Abstract
Objective:
Evidence is limited regarding clinical factors associated with ambulation status over the lifespan of individuals with myelomeningocele. We used longitudinal data from the National Spina Bifida Patient Registry to model population-level variation in ambulation over time and hypothesized that effects of clinical factors associated with ambulation would vary by age and motor level.
Design:
A population-averaged generalized estimating equation was used to estimate the probability of independent ambulation. Model predictors included time (age), race, ethnicity, sex, insurance, and interactions between time, motor level, and the number of orthopedic, noncerebral shunt neurosurgeries, and cerebral shunt neurosurgeries.
Results:
The study cohort included 5371 participants with myelomeningocele. A change from sacral to low-lumbar motor level initially reduced the odds of independent ambulation (OR = 0.24, 95% CI = 0.15–0.38) but became insignificant with increasing age. Surgery count was associated with decreased odds of independent ambulation (orthopedic: OR = 0.65, 95% CI = 0.50–0.85; noncerebral shunt neurosurgery: OR = 0.65, 95% CI = 0.51–0.84; cerebral shunt: OR = 0.90, 95% CI = 0.83–0.98), with increasing effects seen at lower motor levels.
Conclusions:
Our findings suggest that effects of several commonly accepted predictors of ambulation status vary with time. As the myelomeningocele population ages, it becomes increasingly important that study design account for this time-varying nature of clinical reality.
Keywords: Rehabilitation, Myelomeningocele, Spinal Dysraphism, Walking, Wheelchair, Registries, Population Characteristics
Spina bifida is a common congenital condition characterized by varying degrees of incomplete neural tube closure. In the United States, the incidence of spina bifida is approximately 3 in 10,000 live births, with myelomeningocele (MMC), defined by spinal cord protrusion through the unfused vertebral column, being the most common and severe form.1,2 Neurological sequelae including hydrocephalus and tethered cord syndrome as well as orthopedic conditions including scoliosis, hip subluxation or dislocation, and foot or ankle deformities are common within the MMC population.3 To prevent long-term negative impacts on functional mobility, repeated surgical intervention beginning at an early age is common.3,4 Along with surgery, individual mobility is modified by a milieu of personal and environmental factors and evolves over time.4–6 Individuals with MMC identify mobility and ambulation status as important components of independence, employment, and quality of life.7–11
Previous studies have sought to identify factors associated with ambulation with results generally focused on motor (or lesion) level, shunt status (or history of hydrocephalus), hip and knee contractures, spasticity, iliopsoas and quadriceps muscle strength, lower extremity edema, skin infections, surgical intervention, and age.9–15 Although this work has focused attention on important clinical attributes associated with ambulation status, the results are limited by small sample size (often 100 or fewer participants), cross-sectional design, a descriptive nature that is not able to control for confounding factors, and/or focus on a young subset (e.g., 6 yrs and younger) of the MMC population.9–15 Our current epidemiological understanding of ambulation in the MMC population neither includes a full description of ambulatory variation over the lifespan nor addresses the likely complex interaction between important clinical events and their potentially age-dependent effects.4
We sought to describe ambulation status over time in a large, longitudinal cohort of children and adults with MMC who have chosen to participate in the National Spina Bifida Patient Registry (NSBPR).16 This work builds upon previous cross-sectional studies completed at an early phase of data collection in the same registry.10 The large, multicenter study population and increasingly robust longitudinal nature of the data offer the opportunity to explore alternative factors as well as interactions between known associations to more realistically describe ambulation in the MMC population.10 We hypothesized that the effect and significance of clinical factors associated with ambulation status would vary with age and by motor level.
METHODS
Sample/Data Set
This retrospective analysis used longitudinal data from the NSBPR database. At the time of analysis, the NSBPR included 26,715 clinical records gathered between 2009 and 2017 from 35 sites that provide clinical services to patients with spina bifida from across the United States. Registry data collection occurred annually during regular clinic visits, and details of data collection have been previously published.16 All data were collected under each participating institution’s approved institutional review board protocol, and informed consent was signed for all participants. This study conforms to all STrengthening the Reporting of OBservational studies in Epidemiology guidelines and reports the required information accordingly (see Supplemental Checklist, Supplemental Digital Content 1, http://links.lww.com/PHM/A966).
All registry participants 5 yrs and older with MMC were included in analysis. Children younger than 5 yrs were excluded because motor function testing before the age of 5 yrs is unreliable.7
Materials and Outcomes
Variables were chosen a priori within the constraints of the NSBPR based on literature review and investigator expertise. Independent ambulation was defined using the Hoffer ambulation scale (community, household, therapeutic, or nonambulator).17 As expected based on previously published work, there were overall low frequencies of therapeutic and household ambulators, with most individuals being classified as either community ambulators or nonambulators.10 Thus, for the statistical model, household and community ambulators were considered “independent ambulators,” whereas therapeutic and nonambulators were considered “nonindependent ambulators,” consistent with previous studies.18,19
Demographic variables were coded using definitions published by the Centers for Disease Control and Prevention.20 These included race/ethnicity (non-Hispanic white, Hispanic or Latino, non-Hispanic black, other/unknown/refused), sex (male/female), and insurance status (any private, public only, other [state high risk plan, charity care or financial assistance, state spina bifida program, VA Agent Orange/spina bifida plans], uninsured). Given the low frequency of participants in the “other” and uninsured categories, they were collapsed into a “miscellaneous” category for modeling purposes.
