Abstract
Malignant biliary obstruction generally results from primary malignancies of the pancreatic head, bile duct, gallbladder, liver, and ampulla of Vater. Metastatic lesions from other primaries to these organs or nearby lymph nodes are rarer causes of biliary obstruction. The most common primaries include renal cancer, lung cancer, gastric cancer, colorectal cancer, breast cancer, lymphoma, and melanoma. They may be difficult to differentiate from primary hepato-pancreato-biliary cancer based on imaging studies, or even on biopsy. There is also no consensus on the optimal method of treatment, including the feasibility and effectiveness of endoscopic intervention or surgery. A thorough review of the literature on pancreato-biliary metastases and malignant biliary obstruction due to metastatic non-hepato-pancreato-biliary cancer is presented. The diagnostic modality and clinical characteristics may differ significantly depending on the type of primary cancer. Different primaries also cause malignant biliary obstruction in different ways, including direct invasion, pancreatic or biliary metastasis, hilar lymph node metastasis, liver metastasis, and peritoneal carcinomatosis. Metastasectomy may hold promise for some types of pancreato-biliary metastases. This review aims to elucidate the current knowledge in this area, which has received sparse attention in the past. The aging population, advances in diagnostic imaging, and improved treatment options may lead to an increase in these rare occurrences going forward.
Keywords: Bile duct obstruction, Obstructive jaundice, Pancreas, Metastasis, Endoscopic retrograde cholangiopancreatography
Core Tip: Metastases from distant primary cancers are rare causes of biliary obstruction. The most common primaries include renal cancer, lung cancer, gastric cancer, colorectal cancer, breast cancer, lymphoma, and melanoma. Clinical presentation, appropriate immunohistochemical markers for pathological analysis, treatment options, and prognosis may differ depending on the primary cancer. A review of the existing literature and characteristics of metastases from each primary are presented.
INTRODUCTION
Metastatic lesions from other primaries to the pancreatic head, biliary tree, liver, ampulla of Vater, and hilar or peripancreatic lymph nodes have the potential to cause biliary obstruction. The pancreas and biliary tree are rare destinations for metastases. However, such metastases can result from almost every primary, from brain tumor to melanoma of the toe.
In a multicenter analysis of 159 patients with pancreatic metastases, the most common primaries were renal cell carcinoma (38%), lung cancer (2%), colorectal cancer (11%), and sarcoma (6%)[1]. About 41% were isolated metastases, 78% were single masses, and were more or less uniformly distributed throughout the pancreas. About 24% were symptomatic and only ten cases presented with jaundice. Two-thirds were diagnosed by endoscopic ultrasound-fine-needle aspiration (EUS-FNA) and most of the remainder were diagnosed by surgery. Median overall survival was 43 mo, with extrapancreatic metastasis, tumor-related symptoms at diagnosis, and pathology of primary tumors independently predicting survival. In a literature review of 234 subjects, synchronous presentation with the primary tumor and surgical resection were important prognostic factors in addition to tumor-related symptoms at diagnosis and pathology of primary tumors[2]. There were marked differences in the studied subjects: 68% had renal cell carcinoma, 86% were isolated metastases, 79% involved the pancreatic head, and 62% were symptomatic at diagnosis, but extrapancreatic metastases were only observed in 14% of cases. As may be expected, metastases from lung cancer and melanoma had worse prognoses than those from renal cell carcinoma.
Autopsy studies report extremely high rates of pancreatic metastases. An autopsy database study found 81 pancreatic metastases among only 190 pancreatic tumors (43%). Major primaries included the lung (42%), gastrointestinal tract (25%), kidney (5%), and breast (4%)[3]. A Japanese autopsy study found 103 cases of pancreatic metastases among 690 cadavers with malignant tumors, implying that 15% of all metastatic malignancies involve the pancreas[4]. Specifically, pancreatic metastases were found in 35% of gastric cancers, 25% of bladder cancers, 21% of ovarian cancers, 15% of lung cancers, and 9% of renal cell carcinomas and breast cancers. One-third of pancreatic metastases were not visible macroscopically. About 17% involved the pancreas via direct invasion rather than distant metastasis.
Metastases to the bile duct are extremely rare. There are isolated case reports of from primaries including ovarian cancer, colon cancer, rectal cancer, esophageal cancer, gallbladder cancer, hepatocellular carcinoma, breast cancer, and malignant melanoma[5-13].
Herein, hepato-pancreato-biliary metastases from non-hepato-pancreato-biliary malignancies and resulting malignant biliary obstruction (MBO) are discussed. Characteristics of each primary are discussed after a brief overview of diagnosis and treatment of obstructive metastases.
DIAGONSIS
Diagnostic imaging
Computed tomography (CT) is the most common imaging modality used for cancer follow-up. One study found pancreatic metastases in 0.3% of 6623 patients followed up for various malignancies[14]. An analysis of 192 cases in seven imaging studies of pancreatic metastases from various primaries reveals that the most common primaries were renal cell carcinoma (30%), lung (26%), stomach/colon (13%), and breast (10%) (Table 1)[14-20]. Of these, 66% were solitary masses, 27% had multiple nodules, and 7% had diffuse involvement. Isolated pancreatic metastases were observed in 5%-33% of cases. Pancreatic metastases were found synchronously with the primary malignancy in 22%-44% of cases. A mass in the pancreatic head was found in 11%-29% of cases, but only 5%-6% had biliary dilation. Pancreatic duct dilatation was observed in 11%-22% of cases, with pancreatitis rarely observed. CA19-9 was elevated in 8%-28% of cases, but most primaries of such cases were gastrointestinal and may have been unrelated to pancreatic involvement.
Table 1.
Computed tomography findings of pancreatic metastases
|
|
|
|
Primary Malignancy
|
|
|
|
|
Characteristics
|
|||||||
|
Ref.
|
Year
|
Cases
|
Renal
|
Lung
|
Breast
|
Stomach
|
Colon
|
Sarcoma
|
Melanoma
|
Ovary
|
Thyroid
|
Other
|
Solitary mass
|
Multiple nodules
|
Diffuse involvement
|
| Ferrozzi et al[14] | 1997 | 20 | 2 | 6 | 3 | 3 | 1 | 2 | 2 | 1 | 11 | 2 | 7 | ||
| Klein et al [15] | 1998 | 66 | 20 | 15 | 8 | 4 | 5 | 4 | 2 | 1 | 7 | 52 | 11 | 3 | |
| Tsitouridis et al [16] | 2009 | 11 | 1 | 7 | 3 | 7 | 3 | 1 | |||||||
| Angelleli et al [17] | 2012 | 17 | 8 | 4 | 3 | 2 | 7 | 9 | 1 | ||||||
| Shi et al [18] | 2015 | 18 | 3 | 7 | 5 (stomach+colon) | 2 | 1 | 12 | 6 | ||||||
| Choi et al [19] | 2015 | 36 | 17 | 2 | 5 | 7 | 1 | 1 | 1 | 2 | 29 | 7 | |||
| Galia et al [20] | 2018 | 24 | 6 | 8 | 2 | 1 | 4 | 3 | 9 | 13 | 2 | ||||
| Total | 192 | 57 | 49 | 19 | 12 | 8 | 11 | 7 | 5 | 6 | 13 | 127 | 51 | 14 | |
| % of total | 30% | 26% | 10% | 7% | 5% | 6% | 4% | 3% | 3% | 7% | 66% | 27% | 7% | ||
CT characteristics of metastatic lesions were generally concordant with the primary lesions, except some cases of gastric cancer[19]. Metastases from renal cell carcinoma are generally hypervascular with well-defined margins, while most other metastases are hypoattenuating with unclear borders, similar to primary adenocarcinoma[14-19]. In a comparison with primary pancreatic adenocarcinoma, metastases had significantly less pancreatic duct dilatation, vascular involvement, parenchymal atrophy, or peripancreatic fluid[20]. Biliary dilatation was also less frequently observed, possibly due to the small number of metastases located in the pancreatic head in the study.
In summary, most metastases present as solitary masses or multiple nodules on CT and generally do not cause symptoms or biliary obstruction. Renal cell carcinoma metastases may be identified by hypervascular, well-defined lesions, while the absence of pancreatic duct dilatation or parenchymal atrophy may be clues for metastatic disease in general.
Studies on magnetic resonance imaging (MRI) and fluorodeoxyglucose-positron emission tomography/CT (FDG-PET/CT) for diagnosing pancreatic metastases are limited. MRI of metastases of renal cell carcinoma may also be distinguished from primary adenocarcinoma due to their vascularity[21]. One FDG-PET/CT study of 26 lesions in 19 patients found no difference in maximum standardized uptake values between pancreatic metastases and primary pancreatic adenocarcinoma[22]. However, several isodense intrapancreatic nodules missed on contrast CT were discovered by FDG-PET/CT. FDG-PET/CT may also be useful in detecting other unsuspected distant metastases.
Endoscopic ultrasound
Endoscopic ultrasound (EUS) may also provide clues to identify metastatic disease. In a study comparing 28 pancreatic metastases (23 solitary, four multiple, and one diffuse lesion) to 60 cases of pancreatic adenocarcinoma, Hjiioka et al[23] reported that main pancreatic duct dilatation was also observed significantly more frequently in pancreatic adenocarcinoma than in metastatic lesions. Regular borders and absence of retention cysts predicted pancreatic metastases.
In a small study of contrast-enhanced harmonic EUS, pancreatic metastases from renal cell carcinoma and lymphoma were hyperenhancing, while most other metastases were hypoenhancing[24]. While both contrast-enhanced harmonic EUS and elastography may assist in differentiating malignant lesions from benign lesions, there is insufficient evidence in their ability to differentiate metastases from primary adenocarcinoma[25,26].
Fine-needle aspiration
Pancreatic metastatic accounted for 0.9%-2.5% of EUS-FNA samples of the pancreas and 4.7%-7.2% of pancreatic malignancies[27-29]. These figures may be higher than those of other modalities because lesions with typical findings of pancreatic adenocarcinoma may be resected without EUS-FNA. In the largest review of 582 pancreatic metastases diagnosed by EUS-FNA, renal cell carcinoma (34%), lung cancer (15%), colon cancer (10%), melanoma (7%), and breast cancer (6%) were the most common primaries[28].
EUS-FNA is a safe and well-accepted method of diagnosing such metastatic lesions. EUS-FNA for pancreatic metastases and primary adenocarcinoma have reported sensitivities of 75%-94% and 75%-84%, respectively, and specificities of 60%-100% and 97%-100%, respectively[30-33]. No significant complications including tumor seeding of the needle tract have been reported in EUS-FNA of pancreatic metastases[34]. Immunohistochemistry and KRAS mutation analysis can be crucial in reaching the final diagnosis[23].
TREATMENT
Endoscopic biliary drainage
In studies of endoscopic biliary drainage which include metastases from non-hepato-pancreato-biliary primaries, metastases are not necessarily a rare cause of MBO. Metastases were the cause of biliary obstruction in 14% of 1346 patients in 14 such studies[35-49]. The pooled average across six studies limited to distal MBO (n = 395) was 9%, while that of three studies limited to hilar MBO (n = 256) was 22%[40-48]. Gastric cancer (including lymph node metastases) was the cause of 80% of MBO across four studies focused on surgically altered anatomies[49-52]. MBO due to metastases from primaries with better prognoses than primary pancreato-biliary cancer may be able to better generally tolerate and benefit from endoscopic retrograde cholangiopancreatography (ERCP), although they may not be candidates for surgical resection. These figures may be overstated, as some reports which include no metastatic cases did not intentionally exclude them. Selection bias may also be a factor; most reports were from university-affiliated tertiary care centers or cancer institutes.
Studies focused exclusively on biliary drainage for metastatic cancer are mainly limited to those on percutaneous drainage[53,54]. One study on 93 patients achieved clinical success in 73% of patients and found that survival differed significantly depending on the primary tumor[54]. Subsequent chemotherapy prolonged median survival from 1 to 5 mo, with the greatest benefit observed in the 28 cases with colorectal cancer. Another study with 42 cases each of gastric and colorectal cancer found that performance status and absence of peritoneal metastases were associated with longer survival[55].
There is only one study on prognostic factors after ERCP stenting for biliary drainage in metastatic cancers of various origins[56]. Colorectal cancer was the most common primary (25%), and there was only one case of renal cell carcinoma. Technical success was achieved in 91.7% of patients and 67% of successfully cases received subsequent treatment. Performance status and treatment after drainage were independent predictors of overall survival.
One study on metallic stent placement in hilar MBO reported that metastatic disease from other primaries was an independent risk factor for technical failure[57]. The study only included five patients, of which one had pancreatic cancer. The authors reported that the extrinsic nature of the biliary stricture necessitated multiple procedures, including percutaneous drainage. Larger studies have failed to reproduce this result. EUS-guided intervention has also been reported with success in hilar lymph node or hepatic metastases from colorectal, breast, gastric, urogenital, and anal cancer[57]. With respect to endoscopic biliary drainage, it appears acceptable to approach MBO due to metastases in a similar manner as MBO from primary hepato-pancreato-biliary cancer.
Surgery
Metastasectomy accounted for 1.4% of 5745 pancreatic resections across six studies which provided total figures (range: 0.7%-3.1%)[58-63]. A review of 399 metastasectomies found that renal cell carcinoma accounted for 62.6% of cases[64]. Forty percent had symptoms at presentation and, in a separate review, 22% had jaundice[64,65]. About 10% were found synchronously with the primary tumor[64]. Median survival was 50.2 mo after surgery overall, but 71.7 mo for renal cell carcinoma. On the other hand, perioperative mortality was observed in 2.2% of all metastasectomies. Patients with isolated pancreatic metastases had better prognoses than those with other metastases (45 mo vs 26 mo). In the separate review[65], renal cell carcinoma patients had a long post-surgical median survival of 105 mo, compared to 54 mo for colon cancer, 40 mo for sarcoma, 34 mo for ovarian cancer, 26 mo for breast cancer, 14 mo for melanoma, and 6 mo for lung cancer. On the other hand, pooled analysis showed long 5-year post-surgical survival of 61.1% for isolated pancreatic metastases (of which 74% were renal cell carcinomas) and 58.9% for local invasion from colon or gastric cancer[66].
A study of 98 metastasectomies found that old age, non-renal cell carcinomas, vascular invasion, and positive margins were independently associated with increased mortality risk[67]. Resection can generally be considered for isolated metastases. Many reports suggest long-term survival in renal cell carcinoma. Symptomatic relief may also be achieved; all colorectal cancer patients experienced symptomatic relief after metastasectomy in a surgical review[68]. While data is lacking for other primaries, metastasectomy should only be attempted when margin-negative resection can be expected. As melanoma and lung cancer patients have poor prognosis even after surgery, indication for metastasectomy should be considered with caution.
PRIMARY MALIGNANCIES
Renal cell carcinoma
Renal cell carcinoma is the most common primary for pancreatic metastases in CT, EUS-FNA, and surgical series. When such metastases occur, they are discovered synchronously with primary renal cell carcinoma in 7% of cases[69]. There is generally a long time lag of up to 32 years between nephrectomy and pancreatic metastases. Most have multiple extrapancreatic metastases. About half of isolated metastases occur in the pancreatic head. One study suggested a predilection for fatty pancreas[70].
Counterintuitively, many reports note that renal cell carcinoma patients with pancreatic metastases have better prognoses than those without, even when there are concomitant extrapancreatic metastases[69,71-73]. In one study, median overall survival with and without pancreatic metastases were 39 and 23 mo, respectively[73]. The size of isolated metastases, the number of metastatic pancreatic lesions, and time interval from nephrectomy to pancreatic metastasis had no impact on survival[69]. The laterality of the primary renal cancer has no impact on the portion of the pancreas affected. Affinity of indolent types of renal cell carcinomas to the pancreas has been suggested, with characteristic genetic mutations, high sensitivity to antiangiogenic treatment and resistance to immune check point inhibitors[69,74]. The extreme rarity of metastases to the biliary tree may also provide support for this affinity[75,76].
CT of pancreatic metastases are generally hypervascular with well-defined margins[14-16]. The hypointensity on T1-weighted and hyperintensity on T2-weighted imaging make them difficult to differentiate from primary adenocarcinoma on MRI[77]. They can also mimic neuroendocrine tumors on contrast MRI, due to their early contrast enhancement. Diagnosis can be made with high accuracy by EUS-FNA, with immunohistochemistry usually positive for pan-cytokeratin, CD10, EMA, and PAX-8 and negative for synaptophysin, chromogranin, and beta-catenin (nuclear staining)[78].
Despite the large number of reports on pancreatic metastases, there is a surprisingly small number of reports of biliary obstruction. Only two of 307 ERCP cases in eight studies involved metastases from renal cell carcinoma. There are isolated reports of MBO from ampullary or bile duct involvement[79,80]. Simultaneous gastric outlet obstruction can rarely occur[80].
About 80% of pancreatic metastases are resectable[81]. While there are no direct comparative studies, surgery for both single and multiple metastases to the pancreas is generally considered safe and associated with long-term survival[82-84]. A systematic review showed that extrapancreatic disease was a risk factor for recurrence after surgery but had no impact on survival[85]. However, it is not clear whether surgery is required. There was no significant difference in overall survival between tyrosine kinase inhibitors and surgery in one study[86]. Another study reported that the existence of pancreatic metastasis did not affect survival in patients treated with first-line tyrosine kinase inhibitors[87]. Radiotherapy was also effective in a small case series[88].
