Abstract
The conclusions of the European Food Safety Authority (EFSA) following the peer review of the initial risk assessments carried out by the competent authority of the rapporteur Member State, Italy, for the pesticide active substance Aspergillus flavus strain MUCL54911 and the considerations as regards the inclusion of the substance in Annex IV of Regulation (EC) No 396/2005 are reported. The context of the peer review was that required by Regulation (EC) No 1107/2009 of the European Parliament and of the Council. The conclusions were reached on the basis of the evaluation of the representative use of Aspergillus flavus strain MUCL54911 as a biocontrol agent for reduction of aflatoxins contamination on maize (field application). The reliable endpoints, appropriate for use in regulatory risk assessment, are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are identified.
Keywords: Aspergillus flavus strain MUCL54911, peer review, risk assessment, pesticide, biocontrol agent
Summary
Aspergillus flavus strain MUCL54911 is a new active substance for which, in accordance with Article 7 of Regulation (EC) No 1107/2009 of the European Parliament and of the Council, the rapporteur Member State (RMS), Italy, received a first application from Pioneer Hi‐Bred Italia S.r.l. on 2 July 2018 and a second submission on 19 February 2020 for approval. In addition, the applicant submitted an application for inclusion of the substance in Annex IV of Regulation (EC) No 396/2005. Complying with Article 9 of the Regulation, the completeness of the dossier was checked by the RMS and the date of admissibility of the application was recognised as being 14 July 2020.
An initial evaluation of the dossier on Aspergillus flavus strain MUCL54911 was provided by the RMS in the draft assessment report (DAR), and subsequently, a peer review of the pesticide risk assessment on the RMS evaluation was conducted by EFSA in accordance with Article 12 of Regulation (EC) No 1107/2009. The following conclusions are derived.
The use of Aspergillus flavus strain MUCL54911 according to the representative use by field application by tractor‐mounted fertiliser spreader on maize, as a biocontrol agent for reduction of aflatoxins contamination, as proposed at EU level results in a sufficient fungicidal efficacy against the toxigenic strains of Aspergillus flavus.
The assessment of the data package revealed no issues that need to be included as critical areas of concern with respect to the identity, physical and technical properties of the representative formulation. A key issue identified was that information was considered insufficient to unequivocally identify Aspergillus flavus strain MUCL54911 at strain level. It is considered important to have a method using molecular tools that permits to unequivocally identify Aspergillus flavus strain MUCL54911 at strain level.
In the area of mammalian toxicology, a conclusion could not be made on the pathogenicity and infectivity potential of Aspergillus flavus strain MUCL54911. Moreover, the risk assessment for metabolites from non‐dietary exposure could not be finalised due to lack of identification/quantification and toxicological assessment.
In the area of residues, the consumer risk assessment cannot be finalised because a pathogenicity and infectivity potential of viable residues of Aspergillus flavus strain MUCL54911 cannot be excluded. It has to be noted that significant consumer exposure via the diet (viable residues under conditions of the representative use are reported between 9 × 103 CFU/g and 1.3 × 105 CFU/g maize grain) is indicated. Furthermore, for non‐viable residues, several data gaps were identified for qualitative and quantitative information on possible occurrence of metabolites of potential health concern other than those analysed for (aflatoxins B1, B2, G1 and G2, cyclopiazonic acid (CPA), sterigmatocystin (ST) and kojic acid) at harvest under conditions of the representative use. Frozen sample storage stability studies analysing non‐viable residues at harvest in maize grain and data on residues in the rest of the plant at harvest are missing.
Consequently, the consumer risk assessment cannot be finalised for the representative use on maize. Further risk management considerations are required to decide whether Aspergillus flavus strain MUCL54911 can be included into Annex IV of Regulation (EC) No 396/2005.
An assessment not finalised regarding the risk assessment to aquatic organisms has as a contributing factor, the fact that information to address the viability/population dynamics in water/sediment of Aspergillus flavus strain MUCL54911, relating to persistence and multiplication in natural surface water systems was not available. Satisfactory information to demonstrate that, under the conditions of use, any secondary metabolites/toxins produced by Aspergillus flavus strain MUCL54911 will not occur in the environmental compartments in concentrations considerably higher than under natural conditions is missing. Consequently, further data on the persistence, transformation and mobility of these compounds may be needed in order to assess the potential level of environmental exposure including the exposure of groundwater. This has led to other assessments not finalised.
The risk assessment to non‐target organisms: birds, wild mammals, aquatic organisms, honeybees, non‐target arthropods, earthworms, and other soil macro‐ and microorganisms could not be finalised.
Background
Regulation (EC) No 1107/2009 of the European Parliament and of the Council 1 (hereinafter referred to as ‘the Regulation’) lays down, inter alia, the detailed rules as regards the procedure and conditions for approval of active substances. This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States and the applicant(s) for comments on the initial evaluation in the draft assessment report (DAR), provided by the rapporteur Member State (RMS), and the organisation of an expert consultation, where appropriate.
In accordance with Article 12 of the Regulation, EFSA is required to adopt a conclusion on whether an active substance can be expected to meet the approval criteria provided for in Article 4 of the Regulation (also taking into consideration recital (10) of the Regulation) within 120 days from the end of the period provided for the submission of written comments, subject to an extension of 30 days where an expert consultation is necessary, and a further extension of up to 150 days where additional information is required to be submitted by the applicant(s) in accordance with Article 12(3).
Aspergillus flavus strain MUCL54911 is a new active substance for which, in accordance with Article 7 of the Regulation, the RMS, Italy (hereinafter referred to as the ‘RMS’), received a first application from Pioneer Hi‐Bred Italia S.r.l. on 2 July 2018 and a second submission on 19 February 2020 for approval of the active substance Aspergillus flavus strain MUCL54911. In addition, the applicant submitted an application for inclusion of the substance in Annex IV of Regulation (EC) No 396/2005. 2 Complying with Article 9 of the Regulation, the completeness of the dossier was checked by the RMS and the date of admissibility of the application was recognised as being 14 July 2020.
