an alternative to the ergosterol-directed antimycotic agents, they are used only for parenteral administration (29, 57; Sable et al., 37th ICAAC). Despite considerable efforts to modify the lipopeptides chemically or to formulate them to improve oral bioavailability, the level of oral absorption of the echinocandins and pneumocandins is low. Approximately 0.3 to 1% of MK-0991 is orally absorbed in mice (1), while in dogs 9% of the LY303366 dose is orally bioavailable (60; L. Zornes, R. Stafford, M. Novilla, D. Turner, C. Boylan, B. Boyll, T. Butler, Y. Lin, D. Zeckner, W. Turner, and W. L. Current, Program Abstr. 33rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 370, 1993). Thus, we have focused on identifying new (1,3)-β-d-glucan synthase inhibitors with the potential for higher levels of oral absorption compared to those of MK-0991 and LY303366.
Until now, only two chemical classes of compounds, the lipopeptides and papulacandins, have been known to inhibit (1,3)-β-d-glucan synthase. In the 1970s, the echinocandins were the first members of the lipopeptide group to be discovered, and the entire class is often referred to by this term (40, 55). The compounds are cyclic hexapeptides N-linked to a fatty acyl side chain. Later, related fungal fermentation products such as aculeacin A (35), pneumocandin Bo (21), mulundocandin (36, 37, 46), and FR901379 (23) were found. Intrinsically water-soluble and more potent derivatives of pneumocandin Bo were prepared by the addition of amino modifications on the peptide core (4). The most potent derivative, the novel bisamine derivative of pneumocandin Bo, L-733560, had exceptional potency and an expanded spectrum of activity. The compound was used in mode-of-action studies to show that the antifungal activity was due to inhibition of (1,3)-β-d-glucan synthase, an essential enzyme in fungal cell wall assembly (12, 13). The clinical candidate MK-0991 is an aza-substituted derivative of L-733560, has improved pharmacokinetic and safety properties, and has the same mode of action as L-733560 (1, 19; F. A. Bouffard, J. F. Dropinski, J. M. Balkovec, R. M. Black, M. L. Hammond, K. H. Nollstadt, and S. Dreikorn, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F27, 1996). LY303366 is a semisynthetic derivative of the echinocandin B nucleus with a terphenyl head group and a C5 tail (57), while FK463 has a modified lipid tail and a sulfate on the homotyrosine, providing water solubility (K. Maki, Y. Morishita, Y. Iguchi, E. Watabe, K. Otomo, N. Teratani, Y. Watanabe, F. Ikeda, S. Tawara, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, H. Tanaka, K. Sakane, F. Matsumoto, and S. Kuwahara, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-141, 1998).
The second family of (1,3)-β-d-glucan synthase inhibitors are the glycolipid papulacandins (56). These compounds consist of a modified disaccharide linked to two fatty acyl chains. Despite medicinal chemistry efforts to improve the efficacies of these compounds, the papulacandins have not been developed as they have limited potency in animal models (59).
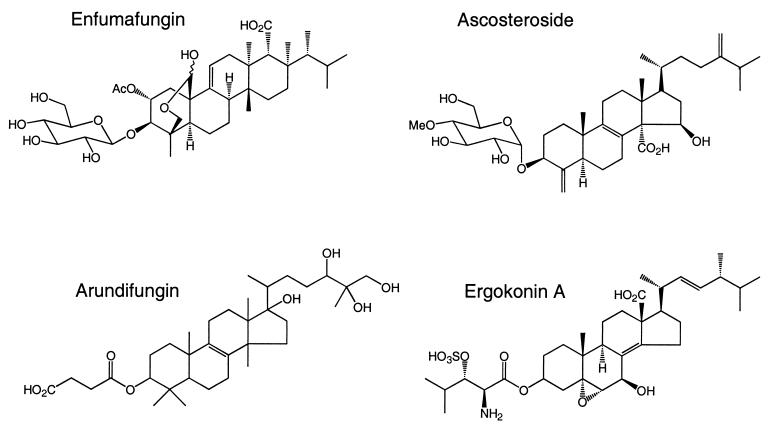
Screening for novel inhibitors to identify new chemical entities that inhibit (1,3)-β-d-glucan synthase but that offer improved pharmacokinetic properties compared to those of the lipopeptides and papulacandins has continued at many laboratories. This paper describes the discovery of a set of diverse acidic terpenoids, shown in Fig. 1, which inhibit β-(1,3)-d-glucan synthase. Two of the compounds, ascosteroside (18, 30), also known as L-767812 (8; J. R. Thompson, S. Dreikorn, J. Onishi, M. Meinz, C. Jue, J. Curotto, and M. Kurtz, Conf. Yeast Genet. Mol. Biol., 1996) and ergokonin A (26), were previously described as antifungal agents, but their modes of action were not identified. The other two compounds, arundifungin (32) and enfumafungin (F. Pelaez, submitted for publication), were recently described as novel natural-product antifungal agents. The discovery that these antifungal agents act by inhibiting (1,3)-β-d-glucan synthase raises the possibility that orally active agents with this fungal-specific mode of action may be developed.
FIG. 1.
Chemical structures of the terpenoid natural products.
MATERIALS AND METHODS
Antifungal compounds and other materials.
Ascosteroside, ergokonin A, arundifungin, enfumafungin, dihydropapulacandin, and L-733560 were purified or prepared at Merck & Co., Inc. (4, 8, 31, 32, 58; Bouffard et al., 36th ICAAC). Amphotericin B and tunicamycin were from Sigma (St. Louis, Mo.), while nikkomycin Z was from Calbiochem (San Diego, Calif.). The lytic enzymes recombinant β-(1,3)-glucanase (L4276) and α-amylase(A2643) were obtained from Sigma (St. Louis, Mo.).
Culture conditions and in vitro antifungal activity.
Antifungal activity was determined with fungal strains from the Merck Culture Collection (MY, MF, and MB; strains) and from the Clinical Culture Collection (CLY strains; Merck & Co., Inc.). The Saccharomyces cerevisiae strains with mutations in FKS1 and FKS2 were constructed at Merck and were isogenic with the wild-type strain, strain W303-1a (Mat a ade2-1 can1-100 his3-11,15 leu2-2,112 trp1-1 ura3-1) is MY2141 in this study. The strains used, with only relevant genotypes specified, were MY2140 (fks1-2), YLIP-267 (fks1-4), and YLIP-325 (fks1-4, fks2::TRP1). The in vitro antifungal susceptibilities of the pathogenic yeasts and molds were determined in a broth microtiter assay by National Committee for Clinical Laboratory Standards (NCCLS) protocols M27-A (38) and M38-P (39), respectively in RPMI medium, with the following modifications used to determine the MIC for Aspergillus. Spores from 3- to 5-day-old slants were suspended and diluted to yield 1 × 103 to 5 × 103 CFU/ml in the test wells. The MICs for yeast were determined on the basis of 100% inhibition of growth, while the MICs for molds were defined as the lowest concentration that significantly reduced growth compared to the growth of the control according to the NCCLS protocol (39). The effects of the compounds against the S. cerevisiae strains were determined in a liquid medium composed of 1% yeast extract, 2% Bacto Peptone, 2% dextrose, and 100 μg of adenine per ml (YPAD). The MICs of the compounds were determined in a liquid microdilution assay in which compounds were diluted from stocks in dimethyl sulfoxide (DMSO) into water and were serially diluted with water in 20-μl aliquots. A total of 180 μl of YPAD inoculated with exponential-phase cells adjusted to 5 × 104 cells per ml was added to the inhibitor-containing wells. After 24 h of incubation at 30°C, the microtiter dishes were examined for growth. The lowest concentration of compound that prevented visible growth was defined as the MIC. All mechanism-of-action studies with Candida albicans MY1055 were conducted with the yeast grown in an enriched synthetic medium, CM (Difco yeast nitrogen base supplemented with 1% Bacto Peptone, 0.5% yeast extract, 10 μM adenine, and 0.1% glucose). The effect of adding osmotic support to the in vitro antifungal activity was determined by adding 0.8 M sorbitol to the CM medium (CMS). The activities of the compounds against exponential-phase C. albicans MY1055 cells was determined by diluting the cells in CM to 5 × 104 cells per ml. The morphological effects of the compounds were determined after treating the yeast with inhibitors at the MICs for 4 h in CM or CMS, staining the cells with neutral red, and examining the cells microscopically as described by Kolotila et al. (25). The quantitative growth-inhibitory effect on the morphology of Aspergillus, defined as a minimum effective concentration (MEC), was determined as described previously with Difco yeast nitrogen base medium (28).