Lung cancer
Lung cancer is the leading cause of cancer death worldwide[89]. About 57% have metastatic disease at diagnosis[90]. About 3% of lung cancer patients develop pancreatic metastases[91]. The frequency of pancreatic metastases depends on the histological subtype, occurring most commonly in small cell carcinoma (10%), followed by adenocarcinoma (2.4%), large cell carcinoma (1.9%), and squamous cell carcinoma (1.1%)[92]. Most cases were asymptomatic[93]. A majority of pancreatic metastases present as solitary lesions (73%) but can also be multiple (12%) or diffuse (15%). Concomitant liver and adrenal metastases are observed in 73% and 69% of cases, respectively.
Small cell lung cancer presents initially with increased serum bilirubin in about 10% of cases, caused by hepatic (6%) or pancreatic (4%) metastases[94]. Obstructive jaundice due to hilar lymph node compression from metastatic small cell lung carcinoma has also been reported[95]. Most reports of metastasis-induced acute pancreatitis come from small cell lung cancer or gastric cancer metastasizing to the pancreatic head, with isolated reports of squamous cell lung cancer, adrenocortical carcinoma, and breast cancer[96-99]. Twenty-six cases from small cell lung cancer have been reported to date[100]. While initially estimated to occur in 0.12% of small cell lung cancer cases, the figure may be higher as 14 cases were reported from a single institution[96,101]. Chemotherapy may provide a survival benefit[96].
At least 23 reports of primary bile duct small cell carcinoma have been reported[102]. While there are no reports of small cell lung cancer metastasizing to the bile duct, there are more than ten reports of biliary obstruction as the initial presentation of small cell lung cancer[103]. In one study, 4.0% of small cell lung cancers presented initially with extrahepatic obstruction due to pancreatic metastases, while another 5.6% had jaundice due to hepatic metastases[104]. While there was good overall response to chemotherapy, the latter group had a worse prognosis.
There are ten reports of squamous cell lung cancer metastasizing to the pancreas[105]. All were single lesions, 67% involved the pancreatic head, 50% had biliary or pancreatic duct dilation, and 30% had obstructive jaundice. There are two reports of lung adenocarcinoma metastasizing to the common bile duct, both causing biliary obstruction[106,107].
Seventeen cases of duodenal metastases from lung cancer have been reported, caused by adenocarcinoma (47%), squamous cell (29%), small cell (12%) or large cell (12%) cancers and arising in all four parts of the duodenum[108,109]. MBO occurred from two metastases occupying the second part (squamous cell and adenocarcinoma), both managed endoscopically[109,110].
CT and MRI may not be contributory in differentiating metastases from primary adenocarcinoma, or in hyperenhancing lesions, neuroendocrine tumors[105]. FDG-PET/CT found abnormal pancreatic accumulations in 1.6%-2.3% of lung cancer cases, discovering metastases from adenocarcinoma and small and large cell lung cancer as small as 6 mm[111]. All had metastases to at least one other organ. While one patient had acute pancreatitis, none developed obstructive jaundice.
As with metastases from other primaries, diagnosis can be made by EUS-FNA[112]. Thyroid transcription factor-1 can aid in differentiating pancreatic metastases from primary pancreatic adenocarcinoma and small cell lung cancer metastases from primary small cell biliary cancer[102,113,114]. KRAS G12C mutations and napsin A are also associated with lung adenocarcinoma, while KRAS G12R mutations, CK20, and CDX2 support the diagnosis of pancreatic adenocarcinoma[114].
In addition to ERCP for MBO and standard treatment for metastatic lung cancer, one study found a survival benefit in resecting pancreatic metastases, with a median survival of 29 mo for curative intent resection vs 8 mo for palliative surgery or medical management[115-117].
Gastric cancer
Most gastric cancers occur in the non-cardia and are commonly associated with Helicobacter pylori infection, leading to geographic differences in incidence. Liver metastases are observed in almost half of metastatic gastric cancer patients[118]. There are only 11 reports of pancreatic metastases, mostly resulting from moderately or poorly differentiated adenocarcinoma[119].
Obstructive jaundice arises most commonly from extrinsic lymph node compression, followed by intraductal metastases which present as band-like wall thickening with enhancement on contrast CT, much like primary cancer of the bile duct[120-122]. A minority arise from direct tumor invasion, extrinsic compression from liver metastases, or peritoneal carcinomatosis. Most result from Borrmann type 3 (63%-72%) or type 2 (21%-24%) adenocarcinomas with antral involvement (60%-98%), after total or partial gastrectomy (79%-89%)[121,123]. Histological composition of the primary tumors varies significantly across studies, with differentiated adenocarcinomas accounting for 9%-90% of total cases[121,123,124]. Obstructive jaundice occurs in 1.4%-2.3% of post-operative patients, with a median interval of 10-15 mo after surgery[125,126].
Drainage for MBO due to gastric cancer can involve two major issues: surgically altered anatomy and concomitant gastric outlet obstruction (GOO). As most affected patients have undergone total or partial gastrectomy, approaching the papilla can be extremely difficult (Figure 1). While percutaneous biliary drainage has largely been successful, poor prognostic factors after drainage include significant liver metastases, hilar strictures and high carbohydrate antigen (CA) 19-9[127,128]. The main cause of stent occlusion was sludge buildup (13%) in one study, with no cases of tumor ingrowth[122]. When ERCP is contraindicated, EUS-guided biliary drainage was comparable to percutaneous drainage in terms of both technical success rate and stent patency period[129]. As in the case of post-operative pancreatic cancer patients, ERCP and stent placement using a double-balloon enteroscope has also been reported for this purpose[49,52,130]. Technical success was reported in all 26 gastric cancer cases across two studies[49,52]. Median time to recurrent biliary obstruction for seven cases in one study was 7.4 mo, with two cases of tumor ingrowth and one case of mucosal hyperplasia[49].
Figure 1.
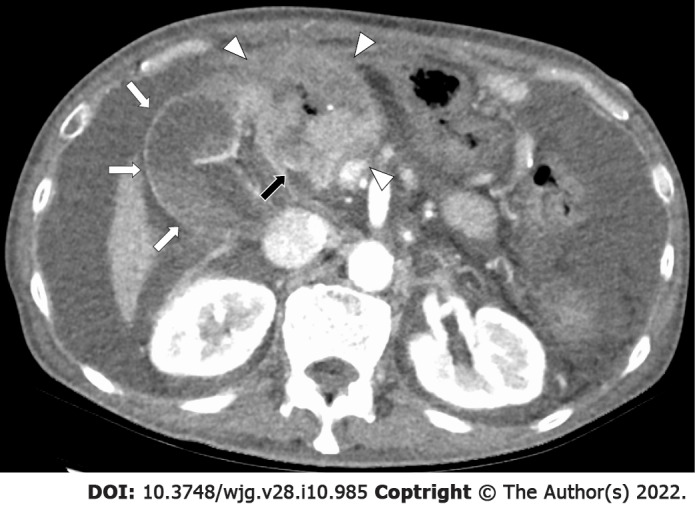
A 69-year-old man presented with obstructive jaundice due to recurrence 18 mo after distal gastrectomy and Roux-en-Y reconstruction for gastric cancer. A recurrent mass with central necrosis (white arrowheads) obstructed the extrahepatic bile duct (black arrow), causing dilatation of intrahepatic bile ducts and gallbladder (white arrows). While endoscopic ultrasound-guided hepaticogastrostomy led to symptomatic relief, the patient died 1 mo later.
After hepatobiliary cancers, gastric cancer is the most common cause of combined MBO and GOO, accounting for 4%-8% of such cases[131-133]. Other rare cases include colon, breast, and renal cancer[131]. The extrahepatic bile duct and first or second parts of the duodenum are most commonly involved. Regardless of the primary site, MBO tends to precede GOO. Double stenting of the duodenum and bile duct has success rates approaching 100%, with only rare reports of post-procedural pancreatitis[133,134]. Metallic stents tended to have longer patency than plastic stents, while more adverse events resulted from EUS-guided drainage when compared with ERCP[133].
Colorectal cancer
Almost half of colorectal cancer patients experience metastatic disease. Metastases to the liver ultimately occur in 25%-30% of affected patients, of which only about 25% can be resected[135-137]. Liver metastases tend to occur more commonly in left-sided cancers and in relatively young patients[137]. Other reported sites of metastases which may cause biliary obstruction include lymph nodes, pancreas, peritoneum, and the extrahepatic bile duct[138,139].
Cytokeratin (CK) 7 negativity and CK20 positivity in EUS-FNA specimens aid in differentiating from primary pancreato-biliary adenocarcinoma, which is generally CK positive and CK20 negative[140]. However, some types of primary pancreatic adenocarcinoma such as the colloid type may be CK7 negative/CK20 positive, complicating the differential diagnosis[30].
Biliary obstruction is associated with poor outcomes not only because it reflects widespread disease, but also because of chemotherapy cannot be performed at the desired dose. One study found jaundice in about 10% of metastatic colorectal cancer patients due to liver metastases (53%) or metastatic lymph nodes (47%)[141]. Endoscopic or percutaneous biliary drainage was only successful in 42% of cases with median overall survival of 1.5 mo, which improved to 9.6 mo when chemotherapy could be restarted. The study was unable to identify predictors of drainage failure, although drainage was attempted less often in cases with hilar involvement. In another study[142], biliary drainage mostly by ERCP was successful in about 65% of cases, allowing 70% of successful cases to restart chemotherapy and improving median survival from 33 to 262 d. A study on both colorectal (n = 32) and gastric cancers (n = 60) found that multiple hepatic metastases and hilar strictures were associated with unsuccessful percutaneous drainage, while poor performance status, multiple liver metastases, ascites, history of treatment with multiple chemotherapy regimens, undifferentiated carcinoma, and high CA 19-9 Levels were associated with poor prognosis[127].
A characteristic almost unique to colorectal cancer is the ability to spread along epithelial surfaces and grow intraductally, mimicking neoplasms of the lung, bladder, or intrahepatic bile ducts[143]. Liver metastases of colorectal cancer can exhibit intrabiliary extension in 3.6%-10.6% of cases, compared to 0.7%-1.9% of metastases from other primaries[144]. Such phenomena are most commonly observed in well-differentiated adenocarcinomas originating in the rectosigmoid[145,146]. Microscopic intrabiliary extension has been reported in up to 40.6% of liver metastases from colorectal cancer[145]. There are also reports of intrabiliary extension from hepatocellular carcinoma as well as liver metastases of neuroendocrine tumor, gastrointestinal tumor, and invasive lobular breast cancer[144,147]. Of the other reports on liver metastases, only the neuroendocrine tumor case presented signs of biliary obstruction[144].
Two patterns of intrabiliary growth were identified by Estrealla et al[144]: Bile duct colonization which replace the biliary epithelium and tumor plugs which may or may not affect the biliary epithelium. Such growth was limited to intrahepatic ducts in 72% of cases, while the remainder involved the hilum. Laboratory, imaging, and histological abnormalities suggesting biliary obstruction were all positively associated with the degree of biliary growth. While intrabiliary extension may mimic intrahepatic cholangiocarcinoma on imaging studies, they can generally be differentiated on immunohistochemistry with CK7 and CK20[140]. In contrast with liver metastases from other primaries, biliary biopsy may be useful in achieving a preoperative diagnosis[138]. Intrabiliary extension is paradoxically associated with a better prognosis, possibly because it occurs more frequently in well-differentiated adenocarcinomas which have longer survival periods[145,146].
As isolated liver and lung metastasectomy have been established in colorectal cancer, resection of isolated pancreatic metastases may also be justifiable in selected patients. A review of 24 studies revealed that out of 37 colorectal cancer cases undergoing pancreatic resection, 19 had disease recurrence, with median survival of 21 mo[68]. In addition, all studied patients experienced symptomatic relief after surgery, which lasted until recurrence of cancer.
Breast cancer
Approximately 6% of breast cancer are metastatic at diagnosis in developed countries, and 20%-30% eventually develop metastases[148]. Most common sites of metastases are bone, lung, liver and brain[149,150]. Jaundice is found in 6%-25% of breast cancer patients with liver metastases, generally resulting from hepatic failure rather than MBO[151]. Some studies suggest worse prognosis when jaundice is present, most likely because chemotherapy must be reduced or discontinued as a result[152]. Liver metastases occur in about 5% of all breast cancer patients and 32% of metastatic cases[152,153].
While about 80% of invasive breast cancers are invasive ductal carcinomas, lobular carcinoma is the most common histological subtype observed in gastrointestinal metastases[154,155]. Pancreatic metastases are found in 5%-13% of autopsies of breast cancer patients[156,157]. In a review of 24 cases of periampullary breast cancer metastases, MBO was observed at initial diagnosis in five cases, with metachronous MBO cases occurring 1-23 years later[158]. Our institution reported eleven cases of obstructive jaundice due to metastatic breast carcinoma, resulting from metastases to hilar or peripancreatic lymph nodes (36%), pancreas (27%), liver (18%), gallbladder (9%), and peritoneal carcinomatosis (9%)[99]. MBO due to metastases to the duodenum have also been reported[159].
Metastases can be difficult to differentiate from primary pancreatic cancer, both clinically and radiologically[28,147,155,160]. CA15-3 elevation may suggest breast cancer metastasis in some cases. As breast cancers are adenocarcinomas, diagnosis by EUS-FNA may require immunohistochemical analysis with monoclonal antibodies such as gross cystic disease fluid protein-15[161]. KRAS point mutation analysis has also been shown to be useful[23].
ERCP has acceptable technical success and adverse event rates, offering a chance for long-term survival if chemotherapy can be resumed[99,162]. Concomitant duodenal strictures may preclude ERCP in 18% of cases and may be treated using EUS-guided interventions (Figure 2)[99]. When resectable, metastatic breast cancer to the pancreas have a median survival of 26 mo[2]. While cases series have suggested potential for improved survival[163], there is insufficient data to determine whether solitary metastatic lesions to the pancreas should be resected. Survival in MBO with extensive metastases may average only 2 mo with palliative care alone[99].
Figure 2.
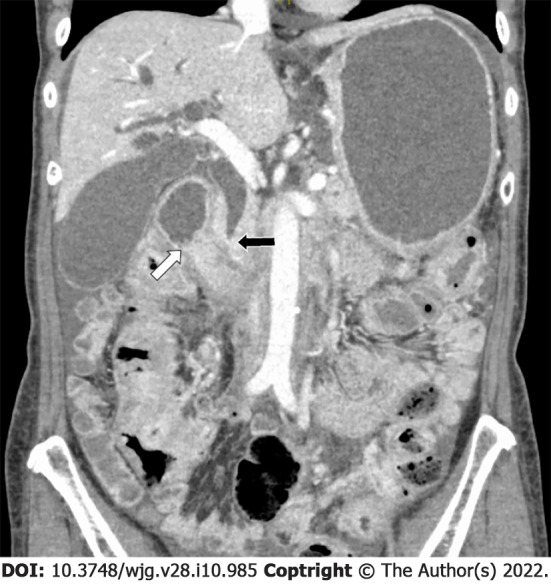
A 62-year-old woman presented with jaundice, nausea, and vomiting 13 years after partial mastectomy for breast cancer. Biopsy-proven duodenal metastases caused both bile duct (black arrow) and duodenal (white arrow) obstruction. Double stenting led to temporary symptomatic relief. The patient subsequently opted for palliative care.
Malignant melanoma and Merkel cell carcinoma
Metastatic melanoma has a poor prognosis with median survival of about 8 mo[164]. Visceral metastases, particularly when multiple, are associated with poor survival[165]. Pancreato-biliary involvement and obstructive jaundice from malignant melanoma can take various forms: primary malignant melanoma of the biliary tract, melanoma of unknown primary arising in the pancreas, pancreato-biliary metastasis, and bile duct compression from hepatic or lymph node metastases.
Autopsy studies on malignant melanoma have shown metastases to the liver in 54%-88%, intestines in 26%-58%, and pancreas in 38%-53% of cases, although discovery rates in the clinical setting are much lower[166]. It should be noted that primaries as well as metastases may not be black, as amelanotic melanoma accounts for up to 27.5% of melanoma cases[167].
Primary malignant melanoma of the biliary tract is a rare type of mucosal melanoma which can only be diagnosed after excluding primaries in other locations including the skin, eye, and gastrointestinal tract. There are 13 reports in the literature, of which 12 presented with jaundice[168]. It tends to present in relatively young males as black, polypoid lesions exhibiting endoluminal growth. Most cases arise in the common bile duct, but can involve intrahepatic bile ducts or the gallbladder. Surgery is the treatment of choice, with a good prognosis if complete resection is achieved. One case was successfully treated with immunotherapy.