The RMS provided its initial evaluation of the dossier on Aspergillus flavus strain MUCL54911 in the DAR, which was received by EFSA on 20 July 2020 (Italy, 2020). The peer review was initiated on 14 October 2020 by dispatching the DAR to the Member States and the applicant, Pioneer Hi‐Bred Italia S.r.l., for consultation and comments. EFSA also provided comments. In addition, EFSA conducted a public consultation on the DAR. The comments received were collated by EFSA and forwarded to the RMS for compilation and evaluation in the format of a reporting table. The applicant was invited to respond to the comments in column 3 of the reporting table. The comments and the applicant’s response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 12(3) of the Regulation were considered in a telephone conference between EFSA and the RMS on 10 February 2021. On the basis of the comments received, the applicant’s response to the comments and the RMS’s evaluation thereof, it was concluded that additional information should be requested from the applicant, and that EFSA should conduct an expert consultation in the areas of effects on human health of the microorganism and of the plant protection product.
The outcome of the telephone conference, together with EFSA’s further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
In accordance with Article 12 of the Regulation, EFSA should adopt a conclusion on whether Aspergillus flavus strain MUCL54911 can be expected to meet the approval criteria provided for in Article 4 of the Regulation, taking into consideration recital (10) of the Regulation.
A final consultation on the conclusions arising from the peer review of the risk assessment took place with Member States via a written procedure in January–February 2022.
This conclusion report summarises the outcome of the peer review of the risk assessment on the active substance and the representative formulation evaluated on the basis of the representative use of Aspergillus flavus strain MUCL54911 as a biocontrol agent for reduction of aflatoxins contamination on maize (field application) as proposed by the applicant. In accordance with Article 12(2) of Regulation (EC) No 1107/2009, risk mitigation options identified in the DAR and considered during the peer review, if any, are presented in the conclusion.
Furthermore, this conclusion also addresses the requirement for an assessment by EFSA under Article 12 of Regulation (EC) No 396/2005, provided that the active substance will be approved under Regulation (EC) No 1107/2009 without restrictions affecting the residue assessment. In the event of a non‐approval of the active substance or an approval with restrictions that have an impact on the residue assessment, the Annex IV proposal from this conclusion might not be relevant any longer and a new assessment under Article 12 of Regulation (EC) No 396/2005 will be required.
A list of the relevant end points for the active substance and the formulation is provided in Appendix A.
A key supporting document to this conclusion is the peer review report (EFSA, 2022), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the DAR;
the reporting table (10 February 2021);
the evaluation table (23 February 2022);
the report of the scientific consultation with Member State experts;
the comments received on the assessment of the additional information;
the comments received on the draft EFSA conclusion.
Given the importance of the DAR, including its revisions (Italy, 2021), and the peer review report, both documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The identity of the microorganism and the properties of the formulated product
Aspergillus flavus strain MUCL54911 is a filamentous fungus deposited at the Belgian Co‐Ordinated Collections of Microorganisms (BCCM/MUCL) under the accession number MUCL54911. It is a wild‐type strain isolated from maize kernels collected in a natural, indigenous Italian population.
The representative formulated product for the evaluation was ‘AF‐X1’, a granule (GR) containing 1 × 108 CFU/kg (0.008 g/kg) Aspergillus flavus strain MUCL54911.
The representative use evaluated comprises field application by tractor‐mounted fertiliser spreader on maize, as a biocontrol agent for reduction of aflatoxins contamination, by competitive exclusion of naturally present toxigenic strains of Aspergillus flavus. Full details of the good agricultural practice (GAP) can be found in the list of end points in Appendix A.
Data were submitted to conclude that the uses of Aspergillus flavus strain MUCL54911 according to the representative use proposed at EU level result in sufficient fungicidal efficacy for the target effect, following the guidance document SANCO/10054/2013 ‐ rev. 3 (European Commission, 2013).
A data gap has been identified for a search of the scientific peer‐reviewed open literature relating to biological properties on the active substance and its relevant metabolites, dealing with side effects on human health published within the 10 years before the date of submission of the dossier, to be conducted and reported in accordance with EFSA guidance on the submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009 (EFSA, 2011).
Conclusions of the evaluation
1. Identity of the microorganism/biological properties/physical and technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion (European Commission, 2000, 2012; EFSA FEEDAP Panel, 2018).
The minimum and maximum content of Aspergillus flavus strain MUCL54911 in the microbial pest control agent (MPCA) was 2.46 × 1010 to 1.32 × 1012 CFU/L; however, a data gap was identified for appropriate QC data regarding the content of Aspergillus flavus strain MUCL54911 in the MPCA to establish a reliable specification range.
The levels of contaminating microorganisms in commercially produced batches comply with the requirements of SANCO/12116/2012 rev.0 (European Commission, 2012).
The identification at strain level of Aspergillus flavus strain MUCL54911 was based on a combination of morphological, chemical, molecular and genetic methods. Aspergillus flavus strain MUCL54911 belongs to the L strain morphotype, to the Vegetative Compatibility Group VCG IT006. Single sequence repeat (SSR) analysis provided additional information to differentiate Aspergillus flavus strain MUCL54911 from other Aspergillus strains; however, this information was considered insufficient to unequivocally identify Aspergillus flavus strain MUCL54911 at strain level. As a consequence, a data gap was identified for a method using molecular tools that permits to unequivocally identify Aspergillus flavus strain MUCL54911 at strain level.
Aspergillus flavus and Aspergillus parasiticus are two closely related fungi, both able to produce aflatoxins. Whether Aspergillus flavus strain MUCL54911 is a non‐toxigenic strain of Aspergillus flavus, remains an open issue and results in several data gaps (see Sections 2, 3, 4.2 and 5).
Aspergillus flavus is a cosmopolitan fungus which occurs naturally in the environment and it is generally considered an opportunistic pathogen able to cause aspergillosis and to produce harmful toxins as secondary metabolites, including carcinogenic aflatoxins and cyclopiazonic acid (CPA). The Vegetative Compatibility Group VCG IT006 was stated by the applicant to be composed exclusively of atoxigenic members, which are characterised by a large deletion in the subtelomere region of chromosome 3 that includes the entire deletion of the aflatoxin biosynthesis gene cluster and genes required for cyclopiazonic acid production. However, data to confirm this statement were not present in the dossier assessed; therefore, there was insufficient evidence to unequivocally demonstrate that Aspergillus flavus strain MUCL54911 does not produce secondary metabolites of potential concern or known mammalian toxicological concern (aflatoxin B1, B2 and CPA). As a consequence, a data gap was identified to address the possibility for the MPCA to contain secondary metabolites of known concern and of potential concern.
Aspergillus flavus is genetically stable in the environment and the recovery of toxigenicity by non‐toxigenic strains of the fungus appears limited based on field investigations reported for North America and Africa, though as the species has sexual life stages, this cannot be entirely excluded. See section 4 for further information on this topic.
