Abstract
BACKGROUND:
Anxiety and depression symptoms are common among cannabis users and could be a risk factor for cannabis use (CU) disorder. Thus, it is critical to understand the neuronal circuits underlying the associations between CU and these symptoms. Alterations in resting-state functional connectivity within and/or between the default mode network and salience network have been reported in CU, anxiety, and depressive disorders and thus could be a mechanism underlying the associations between CU disorder and anxiety/depression symptoms.
METHODS:
Using resting-state functional magnetic resonance imaging, effective connectivities (ECs) among 9 major nodes from the default mode network and salience network were measured using dynamic causal modeling in 2 datasets: the Human Connectome Project (28 CU participants and 28 matched non–drug-using control participants) and a local CU study (21 CU participants and 21 matched non–drug-using control participants) in separate and parallel analyses.
RESULTS:
Relative to the control participants, right amygdala to left amygdala, anterior cingulate cortex to left amygdala, and medial prefrontal cortex to right insula ECs were greater, and left insula to left amygdala EC was smaller in the CU group. Each of these ECs showed a reliable linear relationship with at least one of the anxiety/ depression measures. Most findings on the right amygdala to left amygdala EC were common to both datasets.
CONCLUSIONS:
Right amygdala to left amygdala and anterior cingulate cortex to left amygdala ECs may be related to the close associations between CU and anxiety/depression symptoms. The findings on the medial prefrontal cortex to right insula and left insula to left amygdala ECs may reflect a compensatory mechanism.
Cannabis use (CU), anxiety, and depression often co-occur (1–5). A meta-analysis (2) found increased occurrence of CU among individuals with anxiety disorders even after controlling for other drug use and psychopathology. Another meta-analysis (3) suggested that heavy CU may be associated with increased risk for developing depressive disorders. A recent review (4) concluded that elevated anxiety and anxiety disorders are common in CU and CU disorder. Brain networks during resting state are usually identified using functional connectivity (FC) analysis (6), which reflects the correlation of the functional magnetic resonance imaging (fMRI) blood oxygenation level–dependent time series among brain regions (7). The most commonly studied brain networks include the default mode (DMN), salience (SAN), central executive, striatum, dorsal attention, sensorimotor, visual, and auditory networks (8,9). DMN (13–21), the SAN (11,12,15,16,18,21–23), the central executive network (13,15,19,20,22), and the striatum network (11,22). Alterations in DMN and SAN are the most common findings among these studies. Individuals with CU (14–16,18) or individuals with CU disorder (22,23) had greater FC in DMN (14–16), SAN (15,16,23), or between DMN and SAN (18,22) compared with control subjects. Reviews and meta-analyses on rsfMRI of anxiety disorders (24) and major depressive disorder (25–27) suggest that generalized anxiety and social anxiety disorders are associated with smaller FC in DMN and greater FC in SAN and that major depressive disorder is associated with greater FC in both DMN and SAN.
Several rsfMRI studies investigated anxiety/depression symptoms in CU. Relative to control subjects, individuals with CU had greater FC between left rostral anterior cingulate cortex (ACC) and several other DMN/SAN regions, including right rostral ACC, amygdala, and insula (18). Among individuals with CU, greater FC between bilateral rostral ACC was associated with greater depression symptoms (18). Individuals with
Several studies used resting-state fMRI (rsfMRI) to investigate CU (10–18) and CU disorder (19–23). These studies suggested that CU may be associated with altered FC of the heavy CU had greater FC in the DMN and SAN (i.e., insula) and greater functional anticorrelation between DMN and SAN (16). Also, individuals with CU had a stronger negative association between insula FC and anxiety than control subjects (16). Individuals with CU showed greater FC than control subjects in subcortical SAN regions (28), such as ventral striatum (23). These group differences were most pronounced in individuals with CU who reported greater negative emotionality (23). Although other networks may be involved (17), the majority of these studies suggest that enhanced FC in and/or between DMN and SAN may be related to the association between CU and anxiety/depression symptoms.
Based on these previous studies and motivated by the potential of therapeutically targeting neurocircuit abnormalities that underlie anxiety/depression symptoms and CU disorders (4,5), this study investigated putative neuronal circuits possibly associated with the relationship between CU and anxiety/ depression symptoms. We used rsfMRI-based dynamic causal modeling (DCM) (29) to measure effective (directional) connectivity (EC), such that directional relationships among brain regions can be elucidated. Based on the studies reviewed above, we focused on the DMN and SAN. We also included the amygdala network, consistent with findings of amygdala alterations in anxiety disorders (30), depression (31), and CU (32). Modulated by endocannabinoid signaling, the amygdala may be involved in the regulation of stress, anxiety, and depression (33). We hypothesized that 1) the individuals with CU would show greater strength of ECs within SAN and/or between DMN and SAN regions compared with control subjects, and 2) the strength of these ECs would be associated with greater anxiety/depression symptoms. To test these hypotheses, we investigated 2 separate datasets for independent but parallel analyses in light of the need for reproducibility in rsfMRI research. To identify the EC findings common to both datasets, we also conducted secondary analyses based on the combined datasets.
METHODS AND MATERIALS
Dataset 1
Participants.
The data were from the Human Connectome Project (HCP) 1200 Subjects Data Release (34). Written informed consent was obtained from all participants. Use of HCP data was approved by the Virginia Commonwealth University Institutional Review Board. Dataset 1 included 28 CU participants and 28 matched non–drug-using control participants (control group) (see the Supplement for details about inclusion/exclusion criteria and group matching).
Cannabis Use and Dependence.
The HCP used the self-reported substance use and abuse measures from the Semi-Structured Assessment for the Genetics of Alcoholism (35) to quantify lifetime CU and classify cannabis dependence according to DSM-IV (Table 1). All CU participants met lifetime cannabis dependence benchmarks, but this was not based on clinical interview.
Table 1.
Demographic Information, Cannabis Use and Dependence, Measures of Anxiety and Depression, and Measures of Use of Other Illicit Drugs, Alcohol, and Tobacco and Head Motion for Participants in Dataset 1
| Parameter | CU (n = 28) | Control (n = 28) | Statistical Results | |
|---|---|---|---|---|
| t 54 | p | |||
| Demographic Information | ||||
| Age, Years, Mean ± SD (Range) | 28.4 ± 3.6 (22 to 35) | 27.8 ± 3.5 (22 to 33) | 0.63 | .53 |
| Sex, Female/Male, n | 9/19 | 7/21 | N/A | .77 |
| Race, White/AA/Other, n | 20/4/4 | 15/7/6 | N/A | .27 |
| Education, Years, Mean ± SD (Range) | 14.2 ± 2.1 (11 to 17) | 14.4 ± 1.8 (11 to 17) | 0.38 | .70 |
| Cannabis Use and Dependence | ||||
| Met Lifetime Cannabis Dependence, Yes/No, n | 28/0 | 0/28 | N/A | 0 |
| Ever Used Marijuana, Yes/No, n | 28/0 | 13/15 | N/A | <.0001 |
| Age at First Marijuana Use, Years, n | N/A | .0052 | ||
| ≤14 | 10 | 1 | ||
| 15–17 | 10 | 4 | ||
| 18–20 | 6 | 5 | ||
| ≥21 | 2 | 3 | ||
| N/A | 15 | |||
| Times Used Marijuana, n | N/A | <.0001 | ||
| 0 | 0 | 15 | ||
| 1–10 | 0 | 7 | ||
| 11–100 | 3 | 2 | ||
| 101–999 | 6 | 4 | ||
| ≥1000 | 19 | |||
| Measures of Anxiety and Depression | ||||
| ASR DSM Anxiety Problems Raw Score (Range: 0–14), Mean ± SD (Range) | 3.8 ± 3.5 (0 to 13) | 3.9 ± 2.3 (0 to 9) | 0.126 | .900 |
| ASR DSM Depressive Problems Raw Score (Range: 0–36), Mean ± SD (Range) | 5.1 ± 4.7 (0 to 22) | 4.4 ± 3.2 (0 to 12) | 0.651 | .518 |
| Measures of Use of Other Illicit Drugs, Alcohol, and Tobacco | ||||
| Averaged Times Used Illicit Drugs, <6/≥6, n | 23/5 | 27/1 | N/A | .193 |
| Averaged z Scores for the SSAGA Measures of Alcohol Use, Mean ± SD (Range) | −0.02 ± 0.45 (−1.06 to 0.84) | 0.02 ± 0.44 (−0.71 to 0.99) | 0.336 | .738 |
| Total Times Used/Smoked Any Tobacco in Past 7 Days, Mean ± SD (Range) | 19.1 ± 26.1 (0 to 75) | 16.7 ± 24.5 (0 to 70) | 0.355 | .724 |
| Head Motion | ||||
| Framewise Displacement, mm, Mean ± SD (Range) | 0.153 ± 0.057 (0.081 to 0.289) | 0.154 ± 0.050 (0.061 to 0.275) | 0.070 | .945 |
Fisher exact tests were used to test the group difference in the portion of participants age at first marijuana use <14 years old and times used marijuana >100. All p values are 2-tailed.
