Abstract
Background
‘Long COVID’-associated dyspnoea may persist for months after SARS-CoV-2 infection. Among the causes of persistent dyspnoea, dysfunctional breathing (DB), defined as an erratic or inappropriate ventilation at rest or exercise, has been observed, but little is known about its occurrence and pathophysiology among individuals with ‘long COVID’. We aimed to describe the occurrence and identify clinical predictors of DB among patients following SARS-CoV-2 infection.
Methods
Cardiopulmonary exercise testing (CPET) was performed in 51 SARS-CoV-2 patients (median age, 64 years (IQR, 15)); male, 66.7%) living with ‘long COVID’ and persistent dyspnoea. CPET was classified into three dominant patterns: respiratory limitation with gas exchange abnormalities (RL); normal CPET or O2 delivery/utilisation impairment (D); and DB. Non-parametric and χ2 tests were applied to analyse the association between CPET dominant patterns and demographics, pulmonary function tests and SARS-CoV-2 severity.
Results
Among 51 patients, DB mostly without hyperventilation was found in 29.4% (n=15), RL in 54.9% (n=28) and D in 15.7% (n=8). When compared with RL individuals, patients with DB were younger, had significantly less severe initial infection, a better transfer capacity for carbon monoxide (median 85% (IQR, 28)), higher oxygen consumption (22.9 mL/min/kg (IQR, 5.5)), a better ventilatory efficiency slope (31.6 (IQR, 12.8)), and a higher SpO2 (95% (IQR, 3)).
Conclusions
Our findings suggest that DB without hyperventilation could be an important pathophysiological mechanism of disabling dyspnoea in younger outpatients following SARS-CoV-2 infection, which appears to be a feature of COVID-19 not described in other viral diseases.
Keywords: COVID-19, viral infection
Key messages.
What is already known on this topic
Dysfunctional breathing (DB), mainly of the hyperventilation type has been sporadically described after COVID-19.
What this study adds
Analysis of breathing pattern as assessed by cardiopulmonary exercise testing shows that almost one-third of ‘long COVID-19’ patients complaining of dyspnoea in our retrospective monocentric study had DB. Most DB were characterised by an irregular tidal volume and breathing frequency, without hyperventilation, which has been not been described so far.
How this study might affect research, practice or policy
DB without hyperventilation could be alternative pathophysiological explanation contributing to dyspnoea associated with long COVID-19. In practice, DB recognition in long COVID allows specific physiotherapeutic intervention targeted toward control of breathing.
Introduction
Long-lasting dyspnoea associated with previous SARS-CoV-2 infection is part of the so-called ‘long COVID’ syndrome and may persist for months after acute infection. Estimates of ‘long COVID’ prevalence vary from 2.3% to 40%, depending on the definition and examination time after acute infection.1 Cardiopulmonary exercise testing (CPET) is the most detailed diagnostic procedure to disentangle the mechanisms of dyspnoea by simultaneously evaluating cardiovascular adaptation, ventilation and gas exchange through exercise. Some data on CPET after SARS-CoV-2 infection have been published.2–11 Respiratory limitation (RL) and deconditioning seem to be the main patterns limiting exercise. Based on questionnaires, some authors suspect that hyperventilation syndrome might contribute to dyspnoea in ‘long COVID’. In a case series of patients evaluated with CPET for persistent post-COVID-19 dyspnoea, hyperventilation with an elevated V’E/V’CO2 ratio was described.7 Hyperventilation was further suspected in a larger cohort of patients evaluated with CPET.10
Hyperventilation syndrome is one specific type of dysfunctional breathing (DB), characterised in CPET by low PaCO2 set point resulting in ventilatory inefficiency characterised by high minute ventilation/CO2 output (V’E/V’CO2) with usually a normal dead volume/tidal volume (VD/Vt) ratio.12 However, other types of DB with normal PaCO2 and V’E/V’CO2 have been described, in particular, erratic ventilation with wide and irregular variations of Vt and breathing frequency (BF) over the progression of effort during CPET.13 DB manifesting these characteristics can be distinguished by analysis of ventilation patterns on CPET and may significantly contribute to dyspnoea in people living with ‘long COVID’ patients.12 14 In the present study, we aimed to determine the occurrence of DB in patients with ‘long COVID’-associated dyspnoea, describe the specific ventilation pattern, and identify clinical predictors of DB. Our hypothesis was that a large proportion of individuals had a DB pattern especially in the ambulatory setting when dyspnoea could not be explained by obvious structural findings.
Methods
Patients
We retrospectively included consecutive adult patients complaining of persistent or new-onset dyspnoea (modified Medical Research Council, mMRC≥1) more than 6 weeks after laboratory-confirmed SARS-CoV-2 infection. The data were extracted after review of the consultations of our ‘long COVID’ outpatient clinics of the Hôpital du Valais and Hôpital Riviera Chablais (Switzerland). Patients were referred to clinics by primary care physician or had a follow-up visit after admission for acute COVID-19. Pulmonary function tests and CPET were part of the routine clinical evaluation of patients with persisting dyspnoea.