Whole-cell macromolecular synthesis.
The effects of the acidic terpenoids on macromolecular synthesis were evaluated by treating the cells with increasing concentrations of inhibitors and pulse-labeling the cells with radioactive precursors of specific macromolecules. Exponential-phase C. albicans MY1055 cells in CMS at 106 CFU/ml were treated for 30 min at 30°C with increasing concentrations of inhibitors. The treated cells were pulse-labeled for an additional 30 min with 1 μCi of [3H]N-acetyl-d-1-glucosamine per ml, 0.25 μCi of [U-14C]d-glucose per ml, 1 μCi of [1-14C]sodium acetate per ml, 0.1 μCi of [8-14C]adenine per ml, and 1 μCi of 14C-labeled amino acid per ml. Radiolabeling was quenched by the addition of an equal volume of 10% trichloroacetic acid (TCA). Adenine- and amino acid-labeled acid-insoluble pellets were collected on glass-fiber mats, washed with water, and counted. Acetate-labeled pellets were washed with water and saponified overnight with 5% potassium hydroxide in methanol. The sterols were extracted with 2 volumes of petroleum ether, while the fatty acids were recovered in the petroleum ether extract after acidifying the alkaline methanol extract with 6 N HCl. The organic extracts were analyzed by thin-layer chromatography on silica gel thin-layer chromatography plates in hexane-diethyl ether-glacial acetic acid (80:20:1; vol/vol). The labeled ergosterol and fatty acids were detected by autoradiography and were identified by comparison to standards. The [14C]glucose- and [3H]glucosamine-labeled TCA-insoluble pellets were extracted as follows to N-acetylseparate the alkali-soluble glucan and the alkali-insoluble wall components which contained the β-(1,3)-glucan and the β-(1,6)-glucan linked to chitin (24). The TCA-insoluble pellet was washed with water and was suspended in 1 volume of ethanol-water-diethylether-pyridine (15:15:5:1; vol/vol), which was made basic with 50 μl of concentrated ammonium hydroxide per 100 ml of solvent. After heating for 60 min at 60°C, the pellet was recovered by centrifugation and was washed with solvent. The solvent-extracted pellet was suspended in 1 volume of 6% potassium hydroxide, and the mixture was heated for 90 min at 80°C. The alkali-insoluble and alkali-soluble extracts were separated by centrifugation. Two volumes of a copper sulfate Fehlings solution was added to the alkaline soluble extract to precipitate the mannan (20). The alkaline insoluble wall extract was washed with water and was resuspended in 50 mM Tris (pH 7.4) and treated with 10 U of recombinant β-(1,3)-glucanase per ml for 24 h at 30°C. The radioactivity in the water-insoluble material was recovered from non-enzyme-treated controls, and enzyme-treated samples were collected on glass-fiber mats and washed with water, and the radioactivity was counted.
β-(1,3)-Glucan synthase.
Glucan synthase activity was measured with microsome membranes prepared from exponential-phase C. albicans MY1055 or S. cerevisiae MY2141, MY2140, YLIP-267, or YLIP-325 grown in YPAD at 30°C and harvested at the exponential phase. Microsome membranes were recovered from cell extracts from cells broken with a mini-bead beater as described by Douglas et al. (13). The standard glucan synthase assay, in which bovine serum albumin is present in the buffer, was described by Douglas et al. (13), except that the assay volume was increased from 40 to 100 μl. The conditions for the modified glucan synthase reaction in which bovine serum albumin was eliminated from the assay buffer and Brij 35 was substituted were as follows. The assay was performed in a final volume of 100 μl containing 37.5 mM Tris (pH 7.5), 7.5% glycerol, 2 mM EDTA, 1.5 mM KF, 10 mM dithiothreitol, 20 μM GTP, 0.6 mM UDP-[3H]glucose (68,000 dpm/nmol), 0.0008% Brij 35, 1 U of α-amylase, and 3.2 μg of protein. Test samples were diluted in DMSO such that the final DMSO concentration was 5%. The reaction mixtures were incubated for 2 h at 30°C and were quenched with an equal volume of 20% TCA. The acid-insoluble material was collected by filtration and was washed with water, and the radioactivity was counted. The β-(1,3)-glucan synthase 50% inhibitory concentration (IC50) was defined as the concentration of the compound which inhibited the formation of the TCA-insoluble material by 50%.
RESULTS
Antifungal activity.
The MICs of enfumafungin, ascosteroside, ergokonin A, and arundifungin were compared to those of MK-0991 and L-733560 and are shown in Table 1. The overall antifungal spectra of the four terpenoid compounds were comparable to those of MK-0991 and L-733560, but the potencies of the four compounds varied from 0.5 to >64 μg/ml. Like the lipopeptides, the four terpenoids preferentially inhibited various pathogenic Candida and Aspergillus strains. The most potent compound, enfumafungin, had MICs similar to those of the clinical candidate MK-0991 and L-733560 for multiple Candida strains. Ascosteroside, ergokonin A, and arundifungin were generally less potent than enfumafungin against Candida. One notable exception was the exquisite sensitivity of Candida glabrata to ascosteroside. A common feature of all glucan synthase inhibitors discovered to date is their very poor activity against Cryptococcus strains. Like L-733560 and MK-0991, at less than 32 μg/ml the four acidic terpenoids did not inhibit the growth of Cryptococcus. In addition, at concentrations below 64 μg/ml the natural products did not inhibit bacterial growth (Table 1).
TABLE 1.
Susceptibilities of pathogenic fungi to MK-0991 and L-733560 compared to susceptibilities to enfumafungin, ascosteroside, ergokonin A, and arundifungin
| Strain | MIC (μg/ml)a
|
|||||
|---|---|---|---|---|---|---|
| L-743872 (MK-0991) | L-733560 | Enfumafungin | Ascosteroside | Ergokonin A | Arundifungin | |
| Candida albicans MY1055 | 0.25 | 0.25 | 0.25 | >64 | 1 | 4 |
| Candida albicans MY2301 | 0.5 | 0.25 | 0.5 | >64 | 2 | |
| Candida glabrata CLY574 | 0.5 | 0.5 | 1 | <0.03 | 4 | |
| Candida glabrata MY1381 | 0.5 | 0.5 | 1 | 0.06 | 4 | 2 |
| Candida guilliermondii CLY308 | 0.5 | 1 | 1 | >64 | >32 | |
| Candida guilliermondii CLY346 | 1 | 1 | 2 | >64 | >32 | |
| Candida krusei CLY549 | 1 | 2 | 2 | 16 | >32 | |
| Candida lusitaniae MY1396 | 0.25 | 1 | 1 | 8 | 8 | |
| Candida parapsilosis ATCC 22019 | 1 | 1 | 0.5 | 16 | 2 | |
| Candida tropicalis MY1012 | 0.25 | 0.25 | 0.5 | 16 | 32 | 16 |
| Aspergillus fumigatus MF5668b | 0.25 | 0.125 | ≤0.03 | 4.0 | 4.0 | 1 |
| Aspergillus flavus MF383 | 0.125 | 0.06 | ≤0.03 | 32.0 | 1.0 | |
| Cryptococcus neoformans MY2061 | 16 | 16 | >64 | >64 | >32 | >64 |
| Cryptococcus neoformans MY2062 | 16 | 16 | >64 | 32 | >32 | 32 |
| Staphylococcus aureus MB2865 | 64 | 32 | >64 | >64 | >32 | >64 |
The MICs for the pathogenic fungi were determined in RPMI.