“Time” was defined using participant age (in days). Important time-varying clinical variables included motor level and surgical count. In the NSBPR, the patient’s functional motor level was recorded at each clinic visit. Five categories were used: thoracic (flaccid lower limbs), high-lumbar (hip flexion present), mid-lumbar (knee extension present), low-lumbar (foot dorsiflexion present), and sacral (foot plantar flexion present). If left and right motor levels differed, the more severe (rostral motor level) was used. All neurosurgeries not involving cerebral shunts (e.g., laminectomy, tethered cord release) were categorized as noncerebral shunt neurosurgeries. Running summations of participant cerebral shunt, noncerebral shunt, and orthopedic surgeries were calculated at each visit. Surgeries that occurred before participant registry enrollment were included as baseline surgery counts at the first visit. Initial MMC repair surgeries were not included in surgical counts because all patients underwent surgical repair.
Statistical Analysis
All statistics were performed with Stata 14.2 software (StataCorp, 2015), and α was set at 0.05. Because of nonnormal distributions, demographic and follow-up information was reported using frequencies and medians with interquartile range (IQR). Within-person change in ambulation over time was explored to determine the variation of ambulation status during registry participation.
A population-averaged generalized estimating equation with a logit link function was used to describe the epidemiology of ambulation status (independent vs. nonindependent). Linear combinations of model coefficients were used to estimate odds ratios (ORs) for subpopulations of interest (e.g., effects by motor level at specific ages). The full model included race/ethnicity, sex, insurance status, age (in days), motor level, count of orthopedic surgeries, count of cerebral shunt surgeries, and count of noncerebral shunt surgeries. Time-by-variable interactions were included for both motor level and the three surgical count variables. Interactions between surgical count and motor level were also included. Data were grouped at the individual level to account for within-individual correlation at repeated visits.
After a priori selection of model variables, univariate and bivariate statistics were run. Each variable was sequentially added to the model and interaction terms explored. This resulted in the addition of an interaction term between surgery counts and motor level as well as a quadratic term for age to account for nonlinear effects. Given that all variables were selected before sequential addition to the model, only the final, full model results are reported. Compound symmetry covariance structure was used as within-individual variance was thought to be relatively stable over the limited follow-up duration. Robust standard error estimation (Huber/white/sandwich estimator) was applied to produce valid standard errors even if the hypothesized covariance structure did not hold, and to account for the within-clinic, clustered nature of data collection. After initial data exploration, model diagnostics were checked and overall predictive accuracy assessed by comparing predicted to actual ambulation status within the analysis cohort. RStudio Version 1.1.423 and the ggplot2 package were used for figure plots.21
Because of the complexity of interpreting ORs within a large model with multiple interaction terms, we report raw ORs for all predictors and interactions. We also discuss linear combinations of model-based coefficient estimates for specific values of clinical interest. These linear combinations are not the raw coefficients for each predictor but instead are a summation of all relevant terms from the model. We used linear combinations to explore variation in one domain (e.g., effects of motor level over time in a typical registry participant) while holding other variables (e.g., surgery counts) constant at a clinically meaningful level representative of the study population. For both age as well as motor level, we repeated these calculations at every age within the study population range to produce visual representations of effect size and confidence interval variation over time. Finally, to condense the many effect estimates and interaction terms into a clear summary of our model output and provide a visual demonstration of the reasonableness of our results as compared with measured Hoffer level prevalence within the study population, we used the model to calculate the predicted probability of independent ambulation through the entire study cohort age range and plotted the results.
Sensitivity Analysis
The older adults in our sample who were born before availability of surgical shunting may be more likely to ambulate because of a survival bias. Thus, a sensitivity analysis was performed fitting the model to data without “outlier” visits (i.e., participant age >95th percentile). Results of the second model were compared with those estimated using the full data set.
RESULTS
Demographics and Baseline Characteristics
The analysis included 5371 NSBPR participants with MMC at 5 yrs and older, representing 16,013 clinic visits. The sample was 52% female, and the majority was non-Hispanic white with insurance (Table 1). Their age at entry into the registry ranged from 5 to 88 yrs but skewed younger (median = 12 yrs; IQR = 7–19).
TABLE 1.
Demographic and clinical information for 5371 individuals with myelomeningocele at entry into the registry
| n (%) | |
|---|---|
|
| |
| Ethnicity/race | |
| Non-Hispanic white | 3427 (64) |
| Hispanic or Latino | 1170 (22) |
| Non-Hispanic black | 420 (8) |
| Other (unknown or refused) | 354 (7) |
| Female | 2776 (52) |
| Insurance | |
| Public only | 2672 (50) |
| Any private | 2335 (43) |
| Miscellaneous | 364 (7) |
| Motor level | |
| Thoracic | 1065 (20) |
| High-lumbar | 595 (11) |
| Mid-lumbar | 1662 (31) |
| Low-lumbar | 966 (18) |
| Sacral | 1083 (20) |
| Hoffer status at first visit, n (%) | |
| Community | 2591 (48) |
| Household | 511 (10) |
| Therapeutic | 478 (9) |
| Nonambulatory | 1791 (33) |
The motor level for participants was distributed relatively evenly, with about one-third having a mid-lumbar level (Table 1). Baseline surgical count was low for all three surgical categories, with cerebral shunt surgeries being the most common. Eighty-one percent of participants (n = 4371) underwent at least one previous cerebral shunt surgery (median = 1, IQR = 1–3), and 26% (n = 1388) underwent at least one noncerebral shunt neurosurgery (median = 0, IQR = 0–1). Only 16% (n = 830) underwent at least one previous orthopedic surgery.
