Abstract
Dysregulation of apoptotic machinery is one mechanism by which acute myeloid leukemia (AML) acquires a clonal survival advantage. B-cell lymphoma protein-2 (BCL2) overexpression is a common feature in hematologic malignancies. The selective BCL2 inhibitor, venetoclax (VEN) is used in combination with azacitidine (AZA), a DNAmethyltransferase inhibitor (DNMTi), to treat patients with AML. Despite promising response rates to VEN/AZA, resistance to the agent is common. One identified mechanism of resistance is the upregulation of myeloid cell leukemia-1 protein (MCL1). Pevonedistat (PEV), a novel agent that inhibits NEDD8-activating enzyme, and AZA both upregulate NOXA (PMAIP1), a BCL2 family protein that competes with effector molecules at the BH3 binding site of MCL1. We demonstrate that PEV/AZA combination induces NOXA to a greater degree than either PEV or AZA alone, which enhances VEN-mediated apoptosis. Herein, using AML cell lines and primary AML patient samples ex vivo, including in cells with genetic alterations linked to treatment resistance, we demonstrate robust activity of the PEV/VEN/AZA triplet. These findings were corroborated in preclinical systemic engrafted models of AML. Collectively, these results provide rational for combining PEV/VEN/AZA as a novel therapeutic approach in overcoming AML resistance in current therapies.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous hematopoietic neoplasm characterized by the arrest of differentiation and clonal proliferation of myeloid precursor cells. Despite recent advances, only 24% of patients survive their disease beyond 5 years of diagnosis.1–6 One mechanism by which AML clones acquire a survival advantage is the dysregulation of apoptotic machinery that in normal cells regulates homeostasis in the bone marrow. Apoptosis is controlled at the mitochondrial level via mechanisms regulated by the B-cell lymphoma protein-2 (BCL2) family of proteins.7 This group of proteins is divided into three sub-groups which include pro-apoptotic “BH3-only” proteins BIM, BID, PUMA, NOXA, BAD, BIK, BMF and HRK, and the effector proteins BAX and BAK that are induced by various cell death stimuli to trigger mitochondrial outer membrane permeabilization (MOMP) and apoptosis.8,9 A separate group of anti-apoptotic family members, including BCL2, BCL-XL, MCL1, BCL-W, and BCL2-A1, function to bind and inhibit the pro-apoptotic family members, preventing MOMP and subsequently apoptosis.8,10,11 BCL2 overexpression in human hematologic malignancies is associated with poor responses to conventional chemotherapy.12,13 A deep understanding of mitochondrial protein control of apoptosis has fueled a desire to discover small molecules specifically intended to occupy the hydrophobic BH3 binding site of antiapoptotic proteins and allow initiation of apoptosis by effector molecules. 14,15
Venetoclax (VEN), a selective BCL2 inhibitor, binds BCL2 to directly inhibit sequestration of pro-apoptotic proteins such as the activator BIM. Free BIM can bind to and activate BAX/BAK, inducing conformational changes that result in BAX/BAK homo-oligomerization and mitochondrial outer membrane permeabilization (MOMP), initiating apoptosis. 16,17 VEN has been effective in the clinic for patients with chronic lymphocytic leukemia but has limited efficacy in relapsed-refractory AML as a single agent.18,19 In recent clinical trials in newly-diagnosed AML patients ineligible for intensive chemotherapy, VEN demonstrated an overall response rate of 67% or 48% in combination with DNA methyltransferase inhibitors (DNMTi; AZA or decitabine) or low-dose cytosine arabinoside (LDAC), respectively.20,21 Despite this progress, many patients treated with VEN+DNMTi/LDAC ultimately relapse, and a subset of patients never respond.20–23 One proposed mechanism of resistance to VEN is cellular upregulation of the anti-apoptotic protein myeloid cell leukemia-1 protein (MCL1). VEN in combination with selective MCL1 inhibitors has demonstrated enhanced cytotoxic activity over either agent alone in AML cells in vitro and in xenograft models pre-clinically, but efficacy of this combination in the clinic has yet to be reported.24 Both MCL1-dependent and MCL1-independent mechanisms of VEN resistance are emerging and new approaches aimed at addressing these are moving toward the clinic.25-29
Pevonedistat (PEV) was developed as a targeted inhibitor of NEDD-8 activating enzyme (NAE), which activated the Cullin-RING E3 ubiquitin ligases (CRL) in a process called neddylation. Thus, PEV disrupts the proteasomal- mediated degradation of proteins targeted by the CRL, leading to their accumulation.30 Neddylation is upregulated in AML, and pevonedistat has been tested as a single agent and in combination with the DNMTi, AZA.31-33 In a phase Ib clinical trial of untreated AML patients ≥60 years of age, an intention to treat analysis revealed PEV in combination with AZA induced an overall response rate of 50%.31 Interestingly, PEV and AZA both upregulate NOXA (PMAIP1), a pro-apoptotic BCL2 family protein known to compete with effector molecules at the BH3 binding site of MCL1 to inhibit its anti-apoptotic function.10,34,35 Given the emerging critical role of MCL1 in VEN resistance, we postulated that the combination of PEV/AZA will synergize with VEN in a triple combination with efficacy superior to VEN/AZA or VEN/PEV alone.
Herein, we demonstrate that the triple combination of VEN/PEV/AZA induces robust activity in preclinical models of AML that is superior to either agent alone or as combination doublets. Leveraging AML cell lines and primary AML patient samples ex vivo, we demonstrate that the PEV/AZA combination induces NOXA to a greater extent than PEV or AZA alone to further enhance VEN-mediated apoptosis. Apoptosis induced by the VEN/PEV/AZA combination required PMAIP1, the gene encoding NOXA, since its deletion abrogated the activity of this triplet. Importantly, the VEN/PEV/AZA combination enhanced the kinetics of apoptosis compared to other treatment variations in AML cell lines and patient samples that manifest in vivo to drive durable anti-leukemic activity in cell line-derived and patient-derived xenograft models of AML. Our work provides important mechanistic insight to support the use of these agents in combination in ongoing clinical trials.
Methods
Cell culture and reagents
AML cell lines and patient cells were cultured as previously described, and were short tantem repeats (STR) validated and mycoplasma tested.34 See the Online Supplementary Materials and Methods for reagent details.
Patient samples
Experiments were conducted on primary patient samples provided by the Vanderbilt-Ingram Cancer Center Hematopoietic Malignancies Repository, after obtaining informed consent, and approval of the Vanderbilt University Medical Center Institutional Review Board.