MECs for A. fumigatus, determined in Difco yeast nitrogen base as outlined in the Materials and Methods, were as follows (micrograms per milliliter): L-733560, 0.025; enfumafungin, 0.03; acosteroside, <0.06; ergokonin A, 0.04; arundifungin, 1.
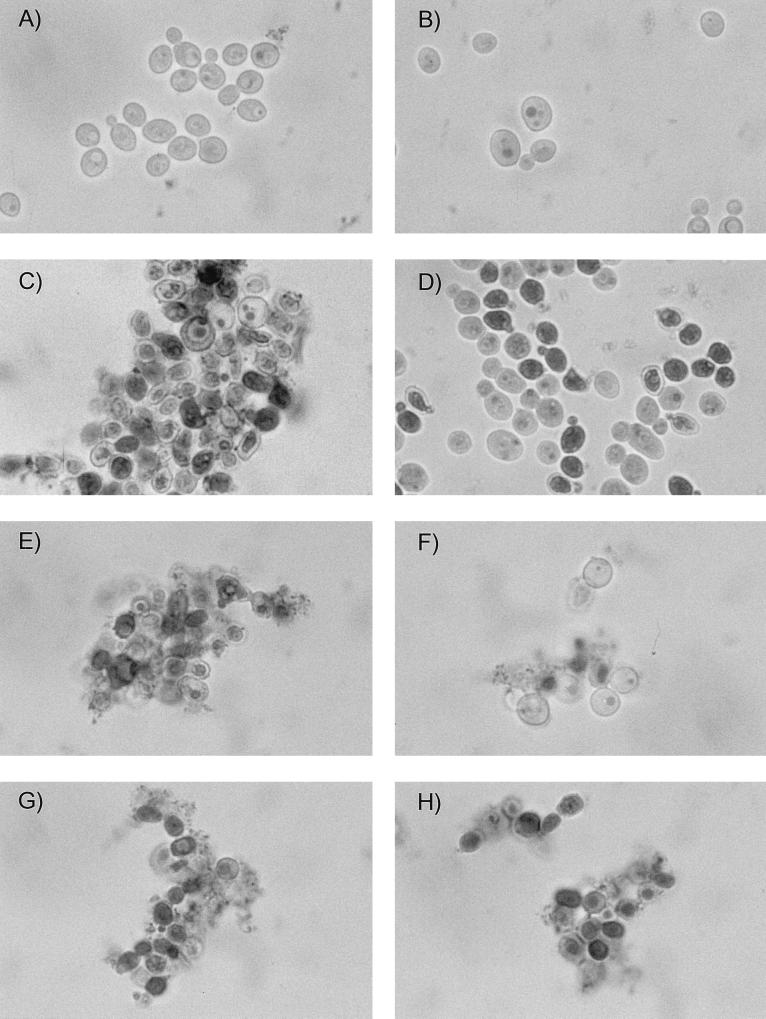
Since the known (1,3)-β-d-glucan synthase inhibitors produce hallmark changes in the morphologies of filamentous fungi and yeast, we examined the effects of the four terpenoids on Aspergillus fumigatus hyphae and on yeast cells. The control compound, L-733560, completely prevented normal polarized growth, as described previously (28). Similar stunted, highly branched hyphae were detected when A. fumigatus was treated with enfumafungin, ergokonin A, or arundifungin (A. Cabello, personal communication, 1999; Pelaez, submitted; F. Vicente, personal communication, 1999). The minimum concentration of a (1,3)-β-d-glucan synthase inhibitor needed to inhibit normal hyphal growth is called the MEC. The results in Table 1 show the activities of the acidic terpenoids as inhibitors of polarized growth in Aspergillus. The MECs (39) ranged from 0.03 to 4 μg/ml and correlated with the MIC determined by the NCCLS M38-P protocol for molds. Enfumafungin and ascosteroside were the most active in producing this effect. In addition to profoundly inhibiting hyphal growth of filamentous fungi, the MICs of L-733560, MK-0991, and the terpenoids altered the normal shape of yeast. Within 4 h of treatment with the compounds at their MICs, C. albicans formed aggregates of either rounded, swollen cells or irregularly shaped cells when they were treated in CM. The individual ovoid cells typically seen in growing yeast populations were absent (Fig. 2). The effect of the acidic terpenoid on the shape and size of Candida cells was indistinguishable from those of MK-0991 and L-733560.
FIG. 2.
Neutral red staining of C. albicans treated with L-733560 and enfumafungin in control and sorbitol-containing medium. Exponential-phase C. albicans MY1055 cells were treated for 4 h with L-733560 or enfumafungin at 0.2 μg/ml (1× the MIC). The growth medium was adjusted to pH 7.0 with 0.1 M phosphate buffer. The cells were stained with an equal volume of a 5-mg/ml solution of neutral red dissolved in water and were examined at ×160 by bright-field microscopy. Control cells in CM (A), control cells in CMS (B), L-733560-treated cells in CM (C), L-733560-treated cells in CMS (D), enfumafungin treated cells in CM (E), enfumafungin-treated cells in CMS (F), amphotericin B-treated cells in CM (G), and amphotericin B-treated cells in CMS (H) are shown.
Another hallmark effect of the β-(1,3)-glucan synthase inhibitor L-733560 is that the anti-Candida activity is fungicidal and that the activity is largely, but not completely, reversed in medium containing osmotic support (28). This is consistent with the role of glucan in maintaining the structural integrity of the cell wall and the preservation of cells by isotonic media. Micrographs of enfumafungin-treated cells are compared to micrographs of L-733560- and amphotericin B-treated cells in Fig. 2. When stained with the vital dye neutral red, greater than 90% of the inhibitor-treated yeast population was stained, indicating that the terpenoids, like L-733560 and amphotericin B, act as fungicidal agents. When yeasts were treated with L-733560 or the acidic terpenoids in medium supplemented with sorbitol as an osmotic stabilizer, a higher proportion of the cells did not stain when the vital dye was applied. The cells accumulated as swollen, round cells (Fig. 2). In contrast, the number of darkly stained cells following treatment with amphotericin B was not decreased in the sorbitol-supplemented medium. The results in Table 2 show that the MICs of the four acidic terpenoids, as well as those of L-733560, were increased at least 10-fold in sorbitol-containing medium. Ascosteroside did not completely inhibit the growth of yeast strain MY1055 but did reduce the growth yields by more than 50%. The minimum concentration required to reduce growth was 0.16 μg/ml and was antagonized in sorbitol-containing medium. In contrast, the antifungal activities of amphotericin B and digitonin, two agents that interfere with membrane permeability properties, were not altered in medium containing sorbitol.
TABLE 2.
Effect of sorbitol on susceptibility of C. albicans MY1055 to acidic terpenoids
| Compound | Antifungal activity (μg/ml)a
|
|
|---|---|---|
| Medium alone | Medium with sorbitol | |
| Amphotericin B | 0.3 | 0.3 |
| Digitonin | 2.5 | 2.5 |
| L-733560 | 0.08 | >5.0 |
| Enfumafungin | 0.04 | >5.0 |
| Ascosteroside | 0.2 | >20.0 |
| Ergokonin A | 0.6 | >20.0 |
| Arundifungin | 5.0 | 50.0 |
The MICs were determined in CM or CMS as outlined in the Materials and Methods. The results for all compounds except ascosteroside are MICs. The results for ascosteroside are not the concentration of compound required to inhibit growth by 100% (MIC) but, rather, are the lowest concentration required to reduce the growth of Candida by 50% compared to the growth of untreated cells.
Preferential inhibition of glucan synthesis in Candida cells.