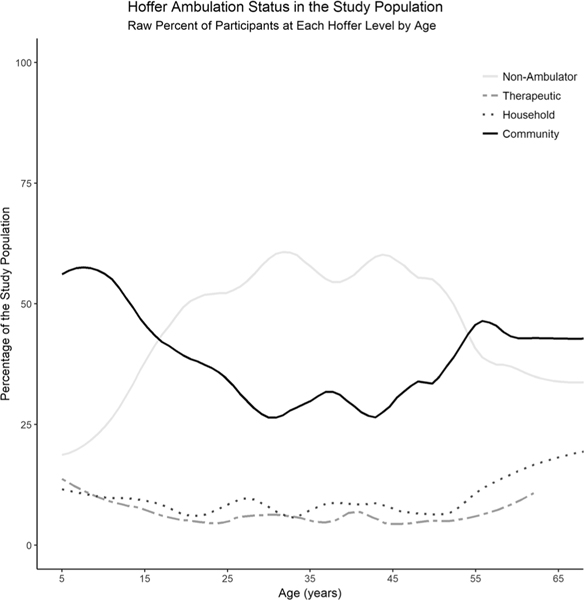
At registry entry, participants were largely classified as either community ambulators or nonambulators (Table 1). However, Hoffer level prevalence across the participant age range varied widely, with community ambulators most prevalent at younger age ranges, nonambulators more prevalent in the early through mid-adulthood yrs, and an unexpected uptick in community ambulators at the extreme upper age range of the study population (Fig. 1).
FIGURE 1.
Community ambulators were most prevalent at younger age ranges, whereas nonambulatory participants were more prevalent in the early through mid-adulthood years. Cumulative percent for the four Hoffer categories add to 100% at each year. Ages that were of low frequency in the sample have less precise prevalence estimates.
Clinical Changes Over the Study Period
Most participants (74%, n = 3988) had at least two visits (median = 3, IQR = 1–4, max = 10) and were followed for a median of 2 yrs (IQR = 0–4.1, max = 8.5). The median time between visits was 12 mos (IQR = 12–15, max = 102). Clinical characteristics were relatively stable for participants with longitudinal data. Less than one-third of participants (n = 1,238) changed functional motor level, with 14% improving and 17% worsening. Only 14% (n = 563) underwent at least one additional cerebral shunt surgery, 6% (n = 221) an additional noncerebral shunt neurosurgery, and 12% (n = 479) an additional orthopedic surgery.
Approximately one-fourth of study participants with longitudinal data (22%, n = 870/3988) had a change in their Hoffer ambulation status; 6% improved and 16% worsened. Most participants who began the study as community ambulators remained community ambulators at study completion (Table 2), and this was similar for nonambulators. Higher rates of change were observed among individuals classified as household or therapeutic ambulators at their first study visit, and more than half of individuals classified as therapeutic ambulators at their first visit worsened to nonambulatory at their last recorded visit (Table 2). Very few individuals (n = 33) improved from nonambulators to community ambulators during their time in the registry.
TABLE 2.
Within-person change in Hoffer status among 3988 individuals with myelomeningocele with at least two study visits
| Ambulatory Status at First Visit, n (Column Percent) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Community n = 1897 | Household n = 385 | Therapeutic n = 373 | Nonambulatory n = 1333 | ||
|
| |||||
| Ambulatory status at last visit | Community | 1641 (87) | 88 (23) | 30 (8) | 33 (2) |
| Household | 155 (8) | 138 (36) | 24 (6) | 35 (2) | |
| Therapeutic | 45 (2) | 62 (16) | 114 (31) | 96 (6) | |
| Nonambulatory | 56 (3) | 97 (25) | 205 (55) | 1358 (89) | |
Model Outcomes
The final generalized estimating equation model accurately predicted independence in ambulation for 84% of the study visits (Wald χ2 (41) = 2317.5, P < 0.001). Residuals were plotted against predicted values and covariates, and no clear deviations from the model mean were identified. Raw model coefficients for all terms included in the final model can be found in Table 3.
TABLE 3.
Generalized estimating equation model coefficients
| OR (95% CI) Full Sample | OR (95% CI) Sensitivity Analysis | |
|---|---|---|
|
| ||
| Time | ||
| Age (in decades) | 0.38 (0.25, 0.56)a | 0.66 (0.55–0.79)a |
| Age2 | 1.18 (1.10–1.28)a | Not applicable |
| Ethnicity and race (ref = non-Hispanic white) | ||
| Hispanic or Latino | 1.13 (0.99–1.31) | 1.11 (0.96–1.28) |
| Non-Hispanic black | 1.45 (1.18–1.77)a | 1.39 (1.13–1.71)b |
| Other, unknown, or refused | 1.01 (0.81–1.28) | 1.02 (0.81–1.28) |
| Female (ref = male) | 1.07 (0.96–1.19) | 1.07 (0.96–1.20) |
| Insurance (ref = any private) | ||
| Public only | 0.73 (0.65–0.80)a | 0.74 (0.67–0.82)a |
| Miscellaneous | 0.74 (0.62–0.88)a | 0.76 (0.64–0.91)b |
| Motor level (ref = sacral) | ||
| Low lumbar | 0.24 (0.15–0.38)a | 0.37 (0.27–0.52)a |
| x Age | 2.03 (1.32–3.11)a | 1.10 (0.90–1.34) |
| x Age2 | 0.85 (0.79–0.93)a | Not applicable |
| Mid lumbar | 0.13 (0.08–0.21)a | 0.15 (0.10–0.20)a |