Isolated metastases to the pancreas are relatively common. While findings on imaging are generally non-specific, isolated reports describe multiple hypoechoic nodules with hyperchoic septa and central necrosis on EUS[169,170]. T2-weighted MRI may help differentiate pancreatic melanoma, which tends to be hypointense, from pancreatic adenocarcinoma, which tends to be hyperintense[171]. If melanoma can be raised in the differential diagnosis, such lesions can be diagnosed by EUS-FNA with the help of immnohistochemical markers such as Human Melanoma Black 45 and Melan A[169,170]. Large analyses suggest a survival benefit for surgical resection[172,173]. Additional support comes from Wood et al[174], who resected eight cases of melanoma metastases to the pancreas and achieved curative resection in six, of which three survived for over five years.
Melanoma of unknown primary, characterized by metastases in lymph nodes or other areas where primary lesions are unlikely to arise without evidence of a separate primary lesion, accounts for 2.2%-3.2% of malignant melanomas[172,173,175]. There are ten reports of isolated pancreatic melanoma with no other lesions. There is no consensus on whether they can be considered primary pancreatic melanoma. They are often resected surgically, justified based on the reports of isolated pancreatic metastases described above[172-175].
There are at least 18 reports of metastatic melanoma to the common bile duct[176]. Painless obstructive jaundice is the usual finding, and prognosis is dismal unless curative resection can be achieved. Five cases of ampullary metastasis causing obstructive jaundice have also been reported, all requiring endoscopic drainage or surgery[177-181].
Merkel cell carcinoma is another type of aggressive skin cancer which has a high recurrence rate after resection. About one-third of Merkel cell carcinoma patients eventually develop metastases, of which 8% develop pancreatic metastases[182]. It can be diagnosed by EUS-FNA and may cause obstructive jaundice when located in the pancreatic head[183]. While a relatively rare disease, the reported incidence is increasing. A high index of suspicion is required for patients with a history of resection and a thorough skin examination should be considered.
Soft tissue sarcoma
Soft tissue sarcomas are mesenchymal tumors which account for less than 1% of all cancers, but are extremely heterogeneous with over 75 subtypes[184]. Metastatic soft tissue sarcomas are generally refractory to chemotherapy and have median survival of less than one year. There are over 50 reports of pancreatic metastases from various types of soft tissue sarcoma, leiomyosarcoma being the most reported subtype[185-188]. Diagnosis was achieved by fine-needle aspiration in 20 cases, of which 11 were conducted by EUS-FNA[189].
In a CT study of 13 leiomyosarcoma cases with pancreatic metastases, 85% were women and primaries were mostly located in the uterus (39%), retroperitoneum (31%), or extremities (23%). Pancreatic metastases developed after a median interval of 24 mo after diagnosis, 38% were multiple, 46% contained necrosis, and 77% were hypovascular in the arterial phase[185]. While 69% had pancreatic head involvement, lesions were small (1.0-3.5 cm) and biliary and pancreatic duct obstruction were only observed in one patient each. Among 27 unique cases contained in two literature reviews, only two of twelve cases with pancreatic head metastases had jaundice[187,188].
There are over 50 cases of primary pancreatic leiomyosarcoma, which is the most common type of pancreatic stromal tumor and occurs most frequently in middle-aged females[189]. Cystic features may lead to misdiagnoses as pseudocysts or cystic neoplasms and to false-negative EUS-FNA results. There are also 23 reports of primary pancreatic carcinosarcoma as well as reports of various subtypes of sarcoma originating in the bile duct as well as the ampulla of Vater[190-194]. More than half of pancreatic carcinosarcomas occur in the pancreatic head, of which about half cause obstructive jaundice[190]. Thus, sarcoma should be included in the differential diagnosis of isolated pancreato-biliary tumors even in the absence of a coexisting primary tumor.
Surgery has been performed for both primary and metastatic sarcoma arising in the pancreas and duodenum[195,196]. The largest case series describes seven out of 17 existing reports of resection for pancreatic metastases, with one postoperative death and recurrence in all other six cases despite margin-free resection in four cases, with a median survival of 21 mo[195]. The benefits of resection therefore remain unclear.
Lymphoma
Biliary obstruction due to lymphoma can occur from primary hepato-pancreato-biliary lymphoma as well as secondary lymphoma directly extending from abdominal lymph nodes. Most involve B-cell non-Hodgkin’s lymphomas (NHL), which cause 1%-2% of all MBOs, with a disproportionately high number of reports from diffuse large B-cell lymphoma[197,198]. MBO occurs in less than 2% of NHL and about 0.5% of Hodgkin’s lymphoma[198,199]. Almost 1% of NHL patients present initially with MBO[200].
NHL involves the pancreas secondarily in over 30% of cases[201]. Primary pancreatic lymphoma is rare, accounting for less than 5% of extranodal NHL[202,203]. While pancreatic lymphoma causes jaundice in up to 42% of cases, biliary and pancreatic ducts may remain unaffected by even large lesions in the pancreatic head[201,204]. Clues favoring lymphoma over pancreatic cancer in imaging studies include absence of calcifications, patency of involved vessels and ducts, poor but homogenous contrast enhancement, and either a well-delineated mass or diffuse involvement[201].
There are about 30 cases of primary lymphoma of the common bile duct[205]. They generally present with obstructive jaundice but often have smooth strictures with negative findings in ERCP brushing cytology or biopsy. One report found 36 reports of primary gallbladder lymphomas, which are less likely to cause MBO than their bile duct counterparts[206]. There are at least 15 reports of primary duodenal lymphoma of various B-cell and T-cell subtypes which presented with obstructive jaundice[207,208]. Three were drained percutaneously and four were treated by ERCP.
MBO from lymphoma usually results from extrinsic compression, although several cases of direct bile duct invasion have been reported[209]. Results of biliary drainage vary significantly across studies, due to the small sample size in each. Ross et al[197] reported technical success in 84% of biliary drainage by ERCP (and 100% of cases without concomitant gastric outlet obstruction due to lymphoma). They recommend plastic stents as strictures in patients presenting initially with MBO resolve with treatment before stent exchange is necessary, while those who develop MBO later in the disease progression do not survive until their first stent exchange is due. Stent-free status was achieved in about one-third of all patients. On the other hand, none of eight patients achieved stent-free status in another study[198]. A third study reported that MBO resolved in all seven patients presenting initially with MBO, regardless of stent placement[200]. Most studies agree that initial presentation with MBO has a much better prognosis than those who develop MBO later on. Those initially presenting with jaundice had significantly improved survival after biliary drainage (21 mo vs 5 mo)[197]. While surgery is an option when a preoperative diagnosis cannot be reached or the lesion appears resectable, chemotherapy and/or involved site radiation therapy is generally considered the standard of care[210].
NHL and Kaposi’s sarcoma should also be included in the differential for biliary obstruction in acquired immunodeficiency syndrome (AIDS) patients, alongside AIDS-related cholangiopathy. Both show hepatic or splenic involvement in about 15% of AIDS patients[211]. AIDS-related NHL more commonly causes MBO by extrinsic compression from lymph nodes or liver involvement rather than from a primary biliary lesion[212]. Primary pancreatic lymphoma is more common in AIDS patients than in the general population, accounting for about 5% of extranodal NHL cases[211]. Hepatic or hilar involvement of Kaposi’s sarcoma, which can occur earlier in the disease course of AIDS, is an even rarer cause of MBO[213].
Leukemia
There are only several reports of leukemia presenting as obstructive jaundice[214-219]. The liver, bile duct, pancreas, or lymph nodes can be involved. Eight of 103 pancreatic metastases found in an autopsy study were cause by leukemia, suggesting that microscopic involvement may not be so rare[4]. Most reports in both adults and children involve acute lymphocytic leukemia. Obstructive jaundice was the presenting symptom in one case[215]. Care is required as obstructive lesions may not be visible on imaging[214]. While endoscopic and surgical treatment may be options to relieve biliary obstruction, aggressive chemotherapy is the standard of care.
Lymph node metastases
Hilar and peripancreatic lymph node metastases are a well-known cause of MBO. Such metastases have received little attention despite their frequency, perhaps because they are common occurrences in hepato-pancreato-biliary cancer. Hilar and distal biliary obstruction were caused by metastatic lymph nodes in 23% and 2%-17% (pooled average of 551 patients across eight studies: 11%) of cases treated mainly by ERCP stenting, respectively[220-228]. This figure may be higher for biliary obstruction in surgically altered anatomies; one report found six cases among 13 patients with surgically altered anatomies treated with metallic biliary stents (46%)[49].
In a study of biliary drainage mainly via ERCP, biliary obstruction due to hilar lymph node metastases resulted mainly from colon (46%), gastric (14%), and breast (14%) cancers[229]. Clinical success was achieved in 86% of cases but required a median of three procedures and percutaneous drainage in 20% of cases. The ability to resume chemotherapy was associated with improved survival in colon and breast cancer patients. A study of 65 patients with distal MBO due to lymph node metastases from gastric (31%), colorectal (18%), lung (11%), breast (8%), and other cancers found that covered metallic stents were had longer stent patency and less stent occlusion or tumor ingrowth than uncovered stents but had higher rates of acute pancreatitis[230]. Neither study noted any unique characteristics for any particular primary. Notably, there was only one case of lymph node metastasis from renal cell cancer, despite being the most common cause of metastases to the pancreas.
Another report found stent occlusion in 50% of lymph node metastasis cases vs 24% in primary biliary tract cancer[231]. While the authors of the study suggested a limited role for metallic stents in biliary obstruction caused by metastatic lymph nodes, no other studies demonstrated a significant difference from primary cancers. Currently, metallic stents are widely used for this purpose. Bilateral stenting in high-grade inoperable strictures leads to lower re-intervention rates without sacrificing technical success or increasing adverse events[232].
It should be noted that none of the studies reported pathological confirmation of lymph node metastases.
Thyroid
Thyroid cancer most commonly metastasizes to the lung and bone. Pancreatic metastases can occur in papillary, follicular, and medullary carcinoma[233]. In a review of 24 reported cases in English and Japanese, the average delay from thyroid cancer diagnosis to pancreatic metastasis is 7 years. Most were solitary lesions, with half arising in the pancreatic head. Biliary obstruction due to metastatic papillary thyroid cancer has been reported[234]. EUS-FNA is commonly used for diagnosis, and solitary metastases are generally resected with good results in selected cases[235].
Gynecological cancers
About 8% of ovarian cancer patients have metastases at diagnosis, but 22% ultimately develop metastases after a median interval of 44 mo after diagnosis[236]. There are 17 reported cases of pancreatic metastases[237]. As BRCA1 and BRCA2 mutations are common risk factors for both ovarian and pancreatic cancers, pancreatic lesions should be evaluated by EUS-FNA to rule out the possibility of double cancers[238]. While MBO generally results from metastatic lymph nodes (Figure 3), obstructive jaundice due to metastasis to the major papilla has been reported[239-241]. Distal pancreatectomy has been reported as an option for cytoreductive surgery in advanced ovarian cancer in a study of six patients with pancreatic metastases[242].
Figure 3.
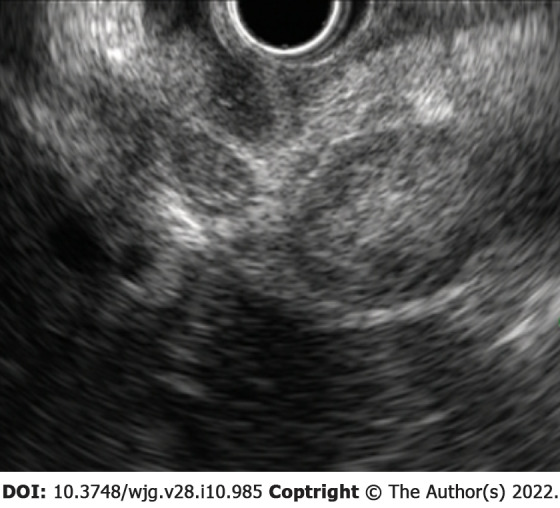
A 69-year-old woman presented with abdominal pain and jaundice 12 mo after surgery for high-grade serous ovarian cancer. Endoscopic ultrasound from the duodenal bulb revealed numerous metastatic lymph nodes obstructing the bile duct by extrinsic compression. Endoscopic biliary drainage was performed, but the patient died 1 mo later.
Less than ten cases of pancreatic metastasis from squamous cell carcinoma of the cervix have been reported[243]. On the other hand, biliary obstruction most often results from external bile duct compression, particularly in the porta hepatis[244]. Cases of periampullary and lymph node metastases requiring percutaneous drainage have been reported[120,245]. Endoscopic drainage has only been reported in one case to date[244].
There are only two reports of pancreatic metastasis of endometrial carcinoma, with no reported obstructive jaundice[246,247]. While endometrial carcinoma is generally discovered early, caution is required for the papillary serious and clear cell types due to their tendency for early metastasis[246].
Urological cancers
While there are no more than ten reports of prostate cancer metastasizing to the pancreas, an autopsy study found pancreatic metastases in 1.4% of cases[248-251]. Diagnosis with immunohistochemical staining for prostatic-specific antigen and successful ERCP have been reported[248].
Pancreatic metastases were reported in four urothelial carcinoma cases, of which two presented with obstructive jaundice[252]. Lesions could generally be diagnosed by EUS-FNA, with the help of immunohistochemistry. Bile duct wall metastasis has been reported from the aggressive micropapillary variant of bladder carcinoma, which was successfully drained by ERCP[253].
Esophageal cancer
There are more than ten reports of esophageal squamous cell carcinoma metastasizing to the pancreas[54,254-259]. Such metastases can be synchronous or metachronous and may present as isolated metastases. Four cases underwent distal pancreatectomy among other treatments, with no short-term recurrence[255-258]. One case presented as a cystic lesion which was diagnosed by EUS-FNA[259]. Five caused MBO, while lesions of all single case reports were located in the pancreatic body or tail. There is one report of MBO from recurrent esophageal cancer after esophagectomy which was first treated by ERCP and subsequently retreated by EUS-guided hepatico-gastrictubestomy when duodenal invasion precluded repeat ERCP[260].
Pure primary squamous cell carcinoma is a rare but important differential diagnosis, of which at least 54 cases have been reported[261]. Slightly over half are located in the head and 55% have metastases at the time of diagnosis. MBO caused by metastatic squamous cell lung carcinoma has also been reported, requiring investigation for a lung primary[262].
Other primaries
There are isolated reports of pancreatic metastases from almost every malignancy, including meningioma, hemangiopericytoma, tonsillar squamous cell carcinoma, adenoid cystic carcinoma, hypopharyngeal carcinoma, thymoma, malignant pleural mesothelioma, pulmonary primitive neuroectodermal tumor, nephroblastoma, gastrointestinal stromal tumor, adrenocortical carcinoma, and testicular teratoma[263-275].
CONCLUSION
Metastases from various primaries can cause MBO. The reported incidence pancreatic metastases vary across studies, depending on the selected modality (Table 2). While most can be diagnosed by EUS-FNA and treated by percutaneous or endoscopic drainage, factors specific to each primary should be kept in mind (Table 3). Surgery may be indicated in isolated metastases. There may be hope for long-term survival if systemic therapy can be resumed after biliary drainage or after margin-free resection of isolated metastases.
Table 2.
Relative frequency of pancreatic metastases by modality/procedure
|
Modality/procedure
|
Total cases
|
Renal
|
Lung
|
Breast
|
Stomach
|
Colon
|
Sarcoma
|
Melanoma
|
Ovary
|
Thyroid
|
Esophagus
|
Lymphoma
|
Other
|
| CT | 192 | 30% | 26% | 10% | 7% | 5% | 6% | 4% | 3% | 3% | 0% | 0% | 7% |
| ERCP | 307 | 1% | 3% | 4% | 27% | 38% | 0% | 2% | 2% | 0% | 2% | 1% | 20% |
| EUS-FNA | 515 | 40% | 20% | 6% | 2% | 9% | 3% | 10% | 3% | 1% | 2% | 0% | 3% |
| Surgery | 399 | 63% | 3% | 3% | 3% | 6% | 7% | 4% | 5% | 0% | 0% | 0% | 6% |
| Autopsy | 184 | 3% | 28% | 4% | 22% | 1% | 2% | 1% | 2% | 1% | 0% | 5% | 31% |
| Weighted average1 | 1597 | 33% | 14% | 5% | 10% | 12% | 4% | 5% | 3% | 1% | 1% | 1% | 11% |
Excludes duplicate data from the same institution during the same period using the same modality. Does not consider possible data duplication across studies of different modalities.
CT: Computed tomography; ERCP: Endoscopic retrograde cholangiopancreatography; EUS-FNA: Endoscopic ultrasound-guided fine-needle aspiration.
Table 3.