Aspergillus flavus grows at temperatures from 12°C to 48°C having the optimal growth temperature ranging from 28°C to 37°C; however, a data gap was identified for the determination of growth temperature range of the strain MUCL54911.
A data gap was identified for information on the resistance or sensitivity to antibiotics.
The supported shelf‐life of the product was 2 years at ambient temperature. A data gap was identified for information regarding the presence of mycotoxins in the sorghum seeds used for the formulation of the microbial pest control product (MPCP).
Acceptable methods are available for the determination of the microorganism in the technical material and formulation; however, a data gap was identified for additional information concerning the accuracy of the method. Acceptable methods are available for the determination of the content of contaminating microorganisms and for the determination of non‐viable residues (aflatoxins, CPA, sterigmatocystin and kojic acid).
Residue definitions were not applicable for Aspergillus flavus strain MUCL54911; therefore, post‐registration monitoring methods are not needed.
2. Mammalian toxicity
Aspergillus flavus strain MUCL54911 was discussed at the Pesticides Peer Review Experts’ TC 64 in November 2021.
General data
As per medical information, Aspergillus spp. are known to be pathogenic to human, causing, especially in immunosuppressed patients, pulmonary manifestations like aspergilloma, invasive pulmonary or tracheobronchial aspergillosis. In the majority (~ 80%) of the cases, aspergillosis is associated with Aspergillus fumigatus and, on rare occasions, with some strains of Aspergillus flavus. Aspergillus flavus is also the cause of allergic manifestations and other clinical syndromes, like chronic granulomatous sinusitis, keratitis, cutaneous aspergillosis, wound infections and osteomyelitis following trauma and inoculation.
It is not known whether these pathogenic properties can be attributed to the specific strain under assessment, since such information is lacking in case reports. However, no adverse health effects or hypersensitivity reactions were reported in personnel involved in laboratory investigations and professionals involved in the use of the product containing the microorganisms during the emergency authorisation (i.e. 120 days) of the formulation based on Aspergillus flavus strain MUCL54911 pursuant article 53 of Regulation (EC) no 1107/2009 3 .
The species Aspergillus flavus is not recommended for the Qualified Presumption of Safety list (EFSA BIOHAZ Panel, 2020).
Toxicity/Infectivity/Pathogenicity studies
The available methods for testing dermal sensitisation are not suitable for testing microorganisms and there are no validated test methods for sensitisation by inhalation. Based on their characteristics, it is considered that all microorganisms including Aspergillus flavus strain MUCL54911 may have the potential to provoke sensitising reactions. Furthermore, there is evidence from literature (Italy, 2021) that Aspergillus flavus is a respiratory sensitiser in humans.
No signs of toxicity have been detected after acute administration of A. flavus strain MUCL54911 by oral and intraperitoneal route in rodents. Pathogenicity and infectiveness of the microorganism were not investigated in these studies. In an acute inhalation toxicity study with intratracheal administration of a single dose level, signs of toxicity and infectivity/pathogenicity were observed and, due to deviations in the experimental protocol 4 , no conclusion could be made.
Overall, no final conclusion could be drawn on the infectivity and pathogenicity potential of the strain MUCL54911 (data gap).
Skin or eye irritation potential has not been identified.
Secondary metabolites/toxins
Aspergillus species are able to produce a number of mycotoxins of known toxicological concern such as aflatoxins and cyclopiazonic acid (CPA), as well as toxins of unknown toxicological relevance (e.g. kojic acid, beta‐nitropropionic acid, aspertoxin, aflatrem and aspergillic acid). Pending identification of the secondary metabolites produced by the strain (see Sections 1 and 4.2), further assessment of the toxicological profile may need to be conducted to identify if metabolites of unknown toxicological relevance have the potential to be of concern (data gap).
Reference values and non‐dietary exposure
Pending further consideration on infectivity/pathogenicity by inhalation, risk assessment from exposure to the microorganism per se cannot be finalised for operators, workers, bystanders and residents (issue not finalised). In the absence of a quantitative risk assessment, the use of personal protective equipment (PPE)/respiratory protective equipment (RPE) for the operators and workers might be considered to reduce the exposure via dermal and inhalation routes (see Table 5).
Table 5.
Risk mitigation measures proposed for the representative uses assessed
| Representative use | Maize |
|
Tractor‐mounted fertiliser spreader, overall | |
| Operator risk | Use of PPE/RPE might be considered to reduce non‐dietary exposure (dermal and inhalation) |
| Worker exposure | Use of PPE/RPE might be considered to reduce non‐dietary exposure (dermal and inhalation) |
Concerning secondary metabolites, the risk assessment for operators, workers, residents and bystanders cannot be finalised in the absence of their identification/quantification in the technical product (see Section 1) and their in situ production after application of the microorganism (see Section 4.2) (issue not finalised).
For kojic acid, detected in some trials in treated crops (see Section 3), an acceptable daily intake (ADI) of 0.01 mg/kg body weight (bw) per day was established based on the information retrieved from the Scientific Committee on Consumer Safety (SCCS) opinion. 5 It is noted that an acute reference dose (ARfD) has not been proposed.
3. Residues
A representative use on maize has been requested for Aspergillus flavus strain MUCL54911. The outdoor field use foresees one treatment at growth stages of BBCH 30 up to BBCH 39. The application has a maximum application rate of 2.5 × 109 CFU/ha.
Six residue trials (two during the 2015 and four during the 2016 growing phase) were performed in Italy and demonstrate that viable residues of Aspergillus flavus strain MUCL54911 at harvest on maize grain are between 9 × 103 and 1.3 × 105 CFU/g seeds following treatment according to the representative use during the growth stage of stem elongation. It is to be noted that no conclusion could be drawn on the infectivity and pathogenicity potential of Aspergillus flavus strain MUCL54911 and this includes oral intake (see respective data gap in Section 2). This data gap is relevant for residues because significant exposure to viable residues can be anticipated.
