Abstract
Numerous components of the immune system, including inflammatory mediators, immune cells and cytokines, have a profound modulatory effect on the homeostatic regulation and regenerative activity of endogenous stem cells and progenitor cells. Thus, understanding how the immune system interacts with stem/progenitor cells could build the foundation to design novel and more effective regenerative therapies. Indeed, utilizing and controlling immune system components may be one of the most effective approaches to promote tissue regeneration. In this review, we first summarize the effects of various immune cell types on endogenous stem/progenitor cells, focusing on the tissue healing context. Then, we present interesting regenerative strategies that control or mimic the effect of immune components on stem/progenitor cells, in order to enhance the regenerative capacity of endogenous and transplanted stem cells. We highlight the potential clinical translation of such approaches for multiple tissues and organ systems, as these novel regenerative strategies could considerably improve or eventually substitute stem cell-based therapies. Overall, harnessing the power of the cross-talk between the immune system and stem/progenitor cells holds great potential for the development of novel and effective regenerative therapies.
Keywords: tissue regeneration, stem/progenitor cell, myeloid cells, T cell, immunotherapy
Graphical Abstract
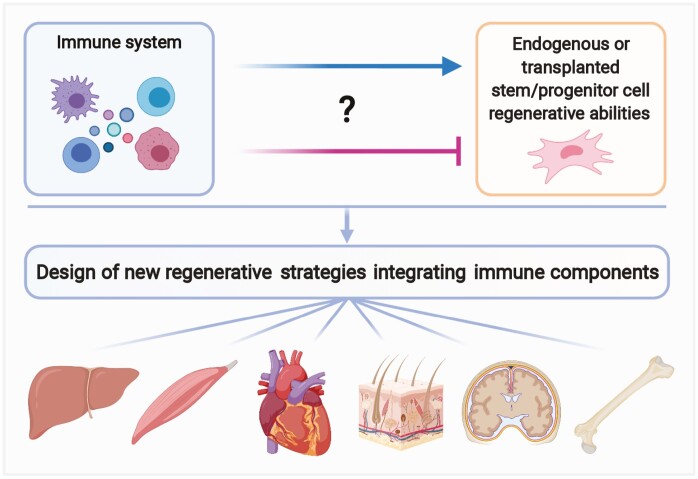
Various immune cell types have been found to modulate the activity of endogenous stem/progenitor cells in animal models of tissue healing. These novel findings may be leveraged for developing better regenerative medicine strategies that integrate immune components. This review highlights mechanisms by which immune cells control endogenous stem/progenitor cells and present regenerative approaches that take advantage of this cross-talk.
Significance Statement.
Regenerative strategies based on stem/progenitor cells have shown modest effectiveness in multiple contexts. While it is well recognized that some stem cell types have an immunomodulatory effect, the immune system also considerably modulates the regenerative activity of stem/progenitor cells. Therefore, components of the immune system could be exploited to improve regenerative therapies. Accordingly, the authors summarize the effects of various immune cells, immunomodulators and immune cell-derived factors on endogenous and transplanted stem cells, and further discuss the potential of novel immune-centric strategies for regenerative medicine applications.
Introduction
Components of the immune system, including inflammatory mediators, immune cells and cytokine networks, are increasingly being recognized as key modulators of tissue homeostasis, repair and regeneration.1,2 While the immune system affects various aspects of the tissue healing process, its role in regulating endogenous stem and progenitor cell populations is becoming evident.3 Following tissue injury, the immune system is rapidly activated and further triggers the recruitment of various immune cells to the site of injury. Signals derived from resident or mobilized immune cells play distinct roles during each stage of the tissue healing process. Notably, particular immune cell types undergo phenotypic and functional changes which ultimately lead to a shift of the microenvironment from a pro-inflammatory to a pro-resolving state. This plasticity and heterogeneity of immune cells can in turn direct stem cell behavior, maintaining a balance between their proliferation and differentiation. Importantly, signals derived from immune cells have been shown to modulate tissue stem/progenitor cells in a positive or negative manner, leading to cell activation and dampening of quiescence or inhibition of regenerative activity.2 Therefore, using and controlling the components of the immune system could be one of the most effective ways to promote tissue regeneration.1
It is well known that certain types of stem cells secrete factors that directly affect the immune system.4 In addition, numerous strategies have been developed to modulate the response of the immune system to transplanted stem cells and reduce transplant rejection. However, we will not discuss the immunomodulatory effect of stem cells or immune tolerance approaches here as they have been reviewed extensively.5-8 Instead, this review discusses the direct role of particular immune cell types in the regulation of endogenous stem/progenitor cells (Fig. 1A). Firstly, we briefly describe their homeostatic function and focus on their role in the repair and regeneration of multiple tissues and organs such as the bone, muscle, skin, heart, liver, and the central nervous system (CNS). Then, we highlight approaches in which delivery of immune mediators, including immune cell-derived factors and immune cells, have been shown to control the regenerative activity of endogenous or transplanted stem/progenitor cells. Such novel strategies could be exploited to complement exogenous stem/progenitor cell transplants or replace them altogether by activating the resident stem/progenitor cell niche instead. Finally, we discuss the translational potential of such immune-centric approaches for regenerative medicine applications.
Figure 1.
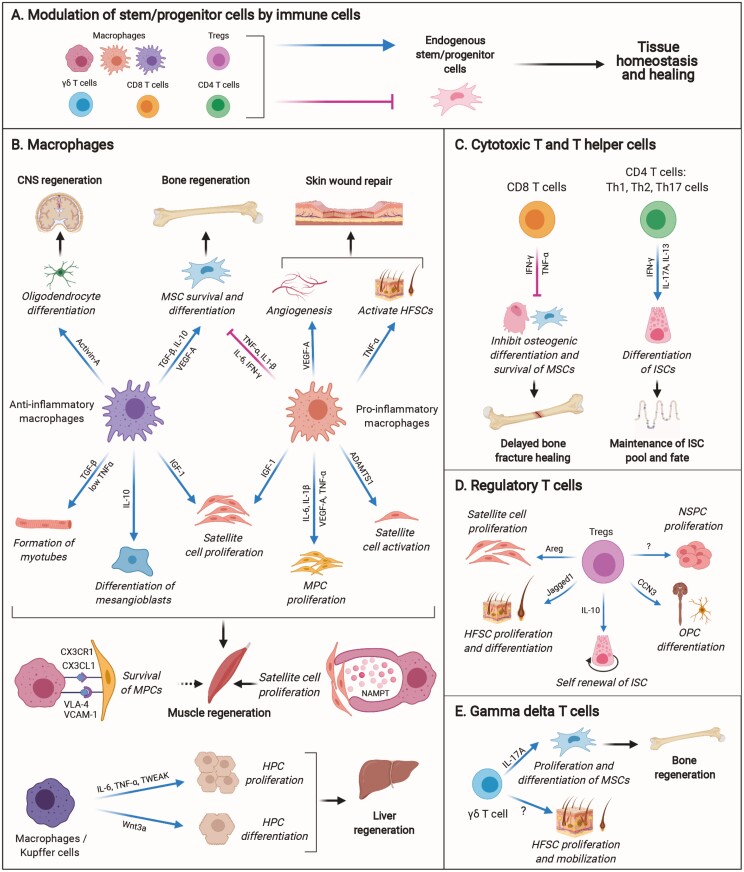
Modulatory effect of immune cells on endogenous stem/progenitor cells. (A) Macrophages and T cells have direct positive or negative effects on endogenous stem/progenitor cells in tissue homeostasis and healing. (B-E) Example of immune cells and their derived factors that exert positive or negative effects on stem/progenitor cells in tissue homeostasis, repair and regeneration. Abbreviations: CNS, central nervous system; FAPs, fibro-adipogenic progenitors; HFSCs, hair follicle stem cells; HPCs, hepatic progenitor cells; HSPCs, hematopoietic stem and progenitor cells; ISCs, intestinal stem cells; MPCs, myogenic precursor cells; MSCs, mesenchymal stem cells; NSPCs, neural stem and progenitor cells; OPCs, oligodendrocyte progenitor cells.
