Abstract
Chagas disease, a worldwide public health concern, is a chronic infection caused by Trypanosoma cruzi. Considering T. cruzi chronic persistence correlates with CD4+ and CD8+ T cell dysfunction and the safety and efficacy profiles of Benznidazol and Nifurtimox, the two drugs currently used for its etiological treatment, are far from ideal, the search of new trypanocidal treatment options is a highly relevant issue. Therefore, the objective of this work was to evaluate the trypanocidal effect and cytokine production induction of three extracts (hexane, dichloromethane and hydroalcoholic) obtained from Clethra fimbriata, a plant traditionally used as a febrifuge in Colombia. Additionally, the extracts’ major components with the highest trypanocidal activity were determined. It was evidenced C. fimbriata hexane extract exhibited the highest activity capable of inhibiting the three parasite developmental stages with an IC50/EC50 of 153.9 ± 29.5 (epimastigotes), 39.3 ± 7.2 (trypomastigotes), and 45.6 ± 10.5 (amastigotes) μg/mL, presenting a low cytotoxicity in VERO cells with a selectivity index ranging from 6.49 to 25.4. Moreover, this extract induced trypomastigote apoptotic death and inhibited parasite cell infection. The extract also induced IFN-γ and TNF production in CD4+ and CD8+ T cells, as well as de novo production of the cytotoxic molecules granzyme B and perforin in CD8+ T cells from healthy donors. Fatty acids and terpenes represented C. fimbriata key compounds. Thus, the trypanocidal activity and cytokine production induction of the hexane extract may be associated with terpene presence, particularly, triterpenes.
Keywords: Chagas disease, Clethra fimbriata, Cytokine production, Tripanocidal, Triterpenes, Trypanosoma cruzi
Chagas disease, Clethra fimbriata, Cytokine production, Tripanocidal, Triterpenes, Trypanosoma cruzi.
1. Introduction
Chagas disease (ChD), caused by Trypanosoma cruzi, is endemic in 21 Latin American countries and it is estimated worldwide around 8 million people are chronically infected [1, 2]. For many years, Chagas disease was only known in Latin America, but due to population migration it expanded to non-endemic countries, becoming a global public-health concern [3, 4, 5]. In Colombia, an estimated of 436,000 individuals are infected, and about 11% of the population is at risk of acquiring the infection [1, 2]. Importantly, climate change will have an influence on a global increase in cases, which has affected the ecotypes and vector behavior. Additionally, T. cruzi reservoirs have moved to new areas, generating disease outbreaks, where they were previously absent [6].
Currently, only two drugs are available for therapeutic use against T. cruzi: Benznidazole® (LAFEPE-Brazil) and Nifurtimox® (Bayer AG) [7, 8]. Treatment with either Benznidazole and Nifurtimox aims to address parasite elimination, reducing the likelihood of cardiac, digestive and combined pathologies and preventing the parasite's transmission chain [9]. Although treatment with both drugs in the acute infection phase and the congenital form is effective, the complexity in developing clinical trials during the chronic infection phase has made it difficult to understand the effectiveness of both drugs during this latter phase [10, 11]. Furthermore, use of these treatments is controversial because they have presented several issues associated with drug toxicity, side effects, need of high doses and prolonged administration time, low compliance to treatment, and the limited availability of medicines in countries where the disease is endemic [12]. Furthermore, clinical isolates with different degrees of in vitro susceptibility to these drugs have been reported [13, 14]. Moreover, recent studies identified a dormant stage of the parasite, contributing to pharmacological response evasion [15].
On the other hand, several studies in patients with Chagas disease dissecting the cellular immune response have described CD4+ and CD8+ effector T cells play a crucial role in the response against T. cruzi. In fact, it has been reported that patients with an advanced stage of the disease exhibit a lower frequency of T cells producing IFN-γ and TNF and a lower proportion of multifunctional T cells than patients at earlier stages of the disease [16, 17]. This phenotype has been also exhibited in T cells of chronically infected mice [18], suggesting that chronic T. cruzi persistence promotes T cell dysfunctionality and heightens the importance of searching for new treatments that can efficiently elicit a protective immune response during chronic T. cruzi infection.
Henceforth, natural products have been considered as a potential innovative source of effective and selective agents for drug development to treat T. cruzi infection [19, 20]. Recently, our research group demonstrated that an ethanol leaf extract from Clethra fimbriata, a Latin American native plant growing from Colombia to Ecuador [21], exhibited activity against T. cruzi epimastigote and trypomastigote stages, inducing IFN-γ and TNF production by CD8+ T cells [22]. However, ethanol extract complexity made it challenging to specify the compounds responsible for these activities. Therefore, in the present study, three less complex extracts (hexane, dichloromethane and hydroalcoholic) with distinct polarity, were prepared to facilitate the compound identification of the extract with the highest activity against the three parasite developmental stages and the cytokine production induction. In addition, the parasite death mechanisms and the extract effect on parasite infectivity were also explored.
2. Materials and methods
2.1. Plant material and extraction
Plant material was collected under the "Permit for wild species specimen collection of biological diversity for research with non-commercial purposes” (Permiso marco de recolección de especímenes de especies silvestres de la diversidad biológica para investigación con fines no comerciales) granted to the Pontificia Universidad Javeriana (Resolution 778 of July 7th, 2017) issued by the “National Environmental Licensing Authority” - Autoridad Nacional de Licencias Ambientales” (ANLA). Additionally, this study was approved by the Research and Ethics Committees of the Facultad de Ciencias at Pontificia Universidad Javeriana. C. fimbriata were collected in Majuy Hill, Via Cota, Cundinamarca, Colombia, and taxonomically identified by the Colombian National Herbarium (voucher specimen number COL 610805). C. fimbriata aerial parts were dried and crushed, followed by extraction by successive maceration (30 extractions) with 1:10 sample to solvent ratio, using hexane (CFHEX), dichloromethane (CFDIC) and ethanol-water (7:3 v/v, CFHA) as solvents. Obtained extracts were concentrated by rota-evaporation at low pressure and the hydroalcoholic extract was lyophilized and stored at 4 °C until use. Prior to biological tests, all extracts were resuspended in ethanol.
2.2. Cell line and parasite maintenance
Green Monkey renal fibroblast-like cells (VERO cells, ATCC CCL-81, Manassas, VA) were cultured in DMEM (Eurobio, Toulouse, France) supplemented with 10% Fetal Bovine Serum (FBS, Eurobio), 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.01 M HEPES (Eurobio). Cells were grown at 37 °C in a humid atmosphere at 5% CO2.
T. cruzi Y-strain epimastigotes (MHOM/BR/00/Y); discrete typing unit (DTU TcII) [23] were maintained in the exponential growth phase in Liver Infusion Tryptose (LIT) medium supplemented with 15% heat inactivated FBS (Eurobio), 100 U/mL penicillin and 100 μg/mL streptomycin (Eurobio), at 28 °C. To obtain the trypomastigotes forms, VERO cells were cultured until achieving a semi-confluent monolayer. Subsequently, cells were incubated with T. cruzi Y-strain trypomastigotes previously obtained from successive passages in a murine model for 12 h. Cells were infected with a 1:10 (cell:parasite) ratio and at 96 h postinfection trypomastigotes were recovered.
2.3. Activity against T. cruzi extracellular forms
Epimastigotes (1 × 106 parasites/well) and trypomastigotes (5 × 105 parasites/well) were seeded in 96-well plates and incubated (48 h for epimastigotes, 24 h for trypomastigotes) with different concentrations of CFHEX, CFIDC and CFHA extracts. As a negative control ethanol (EtOH) (<1%) was used and as positive controls Benznidazole (BZL) (7 μΜ; Sigma-Aldrich, Saint Louis, MO) or Nifurtimox (NFX) (5 μM; Sigma-Aldrich) were employed. Effect on epimastigote viability was estimated by the MTT colorimetric method (MTT, Sigma-Aldrich), where 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazole bromide was metabolically reduced to formazan, and absorbance was acquired by a spectrophotometer at 595 nm [24]. Treatment effect on trypomastigote viability was determined by hemocytometer count [25]. The concentration that inhibited the viability of epimastigotes by 50% (IC50) and eliminated 50% of the trypomastigote population (EC50) were calculated using Prism 6.0 Software (GraphPad, La Jolla, CA, USA) employing non-linear regression. All assays were performed in triplicate and three independent biological replicates were carried out.
2.4. Activity against intracellular forms of T. cruzi
1 × 105 VERO cells were cultured in 6-well plates for 12 h. Subsequently, cells were infected with T. cruzi trypomastigotes at a 1:10 (cell:parasite) ratio. After 12 h of infection, cultures were washed to eliminate un-internalized trypomastigotes, followed by incubation for 48 h at 37 °C and 5% CO2 with different extract concentrations (from 200 to 6.25 μg/mL). Finally, the cultures were washed with PBS (Eurobio), fixed with methanol and stained with Giemsa stain (Sigma-Aldrich). Extract activity was determined by calculating the percentage of infected cells and the number of amastigotes found per infected cell in treated and untreated cultures (association index), by counting 200 randomly distributed cells using a 100 X magnification in a light microscope [26]. The concentration that inhibited 50% of the parasitic population (IC50) was calculated comparing association indices between treated and untreated parasites using Prism 6.0 Software (GraphPad, La Jolla, CA, USA) with a non-linear regression. All assays were performed in triplicate and three independent biological replicates were carried out.