In the four trials during the 2016 growing season, non‐viable residues were determined, namely aflatoxins B1, B2, G1 and G2, cyclopiazonic acid (CPA), sterigmatocystin (ST) and kojic acid in maize grains. These were below the limit of quantification (LOQ) of 0.5 µg/kg, of 25 µg/kg and of 1 µg/kg, for aflatoxins, CPA and ST, respectively. Kojic acid was found at levels ranging from below the LOQ (100 µg/kg) up to 2.332 mg/kg. It is to be noted that the information on the non‐viable residues is not supported by valid storage stability studies for each compound analysed (data gap) Furthermore, as only maize grain was sampled and not for the rest of the plant (i.e. forage and fodder), there is another data gap. In addition, a conclusive characterisation on the potential of Aspergillus flavus strain MUCL54911 to produce other metabolites of potential health concern than those analysed is not possible (see data gaps in Sections 1, 2 and 4.2).
Consequently, the dietary consumer risk assessment cannot be finalised based on the available information on viable and non‐viable residues for the representative use on maize (issue not finalised).
With regard to the five assessment criteria according to the Commission guidance SANCO/11188/2013 Rev. 2 (European Commission, 2015) for potential inclusion in Annex IV of Regulation (EC) No 396/2005, none of the three criteria relevant for microorganisms (criterion III: ‘having no identified hazardous properties’; criterion IV: ‘natural exposure is higher than the one linked to the use as plant protection product’ or criterion V: ‘consumer exposure is not expected’) were considered to be met for of Aspergillus flavus strain MUCL54911 for the following reasons:
Based on the provided information, it cannot be excluded that viable cells of Aspergillus flavus strain MUCL54911 and potentially formed non‐viable residues (metabolites) are of potential health concern (data gap, see Sections 1 and 2);
Aspergillus flavus strain MUCL54911 used as plant protection product leads to a significant increase of viable counts when compared to naturally occurring background levels;
Dietary consumer exposure to viable residues of Aspergillus flavus strain MUCL54911 and to its non‐viable residues (metabolites) cannot be excluded (data gaps, see Sections 1, 2 and 3).
Considering that none of the criteria laid down in the guidance is fulfilled, further risk management considerations are required to decide whether Aspergillus flavus strain MUCL54911 is qualified for being included into Annex IV of Regulation (EC) No 396/2005.
4. Environmental fate and behaviour
Satisfactory information has been provided in relation to potential interference of Aspergillus flavus strain MUCL54911 with the analytical systems for the control of the quality of drinking water provided for in Directive 98/83/EC 6 (see specific Annex VI decision‐making criteria in Part II Commission Regulation (EU) No 546/2011 7 ). As these methods require pathogenic bacteria to be identified and confirmed as absent, it was considered unlikely that filamentous fungi or their conidia would interfere with methodologies used for such determinations.
Sexual stages are present in the lifecycle of Aspergillus flavus. It can form ascospores in laboratory crosses between sexually compatible strains belonging to different vegetative compatibility groups (VCGs). Recombination, leading to toxin‐producing variants, can therefore occur in functional sexual structures. In the field, the formation of ascospores is low (too low to be observed in investigations). Under field conditions, Aspergillus flavus primarily replicates by asexual sporulation to form asexual conidia. In conclusion, in the field, the evidence is that sexual reproduction is uncommon and fastidious. It is the case that atoxigenic strains have been used for more than 20 years in North America and reduction in their efficacy was never observed. The atoxigenic strains are therefore, considered unlikely to ‘mate’ with other VCGs to produce toxigenic variants. The possibility of a recombination event that could result in a virulent strain other than the atoxigenic one is considered unlikely because there is little variation in virulence among Aspergillus flavus genotypes and aflatoxin production has never been identified as being associated with virulence. Furthermore, increased competitiveness is not associated with increased virulence on host plants. The weight of evidence is that there is little or no gene flow between VCGs, so strain MUCL54911 would be unlikely to inherit toxigenic traits. Single Sequence Repeat (SSR) data (as discussed in Section 1) indicate that all VCGs of Aspergillus flavus are reproducing clonally and are stable.
4.1. Fate and behaviour in the environment of the microorganism
Studies on the persistence and multiplication in soil of Aspergillus flavus strain MUCL54911 were carried out at four field trial sites where before and after a single application of the representative product isolates belonging to the same VCG of Aspergillus flavus strain MUCL54911 were assessed with the last soil sampling being about 9 months after application. At two of the trial sites VCG levels had returned to levels from before application. At the other two sites, significant declines from the peak measured occurred. These data are considered sufficient to conclude that Aspergillus flavus strain MUCL54911 will respect the uniform principles criterion of not being expected to persist in soil in concentrations considerably higher than the natural background levels, taking into account repeated applications over the years. Predicted environmental concentrations (PEC) in soil have been calculated (see Appendix A).
With respect to the persistence and multiplication in water published peer‐reviewed literature studies in the dossier indicated that Aspergillus flavus is able to survive in aquatic environments under favourable conditions and has been recovered from both fresh and marine waters. Due to the method of application assessed for the granular product via fertiliser spreader, the Aspergillus flavus strain MUCL54911 applied has the potential to reach surface water by off target bouncing of granules at the time of application and possibly by surface run‐off. PEC surface water has been calculated considering these routes of exposure are possible (see Appendix A). Information to address the viability/population dynamics in water/sediment of Aspergillus flavus strain MUCL54911, to address persistence and multiplication in natural surface water systems was not available. This has therefore been identified as a data gap and assessment not finalised as infectivity/pathogenicity information for aquatic plants other than algae was not available (see Section 5).
Published peer‐reviewed literature studies in the dossier indicate that Aspergillus flavus have been isolated from the air both indoors and outdoors which is what would be expected for a fungus that forms conidia, with this life stage facilitating the organism’s distribution and movement via air.
Regarding mobility, following ca. 5–7 days application of the granules following growth on the nutrients provided by the sorghum kernel that is the granule medium, sporulation will occur producing conidia. Conidia are transported via air or by arthropods. These are the mechanisms by which the strain may colonise topsoil around the granules and the maize plants/maize grains. The organism has been shown to remain in the topsoil layers with limited movement down the soil profile due to lower nutrient levels in subsoils. Dispersion/mobility in the environment of the applied strain will be essentially the same as for the naturally present Aspergillus flavus that lives saprophytically in topsoil and can colonise plants.