| x Age | 1.24 (0.80–1.93) | 1.04 (0.86–1.27) |
| x Age2 | 0.94 (0.87–1.03) | Not applicable |
| High lumbar | 0.04 (0.02–0.08)a | 0.05 (0.03–0.08)a |
| x Age | 1.05 (0.61–1.79) | 0.77 (0.59–0.99)c |
| x Age2 | 0.92 (0.83–1.02) | Not applicable |
| Thoracic | 0.02 (0.01–0.03)a | 0.02 (0.01–0.03)a |
| x Age | 0.98 (0.58–1.67) | 0.88 (0.68–1.14) |
| x Age2 | 0.94 (0.85–1.04) | Not applicable |
| Surgical intervention count | ||
| Cerebral shunt | 0.90 (0.83–0.98)c | 0.90 (0.84–0.95)b |
| x Age | 1.03 (0.97–1.11) | 1.02 (0.99–1.05) |
| x Age2 | 1.00 (0.98–1.01) | Not applicable |
| Noncerebral shunt neurosurgery | 0.65 (0.51–0.84)a | 0.71 (0.59–0.85)a |
| x Age | 1.23 (0.98–1.54) | 1.11 (1.02–1.22)c |
| x Age2 | 0.97 (0.93–1.02) | Not applicable |
| Orthopedic | 0.65 (0.50–0.85)a | 0.74 (0.59–0.93)b |
| x Age | 0.99 (0.78–1.25) | 0.85 (0.77–0.94)b |
| x Age2 | 0.97 (0.90–1.03) | Not applicable |
| Interaction between motor level and surgery | ||
| Cerebral shunt | ||
| x Low lumbar | 0.98 (0.93–1.04) | 0.99 (0.94–1.05) |
| x Mid lumbar | 1.05 (1.00–1.10)c | 1.06 (1.01–1.11)c |
| x High lumbar | 1.00 (0.94–1.06) | 1.01 (0.94–1.07) |
| x Thoracic | 1.01 (0.95–1.08) | 1.02 (0.95–1.08) |
| Noncerebral shunt neurosurgery | ||
| x Low lumbar | 0.95 (0.81–1.11) | 0.94 (0.80–1.11) |
| x Mid lumbar | 0.94 (0.79–1.11) | 0.93 (0.78–1.11) |
| x High lumbar | 1.25 (1.04–1.51)c | 1.24 (1.03–1.49)c |
| x Thoracic | 1.31 (1.09–1.58)c | 1.29 (1.07–1.55)c |
| Orthopedic | ||
| x Low lumbar | 1.39 (1.16–1.66)a | 1.37 (1.14–1.65)b |
| x Mid lumbar | 1.44 (1.18–1.75)a | 1.42 (1.16–1.73)b |
| x High lumbar | 1.60 (1.29–1.99)a | 1.57 (1.23–1.96)a |
| x Thoracic | 1.46 (1.15–1.85)a | 1.43 (1.12–1.83)b |
Please note that only raw coefficients are reported above and are different from the linear combination of effects (that takes into account the multiple interaction terms) reported in the article.
P ≤ 0.001.
P ≤ 0.01.
P ≤ 0.05.
Effects of Time
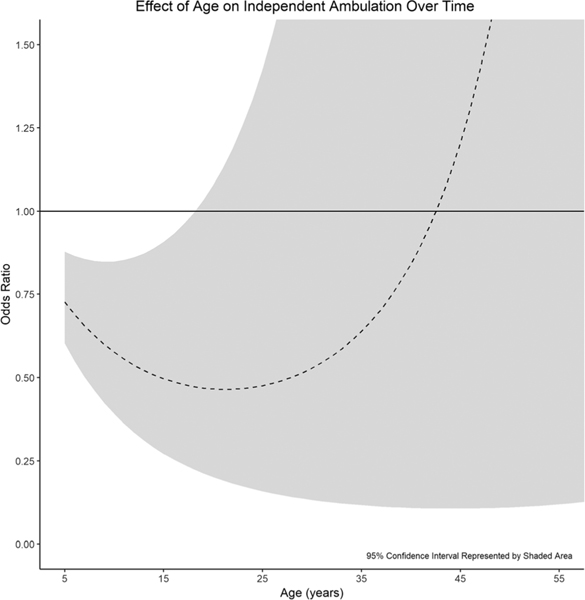
Age (in days) was overall negatively associated with the odds of independent ambulation, with younger participants being more likely to ambulate independently than older participants (Table 3). However, the effect of age on ambulation decreased over time (corresponding to the significant quadratic term). For example, when holding all other variables constant, a “typical” 18-yr-old with a mid-lumbar motor level would have significantly lower expected odds of independent ambulation compared with an 8-yr-old (OR = 0.75, 95% CI = 0.58–0.98, P = 0.034). However, the same individual at 28 would not have significantly lower odds when compared with an 18-yr-old (P = 0.311), although both comparisons represent a change in 10 yrs (Fig. 2).
FIGURE 2.
Increasing age reduces the odds of independent ambulation only into the late teenage years. Thereafter, further increases in age no longer significantly affect the odds of independent ambulation. Note that all effect sizes are reported in terms of the addition of one decade of life.
Effect of Time Invariant Participant Characteristics
All demographic variables except sex were significantly associated with ambulation status (Table 3). Though only a small percentage of the sample (~8%), non-Hispanic black participants had higher odds of independent ambulation relative to non-Hispanic white participants. Individuals who were publicly insured had lower odds of independent ambulation relative to those with private insurance (Table 3).
Effect of Motor Level
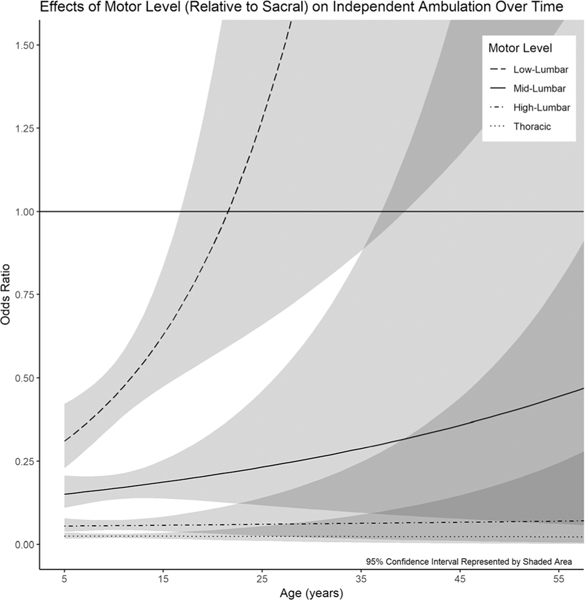
Motor level was significantly associated with ambulation; an individual with a low-lumbar motor level had a lower odds of independent ambulation relative to those with a sacral motor level (Table 3). This was also true for mid-lumbar, high-lumbar, and thoracic motor levels. Notably, the interaction between motor level and age was significant only at the low-lumbar level, with the effect of a change from a sacral to low-lumbar motor level decreasing rapidly with age. By age of 17 yrs, a change from sacral to low-lumbar motor level no longer had a significant effect on ambulation status (P = 0.072; Fig. 3).