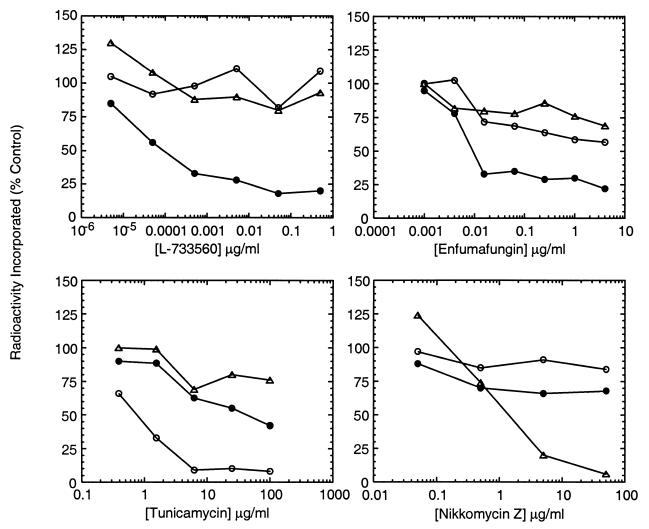
The antifungal spectrum, the change in morphology, and the antagonism of antifungal activity by sorbitol suggested that terpenoids disrupted fungal cell wall glucan assembly. The effects of the compounds on cellular cell wall synthesis were determined in C. albicans. The activities of the compounds were compared to those of L-733560, tunicamycin, and nikkomycin Z, antifungal agents which inhibit β-(1,3)-glucan, mannan, and chitin synthesis, respectively (6, 28, 54). The selective effects of these antifungal agents on cell wall synthesis is shown in the dose-response curves in Fig. 3. The β-(1,3)-glucan synthase inhibitor L-733560 as well as enfumafungin preferentially reduced the level of incorporation of [14C]glucose into the alkali-insoluble cell wall extract by 75%. The concentrations of L-733560 and enfumafungin that maximally inhibited the incorporation of [14C]glucose into the alkali-insoluble cell wall extract did not significantly inhibit the incorporation of [3H]N-acetylglucosamine into this wall extract or the [14C]glucose incorporation into the cell wall extract containing the mannan. In contrast, tunicamycin and nikkomycin selectively inhibited the incorporation of [14C]glucose into the mannan-containing extract and the incorporation of [3H]N-acetylglucosamine into the alkali-insoluble extract, respectively. L-733560 and enfumafungin specifically reduced the level of incorporation of [14C]glucose into the cell wall extract which, in Saccharomyces or Candida, is known to contain β-(1,3)- and β-(1,6)-glucan linked to chitin (5, 24). A selective effect on β-(1,3)-glucan assembly was demonstrated by showing that the alkali-insoluble glucan recovered from inhibitor-treated cells did not contain β-(1,3)-glucan. The proportions of β-(1,3)- and β-(1,6)-glucan in the wall extract were estimated by treating the extracts with β-(1,3)-glucanase as described by Boone et al. (3). Eighty percent of the [14C]glucose in the alkali-insoluble cell wall extract from untreated cells was solubilized by recombinant β-(1,3)-glucanase, indicating that 80% of the radioactivity was due to β-(1,3)-glucan, while the remaining 20% of the radioactivity was assumed to be due to β-(1,6)-glucan. In contrast, the radiolabeled alkali-insoluble cell wall extract recovered from cells treated with L-733560 or the acidic terpenoid at the MIC was completely resistant to recombinant β-(1,3)-glucanase (data not shown). The result indicated that the radiolabeled alkali-insoluble cell wall extract recovered from inhibitor-treated cells consisted entirely of either β-(1,6)-glucan or a form of β-(1,3)-glucan which was not sensitive to recombinant glucanase.
FIG. 3.
Selective inhibition of glucan synthesis by enfumafungin in C. albicans. The effects of L-733560, tunicamycin, nikkomycin Z, and enfumafungin on incorporation of [14C]glucose and [3H]N-acetylglucosamine into wall extracts of C. albicans were determined as described in Materials and Methods. The effects of the compounds on incorporation of radioactivity into alkaline insoluble glucan (●), mannan (○), and chitin (▵) are shown. Data are expressed as the percentage of inhibition compared to that for the control.
To determine whether the antifungal activities of the four acidic terpenoids correlated with the potencies of the compounds as inhibitors of cellular β-(1,3)-glucan assembly, the MICs of the compounds were compared to the effects of the compounds on cellular glucan, chitin, mannan, RNA, and protein synthesis. The MICs of the compounds were determined with the same cell density and medium used for macromolecular labeling, but without the sorbitol (Table 3). Although ascosteroside did not completely inhibit the growth of C. albicans MY1055, at levels as low as 0.2 μg/ml the growth yields were reduced by 50%. The concentrations of L-733560 and the acidic terpenoids that inhibited the incorporation of [14C]glucose into the alkali-insoluble cell wall extract by 50% were comparable to the MICs (Table 3). At concentrations well above those required to inhibit growth, enfumafungin, ergokonin A, and L-733560 inhibited RNA synthesis, which indicated that the compounds had secondary effects at high concentrations. Since glucan and RNA synthesis were inhibited by 50% by 6.25- to 12.5-μg/ml arundifungin, the antifungal activity of this terpenoid is not entirely due to inhibition of glucan synthesis. At the high levels needed to inhibit RNA synthesis, however, none of the compounds inhibited mannan, chitin, or protein synthesis. These labeling results are in contrast to those obtained with the surfactant digitonin, which inhibited the incorporation of all radiolabeled precursors. Thus, on the basis of these results, it was concluded that the antifungal activities of enfumafungin, ascosteroside, and ergokonin, like that of L-733560, were due to selective inhibition of β-(1,3)-glucan assembly.
TABLE 3.
Effects of acidic terpenoids on macromolecular synthesis in C. albicans MY1055a
| Compound | MIC (μg/ml) | Concn (μg/ml) required to inhibit synthesis by 50%
|
||||
|---|---|---|---|---|---|---|
| Glucan | Chitin | Mannan | RNA | Protein | ||
| L-733560 | 0.1 | 0.04 | >0.5 | >0.5 | 20.0 | >20.0 |
| Nikkomycin | 2.0 | >50.0 | 0.2 | >50.0 | >50.0 | >50.0 |
| Tunicamycin | 2.5 | >20.0 | >20.0 | 1.0 | >20.0 | >20.0 |
| Digitonin | 2.5 | 0.6 | 0.6 | 0.6 | 5.0 | 10.0 |
| Enfumafungin | 0.2 | 0.05 | >25.0 | >25.0 | 10.0 | >20.0 |
| Ascosteroside | 0.2 | 0.05 | >50.0 | >50.0 | >40.0 | >40.0 |
| Ergokonin A | 0.7 | 0.2 | >25.0 | >25.0 | 10.0 | >100.0 |
| Arundifungin | 5.0 | 6.25 | >50.0 | >50.0 | 12.5 | >100.0 |
The MICs were determined in CM at the same cell density used in the macromolecular labeling experiments. The MICs of ascosteroside and nikkomycin Z are the lowest concentrations required to reduce the growth of Candida by 50% compared to the growth of untreated cells. The effect on growth relative to that on the growth of untreated control cells was recorded visually after 4 h at 30°C. The effect on macromolecular synthesis was determined as described in Materials and Methods.
Effects of acidic terpenoids on β-(1,3)-glucan synthase of C. albicans.
The effects of the terpenoids on β-(1,3)-glucan synthase, the essential enzyme that forms β-(1,3)-glucan fibrils from UDP-glucose, were evaluated to determine whether these compounds were direct inhibitors of the synthetic enzyme. The standard assay for β-(1,3)-glucan synthase activity measures the incorporation of radioactivity from UDP-[3H]-glucose into TCA-insoluble glucan (13). When microsomes from C. albicans MY1055 were used in this assay, the IC50 of L-733560 was 2 nM, as reported previously (28) (Table 4). In this standard glucan synthase assay, ascosteroside did not inhibit (1,3)-β-d-glucan synthase, and inhibition by enfumafungin was not reproducible. Arundifungin inhibited the enzyme with an IC50 of 10 μg/ml. Since the in vitro antifungal activities of the ascosteroside and enfumafungin were reduced more than 100-fold in medium containing 50% bovine serum albumin (data not shown), we evaluated the effects of these compounds in a modified glucan synthase assay (16). Standard glucan synthase assays include albumin in the buffer to activate glucan synthase (48). Frost et al. (16) showed that glucan synthase activity could be detected in the absence of bovine serum albumin if Brij 35 was included in the reaction buffer. We confirmed that this assay measured the formation of β-(1,3)-glucan; the TCA-insoluble counts were quantitatively solubilized with recombinant glucanase, and the activity was as sensitive to L-733560 as it was in the standard glucan synthase assay conducted in the presence of albumin. In the modified β-(1,3)-glucan synthase assay, the IC50 of L-733560 was 2 nM and inhibition by the four acidic terpenoids was detected (Table 4). Although inhibition was detected, the acidic terpenoids were more than 1,000-fold less active than L-733560 as inhibitors of the Candida glucan synthase. Unlike L-733560, the terpenoid compounds enfumafungin and ascosteroside were 20- and 140-fold less active, respectively, as enzyme inhibitors than as inhibitors of cellular glucan synthesis. The activity of ergokonin A was within 15-fold of its activity as an inhibitor of cellular glucan synthesis, while arundifungin was equally active in both assays.
