Abstract
C-1027, the most potent member of the enediyne antitumor antibiotic family, is produced by Streptomyces globisporus C-1027 and consists of an apoprotein (encoded by the cagA gene) and a nonpeptidic chromophore. The C-1027 chromophore could be viewed as being derived biosynthetically from a benzoxazolinate, a deoxyamino hexose, a β-amino acid, and an enediyne core. By adopting a strategy for cloning of the C-1027 biosynthesis gene cluster by mapping a putative dNDP-glucose 4,6-dehydratase (NGDH) gene to cagA, we have localized 75 kb of contiguous DNA from S. globisporus. DNA sequence analysis of two regions of the cloned gene cluster revealed two genes, sgcA and sgcB, that encode an NGDH enzyme and a transmembrane efflux protein, respectively, and confirmed that the cagA gene resides approximately 14 kb upstream of the sgcAB locus. The involvement of the cloned gene cluster in C-1027 biosynthesis was demonstrated by disrupting the sgcA gene to generate C-1027-nonproducing mutants and by complementing the sgcA mutants in vivo to restore C-1027 production. These results represent the first cloning of a gene cluster for enediyne antitumor antibiotic biosynthesis and provide a starting point for future genetic and biochemical investigations of C-1027 biosynthesis.
The enediyne antibiotics are the focus of intense research activity in the fields of chemistry, biology, and medical sciences because of their unique molecular architectures, biological activities, and modes of action (7, 49). Since the unveiling of the structure of neocarzinostatin chromophore (8) in 1985, the enediyne family has grown steadily. Thus far, there have been three basic groups within the enediyne antibiotic family: (i) the calicheamicin-esperamicin type, which includes the calicheamicins, the esperamicins, and namenamicin; (ii) the dynemicin type; and (iii) the chromoprotein type, consisting of an apoprotein and an unstable enediyne chromophore. The last group includes neocarzinostatin, kedarcidin, C-1027 (Fig. 1), and maduropeptin, whose enediyne chromophore structures have been established, as well as several others whose enediyne chromophore structures are yet to be determined due to their instabilities (49). N1999A2, in contrast to the other chromoproteins, exists as an enediyne chromophore alone, even though its structure is very similar to those of the other chromoprotein chromophores (49).
FIG. 1.
Structures of C-1027 chromophore and the benzenoid diradical intermediate proposed to initiate DNA cleavage.
As a family, the enediyne antibiotics are the most potent, highly active antitumor agents ever discovered. Some members are 1,000 times more potent than adriamycin, one of the most effective, clinically used antitumor antibiotics (55). All members of this family contain a unit consisting of two acetylenic groups conjugated to a double bond or incipient double bond within a 9- or 10-membered ring, i.e., the enediyne core as exemplified by C-1027 in Fig. 1. As the consequence of this structural feature, these compounds share a common mechanism of action: the enediyne core undergoes an electronic rearrangement to form a transient benzenoid diradical, which is positioned in the minor groove of DNA so as to damage DNA by abstracting hydrogen atoms from deoxyriboses on both strands (Fig. 1). Reaction of the resulting deoxyribose carbon-centered radicals with molecular oxygen initiates a process that results in both single-stranded and double-stranded DNA cleavages (7, 18, 34, 47, 49, 53). This novel mechanism of DNA damage has important implications for their application as potent cancer chemotherapeutic agents (7, 44).
Complementary to making structural analogs of microbial metabolites by chemical synthesis, genetic manipulations of the genes governing secondary metabolism offer a promising alternative to preparation of these compounds biosynthetically (5, 16, 20). The success of the latter approach depends critically on the availability of novel genetic systems and on genes that encode novel enzyme activities. The enediynes offer a distinct opportunity to study the biosynthesis of their unique molecular scaffolds and the mechanism of self-resistance to extremely cytotoxic natural products. Elucidation of these aspects should not only provide access to rational engineering of enediyne biosynthesis for novel drug leads and make it possible to construct enediyne-overproducing strains by deregulating the biosynthetic machinery but should also contribute to the general field of combinatorial biosynthesis by expanding the repertoire of novel polyketide synthase (PKS) and deoxysugar biosynthesis genes as well as other genes uniquely associated with enediyne biosynthesis, eventually leading to the making of novel enediynes via combinatorial biosynthesis.
We have been studying the biosynthesis of C-1027 in Streptomyces globisporus C-1027 as a model for the enediyne family of antitumor antibiotics (49). C-1027 consists of a nonpeptidic chromophore and an apoprotein, CagA (also called C-1027AG [36]). The C-1027 chromophore is extremely unstable in the protein-free state, and its structure was initially deduced from an inactive but more stable degradation product (32) and was subsequently confirmed by spectroscopic analysis of the natural product (54) (Fig. 1). While the absolute stereochemistry of the deoxysugar moiety was established by total synthesis (17), the 8S, 9S, 13S, and 17R configurations of the C-1027 chromophore were based only on computer modeling (35). Although no biosynthetic study has been carried out specifically with C-1027, the polyketide origin of the enediyne cores has been implicated by feeding experiments with 13C-labeled acetate for the neocarzinostatin chromophore A (9), dynemicin (50), and esperamicin (21); and deoxysugar biosynthesis has been well characterized in actinomycetes (24, 37). Given the structural similarity of C-1027 to the other enediyne cores and to deoxysugars found in other secondary metabolites, we decided to clone either a PKS or a deoxysugar biosynthesis gene as the first step in the identification of the C-1027 gene cluster from S. globisporus. Furthermore, the CagA apoprotein of C-1027 has been isolated, its amino acid sequence has been determined, and the corresponding cagA gene has been cloned and sequenced (36, 39). Since genes encoding secondary metabolite production in actinomycetes have invariably been found to be clustered in one region of the microbial chromosome (11), we further reasoned that mapping of the cagA gene with either a putative PKS gene, a deoxysugar biosynthesis gene, or both to the same region of the S. globisporus chromosome should be viewed as strong evidence that would support the proposition that the cloned genes constitute the C-1027 biosynthesis gene cluster.