2.5. Cytotoxic activity on VERO cells
VERO cells (5 × 103 cells/well) were seeded in 96-well plates and incubated for 48 h at 37 °C and 5% CO2 with extracts at decreasing concentrations (from 200 to 6.25 μg/mL). MTT colorimetric assay was used to estimate the cytotoxic effect. Doxorubicin (1 μM) was used as positive control and EtOH (<1 %) as a negative control [27, 28]. As previously described, the concentration that inhibited cell viability by 50% (CC50) was calculated. All assays were performed in triplicate and three independent biological replicates were carried out. To evaluate the selectivity of each extract, the selectivity index (SI) ratio between CC50 in VERO cells and IC50 (epimastigote, amastigote) or EC50 (trypomastigote) in T. cruzi stages was used [29].
2.6. Replication inhibition assays
A recovery assay was used to evaluate CFHEX extract effect on T. cruzi replicative processes. Briefly, 3 × 106 epimastigotes/mL were treated with high concentrations of the extract (IC90) for short periods of time (15 min, 30 min, 1h, 2h, and 4h). After incubation, treatments were removed by washing with LIT medium, and 1 × 106 epimastigotes were grown in fresh LIT medium. Recovery of parasitic replication was evaluated daily for 5 consecutive days by hemocytometer cell count [30]. Untreated control parasites were used to evaluate parasite proliferation. All assays were performed in triplicate and three independent biological replicates were carried out.
2.7. Cell invasion assays
To evaluate treatment effect on trypomastigote infective capacity, 3 × 106 trypomastigotes were treated with the selected extract IC50 for 24 h at 37 °C. Following, treatments were removed and 1 × 106 trypomastigotes were incubated with 1 × 105 VERO cells (ratio 1:10 cell/parasite) for 6 h. A control experiment was carried out with a similar setup, but using trypomastigotes without treatment. After incubation, free parasites were removed by washing the monolayer with DMEM medium; subsequently, monolayers were methanol fixed and stained with Giemsa stain. The number of infected cells was estimated by counting 200 randomly distributed cells using 100 X magnification in a light microscope. Three independent experiments were performed in duplicate [31].
2.8. Cell death assay
T. cruzi Y-strain epimastigotes or trypomastigotes (3 × 105) were incubated with the IC50/EC50 and IC90/EC90 of selected extract for 24 h at 37 °C. As a negative control EtOH (<1%) treated parasites were used. For positive control, parasites were treated with NFX EC50. After incubation, parasites were washed with PBS and labeled with Annexin V and propidium iodide (PI) using the FITC-annexin V apoptosis detection kit with PI (Biolegend, San Diego, CA, USA), according to the manufacturer's instructions. Acquisition was performed using a FACSAria II flow cytometer (BD inmunocytometry systems). A total of 20,000 events were acquired in the region previously established as that corresponding to T. cruzi epimastigotes and trypomastigotes. Results were subsequently analyzed using FlowJo 10.6.2 software (Tree Star, Ashland, OR). Double-negative cells were considered intact, whereas double-positive cells were considered in late apoptosis/necrotic cells. Annexin V+/PI− cells were presumably in early apoptosis and the Annexin V−/PI+ were considered necrotic parasites.
2.9. Cytokine production induction
Specifications for antibodies used are listed in Supplementary Table 1. To determine cytokine modulation capacity elicited by the treatments 1 × 106 PBMCs previously obtained by Ficoll-Paque PLUS method using 12 mL of blood from 5 healthy donors, were cultured with the IC50 of selected extract against trypomastigotes or 1/10 CC50 selected extract against PBMCs in the presence of CD28 (1 μg/mL) and CD49d (1 μg/mL) for 12 h at 37 °C, 5% CO2. The last 10 h of culture were performed in the presence of brefeldin A (1 μg/mL) and monensin (1 μg/mL) (BD Pharmingen). Following, cells were labeled with LIVE/DEAD Fixable Aqua for 20 min in the dark at room temperature. After a PBS wash, cells were subsequently stained with anti-CD3 Pacific Blue, anti-CD8 allophycocyanin-H7 and anti-CD4-PerCP-Cy5.5 Abs, followed by fixation and permeation for intracellular staining with anti–IL-2 PE-Dazzle-594, anti–IFN-γ Alexa Fluor 700, anti–TNF allophycocyanin, anti-perforin PE, and anti–granzyme B PE-Cy7 for 30 min at 4 °C. All conjugated antibodies were titrated as previously reported [32]. In each experiment, as a negative control cells were incubated with EtOH (1.28 μL), and as a positive control, cells were stimulated with phorbol 12-myristate 13-acetate (PMA; 5 ng/μL) and ionomycin: (500 ng/μL) (Sigma-Aldrich). At least 50,000 events, gated on live CD3+ cells, were acquired through flow cytometry using a Cytek Aurora flow cytometer (Cytek Biosciences Inc., Fremont, CA, USA). Results were subsequently analyzed using FlowJo 10.6.2 software (Tree Star). The gates for positivity in multicolor panels were determined from the negative control. Multifunctional analyses were performed using a Boolean gating strategy. The data are represented using Pestle version 1.8 and SPICE version 6.0 software (the National Institutes of Health, Bethesda, MD) [33].
2.10. Characterization of chemical composition through HPLC-ESI-QTOF-MS
Metabolite characterization present in the selected extract was carried out using an Agilent Technologies 1260 coupled to a Q-TOF 6545 time-of-flight quadrupole mass analyzer with electrospray ionization (Agilent technologies, Santa Clara, CA USA). To this end, 2 μL of extract were injected at a 0.5 mg/mL concentration into a C8 column (InfinityLab Poroshell 120 EC-C8 (150 × 3.0 mm, 2.7 μm)) at 35 °C using an elution gradient composed of 1% formic acid (v/v) in Milli-Q water (Phase A) and formic acid in 1% methanol (Phase B), with a constant flow of 0.35 mL/min. The elution gradient began at 35% in regard to B for 7 min, it then increased to 90% B, where it was maintained for 18 min. Last, the gradient decreased to 35% of B during a period of 10 min and was maintained for an additional 5 min until the system was rebalanced. Detection by mass spectrometry was performed in negative ESI mode in full scan at 70–1500 m/z. The mass spectrometer source conditions consisted of a capillary voltage of 3.5 kV, a nebulizer gas flow rate of 12.0 L/min, 350 °C source temperature, and a source pressure of 45 psi. Throughout the analysis, two reference masses were used for mass correction: m/z 112.9856 (C2O2F3(NH4)) and m/z 1033.9881 (C18H18O6N3P3F24) in the negative ionization mode.
AutoMS/MS: Five injections with iterative detection were performed with a detection range between 100 to 1500 m/z for MS1 and for MS2 between 50 to 1500 m/z. Compound identification present in the extract was performed using the CEU Mass Mediator tool (http://ceumass.eps.uspceu.es) by matching the observed accurate mass of each compound with the m/z values available online at METLIN (http://metlin.scripps.edu), KEGG (http://genome.jp/kegg), and lipid MAPS (http://lipidMAPS.org) [34].
2.11. Statistical analysis
Significance between two groups was determined using the Mann–Whitney U test. Differences among subject groups were evaluated using Kruskal–Wallis and Dunn's post-test for multiple comparisons. A two-tailed test was employed with a p < 0.05 to establish significant differences. For the statistical analyses GraphPad Prism version 8.1.1 for Mac OS X statistics software (GraphPad Software, San Diego, CA) was used. Co-expression pie charts were compared using 10,000 permutations calculated with SPICE software version 6.0.
3. Results
3.1. In vitro trypanocidal and cytotoxic activity
Three C. fimbriata extracts, CFHEX (yield: 1.24%), CFDIC (yield: 1.5%) and CFHA (yield: 6.7%), were obtained and their anti-T. cruzi and cytotoxic activities were evaluated to select the most promising extract. Although the epimastigote stage does not participate directly in T. cruzi infection in mammals, it has been described as a good initial screening model, due to its greater resistance compared to the trypomastigote and amastigote stages. Thus, the in vitro evaluation of antitrypanosomal activity of crude extracts against T. cruzi epimastigotes revealed both CFHEX and CFDIC extracts inhibited this stage with an IC50 of 153.9 ± 29.5 and 71.3 ± 8.75 μg/mL, respectively (Table 1). The CFHA extract was not active against the epimastigote stage at the concentrations evaluated (from 1.000 to 31.2 μg/mL), therefore, this extract was not considered for further evaluations against other T. cruzi stages.
Table 1.
Clethra fimbriata extracts against Trypanosoma cruzi and cytotoxic effects.
| EXT | [μg/mL] |
SI |
|||||
|---|---|---|---|---|---|---|---|
| IC50 EPI | EC50 TRY | IC50 AMA | CC50 VERO | EPI | TRY | AMA | |
| CFHEX | 153.9 ± 29.5 | 39.3 ± 7.2 | 45.6 ± 10.5 | >1000 | 6.49 | 25.4 | 21.9 |
| CFDIC | 71.3 ± 8.75 | 63.2 ± 7.5 | 95.3 ± 21.2 | 134.5 ± 33.6 | 1.89 | 2.12 | 1.41 |
| NFX | 1.24 ± 0.26 | 0.38 ± 0.16 | 0.34 | >22.9 | 18.4 | 60.2 | 67.3 |
| BNZ | 2.07 ± 0.25 | 1.95 ± 0.23 | ND | >20.8 | 10.0 | 10.6 | ND |
EXT: Extract; EPI: Epimastigote; TRY: Trypomastigote; AMA: Amastigote; SI: Selective index; ND: not determined.