TABLE 4.
Effect of assay conditions on potencies of L-733560 and acidic terpenoids in glucan synthase assay of C. albicans MY1055
| Compound | Glucan synthase IC50 (μg/ml)
|
Concn (μg/ml) required to inhibit whole-cell glucan synthesis to 50% of that for the control | |
|---|---|---|---|
| Standard assay | Modified assay | ||
| L-733560 | 0.001 | 0.002 | 0.04 |
| Enfumafungin | 1–>25 | 1.0 | 0.05 |
| Ascosteroside | >25.0 | 7.0 | 0.05 |
| Ergokonin A | 3.0 | 0.2 | |
| Arundifungin | 10.0 | 6.0 | 6.25 |
Glucan synthase assays were performed as outlined in Materials and Methods.
Effects of acidic terpenoids on Saccharomyces strains with FKS1 mutations.
Since the potencies of enfumafungin and ascosteroside in the enzyme and whole-cell assays for glucan synthesis did not correlate in the C. albicans-based assays, it was not clear that inhibition of cellular glucan synthesis by the terpenoids was due to inhibition of (1,3)-β-d-glucan synthase. To further explore the effects of the four acidic terpenoids on glucan synthase, the terpenoids were tested against S. cerevisiae strains with point mutations in FKS1, the gene encoding the vegetatively expressed large subunit of glucan synthase (29). Mutations in FKS1 have been isolated and characterized in both S. cerevisiae and C. albicans (11, 14, 27; A. Mitchell, C. Douglas, J. d'Ippolito, G. J. Shei, and M. B. Kurtz, Abstr. 1995 Yeast Cell Biol. Meet., abstr. 133, 1995). Since point mutations in this gene lead to specific, high-level resistance to glucan synthase inhibitors both in whole cells and in in vitro enzyme assays (29), it was reasoned that changes in the susceptibilities of these strains to the natural-product inhibitors would suggest effects on the glucan synthase enzyme.
The results in Table 5 show that the strain with the best-characterized S. cerevisiae mutation, fks1-2 (12), previously named R560 (13), was resistant not only to L-733560 and dihydropapulacandin but also to all of the acidic terpenoids. Compared to the wild-type strain, strain MY2141, the mutant strain, strain MY2140, was 20- to 5-fold more resistant to L-733560 and dihydropapulacandin, respectively. Resistance varied with the terpenoid compounds and ranged from 4-fold greater resistance to ergokonin A to 14-fold greater resistance to enfumafungin. Furthermore, the β-(1,3)-glucan synthase from the mutant strain was at least 8-fold less sensitive to the terpenoid compounds than that from the sensitive wild-type strain MY2141 (Table 5).
TABLE 5.
Effect of fks1-2 mutation in S. cerevisiae on sensitivities of cells and glucan synthase to L-733560, papulacandin, and acidic terpenoidsa
| Compound | MY2141 (FKS1 FKS2)
|
MY2140 (fks1-2 FKS2)
|
||
|---|---|---|---|---|
| MIC (μg/ml) | IC50 (μg/ml) for GS | MIC (μg/ml) | IC50 (μg/ml) for GS | |
| L-733560 | 0.31 | 0.4 | 25.0 | 25.0 |
| Papulacandin | 5.0 | 3.0 | 25.0 | 50.0 |
| Enfumafungin | 0.16 | 3.0 | 2.5 | >50.0 |
| Ascosteroside | 10.0 | 10.0 | >100.0 | >100.0 |
| Ergokonin A | 5.0 | 4.0 | 50.0 | 30.0 |
| Arundifungin | 5.0 | 4.0 | 20.0 | >25.0 |
MICs were determined as described in Materials and Methods, and glucan synthase (GS) assays were performed using the Brij 35 conditions.
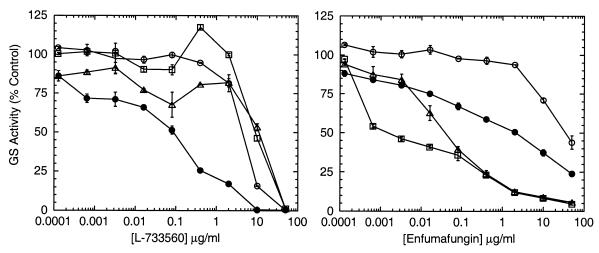
The second mutant allele, fks1-4, differs from the fks1-2 resistance allele in its resistance profile (14) and the location of the point mutation in FKS1 (C. Douglas, unpublished data). In an agar diffusion assay, fks1-4 was resistant to L-733560 and was hypersensitive to dihydropapulacandin (14). The glucan synthase from the fks1-4 mutant was resistant to L-733560 but was not hypersensitive to dihydropapulacandin. Of the four acidic terpenoids tested, enfumafungin had an effect on this strain which was different from those of both L-733560 and dihydropapulacandin. The results in Table 6 show that the fks1-4 strain was approximately 200 times more sensitive to enfumafungin than the wild type and that its glucan synthase was also hypersensitive, as shown in Fig. 4. The shallow slope in the dose-response curve of enfumafungin against the wild-type glucan synthase and the fks1-4 glucan synthase was typical of those of other types of glucan synthase inhibitors, such as L-733560 (11). The hypersensitivity to enfumafungin was independent of the presence of a wild-type copy of FKS2, which is highly homologous to FKS1 and which encodes the large subunit of glucan synthase that is expressed during sporulation but that is not expressed during vegetative growth (33). The greater sensitivity of the FKS2 protein to lipopeptide inhibitors in vitro is the only enzymatic difference between the two enzymes that has been detected thus far (33). Since the glucan synthase from double mutant fks1-4 fks2::TRP was hypersensitive, the hypersensitivity to enfumafungin was due to the alterations in the FKS1 protein produced by the fks1-4 mutation. In contrast, the cells and the glucan synthase from the fks1-4 strain were resistant to L-733560.
TABLE 6.
Susceptibilities of S. cerevisiae fks1-4 mutants to L-733560 and enfumafungin
| Strain | MIC (μg/ml)a
|
|
|---|---|---|
| L-733560 | Enfumafungin | |
| MY2141 (FKS1 FKS2) | 0.31 | 0.31 |
| YLIP267 (fks1-4 FKS2) | 5.0 | 0.02 |
| YLIP325 (fks1-4 fks2::TRP) | 5.0 | 0.02 |
MICs were determined in YPAD as described in Materials and Methods.
FIG. 4.
Effects of FKS1 mutations in S. cerevisiae on the sensitivity of glucan synthase to L-733560 and enfumafungin. Crude membranes from S. cerevisiae MY2141 (FKS1 FKS2) (●), MY2140 (fks1-2 FKS2) (○), YLIP-267 (fks1-4 FKS2) (▵), and YLIP-327 (fks1-4 fks2::TRP) (□) were prepared and assayed for glucan synthase (GS) activity by the Brij 35 assay as described in Materials and Methods. The control activity was determined for each glucan synthase preparation in the absence of compounds to calculate the percentage of activity compared to that for the control.