Knockout cell line generation
Genomic deletion of BBC3 and PMAIP1 were previously described.34 The BAK1/BAX-deficient OCI-AML5 cells were generated using the same protocol except by combining the two CRISPR RNA (crRNA) targeting BAK1/BAX. ATF4-deficient cells were generated by combining the two crRNA (Online Supplementary Table S1).
Cell proliferation assay
For combinatorial studies, cells were treated for 24 hours as previously described.34 For primary cells, compounds were dispensed into a 384-well plate using the Echo 555 liquid handler (Labcyte) and 2,000-8,000 cells/well were incubated for 24 hours or 72 hours for VEN/AZA resistant patient samples and cell viability was measured using the Cell TiterGlo (Promega). Percent viability was defined as the relative luminescence units (RLU) of each well divided by the RLU of cells in dimethyl sulfoxide (DMSO) control and the effective concentration (EC50) to induce 50% cell death were determined by nonlinear regression algorithms using Prism 8.0 (GraphPad).
Western blot
Western blotting was performed as previously described.34 Membranes were incubated with the respective primary antibodies (Online Supplementary Table S2).
Patient-derived xenografts
2×106 primary AML mononuclear cells were engrafted in 7-9- week-old female NSGS (NOD-scid IL2Rgnull3Tg(hSCF/hGMCSF/ hIL3)) mice (The Jackson Laboratory) as described in the Online Supplementary Materials and Methods. Chimerism was assessed weekly in the peripheral blood, and, at time of tissue harvest, in the bone marrow and spleen. Animal experiments were conducted in accordance with guidelines approved by the IACUC at VUMC.
Cell line-derived xenografts
Female fox chase SCID beige mice were employed for the flank xenograft model; female NOD SCID gamma mice were employed in the systemic engraftment of OCI-AML2-Red-Fluc cell line (Charles River Laboratories) and performed as described in the Online Supplementary Materials and Methods. Animal studies were conducted in accordance with the guidelines established by the AbbVie Institutional Animal Care and Use Committee.
Flow cytometry
Red blood cells were lysed with EL buffer on ice (Qiagen), with remaining cells washed and resuspended in 1X phosphate buffered saline (PBS) with 1% bovine serum albumin (BSA) and stained for 15 minutes with the conjugated antibodies listed in the Online Supplementary Table S2. Cells were washed and submitted for flow cytometric analysis using a 3-laser LSRII (Becton Dickinson).
Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde for 48 hours and stored in 70% ethanol, embedded in paraffin and sectioned at 5 μm after bone tissue was decalcified. Sections were dewaxed in Xylene and rehydrated in successive ethanol baths. Standard Mayer’s hematoxylin and eosin (H&E) staining was performed. Antigen retrieval using a standard pH 6 sodium citrate buffer (BioGenex) was performed and sections were stained with monoclonal mouse anti-human CD45 (Dako, M0701, dilution 1:200) using M.O.M. Kit (Vector).
Kinetics of caspase-3/-7 activation and cell death
Activated caspase-3/-7 (aCasp-3/-7+) and cell death (DRAQ7+) was evaluated using an IncuCyte S3 (Sartorius) as described in detail previously.34 Area under the curve (AUC) was calculated over the 48 hours period using Prism 8.0 (GraphPad).
Gene expression
100,000 AML cells (OCI-AML5, MV4-11, THP-1) were cultured in 96-well plates three biological replicates were treated with pevonedistat for 24 hours. QuantiGene Plex assay (Sigma- Aldrich ) was performed as instructed by the manufacturer.
Statistical analysis
An unpaired 2-tailed Student t-test was performed unless otherwise indicated. All statistical analyses were completed using Prism 8.0 software (GraphPad). The effects of VEN/PEV combinatorial activity were calculated using the zero interaction potency (ZIP) synergy model, which compares observed and expected combination effects.36–38
Results
Deletion of PMAIP1 abrogates the synergistic activity of pevonedistat and venetoclax in acute myeloid leukemia cell lines
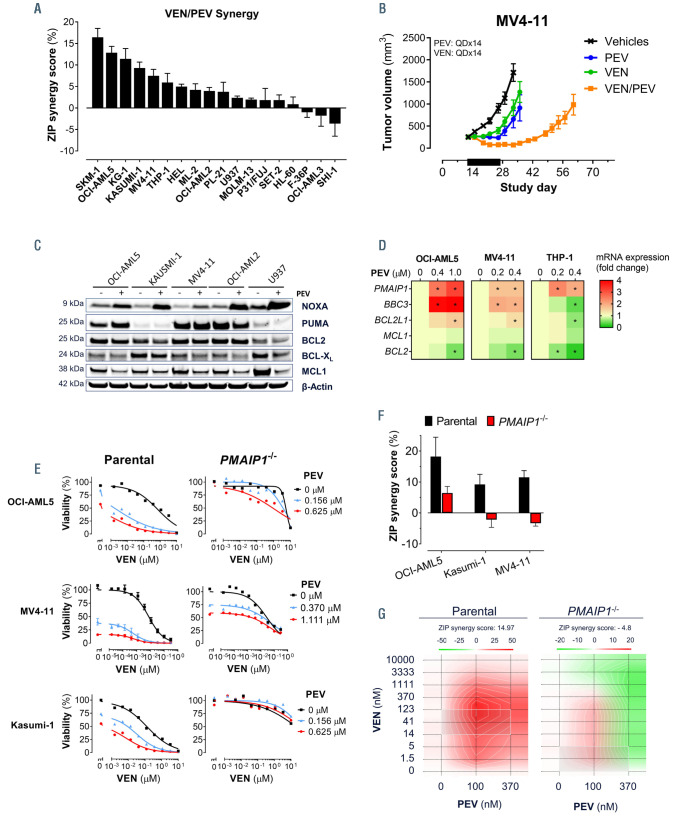
We tested a panel of AML cell lines for the combinatorial activity of VEN and PEV using a 10 by 3 concentration matrix, respectively (Online Supplementary Figure S1A). The two agents were synergistic in 15 of 18 and highly synergistic (d>5%) in nine of 18 AML cell lines (Figure 1A) as assessed by the ZIP synergy model.38 In order to further validate this in vitro observation, a subcutaneous xenograft model of the MV4-11 cell line was established in the flank of immunocompromised mice. The mice were divided into four treatment groups and treated daily for two weeks with either PEV, VEN, or the combination of PEV and VEN. Both VEN and PEV each moderately inhibited MV4-11 tumor growth to similar degrees. However, the two agents together displayed combinatorial activity resulting in pronounced tumor regression and a tumor growth delay (Figure 1B).