AA, African American; ASR, Adult Self-Report; CU, cannabis use; N/A, not applicable; SSAGA, Semi-Structured Assessment for the Genetics of Alcoholism.
Anxiety and Depression Scores.
Anxiety and depression raw scores were based on the Achenbach Adult Self-Report (36). A total of 123 items from Section VIII were administered (37), and items associated with anxiety/depression symptoms generated the anxiety/depression raw scores.
Alcohol and Tobacco Use.
Tobacco and alcohol use were quantified using the methods described in our other work (38).
fMRI Data Acquisition.
Whole-brain gradient-echo, echo-planar fMRI data were acquired with a 32-channel head coil on a modified 3T MAGNETOM Skyra MRI scanner (Siemens Healthcare AG, Erlangen, Germany) (repetition time = 720 ms, echo time = 33.1 ms, flip angle = 52°, bandwidth = 2290 Hz/pixel, in-plane field of view = 208 × 180 mm, 72 slices, 2.0-mm isotropic voxels, multiband acceleration factor of 8) (39). During rsfMRI, participants were instructed to relax and look at a fixation cross, without thinking of anything and without falling asleep. In each session, 2 rsfMRI runs were acquired. Each run had 1200 volumes (14.4 min). Preliminary DCM analysis using the entire 14.4-minute resting-state fMRI scan indicated that the processing time was very long. Thus, we used only the first half of the first run (7.2 min) such that the DCM analysis could be completed within a reasonable time frame (in the order of weeks). This time series (7.2 min) is also comparable to that of dataset 2 (6 min).
fMRI Data Preprocessing.
Per Smith et al. (40), fMRI data were minimally preprocessed to implement gradient distortion correction, rigid body realignment, field map processing, nonlinear normalization to Montreal Neurological Institute space, high-pass filtering with independent component analysis (ICA) denoising, and brain masking. The voxel size of the minimally processed data was 2 × 2 × 2 mm. Subsequent preprocessing steps, including segmentation of the structural images, motion scrubbing, smoothing, default aCompCor fMRI denoising (41,42), and fMRI bandpass filtering (0.008–0.10 Hz), were implemented using the CONN conn_batch_humanconnectomeproject.m script (specifically created for preprocessing HCP minimally processed rsfMRI data) (43). Mean framewise displacement (FD) (44) was used to quantify head motion. The FDs (Table 1) were small for both groups and did not differ between the two groups (t = 0.070, p = .945).
Dataset 2
Participants.
Written informed consent was obtained from all participants. Dataset 2 included 21 CU participants and 21 matched non–drug-using control participants (control group) (see the Supplement for details about inclusion/exclusion criteria and group matching).
Anxiety and Depression Scores.
We combined 1) DSM-5 Self-Rated Level 1 Cross-Cutting Symptom Measure—Adult (45) Anxiety and selected State-Trait Anxiety Inventory (46) items to create a summary anxiety score and 2) DSM-5 Self-Rated Level 1 Cross-Cutting Symptom Measure—Adult Depression and Beck Depression Inventory (47) scores to create a summary depression score. See the Supplement for details.
fMRI Data Acquisition.
A 3T Intera MRI scanner (Philips Healthcare, Best, the Netherlands) with a phased-array SENSE 32-channel receiver head coil was used. A T1-weighted magnetization prepared rapid acquisition gradient-echo structural image was acquired. For the rsfMRI scan, blood oxygenation level–dependent signal was measured with a T2* gradient-echo, echo-planar image sequence (repetition time = 2.0 s, echo time = 28 ms, 37 slices, slice thickness = 3 mm, no gap, field of view = 240 × 240 mm, in-plane resolution = 3 × 3 mm, flip angle = 76°, 180 volumes, 6 min). During rsfMRI, participants were instructed to relax, keep their eyes closed, and remain awake.
rsfMRI Data Preprocessing.
See the Supplement for our local rsfMRI preprocessing pipeline (including the use of ICA-AROMA (48) for removal of head motion–related artifacts). As in dataset 1, FD was used to quantify head motion. The FDs (Table 2) were small for both groups, and FD did not differ between the two groups (t40 = 1.655, p = .11).
Table 2.
Demographic Information, Cannabis Use and Dependence, Measure of Anxiety and Depression, and Measures of Use of Other Illicit Drugs, Alcohol, and Tobacco and Head Motion for Participants in Dataset 2
| Parameter | CU (n = 21) | Control (n = 21) | Statistical Results | |
|---|---|---|---|---|
| t 40 | p | |||
| Demographic Information | ||||
| Age, Years, Mean ± SD (Range) | 23.0 ± 1.4 (20.8 to 25.6) | 22.9 ± 1.9 (20.5 to 26.8) | 0.19 | .85 |
| Sex, Female/Male, n | 12/9 | 9/12 | N/A | .54 |
| Race, White, n | 21 | 21 | N/A | 1 |
| Education, Years, Mean ± SD (Range) | 17.8 ± 0.7 (17 to 19) | 17.6 ± 1.0 (16 to 19) | 0.75 | .46 |
| Cannabis Use and Dependence | ||||
| Lifetime CU, No. of Joints, Mean ± SD (Range) | 1552.5 ± 2315.2 (100 to 8750) | 48.7 ± 9.1 (32.8 to 67.1) | 2.98 | .005 |
| CU per Week, g, Mean ± SD (Range) | 2.37 ± 2.17(0.5 to 10) | N/A | N/A | N/A |
| CU per Week, Days, Mean ± SD (Range) | 4.24 ± 1.44 (2.5 to 7) | N/A | N/A | N/A |
| Age at Onset of CU, Years, Mean ± SD (Range) | 15.4 ± 2.1 (12 to 21) | N/A | N/A | N/A |
| Age at Onset of Heavy CU, Years, Mean ± SD (Range) | 18.3 ± 2.1 (15 to 21.5) | N/A | N/A | N/A |
| Stop Attempts, Mean ± SD (Range) | 0.81 ± 1.25 (0 to 4) | N/A | N/A | N/A |
| Abstinence, Days, Mean ± SD (Range) | 1.20 ± 1.11 (0 to 5) | N/A | N/A | N/A |
| Measure of Anxiety and Depression | ||||
| Anxiety, Mean ± SD (Range) | 22.1 ± 5.7 (13 to 35) | 20.1 ± 4.2 (14 to 26) | 1.295 | .203 |
| Depression, Mean ± SD (Range) | 7.4 ± 4.8 (1 to 17) | 5.7 ± 4.3 (0 to 15) | 1.208 | .234 |
| Measures of Use of Other Illicit Drugs, Alcohol, and Tobacco | ||||
| Lifetime Illicit Drug Use, No. of Occasions, Mean ± SD (Range) | 23.2 ± 22.3 (0 to 80) | 42.0 ± 136.4 (0 to 624) | 0.623 | .537 |
| Alcohol Use and Related Problems (AUDIT), Mean ± SD (Range) | 7.7 ± 4.6 (1 to 20) | 5.8 ± 3.6 (1 to 13) | 1.491 | .144 |
| Cigarette Smoking, % | 52 | 38 | .354 | |
| Head Motion | ||||
| Framewise Displacement, mm, Mean ± SD (Range) | 0.127 ± 0.056 (0.064 to 0.275) | 0.158 ± 0.065 (0.079 to 0.303) | 1.655 | .11 |
AUDIT, Alcohol Use Disorders Identification Test; CU, cannabis use; N/A, Not applicable.