DISCUSSION
Multiple lines of evidence indicate that the acidic terpenoids enfumafungin, ascosteroside, arundifungin, and ergokonin A are new natural-product antifungal agents which inhibit the synthesis of β-(1,3)-glucan. First, cellular glucan synthesis is rapidly inhibited by 50% following treatment of cells with L-733560 or the acidic terpenoids at their MICs. Two of the acidic terpenoids selectively inhibit fungal cell wall glucan assembly at their MICs. Only at concentrations 15-fold (ergokonin A) and >40-fold (enfumafungin) higher than the MICs do these compounds inhibit RNA synthesis. L-733560, which we have previously shown to be a highly specific inhibitor of glucan synthase, begins to show nonspecific effects at 25× its MICs (28). Arundifungin was the least specific in its inhibition; RNA synthesis was inhibited at only 2× the MIC level of arundifungin. The fourth acidic terpenoid, ascosteroside, incompletely inhibited the growth of the test strain but did inhibit glucan assembly at concentrations which reduced growth. Although this compound inhibits cellular glucan synthesis by at least 50%, it is a weaker antifungal agent than enfumafungin, ergokonin, and arundifungin against C. albicans MY1055. Incomplete inhibition of growth, despite initial inhibition of glucan synthesis by ascosteroside, may be related to issues of either compound metabolism or compound efflux. Either one of these mechanisms could reduce the effective concentration of the compound at the target site and reduce the antifungal activity. The results of the macromolecular labeling studies show that the acidic terpenoids are not general metabolic inhibitors, which is in agreement with the results of Gorman et al. (18) who reported that ascosteroside does not inhibit RNA or protein synthesis. They also reported that ascosteroside does not inhibit fungal ergosterol synthesis. Enfumafungin does not inhibit [14C]acetate incorporation into ergosterol, indicating that this terpenoid glycoside does not inhibit sterol biosynthesis either (data not shown).
The second line of evidence that the acidic terpenoids inhibit cell wall glucan synthesis like the β-(1,3)-glucan synthase inhibitor L-733560 does is that all compounds alter the morphologies of filamentous fungi and yeast, which is consistent with a cell wall defect. Aspergillus cultures treated with the acidic terpenoids produced stunted hyphae with bulbous tips, suggestive of a weakened cell wall that expanded under high internal pressure. Similarly, Candida treated with these compounds cannot maintain a normal shape and lose viability. Like L-733560, the antifungal activities of the acidic terpenoids are reduced in sorbitol-supplemented medium and the treated cells grow as large, round cells.
The antifungal spectra of the acidic terpenoids are comparable to those of the known glucan synthase inhibitors L-733560 and papulacandins. Candida and Aspergillus are exquisitely sensitive, while Cryptococcus and bacterial strains are not inhibited. Since genetic and biochemical evidence indicates that C. neoformans has glucan synthase and that the unique FKS1 gene of this organism is essential, it is interesting that all the natural products discovered thus far are poor inhibitors of Cryptococcus growth (53). Recent data suggest that there are significant differences in the biochemical properties of the Cryptococcus enzyme compared to those of the C. albicans and S. cerevisiae enzymes (J. Williamson, personal communication, 1999). These differences may account for the lack of whole-cell activity and suggest that it may be necessary to search for Cryptococcus-specific inhibitors of glucan synthase.
Finally, the differential sensitivities of the S. cerevisiae strains with point mutations in FKS1 support the conclusion that the terpenoids are specific inhibitors of glucan synthase (Table 5). The fact that the fks1-2-containing strain and its enzyme are resistant to L-733560 and to all the terpenoids is consistent with direct inhibition of glucan synthase enzyme activity. This effect is not unique to this high-level-resistant mutant; the L-733560-resistant mutants of C. albicans described previously (27) are also resistant to enfumafungin (data not shown). However, the supersensitivity of the fks1-4-containing strain and its enzyme to one of the terpenoids, enfumafungin, and resistance to L-733560 demonstrate that the interaction of these two inhibitors with the enzyme are likely to differ.
Since the acidic terpenoids, particularly enfumafungin, represent a new group of (1,3)-β-d-glucan synthase inhibitors, studies continue to gain an understanding of how these compounds inhibit FKS1, the integral membrane component of β-(1,3)-glucan synthase. Theoretically, the IC50 of an inhibitor with a single mode of action for the target enzyme should be lower or the same as the concentration needed to inhibit growth. This criterion is met for enfumafungin, ergokonin A, and ascosteroside if measurement of the concentration needed to inhibit whole-cell glucan synthesis is used as the enzyme assay of glucan synthesis. It is also met for L-733560 for both the in vitro enzyme assay and the whole-cell glucan synthesis assay. At present, the IC50s obtained by the glucan synthase assay are higher than those that would be predicted from the whole-cell labeling data or the MICs of the terpenoids. The potency of the most active terpenoid glycoside, enfumafungin, as a glucan synthase inhibitor in the Candida reaction is not affected by the concentration of the substrate, UDP-glucose, showing that this compound does not simply interfere with the reaction by competing with the substrate (data not shown). Considering the potential contribution of protein binding, assay conditions, or compound metabolism on the inhibition of the glucan synthase enzyme, further studies are needed to elucidate the mechanism by which compounds such as enfumafungin inhibit wild-type and mutant glucan synthases.
Terpenoid compounds are abundant in nature and may have diverse biological activities due to specific interactions with proteins (42, 45). For example, the cardenolide glycosides oubain and digitalis inhibit NaK-ATPase, while another type of triterpenoid glycoside affects calcium-dependent potassium channels (34, 49). Other types of terpenoids inhibit DNA synthesis or bacterial protein synthesis ((52). Finally, bile acids and their conjugates were recently shown to bind to specific membrane receptors to affect transcription of the genes involved in cholesterol homeostasis (41). Thus far, terpenoid agents have not been described as specific inhibitors of fungal cell wall assembly, although brassinolides may ultimately be shown to affect plant development through effects on plant cell wall formation (2). In addition to specific interactions with proteins, some terpenoids have surfactant activity and affect protein function by altering the lipid-protein environment. Agents with this mode of action are typically identified through their ability to affect the activities of multiple membrane bound proteins or permeabilize cells (7, 50). On the basis of the evidence presented in this report, we conclude that the acidic terpenoids enfumafungin, ascosteroside, and ergokonin A lack surfactant activity and selectively inhibit glucan synthase.
Thus, ascosteroside, ergokonin A, arundifungin, and enfumafungin represent the first nonlipopeptide and papulacandin-type (1,3)-β-d-glucan synthase inhibitors described in over 20 years. These discoveries resulted from new methodologies, including the isolation of genetically defined strains with unique responses to glucan synthase inhibitors, the development of a simple extraction procedure for the quantitative recovery of fungal cell wall extracts, and modifications to the in vitro glucan synthase assays as well as natural-product screening. With the discovery of the acidic terpenoid natural-product fungal metabolites enfumafungin, ascosteroside, and ergokonin A, an opportunity to develop a new group of antifungal agents which inhibit (1,3)-β-d-glucan synthase may be realized. In addition to the in vitro antifungal activity, two of the compounds, ascosteroside and enfumafungin, have detectable activity against C. albicans in animal models (18; Pelaez, submitted). It is interesting that the oral absorption properties of some terpenoid glycosides have been characterized by others (10, 51). One of these agents, digoxin, is therapeutically effective through an oral route of administration. Characterization of the oral absorption properties of the unique acidic terpenoid glycosides will help in assessments of their potential for development as orally absorbed β-(1,3)-glucan synthase inhibitor antifungal agents.