4.2. Fate and behaviour in the environment of any relevant metabolite formed by the microorganism under relevant environmental conditions
According to the published literature, the species Aspergillus flavus is reported to produce the secondary metabolites aflatoxin B1 and B2, cyclopiazonic acid (with known mammalian toxicological concern) and kojic acid, beta‐nitropropionic acid, aspertoxin, aflatrem and aspergillic (with unknown mammalian toxicological relevance, see Section 2). As already discussed in Section 1, strain MUCL54911 was selected as an atoxigenic strain and in efficacy studies, it reduces the levels of aflatoxins present in maize grain by displacing toxigenic Aspergillus flavus. Therefore, there is some evidence that it may not produce aflatoxins which is consistent with it having been assessed as belonging to the L strain morphotype and VCG IT006. However as already discussed in Sections 1 and 2, evidence is lacking to definitively demonstrate that strain MUCL54911 has the large deletion in the subtelomere region of chromosome 3 that includes the entire deletion of the aflatoxin biosynthesis gene cluster and genes required for cyclopiazonic acid production. Whether strain MUCL54911 can produce kojic acid, beta‐nitropropionic acid, aspertoxin, aflatrem and aspergillic acid is also an open question.
A data gap and assessments not finalised are identified since it is not known to what extent Aspergillus flavus strain MUCL54911 will produce any metabolites following their application. It is not clear if metabolites except aflatoxin B1, aflatoxin B2 and cyclopiazonic acid might fulfil the criteria according to Part B section 7 (iv) of Commission Regulation (EU) No 283/2013 8 namely:
-
–
the relevant metabolite is stable outside the microorganism;
-
–
the relevant metabolite is expected to occur in the environment in concentrations considerably higher than under natural conditions.
-
–
a toxic effect of the relevant metabolite is independent of the presence of the microorganism;
Therefore, data on the potential for Aspergillus flavus strain MUCL54911 to produce metabolites in relation to these criteria are necessary to assess if the further data requirements and the corresponding risk assessment according to Commission Regulation (EU) No 283/2013, part A, section 7 (standard data requirements and assessment mandatory for chemical plant protections active substances) are triggered.
5. Ecotoxicology
Insufficient information was available for assessing the possible infectivity and pathogenicity of Aspergillus flavus strain MUCL54911 to birds. An acute toxicity study on birds exposed to Aspergillus flavus strain MUCL54911 was available and no effects were observed. However, the study duration for the exposure was not considered sufficient to assess infectivity and pathogenicity. With the information available, it was considered that infectivity and pathogenicity to birds could not be excluded. Consequently, a data gap leading to an assessment not finalised was identified for the infectivity and pathogenicity of Aspergillus flavus strain MUCL54911 to birds.
As concluded in Section 2, insufficient information was available to finalise the assessment of infectivity and pathogenicity of Aspergillus flavus strain MUCL54911 in mammals. Consequently, a data gap leading to an assessment not finalised was identified for the infectivity and pathogenicity of Aspergillus flavus strain MUCL54911 to wild mammals.
Adequate information indicating no effects were available for assessing potential infectivity, pathogenicity and adverse effects to fish, freshwater invertebrates and algae from Aspergillus flavus strain MUCL54911 for the representative use. Insufficient data were available for aquatic plants other than algae from the representative use of Aspergillus flavus strain MUCL54911. Consequently, a data gap leading to an assessment not finalised was identified for the infectivity and pathogenicity and adverse effects of Aspergillus flavus strain MUCL54911 to aquatic organisms (aquatic plants other than algae). The RMS disagrees.
Insufficient information was available for assessing the possible infectivity and pathogenicity of Aspergillus flavus strain MUCL54911 to bees. Acute toxicity studies on honeybees exposed to Aspergillus flavus strain MUCL54911 were available indicating low toxicity. However, the study durations for the exposure were not considered sufficient to assess infectivity and pathogenicity. With the information available, it was considered that infectivity and pathogenicity to bees could not be excluded. Consequently, a data gap leading to an assessment not finalised was identified for the infectivity and pathogenicity of Aspergillus flavus strain MUCL54911 to bees.
Three studies on the non‐target arthropods other than bees were available on Aphidius rhopalosiphi, Aleochara bilineata and Typhlodromus pyri exposed to Aspergillus flavus strain MUCL54911 with an application rates 100 times higher than the representative use. A statistical significant difference in mortality was observed between the treated and the control group in the studies with Aphidius rhopalosiphi and Typhlodromus pyri. No effect regarding reproduction was observed. Infectivity and pathogenicity were not observed in the studies. Due to a high control mortality in the prolonged phase (exceeding the trigger of 20%), the study on Typhlodromus pyri may not be fully reliable for assessing infectivity and pathogenicity. Furthermore, it cannot be excluded that due to the limitations in the standard test guidelines, oral exposure could be a potential exposure route for toxicity, infectivity and pathogenicity from Aspergillus flavus strain MUCL54911 to non‐target arthropods that was not investigated in the studies. This resulted in a data gap and an assessment not finalised for the toxicity, infectivity and pathogenicity through the oral route of Aspergillus flavus strain MUCL54911 to non‐target arthropods other than bees.
A reproduction study on earthworm exposed to Aspergillus flavus strain MUCL54911 was available with an application rate 1000 times higher than the representative use. A statistical significant increase in mortality was observed in the treated group compared to the control group. Infectivity and pathogenicity were not investigated in the study. This resulted in a data gap and an assessment not finalised for the infectivity and pathogenicity of Aspergillus flavus strain MUCL54911 to earthworms. The RMS disagrees.
Two reproduction studies on predatory mites Hypoaspis aculeifer and the collembolan Folsomia candida exposed to Aspergillus flavus strain MUCL54911 were available with an application rates 1,000 times higher than the representative use. Toxicity, infectivity and pathogenicity were not observed in the study with Hypoaspis aculeifer. In the study on Folsomia candida, a statistically significant reduction in reproduction was observed and infectivity and pathogenicity were not investigated. Insufficient data were available for assessing the potential effects of Aspergillus flavus strain MUCL54911 on soil microorganisms. This resulted in a data gap and an assessment not finalised for the toxicity and adverse effects of Aspergillus flavus strain MUCL54911 to soil macroorganisms and soil microorganisms, respectively. The RMS disagrees regarding soil microorganisms.
The risk assessment of toxins/secondary metabolites (which it has not been excluded may include aflatoxin B1 and B2, cyclopiazonic acid, kojic acid, beta‐nitropropionic acid, aspertoxin, aflatrem and aspergillic acid, see Section 4.2) could not be finalised for terrestrial non‐target organisms (birds, wild mammals, honeybees, non‐target arthropods, earthworms, other soil macro‐ and microorganisms) and aquatic organisms (fish, freshwater invertebrates, algae and other aquatic plants) for the representative use. Toxicity data were not available for toxins/secondary metabolites (which it has not been excluded may include aflatoxin B1 and B2, cyclopiazonic acid, kojic acid, beta‐nitropropionic acid, aspertoxin, aflatrem and aspergillic acid) to perform a hazard characterisation (resulting in a data gap and assessment not finalised).