One respiratory physician retrospectively collected all data from patients with persisting dyspnoea after SARS-CoV-2 infection and available CPET between 1 June 2020 and 31 March 2021. For safety reasons, patients requiring oxygen therapy did not undergo CPET. Patients with underlying severe respiratory comorbidities diagnosed prior to SARS-CoV-2 infection were excluded. Severity of COVID-19 disease was assessed according to the WHO classification,15 taking into account criteria such as hospital admission, supplemental oxygen, non-invasive or invasive ventilation, and length of stay. Pre-COVID-19 comorbidities were assessed during ‘long COVID-19’ clinic visits.
Patients were assessed using standardised questionnaires (Hospital Anxiety and Depression Scale (HADS) and Chronic Respiratory Questionnaire (CRQ)) and pulmonary function tests (Geratherm Respiratory, Blue Cherry platform software Bad Kissingen, Germany). Spirometry with bronchodilation, static lung volumes measurements and single breath carbon monoxide transfer capacity (TLCO) were performed by trained technicians. Spirometry with bronchodilation was systematically performed to exclude obstructive airways disease contributing to the dyspnoea. We used the Global Lung Initiative (GLI) reference equations for spirometry and TLCO,16 17 and the European Respiratory Society 199318 reference equation for static lung volumes because the new 2021 GLI equations were still not available at the time of the data collection.18
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
CPET protocols
Incremental CPET was performed on a cycle ergometer (Geratherm Ergostik system) to collect gases and flow. Patients received standard explanation about the CPET before the procedure. All CPETs were performed following a systematic order including: a rest phase of 2 min or more (until gas balance was achieved as evaluated by a stable respiratory exchange ratio <1 and stable V’E/V’CO2 over time as well as a stable V’E/V’O2 over time), a warming phase of 2 min of unloaded pedalling, linear ramp increase in load tailored for each patient in order to target an exercise duration of 8–12 min and a recuperation phase of 3–5 min. A facemask was used with the deadspace systematically adjusted to the size of the mask. Pulse oximetry (Konica Minolta Pulsox 300i) and electrocardiogram (Cardiopart 12 Blue ECG Pro Amedtec) were continuously recorded. Finger oximetry was used except in case of poor signal where ear lobe oximetry was used. Blood pressure was measured by a motion-tolerant TANGO M2 Stress Test Monitor (SunTech Medical Morrisville, North Carolina, USA). Arterial blood gases by radial puncture, including lactate concentration, were collected at rest and at peak of exercise within 30 s of exercise termination. The Study of Health in Pomerania (SHIP) reference equations were used to compare observed results with predicted values.19
CPET was analysed using tabulated data, modified Wasserman graphs and additional graph for the analysis of ventilation (ventilation slopes) with the following steps (1) objective determination of maximal effort using at least one predetermined criteria: V’O2 plateau, V’O2≥100% of predicted V’O2, peak ventilatory reserve <15% (1–(peak ventilation/maximum voluntary ventilation)), respiratory exchange ratio >1.05, heart rate ≥100% of predicted heart rate, 6) lactate≥8 mmol/L20; (2) subjective determination of maximal effort if BORG≥7 for dyspnoea and/or leg discomfort20; (3) determination of V’O2/work rate slope as a test quality criteria; (4) graphic determination of V’O2 peak with exercise capacity limitation if V’O2 peak≤84% of predicted V’O2 peak in an objective maximal effort20; (5) graphic determination of anaerobic threshold (AT) using V-slopes as the main criteria and other usual secondary criteria if necessary; (6) graphic determination of respiratory compensation point if applicable; (7) numerical tabulated data analysis and graphical analysis of the different panel in order to chose the dominant pattern.
To assess the objective criteria of maximal effort, the predicted values of heart rate and V’O2 using the SHIP reference equations were compared with the predicted values using the equations provided by the 2019 ERS statement to investigate whether the number of objectively maximal tests were different.20–22
The dominant patterns were then determined in the CPET interpretation:
RL was chosen in the presence of either ventilatory limitation (peak ventilatory reserve <15% of maximal ventilation) or gas exchange abnormalities (hypoxaemia with PaO2 <10 kPa at peak V’O2, or alveolar-arterial difference of O2 of more than 4 kPa at peak V’O2, or increased dead space ventilation (VD/VT>0.28 or >0.30 if aged >40 years at peak V’O2)20
O2 delivery/utilisation impairment was chosen in the absence of RL and in the presence of (1) low end-exercise V’O2 reflecting a low peak work rate and/or a low ∆V’O2 for a given work rate generally associated with a steep ∆HR/V’O2 and consequently a low O2 pulse (∆V’O2/HR ratio) or (2) an early shift to a predominantly anaerobic metabolism as suggested by an early estimated AT. This pattern represents either a cardiac limitation, deconditioning or any other disease leading to impairment of O2 utilisation (eg, myopathies) or O2 delivery (eg, anaemia).13
DB was diagnosed after exclusion of RL and O2 delivery/utilisation impairment and in the presence of either signs of hyperventilation (elevated V’E/V’CO2 slope) or erratic type of breathing (high variability of VT and/or BF) associated or not with sighs. For this, Wasserman panel 9 (VT/V’E) and panel 6 (V’E/V’CO2 slope) of the nine-panel plot (with usual Geratherm filter), together with a specific ventilation slope of VT and BF plotted over V’E with no filter were graphically analysed to determine the ventilatory mechanic response (see online supplemental material for the graph of VT and BF over V’E without filter).14
Normal pattern was diagnosed in the absence of RL, O2 delivery/utilisation impairment and DB.