Modulation of Endogenous Stem and Progenitor Cells by Immune Cells
Macrophages
Monocytes and macrophages are among the first immune cell types that respond to tissue injury.9 In some tissues, a small population of tissue-resident macrophages gets activated upon injury, and secretes chemokines important for the recruitment of neutrophils and monocytes.10 Nevertheless, the majority of macrophages in injured tissues are usually derived from monocytes recruited from the circulation which differentiate into macrophages, depending on the signals within the injured microenvironment.11 These signals can induce dynamic phenotypic alterations in macrophages, as they switch from pro-inflammatory (classically activated “M1”) to anti-inflammatory or pro-repair (alternatively activated “M2”) states.12,13 The M1/M2 designation, however, was initially introduced by studies that observed phenotypic changes in macrophages upon their in vitro stimulation with a defined set of factors which does not recapitulate the complexity of signal integration in vivo.14,15 Macrophages in vivo exist across a continuum of polarization states that are more difficult to classify phenotypically and functionally, but are typically characterized by the expression of cellular and biochemical markers.16 The modulatory role of macrophages on stem/progenitor cell regenerative capacity is evident across multiple species and plays a pivotal role in tissue repair and regeneration. The next paragraphs highlight examples where macrophages modulate the regenerative activity of stem/progenitor cells in various tissues (Fig. 1B).
The contribution of macrophages to skeletal muscle regeneration has been extensively explored.17,18 In vivo studies have shown that depletion of circulating monocytes in the first few hours following muscle injury leads to complete failure in muscle regeneration, while depletion of anti-inflammatory macrophages at later stages negatively impacts the diameter of the regenerating fibers.13 Mechanistically, macrophages have been found to secrete cytokines and produce pro-regenerative molecules such as growth factors and extracellular matrix proteins that support myogenesis during both early and late stages of repair. For example, in vitro studies with human myogenic precursor cells (MPCs) have shown that pro-inflammatory macrophages stimulate MPC proliferation and prevent premature differentiation via their secretion of interleukin (IL)-6, IL‐1β, vascular endothelial growth factor-A (VEGF-A), IL-13 and high levels of tumor necrosis factor alpha (TNF‐α). Furthermore, anti-inflammatory macrophages accelerate myocyte differentiation and myotube formation via secretion of transforming growth factor-beta (TGF-β) and low levels of TNF-α.19
Several macrophage-derived factors have been reported to directly induce satellite cell activation and proliferation. For example, insulin-like growth factor-1 (IGF-1), a key growth factor required for muscle repair and satellite cell proliferation,20 was found to be secreted by macrophages that are recruited to the site of injury.21 Similarly, another study reported that depletion of myeloid cell-derived IGF-1 severely compromised regeneration in vivo.22 Insulin-like growth factor-1 was found to be secreted by both pro- and anti-inflammatory macrophages in the injured muscle. The secreted IGF-1 stimulated the proliferation of activated satellite cells, as well as, promoted macrophage polarization.22 The mechanisms which lead to IGF-1 expression in macrophages are still elusive, but meteorin-like (Metrnl), which is predominantly produced by macrophages in injured muscle, has been shown to have an anti-inflammatory function and promote IGF-1 expression in an autocrine manner.23 Moreover, satellite cells were found to uptake glutamine released from macrophages, promoting their proliferation and differentiation.24 Lastly, macrophages are capable of secreting extracellular matrix enzymes such as ADAMTS1 (a disintegrin and metalloproteinase with thrombospondin type 1 motif), which has been shown to induce satellite cell activation and muscle regeneration in young mice.25 Interlukin-10 is another key cytokine secreted by macrophages that is required to maintain viability, and promote differentiation and fusion of vessel-associated myogenic progenitor cells, mesoangioblasts, into terminally differentiated myofibers.26
Apart from secretion of factors, the direct physical interaction of myogenic cells with macrophages has also been reported to regulate myogenic cell fate. In vitro studies have shown that human macrophages deliver anti-apoptotic signals to myogenic cells via cell-cell adhesion systems. These signals involved the expression of vascular cell adhesion molecule 1 (VCAM-1) and chemokine CX3CL1 present on macrophages, binding to very late antigen 4 (VLA-4) and CX3CR1 on MPCs, respectively, promoting myogenic cell survival.27 Similarly, a study in a zebrafish muscle injury model revealed that macrophages interact in close proximity with satellite cells providing a transient, obligatory niche for the activation of muscle stem cells. Mechanistically, macrophages were found to secrete nicotinamide phosphoribosyltransferase (Nampt), an intracellular enzyme that can act as a cytokine once released in the extracellular milieu. Nampt was shown to bind C-C motif chemokine receptor type 5 (CCR5) on muscle stem cells that led to their proliferation and subsequent muscle regeneration.28
One of the most crucial processes in tissue healing is vascularization, and macrophages have been reported to exert pro-angiogenic effects.29 For example, macrophages were found to be necessary for the differentiation of endothelial-derived progenitors and secretion of pro-angiogenic growth factors after muscle injury in the mouse.30 This led to neo-capillary formation and subsequent vascular remodeling.30 A similar effect has also been demonstrated during the early phase of skin repair, where circulating Ly6C+ inflammatory blood monocytes were found to give rise to a pro-angiogenic VEGF-expressing macrophage subset.31 The function of myeloid cell-derived VEGF-A was necessary and distinct from later-stage epidermal-derived VEGF-A, in promoting wound angiogenesis.31 Macrophages additionally regulate epithelial stem cell activity in the skin during homeostasis and repair. It has been reported that skin-resident macrophage-derived Wnts activate resting hair follicle stem cells (HFSCs), promoting the physiological entry of hair follicular cells into anagen, a new phase of hair follicle growth.32 In addition, both recruited inflammatory macrophages and tissue-resident macrophages play a crucial role in activating HFSCs after skin wounding. In this context, macrophage-derived TNF-α has been shown to activate Lgr5+ HFSCs in a mouse skin injury model, leading to induction of hair follicle anagen re-entry, as well as hair follicle neogenesis.33
