Abstract
Excessive weight gain associated with integrase strand transfer inhibitor (InSTI) antiretrovirals is an emerging issue; however, the metabolic consequences of this effect have not been established. Our objective was to evaluate for InSTI-emergent weight gain and potential associated type 2 diabetes mellitus (T2DM) among a diverse HIV patient cohort. For this retrospective cohort study, we obtained clinical warehouse data for HIV+ patients between fiscal years 2007–17. We compared patients initiated on an InSTI with those started on an alternate regimen. Our primary outcome was percentage weight change from baseline to 24 months postinitiation using the linear mixed-effects model fit by restricted maximum likelihood. Our secondary outcome was incident T2DM as defined by a new prescription for antihyperglycemic medication within 18 months after antiretroviral therapy (ART) start. Diabetes-free survival was estimated using the Kaplan–Meier method, log-rank test, and Cox proportional-hazards model. The cohort included 1,235 individuals initiating ART, 136 (11.0%) with an InSTI. InSTI use in women was significantly associated with greater weight gain compared with non-InSTIs (11.0%, 95% confidence interval, CI: 5.22 to 16.8, p < .01), after adjusting for potential confounding variables. In a univariate analysis, InSTI use was associated with more incident T2DM diagnoses compared with non-InSTI regimens (unadjusted hazard ratio = 3.27, p = .01), although incident T2DM was not associated with weight gain. InSTIs were significantly associated with weight gain among females. We also observed an increased risk of incident diabetes mellitus among both sexes, however, unrelated to weight changes. Further prospective studies will be necessary to confirm this finding and investigate its mechanism.
Keywords: integrase inhibitors, HIV, weight gain, obesity, diabetes mellitus
Introduction
The implementation of effective combination antiretroviral therapy (ART) has led to decreased mortality among persons living with HIV (PLWH) from HIV-associated conditions, such as AIDS and AIDS-defining illnesses.1,2 Today, the predominant cause of mortality among PLWH has shifted toward noncommunicable diseases such as cardiovascular disease (CVD) instead, similar to that of the general population.3 However, compared with their age-matched uninfected counterparts, the burden of cardiometabolic comorbidities is accentuated among PLWH and potentially due to persistent systemic inflammation even after durable viral suppression is achieved.4–7 This concerning trend has prompted an urgent need to pay greater attention to the management of modifiable risk factors for cardiometabolic conditions in the routine care of PLWH.
Among these risk factors, obesity and excess weight are gaining attention due to the increasing prevalence among PLWH on ART.8 Specifically, there has been an accumulating body of evidence on excess weight gain specifically associated with integrase strand transfer inhibitor (InSTI) regimens—the recommended first-line treatment options by most guideline-issuing agencies.9,10 Although initial efficacy and safety trials did not report excess weight gain,11,12 subsequent cohort studies have noted InSTI-emergent excess weight gain in both ART-naive and experienced populations ranging from 3 to 6 kg [body mass index (BMI) of 0.5–1 kg/m2] during the first 12 months of InSTI use.13–16 This weight increase has particularly been noted in women and racial/ethnic minorities, suggesting the presence of vulnerable subgroups.17,18
Concern over InSTI-associated weight gain arises due to the potential for increased risk of metabolic comorbidities, such as type-2 diabetes mellitus (T2DM), which in turn predisposes CVD. Excess weight itself has been strongly associated with insulin resistance in both HIV-uninfected and HIV-infected populations. Approximately 14% increased risk of T2DM has been observed per five pounds (2.27 kg) of weight gained among PLWH compared with 8% in uninfected persons.19 ART itself has also been associated with increased T2DM risk compared with untreated individuals with an over four times greater incidence observed among HIV-infected men compared with those not on ART.20
Currently, guidelines do not recommend switching off InSTIs despite the increasing recognition of InSTI-emergent weight gain because metabolic implications still remain unknown.10 Characterizing factors of InSTI-emergent weight gain and T2DM would help inform the risk/benefit evaluation of these agents and HIV care management guidelines. Given the interplay between excess weight, ART, and T2DM, identifying potential epidemiological associations is a fundamental step toward speculating these mechanisms. Our objective was to conduct a retrospective cohort study evaluating the occurrence of weight gain and T2DM in a racially and sex-diverse urban HIV population upon exposure to ART with an InSTI regimen.
Methods
Databases and cohort development
We obtained data from the Boston Medical Center (BMC) Clinical Data Warehouse for HIV-infected patients ≥18 years who had received outpatient care in the HIV clinic between October 1, 2007 and September 30, 2018. BMC is a Massachusetts safety-net hospital primarily serving an urban and socioeconomically disadvantaged population in the Boston area. The ID clinic at BMC cares for ∼1,600 unique patients annually and receives its funding from Massachusetts Department of Health and Insurance billing. Most patients have Medicare or Medicaid coverage supplemented by the state HIV drug assistance program. Eligible patients were required to be initiated on combination ART with an InSTI, protease inhibitor (PI), or non-nucleoside reverse transcriptase inhibitor (NNRTI)-anchored regimen.
