Abstract
Background
Gestational diabetes mellitus (GDM) is a common endocrine metabolic disease, involving a carbohydrate intolerance of variable severity during pregnancy. The incidence of GDM-related complications and adverse pregnancy outcomes has declined, in part, due to early screening. Machine learning (ML) models are increasingly used to identify risk factors and enable the early prediction of GDM.
Objective
The aim of this study was to perform a meta-analysis and comparison of published prognostic models for predicting the risk of GDM and identify predictors applicable to the models.
Methods
Four reliable electronic databases were searched for studies that developed ML prediction models for GDM in the general population instead of among high-risk groups only. The novel Prediction Model Risk of Bias Assessment Tool (PROBAST) was used to assess the risk of bias of the ML models. The Meta-DiSc software program (version 1.4) was used to perform the meta-analysis and determination of heterogeneity. To limit the influence of heterogeneity, we also performed sensitivity analyses, a meta-regression, and subgroup analysis.
Results
A total of 25 studies that included women older than 18 years without a history of vital disease were analyzed. The pooled area under the receiver operating characteristic curve (AUROC) for ML models predicting GDM was 0.8492; the pooled sensitivity was 0.69 (95% CI 0.68-0.69; P<.001; I2=99.6%) and the pooled specificity was 0.75 (95% CI 0.75-0.75; P<.001; I2=100%). As one of the most commonly employed ML methods, logistic regression achieved an overall pooled AUROC of 0.8151, while non–logistic regression models performed better, with an overall pooled AUROC of 0.8891. Additionally, maternal age, family history of diabetes, BMI, and fasting blood glucose were the four most commonly used features of models established by the various feature selection methods.
Conclusions
Compared to current screening strategies, ML methods are attractive for predicting GDM. To expand their use, the importance of quality assessments and unified diagnostic criteria should be further emphasized.
Keywords: digital health, gestational diabetes mellitus, machine learning, prediction model, prognostic model
Introduction
According to the latest Global Diabetes Map (9th edition) released by the International Diabetes Federation, the number of patients with diabetes during pregnancy is increasing globally, with about 20.4 million (15.8%) women suffering from hyperglycemia; among them, 83.6% of cases were due to gestational diabetes mellitus (GDM) [1]. GDM, a common metabolic disease, is usually a transient disorder during pregnancy that resolves at delivery. Pregnant women with GDM are at greater risk of adverse pregnancy outcomes that threaten a normal birth. An oral glucose tolerance test (OGTT) is typically recommended to screen for GDM between the 24th and 28th weeks of gestation. Physicians usually measure the fasting plasma glucose concentration 1 to 2 hours after the patient ingests glucose [2]. The American Diabetes Association recommends that women be screened at the first prenatal examination to aid with the early identification of hyperglycemia risk. Nonetheless, GDM screening recommendations are controversial among international organizations regarding four aspects: (1) universal versus selective screening, (2) early pregnancy screening (ie, before pregnancy or at the first prenatal visit versus screening at 24-28 gestational weeks), (3) a one-step versus two-step approach, and (4) inconsistent diagnostic criteria (Table S1 in Multimedia Appendix 1) [3].
Machine learning (ML) methods have become favorable tools for disease prevention and management. For instance, the multivariate logistic regression (LR) model is a recognized ML algorithm for predicting diabetes and its complications. Furthermore, other methods, such as random forest (RF), extreme gradient boosting (XGBoost), and light gradient boosting machine (LightGBM), are also applied to diabetes-related problems. A growing number of studies have used such methods to identify risk factors of GDM and construct early prediction models for the disease [4,5]. ML presents a powerful tool for analyzing large amounts of diverse health care data and augmenting doctors’ capabilities. However, ML has limitations that can lead to inaccurate predictions in some clinical scenarios, and the significance of its assessment was highlighted in a real-world study [6]. The US Food and Drug Administration (FDA) has issued guidance on software as a medical device that explains risk stratification and the analytical and clinical validation required of artificial intelligence (AI) tools in health care. IDx-DR, the first FDA-approved ML application to help make screening decisions, achieved high sensitivity (87%) and specificity (91%) for diabetic retinopathy in primary care clinics [7]. Most of the published prognostic models for GDM also showed acceptable discrimination and calibration [8], but they vary in quality and perform inconsistently.
Few systematic analyses of ML models for GDM are currently available. Here, we conducted a thorough meta-analysis of the predictive value of ML in GDM using a quality evaluation by the Prediction Model Risk of Bias Assessment Tool (PROBAST) and compared ML models with universal and selective screening methods. Essentially, we wondered if ML could be a new GDM screening option.
Methods
Research Design
This study was conducted according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Table S2 in Multimedia Appendix 1) [9].
Search Methods
The PubMed, Web of Science, IEEE Xplore, and China National Knowledge Infrastructure databases were searched for articles published in English or Chinese between August 2019 and October 2020. We built up the search strategy according to the PICO (population, intervention, control, and outcomes) principle; for our study, “P” represents GDM populations, “I” represents ML methods as interventions, “C” represents gold standards as controls, and “O” represents the outcomes of prediction and diagnosis, such as sensitivity, specificity, and accuracy (Table S3 in Multimedia Appendix 1). The details of the search keywords are listed in Textbox S1 in Multimedia Appendix 1. Additionally, the reference list of each identified study was manually searched to identify any additional studies. NoteExpress 3.2 (Aegean) [10] and EndNote X7 (Clarivate) [11] were employed to manage the studies and remove duplicate items.
Inclusion and Exclusion Criteria
All studies included had to meet the following criteria: (1) published in English or Chinese; (2) included pregnant women from the general population, with a clear definition for GDM diagnosis; (3) included ML models for GDM prediction, with a clear description of the ML models; and (4) showed the performance of ML models, including sufficient data to enable the inference of sensitivity and specificity.
Articles in other languages, other types of articles (eg, reports and reviews), or those that used other measures for GDM detection were excluded. Four investigators (LY, WH, YW, and CG) participated in the literature screening to review all the studies that met the inclusion criteria. Each chosen article was screened at least twice, and disagreements were resolved by the reviewer (ZZ). Studies providing the most detailed information of variables and outcome indicators were kept for reference.
Data Extraction
Data extraction was performed independently by two investigators (LY and LZ) according to the existing literature and the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) standardized protocol [12]. A total of 25 studies were ultimately selected for the analysis. The following data were extracted from each study: (1) demographic information (ie, the country in which the data were gathered, the setting, the data source, the study design, the prediction temporality, and the outcome definition); (2) the data division method, the feature selection algorithms, the features of the model training, the ML prediction model type, and the model validation and application; (3) prediction outcomes, including accuracy, sensitivity, specificity, and area under the receiver operating characteristic curve (AUROC); and (4) funding and ethics approval.
Quality and Bias Assessments
The PROBAST [13], which includes a total of 20 signaling questions in four domains (ie, participants, predictors, outcome, and analysis), was used as a tool for assessing the risk of bias and applicability of each included study.
