Abstract
Mitochondria are critical organelles in the regulation of intrinsic apoptosis. As a general feature of blood cancers, different antiapoptotic members of the BCL-2 protein family localize at the outer mitochondrial membrane to sequester variable amounts of proapoptotic activators, and hence protect cancer cells from death induction. However, the impact of distinct anti-apoptotic members on apoptosis prevention, a concept termed anti-apoptotic dependence, differs remarkably across disease entities. Over the last two decades, several genetic and functional methodologies have been established to uncover the anti-apoptotic dependencies of the majority of blood cancers, inspiring the development of a new class of small molecules called BH3 mimetics. In this review, we highlight the rationale of targeting mitochondrial apoptosis in hematology, and provide a comprehensive map of the anti-apoptotic dependencies that are currently guiding novel therapeutic strategies. Cell-extrinsic and -intrinsic mechanisms conferring resistance to BH3 mimetics are also examined, with insights on potential strategies to overcome them. Finally, we discuss how the field of mitochondrial apoptosis might be complemented with other dimensions of precision medicine for more successful treatment of ‘highly complex’ hematologic malignancies.
Introduction
Prevention of programmed cell death is a hallmark of cancer cells and efforts to re-establish pro-death pathways have been the mainstay of research in the field of anti-cancer therapeutics.1 Among modalities of programmed cell death, apoptosis is the best characterized in terms of triggering stimuli, sequencing of biochemical events, intracellular organelles involved, and morphological changes.2 Two interconnected forms of apoptosis have been described: the extrinsic and the intrinsic (i.e., mitochondrial) pathways. The former is triggered on the cell surface by the engagement of death receptors, such as tumor necrosis factor receptor and tumor necrosis factor-related apoptosis-inducing ligand receptor, and proceeds through caspase-8/10 activation and BID cleavage.3 The latter is induced by oncogenic signaling, nutrient deprivation, genotoxic drugs and other cellular stressors, and is regulated at the level of the outer mitochondrial membrane by pro- and anti-apoptotic BCL-2 family members.4
Overview of the BCL-2 family
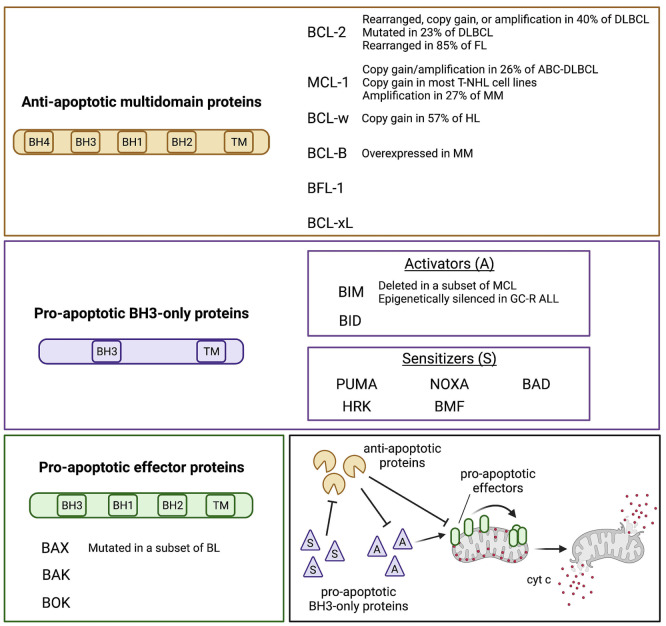
The BCL-2 family members are distinguished into three main categories: antiapoptotic, pro-apoptotic BH3-only, and pro-apoptotic effectors4 (Figure 1). BCL-2, MCL-1, BCL-w, BCL-B, BCL-xL, and BFL-1 are the main pro-survival relatives and contain all four BH domains.4,5 BID, BIM, PUMA, NOXA, BAD, HRK, and BMF are the pro-apoptotic BH3-only proteins, and only share the BH3 domain with the rest of the family. Among these, BID and BIM can directly activate the pro-apoptotic effectors, while the others act as sensitizers by antagonizing the pro-survival members.6 BAX, BAK, and BOK contain three out of four BH domains and represent the pro-apoptotic effector proteins that form homodimers and heterodimers through the outer mitochondrial membrane.7 Overall, the mitochondrial cascade of apoptosis starts when a death stimulus triggers the translocation of BH3-only proteins to the mitochondrial surface. This leads to subsequent displacement of further pro-apoptotic activators and effectors from the pro-survival members, promoting BAX/BAK assembly through the outer mitochondrial membrane. These pore-like structures provoke the cytoplasmic leakage of cytochrome c and other apoptogenic factors, which in turn activate the executioner caspases, and disrupt the mitochondrial transmembrane potential that is necessary for oxidative phosphorylation (OxPHOS).8 BH3-only proteins have different interaction modalities for each anti-apoptotic member. BAD, HRK and NOXA bind preferentially to selected anti-apoptotic members, whereas BIM, BID, PUMA and BMF interact indiscriminately with all of them. BAD binds BCL-2, BCL-w and BCL-xL, HRK binds BCL-xL, while NOXA preferentially binds MCL-1 and BFL-1.6
In hematologic malignancies, there is selective pressure for upregulating the pro-survival members via genetic and non-genetic mechanisms. For example, in both follicular lymphoma (FL) and double-hit diffuse large-B cell lymphoma (DLBCL), t(14;18) juxtaposes BCL2 to the immunoglobulin heavy chain (IGH) locus, increasing BCL-2 protein levels.9,10 In chronic lymphocytic leukemia (CLL) with del(13q), the lack of microRNA 15/16 derepresses BCL-2 expression.11 Multiple myeloma (MM) and selected subtypes of DLBCL harbor the 1q21 amplification, which leads to MCL-1 overexpression.12,13 On the other hand, pro-apoptotic BH3-only members are sometimes downregulated. BIM is epigenetically silenced in a subset of patients with acute lymphoblastic leukemia (ALL), leading to glucocorticoid resistance and poorer outcomes.14 Similarly, a subgroup of patients with mantle cell lymphoma expresses low levels of BIM and is less likely to achieve complete response to standard treatments.15 All of these mechanisms converge towards apoptosis evasion, a common denominator for cancer cells.
Figure 1.
The BCL-2 protein family. The three subgroups of the BCL-2 protein family and their recurrent genetic alterations are shown in the colored boxes. A schematic of the interactions occurring among the BCL-2 family members is represented in the lower right box, based on the “indirect activation” model. Overall, the antiapoptotic proteins sequester the pro-apoptotic BH3-only activators (A) and effectors, preventing the initiation of the apoptotic cascade. By contrast, the pro-apoptotic sensitizers (S) antagonize the anti-apoptotic members thus freeing the activators, which in turn trigger the polymerization of the effectors. This creates pore-like structures at the outer mitochondrial membrane which favor the release of cytochrome c (cyt c) and other apoptogenic factors. While the model shown here has been widely adopted to define the concept of apoptotic priming, recent evidence suggests that, at least in selected cancer types, BAX and BAK activation may only require that these members are freed from the anti-apoptotics, with no need of direct interaction with pro-apoptotic members. DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; ABC: activated B-cell; T-NHL: T-cell non-Hodgkin lymphoma; MM: multiple myeloma; HL: Hodgkin lymphoma; MCL: mantle cell lymphoma; GC-R: ALL glucocorticoid-resistant acute lymphoblastic leukemia; BL: Burkitt lymphoma.
Evasion from mitochondrial apoptosis
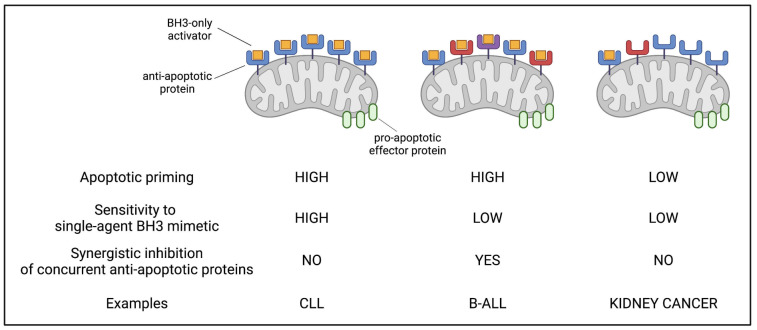
Solid tumors and hematologic malignancies evade mitochondrial apoptosis in markedly different ways (Figure 2), perhaps reminiscent of the biology of the normal tissue counterparts.16 Diseases such as kidney, colorectal, and cervical cancer show an extremely poor proclivity to activate intrinsic apoptosis, even when strong pro-apoptotic stressors are directly applied on cancer cell mitochondria. Such low apoptotic priming, defined by the near absence of BH3-only activators on the mitochondrial surface, is a major factor contributing to the chemoresistance of solid tumors, and suggests targeting solely this pathway might not be successful against these diseases.16 By contrast, mitochondria of blood cancer cells struggle to maintain the integrity of the outer mitochondrial membrane due to its occupation by several proapoptotic activators (i.e., high mitochondrial priming). In this type of cancers, apoptotic evasion is based on the activity of several anti-apoptotic proteins aiming at buffering large amounts of pro-apoptotics that dynamically shuttle between the cytoplasm and the mitochondria. 16 The need for an efficient anti-apoptotic arsenal creates a specific vulnerability that is being successfully targeted by venetoclax and other BH3 mimetics.17 This class of small molecules targets the interaction interface between the anti- and pro-apoptotic members thus allowing the latter to initiate the apoptotic cascade. While in some cases a single anti-apoptotic protein is the only barrier to apoptotic triggering, in others multiple antiapoptotic members act in concert to oppose outer mitochondrial membrane permeabilization18-20 (Figure 2).