FIGURE 3.
By the third decade, the effect of a low-lumbar motor level on independent ambulation is not significantly different from that of a sacral motor level.
Effect of Surgeries
Additional surgeries were associated with a reduced odds of independent ambulation, and this held for all types of surgery. The effect of an additional surgery (regardless of surgery type) on ambulation did not change significantly over time; meaning surgery was associated with the same effect regardless of participant age (Table 3).
Interaction Between Surgery and Motor Level
The effect of an additional surgery was influenced by motor level. Those with lower motor levels (and thus those most likely to be independent ambulators) had the largest reductions in odds of independent ambulation associated with surgical intervention. For a typical 12-yr-old (median age of study participants), orthopedic surgery was associated with a reduced odds of independent ambulation but effects were entirely limited to the sacral motor group (OR = 0.64, 95% CI = 0.51–0.80, P < 0.001), losing significance for low-lumbar motor levels and above. For the same 12-yr-old, effects of noncerebral shunt neurosurgery similarly lost significance at the high-lumbar and thoracic motor levels. Effects of cerebral shunt surgery also varied by motor level, but variation was less pronounced, and reduced odds of independent ambulation associated with shunt surgery persisted through the thoracic motor level (OR = 0.95, 95% CI = 0.91–0.99, P = 0.019).
Predicted Probability of Independent Ambulation
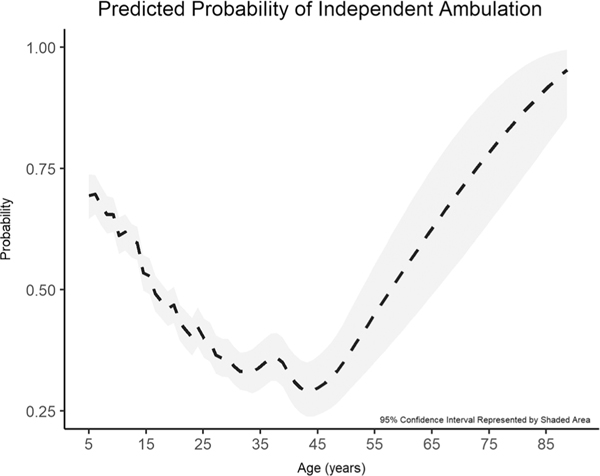
The probability of independent ambulation varied widely over the age range of the study population, starting at nearly 70% for a “typical” 5-yr-old, dropping into the 30% to 40% range by the fourth and fifth decades, and rising steeply again at the far upper end of the study population age range (Fig. 4). Notably, as discussed hereinafter in the sensitivity analysis, the predicted probability at the upper age range is describing a very small (<5%) subset of the study population.
FIGURE 4.
Probability of independent ambulation varied widely over the age range of the study population.
Sensitivity Analysis
The 95th percentile of age at first study visit was 36.7 yrs. Two hundred sixty-seven participants (representing 4.97% of participants with 619 visits) who were older than 36.7 yrs (median age = 43.4, IQR = 39.9–48.8) at their first study visit were dropped. Demographics and clinical characteristics were generally similar between the full and sensitivity analysis cohorts (data not shown). However, lifetime rates of surgical intervention differed significantly. Among the oldest 95th percentile of study participants, 15% had ever undergone orthopedic surgical intervention (as compared with 24% of the younger cohort, P < 0.001), 36% had ever undergone noncerebral shunt neurosurgery (as compared with 29% of the younger cohort, P < 0.001), and 71% had ever had any type of cerebral shunt procedure (as compared with 84% of the younger cohort, P < 0.001).
As in the full cohort, the generalized estimating equation model continued to accurately predict ambulation status 84% of the time (Wald χ2 (33) = 2217.68, P < 0.001). No variables that were significant in the full cohort analysis lost significance in the sensitivity analysis although the quadratic term for time was no longer significant and was dropped from the sensitivity analysis model (Table 3).
Notably, the effect of orthopedic and noncerebral shunt neurosurgery did vary over time in the younger sensitivity analysis cohort. This was in contrast to the larger model where effect of surgical intervention was constant over time. For orthopedic surgery, reduced odds of independent ambulation associated with surgical intervention increased over time. For instance, an additional orthopedic intervention in a “typical” 5-yr-old with a low-lumbar motor level was not significantly associated with a change in ambulation status (P = 0.317); however, an additional orthopedic surgery in a 20-yr-old with otherwise similar clinical characteristics was associated with a reduced odds of independent ambulation (OR = 0.73, 95% CI = 0.65–0.83, P < 0.001). For noncerebral shunt neurosurgery, the opposite was true, and increasing age was associated with increasing odds of independent ambulation associated with surgical intervention, with greater increases seen with more rostral motor levels. For example, an additional noncerebral shunt neurosurgery procedure on a “typical” 12-yr-old with a low-lumbar motor level was associated with significantly lower expected odds of independent ambulation (OR = 0.76, 95% CI = 0.66–0.87, P < 0.001). In contrast, an additional noncerebral shunt neurosurgery in a 30-yr-old with otherwise similar clinical characteristics was no longer significantly associated with a change in ambulation status (P = 0.380) and, in a 30-yr-old with a high-lumbar motor level, was associated with increased odds of independent ambulation (OR = 1.21, 95% CI = 1.03–1.43, P = 0.022).