REFERENCES
- 1.Abruzzo G K, Flattery A M, Gill C J, Kong L, Smith J G, Pikounis V B, Balkovec J M, Bouffard A F, Dropinski J F, Rosen H, Kropp H, Bartizal K. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1997;41:2333–2338. doi: 10.1128/aac.41.11.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altmann T. A tale of dwarfs and drugs: brassinosteroids to the rescue. Trends Genet. 1998;14:490–495. doi: 10.1016/s0168-9525(98)01598-4. [DOI] [PubMed] [Google Scholar]
- 3.Boone C, Sommer S S, Hensel A, Bussey H. Yeast KRE genes provide evidence for a pathway of cell wall β-glucan assembly. J Cell Biol. 1990;110:1833–1843. doi: 10.1083/jcb.110.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouffard F A, Zambias R A, Dropinski J F, Balkovec J M, Hammond M L, Abruzzo G K, Bartizal K F, Marrinan J A, Kurtz M B, McFadden D C, Nollstadt K H, Powles M A, Schmatz D M. Synthesis and antifungal activity of novel cationic pneumocandin Bo derivatives. J Med Chem. 1994;37:222–225. doi: 10.1021/jm00028a003. [DOI] [PubMed] [Google Scholar]
- 5.Brown J A, Catley B J. Monitoring polysaccharide synthesis in Candida albicans. Carbohydr Res. 1992;227:195–202. [Google Scholar]
- 6.Cabib E. Differential inhibition of chitin synthetases 1 and 2 from Saccharomyces cerevisiae by polyoxin D and nikkomycins. Antimicrob Agents Chemother. 1991;35:170–173. doi: 10.1128/aac.35.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christian D A, Hadwiger L A. Pea saponins in the Pea-Fusarium solani interaction. Exp Mycol. 1989;13:419–427. [Google Scholar]
- 8.Clapp, W. H., G. H. Harris, G. F. Bills, J. E. Curotto, A. W. Dombrowski, S. J. Driekorn, M. B. Kurtz, M. S. Meinz, J. C. Onishi, J. D. Polishook, S. L. Streicher, J. R. Thompson, M. Williams, and D. L. Zink. 28 January 1997. U.S. patent 5,597,806.
- 9.Current W L, Tang J, Boylan C, Watson P, Zechkner D, Turner W, Rodriguez M, Dixon C, Ma D, Radding J A. Glucan biosynthesis as a target for antifungals: the echinocandin class of antifungal agents. In: Dixon G K, Copping L G, Hollomon D W, editors. The discovery and mode of action of antifungal drugs. Oxford, United Kingdom: BIOS Scientific Publishers, Ltd.; 1995. pp. 143–160. [Google Scholar]
- 10.Das G. Beta-methyl digoxin: a better absorbable digoxin. Int J Clin Pharmacol. 1989;27:521–525. [PubMed] [Google Scholar]
- 11.Douglas C M, D'Ippolito J A, Shei G J, Meinz M, Onishi J, Marrinan J A, Li W, Abruzzo G K, Flattery A, Bartizal K, Mitchell A, Kurtz M B. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob Agents Chemother. 1997;41:2471–2479. doi: 10.1128/aac.41.11.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas C M, Foor F, Marrinan J A, Morin N, Nielsen J B, Dahl A M, Mazur P, Baginsky W, Li W, El-Sherbeini M, Clemas J A, Mandala S M, Frommer B R, Kurtz M B. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-β-d-glucan synthase. Proc Natl Acad Sci USA. 1994;91:12907–12911. doi: 10.1073/pnas.91.26.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas C M, Marrinan J A, Li W, Kurtz M B. A Saccharomyces cerevisiae mutant with echinocandin resistant 1,3-β-d-glucan synthase activity. J Bacteriol. 1994;176:5686–5696. doi: 10.1128/jb.176.18.5686-5696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.el-Sherbeini M, Clemas J A. Nikkomycin Z supersensitivity of an echinocandin-resistant mutant of Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1995;39:200–207. doi: 10.1128/aac.39.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forche, E., H. Augustiniak, G. Hofle, and H. Reichenbach. 1991. Ergokonin: a new antibiotic from Trichoderma koningii. Planta Med. 2.
- 16.Frost D J, Brandt K, Capobianco J, Goldman R. Characterization of (1,3)-β-glucan synthase in Candida albicans—microsomal assay from the yeast or mycelial morphological forms and a permeabilized whole-cell assay. Microbiology. 1994;140:2239–2246. doi: 10.1099/13500872-140-9-2239. [DOI] [PubMed] [Google Scholar]
- 17.Gallis H A, Drew R H, Pickard W W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 18.Gorman J A, Chang L P, Clark J, Gustavson D R, Lam K S, Mamber S W, Pirnik D, Ricca C, Fernandes P B, O'Sullivan J. Ascosteroside, a new antifungal agent from Ascotricha amphitricha. I. Taxonomy, fermentation and biological activities. J Antibiot (Tokyo) 1996;49:547–552. doi: 10.7164/antibiotics.49.547. [DOI] [PubMed] [Google Scholar]
- 19.Hajdu R, Thompson R, Sundelof J G, Pelak B A, Bouffard F A, Dropinski J F, Kropp H M. Preliminary animal pharmacokinetics of the parenteral antifungal agent MK-0991 (L-743872) Antimicrob Agents Chemother. 1997;41:2339–2344. doi: 10.1128/aac.41.11.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanson B A, Lester R L. Effects of inositol starvation on phospholipid and glycan synthesis in Saccharomyces cerevisiae. J Bacteriol. 1980;1980:79–89. doi: 10.1128/jb.142.1.79-89.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hensens O D, Liesch J M, Zink D L, Smith J L, Wichmann C F, Schwartz R E. Pneumocandins from Zalerion arboricola. III. Structure elucidation. J Antibiot. 1992;45:1875–1885. doi: 10.7164/antibiotics.45.1875. [DOI] [PubMed] [Google Scholar]
- 22.Hiemenz J W, Walsh T J. Lipid formulations of amphotericin B. J Liposome Res. 1998;8:443–467. [Google Scholar]
- 23.Iwamoto T, Fujie A, Sakamoto K, Tsurumi Y, Shigematsu N, Yamashita M, Hashimoto S, Okuhara M, Kohsaka M. WF11899A, B and C, novel antifungal lipopeptides. I. Taxonomy, fermentation, isolation and physico-chemical properties. J Antibiot (Tokyo) 1994;47:1084–91. doi: 10.7164/antibiotics.47.1084. [DOI] [PubMed] [Google Scholar]
- 24.Kollar R, Reinhold B B, Petrakova E, Yeh H J C, Ashwell G, Drgonova J, Kapteyn J C, Klis F M, Cabib E. Architecture of the yeast cell wall. β(1,6)-Glucan interconnects mannprotein, β(1,3)-glucan and chitin. J Biol Chem. 1997;272:17762–17775. doi: 10.1074/jbc.272.28.17762. [DOI] [PubMed] [Google Scholar]
- 25.Kolotila M P, Smith C W, Rogers A L. Candidacidal activity of macrophages from three mouse strains as demonstrated by a new method: neutral red staining. J Med Vet Mycol. 1987;25:283–290. doi: 10.1080/02681218780000321. [DOI] [PubMed] [Google Scholar]
- 26.Kumeda Y, Asao T, Iida A, Wada S, Futami S, Fujita T. Effects of ergokonin a produced by Trichoderma viride on the growth and morphological development of fungi. J Antibacteriol Antifungal Agents Jpn. 1994;22:663–670. [Google Scholar]
- 27.Kurtz M B, Abruzzo G, Flattery A, Bartizal K, Marrinan J A, Li W, Milligan J, Nollstadt K, Douglas C M. Characterization of echinocandin-resistant mutants of Candida albicans: genetic, biochemical, and virulence studies. Infect Immun. 1996;64:3244–3251. doi: 10.1128/iai.64.8.3244-3251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtz M B, Douglas C, Marrinan J, Nollstadt K, Onishi J, Dreikorn S, Milligan J, Mandala S, Thompson J, Balkovec J M, Bouffard F A, Dropinski J F, Hammond M L, Zambias R A, Abruzzo G, Bartizal K, Mcmanus O B, Garcia M L. Increased antifungal activity of L-733,560, a water-soluble, semisynthetic pneumocandin, is due to enhanced inhibition of cell-wall synthesis. Antimicrob Agents Chemother. 1994;38:2750–2757. doi: 10.1128/aac.38.12.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurtz M B, Douglas C M. Lipopeptide inhibitors of fungal glucan synthase. J Med Vet Mycol. 1997;35:79–86. doi: 10.1080/02681219780000961. [DOI] [PubMed] [Google Scholar]
- 30.Leet J E, Huang S, Klohr S E, McBrien K D. Ascosteroside, a new antifungal agent from Ascotricha amphitricha. II. Isolation and structure elucidation. J Antibiot (Tokyo) 1996;49:553–559. doi: 10.7164/antibiotics.49.553. [DOI] [PubMed] [Google Scholar]
- 31.Liesch, J. M., M. Meinz, J. C. Onishi, S. A. Morris, R. E. Schwartz, G. F. Bills, R. A. Giacobbe, W. S. Horn, D. L. Zink, A. Cabello, M. T. Diez, I. Martin, F. Pelaez, and F. Vicente. 26 May 1998. U.S. patent 5,756,472.