Genetic and functional approaches to detect anti-apoptotic dependencies
Over the last 15 years, several genetic and functional methodologies have been set up to derive anti-apoptotic dependencies in cancer. Genetic knock-out of selected anti-apoptotic proteins using CRISPR-Cas9 or related gene-editing techniques have pointed out the anti-apoptotic role of MCL-1 and BCL-w in myc-driven lymphomas. 21-23 Moreover, doxycycline-inducible silencing RNA (siRNA) targeting specific pro-survival proteins has been successfully transfected in human lymphoma cell lines to evaluate which member has the greatest impact on in vitro cell survival.24 While the strength of these approaches lies in their ability to accurately inform about the role of a selected BCL-2 family gene, they are poorly applicable to primary cells from cancer patients. As their use is mostly limited to cancer cell lines, genetic approaches are precious to infer general principles of apoptotic regulation in a given cancer type, but lack scalability to large numbers of patient-derived samples. This is of relevance because some of the most common hematologic malignancies such as acute myeloid leukemia (AML) and DLBCL show heterogeneity of anti-apoptotic dependencies across patients, and perhaps even within patients over the course of their disease.19,25,26
Functional approaches provide higher scalability but less molecular insight. They are mainly based on in vitro and ex vivo pharmacological targeting of pro-survival BH3 proteins, and on BH3 profiling.27-30 Side-by-side comparisons of different BH3 mimetics targeting distinct antiapoptotic proteins have been performed on cancer cell lines and primary samples, and several readouts such as intracellular ATP content, annexin V externalization, and caspase 3/7 activation have been utilized to detect differences in cell viability.27,28 These pharmacological assays inform about the anti-apoptotic dependencies of tumor samples, working at the same time as apoptosis-tailored drug sensitivity screens with potential impact on precision strategies. Long BH3-mimetic incubation times (i.e., more than 18-24 hours), which may be needed to detect many of the cell-death readouts, is a common shortcoming of these assays as primary cells do not often survive long-term in ex vivo cultures.31 Moreover, it has been reported that in vitro-cultured primary cancer cells lose similarities with the original tumor over time,32 thus weakening the reliability of the results. Off-target effects of some BH3 mimetics, for example BCL-2-independent inhibition of OxPHOS by venetoclax,33 might be an additional limitation of these assays when the primary aim is to study the biology of the disease rather than the drug efficacy. A clinically applicable declination of such approaches, called the BH3-mimetic toolkit, has been employed on MM samples using CD138 loss as a flow cytometry readout of cancer cell death. Venetoclax (a BCL-2 inhibitor), A1155463, A1331852 (both BCL-xL inhibitors), and A1210477 (a MCL-1 inhibitor) were tested at multiple concentrations, and three dependency groups were derived in an unbiased way using analytical tools.30 BH3 profiling is a different functional technique based on exposing cancer cell mitochondria to an array of pro-apoptotic peptides with distinct binding modalities for different anti-apoptotic members. In this assay, the anti-apoptotic addiction of cancer cells can be inferred by the pattern of cytochrome c release upon peptide incubation. 34 Due to the short incubation time, BH3 profiling averts the risk of artifactual genetic selections or functional modifications that could potentially occur during prolonged ex vivo culture. In addition, the specificity of treating peptides that act directly on the mitochondrial surface renders this assay particularly focused on the BCL-2 family dynamics, without off-target effects that might instead be encountered using small molecules. A potential downside is that BH3 profiling is an organelle-centered method that does not take into account how other cellular components might react to peptide-induced cytochrome c release. For instance, defective activation of downstream cytosolic caspases, which sometimes occurs in solid tumors due to somatic mutations,35 may blunt the apoptotic response triggered by cytochrome c leakage. BH3 profiling also functions as a platform to set up an additional assay, named dynamic BH3 profiling, which measures changes in apoptotic priming and anti-apoptotic dependencies triggered by drug candidates. In this case, the incubation with pro-apoptotic peptides is preceded by ex vivo treatment with a panel of drugs, with the aim of identifying those that most efficiently lower the threshold for cytochrome c release.36,37 Dynamic BH3 profiling has proven useful to assess the impact of Bruton tyrosine kinase (BTK) inhibitors on BCL-2 dependence of CLL cells,38 and as a functional precision medicine strategy for T-cell prolymphocytic leukemia.39
Figure 2.
Different forms of evasion from mitochondrial apoptosis. Three distinct scenarios are depicted. In cancers such as chronic lymphocytic leukemia, large amounts of pro-apoptotic activators are sequestered (i.e., high priming) by a single anti-apoptotic relative. In other hematologic cancers (e.g., B-cell acute lymphoblastic leukemia), apoptotic priming is high, but the pro-apoptotics are concurrently sequestered by multiple anti-apoptotic relatives (e.g., BCL-2, MCL-1 and BCL-xL). In solid tumors, pro-apoptotic members are mostly not bound to the anti-apoptotic proteins, and hence sensitivity to BH3 mimetics is generally low. CLL: chronic lymphocytic leukemia; B-ALL: B-cell acute lymphoblastic leukemia.
The anti-apoptotic map of hematologic malignancies
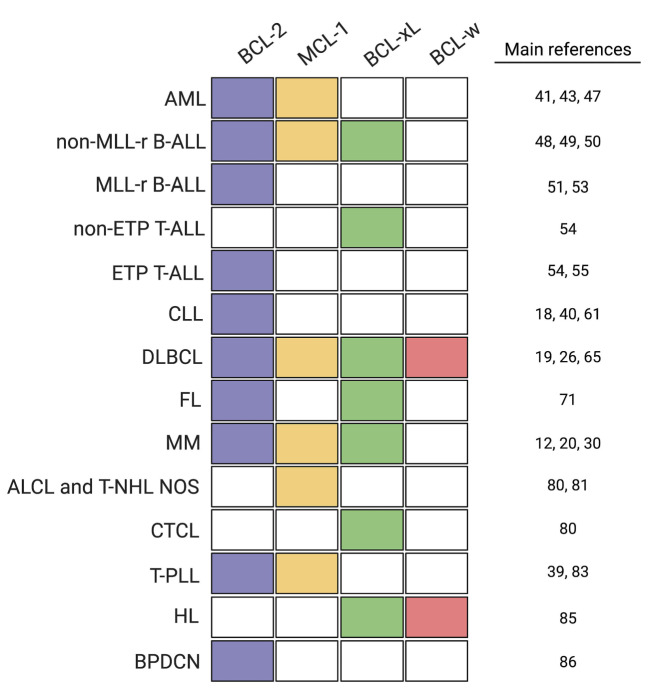
Extensive preclinical research and clinical observations are building our knowledge on how cancer cells evade apoptosis. The anti-apoptotic map of hematologic malignancies (Figure 3) has recently guided highly effective treatments for CLL and AML,40,41 and is currently inspiring novel antineoplastic regimens for other types of blood disorders. This section focuses on key preclinical findings relevant to apoptosis avoidance. Clinical results of BH3 mimetics in hematology will only be touched on, as they have been recently reviewed by Roberts and colleagues.42
Figure 3.
The anti-apoptotic map of hematologic malignancies. The colored map shows the antiapoptotic dependencies of several hematologic malignancies, based on preclinical data and clinical results (see references). With the exception of follicular lymphoma, in which the BCL-xL dependence is driven by microenvironmental stimuli, all the other anti-apoptotic dependencies depicted here refer to cell-intrinsic dependencies. AML: acute myeloid leukemia; MLL-r B-ALL: MLL-rearranged B-cell acute lymphoblastic leukemia; ETP T-ALL: early T-cell acute lymphoblastic leukemia; CLL: chronic lymphocytic leukemia; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; MM multiple myeloma; ALCL: anaplastic large T-cell lymphoma; T-NHL NOS: T-cell non- Hodgkin lymphoma not otherwise specified; CTCL: cutaneous T-cell lymphoma; T-PLL: T-cell prolymphocytic leukemia; HL: Hodgkin lymphoma; BPDCN: blastic plasmacytoid dendritic cell neoplasia.