We only included patients who were both new to the ID clinic at BMC and had no prior antiretroviral prescription in our records. We collected demographic data and HIV-related clinical variables (Supplementary Material S1), as well as recorded height and weight data. Sex was defined as biological sex at birth as gender identities were not available as structured variables in the electronic medical record (EMR) at the time of data capture. We extracted medication history, laboratory data, as well as ICD-9/10 codes corresponding to diagnoses of cardiometabolic risk factors [e.g., diabetes mellitus (DM), hyperlipidemia, weight gain, and hypertension]. We excluded individuals who were incarcerated. This study was approved by the Boston University/BMC Institutional Review Board.
Exposures and outcomes
The primary outcome measure was percentage change in baseline weight and BMI in the first 24 months post-ART initiation. For this outcome, we limited the cohort to individuals who had both baseline and at least one follow-up weight and height measurement. Baseline anthropomorphic measures were defined as the measurement (e.g., weight or height) within 3 months before ART initiation. All measurements in the time window were included in analysis following manual review of data to remove irreconcilable measurements potentially due to data entry errors (e.g., incorrect units between kg and lbs). The secondary outcome was the occurrence of T2DM as defined by the new prescription for an antihyperglycemic agent in the first 18 months post-ART initiation.
We also evaluated changes in hemoglobin A1c (HbA1C) and glucose measurements during the first 18 months of treatment. The main exposure was use of InSTI versus non-InSTI-anchored initial ART regimen. Non-InSTI was defined as use of PIs or NNRTIs. Patients who switched the anchor medication of their ART (i.e., InSTI, PI, or NNRTI) during the first 12 months following initiation were excluded to minimize confounding outcomes. In addition, patients who were on antihyperglycemic medication or had an ICD billing code for the diagnosis of diabetes before the study period were excluded in the analysis for both primary and secondary outcomes because of the potential modulating effect of comorbid diabetes and antihyperglycemic medication on weight.
Statistical analyses
Baseline characteristics between InSTI and non-InSTI cohorts were compared using a two-sample Student's t-test for continuous variables and two-sided Fisher's exact test for categorical variables. Our primary outcome was percentage weight change from baseline weight in the first 24 months post-ART-initiation. We first conducted a univariate analysis using a linear mixed-effects model to determine if there were other confounding variables that may affect the association between ART and weight gain.
We identified covariates for the multivariable model based on variables that significantly differed between groups on the univariate model as well as variables identified as confounders in previous weight gain studies13,14,16 (e.g., year of ART initiation, age, sex, race, ethnicity, and baseline CD4 T cell lymphocyte count and HIV-1 viral load). In this multivariable model, we adjusted for these identified covariates using linear mixed-effects model fit by restricted maximum likelihood. Our secondary outcome was the presence of a new antihyperglycemic prescription in the first 18 months of ART initiation, which we used as a surrogate identifier for a new DM diagnosis.
We defined incident DM as progression-free survival (PFS) measured starting from the first day of ART initiation until a patient experienced DM or died without documented progression, whichever date was earliest, or otherwise was censored at the date of last follow-up. PFS rates were estimated by the Kaplan–Meier method, with InSTI versus non-InSTI comparison obtained by a log-rank test. We performed multivariable analyses using a Cox proportional hazards model. Statistical analyses were performed using R version 3.6.2.
Results
Cohort characteristics
One thousand two hundred thirty-five patients were included in the total cohort (Table 1); 1,099 who were initiated on non-InSTIs compared with 136 initiated on InSTIs during the study period of FY 2007 to 2017. InSTIs commenced were primarily dolutegravir (DTG) in 84/136 patients (61.8%), followed by raltegravir (RAL) 25.7% and elvitegravir (EVG) 12.5%.
Table 1.