Characteristics of malignant biliary obstruction caused by various primary malignancies
|
|
Unique characteristics/diagnostic clues
|
Major causes of malignant biliary obstruction
|
| Renal cell carcinoma | Enhancing lesion on imaging. Most pancreatic metastases resectable and associated with a good prognosis. Can arise up to 32 years after diagnosis of primary tumor | Pancreatic metastasis (biliary obstruction rare) |
| Lung cancer | Most reported in small cell lung cancer. Possible primary small cell biliary cancer | Pancreatic metastasis |
| Gastric cancer | Most cases present after surgery for Borrmann 3 antral lesions. Possible need for double stenting due to gastric outlet obstruction | Lymph nodes, liver metastasis, direct invasion |
| Colorectal cancer | Intraductal growth can mimic bile duct cancer | Liver metastasis, lymph nodes |
| Breast cancer | Most reported in invasive lobular carcinoma. Possible duodenal obstructionCan arise up to 32 years after diagnosis of primary tumor | Lymph nodes, pancreatic metastasis |
| Melanoma | Possible pancreato-biliary primary | Pancreatic metastasis |
| Lymphoma | More common in non-Hodgkin's lymphoma. Possible pancreato-biliary primary | Lymph nodes |
| Sarcoma | Possible pancreato-biliary primary | Pancreatic metastasis |
Footnotes
Conflict-of-interest statement: The author has no financial disclosures or conflicts of interest to declare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 20, 2021
First decision: July 3, 2021
Article in press: February 15, 2022
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang XF S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
References
- 1.Ito T, Takada R, Omoto S, Tsuda M, Masuda D, Kato H, Matsumoto T, Moriyama I, Okabe Y, Shiomi H, Ishida E, Hatamaru K, Hashimoto S, Tanaka K, Kawamoto H, Yanagisawa A, Katayama T, Yazumi S Biliopancreatic Study Group. Analysis of Prognostic Factors in Pancreatic Metastases: A Multicenter Retrospective Analysis. Pancreas. 2018;47:1033–1039. doi: 10.1097/MPA.0000000000001132. [DOI] [PubMed] [Google Scholar]
- 2.Masetti M, Zanini N, Martuzzi F, Fabbri C, Mastrangelo L, Landolfo G, Fornelli A, Burzi M, Vezzelli E, Jovine E. Analysis of prognostic factors in metastatic tumors of the pancreas: a single-center experience and review of the literature. Pancreas. 2010;39:135–143. doi: 10.1097/MPA.0b013e3181bae9b3. [DOI] [PubMed] [Google Scholar]
- 3.Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary tumors of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004;444:527–535. doi: 10.1007/s00428-004-0987-3. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura E, Shimizu M, Itoh T, Manabe T. Secondary tumors of the pancreas: clinicopathological study of 103 autopsy cases of Japanese patients. Pathol Int. 2001;51:686–690. doi: 10.1046/j.1440-1827.2001.01258.x. [DOI] [PubMed] [Google Scholar]
- 5.Shijo M, Fukase K, Ohtsuka H, Ariake K, Masuda K, Ishida M, Mizuma M, Nakagawa K, Hayashi H, Morikawa T, Motoi F, Naitoh T, Unno M. Metastasis of ovarian cancer to the bile duct: a case report. Surg Case Rep. 2019;5:100. doi: 10.1186/s40792-019-0659-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawakatsu S, Kaneoka Y, Maeda A, Takayama Y, Fukami Y, Onoe S. Intrapancreatic bile duct metastasis from colon cancer after resection of liver metastasis with intrabiliary growth: a case report. World J Surg Oncol. 2015;13:254. doi: 10.1186/s12957-015-0676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa Y, Maeda A, Seita K, Kaneoka Y. Lower bile duct metastasis from rectal cancer after surgery for liver metastasis and intrahepatic bile duct metastasis: a case report. BMC Surg. 2020;20:137. doi: 10.1186/s12893-020-00799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rana SS, Kapoor R, Gupta P, Gupta R. Extrahepatic biliary obstruction due to bile duct metastasis from primary esophageal squamous cell carcinoma: a rare cause of jaundice. Ann Gastroenterol. 2019;32:528. doi: 10.20524/aog.2019.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akiyama H, Sato T, Toyoda S, Yamada H. An extrahepatic bile duct metastasis from a gallbladder cancer mimicking Mirizzi's syndrome. Am J Gastroenterol. 1999;94:508–510. doi: 10.1111/j.1572-0241.1999.885_n.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim YC, Park MS. Distal common bile duct metastasis from hepatocellular carcinoma. Hepatology. 2012;55:1638–1639. doi: 10.1002/hep.25502. [DOI] [PubMed] [Google Scholar]
- 11.Satake M, Furutani T, Ozawa H, Konishi T, Yasunaga M. [A case of extrahepatic bile duct metastasis from gastric cancer] Nihon Shokakibyo Gakkai Zasshi. 2013;110:412–418. [PubMed] [Google Scholar]
- 12.Tang J, Zhao GX, Deng SS, Xu M. Rare common bile duct metastasis of breast cancer: A case report and literature review. World J Gastrointest Oncol. 2021;13:147–156. doi: 10.4251/wjgo.v13.i2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verbanck JJ, Rutgeerts LJ, van Aelst FJ, Tytgat JH, Decoster JM, Noyez DN, Theunynck PJ, Geboes KJ. Primary malignant melanoma of the gallbladder, metastatic to the common bile duct. Gastroenterology. 1986;91:214–218. doi: 10.1016/0016-5085(86)90461-0. [DOI] [PubMed] [Google Scholar]
- 14.Ferrozzi F, Bova D, Campodonico F, Chiara FD, Passari A, Bassi P. Pancreatic metastases: CT assessment. Eur Radiol. 1997;7:241–245. doi: 10.1007/s003300050144. [DOI] [PubMed] [Google Scholar]
- 15.Klein KA, Stephens DH, Welch TJ. CT characteristics of metastatic disease of the pancreas. Radiographics. 1998;18:369–378. doi: 10.1148/radiographics.18.2.9536484. [DOI] [PubMed] [Google Scholar]
- 16.Tsitouridis I, Diamantopoulou A, Michaelides M, Arvanity M, Papaioannou S. Pancreatic metastases: CT and MRI findings. Diagn Interv Radiol. 2010;16:45–51. doi: 10.4261/1305-3825.DIR.1996-08.1. [DOI] [PubMed] [Google Scholar]
- 17.Angelelli G, Mancini M, Pignataro P, Pedote P, Scardapane A. Multidetector computed tomography in the study of pancreatic metastases. Radiol Med. 2012;117:369–377. doi: 10.1007/s11547-011-0736-z. [DOI] [PubMed] [Google Scholar]
- 18.Shi HY, Zhao XS, Miao F. Metastases to the Pancreas: Computed Tomography Imaging Spectrum and Clinical Features: A Retrospective Study of 18 Patients With 36 Metastases. Medicine (Baltimore) 2015;94:e913. doi: 10.1097/MD.0000000000000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi TW, Kim SH, Shin CI, Han JK, Choi BI. MDCT findings of pancreatic metastases according to primary tumors. Abdom Imaging. 2015;40:1595–1607. doi: 10.1007/s00261-014-0299-2. [DOI] [PubMed] [Google Scholar]
- 20.Galia M, Albano D, Picone D, Terranova MC, Agrusa A, Di Buono G, Licata A, Lo Re G, La Grutta L, Midiri M. Imaging features of pancreatic metastases: A comparison with pancreatic ductal adenocarcinoma. Clin Imaging. 2018;51:76–82. doi: 10.1016/j.clinimag.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Triantopoulou C, Kolliakou E, Karoumpalis I, Yarmenitis S, Dervenis C. Metastatic disease to the pancreas: an imaging challenge. Insights Imaging. 2012;3:165–172. doi: 10.1007/s13244-011-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu S, Zhang J, Zuo C, Cheng C, Liu Q, Sun G. (18)F-FDG-PET/CT findings in pancreatic metastasis. Radiol Med. 2015;120:887–898. doi: 10.1007/s11547-014-0473-1. [DOI] [PubMed] [Google Scholar]
- 23.Hijioka S, Matsuo K, Mizuno N, Hara K, Mekky MA, Vikram B, Hosoda W, Yatabe Y, Shimizu Y, Kondo S, Tajika M, Niwa Y, Tamada K, Yamao K. Role of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in diagnosing metastasis to the pancreas: a tertiary center experience. Pancreatology. 2011;11:390–398. doi: 10.1159/000330536. [DOI] [PubMed] [Google Scholar]
- 24.Fusaroli P, D'Ercole MC, De Giorgio R, Serrani M, Caletti G. Contrast harmonic endoscopic ultrasonography in the characterization of pancreatic metastases (with video) Pancreas. 2014;43:584–587. doi: 10.1097/MPA.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Jin H, Liao D, Qian B, Zhang Y, Xu M, Han S. Contrast-enhanced harmonic endoscopic ultrasonography for the differential diagnosis of pancreatic masses: A systematic review and meta-analysis. Mol Clin Oncol. 2019;11:425–433. doi: 10.3892/mco.2019.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iglesias-Garcia J, Lindkvist B, Lariño-Noia J, Abdulkader-Nallib I, Dominguez-Muñoz JE. Differential diagnosis of solid pancreatic masses: contrast-enhanced harmonic (CEH-EUS), quantitative-elastography (QE-EUS), or both? United European Gastroenterol J. 2017;5:236–246. doi: 10.1177/2050640616640635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raymond SLT, Yugawa D, Chang KHF, Ena B, Tauchi-Nishi PS. Metastatic neoplasms to the pancreas diagnosed by fine-needle aspiration/biopsy cytology: A 15-year retrospective analysis. Diagn Cytopathol. 2017;45:771–783. doi: 10.1002/dc.23752. [DOI] [PubMed] [Google Scholar]
- 28.Smith AL, Odronic SI, Springer BS, Reynolds JP. Solid tumor metastases to the pancreas diagnosed by FNA: A single-institution experience and review of the literature. Cancer Cytopathol. 2015;123:347–355. doi: 10.1002/cncy.21541. [DOI] [PubMed] [Google Scholar]
- 29.Waters L, Si Q, Caraway N, Mody D, Staerkel G, Sneige N. Secondary tumors of the pancreas diagnosed by endoscopic ultrasound-guided fine-needle aspiration: a 10-year experience. Diagn Cytopathol. 2014;42:738–743. doi: 10.1002/dc.23114. [DOI] [PubMed] [Google Scholar]
- 30.Ioakim KJ, Sydney GI, Michaelides C, Sepsa A, Psarras K, Tsiotos GG, Salla C, Nikas IP. Evaluation of metastases to the pancreas with fine needle aspiration: A case series from a single centre with review of the literature. Cytopathology. 2020;31:96–105. doi: 10.1111/cyt.12793. [DOI] [PubMed] [Google Scholar]
- 31.Giovannini M, Seitz JF, Monges G, Perrier H, Rabbia I. Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy. 1995;27:171–177. doi: 10.1055/s-2007-1005657. [DOI] [PubMed] [Google Scholar]
- 32.Ardengh JC, Lopes CV, Kemp R, Venco F, de Lima-Filho ER, dos Santos JS. Accuracy of endoscopic ultrasound-guided fine-needle aspiration in the suspicion of pancreatic metastases. BMC Gastroenterol. 2013;13:63. doi: 10.1186/1471-230X-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, Vickers SM, Wilcox CM. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663–2668. doi: 10.1111/j.1572-0241.2003.08666.x. [DOI] [PubMed] [Google Scholar]
- 34.Matsui T, Nishikawa K, Yukimoto H, Katsuta K, Nakamura Y, Tanaka S, Oiwa M, Nakahashi H, Shomi Y, Haruki Y, Taniguchi K, Shimomura M, Isaji S. Needle tract seeding following endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: a report of two cases. World J Surg Oncol. 2019;17:134. doi: 10.1186/s12957-019-1681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridtitid W, Rerknimitr R, Janchai A, Kongkam P, Treeprasertsuk S, Kullavanijaya P. Outcome of second interventions for occluded metallic stents in patients with malignant biliary obstruction. Surg Endosc. 2010;24:2216–2220. doi: 10.1007/s00464-010-0931-3. [DOI] [PubMed] [Google Scholar]
- 36.Flores Carmona DY, Alonso Lárraga JO, Hernández Guerrero A, Ramírez Solís ME. Comparison of covered and uncovered self-expandable stents in the treatment of malignant biliary obstruction. Rev Esp Enferm Dig. 2016;108:246–249. doi: 10.17235/reed.2016.4161/2015. [DOI] [PubMed] [Google Scholar]
- 37.Kaw M, Singh S, Gagneja H. Clinical outcome of simultaneous self-expandable metal stents for palliation of malignant biliary and duodenal obstruction. Surg Endosc. 2003;17:457–461. doi: 10.1007/s00464-002-8541-3. [DOI] [PubMed] [Google Scholar]
- 38.Kahaleh M, Talreja JP, Loren DE, Kowalski TE, Poneros JM, Degaetani M, Raijman I, Sejpal DV, Patel S, Rosenkranz L, McNamara KN, Brijbassie A, Wang AY, Gaidhane M, Sethi A, Stevens PD. Evaluation of a fully covered self-expanding metal stent with flared ends in malignant biliary obstruction: a multicenter study. J Clin Gastroenterol. 2013;47:e96–100. doi: 10.1097/MCG.0b013e3182951a32. [DOI] [PubMed] [Google Scholar]
- 39.McDougall NI, Edmunds SE. An audit of metal stent palliation for malignant biliary obstruction. J Gastroenterol Hepatol. 2001;16:1051–1054. doi: 10.1046/j.1440-1746.2001.02582.x. [DOI] [PubMed] [Google Scholar]
- 40.Haag GM, Herrmann T, Jaeger D, Stremmel W, Schemmer P, Sauer P, Gotthardt DN. Outcomes and risk factors for cancer patients undergoing endoscopic intervention of malignant biliary obstruction. BMC Gastroenterol. 2015;15:171. doi: 10.1186/s12876-015-0399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deprez PH, Moreels TG, Aouattah T, Piessevaux H, Pérez-Cuadrado-Robles E. A new 12-French plastic stent for unresectable distal malignant biliary obstruction. Endoscopy. 2020;52:474–482. doi: 10.1055/a-1120-8498. [DOI] [PubMed] [Google Scholar]
- 42.van Berkel AM, Bruno MJ, Bergman JJ, van Deventer SJ, Tytgat GN, Huibregtse K. A prospective randomized study of hydrophilic polymer-coated polyurethane vs polyethylene stents in distal malignant biliary obstruction. Endoscopy. 2003;35:478–482. doi: 10.1055/s-2003-39666. [DOI] [PubMed] [Google Scholar]
- 43.van Berkel AM, Boland C, Redekop WK, Bergman JJ, Groen AK, Tytgat GN, Huibregtse K. A prospective randomized trial of Teflon vs polyethylene stents for distal malignant biliary obstruction. Endoscopy. 1998;30:681–686. doi: 10.1055/s-2007-1001388. [DOI] [PubMed] [Google Scholar]
- 44.Lee TH, Choi JH, Park do H, Song TJ, Kim DU, Paik WH, Hwangbo Y, Lee SS, Seo DW, Lee SK, Kim MH. Similar Efficacies of Endoscopic Ultrasound-guided Transmural and Percutaneous Drainage for Malignant Distal Biliary Obstruction. Clin Gastroenterol Hepatol. 2016;14:1011–1019.e3. doi: 10.1016/j.cgh.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 45.Khashab MA, Valeshabad AK, Afghani E, Singh VK, Kumbhari V, Messallam A, Saxena P, El Zein M, Lennon AM, Canto MI, Kalloo AN. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci. 2015;60:557–565. doi: 10.1007/s10620-014-3300-6. [DOI] [PubMed] [Google Scholar]