DISCUSSION
We studied a large, national population of predominantly young participants with MMC and a bimodal baseline ambulatory distribution at the community and nonambulatory levels. We demonstrated, for the first time to our knowledge, that the effects of major clinical predictors of ambulation, such as motor level (and possibly surgical intervention per the sensitivity analysis), are not constant over time. We found that significant effects of age were limited to roughly the first two decades of life; subsequent to the second decade, increasing age was no longer associated with changes in ambulation status. Similarly, changes in motor level (especially for lower motor levels) were generally most pronounced at younger age ranges, and a move from sacral to low lumbar motor levels no longer affected ambulation status by the late teenage years.
Clinically, there is no clear explanation for why the effect of age in and of itself would decay over time and disappear entirely after the second decade. It is likely that this finding is at least partially explained by underlying clinical changes in the MMC population not captured by our data set and that typically occur at specific age ranges. Age in our model may be functioning, at least in part, as a proxy for these age- associated clinical changes. For instance, individuals in their teenage years may begin voluntarily transitioning toward the more efficient use of a wheelchair as they enter settings associated with increased demands on physical mobility, such as a high school campus. Because the NSBPR does not capture qualitative data associated with elective transitions in ambulation status or reasons behind those choices, we were unable to explicitly include these as model variables.9 In addition, whether contributing to the move away from independent ambulation and/or exacerbated as a result thereof, obesity may play a role in lowering the prevalence of independent ambulation in the late adolescent and young adult years.22 Finally, the increased probability of independent ambulation noted at the upper age range supports the existence of a strong survival bias present in older adults from the “preshunt” era—individuals who likely are clinically exceptional and not necessarily representative of the larger, younger, MMC population.
With this survival bias in mind, we dropped individuals at the oldest extreme of the study population and found a new significant interaction between effects of orthopedic surgery and noncerebral shunt neurosurgery with time. We believe that these findings likely highlight the clinical reality that surgical intent as well as specific procedures and associated complication rates generally vary with age.23 Although the data set does not capture surgical intent, goals of surgical intervention in the MMC population move through several age-dependent phases, from initially correcting congenital deformity in the first years after birth (e.g., clubfoot repair), toward a focus on improving function in older children and adolescents (e.g., tendon transfer with osteotomy), and finally attempting to reduce intractable pain and/or limit neurological decline (e.g., scoliosis correction or tethered cord release) in older adults.23–26 Unfortunately, because of registry data constraints, our model cannot distinguish between functional decline as the impetus for surgical intervention, and alternatively, functional decline as a result of surgical intervention. Much of the reduction in odds of ambulation associated with surgical intervention may reflect a reality that a preexisting functional decline prompts surgical intervention to slow or reverse the decline.
Considering those caveats, our results align with previously published literature by confirming the multifactorial nature of ambulation status in the MMC population.10,11 Similar to previously published work, we found that more rostral motor level and multiple types of surgical intervention were generally associated with a reduction in the odds of independent ambulation.4,10 Perhaps because of the large, diverse study population, we found that insurance status, race, and ethnicity played a role in predicting independent ambulation, suggesting possible socioeconomic and/or genetic effects. However, our model was neither designed nor powered to adequately explore specific questions of racial and ethnic effect on independent ambulation, and further work is merited to more fully investigate the myriad potential confounders. Similar to previously published work, we found that age played a significant role in ambulation status.4,10,27 However, we believe that the significant findings associated with age are likely largely a result of age-related clinical events not otherwise accounted.
Limitations and Future Directions
There are several additional factors that may affect the conclusions and generalizability of our findings. First, the NSBPR registry is comprised primarily of participants who attend large, multidisciplinary academic clinics; as such, individuals enrolled in the registry may not reflect those with MMC who receive care in the community. Second, as a patient registry data set, the NSBPR is subject to pitfalls including selection bias that are common to registry data.28 Third, because motor level was used as a primary predictor in the model, children younger than 5 yrs were excluded from analysis secondary to concern for unreliable clinical motor examinations. Although we believe excluding children younger than 5 yrs improved the internal validity of our results, these children do comprise an important group within the MMC population for whom our study results may not be generalizable.
The NSBPR is also a relatively new patient registry, and as such, individual longitudinal follow-up is limited (as seen in our results with a median individual follow-up of 2 yrs). This limited follow-up leaves little room for individual participant clinical variation over time and constrains statistical methods and resulting conclusions. As a result, our current analysis is focused on population-level changes and cannot infer or predict how a clinical event will affect a particular individual. As the NSBPR continues to collect longitudinal data, further, more complex analyses focused at the individual level have the potential to work toward a clinically powerful predictive tool designed to help clinicians and families make more informed decisions using current clinical characteristics to predict future functional ability.
Furthermore, the small numbers of individuals categorized as either home or therapeutic ambulators within the NSBPR (Fig. 1) led us to collapse Hoffer status into a binary outcome in an effort to improve statistical power and simplify interpretation. The tradeoff, however, is a loss of our ability to directly comment on individual Hoffer categories using the model. Similarly, we were able to comment on effects associated with public versus private insurance, but further inference regarding effects of less common insurance categories was not possible. In this vein, both because all registry participants by definition have access to large multidisciplinary clinics and also secondary to data availability limitations, we were largely unable to comment on any effects associated with the social determinants of health. Further studies are warranted to assess the degree to which socioeconomic status and access to care affect ambulation status over time.