Baseline Characteristics of Patients Antiretroviral Therapy Initiation
| Median (IQR/%) |
p a | |||
|---|---|---|---|---|
| Total (N = 1,235) | Non-INSTIs (N = 1,099) | INSTIs (N = 136) | ||
| Age (years) | 41.54 (33.8–49.0) | 41.57 (34.0–48.7) | 40.82 (32.8–52.3) | .14 |
| Gender: female | 469 (38.0%) | 421 (38.3%) | 48 (35.3%) | .51 |
| Race: white | 268 (21.7%) | 235 (21.4%) | 33 (24.3%) | .61 |
| Black/African American | 673 (54.5%) | 602 (54.8%) | 71 (52.2%) | |
| Asian | 18 (1.5%) | 16 (1.5%) | 2 (1.5%) | |
| Ethnicity: Hispanic or Latinx | 237 (19.2%) | 213 (19.4%) | 24 (17.6%) | .73 |
| Year of ART initiation | 2008 (2004–2012) | 2007 (2004–2011) | 2015 (2014–2016) | <2.2e-16 |
| InSTI agent: Raltegravir | 35 (25.7%) | |||
| Dolutegravir | 84 (61.8%) | |||
| Elvitegravir | 17 (12.5%) | |||
| Weight (kg) | 74.98 (65.31–84.55 | 75.00 (65.91–84.45) | 74.55 (64.22–85.18) | .70 |
| BMI (kg/m2)b | 25.55 (22.9–28.9) | 25.57 (23–29) | 25.54 (22.7–28.6) | .79 |
| HbA1Cc (%) | 5.7 (5.5–6.1) | 5.8 (5.5–6.2) | 5.5 (5.2–5.6) | .31 |
| CD4 cell countd (cells/mm3) | 323 (171.2–491.0) | 321.5 (187.8–478.2) | 334 (34.5–594.5) | .84 |
p-Value for continuous variable is obtained from two-sample Student's t-test; p-value for categorical variable is obtained from two-sided Fisher's exact test.
Missingness: b612 observations were available for baseline weight/BMI with 489 among non-InSITs and 123 among InSTIs; c66 observations were available for HbA1C with 59 among non-InSTIs and 7 among InSTIs; d1,230 observations were available for CD4 with 1,096 among non-InSTIs and 134 among InSTIs.
ART, antiretroviral therapy; BMI, body mass index; HbA1C, hemoglobin A1c; InSTI, integrase strand transfer inhibitor; IQR, interquartile range.
Median age at ART initiation was similar between groups at 41.5 years (interquartile range, IQR: 33.8–49.0), as were other baseline characteristics. Four hundred sixty-nine (38.0%) patients of the total cohort were female and 673 (54.5%) of black race. Baseline weight was similar between the InSTI and non-InSTI groups (74.55 vs. 75.00 kg, p = .70). Baseline CD4 count did not differ greatly between groups and was 323 cells/mm3 (IQR: 171–491) among the whole cohort.
Weight change in ART-naive patients postinitiation
Longitudinal analysis of weight gain in relation to ART class was limited to individuals who had both baseline weight measurements before ART initiation and at least 18 ± 3 months postinitiation; characteristics for this limited cohort are shown in Supplementary Table S2. The number of weight measurements available postbaseline per patient ranged from 1 to 106, with an average of 13.4 measurements per individual. In univariate analyses (Table 2 and Supplementary Tables S3 and S4), InSTI use was associated with 2.6% increase in weight over 24 months, although this was not statistically significant (95% confidence interval, CI: −0.08 to 5.32, p = .06).
Table 2.
Univariate Model of 24-Month Weight Gain in Antiretroviral Therapy-Naive Patients
| Factor | Change in weight from baseline (%) | Standard error | 95% CI | p |
|---|---|---|---|---|
| Integrase inhibitor | 2.62 | 1.38 | −0.08 to 5.32 | 0.06 |
| Year of ART initiation | 0.08 | 1.44 | −2.62 to 2.90 | 1.0 |
| Age | 0.07 | 0.05 | −0.02 to 0.168 | 0.13 |
| Sex: female | 1.31 | 1.08 | −0.82 to 3.42 | 0.23 |
| Race: white | −1.37 | 1.24 | −3.81 to 1.06 | 0.27 |
| Ethnicity: Hispanic or Latinx | 0.60 | 1.93 | −3.18 to 4.38 | 0.76 |
| NRTI backbone: TDF | 3.09 | 4.05 | −4.85 to 11.0 | 0.45 |
| CD4 count | −0.01 | 0.00 | −0.01 to −0.01 | 0.01 |
| HIV-1 RNA viral load closest to ART initiation | 0.00 | 0.00 | 0.00 to 0 | 0.06 |
CI, confidence interval; TDF, tenofovir disoproxil fumarate.