- 46.Chennat J, Waxman I. Initial performance profile of a new 6F self-expanding metal stent for palliation of malignant hilar biliary obstruction. Gastrointest Endosc. 2010;72:632–636. doi: 10.1016/j.gie.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 47.Polydorou AA, Cairns SR, Dowsett JF, Hatfield AR, Salmon PR, Cotton PB, Russell RC. Palliation of proximal malignant biliary obstruction by endoscopic endoprosthesis insertion. Gut. 1991;32:685–689. doi: 10.1136/gut.32.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawakubo K, Kawakami H, Toyokawa Y, Otani K, Kuwatani M, Abe Y, Kawahata S, Kubo K, Kubota Y, Sakamoto N. Risk factors for technical failure of endoscopic double self-expandable metallic stent placement by partial stent-in-stent method. J Hepatobiliary Pancreat Sci. 2015;22:79–85. doi: 10.1002/jhbp.170. [DOI] [PubMed] [Google Scholar]
- 49.Yamauchi H, Kida M, Okuwaki K, Miyazawa S, Iwai T, Imaizumi H, Eiji M, Hasegawa R, Koizumi W. A Case Series: Outcomes of Endoscopic Biliary Self-Expandable Metal Stent for Malignant Biliary Obstruction with Surgically Altered Anatomy. Dig Dis Sci. 2016;61:2436–2441. doi: 10.1007/s10620-016-4148-8. [DOI] [PubMed] [Google Scholar]
- 50.Takeda T, Sasaki T, Mie T, Furukawa T, Kanata R, Kasuga A, Matsuyama M, Ozaka M, Sasahira N. The safety and efficacy of self-expandable metallic stent placement for malignant biliary obstruction with surgically altered anatomy. Scand J Gastroenterol. 2021;56:94–102. doi: 10.1080/00365521.2020.1847317. [DOI] [PubMed] [Google Scholar]
- 51.Park JH, Song HY, Kim SH, Shin JH, Kim JH, Kim BS, Yook JH. Metallic stent placement in patients with recurrent malignant obstruction in the surgically altered stomach. Ann Surg Oncol. 2014;21:2036–2043. doi: 10.1245/s10434-014-3566-0. [DOI] [PubMed] [Google Scholar]
- 52.Tanisaka Y, Ryozawa S, Mizuide M, Fujita A, Ogawa T, Tashima T, Noguchi T, Suzuki M, Katsuda H, Araki R. Usefulness of self-expandable metal stents for malignant biliary obstruction using a short-type single-balloon enteroscope in patients with surgically altered anatomy. J Hepatobiliary Pancreat Sci. 2021;28:272–279. doi: 10.1002/jhbp.889. [DOI] [PubMed] [Google Scholar]
- 53.Bear HD, Turner MA, Parker GA, Lawrence W Jr, Horsley JS 3rd, Messmer JM, Cho SR. Treatment of biliary obstruction caused by metastatic cancer. Am J Surg. 1989;157:381–5; discussion 385. doi: 10.1016/0002-9610(89)90580-1. [DOI] [PubMed] [Google Scholar]
- 54.Vandenabeele LAM, Dhondt E, Geboes KP, Defreyne L. Percutaneous stenting in malignant biliary obstruction caused by metastatic disease: clinical outcome and prediction of survival according to tumor type and further therapeutic options. Acta Gastroenterol Belg. 2017;80:249–255. [PubMed] [Google Scholar]
- 55.Ferraz Gonçalves JA, Rosendo E, Sousa L, Lopes AR, Leão I, Queirós R, Marote S, Sousa MJ. Complications of Biliary Drainage in Patients with Malignant Biliary Obstruction. J Gastrointest Cancer. 2020 doi: 10.1007/s12029-020-00541-6. [DOI] [PubMed] [Google Scholar]
- 56.Yuan P, Zhang L, Li S, Li X, Wu Q. Clinical results after biliary drainage by endoscopic retrograde cholangiopancreatography for analysis of metastatic cancer survival and prognostic factors. Surg Endosc. 2020 doi: 10.1007/s00464-020-08121-2. [DOI] [PubMed] [Google Scholar]
- 57.De Cassan C, Bories E, Pesenti C, Caillol F, Godat S, Ratone JP, Delpero JR, Ewald J, Giovannini M. Use of partially covered and uncovered metallic prosthesis for endoscopic ultrasound-guided hepaticogastrostomy: Results of a retrospective monocentric study. Endosc Ultrasound. 2017;6:329–335. doi: 10.4103/2303-9027.209869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Konstantinidis IT, Dursun A, Zheng H, Wargo JA, Thayer SP, Fernandez-del Castillo C, Warshaw AL, Ferrone CR. Metastatic tumors in the pancreas in the modern era. J Am Coll Surg. 2010;211:749–753. doi: 10.1016/j.jamcollsurg.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwak JH, Heo JS, Park JY, Choi DW, Choi SH, Lee HS. Outcomes of pancreaticoduodenectomy in patients with metastatic cancer. Korean J Hepatobiliary Pancreat Surg. 2014;18:147–151. doi: 10.14701/kjhbps.2014.18.4.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sperti C, Pasquali C, Liessi G, Pinciroli L, Decet G, Pedrazzoli S. Pancreatic resection for metastatic tumors to the pancreas. J Surg Oncol. 2003;83:161–6; discussion 166. doi: 10.1002/jso.10262. [DOI] [PubMed] [Google Scholar]
- 61.Endo Y, Noda H, Watanabe F, Kato T, Kakizawa N, Ichida K, Kasahara N, Rikiyama T. A Retrospective Analysis of Preoperative Evaluation and Surgical Resection for Metastatic Tumors of the Pancreas. Indian J Surg Oncol. 2019;10:251–257. doi: 10.1007/s13193-019-00905-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dar FS, Mukherjee S, Bhattacharya S. Surgery for secondary tumors of the pancreas. HPB (Oxford) 2008;10:498–500. doi: 10.1080/13651820802356598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.You DD, Choi DW, Choi SH, Heo JS, Kim WS, Ho CY, Lee HG. Surgical resection of metastasis to the pancreas. J Korean Surg Soc. 2011;80:278–282. doi: 10.4174/jkss.2011.80.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adler H, Redmond CE, Heneghan HM, Swan N, Maguire D, Traynor O, Hoti E, Geoghegan JG, Conlon KC. Pancreatectomy for metastatic disease: a systematic review. Eur J Surg Oncol. 2014;40:379–386. doi: 10.1016/j.ejso.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 65.Reddy S, Wolfgang CL. The role of surgery in the management of isolated metastases to the pancreas. Lancet Oncol. 2009;10:287–293. doi: 10.1016/S1470-2045(09)70065-8. [DOI] [PubMed] [Google Scholar]
- 66.Hung JH, Wang SE, Shyr YM, Su CH, Chen TH, Wu CW. Resection for secondary malignancy of the pancreas. Pancreas. 2012;41:121–129. doi: 10.1097/MPA.0b013e31821fc8f2. [DOI] [PubMed] [Google Scholar]
- 67.Lee SR, Gemenetzis G, Cooper M, Javed AA, Cameron JL, Wolfgang CL, Eckhauser FE, He J, Weiss MJ. Long-Term Outcomes of 98 Surgically Resected Metastatic Tumors in the Pancreas. Ann Surg Oncol. 2017;24:801–807. doi: 10.1245/s10434-016-5619-z. [DOI] [PubMed] [Google Scholar]
- 68.Sperti C, Moletta L, Patanè G. Metastatic tumors to the pancreas: The role of surgery. World J Gastrointest Oncol. 2014;6:381–392. doi: 10.4251/wjgo.v6.i10.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sellner F. Isolated Pancreatic Metastases of Renal Cell Carcinoma-A Paradigm of a Seed and Soil Mechanism: A Literature Analysis of 1,034 Observations. Front Oncol. 2020;10:709. doi: 10.3389/fonc.2020.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fahlbusch T, Luu AM, Braumann C, Lukas C, Uhl W, Künzli BM. Lipomatous pancreas facilitates late onset of renal cell carcinoma metastases. Acta Chir Belg. 2020:1–6. doi: 10.1080/00015458.2020.1765672. [DOI] [PubMed] [Google Scholar]
- 71.Kalra S, Atkinson BJ, Matrana MR, Matin SF, Wood CG, Karam JA, Tamboli P, Sircar K, Rao P, Corn PG, Tannir NM, Jonasch E. Prognosis of patients with metastatic renal cell carcinoma and pancreatic metastases. BJU Int. 2016;117:761–765. doi: 10.1111/bju.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grassi P, Doucet L, Giglione P, Grünwald V, Melichar B, Galli L, De Giorgi U, Sabbatini R, Ortega C, Santoni M, Bamias A, Verzoni E, Derosa L, Studentova H, Pacifici M, Coppa J, Mazzaferro V, de Braud F, Porta C, Escudier B, Procopio G. Clinical Impact of Pancreatic Metastases from Renal Cell Carcinoma: A Multicenter Retrospective Analysis. PLoS One. 2016;11:e0151662. doi: 10.1371/journal.pone.0151662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grassi P, Verzoni E, Mariani L, De Braud F, Coppa J, Mazzaferro V, Procopio G. Prognostic role of pancreatic metastases from renal cell carcinoma: results from an Italian center. Clin Genitourin Cancer. 2013;11:484–488. doi: 10.1016/j.clgc.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 74.Singla N, Xie Z, Zhang Z, Gao M, Yousuf Q, Onabolu O, McKenzie T, Tcheuyap VT, Ma Y, Choi J, McKay R, Christie A, Torras OR, Bowman IA, Margulis V, Pedrosa I, Przybycin C, Wang T, Kapur P, Rini B, Brugarolas J. Pancreatic tropism of metastatic renal cell carcinoma. JCI Insight. 2020;5 doi: 10.1172/jci.insight.134564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koga H, Yane K, Maguchi H, Takahashi K, Katanuma A, Kin T, Ambo Y, Omori Y, Shinohara T. Cystic Duct Metastasis from Renal Cell Carcinoma. Intern Med. 2018;57:213–218. doi: 10.2169/internalmedicine.9228-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miyagishima T, Ohnishi S, Chuma M, Kishimoto A, Kumagai K, Ishizuka J, Kobayashi T, Kamiya K, Fujimoto N, Kamiyama T, Ogasawara K, Hata Y, Takahashi T. Intraluminal tumor of the common bile duct as a metastasis of renal cell carcinoma. Intern Med. 1996;35:720–723. doi: 10.2169/internalmedicine.35.720. [DOI] [PubMed] [Google Scholar]
- 77.Sikka A, Adam SZ, Wood C, Hoff F, Harmath CB, Miller FH. Magnetic resonance imaging of pancreatic metastases from renal cell carcinoma. Clin Imaging. 2015;39:945–953. doi: 10.1016/j.clinimag.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 78.Pannala R, Hallberg-Wallace KM, Smith AL, Nassar A, Zhang J, Zarka M, Reynolds JP, Chen L. Endoscopic ultrasound-guided fine needle aspiration cytology of metastatic renal cell carcinoma to the pancreas: A multi-center experience. Cytojournal. 2016;13:24. doi: 10.4103/1742-6413.192191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hasan S, Khan Z, Khan MS, Darr U, Javaid T, Ahmed R, Nawras A. Renal Cell Carcinoma Presenting as an Ampullary Mass: A Case Report and Review of Literature. Gastroenterology Res. 2018;11:231–234. doi: 10.14740/gr981w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iqbal R, Wiadji E. Metastatic renal cell carcinoma presenting as jaundice with biliary and gastric outlet obstruction. A case report. J Surg Case Rep. 2021;2021:rjaa591. doi: 10.1093/jscr/rjaa591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bassi C, Butturini G, Falconi M, Sargenti M, Mantovani W, Pederzoli P. High recurrence rate after atypical resection for pancreatic metastases from renal cell carcinoma. Br J Surg. 2003;90:555–559. doi: 10.1002/bjs.4072. [DOI] [PubMed] [Google Scholar]
- 82.Jaen-Torrejimeno I, Rojas-Holguín A, López-Guerra D, Ramia JM, Blanco-Fernández G. Pancreatic resection for metastatic renal cell carcinoma. A systematic review. HPB (Oxford) 2020;22:479–486. doi: 10.1016/j.hpb.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 83.Malleo G, Salvia R, Maggino L, Marchegiani G, D'Angelica M, DeMatteo R, Kingham P, Pulvirenti A, Sereni E, Jarnagin WR, Bassi C, Allen PJ, Butturini G. Long-term Outcomes After Surgical Resection of Pancreatic Metastases from Renal Clear-Cell Carcinoma. Ann Surg Oncol. 2021;28:3100–3108. doi: 10.1245/s10434-021-09649-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sellner F, Tykalsky N, De Santis M, Pont J, Klimpfinger M. Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: an indication for pancreatic surgery. Ann Surg Oncol. 2006;13:75–85. doi: 10.1245/ASO.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 85.Schwarz L, Sauvanet A, Regenet N, Mabrut JY, Gigot JF, Housseau E, Millat B, Ouaissi M, Gayet B, Fuks D, Tuech JJ. Long-term survival after pancreatic resection for renal cell carcinoma metastasis. Ann Surg Oncol. 2014;21:4007–4013. doi: 10.1245/s10434-014-3821-4. [DOI] [PubMed] [Google Scholar]
- 86.Santoni M, Conti A, Partelli S, Porta C, Sternberg CN, Procopio G, Bracarda S, Basso U, De Giorgi U, Derosa L, Rizzo M, Ortega C, Massari F, Iacovelli R, Milella M, Di Lorenzo G, Buti S, Cerbone L, Burattini L, Montironi R, Santini D, Falconi M, Cascinu S. Surgical resection does not improve survival in patients with renal metastases to the pancreas in the era of tyrosine kinase inhibitors. Ann Surg Oncol. 2015;22:2094–2100. doi: 10.1245/s10434-014-4256-7. [DOI] [PubMed] [Google Scholar]
- 87.Chrom P, Stec R, Bodnar L, Szczylik C. Prognostic Significance of Pancreatic Metastases from Renal Cell Carcinoma in Patients Treated with Tyrosine Kinase Inhibitors. Anticancer Res. 2018;38:359–365. doi: 10.21873/anticanres.12230. [DOI] [PubMed] [Google Scholar]
- 88.Saito J, Yamanaka K, Sato M, Mori N, Sekii K, Yoshioka T, Itatani H, Nakatsuka S. Four cases of advanced renal cell carcinoma with pancreatic metastasis successfully treated with radiation therapy. Int J Clin Oncol. 2009;14:258–261. doi: 10.1007/s10147-008-0833-8. [DOI] [PubMed] [Google Scholar]
- 89.Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med. 2020; 41: 1-24. [DOI] [PubMed] [Google Scholar]
- 90.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) SEER Cancer Statistics Review, 1975-2016. [cited March 6, 2021]. Available from: https://seer.cancer.gov/csr/1975_2016/
- 91.Maeno T, Satoh H, Ishikawa H, Yamashita YT, Naito T, Fujiwara M, Kamma H, Ohtsuka M, Hasegawa S. Patterns of pancreatic metastasis from lung cancer. Anticancer Res. 1998;18:2881–2884. [PubMed] [Google Scholar]
- 92.Liratzopoulos N, Efremidou EI, Papageorgiou MS, Romanidis K, Minopoulos GJ, Manolas KJ. Extrahepatic biliary obstruction due to a solitary pancreatic metastasis of squamous cell lung carcinoma. Case report. J Gastrointestin Liver Dis. 2006;15:73–75. [PubMed] [Google Scholar]
- 93.Matsukuma S, Suda K, Abe H, Ogata S, Wada R. Metastatic cancer involving pancreatic duct epithelium and its mimicry of primary pancreatic cancer. Histopathology. 1997;30:208–213. doi: 10.1046/j.1365-2559.1997.d01-604.x. [DOI] [PubMed] [Google Scholar]
- 94.Gonlugur U, Mirici A, Karaayvaz M. Pancreatic involvement in small cell lung cancer. Radiol Oncol. 2014;48:11–19. doi: 10.2478/raon-2013-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Castro-Pocas FM, Araújo TP, Ferreira ML, Saraiva MM. The role of endoscopic ultrasound in a case of lung cancer with jaundice. Endosc Ultrasound. 2018;7:279–281. doi: 10.4103/2303-9027.193570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu M, Zheng L, Han D, Wang Y, Ren L, Lu Y, Zhang S. Systemic Chemotherapy in Metastasis-Induced Acute Pancreatitis Patients With Small Cell Lung Cancer. Pancreas. 2019;48:1303–1306. doi: 10.1097/MPA.0000000000001442. [DOI] [PubMed] [Google Scholar]