We report here on the cloning and sequencing of two genes, sgcA (S. globisporus C-1027) and sgcB, that encode a dNDP-glucose 4,6-dehydratase (NGDH) and a transmembrane efflux protein, respectively. The sgc-sgcAB loci are indeed clustered with the cagA gene, leading to the localization of a 75-kb gene cluster from S. globisporus. The involvement of the cloned gene cluster in C-1027 biosynthesis was demonstrated by disrupting the sgcA gene to generate C-1027-nonproducing mutants and by complementing the sgcA mutants in vivo to restore C-1027 production. Our results, together with a similar effort on the calicheamicin gene cluster in the laboratory of Thorson et al. (49), represent the first cloning of a gene cluster for enediyne antitumor antibiotic biosynthesis.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Escherichia coli DH5α was used as a general host for routine subcloning (40). E. coli XL 1-Blue MR (Stratagene, La Jolla, Calif.) was used as the transduction host for cosmid library construction. E. coli S17-1 was used as the donor host for E. coli-S. globisporus conjugation (30). Micrococcus luteus ATCC 9431 was used as the test organism for assay of the antibacterial activity of C-1027 (13). The pGEM-3zf, -5zf, and -7zf and pGEM-T vectors were from Promega (Madison, Wis.). The S. globisporus strains and other plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in the study
| Strain or plasmid | Relevant characteristicsa | Reference |
|---|---|---|
| S. globisporus | ||
| C-1027 | Wild type | 13 |
| AF40 | Mutant resulted from acriflavine treatment of S. globisporus C-1027, C-1027 nonproducing | 27 |
| AF44 | Mutant resulted from acriflavine treatment of S. globisporus C-1027, C-1027 nonproducing | 27 |
| AF67 | Mutant resulted from acriflavine treatment of S. globisporus C-1027, C-1027 nonproducing | 27 |
| SB1001 | sgcA-disrupted mutant resulted from integration of pBS1012 into S. globisporus C-1027 Aprr, C-1027 nonproducing | This work |
| SB1002 | sgcA-disrupted mutant resulted from integration of pBS1013 into S. globisporus C-1027 Aprr, C-1027 nonproducing | This work |
| Plasmids | ||
| pOJ446 | E. coli-Streptomyces shuttle cosmid, Aprr | 4 |
| pOJ260 | E. coli vector, nonreplicating in Streptomyces, Aprr | 4 |
| pKC1139 | E. coli-Streptomyces shuttle vector, temperature-sensitive replication origin carrier, Aprr | 4 |
| pWHM3 | E. coli-Streptomyces shuttle vector, Thr | 51 |
| pWHM79 | ermE* promoter in pGEM-3zf | 43 |
| pBS1001 | 0.75-kb PCR product amplified from S. globisporus with type I PKS primers in pGEM-T | This work |
| pBS1002 | 0.55-kb PCR product amplified from S. globisporus with NGDH gene primers in pGEM-T | This work |
| pBS1003 | 0.73-kb PCR product amplified from pBS1005 with cagA primers in pGEM-T | This work |
| pBS1004 | pOJ446 S. globisporus genomic library cosmid | This work |
| pBS1005 | pOJ446 S. globisporus genomic library cosmid | This work |
| pBS1006 | pOJ446 S. globisporus genomic library cosmid | This work |
| pBS1007 | 3.0-kb BamHI fragment from pBS1005 in pGEM-3zf, sgcA sgcB | This work |
| pBS1008 | 4.0-kb BamHI fragment from pBS1005 in pGEM-3zf, cagA | This work |
| pBS1009 | 1.0-kb KpnI truncated fragment of sgcA from pBS1007 in pGEM-3zf | This work |
| pBS1010 | 0.75-kb SacII-SphI internal fragment of sgcA from pBS1009 in pGEM-5zf | This work |
| pBS1011 | 0.75-kb SacI-SphI internal fragment of sgcA from pBS1010 in pGEM-3zf | This work |
| pBS1012 | 0.75-kb EcoRI-HindIII internal fragment of sgcA from pBS1010 in pOJ260 | This work |
| pBS1013 | 0.75-kb EcoRI-HindIII internal fragment of sgcA from pBS1010 in pKC1139 | This work |
| pBS1014 | 2.0-kb EcoRI-SphI fragment from pBS1007 in the SmaI-SphI sites of pWHM79, ermE* sgcA | This work |
| pBS1015 | 2.5-kb EcoRI-HindIII fragment from pBS1014 in pWHM3, ermE* sgcA | This work |
| pBS1016 | Self-ligation of the 5.2-kb KpnI fragment from pBS1007 | This work |
| pBS1017 | 0.45-kb EcoRI-SacI fragment from pWHM79 in EcoRI-SacI sites of pBS1016, ermE* sgcB | This work |
| pBS1018 | 2.5-kb EcoRI-HindIII fragment from pBS1017 in pKC1139, ermE* sgcB | This work |
Apr, apramycin; r, resistant; Th, thiostrepton.
Biochemicals and chemicals.
Ampicillin, apramycin, nalidixic acid, and thiostrepton were from Sigma (St. Louis, Mo.). Unless specified otherwise, restriction enzymes and other molecular biology reagents were from standard commercial sources.
Media and culture conditions.
E. coli strains carrying plasmids were grown in Luria-Bertani (LB) medium and were selected with appropriate antibiotics (40). S. globisporus strains were grown on ISP-4 (Difco Laboratories, Detroit, Mich.) or R2YE (12) at 28°C for sporulation and in Trypticase soy broth (TSB) (12) supplemented with 5 mM MgCl2 and 0.5% glycine at 28°C and 250 rpm for isolation of genomic DNA. For transformation, S. globisporus strains were grown in YEME (12) for preparation of protoplasts and on R2YE for protoplast regeneration. For conjugation, both the E. coli S17-1 donors and the S. globisporus recipients (upon germination in TSB) were prepared in LB, and donors and recipients were grown on either ISP-4 medium with 0.05% yeast extract and 0.1% tryptone or AS-1 medium (2, 4) at 30°C for isolation of exconjugants.
For C-1027 production, S. globisporus strains were grown either on R2YE or on ISP-4 agar medium at 28°C or in liquid medium by a two-stage fermentation. For liquid culture, the seed inoculum was prepared by inoculating 50 ml of medium [consisting of 2% glycerol, 2% dextrin, 1% fish meal, 0.5% peptone, 0.2% (NH4)2SO4, and 0.2% CaCO3 (pH 7.0)] with an aliquot of the spore suspension and incubating the mixture at 28°C and 250 rpm for 2 days. To a fresh 50 ml of the same medium was then added the seed culture (5%), and incubation was continued at 28°C and 250 rpm for 3 to 6 days (13). The fermentation supernatants were harvested by centrifugation (Eppendorf 5415C centrifuge, 4°C, 10 min, 14,000 rpm) on days 3, 4, and 5 and were assayed for their antibacterial activities against M. luteus (13).
DNA isolation and manipulation.
Plasmid preparation and DNA extraction were carried out by using commercial kits (Qiagen, Santa Clarita, Calif.). Total S. globisporus DNA was isolated by protocols described in the literature (12, 38). Restriction endonuclease digestion and ligation were done by standard methods (40). For Southern analysis, digoxigenin labeling of DNA probes, hybridization, and detection were performed by the protocols provided by the manufacturer (Boehringer Mannheim Biochemicals, Indianapolis, Ind.).
DNA sequencing.
Automated DNA sequencing was carried out on an ABI Prism 377 DNA Sequencer with the ABI Prism dye terminator cycle sequencing ready reaction kit and AmpliTaq DNA polymerase FS (Perkin-Elmer/ABI, Foster City, Calif.). Sequencing service was provided by either the DBS Automated DNA Sequencing Facility, University of California at Davis, or Davis Sequencing Inc. (Davis, Calif.). Data were analyzed by ABI Prism Sequencing 2.1.1 software and the Genetics Computer Group program (Madison, Wis.).
PCR.
Primers were synthesized at the Protein Structure Laboratory, University of California at Davis. PCR was carried out on a Gene Amp PCR System 2400 (Perkin-Elmer/ABI) with Taq polymerase and buffer from Promega. A typical PCR mixture consisted of 5 ng of S. globisporus genomic or plasmid DNA as template, 25 pmol of each primers, 25 μM deoxynucleoside triphosphates, 5% dimethyl sulfoxide, 2 U of Taq polymerase, and 1× buffer with or without 20% glycerol in a final volume of 50 μl. The PCR temperature program was as follows: initial denaturation at 94°C for 5 min and 24 to 36 cycles of 45 s at 94°C, 1 min at 60°C, and 2 min at 72°C, followed by an additional 7 min at 72°C.
For type II PKS, the following two pairs of degenerate primers were used: primers 5′-AGC TCC ATC AAG TCS ATG RTC GG-3′ (forward) and 5′-CC GGT GTT SAC SGC GTA GAA CCA GGC G-3′ (reverse) and primers 5′-GAC ACV GCN TGY TCB TCV-3′ (forward) and 5′-RTG SGC RTT VGT NCC RCT-3′ (B, C + G + T; N, A + C + G + T; R, A + G; S, C + G; V, A + C + G; Y, C + T) (reverse) (41). No product was amplified under all conditions tested. For type I PKS, the following pair of degenerate primers was used: 5′-GCS TCC CGS GAC CTG GGC TTC GAC TC-3′ (forward) and 5′-AG SGA SGA SGA GCA GGC GGT STC SAC-3′ (S, G + C) (reverse) (19). A distinctive product with the predicted size of 0.75 kb was amplified in the presence of 20% glycerol and was cloned into pGEM-T by the protocol provided by the manufacturer (Promega) to yield pBS1001.
For NGDH, the following pair of degenerate primers was used: 5′-CS GGS GSS GCS GGS TTC ATC GG-3′ (forward) and 5′-GG GWR CTG GYR SGG SCC GTA GTT G-3′ (R, A + G; S, C + G; W, A + T; Y, C + T) (reverse) (6). A distinctive product with the predicted size of 0.55 kb was amplified and was cloned into pGEM-T to yield pBS1002.