Additionally the presence of ECG sign in favour of ischaemia and the presence of arrhythmia during continuous ECG were recorded and reported if present
bmjresp-2021-001126supp001.pdf (279.3KB, pdf)
Three CPET patterns were finally determined. (1) The RL pattern; (2) the DB pattern; (3) the D pattern grouped together normal CPETs and CPETs with O2 delivery/utilisation impairment because the number of CPET in the group of normal CPET and CPETs with O2 delivery/utilisation impairment was too low to run independent statistical analysis
Statistical analysis
All authors had full access to the database population. Non-parametric equality-of-median tests and χ2 tests were used to compare continuous or categorical variables as appropriate. Level of statistical difference of p≤0.05 was considered as significant. Results are given in mean (SD) or median (IQR). All statistical analysis were performed with STATA V.11.
Results
A total of 51 patients living with ‘long COVID’-associated dyspnoea were included in the study. Mean delay between the resolution of acute symptoms and the clinical and functional examination was 119 days (SD, 89). Overall, median age was 64 years (IQR, 15); 66.7% were male. Thirty-one per cent had no previous comorbidity and 40% reported never smoking. Median body mass index was 28.3 (IQR, 6.5). DB dominant pattern was found in 29.4% (n=15), RL in 54.9% (n=28) and D in 15.7% (n=8). The D pattern included two patients with normal CPET and six patients with O2 delivery/utilisation impairment pattern.
Baseline characteristics and SARS-CoV-2 severity parameters are shown in table 1 according to the dominant CPET patterns. DB patients were younger (median age, 54 (IQR, 22) years) with less severe acute COVID-19. While 96.4% of patients with RL were hospitalised at the time of acute infection for severe COVID-19, only 40% of those with DB were hospitalised. Radiological examinations at the time of pulmonary function testing were normal for DB and D patients. Chest X-ray or CT of patients with RL all showed interstitial infiltrates of varying severity.
Table 1.
Baseline characteristics and SARS-CoV-2 severity
| Respiratory limitation n=28 (54.9%) | O2 delivery/utilisation impairment or normal n=8 (15.7%) | Dysfunctional breathing n=15 (29.4%) | P value | |
| Age, median (25th–75th) | 67 (61–74) | 58 (44–66) | 54 (43–65) | 0.047 |
| Women, n (%) | 5 (17.9) | 5 (62.5) | 7 (46.7) | 0.026 |
| BMI, median (25th–75th) | 28.6 (25.5–31.2) | 28.2 (26.0–30.8) | 26.3 (24.9–32.2) | 0.977 |
| Comorbidities | ||||
| Cardiovascular disease, n (%) | 10 (35.7) | 1 (12.5) | 1 (6.7) | 0.073 |
| Diabetes, n (%) | 9 (32.1) | 1 (12.5) | 2 (13.3) | 0.278 |
| Hypertension, n (%) | 15 (53.6) | 2 (25.0) | 2 (13.3) | 0.025 |
| Obesity, n (%) | 10 (35.7) | 2 (25.0) | 6 (40.0) | 0.772 |
| Chronic lung disease, n (%) | 4 (14.3) | 0 | 3 (20.0) | 0.411 |
| Never smoker, n (%) | 7 (25.9) | 6 (75.0) | 7 (46.7) | 0.151 |
| SARS-CoV-2 severity | ||||
| Hospitalised, n (%) | 27 (96.4) | 3 (37.5) | 6 (40.0) | <0.001 |
| Intensive care unit admitted, n (%) | 18 (64.3) | 2 (25.0) | 2 (13.3) | 0.003 |
| WHO severity classification | ||||
| Mild/moderate, n (%) | 3 (10.7) | 6 (75.0) | 10 (66,7) | <0.001 |
| Severe/critical, n (%) | 25 (89.3) | 2 (25.0) | 5 (33.3) |
BMI, body mass index.