Statistical Analysis
The performance of each ML model was described using the primary outcome measures of discrimination and calibration. Model discrimination or concordance index (C-index) is similar to the AUROC [14] and indicates its diagnostic or prognostic discrimination ability as none (AUROC ≤0.6), poor (AUROC >0.6 to 0.7), fair (AUROC >0.7 to 0.8), good (AUROC >0.8 to 0.9), or optimum (AUROC >0.9 to 1). Model calibration is a metric of goodness of fit that assesses the agreement between observed and predicted outcomes and reflects the stability of the model via calibration plots. The diagnostic odds ratio (DOR) was also calculated via the following equation:
| DOR = PLR / NLR (1) |
where PLR is the positive likelihood ratio and NLR is the negative likelihood ratio. The PLR and NLR were calculated to express how frequently the model predicted GDM among the individuals with GDM versus among those without GDM using the following equations:
| PLR = Sensitivity / (1 − Specificity) (2) |
| NLR = (1 – Sensitivity) / Specificity (3) |
In this meta-analysis, the Meta-DiSc software program (version 1.4) [15] was used to calculate the pooled estimates of AUROC, sensitivity, specificity, PLR, NLR, and DOR. It was used to summarize the data from the included studies and graphically investigate the homogeneity among the studies. The I2 test was used to assess the statistical heterogeneity among the included studies. An I2 value of more than 75% indicated high heterogeneity among the studies [16]. The analysis of the included studies was divided into primary and subgroup analyses to judge the performances of the ML methods in predicting GDM in different clinical scenarios. Sensitivity analysis, subgroup analyses, and a meta-regression were also conducted to gain insight into potential sources of interstudy heterogeneity due to selector or inclusion criteria bias. The abilities of the different ML algorithms (eg, LR, Bayesian model, TreeNet, and GA-CatBoost [genetic algorithm category boosting]) for predicting GDM are discussed in the Subgroup Analysis section. The four predictive models with the highest and the lowest values were excluded from the sensitivity analysis to assess the impact of outliers on pooled sensitivity and specificity.
Results
Study Selection
A total of 27,071 studies were initially identified; of those, 1256 (4.6%) that discussed GDM were subjected to abstract screening. A total of 67 studies were subjected to full-text review; of those, 25 (37%) were included in the meta-analysis [17-33]. Figure 1 shows the PRISMA flow diagram of the study selection process.
Figure 1.
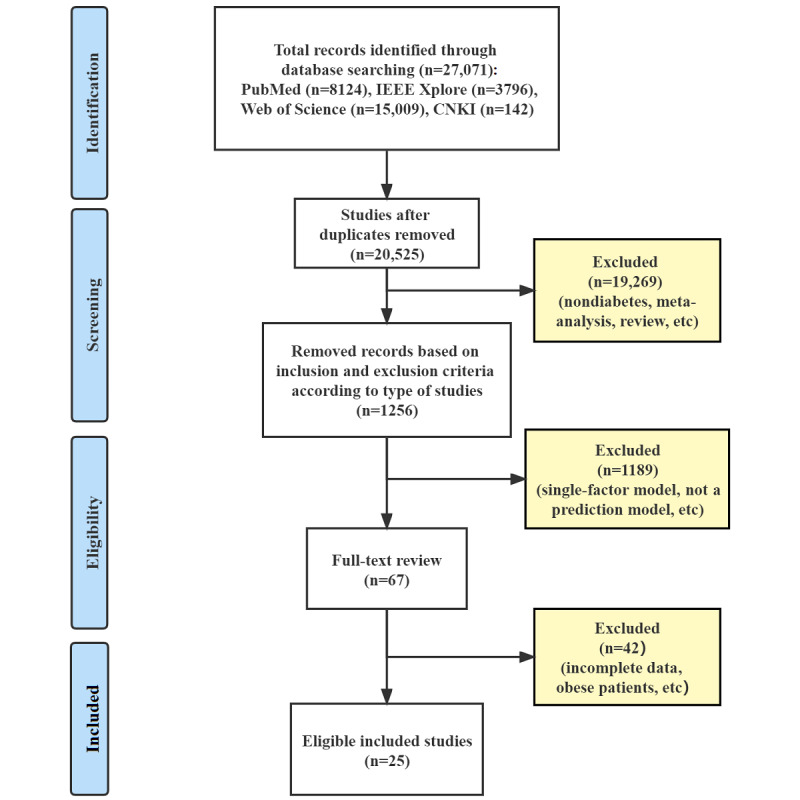
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram for study selection. CNKI: China National Knowledge Infrastructure.
Study Characteristics
The articles’ years of publication ranged from 2004 to 2020; 10 out of 25 (40%) were published in 2020 (Figure S1 in Multimedia Appendix 1). All studies included women older than 18 years without a history of heart or cerebrovascular disease or vital organ dysfunction. Out of 25 studies, 9 (36%) included patients with a history of GDM, while other studies excluded those cases with a history of GDM (Tables S4-S6 in Multimedia Appendix 1). The source data for ML training were mostly obtained from medical centers and maternity hospitals; some also included self-administered questionnaires. Out of 25 studies, 9 (36%) were conducted using data from a population-based prospective cohort or multicenter study. The sample size of the included studies varied from 134 to 66,687 participants.
Feature selection is an important step for ML training. Xiong et al [19] developed a prediction model for GDM risk in the first 19 weeks of gestation with several hepatic, renal, and coagulation function measures; they observed that a cutoff of prothrombin time and activated partial thromboplastin time could reliably predict GDM with a sensitivity of 88.3%, a specificity of 99.47%, and an AUROC of 94.2%. Maternal age, family history of diabetes, BMI, and fasting blood glucose were the four most commonly used features of the established models, whereas pregnancy-associated plasma protein A, leptin, lipocalin-2, adiponectin, weight gain, and soft drink intake during pregnancy were used in only one or two models each. Table 1 [17-41] summarizes the most frequent features included in the prognostic models.
Table 1.
The most frequent factors included in risk prediction models for gestational diabetes mellitus.
| Study first author, year | Factors included in models | ||||||||||||
|
|
MAa (n=19) | FHDb (n=14) | BMI (n=12) | FPGc (n=11) | PBMId (n=8) | HDe (n=8) | Ethnicity (n=6) | TGf (n=5) | HbA1cg (n=4) | SBPh (n=3) | Height (n=3) | hsCRPi (n=3) | |
| Gao, 2020 [21] | ✓j | ✓ | ✓ |
|
|
|
|
|
|
✓ | ✓ |
|
|
| Liu, 2020 [17] | ✓ |
|
|
✓ | ✓ |
|
|
|
|
|
|
|
|
| Miao, 2020 [28] |
|
✓ |
|
✓ |
|
|
|
✓ |
|
|
|
|
|
| Tan, 2020 [41] | ✓ | ✓ |
|
✓ | ✓ |
|
|
|
|
|
|
|
|
| Wu, 2020 [18] | ✓ | ✓ |
|
✓ |
|
✓ |
|
|
|
|
|
|
|
| Xiong, 2020 [19] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Ye, 2020 [20] | ✓ |
|
✓ | ✓ | ✓ | ✓ |
|
✓ | ✓ |
|
|
|
|
| Zhang, 2020 [39] | ✓ | ✓ |
|
✓ | ✓ | ✓ |
|
|
✓ | ✓ |
|
✓ | |
| Snyder, 2020 [40] | ✓ |
|
|
|
✓ |
|
✓ |
|
|
|
|
|
|
| Cui, 2019 [25] | ✓ | ✓ |
|
|
✓ |
|
|
|
|
|
|
|
|
| Zheng, 2019 [24] | ✓ |
|
✓ | ✓ |
|
|
|
✓ |
|
|
|
|
|
| Nombo, 2018 [26] |
|
✓ | ✓ |
|
|
|
|
|
|
|
|
|
|
| Sweeting, 2018 [27] |
|
✓ | ✓ |
|
|
✓ | ✓ | ✓ |
|
|
|
|
|
| Xiao, 2018 [38] | ✓ |
|
✓ | ✓ |
|
|
|
✓ | ✓ |
|
✓ |
|
|
| Huang, 2017 [23] | ✓ | ✓ |
|
✓ | ✓ |
|
|
|
|
|
|
|
|
| Wu, 2017 [22] | ✓ |
|
✓ | ✓ |
|
|
|
|
|
|
✓ | ✓ | |
| Gabbay-Benziv, 2015 [30] | ✓ |
|
✓ |
|
|
✓ | ✓ |
|
|
✓ |
|
|
|
| Thériault, 2015 [29] | ✓ | ✓ | ✓ |
|
|
✓ | ✓ |
|
✓ |
|
|
✓ | |
| Eleftheriades, 2014 [31] | ✓ |
|
|
|
|
|
|
|
|
|
|
|
|
| Pintaudi, 2013 [32] |
|
✓ |
|
✓ | ✓ |
|
|
|
|
|
|
|
|
| Savona-Ventura, 2013 [33] | ✓ |
|
|
✓ |
|
|
|
|
|
|
|
|
|
| Tran, 2013 [34] | ✓ |
|
✓ |
|
|
|
|
|
|
|
|
|
|
| Teede, 2011 [35] | ✓ | ✓ |
|
|
|
✓ | ✓ |
|
|
|
|
|
|
| Vanleeuwen, 2009 [36] |
|
✓ | ✓ |
|
|
✓ | ✓ |
|
|
|
|
|
|
| Caliskan, 2004 [37] | ✓ | ✓ | ✓ |
|
|
|
|
|
|
|
|
|
|
aMA: maternal age.