Acute myeloid leukemia and myelodysplastic syndromes
Regulation of intrinsic apoptosis in AML shows both intratumor and interpatient heterogeneity. In 2014, Pan and co-workers identified a BCL-2 dependence for roughly 80% of primary AML cases, with rapid apoptotic triggering upon ex vivo exposure to venetoclax. BCL-2 protein expression correlated with sensitivity to venetoclax, whereas BCL-xL and, to a lesser extent MCL-1, showed anti-correlation with susceptibility to BCL-2 inhibition.43 A phase II trial evaluating the activity of venetoclax as a single agent in high-risk AML demonstrated an overall response rate of 19%, with particularly favorable responses among patients carrying isocitrate dehydrogenase 1/2 (IDH1/2) mutations.41 BH3 profiling identified patients who were more likely to stay on venetoclax therapy longer than 30 days, working as a predictive functional assay with potential clinical applications.41 Bone marrow cells from patients with high-risk myelodysplastic syndromes are also sensitive to BCL-2 antagonism. In vitro treatment with ABT-737 or venetoclax depletes the myelodysplastic syndrome progenitor compartment, and decreases the colony-forming capacity and the percentage of CD34+ cells.44 The combination of venetoclax plus intensive chemotherapy led to complete remission in 82% of patients with newly-diagnosed AML and highrisk myelodysplastic syndromes.45 Despite the meaningful clinical activity of venetoclax-based regimens in these settings, about 20% of patients are primarily refractory. Among AML patients achieving complete remission with venetoclax plus azacytidine, the median duration of response was only 11.3 months.46 This suggests that a subset of AML cases is not BCL-2 dependent, and that additional groups of patients may harbor subclonal dependencies to different anti-apoptotic proteins. A recent study addressed this point and found that BCL-2 dependence decreases through stages of AML morphological maturation.47 Indeed, lower BCL-2 expression, at both mRNA and protein levels, was observed in French- American-British (FAB) M5 AML compared to FABM0/ M1/M2 leukemia. A combination of venetoclax plus azacitidine failed to inhibit OxPHOS in monocytic AML, with a modest impact on cell viability.47 By contrast, the MCL-1 inhibitor VU661013 combined with azacitidine significantly suppressed OxPHOS in monocytic cases and was more effective than venetoclax-based treatments in inducing cell death. Genetic knockdown of MCL-1 was sufficient to trigger apoptosis in primary monocytic AML specimens, further indicating their reliance on MCL-1 to maintain survival.47 Accordingly, 62% of patients with FAB-M5 AML were refractory to venetoclax-azacitidine, whereas only 8% of non-FAB-M5 cases did not respond to this regimen.47 A side-by-side comparison of BH3 mimetics in mediating AML cell killing confirmed that a subset of immortalized and primary AML cells is highly sensitive (low micromolar/nanomolar range) to the MCL- 1 inhibitor S63845, but not to BCL-xL or BCL-2 inhibitors.27
Acute lymphoblastic leukemia
B-cell ALL shows concurrent dependence on BCL-2 and BCL-xL, with MCL-1 being identified as a further antiapoptotic member able to confer resistance to single or dual BCL-2/BCL-xL antagonism.48-50 Indeed, sensitivity to venetoclax, which was predicted by BCL2 gene expression level as well as BH3 profiling, was highly heterogeneous in a panel of B-ALL cell lines and patient-derived xenograft, with EC50 values ranging from 1.8 nM to 5.5 mM.49 Accordingly, venetoclax was effective in vivo in only a minority of B-ALL xenografts, whereas combined inhibition of BCL-2 and BCL-xL resulted in synergistic killing of most B-ALL in vivo models.51 A recently reported phase I trial of the combination of venetoclax with low-dose navitoclax (dual BCL-2/BCL-xL inhibitor) plus chemotherapy in relapsed/refractory (R/R) B-ALL has shown encouraging results, with a complete remission rate of 60%.52 Despite the heterogeneity and the high degree of anti-apoptotic co-dependencies in B-ALL, the subgroup carrying the t(4;11) translocation proved uniformly BCL-2-dependent.51,53 The fusion protein MLL/AF4 activates BCL2 transcription via DOT1L-mediated H3K79me2/3, without altering the expression level of other anti-apoptotic members. This renders MLL-rearranged cells susceptible to venetoclax-induced apoptosis in vitro, and sensitive to venetoclax-based combinations in vivo.53
The anti-apoptotic dependency of T-ALL reflects the maturation stage of lymphoblasts. Early T-cell progenitor (ETP) ALL cells most closely resemble early thymic, CD4– /CD8– T cells, and are dependent upon BCL-2.54 Instead, non-ETP T-ALL cells have a gene expression and phenotypic profile resembling that of more mature, CD4+/CD8+ T cells, expressing abundant BCL-xL protein levels, and having functional dependency upon BCL-xL.54 While ETP-ALL patient-derived xenograft models are very sensitive to venetoclax in vivo, the non-ETP counterpart is relatively resistant.54 Moreover, sensitivity to long-term BCL-2 inhibition in ETP-ALL might be compromised by microenvironment-derived signals. More specifically, the spleen has been identified as a sanctuary site for residual ETP lymphoblasts following venetoclax treatment. Such surviving cells display decreased BCL-2 expression and reduced BCL-2 dependence, with requirement of concomitant MCL-1 inhibition to evoke robust cell death.55
Chronic lymphocytic leukemia
In the light of its broad heterogeneity in terms of chromosomal aberrations, gene mutations, clinical characteristics, and drug response profiles,56-58 CLL shows surprisingly homogeneous anti-apoptotic regulation.18 More than three decades of basic discoveries have pointed out that BCL-2 is overexpressed in CLL cells compared to normal B lymphocytes due to gene promoter hypomethylation, 59 miR15/16 downregulation,11 and, more rarely, BCL- 2 translocation.60 Such genetic bases for BCL-2 addiction have been functionally confirmed by BH3 profiling, which revealed a clear-cut BCL-2 dependence of CLL cells regardless of TP53 status and previous lines of therapy.18,61 This set the stage for the clinical introduction of venetoclax, the first approved selective BCL-2 inhibitor, which achieved an overall response rate of 79% with 20% of complete remissions in the R/R CLL setting.40
While cell-intrinsic genetic programs seem to be responsible for the exceedingly high BCL-2 dependency of CLL cells, signals from the microenvironment can add further layers of anti-apoptotic protection. Indeed, anti-gen stimulation and CXCL12, both converging on BTK, decrease apoptotic priming and BCL-2 dependence.62 As a consequence, ibrutinib and other inhibitors of the B-cell receptor signaling pathway increase BCL-2 dependence by impeding extrinsic signals to upmodulate MCL-1 and BCL-xL which would ultimately favor additional pro-survival forces.38 The CLARITY study, exploring the combination of ibrutinib and venetoclax in R/R CLL, found 51% complete remissions with a high rate of minimal residual disease eradication, which enabled treatment cessation in a subset of patients.63
B-cell non-Hodgkin lymphoma
DLBCL, the most common type of B-cell non- Hodgkin lymphoma (NHL), is remarkably heterogeneous in terms of mutational landscape and clinical pictures. 64 Anti-apoptotic dependencies show heterogeneity as well, and do not correlate with cell of origin.19,26,65 Loss of the anti-apoptotic BCL-w was reported to delay MYC-driven lymphoma development in Em-Myc transgenic mice by augmenting MYC-induced apoptosis.22 Moreover, BCL-w is overexpressed in a subset of DLBCL characterized by shorter overall survival, suggesting a primary role for apoptosis evasion.22 Despite this, a recent study found that CRISPR/Cas9-mediated loss of BCL-w did not trigger apoptosis in DLBCL cell lines, nor did it increase the sensitivity to BH3 mimetics targeting additional pro-survival proteins, casting doubts about the role of BCL-w in human DLBCL.28 MYC-driven lymphomagenesis is also sustained by MCL-1, which is highly expressed in activated B-cell DLBCL.66 MCL1 copy number abnormalities were detectable in 25.7% of activated B-cell -DLBCL versus