Macrophages are also important for bone regeneration driven by stem cells. For example, in the absence of macrophages, there is a significant reduction in the number of bone marrow mesenchymal stem cells (BM-MSCs) and their osteoblastic differentiation capacity.34 Macrophage-deficient mice exhibited reduced rates of bone formation, associated with a decrease in the abundance of osteoblasts along the bone surfaces.34 The mechanisms are still not fully understood, but the secretion of cytokines by macrophages likely modulates stem and progenitor cells during bone regeneration. For example, an in vitro study with human MSCs reported that pro-inflammatory macrophages secreted TNF‐α, IL‐1β, IL‐6, and interferon-gamma (IFN-γ) that was detrimental to the viability and function of MSCs.35 Furthermore, anti-inflammatory macrophages secreted IL‐10, TGF‐β and VEGF-A that improved the survival and differentiation potential of MSCs.35 Other in vitro studies have shown that cytokines IL-6 and Oncostatin M, which are classically produced by inflammatory macrophages, promote osteoblast differentiation and matrix mineralization, while suppressing adipogenesis, in human BM-MSCs and adipose tissue-derived MSCs (AT-MSCs).36,37 Furthermore, an in vivo study reported that macrophages are an important source of IL-1β in the injured bone microenvironment.38 This was found to significantly inhibit proliferation, migration and osteoblastic differentiation of mouse bone marrow-derived and compact bone-derived MSCs.38
In the liver, macrophages are the most abundant immune cell type, consisting of resident macrophages—Kupffer cells, and infiltrating monocyte-derived macrophages.39,40 Both macrophage types are an important source of cytokines such as TNF-α, IL-6, and TNF-like weak inducer of apoptosis (TWEAK), which stimulate proliferation of hepatic progenitor cells (HPCs), termed “oval cells” during liver injury and regeneration.41-46 Furthermore, Kupffer cells were found to be required for the parenchymal invasion/migration of expanding HPCs, which is important for their biliary organization and phenotypic orientation after liver injury.47 Lastly, the phagocytosis of tissue debris by macrophages was shown to induce expression of Wnt3a during liver injury, which in turn inhibited Notch signaling in HPCs, and promoted their differentiation into hepatocytes for regeneration.48
The effects of macrophages on brain repair and CNS regeneration have been investigated as well. For example, bone marrow-derived monocytes/macrophages were shown to accumulate in the CNS and alter neural stem/progenitor cells (NSPCs) following irradiation injury in mice.49 This is speculated to be critical for brain repair, although the exact mechanism remains elusive. Interestingly, a study investigating CNS remyelination in a multiple sclerosis mouse model discovered that anti-inflammatory macrophages secreted activin-A that was shown to drive oligodendrocyte differentiation.50
Cytotoxic T Cells and T Helper Cells
T cells including cytotoxic T cells (CD8 T cells) and T helper cells (CD4 T cells) have been found to be important actors in tissue repair and regeneration (Fig. 1C). For instance, in the context of bone injury, a study in humans revealed that delayed bone fracture healing was associated with significantly enhanced levels of CD8 effector memory T cells.51 High numbers of these T cells resulted in elevated concentrations of IFN-γ and TNF-α in the peripheral blood. Interestingly, in vitro analysis showed that these cytokines inhibited the survival of human BM-MSCs, suggesting their inhibitory effect on osteogenic differentiation of bone-forming cells in vivo.51 In the context of intestinal homeostasis, CD4 T helper cells have been shown to control Lgr5+ intestinal stem cell (ISC) activity. Interestingly, Lgr5+ ISCs were shown to express MHCII, thus the cells could present antigens and interact with T helper cells. Due to this interaction, T helper (Th) 1, Th2, and Th17 cells were found to suppress intestinal stem cell renewal and promote differentiation through the secretion of IFN-γ, IL-13, and IL-17A, respectively.52 Consequently, this crosstalk affected the fate of the ISCs and was required to maintain the balance between ISC renewal and differentiation during homeostasis and inflammation.52
Regulatory T Cells
A distinctive feature of regulatory T cells (Tregs) is their ability to suppress and control inflammation.53 However, Tregs are not only essential for immune homeostasis, as they also display numerous non-immune functions such as modulation of stem/progenitor cell activity (Fig. 1D). Indeed, Tregs have emerged as critical players in the tissue repair and regeneration processes.53 For example, Tregs residing in close contact with hematopoietic stem and progenitor cells (HSPC) in the bone marrow control HSPC quiescence and pool size.54 More recently, Tregs have been shown to directly control the activity of many other types of stem/progenitor cells, in the context of tissue healing. For instance, Tregs in the intestine were found to promote the renewal of the Lgr5+ ISC pool via secretion of IL-10.52 In the context of muscle regeneration, it was shown that IL-33, expressed by fibro/adipocyte progenitors (FAPs), promotes accumulation of Tregs that upregulate the expression of its receptor ST2 post injury.55 In addition, Tregs accumulating in injured muscle have been reported to secrete the growth factor amphiregulin which was found to enhance satellite cell differentiation.56 Interestingly, Tregs may also enhance satellite cell proliferation, as another study found that Treg recruitment to injured muscle was limited to the time period of satellite cell expansion (from 3 to 5 days post injury).57 Moreover, this study showed that in vitro-induced Tregs promoted satellite cell expansion and inhibited their myogenic differentiation.57 Overall, it is clear that Tregs play a critical role in influencing the muscle regenerative process in the mouse via their effect on muscle stem/progenitor cells.
Tregs in the skin have also been shown to promote tissue homoeostasis and repair. The important role of Tregs was clearly shown by the delayed wound re-epithelialization and wound closure following systemic Treg ablation in the mouse.58 A similar study reported that there is a significant reduction in hair regrowth in the absence of Tregs.59 Interestingly, Tregs were found to reside in the bulge region of the hair follicle, and preferentially localize to the HFSC niche. Expression of the Notch ligand Jagged-1 (Jag1) by Tregs was shown to induce HFSC proliferation.59 In addition, it has been demonstrated that Tregs promote skin repair by facilitating the differentiation of HFSCs to epithelial cells following skin injury via the IL-17-CXCL5 axis.60