Methods Common for Both Datasets
Group ICA.
We used ICA (see Supplement) to verify and define the hypothesized canonical networks. For each dataset, the group ICA was conducted across all the CU and control participants combined from that dataset. The results of ICA (Figure 1) thus constrained the subsequent selection and localization of network nodes for EC analysis.
Figure 1.
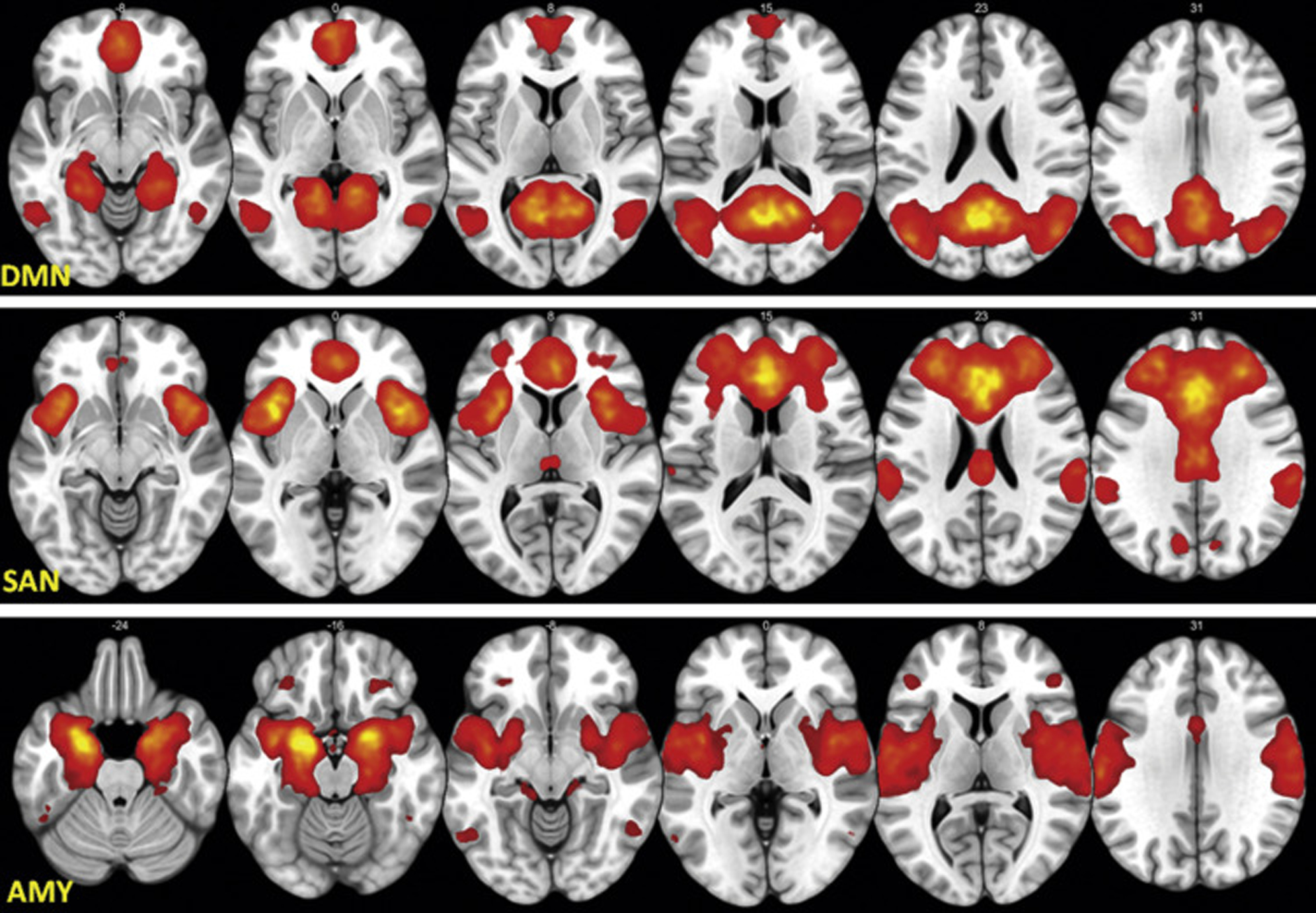
The group average z map from dataset 1+2 for default mode network (DMN) (top panel), salience network (SAN) (middle panel), and amygdala (AMY) (bottom panel) resting-state networks found by independent component analysis and used to constrain and refine the dynamic causal modeling nodes. The left side in the figure represents the left hemisphere of the brain, and the right side represents the right hemisphere of the brain.
Standard Scores for Anxiety and Depression.
In each dataset, both anxiety and depression scores were converted to z scores. We created an additional single dependent variable (composite anxiety/depression z score) from the mean of anxiety z score and depression z score for each participant, representing the average degree of anxiety and depression (49–51).
Spectral DCM.
Spectral DCM (29), as implemented in SPM12 revision 7487 (http://www.fil.ion.ucl.ac.uk/spm/), was used to measure EC. See the Supplement for further description of DCM.
A Priori Selected DCM Nodes.
The DCM nodes (regions of interest) were determined in 2 steps. In step 1, we determined which anatomical brain regions should be considered as possible candidate DCM regions based on the aggregate literature of brain functional abnormalities in phenotypes related to CU, anxiety, and depression (30–32). In step 2, we further determined whether a candidate region should be selected as a final region based on the actual FC results (i.e., if the putative region empirically showed up as part of the FC network specific to the participants) and, if so, where the DCM node (a sphere) should be placed within the final region (the center of the sphere was located at the maximum significance of the FC within the final region). The putative regions found in the validated networks were 4 DMN regions, i.e., medial prefrontal cortex (mPFC), posterior cingulate cortex, left lateral parietal, and right lateral parietal; 3 SAN regions, i.e., ACC, left insula, and right insula; and 2 amygdala regions, i.e., left amygdala and right amygdala (see Supplement for atlas details). Each of the 9 putative a priori regions were found empirically to be within each of the 3 group-averaged brain networks (i.e., from dataset 1, from dataset 2, and the mean from combined dataset 1+2) (see Supplement), and therefore these regions were selected for further refinement in step 2. In step 2, each final DCM node was defined as an 8-mm-radius sphere centered at the local maximum z value within each selected brain region in the mean network from dataset 1+2 Figure (2) in Montreal Neurological Institute space. The same nodes were used for each participant.
Figure 2.