12.5% of germinal-center DLBCL, and constitutive STAT3 signaling further contributes to MCL-1 upregulation in selected cases.66 The MCL-1 antagonist AZD5991, currently in clinical testing, curtails tumor growth and disrupts mitochondrial metabolism in MCL-1-addicted DLBCL cells via TP53- and BAXdependent mechanisms.13 BCL2 is also frequently deregulated in DLBCL due to the t(14;18) chromosomal translocation, gene mutations, copy number alterations and amplifications.67 Such genetic events confer poor prognosis especially when combined with MYC amplification. 10 While in some cases BCL2 dysregulation is associated with functional dependency on BCL-2 and high sensitivity to venetoclax, in others targeting additional pro-survival members is needed to achieve apoptosis. Indeed, several B-cell receptor-dependent DLBCL lines with genetic bases for BCL-2 dysregulation require dual targeting of PI3Ka/d, which in turns decreases MCL-1 abundance, and BCL-2 to commit cell death.68 Anti-apoptotic heterogeneity and frequent co-dependencies account for the disappointing results of singleagent venetoclax against DLBCL, with an overall response rate of only 18% in R/R cases.69 Less common subtypes of B-NHL, including follicular lymphoma and mantle cell lymphoma, display better clinical responses to BCL-2 inhibition.69,70 However, durable remissions are not frequent and may require combination therapies. At least in follicular lymphoma, in which high BCL-2 expression caused by the t(14;18) translocation has ever been considered the pathogenic hallmark, concurrent antagonism of microenvironment-induced BCLxL is needed to efficiently trigger apoptosis.71
Multiple myeloma
At least one-third of MM cases are predominantly MCL-1 dependent, whereas the others are either BCL-2 dependent or characterized by BCL-2/MCL-1 co-dependence. 12,20,30,72 Moreover, a minority of MM cell lines and primary samples show some degree of BCL-xL dependence. 30,72 MCL-1 addiction can be driven by genetic alteration and microenvironmental cues. The MCL1 gene is located on 1q21, which is amplified in 43-72% of MM patients depending on disease status.73 1q21 copy gain directly correlates with MCL1 transcript abundance and MCL-1 protein expression.12 Importantly, plasma cells from 1q21-amplified cases are especially sensitive to MCL-1 inhibition, while proving relatively resistant to venetoclax.12 Interleukin-6 released by bone marrow stromal cells can further amplify MCL-1 dependence through MCL-1 transcriptional upregulation and BIM phosphorylation. Such events switch the BIM binding partner from BCL-2 and BCL-xL to MCL-1.74,75 Blocking the interleukin- 6 signaling pathway decreases MCL-1 dependence and enhances sensitivity to venetoclax.76,77 On the other hand, BCL-2-dependent cases are enriched among MM with the t(11;14)(q13;q32) translocation, which is detected in 15% to 20% of cases.78 In the phase I study of venetoclax monotherapy in R/R MM, the overall response rate was 21% in the whole population, but reached up to 40% in the subgroup harboring t(11;14).79 High BCL2:MCL1 and BCL2:BCL2L1 mRNA expression ratios correlated with venetoclax sensitivity.79 Given the anti-apoptotic heterogeneity and the frequent co-dependencies of MM cells, dual targeting of BCL-2 and MCL-1 has been assessed in preclinical studies, showing a profound synergism potentially translatable to the clinic.77 Moreover, the BH3- mimetic toolkit revealed an increase in MCL-1 addiction from 33% at diagnosis to 69% at relapse, indicating temporal remodeling of cellular dependencies.30 This assay also identified a subset of newly diagnosed MM patients not sensitive to any of the BH3 mimetics, highlighting that apoptosis targeting might not always be a suitable therapeutic option.30
T-cell non-Hodgkin lymphoma
Protein expression analyses identified MCL-1 as the major anti-apoptotic member in cell lines and primary samples of systemic T-cell NHL.80,81 Copy number gains involving the MCL1 locus were found in ten out of 21 TNHL cell lines.80 Accordingly, loss of a single MCL1 allele delayed tumor formation in T-NHL mouse models and compromised the viability of neoplastic T cells,81 BH3 profiling confirmed that most T-NHL cell lines, especially those from anaplastic T-cell lymphomas and other peripheral T-cell lymphomas, are MCL-1 dependent.80 The development of several MCL-1 antagonists has recently allowed the translation of these biological findings into pharmacological approaches. Indeed, AZD5991 reduced tumor volumes in vivo, and synergized with cyclophosphamide, vincristine, doxorubicin and prednisone to improve survival of mice with T-NHL.80 By contrast, cutaneous T-NHL cases are primarily BCL-xL dependent, and some of them carry copy number alteration of the BCL2L1 gene.80 A proteolysis targeting chimera that targets BCL-xL for degradation has been developed to effectively kill cutaneous T-NHL cells in vitro and in vivo, without causing significant thrombocytopenia as previously reported for navitoclax.82
T-cell prolymphocytic leukemia
As compared to CLL, T-cell prolymphocytic leukemia is less primed for apoptosis, less BCL-2 dependent, and often BCL-2/MCL-1 co-dependent.39,83 BCL-xL dependence is usually more pronounced in T-cell prolymphocytic leukemia than in CLL, but remains of secondary importance when compared with BCL-2 and MCL-1 dependence.39 Clinical reports suggest that venetoclax monotherapy is often inadequate to get durable disease control.84 As demonstrated by dynamic BH3 profiling, inhibitors of histone deacetylase and the JAK/STAT pathway increase apoptotic priming and BCL-2 dependence, strengthening the pro-apoptotic effect of venetoclax in vitro and in vivo.39
Hodgkin lymphoma
As compared to normal B lymphocytes, Hodgkin lymphoma cells express higher levels of BCL-w and BCL-xL mRNA.85 BCL-w expression further increases in advanced stages and in the R/R setting.85 Fluorescence in situ hybridization analyses on Hodgkin lymphoma samples showed that copy number gains of chromosome 14 and amplifications of the BCL-w containing region were common events in the pathogenesis of Hodgkin lymphoma and led to almost invariably high BCL-w protein expression as detected by immunohistochemistry. Genetic knockdown of BCL-w or pharmacological antagonism of BCL-xL significantly reduced Hodgkin lymphoma cell viability, pointing to a primary role for these anti-apoptotic proteins in sustaining Hodgkin lymphoma growth.85
Blastic plasmacytoid dendritic cell neoplasm
Preclinical data indicate that blastic plasmacytoid dendritic cell neoplasm is primarily BCL-2 dependent. Blastic plasmacytoid dendritic cell neoplasm cells collected from patient-derived xenografts or directly from patients’ skin and bone marrow showed higher BCL-2 dependency compared with randomly selected AML cases.86 In vivo experiments confirmed these findings and, to date, several reports have been published about patients with relapsed blastic plasmacytoid dendritic cell neoplasm successfully treated with single-agent venetoclax.87,88 Such a therapeutic approach is currently being investigated in a phase I clinical trial (NCT03485547).
Resistance to BH3 mimetics: a tale of cellular interactions, mitochondrial biology and mutational pressure
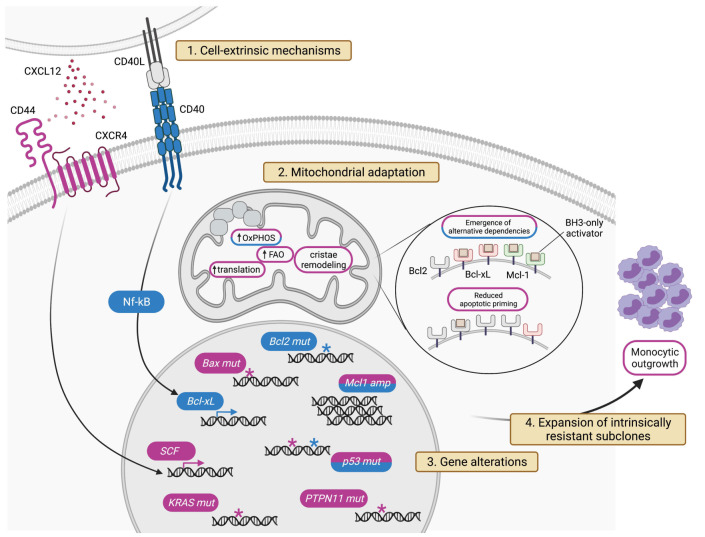
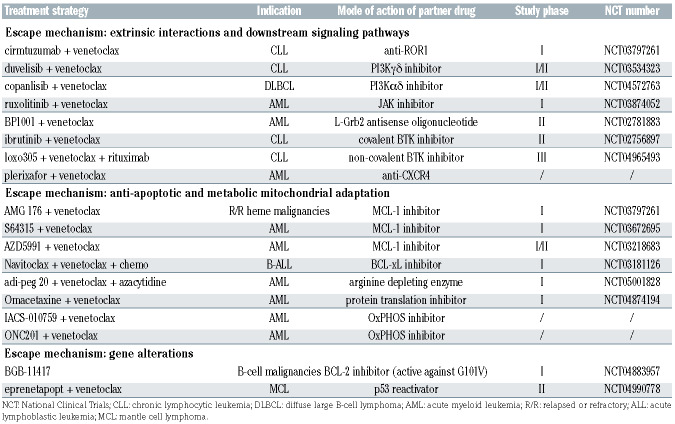
With the growing use of venetoclax in clinical practice, several resistance mechanisms have been outlined, especially in the context of CLL and AML which currently represent the major indications for BCL-2 inhibition. Three broad concepts are emerging. First, clinical progressions on venetoclax are mostly underpinned by “polyclonal patterns”, whereby different leukemic clones within the same patient take distinct paths to survive BCL-2 inhibition independent of each other.89 Secondly, the general biological principles driving resistance to one specific BH3 mimetic are shared with other BCL-2 family antagonists. Although this section is mainly focused on the mechanisms of resistance to venetoclax, the only Food and Drug Administration-approved BH3 mimetic so far, early data are piling up about similar escape trajectories occurring in cells treated with MCL-1 or BCL-xL antagonists.25 Thirdly, resistance to BH3 mimetics is highly “multimodal”, as multiple cellular components can be rewired to undermine the efficacy of BCL-2 family antagonism. The four major modalities of acquisition of resistance to venetoclax are based on cell-extrinsic interactions,90,91 mitochondrial adaptation,92 genomic alterations,89 and, as previously highlighted for the monocytic escape of AML, the emergence of intrinsically resistant clones47 (Figure 4). While such variety of resistance mechanisms contrasts with the simpler mutational evolution often observed in patients treated with kinase inhibitors,93 it offers several clues to plan at best subsequent therapies, and to design strategic drug combinations as well (Table 1).