bFHD: family history of diabetes.
cFPG: fasting plasma glucose.
dPBMI: prepregnancy BMI.
eHD: history of diabetes.
fTG: triglyceride.
gHbA1c: hemoglobin A1c.
hSBP: systolic blood pressure.
ihsCRP: high-sensitivity C-reaction protein.
jA checkmark (✓) indicates that the factor was included.
The LR model was the most universally used model in the 25 studies (n=17, 68%) for predicting GDM risk, while 5 (20%) studies assessed the performance of other ML methods (ie, GA-CatBoost, XGBoost, Bayesian model, TreeNet, gradient-boosting decision tree [GBDT], adaptive boosting [AdaBoost], LightGBM, Vote, and RF). For measuring deep learning performance, AUROC and the Youden index were most commonly used. AUROC was used in studies that did not provide the C-index. Out of 25 studies, 2 (8%) did not report metrics of model discrimination. Of the 25 studies, only 7 (28%) presented calibration measures. Internal validation was performed in 13 studies (52%) using random split or k-fold cross-validation and bootstrapping. Only 4 studies out of 25 (16%) performed external validation.
Quality Assessment
Items from the PROBAST checklists (Multimedia Appendix 2) were used to assess the risk of bias and applicability of the prognostic prediction model studies. According to the criteria, the biases of participants in 4 out of 25 (16%) studies [30,31,33,36] were moderate, mainly due to debatable criteria, while biases in the other studies were low. Out of 25 studies, 24 (96%) study groups had a low bias of predictors, while 1 (4%) [32] had a moderate risk of bias because the prediction assessment was created with knowledge of the outcome data. The bias of outcome in 6 (24%) studies [22,30,33,35-37] was moderate due to the diagnostic criteria, while the others were low. A total of 8 (32%) groups had a moderate bias of analysis [21,23,27,31-33,36,37] and 1 (4%) [30] showed a high risk of bias due to an unreasonable number of participants with outcomes. The overall bias rating of 10 (40%) groups [21-23,27,30,32,33,35-37] was moderate. Overall concerns regarding the applicability rating of 7 (28%) studies [21-23,27,29,32,33] were moderate because of excessive features in models making it difficult to collect data in actual use, whereas others were low (Table S7 in Multimedia Appendix 1).
Performance of ML Models for GDM Prediction
The overall pooled AUROC for ML models for predicting GDM was 0.8492 (Figure 2). Additional values were as follows: sensitivity 0.69 (95% CI 0.68-0.69; P<.001; I2=99.6%; Figure 3); specificity 0.75 (95% CI 0.75-0.75; P<.001; I2=100%; Figure 4); DOR 13.78 (95% CI 9.53-19.94; P<.001; I2=99.1%); PLR 4.02 (95% CI 3.13-5.17; P<.001; I2=99.6%); and NLR 0.31 (95% CI 0.26-0.38; P<.001; I2=98.7%).
Figure 2.
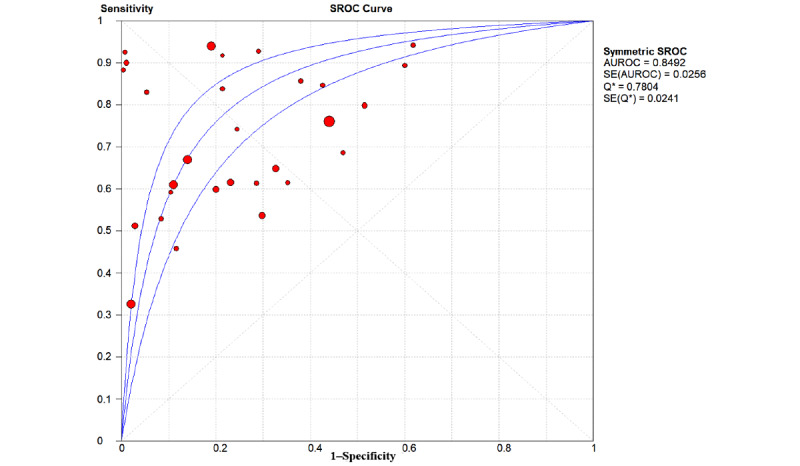
The overall pooled area under the receiver operating characteristic curve (AUROC) of machine learning models for gestational diabetes mellitus prediction. Q*: the sensitivity at the intersection of the SROC curve and the straight line (sensitivity=specificity); SROC: summary receiver operating characteristic.
Figure 3.
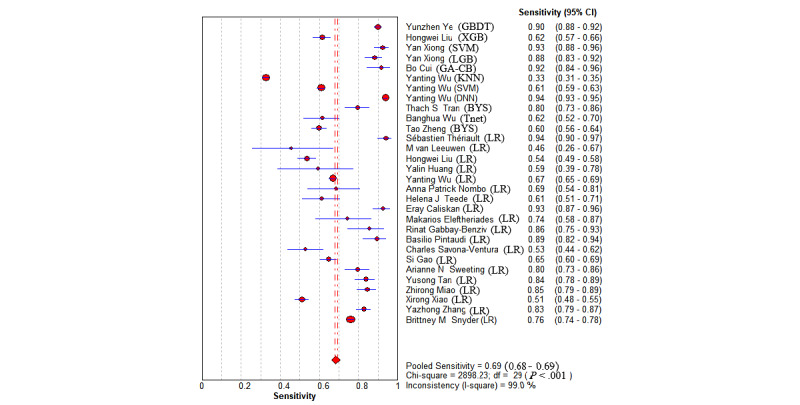
The overall pooled sensitivity of machine learning models for gestational diabetes mellitus prediction. First authors for each study are listed along the y-axis. The vertical red dotted lines are the 95% CIs of the pooled sensitivity. BYS: Bayesian; DNN: deep neural network; GA-CB: GA-CatBoost (genetic algorithm category boosting); GBDT: gradient-boosting decision tree; KNN: k-nearest neighbors; LGB: LightGBM (light gradient boosting machine); LR: logistic regression; SVM: support vector machine; Tnet: TreeNet; XGB: XGBoost (extreme gradient boosting).
Figure 4.