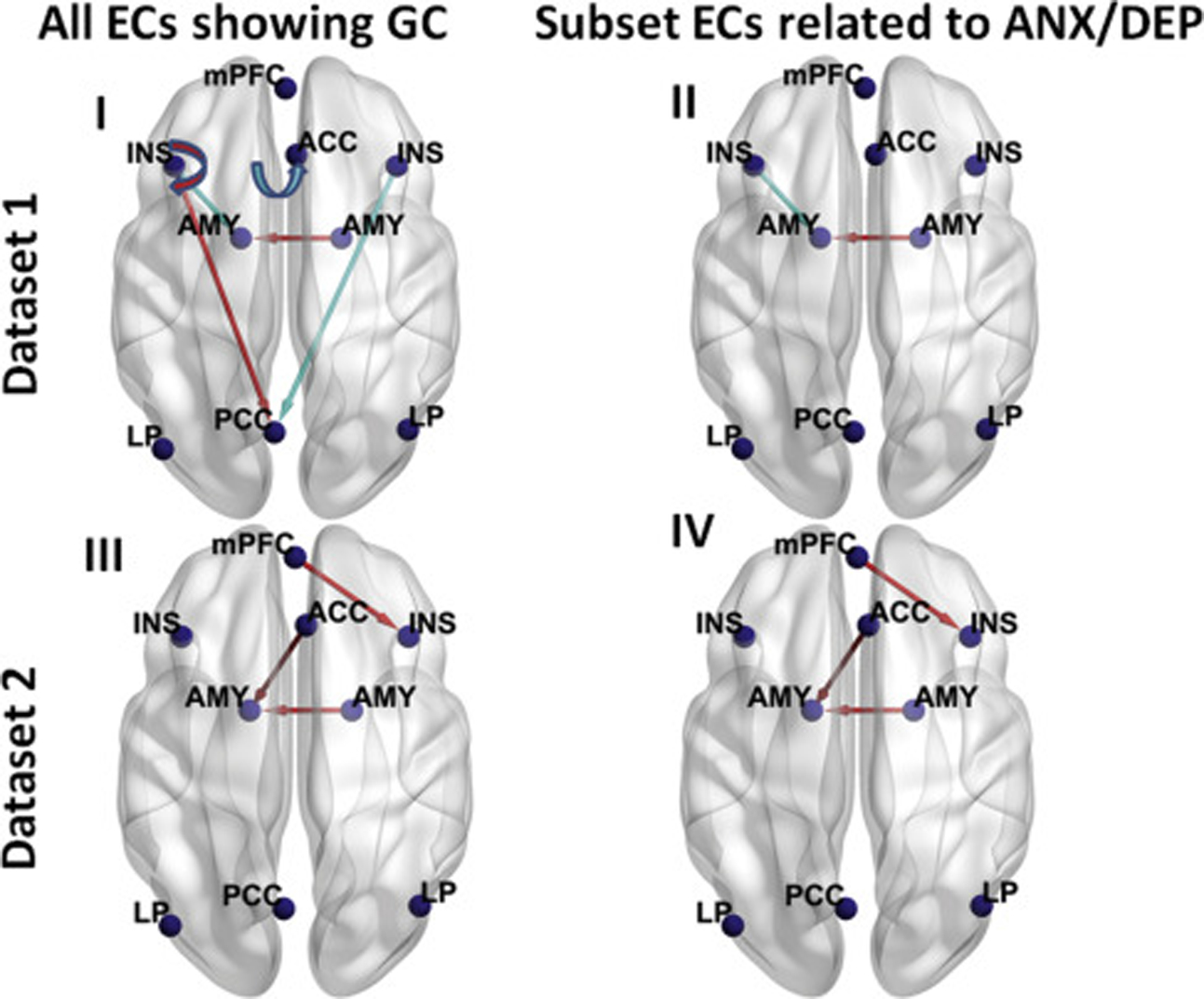
Lines with arrows representing the group difference (GC) (cannabis use [CU] minus control) in effective connectivities (ECs) in dataset 1 (n = 28 for CU group and n = 28 for control group) (top panels) and dataset 2 (n = 21 for CU group and n = 21 for control group) (bottom panels), visualized with the BrainNet Viewer (http://www.nitrc.org/projects/bnv/) (72). For each dataset, all ECs showing group differences are shown in the left panels, and subset ECs showing group differences that are linearly related to at least 1 of the 3 negative affect z scores (anxiety [ANX], depression [DEP], and composite ANX/DEP z scores) are shown in the right panels. A semicircular line with arrow denotes self-connection. A red line denotes that this EC was greater in the CU group than the control group, and a light blue line denotes that this EC was smaller in the CU group than the control group. The Montreal Neurological Institute coordinates (mm) of the 9 dynamic causal modeling nodes are as follows: medial prefrontal cortex (mPFC) (−2, 54, −4), posterior cingulate cortex (PCC) (−4, −64, 22), left lateral parietal (LP) (−43, −75, 26), right LP (47, −69, 26), anterior cingulate cortex (ACC) (4, 30, 28), left insula (INS) (−36, 22, 2), right insula (INS) (36, 20, 6), left amygdala (AMY) (−23, −2, −20), and right AMY (20, 0, −20). The left side in the figure represents the left hemisphere of the brain, and the right side represents the right hemisphere of the brain.
DCM Parametric Empirical Bayes Analysis
For each participant in each dataset, a fully connected DCM (each node connects to itself and all other nodes) was specified and estimated. The parametric empirical Bayes (PEB) approach (52) was used to conduct group-level analyses for the ECs. In PEB, group-level analyses are conducted using Bayesian posterior inference (53), which does not need to contend with the multiple-comparison problem because of the lack of false positives (53). Bayesian posterior probability (Bayesian-PP) is used as an indicator of the confidence in whether the mean of an EC within a group is different from zero (or the mean of another group) or the confidence in the degree of linear relationship between variables. The Bayesian-PP (0 ≤ Bayesian-PP ≤ 1) is the conditional probability that is computed by PEB using Bayes rule after the available information (the likelihood function and the prior probability density of the model parameters) is taken into account. The higher the Bayesian-PP, the greater the confidence (see Supplement for computational details). Here, an EC finding was considered reliable if Bayesian-PP was >0.95.
For each dataset separately, 3 kinds of PEB analyses were conducted: 1) testing the group difference in each EC between the CU and control groups; 2) testing the linear relationship between each EC and the anxiety/depression score across both CU and control participants using linear regression; and 3) testing the linear relationship between each EC and the CU parameters in the CU participants (for dataset 2 only, owing to availability). See the Supplement for further description of linear regression within PEB. To harmonize the EC results from the two datasets, we conducted several secondary DCM analyses (see Supplement for methods and results).
RESULTS
Nonimaging Results
Tables 1 and 2 summarize the demographics, CU, anxiety and depression scores, and usage of other illicit drugs, alcohol, and tobacco. For either dataset, there was no significant difference between for CU and control groups in these measures except for CU. See the Supplement for the correlation between the anxiety and depression scores.
DCM Results From Dataset 1
Group Comparison.
The group difference (CU minus control), together with average EC across both CU and control groups, for each EC and the corresponding Bayesian-PP are shown in Table S5. Among the 81 ECs, 6 ECs (including the right amygdala to left amygdala EC) showed reliable (Bayesian-PP = 1) group differences (Figure 2 [I]), which were preserved after alcohol and tobacco use were included as covariates (Table S5). These group differences were also preserved after each of the negative affect z scores was included as a covariate (Table S5).
Linear Relationships Between EC and Each of the 3 Negative Affect z Scores for CU and Control Participants Combined.
For each of the 3 analyses, the linear regression coefficient (β) for each EC and corresponding Bayesian-PP are shown in Table S6. The 9 ECs showing a reliable (Bayesian-PP = 1) linear relationship with at least 1 of the 3 negative affect z scores are depicted in Figure 3 (I). Two of these 9 ECs also showed reliable group difference (Figure 2 [II]). For 7 of these 9 ECs, the reliable linear relationships were preserved after the group was included as a covariate (Table S6). The linear relationship between the left insula to left amygdala EC and the anxiety z score and the linear relationship between the right amygdala to left amygdala EC and the composite anxiety/depression z score disappeared after the group was included as a covariate.
Figure 3.