In order to explore the molecular drivers behind the VEN/PEV synergistic activity, we measured changes in the BCL2 family proteins and gene expression in AML cell lines. Treatment with PEV caused a robust upregulation in NOXA protein levels in all five AML cell lines (Figure 1C), and increased gene expression of its transcript, PMAIP1, in the three of the AML cell lines assessed (Figure 1D). The BH3-only protein PUMA was inconsistently induced across these cell lines at the protein or transcript level (Figure 1C and D). In contrast to the BH3-only pro-apoptotic members, MCL1 was downregulated in all cell lines treated with PEV (Figure 1C). In order to establish the importance of NOXA for either PEV or VEN/PEV activity, we utilized three PMAIP1-deficient AML cell lines.34 Deletion of PMAIP1 reduced PEVinduced death in OCI-AML5, Kasumi-1, and MV4-11 cells (Figure 1E; Online Supplementary Figure S1B) and abrogated the synergistic activity of VEN/PEV in all three cell lines (Figure 1F and G). These findings illuminate the importance of NOXA for the synergistic activity between VEN and PEV.
Venetoclax/pevonedistat/azacitidine induces BAX/BAK-dependent apoptosis in acute myeloid leukemia cell lines
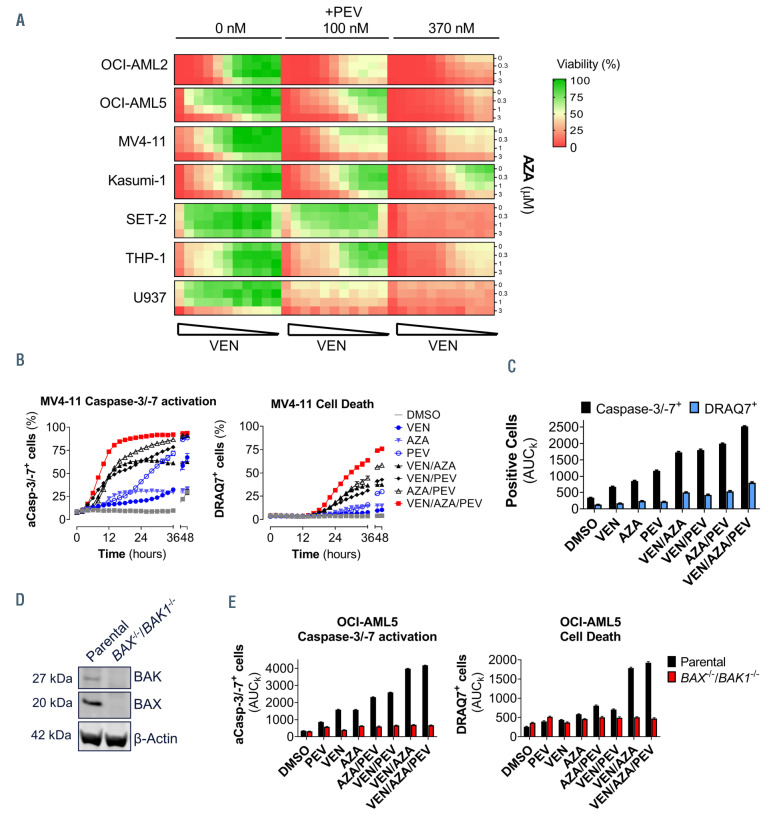
Previously, we demonstrated that NOXA is essential for the combinatorial activity between VEN and AZA in AML cell lines, providing an insight into the mechanism of VEN/AZA clinical activity in AML patients.34 We explored the impact of adding PEV to the VEN/AZA combination to AML cell lines in vitro. A panel of seven AML cell lines were treated with ten doses of VEN and three doses of AZA. Upon the addition of PEV at 100 nM or 370 nM, the overall cell viability decreased in the VEN/AZA combination matrix, indicating an added benefit of PEV to the VEN/AZA cell killing in all seven cell lines (Figure 2A), including in THP-1 cells where VEN/AZA was not synergistic but VEN/PEV was (Figure 1A).34 Similarly, the addition of AZA to VEN/PEV increased cell death in SET-2 and U937 cells (Figure 2A). Thus, VEN/PEV/AZA triple combination improves the activity observed with either the VEN/PEV or VEN/AZA treatments, overcoming the inherent resistance of some AML cells to these treatment doublets.
In order to explore the kinetics of cell death with the VEN/AZA/PEV combination, caspase-3/-7 activation and cell death (DRAQ7 uptake) were measured over time following the addition of individual agents, the double combinations, or the triple combination. The triple VEN/AZA/PEV treatment induced a more rapid activation of caspase-3/-7 and cell death in MV4-11 (Figure 2B) and Kasumi-1 (Online Supplementary Figure 2A) compared to other variations of this treatment combination. Similarly, the overall levels of caspase-3/-7 and cell death over the 48-hour period, as measured by the area under the kinetic curve (AUCk), was significantly higher than the singlet or doublet treatments (Figure 2C). We asked whether the cell death induced by the triplet is dependent upon the intrinsic apoptosis signaling pathway. OCI-AML5 cells deficient in both BAX and BAK1 were generated using CRISPRCas9 clonal gene editing and the absence of BAX and BAK protein expression confirmed (Figure 2D). The triplet combination treatment in parental OCI-AML5 cells induced significant caspase-3/-7 activation and cell death, that was completely abrogated by BAX/BAK1 deletion (Figure 2E; Online Supplementary Figure S2B).
Figure 1.
Deletion of PMAIP1 abrogates the synergistic activity of pevonedistat and venetoclax in acute myeloid leukemia cell lines. (A) Synergistic activity between venetoclax (VEN) and pevonedistat (PEV) as determined by the zero interaction potency (ZIP) model following 24 hours of treatment of a panel of acute myeloid leukemia (AML) cell lines. (B) Tumor growth over time in the MV4-11 subcutaneous xenograft model treated (black bar) with vehicle control, VEN (50 mg/kg; 14-day daily [QDx14]; orally [PO]), PEV (60 mg/kg; QDx14; intraperitoneal [IP]), or the two agents in combination (QD×14). Data are presented as the mean tumor volume ± standard error of the mean (SEM) from eight mice per treatment group. (C) Western blot analysis of NOXA, PUMA, BCL2, BCL-XL, and MCL1 proteins in a panel of AML cell lines treated with PEV (OCI-AML5: 1 mM; Kasumi-1: 1.5 mM; MV4-11: 0.4 mM; OCI-AML2: 0.15 mM; U937: 2 mM) for 24 hours. (D) Gene expression analysis of PMAIP1, BBC3, BCL2L1, MCL1, and BCL2 in OCI-AML5, MV4-11, and THP-1 cells following treatment with PEV at the indicated concentrations (OCI-AML5: 0.4 mM and 1 mM; MV4-11: 0.2 mM and 0.4 mM) after 24 hour of treatment relative to the dimethyl sulfoxide (DMSO) control cells (t-test, *P<0.05, n=3). (E) Cell viability of three parental or PMAIP1-/- AML cell lines after 24 hours of treatment with VEN and PEV in combination (n=3). (F) Synergy measured by ZIP model metric for the VEN and PEV combination in three parental or PMAIP1-/- AML cell lines after 24 hours of treatment (n=3) (lower panel). (G) Visualization of the calculated 2D ZIP synergy map for the parental and PMAIP1-/- MV4/11 from (F).