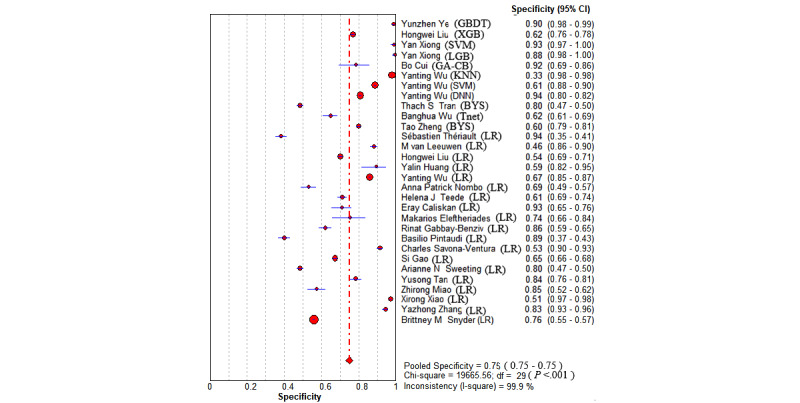
The overall pooled specificity of machine learning for gestational diabetes mellitus prediction. First authors for each study are listed along the y-axis. The vertical red dotted line is the 95% CI of the pooled specificity. BYS: Bayesian; DNN: deep neural network; GA-CB: GA-CatBoost (genetic algorithm category boosting); GBDT: gradient-boosting decision tree; KNN: k-nearest neighbors; LGB: LightGBM (light gradient boosting machine); LR: logistic regression; SVM: support vector machine; Tnet: TreeNet; XGB: XGBoost (extreme gradient boosting).
Sensitivity Analysis
After the exclusion of the 4 (16%) models with the lowest and highest sensitivity and specificity, the random effects meta-analysis produced estimated pooled sensitivity of 0.73 (95% CI 0.72-0.74; P<.001; I2=98.3%) and pooled specificity of 0.73 (95% CI 0.72-0.73; P<.001; I2=99.8%). Therefore, the pooled estimates were deemed insensitive to the exclusion of outliers (Figure S2 in Multimedia Appendix 1).
Subgroup Analysis
The comparison of the GDM prediction performance results is shown in Table 2; forest plots are shown in Figures S3-S8 in Multimedia Appendix 1.
Table 2.
The comparison of performance of machine learning models in gestational diabetes mellitus (GDM) prediction applied to different subgroups.
| Subgroup | Models (N=30), n (%) | AUROCa | Sensitivity (95% CI) | Specificity (95% CI) | PLRb (95% CI) | NLRc (95% CI) | DORd (95% CI) |
| Overall | 30 (100) | 0.8492 | 0.69 (0.68-0.69) | 0.75 (0.75-0.75) | 4.02 (3.13-5.17) | 0.31 (0.26-0.38) | 13.78 (9.53-19.94) |
| 0-13 weeks before diagnosis | 16 (53) | 0.8667 | 0.74 (0.73-0.75) | 0.64 (0.64-0.64) | 3.89 (2.92-5.19) | 0.28 (0.22-0.36) | 16.55 (9.52-28.77) |
| 14-28 weeks before diagnosis | 14 (47) | 0.8365 | 0.64 (0.63-0.65) | 0.85 (0.84-0.85) | 3.90 (2.76-5.53) | 0.35 (0.25-0.48) | 11.67 (7.59-18.02) |
| With GDM history | 11 (37) | 0.8759 | 0.67 (0.66-0.68) | 0.85 (0.85-0.86) | 5.29 (3.39-8.25) | 0.28 (0.18-0.44) | 19.82 (11.49-34.13) |
| Without GDM history | 19 (63) | 0.8330 | 0.70 (0.66-0.68) | 0.65 (0.64-0.65) | 3.12 (2.52-3.86) | 0.35 (0.30-0.41) | 8.27 (5.14-13.29) |
| Logistic regression | 19 (63) | 0.8151 | 0.71 (0.70-0.72) | 0.67 (0.67-0.67) | 3.04 (2.37-3.89) | 0.37 (0.32-0.43) | 8.73 (5.99-12.73) |
| Non–logistic regression | 11 (37) | 0.8891 | 0.66 (0.65-0.67) | 0.85 (0.85-0.86) | 6.80 (4.45-10.37) | 0.24 (0.15-0.38) | 31.85 (15.93-63.69) |
aAUROC: area under receiver operating characteristic curve.
bPLR: positive likelihood ratio.
cNLR: negative likelihood ratio.
dDOR: diagnostic odds ratio.
In this study, 19 prediction models were established using the LR models [17,18,20-23,26-33,35-39], and the overall pooled AUROC for the LR models for predicting GDM was 0.8151 (Figure 5). The overall pooled AUROC for non-LR models to predict GDM was 0.8891 (Figure 6), the highest value among these subgroups. Further analysis of these non-LR methods showed that two support vector machine (SVM) models [19,20] achieved AUROC values of 0.82 and 0.98, respectively (Figure S9 in Multimedia Appendix 1), while two Bayesian models [24,34] achieved AUROC values of 0.766 and 0.71, respectively (Figure S10 in Multimedia Appendix 1). Interestingly, Ye et al [20] developed eight common ML methods—GBDT, AdaBoost, LightGBM, LR, Vote, XGBoost, decision tree (DT), and RF—and two common regression models to predict the occurrence of GDM with a data set of 822,242 patients. GBDT, AdaBoost, and LightGBM (AUROC 0.70-0.75) were the top three models, while DT and RF were the worst models (AUROC 0.5-0.68) in that study. The capabilities of three ML methods were compared using data from 490 people [21]. The deep neural network model achieved the highest AUROC of 0.92, while the SVM and k-nearest neighbors (KNN) models achieved AUROC values of 0.82 and 0.68, respectively. XGBoost, LightGBM, GA-CatBoost, and TreeNet were used in 4 out of 25 (16%) studies and achieved AUROC values of 0.742, 0.942, 0.872, and 0.676, respectively [17,19,21,25] (Table 2).
Figure 5.
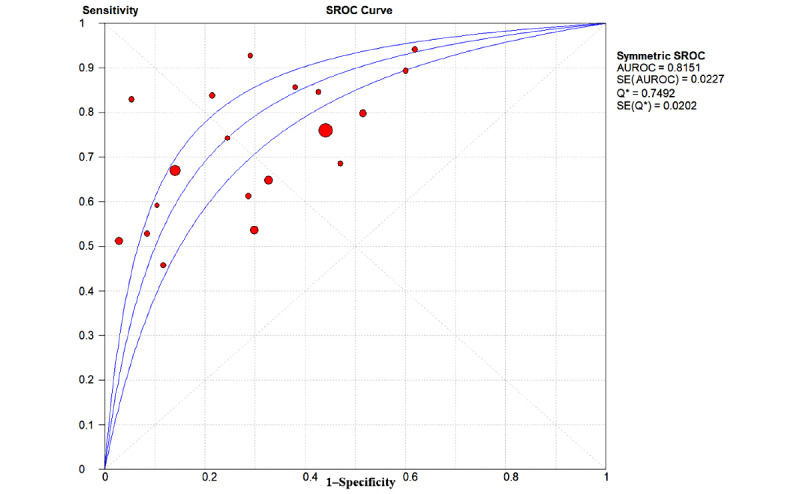
The overall pooled area under the receiver operating characteristic curve (AUROC) of logistic regression models for gestational diabetes mellitus prediction. Q*: the sensitivity at the intersection of the SROC curve and the straight line (sensitivity=specificity); SROC: summary receiver operating characteristic.
Figure 6.