Pulmonary function and CPET
Pulmonary function tests and CPET results are shown in table 2. Patients with a DB pattern were tested later (median, 153 days (IQR, 119)) after initial infection than those with D (median, 110 days (IQR, 120)) or RL (median 58 days (IQR, 39); p<0.001). Most DB patients had normal dynamic and static lung function and some had a mildly reduced diffusion capacity. Oxygen consumption and workload at peak exercise were within the normal range for DB patients. Forty per cent of DB patients had a TLCO <80% of the normal predicted but their CPET pattern had no RL or O2 delivery/utilisation impairment (exclusion criteria). By contrast, reduced TLCO was found in 100% of RL patients, which was always associated with gas exchange abnormalities or ventilatory limitation as defined previously.
Table 2.
Pulmonary function and cardiopulmonary exercise tests
| Respiratory limitation n=28 (54.9%) | O2 delivery/utilisation impairment or normal n=8 (15.7%) | Dysfunctional breathing n=15 (29.4%) | P value | |
| Time from diagnosis to PFTs and CPET, days median (25th–75th) | 58 (45–84) | 110 (76–196) | 153 (115–234) | <0.001 |
| PFTs and ABG | ||||
| FEV1 % predicted, median (25th–75th) | 75 (63–87) | 99 (87–107) | 98 (86–108) | 0.002 |
| FVC % predicted, median (25th–75th) | 70 (63–84) | 98.5 (87–107) | 103 (95–106) | <0.001 |
| TLCO % predicted, median (25th–75th) | 42 (34–49) | 71 (60–88) | 85 (64–92) | <0.001 |
| TLC % predicted, median (25th–75th) | 72 (63–85) | 99 (86–108) | 106 (95–110) | <0.001 |
| pH, median (25th–75th) | 7.43 (7.41–7.45) | 7.43 (7.40–7.44) | 7.43 (7.41–7.45) | 0.840 |
| PaO2, kPa, median (25th–75th) | 9.4 (8.5–10.3) | 11.5 (10.4–12.6) | 12.0 (10.6–12.4) | <0.001 |
| PaCO2, kPa, median (25th–75th) | 4.6 (4.1–5.0) | 4.7 (4.6–5.0) | 4.7 (4.5–4.9) | 0.889 |
| 6MWD, m, median (25th-75th) | 403 (337–439) | 480 (375–525) | 546 (520–600) | 0.012 |
| CPET | ||||
| Peak V’O2 mL/min/kg, median (25th–75th) | 13.3 (10.2–16.4) | 15.2 (13.7–20.5) | 22.9 (20.0–25.5) | <0.001 |
| Peak V’O2 % predicted | 53 (40–72) | 65 (59–84) | 87 (81–101) | 0.002 |
| Workload, W, median (25th-75th) | 90 (60–113) | 117 (86–189) | 158 (126–174) | <0.001 |
| Breathing reserve %, median (25th–75th) | 22 (10–37) | 47 (29–52) | 29 (23–37) | 0.189 |
| V’E/V’CO2 slope, median (25th–75th) | 39.8 (37.6–45.1) | 30.3 (26.5–32.1) | 31.6 (26.2–39.0) | 0.016 |
| Anaerobic threshold % of predicted V’O2 max, median (25th–75th) | 44 (27–51) | 44 (39–51) | 53 (49–67) | 0.012 |
| SpO2 at peak %, median (25th–75th) | 88 (84–92) | 95 (91–95) | 95 (94–97) | <0.001 |
| PaO2 at peak (kPa), median (25th–75th) | 8.4 (7.1–9.4) | 12.8 (12.5–13.3) | 12.6 (12.2–14.7) | <0.001 |
| VD/VT ratio at peak, median (25th–75th) | 0.40 (0.36–0.44) | 0.24 (0.18–0.31) | 0.22 (0.20–0.24) | 0.002 |
| Peak heart rate bpm, median (25th–75th) | 129 (115–144) | 152 (128–171) | 152 (144–176) | 0.004 |
| OUES slope, median (25th–75th) | 1.23 (0.92–1.79) | 1.43 (1.11–1.79) | 2.06 (1.51–2.43) | 0.274 |
6MWD 6 min walk distance, CPET, cardiopulmonary exercise tests; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; OUES, oxygen uptake extraction slope; PFT, pulmonary function tests; TLCO, transfer capacity for carbon monoxide; VD/VT, dead volume/tidal volume; V’O2, oxygen consumption V’E/V’CO2 ventilatory efficiency.
Regarding quality criteria of CPET, 42 tests (82.4%) were objectively maximal according to the previously described criteria. The number of objectively maximal tests was identical when comparing the SHIP equation with the two separate prediction equations suggested in the recent ERS statement for V’O2 and heart rate.20–22 All tests were performed until the patient decided to stop (advised to perform until exhaustion in the general instructions). No tests were stopped for medical reasons (eg, ischaemia). Median Borg scale at end exercise was 8 (IQR, 4). Mean V’O2/work slope was 8.99 (SD, 1.95) mL/min/kg/W and mean step increments (work/time) was 13.86 (3.56) W/min.