Regarding the harmful effects T. cruzi stages exert during mammal infection, it was observed that CFHEX and CFDIC extracts induced death and inhibition of T. cruzi during the trypomastigote and amastigote stages with an EC50 of 39.3 ± 7.2 and 63.2 ± 7.5 μg/mL for the trypomastigote stage and IC50 of 45.6 ± 10.5 and 95.3 ± 21.2 μg/mL in the amastigote stage, respectively (Table 1). The trypanocidal effect was classified according to Osorio et al. in 2007 as highly active (IC50 < 10 μg/mL), active (10 < IC50 < 50 μg/mL), moderately active (50 < IC50 < 100 μg/mL) or non-active (IC50 > 100 μg/mL) [35]. Thus, the CFHEX extract was classified as active, while the CFDIC extract was classified as moderately active.
Cytotoxicity analysis from the three extracts evaluated in VERO cells demonstrated that only the CFDIC extract presented moderate toxicity with a CC50 of 134.5 ± 33.6 μg/mL (Table 1) and the lowest SI in the three parasitic stages (epimastigotes: 1.89, trypomastigotes: 2.12 and amastigotes: 1.41). On the other hand, the low cytotoxicity of the CFHEX extract on VERO cells (CC50 > 1,000 μg/mL) provided a better selectivity against the three T. cruzi stages with SI of 6.49, 25.4 and 21.9 against epimastigotes, trypomastigotes and amastigotes, respectively. Based on the trypanocidal activity and selectivity indices, the CFHEX extract was selected for further evaluations.
3.2. Effects on T. cruzi replication and infectivity
CFHEX extract effects on epimastigote replication and T. cruzi trypomastigote infection capacity were evaluated. For the replication assay the parasites were treated with 690 μg/mL (IC90) of the CFHEX extract. After incubation and treatment removal, parasite and the control replication was assessed daily for five consecutive days. The treated parasites recovered their replication capacity and no significant differences were observed compared to untreated parasites (Figure 1A). These results established that CFHEX did not induce restrictive effects on T. cruzi epimastigote replication. Moreover, trypomastigote infectivity treated with 39.3 μg/mL (EC50) CFHEX extract was evaluated according to materials and methods. Treated trypomastigotes were incubated with VERO cells and the number of infected cells was counted. It was observed exposure of trypomastigotes to CFHEX EC50 significantly decreased the infective capacity of this stage in 25.64%, from an average of 39 infected cells/200 random cells in the untreated control to 29 infected cells/200 random cells in the treated cultures (p < 0.05) (Figure 1B).
Figure 1.
Analysis of Growth Recovery of epimastigotes and infectivity of trypomastigotes treated with CFHEX extract. (A) Growth curve of epimastigotes after CFHEX extract treatment during 15 min, 30 min, 1h, 2h, and 4h. Data are presented as the median ± range of 3 independent experiments. (B) Number of amastigote infected VERO cells stained with Giemsa stain of 200 randomly selected cells. Data are presented as the median and range. Each dot represents an independent biological replica. p values were calculated using a Mann-Whitney U test. ∗p < 0.05.
3.3. Determination of T. cruzi death mechanisms
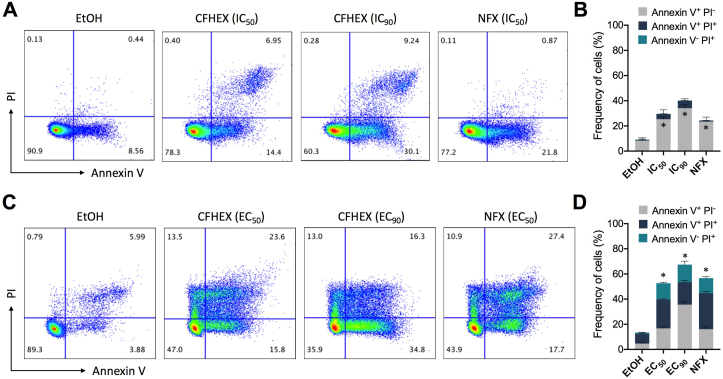
To determine cell death mechanisms, parasites were labeled with Annexin V and PI. In the assay with epimastigotes cultured during 24h with ethanol (vehicle control), most cells were negative for Annexin V and PI staining (91.05 ± 2.99%), evidencing cell viability (Figure 2A and B). On the contrary, cultures treated with IC50 of the CFHEX extract demonstrated a significant increase in the frequency of early apoptotic parasites (Annexin V+, PI−) compared with the control. As expected, this effect was amplified when an IC90 of the CFHEX extract was used (Figure 2A and B). Regarding the frequency of necrotic (Annexin V− PI+) and late apoptotic (Annexin V+ PI+) parasites, no significant differences were observed (Figure 2A and B). When parasites were treated with NFX IC50, the main cell death mechanism observed was early apoptosis (23.58% ± 2.67%), but unlike treatment with the extract, a low frequency of parasites were in late apoptotis (Figure 2A and B). When cell death was evaluated in the trypomastigote stage, parasites treated with ethanol for 24 h were negative for Annexin V and PI staining (87.04 ± 2.92%) (Figure 2C and D), while trypomastigotes treated during 24 h with the EC50 of the CFHEX extract, presented a significant (p < 0.05) increase in the frequency of early apoptotic (16.66 ± 1.09%), necrotic (12.78 ± 0.95%), and late apoptotic (22.48 ± 1.48%) parasites (Figure 2C and D). Likewise, a similar behavior was observed when the trypomastigotes were treated with the extract's EC90, however, a significant increase in the Annexin V+ population was also observed in comparison with the trypomastigotes treated with the EC50 (Figure 2D). When parasites were treated with the NFX EC50 a similar trend was observed, revealing parasite cell death could be mainly attributed to apoptotic events (Figure 2C and D).
Figure 2.
Parasite death induction. Frequency of death (Annexin V+, PI−; Annexin V+, PI+; Annexin V−, PI+) of epimastigote (A and B) and trypomastigote stage (C and D) after 24 h of treatment with ethanol (vehicle control), CFHEX (IC50 or EC50 and IC90 or EC90) or Nifurtimox (IC50 or EC50). Parasites were double stained with Annexin V and PI and analyzed by flow cytometry. In representative flow cytometry analysis, four populations were indicated as necrosis (Annexin V−, PI+), late apoptosis (Annexin V+, PI+), apoptosis (Annexin V+, PI−) and viable (Annexin V−, PI−) parasites (A and C). Frequency of epimastigotes (B) and trypomastigotes (D) in apoptosis, late apoptosis and necrosis after treatment with ethanol, CFHEX extract and nifurtimox (NFX). Data are presented as the median value and range of two independent experiments. Significant differences of each treatment in comparison with control are shown with an asterisk (∗p < 0.05). In B significant differences were only observed in early apoptotic parasites (grey bars). In D significant differences were identified in all populations, early and late apoptotic and necrotic parasites.
3.4. CFHEX extract induction of cytokine production in CD4+ and CD8+ T cells
Based on previous studies that have shown lipopolysaccharide (LPS) induces inflammatory cytokine release [36], LPS presence in the CFHEX extract was verified by a commercial limulus amebocyte lysate kit (Lonza, Walkersville, MD USA), where LPS was not detected in the extract (data not shown). Furthermore, given the importance of the immune response in T. cruzi infection control [16, 37, 38, 39]; it was evaluated whether the CFHEX extract could induce IFN-γ, TNF, or IL-2 production, in addition to perforin or granzyme B liberation in T cell population as well (Figure 3A). Consequently, cytokine secretion by CD4+ and CD8+ T cells stimulated with the positive control (PMA/Ionomycin) and hexane extract was evaluated. T cells stimulated with the PMA/Ionomycin exhibited a high frequency of CD4+ and CD8+ T cells producing IFN-γ, TNF or IL-2 compared with the negative control (data not shown). Likewise, T cells treated with two concentrations (39 μg/mL and 50 μg/mL) of the extract showed an increase in the frequency of CD4+ T cells producing IFN-γ or TNF compared with the negative control (EtOH); however, no differences were observed in IL-2 production (Figure 3B). Following, a Boolean gating approach was used to determinate T cell functional profiles with one (IFN-γ+ or TNF+) or two (IFN-γ+/TNF+) functions. As expected, it was established that CD4+ T cells produced both cytokines after PMA/Ionomycin stimulation (Figure 3C). Notably, CD4+ T cells producing IFN-γ and TNF were also detected after stimulation with both concentrations of the extract (Figure 3C). The same trend was observed when CD8+ T cells were evaluated, since both concentrations of the CFHEX extract induced production of IFN-γ or TNF but not IL-2 (Figure 3D). Additionally, when the cytolytic machinery of CD8+ T cells was evaluated, it was observed both concentrations of the CFHEX extract also induced cytotoxic molecule production (Figure 3E). Moreover, it was noted when cells were stimulated with the extract in comparison with the control, a frequency of CD8+ T cells were capable of producing granzyme B and perforin de novo. Nevertheless, a higher production of perforin was detected when the lowest concentration of the extract was used (Figure 3E). CD8+ T extract stimulated cells produced mainly one and two cytokines. However, it was distinguished a percentage of cells were capable of simultaneously producing three cytokines (Figure 3F), demonstrating the extract induced multifunctional CD8+ T cells. Interestingly, it was observed that the extract chiefly induced a cytotoxic response with a high frequency of perforin and granzyme producing cells (Figure 3F and G). The most prevalent population of CD8+ T cells simultaneously expressing three or two markers were granzyme B+, perforin+, TNF and granzyme B+, perforin+, respectively, indicating that the CFHEX induced mainly a cytotoxic response (Figure 3G). In summary, these results demonstrated CFHEX extract induced IFN-γ or TNF production by CD4+ and CD8+ T cells, in addition to a proportion of multifunctional CD4+ and CD8+ T cells.