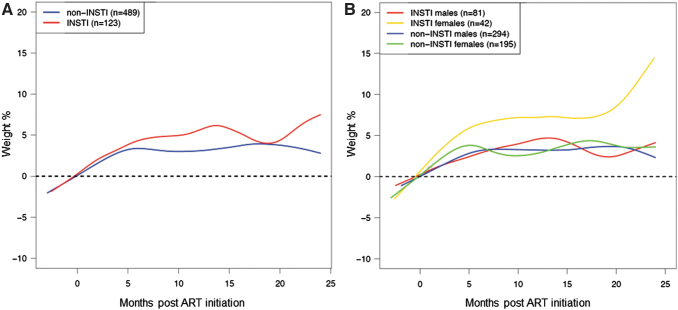
Baseline CD4 count was significantly associated with weight gain (95% CI: −2.71 to −0.01, p = .01), and thus, this was one of the variables included in the multivariable model. In the multivariable analysis of weight gain at 24 months, adjusted for year of ART initiation, age at baseline, ethnicity, NRTI backbone, CD4, and baseline HIV viral load, InSTI compared with non-InSTIs use in females was associated with a significantly greater percentage increase in weight from baseline to 24 months (Table 3 and Fig. 1) (95% CI: 5.22 to 16.8, p < .01). This change was observed in both white (10.6%, 95% CI: 2.83 to 18.4) and nonwhite (11.0% 95% CI: 5.17 to 16.9) women (Table 3). Distribution of the individual measurements depicting the variability is shown in Supplementary Figure S5.
Table 3.
Adjusted Multivariate Analysis of Weight Gain Associated with Integrase Strand Transfer Inhibitors
| Cohort characteristica | Percent weight gainb (%) | 95% CI | Absolute weight gaina (kg) | 95% CI |
|---|---|---|---|---|
| Males | 1.87 | −2.10 to 5.84 | 1.51 | −1.34 to 4.37 |
| White | 1.68 | −3.88 to 7.24 | 1.30 | −2.70 to 5.30 |
| Nonwhite | 2.08 | −2.52 to 6.68 | 1.70 | −1.61 to 5.01 |
| Females | 10.96 | 5.22 to 16.75 | 6.66 | 2.51 to 10.82 |
| White | 10.62 | 2.83 to 18.41 | 6.32 | 0.71 to 11.92 |
| Nonwhite | 11.02 | 5.17 to 16.88 | 6.71 | 2.50 to 10.93 |
Both Hispanic and non-Hispanic ethnicities.
InSTI effect after adjustment, ART initiation year, age, ethnicity, NRTI backbone, CD4 T cell count, and HIV-1 quantitative viral load at baseline.
FIG. 1.
Smooth spline of percent weight gain in the first 24 months of ART initiation by ART type (A) and ART type and gender (B). ART, antiretroviral therapy; InSTI, integrase strand transfer inhibitor.
In a piecewise linear fit model of percentage weight gain in 6-month intervals (Supplementary Fig. S6), the greatest slope of weight gain was in the 6 months immediately after ART initiation among women (slope estimate: 0.88 at 0–6 months vs. 0.15 at 6–18 months vs. 0.52 at 18–24 months). We did not find a significant difference in weight gain by InSTI versus non-InSTI subgroups by race, ethnicity, or NRTI backbone [tenofovir disoproxil fumarate (TDF) vs. tenofovir alafenamide (TAF) vs. abacavir (ABC)].
Diabetes outcomes in ART-naive patients post-ART initiation
In unadjusted analyses, we found InSTI use to be significantly associated with increased risk of new prescription for antihyperglycemic medication at 18 months postinitiation [unadjusted hazard ratio (HR) = 3.27, p = .01; Table 4 and Fig. 2], however, this was not significant in a multivariable model (HR = 1.30, p = .728; Table 5). Other variables, such as age at ART initiation, sex, race, ethnicity, and NRTI backbone, were not associated with T2DM risk in the multivariable model (Table 5). Of note, female sex was associated with an HR of 2.72 for incident antihyperglycemic prescription, although this was not significant despite the difference in InSTI-emergent weight gain between sexes.
Table 4.
Univariate Model of Type 2 Diabetes Mellitus Incidence (Defined by Incident Antihyperglycemic Medication Prescription at 18 Months Postinitiation) by Integrase Strand Transfer Inhibitor Versus Nonintegrase Strand Transfer Inhibitor
| HR | Standard error | 95% CI | p | |
|---|---|---|---|---|
| InSTI (vs. non-InSTI) | 3.27 | 0.48 | 2.33 to 4.22 | 0.01 |
HR, hazard ratio.
FIG. 2.