- 97.Kawaguchi S, Ohtsu T, Terada S, Endo S. Obstructive pancreatitis secondary to a pancreatic metastasis from lung cancer treated with nasopancreatic drainage. Clin J Gastroenterol. 2019;12:382–386. doi: 10.1007/s12328-019-00944-4. [DOI] [PubMed] [Google Scholar]
- 98.Mori T, Kondo H, Naitoh I, Koyama T, Takenaka Y, Komai H, Araki S, Kitagawa M, Nishigaki N, Tanaka Y, Itoh K, Hasegawa C, Kawai T, Hayashi K. Endoscopic Ultrasonography-guided Fine-needle Aspiration Revealed Metastasis-induced Acute Pancreatitis in a Patient with Adrenocortical Carcinoma. Intern Med. 2019;58:2645–2649. doi: 10.2169/internalmedicine.2450-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Okamoto T, Nakamura K, Fukuda K. Endoscopic retrograde cholangiopancreatography for bile duct obstruction due to metastatic breast cancer. Dig Endosc. 2020;32:1118. doi: 10.1111/den.13835. [DOI] [PubMed] [Google Scholar]
- 100.Chowhan NM, Madajewicz S. Management of metastases-induced acute pancreatitis in small cell carcinoma of the lung. Cancer. 1990;65:1445–1448. doi: 10.1002/1097-0142(19900315)65:6<1445::aid-cncr2820650632>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 101.Stewart KC, Dickout WJ, Urschel JD. Metastasis-induced acute pancreatitis as the initial manifestation of bronchogenic carcinoma. Chest. 1993;104:98–100. doi: 10.1378/chest.104.1.98. [DOI] [PubMed] [Google Scholar]
- 102.Krasinskas AM, Chiosea SI, Pal T, Dacic S. KRAS mutational analysis and immunohistochemical studies can help distinguish pancreatic metastases from primary lung adenocarcinomas. Mod Pathol. 2014;27:262–270. doi: 10.1038/modpathol.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ochi N, Takigawa N, Yasugi M, Ishida E, Kawamoto H, Taniguchi A, Harada D, Hayashi E, Toda H, Yanai H, Tanimoto M, Kiura K. Obstructive jaundice at the initial presentation in small-cell lung cancer. Int Med Case Rep J. 2010;3:9–12. doi: 10.2147/imcrj.s8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johnson DH, Hainsworth JD, Greco FA. Extrahepatic biliary obstruction caused by small-cell lung cancer. Ann Intern Med. 1985;102:487–490. doi: 10.7326/0003-4819-102-4-487. [DOI] [PubMed] [Google Scholar]
- 105.Lu T, Li X, Zhou Y. Pancreatic metastasis from squamous cell lung cancer: computed tomography and magnetic resonance imaging findings. J Int Med Res. 2021;49:300060521996188. doi: 10.1177/0300060521996188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cha IH, Kim JN, Kim YS, Ryu SH, Moon JS, Lee HK. Metastatic common bile duct cancer from pulmonary adenocarcinoma presenting as obstructive jaundice. Korean J Gastroenterol. 2013;61:50–53. doi: 10.4166/kjg.2013.61.1.50. [DOI] [PubMed] [Google Scholar]
- 107.Ochi N, Goto D, Yamane H, Yamagishi T, Honda Y, Monobe Y, Kawamoto H, Takigawa N. Obstructive jaundice caused by intraductal metastasis of lung adenocarcinoma. Onco Targets Ther. 2014;7:1847–1850. doi: 10.2147/OTT.S68757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahmed A, Nasir UM, Delle Donna P, Swantic V, Ahmed S, Lenza C. A Rare Presentation of Poorly Differentiated Lung Carcinoma with Duodenal Metastasis and Literature Review. Case Rep Gastroenterol. 2020;14:186–196. doi: 10.1159/000506927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O'Neill RS, Duong T, Dionela W, Rogge C, Brungs D. Pancreatitis and Biliary Obstruction Secondary to Duodenal Metastasis from Rapidly Progressing Lung Adenocarcinoma Treated with Common Bile Duct Stenting. Case Rep Oncol. 2020;13:962–967. doi: 10.1159/000508745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Misra SP, Dwivedi M, Misra V, Dharmani S, Gupta M. Duodenal metastases from squamous cell carcinoma of the lung: endoscopic management of bleeding and biliary and duodenal obstruction. Indian J Gastroenterol. 2004;23:185–186. [PubMed] [Google Scholar]
- 111.Sato M, Okumura T, Kaito K, Kiyoshima M, Asato Y, Uchiumi K, Iijima H, Hashimoto I, Kaburagi T, Amemiya R. Usefulness of FDG-PET/CT in the detection of pancreatic metastases from lung cancer. Ann Nucl Med. 2009;23:49–57. doi: 10.1007/s12149-008-0205-5. [DOI] [PubMed] [Google Scholar]
- 112.DeWitt J, Jowell P, Leblanc J, McHenry L, McGreevy K, Cramer H, Volmar K, Sherman S, Gress F. EUS-guided FNA of pancreatic metastases: a multicenter experience. Gastrointest Endosc. 2005;61:689–696. doi: 10.1016/s0016-5107(05)00287-7. [DOI] [PubMed] [Google Scholar]
- 113.Yatabe Y, Mitsudomi T, Takahashi T. TTF-1 expression in pulmonary adenocarcinomas. Am J Surg Pathol. 2002;26:767–773. doi: 10.1097/00000478-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 114.Wu PS. Pancreatic metastasis from non-small cell lung carcinoma diagnosed on EUS biopsy: report of a rare case and potential pitfall. Int J Clin Exp Pathol. 2020;13:2412–2414. [PMC free article] [PubMed] [Google Scholar]
- 115.Bestari MB, Agustanti N. Obstructive jaundice due to pancreatic metastasis from non-small cell lung cancer. Acta Med Indones. 2013;45:216–219. [PubMed] [Google Scholar]
- 116.Singh D, Vaidya OU, Sadeddin E, Yousef O. Role of endoscopic ultrasound and endoscopic retrograde cholangiopancreatography in isolated pancreatic metastasis from lung cancer. World J Gastrointest Endosc. 2012;4:328–330. doi: 10.4253/wjge.v4.i7.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.DeLuzio MR, Moores C, Dhamija A, Wang Z, Cha C, Boffa DJ, Detterbeck FC, Kim AW. Resection of oligometastatic lung cancer to the pancreas may yield a survival benefit in select patients--a systematic review. Pancreatology. 2015;15:456–462. doi: 10.1016/j.pan.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 118.Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. Metastatic spread in patients with gastric cancer. Oncotarget. 2016;7:52307–52316. doi: 10.18632/oncotarget.10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yokoyama Y, Sakata H, Uekusa T, Tajima Y, Ishimaru M. Solitary pancreatic metastasis of gastric cancer with synchronous pancreatic ductal carcinoma: A case report. Int J Surg Case Rep. 2020;70:164–167. doi: 10.1016/j.ijscr.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iwasaki M, Furuse J, Yoshino M, Konishi M, Kawano N, Kinoshita T, Ryu M. Percutaneous transhepatic biliary drainage for the treatment of obstructive jaundice caused by metastases from nonbiliary and nonpancreatic cancers. Jpn J Clin Oncol. 1996;26:465–468. doi: 10.1093/oxfordjournals.jjco.a023265. [DOI] [PubMed] [Google Scholar]
- 121.Lee BH, Chin SY, Kim SA, Kim KH, Do YS. Obstructive jaundice in gastric carcinoma: cause, site, and relationship to the primary lesion. Abdom Imaging. 1995;20:307–311. doi: 10.1007/BF00203359. [DOI] [PubMed] [Google Scholar]
- 122.Lee J, Gwon DI, Ko GY, Kim JW, Sung KB. Biliary intraductal metastasis from advanced gastric cancer: radiologic and histologic characteristics, and clinical outcomes of percutaneous metallic stent placement. Eur Radiol. 2016;26:1649–1655. doi: 10.1007/s00330-015-3995-6. [DOI] [PubMed] [Google Scholar]
- 123.Migita K, Watanabe A, Yoshioka T, Kinoshita S, Ohyama T. Clinical outcome of malignant biliary obstruction caused by metastatic gastric cancer. World J Surg. 2009;33:2396–2402. doi: 10.1007/s00268-009-0186-0. [DOI] [PubMed] [Google Scholar]
- 124.Hong HP, Seo TS, Cha IH, Yu JR, Mok YJ, Oh JH, Kwon SH, Kim SS, Kim SK. Percutaneous placement of self-expandable metallic stents in patients with obstructive jaundice secondary to metastatic gastric cancer after gastrectomy. Korean J Radiol. 2013;14:789–796. doi: 10.3348/kjr.2013.14.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Song JH, Yoon KY, Lee SH. Prognosis of Malignant Obstructive Jaundice Following Surgery for Gastric Carcinoma. Cancer Res Treat. 2003;35:130–134. doi: 10.4143/crt.2003.35.2.130. [DOI] [PubMed] [Google Scholar]
- 126.Kim GE, Shin HS, Seong JS, Loh JJ, Suh CO, Lee JT, Roh JK, Kim BS, Kim WH, Kim MW. The role of radiation treatment in management of extrahepatic biliary tract metastasis from gastric carcinoma. Int J Radiat Oncol Biol Phys. 1994;28:711–717. doi: 10.1016/0360-3016(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 127.Kasuga A, Ishii H, Ozaka M, Matsusaka S, Chin K, Mizunuma N, Yukisawa S, Matsueda K, Furuse J. Clinical outcome of biliary drainage for obstructive jaundice caused by colorectal and gastric cancers. Jpn J Clin Oncol. 2012;42:1161–1167. doi: 10.1093/jjco/hys168. [DOI] [PubMed] [Google Scholar]
- 128.Makino T, Fujitani K, Tsujinaka T, Hirao M, Kashiwazaki M, Nakamori S, Ikenaga M, Mishima H, Masuda N, Sawamura T. Role of percutaneous transhepatic biliary drainage in patients with obstructive jaundice caused by local recurrence of gastric cancer. Hepatogastroenterology. 2008;55:54–57. [PubMed] [Google Scholar]
- 129.Ogura T, Okuda A, Miyano A, Imanishi M, Nishioka N, Yamada M, Yamda T, Kamiyama R, Masuda D, Higuchi K. EUS-guided vs percutaneous biliary access in patients with obstructive jaundice due to gastric cancer. Dig Liver Dis. 2019;51:247–252. doi: 10.1016/j.dld.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 130.Shimatani M, Matsushita M, Takaoka M, Kusuda T, Fukata N, Koyabu M, Uchida K, Okazaki K. "Short" double balloon enteroscope for endoscopic retrograde cholangiopancreatography with conventional sphincterotomy and metallic stent placement after Billroth II gastrectomy. Endoscopy. 2009;41 Suppl 2:E19–E20. doi: 10.1055/s-0028-1103466. [DOI] [PubMed] [Google Scholar]
- 131.Mutignani M, Tringali A, Shah SG, Perri V, Familiari P, Iacopini F, Spada C, Costamagna G. Combined endoscopic stent insertion in malignant biliary and duodenal obstruction. Endoscopy. 2007;39:440–447. doi: 10.1055/s-2007-966327. [DOI] [PubMed] [Google Scholar]
- 132.Kim KO, Kim TN, Lee HC. Effectiveness of combined biliary and duodenal stenting in patients with malignant biliary and duodenal obstruction. Scand J Gastroenterol. 2012;47:962–967. doi: 10.3109/00365521.2012.677956. [DOI] [PubMed] [Google Scholar]
- 133.Hamada T, Nakai Y, Lau JY, Moon JH, Hayashi T, Yasuda I, Hu B, Seo DW, Kawakami H, Kuwatani M, Katanuma A, Kitano M, Ryozawa S, Hanada K, Iwashita T, Ito Y, Yagioka H, Togawa O, Maetani I, Isayama H. International study of endoscopic management of distal malignant biliary obstruction combined with duodenal obstruction. Scand J Gastroenterol. 2018;53:46–55. doi: 10.1080/00365521.2017.1382567. [DOI] [PubMed] [Google Scholar]
- 134.Nakai Y, Hamada T, Isayama H, Itoi T, Koike K. Endoscopic management of combined malignant biliary and gastric outlet obstruction. Dig Endosc. 2017;29:16–25. doi: 10.1111/den.12729. [DOI] [PubMed] [Google Scholar]
- 135.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. doi: 10.1186/1471-2407-14-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. doi: 10.1186/s12885-017-3925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Inchingolo R, Nestola M, Nunes TF, Spiliopoulos S, Nardella M. Biliary involvement in liver metastases: long-term experience with biliary biopsy from a single center. Radiol Bras. 2021;54:15–20. doi: 10.1590/0100-3984.2020.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tanemura A, Mizuno S, Okura Y, Inoue H, Takaki H, Nishimura K, Uchida K, Isaji S. Margin-negative limited resection of metastatic pancreatic tumors from rectal cancer preoperatively diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsies: report of two cases. Surg Today. 2014;44:366–372. doi: 10.1007/s00595-012-0407-2. [DOI] [PubMed] [Google Scholar]
- 140.Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13:962–972. doi: 10.1038/modpathol.3880175. [DOI] [PubMed] [Google Scholar]
- 141.Nichols SD, Albert S, Shirley L, Schmidt C, Abdel-Misih S, El-Dika S, Groce JR, Wu C, Goldberg RM, Bekaii-Saab T, Bloomston M. Outcomes in patients with obstructive jaundice from metastatic colorectal cancer and implications for management. J Gastrointest Surg. 2014;18:2186–2191. doi: 10.1007/s11605-014-2670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sellier F, Bories E, Sibertin-Blanc C, Griffiths K, Dahan L, Giovannini M, Gaudart J, Seitz JF, Laugier R, Caillol F, Grandval P. Clinical outcome after biliary drainage for metastatic colorectal cancer: Survival analysis and prognostic factors. Dig Liver Dis. 2018;50:189–194. doi: 10.1016/j.dld.2017.09.121. [DOI] [PubMed] [Google Scholar]
- 143.Riopel MA, Klimstra DS, Godellas CV, Blumgart LH, Westra WH. Intrabiliary growth of metastatic colonic adenocarcinoma: a pattern of intrahepatic spread easily confused with primary neoplasia of the biliary tract. Am J Surg Pathol. 1997;21:1030–1036. doi: 10.1097/00000478-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 144.Estrella JS, Othman ML, Taggart MW, Hamilton SR, Curley SA, Rashid A, Abraham SC. Intrabiliary growth of liver metastases: clinicopathologic features, prevalence, and outcome. Am J Surg Pathol. 2013;37:1571–1579. doi: 10.1097/PAS.0b013e318293ddf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kubo M, Sakamoto M, Fukushima N, Yachida S, Nakanishi Y, Shimoda T, Yamamoto J, Moriya Y, Hirohashi S. Less aggressive features of colorectal cancer with liver metastases showing macroscopic intrabiliary extension. Pathol Int. 2002;52:514–518. doi: 10.1046/j.1440-1827.2002.01382.x. [DOI] [PubMed] [Google Scholar]
- 146.Latorre Fragua RA, Manuel Vazquez A, Rodrigues Figueira Y, Ramiro Pérez C, López Marcano AJ, de la Plaza Llamas R, Ramia Ángel JM. Intrabiliary metastases in colorectal cancer: a systematic review. J Hepatobiliary Pancreat Sci. 2019;26:270–280. doi: 10.1002/jhbp.635. [DOI] [PubMed] [Google Scholar]
- 147.Ikeda Y, Matsumata T, Adachi E, Hayashi H, Takenaka K, Sugimachi K. Hepatocellular carcinoma of the intrabiliary growth type. Int Surg. 1997;82:76–78. [PubMed] [Google Scholar]
- 148.American Cancer Society. Breast Cancer Facts & Figures 2019-2020. Atlanta: American Cancer Society, Inc. 2019. [cited 17 March 2021]. Available from https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf .
- 149.Xiao W, Zheng S, Yang A, Zhang X, Zou Y, Tang H, Xie X. Breast cancer subtypes and the risk of distant metastasis at initial diagnosis: a population-based study. Cancer Manag Res. 2018;10:5329–5338. doi: 10.2147/CMAR.S176763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liang Y, Zhang H, Song X, Yang Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin Cancer Biol. 2020;60:14–27. doi: 10.1016/j.semcancer.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 151.Meyer JE, Messer RJ, Patel VC. Diagnosis and treatment of obstructive jaundice secondary to liver metastases. Cancer. 1978;41:773–775. doi: 10.1002/1097-0142(197802)41:2<773::aid-cncr2820410252>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 152.Hoe AL, Royle GT, Taylor I. Breast liver metastases--incidence, diagnosis and outcome. J R Soc Med. 1991;84:714–716. doi: 10.1177/014107689108401207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Winston CB, Hadar O, Teitcher JB, Caravelli JF, Sklarin NT, Panicek DM, Liberman L. Metastatic lobular carcinoma of the breast: patterns of spread in the chest, abdomen, and pelvis on CT. AJR Am J Roentgenol. 2000;175:795–800. doi: 10.2214/ajr.175.3.1750795. [DOI] [PubMed] [Google Scholar]
- 154.Derouane F, Yombi JC, Baurain JF, Danse E, Komuta M, Yildiz H. When a metastatic breast cancer is mimicking a pancreatic cancer: case report and review of the literature. Acta Clin Belg. 2020;75:301–307. doi: 10.1080/17843286.2019.1607990. [DOI] [PubMed] [Google Scholar]
- 155.Apodaca-Rueda M, Chaim FHM, Garcia MDS, Saito HPA, Gestic MA, Utrini MP, Callejas-Neto F, Chaim EA, Cazzo E. Solitary pancreatic metastasis from breast cancer: case report and review of literature. Sao Paulo Med J. 2019;137:201–205. doi: 10.1590/1516-3180.2017.0144260617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cifuentes N, Pickren JW. Metastases from carcinoma of mammary gland: an autopsy study. J Surg Oncol. 1979;11:193–205. doi: 10.1002/jso.2930110303. [DOI] [PubMed] [Google Scholar]
- 157.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23:175–180. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 158.Lin Y, Wong SI, Wang Y, Lam C, Peng X. Periampullary Metastases from Breast Cancer: A Case Report and Literature Review. Case Rep Oncol Med. 2019;2019:3479568. doi: 10.1155/2019/3479568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao R, Li Y, Yu X, Yang W, Guo X. Duodenal metastasis from recurrent invasive lobular carcinoma of breast: a case report and literature review. Int J Clin Oncol. 2012;17:160–164. doi: 10.1007/s10147-011-0258-7. [DOI] [PubMed] [Google Scholar]
- 160.Molino C, Mocerino C, Braucci A, Riccardi F, Trunfio M, Carrillo G, Vitale MG, Cartenì G, De Sena G Breast Unit Cardarelli Hospital, Naples, Italy. Pancreatic solitary and synchronous metastasis from breast cancer: a case report and systematic review of controversies in diagnosis and treatment. World J Surg Oncol. 2014;12:2. doi: 10.1186/1477-7819-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kitamura N, Murata S, Abe H, Hanasawa K, Tsukashita S, Tani T. Obstructive jaundice in a metastatic tumor of the pancreas from breast cancer: a case report. Jpn J Clin Oncol. 2003;33:93–97. doi: 10.1093/jjco/hyg018. [DOI] [PubMed] [Google Scholar]
- 162.Mitchell PL, Harvey VJ, Lane MR, Evans BD, Thompson PI, Hamilton I. Palliation of biliary obstruction in patients with advanced breast cancer using endoscopic stents. Br J Surg. 1993;80:1188–1189. doi: 10.1002/bjs.1800800942. [DOI] [PubMed] [Google Scholar]
- 163.Crippa S, Bonardi C, Bovo G, Mussi C, Angelini C, Uggeri F. Pancreaticoduodenectomy for pancreatic metastases from breast carcinoma. JOP. 2004;5:377–383. [PubMed] [Google Scholar]
- 164.Uprety D, Bista A, Chennamadhavuni A, Niroula A, Jafri SIM, Smith A, Arjyal L. Survival trends among patients with metastatic melanoma in the pretargeted and the post-targeted era: a US population-based study. Melanoma Res. 2018;28:56–60. doi: 10.1097/CMR.0000000000000394. [DOI] [PubMed] [Google Scholar]
- 165.Wilson MA, Zhong J, Rosenbaum BE, Utter K, Moran U, Darvishian F, Polsky D, Berman RS, Shapiro RL, Pavlick AC, Osman I. Impact of initial stage on metastatic melanoma survival. Melanoma Res. 2019;29:281–288. doi: 10.1097/CMR.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kohler B, Riemann JF. Obstructive jaundice due to an intraductal melanoma metastasis. Endoscopy. 1987;19:79–80. doi: 10.1055/s-2007-1018242. [DOI] [PubMed] [Google Scholar]
- 167.Gong HZ, Zheng HY, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221–230. doi: 10.1097/CMR.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 168.Cameselle-García S, Pérez JLF, Areses MC, de Castro JDF, Mosquera-Reboredo J, García-Mata J. Primary malignant melanoma of the biliary tract: A case report and literature review. World J Clin Cases. 2019;7:2302–2308. doi: 10.12998/wjcc.v7.i16.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kanno Y, Noda Y, Koshita S, Ogawa T, Masu K, Oikawa M, Okada T, Akazawa N, Sawai T, Ito K. Surgically resected pancreatic metastasis from nasal malignant melanoma: case report and literature review. Clin J Gastroenterol. 2019;12:372–381. doi: 10.1007/s12328-019-00936-4. [DOI] [PubMed] [Google Scholar]
- 170.Nakamura Y, Yamada R, Kaneko M, Naota H, Fujimura Y, Tabata M, Kobayashi K, Tanaka K. Isolated pancreatic metastasis from malignant melanoma: a case report and literature review. Clin J Gastroenterol. 2019;12:626–636. doi: 10.1007/s12328-019-00996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Solomons G, Gibson RN, Tello RJ. Pancreatic head melanoma producing obstructive jaundice: magnetic resonance characteristics aiding differentiation from adenocarcinoma. Australas Radiol. 2000;44:471–473. doi: 10.1046/j.1440-1673.2000.00858.x. [DOI] [PubMed] [Google Scholar]
- 172.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–1678. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 173.Kamposioras K, Pentheroudakis G, Pectasides D, Pavlidis N. Malignant melanoma of unknown primary site. To make the long story short. A systematic review of the literature. Crit Rev Oncol Hematol. 2011;78:112–126. doi: 10.1016/j.critrevonc.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 174.Wood TF, DiFronzo LA, Rose DM, Haigh PI, Stern SL, Wanek L, Essner R, Morton DL. Does complete resection of melanoma metastatic to solid intra-abdominal organs improve survival? Ann Surg Oncol. 2001;8:658–662. doi: 10.1007/s10434-001-0658-4. [DOI] [PubMed] [Google Scholar]
- 175.Jin Y, Ran C, Li F, Cheng N. Melanoma of unknown primary in the pancreas: should it be considered primary? BMC Surg. 2020;20:76. doi: 10.1186/s12893-020-00731-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Colovic RB, Grubor NM, Jovanovic MD, Micev MT, Colovic NR. Metastatic melanoma to the common bile duct causing obstructive jaundice: a case report. World J Gastroenterol. 2007;13:813–815. doi: 10.3748/wjg.v13.i5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sans M, Llach J, Bordas JM, Andreu V, Campo A, Castells A, Mondelo E, Terés J, Rodés J. Metastatic malignant melanoma of the papilla of Vater: an unusual case of obstructive cholestasis treated with biliary prostheses. Endoscopy. 1996;28:791–792. doi: 10.1055/s-2007-1005618. [DOI] [PubMed] [Google Scholar]
- 178.Caballero-Mendoza E, Gallo-Reynoso S, Arista-Nasr J, Angeles-Angeles A. Obstructive jaundice as the first clinical manifestation of a metastatic malignant melanoma in the ampulla of vater. J Clin Gastroenterol. 1999;29:188–189. doi: 10.1097/00004836-199909000-00017. [DOI] [PubMed] [Google Scholar]
- 179.Marks JA, Rao AS, Loren D, Witkiewicz A, Mastrangelo MJ, Berger AC. Malignant melanoma presenting as obstructive jaundice secondary to metastasis to the Ampulla of Vater. JOP. 2010;11:173–175. [PubMed] [Google Scholar]
- 180.Nakayama H, Miyazaki S, Kikuchi H, Saito N, Shimada H, Sakai S, Suzuki M, Kimura K. Malignant vaginal melanoma with metastases to the papilla of Vater in a dialysis patient: a case report. Intern Med. 2011;50:345–349. doi: 10.2169/internalmedicine.50.4564. [DOI] [PubMed] [Google Scholar]
- 181.Bendic A, Glavina Durdov M, Stipic R, Karaman I. Melanoma in the ampulla of Vater. Hepatobiliary Pancreat Dis Int. 2013;12:106–108. doi: 10.1016/s1499-3872(13)60016-8. [DOI] [PubMed] [Google Scholar]
- 182.Lewis CW, Qazi J, Hippe DS, Lachance K, Thomas H, Cook MM, Juhlin I, Singh N, Thuesmunn Z, Takagishi SR, McEvoy A, Doolittle-Amieva C, Bhatia S, Paulson KG, O'Malley RB, Wang CL, Nghiem P. Patterns of distant metastases in 215 Merkel cell carcinoma patients: Implications for prognosis and surveillance. Cancer Med. 2020;9:1374–1382. doi: 10.1002/cam4.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Elkafrawy A, Numan L, Albawaliz A, Liu C, Bahaj W, Tawfik O, Hamid F. A Rare Case of Metastatic Merkel Cell Carcinoma to the Stomach and Pancreas Presenting With Upper Gastrointestinal Bleeding and Obstructive Jaundice. ACG Case Rep J. 2021;8:e00523. doi: 10.14309/crj.0000000000000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ratan R, Patel SR. Chemotherapy for soft tissue sarcoma. Cancer. 2016;122:2952–2960. doi: 10.1002/cncr.30191. [DOI] [PubMed] [Google Scholar]
- 185.Meyer M, Seetharam M. First-Line Therapy for Metastatic Soft Tissue Sarcoma. Curr Treat Options Oncol. 2019;20:6. doi: 10.1007/s11864-019-0606-9. [DOI] [PubMed] [Google Scholar]
- 186.Suh CH, Keraliya A, Shinagare AB, Kim KW, Ramaiya NH, Tirumani SH. Multidetector computed tomography features of pancreatic metastases from leiomyosarcoma: Experience at a tertiary cancer center. World J Radiol. 2016;8:316–321. doi: 10.4329/wjr.v8.i3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Akatsu T, Shimazu M, Morii T, Morioka H, Yabe H, Kitajima M. Pancreatic metastasis from musculoskeletal sarcoma: a case report with malignant fibrous histiocytoma and review of the literature. Dig Dis Sci. 2007;52:1958–1963. doi: 10.1007/s10620-006-9424-6. [DOI] [PubMed] [Google Scholar]
- 188.Robert PE, Orry D, Mor C, Rosset P, Guyetant S, Salame E, de Calan L. Resectable pancreatic metastasis of left thighbone leiomyosarcoma: case report and literature review. J Gastrointest Cancer. 2012;43:40–43. doi: 10.1007/s12029-010-9236-y. [DOI] [PubMed] [Google Scholar]
- 189.Varghese L, Ngae MY, Wilson AP, Crowder CD, Gulbahce HE, Pambuccian SE. Diagnosis of metastatic pancreatic mesenchymal tumors by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol. 2009;37:792–802. doi: 10.1002/dc.21104. [DOI] [PubMed] [Google Scholar]
- 190.Fadaee N, Sefa T, Das A, Rajkomar K. Pancreatic leiomyosarcoma: a diagnostic challenge and literature review. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2019-231529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ruess DA, Kayser C, Neubauer J, Fichtner-Feigl S, Hopt UT, Wittel UA. Carcinosarcoma of the Pancreas: Case Report With Comprehensive Literature Review. Pancreas. 2017;46:1225–1233. doi: 10.1097/MPA.0000000000000904. [DOI] [PubMed] [Google Scholar]
- 192.Herrera-Goepfert R. Postradiation Synovial Sarcoma of the Common Bile Duct: A Previously Unreported Anatomic Site. Int J Surg Pathol. 2018;26:469–474. doi: 10.1177/1066896917752863. [DOI] [PubMed] [Google Scholar]
- 193.Miyabe K, Masaki A, Nakazawa T, Ando T, Hayashi K, Naitoh I, Okumura F, Shimizu S, Kondo H, Yoshida M, Yamashita H, Umemura S, Hori Y, Ge Y, Ohara H, Joh T, Inagaki H. Histiocytic sarcoma of the bile duct. Intern Med. 2014;53:707–712. doi: 10.2169/internalmedicine.53.1384. [DOI] [PubMed] [Google Scholar]
- 194.Guzmán Calderón E. Carcinosarcoma of the ampulla of Vater. Rev Esp Enferm Dig. 2019;111:649–650. doi: 10.17235/reed.2019.5932/2018. [DOI] [PubMed] [Google Scholar]
- 195.Huddy JR, Sodergren MH, Deguara J, Thway K, Jones RL, Mudan SS. Pancreaticoduodenectomy for the Management of Pancreatic or Duodenal Metastases from Primary Sarcoma. Anticancer Res. 2018;38:4041–4046. doi: 10.21873/anticanres.12693. [DOI] [PubMed] [Google Scholar]
- 196.Zhang H, Jensen MH, Farnell MB, Smyrk TC, Zhang L. Primary leiomyosarcoma of the pancreas: study of 9 cases and review of literature. Am J Surg Pathol. 2010;34:1849–1856. doi: 10.1097/PAS.0b013e3181f97727. [DOI] [PubMed] [Google Scholar]
- 197.Ross WA, Egwim CI, Wallace MJ, Wang M, Madoff DC, Lee JH. Outcomes in lymphoma patients with obstructive jaundice: a cancer center experience. Dig Dis Sci. 2010;55:3271–3277. doi: 10.1007/s10620-010-1310-6. [DOI] [PubMed] [Google Scholar]
- 198.Odemiş B, Parlak E, Başar O, Yüksel O, Sahin B. Biliary tract obstruction secondary to malignant lymphoma: experience at a referral center. Dig Dis Sci. 2007;52:2323–2332. doi: 10.1007/s10620-007-9786-4. [DOI] [PubMed] [Google Scholar]
- 199.Boddie AW Jr, Eisenberg BL, Mullins JD, Schlichtemeier AL. The diagnosis and treatment of obstructive jaundice secondary to malignant lymphoma: a problem in multidisciplinary management. J Surg Oncol. 1980;14:111–123. doi: 10.1002/jso.2930140204. [DOI] [PubMed] [Google Scholar]
- 200.Fidias P, Carey RW, Grossbard ML. Non-Hodgkin's lymphoma presenting with biliary tract obstruction. A discussion of seven patients and a review of the literature. Cancer. 1995;75:1669–1677. doi: 10.1002/1097-0142(19950401)75:7<1669::aid-cncr2820750718>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 201.Behrns KE, Sarr MG, Strickler JG. Pancreatic lymphoma: is it a surgical disease? Pancreas. 1994;9:662–667. [PubMed] [Google Scholar]
- 202.Teefey SA, Stephens DH, Sheedy PF 2nd. CT appearance of primary pancreatic lymphoma. Gastrointest Radiol. 1986;11:41–43. doi: 10.1007/BF02035029. [DOI] [PubMed] [Google Scholar]
- 203.Sweeney AD, Fisher WE, Wu MF, Hilsenbeck SG, Brunicardi FC. Value of pancreatic resection for cancer metastatic to the pancreas. J Surg Res. 2010;160:268–276. doi: 10.1016/j.jss.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 204.Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: differential aspects. AJR Am J Roentgenol. 2000;174:671–675. doi: 10.2214/ajr.174.3.1740671. [DOI] [PubMed] [Google Scholar]
- 205.Wong DL, Deschner BW, King LC, Glazer ES. Primary Diffuse Large B Cell Lymphoma of the Common Bile Duct. J Gastrointest Surg. 2020;24:2376–2378. doi: 10.1007/s11605-020-04569-8. [DOI] [PubMed] [Google Scholar]
- 206.Mani H, Climent F, Colomo L, Pittaluga S, Raffeld M, Jaffe ES. Gall bladder and extrahepatic bile duct lymphomas: clinicopathological observations and biological implications. Am J Surg Pathol. 2010;34:1277–1286. doi: 10.1097/PAS.0b013e3181e9bb8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Peter U, Honegger H, Koelz HR. Obstructive jaundice caused by primary duodenal lymphoma. Case report and review of the literature. Digestion. 2007;75:124–125. doi: 10.1159/000104828. [DOI] [PubMed] [Google Scholar]
- 208.Sato J, Ishiwatari H, Ashida R, Sasaki K, Fujie S, Kaneko J, Satoh T, Matsubayashi H, Kishida Y, Yoshida M, Ito S, Kawata N, Imai K, Kakushima N, Takizawa K, Hotta K, Uesaka K, Ono H. Primary non-ampullary duodenal follicular lymphoma presenting with obstructive jaundice. Clin J Gastroenterol. 2020;13:214–218. doi: 10.1007/s12328-019-01033-2. [DOI] [PubMed] [Google Scholar]
- 209.Ito Y, Miyauchi M, Nakamura T, Takahara N, Nakai Y, Taoka K, Toyama K, Shinozaki-Ushiku A, Koike K, Kurokawa M. Significance of biopsy with ERCP for diagnosis of bile duct invasion of DLBCL. Int J Hematol. 2019;110:381–384. doi: 10.1007/s12185-019-02661-7. [DOI] [PubMed] [Google Scholar]
- 210.Bouvet M, Staerkel GA, Spitz FR, Curley SA, Charnsangavej C, Hagemeister FB, Janjan NA, Pisters PW, Evans DB. Primary pancreatic lymphoma. Surgery. 1998;123:382–390. [PubMed] [Google Scholar]
- 211.Gore RM, Miller FH, Yaghmai V. Acquired immunodeficiency syndrome (AIDS) of the abdominal organs: imaging features. Semin Ultrasound CT MR. 1998;19:175–189. doi: 10.1016/s0887-2171(98)90059-2. [DOI] [PubMed] [Google Scholar]
- 212.Miller FH, Gore RM, Nemcek AA Jr, Fitzgerald SW. Pancreaticobiliary manifestations of AIDS. AJR Am J Roentgenol. 1996;166:1269–1274. doi: 10.2214/ajr.166.6.8633429. [DOI] [PubMed] [Google Scholar]
- 213.Flores Córdova E, Mathew M, Mutneja H, Gonzalez Caldito E, Demetria M. An Uncommon Presentation of Extrahepatic Cholestasis due to Single Biliary Stricture From Kaposi Sarcoma. Cureus. 2020;12:e8913. doi: 10.7759/cureus.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Siddique MN, Popalzai M, Aoun N, Maroun R, Awasum M, Dai Q. Precursor B-cell acute lymphoblastic leukemia presenting as obstructive jaundice: a case report. J Med Case Rep. 2011;5:269. doi: 10.1186/1752-1947-5-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Agarwal A, Dadu T, Bhalla VP, Malhotra V. Myeloid sarcoma of bile ducts presenting as obstructive jaundice - A case report. Indian J Pathol Microbiol. 2019;62:602–604. doi: 10.4103/IJPM.IJPM_371_18. [DOI] [PubMed] [Google Scholar]