- 32.Liesch, J. M., M. Meinz, J. C. Onishi, R. E. Schwartz, G. F. Bills, R. A. Giacobbe, D. L. Zink, A. Cabello, M. T. Diez, I. Martin, F. Pelaez, and F. Vicente. 27 January 1998. U.S. patent 5,712,109.
- 33.Mazur P, Morin N, Baginsky W, El-Sherbeini M, Clemas J A, Nielsen J B, Foor F. Differential expression and function of two homologous subunits of yeast 1,3-β-d-glucan synthase. Mol Cell Biol. 1995;15:5671–5681. doi: 10.1128/mcb.15.10.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McManus O B, Harris G H, Giangiacomo K M, Feigenbaum P, Reuben J P, Addy M E, Burka J F, Kaczorowski G J, Garcia M L. An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry. 1993;32:6128–6133. doi: 10.1021/bi00075a002. [DOI] [PubMed] [Google Scholar]
- 35.Mizuno K, Yagi A, Satoi S, Takada M, Hayashi M. Studies on aculeacin. I. Isolation and characterization of aculeacin A. J Antibiot. 1977;30:297–302. doi: 10.7164/antibiotics.30.297. [DOI] [PubMed] [Google Scholar]
- 36.Mukhopadhyay T, Ganguli B N, Fehlhaber H W, Kogler H, Vertesy L. Mulundocandin, a new lipopeptide antibiotic. II. Structure elucidation. J Antibiot. 1987;40:281–289. doi: 10.7164/antibiotics.40.281. [DOI] [PubMed] [Google Scholar]
- 37.Mukhopadhyay T, Roy K, Bhat R G, Sawant S N, Blumbach J, Ganguli B N, Fehlhaber H W, Kogler H. Deoxymulundocandin—a new echinocandin type antifungal antibiotic. J Antibiot. 1992;45:618–623. doi: 10.7164/antibiotics.45.618. [DOI] [PubMed] [Google Scholar]
- 38.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 39.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; proposed standard. NCCLS document M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 40.Nyfeler R, Keller S W. Metabolites of microorganisms. 143. Echinocandin B, a novel polypeptide-antibiotic from Aspergillus nidulans var. echinulatus: isolation and structural components. Helv Chim Acta. 1974;57:2459–2477. doi: 10.1002/hlca.19740570818. [DOI] [PubMed] [Google Scholar]
- 41.Parks D J, Blanchard S G, Bledsoe R K, Chandra G, Consler T G, Kliewer S A, Stimmel J B, Willson T M, Zavacki A M, Moore D D, Lehmann J M. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 42.Revelli A, Massobrio M, Tesaric J. Nongenomic actions of steroid hormones in reproductive tissues. Endocrine Rev. 1998;19:3–17. doi: 10.1210/edrv.19.1.0322. [DOI] [PubMed] [Google Scholar]
- 43.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez L J, Rex J H, Anaissie E J. Update on invasive candidiasis. Adv Pharmacol. 1996;137:349–400. doi: 10.1016/s1054-3589(08)60955-2. [DOI] [PubMed] [Google Scholar]
- 45.Rouhi A M. Researchers unlocking potential of diverse, widely distributed saponins. Chem Eng News. 1995;1995(11 September):28–35. [Google Scholar]
- 46.Roy K, Mukhopadhyay T, Reddy G C, Desikan K R, Ganguli B N. Mulundocandin, a new lipopeptide antibiotic. I. Taxonomy, fermentation, isolation and characterization. J Antibiot (Tokyo) 1987;40:275–280. doi: 10.7164/antibiotics.40.275. [DOI] [PubMed] [Google Scholar]
- 47.Sheehan D J, Hitchcock C A, Sibley C M. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40–79. doi: 10.1128/cmr.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shematek E M, Braatz J A, Cabib E. Biosynthesis of the yeast cell wall. I. Preparation and properties of β-(1,3)-glucan synthetase. J Biol Chem. 1980;255:888–894. [PubMed] [Google Scholar]
- 49.Smith T W. Digitalis, mechanisms of action and clinical use. N Engl J Med. 1988;318:358–365. doi: 10.1056/NEJM198802113180606. [DOI] [PubMed] [Google Scholar]
- 50.Sohn R, Marinetti G V. Effect of detergents on the enzyme activities of the rat liver plasma membrane. Chem Phys Lipids. 1974;12:17–30. doi: 10.1016/0009-3084(74)90065-6. [DOI] [PubMed] [Google Scholar]
- 51.Strobach H, Wirth K E, Rojsathaporn. Naunyn-Schmied K. Absorption, metabolism and elimination of strophanthus glycosides in man. Naunyn Schmiedebergs Arch Pharmacol. 1986;334:496–500. doi: 10.1007/BF00569392. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka N, Kinoshita T, Masukawa H S O. Mechanism of inhibition of protein synthesis by fusidic acid and related steroidal antibiotics. J Biochem. 1969;65:459–464. doi: 10.1093/oxfordjournals.jbchem.a129034. [DOI] [PubMed] [Google Scholar]
- 53.Thompson J R, Douglas C M, Li W, Jue C K, Pramanik B, Yuan X, Rude T H, Toffaletti D L, Perfect J R, Kurtz M. A glucan synthase FKS1 homolog in Cryptococcus neoformans is single copy and encodes an essential function. J Bacteriol. 1999;181:444–453. doi: 10.1128/jb.181.2.444-453.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tkacz J A, Lampen J O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975;65:248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- 55.Traber R, Keller-Juslen C, Loosli H R, Kuhn M, von Warburg A. 129. Cyclopeptid-antibiotika aus Aspergillus-arten. Struktur der echinocandine C undD. Helv Chim Acta. 1979;62:1252–1267. [Google Scholar]
- 56.Traxler P, Gruner J, Auden J A. Papulacandins, a new family of antibiotics with antifungal activity. I. Fermentation, isolation, chemical and biological characterization of papulacandins A, B, C, D and E. J Antibiot. 1977;30:289–296. doi: 10.7164/antibiotics.30.289. [DOI] [PubMed] [Google Scholar]
- 57.Turner W W, Current W. Echinocandin antifungal agents. In: Strohl W R, editor. Biotechnology of antibiotics. New York, N.Y: Marcel-Dekker, Inc.; 1997. pp. 315–334. [Google Scholar]
- 58.VanMiddlesworth F, Omstead M N, Schmatz D, Bartizal K, Fromtling R, Nollstadt K, Honeycut S, Zweerink M, Garrity G, Bills G. L-687,781, a new member of the papulacandin family of β-1,3-d-glucan synthesis inhibitors. I. Fermentation, isolation, and biological activity. J Antibiot. 1991;44:45–51. doi: 10.7164/antibiotics.44.45. [DOI] [PubMed] [Google Scholar]
- 59.Yeung C M, Klein L L, Lartey P A. Preparation and antifungal activity of fusacandin analogs: C-6′ sidechain esters. Bioorg Med Chem Lett. 1996;6:819–822. [Google Scholar]
- 60.Zornes L L, Stratford R E. Development of a plasma high-performance liquid chromatographic assay for LY303366, a lipopeptide antifungal agent, and its application in a dog pharmacokinetic study. J Chromatogr B Biomed Sci Appl. 1997;695:381–387. doi: 10.1016/s0378-4347(97)00184-9. [DOI] [PubMed] [Google Scholar]