In the context of brain repair, Tregs have been shown to interact with NSPCs and oligodendrocyte progenitor cells (OPCs). For example, Treg depletion was found to impair remyelination and oligodendrocyte differentiation in a mouse model of lysolecithin-mediated demyelination.61 Mechanistically, Tregs were found to promote OPC differentiation and myelination in vitro via secretion of cellular communication network factor 3 (CCN3).61 The protective and reparative role of Tregs following brain injury has also been demonstrated in response to an ischemic stroke.62 Following ischemic stroke in the mouse brain, neurogenesis is induced through the proliferation of NSPCs. While the mechanisms are still elusive, this process is in part regulated by Tregs, as their depletion following ischemia impaired neurogenesis and reduced NSPC proliferation.63
Interestingly, the tissue-specific role of Tregs is also supported by a study performed on zebrafish, which demonstrated that Tregs migrate to the site of organ damage and express organ-specific regenerative factors. Specifically, Tregs were found to express the regenerative factors neurotrophin 3 in the spinal cord, neuregulin 1 in the heart and igf1 in the retina, which resulted in the proliferation of tissue-resident precursor cells in all the respective tissues.64
Gamma Delta T Cells
Gamma delta (γδ) T cells represent a major T-cell population in many tissues.65,66 While the role of these cells in tissue healing is still elusive, γδ T cells have been reported to promote tissue repair by interacting with particular tissue-resident stem cells (Fig. 1E). For example, IL-17A-producing γδ T cells were shown to stimulate bone regeneration by accelerating osteogenesis in the mouse.67 Mechanistically, IL-17A promoted proliferation and differentiation of injury associated MSCs, and consequently enhanced osteoblast function after bone injury.67 On the other hand, in the skin it was shown that γδ T cells are activated by IL-1α and IL-7 released from damaged keratinocytes, upon injury.68 Although the mechanisms are still not well understood, a downstream effect of activated γδ T cells was the proliferation of HFSCs and their mobilization for epidermal wound repair.68
Other Immune Cells
While the effect of macrophages and T cells on endogenous stem/progenitor cells is now well recognized, other immune cells, including natural killer (NK) cells, neutrophils and eosinophils likely modulate tissue-resident stem/progenitor cells as well. For example, NK cells have been shown to play an important role in bone regeneration via secretion of chemokines such as neutrophil activating peptide 2 (also known as CXCL7), CXCL2, CXCL3, CCL5, and IL-8, which were shown to stimulate MSC mobilization.69 Similarly, neutrophils are an important regulator of HSPC mobilization as it was demonstrated that the depletion of neutrophils impaired IL-8 induced mobilization of HSPCs.70 Regarding the role of eosinophils, it was found that these cells are rapidly recruited at sites of muscle injury and secrete IL-4 to activate the regenerative actions of muscle resident FAPs. The activation of the IL-4/IL-13 signaling pathway in FAPs was shown to promote their proliferation, supporting myogenesis, while inhibiting their differentiation into adipocytes.71
Recently, studies have displayed emerging roles for platelets as immune cells.72 Indeed, platelets are the first cells to accumulate at sites of vascular injury. In addition to primary homeostasis, platelets are critical for the tissue healing process 73 as they secrete a variety of cytokines and growth factors.74 For instance, the chemokine stromal cell-derived factor 1 (SDF-1) is secreted by platelets and has been shown to support CD34+ stem cell differentiation into endothelial progenitor cells that migrate and accumulate toward the endothelium.75,76 Similarly, another study has shown that platelet-derived fibroblast growth factor-2 promotes proliferation and migration of MSCs to the endothelium following vascular injury.77 Platelets have also been reported to enhance the recruitment and mobilization of smooth muscle progenitor cells to a vascular injury site via the SDF-1/CXCR4 axis.78
Extracellular Vesicles Derived from Immune Cells
Apart from exerting their effects via secreted molecules or direct cell-to-cell contact, immune cells may communicate with target cells via the release of extracellular vesicles (EVs).79 Although the regulatory effects of stem cell-derived EVs on immune cells have been extensively studied,80 fewer studies have explored the effect of immune-cell derived EVs, specifically on stem/progenitor cells. However, it is likely that immune cells modulate stem/progenitor cells via EVs. For instance, in vitro studies have shown that lipopolysaccharide-stimulated human primary monocytes release exosomes that stimulate osteogenic differentiation,81 or cytokine secretion and upregulation of matrix metalloproteinase genes in MSCs,82 suggesting a potential function in bone remodeling.82 Similarly, EVs derived from platelet lysates and dendritic cells have been found to promote BM-MSC osteogenic differentiation and migration, respectively.83,84 Another interesting study demonstrated that macrophage-derived EVs were essential for the restoration of intestinal homeostasis in response to radiation-induced intestinal injury, using both in vitro and in vivo assays.85 In particular, WNTs 5a, 6, and 9a, packaged in EVs secreted by macrophages were crucial for Lgr5+ ISC survival, self-renewal and proliferation.
Improving the Regenerative Activity of Endogenous and Transplanted Stem Cells with Immune Cells and Immune Cell-Derived Factors
The sections above highlighted how tissue-resident and recruited immune cells modulate the regenerative activity of endogenous stem/progenitor cells. The following sections describe examples of strategies that have taken advantage of delivering immune cells, immunomodulators and immune cell-derived factors, to improve the regenerative activity of endogenous and transplanted stem/progenitor cells (Fig. 2A).
Figure 2.
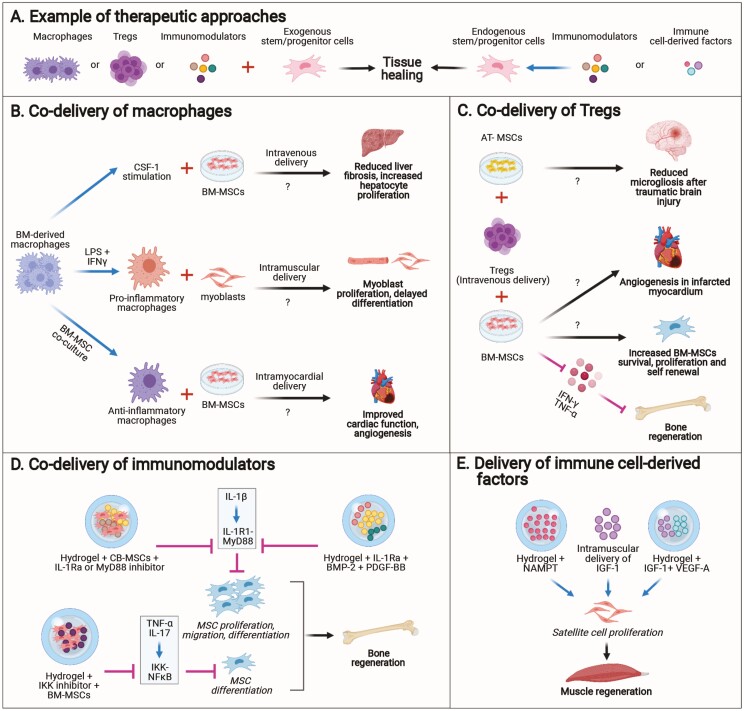
Approaches that use or control immune components to stimulate the regenerative activity of endogenous and transplanted stem/progenitor cells. (A) Example of the approaches. (B) Co-delivery of macrophages with MSCs or myoblasts. (C) Co-delivery of Tregs with MSCs. (D) Co-delivery of immunomodulators with stem cells or with growth factors that act on endogenous stem/progenitor cells. (E) Delivery of immune cell-derived factors to promote the regenerative activity of endogenous stem/progenitor cells. Abbreviations: MSCs, mesenchymal stem cells; AT-MSCs, adipose tissue-derived MSCs; BM-MSCs, bone-marrow-derived MSCs; CB-MSCs, compact bone-derived MSCs.