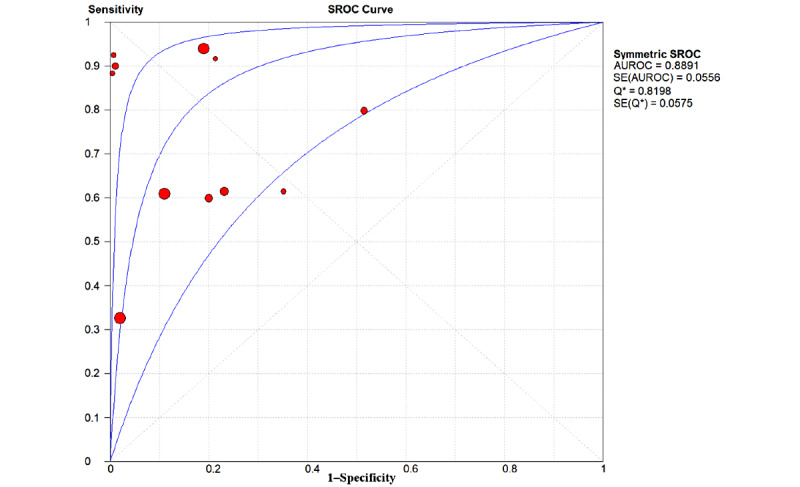
The overall pooled area under the receiver operating characteristic curve (AUROC) of non–logistic regression models for gestational diabetes mellitus prediction. Q*: the sensitivity at the intersection of the SROC curve and the straight line (sensitivity=specificity); SROC: summary receiver operating characteristic.
Meta-regression
The meta-regression analysis was conducted due to the high level of interstudy heterogeneity [42]. Sample size, country where the data were collected, publication year, ML methods used, and model quality did not affect diagnostic accuracy (P=.13). The antilogarithm transformations of the resulting estimated parameters could be interpreted as a relative DOR of the corresponding covariate, indicating the change in diagnostic performance of the test under study per unit increase in the covariate (Table S8 in Multimedia Appendix 1).
Discussion
Principal Findings
This study was a pilot meta-analysis evaluating the performance of ML models for predicting GDM. Its overall pooled estimation of 25 studies showed that ML models achieved high accuracy in early recognition of GDM patients. ML models could forecast based on data from 8 to 24 weeks’ gestation. There was even a model that used prepregnancy features to predict the outcome up to 28 weeks in advance, suggesting the significance of ML models for GDM prediction. Compared to the census or existing screening methods, ML methods have certain advantages. Universal screening leads to 100% detection for physicians who usually make decisions based on an OGTT test, which may place an unnecessary burden on individual women and health care resources. Current selective screening strategies are based on a list of risk factors and have fixed sensitivity (±65%) and specificity (±80%). Although the ML methods do not provide greater benefit than current available screening strategies, an advantage is that a preferred trade-off between sensitivity and specificity can be selected [43]. The choice of statistical method is more to compute a quantitative measure of existing data than to predict unknown data in a general and feasible way [44].
According to the subgroup analysis, models created using non-LR methods achieved the highest AUROC, suggesting that researchers should test more candidate models. One study aimed to review and compare the predictive performances of LR and other ML algorithms for developing or validating a multivariable prognostic prediction model for pregnancy care; that study also recommended a reanalysis of existing LR models for several pregnancy outcomes by comparing them with those algorithms that apply standard guidelines [45]. Among those non-LR models, ensemble methods, like LightGBM and GA-CatBoost, that are composed of multiple weaker models and are independently trained had a satisfactory result. Variables in the GBDT model underscored the advantage of identifying nonlinear relationships. The SVM model also achieved superior outcomes; that method builds a model that assigns new examples to one category or the other, making it a nonprobabilistic binary linear classifier. Methods like KNN, DT, and RF did not perform as well as the LightGBM and GA-CatBoost methods, which may be due to the fact that DT classifications are based on a single condition at the bottom, so small changes can lead to mistakes. For RF, the high dimension of medical data complicates the classification and prediction. Similarly, KNN cannot be used in high-dimensional feature spaces. Some researchers [23] found that the difference between two methods had no statistical significance, since LR models are suitable for simple data with linear relationships between variables and outcomes. Our study also found that LR models were conducive to achieving more stable performance according to the summary receiver operating characteristic curve. The subgroup of 0 to 13 weeks before diagnosis achieved the highest pooled sensitivity, while the subgroup of 14 to 28 weeks before diagnosis achieved the highest specificity, meaning that ML may assist clinicians identify more patients in early screening and avoid excessive misdiagnosis in the second trimester.
The feature selection was also crucial for model performance and interpretation. Among the 25 studies, maternal age was used as a feature in 19 studies, as was previously reported and validated in our study. One of the included studies reported that the incidence of GDM increases after 25 years of age, the main reason being that the function of islet β-cells decreases with age, so the insulin antagonism of older adult pregnant women is aggravated [46]. Eight models considered GDM history to be a vital factor for predicting GDM. A DOR value of 21.09 appeared when a GDM history was included as a risk factor for predicting future GDM. Previous research discovered that women with GDM were more likely to have a family history of type 2 diabetes mellitus and a history of GDM, partially due to overlapping genetic bases between the diseases [18]. The nonsignificant association of GDM with a GDM history in other studies was a result of the overwhelming proportion of nulliparous women in their studies who had no risk of developing GDM. The association between GDM and blood lipid indexes, including triglyceride (TG), high-density lipoprotein, and low-density lipoprotein (LDL), has been studied, and TG level had the closest relationship with GDM [47]. Our research also found that although the levels of TG, total cholesterol, and LDL in the GDM group were higher than those in normal pregnant women in most included studies, only TG level was a high-risk factor of GDM after the feature selection. A novel model that included ultrasound data of maternal fat distribution and serum inflammatory factors observed that pregnant women with GDM had greater visceral fat thickness and subcutaneous fat thickness; the model also demonstrated that increased subcutaneous and visceral fat may lead to increased insulin resistance in muscle and adipose tissue [36]. Sweeting et al [27] observed higher leptin and lipocalin-2 levels and lower adiponectin levels in women who developed GDM and proposed adipokines as GDM features.
Strengths and Limitations
The main strength of the study is that its methodology was logical and described in sufficient detail to be reproducible. Almost all published prognostic models for GDM were included in this meta-analysis, which enabled their comparison. The data collection table was based on the characteristics of the GDM prediction models. Additionally, the novel PROBAST was used to assess the risk of bias and applicability of prognostic prediction model studies. The Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool is a widely used tool for estimating the bias and applicability of primary diagnostic accuracy studies, but is not perfectly suited for predictive models [48]. An increasing number of researchers prefer the PROBAST to the QUADAS tool for assessing the bias of AI-based models in systematic reviews as well as meta-analyses; this is the case because more details of the model, such as data source, processing, number of events per variable, feature selection, model development, and model validation, were checked intensively [49-52]. We found that 14 development studies had a risk of bias in methodological quality or applicability, which may lead to overfitted prediction models. It is noteworthy that the quality of recent models is higher than that of those published earlier according to the PROBAST. Some bias could be prevented if the studies reported their research according to the TRIPOD initiative [12].
Despite our study’s confirmation that ML models have promising prediction ability for GDM, there are some limitations to our research. The main limitations arose from the interstudy heterogeneity. First, the sample sizes and distributions differed among studies, affecting each model’s performance and applicability. There were also a heterogenous variety of feature selection methods. Some researchers preferred the features that have a statistically significant association with GDM, while others included the factors based on existing knowledge from previously established models in combination with predictor reliability, consistency, applicability, availability, and cost. Second, the performance of a low-quality model might be overestimated when the analysis of the internal bias of the model is ignored. As some studies have bias to various degrees, the results of the studies in this analysis must be applied with caution. It should be noticed that the PROBAST is more likely to identify bias in prediction models than other tools designed for conventional diagnostic methods. The other limitation is that few models underwent external validation to test their extensibility. However, a previous study [8] performed an external validation of 12 published GDM prediction models and suggested that most of the published models showed acceptable discrimination and calibration, but the author pointed out possible heterogeneity in these models due to variations in GDM incidence in different populations.