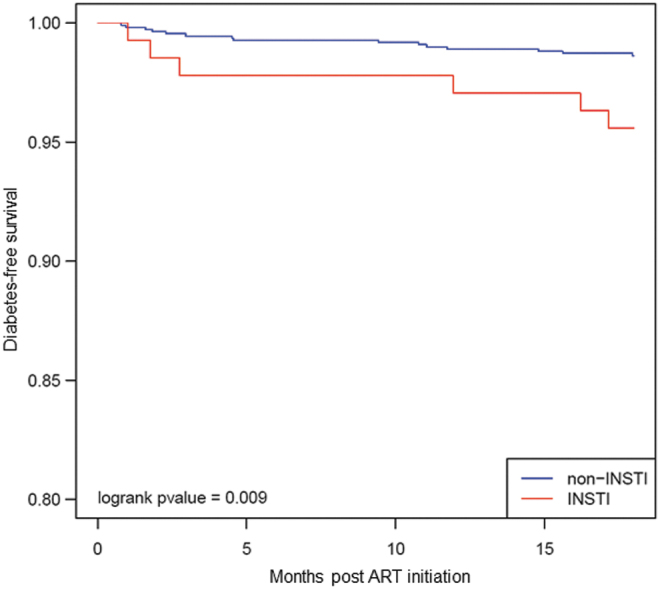
Kaplan–Meier curve depicting the probability of incident antihyperglycemic medication and type of ART regimen in the first 18 months of treatment.
Table 5.
Multivariate Model of Type 2 Diabetes Mellitus Incidence (Defined by Incident Antihyperglycemic Medication Prescription at 18 Months Postinitiation) by Integrase Strand Transfer Inhibitor Versus Nonintegrase Strand Transfer Inhibitor
| Factor | HR | Standard error | 95% CI | p |
|---|---|---|---|---|
| InSTI use (vs. non-InSTI) | 1.30 | 0.76 | −0.19 to 2.80 | 0.73 |
| ART initiation year 2010–2012 | 0.77 | 0.93 | −1.04 to 2.59 | 0.78 |
| ART initiation year 2012–2014 | 1.44 | 0.79 | −0.11 to 2.98 | 0.65 |
| ART initiation year 2014–2016 | 1.25 | 0.97 | −0.65 to 3.15 | 0.82 |
| ART initiation year 2016–2018 | 2.66 | 1.14 | 0.43 to 4.88 | 0.39 |
| Age at ART initiation | 1.03 | 0.02 | 0.99 to 1.07 | 0.19 |
| Sex: female | 2.72 | 0.54 | 1.66 to 3.78 | 0.06 |
| Race: white | 0.58 | 0.78 | −0.96 to 2.11 | 0.48 |
| Ethnicity: Hispanic or Latinx | 1.42 | 0.62 | 0.21 to 2.63 | 0.57 |
| NRTI backbone: TAF | 1.60 | 1.03 | −0.42 to 3.62 | 0.65 |
| NRTI backbone: ABC | 2.67 | 0.72 | 1.26 to 4.08 | 0.17 |
| NRTI backbone: other | 0.00 | 4,795.79 | −9,399.73 to 9,399.74 | 1.00 |
| CD4 at ART initiation | 1.00 | 0.01 | 1.00 to 1.00 | 0.20 |
| HIV-1 RNA viral load at ART initiation | 1.00 | 0.00 | 1.00 to 1.00 | 0.456 |
ABC, abacavir; TAF, tenofovir alafenamide.
We attempted to investigate change in HbA1C between InSTI and non-InSTI groups, but there were too few measurements to conduct this analysis (Table 1), likely limited due to yearly HbA1C monitoring of nondiabetic patients as per HIV guidelines. We also examined change in random glucose measurements between groups over time and found no difference between the InSTI and non-InSTI groups (data not shown).
We did not find an association between incident antihyperglycemic prescriptions and weight gain (Supplementary Fig. S7). We also grouped weight gain into discrete categories by amount of weight gain gained [≤5 lbs (2.27 kg), 6 (2.72 kg) - 10 (4.55 kg) lbs, and >10 (4.55 kg) lbs] and found no association with T2DM risk (p = .70, Supplementary Table S8).
Discussion
In this retrospective study of a racially and sex-diverse cohort of PLWH at a Massachusetts safety net hospital, we found InSTI-emergent weight gain of ∼11% of baseline weight among females at 24 months post-InSTI initiation, corresponding to an median absolute weight increase of 6.18 kg. Among the entire cohort, we also found a threefold greater incidence in T2DM in univariate analysis as reflected by new antihyperglycemic prescriptions at 18 months post-InSTI initiation, implying that InSTI use may be associated with metabolic abnormalities. However, our data did not demonstrate a relationship between weight gain and T2DM incidence, suggesting that the mechanism of InSTI-associated metabolic dysregulation and weight gain may be independent.
In recent years, it has become apparent that the trend of weight gain associated with ART use—in particular InSTIs—exceeds far beyond the “return to health” phenomenon previously seen in those recovering from AIDS wasting syndrome and appear to have a different lipodystrophy phenotype as seen with older regimens.21 In the context of the emerging obesity epidemic among PLWH, the possible contribution of InSTIs to excessive weight gain has garnered significant attention as a contributing risk factor.