- 216.Fleming DR, Slone SP. CML blast crisis resulting in biliary obstruction following BMT. Bone Marrow Transplant. 1997;19:853–854. doi: 10.1038/sj.bmt.1700748. [DOI] [PubMed] [Google Scholar]
- 217.Malpica A, Phillips CC, Estrada RE, Banez EI. Plasma cell leukemia presenting as a pancreatic mass. Arch Pathol Lab Med. 1993;117:844–845. [PubMed] [Google Scholar]
- 218.Patel KJ, Latif SU, de Calaca WM. An unusual presentation of precursor T cell lymphoblastic leukemia/Lymphoma with cholestatic jaundice: case report. J Hematol Oncol. 2009;2:12. doi: 10.1186/1756-8722-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Daniel SV, Vani DH, Smith AM, Hill QA, Menon KV. Obstructive jaundice due to a pancreatic mass: a rare presentation of acute lymphoblastic leukaemia in an adult. JOP. 2010;11:72–74. [PubMed] [Google Scholar]
- 220.De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral vs bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547–553. doi: 10.1067/mge.2001.113381. [DOI] [PubMed] [Google Scholar]
- 221.Isayama H, Kawabe T, Nakai Y, Ito Y, Togawa O, Kogure H, Yashima Y, Yagioka H, Matsubara S, Sasaki T, Sasahira N, Hirano K, Tsujino T, Tada M, Omata M. Management of distal malignant biliary obstruction with the ComVi stent, a new covered metallic stent. Surg Endosc. 2010;24:131–137. doi: 10.1007/s00464-009-0537-9. [DOI] [PubMed] [Google Scholar]
- 222.Isayama H, Kawabe T, Nakai Y, Tsujino T, Sasahira N, Yamamoto N, Arizumi T, Togawa O, Matsubara S, Ito Y, Sasaki T, Hirano K, Toda N, Komatsu Y, Tada M, Yoshida H, Omata M. Cholecystitis after metallic stent placement in patients with malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2006;4:1148–1153. doi: 10.1016/j.cgh.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 223.Hamada T, Isayama H, Nakai Y, Togawa O, Takahara N, Uchino R, Mizuno S, Mohri D, Yagioka H, Kogure H, Matsubara S, Yamamoto N, Ito Y, Tada M, Koike K. Antireflux Metal Stent as a First-Line Metal Stent for Distal Malignant Biliary Obstruction: A Pilot Study. Gut Liver. 2017;11:142–148. doi: 10.5009/gnl15579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Isayama H, Kawakubo K, Nakai Y, Inoue K, Gon C, Matsubara S, Kogure H, Ito Y, Tsujino T, Mizuno S, Hamada T, Uchino R, Miyabayashi K, Yamamoto K, Sasaki T, Yamamoto N, Hirano K, Sasahira N, Tada M, Koike K. A novel, fully covered laser-cut nitinol stent with antimigration properties for nonresectable distal malignant biliary obstruction: a multicenter feasibility study. Gut Liver. 2013;7:725–730. doi: 10.5009/gnl.2013.7.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Katsinelos P, Paroutoglou G, Chatzimavroudis G, Terzoudis S, Zavos C, Gelas G, Pilpilidis I, Kountouras J. Prospective randomized study comparing double layer and Tannenbaum stents in distal malignant biliary stenosis. Acta Gastroenterol Belg. 2010;73:445–450. [PubMed] [Google Scholar]
- 226.Piñol V, Castells A, Bordas JM, Real MI, Llach J, Montañà X, Feu F, Navarro S. Percutaneous self-expanding metal stents vs endoscopic polyethylene endoprostheses for treating malignant biliary obstruction: randomized clinical trial. Radiology. 2002;225:27–34. doi: 10.1148/radiol.2243011517. [DOI] [PubMed] [Google Scholar]
- 227.Shimizu S, Naitoh I, Nakazawa T, Hayashi K, Miyabe K, Kondo H, Yoshida M, Yamashita H, Umemura S, Hori Y, Ohara H, Joh T. Predictive factors for pancreatitis and cholecystitis in endoscopic covered metal stenting for distal malignant biliary obstruction. J Gastroenterol Hepatol. 2013;28:68–72. doi: 10.1111/j.1440-1746.2012.07283.x. [DOI] [PubMed] [Google Scholar]
- 228.Park JK, Woo YS, Noh DH, Yang JI, Bae SY, Yun HS, Lee JK, Lee KT, Lee KH. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: prospective randomized controlled study. Gastrointest Endosc. 2018;88:277–282. doi: 10.1016/j.gie.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 229.Van Laethem JL, De Broux S, Eisendrath P, Cremer M, Le Moine O, Devière J. Clinical impact of biliary drainage and jaundice resolution in patients with obstructive metastases at the hilum. Am J Gastroenterol. 2003;98:1271–1277. doi: 10.1111/j.1572-0241.2003.07504.x. [DOI] [PubMed] [Google Scholar]
- 230.Kawakubo K, Isayama H, Nakai Y, Togawa O, Sasahira N, Kogure H, Sasaki T, Matsubara S, Yamamoto N, Hirano K, Tsujino T, Toda N, Tada M, Omata M, Koike K. Efficacy and safety of covered self-expandable metal stents for management of distal malignant biliary obstruction due to lymph node metastases. Surg Endosc. 2011;25:3094–3100. doi: 10.1007/s00464-011-1675-4. [DOI] [PubMed] [Google Scholar]
- 231.Okamoto T, Yanagisawa S, Fujioka S, Gocho T, Yanaga K, Kakutani H, Tajiri H. Is metallic stenting worthwhile for biliary obstruction due to lymph node metastases? J Surg Oncol. 2006;94:614–618. doi: 10.1002/jso.20614. [DOI] [PubMed] [Google Scholar]
- 232.Ashat M, Arora S, Klair JS, Childs CA, Murali AR, Johlin FC. Bilateral vs unilateral placement of metal stents for inoperable high-grade hilar biliary strictures: A systemic review and meta-analysis. World J Gastroenterol. 2019;25:5210–5219. doi: 10.3748/wjg.v25.i34.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Murakami Y, Shimura T, Okada R, Kofunato Y, Ishigame T, Yashima R, Nakano K, Suzuki S, Takenoshita S. Pancreatic metastasis of papillary thyroid carcinoma preoperatively diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsy: a case report with review of literatures. Clin J Gastroenterol. 2018;11:521–529. doi: 10.1007/s12328-018-0875-z. [DOI] [PubMed] [Google Scholar]
- 234.Tramontin MY, Faria PAS, Nascimento CMD, Barbosa CA, Barros MFRP, Barros ARG, Carvalho RC, Castro Neto AKP, Andrade FA, Corbo R, Vaisman F, Bulzico D. Cholestatic syndrome as initial manifestation of pancreatic metastasis of papillary thyroid carcinoma: case report and review. Arch Endocrinol Metab. 2020;64:179–184. doi: 10.20945/2359-3997000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Paspala A, Kostakis ID, Gaitanidis A, Prodromidou A, Schizas D, Machairas N. Long-Term Outcomes After Hepatic and Pancreatic Resections for Metastases from Thyroid Cancer: a Systematic Review of the Literature. J Gastrointest Cancer. 2019;50:9–15. doi: 10.1007/s12029-018-00196-4. [DOI] [PubMed] [Google Scholar]
- 236.Cormio G, Rossi C, Cazzolla A, Resta L, Loverro G, Greco P, Selvaggi L. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer. 2003;13:125–129. doi: 10.1046/j.1525-1438.2003.13054.x. [DOI] [PubMed] [Google Scholar]
- 237.Chan S, Wassef W. Endoscopic Ultrasound-Guided Fine-Needle Aspiration for Diagnosis of Pancreatic Metastases Secondary to Ovarian Carcinoma. ACG Case Rep J. 2021;8:e00544. doi: 10.14309/crj.0000000000000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rosenblatt M, Zafaranloo S, Tancer ML. Carcinoma of the ovary presenting as obstructive jaundice. Gynecol Oncol. 1989;32:385–389. doi: 10.1016/0090-8258(89)90647-1. [DOI] [PubMed] [Google Scholar]
- 240.Gupta A, Noba AL, Gupta S, Arora VK, Rathi V, Kumar S. Papillary cystadenocarcinoma of ovary presenting as obstructive jaundice: a rare presentation. Oman Med J. 2012;27:159–160. doi: 10.5001/omj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Takasaki Y, Irisawa A, Shibukawa G, Sato A, Abe Y, Yamabe A, Arakawa N, Maki T, Yoshida Y, Igarashi R, Yamamoto S, Ikeda T, Soeta N, Saito T, Hojo H. A Case of Obstructive Jaundice Caused by Metastasis of Ovarian Cancer to the Duodenal Major Papilla. Clin Med Insights Case Rep. 2018;11:1179547618791571. doi: 10.1177/1179547618791571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Yildirim Y, Sanci M. The feasibility and morbidity of distal pancreatectomy in extensive cytoreductive surgery for advanced epithelial ovarian cancer. Arch Gynecol Obstet. 2005;272:31–34. doi: 10.1007/s00404-004-0657-3. [DOI] [PubMed] [Google Scholar]
- 243.Manuel V, Rocha E, Fortini G, Pascoal Z, Netto R, Rengel L, Birolini C, Utiyama EM. Uterine cancer presenting as obstructive jaundice. Int J Womens Health. 2016;8:261–263. doi: 10.2147/IJWH.S108587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Levey JM. Endoscopic biliary drainage for metastatic squamous cell carcinoma of the cervix. Gastrointest Endosc. 2000;51:97–99. doi: 10.1016/s0016-5107(00)70401-9. [DOI] [PubMed] [Google Scholar]
- 245.Ahmmad E, Abdulkarim AS, Dirweesh A. Peri-Ampullary Metastasis From Endometrial Adenocarcinoma: A Rare Etiology of Obstructive Jaundice. Gastroenterology Res. 2019;12:37–39. doi: 10.14740/gr1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ghandili S, Bardenhagen J, Izbicki JR. Pancreatic Metastasis from Endometrial Carcinoma. J Gastrointest Surg. 2019;23:377–378. doi: 10.1007/s11605-018-3934-3. [DOI] [PubMed] [Google Scholar]
- 247.Fragulidis GP, Vezakis A, Chondrogiannis K, Mellou A, Melemeni A, Polydorou A. Clinical presentation and management of gastro-intestinal and pancreatic secondary metastatic tumors. J BUON. 2015;20:1009–1014. [PubMed] [Google Scholar]
- 248.Jacob J, Chargari C, Bauduceau O, Fayolle M, Ceccaldi B, Prat F, Le Moulec S, Vedrine L. Pancreatic metastasis from prostate cancer. Case Rep Med. 2010;2010:826273. doi: 10.1155/2010/826273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Desai B, Elatre W, Quinn DI, Jadvar H. FDG PET/CT demonstration of pancreatic metastasis from prostate cancer. Clin Nucl Med. 2011;36:961–962. doi: 10.1097/RLU.0b013e3182291d1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ando T, Watanabe K, Mizusawa T, Sakai T, Katagiri A. Pancreatic metastasis from locally recurrent neuroendocrine differentiated prostate cancer after radical prostatectomy. Urol Case Rep. 2020;31:101155. doi: 10.1016/j.eucr.2020.101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 252.Chambers M, Krall K, Hébert-Magee S. Falling under the umbrella cells: A single institutional experience and literature review of urothelial carcinoma presenting as a primary pancreatic mass on endoscopic ultrasound-guided fine-needle aspiration. Cytojournal. 2017;14:6. doi: 10.4103/1742-6413.202601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hong SP, Park SW, Lee SJ, Chung JP, Song SY, Chung JB, Kang JK, Cho NH. Bile duct wall metastasis from micropapillary variant transitional cell carcinoma of the urinary bladder mimicking primary hilar cholangiocarcinoma. Gastrointest Endosc. 2002;56:756–760. doi: 10.1067/mge.2002.129083. [DOI] [PubMed] [Google Scholar]
- 254.Esfehani MH, Mahmoodzadeh H, Alibakhshi A, Safavi F. Esophageal squamous cell carcinoma with pancreatic metastasis: a case report. Acta Med Iran. 2011;49:760–762. [PubMed] [Google Scholar]
- 255.Park C, Jang JY, Kim YH, Hwang EJ, Na KY, Kim KY, Park JH, Chang YW. A case of esophageal squamous cell carcinoma with pancreatic metastasis. Clin Endosc. 2013;46:197–200. doi: 10.5946/ce.2013.46.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sawada T, Adachi Y, Noda M, Akino K, Kikuchi T, Mita H, Ishii Y, Endo T. Hepatic portal venous gas in pancreatic solitary metastasis from an esophageal squamous cell carcinoma. Hepatobiliary Pancreat Dis Int. 2013;12:103–105. doi: 10.1016/s1499-3872(13)60015-6. [DOI] [PubMed] [Google Scholar]
- 257.Okamoto H, Hara Y, Chin M, Hagiwara M, Onodera Y, Horii S, Shirahata Y, Kamei T, Hashizume E, Ohuchi N. An extremely rare case of pancreatic metastasis of esophageal squamous cell carcinoma. World J Gastroenterol. 2014;20:593–597. doi: 10.3748/wjg.v20.i2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Koizumi W, Kitago M, Shinoda M, Yagi H, Abe Y, Oshima G, Hori S, Inomata K, Kawakubo H, Kawaida M, Kitagawa Y. Successful resection of pancreatic metastasis from oesophageal squamous cell carcinoma: a case report and review of the literature. BMC Cancer. 2019;19:320. doi: 10.1186/s12885-019-5549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yoon E, Nassar Y, Tejada-Almonte J, Mansoor MS, Umrau K, Hida S. An Entirely Atypical Presentation of Esophageal Squamous Cell Cancer with Pancreatic and Bone Metastases. Case Rep Gastroenterol. 2019;13:423–429. doi: 10.1159/000494749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Maehara K, Hijioka S, Saito Y. Endoscopic ultrasound-guided hepatico-gastrictubestomy for bile duct stent obstruction in a patient with recurrent cancer after esophageal cancer surgery with gastric tube reconstruction. Dig Endosc. 2021;33:466–467. doi: 10.1111/den.13901. [DOI] [PubMed] [Google Scholar]
- 261.Ntanasis-Stathopoulos I, Tsilimigras DI, Georgiadou D, Kanavidis P, Riccioni O, Salla C, Psaltopoulou T, Sergentanis TN. Squamous cell carcinoma of the pancreas: A systematic review and pooled survival analysis. Eur J Cancer. 2017;79:193–204. doi: 10.1016/j.ejca.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 262.Machairas N, Paspala A, Schizas D, Ntomi V, Moris D, Tsilimigras DI, Misiakos EP, Machairas A. Metastatic squamous cell carcinoma to the pancreas: Report of an extremely rare case. Mol Clin Oncol. 2019;10:144–146. doi: 10.3892/mco.2018.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tanabe S, Soeda S, Mukai T, Oki S, Yun K, Miyahara S. A case report of pancreatic metastasis of an intracranial angioblastic meningioma (hemangiopericytoma) and a review of metastatic tumor to the pancreas. J Surg Oncol. 1984;26:63–68. doi: 10.1002/jso.2930260114. [DOI] [PubMed] [Google Scholar]
- 264.Ramos LR, Marques PP, Loureiro R, Brito MJ, de Freitas J. Pancreatic metastasis of a meningeal hemangiopericytoma: a rare cause of obstructive jaundice. Endoscopy. 2014;46 Suppl 1 UCTN:E135–E136. doi: 10.1055/s-0033-1359158. [DOI] [PubMed] [Google Scholar]
- 265.Glass R, Andrawes SA, Hamele-Bena D, Tong GX. Metastatic tonsillar squamous cell carcinoma masquerading as a pancreatic cystic tumor and diagnosed by EUS-guided FNA. Diagn Cytopathol. 2017;45:1042–1045. doi: 10.1002/dc.23769. [DOI] [PubMed] [Google Scholar]
- 266.Falk GA, El-Hayek K, Morris-Stiff G, Tuthill RJ, Winans CG. Adenoid cystic carcinoma of the base of the tongue: Late metastasis to the pancreas. Int J Surg Case Rep. 2011;2:1–3. doi: 10.1016/j.ijscr.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.David D, Masineni SN, Giorgadze T. Fine-needle aspiration diagnosis of high grade adenoid cystic carcinoma metastatic to the pancreas. Diagn Cytopathol. 2015;43:117–120. doi: 10.1002/dc.23128. [DOI] [PubMed] [Google Scholar]
- 268.Kucur C, Durmus K, Ozer E, Kurt H, Yearsley MM. Hypopharyngeal Carcinoma With Pancreatic Metastasis. J Craniofac Surg. 2015;26:1431–1433. doi: 10.1097/SCS.0000000000001615. [DOI] [PubMed] [Google Scholar]
- 269.Passuello N, Pozza G, Blandamura S, Valmasoni M, Sperti C. Thymoma metastatic to liver and pancreas: case report and review of the literature. J Int Med Res. 2017;45:868–874. doi: 10.1177/0300060516680673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lin YT, Wu BS, Yang SF, Chen HC. Isolated pancreatic metastasis of a malignant pleural mesothelioma. Kaohsiung J Med Sci. 2009;25:395–400. doi: 10.1016/S1607-551X(09)70533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Shi L, Guo Z, Wu X. Primary pulmonary primitive neuroectodermal tumor metastasis to the pancreas: a rare case with seven-year follow-up. Diagn Pathol. 2013;8:51. doi: 10.1186/1746-1596-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Dokmak S, Cabral C, Couvelard A, Aussilhou B, Belghiti J, Sauvanet A. Pancreatic metastasis from nephroblastoma: an unusual entity. JOP. 2009;10:396–399. [PubMed] [Google Scholar]
- 273.Hagiwara N, Matsutani T, Nomura T, Fujita I, Kanazawa Y, Ueda J, Arai H, Kakinuma D, Kanno H, Naito Z, Uchida E. Pancreatic Metastasis from Gastrointestinal Stromal Tumor of the Stomach: A Case Report. J Nippon Med Sch. 2016;83:133–138. doi: 10.1272/jnms.83.133. [DOI] [PubMed] [Google Scholar]
- 274.Baur J, Schedelbeck U, Pulzer A, Bluemel C, Wild V, Fassnacht M, Steger U. A case report of a solitary pancreatic metastasis of an adrenocortical carcinoma. BMC Surg. 2015;15:93. doi: 10.1186/s12893-015-0076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Rentsch I, Gärtner U, Müller P, Kerk L. Endoscopic retrograde cholangiopancreaticography (ERCP) in obstructive jaundice caused by metstatic testicular teratoma. Endoscopy. 1977;9:101–103. doi: 10.1055/s-0028-1098499. [DOI] [PubMed] [Google Scholar]