Figure 4.
Mechanisms of venetoclax resistance. The figure depicts the four major modalities of resistance to venetoclax: cell-extrinsic mechanisms, outer and inner mitochondrial adaptation, genomic alterations, and expansion of intrinsically resistant subclones. Mechanisms highlighted in purple have been described in acute myeloid leukemia. Those highlighted in blue have been reported in chronic lymphocytic leukemia. See text for pathway details. SCF: stem cell factors; OxPHOS: oxidative phosphorylation; FAO: fatty acid oxidation.
Table 1.
Treatment strategies that may prevent/overcome escape from venetoclax based on preclinical mechanistic predictions.
Cell-extrinsic interactions
In AML, CXCL12 released by surrounding stromal cells protects the leukemic clone from BCL-2 inhibition by activating the CD44/CXCR4 complex, which in turn induces the transcription of several pro-survival embryonic stem-cell core transcription factors.90 Likewise, in CLL CD40 ligation enhances non-BCL-2 anti-apoptotic dependencies by inducing BCL-xL transcription via the canonical as well as the non-canonical NF-kB pathway.91 Induction of pro-survival members by cell-extrinsic factors may account for the relatively less durable response to venetoclax observed among CLL patients with >5 cm lymph nodes, where most of the cell-to-cell and paracrine interactions take place.40
Mitochondrial adaptations
Mitochondria are active players in the initiation of venetoclax resistance due to anti-apoptotic remodeling, occurring at the outer mitochondrial membrane, and metabolic reprogramming, occurring at the inner mitochondrial membrane. Functional shifts towards alternative antiapoptotic defenses render leukemic mitochondria progressively less vulnerable to BCL-2 antagonism, with dual BCL-2 and MCL-1 antagonism outperforming the individual targeting.25,92,94 Moreover, a global reduction of mitochondrial apoptotic priming is quite common in AML acquiring resistance to different types of BH3 mimetics.25 As apoptotic regulation is tightly connected with bioenergetic processes, the upmodulation of mitochondrial metabolic pathways confers resistance to BH3 mimetics. While in venetoclax-sensitive AML stem cells OxPHOS is suppressed through the inhibition of amino acid metabolism, in resistant clones it is restored through the enhancement of fatty acid oxidation.95 In this context, the role of BCL-2 remains unclear because venetoclax is reported to inhibit OxPHOS independently of BCL-2 expression.96 Thus, resistant cells might have evolved the ability to restore OxPHOS, bypassing the inhibitory effect of venetoclax. In addition, resistant AML stem cells show elevated nicotinamide metabolism which promotes the uptake and catabolism of amino acids, as well as the conversion of fatty acids into the tricarboxylic acid cycle intermediates 2-oxoglutarate and malate. Indeed, inhibitors of nicotinamide phosphoribosyltransferase specifically target leukemic cells with acquired resistance to venetoclax.97 The modulation of mitochondrial cristae ultrastructure also impacts on bioenergetics and BH3 mimetic sensitivity. The mitochondrial chaperonin CLPB has been recently identified as a crucial interactor of the cristae-shaping protein OPA1, and its downregulation alters cristae architecture and renders cytochrome c more prone for cytosolic release.98 An independent genome-wide CRISPR screen identified genes involved in mitochondrial translation as an additional circuit to bypass BCL-2 antagonism, and antibiotics targeting the mitochondrial ribosomes effectively overcome venetoclax resistance.99
Genomic alterations
Emergence of subclones harboring BCL2 mutations is increasingly described in CLL patients progressing on venetoclax, with the most frequent being Gly101Val and substitutions at Asp103.89 These BCL-2 structural variants decrease venetoclax binding affinity by several folds. Interestingly, BCL-2 mutants maintain the ability to bind and sequester BIM, thus preserving their fundamental role in the regulation of apoptotic balance.89 In other cases, focal amplification of MCL1 or larger chromosomal gains at 1q drive venetoclax resistance. In this context, MCL-1 overexpression takes control of the anti-apoptotic dependencies at the expense of BCL-2.92 A recent work indicates that also BAX variants, including missense, nonsense, frameshift, and splice site mutations, emerge in AML progressing on venetoclax.100 In AML cell lines, BAX but not BAK1 loss is associated with resistance to BCL-2 and MCL-1 antagonists.100 A mutation at BAX c.370 was detected in a case of mantle cell lymphoma that relapsed on venetoclax.101 In contrast to BCL2 mutations, BAX alterations function as a more downstream resistance mechanism potentially affecting sensitivity to a wider range of BH3 mimetics, with important implications when MCL-1 and BCL-xL inhibitors become available for clinical practice. Mutations in genes that do not encode BCL-2 family members have also been implicated in resistance mechanisms. Although TP53-disrupted AML and CLL cells are sensitive to acute venetoclax treatment, they are capable of escaping chronic BCL-2 inhibition, partly because of an increased threshold for BAK/BAX activation.102 Moreover, lack of TP53 activity reduces the transcription of the pro-apoptotic genes PUMA and NOXA, potentially heightening the apoptotic threshold and impairing the efficacy of individual BH3 mimetics when used at suboptimal doses or over long periods of time.102 In AML, KRAS and PTPN11 mutations also decrease sensitivity to venetoclax. Mutant KRAS downregulates BCL-2 and BAX, while it upregulates MCL-1 and BCL2A1, possibly through the activation of the NFkB pathway. Similarly, mutant PTPN11 increases the expression of BCL-xL, MCL-1 and its phosphorylated form, potentially targetable by the correspondent inhibitors.103
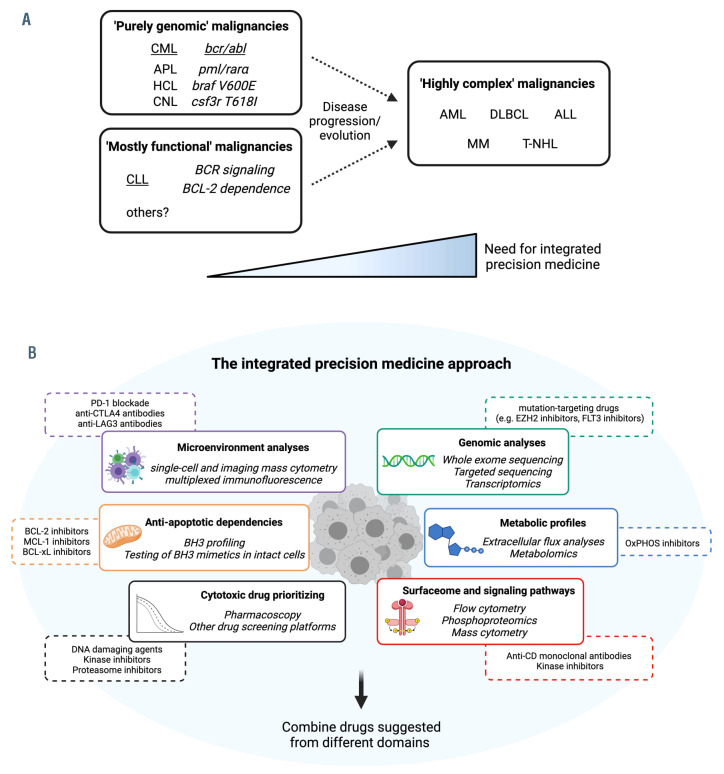
Figure 5.