Azacitidine- and pevonedistat-induced NOXA contributes to the combinatorial activity with venetoclax
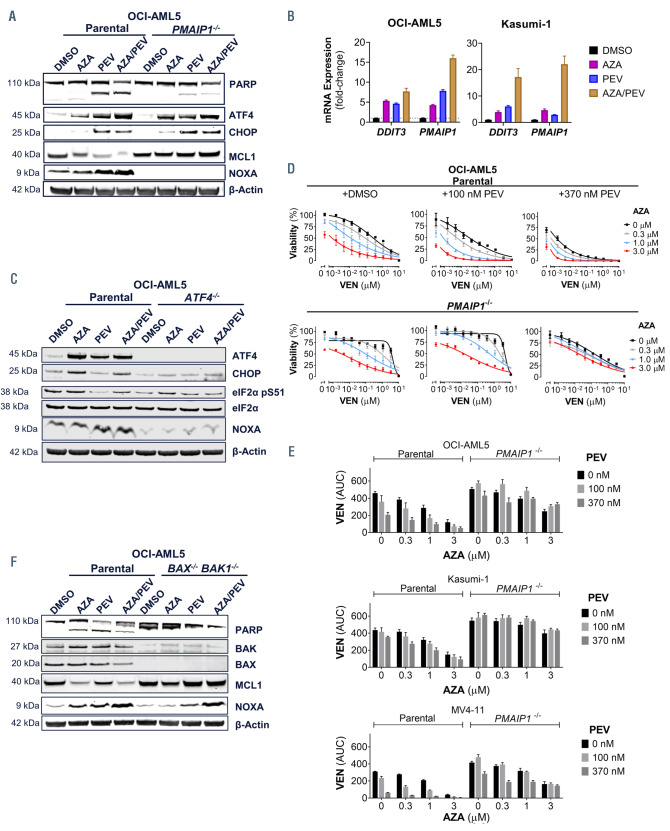
NOXA is important for efficient VEN, PEV or AZA single- agent activity, as well as for VEN/AZA34 or VEN/PEV synergy (Figure 1F).35 However, PMAIP1 is induced by AZA or PEV through distinct mechanisms. AZA treatment induces the integrated stress response (ISR) pathway to upregulate PMAIP1, whereas PEV stabilizes the transcription factor ATF4 to enhance PMAIP1 expression.34,39 In order to understand the effects of combining AZA and PEV on the ISR’s ATF4-NOXA axis, we treated the parental and PMAIP1 deficient OCI-AML5 cell lines for 24 hours with either agent alone or in combination. In parental cells, AZA or PEV alone or in combination induced ATF4 or its transcriptional targets CHOP and NOXA and led to apoptosis, as measured by levels of cleaved PARP (Figure 3A). Similarly, AZA/PEV combination induced DDIT3 and PMAIP1 transcripts to a greater level than the single agent treatments in OCI-AML5 and Kasumi-1 cells (Figure 3B). In contrast, the PMAIP1-deficient OCI-AML5 cells displayed reduced levels of cleaved PARP, while the ATF4 and CHOP induction was not affected by the gene deletion (Figure 3A). Upon ATF4 ablation in OCI-AML5, either agent alone, or the combination, was unable to induce CHOP or NOXA (Figure 3C). Furthermore, unlike AZA, PEV did not induce significant phosphorylation of eIF2a at Ser 51, a marker of ISR activation, while still inducing ATF4 and NOXA. This suggests PEV stabilizes ATF4 in the absence of ISR. Thus, ATF4 is required for the induction of NOXA by AZA/PEV. In order to understand if NOXA is critical for the PEV/VEN-mediated sensitization of AML cell lines to VEN, we treated the parental and PMAIP1-deficient cell lines with the VEN/PEV/AZA triple combination and measured cell viability after 24 hours. Relative to the parental cell lines, PMAIP1-deficiency reduced the potency of VEN-mediated cell death alone or in combination with AZA, PEV, or AZA/PEV in OCI-AML5 (Figure 3D and 3E), Kasumi-1 (Online Supplementary Figure 3A) and MV4-11 (Online Supplementary Figure 3B) cell lines. NOXA can promote the degradation of MCL1 by the proteosome and executioner caspases can also cleave MCL1 during apoptosis. 40 In order to understand the mechanism of MCL1 loss upon treatment with either AZA or PEV, the OCI-AML5 cell line deficient in BAX and BAK1 were treated with these agents (Figure 3F). MCL1 protein degradation induced by either AZA or PEV was not observed in the OCI-AML5 cell lines deficient in either PMAIP1 (Figure 3A), or BAX/BAK1 (Figure 3E). In the BAX-/- BAK1-/- cells, MCL1 was not degraded despite NOXA upregulation (Figure 3F), thus MCL1 degradation by either AZA or PEV requires both sensitization by NOXA and activation of apoptosis by BAX/BAK. Taken together, these data demonstrate that NOXA can be induced by both AZA and PEV and that it has a central role in driving their combinatorial activity with VEN.
Figure 2.
