This case series characterizes the predominant autoantigen in Orf-induced immunobullous disease and further describes this clinical entity.
Key Points
Question
What is the predominant autoantigen in orf-induced immunobullous disease?
Findings
In this case series of 5 patients, autoantibodies in orf-induced immunobullous disease were mainly directed against laminin 332. Orf-induced anti–laminin 332 pemphigoid was characterized by (1) predominant skin involvement with tense blisters and erythema, (2) relatively young age compared with bullous pemphigoid and mucous membrane pemphigoid, (3) limited disease course, and (4) IgG1 and IgG3 as predominant autoantibody subclasses.
Meaning
Orf-induced anti–laminin 332 pemphigoid can be regarded as a clinically and immunologically distinct entity; the present study highlights the importance of testing for serum anti–laminin 332 IgG in all patients with suspected orf-induced immunobullous disease.
Abstract
Importance
Ecthyma contagiosum, or orf, is a viral zoonotic infection caused by Poxviridae. Although human orf infection is considered to follow a self-limited course, various immunological reactions may be triggered, including immunobullous diseases. In the majority of the latter cases, the antigenic target remained enigmatic.
Objective
To characterize the predominant autoantigen in orf-induced immunobullous disease and further describe this clinical entity.
Design, Setting, and Participants
This multicenter case series sought to provide detailed clinical, histopathological and immunological characteristics of a patient with orf-induced pemphigoid. Based on this index patient, serological analyses were conducted of 4 additional patients with previously reported orf-induced immunobullous disease. Immunoblotting with extracellular matrix and a recently established indirect immunofluorescence assay for detection of serum anti–laminin 332 IgG were performed.
Exposures
The disease course and clinical characteristics of orf-induced immunobullous disease were observed.
Main Outcomes and Measures
Orf-induced immunobullous disease is primarily characterized by anti–laminin 332 autoantibodies, predominant skin involvement, and a self-limiting course. The study provides further details on epidemiological, clinical, immunopathological, diagnostic, and therapeutic aspects of orf-induced immunobullous disease.
Results
In all 5 patients, IgG1 and/or IgG3 autoantibodies against laminin 332 were identified. The α3, β3, and γ2 chains were recognized in 2, 4, and 1 patient(s), respectively.
Conclusions and Relevance
In this case series, laminin 332, a well-known target antigen in mucous membrane pemphigoid, was a major autoantigen in orf-induced immunobullous disease, even though predominant mucosal lesions were lacking in this autoimmune blistering disease. Orf-induced anti–laminin 332 pemphigoid is proposed as distinct clinical entity.
Introduction
Orf, also known as ecthyma contagiosum, is a zoonotic viral infection that primarily affects small ruminants caused by the orf virus of the Poxviridae family. While orf can lead to severe complications in animals, it is deemed to follow a benign course with spontaneous resolution in humans. However, human orf has recently generated considerable interest owing to increasing evidence of its role in inducing autoimmune bullous diseases (AIBDs).1,2,3,4,5,6,7 Previously reported cases of orf-induced AIBDs have all been characterized as subepidermal blistering disorders. Hereby, all but 1 patient showed mucocutaneous lesions with predominant skin involvement.1,3 The specific target antigen has so far remained elusive in most patients with orf-induced AIBDs.
Methods
Direct immunofluorescence (IF) of perilesional biopsies and indirect IF microscopy on normal human skin split with a 1-molar sodium chloride solution (1 M NaCl) were performed as described previously.8 Indirect IF was performed using skin lacking laminin 332 or type VII collagen (Col7) derived from patients with junctional and dystrophic epidermolysis bullosa deficient of laminin 332 and Col7, respectively.5,9 For the detection of serum anti–laminin 332 reactivity, a recently developed indirect IF test based on the recombinant expression of laminin 332 (Euroimmun)10 and immunoblotting with extracellular matrix of cultured human keratinocytes were used (eMethods in the Supplement). Reactivity against the p200 protein and Col7 was analyzed by immunoblotting (eMethods in the Supplement).