Figure 3.
CFHEX extract induction of cytokine production in T cells. (A) Representative dot plots of the gating strategy for selection of CD3+, CD4+ and CD8+ T cells; before, cell doublets were excluded, and live cells were selected. Following, CD4+ and CD8+ T cells were selected to evaluate IFN-γ, TNF and IL-2 production after cell stimulation with EtOH (negative control), PMA/Ionomycin (positive control), IC50 of CFHEX extract against trypomastigotes (39 μg/mL) or 1/10 IC50 of CFHEX against PBMCs (50 μg/mL). Applied gates were aimed to identify cytokine production by CD4+ or CD8+ T cells defined according to cells cultured with 0.13% ETOH for each individual. Frequency of CD4+ (B) or CD8+ (D) T cells producing IFN-γ, TNF or IL-2 after 12 h of culture with EtOH and 39 μg/mL or 50 μg/mL of CFHEX. The bars and error bars indicate the median and range. (C) Functional profile of CD4+ T cells following stimulation with PMA/Ionomycin, and 39 μg/mL or 50 μg/mL of CFHEX. The functional profiles are grouped and color-coded according to cytokine productions. (E) Frequency of CD8+ T cells producing de novo perforin and granzyme. To this end the frequency of cytokine production by cells stimulated with EtOH was subtracted from the frequency of cytokine production by stimulated cells. (F) Functional profile of CD8+ T cells following stimulation with PMA/Ionomycin or CFHEX extract. Green box: simultaneous production of 4 cytokines. Red box: simultaneous production of 3 cytokines, Blue box: simultaneous production of 2 cytokines, grey box: one cytokine produced Grey arch: Granzyme B, Blue arch: Perforin production (G) Response summary in CD8+ cells based upon granzyme B, IFN-γ, IL-2, perforin, and TNF, broken down into the relative contribution of each functional combination. Combinations not contributing to the functional profile are not shown. The p values were calculated using the Mann-Whitney U test. The p values of the permutation test in the co-expression analysis (C and F) are shown in the pie charts. ∗p < 0.05, ∗∗p < 0.01.
3.5. Characterization of the extract's chemical composition
Taking into account the biological activities evidenced by the CFHEX extract, major chemical component characterization present in this extract was carried out by HPLC-ESI-QTOF-MS, as previously described. Table 2 illustrates identified structures in the extract with relative abundances greater than 1%.
Table 2.
Clethra fimbriata hexanic extract major component characterization as determined by HPLC-ESI-QTOF-MS analyses.
| Compound name | CT | MF | MW g/mol | RT (min) | ME (ppm) | RA (%) | OI | Conf. |
|---|---|---|---|---|---|---|---|---|
| Unknown | Fatty acid | C23 H32 O2 | 339.2339 | 10.79 | 3 | 7.49 | [M-H]− | Putative |
| Dihydroursolic acid | Triterpen | C30 H50 O3 | 457.3695 | 12.245 | 1.68 | 6.09 | [M-H]− | MS/MS |
| Camelliagenin A | Triterpen | C30 H50 O4 | 473.3651 | 18.154 | 3.1 | 5.03 | [M-H]− | Putative |
| Betulinic acid | Triterpen | C30 H48 O3 | 455.3544 | 10.695 | 2.85 | 3.36 | [M-H]− | MS/MS |
| Jasmone | Monoterpen | C11 H16 O | 163.1128 | 10.788 | 0.16 | 3.29 | [M-H]− | Putative |
| Isopalmitic acid | Fatty acid | C16 H32 O2 | 255.2336 | 11.441 | 2.47 | 3.22 | [M-H]− | MS/MS |
| Methoxy-heptadecanoic acid | Fatty acid | C18 H34 O2 | 281.2494 | 11.756 | 3.24 | 3.1 | [M-H]-[H20] | MS/MS |
| Methyl-heptadecanoic acid | Fatty acid | C18 H36 O2 | 283.265 | 12.642 | 3.15 | 2.86 | [M-H]− | MS/MS |
| Linoleic acid | Fatty acid | C18 H32 O2 | 279.2336 | 11.127 | 2 | 2.39 | [M-H]− | Putative |
| keto stearic acid | Fatty acid | C18 H34 O3 | 446.3779 | 15.308 | 3 | 1.95 | [M-H]-[H20] | MS/MS |
| Octadeacanoic acid | Fatty acid | C18 H34 O4 | 295.2287 | 9.627 | 1.81 | 1.67 | [M-H]-[H20] | MS/MS |
| Hexacosanoic acid | Fatty acid | C26 H52 O2 | 395.3907 | 13.39 | 3 | 1.63 | [M-H]− | Putative |
| Unknown | Alkaloid | C12 H21 N | 224.166 | 10.511 | 2 | 1.61 | [M-H]− | Putative |
| Ursolic acid | Triterpen | C30 H48 O3 | 455.3543 | 10.851 | 1 | 1.4 | [M-H]− | MS/MS |
| alpha-Linolenic acid | Fatty acid | C18 H30 O2 | 277.2179 | 10.666 | 2 | 1.37 | [M-H]− | Putative |
| Messagenin | Triterpen | C29 H48 O3 | 443.3545 | 18.456 | 2.99 | 1.33 | [M-H]− | MS/MS |
| α-tocopherol acetate | Fatty acid | C31 H52 O3 | 471.3859 | 23.215 | 3.5 | 1.28 | [M-H]− | MS/MS |
CT: Compound type, MF: Molecular Formula, MW: Molecular Weight, RT: Retention Time, ME: Mass Error, RA: Relative Abundance, OI: Observed Ion, Conf: Confirmation.
C. fimbriata major compounds are represented mainly by two compound types: fatty acids and terpenes, which represent 26.96% and 20.5% of the CFHEX extract composition, respectively. Other types of compounds such as alkaloids (relative abundance: 1.61%) and vitamins (relative abundance: 1.28%) were also found as major constituents of CFHEX.
Regarding the fatty acids established, saturated fatty acids such as Isopalmitic acid, Methoxy-heptadecanoic acid, Methyl-heptadecanoic acid, keto stearic acid, Octadeacanoic acid and Hexacosanoic acid were chiefly observed, followed by, unsaturated fatty acids such as Linoleic and Linolenic acids. On the other hand, compounds like Camelliagenin A, Messagenin and Betulinic, Ursolic and Dihydroursolic acids, all categorized as triterpenoids represented the majority of the terpenic fraction found in CFHEX.
4. Discussion
All along history plants have been used in the treatment of multiple organic disorders and recently they have been instituted as novel natural products with specific bioactivities. In developing countries plants play an important role to manage primary needs of medical care [40]. Historically natural products obtained from plants and their structural analogues, have greatly contributed to the pharmacotherapy of various diseases. Some representative examples are paclitaxel and vinblastine as antitumor agents [41], galantamine and apomorphine (derived from morphine) alkaloids used in the treatment of Alzheimer's and Parkinson's, respectively [42, 43] and artemisinin and its derivatives, artemether and artemisone, as antimalarial agents [44].
Our group has been studying various Colombian native plants as a source of new metabolites that in the future may be considered as alternative treatments for Chagas disease. Thus, we previously described a C. fimbriata ethanolic extract obtained from leaves, rich in flavonoids and triterpenes that was active against epimastigotes and trypomastigotes stages of T. cruzi parasite [22]. The present work evaluated the use of three different solvents, hexane, dichloromethane and hydroalcoholic to extract with greater precision C. fimbriata chemical components associated with trypanocidal activity. Thus, low polarity compounds such as terpenes, phytosterols and fatty acids were grouped in the hexane and dichloromethane extracts and high polarity compounds like flavonoids, tannins and saponins were present in the hydroalcoholic mixture.