Clinical Implications
Although several GDM scoring systems have been developed, none are widely recommended by current guidelines. Based on the discussion above, several items must be considered in order to maximize the advantages of ML models for predicting GDM in clinical practice for model researchers or for decision makers. For the former, we recommend that the decision concerning which feature selection methods and ML algorithms to use should be based on clinical need rather than accuracy. A model with excess features that are difficult to obtain in routine medicine is unlikely to be applied broadly. Researchers should also provide the process of data preprocessing and outcomes of validation, discrimination, calibration, and classification to elaborate the performance of models from multiple perspectives. For decision makers, we recommend that data sources, such as a population-based cohort designed for GDM research with a unified international diagnostic criterion, promote the ML methods in this target. Studies revealed that although electronic health records provide various data, including time series and images for novel ML methods, they have inherent biases that are influenced by the interaction of the patient with the health care system. In contrast, community-based predictions may robustly capture more asymptomatic high-risk cases [53]. The incidence of GDM based on the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) (22.94%) and the National Institute for Health and Care Excellence (21.72%) is over 3-fold higher than that based on the criteria from the 7th edition of the Chinese obstetrics and gynecology textbook (6.08%) published by the People’s Medical Publishing House [54]. Some experts in China have advocated implementation of the IADPSG criteria because they believe that it will guide researchers to better understand the prevalence of GDM in different regions and ensure that the country’s standards will be aligned with international ones. Nevertheless, researchers doubt that the IADPSG findings will apply to all populations, since those criteria were applied to mainly Caucasian women. All in all, it would indeed be helpful to unify the GDM diagnostic criteria as soon as possible. This meta-analysis reported the advantages of ML models and the factors requiring attention. A similar meta-analysis of ML models and deep learning algorithms used to detect patients at risk of developing diabetes reported that AI-based automated tools provide substantial benefits for reducing screening costs and can replace earlier treatments [55].
Conclusions
In conclusion, ML methods demonstrate high performance and will be a more selective and cost-effective screening method for GDM. The importance of quality assessment and unified diagnostic criteria should be further emphasized.
Acknowledgments
This work was supported by grants from the National Key R&D Program of China (grants 2018YFC1314900 and 2018YFC1314902), Nantong “226 Project,” Excellent Key Teachers in the “Qing Lan Project” of Jiangsu Colleges and Universities, Science and Technology Project of Nantong City (grant MS12020037), Jiangsu Students’ Platform for Innovation and Entrepreneurship Training Program (grant 201910304108Y), and Jiangsu Postgraduate Research and Innovation Program (grant KYCX20_2836).
Abbreviations
- AdaBoost
adaptive boosting
- AI
artificial intelligence
- AUROC
area under the receiver operating characteristic curve
- C-index
concordance index
- DOR
diagnostic odds ratio
- DT
decision tree
- FDA
Food and Drug Administration
- GA-CatBoost
genetic algorithm category boosting
- GBDT
gradient-boosting decision tree
- GDM
gestational diabetes mellitus
- IADPSG
International Association of the Diabetes and Pregnancy Study Groups
- KNN
k-nearest neighbors
- LDL
low-density lipoprotein
- LightGBM
light gradient boosting machine
- LR
logistic regression
- ML
machine learning
- NLR
negative likelihood ratio
- OGTT
oral glucose tolerance test
- PICO
population, intervention, control, and outcomes
- PLR
positive likelihood ratio
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROBAST
Prediction Model Risk of Bias Assessment Tool
- QUADAS
Quality Assessment of Diagnostic Accuracy Studies
- RF
random forest
- SVM
support vector machine
- TG
triglyceride
- TRIPOD
Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis
- XGBoost
extreme gradient boosting
Supplementary materials.
Checklists from the Prediction Model Risk of Bias Assessment Tool (PROBAST).
Footnotes
Authors' Contributions: HW supervised this study. LY, WH, YW, LZ, and CG performed literature retrieval and data extraction. KJ and YL provided technical support. ZZ performed experimental design and data processing and wrote the manuscript.
Conflicts of Interest: None declared.
References
- 1.Yuen L, Saeedi P, Riaz M, Karuranga S, Divakar H, Levitt N, Yang X, Simmons D. Projections of the prevalence of hyperglycaemia in pregnancy in 2019 and beyond: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019 Nov;157:107841. doi: 10.1016/j.diabres.2019.107841.S0168-8227(19)31245-8 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013 Dec 19;37(Supplement_1):S81–S90. doi: 10.2337/dc14-s081. [DOI] [PubMed] [Google Scholar]
- 3.Caissutti C, Berghella V. Scientific evidence for different options for GDM screening and management: Controversies and review of the literature. Biomed Res Int. 2017;2017:2746471. doi: 10.1155/2017/2746471. doi: 10.1155/2017/2746471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J. Prediction of Gestational Diabetes Based on Machine Learning [master's thesis; document in Chinese] Chengdu, China: Southwestern University of Finance and Economics; 2019. https://tinyurl.com/yck82cvx . [Google Scholar]
- 5.Artzi NS, Shilo S, Hadar E, Rossman H, Barbash-Hazan S, Ben-Haroush A, Balicer RD, Feldman B, Wiznitzer A, Segal E. Prediction of gestational diabetes based on nationwide electronic health records. Nat Med. 2020 Jan;26(1):71–76. doi: 10.1038/s41591-019-0724-8.10.1038/s41591-019-0724-8 [DOI] [PubMed] [Google Scholar]
- 6.Ngiam KY, Khor IW. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019 May;20(5):e262–e273. doi: 10.1016/S1470-2045(19)30149-4.S1470-2045(19)30149-4 [DOI] [PubMed] [Google Scholar]
- 7.Changes to Existing Medical Software Policies Resulting From Section 3060 of the 21st Century Cures Act: Guidance for Industry and Food and Drug Administration Staff. Rockville, MD: US Food and Drug Administration; 2019. Sep, https://tinyurl.com/yy8zuajc . [Google Scholar]
- 8.Lamain-de Ruiter M, Kwee A, Naaktgeboren CA, de Groot I, Evers IM, Groenendaal F, Hering YR, Huisjes AJM, Kirpestein C, Monincx WM, Siljee JE, Van 't Zelfde A, van Oirschot CM, Vankan-Buitelaar SA, Vonk MAAW, Wiegers TA, Zwart JJ, Franx A, Moons KGM, Koster MPH. External validation of prognostic models to predict risk of gestational diabetes mellitus in one Dutch cohort: Prospective multicentre cohort study. BMJ. 2016 Aug 30;354:i4338. doi: 10.1136/bmj.i4338. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=27576867 . [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. https://dx.plos.org/10.1371/journal.pmed.1000097 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NoteExpress. [2020-11-01]. http://www.inoteexpress.com/wiki/index.php/%E9%A6%96%E9%A1%B5 .