Despite important respiratory discomfort and high Borg scale scores, DB patients exhibited normal breathing reserve at peak exercise. Mean exercise capacity (V’O2) was normal in DB population (>84%) compared with the reference equation. In other groups it was on average diminished. Rapid and chaotic changes in VT and BF during incremental exercise were identified in DB patients by a combined analysis of ventilation slopes and the VT/V’E graph (figure 1A–C). Interestingly, the overall V’E progression during testing was adjusted to the metabolic need, while V’E/V’CO2 and baseline PaCO2 were within the normal range in DB, thus reducing the likelihood of hyperventilation.
Figure 1.
Ventilation slopes and Wasserman panel (VT/V’E). (A) Normal subject. (B) Respiratory limitation showing a regular, but limited increase of tidal volume with high breathing frequency. (C) Dysfunctional breathing with an erratic pattern. Plots of tidal volume (VT on the right y-axis) and breathing frequency (BF on the left y-axis) against minute ventilation (V’E on the x-axis) during incremental exercise testing. Data are not filtered in the ventilation slopes. Geratherm Respiratory combined filter is used in the Wasserman panel (VT/V’E) (see online supplemental material). BF, breathing frequency; VT, tidal volume; V’E, minute ventilation.
Health-related quality of life
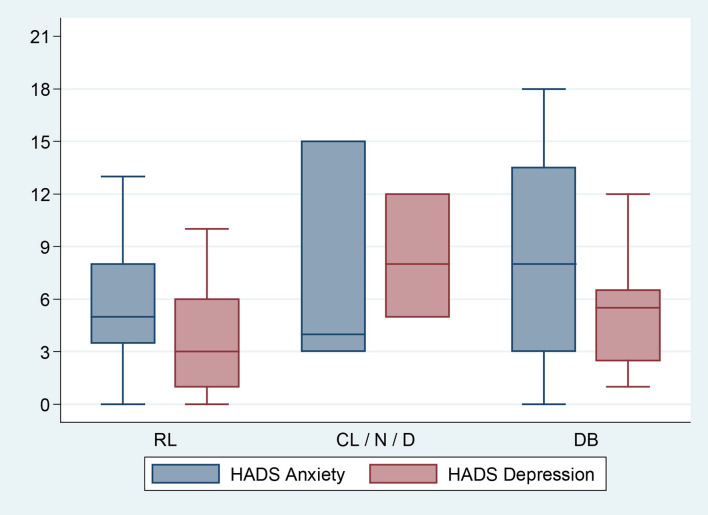
The HADS scores were statistically similar among the three patterns. Overall, 37.1% of patients had an anxiety score >7, indicating a possible or certain anxiety, but without statistically significant differences between patterns (RL 33%, D 33%, DB 50%; p=0.693) (figure 2). The scores of the four domains of the Chronic Respiratory Questionnaire were also similar. Interestingly, patients with RL tended to report higher scores than patients with DB, which could indicate a lower burden of symptoms.
Figure 2.
Hospital Anxiety and Depression Scale (HADS). Higher scores denote a higher risk of anxiety or depression. <7: no anxiety or depression; 8–10: possible anxiety or depression; 11–21: high probability of anxiety or depression. No statistical differences between groups (p>0.05). CL/N/D, cardiac limitation or normal cardiopulmonary exercise testing or deconditioning; DB, dysfunctional breathing; RL, respiratory limitation group.
Discussion
We found that DB evaluated by CPET occurs in almost one-third of the patients living with ‘long COVID’ and complaining of dyspnoea in our centre. DB was associated with younger age and previous mild/moderate acute COVID. Additionally, DB was still recognisable more than 200 days after SARS-CoV-2 infection in patients complaining of persistent dyspnoea. Although DB has already been suspected in patients living with ‘long COVID’, this is the first study to our knowledge to describe an erratic type of breathing mainly without hyperventilation best corresponding to the periodic deep sighing type of breathing.12 The retrospective nature of the study and the design (specialised respiratory physicians) does not allow a true estimation of the prevalence of DB after SARS-CoV-2 infection. By design, our study categorised patients into three dominant mutually exclusive CPET patterns, which may lead to an underestimation of DB in those with RLs and O2 delivery/utilisation impairment. However, since patients with underlying respiratory disease or unable to perform the CPET for safety reasons including oxygen use were excluded from the analysis, the prevalence of DB reported in this study could also be overestimated because excluded patients would most likely have a predominant RL pattern. The 15 patients diagnosed with DB had a normal predicted peak V’O2 consumption. Despite this, they experienced a reduced quality of life as evaluated by the HADS and CRQ and some level of disabling dyspnoea. This is important for rehabilitation programme for DB patients since improvement in exercise capacity is probably not the right target to relieve the feeling of dyspnoea. It is also important for diagnosis because a normal exercise capacity should not lead to the conclusion of normality and false reassurance when there is a clear DB pattern associated. Even though some patients with DB exhibited lowered TLCO, chest imaging always excluded a significant interstitial disease and RL per se was not the exercise-limiting factor in these patients. In our results, DB group had statistically significantly better exercise capacity (V’O2), lung volumes (FVC, TLC), diffusion capacity (TLCO) and oxygenation (PaO2), than RL group. However, these differences should be interpreted with caution because patients in the RD group were tested later than those in the RL group. In RL group lung function and exercise capacity might improve over time, thus reducing the differences between groups and allowing DB pattern to occur.