Finally, our results are only applicable to the subset of individuals with MMC who belong to a larger, more heterogeneous spina bifida population. Future studies are needed to study ambulation in the non-MMC spina bifida population as well as transfers in wheelchair users.
CONCLUSIONS
To our knowledge, this is the first study of the MMC population that begins to incorporate the time-varying nature of clinical reality into our understanding of functional ability. Our findings suggest that effects of several commonly accepted predictors of ambulation status do vary with time. As a growing proportion of the MMC population enters adulthood and studies include a more heterogeneous range of participant ages, it becomes increasingly important to recognize the time-varying nature of participant clinical conditions and account for this variation during study design. We believe that our work represents a step toward better understanding functional status as well as appropriate analysis methods in the MMC population. We hope that this understanding adds to a foundation from which to build toward both identifying areas where intervention is most likely to improve clinical outcome as well as toward more individualized care with tools designed to predict future function.
Supplementary Material
What Is Known
Evidence regarding clinical factors associated with ambulation status of individuals with MMC is limited by small sample size (often 100 or fewer participants), cross-sectional design, and/or focus on a young subset (e.g., 6 yrs and younger) of the MMC population.
What Is New
We used longitudinal data from the National Spina Bifida Patient Registry to model population-level variation in ambulation over time. We found that effects on ambulation status of commonly accepted clinical factors such as age and motor level do vary significantly with time.
To Claim CME Credits:
Complete the self-assessment activity and evaluation online at http://www.physiatry.org/JournalCME
CME Objectives:
Upon completion of this article, the reader should be able to: (1) Describe general trends in ambulation status by age in the myelomeningocele population; (2) Recognize the nuances of cause and effect underlying the relationship between surgical intervention and ambulation status; (3) Explain why variation of clinical effect over time within myelomeningocele population matters.
Level:
Advanced
Accreditation:
The Association of Academic Physiatrists is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
The Association of Academic Physiatrists designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
ACKNOWLEDGMENTS
The authors thank the many individuals with spina bifida and their family members who participated in this research, without whom the NSBPR would not be possible. The NSBPR has also been successful because of the contributions of the Centers for Disease Control and Prevention, the Spina Bifida Association, and all members of the NSBPR Coordinating Committee. Members of this Committee during the collection of the data reported are listed in alphabetical order and were Richard Adams, Texas Scottish Rite Hospital for Children, Dallas; Pat Beierwaltes, Children’s Hospital of Michigan, Detroit; Timothy Brei, Riley Hospital for Children, Indianapolis; Robin Bowman, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago; Heidi Castillo, Cincinnati Children’s Hospital Medical Center, Cincinnati and Texas Children’s Hospital, Houston; James Chinarian, Children’s Hospital of Michigan, Detroit; Mark Dias, Hershey Medical Center, Hershey; Brad Dicianno, University of Pittsburgh Medical Center, Pittsburgh; Nienke Dosa, Upstate Golisano Children’s Hospital, Syracuse; Carlos Estrada, Boston Children’s Hospital, Boston; Kurt Freeman, Oregon Health and Science University, Portland; David Joseph, Children’s Hospital of Alabama, Birmingham; Pamela Murphy, District Medical Group Children’s Rehabilitative Services, Phoenix; Jacob Neufeld, Children’s Hospital and Research Center at Oakland, Oakland, University of California at San Francisco Benioff Children’s Hospital, San Francisco, and St. Luke’s Boise Medical Center, Boise; Joseph O’Neil, Riley Hospital for Children, Indianapolis; Michael Partington, Gillette Children’s Specialty Healthcare, St. Paul; Paula Peterson, Primary Children’s Medical Center, Salt Lake City; Elaine Pico, Children’s Hospital and Research Center at Oakland, Oakland and University of California at San Francisco Benioff Children’s Hospital, San Francisco; Karen Ratliff-Schaub, Nationwide Children’s Hospital, Columbus; Kathleen Sawin, Children’s Hospital of Wisconsin, Milwaukee; Kathryn Smith, Children’s Hospital Los Angeles, Los Angeles; Stacy Tanaka, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Vanderbilt; Jeffrey Thomson, Connecticut Children’s Medical Center, Hartford and Shriners Hospitals for Children Springfield, Springfield; William Walker, Seattle Children’s Hospital, Seattle; John Wiener, Duke University Medical Center, Durham; Pamela Wilson, Children’s Hospital Colorado, Denver; and Hadley Wood, Cleveland Clinic, Cleveland.
This project was funded by the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, Georgia (Grant Number U01DD001078). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ajpmr.com).
Contributor Information
W. Austin Davis, Department of Physical Medicine and Rehabilitation, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania.
Christina K. Zigler, Department of Population Health Sciences, Duke University School of Medicine, Durham, North Carolina.
Theresa M. Crytzer, Veterans Affairs Pittsburgh Healthcare System, Human Engineering Research Laboratories, Pittsburgh, Pennsylvania; Department of Rehabilitation Science and Technology, School of Health and Rehabilitation Sciences, University of Pittsburgh, Pittsburgh, Pennsylvania..
Sara Izzo, Department of Physical Medicine and Rehabilitation, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania.
Amy J. Houtrow, Department of Physical Medicine and Rehabilitation, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania.
Brad E. Dicianno, Department of Physical Medicine and Rehabilitation, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania; Veterans Affairs Pittsburgh Healthcare System, Human Engineering Research Laboratories, Pittsburgh, Pennsylvania; Department of Rehabilitation Science and Technology, School of Health and Rehabilitation Sciences, University of Pittsburgh, Pittsburgh, Pennsylvania..