For cagA, the following pair of primers whose sequences were homologous to the sequences flanking the cagA coding region were used: 5′-AG GTG GAG GCG CTC ACC GAG-3′ (forward) and 5′-G GGC GTC AGG CCG TAA GAA G-3′ (reverse) (39). A distinctive product with the predicted size of 0.73 kb was amplified from pBS1005 and was cloned into pGEM-T to yield pBS1003.
Genomic library construction and screening.
S. globisporus genomic DNA was partially digested with MboI to yield a smear of about 60 kb, as monitored by electrophoresis on a 0.3% agarose gel. This sample was dephosphorylated upon treatment with shrimp alkaline phosphatase and was ligated into the E. coli-Streptomyces shuttle vector pOJ446 (4) that was prepared by digestion with HpaI, shrimp alkaline phosphatase treatment, and additional digestion with BamHI. The resulting ligation mixture was packaged with the Gigapack II XL two-component packaging extract (Stratagene). The package mixture was transduced into E. coli XL 1-Blue MR. The transduced cells were spread onto LB plates containing apramycin (100 μg/ml), and the plates were incubated at 37°C overnight. The titer of the primary library was approximately 6,000 CFU per μg of DNA. Restriction enzyme analysis of 12 randomly selected cosmids confirmed that the average size of the inserts was about 35 to 45 kb (38).
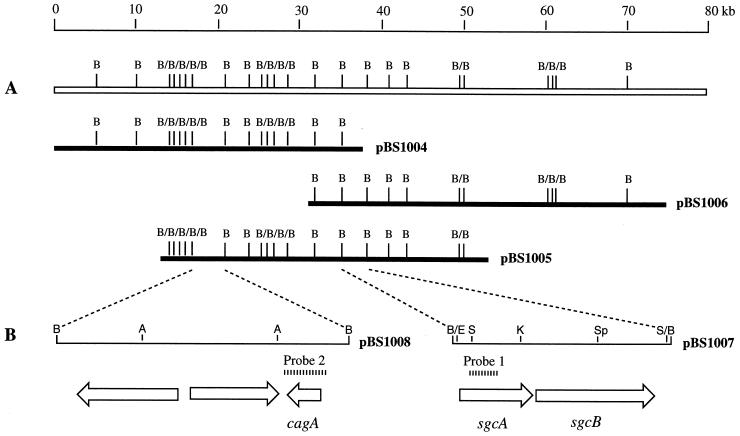
To screen the genomic library, colonies from five LB plates containing apramycin (100 μg/ml; with approximately 2,000 colonies per plate) were transferred to nylon transfer membranes (Micro Separations, Inc., Westborough, Mass.) and were screened by colony hybridization with the PCR-amplified 0.55-kb NGDH fragment from pBS1002 as a probe. The positive cosmid clones were rescreened by PCR with primers specific for NGDH, and their identities were confirmed by Southern hybridization (40). Further restriction enzyme mapping and chromosomal walking of these overlapping cosmids led to the genetic localization of the 75-kb sgc gene cluster, as represented by pBS1004, pBS1005, and pBS1006 (Fig. 2A). A 3.0-kb BamHI fragment from pBS1005 that hybridized to the NGDH probe was cloned into the same sites of pGEM-3zf to yield pBS1007. Similarly, a 4.0-kb BamHI fragment from pBS1005 that hybridizes to the PCR-amplified 0.73-kb cagA probe from pBS1003 was cloned into the same sites of pGEM-3zf to yield pBS1008 (Fig. 2B).
FIG. 2.
Restriction map of the 75-kb sgc gene cluster from S. globisporus as represented by three cosmid clones (A), and genetic organization of the sgcA, sgcB, and cagA genes, showing that they are clustered in the sgc gene cluster (B). Probe 1, the 0.55-kb NDGH gene fragment from pBS1002; probe 2, the 0.73-kb cagA fragment from pBS1003. A, ApaI; B, BamHI; E, EcoRI; K, KpnI, S, SacII; Sp, SphI.
Generation of sgcA mutants by insert-directed homologous recombination in S. globisporus.
A 1.0-kb KpnI fragment from pBS1007, which contained the C-terminal truncated sgcA, was subcloned into pGEM-3zf to yield pBS1009. An internal fragment of sgcA was moved sequentially as a 0.75-kb SacII-SphI fragment from pBS1009 into the same sites of pGEM-5zf to yield pBS1010 and as a 0.75-kb SacI-SphI fragment from pBS1010 into the same sites of pGEM-3zf to yield pBS1011. The latter plasmid was digested with EcoRI and HindIII, and the resulting 0.75-kb EcoRI-HindIII fragment was cloned into the same sites of pOJ260 (4) and pKC1139 (4) to yield pBS1012 and pBS1013, respectively.
Introduction of pBS1012 and pBS1013 into S. globisporus was carried out by either polyethylene glycol (PEG)-mediated protoplast transformation (12) or E. coli-S. globisporus conjugation (4, 28, 29); methods for both of these procedures were recently developed in our laboratory (W. Liu and B. Shen, unpublished data). In brief, for transformation, pBS1012 and pBS1013 were propagated in E. coli ET12567 (26), and the resulting double-stranded plasmid DNA was denatured by alkaline treatment (10). The latter DNA (5 μl) and 200 μl of 25% PEG 1000 in P buffer (12) were sequentially added to 50 μl of S. globisporus protoplasts (109) in P buffer. The resulting suspension was mixed immediately and was spread onto R2YE plates. After incubation at 28°C for 16 to 20 h, the plates were overlaid with soft R2YE (0.7% agar) containing apramycin (final concentration, 100 μg/ml); incubation continued until colonies appeared (in 5 to 7 days). For conjugation, E. coli S17-1(pBS1012) or E. coli S17-1 (pBS1013) was grown to an optical density at 600 nm of 0.3 to 0.4. Cells from a 20-ml culture were pelleted by centrifugation, washed in LB, and resuspended in 2 ml of LB as the E. coli donors. S. globisporus spores (103 to 109) were washed, resuspended in TSB, and incubated at 50°C for 10 min to activate germination. After additional incubation at 37°C for 2 to 5 h, the spores were pelleted and resuspended in LB as the S. globisporus recipients. The donors (100 μl) and recipients (100 μl) were mixed and spread equally onto two modified ISP-4 or AS-1 plates freshly supplemented with 10 mM MgCl2 (see Media and culture conditions). The plates were incubated at 28°C for 16 to 22 h. After removal of most of the E. coli S17-1 donors by washing the surface with sterile water, the plates were overlaid with 3 ml of soft LB (0.7% agar) containing nalidixic acid (final concentration, 50 μg/ml) and apramycin (final concentration, 100 μg/ml) and were incubated at 28°C until exconjugants appeared (in approximately 5 days).
Unlike pBS1012, which is a Streptomyces nonreplicating plasmid, pBS1013 bears a temperature-sensitive Streptomyces replication origin (4, 33) that is unable to replicate at temperatures above 34°C (Table 1), while the S. globisporus wild-type strain grows normally up to 37°C. Thus, spores of S. globisporus(pBS1013) from either the transformants or the exconjugants were spread onto R2YE plates containing apramycin (100 μg/ml). The plates were incubated directly at 37°C, and mutants that resulted from single-crossover homologous recombination between pBS1013 and the S. globisporus chromosome were readily obtained in 7 to 10 days. Alternatively, the plates were first incubated at 28°C for 2 days until pinpoint-size colonies became visible and were then shifted to 37°C to continue incubation. Mutants resulting from single-crossover homologous recombination grew out of the original pinpoint-size colonies as easily distinguishable sectors in 7 to 10 days.
Construction of sgcA and sgcB expression plasmids.
pBS1007 was digested with EcoRI and was made blunt ended by treatment with the Klenow fragment of DNA polymerase I. Upon additional digestion with SphI, the resulting 2.0-kb blunt-ended SphI fragment containing the intact sgcA gene was cloned into the SmaI-SphI sites of pWHM79 (43) to yield pBS1014. The latter was digested with EcoRI and HindIII, and the resulting 2.5-kb EcoRI-HindIII fragment was cloned into the same sites of pWHM3 (51) to yield pBS1015, in which the expression of sgcA is under the control of the ermE* promoter (3).