Dyspnoea after SARS-CoV-2 is a frequent symptom following various grades of acute disease severity. Nevertheless, the mechanisms explaining dyspnoea after severe or mild SARS-CoV-2 infection may obviously differ. Some studies have reported on cardiopulmonary adaptation to exercise assessed by CPET in patients living with ‘long COVID’. V’O2 peak is diminished up to 6 months after hospitalisation for SARS-CoV-2 infection.2–6 9 11 23 The severity of the disease seems to correlate with the V’O2 peak (the more severe the disease, the lower the V’O2 peak).8 9 RL and O2 delivery/utilisation impairment (mainly cardiac limitation or deconditioning) have been described and deconditioning seems to be the main cause of exercise limitation after hospitalisation for SARS-CoV-2 infection.2–6 9 11 23 These findings were expected because CPET have been performed mainly in patients after hospitalisation for SARS-CoV-2 pneumonia associated or not with acute respiratory distress syndrome. In these patients RL pattern is logical until the parenchymal sequelae are resolved. Furthermore deconditioning is frequent after hospitalisation with prolonged bed rest and severe disease. In line with our results, V’O2 peak was not correlated with dyspnoea in 156 patients prospectively evaluated after SARS-CoV-2 infection.9 A case series of eight patients with hyperventilation syndrome as evaluated by CPET was described 3 months after mild ambulatory SARS-CoV-2 infection.7 A large cohort of mainly hospitalised patients evaluated by CPET added evidence that hyperventilation was a probable mechanism of persistent dyspnoea after SARS-CoV-2 infection.10 Hyperventilation seems to emerge as one type of DB that could contribute to dyspnoea after SARS-CoV-2 infection. However, to our knowledge no study so far detected other types of DB evaluated by CPET after SARS-CoV-2.
DB can be defined as a neural breathing disorder originating from the central nervous system, where an abnormal breathing drive results in respiratory discomfort in the absence or in excess of the magnitude of underlying cardiorespiratory disease.12
The lack of gold standard to diagnose DB is an acknowledged problem. The Nijmegen questionnaire was initially validated for hyperventilation syndrome and its use is extrapolated in other type of breathing without evidence.12 Most of our patients were complaining of deep sighs with erratic type of breathing and lacked the typical symptoms associated with hyperventilation and it appeared unlikely that the Nijmegen was an appropriate instrument for the diagnosis of DB in these patients.
CPET is the most exhaustive method for the investigation of dyspnoea as it can determine exercise capacity, as well as the dominant causes of exercise limitation, including DB. In DB, an abnormally high BF for the work rate and an erratic pattern of both VT and BF in response to an increasing work rate can be recognised using the ventilation panels (panel 9 of Wasserman or ventilation slopes) from most CPET software. We also used a specific graph to study ventilation, which plots VT and BF over V’E but without any filter applied which may mask the erratic pattern of ventilation. This allows for a qualitative impression of the dispersion of VT and BF over the test (see online supplemental material). Despite sharing similar symptoms with the DB hyperventilation syndrome (such as dyspnoea, sighing, yawning), DB with a chaotic ventilatory pattern observed at CPET can be distinguished from hyperventilation syndrome. Indeed V’E/V’CO2 and PaCO2 are mostly normal in DB with a chaotic ventilatory pattern, while they are expected to be abnormal in the hyperventilation syndrome. The association between the specific chaotic ventilatory pattern of DB and the absence of a cardiac or respiratory cause for exercise termination is highly suggestive of the diagnosis.14
We identified DB mostly without hyperventilation as an explanation of persisting dyspnoea after SARS-Cov-2 infection. Indeed, hyperventilation type of DB with inappropriately elevated V’E/V’CO2 ratio in the absence of VD/VT elevation was observed in only one patient. In this context, the V’E/V’CO2 slope is suggestive of a low PaCO2 setpoint during exercise, which represent alveolar hyperventilation.24 25 All other patients presented DB with a chaotic ventilatory pattern. Some of these patients described a sensation of air hunger and presented sighing at rest, which were associated with deep sighing during exercise, as objectively evidenced by CPET. As explained above no other physiological limitations suggesting an underlying organic disease were present.12
A classification of five types of DB has been proposed: (1) hyperventilation syndrome; (2) periodic deep sighing; (3) thoracic dominant breathing; (4) forced abdominal expiration and (5) thoraco-abdominal asynchrony.12 These patterns have been described at rest and cannot be transposed to our data, which also concern ventilation during exercise. Regardless of this, dyspnoeic ‘long COVID’ patients with DB exhibited a chaotic ventilatory pattern at rest (during the rest phase of CPET) and during exercise close to the periodic deep sighing category. Moreover, patients frequently reported periodic deep sighing together with yawning.