6. Overview of the risk assessment of the organism or metabolite compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1, 2, 3–4, 5)
Table 1.
Soil
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Aspergillus flavus strain MUCL54911 | A data gap and an issue not finalised was identified for earthworms, other non‐target soil macro‐ and microorganisms. |
| Toxins/secondary metabolites (which it has not been excluded may include aflatoxin B1 and B2, cyclopiazonic acid, kojic acid, beta‐nitropropionic acid, aspertoxin, aflatrem and aspergillic acid) | A data gap and an issue not finalised was identified for earthworms, other non‐target soil macro‐ and microorganisms. |
Table 2.
Groundwater a
| Compound (name and/or code) |
> 0.1 μg/L at 1 m depth for the representative uses b Step 2 |
Biological (pesticidal) activity/relevance Step 3a. |
Hazard identified Steps 3b. and 3c. |
Consumer RA triggered Steps 4 and 5 |
Human health relevance |
|---|---|---|---|---|---|
| Toxins/secondary metabolites (which it has not been excluded may include aflatoxin B1 and B2, cyclopiazonic acid, kojic acid, beta‐nitropropionic acid, aspertoxin, aflatrem and aspergillic acid) | Open assessment not finalised | Open |
Yes for aflatoxin B1 and B2, cyclopiazonic acid open for other compounds |
Open |
Yes for aflatoxin B1 and B2, cyclopiazonic acid open for other compounds |
Assessment according to European Commission guidance of the relevance of groundwater metabolites (2003).
FOCUS scenarios or relevant lysimeter.
Table 3.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Aspergillus flavus strain MUCL54911 | A data gap and an issue not finalised was identified for aquatic plants other than algae. |
| Toxins/secondary metabolites (which it has not been excluded may include aflatoxin B1 and B2, cyclopiazonic acid, kojic acid, beta‐nitropropionic acid, aspertoxin, aflatrem and aspergillic acid) | A data gap and an issue not finalised was identified for all aquatic organisms. |
Table 4.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| Aspergillus flavus strain MUCL54911 | Level of 1.01 × 108 CFU/animal caused signs of toxicity and pathogenicity in rodents |
7. Particular conditions proposed to be taken into account by risk managers for the representative use evaluated
Risk mitigation measures (RMMs) identified following consideration of Member State (MS) and/or applicant’s proposal(s) during the peer review, if any, are presented in this section. These measures applicable for human health and/or the environment leading to a reduction of exposure levels of operators, workers, bystanders/residents, environmental compartments and/or non‐target organisms for the representative uses are listed below. The list may also cover any RMMs as appropriate, leading to an acceptable level of risks for the respective non‐target organisms.
It is noted that final decisions on the need of RMMs to ensure the safe use of the plant protection product containing the concerned active substance will be taken by risk managers during the decision‐making phase. Consideration of the validity and appropriateness of the RMMs remains the responsibility of MSs at product authorisation, taking into account their specific agricultural, plant health and environmental conditions at national level.
8. Concerns and related data gaps for the representative use evaluated
8.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for one or more of the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011 9 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following issues or assessments that could not be finalised have been identified, together with the reasons including the associated data gaps where relevant, which are reported directly under the specific issue to which they are related:
-
The non‐dietary risk assessment for operators, workers, bystanders and residents could not be concluded considering the following data gaps:
Lack of sufficient investigations on the pathogenicity/infectivity in the available toxicity studies with Aspergillus flavus strain MUCL54911 (see Section 2).
Pending identification and quantification of secondary metabolites both in the product (see Section 1) and produced in situ (see Section 4.2), further toxicological data/information on these metabolites might be needed to finalise the risk assessment Section 2).
-
The consumer dietary risk assessment could not be concluded considering the following data gaps:
Due to the lack of data on pathogenicity/infectivity, a potential health concern of viable residues of Aspergillus flavus strain MUCL54911 at harvest for dietary consumption cannot be excluded (see Sections 2 and 3).
Further information on qualitative and quantitative occurrence of metabolites of potential health concern linked to the representative use of Aspergillus flavus strain MUCL54911 on maize grain and rest of the plant and regarding their potential presence at harvest is required (see Sections 1, 2 and 3).
Storage stability information for relevant metabolites of Aspergillus flavus strain MUCL54911 (see Section 3).
-
Levels of secondary metabolites that could be present in the product or formed in situ in the environment after application that could result in exposure to groundwater and other non‐target organisms/their potential toxicity, and consequently, the risk assessment for non‐target terrestrial organisms (birds, wild mammals, bees, non‐target arthropods, earthworms, other soil macro‐ and microorganisms) and non‐target aquatic organisms (fish, freshwater invertebrates, and aquatic plants) could not be concluded considering the identified data gap:
Pending further investigations on the production of toxins/secondary metabolites (which it has not been excluded may include aflatoxin B1 and B2, cyclopiazonic acid, kojic acid, beta‐nitropropionic acid, aspertoxin, aflatrem and aspergillic acid) and levels present in the environment after application, further considerations will have to be given to their potential toxicity in order to conclude on the risk assessment for terrestrial non‐target organisms (birds, wild mammals, bees, non‐target arthropods, earthworms, other soil macro‐ and microorganisms) and aquatic organisms (fish, freshwater invertebrates, algae and other aquatic plants) and on their groundwater exposure assessment (see Sections 4.2 and 5).
-
The ecotoxicological assessment of the representative use could not be concluded considering the identified data gaps:
Data and information for the assessment of the potential infectivity, pathogenicity and adverse effects to non‐target terrestrial organisms (birds, wild mammals, bees, non‐target arthropods, earthworms and other soil macroorganisms (regarding toxicity) and soil microorganisms) and aquatic organisms (aquatic plants other than algae) from Aspergillus flavus strain MUCL54911 (see Section 5).
Information to address the viability/population dynamics in water/sediment of Aspergillus flavus strain MUCL54911, to address persistence and multiplication in natural surface water systems was not available (see Section 4.1).
8.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following critical areas of concern are identified, together with any associated data gaps, where relevant, which are reported directly under the specific critical area of concern to which they are related:
Critical area of concern was not identified.