Adding azacitidine to venetoclax/pevonedistat treatment significantly decreases acute myeloid leukemia cell line viability in vitro that is dependent on BAX/BAK-mediated apoptosis. (A) Cell viability matrix measure in a panel of acute myeloid leukemia (AML) cell lines following 24 hours of treatment with venetoclax (VEN) (10 mM top dose 1:3 dilution, except OCI-AML2, MV4-11 top dose of 300 nM) and azacitidine (AZA) (0.3 and 1 mM) and pevonedistat (PEV) at the indicated doses. (B) Activated caspase-3/-7 positive cells (aCasp-3/-7+) or dead cells (DRAQ7+) were counted over time following treatment with the VEN (1 nM), AZA (1 mM), PEV (100 nM), or indicated combinations of these compounds in MV4-11 cells (n=5). (C) Area under the kinetic curves (AUCk) from (B) was calculated over a 48-hour period and plotted as total positive cells (n=5). (D) Western blot analysis of BAX and BAK expression in the parental and BAX-/-/BAK1-/- OCI-AML5 cell lines. (E) OCIAML5 cells were treated with VEN (10 nM), AZA (3 mM), PEV (100 nM) or indicated combinations, and the AUC of the total aCasp-3/-7+ or DRAQ7+ cells calculated over a 48-hour time period were plotted as total aCasp-3/-7+ (left) or DRAQ7+ (right) cells positive cells (n=5).
Figure 3.
Azacitidine- and pevonedistat-induced NOXA contributes to the combinatorial activity with venetoclax. (A) Western blot analysis of total PARP, ATF4, CHOP, and NOXA protein in the parental and the PMAIP1-/- OCI-AML5 cell line treated with azacitidine (AZA) (1 mM), pevonedistat (PEV) (0.37 mM) or both for 24 hours. b-actin was used a protein loading control. (B) DDIT3 and PMAIP1 gene expression was measured in cell lines from (A) under the same conditions. (C) Western blot analysis of ATF4, CHOP, eIF2a, phosphor eIF2a Ser51 and NOXA protein in the parental and the ATF4-/- OCI-AML5 cell line treated as indicated in (A). (D) Cell viability of OCI-AML5 cell line following 24 hours of treatment with venetoclax (VEN) (10 mM top dose 1:3 dilution) and AZA (0.3 mM, 1 mM and 3 mM) and PEV (370 nM) (n=3). (E) Cell viability as measured by area under the curve (AUC) of the VEN dose response curve (from C and Online Supplementary Figures S3A and B) of three parental or PMAIP1-/- acute myeloid leukemia (AML) cell lines following 24 hours of treatment with VEN (10 mM top dose 1:3 dilution, MV4-11 top dose of 0.3 mM) and AZA (0.3 mM, 1 mM and 3 mM) and PEV 100 nM and 370 nM (n=3). (F) Western blot analysis of total PARP, BAX, BAK, MCL1, NOXA protein in the parental and the BAX-/-/BAK1-/- OCI-AML5 cell line treated as indicated in (A). DMSO: dimethyl sulfoxide.
Figure 4.
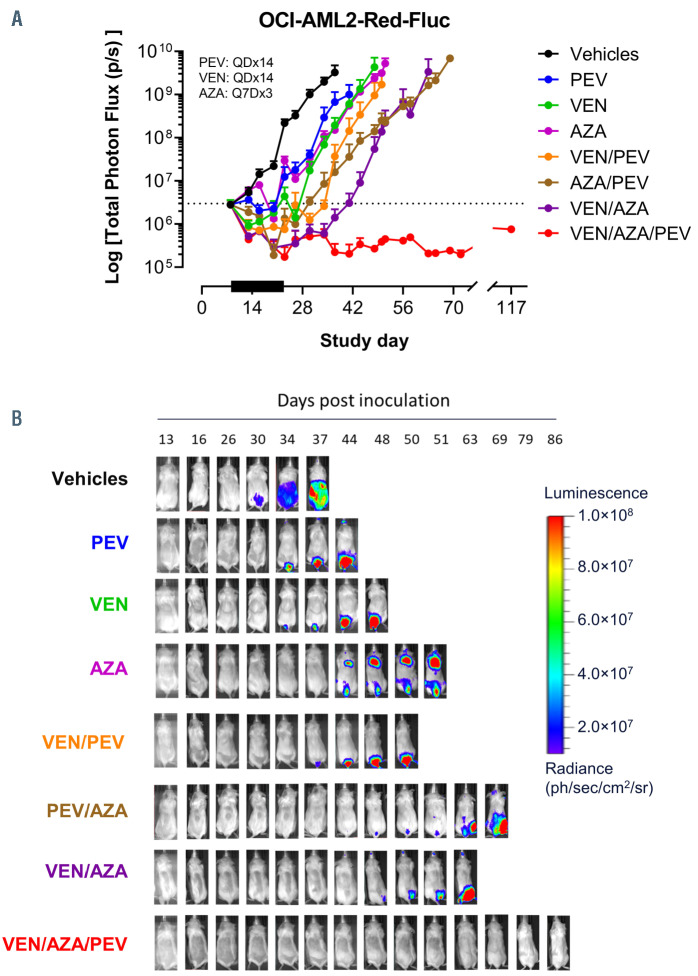
The venetoclax/pevonedistat/azacitidine triple-combination treatment induces durable responses in a systemic xenograft model of acute myeloid leukemia. (A) Tumor growth from whole-body ROI bioluminescent signal (total photons/second) of systemically engrafted OCI-AML2-Red-Fluc tumor cells measured over time and treated (black bar) with vehicle control, venetoclax (VEN) (50 mg/kg, 14-day daily [QDx14], orally [PO]), AZA (8 mg/kg, every 7 days for three treatments [Q7D×3], intravenously [IV]), pevonedistat (PEV) (60 mg/kg, QD×14, intraperitoneal [IP]) or the combinations of the two or all three agents (n=6-8 mice per treatment group). (B) Representative images of animals’ bioluminescent signal of the treatment cohorts from panel (A) showing significant delay in in vivo acute myeloid leukemia (AML) growth with either single- or dual-treatment combinations, while no growth (BLI signal) was detected in the triple-combination treatment.
Figure 5.