In our study, we analysed patients with a broad range of COVID-19 severity. The reduced peak V’O2 caused by an RL was dominant in the most severe cases of COVID-19. The RL was mainly explained by gas exchange abnormalities with some patients having restrictive ventilation abnormalities. Additionally, most of these patients exhibited signs of O2 delivery/utilisation impairment, including a low AT. This could be due to concomitant deconditioning or because exercise induced hypoxaemia associated with haemoglobin oxygen desaturation is in itself a cause of O2 delivery impairment leading to lower V’O2 peak, lower O2 pulse and lower AT threshold. The sequelae of acute respiratory distress syndrome in severe COVID-19 survivors have been described previously.26 Due to our study design, which categorised patients according to the dominant pattern, the number of patients with O2 delivery/utilisation impairment without RL was limited (six patients). In this category cardiac condition limiting exercise were excluded with normal echocardiography and only one patient exhibited rapid atrial fibrillation stopping the exercise with an obvious cardiac limitation. No patients had signs of ischaemia during the analysis. In the absence of cardiac condition and RL, an O2 delivery/utilisation impairment pattern is highly suggestive of deconditioning which seemed to be the exercise-limiting factor for the remaining patients of this group (five patients). Due to the low number of patients with the O2 delivery/utilisation impairment pattern, we had to regroup them with the normal group (two patients) in order to run statistical analysis. This is of course a limitation because these two patterns are different and further studies are needed in order to have a better understanding of this population.
DB could possibly coexist with RL in patients with initial severe SARS-CoV-2 infection. However, we felt we did not have the experience at that time to evaluate what an ‘out of proportion’ dyspnoea would be for patients presenting with RL after SARS-CoV-2. Studies assessing this aspect would be of value to improve the care of ‘long COVID’ patients who could benefit from a targeted intervention.
Involvement of the respiratory centres in the brainstem in COVID-19 has also been hypothesised, to account for the instability of breathing control in DB. Some authors have postulated that an inflammatory or micro-angiopathic insult to the pre-Bötzinger complex during the acute phase of SARS-CoV-2 infection may explain dysregulation of the ventilatory drive.27 The link between DB and brainstem dysfunction is only a hypothesis that should be verified with further studies.
A proper and quick diagnosis of DB is important as these patients could benefit from specific physiotherapy techniques addressing voluntary breathing control. So far there are no randomised controlled studies of DB management in patients with ‘long COVID’.
Strengths and limitations
Limitations of our study are the lack of a universal gold standard for the diagnosis of DB,12 14 28 the small number of patients included which lead to the need of the merging of two different patterns (normal and O2 delivery/utilisation impairment), the single centre analysis of our study which could limit the generalisability of our results and the varying time elapsed between the diagnosis and the CPET evaluation. These last points may preclude a more definitive interpretation of the difference in CPET, PFT and arterial blood gas parameters among the groups. The strengths of our study are the comprehensive analysis of CPET describing a new pattern of DB, the inclusion of both hospitalised and ambulatory patients and the reasonable time lag separating acute infection from assessment.
Conclusions
DB without hyperventilation with an erratic type of breathing and deep sighs seems to be another important feature that could explain persisting dyspnoea in long COVID patients. Our monocentric experience found a high occurrence of this condition that needs to be confirmed in other centres. The pathophysiology of DB after SARS-CoV-2 infection is unknown. A prompt diagnosis is needed in order to offer a specific respiratory training. Further studies including a larger number of patients and a longer time window for CPET examination are needed to better understand the prognostic, natural history and potential treatment of DB in ‘long COVID’ patients.
Acknowledgments
We would like to thank all patients who agreed to take part in the study.
Footnotes
IF and LG contributed equally.
Contributors: IF, LG and P-OB designed the study and drafted the manuscript. IF and LG contributed equally as first co-author. PV, AV-C, MA, GG and DL revised the work, contributed to critical appraisal and enriched the literature search. P-OB takes the full responsibility for the work and conduct of the study, had access to the data, and control the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and local ethical committee (Swiss ethics) accepted this retrospective collection of data (BASEC number: 2021-01028). Participants gave informed consent to participate in the study before taking part.