- 11.Eapen B. EndNote 7.0. Indian J Dermatol Venereol Leprol. 2006;72(2):165–166. doi: 10.4103/0378-6323.25654. [DOI] [PubMed] [Google Scholar]
- 12.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. Eur J Clin Invest. 2015 Feb;45(2):204–214. doi: 10.1111/eci.12376. [DOI] [PubMed] [Google Scholar]
- 13.Moons KG, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, Reitsma JB, Kleijnen J, Mallett S. PROBAST: A tool to assess risk of bias and applicability of prediction model studies: Explanation and elaboration. Ann Intern Med. 2019 Jan 01;170(1):W1. doi: 10.7326/m18-1377. [DOI] [PubMed] [Google Scholar]
- 14.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW. Assessing the performance of prediction models. Epidemiology. 2010;21(1):128–138. doi: 10.1097/ede.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006 Jul 12;6:31. doi: 10.1186/1471-2288-6-31. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/1471-2288-6-31 .1471-2288-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 06;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. http://europepmc.org/abstract/MED/12958120 .327/7414/557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Li J, Leng J, Wang H, Liu J, Li W, Liu H, Wang S, Ma J, Chan JC, Yu Z, Hu G, Li C, Yang X. Machine learning risk score for prediction of gestational diabetes in early pregnancy in Tianjin, China. Diabetes Metab Res Rev. 2021 Jul 09;37(5):e3397. doi: 10.1002/dmrr.3397. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Zhang C, Mol BW, Kawai A, Li C, Chen L, Wang Y, Sheng J, Fan J, Shi Y, Huang H. Early prediction of gestational diabetes mellitus in the Chinese population via advanced machine learning. J Clin Endocrinol Metab. 2021 Mar 08;106(3):e1191–e1205. doi: 10.1210/clinem/dgaa899. http://europepmc.org/abstract/MED/33351102 .6031346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong Y, Lin L, Chen Y, Salerno S, Li Y, Zeng X, Li H. Prediction of gestational diabetes mellitus in the first 19 weeks of pregnancy using machine learning techniques. J Matern Fetal Neonatal Med. 2020 Aug 06;:1–7. doi: 10.1080/14767058.2020.1786517. [DOI] [PubMed] [Google Scholar]
- 20.Ye Y, Xiong Y, Zhou Q, Wu J, Li X, Xiao X. Comparison of machine learning methods and conventional logistic regressions for predicting gestational diabetes using routine clinical data: A retrospective cohort study. J Diabetes Res. 2020;2020:4168340. doi: 10.1155/2020/4168340. doi: 10.1155/2020/4168340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao S, Leng J, Liu H, Wang S, Li W, Wang Y, Hu G, Chan JCN, Yu Z, Zhu H, Yang X. Development and validation of an early pregnancy risk score for the prediction of gestational diabetes mellitus in Chinese pregnant women. BMJ Open Diabetes Res Care. 2020 Apr;8(1):e000909. doi: 10.1136/bmjdrc-2019-000909. https://drc.bmj.com/lookup/pmidlookup?view=long&pmid=32327440 .8/1/e000909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu B, Huang H, Yao Q, Deng R, Li H, Liang T. Application of big data and artificial intelligence methods in the prediction of gestational diabetes mellitus. Chin J Health Inf Manag. 2017;14(6):832–837. doi: 10.3969/j.issn.1672-5166.2017.06.016. [DOI] [Google Scholar]
- 23.Huang Y. Construction of a Gestational Diabetes Risk Model Based on the Guangxi Birth Cohort and Analysis of its Impact on Pregnancy Outcomes [master's thesis; document in Chinese] Guangxi, China: Guangxi Medical University; 2017. [2020-09-14]. https://tinyurl.com/2p8tredx . [Google Scholar]
- 24.Zheng T, Ye W, Wang X, Li X, Zhang J, Little J, Zhou L, Zhang L. A simple model to predict risk of gestational diabetes mellitus from 8 to 20 weeks of gestation in Chinese women. BMC Pregnancy Childbirth. 2019 Jul 19;19(1):252. doi: 10.1186/s12884-019-2374-8. https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-019-2374-8 .10.1186/s12884-019-2374-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui B. Study of Cascade GA-CatBoost in Predictive Diagnosis of Gestational Diabetes Mellitus [master's thesis; document in Chinese] Taiyuan, China: Taiyuan University of Technology; 2019. [2022-01-25]. http://150.138.141.24/kcms/detail/detail.aspx?filename=1019872456.nh&dbcode=CMFD&dbname=CMFDREF . [Google Scholar]
- 26.Nombo AP, Mwanri AW, Brouwer-Brolsma EM, Ramaiya KL, Feskens EJ. Gestational diabetes mellitus risk score: A practical tool to predict gestational diabetes mellitus risk in Tanzania. Diabetes Res Clin Pract. 2018 Nov;145:130–137. doi: 10.1016/j.diabres.2018.05.001.S0168-8227(18)30684-3 [DOI] [PubMed] [Google Scholar]
- 27.Sweeting A, Wong J, Appelblom H, Ross G, Kouru H, Williams P, Sairanen M, Hyett J. A novel early pregnancy risk prediction model for gestational diabetes mellitus. Fetal Diagn Ther. 2019;45(2):76–84. doi: 10.1159/000486853.000486853 [DOI] [PubMed] [Google Scholar]
- 28.Miao Z, Wu H, Zhang Y, Gao Y, Jiang Y, Zhao Y, Yang H, Zhang J, Guo X. The values of early pregnancy glucose and lipid metabolism indexes in predicting gestational diabetes mellitus with different prepregnancy body mass index. Chin J Diabetes. 2020;12(7):462–468. doi: 10.3760/cma.j.cn115791-20200401-00189. [DOI] [Google Scholar]
- 29.Thériault S, Giguère Y, Massé J, Girouard J, Forest J. Early prediction of gestational diabetes: A practical model combining clinical and biochemical markers. Clin Chem Lab Med. 2016 Mar;54(3):509–518. doi: 10.1515/cclm-2015-0537./j/cclm.ahead-of-print/cclm-2015-0537/cclm-2015-0537.xml [DOI] [PubMed] [Google Scholar]
- 30.Gabbay-Benziv R, Doyle L, Blitzer M, Baschat A. First trimester prediction of maternal glycemic status. J Perinat Med. 2015 May;43(3):283–289. doi: 10.1515/jpm-2014-0149./j/jpme.ahead-of-print/jpm-2014-0149/jpm-2014-0149.xml [DOI] [PubMed] [Google Scholar]
- 31.Eleftheriades M, Papastefanou I, Lambrinoudaki I, Kappou D, Lavranos D, Akalestos A, Souka AP, Pervanidou P, Hassiakos D, Chrousos GP. Elevated placental growth factor concentrations at 11-14 weeks of gestation to predict gestational diabetes mellitus. Metabolism. 2014 Nov;63(11):1419–1425. doi: 10.1016/j.metabol.2014.07.016.S0026-0495(14)00236-4 [DOI] [PubMed] [Google Scholar]
- 32.Pintaudi B, Di Vieste G, Corrado F, Lucisano G, Pellegrini F, Giunta L, Nicolucci A, D’Anna R, Di Benedetto A. Improvement of selective screening strategy for gestational diabetes through a more accurate definition of high-risk groups. Eur J Endocrinol. 2013 Nov 22;170(1):87–93. doi: 10.1530/eje-13-0759. https://eje.bioscientifica.com/view/journals/eje/170/1/87.xml . [DOI] [PubMed] [Google Scholar]
- 33.Savona-Ventura C, Vassallo J, Marre M, Karamanos BG, MGSD-GDM Study Group A composite risk assessment model to screen for gestational diabetes mellitus among Mediterranean women. Int J Gynaecol Obstet. 2013 Mar;120(3):240–244. doi: 10.1016/j.ijgo.2012.10.016.S0020-7292(12)00593-0 [DOI] [PubMed] [Google Scholar]
- 34.Tran TS, Hirst JE, Do MAT, Morris JM, Jeffery HE. Early prediction of gestational diabetes mellitus in Vietnam: Clinical impact of currently recommended diagnostic criteria. Diabetes Care. 2013 Mar;36(3):618–624. doi: 10.2337/dc12-1418. http://europepmc.org/abstract/MED/23160727 .dc12-1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teede H, Harrison C, Teh W, Paul E, Allan C. Gestational diabetes: Development of an early risk prediction tool to facilitate opportunities for prevention. Aust N Z J Obstet Gynaecol. 2011;51(6):499–504. doi: 10.1111/j.1479-828x.2011.01356.x. [DOI] [PubMed] [Google Scholar]