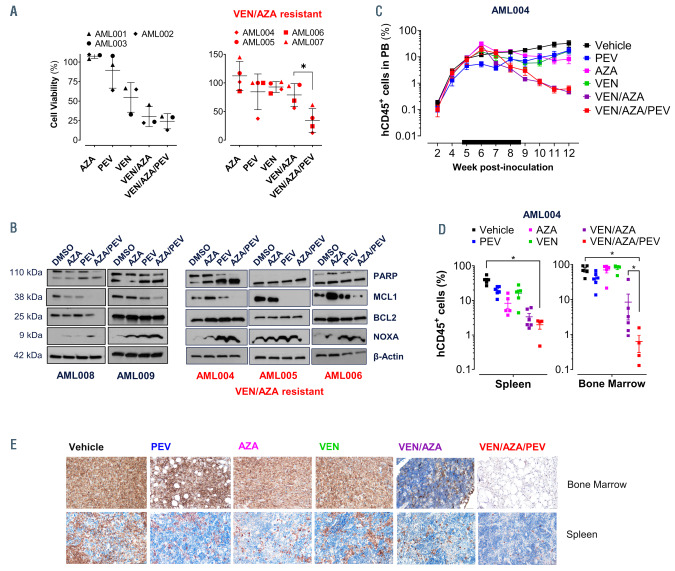
The venetoclax/pevonedistat/azacitidine triple-combination treatment is efficacious in preclinical primary acute myeloid leukemia models. (A) Primary acute myeloid leukemia (AML) samples from seven different patients with different mutational profiles treated ex vivo for 24 or 72 hours (venetoclax/azacitidine [VEN/AZA] resistant patients) with pevonedistat (PEV) (0.3 mM), AZA (0.3 mM) and VEN (0.01 mM) alone, and combinations. (B) Western blot analysis of a primary AML patient samples after 12 hours (AML008) or 24 hours (AML004, AML005, AML006 and AML009) of treatment with dimethyl sulfoxide (DMSO) control, AZA alone, PEV alone, and PEV/AZA in combination. Protein levels of BCL2 family members MCL1, BCL2, NOXA, cleaved PARP, and b-actin. (C) Mice were injected with 2x106 patient primary AML 004 cells via tail vein on day 1, 24 hours after cesium irradiation and were treated (black bar) with vehicle, PEV (30 mg/kg; every other day for 28 days [QOD×28]), AZA (1.5 mg/kg; once every day for 7 days [QD×7]), VEN (15 mg/kg; once every day for 28 days [QDx28]), VEN/AZA or triple combination VEN/AZA/PEV. Human CD45 positive (hCD45+) cells were measured weekly by flow cytometry in peripheral blood (PB) from week 2 through week 12 post-transplant (t-test, *P<0.05). (D) Percent of hCD45+ cells in bone marrow and spleen tissue were measured via flow cytometry on day of tissue harvest week 12 post-transplant. (E) Immunohistochemistry of bone marrow (femur) and spleen (20X), stained with monoclonal antibody for hCD45 in experimental mice.
The venetoclax/pevonedistat/azacitidine combination treatment is highly active in preclinical models of acute myeloid leukemia
The in vivo efficacy of the triple combination of PEV, VEN and AZA was tested in a systemic murine model that allowed monitoring the tumor burden of xenografted bioluminescent OCI-AML2-Red-Fluc cells. Animals were distributed into eight treatment cohorts and treated with either vehicle alone, VEN (50 mg/kg, QD×14, orally [PO]), 5-Aza (8 mg/kg, Q7D×3, intravenous [IV]), PEV (60 mg/kg, QD×14, intraperitoneal [IP]) or the combinations of the two or all three agents. Animals were imaged at regular intervals until they reached endpoint (1×1010 photons/second). The single-agent treatments were active in this model, and the doublets were more efficacious than the single-agents (Figure 4A). Most notably, the triplet combination of PEV, VEN and AZA demonstrated enhanced tumor growth inhibition compared to the singlet or doublet treatment cohorts, driving durable responses with no evidence of tumor growth (zero of eight mice) by day 117, 93 days after treatment had ceased (Figure 4A and B). Importantly, none of the cohorts experienced a significant decrease in body weight (Online Supplementary Figure 4A).
In order to further explore the utility of the VEN/AZA/PEV triple combination, we treated a panel of primary AML patient samples with different mutational, karyotype profiles, and treatment failure (Online Supplementary Table S3) with PEV, AZA and VEN alone, and combinations of these agents (Figure 5A). Improved combinatorial activity was noted in the triplet combination for all patient samples when compared to VEN/AZA. In order to test whether PEV can also improve efficacy in patient samples with significant resistance to VEN/AZA in vitro, we tested four patients with in vitro resistance to the doublet. In this subset of patients, the triplet combination also demonstrated superior efficacy compared to VEN/AZA alone (Figure 5A). Western blot analysis of five primary AML patient samples treated with PEV/AZA in combination for 12 or 24 hours increased NOXA protein expression further than either drug alone, and coincided with a decrease in MCL1 protein without impacting BCL2 expression (Figure 5B). In order to validate our ex vivo findings in a patient-derived xenograft model, NSGS mice were transplanted with de novo cells from patient AML 004. Next generation sequencing of this patient’s AML revealed a complex mutational profile including mutations in FLT3- ITD, NPM1, IDH2, and DNMT3A. Chimerism was established, and 5 weeks post-transplant we began treating the mice with PEV, AZA, VEN, combination VEN/AZA (the current standard of care) or the triplet combination at subtherapeutic doses of 30 mg/kg of PEV, 1.5 mg/kg of AZA, and 15 mg/kg of VEN for 28 days. Vehicle-treated mice rapidly succumbed to leukemia by week 12 post-transplant (Figure 5C). At this point, the experiment was concluded, and tissue was harvested for evaluation of human chimerism in the bone marrow and spleen (Figure 5D). The triplet combination resulted in decreases of tumor burden in the bone marrow (0.6±0.3%) beyond any single agent treatment (P<0.05; VEN: 79.0±7.8%; PEV:
40.8±7.5%; AZA: 69.9±12.6%) or VEN/AZA alone (P<0.05; 8.5±5.8%) (Figure 5D). Staining with anti-hCD45 antibody revealed lowest AML chimerism in the group treated with the triplet combination (Figure 5E). Normal tissues were unaffected in this experiment, and combination of the three agents neither led to any significant effects on the weight of the mice (Online Supplementary Figure S4B), nor exhibited signs of stress. Ex vivo study of the triplet combination did not lead to a significant reduction in total colony formation in normal human CD34+ cells (Online Supplementary Figure S5).
Discussion
Novel drug combinations of VEN with DNMTi or LDAC, have led to dramatic improvements in responses in patients with AML.20,22 Despite these advances, some patients do not respond or will relapse on this therapy emphasizing a need to develop novel treatment strategies. We explored the impact of adding the NAE inhibitor, PEV, to the clinically relevant treatment regimen of VEN/AZA in experimental models of AML. We demonstrate that the VEN/PEV/AZA triple combination requires the induction of the BH3-only protein NOXA to enhance the kinetics and depth of apoptosis in AML cell lines in vitro when compared to other treatment variations of these drug components. These observations are reflected in vivo, where the VEN/PEV/AZA combination triplet induces durable anti-leukemic responses in systemic cell line-derived and patient-derived xenograft models of AML.