References
- 1.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021;27:626–31. 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blokland IJ, Ilbrink S, Houdijk H, et al. [Exercise capacity after mechanical ventilation because of COVID-19: Cardiopulmonary exercise tests in clinical rehabilitation]. Ned Tijdschr Geneeskd 2020;164:D5253. [PubMed] [Google Scholar]
- 3.Gao Y, Chen R, Geng Q, et al. Cardiopulmonary exercise testing might be helpful for interpretation of impaired pulmonary function in recovered COVID-19 patients. Eur Respir J 2021;57:2004265. 10.1183/13993003.04265-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-Term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 2021;31:100683. 10.1016/j.eclinm.2020.100683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohr A, Dannerbeck L, Lange TJ, et al. Cardiopulmonary exercise pattern in patients with persistent dyspnoea after recovery from COVID-19. Multidiscip Respir Med 2021;16:732. 10.4081/mrm.2021.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debeaumont D, Boujibar F, Ferrand-Devouge E, et al. Cardiopulmonary Exercise Testing to Assess Persistent Symptoms at 6 Months in People With COVID-19 Who Survived Hospitalization: A Pilot Study. Phys Ther 2021;101:pzab099. 10.1093/ptj/pzab099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motiejunaite J, Balagny P, Arnoult F, et al. Hyperventilation: a possible explanation for long-lasting exercise intolerance in mild COVID-19 survivors? Front Physiol 2020;11:614590. 10.3389/fphys.2020.614590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdallah SJ, Voduc N, Corrales-Medina VF, et al. Symptoms, pulmonary function, and functional capacity four months after COVID-19. Ann Am Thorac Soc 2021;18:1912–7. 10.1513/AnnalsATS.202012-1489RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skjørten I, Ankerstjerne OAW, Trebinjac D, et al. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J 2021;58:2100996. 10.1183/13993003.00996-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motiejunaite J, Balagny P, Arnoult F, et al. Hyperventilation as one of the mechanisms of persistent dyspnoea in SARS-CoV-2 survivors. Eur Respir J 2021;58:2101578. 10.1183/13993003.01578-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rinaldo RF, Mondoni M, Parazzini EM, et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur Respir J 2021;58:2100870. 10.1183/13993003.00870-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulding R, Stacey R, Niven R, et al. Dysfunctional breathing: a review of the literature and proposal for classification. Eur Respir Rev 2016;25:287–94. 10.1183/16000617.0088-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neder J, Berton D AR. Abnormal patterns of response to incremental CPET. In: Sheffield ERS. In: Palange P, Laveneziana P, Neder JA, eds. Clinical exercise testing (ERS monograph, 2018: 34–58. [Google Scholar]
- 14.Ionescu MF, Mani-Babu S, Degani-Costa LH, et al. Cardiopulmonary exercise testing in the assessment of dysfunctional breathing. Front Physiol 2020;11:620955. 10.3389/fphys.2020.620955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO . Clinical management of COVID-19. WHO/2019-nCoV/clinical/2020, 2015. [Google Scholar]
- 16.Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: global lung function initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017;50:1700010. 10.1183/13993003.00010-2017 [DOI] [PubMed] [Google Scholar]
- 17.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324–43. 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party standardization of lung function tests, European community for steel and coal. official statement of the European respiratory Society. Eur Respir J Suppl 1993;16:5–40. [PubMed] [Google Scholar]
- 19.Koch B, Schäper C, Ittermann T, et al. Reference values for cardiopulmonary exercise testing in healthy volunteers: the SHIP study. Eur Respir J 2009;33:389–97. 10.1183/09031936.00074208 [DOI] [PubMed] [Google Scholar]
- 20.Radtke T, Crook S, Kaltsakas G, et al. ERS statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur Respir Rev 2019;28:180101. 10.1183/16000617.0101-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fairbarn MS, Blackie SP, McElvaney NG, et al. Prediction of heart rate and oxygen uptake during incremental and maximal exercise in healthy adults. Chest 1994;105:1365–9. 10.1378/chest.105.5.1365 [DOI] [PubMed] [Google Scholar]
- 22.Jones NL, Makrides L, Hitchcock C, et al. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis 1985;131:700–8. 10.1164/arrd.1985.131.5.700 [DOI] [PubMed] [Google Scholar]
- 23.Clavario P, Marzo D, Lotti R. Assessment of functional capacity with cardiopulmonary exercise testing in non-severe COVID-19 patients at three months follow-up. MedRxiv [preprint] 2020. [Google Scholar]
- 24.Ward SA. Ventilation/carbon dioxide output relationships during exercise in health. Eur Respir Rev 2021;30:200160. 10.1183/16000617.0160-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson M, Ionescu MF, Sylvester K, et al. Minute ventilation/carbon dioxide production in patients with dysfunctional breathing. Eur Respir Rev 2021;30:200182. 10.1183/16000617.0182-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mart MF, Ware LB. The long-lasting effects of the acute respiratory distress syndrome. Expert Rev Respir Med 2020;14:577–86. 10.1080/17476348.2020.1743182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gandhi S, Srivastava AK, Ray U, et al. Is the collapse of the respiratory center in the brain responsible for respiratory breakdown in COVID-19 patients? ACS Chem Neurosci 2020;11:1379–81. 10.1021/acschemneuro.0c00217 [DOI] [PubMed] [Google Scholar]
- 28.Barker N, Everard ML. Getting to grips with 'dysfunctional breathing'. Paediatr Respir Rev 2015;16:53–61. 10.1016/j.prrv.2014.10.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2021-001126supp001.pdf (279.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request.