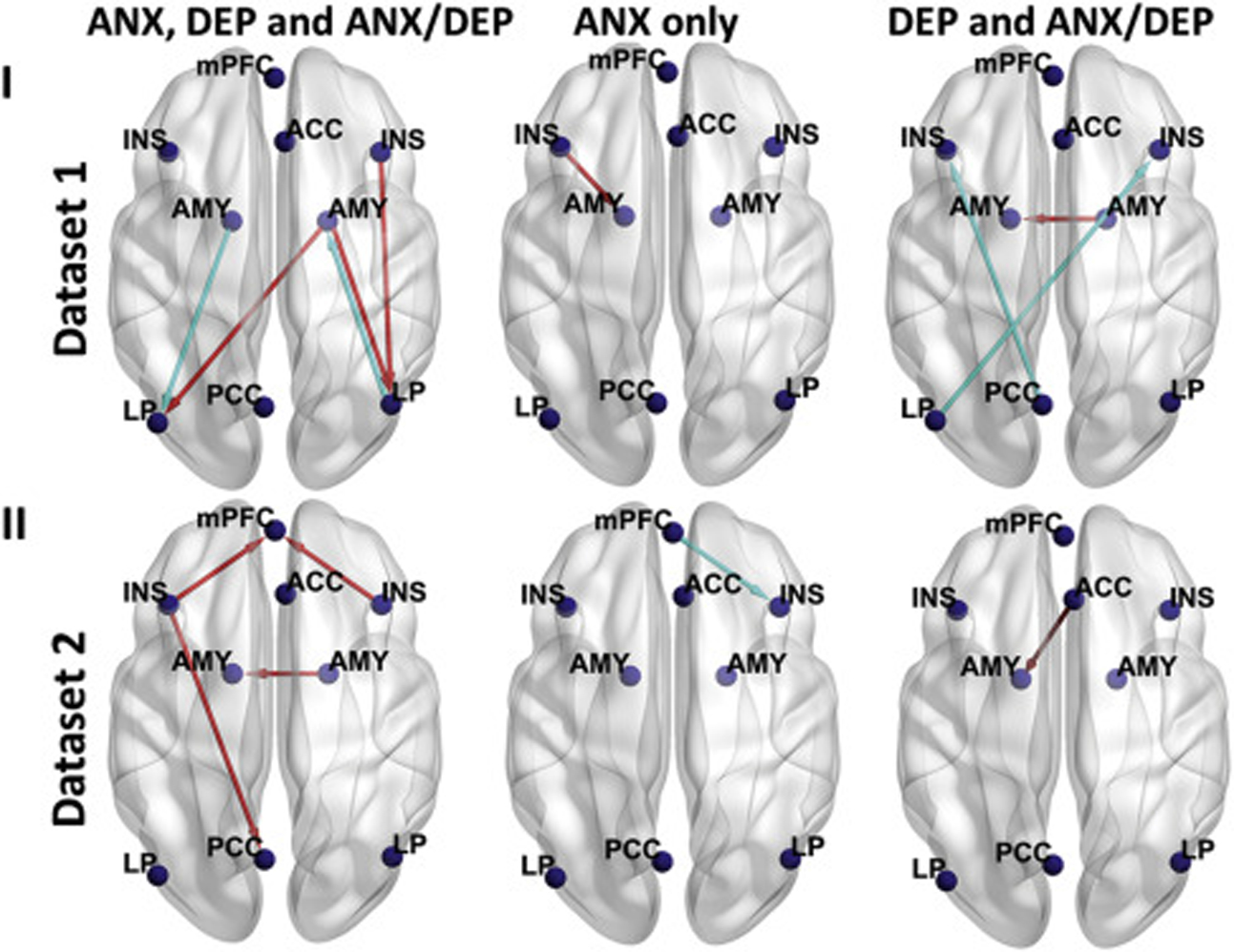
Lines with arrows representing effective connectivities (ECs) that showed linear relationships with at least 1 of the 3 negative affect z scores (anxiety [ANX], depression [DEP], and composite ANX/DEP z scores) across all participants (in each dataset, both cannabis use [CU] and control participants) in dataset 1 (n = 56) (top panels), dataset 2 (n = 42) (bottom panels). For each dataset, ECs showing a linear relationship with all 3 negative affect z scores are shown in the left panel, EC showing a linear relationship with ANX z score only is shown in the middle panel, and ECs showing a linear relationship with both DEP and composite ANX/DEP z scores are shown in the right panel. For both datasets, none of the ECs showed a linear relationship with any of the negative affect z scores other than shown in the figure. A red line denotes that this EC had a positive linear relationship with at least 1 of the 3 negative affect z scores, and a light blue line denotes that this EC had a negative linear relationship with at least 1 of the 3 negative affect z scores. The left side in the figure represents the left hemisphere of the brain, and the right side represents the right hemisphere of the brain. ACC, anterior cingulate cortex; AMY, amygdala; INS, insula; LP, lateral parietal; mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex.
DCM Results From Dataset 2
Group Comparison.
The group difference (CU minus control), together with the average EC across both CU and control groups, in each EC and corresponding Bayesian-PP are shown in Table S7. Among the 81 ECs, 3 ECs (including the right amygdala to left amygdala EC) showed a reliable (Bayesian-PP = 1) group difference (Figure 2 [III]), which were preserved after alcohol and tobacco use were included as covariates (Table S7). The group differences on mPFC to right insula and ACC to left amygdala ECs disappeared after the anxiety z score was used as a covariate; the group difference on right amygdala to left amygdala EC disappeared after the anxiety z score or the composite anxiety/depression z score was used as a covariate.
Linear Relationships Between EC and Each of the 3 Negative Affect z Scores for CU and Control Participants Combined.
The results of these 3 linear regression analyses are shown in Table S8. Six ECs showing a reliable (Bayesian-PP = 1) linear relationship with at least 1 of the 3 negative affect z scores are depicted in Figure 3 (II). Three of these 6 ECs also showed a reliable group difference [Figure 2 (IV)]. The linear relationship between the ACC to left amygdala EC and the composite anxiety/depression z score disappeared after the group was included as a covariate (Table S8).
Linear Relationship Between EC and CU Measures in CU Participants.
The results of this linear regression analysis are shown in Table S9 and Figure S4. The right amygdala to left amygdala EC, which also demonstrated reliable group differences, showed a reliable (Bayesian-PP = 1) negative regression on the days of CU per week.
Major DCM Results
Four ECs (i.e., right amygdala to left amygdala, left insula to left amygdala, ACC to left amygdala, and mPFC to right insula ECs) showed a reliable group difference (Figure 2) and reliable linear relationship with at least one of the negative affect measures (Figure 3). The major results on these ECs are summarized in Table 3.
Table 3.
Summary of ECs Showing Both Group Differences, Linear Relationship With at Least 1 of the 3 Negative Affect z Scores in Dataset 1, Dataset 2, and Dataset 1+2, and Linear Relationship With at Least 1 of the CU Measures in Dataset 2
| EC | Results on EC | Found in |
|---|---|---|
| Right AMY → Left AMY | CU greater than control Across all participants (both CU and control participants) in each dataset, greater EC was associated with greater DEP z score and greater composite ANX/DEP z score |
Dataset 1, dataset 2 |
| Across all participants (both CU and control participants) in dataset 2, greater EC was associated with greater ANX z score | Dataset 2 | |
| Left INS → Left AMY | CU smaller than control Across all participants (both CU and control participants) in dataset 1, smaller EC was associated with smaller ANX z score |
Dataset 1 |
| ACC → Left AMY | CU greater than control Across all participants (both CU and control participants) in dataset 2, greater EC was also associated with greater DEP z score and greater composite ANX/DEP z score |
Dataset 2 |
| mPFC → Right INS | CU greater than control Across all participants (both CU and control participants) in dataset 2, greater EC was also associated with smaller ANX z score |
Dataset 2 |
Dataset 1: n = 28 for CU group, n = 28 for control group, and n = 56 for both groups combined; dataset 2: n = 21 for CU group, n = 21 for control group, and n = 42 for both groups combined; dataset 1+2: n = 49 for CU group, n = 49 for control group, and n = 98 for both groups combined.