Although the addition of VEN to AZA in newly diagnosed AML patients ineligible for intensive chemotherapy improved overall survival (14.7 months VEN/AZA vs. 9.6 months AZA), a subset of AML patients present with shorter durations of response or never respond to treatment. 23,41 A previous report suggested the potential of PEV to synergize with VEN in cell lines in vitro.35 In order to build upon these observations and to explore the mechanism of this interaction, we extensively explored the potential combinatorial activity extensively with, and without AZA. VEN and AZA are effective in most AML cell lines in vitro and reflect clinical observations of diminished responses in cases of mutant TP53.34,41,42 Adding PEV to the VEN/AZA treatment paradigm enhanced cell death in AML cell lines and patient samples, consistent with our previous finding of NOXA induction in AML exposed to AZA,34 and consistent with synergistic NOXA induction with the combination of PEV and AZA. This coincided with diminished MCL1 protein which the primary target of NOXA in the mitochondria. To that end, we saw enhanced killing of AML in cell lines and patient samples in which VEN/PEV or then VEN/AZA combination activity was limited.
In order to capture the response heterogeneity of AML we tested the triple combination in a panel of primary AML samples from patients found ultimately to be refractory to conventional chemotherapy, and/or VEN/DNMTi treatment (Online Supplementary Table S3). The triplet combination was found to effectively kill the malignant AML cells in ex vivo assays and in a disseminated AML patient-derived xenograft (PDX) model (AML004). Of note, we observed ex vivo activity of all three agents in the AML003 and AML005 patient cells even though in the clinic these patients eventually failed VEN/DNMTi treatment. Considering the heterogeneity that characterizes AML and the clonal selection that occurs under existing treatment strategies that can eventually contribute to disease relapse,43,44 we speculate that the breadth of activity observed with VEN/PEV/AZA across AML cell lines and patient samples harboring differing genetic mutations may enable more durable responses in AML patients.
An additional feature of the combinatorial activity of the VEN/PEV/AZA treatment was the increased rate of caspase-3/-7 activation and the resulting AML cell death over either agent as a monotherapy or as a combination doublet. These in vitro observations translated in vivo where the VEN/PEV/AZA treatment drove durable antileukemic activity in a systemic model of OCI-AML2, with no evidence of tumor growth observed at 93 days posttreatment cessation. Utilizing a PDX model of AML, we demonstrated that the VEN/PEV/AZA combination reduced the leukemic burden within the spleen and bone marrow to a greater extent than VEN, AZA or VEN/AZA in combination. In efforts to minimize potential toxicity, we lowered the doses of each agent used in this study, which if administered alone may be sub-therapeutic. However, even at these low dosages, the VEN/PEV/AZA treatment proved safe and effective, offering an opportunity to potentially mitigate hematologic toxicity seen with azanucleosides or LDAC, and in recent combination trials with VEN.23,45,46
PMAIP1 induction is critical to the activity of AZA and PEV, and their respective synergy with VEN;34,35,39 however; the mechanism by which AZA and PEV induce PMAIP1 (NOXA) expression are distinct. PEV inhibition of NAE and the subsequent inactivation of CRL, leads to an accumulation of the CRL substrates.47 Importantly, CRL substrates include the transcription factors MYC and ATF4, which transcriptionally induce the expression of PMAIP1.35,39 Recently, we showed that AZA induced cellular stress and ATF4/NOXA through the upstream activation of the ISR pathway and eIF2a phosphorylation.34 Here, we observed that ATF4, and its transcriptional targets CHOP and NOXA were all induced by either AZA or PEV, which when combined, enhanced NOXA expression further in AML cells and was associated with elevated PARP cleavage and cell death. Deletion of PMAIP1 in this combination decreased the magnitude of apoptosis, measured by PARP cleavage, but not the ATF4/CHOP induction. This implies that NOXA, and not ATF4 or its other targets, is responsible for the ensuing apoptosis. Furthermore, deleting PMAIP1 resulted in significant decrease in cell death induced by the VEN/AZA/PEV triple combination, indicating a critical role for NOXA in the apoptosis-induction mechanism of this combination. NOXA has its greatest affinity for MCL1 over BCL-XL and BCL2, and when upregulated, serves to reduce the anti-apoptotic function of MCL1,48,49 subsequently priming cells to venetoclax-mediated apoptosis. When AZA and PEV are combined, their additive effects on NOXA induction increases the kinetics and depth of venetoclaxmediated caspase-3/-7 activation and cell death that proceeds in a BAX/BAK-dependent manner.
While treatment with VEN/AZA in patients with AML has been successful, additional therapies are needed to prevent or rescue patients from relapse of their disease. Our studies provide mechanistic insight as to how addition of PEV to VEN/AZA is synergistic and suggest that this triple combination will be a promising treatment strategy. Clinical trials, including NCT03863257 and NCT04172844, are ongoing to assess the safety and efficacy of the triplet combination in patients with AML. The question of whether sequential dosing with PEV/AZA in cases of VEN resistance, or in cases of de novo MCL1 dependence, is tenable and effective in the clinic remains, and should be explored in future studies.
Supplementary Material
Funding Statement
Funding: BNS receives support from the Department of Health and Human Services National Institutes of Health National Cancer Institute under grant number 5K12CA090625. MRS is a Leukemia and Lymphoma Society Clinical Scholar and is supported by the E.P. Evans Foundation, Adventure Allie Fund, the Biff Ruttenberg Foundation, the Beverly and George Rawlings Directorship, and NIHP30 CA068485-19.