Alternatively, pBS1007 was digested with KpnI, which removed most of the sgcA gene, and the 5.2-kb KpnI fragment was recovered and self-ligated to yield pBS1016. The ermE* promoter was subcloned from pWHM79 (43) as a 0.45-kb EcoRI-SacI fragment and was cloned into the same sites of pBS1016 to yield pBS1017. The latter was digested with EcoRI and HindIII, and the resulting 2.5-kb EcoRI-HindIII fragment was cloned into the same sites of pKC1139 to yield pBS1018, in which the expression of sgcB is under the control of the ermE* promoter.
Determination of C-1027 production.
The production of C-1027 was detected by assaying its antibacterial activity against M. luteus (13). From the liquid culture, fermentation supernatant (180 μl) was added to stainless steel cylinders placed on LB plates preseeded with an overnight M. luteus culture (0.01% [vol/vol]). From solid culture, a small square block (0.5 by 0.5 by 0.5 cm3) of agar from either R2YE or ISP-4 medium was directly placed onto M. luteus-seeded LB plates. The plates were incubated at 37°C for 24 h, and C-1027 production was estimated by measuring the sizes of the inhibition zones.
Nucleotide sequence accession number.
The nucleotide sequence reported here has been deposited in the GenBank database with the accession no. AF201913.
RESULTS
No PKS gene was amplified from S. globisporus by PCR.
On the assumption that the C-1027 enediyne core is of polyketide origin, the PCR approach was adopted to screen S. globisporus for any putative PKS genes, although it is far from certain a priori if the biosynthesis of the enediyne core invokes a PKS and, if so, whether the enediyne PKS will exhibit a type I or type II structural organization. PCR methods for cloning of either type I or type II PKS genes have been developed, and these methods have proven to be very effective for the cloning of PKS genes from various polyketide-producing actinomycetes (19, 41). While no distinctive product was amplified under all conditions examined with both pairs of primers designed for type II PKS, a single product with the expected size of 0.75 kb was readily amplified by PCR from S. globisporus with primers designed for type I PKS, and the product was subsequently cloned (pBS1001). Intriguingly, sequence analysis of six randomly selected pBS1001 clones yielded an identical product—indicative of a specific PCR amplification—the deduced amino acid sequence of which, however, showed no homology to known PKSs (data not shown), excluding the possibility of using PKS as a probe to identify the sgc biosynthesis gene cluster.
Cloning of a putative NGDH gene from S. globisporus by PCR.
The biosynthesis of various deoxyhexoses shares a common key intermediate—4-keto-6-deoxyglucose nucleoside diphosphate or its analogs—whose formation from glucose nucleoside diphosphate is catalyzed by the NGDH enzyme, an NAD+-dependent oxidoreductase (24, 37). The PCR method was adopted to clone the putative NGDH gene from S. globisporus with primers designed according to the homologous regions of various NGDH enzymes from actinomycetes (6), resulting in the amplification of a single product with the expected size of 0.55 kb (pBS1002). Sequence analysis of pBS1002 confirmed its identity as a part of a putative NGDH gene.
To clone the complete NGDH gene, an S. globisporus genomic library, constructed in the E. coli-Streptomyces shuttle vector pOJ446 (4, 38), was analyzed by Southern hybridization with the PCR-amplified 0.55-kb fragment from pBS1002 as a probe. Of the 10,000 colonies screened, 36 positive colonies were identified, and 9 of them were confirmed by PCR to harbor the NGDH gene. Restriction enzyme mapping showed that all of them contained a single 3.0-kb BamHI fragment that hybridized to the NGDH probe. Additional chromosomal walking from this locus eventually led to the localization of the 75-kb sgc gene cluster, covered by 18 overlapping cosmids as represented by pBS1004, pBS1005, and pBS1006 (Fig. 2A). The 3.0-kb BamHI fragment was subcloned (pBS1007) (Fig. 2B), and its nucleotide (nt) sequence was determined.
Analysis of DNA sequences of the sgcA and sgcB genes.
Two complete open reading frames (ORFs) (sgcA and sgcB) were identified within the 3.0-kb BamHI fragment of pBS1007, the 3,035-nt sequence of which is shown in Fig. 3. The sgcA gene most likely begins with an ATG at nt 101, preceded by a probable ribosome binding site (RBS), GGAGG, and ends with a TGA stop codon at nt 1099. sgcA should therefore encode a 332-amino-acid protein with a molecular weight of 36,341 and an isoelectric point of 6.01. A Gapped-BLAST search showed that the deduced sgcA gene product is highly homologous to various putative and known NGDH enzymes from antibiotic-producing actinomycetes, including Gdh from the erythromycin biosynthesis gene cluster in Saccharopolyspora erythraea (64% identity and 70% similarity) (23), MtmE from the mithramycin biosynthesis gene cluster in Streptomyces argillaceus (64% identity and 68% similarity) (25), and TylA2 from the tylosin biosynthesis gene cluster in Streptomyces fradiae (62% identity and 68% similarity) (31) (Fig. 4). A conserved sequence of 14 amino acid residues close to the N termini can be easily identified in these proteins. This sequence has been described as a βαβ fold with an NAD+-binding motif, GxGxxG (Fig. 4, boxed), consistent with its biochemical role in deoxyhexose biosynthesis (24, 37). The function of Gdh and MtmE as TDP-glucose 4,6-dehydratases, requiring NAD+ as a cofactor, has been confirmed by an enzyme assay following expression of the gdh (23) and mtmE (25) genes in E. coli, respectively, and by purification of the Gdh protein from S. erythraea (51). From these data, it is reasonable to suggest that sgcA encodes the NGDH enzyme required for the biosynthesis of the 4,6-dideoxy-4-dimethylamino-5-methylrhamnose moiety of the C-1027 chromophore.
FIG. 3.
DNA and deduced amino acid sequences of the 3.0-kb BamHI fragment from pBS1007 showing the sgcA and sgcB genes. Possible RBSs are boxed. The presumed translational start and stop sites are in boldface type. Restriction enzyme sites of interest are underlined. The amino acids, according to which the degenerated PCR primer were designed for amplification of the NDGH gene from S. globisporus, are underlined.
FIG. 4.
Amino acid sequence alignment of SgcA with three other dNDP-glucose 4,6-dehydratases. Gdh, TDP-glucose 4,6-dehydratase of S. erythraea (AAA68211); MtmE, TDP-glucose 4,6-dehydratase in the mithramycin pathway of S. argillaceus (CAA71847); TylA2, TDP-glucose 4,6-dehydratase in the tylosin pathway of S. fradiae (S49054). Protein accession numbers are given in parentheses. The αβα fold with the NAD+-binding motif of GxGxxG is boxed.
Transcribed in the same direction as sgcA, the sgcB gene is located 43 nt downstream of sgcA. It should begin with a GTG at nt 1143, preceded by a probable RBS, AGGAG, and should end with a TGA at nt 2708 (Fig. 3). Correspondingly, sgcB should therefore encode a 521-amino-acid protein with a molecular weight of 52,952 and an isoelectric point of 4.64. Comparison of the deduced sgcB product with sequences in nucleotide sequence databases revealed that SgcB is closely related to a family of membrane efflux pumps, such as LfrA from Mycobacterium smegmatis (43% identity and 50% similarity; protein accession no. AAC43550) (48), OrfA from Streptomyces cinnamomeus (42% identity and 47% similarity; protein accession no. AAB71209) (45), and RifP from the rifamycin biosynthesis gene cluster in Amycolatopsis mediterranei (35% identity and 44% similarity; protein accession no. AAC01725) (1). These proteins are membrane-localized transporters involved in the transport of antibiotics (conferring resistance), sugars, and other substances. While direct evidence that RifP confers rifamycin resistance in A. mediterranei by transporting it out of the cells is lacking (1), it has been proven that LfrA uses the transmembrane proton gradient in an antiporter mode to drive the efflux of intracellular antibiotics, resulting in fluoroquinolone resistance in M. smegmatis (48). On the basis of the high degree of amino acid sequence conservation, an equivalent role could be proposed for SgcB, which confers resistance by exporting C-1027 from S. globisporus.