ACC, anterior cingulate cortex; AMY, amygdala; ANX, anxiety; CU, cannabis use; DEP, depression; EC, effective connectivity; INS, insula; mPFC, medial prefrontal cortex.
DISCUSSION
Our analyses in 2 different datasets identified 4 ECs showing both reliable group differences (right amygdala to left amygdala, ACC to left amygdala, mPFC to right insula ECs were greater in the CU group; left insula to left amygdala EC was smaller in the CU group) and a reliable linear relationship with one or more anxiety/depression measures. Most of the main results are consistent with the results of the secondary analyses on the combined dataset 1+2 (see Supplement).
Right Amygdala to Left Amygdala EC
In both datasets, right amygdala to left amygdala EC was greater in the CU group than the control group and was reliably and positively associated with both the depression and the composite anxiety/depression z scores. Specific for dataset 2, this EC was also reliably and positively associated with the anxiety z score. We are not aware of published studies reporting FC/EC between the bilateral amygdalae in CU- or anxiety/depression–related disorders. However, a meta-analysis (54) in humans and primates showed robust FC between bilateral amygdalae. Just as bilateral amygdalae show similar connectivity during resting state (55), enhanced right amygdala to left amygdala EC could be related to consolidation of emotional memory (56,57). More research is needed to determine the relevance to the present study.
ACC to Left Amygdala EC (Specific to Dataset 2)
ACC to left amygdala EC was greater in the CU group than the control group. Cannabis-related enhanced ACC to amygdala FC has been reported during the resting state (18). Across both CU and control participants, greater EC was associated with greater depression z score and greater composite anxiety/ depression z score. Consistently, anxious individuals showed enhanced ACC to amygdala FC during processing of fearful faces, and this FC correlated positively with self-reported anxious symptoms (58). As part of an aversive amplification circuit (58), the ACC and amygdala are thought to be necessary for recognition and expression of social fear (59,60). ACC and insula are regions associated with the amygdala for control of autonomic behavior (61). Greater change in ACC to amygdala FC is associated with greater autonomic measure of fear conditioning (62). Thus, the enhanced ACC to left amygdala EC may be related to recognizing social fear or autonomic response.
mPFC to Right Insula EC (Specific to Dataset 2)
mPFC to right insula EC was greater in the CU group than the control group. Across both CU and control participants, greater EC was associated with smaller anxiety z score. While the enhancement in the two ECs discussed above is associated with increased anxiety/depression symptoms in CU, the two groups did not differ in the anxiety/depression measures. These results support a compensatory mechanism in CU that has been discussed in the CU literature (38,63–65). Different from right amygdala to left amygdala and ACC to left amygdala ECs, mPFC to right insula EC is associated with reduced anxiety in the CU group and thus is possibly related to a compensatory mechanism in CU. Consistently, the mPFC-insula circuit has been suggested as a target for interventions (66) that may reduce anxiety and depression symptoms (67).
Left Insula to Left Amygdala EC (Specific to Dataset 1)
Left insula to left amygdala EC was smaller in the CU group than the control group. Across both CU and control participants, smaller EC was associated with smaller anxiety z score, suggesting that this may be another EC reflecting a compensatory mechanism in CU. The linear regression results are consistent with a study (68) showing that the resting-state FC and structural connectivity between left insula and left amygdala were positively correlated with anxiety. The FC between the left insula to left amygdala self-connection EC has been suggested to be related to the adjustment of autonomic behavioral responding (68).
For dataset 1, the group differences on ECs were preserved after each of the 3 anxiety/depression z scores was included as a covariate, suggesting that the group differences found in dataset 1 were mainly driven by CU. Differently in dataset 2, the group differences on ECs were preserved after the depression (or composite anxiety/depression) z score was included as a covariate but disappeared after the anxiety z score was included as a covariate. These results suggest that the group difference in dataset 2 was mainly driven by anxiety. Thus, the driving factors were different: CU for dataset 1 (in which the CU participants had relatively longer CU history) and anxiety for dataset 2 (in which the CU participants had relatively shorter CU history). This supports our suggestion (69) that the effects of stress and CU on this particular EC are similar, resulting in greater connectivity with the amygdala.
We have shown that the finding about right amygdala to left amygdala EC was reproducible across 2 different sets of rsfMRI data, which were acquired from different scanners and different cohorts and were preprocessed using different pipelines. This finding suggests that these rsfMRI results can be replicated in individuals with CU. Neurocircuit findings related to anxiety/depression symptoms and CU could be considered as therapeutic targets (4,5). If the findings in this study are replicated in future studies, amygdala-targeted interventions, e.g., propranolol (a β-adrenergic receptor antagonist) (70), may reduce anxiety in cannabis users, which could in turn reduce CU, as cannabis is often used to cope with stress/anxiety (71).
Limitations
First, in dataset 1, although the Semi-Structured Assessment for the Genetics of Alcoholism demonstrates good reliability and validity and has been extensively used in studies of substance use disorders (https://cogastudy.org/ssaga-i-and-ssaga-ii-information), the CU participants were neither clinically referred/interviewed nor recruited by virtue of substance use disorder. Although recent cannabis dependence as inferred from Semi-Structured Assessment for the Genetics of Alcoholism responses were consistent with urine toxicology results, the CU of dataset 1 may nevertheless have been milder compared with a specifically recruited or clinically referred sample. Second, because the urine testing in both datasets was not quantitative, we were unable to examine whether there were dose-response relationships with the EC findings. However, for dataset 1, we have discussed elsewhere (38) that psychiatric symptoms due to withdrawal were unlikely to contribute to the group differences in EC. In dataset 2, the average cannabis abstinence duration was about 1 day, and the right amygdala to left amygdala EC was not related to the abstinence duration. These results suggest that withdrawal-related symptoms were also unlikely to affect the main findings in dataset 2. Third, historical information on other substance use disorders was not available in dataset 1, and thus previous abuse of other drugs might explain differences in EC. However, as discussed in our other work (38), previous use of other drugs likely did not affect the DCM findings significantly because of the exclusion of participants with positive urine screens for other drugs and the lack of group difference in the use of other drugs. Fourth, the DCM nodes used in this study were a priori selected from previously established brain networks and constrained by the actual sample-specific networks empirically found by the ICA analysis. Thus, it is possible that other neural connectivities, which may be also altered in individuals with CU or related to anxiety and depression symptoms, were not identified because the connecting regions were not included as DCM nodes. Fifth, across the datasets, the anxiety and depression scores were obtained using different methods. Sixth, although we matched the groups in alcohol usage in both datasets and excluded participants with breath alcohol concentration greater than 0.05 g/210 L in dataset 1, we cannot rule out confounding effects of alcohol use. In dataset 2, breath alcohol concentrations were not obtained. However, all participants were instructed to abstain from any alcohol 24 hours before the fMRI scan. Further, in dataset 2, anyone with a history of a psychiatric disorder except for anxiety and depression was excluded. Thus, this approach could reduce the generalizability of the findings in dataset 2. Nevertheless, right amygdala to left amygdala EC abnormalities in dataset 2 were also present in dataset 1 (in which this exclusion criterion was not applied). Finally, the two datasets were different in several aspects (e.g., scanner, preprocessing, and cohort). For two similar datasets, one might consider future studies generating a predictive model to classify cannabis users versus control subjects (using methods such as logistic regression) using EC features from one dataset and then use that model to classify participants (cannabis users vs. controls) in the other dataset.