REFERENCES
- 1.Copp AJ, Adzick NS, Chitty LS, et al. : Spina bifida. Nat Rev Dis Primers 2015;1:15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Data and Statistics | Spina Bifida | NCBDDD | CDC. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/ncbddd/spinabifida/data.html. Published September 13, 2018. Accessed October 16, 2018
- 3.Dicianno BE, Kurowski BG, Yang JMJ, et al. : Rehabilitation and medical management of the adult with spina bifida. Am J Phys Med Rehabil 2008;87:1027–50 [DOI] [PubMed] [Google Scholar]
- 4.Alabi NB, Thibadeau J, Wiener JS, et al. : Surgeries and health outcomes among patients with spina bifida. Pediatrics 2018;142:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloemen MAT, Verschuren O, van Mechelen C, et al. : Personal and environmental factors to consider when aiming to improve participation in physical activity in children with spina bifida: a qualitative study. BMC Neurol 2015;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson KL, Dudgeon B, Kuehn C, et al. : Assistive technology use among adolescents and young adults with spina bifida. Am J Public Health 2007;97:330–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoenmakers MA, Uiterwaal CS, Gulmans VA, et al. : Determinants of functional independence and quality of life in children with spina bifida. Clin Rehabil 2005;19:677–85 [DOI] [PubMed] [Google Scholar]
- 8.Bakaniene I, Prasauskiene A, Vaiciene-Magistris N: Health-related quality of life in children with myelomeningocele: a systematic review of the literature. Child Care Health Dev 2016;42:625–43 [DOI] [PubMed] [Google Scholar]
- 9.Dicianno BE, Bellin MH, Zabel AT: Spina bifida and mobility in the transition years. Am J Phys Med Rehabil 2009;88:1002–6 [DOI] [PubMed] [Google Scholar]
- 10.Dicianno BE, Karmarkar A, Houtrow A, et al. : Factors associated with mobility outcomes in a National Spina Bifida Patient Registry. Am J Phys Med Rehabil 2015;94:1015–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartonek A, Saraste H: Factors influencing ambulation in myelomeningocele: a cross-sectional study. Dev Med Child Neurol 2001;43:253–60 [DOI] [PubMed] [Google Scholar]
- 12.Danielsson AJ, Bartonek A, Levey E, et al. : Associations between orthopaedic findings, ambulation and health-related quality of life in children with myelomeningocele. J Child Orthop 2008;2:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartonek A: Motor development toward ambulation in preschool children with myelomeningocele—a prospective study. Pediatr Phys Ther 2010;22:52–60 [DOI] [PubMed] [Google Scholar]
- 14.Oakeshott P, Hunt GM, Poulton A, et al. : Open spina bifida: birth findings predict long-term outcome. Arch Dis Child 2012;97:474–6 [DOI] [PubMed] [Google Scholar]
- 15.Bartonek Å, Saraste H, Samuelsson L, et al. : Ambulation in patients with myelomeningocele: a 12-year follow-up. J Pediat Orthop 1999;19:202–6 [DOI] [PubMed] [Google Scholar]
- 16.Thibadeau JK, Ward EA, Soe MM, et al. : Testing the feasibility of a National Spina Bifida Patient Registry. Birth Defects Res Part A Clin Mol Teratol 2013;97:36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffer MM, Feiwell E, Perry R, et al. : Functional ambulation in patients with myelomeningocele. J Bone Joint Surg Am 1973;55:137–48 [PubMed] [Google Scholar]
- 18.van den Berg-Emons HJG, Bussmann JBJ, Meyerink HJ, et al. : Body fat, fitness and level of everyday physical activity in adolescents and young adults with meningomyelocele. J Rehabil Med 2003;35:271–5 [DOI] [PubMed] [Google Scholar]
- 19.Bruinings AL, van den Berg-Emons HJG, Buffart LM, et al. : Energy cost and physical strain of daily activities in adolescents and young adults with myelomeningocele. Dev Med Child Neurol 2007;49:672–7 [DOI] [PubMed] [Google Scholar]
- 20.Data Documentation, Codebook, and Frequencies. National Spina Bifida Patient Registry 2009–2017. Dataset 6, Version 2.5/2.6 of GroundZero EMR. Extracted from April 29, 2018 data transmission [Google Scholar]
- 21.Create Elegant Data Visualizations Using the Grammar of Graphics. Available at: https://ggplot2.tidyverse.org/index.html. Accessed January 13, 2019
- 22.Ogden CL, Carroll MD, Kit BK, et al. : Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311:806–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baghdadi T, Abdi R, Bashi RZ, et al. : Surgical management of hip problems in myelomeningocele: a review article. Arch Bone Jt Surg 2016;4:197–203 [PMC free article] [PubMed] [Google Scholar]
- 24.Park KB, Park HW, Joo SY, et al. : Surgical treatment of calcaneal deformity in a select group of patients with myelomeningocele. J Bone Joint Surg Am 2008;90:2149–59 [DOI] [PubMed] [Google Scholar]
- 25.Matsuda S, Yamaguchi S, Kajihara Y, et al. : Neurologic decline in an older patient with repaired myelomeningocele complicated with lumbar canal stenosis. World Neurosurgery 2017;103:952.e1–4 [DOI] [PubMed] [Google Scholar]
- 26.George TM, Fagan LH: Adult tethered cord syndrome in patients with postrepair myelomeningocele: an evidence-based outcome study. J Neurosurg 2005;102(2 suppl):150–6 [DOI] [PubMed] [Google Scholar]
- 27.Pauly M, Cremer R: Levels of mobility in children and adolescents with spina bifida—clinical parameters predicting mobility and maintenance of these skills. Eur J Pediatr Surg 2012;23:110–4 [DOI] [PubMed] [Google Scholar]
- 28.Schechter MS: Patient registry analyses: seize the data, but caveat lector. J Pediatr 2008;153:733–5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.