The results obtained in the present investigation, indicated the CFHEX extract inhibited all three stages of T. cruzi with an IC50 of 153.9 ± 29.5 μg/mL and 45.6 ± 10.5 μg/mL against epimastigotes and amastigotes, respectively, and EC50 of 39.3 ± 7.2 μg/mL against trypomastigotes. Additionally, it is important to take into account this extract's selectivity index, since some research suggests that treatments with selectivity indexes greater than 3 can be considered selective [29]. Compared to other reported extracts with trypanocidal activity, the CFHEX extract presented a greater or similar selectivity. For instance, the petroleum ether and methanol extracts obtained from Tetraselmis suecica and Khaya anthotheca plants attained SI of 6.20 and 11.2, using the trypomastigote stage and macrophages (J774) and VERO cells, respectively [45, 46]. Valencia and collaborators in 2011 described in the U937 cellular model, Hieronyma antioquensis hexane extract presented selectivity indices of 7 against trypomastigotes and 27 against amastigotes [29]. Hence, the CFHEX extract with an SI of 25.4 and 21.9 against trypomastigotes and amastigotes, respectively, can be considered a promising source for future research, since more than 20 fold of the extract's concentration is required to inhibit the cellular model in comparison with the parasitic stage.
Interestingly, in the analysis of the concentrations required to inhibit each parasitic stage, a decrease in the concentrations necessary to inhibit trypomastigotes and amastigotes in comparison to the epimastigotes was observed. These differences are consistent with numerous investigations in which the epimastigote stage is considered the stage of least susceptibility [47, 48, 49]. For example, the hydroalcoholic extract obtained from Arrabidaea chica leaves against epimastigotes presented an IC50 of 213.8 μg/mL and against trypomastigotes an EC50 of 24.8 μg/mL [49]. Similar effects were observed in Syzygium aromaticum essential oils, which presented an IC50 of 99.5 μg/mL for epimastigotes and an EC50 of 57.5 μg/mL for trypomastigotes [48]. However, the susceptibility of the amastigote stage is still controversial. The diterpene geranylgeraniol presented an IC50 of 12.5 μg/mL against T. cruzi epimastigotes, while its IC50 against the forms of amastigotes was 2.0 μg/mL, displaying greater susceptibility in amastigotes [50]. On the other hand, compounds such as the flavonoid eupatorin isolated from Stevia satureifolia leaves, lost their trypanocidal capacity against the amastigote stage [27], which, highlights the importance of CFHEX capacity of inhibiting the T. cruzi's trypomastigote and amastigote stages.
Extract effect differences among each stage may be related to the diverse metabolic pathways and membrane compositions in the various developmental forms of T. cruzi [51, 52]. Furthermore, the differential susceptibility could be attributed to differences in the parasite environment, since epimastigotes and trypomastigotes are free forms exposed to the external environment, whereas amastigote forms reside within the host cell. Moreover, ease of treatment diffusion into biological membranes cannot be excluded, which can influence activity results among different stages. Notably, the results obtained in amastigotes with the CFHEX extract suggest the presence of substances capable of absorption. Additionally, they were able to inhibit amastigote formation within cells, reducing the amount of trypomastigotes released to the environment, which represents an important advance from that observed in epimastigotes and trypomastigotes.
Extract treatment effects can be broadly grouped into two categories: trypanocidal effects (parasite elimination) [49, 51, 53, 54] and cell cycle arrest (inhibition of replication) [55, 56]. The exposure of epimastigotes to high CFHEX extract concentrations for short periods of time do not induce a delay in the parasite's replicative cycle, suggesting a trypanocidal effect of CFHEX at this stage. However, longer treatment time intervals are required to verify any effect on the replicative cycle. Contrary to what was observed with exposure of drugs, such as lovastatin and ketoconazole, which at 100 μM and 120 μM in a time-dependent manner induced loss to resume growth after cessation of pharmacological stress [30]. In addition, trypomastigotes treated with CFHEX decreased their infective capacity. These findings suggest, in addition to a trypanocidal effect on the trypomastigote stage, a decrease in their infective capacity. Therefore, it is possible that CFHEX may have inhibitory effects on proteins important for the adhesion and invasion process such as gp83, trans-sialidase, and proteases, such as cruzipain, oligopeptidase B and oligopeptidase Tc80, among others involved in the early stages of the parasite cellular invasion process [57]. However, further studies are required to test this hypothesis.
Trypanocidal effects depend directly on the chemical components present in the extracts. CFHEX extract chemical composition includes triterpenoids, which agrees with that observed by Castañeda et al. in 2021, where C. fimbriata major constituent putative characterization of the ethanol extract identified compounds belonging to the triterpene group [22]. These compounds have been reported in numerous investigations as trypanocidal agents. Almeida et al. in 2016 evaluated the trypanocidal effects of Copernicia prunifera plant extracts rich in dammarane-type compounds, finding that triterpene (24R) -methyldammara-20,25-dien-3α-ol eliminated trypomastigotes with an EC50 of 35.2 μM. Additionally, when cytotoxicity was assessed in mammalian conjunctive cells, a low toxicity was observed, reaching a selectivity index of 5.7 [58]. Other triterpenes such as α and β amyrin together with their semi-synthetic derivatives have been described as having trypanocidal activities on trypomastigotes and amastigotes, whose mechanisms of action seems to be related to mitochondrial membrane potential changes and ultrastructural features suggesting autophagy processes [59].
Among the major triterpenes characterized in CFHEX, two stand out due to their previously described biological activities. Ursolic acid, a pentacyclic triterpenoid (C30H48O3), which has been described with anti-inflammatory, anti-oxidant, anti-apoptotic, and anti-carcinogenic effects [60]. Additionally, various investigations have observed trypanocidal effects associated with this triterpene. Vanrell et al. in 2020 reported that RAW macrophage infected with trypomastigotes (Y-strain), which were treated with ursolic acid (10 μM) induced a significant amastigote reduction compared with untreated cultures [61]. Other studies carried out in acute infection models in BALB/C albino mice, found that ursolic acid oral treatment at a concentration of 20 mg/kg/day resulted in 60% reduction of parasitemia at the parasitemic peak after infection of male mice with Y-strain trypomastigotes [62]. This triterpene is currently being studied mainly in encapsulation systems, which is expected to improve its trypanocidal and absorption capacities in biological systems [63, 64]. On the other hand, betulinic acid (C30H48O3), a pentacyclic lupane-type triterpene, widely found in the medicinal herbs and plants, exhibits a variety of biological properties, such as inhibition of human immunodeficiency virus, anti-bacterial, anti-malarial, anti-inflammatory, anthelmintic, antinociceptive, and anti-cancer activities [65]. Furthermore, it has been demonstrated that betulinic acid inhibits T. cruzi growth at the three stages without displaying toxicity in LLC-MK2 cells at the concentrations used (200–1,600 μM). Possible parasite cell death mechanisms showed alterations in mitochondrial membrane potential, alterations in cell membrane integrity, increased formation of reactive oxygen species and increased swelling of the reservosomes, effects associated with cell deaths by necrosis [66]. Although, death mechanisms induced by betulinic and ursolic acid have not been fully studied, it has been reported that epimastigotes and trypomastigotes treated with betulinic acid show necrosis induction, with increased reactive oxygen species production, loss of mitochondrial integrity and swelling of the reservosomes [66, 67]. Regarding ursolic acid, to the best of our knowledge, no mechanism of induced death has been established in T. cruzi. However, it has been observed in Gemcitabine-resistant pancreatic cancer cells that ursolic acid treatment reduces cell viability through cell cycle arrest and endoplasmic reticulum stress, resulting in apoptosis and autophagy in a dose-dependent manner [68]. Together, these findings partially support death mechanisms induced by CFHEX, in which necrosis and apoptosis induction was observed, although it was preferentially apoptotic. The induction of the two types of cell deaths can be accounted by the extract's complexity, where death mechanisms can be influenced by the diversity of the structures within the extract, as well as an additive or synergistic effect between the compounds. More specific studies are required to evaluate the cell death mechanisms for each component.
The relevance of both CD4+ and CD8+ T cells in T. cruzi infection control has been demonstrated in humans and in murine models [37, 69, 70]. T cells secrete cytokines and display cytotoxic activity via granule release containing perforin and granzymes. IFN-γ is critical in orchestrating Th1 responses, which have been considered more effective in eliminating intracellular pathogens, such as T. cruzi. Both IFN-γ and TNF are activators of inducible nitric oxide synthase, which produce nitric oxide, largely responsible for macrophage-mediated T. cruzi elimination [71]. Other cytotoxic molecules, such as perforin and granzyme B also play an important role in T. cruzi infection control, promoting superoxide and inactivate oxidative defense enzyme generation, resulting in mitochondrial swelling, transmembrane potential dissipation, membrane blebbing, phosphatidylserine exposure, DNA damage and chromatin condensation [72]. Previous reports have suggested T cell's response defined by a polyfunctional activity, is crucial for determining the clinical outcome of chronic infections [18, 73, 74, 75]. In advanced T. cruzi infection phases, a lower frequency of T cells producing IFN-γ and TNF and a lower proportion of multifunctional T cells in comparison with the early phases of the infection have been described [16, 17], suggesting that chronic parasite persistence promotes T cell dysfunction. These facts highlight the importance of searching for new therapeutic strategies that efficiently stimulate the immune response during T. cruzi infection.
In this work, although it was observed that CFHEX extract induces IFN-γ and TNF production by CD4+ and CD8+ T cells from HDs, as well as de novo production of cytotoxic molecules granzyme B and perforin in CD8+ T cells, it is important to further investigate the type of compounds that may be mediating these effects. This to evaluate whether in chronic chagasic patients it is possible to find a similar effect and to propose long-term immunotherapeutic strategies. Hence, investigations have suggested triterpene plays a role in immune response modulation processes. Additionally, an increase in TNF production in mice treated with betulinic acid in a dose-dependent manner has been described [76]. Likewise, betulinic acid at a high concentration (50 μg/mL) derived from Ziziphus jujube barks presented an increased TNF level in human lymphocytes and macrophages [77], displaying its possible role in immune response modulation. Treatment with other triterpenes, such as Centella asiatica madecassic acid increased CD4+ and CD8+ T cell number and frequency of IFN–γ production by T cells compared with the untreated group of mice [78].