Conclusions
These findings suggest that the enhanced right amygdala to left amygdala and ACC to left amygdala ECs found in the CU participants could be related to comorbidity between CU, anxiety, and depression symptoms. The findings on the mPFC to right insula and left insula to left amygdala ECs may reflect a compensatory mechanism in CU. It is unclear if the alterations on these ECs preexisted or were due to CU.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
We thank the anonymous reviewers for their constructive comments, which have resulted in significant improvement of this article.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsc.2020.09.015.
Contributor Information
Liangsuo Ma, Institute for Drug and Alcohol Studies, Virginia Commonwealth University, Richmond, Virginia; Department of Radiology, Virginia Commonwealth University, Richmond, Virginia.
John M. Hettema, Department of Psychiatry, Virginia Commonwealth University, Richmond, Virginia Department of Psychiatry, Texas A&M University Health Science Center, Bryan, Texas.
Janna Cousijn, Neuroscience of Addiction lab, Department of Psychology, University of Amsterdam, Amsterdam, Netherlands..
James M. Bjork, Institute for Drug and Alcohol Studies, Virginia Commonwealth University, Richmond, Virginia Department of Psychiatry, Virginia Commonwealth University, Richmond, Virginia.
Joel L. Steinberg, Institute for Drug and Alcohol Studies, Virginia Commonwealth University, Richmond, Virginia Department of Psychiatry, Virginia Commonwealth University, Richmond, Virginia.
Lori Keyser-Marcus, Institute for Drug and Alcohol Studies, Virginia Commonwealth University, Richmond, Virginia; Department of Psychiatry, Virginia Commonwealth University, Richmond, Virginia.
Kyle Woisard, Institute for Drug and Alcohol Studies, Virginia Commonwealth University, Richmond, Virginia.
QiQi Lu, Department of Statistical Sciences and Operations Research, Virginia Commonwealth University, Richmond, Virginia.
Roxann Roberson-Nay, Department of Psychiatry, Virginia Commonwealth University, Richmond, Virginia.
Antonio Abbate, Department of Internal Medicine, Virginia Commonwealth University, Richmond, Virginia.
F. Gerard Moeller, Institute for Drug and Alcohol Studies, Virginia Commonwealth University, Richmond, Virginia; Department of Psychiatry, Virginia Commonwealth University, Richmond, Virginia; Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, Virginia; Department of Neurology, Virginia Commonwealth University, Richmond, Virginia.
REFERENCES
- 1.van der Pol P, Liebregts N, de Graaf R, Ten Have M, Korf DJ, van den Brink W, et al. (2013): Mental health differences between frequent cannabis users with and without dependence and the general population. Addiction 108:1459–1469. [DOI] [PubMed] [Google Scholar]
- 2.Kedzior KK, Laeber LT (2014): A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population—a meta-analysis of 31 studies. BMC Psychiatry 14:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lev-Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, Rehm J (2014): The association between cannabis use and depression: A systematic review and meta-analysis of longitudinal studies. Psychol Med 44:797–810. [DOI] [PubMed] [Google Scholar]
- 4.Walukevich-Dienst K, Crapanzano KA, Lewis EM, Buckner JD (2019): Cannabis and anxiety: A biopsychosocial mode. Curr Addict Rep 6:456–465. [Google Scholar]
- 5.Baker AL, Hides L, Lubman DI (2010): Treatment of cannabis use among people with psychotic or depressive disorders: A systematic review. J Clin Psychiatry 71:247–254. [DOI] [PubMed] [Google Scholar]
- 6.Friston KJ (2011): Functional and effective connectivity: A review. Brain Connect 1:13–36. [DOI] [PubMed] [Google Scholar]
- 7.Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- 8.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. (2009): Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole DM, Smith SM, Beckmann CF (2010): Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behan B, Connolly CG, Datwani S, Doucet M, Ivanovic J, Morioka R, et al. (2014): Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology 84:131–137. [DOI] [PubMed] [Google Scholar]
- 11.Blanco-Hinojo L, Pujol J, Harrison BJ, Macia D, Batalla A, Nogue S, et al. (2017): Attenuated frontal and sensory inputs to the basal ganglia in cannabis users. Addict Biol 22:1036–1047. [DOI] [PubMed] [Google Scholar]
- 12.Buchy L, Cannon TD, Anticevic A, Lyngberg K, Cadenhead KS, Cornblatt BA, et al. (2015): Evaluating the impact of cannabis use on thalamic connectivity in youth at clinical high risk of psychosis. BMC Psychiatry 15:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng H, Skosnik PD, Pruce BJ, Brumbaugh MS, Vollmer JM, Fridberg DJ, et al. (2014): Resting state functional magnetic resonance imaging reveals distinct brain activity in heavy cannabis users—a multi-voxel pattern analysis. J Psychopharmacol 28:1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, et al. (2014): Long-term effects of marijuana use on the brain. Proc Natl Acad Sci U S A 111:16913–16918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Larson MP, Rogowska J, Yurgelun-Todd D (2015): Aberrant orbitofrontal connectivity in marijuana smoking adolescents. Dev Cogn Neurosci 16:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pujol J, Blanco-Hinojo L, Batalla A, Lopez-Sola M, Harrison BJ, Soriano-Mas C, et al. (2014): Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res 51:68–78. [DOI] [PubMed] [Google Scholar]
- 17.Subramaniam P, Rogowska J, DiMuzio J, Lopez-Larson M, McGlade E, Yurgelun-Todd D (2018): Orbitofrontal connectivity is associated with depression and anxiety in marijuana-using adolescents. J Affect Disord 239:234–241. [DOI] [PubMed] [Google Scholar]
- 18.Shollenbarger S, Thomas AM, Wade NE, Gruber SA, Tapert SF, Filbey FM, et al. (2019): Intrinsic frontolimbic connectivity and mood symptoms in young adult cannabis users. Front Public Health 7:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camchong J, Lim KO, Kumra S (2017): Adverse effects of cannabis on adolescent brain development: A longitudinal study. Cereb Cortex 27:1922–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thijssen S, Rashid B, Gopal S, Nyalakanti P, Calhoun VD, Kiehl KA (2017): Regular cannabis and alcohol use is associated with resting-state time course power spectra in incarcerated adolescents. Drug Alcohol Depend 178:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wetherill RR, Fang Z, Jagannathan K, Childress AR, Rao H, Franklin TR (2015): Cannabis, cigarettes, and their co-occurring use: Disentangling differences in default mode network functional connectivity. Drug Alcohol Depend 153:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F, Zimmermann K, Xin F, Scheele D, Dau W, Banger M, et al. (2018): Shifted balance of dorsal versus ventral striatal communication with frontal reward and regulatory regions in cannabis-dependent males. Hum Brain Mapp 39:5062–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manza P, Tomasi D, Volkow ND (2018): Subcortical local functional hyperconnectivity in cannabis dependence. Biol Psychiatry Cogn Neurosci Neuroimaging 3:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Northoff G (2020): Anxiety disorders and the brain’s resting state networks: From altered spatiotemporal synchronization to psychopathological symptoms. Adv Exp Med Biol 1191:71–90. [DOI] [PubMed] [Google Scholar]
- 25.Northoff G (2016): How do resting state changes in depression translate into psychopathological symptoms? From ‘spatiotemporal correspondence’ to ‘spatiotemporal psychopathology. Curr Opin Psychiatry 29:18–24. [DOI] [PubMed] [Google Scholar]
- 26.Dutta A, McKie S, Deakin JF (2014): Resting state networks in major depressive disorder. Psychiatry Res 224:139–151. [DOI] [PubMed] [Google Scholar]
- 27.Zhou M, Hu X, Lu L, Zhang L, Chen L, Gong Q, et al. (2017): Intrinsic cerebral activity at resting state in adults with major depressive disorder: A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 75:157–164. [DOI] [PubMed] [Google Scholar]
- 28.Menon V (2015): Salience network. In: Toga AW, editor. Brain Mapping: An Encyclopedic Reference, vol. 2. London: Academic Press, 597–611. [Google Scholar]