Co-delivery of Macrophages
Several studies have shown the potential of macrophages in improving the activity of stem/progenitor cells for tissue regeneration (Fig. 2B). For example, intramuscular co-injection of myoblasts with pro-inflammatory macrophages in immunodeficient alymphoid mice improved the effect of the injected myoblasts and subsequent muscle regeneration, by extending the window of myoblast proliferation, delaying differentiation and increasing their migration.86 Similarly, the adjuvant function of macrophages in stem cell-induced cardiac repair of myocardial infarction has also been investigated in the rat. For example, one study explored how bone marrow-derived macrophages (BMDMs), when polarized to an anti-inflammatory state upon coculture with BM-MSCs, could be used a cellular adjuvant for transplantation. Bone marrow-derived macrophages were co-injected into the peri-infarct area of rats after myocardial infarction, along with these anti-inflammatory cocultured BMDMs, and this strategy led to improved cardiac function 2 weeks post treatment. This was accompanied by enhanced angiogenesis and increased anti-inflammatory macrophages, suggesting the possible use of BMDMs as an adjuvant to stem cell therapy for cardiac repair.87 Given the importance of macrophages in liver regeneration, the therapeutic potential of exogenous syngeneic macrophages was investigated in a mouse model of chronic liver injury.88 Mouse bone marrow cells were cultured with colony stimulating factor-1 (CSF-1) to generate differentiated macrophages which were then injected via the hepatic portal vein into the injured mice. This led to an expansion of HPCs and reduced liver fibrosis, accompanied by an upregulation of IL-10, TWEAK, IGF-1, and VEGF-A, demonstrating the anti-inflammatory and pro-regenerative effects of this therapeutic strategy.88 Another novel approach has been tested in mice as a potential treatment for liver cirrhosis. A combination therapy involving the intravenous co-injection of MSCs and CSF‐1-induced bone marrow‐derived macrophages (id‐BMMs) was administered in a mouse model of liver cirrhosis. This strategy was significantly more effective at reducing liver fibrosis, elevating hepatocyte proliferation and decreasing blood levels of liver enzymes, compared to monotherapy with MSCs or id‐BMMs.89 Furthermore, this combinatorial approach was found to improve resolution of liver fibrosis by inducing the migration of host macrophages and neutrophils into the damaged liver. This was accompanied by an increase in the frequency of M2-like “anti-inflammatory” CD206-positive macrophages, and a concomitant upregulation of antifibrotic and pro-regenerative factors within the first week after cell administration.89
Co-delivery of Tregs
Similar to macrophages, Tregs have also been used to enhance the regenerative activity of stem/progenitor cells (Fig. 2C). For instance, it was shown that implantation of autologous BM-MSCs in mouse calvarial bone defects only led to partial regeneration. However, intravenous infusion of Tregs administered 2 days prior to BM-MSC transplantation could significantly improve bone regeneration and lead to complete repair of the bone defects.90 This effect was attributed to the Treg-mediated inhibition of IFN-γ and TNF-α that prevented apoptosis of BM-MSC transplants and likely facilitated their differentiation.90 In addition, the protective and supportive role of Tregs upon co-transplantation with BM-MSCs has been described in other animal models. For example, co-intramyocardial injection of autologous Tregs with BM-MSCs in a porcine model of chronic ischemia increased MSC survival rate, proliferation and self-renewal, as well as augmented capillary density.91 Another study demonstrated the potential benefits of a combination therapy using Tregs and AT-MSCs in mitigating the neuroinflammatory response after traumatic brain injury in rats.92 In comparison to monotherapy, a staggered regimen of Treg infusion at 24 hours, followed by MSCs at 72 hours post injury, significantly reduced microgliosis in the brain 2 weeks post traumatic brain injury. The beneficial role of Tregs has been attributed to their ability to dampen the immune responses in the recipient, leading to a more favorable environment upon MSC infusion 48 hours later.92
The role of Tregs in conferring neuroprotection and regulating neurogenesis after an ischemic stroke has been investigated as well. For instance, it was reported that the direct injection of in vitro-activated Tregs into the left lateral ventricle of the adult mouse brain post ischemia significantly promoted NSPC proliferation in the subventricular zone.93 The effect of Tregs was found to be via IL-10 signaling in NSPCs that promoted their proliferation.93 Interestingly, other studies in mice and rats have shown that a small population of Tregs is naturally present within bone marrow-derived stem cell transplants used for stroke therapies.94,95 These Tregs have been shown to have an important immunomodulatory and neuroprotective role, leading to increased myelin production by OPCs post-ischemic stroke injury.94,95
Delivery Of Immune Cell-Derived Factors and Mediators
As highlighted earlier, an important parameter that can modulate the regenerative activity of stem/progenitor cells is the presence of immune mediators such as cytokines in the injured tissue. Thus, controlling the effect of cytokines has been explored to create a more optimal microenvironment for endogenous and transplanted stem/progenitor cells (Fig. 2D). For instance, IL-1 receptor 1(IL-1R1)/MyD88 signaling triggered by the pro-inflammatory cytokine IL-1β in mouse injured bone was found to inhibit the proliferation and osteoblastic differentiation of transplanted compact bone-derived MSCs (CB-MSCs).38 Therefore, to enhance the regenerative activity of bone-derived MSCs, the cells were delivered via a hydrogel functionalized with an inhibitor of the IL-1R1/MyD88 signaling pathway. This strategy could considerably improve the regenerative capacity of the stem cells in a mouse model of bone regeneration.38 Similarly, it was reported that the IκB kinase (IKK)/NF-κB pathway triggered by TNF-α and IL-17 impairs the osteogenic differentiation of BM-MSCs by promoting β-catenin ubiquitination and degradation.96 Hence, transplantation of BM-MSCs with an IKK small-molecule inhibitor was used to enhance the regenerative activity of the stem cells in a rat bone regeneration model.96 Creating an optimal microenvironment for stem/progenitor cells may also be useful for regenerative strategies based on growth factors that target stem/progenitor cells. For example, IL-1R1 signaling has been shown to decrease the responsiveness of MSCs and osteoblasts to growth factors such as bone morphogenetic protein-2 (BMP-2) and platelet-derived growth factor-BB (PDGF-BB).97 Thus, a sustained co-delivery of BMP-2 and/or PDGF-BB with IL-1 receptor antagonist was shown to increase the regenerative effect of the growth factors in mouse bone regeneration models.97
As mentioned above, macrophage-derived factors such as IGF-1, VEGF-A and Nampt were found to directly promote muscle regeneration by promoting the activity of satellite cells. Thus, the therapeutic potential of these macrophage-derived factors has been explored for muscle regeneration (Fig. 2E). For example, delivering a single dose of NAMPT via a hydrogel led to improved muscle regeneration in a mouse model of volumetric muscle loss.28 This was shown to correlate with an increase in the number of proliferating PAX7+ muscle satellite cells and centrally nucleated de novo muscle fibers. Similarly, it has been shown that intramuscular delivery of IGF-1 significantly increases muscle fiber size and volume in mice that have impaired macrophage mobilization (CCR2 knockout).21 In another study, delivering IGF-1 together with VEGF-A through an injectable hydrogel further enhanced skeletal muscle fiber regeneration through increased myoblast proliferation, improved angiogenesis, reinnervation and reduction in apoptosis.98
Platelet rich plasma (PRP) has been extensively used therapeutically to promote tissue healing and stem/progenitor cell activity as it releases pro-regenerative factors upon activation.99 In particular, PRP has been used in combination with stem cells for bone regeneration applications.100 For example, PRP was shown to significantly enhance the regenerative effect of MSCs by promoting their proliferation and osteogenic differentiation in rabbits.101 Similarly, a combination of PRP with BM-MSCs could improve the viability and proliferative capacity of BM-MSCs, as well as their capacity to produce pro-angiogenic factors such as VEGF and PDGF-BB.102 Moreover, a combination of AT-MSCs and PRP was shown to significantly promote periodontal tissue regeneration in a canine model.103