Collectively, our results demonstrated C. fimbriata hexane extract selectively inhibited all three stages of T. cruzi, an effect mediated by apoptosis. Furthermore, this extract increased the frequency of CD4+ T producing IFN-γ or TNF, induced an augmentation of multifunctional CD4+ and CD8+ T cells, and prompted a CD8+ T cell cytotoxic response. Moreover, this extract induced trypomastigote apoptotic death and inhibited parasite cell infection. C. fimbritata's extract trypanocidal effect and activation of CD4+ and CD8+ T cell cytokine production, suggest a novel source of compounds, which in the future may contribute to new alternatives for T. cruzi infection control.
5. Conclusions
Hexane extract obtained from the C. fimbriata has the ability to effectively and selectively inhibit all three stages of T. cruzi, mainly inducing parasite death by apoptosis. Additionally, this extract increases the frequency of CD4+ and CD8+ T cells that produce IFN-γ or TNF, and most importantly, it also increases the frequency of multifunctional CD4+ and CD8+ T cells, and induces the CD8+ T cells cytotoxic response. These biological effects can be associated with the presence of triterpenic-type compounds, one of the two main extract constituents.
Declarations
Author contribution statement
Daniel Pardo-Rodriguez: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Paola Lasso, José Mateus: Conceived and designed the experiments; Analyzed and interpreted the data.
John Mendez, Concepción J. Puerta, Adriana Cuéllar: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Jorge Robles, Claudia Cuervo: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Vicerrectoría de Investigación from the Pontificia Universidad Javeriana (Proposal ID 7733). DPR was supported by Ph.D. scholarships from MinCiencias "Ministerio de Ciencia, Tecnología e Innovación, Colombia"; Convocatoria 755 - 2016, Formación de Capital Humano de Alto Nivel para el Departamento de Tolima.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to the Pontificia Universidad Javeriana for assistance with the editing of the manuscript (ID Proposal 9639) and publication (ID Proposal 9960). This work was supported by the Vicerrectoría de Investigación from the Pontificia Universidad Javeriana and belongs to the projects “Evaluación de la capacidad tripanocida de fracciones de Clethra fimbriata, Siparuna sessiliflora y Ageratina vacciniifolia sobre Trypanosoma cruzi y el efecto en la producción de citocinas por linfocitos T CD8+ de individuos sanos”, ID Proposal 7733.
Footnotes
This article is a part of "Ethnopharmacology – challenges for the future" Special issue.
Contributor Information
Jorge Robles, Email: jrobles@javeriana.edu.co.
Claudia Cuervo, Email: claudia.cuervo@javeriana.edu.co.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Rassi A., Rassi A., Marin-Neto J.A. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Weekly epidemiological record (WER) WHO. 2015;90:33–40. [Google Scholar]
- 3.Pérez-Molina J.A., Molina I. Chagas disease. Lancet. 2017;6736:1–13. [Google Scholar]
- 4.Rassi A., Rassi A., Marcondes de Rezende J. American trypanosomiasis (chagas disease) Infect. Dis. Clin. 2012;26:275–291. doi: 10.1016/j.idc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Lidani K.C.F., Andrade F.A., Bavia L., Damasceno F.S., Beltrame M.H., Messias-Reason I.J., Sandri T.L. Chagas disease: from discovery to a worldwide health problem. Front. Public Health. 2019;7:1–13. doi: 10.3389/fpubh.2019.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abad-Franch F., Monteiro F.A., Jaramillo O. N., Gurgel-Gonçalves R., Dias F.B.S., Diotaiuti L. Ecology, evolution, and the long-term surveillance of vector-borne Chagas disease: a multi-scale appraisal of the tribe Rhodniini (Triatominae) Acta Trop. 2009;110:159–177. doi: 10.1016/j.actatropica.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Dias J.C.P., Coura J.R., Yasuda M.A.S. 2014. The Present Situation, Challenges, and Perspectives Regarding the Production and Utilization of Effective Drugs against Human Chagas Disease. [DOI] [PubMed] [Google Scholar]
- 8.Patterson S., Wyllie S. Nitro drugs for the treatment of trypanosomatid diseases: past, present, and future prospects. Trends Parasitol. 2014;30:289–298. doi: 10.1016/j.pt.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marín C., Ramírez-Macías I., López-Céspedes A., Olmo F., Villegas N., Díaz J.G., Rosales M.J., Gutiérrez-Sánchez R., Sánchez-Moreno M. In vitro and in vivo trypanocidal activity of flavonoids from Delphinium staphisagria against Chagas disease. J. Nat. Prod. 2011;74:744–750. doi: 10.1021/np1008043. [DOI] [PubMed] [Google Scholar]
- 10.Urbina J.A. Recent clinical trials for the etiological treatment of chronic chagas disease: advances, challenges and perspectives. J. Eukaryot. Microbiol. 2015;62:149–156. doi: 10.1111/jeu.12184. [DOI] [PubMed] [Google Scholar]
- 11.Urbina J.A. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop. 2010;115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Burki T. Production of drugs for Chagas disease predicted to fall short. Lancet Infect. Dis. 2011;11:901–902. [Google Scholar]
- 13.Mejía-Jaramillo A.M., Fernández G.J., Montilla M., Nicholls R.S., Triana-Chávez O. Sensibilidad al benzonidazol de cepas de Trypanosoma cruzi sugiere la circulación de cepas naturalmente resistentes en Colombia. Biomédica - Rev. Del Inst. Nac. Salud. 2012;32:196–205. doi: 10.1590/S0120-41572012000300007. [DOI] [PubMed] [Google Scholar]
- 14.Mejia A.M., Hall B.S., Taylor M.C., Gómez-Palacio A., Wilkinson S.R., Triana-Chávez O., Kelly J.M. Benznidazole-resistance in Trypanosoma cruzi is a readily acquired trait that can arise independently in a single population. J. Infect. Dis. 2012;206:220–228. doi: 10.1093/infdis/jis331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sánchez-Valdéz F.J., Padilla A., Wang W., Orr D., Tarleton R.L. Spontaneous dormancy protects Trypanosoma cruzi during extended drug exposure. Elife. 2018;7 doi: 10.7554/eLife.34039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lasso P., Mateus J., Pavía P., Rosas F., Roa N., Thomas M.C., López M.C., González J.M., Puerta C.J., Cuéllar A. Inhibitory receptor expression on CD8 + T cells is linked to functional responses against Trypanosoma cruzi antigens in chronic chagasic patients. J. Immunol. 2015;195:3748–3758. doi: 10.4049/jimmunol.1500459. [DOI] [PubMed] [Google Scholar]
- 17.Albareda M.C., Olivera G.C., Laucella S.A., Gabriela M., Fernandez E.R., Lococo B., Viotti R., Tarleton R.L., Postan M., Nacional I., Fatala D.P.M., Aires B. Chronic human infection with Trypanosoma cruzi drives CD4+ T cells to immune senescence. J. Immunol. 2009;183:4103–4108. doi: 10.4049/jimmunol.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mateus J., Guerrero P., Lasso P., Cuervo C., González J.M., Puerta C.J., Cuéllar A. An animal model of acute and chronic chagas disease with the reticulotropic Y strain of Trypanosoma cruzi that depicts the multifunctionality and dysfunctionality of T cells. Front. Immunol. 2019;10:1–17. doi: 10.3389/fimmu.2019.00918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt T.J., Khalid S.A., Romanha A., Alves T.M., Biavatti M., Brun R., Da Costa F.B., de Castro S.L., Ferreira V.F., de Lacerda M.V.G., Lago J.H.G., Leon L.L., Lopes N.P., das Neves Amorim R., Niehues M., Ogungbe I.V. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases - Part I. Curr. Med. Chem. 2012;19:2128–2175. doi: 10.2174/092986712800229023. [DOI] [PubMed] [Google Scholar]
- 20.Santos S.S., De Araújo R.V., Giarolla J., El Seoud O., Ferreira E.I. Searching for drugs for Chagas disease, leishmaniasis and schistosomiasis : a review. Int. J. Antimicrob. Agents. 2020;55:105906. doi: 10.1016/j.ijantimicag.2020.105906. [DOI] [PubMed] [Google Scholar]
- 21.Bernal R., Gradstein R.S., Celis M. Universidad Nacional de Colombia - Sede Bogotá Facultad de Ciencias, Instituto de Ciencias Naturales; Bogotá: 2016. Catálogo de plantas y líquenes de Colombia, Volumen I. [Google Scholar]