The cagA gene is clustered with the sgcA-sgcB loci.
To determine if cagA is clustered with the sgcA and sgcB loci, PCR primers were designed according to the flanking regions of cagA (39). A single product with the predicted size of 0.73 kb was indeed amplified from several of the overlapping cosmids (which cover the 75-kb sgc cluster), including pBS1004 and pBS1005, the identity of which as cagA was confirmed by sequencing. Restriction enzyme mapping and Southern hybridization analysis localized cagA to a single 4.0-kb BamHI fragment that is approximately 14 kb upstream of the sgcA-sgcB loci (Fig. 2B). The 4.0-kb BamHI fragment was subcloned (pBS1008), and its nt sequence was determined, revealing the cagA gene along with two additional ORFs (data not shown) (Fig. 2). As reported earlier, cagA encodes a 142-amino-acid protein that is processed by cleavage of a 32-amino-acid lead peptide to yield the mature CagA apoprotein (39).
Disruption of the sgcA gene in S. globisporus.
To examine if the cloned sgc cluster encodes C-1027 biosynthesis, sgcA was insertionally disrupted by a single-crossover homologous recombination event to generate C-1027-nonproducing mutant strains (Fig. 5A). Two plasmids were used: pBS1012 (a pOJ260 derivative) and pBS1013 (a pKC1139 derivative). Each of these plasmids contains a 0.75-kb internal fragment from sgcA (Table 1). After introduction of pBS1012 into S. globisporus either by PEG-mediated protoplast transformation or by E. coli-S. globisporus conjugation, transformants or exconjugants that were resistant to apramycin were isolated in all cases. Since pBS1012 is derived from the Streptomyces nonreplicating plasmid of pOJ260, these isolates must have resulted from integration of pBS1012 into the S. globisporus chromosome by homologous recombination. Plasmid pBS1013 was similarly introduced into S. globisporus. However, since pBS1013 is derived from pKC1139, which carries the temperature-sensitive Streptomyces replication origin from pSG5 and which can replicate normally at 28°C (33), these isolates were subjected to incubation at the nonpermissive temperature of 37°C to eliminate free plasmids from the host cells. As expected, for all recombinants except for the recombinants that continue to grow at 37°C normal growth stopped, which is indicative of integration of pBS1013 into S. globisporus by homologous recombination. The apramycin-resistant strains S. globisporus SB1001 and S. globisporus SB1002 were chosen as representatives of mutant strains with disrupted sgcA genes that resulted from integration of pBS1012 and pBS1013, respectively.
FIG. 5.
Disruption of sgcA by single-crossover homologous recombination. (A) Construction of sgcA disruption mutant and restriction maps of the wild-type S. globisporus C-1027 and S. globisporus SB1001 mutant strains showing predicted fragment sizes upon BamHI digestion. Southern analysis of S. globisporus C-1027 (lane 1) and S. globisporus SB1001 (lanes 2, 3, and 4; three individual isolates, respectively) genomic DNAs digested with BamHI by using the pOJ260 vector (B) or the 0.75-kb SacII-KpnI fragment of sgcA from pBS1012 (C) as a probe, respectively. B, BamHI; K, KpnI; S, SacII.
To confirm that targeted sgcA disruption had occurred by a single-crossover homologous recombination event, Southern analysis of the DNA from the mutant strains was performed as exemplified for S. globisporus SB1001 with either pOJ260 or the 0.75-kb SacII-KpnI internal fragment of sgcA from pBS1010 as a probe. As shown in Fig. 5B, a distinctive band of the predicted size of 6.3 kb was detected with the pOJ260 vector as a probe in all mutant strains (lanes 2, 3, and 4); this band was absent from the wild-type strain (lane 1). Complementarily, when the 0.75-kb SacII-KpnI internal fragment of sgcA was used as a probe (Fig. 5C), the 3.0-kb band in the wild-type strain (lane 1) was split into two fragments of 6.3 and 1.0 kb in the mutant strains (lanes 2, 3, and 4), as would be expected for disruption of sgcA by a single-crossover homologous recombination event.
S. globisporus SB1001 and S. globisporus SB1002 are C-1027-nonproducing mutants.
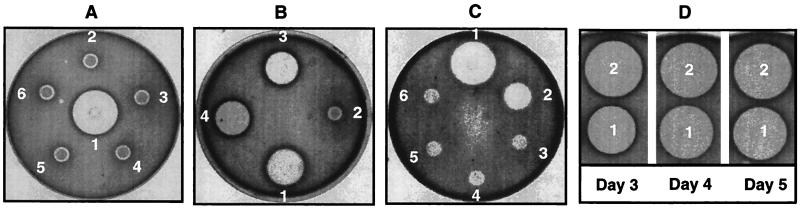
No apparent difference in growth characteristics and morphologies between the wild-type S. globisporus and the mutant S. globisporus SB1001 and S. globisporus SB1002 strains was observed. While C-1027 production in the wild-type S. globisporus strain could be detected on day 3, peaked on day 5, and continued for a few more days, as judged by assaying the antibacterial activity of the culture supernatant against M. luteus (13), C-1027 production was completely abolished in the sgcA mutant strains S. globisporus SB1001 and S. globisporus SB1002 (Fig. 6A). The phenotype of the latter strain was identical to those of the AF40, AF44, and AF67 mutants, which are C-1027-nonproducing S. globisporus strains that have been characterized previously (Fig. 6A and C) (27).
FIG. 6.
Determination of C-1027 production in various S. globisporus strains by assaying their antibacterial activities against M. luteus. (A) Dots: 1, S. globisporus C-1027; 2, 3, and 4, S. globisporus SB1001 (three individual isolates, respectively); 5, S. globisporus AF67; 6, S. globisporus AF40. (B) Dots: 1, S. globisporus C-1027; 2, S. globisporus SB1001(pWHM3); 3 and 4, S. globisporus SB1001(pBS1015) (two individual isolates, respectively). Both S. globisporus SB1001(pWHM3) and S. globisporus SB1001(pBS1015) were grown in the presence of 5 μg of thiostrepton per ml. (C) Dots: 1, S. globisporus C-1027; 2, S. globisporus SB1001(pBS1015); 3, S. globisporus SB1001; 4, S. globisporus SB1001(pWHM3); 5, S. globisporus AF40; 6, S. globisporus AF44. All S. globisporus strains were grown in the absence of thiostrepton. (D) Dots: 1, S. globisporus(pKC1139); 2, S. globisporus(pBS1018).
In vivo complementation of S. globisporus SB1001.
The ability of the wild-type sgcA gene to complement the disrupted sgcA gene was tested with S. globisporus SB1001. The construction of pBS1015, in which the expression of sgcA is under the control of the constitutive ermE* promoter, was described in Materials and Methods. Both the pBS1015 construct and the pWHM3 vector as a control were introduced by transformation into the S. globisporus SB1001 mutant strains. Culture supernatants from each transformant were bioassayed against M. luteus for C-1027 production. pBS1015 restored C-1027 production to S. globisporus SB1001 to the wild-type level; no C-1027 production was detected in the control in which pWHM3 was introduced into S. globisporus SB1001 (Fig. 6B and C). A significant reduction of C-1027 production was observed when S. globisporus SB1001(pBS1015) was cultured under identical conditions but without thiostrepton (Fig. 6B versus C), indicative of the possibility that pBS1015 may be unstable in S. globisporus SB1001 in the absence of antibiotic selection pressure.
Expression of sgcB in S. globisporus.
The effect of sgcB on C-1027 production was tested in the wild-type S. globisporus strain. The construction of pBS1018, in which the expression of sgcB is under the control of the constitutive ermE* promoter, was described in Materials and Methods. pBS1018 and the pKC1139 vector as a control were each introduced by conjugation into S. globisporus. Culture supernatants from each exconjugant were harvested on days 3, 4, and 5 and were assayed for C-1027 production by determining the antibacterial activity against M. luteus. While no apparent difference for C-1027 production was observed between the S. globisporus and S. globisporus(pKC1139) strains, a significant increase in C-1027 production (150% ± 25%) was evident in the early stage of S. globisporus(pBS1018) fermentation (Fig. 6D, day 3). However, such an effect on C-1027 production leveled off as the fermentation proceeded and became insignificant when the culture reached the late stationary phase of fermentation (Fig. 6D, days 4 and 5).
DISCUSSION
Our inability to clone the putative enediyne PKS gene by PCR with degenerate primers designed according to the highly conserved amino acid sequences of either type I or type II PKSs or by DNA hybridization with homologous type I or type II PKS as probes (data not shown) was unexpected, since feeding experiments by incorporation of [1-13C]- and [1,2-13C]acetate into the enediyne cores of esperamicin (21), dynemicin (50), and neocarzinostatin (9) supported their polyketide origin. Although the enediyne cores are structurally distinct from either the reduced or the aromatic polyketides, the biosynthesis of which is well characterized by type I or type II PKS, respectively, it could be imagined that an enediyne PKS catalyzes the biosynthesis of a polyunsaturated linear heptaketide intermediate that is subsequently cyclized into the enediyne core structure (14, 46, 49). Alternatively, Hensens and coworkers (9) proposed a fatty acid origin for the enediyne core that was also consistent with the isotope labeling results. Those investigators suggested that oleate is a precursor that is shortened by a loss of carbons from both ends and that is desaturated via the oleate-crepenynate pathway to furnish the enediyne core (9). The latter pathway resembles polyacetylene biosynthesis in higher plants and fungi and requires an acetylene-forming enzyme; a plant gene encoding such an enzyme was identified recently (22). We have now completed the DNA sequence analysis of approximately 60 kb of the sgc gene cluster but have failed to reveal any gene that resembles PKS. Although our current data fall short in predicting the mechanism for the biosynthesis of the C-1027 enediyne core, they are sufficient to exclude the involvement of a PKS enzyme similar to those that have been characterized for aromatic or reduced polyketide biosynthesis.
Although little is known about the resistance mechanism for the enediyne antibiotics in general, the apoproteins of the chromoprotein type of enediynes could be viewed as resistance elements that confer self-resistance to the producing organisms by drug sequestration (49). Such a resistance mechanism is in fact well established in antibiotic-producing actinomycetes, for example, BlmA, the bleomycin-binding protein from Streptomyces verticillus (42). Given the fact that antibiotic production genes have invariably been found to be clustered in one region of the microbial chromosome and to consist of structural, resistance, and regulatory genes, we adopted a strategy to clone the sgc gene cluster by mapping a putative C-1027 structural gene to the previously cloned cagA gene, considered a resistance gene that encodes the C-1027 apoprotein.
We chose NGDH as the putative C-1027 structural gene on the basis of the 4,6-dideoxy-4-dimethylamino-5-methylrhamnose moiety of the C-1027 chromophore. It has been well established that all deoxyhexoses could be derived from the common intermediate of 4-keto-6-deoxyglucose nucleoside diphosphate, the biosynthesis of which from glucose nucleoside diphosphate is catalyzed by an NGDH enzyme. We cloned the NGDH gene from S. globisporus by PCR and used it as a probe to screen an S. globisporus genomic library, resulting in the isolation of the 75-kb sgc gene cluster. DNA sequence analysis of a 3.0-kb BamHI fragment of the sgc cluster confirmed the presence of the NGDH protein, encoded by sgcA, along with sgcB, which encodes a transmembrane efflux protein (Fig. 3). The cagA gene indeed resides approximately 14 kb upstream of sgcA (Fig. 2); DNA sequence analysis of a 4.0-kb BamHI fragment confirmed the identity of cagA along with two additional ORFs (data not shown). These results underline once again the effectiveness of cloning natural-product biosynthesis gene clusters by exploiting the clustering phenomenon between resistance and structural genes.
The involvement of the cloned gene cluster in C-1027 biosynthesis was demonstrated by disrupting the sgcA gene to generate S. globisporus mutants, the ability of which to produce C-1027 was completely abolished (Fig. 6A) and by complementing the sgcA mutants in vivo upon expression of sgcA in trans to restore C-1027 production (Fig. 6B and 6C). These data unambiguously establish that sgcA is essential for C-1027 production and thus support the conclusion that the cloned gene cluster encodes C-1027 biosynthesis. It should be pointed out that although the sgcA mutants S. globisporus SB1001 and S. globisporus SB1002 were characterized as C-1027 nonproducing on the basis of the antibacterial assay alone (Fig. 6A), this phenotype was identical to that of the controls of the AF40, AF44, and AF67 mutants (Fig. 6A and C). The latter strains were isolated previously upon random mutagenization of the wild-type S. globisporus strain with acriflavine and were confirmed to be C-1027 nonproducing by both the antibacterial bioassay and an antitumor spermatogonial assay (27), providing strong support to the current study. Gene disruption and complementation in S. globisporus were made possible by the recently developed genetic system that allowed us to introduce plasmid DNA into S. globisporus via either PEG-mediated protoplast transformation (12) or E. coli-S. globisporus conjugation (4, 28, 29) for analysis of the sgc biosynthesis gene cluster in vivo. Given the difficulties encountered with calicheamicin biosynthesis in Micromonospora echinospora, into which all attempts to introduce plasmid DNA have failed (49), the latter results underscore the importance of selecting C-1027 as a model system for enediyne biosynthesis so that many of the genetic tools developed for Streptomyces species can now be directly applied to the study of enediyne biosynthesis.
Finally, the function of sgcB was probed by examining C-1027 production, following expression of the gene in the wild-type S. globisporus strain. Comparison of the deduced amino acid sequence with sequences in protein sequence databases suggested that SgcB is a transmembrane efflux protein, conferring resistance by exporting C-1027 out of the cell. Hence, in addition to CagA, SgcB could be viewed as the second resistance element identified for C-1027 biosynthesis. Multiple resistance genes have been identified in numerous antibiotic biosynthesis gene clusters (11). It could be imagined that CagA and SgcB function cooperatively to provide resistance. The C-1027 chromophore is first sequestered by binding to the preaproprotein CagA to form a complex, which is then transported out of the cell by the efflux pump SgcB and which is processed by removing the leader peptide to yield the chromoprotein, although we do not have any experimental data to substantiate this speculation. Since it is known that yields for antibiotic production could be profoundly altered by the introduction of extra copies of regulatory, resistance, or structural genes into wild-type organisms (15), we tested the effect of overexpressing sgcB in S. globisporus on C-1027 production. While no apparent adverse effect on C-1027 production was observed upon introduction of the pKC1139 vector into S. globisporus (data not shown), a significant increase in C-1027 production (150% ± 25%) was observed in the early stage of S. globisporus(pBS1017) fermentation (Fig. 6D, day 3), supporting the predicted function for SgcB in C-1027 biosynthesis. We propose that C-1027 resistance could be a limiting factor at the onset of C-1027 production, which is attenuated by the extra copy of the plasmid-borne sgcB, and overexpression of sgcB under the control of the constitutive ermE* promoter results in increase of C-1027 production. However, as the S. globisporus(pBS1017) fermentation proceeds to its stationary phase, C-1027 resistance is no longer a limiting factor for overall C-1027 production, and the effect of the extra copy of SgcB on C-1027 production consequently becomes insignificant (Fig. 6D, day 5).
In conclusion, genetic analysis of enediyne biosynthesis has heretofore met with little success, despite considerable effort (49). The localization of the sgc gene cluster and characterization of the sgcA and sgcB genes have now provided an excellent starting point for future genetic and biochemical investigations of C-1027 biosynthesis, and gene disruption and overexpression in S. globisporus demonstrated the potential to construct enediyne-overproducing strains and to produce novel enediynes that may have enhanced potency as novel anticancer drugs by combinatorial biosynthesis and targeted mutagenesis. We envisage that the results from C-1027 biosynthesis should facilitate the cloning and characterization of biosynthesis gene clusters of other enediyne antibiotics in Streptomyces as well as in other actinomycetes and could have a great impact on the overall field of combinatorial biosynthesis.
ACKNOWLEDGMENTS
We thank Yuan Li, Institute of Medicinal Biotechnology, The Chinese Academy of Medical Science, Beijing, China, for the wild-type S. globisporus and the AF40, AF44, and AF67 C-1027-nonproducing mutant strains; Douglas J. MacNeil, Merck Research Laboratories, for a culture of E. coli ET12567; C. Richard Hutchinson, University of Wisconsin, Madison, for providing pWHM3 and pWHM79 and for helpful discussion and constant encouragement; and Cesar Sanchez, Scott Standage, and Steven Christenson for critical reading of the manuscript.
This work was supported in part by a grant from the Cancer Research Coordinating Committee, University of California, the National Institutes of Health (grant CA78747), and the Searle Scholars Program/The Chicago Community Trust.
REFERENCES
- 1.August P R, Tang L, Yoon Y J, Ning S, Mueller R, Yu T W, Taylor M, Hoffmann D, Kim C G, Zhang X, Hutchinson C R, Floss H G. Biosynthesis of the ansamycin antibiotic rifamycin. Deductions from the molecular analysis of the rif biosynthetic gene cluster of Amycolatopsis mediterranei S699. Chem Biol. 1998;5:69–79. doi: 10.1016/s1074-5521(98)90141-7. [DOI] [PubMed] [Google Scholar]
- 2.Baltz R H. Genetic recombination in Streptomyces fradiae by protoplast fusion and cell regeneration. Dev Ind Microbiol. 1980;21:43–54. doi: 10.1099/00221287-107-1-93. [DOI] [PubMed] [Google Scholar]
- 3.Bibb M J, White J, Ward J M, Janssen G R. The mRNA for the 23S rRNA methylase encoded by the ermE gene of Saccharopolyspora erythraea is translated in the absence of a conventional ribosome-binding site. Mol Microbiol. 1994;14:533–545. doi: 10.1111/j.1365-2958.1994.tb02187.x. [DOI] [PubMed] [Google Scholar]
- 4.Bierman M, Logan R, O'Brien K, Seno E T, Rao R N, Schoner B E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116:43–69. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 5.Cane D E, Walsh C T, Khosla C. Harnessing the biosynthetic code: combinations, permutations, and mutations. Science. 1998;282:63–68. doi: 10.1126/science.282.5386.63. [DOI] [PubMed] [Google Scholar]
- 6.Decker H, Gaisser S, Pelzer S, Schneider P, Westrich L, Wohlleben W, Bechthold A. A general approach for cloning and characterizing dNDP-glucose dehydratase genes from actinomycetes. FEMS Lett. 1996;141:195–201. doi: 10.1111/j.1574-6968.1996.tb08384.x. [DOI] [PubMed] [Google Scholar]
- 7.Doyle T W, Borders D B, editors. Enediyne antibiotics as antitumor agents. New York, N.Y: Marcel Dekker, Inc.; 1995. [Google Scholar]
- 8.Edo K, Mizugaki M, Koide Y, Seto H, Furihata K, Otake N, Ishida N. The structure of neocarzinostatin chromophore possessing a novel bicyclo[7,3,0]dodecadiyne system. Tetrahedron Lett. 1985;26:331–340. [Google Scholar]
- 9.Hensens O D, Giner J-L, Goldberg I H. Biosynthesis of NCS chrom A, the chromophore of the antitumor antibiotic neocarzinostatin. J Am Chem Soc. 1989;111:3295–3299. [Google Scholar]
- 10.Ho S-H, Chater K F. Denaturation of circular or linear DNA facilitates targeted integrative transformation of Streptomyces coelicolor A3(2): possible relevance to other organisms. J Bacteriol. 1997;179:122–127. doi: 10.1128/jb.179.1.122-127.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopwood D A. Genetic contributions to understanding polyketide synthases. Chem Rev. 1997;97:2465–2497. doi: 10.1021/cr960034i. [DOI] [PubMed] [Google Scholar]
- 12.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, United Kingdom: John Innes Foundation; 1985. [Google Scholar]
- 13.Hu J, Xue Y-C, Xie M-Y, Zhang R, Otani T, Minami Y, Yamada Y, Marunaka T. A new macromolecular antitumor antibiotic C-1027 I. Discovery, taxonomy of producing organism, fermentation and biological activity. J Antibiot. 1988;41:1575–1579. doi: 10.7164/antibiotics.41.1575. [DOI] [PubMed] [Google Scholar]
- 14.Hu Z, Bao K, Zhou X, Zhou Q, Hopwood D A, Kieser T, Deng Z. Repeated polyketide synthase modules involved in the biosynthesis of a heptaene macrolide by Streptomyces sp. FR-008. Mol Microbiol. 1994;14:163–172. doi: 10.1111/j.1365-2958.1994.tb01276.x. [DOI] [PubMed] [Google Scholar]
- 15.Hutchinson C R. Drug synthesis by genetically engineered microorganisms. Bio/Technology. 1994;12:375–380. doi: 10.1038/nbt0494-375. [DOI] [PubMed] [Google Scholar]
- 16.Hutchinson C R, Fujii I. Polyketide synthase gene manipulation: a structure-function approach in engineering novel antibiotics. Annu Rev Microbiol. 1995;49:201–238. doi: 10.1146/annurev.mi.49.100195.001221. [DOI] [PubMed] [Google Scholar]
- 17.Iida K, Ishii T, Hirama M. Synthesis and absolute stereochemistry of the aminosugar moiety of antibiotic C-1027 chromophore. Tetrahedron Lett. 1993;34:4079–4082. [Google Scholar]
- 18.Ikemoton N, Kumar R A, Ling T T, Ellestad G A, Danishefky S J. Calicheamicin-DNA complexes—war head alignment and saccharide recognition of the minor groove. Proc Natl Acad Sci USA. 1995;92:10506–10510. doi: 10.1073/pnas.92.23.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kakavas S J, Katz L, Stassi D L. Identification and characterization of the niddamycin polyketide synthase genes from Streptomyces caelestis. J Bacteriol. 1997;179:7515–7522. doi: 10.1128/jb.179.23.7515-7522.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz L, Donadio S. Polyketide synthesis: prospects for hybrid antibiotics. Annu Rev Microbiol. 1993;47:875–912. doi: 10.1146/annurev.mi.47.100193.004303. [DOI] [PubMed] [Google Scholar]
- 21.Lam K S, Veitch J A, Golik J, Krishnan B, Klohr S E, Volk K J, Forenza S, Doyle T W. Biosynthesis of esperamicin-A(1), an enediyne antitumor antibiotic. J Am Chem Soc. 1993;115:12340–12345. [Google Scholar]
- 22.Lee M, Lenman M, Banas A, Bafor M, Singh S, Schweizer M, Nilsson R, Liljenberg C, Dahlqvist A, Gummeson P, Sjodahl S, Green A, Stymne S. Identification of non-heme diiron proteins that catalyze triple bond and epoxy group formation. Science. 1998;280:915–918. doi: 10.1126/science.280.5365.915. [DOI] [PubMed] [Google Scholar]
- 23.Linton K J, Jarvis B W, Hutchinson C R. Cloning of the genes encoding thymidine diphosphoglucose 4,6-dehydratase and thymidine diphospho-4-keto-6-deoxyglucose 3,5-epimerase from the erythromycin-producing Saccharopolyspora erythraea. Gene. 1995;153:33–40. doi: 10.1016/0378-1119(94)00809-7. [DOI] [PubMed] [Google Scholar]
- 24.Liu H-W, Thorson J S. Pathways and mechanisms in the biosynthesis of novel deoxysugars by bacteria. Annu Rev Microbiol. 1994;48:223–256. doi: 10.1146/annurev.mi.48.100194.001255. [DOI] [PubMed] [Google Scholar]
- 25.Lombo F, Siems K, Brana A F, Mendez C, Bindseil K, Salas J A. Cloning and insertional inactivation of Streptomyces argillaceus genes involved in the earliest steps of biosynthesis of the sugar moieties of the antitumor polyketide mithramycin. J Bacteriol. 1997;179:3354–3357. doi: 10.1128/jb.179.10.3354-3357.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacNeil D J, Gewain K M, Ruby C L, Dezney G, Gibbons P H, MacNeil T. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene. 1992;111:61–68. doi: 10.1016/0378-1119(92)90603-m. [DOI] [PubMed] [Google Scholar]
- 27.Mao X, Li Y, Shi L. With random amplified polymorphic DNA technique to study antibiotic biosynthesis gene cloning and expression. Chinese J Biotechnol. 1997;13:195–199. [Google Scholar]
- 28.Matsushima P, Baltz R H. A gene cloning system for ‘Streptomyces toyocaensis.’. Microbiology. 1996;142:261–267. doi: 10.1099/13500872-142-2-261. [DOI] [PubMed] [Google Scholar]
- 29.Matsushima P, Broughton M C, Turner J R, Baltz R H. Conjugal transfer of cosmid DNA from Escherichia coli to Saccharopolyspora spinosa: effects of chromosomal insertions on macrolide A83543 production. Gene. 1994;146:39–45. doi: 10.1016/0378-1119(94)90831-1. [DOI] [PubMed] [Google Scholar]
- 30.Mazodier H, Petter R, Thompson C. Intergeneric conjugation between Escherichia coli and Streptomyces species. J Bacteriol. 1989;171:3583–3585. doi: 10.1128/jb.171.6.3583-3585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merson-Davies L A, Cundliffe E. Analysis of five tylosin biosynthetic genes from the tyllBA region of the Streptomyces fradiae genome. Mol Microbiol. 1994;13:349–355. doi: 10.1111/j.1365-2958.1994.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 32.Minami Y, Yoshida K, Azuma R, Saeki M, Otani T. Structure of an aromatization product of C-1027 chromophore. Tetrahedron Lett. 1993;34:2633–2636. [Google Scholar]
- 33.Muth G, Nussbaumer B, Wohlleben W, Puhler A. A vector system with temperature-sensitive replication for gene disruption and mutational cloning in streptomycetes. Mol Gen Genet. 1989;219:341–348. [Google Scholar]
- 34.Myers A G, Kort M E, Hammond M. A comparison of DNA cleavage by neocarzinostatin chromophore and its aglycon: evaluating the role of the carbohydrate residue. J Am Chem Soc. 1997;119:2965–2972. [Google Scholar]
- 35.Okuno Y, Otsuka M, Sugiura Y. Computer modeling analysis for enediyne chromophore-apoprotein complex of macromolecular antibiotic C-1027. J Med Chem. 1994;37:2266–2273. doi: 10.1021/jm00041a004. [DOI] [PubMed] [Google Scholar]
- 36.Otani T, Yasuhara T, Minami Y, Shimazu T, Zhang R, Xie M. Purification and primary structure of C-1027-AG, a selective antagonist of antitumor antibiotic C-1027, from Streptomyces globisporus. Agri Biol Chem. 1991;55:407–417. [PubMed] [Google Scholar]
- 37.Piepersberg W. Molecular biology, biochemistry, and fermentation of aminoglycoside antibiotics. In: Strohl W R, editor. Biotechnology of antibiotics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 81–163. [Google Scholar]
- 38.Rao R N, Richardson M A, Kuhstoss S. Cosmid shuttle vectors for cloning and analysis of Streptomyces DNA. Methods Enzymol. 1987;153:166–198. doi: 10.1016/0076-6879(87)53053-1. [DOI] [PubMed] [Google Scholar]
- 39.Sakata N, Ikeno S, Hori M, Hamada M, Otani T. Cloning and nucleotide sequencing of the antitumor antibiotic C-1027 apoprotein gene. Biosci Biotechnol Biochem. 1992;56:1592–1595. doi: 10.1271/bbb.56.1592. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 41.Seow K-T, Meurer G, Gerlitz M, Wendt-Pienkowski E, Hutchinson C R, Davies J. A study of chain-length determination in iterative type II polyketide synthases using genes cloned from soil DNA. J Bacteriol. 1997;179:7360–7368. doi: 10.1128/jb.179.23.7360-7368.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen B, Du L, Sanchez C, Chen M, Edwards D J. Bleomycin biosynthesis in Streptomyces verticillus ATCC 15003: a model of hybrid peptide and polyketide biosynthesis. Bioorg Chem. 1999;27:155–171. [Google Scholar]
- 43.Shen B, Hutchinson C R. Deciphering the mechanism for the assembly of aromatic polyketides by a bacterial polyketide synthase. Proc Natl Acad Sci USA. 1996;93:6600–6604. doi: 10.1073/pnas.93.13.6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sievers E L, Appelbaum F R, Spielberger R T, Forman S J, Flowers D, Smith F O, Shannon-Dorcy K, Berger M S, Bernstein I D. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood. 1999;93:3678–3684. [PubMed] [Google Scholar]
- 45.Sommer P, Bormann C, Gotz F. Genetic and biochemical characterization of a new extracellular lipase from Streptomyces cinnamomeus. Appl Environ Microbiol. 1997;63:3553–3560. doi: 10.1128/aem.63.9.3553-3560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spaink H P, Sheeley D M, van Brussel A A N, Glushka J, York W S, Tak T, Geiger O, Kennedy E P, Reinhold V N, Lugtenberg B J J. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature. 1991;354:125–130. doi: 10.1038/354125a0. [DOI] [PubMed] [Google Scholar]
- 47.Stassinopoulos A, Ji J, Gao X, Goldberg I H. Solution structure of a two-base DNA bulge complexed with an enediyne cleaving analog. Science. 1996;272:1943–1946. doi: 10.1126/science.272.5270.1943. [DOI] [PubMed] [Google Scholar]
- 48.Takiff H E, Cimino M, Musso M C, Weisbrod T, Martinez R, Delgado M B, Salazar L, Bloom B R, Jacobs W R., Jr Efflux pump of the proton antiporter family confers low-level fluoroquinolone resistance in Mycobacterium smegmatis. Proc Natl Acad Sci USA. 1996;93:362–366. doi: 10.1073/pnas.93.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thorson J S, Shen B, Whitwam R E, Liu W, Li Y, Ahlert J. Enediyne biosynthesis and self-resistance: a progress report. Bioorg Chem. 1999;27:172–188. [Google Scholar]
- 50.Tokiwa Y, Miyoshi-Saitosh M, Kobayashi H, Sunaga R, Konishi M, Oki T, Iwasaki S. Biosynthesis of dynemicin A, a 3-ene-1,5-diyne antitumor antibiotic. J Am Chem Soc. 1992;114:4107–4110. [Google Scholar]
- 51.Vara J, Lewandowska-Sharbek M, Wang Y-G, Donadio S, Hutchinson C R. Cloning of genes governing the deoxysugar portion of the erythromycin biosynthesis pathway in Saccharopolyspora erythraea (Streptomyces erythreus) J Bacteriol. 1989;171:5872–5881. doi: 10.1128/jb.171.11.5872-5881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vara J A, Hutchinson C R. Purification of thymidine-diphospo-d-glucose 4,6-dehydratase from an erythromycin-producing strain of Saccharopolyspora erythraea by high resolution liquid chromatography. J Biol Chem. 1988;263:14992–14995. [PubMed] [Google Scholar]
- 53.Xu Y, Zhen Y, Goldberg I H. Enediyne C-1027 induces the formation of novel covalent DNA interstrand cross-links and monoadducts. J Am Chem Soc. 1997;119:1133–1134. [Google Scholar]
- 54.Yoshida K, Minami Y, Azuma R, Saeki M, Otani T. Structure and cycloaromatization of a novel enediyne, C-1027 chromophore. Tetrahedron Lett. 1993;34:2637–2640. [Google Scholar]
- 55.Zhen Y-S, Ming X-Y, Yu B, Otani T, Saito H, Yamada Y. A new macromolecular antitumor antibiotic, C-1027. III. Antitumor activity. J Antibiot. 1989;42:1294–1298. doi: 10.7164/antibiotics.42.1294. [DOI] [PubMed] [Google Scholar]