- 36.van Leeuwen M, Opmeer B, Zweers E, van Ballegooie E, ter Brugge HG, de Valk HW, Visser GHA, Mol BWJ. Estimating the risk of gestational diabetes mellitus: A clinical prediction model based on patient characteristics and medical history. BJOG. 2010 Jan;117(1):69–75. doi: 10.1111/j.1471-0528.2009.02425.x.BJO2425 [DOI] [PubMed] [Google Scholar]
- 37.Caliskan E, Kayikcioglu F, Oztürk N, Koc S, Haberal A. A population-based risk factor scoring will decrease unnecessary testing for the diagnosis of gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2004 Jun;83(6):524–530. doi: 10.1111/j.0001-6349.2004.00389.x. doi: 10.1111/j.0001-6349.2004.00389.x.AOG389 [DOI] [PubMed] [Google Scholar]
- 38.Xiao X, Dong X, Li X, Yin C. The early predictive value of the basic characteristics of pregnant women and 14-20 weeks of glucose and lipid metabolism indicators for gestational diabetes. Chin J Pract Gynecol Obstet. 2018;34(04):429–432. doi: 10.19538/j.fk2018040120. [DOI] [Google Scholar]
- 39.Zhang Y, Zhou L, Tian L, Li X, Zhang G, Qin J, Zhang D, Fang H. A mid-pregnancy risk prediction model for gestational diabetes mellitus based on the maternal status in combination with ultrasound and serological findings. Exp Ther Med. 2020 Jul;20(1):293–300. doi: 10.3892/etm.2020.8690. http://europepmc.org/abstract/MED/32536997 .ETM-0-0-8690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snyder BM, Baer RJ, Oltman SP, Robinson JG, Breheny PJ, Saftlas AF, Bao W, Greiner AL, Carter KD, Rand L, Jelliffe-Pawlowski LL, Ryckman KK. Early pregnancy prediction of gestational diabetes mellitus risk using prenatal screening biomarkers in nulliparous women. Diabetes Res Clin Pract. 2020 May;163:108139. doi: 10.1016/j.diabres.2020.108139. http://europepmc.org/abstract/MED/32272192 .S0168-8227(20)30389-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan Y. Clinical research on gestational diabetes models in Changchun, China. Matern Child Health Care China. 2020;35(16):2957–2959. doi: 10.19829/j.zgfybj.issn.1001-4411.2020.16.007. [DOI] [Google Scholar]
- 42.Lijmer JG, Bossuyt PMM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med. 2002 Jun 15;21(11):1525–1537. doi: 10.1002/sim.1185. [DOI] [PubMed] [Google Scholar]
- 43.Meertens LJE, Scheepers HCJ, van Kuijk SMJ, Roeleveld N, Aardenburg R, van Dooren IMA, Langenveld J, Zwaan IM, Spaanderman MEA, van Gelder MMHJ, Smits LJM. External validation and clinical utility of prognostic prediction models for gestational diabetes mellitus: A prospective cohort study. Acta Obstet Gynecol Scand. 2020 Jul;99(7):891–900. doi: 10.1111/aogs.13811. doi: 10.1111/aogs.13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bzdok D, Altman N, Krzywinski M. Statistics versus machine learning. Nat Methods. 2018 Apr;15(4):233–234. doi: 10.1038/nmeth.4642. http://europepmc.org/abstract/MED/30100822 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sufriyana H, Husnayain A, Chen Y, Kuo C, Singh O, Yeh T, Wu Y, Su EC. Comparison of multivariable logistic regression and other machine learning algorithms for prognostic prediction studies in pregnancy care: Systematic review and meta-analysis. JMIR Med Inform. 2020 Nov 17;8(11):e16503. doi: 10.2196/16503. https://medinform.jmir.org/2020/11/e16503/ v8i11e16503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Du L. The influence of aging factors on pregnancy outcome [journal in Chinese] Clinical Medical Engineering. 2011;18(3):365–366. doi: 10.3969/j.issn.1674-4659.2011.03.0365. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2011&filename=YBQJ201103024&uniplatform=NZKPT&v=ZjGI9pLHMoYOTqhH45yoRzjKsn4UteRtFFGlYTV9WsJ9og26IiQjwPeTuaH6dkss%20[accessed%202020-11-1]%20l%20confirm%20that%20other%20references%20are%20correct.%20Best%20regards,%20Zheqing%20Zhang . [DOI] [Google Scholar]
- 47.Niromanesh S, Shirazi M, Dastgerdy E, Sharbaf FR, Shirazi M, Khazaeipour Z. Association of hypertriglyceridaemia with pre-eclampsia, preterm birth, gestational diabetes and uterine artery pulsatility index. Natl Med J India. 2012;25(5):265–267. [PubMed] [Google Scholar]
- 48.Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MMG, Sterne JAC, Bossuyt PMM, QUADAS-2 Group QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011 Oct 18;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. https://www.acpjournals.org/doi/abs/10.7326/0003-4819-155-8-201110180-00009?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed .155/8/529 [DOI] [PubMed] [Google Scholar]
- 49.Palazón-Bru A, Mares-García E, López-Bru D, Mares-Arambul E, Folgado-de la Rosa DM, de Los Ángeles Carbonell-Torregrosa M, Gil-Guillén VF. A critical appraisal of the clinical applicability and risk of bias of the predictive models for mortality and recurrence in patients with oropharyngeal cancer: Systematic review. Head Neck. 2020 Apr;42(4):763–773. doi: 10.1002/hed.26025. [DOI] [PubMed] [Google Scholar]
- 50.Di Tanna Gl, Wirtz H, Burrows Kl, Globe G. Evaluating risk prediction models for adults with heart failure: A systematic literature review. PLoS One. 2020;15(1):e0224135. doi: 10.1371/journal.pone.0224135. https://dx.plos.org/10.1371/journal.pone.0224135 .PONE-D-19-18060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagendran M, Chen Y, Lovejoy CA, Gordon AC, Komorowski M, Harvey H, Topol EJ, Ioannidis JPA, Collins GS, Maruthappu M. Artificial intelligence versus clinicians: Systematic review of design, reporting standards, and claims of deep learning studies. BMJ. 2020 Mar 25;368:m689. doi: 10.1136/bmj.m689. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=32213531 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dretzke J, Chuchu N, Agarwal R, Herd C, Chua W, Fabritz L, Bayliss S, Kotecha D, Deeks JJ, Kirchhof P, Takwoingi Y. Predicting recurrent atrial fibrillation after catheter ablation: A systematic review of prognostic models. Europace. 2020 May 01;22(5):748–760. doi: 10.1093/europace/euaa041. http://europepmc.org/abstract/MED/32227238 .5813809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silva KD, Lee WK, Forbes A, Demmer RT, Barton C, Enticott J. Use and performance of machine learning models for type 2 diabetes prediction in community settings: A systematic review and meta-analysis. Int J Med Inform. 2020 Nov;143:104268. doi: 10.1016/j.ijmedinf.2020.104268.S1386-5056(20)31167-9 [DOI] [PubMed] [Google Scholar]
- 54.He Z, Xie H, Liang S, Tang Y, Ding W, Wu Y, Ming W. Influence of different diagnostic criteria on gestational diabetes mellitus incidence and medical expenditures in China. J Diabetes Investig. 2019 Sep;10(5):1347–1357. doi: 10.1111/jdi.13008. doi: 10.1111/jdi.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Islam MM, Yang H, Poly TN, Jian W, Li YCJ. Deep learning algorithms for detection of diabetic retinopathy in retinal fundus photographs: A systematic review and meta-analysis. Comput Methods Programs Biomed. 2020 Jul;191:105320. doi: 10.1016/j.cmpb.2020.105320.S0169-2607(19)31101-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.
Checklists from the Prediction Model Risk of Bias Assessment Tool (PROBAST).