- 22.Castañeda J.S., Suta-Velásquez M., Mateus J., Pardo-Rodriguez D., Puerta C.J., Cuéllar A., Robles J., Cuervo C. Preliminary chemical characterization of ethanolic extracts from Colombian plants with promising anti - Trypanosoma cruzi activity. Exp. Parasitol. 2021;223:108079. doi: 10.1016/j.exppara.2021.108079. [DOI] [PubMed] [Google Scholar]
- 23.Pavia P.X., Thomas M.C., López M.C., Puerta C.J. Molecular characterization of the short interspersed repetitive element SIRE in the six discrete typing units (DTUs) of Trypanosoma cruzi. Exp. Parasitol. 2012;132:144–150. doi: 10.1016/j.exppara.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Muelas-Serrano S., Nogal-Ruiz J., Gomez-Barrio A. Setting of a colorimetric method to determine the viability of Trypanosoma cruzi epimastigotes. Parasitol. Res. 2000;86:999–1002. doi: 10.1007/pl00008532. [DOI] [PubMed] [Google Scholar]
- 25.Matsuo A.L., Silva L.S., Torrecilhas A.C., Pascoalino B.S., Ramos T.C., Rodrigues E.G., Schenkman S., Caires A.C.F., Travassos L.R. In vitro and in vivo trypanocidal effects of the cyclopalladated compound 7a, a drug candidate for treatment of Chagas’ disease. Antimicrob. Agents Chemother. 2010;54:3318–3325. doi: 10.1128/AAC.00323-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meira C.S., Guimarães E.T., Dos Santos J.A.F., Moreira D.R.M., Nogueira R.C., Tomassini T.C.B., Ribeiro I.M., De Souza C.V.C., Ribeiro Dos Santos R., Soares M.B.P. In vitro and in vivo antiparasitic activity of Physalis angulata L. concentrated ethanolic extract against Trypanosoma cruzi. Phytomedicine. 2015;22:969–974. doi: 10.1016/j.phymed.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Beer M.F., Frank F.M., Germán Elso O., Ernesto Bivona A., Cerny N., Giberti G., Luis Malchiodi E., Susana Martino V., Alonso M.R., Patricia Sülsen V., Cazorla S.I. Trypanocidal and leishmanicidal activities of flavonoids isolated from Stevia satureiifolia var. satureiifolia. Pharm. Biol. 2016;54:2188–2195. doi: 10.3109/13880209.2016.1150304. [DOI] [PubMed] [Google Scholar]
- 28.Moreno É.M., Leal S.M., Stashenko E.E., García L.T. Induction of programmed cell death in Trypanosoma cruzi by Lippia alba essential oils and their major and synergistic terpenes (citral, limonene and caryophyllene oxide) BMC Compl. Alternative Med. 2018;18:1–16. doi: 10.1186/s12906-018-2293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valencia L., Muñoz D.L., Robledo S.M., Echeverri F. Actividad tripanocida y citotóxica de extractos de plantas Colombianas. Biomedica. 2011;31:552–559. doi: 10.1590/S0120-41572011000400010. [DOI] [PubMed] [Google Scholar]
- 30.Kessler R.L., Soares M.J., Probst C.M., Auré M., Krieger L., Goldberg A.C. Trypanosoma cruzi response to sterol biosynthesis inhibitors: morphophysiological alterations leading to cell death. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz-Soto J.C., Lasso P., Guzmán F., Forero-Shelton M., Thomas M. del C., López M.C., Guhl F., Cuellar A., Puerta C.J., González J.M. Rabbit serum against K1 peptide, an immunogenic epitope of the Trypanosoma cruzi KMP-11, decreases parasite invasion to cells. Acta Trop. 2012;123:224–229. doi: 10.1016/j.actatropica.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Mateus J., Lasso P., González J.M., Puerta C.J., Cuéllar A. Design of a multicolor panel to assess intracellular and surface molecules by flow cytometry. Biomedica. 2013;33:660–672. doi: 10.7705/biomedica.v33i4.1709. [DOI] [PubMed] [Google Scholar]
- 33.Roeder M., Nozzi J., Nason M. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytom. A. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gil-De-La-Fuente A., Godzien J., Saugar S., Garcia-Carmona R., Badran H., Wishart D.S., Barbas C., Otero A. CEU Mass Mediator 3.0: a metabolite annotation tool. J. Proteome Res. 2019;18:797–802. doi: 10.1021/acs.jproteome.8b00720. [DOI] [PubMed] [Google Scholar]
- 35.Osorio E., Arango G.J., Jiménez N., Alzate F., Ruiz G., Gutiérrez D., Paco M.A., Giménez A., Robledo S. Antiprotozoal and cytotoxic activities in vitro of Colombian Annonaceae. J. Ethnopharmacol. 2007;111:630–635. doi: 10.1016/j.jep.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Ulmer A.J., Flad H.D., Rietschel T., Mattern T. Induction of proliferation and cytokine production in human T lymphocytes by lipopolysaccharide (LPS) Toxicology. 2000;152:37–45. doi: 10.1016/s0300-483x(00)00290-0. [DOI] [PubMed] [Google Scholar]
- 37.Rottenberg M.E., Bakhiet M., Olsson T., Kristensson K., Mak T., Wigzell H., Orn A. Differential susceptibilities of mice genomically deleted of CD4 and CD8 to infections with Trypanosoma cruzi or Trypanosoma brucei. Infect. Immun. 1993;61:5129–5133. doi: 10.1128/iai.61.12.5129-5133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mateus J., Pérez-Antón E., Lasso P., Egui A., Roa N., Carrilero B., González J.M., Thomas M.C., Puerta C.J., López M.C., Cuéllar A. Antiparasitic treatment induces an improved CD8+ T cell response in chronic chagasic patients. J. Immunol. 2017;198:3170–3180. doi: 10.4049/jimmunol.1602095. [DOI] [PubMed] [Google Scholar]
- 39.Hoft D.F., Eickhoff C.S. Type 1 immunity provides optimal protection against both mucosal and systemic Trypanosoma cruzi challenges. Infect. Immun. 2002;70:6715–6725. doi: 10.1128/IAI.70.12.6715-6725.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Andel T., Carvalheiro L.G. Why urban citizens in developing countries use traditional medicines: the case of Suriname, Evidence-Based Complement. Alternative Med. 2013;2013 doi: 10.1155/2013/687197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demain A.L., Vaishnav P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011;4:687–699. doi: 10.1111/j.1751-7915.2010.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Razay G., Wilcock G.K. Galantamine in Alzheimer’s disease. Expert Rev. Neurother. 2008;8:9–17. doi: 10.1586/14737175.8.1.9. [DOI] [PubMed] [Google Scholar]
- 43.Carbone F., Djamshidian A., Seppi K., Poewe W. Apomorphine for Parkinson’s disease: efficacy and safety of current and new formulations. CNS Drugs. 2019;33:905–918. doi: 10.1007/s40263-019-00661-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ansari M., Saify Z., Sultana N., Ahmad I., Saeed-Ul-Hassan S., Tariq I., Khanum M. Malaria and artemisinin derivatives: an updated review. Mini Rev. Med. Chem. 2013;13:1879–1902. doi: 10.2174/13895575113136660097. [DOI] [PubMed] [Google Scholar]
- 45.Obbo C.J.D., Kariuki S.T., Gathirwa J.W., Olaho-Mukani W., Cheplogoi P.K., Mwangi E.M. In vitro antiplasmodial, antitrypanosomal and antileishmanial activities of selected medicinal plants from Ugandan flora: refocusing into multi-component potentials. J. Ethnopharmacol. 2019;229:127–136. doi: 10.1016/j.jep.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 46.Veas R., Rojas-Pirela M., Castillo C., Olea-Azar C., Moncada M., Ulloa P., Rojas V., Kemmerling U. Microalgae extracts: potential anti-Trypanosoma cruzi agents? Biomed. Pharmacother. 2020;127:110178. doi: 10.1016/j.biopha.2020.110178. [DOI] [PubMed] [Google Scholar]
- 47.Azeredo C.M.O., Santos T.G., de N.S. Maia B.H.L., Soares M.J. In vitro biological evaluation of eight different essential oils against Trypanosoma cruzi, with emphasis on Cinnamomum verum essential oil. BMC Compl. Alternative Med. 2014;14:1–8. doi: 10.1186/1472-6882-14-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santoro G.F., Cardoso M.G., Guimarães L.G.L., Mendonça L.Z., Soares M.J. Trypanosoma cruzi: activity of essential oils from Achillea millefolium L., Syzygium aromaticum L. and Ocimum basilicum L. on epimastigotes and trypomastigotes. Exp. Parasitol. 2007;116:283–290. doi: 10.1016/j.exppara.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 49.Miranda N., Gerola A.P., Novello C.R., Ueda-Nakamura T., de Oliveira Silva S., Dias-Filho B.P., Hioka N., de Mello J.C.P., Nakamura C.V. Pheophorbide a, a compound isolated from the leaves of Arrabidaea chica, induces photodynamic inactivation of Trypanosoma cruzi. Photodiagnosis Photodyn. Ther. 2017;19:256–265. doi: 10.1016/j.pdpdt.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Alegre-Gómez S., Sainz P., Simões M.F., Rijo P., Moiteiro C., González-Coloma A., Martínez-Díaz R.A. Antiparasitic activity of diterpenoids against Trypanosoma cruzi. Planta Med. 2017;83:306–311. doi: 10.1055/s-0042-115646. [DOI] [PubMed] [Google Scholar]