Because all analyses were performed as part of an extensive routine diagnostic workup, ethical approval was not required by University of Lübeck. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Results
Index Case
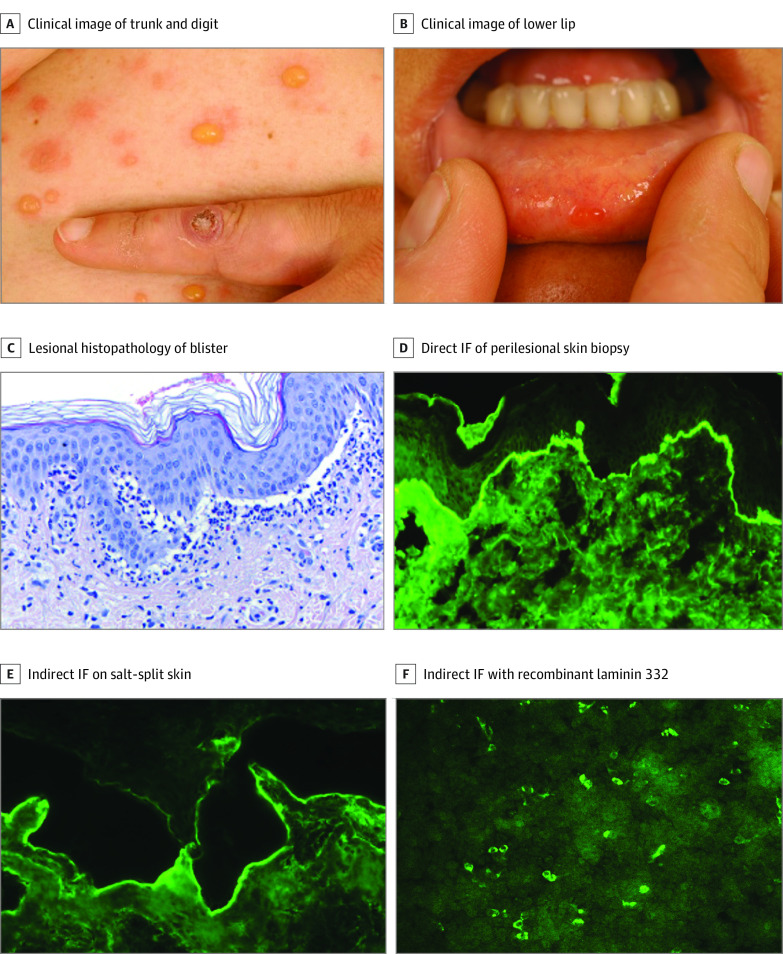
A patient in their late 30s presented with a 1-week history of tense blisters predilected to the trunk and extremities and mucosal erosions on the soft palate and lower lip. On the left index finger, a solitary, firm, violaceous nodule was present (Figure 1A and B). This lesion reportedly arose about 6 weeks prior to presentation, shortly after bottle-feeding a lamb with typical orf lesions. Polymerase chain reaction analysis of the inciting lesion yielded parapoxvirus DNA. Lesional histopathology of a blister showed a subepidermal splitting and a lymphocytic infiltrate in the upper dermis with admixed eosinophils (Figure 1C). Direct IF microscopy of a biopsy in close vicinity of a blister demonstrated linear depositions of IgG, complement C3, and, to a lesser extent, IgA along the basement membrane zone (Figure 1D). Upon splitting with 1 M NaCl, C3 labeled the dermal side of the artificial blister (data not shown). Concordantly, indirect IF microscopy on salt-split human skin revealed IgG deposits at the blister floor (Figure 1E). Based on these findings, the provisional diagnosis of orf-induced AIBD was made with Col7, laminin 332, and p200 protein as putative target antigens. Immunoblotting with human dermis, the recombinant NC1 domain of Col7, and extracellular matrix of cultured human keratinocytes was unreactive for IgG4 autoantibodies. Treatment with oral prednisone (0.5 mg/kg tapering) and dapsone (1.0 mg/kg/d) combined with topical clobetasol propionate, 0.05%, ointment led to complete remission within 2 weeks. Two months after therapy initiation, no circulating autoantibodies were detectable by indirect IF on human salt-split skin.
Figure 1. Orf-Induced Anti–Laminin 332 (Bullous) Pemphigoid of the Index Patient (Case 1).
A, Tense blisters and vesicles on the trunk. On the dorsum of the left second digit, a solitary crusted nodule is seen, representing the original orf lesion. B, A small vesicle on the inner side of the lower lip. C, Histopathology (hematoxylin-eosin) of an abdominal lesional skin biopsy reveals subepidermal splitting and a dense inflammatory infiltrate at the dermal-epidermal junction (DEJ) predominant of neutrophils forming microabscesses as well as eosinophils and lymphocytes. D, Direct immunofluorescence (IF) microscopy of perilesional skin with linear deposition of IgG along the DEJ in an n-serrated pattern. E, Indirect IF on normal salt-split skin with IgG deposits along the dermal side of the artificial split. F, IgG1 against recombinant laminin 332 using the Biochip mosaic.
Reactivity Against Laminin 332
By indirect IF with skin deficient for laminin 332 or Col7, serum of the index patient (case 1) showed circulating IgG against Col7-deficient but not laminin 332-deficient skin (Figure 2). When the serum was subjected to indirect IF with recombinant laminin 332, IgG1 and IgG3 reactivity against the heterotrimer and the β3 chain was detected (Figure 1F; eFigure 1 in the Supplement). This reactivity was also seen by immunoblotting with extracellular matrix of cultured human keratinocytes (eFigure 2 in the Supplement; Table).
Figure 2. Indirect Immunofluorescence of Orf-Induced Anti–Laminin 332 (Bullous) Pemphigoid of the Index Patient (Case 1).
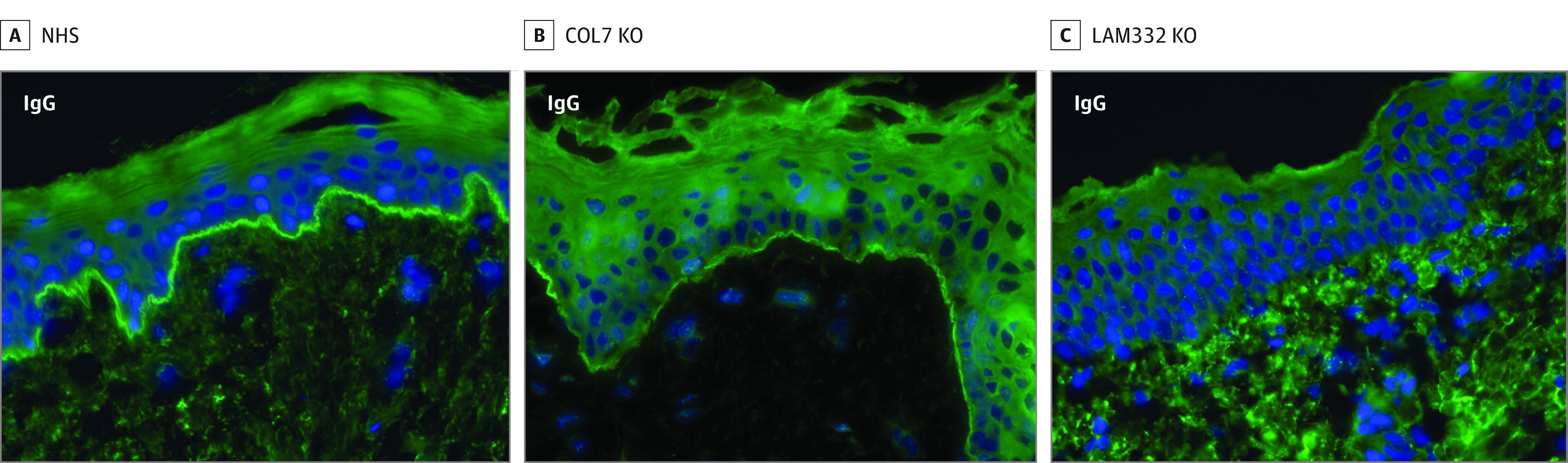
Linear deposition of IgG at the dermal-epidermal junction on normal human skin (NHS) (A) and type VII collagen-deficient knockout skin (COL7 KO) (B). In contrast, no serum IgG antibodies are seen with laminin-332 knockout skin (LAM332 KO) (C).
Table. Clinical and Immunological Features of the Index Patient and 4 Previously Reported Cases of Orf-Induced Pemphigoid.
| Patient No./sex/age | Interval between orf and blister formation, wk | Direct IF BMZ | Indirect IF on salt-split skin | Clinical featuresa | Laminin 332 Biochip | IB extracellular matrix | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Skin | Mucous membranes | IgG1 | IgG3 | IgG1 | IgG3 | |||||
| 1/30s | 5 | C3, IgG, IgA (n-serrated) | IgG, C3 dermal side | ++ | + | α3β3γ2+ | α3β3γ2+ | β3+ | β3+ | Index patient |
| Oral | β3+ | β3+ | α3 (Weak) | |||||||
| 2/M/31 | 4 | C3, IgG | IgG, dermal side | ++ | + | α3β3γ2+ | α3β3γ2+ | γ2+ | α3+ | White et al,4 2008 |
| Oral, ocular | β3+ | |||||||||
| 3/F/48 | 2 | C3, IgG | IgG, dermal side | ++ | + | α3β3γ2+ | α3β3γ2+ | NP | β3+ | White et al,4 2008 |
| Oral | ||||||||||
| 4/F/52b | Several | IgG, C3, IgA (n-serrated) | IgG, dermal side | ++ | + | α3β3γ2+ | α3β3γ2+ | NP | β3+ | van den Bos et al,5 2012 |
| Oral | ||||||||||
| 5/M/41c | 4 | IgG, C3 | IgG, dermal side | ++ | + | α3β3γ2+ | α3β3γ2+ | NP | Negative | Zuelgaray et al,6 2018 |
| Oral, nasal | ||||||||||
Abbreviations: BMZ, basement membrane zone; F, female; IB, immunoblot; IF, immunofluorescence; M, male; NP, not performed.
The plus symbol indicates the presence of cutaneous and/or mucosal lesions; ++ indicates predominant involvement of the skin compared with the mucous membranes.
Reactivity against laminin 332 has previously been described in this case.
Previously described as orf-induced epidermolysis bullosa acquisita; in the current study, no anti–type VII collagen reactivity was present by enzyme-linked immunosorbent assay (Euroimmun) and IB with dermal extract (data not shown).
Subsequently, available serum samples of 5 additional patients with orf-triggered AIBD that had been reported in the literature before (cases 2-5, case 124,5,6,7) were reassayed by indirect IF using the Biochip mosaic (Euroimmun) and, in part, immunoblotting with extracellular matrix of cultured human keratinocytes. All serum samples except case 12 revealed IgG autoantibodies against laminin 332 (Table; eFigures 3-6 in the Supplement).7
Discussion
Although human orf is a self-limiting condition, it has been recently associated with AIBD1,3,4,5,6,7 (Table; eTable in the Supplement). In most cases, the AIBD commenced 2 to 4 weeks after the emergence of the orf lesion. The median age of individuals with orf-induced AIBD was 46.4 years. In 8 of the 13 cases (62%) known to be described so far, mucosal lesions were present, while predominant mucosal involvement has only been reported in 1 (Table; eTable in the Supplement). The latter patient was consequently diagnosed with orf-induced mucous membrane pemphigoid (MMP).3,11 All patients with mucosal involvement presented with oral lesions followed by involvement of nostrils (3 of 13 [23%]) and conjunctivae (2 of 13 [15%]). To date, the target antigen in orf-triggered AIBD has remained unknown in all but 3 cases, which included reactivity against Col7 (cases 5 and 12)6,7 and against laminin 332 (case 4).5
Here, we describe in detail a patient with orf-induced anti–laminin 332 (bullous) pemphigoid and detected serum anti–laminin 332 reactivity in another 4 previously reported patients with orf-induced AIBD. Our findings of circulating anti–laminin 332 IgG in these patients by a recently established indirect IF test10 was corroborated by (1) the lack of serum reactivity with laminin 332-deficient skin, (2) detection of laminin 332–specific IgG also by immunoblotting with extracellular extract of cultured keratinocytes, and (3) laminin 332 reactivity in the only previously reported orf-induced AIBD with anti–laminin 332 antibodies (case 4). In case 5, low levels of serum autoantibodies against Col7 have originally been reported,6 which were undetectable in the present study. We hypothesize that in case 5, laminin 332 was the predominant target antigen, and low levels of anti-Col7 antibodies had become undetectable during storage or transport. Concomitant autoantibodies against laminin 332 and Col7 have been previously reported in AIBD and may be attributed to intermolecular epitope spreading.12 In the only other known case of orf-induced AIBD with Col7-specific antibodies (case 12),7 we were unable to detect anti–laminin 332 reactivity. Thus, this patient can be classified as having orf-induced epidermolysis bullosa acquisita.
Laminin 332 has been described as target antigen in 10% to 25% of patients with MMP.13 Two main differences between anti–laminin 332 reactivity in MMP and orf-induced (bullous) pemphigoid can be recognized. Mucosal involvement in anti–laminin 332 MMP, by definition, predominates over skin lesions.11 By contrast, in orf-induced (bullous) pemphigoid, mucosal lesions are present in about 62% of patients but do not predominate, except in 1 case of orf-induced MMP in which the target antigen remained elusive (case 13, eTable in the Supplement).3 The anti–laminin 332 IgG subclass is the second main difference between anti–laminin 332 MMP and orf-induced (bullous) pemphigoid. In latter patients, IgG1 and IgG3 comprise the main autoantibody IgG subclasses, whereas IgG4 autoantibodies can nearly always be detected in anti–laminin 332 MMP.10,14 This is in line with the fact that viral infections generally lead to IgG1 and IgG3 subclasses which, in contrast to IgG4, are potent complement activators. Compared with the shorter half-life of IgG3, IgG4 is usually formed after repeated or long-term antigen exposure.15 The observed difference in the autoantibody subclasses could be a possible explanation for the predominant skin involvement in orf-induced AIBD.
Limitations
Limitations of the study include its retrospective design and the low number of cases.
Conclusions
In this case series, autoantibodies in orf-induced immunobullous disease were predominantly directed against laminin 332, which highlights the importance of testing for serum anti–laminin 332 IgG1 and IgG3 in all patients with this disease (eDiscussion in the Supplement).
eMethods.
eDiscussion.
eFigure 1. Serological diagnosis of orf-induced anti-laminin 332 pemphigoid of the index patient (Case 1)
eFigure 2. Immunoblot analysis of orf-induced (bullous) pemphigoid
eFigure 3. IgG1 reactivity against laminin 332 by IF microscopy using Biochip™ mosaic
eFigure 4. Detection of IgG3 autoantibodies against laminin 332 by IF microscopy using Biochip™ mosaic
eFigure 5. Positive and negative controls for IgG3 reactivity against laminin 332 by IF microscopy using Biochip™ mosaic
eFigure 6. Analysis of IgG subclass-specific autoantibodies against laminin 332 of the index patient (Case 1) using Biochip™ mosaic
eTable. Clinical and immunological aspects of the 7 other hitherto published cases of orf-induced pemphigoid
eReferences.