Integrated precision medicine for ‘highly complex’ hematologic malignancies. (A) Classification of hematologic malignancies based on their molecular/functional complexity and treatment requirements. In chronic myeloid leukemia, the prototype of ‘purely genomic’ malignancies, a single genomic aberration drives disease biology and treatment modalities. In chronic lymphocytic leukemia, a model for ‘mostly functional’ malignancies, a few functional pathways (Bcell receptor signaling, BCL-2 dependence) sustain leukemic growth and have proven to be the best drug targets so far. In acute myeloid leukemia and other ‘highly complex’ malignancies, multiple driver mutations and functional oncogenic pathways co-occur to promote cancer growth and escape treatments. This category might be approached with an integrated precision medicine strategy. (B) Integrated precision medicine is based on interrogation of different static (i.e., microenvironment, surfaceome, genomics) and functional (i.e., anti-apoptotic and metabolic dependencies, signaling pathways, drug sensitivity) domains through dedicated assays (italics). Each of these assays will provide information about different tumor-specific vulnerabilities (e.g., BH3 profiling might highlight BCL-2 dependence, extracellular flux analysis might indicate oxidative phosphorylation utilization, microenvironment analysis might demonstrate CTLA-4-based interactions). Eventually, a combination of drugs targeting vulnerabilities from different domains is suggested, with potential benefits against ‘highly complex’ malignancies. CML: chronic myeloid leukemia; APL: acute promyelocytic leukemia; HCL: hairy cell leukemia; CNL: chronic neutrophilic leukemia; AML: acute myeloid leukemia; DLBCL: diffuse large B-cell lymphoma; ALL: acute lymphoblastic leukemia; MM: multiple myeloma; T-NHL: T-cell non-Hodgkin lymphoma; OxPHOS: oxidative phosphorylation.
Anti-apoptotic profiles at the forefront of integrated precision medicine
Until very recently, the paradigm of precision medicine in clinical oncology consisted in matching the right drug with the right patient based on a tumor’s genomic signature. Results from the NCI-MATCH trial indicated, however, that only 37.6% of enrolled patients had actionable alterations and only 17.8% were assigned to a treatment arm.104 Furthermore, resistance-conferring tumor mutations were found in 71.3% of specimens, thus lowering the chance of meaningful responses.104 Overall, the cooccurrence of multiple driver mutations, the poor ability to predict which mutation is a driver and which is a mere bystander, the complexity of resistance mechanisms, and the paucity of available drugs compared to the variety of genomic alterations limit the success rate of genomicdriven precision medicine. The emergence of mitochondrial apoptosis as a cancer vulnerability highlights that successful targets can be found outside genomic alterations, and that genomic alterations not always predict response to mitochondrial targeting. Indeed, BCL-2 rearrangements or expression level do not always correlate with venetoclax response. By contrast, functional assays were able to identify tumors that were more likely to respond to BCL-2 inhibition in the clinic.20,49 Moreover, in the context of CLL, results of BH3 profiling correlated with lymphocyte count reduction upon venetoclax initiation in vivo.61 Although the reliability of these approaches in predicting clinical endpoints is still under investigation (e.g., NCT03943342, NCT03214562, NCT03709758), it looks clear that targeting functional cell biology domains (e.g., mitochondrial apoptosis, B-cell receptor signaling, immune interactions), in addition to the static mutational repertoire, provides therapeutic benefits in hematology. Nevertheless, there should be awareness that mitochondrial apoptosis is only one of the functional domains of cancer cell biology and, for most hematologic malignancies, mitochondrial targeting alone does not provide deep and durable remissions.
Blood cancers might be currently classified into three categories based on their underpinnings and treatment requirements. The rare ‘purely genomic’ malignancies have a single genetic abnormality that almost entirely sustains the neoplastic growth. In this case, genomic-driven precision medicine is a highly effective therapeutic strategy and has already gained success. CML is the prototype of these diseases that are effectively treated with molecules targeting their genetic hallmarks.105 The ‘mostly functional’ malignancies, such as CLL, are particularly vulnerable to the targeting of selected oncogenic pathways that have been discovered through cell biology experiments rather than mutational analyses.106 While CLL cells have several mutations across their genome, the targets of the most effective drugs, such as ibrutinib and venetoclax, are never mutated.106 In this category, knowledge built on perturbation of live cells proved perhaps more useful than DNA sequencing in terms of therapeutic applications, and targeting only one functional domain at a time yields considerable results.40 The ‘highly complex’ malignancies, such as AML and DLBCL, are those in which different genetic drivers frequently co-occur, and multiple functional domains simultaneously sustain cancer cell survival.107 In this context, single-agent treatments rarely provide impressive results due to intrinsic and adaptive resistance. An integrated precision medicine approach encompassing genetic and functional testing might be needed to improve the outcome of this category, especially in the R/R setting in which tumor heterogeneity is further amplified.107 As illustrated in Figure 5, analysis on an individual basis of anti-apoptotic dependencies together with complementary static and functional measurements might help to reach this goal in the future. A prerequisite for this approach will be to design clinically applicable ex vivo assays, each with the ability to interrogate one specific domain of cancer cell biology. Because cancer cells usually maintain BAX and BAK expression, and hence are susceptible to the restoration of mitochondrial apoptosis, rational targeting of extra-mitochondrial vulnerabilities through integrated precision medicine might eventually increase apoptotic priming and enhance the effectiveness of concomitant BCL-2 family antagonism.
Concluding remarks
The clinical success of venetoclax in CLL and AML generated considerable enthusiasm on targeting apoptosis in hematology. Basic discoveries in the field will be further rewarded with the possible clinical introduction of MCL- 1 and BCL-xL inhibitors, which are currently under investigation. Different methods have been established to derive tumor-specific anti-apoptotic dependencies, with potential clinical applications. In particular, functional precision medicine platforms such as BH3 profiling and the BH3-mimetic toolkit have proven promising to prioritize apoptosis-targeting agents in a clinically appropriate timeframe. In several studies, they predicted clinical results more accurately than the expression of the BCL-2 family proteins or other static measurements. Moreover, these approaches are somehow breaking the paradigm of precision medicine as an omics-based concept, and pave the way for novel companion diagnostic assays based on ex vivo perturbation of live cells. To pursue this path, clinical and technical efforts will be needed to obtain adequate amounts of live cancer cells from patients, and to standardize ex vivo protocols aiming at minimizing interlaboratory variability. Despite the undoubted advantages that BH3 domain-related pharmacology has been providing for some hematologic malignancies, it is clear that a multitude of other genetic and functional dependencies exists in complex cancers. In such cases, different angles of cell biology need to be explored simultaneously to instruct more effective combination strategies.
Acknowledgments
This research received no external funding. The figures were created with Biorender.com.