- 51.Andrade M.A., Cardoso M.D.G., Gomes M. de S., de Azeredo C.M.O., Batista L.R., Soares M.J., Rodrigues L.M.A., Figueiredo A.C.S. Biological activity of the essential oils from Cinnamodendron dinisii and Siparuna guianensis. Braz. J. Microbiol. 2015;46:189–194. doi: 10.1590/S1517-838246120130683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berná L., Chiribao M.L., Greif G., Rodriguez M., Alvarez-Valin F., Robello C. Transcriptomic analysis reveals metabolic switches and surface remodeling as key processes for stage transition in Trypanosoma cruzi. PeerJ. 2017;2017:1–32. doi: 10.7717/peerj.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baldissera M.D., Da Silva A.S., Oliveira C.B., Santos R.C.V., Vaucher R.A., Raffin R.P., Gomes P., Dambros M.G.C., Miletti L.C., Boligon A.A., Athayde M.L., Monteiro S.G. Trypanocidal action of tea tree oil (Melaleuca alternifolia) against Trypanosoma evansi in vitro and in vivo used mice as experimental model. Exp. Parasitol. 2014;141:21–27. doi: 10.1016/j.exppara.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 54.De Souza E.M., da Silva P.B., Nefertiti A.S.G., Ismail M.A., Arafa R.K., Tao B., Nixon-Smith C.K., Boykin D.W., Soeiro M.N.C. Trypanocidal activity and selectivity in vitro of aromatic amidine compounds upon bloodstream and intracellular forms of Trypanosoma cruzi. Exp. Parasitol. 2011;127:429–435. doi: 10.1016/j.exppara.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Elias M.C., Crispim M., Trujillo-ferrara J.G., Silber M. A Fluorinated Phenylbenzothiazole arrests the Trypanosoma host cells. Antimicrob. Agents Chemother. 2020;64:1–16. doi: 10.1128/AAC.01742-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuma A.A., Mendes I.C., Reignault L.C., Elias M.C., De Souza W., Machado C.R., Motta M.C.M. How Trypanosoma cruzi handles cell cycle arrest promoted by camptothecin, a topoisomerase I inhibitor. Mol. Biochem. Parasitol. 2014;193:93–100. doi: 10.1016/j.molbiopara.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 57.De Souza W., De Carvalho T.M.U., Barrias E.S. Review on Trypanosoma cruzi: host cell interaction. Int. J. Cell Biol. 2010;2010 doi: 10.1155/2010/295394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Almeida B.C., Araújo B.Q., Carvalho A.A., Freitas S.D.L., Maciel D. da S.A., Ferreira A.J.S., Tempone A.G., Martins L.F., Alexandre T.R., Chaves M.H., Lago J.H.G. Antiprotozoal activity of extracts and isolated triterpenoids of ‘carnauba’ (Copernicia prunifera) wax from Brazil. Pharm. Biol. 2016;54:3280–3284. doi: 10.1080/13880209.2016.1224257. [DOI] [PubMed] [Google Scholar]
- 59.Bossolani G.D.P., Ueda-Nakamura T., Silva S.O., Dias Filho B.P., Costa T.O.G., Quintanilla R.H.R., Martinez S.T., Veiga V.F., Pinto A.C., Nakamura C.V. Anti-trypanosoma activity and synergistic effects of natural and semi-synthetic triterpenes and predominant cell death through autophagy in amastigote forms. J. Braz. Chem. Soc. 2017;28:2473–2489. [Google Scholar]
- 60.Seo D.Y., Lee S.R., Heo J.W., No M.H., Rhee B.D., Ko K.S., Kwak H.B., Han J. Ursolic acid in health and disease. KOREAN J. PHYSIOL. PHARMACOL. 2018;22:235–248. doi: 10.4196/kjpp.2018.22.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vanrell M.C., Martinez S.J., Muñoz L.I., Salassa B.N., Romano P.S. 1st Int. Electron. Conf. Biomol. Nat. Bio-Inspired Ther. Hum. Dis. 2020. Ursolic acid promotes clearance of Trypanosoma cruzi amastigotes in the host cell; pp. 1–12. [Google Scholar]
- 62.Da Silva Ferreira D., Esperandim V.R., Toldo M.P.A., Kuehn C.C., Do Prado Júnior J.C., Cunha W.R., Silva M.L.A.e., de Albuquerque S. In vivo activity of ursolic and oleanolic acids during the acute phase of Trypanosoma cruzi infection. Exp. Parasitol. 2013;134:455–459. doi: 10.1016/j.exppara.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Vargas de Oliveira E.C., Carneiro Z.A., de Albuquerque S., Marchetti J.M. Development and evaluation of a nanoemulsion containing ursolic acid: a promising trypanocidal agent. AAPS PharmSciTech. 2017;18:2551–2560. doi: 10.1208/s12249-017-0736-y. [DOI] [PubMed] [Google Scholar]
- 64.Quezada C.Q., Azevedo C.S., Charneau S., Santana J.M., Chorilli M., Carneiro M.B., Bastos I.M.D. Advances in nanocarriers as drug delivery systems in Chagas disease. Int. J. Nanomed. 2019;14:6407–6424. doi: 10.2147/IJN.S206109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghaffari Moghaddam M., Ahmad F. Bin H., Samzadeh-Kermani A. Biological activity of betulinic acid: a review. Pharmacol. Pharm. 2012;3:119–123. [Google Scholar]
- 66.Sousa P.L., Souza R.O. da S., Tessarolo L.D., de Menezes R.R.P.P.B., Sampaio T.L., Canuto J.A., Martins A.M.C. Betulinic acid induces cell death by necrosis in Trypanosoma cruzi. Acta Trop. 2017;174:72–75. doi: 10.1016/j.actatropica.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Meira C.S., Barbosa-Filho J.M., Lanfredi-Rangel A., Guimarães E.T., Moreira D.R.M., Soares M.B.P. Antiparasitic evaluation of betulinic acid derivatives reveals effective and selective anti-Trypanosoma cruzi inhibitors. Exp. Parasitol. 2016;166:108–115. doi: 10.1016/j.exppara.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 68.Lin J.H., Chen S.Y., Lu C.C., Lin J.A., Yen G.C. Ursolic acid promotes apoptosis, autophagy, and chemosensitivity in gemcitabine-resistant human pancreatic cancer cells. Phyther. Res. 2020;34:2053–2066. doi: 10.1002/ptr.6669. [DOI] [PubMed] [Google Scholar]
- 69.Tzelepis F., De Alencar B.C.G., Penido M.L.O., Gazzinelli R.T., Persechini P.M., Rodrigues M.M. Distinct kinetics of effector CD8+ cytotoxic T cells after infection with Trypanosoma cruzi in naïve or vaccinated mice. Infect. Immun. 2006;74:2477–2481. doi: 10.1128/IAI.74.4.2477-2481.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar S., Tarleton R.L. The relative contribution of antibody production and CD8+ T cell function to immune control of Trypanosoma cruzi. Parasite Immunol. 1998;20:207–216. doi: 10.1046/j.1365-3024.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- 71.Cardoni R.L., Rottenberg M.E., Segura E.L. Increased production of reactive oxygen species by cells from mice acutely infected with Trypanosoma cruzi. Cell. Immunol. 1990;128:11–21. doi: 10.1016/0008-8749(90)90002-9. [DOI] [PubMed] [Google Scholar]
- 72.Dotiwala F., Mulik S., Polidoro R.B., Ansara J.A., Walch M., Gazzinelli R.T., Lieberman J. Killer lymphocytes use granulysin, perforin and granzymes to kill intracellular parasites. Nat. Med. 2016;22:210–216. doi: 10.1038/nm.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lasso P., Mateus J., Pavía P., Rosas F., Roa N., Thomas M.C., López M.C., González J.M., Puerta C.J., Cuéllar A. Inhibitory receptor expression on CD8 + T cells is linked to functional responses against Trypanosoma cruzi antigens in chronic chagasic patients. J. Immunol. 2015;195:3748–3758. doi: 10.4049/jimmunol.1500459. [DOI] [PubMed] [Google Scholar]
- 74.Darrah P.A., Patel D.T., De Luca P.M., Lindsay R.W.B., Davey D.F., Flynn B.J., Hoff S.T., Andersen P., Reed S.G., Morris S.L., Roederer M., Seder R.A. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 75.Betts M.R., Nason M.C., West S.M., De Rosa S.C., Migueles S.A., Abraham J., Lederman M.M., Benito J.M., Goepfert P.A., Connors M., Roederer M., Koup R.A. Immunobiology HIV nonprogressors preferentially maintain highly functional. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yi J.E., Obminska-Mrukowicz B., Yuan L.Y., Yuan H. Immunomodulatory effects of betulinic acid from the bark of white birch on mice. J. Vet. Sci. 2010;11:305–313. doi: 10.4142/jvs.2010.11.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dash S.K., Chattopadhyay S., Tripathy S., Dash S.S., Das B., Mandal D., Mahapatra S.K., Bag B.G., Roy S. Self-assembled betulinic acid augments immunomodulatory activity associates with IgG response. Biomed. Pharmacother. 2015;75:205–217. doi: 10.1016/j.biopha.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H., Zhang M., Tao Y., Wang G., Xia B. Madecassic acid inhibits the mouse colon cancer growth by inducing apoptosis and immunomodulation. JBUON. 2014;19:372–376. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.