8.3. Overview of the concerns identified for each representative use considered (Table 6)
Table 6.
Overview of concerns reflecting the issues not finalised, critical areas of concerns and the risks identified that may be applicable for some but not for all uses or risk assessment scenarios
| Representative use | Maize | |
|---|---|---|
|
Tractor‐mounted fertiliser spreader, overall |
||
| Operator risk | Risk identified | |
| Assessment not finalised | X1 | |
| Worker risk | Risk identified | |
| Assessment not finalised | X1 | |
| Resident/bystander risk | Risk identified | |
| Assessment not finalised | X1 | |
| Consumer risk | Risk identified | |
| Assessment not finalised | X2 | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | |
| Assessment not finalised | X3,4 | |
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | |
| Assessment not finalised | X3,4 | |
| Risk to aquatic organisms | Risk identified | |
| Assessment not finalised | X3,4 | |
| Groundwater exposure to active substance | Legal parametric value breached | |
| Assessment not finalised | ||
| Groundwater exposure to metabolites | Legal parametric value breached a | |
| Parametric value of 10 µg/L a breached | ||
| Assessment not finalised | X3 | |
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 7, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 6.)
9. List of other outstanding issues
Remaining data gaps not leading to critical areas of concern or issues not finalised but considered necessary to comply with the data requirements, and which are relevant for some or all of the representative uses assessed at EU level. Although not critical, these data gaps may lead to uncertainties in the assessment and are considered relevant.
These data gaps refer only to the representative use assessed and are listed in the order of the sections:
Appropriate QC data regarding the content of Aspergillus flavus strain MUCL54911 in the MPCA to establish a reliable specification range (see Section 1).
Method using molecular tools that permits to unequivocally identify Aspergillus flavus strain MUCL54911 at strain level (see Section 1).
Determination of growth temperature range of Aspergillus flavus strain MUCL54911 (see Section 1).
Information on the resistance or sensitivity to antibiotics of Aspergillus flavus strain MUCL54911 (see Section 1).
Information regarding the presence of mycotoxins in the sorghum seeds used for the formulation of the MPCP (see Section 1).
Additional information concerning the accuracy of the method for the determination of the microorganism in the technical material and formulation (see Section 1).
Abbreviations
- Λ
Wavelength
- Ε
decadic molar extinction coefficient
- µg
Microgram
- µm
micrometer (micron)
- AMA
Amphibian Metamorphosis Assay
- ADI
acceptable daily intake
- BCCM
Belgian Co‐Ordinated Collections of Microorganisms
- Bw
body weight
- CABI
Centre for Agricultural Bioscience International
- CFU
colony forming units
- CHO
Chinese hamster ovary cells
- CL
confidence limits
- Cm
Centimetre
- D
Day
- DAR
draft assessment report
- DNA
deoxyribonucleic acid
- EC50
effective concentration
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- G
Gram
- GAP
Good Agricultural Practice
- H
hour(s)
- Ha
Hectare
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- Iv
Intravenous
- Kg
Kilogram
- L
Litre
- LOQ
limit of quantification
- M
Metre
- M
Mol
- Mg
Milligram
- mL
Millilitre
- Mm
millimetre (also used for mean measured concentrations)
- MPCP
microbial pest control product
- MUCL
Mycothèque de l’Université Catholique de Louvain
- Ng
Nanogram
- Pa
Pascal
- PEC
predicted environmental concentration
- PECsw
predicted environmental concentration in surface water
- pH
pH‐value
- PPE
personal protective equipment
- Ppm
parts per million (10–6)
- Ppp
plant protection product
- r2
coefficient of determination
- RMS
Rapporteur Member State
- RPE
respiratory protective equipment
- S
svedberg, S (10‐13 s)
- SMILES
simplified molecular‐input line‐entry system
- SPG
specific protection goal
- SSR
simple sequence repeat
- WHO
World Health Organization
Appendix A – List of end points for the active substance and the representative formulation
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7202
Appendix B – Used compound codes
| Code/trivial name a | IUPAC name/SMILES notation/InChiKey b | Structural formula c |
|---|---|---|
|
aflatoxin B1 (AFB1) |
4‐methoxy‐2,3,6a,9a‐tetrahydrocyclopenta[c]furo[3',2':4,5]furo[2,3‐h][1]benzopyran‐1,11‐dione COc1cc2OC3OC=CC3c2c4OC(=O)C5=C(CCC5=O)c14 OQIQSTLJSLGHID‐UHFFFAOYSA‐N |
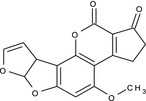
|
|
aflatoxin B2 (AFB2) |
4‐methoxy‐2,3,6a,8,9,9a‐hexahydrocyclopenta[c]furo[3',2':4,5]furo[2,3‐h][1]benzopyran‐1,11‐dione COc1cc2OC3OCCC3c2c4OC(=O)C5=C(CCC5=O)c14 WWSYXEZEXMQWHT‐UHFFFAOYSA‐N |
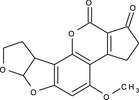
|
|
aflatoxin G1 (AFG1) |
5‐methoxy‐3,4,7a,10a‐tetrahydro‐1H,12H‐furo[3',2':4,5]furo[2,3‐h]pyrano[3,4‐c][1]benzopyran‐1,12‐dione COc1cc2OC3OC=CC3c2c4OC(=O)C5=C(CCOC5=O)c14 XWIYFDMXXLINPU‐UHFFFAOYSA‐N |
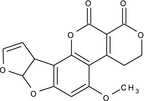
|
|
aflatoxin G2 (AFG2) |
5‐methoxy‐3,4,7a,9,10,10a‐hexahydro‐1H,12H‐furo[3',2':4,5]furo[2,3‐h]pyrano[3,4‐c][1]benzopyran‐1,12‐dione COc1cc2OC3OCCC3c2c4OC(=O)C5=C(CCOC5=O)c14 WPCVRWVBBXIRMA‐UHFFFAOYSA‐N |
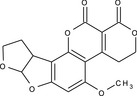
|
|
cyclopiazonic acid (CPA) |
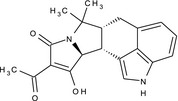