References
- 1.Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17(7):395-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tummers B, Green DR. Caspase-8: regulating life and death. Immunol Rev. 2017;277(1):76-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25(1):65-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamouda MA, Jacquel A, Robert G, et al. BCL-B (BCL2L10) is overexpressed in patients suffering from multiple myeloma (MM) and drives an MM-like disease in transgenic mice. J Exp Med. 2016;213(9): 1705-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2(3): 183-192. [DOI] [PubMed] [Google Scholar]
- 7.Moldoveanu T, Czabotar PE. BAX, BAK, and BOK: a coming of age for the BCL-2 family effector proteins. Cold Spring Harb Perspect Biol. 2020;12(4):a036319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H, Tu HC, Ren D, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36(3):487-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devan J, Janikova A, Mraz M. New concepts in follicular lymphoma biology: from BCL2 to epigenetic regulators and non-coding RNAs. Semin Oncol. 2018;45(5-6):291-302. [DOI] [PubMed] [Google Scholar]
- 10.Riedell PA, Smith SM. Double hit and double expressors in lymphoma: definition and treatment. Cancer. 2018;124(24):4622-4632. [DOI] [PubMed] [Google Scholar]
- 11.Pekarsky Y, Balatti V, Croce CM. BCL2 and miR-15/16: from gene discovery to treatment. Cell Death Differ. 2018;25(1):21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slomp A, Moesbergen LM, Gong JN, et al. Multiple myeloma with 1q21 amplification is highly sensitive to MCL-1 targeting. Blood Adv. 2019;3(24):4202-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu T, Lam V, Thieme E, et al. Pharmacologic targeting of Mcl-1 induces mitochondrial dysfunction and apoptosis in B-cell lymphoma cells in a TP53- and BAX-dependent manner. Clin Cancer Res. 2021;27(17):4910-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachmann PS, Piazza RG, Janes ME, et al. Epigenetic silencing of BIM in glucocorticoid poor-responsive pediatric acute lymphoblastic leukemia, and its reversal by histone deacetylase inhibition. Blood. 2010;116(16): 3013-3022. [DOI] [PubMed] [Google Scholar]
- 15.Wang JD, Katz SG, Morgan EA, Yang DT, Pan X, Xu ML. Proapoptotic protein BIM as a novel prognostic marker in mantle cell lymphoma. Hum Pathol. 2019;93:54-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni Chonghaile T, Sarosiek KA, Vo TT, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334(6059): 1129-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerella C, Dicato M, Diederich M. BH3 mimetics in AML therapy: death and beyond? Trends Pharmacol Sci. 2020;41(11): 793-814. [DOI] [PubMed] [Google Scholar]
- 18.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117(1):112-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jong MRW, Langendonk M, Reitsma B, et al. Heterogeneous pattern of dependence on anti-apoptotic BCL-2 family proteins upon CHOP treatment in diffuse large B-cell lymphoma. Int J Mol Sci. 2019;20(23):6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Touzeau C, Ryan J, Guerriero J, et al. BH3 profiling identifies heterogeneous dependency on Bcl-2 family members in multiple myeloma and predicts sensitivity to BH3 mimetics. Leukemia. 2016;30(3):761-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grabow S, Delbridge AR, Aubrey BJ, Vandenberg CJ, Strasser A. Loss of a single Mcl-1 allele inhibits MYC-driven lymphomagenesis by sensitizing pro-B cells to apoptosis. Cell Rep. 2016;14(10):2337-2347. [DOI] [PubMed] [Google Scholar]
- 22.Adams CM, Kim AS, Mitra R, Choi JK, Gong JZ, Eischen CM. BCL-W has a fundamental role in B cell survival and lymphomagenesis. J Clin Invest. 2017;127(2):635-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dengler MA, Teh CE, Thijssen R, et al. Potent efficacy of MCL-1 inhibitor-based therapies in preclinical models of mantle cell lymphoma. Oncogene. 2020;39(9):2009-2023. [DOI] [PubMed] [Google Scholar]
- 24.Kelly GL, Grabow S, Glaser SP, et al. Targeting of MCL-1 kills MYC-driven mouse and human lymphomas even when they bear mutations in p53. Genes Dev. 2014;28(1):58-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatt S, Pioso MS, Olesinski EA, et al. Reduced mitochondrial apoptotic priming drives resistance to BH3 mimetics in acute myeloid leukemia. Cancer Cell. 2020;38(6): 872-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith VM, Dietz A, Henz K, et al. Specific interactions of BCL-2 family proteins mediate sensitivity to BH3-mimetics in diffuse large B-cell lymphoma. Haematologica. 2020;105(8):2150-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ewald L, Dittmann J, Vogler M, Fulda S. Side-by-side comparison of BH3-mimetics identifies MCL-1 as a key therapeutic target in AML. Cell Death Dis. 2019;10(12):917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diepstraten ST, Chang C, Tai L, et al. BCLW is dispensable for the sustained survival of select Burkitt lymphoma and diffuse large B-cell lymphoma cell lines. Blood Adv. 2020;4(2):356-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan J, Montero J, Rocco J, Letai A. iBH3: simple, fixable BH3 profiling to determine apoptotic priming in primary tissue by flow cytometry. Biol Chem. 2016;397(7):671-678. [DOI] [PubMed] [Google Scholar]
- 30.Gomez-Bougie P, Maiga S, Tessoulin B, et al. BH3-mimetic toolkit guides the respective use of BCL2 and MCL1 BH3-mimetics in myeloma treatment. Blood. 2018;132(25): 2656-2669. [DOI] [PubMed] [Google Scholar]
- 31.Samson DJ, Seidenfeld J, Ziegler K, Aronson N. Chemotherapy sensitivity and resistance assays: a systematic review. J Clin Oncol. 2004;22(17):3618-3630. [DOI] [PubMed] [Google Scholar]
- 32.Shah N, Oseth L, Tran H, Hirsch B, LeBien TW. Clonal variation in the B-lineage acute lymphoblastic leukemia response to multiple cytokines and bone marrow stromal cells. Cancer Res. 2001;61(13):5268-5274. [PubMed] [Google Scholar]
- 33.Pollyea DA, Stevens BM, Jones CL, et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med. 2018;24(12):1859-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Gaizo Moore V, Letai A. BH3 profiling-- measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett. 2013;332(2):202-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghavami S, Hashemi M, Ande SR, et al. Apoptosis and cancer: mutations within caspase genes. J Med Genet. 2009;46(8):497-510. [DOI] [PubMed] [Google Scholar]
- 36.Montero J, Sarosiek KA, DeAngelo JD, et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell. 2015;160(5):977-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montero J, Letai A. Dynamic BH3 profilingpoking cancer cells with a stick. Mol Cell Oncol. 2016;3(3):e1040144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng J, Isik E, Fernandes SM, Brown JR, Letai A, Davids MS. Bruton's tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia. Leukemia. 2017;31(10):2075-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herbaux C, Kornauth C, Poulain S, et al. BH3 profiling identifies ruxolitinib as a promising partner for venetoclax to treat Tcell prolymphocytic leukemia. Blood. 2021;137(25):3495-3506. [DOI] [PubMed] [Google Scholar]
- 40.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts AW, Wei AH, Huang DCS. BCL2 and MCL1 inhibitors for hematologic malignancies. Blood. 2021;138(13):1120-1136. [DOI] [PubMed] [Google Scholar]
- 43.Pan R, Hogdal LJ, Benito JM, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4(3):362-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jilg S, Reidel V, Müller-Thomas C, et al. Blockade of BCL-2 proteins efficiently induces apoptosis in progenitor cells of highrisk myelodysplastic syndromes patients. Leukemia. 2016;30(1):112-123. [DOI] [PubMed] [Google Scholar]
- 45.Kadia TM, Reville PK, Borthakur G, et al. Venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a cohort from a singlecentre, single-arm, phase 2 trial. Lancet Haematol. 2021;8(8):e552-e561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pei S, Pollyea DA, Gustafson A, et al. Monocytic subclones confer resistance to venetoclax-based therapy in patients with acute myeloid leukemia. Cancer Discov. 2020;10(4):536-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood. 2008;111(4): 2300-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seyfried F, Demir S, Horl RL, et al. Prediction of venetoclax activity in precursor B-ALL by functional assessment of apoptosis signaling. Cell Death Dis. 2019;10(8):571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alford SE, Kothari A, Loeff FC, et al. BH3 inhibitor sensitivity and Bcl-2 dependence in primary acute lymphoblastic leukemia cells. Cancer Res. 2015;75(7):1366-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khaw SL, Suryani S, Evans K, et al. Venetoclax responses of pediatric ALL xenografts reveal sensitivity of MLLrearranged leukemia. Blood. 2016;128(10): 1382-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pullarkat VA, Lacayo NJ, Jabbour E, et al. Venetoclax and navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov. 2021;11(6):1440-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benito JM, Godfrey L, Kojima K, et al. MLLrearranged acute lymphoblastic leukemias activate BCL-2 through H3K79 methylation and are sensitive to the BCL-2-specific antagonist ABT-199. Cell Rep. 2015;13(12): 2715-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chonghaile TN, Roderick JE, Glenfield C, et al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov. 2014;4(9):1074-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Grande A, Peirs S, Donovan PD, et al. The spleen as a sanctuary site for residual leukemic cells following ABT-199 monotherapy in ETP-ALL. Blood Adv. 2021;5(7):1963-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moia R, Patriarca A, Schipani M, Gaidano G. The biology of chronic lymphocytic leukemia: diagnostic and prognostic implications. Cancer J. 2021;27(4):266-274. [DOI] [PubMed] [Google Scholar]
- 57.Gohil SH, Wu CJ. Dissecting CLL through high-dimensional single-cell technologies. Blood. 2019;133(13):1446-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mavridou D, Psatha K, Aivaliotis M. Proteomics and drug repurposing in CLL towards precision medicine. Cancers (Basel). 2021;13(14):3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanada M, Delia D, Aiello A, Stadtmauer E, Reed JC. Bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993;82(6):1820-1828. [PubMed] [Google Scholar]