Lastly, the potential of delivering macrophage-derived EVs for stem/progenitor cell-mediated tissue healing has also been explored. For example, a study showed that intravenous delivery of macrophage-derived EV-packaged WNTs in mice irradiated around the gastrointestinal tracts rescued the ISCs from radiation toxicity.85 Additionally, another study showed that EVs isolated from supernatants of an in vitro cultured mouse macrophage cell line were enriched with WNTs.104 Intradermal delivery of these EVs in mice promoted hair follicle growth and dermis thickness, through the stimulation of growth factor production by dermal papilla cells, which provide a niche for epithelial progenitor cells involved in hair follicle regeneration.104
Current Status and Future Directions
It is becoming evident that various components of the immune system play a major role in regulating the regenerative activity of stem/progenitor cells in multiple tissues and organs. This has been leveraged for therapeutic benefit to design novel regenerative strategies. For example, Ixmyelocel-T is an autologous multicellular therapy manufactured from bone marrow aspirate consisting of 2 key cell types, namely, anti-inflammatory CD14+ macrophages and CD90+ MSCs. The therapy has shown promising results in early phase clinical trials for improving patient outcomes in ischemic dilated cardiomyopathy and critical limb ischemia, although the exact mechanisms of action are not known.105-107 Similarly, another trial aims to evaluate the effects of co-delivering MSCs and Tregs for treating end-stage liver disease,108 as studies have shown how the crosstalk between these 2 cell types can be exploited in the context of acute liver injury.109
Although the studies mentioned above are promising approaches for tissue repair and regeneration, their clinical application is still in its infancy. Moreover, it is essential to evaluate the safety and potential risks or side-effects associated with delivering immune cells and their derivatives. For instance, we need to understand the potential effects of such therapies on endogenous immune cell populations and lymphoid organs. It is also important to evaluate any aggressive systemic inflammatory responses such as cytokine storms, potential changes in the host’s ability to respond to infection, as well as susceptibility to tumor formation and malignancy. In addition, age, the so called “immune aging”, and gender may influence the outcome of such therapies. Furthermore, to ensure successful clinical translation, we need to determine the optimal route and dose of administration, establish quality control measures, and understand immunological compatibility between patients. Thus, more research is required to understand the behavior of transplanted immune cells or mediators, their homing to specific niches within the host and their crosstalk with the tissue microenvironment. It is worth noting that some studies have shown the potential benefit of controlling the behavior of immune cells, for instance by engineering them with “safety switches”, or suicide genes that lead to conditional cell death post-transplantation,110 or using biomaterials with desirable physicochemical properties that favorably regulate the cellular responses.111
In conclusion, our understanding of the interaction between the immune system and endogenous stem/progenitor cells provides an exciting new avenue for designing a new generation of regenerative strategies. Such immune-centric approaches could improve therapies based on adult stem/progenitor cells such as MSCs, which have demonstrated relatively modest success for regenerative medicine applications in clinical trials.112 Furthermore, the use of exogenous stem/progenitor cells might be substituted with the delivery of the appropriate immune cells, immunomodulators, or immune cell-derived factors which are able to stimulate the endogenous stem/progenitor cell pools in the target tissue or organ. These novel strategies support the potential of supplementing cell therapies with a combinatorial approach, utilizing the cells of the immune system, or its downstream effectors/ signaling molecules. Nevertheless, despite growing evidence in pre-clinical research supporting the use of immune components, the mechanisms by which the immune system regulates stem/progenitor cells during the tissue homeostasis and healing are still elusive. Therefore, substantial basic research is needed to understand the subtle interactions between the components of the immune system and stem/progenitor cells in the context of tissue repair and regeneration. This would provide key insights into developing pioneering regenerative approaches for treating tissue injuries and disorders.
Acknowledgments
This work was partially funded by the National Health and Medical Research Council (APP1140229 and APP1176213) and the Medical Research Future Fund (APP1202105) to M.M.M. The Australian Regenerative Medicine Institute is supported by grants from the State Government of Victoria and the Australian Government. All figures were created with the help of BioRender software (www.biorender.com).
Conflict of Interest
The authors declared no potential conflicts of interest.
Author Contributions
Y.K.A., B.N., and M.M.M wrote the manuscript. S.D. provided input for Section 2. Y.A. and B.N. made the figures. M.M.M. supervised the writing.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Julier Z, Park AJ, Briquez PS, et al. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13-28. [DOI] [PubMed] [Google Scholar]
- 2. Aurora AB, Olson EN.. Immune modulation of stem cells and regeneration. Cell Stem Cell. 2014;15:14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Naik S, Larsen SB, Cowley CJ, et al. Two to Tango: dialog between immunity and stem cells in health and disease. Cell. 2018;175:908-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin L, Du L.. The role of secreted factors in stem cells-mediated immune regulation. Cell Immunol. 2018;326:24-32 [DOI] [PubMed] [Google Scholar]
- 5. Alpdogan O, van den Brink MRM.. Immune tolerance and transplantation. Semin Oncol. 2012;39:629-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chidgey AP, Layton D, Trounson A, et al. Tolerance strategies for stem-cell-based therapies. Nature. 2008;453:330-337. [DOI] [PubMed] [Google Scholar]
- 7. Wang M, Yuan Q, Xie L.. Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem Cells Int. 2018;2018:3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ayala-Cuellar AP, Kang J-H, Jeung E-B, et al. Roles of mesenchymal stem cells in tissue regeneration and immunomodulation. Biomol Ther. 2019;27:25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oishi Y, Manabe I.. Macrophages in inflammation, repair and regeneration. Int Immunol. 2018;30:511-528. [DOI] [PubMed] [Google Scholar]
- 10. Brigitte M, Schilte C, Plonquet A, et al. Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis Rheum. 2010;62:268-279. [DOI] [PubMed] [Google Scholar]
- 11. Davies LC, Jenkins SJ, Allen JE, et al. Tissue-resident macrophages. Nat Immunol. 2013;14:986-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wynn TA, Vannella KM.. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ogle ME, Segar CE, Sridhar S, et al. Monocytes and macrophages in tissue repair: implications for immunoregenerative biomaterial design. Exp Biol Med. 2016;241:1084-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gosselin D, Link VM, Romanoski CE, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mosser DM, Edwards JP.. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tidball JG, Villalta SA.. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173-R1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brunelli S, Rovere-Querini P.. The immune system and the repair of skeletal muscle. Pharmacol Res. 2008;58:117-121. [DOI] [PubMed] [Google Scholar]
- 19. Saclier M, Yacoub-Youssef H, Mackey AL, et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31:384-396. [DOI] [PubMed] [Google Scholar]
- 20. Ahmad SS, Ahmad K, Lee EJ, et al. Implications of insulin-like growth factor-1 in skeletal muscle and various diseases. Cells. 2020;9:1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu H, Huang D, Saederup N, et al. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 2011;25:358-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tonkin J, Temmerman L, Sampson RD, et al. Monocyte/macrophage-derived IGF-1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Mol Ther. 2015;23:1189-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baht GS, Bareja A, Lee DE, et al. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat Metab. 2020;2:278-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shang M, Cappellesso F, Amorim R, et al. Macrophage-derived glutamine boosts satellite cells and muscle regeneration. Nature. 2020;587:626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Du H, Shih C-H, Wosczyna MN, et al. Macrophage-released ADAMTS1 promotes muscle stem cell activation. Nat Commun. 2017;8:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bosurgi L, Corna G, Vezzoli M, et al. Transplanted mesoangioblasts require macrophage IL-10 for survival in a mouse model of muscle injury. J Immunol. 2012;188:6267. [DOI] [PubMed] [Google Scholar]
- 27. Sonnet C, Lafuste P, Arnold L, et al. Human macrophages rescue myoblasts and myotubes from apoptosis through a set of adhesion molecular systems. J Cell Sci. 2006;119:2497. [DOI] [PubMed] [Google Scholar]
- 28. Ratnayake D, Nguyen PD, Rossello FJ, et al. Macrophages provide a transient muscle stem cell niche via NAMPT secretion. Nature. 2021;591:281-287. [DOI] [PubMed] [Google Scholar]
- 29. Okuno Y, Nakamura-Ishizu A, Kishi K, et al. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood. 2011;117:5264-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zordan P, Rigamonti E, Freudenberg K, et al. Macrophages commit postnatal endothelium-derived progenitors to angiogenesis and restrict endothelial to mesenchymal transition during muscle regeneration. Cell Death Dis. 2014;5:e1031-e1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Willenborg S, Lucas T, van Loo G, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120:613-625. [DOI] [PubMed] [Google Scholar]
- 32. Castellana D, Paus R, Perez-Moreno M.. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol. 2014;12:e1002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X, Chen H, Tian R, et al. Macrophages induce AKT/β-catenin-dependent Lgr5(+) stem cell activation and hair follicle regeneration through TNF. Nat Commun. 2017;8:14091-14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. L V, Baht GS, Whetstone H, et al. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res. 2015;30:1090-1102. [DOI] [PubMed] [Google Scholar]
- 35. Freytes DO, Kang JW, Marcos-Campos I, et al. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114:220-229. [DOI] [PubMed] [Google Scholar]
- 36. Guihard P, Danger Y, Brounais B, et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells. 2012;30:762-772. [DOI] [PubMed] [Google Scholar]
- 37. Song HY, Jeon ES, Kim JI, et al. Oncostatin M promotes osteogenesis and suppresses adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells. J Cell Biochem. 2007;101:1238-1251. [DOI] [PubMed] [Google Scholar]
- 38. Martino MM, Maruyama K, Kuhn GA, et al. Inhibition of IL-1R1/MyD88 signalling promotes mesenchymal stem cell-driven tissue regeneration. Nat Commun. 2016;7:11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wen Y, Lambrecht J, Ju C, et al. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol. 2021;18:45-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ju C, Tacke F.. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol. 2016;13:316-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lowes KN, Croager EJ, Olynyk JK, et al. Oval cell-mediated liver regeneration: role of cytokines and growth factors. J Gastroenterol Hepatol. 2003;18:4-12. [DOI] [PubMed] [Google Scholar]
- 42. Viebahn CS, Benseler V, Holz LE, et al. Invading macrophages play a major role in the liver progenitor cell response to chronic liver injury. J Hepatol. 2010;53:500-507. [DOI] [PubMed] [Google Scholar]
- 43. Xiang S, Dong H-H, Liang H-F, et al. Oval cell response is attenuated by depletion of liver resident macrophages in the 2-AAF/partial hepatectomy rat. PLoS One. 2012;7:e35180-e35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Elsegood CL, Chan CW, Degli-Esposti MA, et al. Kupffer cell-monocyte communication is essential for initiating murine liver progenitor cell-mediated liver regeneration. Hepatology. 2015;62:1272-1284. [DOI] [PubMed] [Google Scholar]
- 45. Jakubowski A, Ambrose C, Parr M, et al. TWEAK induces liver progenitor cell proliferation. J Clin Invest. 2005;115:2330-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tirnitz-Parker JE, Viebahn CS, Jakubowski A, et al. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology. 2010;52:291-302. [DOI] [PubMed] [Google Scholar]
- 47. Van Hul N, Lanthier N, Español Suñer R, et al. Kupffer cells influence parenchymal invasion and phenotypic orientation, but not the proliferation, of liver progenitor cells in a murine model of liver injury. Am J Pathol. 2011;179:1839-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boulter L, Govaere O, Bird TG, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dietrich J, Baryawno N, Nayyar N, et al. Bone marrow drives central nervous system regeneration after radiation injury. J Clin Invest. 2018;128:281-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miron VE, Boyd A, Zhao J-W, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reinke S, Geissler S, Taylor WR, et al. Terminally differentiated CD8+ T cells negatively affect bone regeneration in humans. Sci Transl Med. 2013;5:177ra-17136. [DOI] [PubMed] [Google Scholar]
- 52. Biton M, Haber AL, Rogel N, et al. T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell. 2018;175:1307-1320.e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li J, Tan J, Martino MM, et al. Regulatory T-cells: potential regulator of tissue repair and regeneration. Front Immunol. 2018;9:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fujisaki J, Wu J, Carlson AL, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kuswanto W, Burzyn D, Panduro M, et al. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity. 2016;44:355-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Burzyn D, Kuswanto W, Kolodin D, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Castiglioni A, Corna G, Rigamonti E, et al. FOXP3+ T cells recruited to sites of sterile skeletal muscle injury regulate the fate of satellite cells and guide effective tissue regeneration. PLoS One. 2015;10:e0128094-e0128094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nosbaum A, Prevel N, Truong H-A, et al. Cutting edge: regulatory T cells facilitate cutaneous wound healing. J Immunol. 2016;196:2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ali N, Zirak B, Rodriguez RS, et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell. 2017;169:1119-1129.e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mathur AN, Zirak B, Boothby IC, et al. Treg-cell control of a CXCL5-IL-17 inflammatory axis promotes hair-follicle-stem-cell differentiation during skin-barrier repair. Immunity. 2019;50:655-667.e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dombrowski Y, O, ’Hagan, Dittmer M, et al. Regulatory T cells promote myelin regeneration in the central nervous system Nat Neurosci. 2017;20:674-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ito M, Komai K, Mise-Omata S, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565:246-250. [DOI] [PubMed] [Google Scholar]
- 63. Saino O, Taguchi A, Nakagomi T, et al. Immunodeficiency reduces neural stem/progenitor cell apoptosis and enhances neurogenesis in the cerebral cortex after stroke. J Neurosci Res. 2010;88:2385-2397. [DOI] [PubMed] [Google Scholar]
- 64. Hui SP, Sheng DZ, Sugimoto K, et al. Zebrafish regulatory T cells mediate organ-specific regenerative programs. Dev Cell. 2017;43:659-672.e655. [DOI] [PubMed] [Google Scholar]
- 65. Jameson J, Ugarte K, Chen N, et al. A role for skin γδ T cells in wound repair. Science. 2002;296:747. [DOI] [PubMed] [Google Scholar]
- 66. Nielsen MM, Witherden DA, Havran WL.. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol. 2017;17:733-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ono T, Okamoto K, Nakashima T, et al. IL-17-producing γδ T cells enhance bone regeneration. Nat Commun. 2016;7:10928-10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee P, Gund R, Dutta A, et al. Stimulation of hair follicle stem cell proliferation through an IL-1 dependent activation of γδT-cells. Elife. 2017;6:e28875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Almeida CR, Caires HR, Vasconcelos DP, et al. NAP-2 secreted by human NK cells can stimulate mesenchymal stem/stromal cell recruitment. Stem Cell Rep. 2016;6:466-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pruijt JFM, Verzaal P, van Os R, et al. Neutrophils are indispensable for hematopoietic stem cell mobilization induced by interleukin-8 in mice. Proc Natl Acad Sci USA. 2002;99:6228-6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Heredia Jose E, Mukundan L, Chen Francis M, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Morrell CN, Aggrey AA, Chapman LM, et al. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123:2759-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Eisinger F, Patzelt J, Langer HF.. The platelet response to tissue injury. Front Med. 2018;5:317-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(Suppl 1):S13-S33. [DOI] [PubMed] [Google Scholar]
- 75. Stellos K, Langer H, Daub K, et al. Platelet-derived stromal cell–derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation. 2008;117:206-215. [DOI] [PubMed] [Google Scholar]
- 76. de Boer H, C, Verseyden C, Ulfman LH, et al. Fibrin and activated platelets cooperatively guide stem cells to a vascular injury and promote differentiation towards an endothelial cell phenotype. Arterioscler Thromb Vasc Biol. 2006;26:1653-1659. [DOI] [PubMed] [Google Scholar]
- 77. Langer HF, Stellos K, Steingen C, et al. Platelet derived bFGF mediates vascular integrative mechanisms of mesenchymal stem cells in vitro. J Mol Cell Cardiol. 2009;47:315-325. [DOI] [PubMed] [Google Scholar]
- 78. Zernecke A, Schober A, Bot I, et al. SDF-1α/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784-791. [DOI] [PubMed] [Google Scholar]
- 79. Wang Y, Zhao M, Liu S, et al. Macrophage-derived extracellular vesicles: diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 2020;11:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xie M, Xiong W, She Z, et al. Immunoregulatory effects of stem cell-derived extracellular vesicles on immune cells. Front Immunol. 2020;11:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ekström K, Omar O, Granéli C, et al. Monocyte exosomes stimulate the osteogenic gene expression of mesenchymal stem cells PLoS One. 2013;8:e75227-e75227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gebraad A, Kornilov R, Kaur S, et al. Monocyte-derived extracellular vesicles stimulate cytokine secretion and gene expression of matrix metalloproteinases by mesenchymal stem/stromal cells FEBS J. 2018;285:2337-2359. [DOI] [PubMed] [Google Scholar]
- 83. Torreggiani E, Perut F, Roncuzzi L, et al. Exosomes: novel effectors of human platelet lysate activity. Eur Cell Mater. 2014;28:137-151; discussion 151. [DOI] [PubMed] [Google Scholar]
- 84. Silva AM, Almeida MI, Teixeira JH, et al. Dendritic cell-derived extracellular vesicles mediate mesenchymal stem/stromal cell recruitment. Sci Rep. 2017;7:1667-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Saha S, Aranda E, Hayakawa Y, et al. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat Commun. 2016;7:13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bencze M, Negroni E, Vallese D, et al. Proinflammatory macrophages enhance the regenerative capacity of human myoblasts by modifying their kinetics of proliferation and differentiation. Mol Ther. 2012;20:2168-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lim Sy, Cho DI, Jeong HY, et al. Adjuvant role of macrophages in stem cell-induced cardiac repair in rats. Exp Mol Med. 2018;50:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Thomas JA, Pope C, Wojtacha D, et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53:2003-2015. [DOI] [PubMed] [Google Scholar]
- 89. Watanabe Y, Tsuchiya A, Seino S, et al. Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice. Stem Cells Transl Med. 2019;8:271-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Y L, Wang L, Kikuiri T, et al. Mesenchymal stem cell–based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011;17:1594-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhou Y, Singh AK, Hoyt RF Jr., et al. Regulatory T cells enhance mesenchymal stem cell survival and proliferation following autologous cotransplantation in ischemic myocardium. J Thorac Cardiovasc Surg. 2014;148:1131-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Caplan HW, Prabhakara KS, Toledano Furman NE, et al. Combination therapy with Treg and mesenchymal stromal cells enhances potency and attenuation of inflammation after traumatic brain injury compared to monotherapy: treg and MSC to treat neuroinflammation and TBI. Stem Cells. 2021;39:358-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang J, Xie L, Yang C, et al. Activated regulatory T cell regulates neural stem cell proliferation in the subventricular zone of normal and ischemic mouse brain through interleukin 10. Front Cell Neurosci. 2015;9:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zarriello S, Neal EG, Kaneko Y, et al. T-regulatory cells confer increased myelination and stem cell activity after stroke-induced white matter injury. J Clin Med. 2019;8:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Neal EG, Acosta SA, Kaneko Y, et al. Regulatory T-cells within bone marrow-derived stem cells actively confer immunomodulatory and neuroprotective effects against stroke. J Cereb Blood Flow Metab. 2019;39:1750-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chang J, Liu F, Lee M, et al. NF-κB inhibits osteogenic differentiation of mesenchymal stem cells by promoting β-catenin degradation. Proc Natl Acad Sci USA. 2013;110:9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Julier Z, Karami R, Nayer B, et al. Enhancing the regenerative effectiveness of growth factors by local inhibition of interleukin-1 receptor signaling. Sci Adv. 2020;6:eaba7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Borselli C, Storrie H, Benesch-Lee F, et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci USA. 2010;107:3287-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Alves R, Grimalt R.. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disorders. 2018;4:18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fernandes G, Yang S.. Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering. Bone Res. 2016;4:16036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yin N, Wang Y, Ding L, et al. Platelet-rich plasma enhances the repair capacity of muscle-derived mesenchymal stem cells to large humeral bone defect in rabbits. Sci Rep. 2020;10:6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. El Backly RM, Zaky SH, Muraglia A, et al. A platelet-rich plasma-based membrane as a periosteal substitute with enhanced osteogenic and angiogenic properties: a new concept for bone repair. Tissue Eng Part A. 2012;19:152-165. [DOI] [PubMed] [Google Scholar]
- 103. Tobita M, Uysal CA, Guo X, et al. Periodontal tissue regeneration by combined implantation of adipose tissue-derived stem cells and platelet-rich plasma in a canine model. Cytotherapy. 2013;15:1517-1526. [DOI] [PubMed] [Google Scholar]
- 104. Rajendran RL, Gangadaran P, Seo CH, et al. Macrophage-derived extracellular vesicle promotes hair growth. Cells. 2020;9:856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Henry TD, Schaer GL, Demaria A, et al. The ixCELL-DCM trial: rationale and design. Cell Transplant. 2016;25:1689-1699. [DOI] [PubMed] [Google Scholar]
- 106. Patel AN, Henry TD, Quyyumi AA, et al. Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet. 2016;387:2412-2421. [DOI] [PubMed] [Google Scholar]
- 107. Powell RJ, Marston WA, Berceli SA, et al. Cellular therapy with Ixmyelocel-T to treat critical limb ischemia: the randomized, double-blind, placebo-controlled RESTORE-CLI trial. Mol Ther. 2012;20:1280-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Safety and Efficacy Study of Co-transfering of Mesenchymal Stem Cell and Regulatory T Cells in Treating End-stage Liver Disease . Accessed June 14, 2021. https://ClinicalTrials.gov/show/NCT03460795.
- 109. Gazdic M, Markovic BS, Arsenijevic A, et al. Crosstalk between mesenchymal stem cells and T regulatory cells is crucially important for the attenuation of acute liver injury. Liver Transpl. 2018;24:687-702. [DOI] [PubMed] [Google Scholar]
- 110. Stasi A D, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dellacherie MO, Seo BR, Mooney DJ.. Macroscale biomaterials strategies for local immunomodulation. Nat Rev Mater. 2019;4:379-397. [Google Scholar]
- 112. Fan XL, Zhang Y, Li X, et al. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77:2771-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.