Similar to prior studies,15–18 we found marked weight gain among females initiated on InSTIs compared with other regimens. As the median BMI for our population was already within the overweight category, our observed 11% increase in baseline weight could push many patients into obese BMI categories. Interestingly, we did not observe this trend among men where weight gain did not change significantly with InSTI use. The reason for the sex difference in susceptibility to InSTI weight gain in our population is unclear, however, obesity is more prevalent among women in the general population.22 This pattern may simply reflect greater susceptibility to weight gain among females compared with men, particularly in midlife, compounded with obesogenic effects of InSTI use.
This increased susceptibility to excess adiposity in women is thought to be related to hormonal effects on diet, energy expenditure, as well as body fat composition and distribution.23,24 Whether this age-related weight effect is synergistic or simply additive to InSTI-specific weight effects will require further investigation. Regardless, sources of metabolic dysfunction remain clinically relevant to females who may be more vulnerable to CVD due to obesity.25,26 A greater understanding of the mechanism of InSTI weight gain, whether acting directly on adipose tissue27 or through modulation of the melanocortin system, as experienced with antipsychotic medication,28 may be useful in further elucidating these sex-based differences.
We also identified an over threefold risk of T2DM in univariate analysis of patients on InSTIs as reflected by new prescriptions for antihyperglycemic medications. Earlier studies have demonstrated conflicting evidence of the risk of T2DM with InSTI use. Our findings are consistent with recent analysis of Women's Interagency Health study29 data demonstrating increases in HbA1C levels following InSTI switch, although we observed increased risk of T2DM in both males and females present in our cohort. In contrast, an overall increase in incident T2DM following InSTI initiation was not observed in a recent French cohort study.30
A possible factor accounting for the difference in findings between this study and ours was subgroups known to be at greater risk for diabetes such as females and black race were enriched in our cohort. Ultimately, our finding of adverse metabolic consequences of this first-line antiretroviral class merits concern in an aging HIV population already with a higher prevalence of CVD compared with uninfected counterparts, and understanding the underlying mechanism by which InSTI adversely affects weight and insulin resistance is crucial to strategizing intervention.
A commonly acknowledged mechanism in driving insulin resistance is the role of weight gain.19,31–33 Interestingly, we did not find an association between weight gain and incident T2DM diagnoses in our cohort. This could be explained by our relatively small sample size of patients with insufficient power to determine this association or could also suggest a mechanism through which InSTIs may lead to T2DM independent of weight gain. Although ART has long been associated with an increased risk of T2DM in numerous epidemiologic studies, many were studied in the early or pre-InSTI era.20,34,35 One of the mechanisms underlying “lean” diabetes has been speculated to be due to pancreatic secretory dysfunction.36 For example, PIs have been shown to inhibit the glucose transporter GLUT-437 and potentially causing pancreatic beta cell dysfunction.38
Our data suggest that InSTIs may similarly be associated with metabolic dysregulation, although the specific mechanism for this class is yet to be determined. In vitro studies and ex vivo animal models have found that in addition to adipocyte hypertrophy, InSTIs can induce adipose tissue dysfunction characterized by tissue fibrosis, mitochondrial toxicity, and oxidative stress, leading to downstream insulin resistance that supports a plausible mechanism for InSTI-associated metabolic dysfunction.27 Depending on whether or not there are similarities between InSTI-associated weight gain and insulin resistance, interventions for the latter may require a different management approach beyond weight management and lifestyle measures.
The observation of weight gain with InSTI has been alternatively suggested to be due to the switch from TDF to TAF, with the latter reformulated with many of the newer InSTIs in single-tablet formulations, rather than the sole effect of InSTIs. Unlike prior studies,15 we did not find a significant association between TAF use as a contributor to weight gain, either independently or in association with InSTI use. We were likely limited by a small sample size of patients on TAF during the period in which we obtained clinical data.
Our study had several other limitations. We relied greatly on EMR data, and thus, inaccurate recording is a possible limitation of these findings. We attempted to mitigate this by manually reviewing samples of extreme weight measurements and either excluded those patients or corrected the values. In addition, we chose to use incident antihyperglycemic prescription to reflect T2DM diagnosis as we felt billing codes may in fact underestimate the true incidence of new DM if not placed as a new diagnosis by the physician. We also attempted to examine surrogate markers, such as glucose measurements and HbA1C, but did not find enough data points available to accurately assess temporal changes in different ART groups as HbA1c at that time was not recommended to be routinely checked more than once annually.