References
- 1.Ravandi F, Ritchie EK, Sayar H, et al. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2015;16(9):1025-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes JE, Goldberg SL, Feldman EJ, et al. Phase II, multicenter, randomized trial of CPX-351 (cytarabine:Daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML. Cancer. 2015;121(2):234-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lancet JE, Cortes JE, Hogge DE, et al. Phase II, multicenter, randomized, open label trial of CPX-351 (cytarabine:daunorubicin) liposome injection versus cytarabine and daunorubicin in patients with untreated AML 60-75 years of age. Blood. 2014; 123(21):3239-3246.24687088 [Google Scholar]
- 5.Shah A, Andersson TML, Rachet B, Björkholm M, Lambert PC. Survival and cure of acute myeloid leukaemia in England, 1971-2006: a population-based study. Br J Haematol. 2013;162(4):509-516. [DOI] [PubMed] [Google Scholar]
- 6.Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019; 36:70-87. [DOI] [PubMed] [Google Scholar]
- 7.Yang E, Korsmeyer SJ. Molecular thanatopsis: A discourse on the BCL2 family and cell death. Blood. 1996;88(2):386-401. [PubMed] [Google Scholar]
- 8.Merino D, Kelly GL, Lessene G, Wei AH, Roberts AW, Strasser A. BH3-mimetic drugs: blazing the trail for new cancer medicines. Cancer Cell. 2018;34(6):879-891. [DOI] [PubMed] [Google Scholar]
- 9.Adams JM, Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25(1):27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122(4):437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Gaizo Moore V, Letai A. BH3 profilingmeasuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett. 2013; 332(2):202-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campos L, Rouault JP, Sabido O, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81(11):3091-3096. [PubMed] [Google Scholar]
- 13.Robertson LE, Plunkett W, McConnell K, Keating MJ, McDonnell TJ. Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia. 1996; 10(3):456-459. [PubMed] [Google Scholar]
- 14.Pan R, Hogdal LJ, Benito JM, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid Leukemia. Cancer Discov. 2014;4(3):362-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niu X, Wang G, Wang Y, et al. Acute myeloid leukemia cells harboring MLL fusion genes or with the acute promyelocytic leukemia phenotype are sensitive to the Bcl-2-selective inhibitor ABT-199. Leukemia. 2014;28(7):1557-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarosiek KA, Chi X, Bachman JA, et al. BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response. Mol Cell. 2013;51(6):751-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008;27(01):S149-S157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016; 6(10):1106-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for patients with untreated AML ineligible for intensive chemotherapy: phase 3 randomized placebo- controlled trial. Blood. 2020; 135(24):2137-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei AH, Strickland SA, Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: Results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. [DOI] [PubMed] [Google Scholar]
- 24.Ramsey HE, Fischer MA, Lee T, et al. A novel MCL1 inhibitor combined with venetoclax rescues venetoclax-resistant acute myelogenous Leukemia. Cancer Discov. 2018;8(12):1566-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Glytsou C, Zhou H, et al. Targeting mitochondrial structure sensitizes acute myeloid Leukemia to venetoclax treatment. Cancer Discov. 2019; 9(7):890-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nechiporuk T, Kurtz SE, Nikolova O, et al. The TP53 apoptotic network is a primary mediator of resistance to BCL2 inhibition in AML cells. Cancer Discov. 2019;9(7):910-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savona MR, Rathmell JC. Mitochondrial homeostasis in AML and gasping for response in resistance to BCL2 blockade. Cancer Discov. 2019;9(7):831-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer MA, Friedlander SY, Arrate MP, et al. Venetoclax response is enhanced by selective inhibitor of nuclear export compounds in hematologic malignancies. Blood Adv. 2020;4(3):586-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CC, Yang CF, Yang MH, et al. Pretreatment prognostic factors and treatment outcome in elderly patients with de novo acute myeloid leukemia. Ann Oncol. 2005;16(8):1366-1373. [DOI] [PubMed] [Google Scholar]
- 30.Soucy TA, Smith PG, Milhollen MA, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458(7239):732-736. [DOI] [PubMed] [Google Scholar]
- 31.Swords RT, Coutre S, Maris MB, et al. Pevonedistat, a first-in-class NEDD8-activating enzyme inhibitor, combined with azacitidine in patients with AML. Blood. 2018;131(13):1415-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nawrocki ST, Griffin P, Kelly KR, Carew JS. MLN4924: a novel first-in-class inhibitor of NEDD8-activating enzyme for cancer therapy. Expert Opin Investig Drugs. 2012;21(10):1563-1573. [DOI] [PubMed] [Google Scholar]
- 33.Khalife J, Radomska HS, Santhanam R, et al. Pharmacological targeting of miR-155 via the NEDD8-activating enzyme inhibitor MLN4924 (Pevonedistat) in FLT3- ITD acute myeloid leukemia. Leukemia. 2015;29(10):1981-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin S, Cojocari D, Purkal JJ, et al. 5- Azacitidine induces NOXA to prime AML cells for venetoclax-mediated apoptosis. Clin Cancer Res. 2020;26(13):3371-3383. [DOI] [PubMed] [Google Scholar]
- 35.Knorr KL, Schneider PA, Meng XW, et al. MLN4924 induces Noxa upregulation in acute myelogenous leukemia and synergizes with Bcl-2 inhibitors. Cell Death Differ. 2015;22(12):2133-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yadav B, Pemovska T, Szwajda A, et al. Quantitative scoring of differential drug sensitivity for individually optimized anticancer therapies. Sci Rep. 2014;4:5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ianevski A, Giri AK, Aittokallio T. SynergyFinder 2.0: visual analytics of multi-drug combination synergies. Nucleic Acids Res. 2020;48(W1):W488-W493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yadav B, Wennerberg K, Aittokallio T, Tang J. Searching for drug synergy in complex dose-response landscapes using an interaction potency model. Comput Struct Biotechnol J. 2015;13:504-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Jiang Y, Wu J, et al. NEDD8-activating enzyme inhibitor, MLN4924 (Pevonedistat) induces NOXA-dependent apoptosis through up-regulation of ATF-4. Biochem Biophys Res Commun. 2017; 488(1):1-5. [DOI] [PubMed] [Google Scholar]
- 40.Senichkin V V., Streletskaia AY, Gorbunova AS, Zhivotovsky B, Kopeina GS. Saga of Mcl-1: regulation from transcription to degradation. Cell Death Differ. 2020; 27(2):405-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chyla BJ, Harb J, Mantis C, et al. Response to venetoclax in combination with low intensity therapy (LDAC or HMA) in untreated patients with acute myeloid leukemia patients with IDH, FLT3 and other mutations and correlations with BCL2 family expression. Blood. 2019; 134(Suppl 1):S546. [Google Scholar]
- 42.Pei S, Pollyea DA, Gustafson A, et al. Monocytic subclones confer resistance to venetoclax-based therapy in patients with acute myeloid leukemia. AACR Journals.org Cancer Discov. 2020;10(4): 101-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136-1152. [DOI] [PubMed] [Google Scholar]
- 44.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(2):216-228. [DOI] [PubMed] [Google Scholar]
- 46.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J Clin Oncol. 2002; 20(10):2429-2440. [DOI] [PubMed] [Google Scholar]
- 47.Emanuele MJ, Elia AE, Xu Q, et al. Global identification of modular cullin-RING ligase substrates. Cell 2011;147(2):459-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith AJ, Dai H, Correia C, et al. Noxa/Bcl- 2 protein interactions contribute to bortezomib resistance in human lymphoid cells. J Biol Chem. 2011;286(20):17682-17692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez-Bougie P, Wuilleme-Toumi S, Menoret E, et al. Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res. 2007;67(11):5418-5424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.