- 29.Friston KJ, Kahan J, Biswal B, Razi A (2014): A DCM for resting state fMRI. Neuroimage 94:396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bas-Hoogendam JM, Groenewold NA, Aghajani M, Freitag GF, Harrewijn A, Hilbert K, et al. (2020): ENIGMA-anxiety working group: Rationale for and organization of large-scale neuroimaging studies of anxiety disorders [published online ahead of print Jul 3]. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rakesh D, Allen NB, Whittle S (2020): Balancing act: Neural correlates of affect dysregulation in youth depression and substance use—a systematic review of functional neuroimaging studies. Dev Cogn Neurosci 42:100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volkow ND, Hampson AJ, Baler RD (2017): Don’t worry, be happy: Endocannabinoids and cannabis at the intersection of stress and reward. Annu Rev Pharmacol Toxicol 57:285–308. [DOI] [PubMed] [Google Scholar]
- 33.Ramikie TS, Nyilas R, Bluett RJ, Gamble-George JC, Hartley ND, Mackie K, et al. (2014): Multiple mechanistically distinct modes of endocannabinoid mobilization at central amygdala glutamatergic synapses. Neuron 81:1111–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K, et al. (2013): The WU-Minn Human Connectome Project: An overview. Neuroimage 80:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI Jr, et al. (1994): A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. J Stud Alcohol 55:149–158. [DOI] [PubMed] [Google Scholar]
- 36.Achenbach TM (2009): The Achenbach System of Empirically Based Assessment (ASEBA): Development, Findings, Theory, and Applications. Burlington: University of Vermont, Research Center of Children, Youth & Families. [Google Scholar]
- 37.Cheng W, Rolls ET, Ruan H, Feng J (2018): Functional connectivities in the brain that mediate the association between depressive problems and sleep quality. JAMA Psychiatry 75:1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma L, Steinberg JL, Bjork JM, Wang Q, Hettema JM, Abbate A, et al. (2020): Altered effective connectivity of central autonomic network in response to negative facial expression in adults with cannabis use disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 5:84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, et al. (2013): Function in the human connectome: Task-fMRI and individual differences in behavior. Neuroimage 80:169–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, et al. (2013): Resting-state fMRI in the Human Connectome Project. Neuroimage 80:144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behzadi Y, Restom K, Liau J, Liu TT (2007): A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH (2014): Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage 96:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitfield-Gabrieli S, Nieto-Castanon A (2012): Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141. [DOI] [PubMed] [Google Scholar]
- 44.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American Psychiatric Association (2013): DSM-5 Self-Rated Level 1 Cross-Cutting Symptom Measure—Adult. Available at: https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed May 18, 2013.
- 46.Spielberger CD, Gorsuch RL, Lushene RE (1970): Manual for the State-Trait Anxiety Inventory (Self-Evaluation Questionnaire). Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- 47.Beck AT, Steer RA, Brown GK (1996): Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- 48.Pruim RHR, Mennes M, Buitelaar JK, Beckmann CF (2015): Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage 112:278–287. [DOI] [PubMed] [Google Scholar]
- 49.Kendler KS, Heath AC, Martin NG, Eaves LJ (1987): Symptoms of anxiety and symptoms of depression. Same genes, different environments? Arch Gen Psychiatry 44:451–457. [DOI] [PubMed] [Google Scholar]
- 50.Hettema JM (2008): What is the genetic relationship between anxiety and depression? Am J Med Genet C Semin Med Genet 148C:140–146. [DOI] [PubMed] [Google Scholar]
- 51.Kalin NH (2020): The critical relationship between anxiety and depression. Am J Psychiatry 177:365–367. [DOI] [PubMed] [Google Scholar]
- 52.Friston KJ, Litvak V, Oswal A, Razi A, Stephan KE, van Wijk BC, et al. (2016): Bayesian model reduction and empirical Bayes for group (DCM) studies. Neuroimage 128:413–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friston KJ, Penny W (2003): Posterior probability maps and SPMs. Neuroimage 19:1240–1249. [DOI] [PubMed] [Google Scholar]
- 54.Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT (2010): Meta-analytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Hum Brain Mapp 31:173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung WH, Lee S, Lerman C, Kable JW (2018): Amygdala functional and structural connectivity predicts individual risk tolerance. Neuron 98:394–404, e394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGaugh JL, Roozendaal B (2002): Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol 12:205–210. [DOI] [PubMed] [Google Scholar]
- 57.Tyng CM, Amin HU, Saad MNM, Malik AS (2017): The influences of emotion on learning and memory. Front Psychol 8:1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson OJ, Krimsky M, Lieberman L, Allen P, Vytal K, Grillon C (2014): Towards a mechanistic understanding of pathological anxiety: The dorsal medial prefrontal-amygdala ‘aversive amplification’ circuit in unmedicated generalized and social anxiety disorders. Lancet Psychiatry 1:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toyoda H, Li XY, Wu LJ, Zhao MG, Descalzi G, Chen T, et al. (2011): Interplay of amygdala and cingulate plasticity in emotional fear. Neural Plast 2011:813749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jhang J, Lee H, Kang MS, Lee HS, Park H, Han JH (2018): Anterior cingulate cortex and its input to the basolateral amygdala control innate fear response. Nat Commun 9:2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagai M, Dote K, Kato M, Sasaki S, Oda N, Kagawa E, et al. (2017): The insular cortex and takotsubo cardiomyopathy. Curr Pharm Des 23:879–888. [DOI] [PubMed] [Google Scholar]
- 62.Schultz DH, Balderston NL, Helmstetter FJ (2012): Resting-state connectivity of the amygdala is altered following Pavlovian fear conditioning. Front Hum Neurosci 6:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma L, Steinberg JL, Bjork JM, Keyser-Marcus L, Vassileva J, Zhu M, et al. (2018): Fronto-striatal effective connectivity of working memory in adults with cannabis use disorder. Psychiatry Res Neuroimaging 278:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skalski LM, Towe SL, Sikkema KJ, Meade CS (2016): The impact of marijuana use on memory in HIV-infected patients: A comprehensive review of the HIV and marijuana literatures. Curr Drug Abuse Rev 9:126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solowij N, Battisti R (2008): The chronic effects of cannabis on memory in humans: A review. Curr Drug Abuse Rev 1:81–98. [DOI] [PubMed] [Google Scholar]
- 66.Pavuluri M, May A (2015): I feel, therefore, I am: The insula and its role in human emotion, cognition and the sensory-motor system. AIMS Neurosci 2:18–27. [Google Scholar]
- 67.Hofmann SG, Gomez AF (2017): Mindfulness-based interventions for anxiety and depression. Psychiatr Clin North Am 40:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baur V, Hanggi J, Langer N, Jancke L (2013): Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol Psychiatry 73:85–92. [DOI] [PubMed] [Google Scholar]
- 69.Ma L, Del Buono MG, Moeller FG (2019): Cannabis use as a risk factor for Takotsubo (stress) cardiomyopathy: Exploring the evidence from brain-heart link. Curr Cardiol Rep 21:121. [DOI] [PubMed] [Google Scholar]
- 70.Kindt M, Soeter M, Vervliet B (2009): Beyond extinction: Erasing human fear responses and preventing the return of fear. Nat Neurosci 12:256–258. [DOI] [PubMed] [Google Scholar]
- 71.Cuttler C, Spradlin A, McLaughlin RJ (2018): A naturalistic examination of the perceived effects of cannabis on negative affect. J Affect Disord 235:198–205. [DOI] [PubMed] [Google Scholar]
- 72.Xia M, Wang J, He Y (2013): BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One 8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