- 60.Fang H, Reichard KK, Rabe KG, et al. IGH translocations in chronic lymphocytic leukemia: clinicopathologic features and clinical outcomes. Am J Hematol. 2019;94(3):338-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson MA, Deng J, Seymour JF, et al. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood. 2016;127(25):3215-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davids MS, Deng J, Wiestner A, et al. Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood. 2012;120(17):3501-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hillmen P, Rawstron AC, Brock K, et al. Ibrutinib plus venetoclax in relapsed/refractory chronic lymphocytic leukemia: the CLARITY study. J Clin Oncol. 2019;37(30): 2722-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morin RD, Arthur SE, Hodson DJ. Molecular profiling in diffuse large B-cell lymphoma: why so many types of subtypes? Br J Haematol. 2022;196(4):814-829. [DOI] [PubMed] [Google Scholar]
- 65.Rys RN, Wever CM, Geoffrion D, et al. Apoptotic blocks in primary non-Hodgkin B cell lymphomas identified by BH3 profiling. Cancers (Basel). 2021;13(5):1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wenzel SS, Grau M, Mavis C, et al. MCL1 is deregulated in subgroups of diffuse large Bcell lymphoma. Leukemia. 2013;27(6):1381-1390. [DOI] [PubMed] [Google Scholar]
- 67.Klanova M, Klener P. BCL-2 Proteins in pathogenesis and therapy of B-cell non- Hodgkin lymphomas. Cancers (Basel). 2020;12(4):938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bojarczuk K, Wienand K, Ryan JA, et al. Targeted inhibition of PI3Kalpha/delta is synergistic with BCL-2 blockade in genetically defined subtypes of DLBCL. Blood. 2019;133(1):70-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non- Hodgkin lymphoma. J Clin Oncol. 2017;35(8):826-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davids MS, Roberts AW, Kenkre VP, et al. Long-term follow-up of patients with relapsed or refractory non-Hodgkin lymphoma treated with venetoclax in a phase I, first-in-human study. Clin Cancer Res. 2021;27(17):4690-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Serrat N, Guerrero-Hernandez M, Matas-Cespedes A, et al. PI3Kdelta inhibition reshapes follicular lymphoma-immune microenvironment cross talk and unleashes the activity of venetoclax. Blood Adv. 2020;4(17):4217-4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Punnoose EA, Leverson JD, Peale F, et al. Expression profile of BCL-2, BCL-XL, and MCL-1 predicts pharmacological response to the BCL-2 selective antagonist venetoclax in multiple myeloma models. Mol Cancer Ther. 2016;15(5):1132-1144. [DOI] [PubMed] [Google Scholar]
- 73.Hanamura I, Stewart JP, Huang Y, et al. Frequent gain of chromosome band 1q21 in plasma-cell dyscrasias detected by fluorescence in situ hybridization: incidence increases from MGUS to relapsed myeloma and is related to prognosis and disease progression following tandem stem-cell transplantation. Blood. 2006;108(5):1724-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wuilleme-Toumi S, Robillard N, Gomez P, et al. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia. 2005;19(7):1248-1252. [DOI] [PubMed] [Google Scholar]
- 75.Gupta VA, Matulis SM, Conage-Pough JE, et al. Bone marrow microenvironment-derived signals induce Mcl-1 dependence in multiple myeloma. Blood. 2017;129(14):1969-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang M, Wu D, Liu P, Deng J. Silence of MCL-1 upstream signaling by shRNA abrogates multiple myeloma growth. Exp Hematol Oncol. 2014;3(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Algarin EM, Diaz-Tejedor A, Mogollon P, et al. Preclinical evaluation of the simultaneous inhibition of MCL-1 and BCL-2 with the combination of S63845 and venetoclax in multiple myeloma. Haematologica. 2020; 105(3):e116-e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Touzeau C, Maciag P, Amiot M, Moreau P. Targeting Bcl-2 for the treatment of multiple myeloma. Leukemia. 2018;32(9):1899-1907. [DOI] [PubMed] [Google Scholar]
- 79.Kumar S, Kaufman JL, Gasparetto C, et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130(22):2401-2409. [DOI] [PubMed] [Google Scholar]
- 80.Koch R, Christie AL, Crombie JL, et al. Biomarker-driven strategy for MCL1 inhibition in T-cell lymphomas. Blood. 2019;133(6):566-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spinner S, Crispatzu G, Yi JH, et al. Re-activation of mitochondrial apoptosis inhibits T-cell lymphoma survival and treatment resistance. Leukemia. 2016;30(7):1520-1530. [DOI] [PubMed] [Google Scholar]
- 82.He Y, Koch R, Budamagunta V, et al. DT2216-a Bcl-xL-specific degrader is highly active against Bcl-xL-dependent T cell lymphomas. J Hematol Oncol. 2020;13(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith VM, Lomas O, Constantine D, et al. Dual dependence on BCL2 and MCL1 in Tcell prolymphocytic leukemia. Blood Adv. 2020;4(3):525-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kornauth C, Herbaux C, Boidol B, et al. Rationale for the combination of venetoclax and ibrutinib in T-prolymphocytic leukemia. Haematologica. 2021;106(8):2251-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adams CM, Mitra R, Vogel AN, Liu J, Gong JZ, Eischen CM. Targeting BCL-W and BCLXL as a therapeutic strategy for Hodgkin lymphoma. Leukemia. 2020;34(3):947-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Montero J, Stephansky J, Cai T, et al. Blastic plasmacytoid dendritic cell neoplasm is dependent on BCL2 and sensitive to venetoclax. Cancer Discov. 2017;7(2):156-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beziat G, Ysebaert L, Gaudin C, Steinmeyer Z, Balardy L. Venetoclax to treat relapsed blastic plasmacytoid dendritic cell neoplasm: a case-report and review of literature. Leuk Res. 2019;85:106199. [DOI] [PubMed] [Google Scholar]
- 88.Agha ME, Monaghan SA, Swerdlow SH. Venetoclax in a patient with a blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 2018;379(15):1479-1481. [DOI] [PubMed] [Google Scholar]
- 89.Blombery P, Thompson ER, Nguyen T, et al. Multiple BCL2 mutations cooccurring with Gly101Val emerge in chronic lymphocytic leukemia progression on venetoclax. Blood. 2020;135(10):773-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu X, Munoz-Sagredo L, Streule K, et al. CD44 loss of function sensitizes AML cells to the BCL-2 inhibitor venetoclax by decreasing CXCL12-driven survival cues. Blood. 2021;138(12):1067-1080. [DOI] [PubMed] [Google Scholar]
- 91.Haselager M, Thijssen R, West C, et al. Regulation of Bcl-XL by non-canonical NFkappaB in the context of CD40-induced drug resistance in CLL. Cell Death Differ. 2021;28(5):1658-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guieze R, Liu VM, Rosebrock D, et al. Mitochondrial reprogramming underlies resistance to BCL-2 inhibition in lymphoid malignancies. Cancer Cell. 2019;36(4):369-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bixby D, Talpaz M. Mechanisms of resistance to tyrosine kinase inhibitors in chronic myeloid leukemia and recent therapeutic strategies to overcome resistance. Hematology Am Soc Hematol Educ Program. 2009;461-476. [DOI] [PubMed] [Google Scholar]
- 94.Haselager MV, Kielbassa K, Ter Burg J, et al. Changes in Bcl-2 members after ibrutinib or venetoclax uncover functional hierarchy in determining resistance to venetoclax in CLL. Blood. 2020;136(25):2918-2926. [DOI] [PubMed] [Google Scholar]
- 95.Stevens BM, Jones CL, Pollyea DA, et al. Fatty acid metabolism underlies venetoclax resistance in acute myeloid leukemia stem cells. Nat Cancer. 2020;1(12):1176-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roca-Portoles A, Rodriguez-Blanco G, Sumpton D, et al. Venetoclax causes metabolic reprogramming independent of BCL-2 inhibition. Cell Death Dis. 2020;11(8):616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jones CL, Stevens BM, Pollyea DA, et al. Nicotinamide metabolism mediates resistance to venetoclax in relapsed acute myeloid leukemia stem cells. Cell Stem Cell. 2020;27(5):748-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen X, Glytsou C, Zhou H, et al. Targeting mitochondrial structure sensitizes acute myeloid leukemia to venetoclax treatment. Cancer Discov. 2019;9(7):890-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharon D, Cathelin S, Mirali S, et al. Inhibition of mitochondrial translation overcomes venetoclax resistance in AML through activation of the integrated stress response. Sci Transl Med. 2019;11(516): eaax2863. [DOI] [PubMed] [Google Scholar]
- 100.Moujalled DM, Brown FC, Pomilio G, et al. Acquired mutations in BAX confer resistance to BH3 mimetics in acute myeloid leukemia. Blood. 2020;136(Suppl 1):7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thompson ER, Nguyen T, Kankanige Y, et al. High clonal complexity of resistance mechanisms occurring at progression after single-agent targeted therapy strategies in chronic lymphocytic leukemia. Blood. 2020;136(Suppl 1):15-16. [Google Scholar]
- 102.Thijssen R, Diepstraten ST, Moujalled D, et al. Intact TP-53 function is essential for sustaining durable responses to BH3-mimetic drugs in leukemias. Blood. 2021;137(20): 2721-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang H, Nakauchi Y, Kohnke T, et al. Integrated analysis of patient samples identifies biomarkers for venetoclax efficacy and combination strategies in acute myeloid leukemia. Nat Cancer. 2020;1(8):826-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Flaherty KT, Gray RJ, Chen AP, et al. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: National Cancer Institute Molecular Analysis for Therapy Choice (NCIMATCH). J Clin Oncol. 2020;38(33):3883-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hehlmann R. Chronic myeloid leukemia in 2020. Hemasphere. 2020;4(5):e468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Letai A. Functional precision cancer medicine- moving beyond pure genomics. Nat Med. 2017;23(9):1028-1035. [DOI] [PubMed] [Google Scholar]
- 107.Adashek JJ, Subbiah V, Kurzrock R. From tissue- agnostic to N-of-one therapies: (r)evolution of the precision paradigm. Trends Cancer. 2021;7(1):15-28. [DOI] [PubMed] [Google Scholar]