We acknowledge that many individuals had undetectable HIV-1 viral load at ART initiation, which is potentially due to limitations of obtaining accurate EMR data from our institution (i.e., patients transferring into care following diagnosis). However, we controlled for this finding in multivariate analysis and reported similar CD4 counts between groups, suggesting that both cohorts were at equivalent stages of disease upon initiating ART. Other variables that may have affected weight such as substance use were not captured as they were not reliably available as structured data elements from the clinical data warehouse. Furthermore, we could only capture biological sex at birth as gender identity was not available as a structural variable at the time of data capture.
As this was a single-center study, we were limited by sample size, which may have affected our ability to detect outcomes in the multivariable model and by the generalizability to less urban and diverse populations. In particular, we had a relatively small number of patients on InSTIs as they are relatively newer agents, and few met the inclusion criteria of interrupted 12-month usage. Finally, as this was a retrospective study, we were unable to determine the causality of effect as to whether incident DM was due to weight gain or the direct effect of InSTIs. These critical insights will require further mechanistic and prospective studies to determine the direction of association.
Conclusion
Our observations support the association between InSTIs and excess weight gain in females. These data also suggest that InSTI use is associated with incident T2DM compared with alternate regimen. Whether increased DM incidence and weight gain in PLWH subsequently leads to augmented CVD risk will require long-term prospective studies. Regardless, this increased DM risk and its potential downstream metabolic consequences of InSTI use argue for enhanced surveillance of insulin resistance, and aggressive prevention and mitigation strategies in certain populations, with special focus on women. High priority should be placed on future investigation, including assessing the reversibility of these effects as well as effective integrative approaches for weight management for those who maintain use of InSTIs.
Supplementary Material
Acknowledgments
We acknowledge the BMC Clinical Translational Science Institute and Clinical Data Warehouse for their assistance in providing data for this project.
Authors' Contributions
A.A. and A.O. conceptualized this study, performed data extraction and cleaning, and prepared the article. W.J. and L.F.W. performed all the statistical analyses. S.V. assisted with data extraction and cleaning. M.S. and N.H.L. assisted with the study design and article preparation.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
A.A. and N.H.L. are recipients of an independent educational grant funded by Gilead Sciences and are site investigators for studies funded by GlaxoSmithKline/ViiV and Merck. A.A., A.O., M.S., and N.H.L. are supported by R01-grant (AG060890). L.F.W. and W.J. are supported by the Brown-BUSM Center for AIDS Research (CFAR) grant (P30-AI042853). M.S. is supported by a K24 grant (K24AI145661) mentorship grant. This study was not supported by any other external funding source.
Supplementary Material
References
- 1. Smith C, Sabin CA, Lundgren JD, et al. : Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS 2010;24:1537–1548. [DOI] [PubMed] [Google Scholar]
- 2. Smith CJ, Ryom L, Weber R, et al. : Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet 2014;384:241–248. [DOI] [PubMed] [Google Scholar]
- 3. Farahani M, Mulinder H, Farahani A, Marlink R: Prevalence and distribution of non-AIDS causes of death among HIV-infected individuals receiving antiretroviral therapy: A systematic review and meta-analysis. Int J STD AIDS 2017;28:636–650. [DOI] [PubMed] [Google Scholar]
- 4. Zicari S, Sessa L, Cotugno N, et al. : Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 2019;11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peterson TE, Baker JV: Assessing inflammation and its role in comorbidities among persons living with HIV. Curr Opin Infect Dis 2019;32:8–15. [DOI] [PubMed] [Google Scholar]
- 6. Vance DE, Mugavero M, Willig J, Raper JL, Saag MS: Aging with HIV: A cross-sectional study of comorbidity prevalence and clinical characteristics across decades of life. J Assoc Nurses AIDS Care 2011;22:17–25. [DOI] [PubMed] [Google Scholar]
- 7. Hasse B, Ledergerber B, Furrer H, et al. : Morbidity and aging in HIV-infected persons: The Swiss HIV cohort study. Clin Infect Dis 2011;53:1130–1139. [DOI] [PubMed] [Google Scholar]
- 8. Koethe JR, Jenkins CA, Lau B, et al. : Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 2016;32:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Maryland: Department of Health and Human Services. [Google Scholar]
- 10. Saag MS, Gandhi RT, Hoy JF, et al. : Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society–USA Panel. JAMA 2020;324:1651–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raffi F, Rachlis A, Stellbrink H-J, et al. : Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013;381:735–743. [DOI] [PubMed] [Google Scholar]
- 12. Sax PE, Pozniak A, Montes ML, et al. : Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): A randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet 2017;390:2073–2082. [DOI] [PubMed] [Google Scholar]
- 13. Bourgi K, Jenkins CA, Rebeiro PF, et al. : Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc 2020;23:e25484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bourgi K, Rebeiro PF, Turner M, et al. : Greater weight gain in treatment naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2019;70:1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Venter WDF, Moorhouse M, Sokhela S, et al. : Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019;381:803–815. [DOI] [PubMed] [Google Scholar]
- 16. Sax PE, Erlandson KM, Lake JE, et al. : Weight gain following initiation of antiretroviral therapy: Risk factors in randomized comparative clinical trials. Clin Infect Dis 2020;71:1379–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kerchberger AM, Sheth AN, Angert CD, et al. : Weight gain associated with integrase stand transfer inhibitor use in women. Clin Infect Dis 2020;71:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lake JE, Wu K, Bares SH, et al. : Risk factors for weight gain following switch to integrase inhibitor-based antiretroviral therapy. Clin Infect Dis 2020;71:e471–e477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herrin M, Tate JP, Akgun KM, et al. : Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr 2016;73:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown TT, Cole SR, Li X, et al. : Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005;165:1179–1184. [DOI] [PubMed] [Google Scholar]
- 21. Kumar S, Samaras K: The impact of weight gain during HIV treatment on risk of pre-diabetes, diabetes mellitus, cardiovascular disease, and mortality. Front Endocrinol (Lausanne) 2018;9:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hales CM CM, Fryar CD, Ogden, CL: Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics. 2020. [PubMed] [Google Scholar]
- 23. Lovejoy JC: The influence of sex hormones on obesity across the female life span. J Womens Health 1998;7:1247–1256. [DOI] [PubMed] [Google Scholar]
- 24. Kapoor E, Collazo-Clavell ML, Faubion SS: Weight gain in women at midlife: A concise review of the pathophysiology and strategies for management. Mayo Clin Proc 2017;92:1552–1558. [DOI] [PubMed] [Google Scholar]
- 25. Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB: Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch Intern Med 2002;162:1867–1872. [DOI] [PubMed] [Google Scholar]
- 26. Willett WC, Manson JE, Stampfer MJ, et al. : Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. JAMA 1995;273:461–465. [DOI] [PubMed] [Google Scholar]
- 27. Gorwood J, Bourgeois C, Pourcher V, et al. : The integrase inhibitors dolutegravir and raltegravir exert pro-adipogenic and profibrotic effects and induce insulin resistance in human/simian adipose tissue and human adipocytes. Clin Infect Dis 2020;71:e549–e560. [DOI] [PubMed] [Google Scholar]
- 28. Domingo P, Villarroya F, Giralt M, Domingo JC: Potential role of the melanocortin signaling system interference in the excess weight gain associated to some antiretroviral drugs in people living with HIV. Int J Obes (Lond) 2020;44:1970–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Summers NA, Lahiri CD, Angert CD, et al. : Metabolic changes associated with the use of integrase strand transfer inhibitors among virally controlled women. J Acquir Immune Defic Syndr 2020;85:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ursenbach A, Max V, Maurel M, et al. : Incidence of diabetes in HIV-infected patients treated with first-line integrase strand transfer inhibitors: A French multicentre retrospective study. J Antimicrob Chemother 2020;75:3344–3348. [DOI] [PubMed] [Google Scholar]
- 31. Maggio CA, Pi-Sunyer FX: Obesity and type 2 diabetes. Endocrinol Metab Clin North Am 2003;32:805–822, viii. [DOI] [PubMed] [Google Scholar]
- 32. Gallagher EJ, LeRoith D, Karnieli E: The metabolic syndrome—From insulin resistance to obesity and diabetes. Endocrinol Metab Clin North Am 2008;37:559–579, vii. [DOI] [PubMed] [Google Scholar]
- 33. Resnick HE, Valsania P, Halter JB, Lin X: Relation of weight gain and weight loss on subsequent diabetes risk in overweight adults. J Epidemiol Community Health 2000;54:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Samad F, Harris M, Puskas CM, et al. : Incidence of diabetes mellitus and factors associated with its development in HIV-positive patients over the age of 50. BMJ Open Diabetes Res Care 2017;5:e000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nduka CU, Stranges S, Kimani PK, Sarki AM, Uthman OA: Is there sufficient evidence for a causal association between antiretroviral therapy and diabetes in HIV-infected patients? A meta-analysis. Diabetes Metabol Res Rev 2017;33:e2902. [DOI] [PubMed] [Google Scholar]
- 36. George AM, Jacob AG, Fogelfeld L: Lean diabetes mellitus: An emerging entity in the era of obesity. World J Diabetes 2015;6:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koster JC, Remedi MS, Qiu H, Nichols CG, Hruz PW: HIV protease inhibitors acutely impair glucose-stimulated insulin release. Diabetes 2003;52:1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sims EA-OX, Park G, Mather KJ, Mirmira RA-O, Liu Z, Gupta SA-O: Immune reconstitution in ART treated, but not untreated HIV infection, is associated with abnormal beta cell function. PLoS One 13:e0197080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.