References
- 1.Murphy JK, Ralfs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134(5):929-930. doi: 10.1111/j.1365-2133.1996.tb06328.x [DOI] [PubMed] [Google Scholar]
- 2.Macfarlane AW. Human orf complicated by bullous pemphigoid. Br J Dermatol. 1997;137(4):656-657. doi: 10.1111/j.1365-2133.1997.tb03813.x [DOI] [PubMed] [Google Scholar]
- 3.van Lingen RG, Frank RG, Koopman RJ, Jonkman MF. Human orf complicated by mucous membrane pemphigoid. Clin Exp Dermatol. 2006;31(5):711-712. doi: 10.1111/j.1365-2230.2006.02162.x [DOI] [PubMed] [Google Scholar]
- 4.White KP, Zedek DC, White WL, et al. Orf-induced immunobullous disease: a distinct autoimmune blistering disorder. J Am Acad Dermatol. 2008;58(1):49-55. doi: 10.1016/j.jaad.2007.08.029 [DOI] [PubMed] [Google Scholar]
- 5.van den Bos RR, Middelburg T, van Biezen P, van der Eijk AA, Pas HH, Diercks GF. Orf-induced pemphigoid with antilaminin-332 antibodies. Br J Dermatol. 2012;167(4):956-958. doi: 10.1111/j.1365-2133.2012.11005.x [DOI] [PubMed] [Google Scholar]
- 6.Zuelgaray E, Salle de Chou C, Gottlieb J, et al. Human orf complicated by epidermolysis bullosa acquisita. Br J Dermatol. 2018;178(2):547-550. doi: 10.1111/bjd.15496 [DOI] [PubMed] [Google Scholar]
- 7.Daneshpazhooh M, Mahmoudi H, Toosi R, Tavakolpour S, Schmidt E, Zillikens D. Post-orf epidermolysis bullosa acquisita. J Eur Acad Dermatol Venereol. 2019;33(3):e118-e119. doi: 10.1111/jdv.15299 [DOI] [PubMed] [Google Scholar]
- 8.van Beek N, Krüger S, Fuhrmann T, et al. Multicenter prospective study on multivariant diagnostics of autoimmune bullous dermatoses using the BIOCHIP technology. J Am Acad Dermatol. 2020;83(5):1315-1322. doi: 10.1016/j.jaad.2020.01.049 [DOI] [PubMed] [Google Scholar]
- 9.Vodegel RM, de Jong MC, Pas HH, Yancey KB, Jonkman MF. Anti-epiligrin cicatricial pemphigoid and epidermolysis bullosa acquisita: differentiation by use of indirect immunofluorescence microscopy. J Am Acad Dermatol. 2003;48(4):542-547. doi: 10.1067/mjd.2003.99 [DOI] [PubMed] [Google Scholar]
- 10.Goletz S, Probst C, Komorowski L, et al. A sensitive and specific assay for the serological diagnosis of antilaminin 332 mucous membrane pemphigoid. Br J Dermatol. 2019;180(1):149-156. doi: 10.1111/bjd.17202 [DOI] [PubMed] [Google Scholar]
- 11.Rashid H, Meijer JM, Diercks GFH, et al. Assessment of diagnostic strategy for mucous membrane pemphigoid. JAMA Dermatol. 2021;157(7):780-787. doi: 10.1001/jamadermatol.2021.1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishida E, Nishio E, Murashima H, Ishii N, Hashimoto T, Morita A. Case of epidermolysis bullosa acquisita with concomitant anti-laminin-332 antibodies. J Dermatol. 2018;45(4):472-474. doi: 10.1111/1346-8138.14169 [DOI] [PubMed] [Google Scholar]
- 13.Schmidt E, Rashid H, Marzano AV, et al. European guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology—part II. J Eur Acad Dermatol Venereol. 2021;35(10):1926-1948. doi: 10.1111/jdv.17395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu R, Lazarova Z, Yee C, Yancey KB. Noncomplement fixing, IgG4 autoantibodies predominate in patients with anti-epiligrin cicatricial pemphigoid. J Invest Dermatol. 1997;109(4):557-561. doi: 10.1111/1523-1747.ep12337073 [DOI] [PubMed] [Google Scholar]
- 15.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eDiscussion.
eFigure 1. Serological diagnosis of orf-induced anti-laminin 332 pemphigoid of the index patient (Case 1)
eFigure 2. Immunoblot analysis of orf-induced (bullous) pemphigoid
eFigure 3. IgG1 reactivity against laminin 332 by IF microscopy using Biochip™ mosaic
eFigure 4. Detection of IgG3 autoantibodies against laminin 332 by IF microscopy using Biochip™ mosaic
eFigure 5. Positive and negative controls for IgG3 reactivity against laminin 332 by IF microscopy using Biochip™ mosaic
eFigure 6. Analysis of IgG subclass-specific autoantibodies against laminin 332 of the index patient (Case 1) using Biochip™ mosaic
eTable. Clinical and immunological aspects of the 7 other hitherto published cases of orf-induced pemphigoid
eReferences.