(6aR,11aS,11bR)‐10‐acetyl‐11‐hydroxy‐7,7‐dimethyl‐2,6,6a,7,11a,11b‐hexahydro‐9H‐pyrrolo[1',2':2,3]isoindolo[4,5,6‐cd]indol‐9‐one CC(=O)C1=C(O)[C@@H]2[C@@H]3[C@@H](Cc4cccc5[nH]cc3c45)C(C)(C)N2C1=O SZINUGQCTHLQAZ‐DQYPLSBCSA‐N |
|
|
sterigmatocystin (ST) |
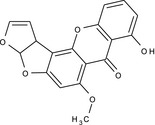
8‐hydroxy‐6‐methoxy‐3a,12c‐dihydro‐7H‐furo[3',2':4,5]furo[2,3‐c]xanthen‐7‐one COc1cc2OC3OC=CC3c2c4Oc5cccc(O)c5C(=O)c14 UTSVPXMQSFGQTM‐UHFFFAOYSA‐N |
|
| aspergillic acid |
6‐(butan‐2‐yl)‐1‐hydroxy‐3‐(2‐methylpropyl)pyrazin‐2(1H)‐one CCC(C)C1=CN=C(CC(C)C)C(=O)N1O IUZCDJYHMMWBBE‐UHFFFAOYSA‐N |

|
|
kojic acid (KA) |
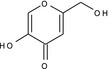
5‐hydroxy‐2‐(hydroxymethyl)‐4H‐pyran‐4‐one OCC1=CC(=O)C(=CO1)O BEJNERDRQOWKJM‐UHFFFAOYSA‐N |
|
| beta‐nitropropionic acid |
3‐nitropropanoic acid OC(=O)CC[N+]([O‐])=O WBLZUCOIBUDNBV‐UHFFFAOYSA‐N |

|
| Aspertoxin |
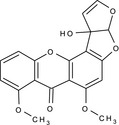
12c‐hydroxy‐6,8‐dimethoxy‐3a,12c‐dihydro‐7H‐furo[3',2':4,5]furo[2,3‐c]xanthen‐7‐one COc1cccc2Oc3c(c(OC)cc4OC5OC=CC5(O)c34)C(=O)c12 QRARGUIFAGCOOA‐UHFFFAOYSA‐N |
|
| Aflatrem |
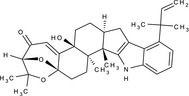
(3R,5bS,7aS,13bS,13cR,15aS)‐5b‐hydroxy‐2,2,13b,13c‐tetramethyl‐9‐(2‐methylbut‐3‐en‐2‐yl)‐2,3,5b,6,7,7a,8,13,13b,13c,14,15‐dodecahydro‐4H‐3,15a‐epoxy[1]benzoxepino[6',7':6,7]indeno[1,2‐b]indol‐4‐one C=CC(C)(C)c1cccc2[NH]c3c(C[C@@H]4CC[C@@]5(O)C6=CC(=O)[C@@H]7O[C@]6(OC7(C)C)CC[C@]5(C)[C@@]34C)c12 YVDJBQQJIDPRKP‐SLUQHKSNSA‐N |
|
The metabolite name in bold is the name used in the conclusion when are known to be produced by the species.
ACD/Name 2020.2.1 ACD/Labs 2020 Release (File version N15E41, Build 116563, 15 June 2020).
ACD/ChemSketch 2020.2.1 ACD/Labs 2020 Release (File version C25H41, Build 121153, 22 March 2021).
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Alvarez F, Arena M, Auteri D, Binaglia M, Castoldi A, Chiusolo A, Colagiorgi A, Colas M, Crivellente F, De Lentdecker C, Egsmose M, Fait G, Ferilli F, Gouliarmou V, Herrero Nogareda L, Ippolito A, Istace F, Jarrah S, Kardassi D, Kienzler A, Lanzoni A, Lava R, Leuschner R, Linguadoca A, Lythgo C, Mangas I, Miron I, Molnar T, Padovani L, Parra Morte JM, Serafimova R, Sharp R, Szentes C, Terron A, Theobald A, Tiramani M and Villamar‐Bouza L, 2022. Conclusion on the peer review of the pesticide risk assessment of the active substance Aspergillus flavus strain MUCL54911. EFSA Journal 2022;20(3):7202, 21 pp. 10.2903/j.efsa.2022.7202
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00506
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: EFSA wishes to thank the rapporteur Member State, Italy, for the preparatory work on this scientific output. EFSA wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Approved: 24 February 2022
Notes
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
See experts’ consultation 6.1. Report of pesticide peer review TC 64 aspergillus flavus strain MUCL54911 – NAS 1107/2009 (November 2021).
SCCS (Scientific Committee on Consumer Safety), Opinion on kojic acid, 26‐27 June 2012. Available at: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_098.pdf
Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. OJ L 330, 5.12.98, p. 32‐54.
Commission Regulation (EU) 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Regulation (EU) 283/2013 of 1 March 2013 setting out the data requirements for active substances in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, p. 1–84.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- EFSA (European Food Safety Authority) , 2011. Submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009. EFSA Journal 2011;9(2):2092, 49 pp. 10.2903/j.efsa.2011.2092 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2022. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance Aspergillus flavus strain MUCL54911. Available online: www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2020. Scientific Opinion on the update of the list of QPS recommended biological agents intentionally added to food or feed as notified to EFSA (2017–2019). EFSA Journal 2020;18(2):5966, 56 pp. 10.2903/j.efsa.2020.5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2018. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA Journal 2012;10(6):2740, 10 pp. 10.2903/j.efsa.2012.2740 [DOI] [Google Scholar]
- European Commission , 2000. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000.
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council. Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission , 2012. Working Document on Microbial Contaminant Limits for Microbial Pest Control Products. SANCO/12116/2012 –rev. 0, September 2012.
- European Commission , 2013. Guidance document on data requirements on efficacy for the dossier to be submitted for the approval of new active substances contained in plant protection products. SANCO/10054/2013‐rev. 3, 11 July 2013.
- European Commission , 2015. Guidance document on criteria for the inclusion of active substances into Annex IV of Regulation (EC) No 396/2005. SANCO/11188/2013‐rev. 2, 14 September 2015.
- Italy , 2020. Draft Assessment Report (DAR) on the active substance Aspergillus flavus strain MUCL54911 prepared by the rapporteur Member State Italy, in the framework of Regulation (EC) No 1107/2009, July 2020. Available online: www.efsa.europa.eu
- Italy , 2021. Revised Draft Assessment Report (DAR) on Aspergillus flavus strain MUCL54911 prepared by the rapporteur Member State Italy in the framework of Regulation (EC) No 1107/2009, September 2021. Available online: www.efsa.europa.eu
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation


